Abstract
In articular cartilage-repair, grafts usually fuse unsatisfactorily with surrounding host cartilage. Enzymatic dissociation of cartilaginous matrix to free chondrocytes may benefit fusion. We tested such a hypothesis with human cartilage in vitro, and with porcine cartilage in vivo. Human articular cartilage was collected from knee surgeries, cut into disc-and-ring sets, and randomly distributed into three groups: disc-and-ring sets in Group 1 were left untreated; in Group 2 only discs, and in Group 3 both discs and rings were treated with enzyme. Each disc-and-ring reassembly was cultured in a perfusion system for 14 days; expression of cartilage marker proteins and genes was evaluated by immunohistochemistry and PCR. Porcine articular cartilage from knees was similarly fashioned into disc-and-ring combinations. Specimens were randomly distributed into a control group without further treatment, and an experimental group with both disc and ring treated with enzyme. Each disc-and-ring reassembly was transplanted into subcutaneous space of a nude mouse for 30 days, and retrieved to examine disc-ring interface. In in vitro study with human cartilage, a visible gap remained at disc-ring interfaces in Group 1, yet became indiscernible in Group 2 and 3. Marker genes, including type II collagen, aggrecan and Sox 9, were well expressed by chondrocytes in all specimens, indicating that chondrocytes’ phenotype retained regardless of enzymatic treatment. Similar results were found inin vivo study with porcine cartilage. Enzymatic dissociation of cartilaginous matrix promotes fusion of adjacent cartilage. The clinical relevance may be a novel method to facilitate integration of repaired cartilage in joints.
Keywords: Cartilage repair, Cartilaginous matrix, Enzymatic treatment, Cartilage fusion
Abbreviations: cDNA, complementary deoxyribonucleic acid; DMMB, 1,9-dimethyl methylene blue; DNA, deoxyribonucleic acid; GAG, glycosaminoglycan; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; H&E, hematoxylin and eosin; PBS, phosphate-buffered saline; PCR, polymerase chain reaction; RNA, ribonucleic acid; Sox 9, SRY-box transcription factor 9
Highlights
-
•
Cartilage repair-patches fuse poorly to surrounding host cartilage.
-
•
Collagenase treatment of adjacent cartilaginous tissues facilitates their fusion.
-
•
Collagenase treatment of cartilage promotes chondrocyte proliferation and presentation.
-
•
Collagenase treatment does not affect phenotypes of chondrocytes.
1. Introduction
The fact that articular cartilage repairs poorly after injury has not changed much since its discovery in the 19th century. Surgical repair of focal defects of the articular surface has been suggested to prevent subsequent catastrophic osteoarthritis. Untreated lesions in weight bearing joints such as the knee impair quality of life as much as severe osteoarthritis. The World Health Organization has reported osteoarthritis as the highest-ranking disease among the musculoskeletal diseases [1,2]. Although many surgical modalities have been advocated, those that use cartilaginous grafts to replace the defects have yielded better clinical outcomes.
The primary goal in repairing focal cartilaginous defects is to restore a smooth articular surface. If the defect is repaired by patching it with a cartilage graft, the surface of the patch is expected to flush well with the surrounding native cartilage without humping, dimpling or leaving a gap in between. It is therefore important to ensure fusion between the repair and the native cartilage [[3], [4], [5]]. However, one of the major obstacles in current techniques of cartilage repair is the unsatisfactory integration of the implanted with the host tissues [6,7]. Mosaicplasty using an osteochondral autograft to replace the cartilage defect was once reported to yield a good clinical outcome, but these transplants were later found to have no host-graft healing at all [8]. Newer clinical techniques using engineered cartilage as the repair material have also encountered the same difficulty with incomplete integration, resulting in a split between the matrix-based autologous chondrocyte implantation and the host cartilage at one year postoperation [9].
The reasons for such poor integration of cartilaginous tissue remains unclear. Articular cartilage is an avascular tissue composed of chondrocytes embedded in a dense extracellular matrix [10]. The chondrocytes are securely confined in the matrix with minimal motility, making it difficult, if not impossible, for them to migrate to the boundary area and bond the implanted cartilage with the surrounding host cartilage. The avascular nature of cartilage precludes a primary inflammatory process to occur at the junctional region, indicating that remodeling of the matrix may not happen. Many studies have investigated various methods to promote fusion between the cartilaginous matrix of the adjacent cartilage. Peretti et al. used isolated chondrocytes and devitalized cartilage to bond adjacent cartilage in animal models [11,12]. Pabbruwe et al. also relied on colonization of chondrocytes at the cartilaginous interface, using a collagen membrane as a scaffold, to induce integration. However, these procedures are complicated and may be impractical for clinical application.
The findings of previous studies attempting to fuse adjacent cartilage suggest that loosening the matrix and recruiting free chondrocytes around the boundary region might be the key for integration of cartilaginous tissue. The rationale is to create an environment similar to tissue-remodeling induced by the inflammatory process during healing. We therefore designed an enzyme-treatment model to enzymatically dissociate the matrix of cartilage implants, and hypothesized that such treatment would benefit the fusion of the implanted cartilage with the native host-cartilage.
2. Materials and methods
This study was conducted in the corresponding author's hospital after approval by its Research Ethics Review Committee. The protocol for the animal experiments was approved by the Institutional Animal Experiment Committee of the hospital.
2.1. Articular cartilage collection and experimental design
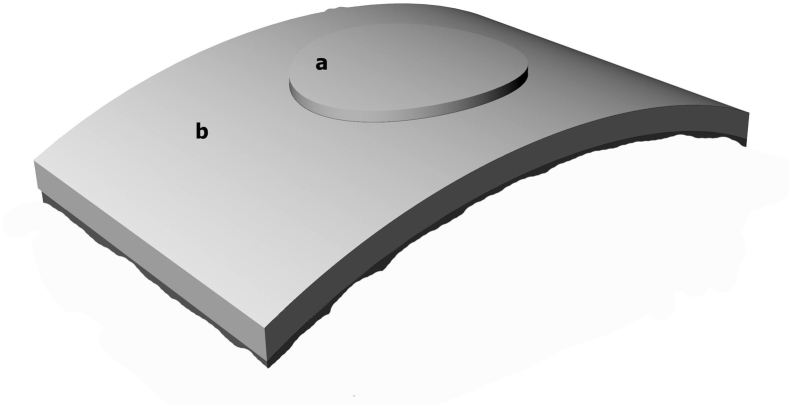
The first part of this study used human cartilage-samples and in vitro cultivation. Specimens of human articular cartilage were collected from osteoarthritic knees during total prosthetic arthroplasty. A total of 8 patients were involved, all of whom signed an informed consent before the surgery. Full-thickness cartilage flake was collected from the spared portion of the operated knee. A 5-mm disc was cut from its center, forming 2 parts including the disk and the remainder of the flake as an outer ring (Fig. 1). Specimens were randomly and evenly distributed into three groups. The specimens in Group 1 served as the control, receiving no enzymatic treatment. Only the discs in Group 2, and both the discs and rings in Group 3, were enzymatically treated with immersion in 0.64 mg/mL collagenase (Liberase, Roche Diagnostics GmbH, Germany) for 20 min at 37 °C. All specimens were rinsed thoroughly with Hank's Balanced Salt Solution (HBSS, Life Technologies, Inc., Gaithersburg, MD). The disc and the ring from the same flake were re-assembled. Half of the assembled specimens in each group were retained for prompt assays; the other specimens were cultured for 14 days in a perfuse culture system [13] containing Dulbecco modified Eagle's medium (DMEM, Life Technologies, Inc., Gaithersburg, MD) supplemented with 10% fetal bovine serum (FBS, Gibco, Grand Island, NY) at 37 °C with a perfusion rate of 10 mL/min. The sample sizes were three in each group. The cartilage at the disc-ring interface was examined, including histochemical staining for the quantitation of glycosaminoglycan (GAG) content and real-time RT-PCR for the expression of marker genes for cartilage.
Fig. 1.
Schematic diagrams of disc-ring assembly used to test for fusion between the two components. At the time of examination, assembled specimens were sectioned along the diameter of the disc for light microscopy. a = disc part, b = ring part.
The second part of this study used porcine cartilage-samples and in vivo cultivation. Full-thickness cartilage specimens were harvested aseptically from the knee joints of one-year-old adult pigs in a laminar flow workstation and cut into a disc-and-ring configuration as aforementioned (Fig. 1). Half of the specimens were retained as the control group, in which the disk and ring of each set were re-assembled immediately without additional treatment. The other specimens served as the experimental group, in which both the disc and the ring of the same set were enzymatically treated, and washed thoroughly with HBSS similarly as for the human specimens. The sample sizes are three in each group. Each set of assembled specimens was buried in the subcutaneous space on the back of a 20-week-old nude mouse under anesthesia with 0.1 mL Zoletil-50 (VIRBAC, France) and 0.1 mL Xylazine (10 mg/mL, SigmaAldrich, St. Louis, MO) per 100 g body-weight. The specimens were retrieved after 30 days, and the tissue at the disc-ring interface was examined by histochemical staining.
2.2. RNA extraction and PCR
Total RNA was extracted from examined tissue with Trizol reagent (Invitrogen, Life Technologies, Gaithersburg, MD). Briefly, the lysates of the cartilaginous samples were extracted with chloroform, and the total RNA was precipitated with isopropanol. The concentration of total RNA was measured by absorbance at 260 nm 2 μg of total RNA were reverse transcribed using oligo (dT)20 as the primer and reverse transcriptase (Invitrogen, Life Technologies, Gaithersburg, MD). The amount of cDNA was measured by polymerase chain reaction (PCR). The first-strand cDNA product was subjected to PCR using oligonucleotide primer pairs for type I collagen, type II collagen, aggrecan, Sox 9, and GAPDH as the housekeeping gene (Table 1). The PCR protocol included an initial denaturation at 95 °C for 3 min, followed by 25 cycles of 94 °C for 30 s, 55 °C for 30 s and 72 °C for 45 s. The products were analyzed by electrophoresis in 1% agarose gel containing ethidium bromide and visualized with an ultraviolet camera.
Table 1.
Primer pairs for the polymerase chain reaction (PCR) assay.
| Target gene | Sequences of primer |
|---|---|
| Type I collagen | sense- ATGCTCAGCTTTGTGGATACG |
| antisense- CAGCAGGTCCTTGGAAACCTT | |
| Type II collagen | sense-CAACCAGATTGAGAGCATCC |
| antisense-TGTTTCGTGCAGCCATCCTT | |
| Aggrecan | sense-CAGTGCCATCATTGC |
| antisense-TCCTTGTCTCCATAGC | |
| Sox 9 | sense-ATCTGAAGAAGGAGAGCGAG |
| antisense-TCAGAAGTCTCCAGAGCTTG | |
| GAPDH | sense-ACCACAGTCCATGCCATCAC |
| antisense-TCCACCACCCTGTTGCTGTA |
2.3. Quantitation of cell numbers and GAG
The cell numbers were measured by total DNA content assay. The amount of GAG in the specimens was measured by the 1,9-dimethyl methylene blue (DMMB) assay [1]. Immediately after the enzymatic treatment, half of the specimens were retained as the Day 0 sample and sent for assay without further culture. The other specimens were cultured in a perfusion culture system for 14 days as aforementioned before they were collected for the assay. Specimens subjected to analysis were washed with phosphate-buffered saline (PBS) and digested with papain overnight at 60 °C. The amount of DNA in a sample was measured by absorbance at 260/280 nm for normalization. After centrifugation, the supernatant was collected for incubation with DMMB and was shaken for 30 min at room temperature. After centrifugation, the supernatant was discarded and the lysate was incubated in decomposing solution containing 50 mM sodium acetate solution buffered with 10% propan-1-ol and shaken for 30 min at room temperature. Absorbance was measured at 656 nm.
2.4. Histochemical staining
The specimens were fixed in 4% paraformaldehyde/PBS, sequentially dehydrated in grade-alcohol and embedded in paraffin. Sections of 5-μm thickness were obtained from the paraffin block, de-paraffinized with xylene, sequentially rehydrated in grade-alcohols, stained with hematoxylin and eosin (H&E), and viewed under light-microscope.
2.5. Data analysis
Quantitative data were analyzed with the Student's t-test. The p values < 0.05 were regarded statistically significant.
3. Results
3.1. Gene expression
The marker genes for hyaline cartilage, including the type II collagen, aggrecan and Sox 9 genes, were expressed by cells of all three groups. The marker gene for fibrous cartilage, specifically the type I collagen gene, was expressed at comparable levels among all three groups. GAPDH mRNA expression levels were assessed by standard PCR (Fig. 2).
Fig. 2.
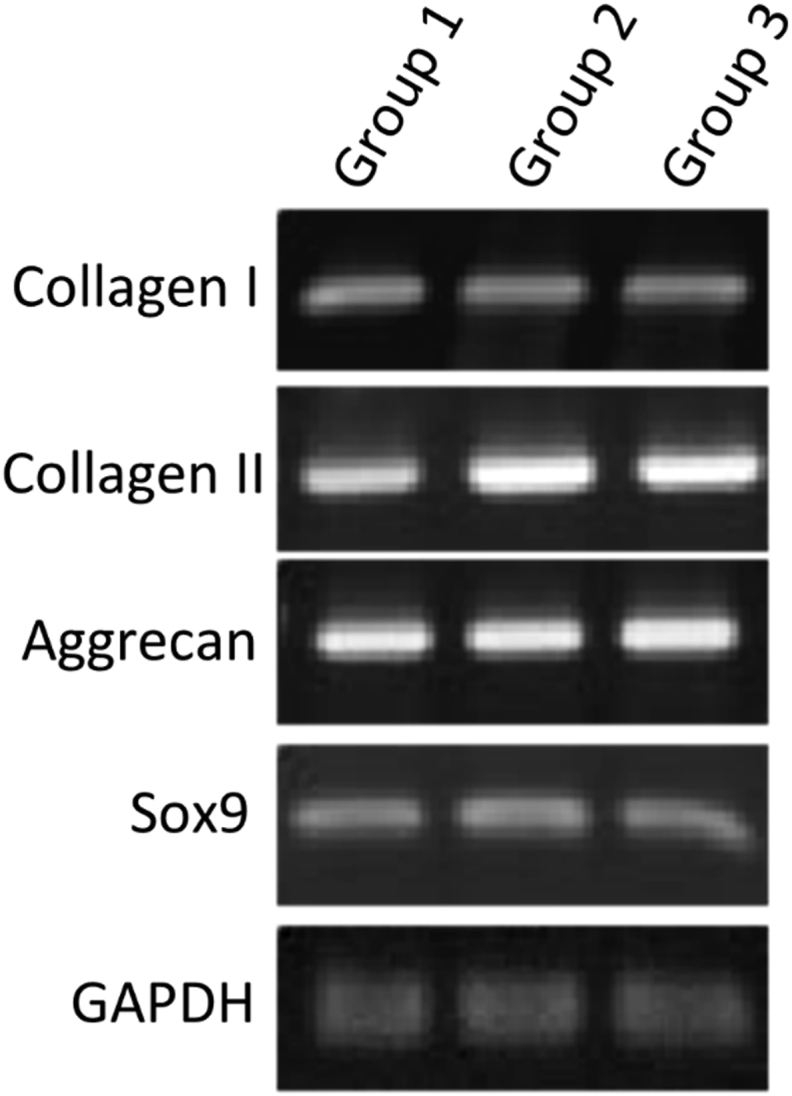
Characterization of cartilage marker-gene expression. Relative gene expression of cartilage markers was monitored by PCR: specimens in Group 1 were not enzymatically treated, specimens in Group 2 had only the disc part treated with enzyme, and specimens in Group 3 had both the disc and ring parts treated. The hyaline cartilage markers included type II collagen, aggrecan and Sox 9. Type I collagen served as the fibrous cartilage marker and was monitored concomitantly. The expressions of GAPDH mRNA were assessed by standard RT-PCR.
3.2. Cell proliferation and GAG content in cartilage
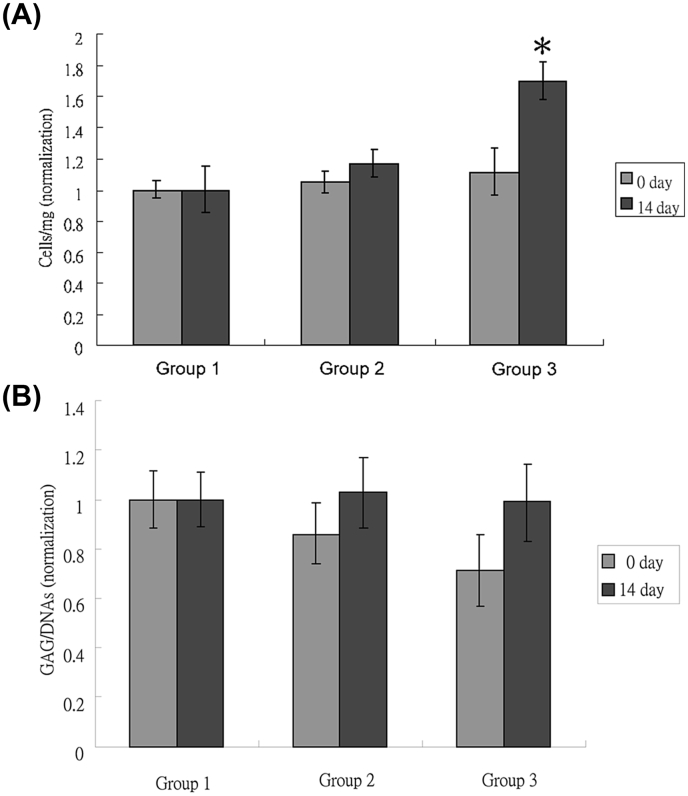
The cell proliferation assay was done with determination of the cell numbers in each cartilage sample by DNA content. The cell numbers of Group 1 had similar equity on day 0 and on day 14. The cell numbers in Group 2 were insignificantly higher on day 14 than on day 0. The cell numbers in Group 3 was significantly higher on day 14 than on day 0 (Fig. 3A).
Fig. 3.
(A) The cell numbers in the cartilage samples were measured by DNA content assay (B) The content of GAG in the cartilage samples was measured by DMMB colorimetric assay, and was normalized by DNA content (n = 3, p > 0.05). After the enzymatic treatment, the cartilage samples were harvested before cultivation (0 day) and after 14 days of cultivation in a perfusion culture system (14 day).
The total GAG content in each cartilage sample was measured by DMMB colorimetric assay. The content in Group 1 remained constant on day 0 and on day 14 without a significant change. The contents in Group 2 and 3 were both less than that in Group 1 on day 0, whereas both increased to be comparable with that in Group 1 on day14 (Fig. 3B).
3.3. Interface of gap
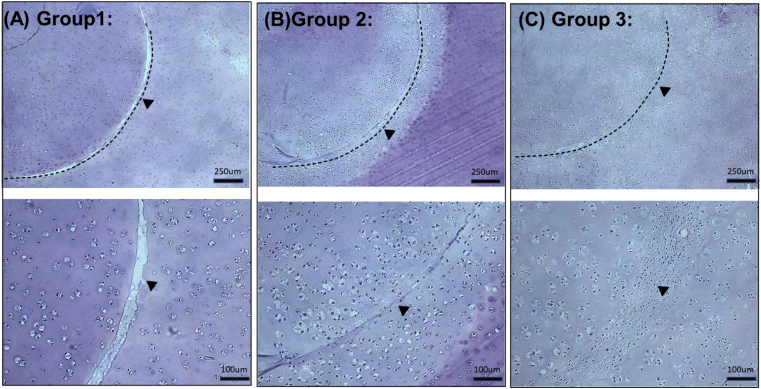
The disc-ring interfaces of human specimens after 14 days of in vitro culturing were observed with H&E stain under light microscopy. The disk-ring gaps were easily identified visually in the specimens of Group 1, and were narrower in the specimens of Group 2. In the Group 3 specimens the discs fused well with the rings without an visually identifiable fissure (Fig. 4).
Fig. 4.
The light microscopic appearance of the disc-ring interface after 14 days of in vitro culturing (human specimen). Dotted lines and arrows indicate the original disc-ring junctions (A) Group 1 (B) Group 2 (C) Group 3.
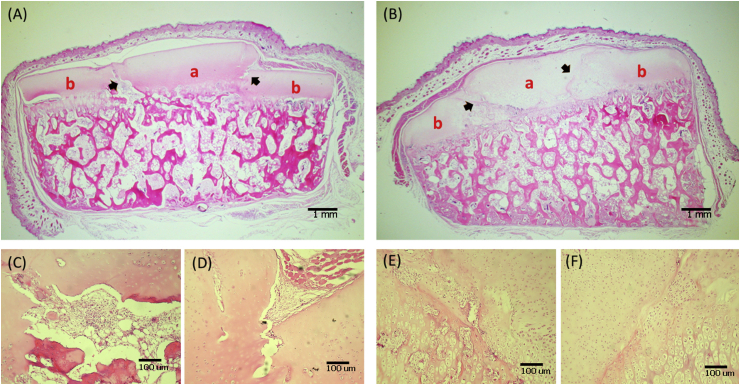
The disc-ring interfaces of the porcine specimens were similarly inspected after 30 days of in vivo culturing. The specimens in the control group had a significantly visible gap in the interface, whereas the tissues of specimens in the experimental group fused to an integrity without identifiable interface (Fig. 5).
Fig. 5.
The light-microscopic appearance of the disc-ring interface after 30 days of in vivo culturing (porcine specimen) (A) Control group (B) Experimental group (C, D) Close-up view of the junctional regions (arrows) in (A) (E, F) close-up view of the junctional regions (arrows) in (B). a = disc part, b = ring part.
4. Discussion
Repair of articular cartilage-defect is an unmet clinical need for its poor healing capability [14]. The current standard of treatment for such defects is primarily repair by autograft or allograft transplantation using engineered cartilage fragments, scaffolds or cells, including chondrocytes and mesenchymal stem cells [[15], [16], [17]]. In order to constitute a functional joint with a smooth articular surface, the surface of the repaired cartilage must be flush and continuous with the surrounding host cartilage, without any step or gap.
The extracellular matrix serves as the fundamental biomechanical underpinnings of cartilage, and provides biological support to the chondrocytes housed within [18,19]. It works as a reservoir for nutrients, growth factors, cytokines, and modulators for the maintenance and turnover of chondrocytes, and also as the physical scaffolding that shapes the tissue and provides strong shelter around the chondrocytes [19,20]. However, the fibrous matrix also confines the chondrocytes from migrating within the tissue [10]. Additionally, the cartilage is an avascular tissue and therefore lacks the inflammatory process evoked by the blood cells [21]. Consequently, both the host and the implanted cartilage have minimal potential to remodel, and thus cannot fuse properly without intervention. A possible solution would be the release of chondrocytes, either of the host or of the implanted cartilage, from the matrix, which would then be re-constituted by the free chondrocytes.
A study by Krych et al. [[3], [4], [5]] about the osteochondral defect in a rabbit model showed that the long term digestion might have been slightly beneficial; however, there was no new matrix generated and therefore no good connection occurred between the implanted gelatin sponge and the native cartilage. In our experiments, the extracellular matrix of the cartilage was not completely digested; instead, it was dissociated only in order to mobilize the chondrocytes. The chondrocytes were not insulted by the enzymatic treatment and preserved their phenotype, whereas the gross structure of the matrix remained intact as we observed under microscopy. The protocol for such treatment was optimized in a provisional experiment, in which the optimal time for enzymatic exposure was verified by courses of different length. The depth of matrix-dissociation after 40-min exposure to the enzyme was only 1.06 folds of that after 20-min exposure; all chondrocytes remained alive after either time-course of treatment (data not shown) [13]. In the present study, the implant (represented by the disk) and the native (represented by the ring) cartilage would be fuse well using the 20-min regimen that the cartilaginous matrix was only partial digested by collagenase. The partial digestion was supposed to loosed the extracellular matrix of cartilage without affecting the phenotypes of the chondrocytes and might promote the chondrocytes to secret more the extracellular matrix to fuse the gap.
Articular cartilage is composed of simply chondrocytes within the extracellular matrix [22]. The matrix plays an important role in regulating chondrocyte-functions—through cell–matrix interaction, organization of the cytoskeleton, and integrin-mediated signaling—to maintain cartilage homeostasis [23]. The composition and structure of the matrix change during the progression of osteoarthritis due to many factors. Such change of the matrix affects the biology of the resident chondrocytes [22,24]. Type II collagen is the major collagenous constituent of the articular cartilage, whereas aggrecan is a cartilage-specific proteoglycan that combines with hyaluronan via link proteins to form large GAG aggregates [10,22]. Sox 9 is a nuclear transcription factor and is the earliest marker expressed by chondrocytes undergoing the process of condensation during chondrogenesis [25]. The expression of Sox9 requires the presence of cartilage-specific matrix proteins, primarily collagen II and other matrix proteins, including collagen IX, X, and XI and cartilage-derived retinoic acid-sensitive protein [26]. Collagen I is abundant in human tissues such as skin, tendon and bone, as well as in tissues surrounding internal organs [27,28] yet not in the articular cartilage. The abnormally excessive presentation of collagen I the chondrocytes may indicate the transition of these cells from chondrogenic to fibroblastic [29]. The chondrocytes in our specimens remained chondrogenic throughout the experiments regardless of the enzymatic treatment, as shown by their preserved ability to express the cartilage marker proteins, specifically collagen II, aggrecan and Sox9 (Fig. 2). Whether the enzyme did not affect the chondrocytes at all or the time of exposure was not long enough, was not investigated. However, all chondrocytes expressed collagen I in similar amounts, also regardless of enzymatic treatment.
The extracellular matrix is produced by cells and it, in return, provides structural and biochemical support to the cells [20,22]. The articular cartilage is a hypocellular tissue with the volumetric ratio of the matrix to the resident chondrocytes more than 10:1 [23,30,31]. The matrix also constitutes the mechanical functions of articular cartilage. GAG is abundant in the extracellular matrix of articular cartilage, and changes in the GAG content significantly alter the physical properties of the cartilage [10,22,31]. We monitored the cell numbers and GAG contents in the matrix during the remodeling process when fusion was taking place. The cell numbers in both groups after partial enzyme-digestion significantly increased from day 0 to day 14. The fluctuation of GAG content in the matrix did not change the phenotype of the chondrocytes. Enzymatic dissociation caused the matrix to loose its GAG network, but such loss was recovered by the chondrocytes (Fig. 3).
The repair of defects in articular cartilage has been challenging in clinical practice. Unsatisfactory outcomes obtained after the first technique initially introduced in the late 1980's has evoked the development of new techniques [32,33]. The modern strategies usually involve patching the defects with autologous chondral or osteochondral grafts or with regenerated neocartilage [[34], [35], [36]]. To restore joint function by means of these techniques, the patch has to fill the defect with its surface flush with the gross articular surface, without any raised or lowered areas, and to integrate with the surrounding host cartilage without a gap. Such integration between the graft with the native cartilage, in order to constitute a smooth, continuous articulating surface, has been regarded as a critical factor in many scoring systems to evaluate the outcome of cartilage repair [37], including the Visual Histological Score for Cartilage Repair proposed by the International Cartilage Repair Society [38]. The results of our experiment show that enzymatic dissociation of the matrix is necessary for the grossly contacted cartilage to histologically fuse. The matrix-remodeling process, which includes degradation, deposition and other modification of the matrix-components, is the primary determinant of fusion at the interface [39]. We found that enzymatic treatment induced the remodeling process of the matrix, resulting in components of the matrix being lost and recovered (Fig. 3). The tissues fused better when both sides of the interface were treated enzymatically than when only one side was treated (Fig. 4). Because the phenotype of the chondrocytes was not altered by the treatment and the molecules in the matrix did not link spontaneously, a reasonable explanation for the matrix-fusion is that it resulted from the remodeling process governed by the chondrocytes, which were the only cells around (Fig. 2, Fig. 4, Fig. 5). Although the matrix lost some GAG during enzymatic dissociation, the GAG amount recovered along with the later synthesis of refilling substrate by the chondrocytes (Fig. 3). Further studies about the details of such remodeling of the matrix would be of interest.
One concern of translating our enzymatic dissociation to the clinical setting would be the potential damage to the native cartilage by the enzyme. Because our technique is intended to be applied on the repair of focal cartilage defects by means of patching them with natural or engineered cartilaginous grafts, a simple devise is being designed to contain the enzyme solution at the defect site. It resembles an oxygen mask that can cover the cartilage defect including the patching graft during surgery, avoiding the contact of the enzyme solution with the surrounding native cartilage. Besides, the time of treatment was optimized in our earlier study to minimize possible adverse effects by the enzyme to the treated cartilage [13]. Longer exposure to the enzyme may cause the cartilaginous matrix to breakdown extensively and results in the loss of the chondrocytes at large consequently.
To accelerate the integration of the regenerative cartilage and native cartilage is a critical issue of postoperative evaluation. The partial enzyme digestion may enhance the fusion of the transplanted and the native cartilage by facilitating the remodeling of extracellular matrix. It most likely works not only by loosing the extracellular matrix of cartilage but also by stimulating the cell proliferation.
5. Conclusions
We conclude that enzymatic dissociation of cartilaginous tissue facilitates its fusion with the adjacent cartilage. Such fusion, it is hoped, will be of clinical relevance in repairing cartilage defects by implantation of chondral grafts or engineered neocartilage.
Declaration of competing interest
The authors have declared that they have no conflict of interest.
Acknowledgements
This research was supported by the Ministry of Science & Technology, Taiwan under Grant 101-2314-B-002-111-MY2. We thank the staff in the Second Core Lab, Department of Medical Research, National Taiwan University Hospital for their technical support during this study.
Footnotes
Peer review under responsibility of the Japanese Society for Regenerative Medicine.
Contributor Information
Ching-Chuan Jiang, Email: ccj@ntu.edu.tw.
Chang-Hsun Hsieh, Email: wolf0220@gmail.com.
Chun-Jen Liao, Email: cjliao@twbm.com.tw.
Wen-Hsiang Chang, Email: wenhsiang@twbm.com.tw.
Wei-Ju Liao, Email: weijuliao@twbm.com.tw.
Jyy-Jih Tsai-Wu, Email: jjtsai@ntuh.gov.tw.
Hongsen Chiang, Email: hongsen@ntu.edu.tw.
References
- 1.Bitton R. The economic burden of osteoarthritis. Am J Manag Care. 2009;15(8 Suppl):S230–S235. [PubMed] [Google Scholar]
- 2.Felson D.T. Clinical practice. Osteoarthritis of the knee. N Engl J Med. 2006;354(8):841–848. doi: 10.1056/NEJMcp051726. [DOI] [PubMed] [Google Scholar]
- 3.Niethammer T.R., Limbrunner K., Betz O.B., Gulecyuz M.F., Pietschmann M.F., Feist M. Analysis of the autologous chondrocyte quality of matrix-based autologous chondrocyte implantation in the knee joint. Int Orthop. 2016;40(1):205–212. doi: 10.1007/s00264-015-2825-4. [DOI] [PubMed] [Google Scholar]
- 4.Lee Y.H., Suzer F., Thermann H. Autologous matrix-induced chondrogenesis in the knee: a review. Cartilage. 2014;5(3):145–153. doi: 10.1177/1947603514529445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vedicherla S., Buckley C.T. Cell-based therapies for intervertebral disc and cartilage regeneration - current concepts, parallels and perspectives. J Orthop Res. 2016:8–22. doi: 10.1002/jor.23268. [DOI] [PubMed] [Google Scholar]
- 6.Doran P.M. Cartilage tissue engineering: what have we learned in practice? Methods Mol Biol. 2015;1340:3–21. doi: 10.1007/978-1-4939-2938-2_1. [DOI] [PubMed] [Google Scholar]
- 7.Wang C.C., Yang K.C., Lin K.H., Liu Y.L., Yang Y.T., Kuo T.F. Expandable scaffold improves integration of tissue engineered cartilage: an in vivo study in a rabbit model. Tissue Eng. 2016:873–884. doi: 10.1089/ten.TEA.2015.0510. [DOI] [PubMed] [Google Scholar]
- 8.Lane J.G., Massie J.B., Ball S.T., Amiel M.E., Chen A.C., Bae W.C. Follow-up of osteochondral plug transfers in a goat model: a 6-month study. Am J Sports Med. 2004;32(6):1440–1450. doi: 10.1177/0363546504263945. [DOI] [PubMed] [Google Scholar]
- 9.Trattnig S., Ba-Ssalamah A., Pinker K., Plank C., Vecsei V., Marlovits S. Matrix-based autologous chondrocyte implantation for cartilage repair: noninvasive monitoring by high-resolution magnetic resonance imaging. Magn Reson Imaging. 2005;23(7):779–787. doi: 10.1016/j.mri.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 10.Sophia Fox A.J., Bedi A., Rodeo S.A. The basic science of articular cartilage: structure, composition, and function. Sport Health. 2009;1(6):461–468. doi: 10.1177/1941738109350438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Peretti G.M., Randolph M.A., Caruso E.M., Rossetti F., Zaleske D.J. Bonding of cartilage matrices with cultured chondrocytes: an experimental model. J Orthop Res. 1998;16(1):89–95. doi: 10.1002/jor.1100160115. [DOI] [PubMed] [Google Scholar]
- 12.Peretti G.M., Zaporojan V., Spangenberg K.M., Randolph M.A., Fellers J., Bonassar L.J. Cell-based bonding of articular cartilage: an extended study. J Biomed Mater Res. 2003;64(3):517–524. doi: 10.1002/jbm.a.10367. [DOI] [PubMed] [Google Scholar]
- 13.Liao C.J., Lin Y.J., Chiang H., Chiang S.F., Wang Y.H., Jiang C.C. Injecting partially digested cartilage fragments into a biphasic scaffold to generate osteochondral composites in a nude mice model. J Biomed Mater Res. 2007;81(3):567–577. doi: 10.1002/jbm.a.31035. [DOI] [PubMed] [Google Scholar]
- 14.Minas T. A primer in cartilage repair. J Bone Joint Surg Br. 2012;94(11 Suppl A):141–146. doi: 10.1302/0301-620X.94B11.30679. [DOI] [PubMed] [Google Scholar]
- 15.Triche R., Mandelbaum B.R. Overview of cartilage biology and new trends in cartilage stimulation. Foot Ankle Clin. 2013;18(1):1–12. doi: 10.1016/j.fcl.2012.12.001. [DOI] [PubMed] [Google Scholar]
- 16.Woodfield T.B., Bezemer J.M., Pieper J.S., van Blitterswijk C.A., Riesle J. Scaffolds for tissue engineering of cartilage. Crit Rev Eukaryot Gene Expr. 2002;12(3):209–236. doi: 10.1615/critreveukaryotgeneexpr.v12.i3.40. [DOI] [PubMed] [Google Scholar]
- 17.Monteiro N., Martins A., Reis R.L., Neves N.M. Nanoparticle-based bioactive agent release systems for bone and cartilage tissue engineering. Regen Ther. 2015;1:109–118. doi: 10.1016/j.reth.2015.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wilusz R.E., Sanchez-Adams J., Guilak F. The structure and function of the pericellular matrix of articular cartilage. Matrix Biol. 2014;39:25–32. doi: 10.1016/j.matbio.2014.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kwon H., Paschos N.K., Hu J.C., Athanasiou K. Articular cartilage tissue engineering: the role of signaling molecules. Cell Mol Life Sci. 2016;73(6):1173–1194. doi: 10.1007/s00018-015-2115-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gentili C., Cancedda R. Cartilage and bone extracellular matrix. Curr Pharmaceut Des. 2009;15(12):1334–1348. doi: 10.2174/138161209787846739. [DOI] [PubMed] [Google Scholar]
- 21.Smith C.A., Richardson S.M., Eagle M.J., Rooney P., Board T., Hoyland J.A. The use of a novel bone allograft wash process to generate a biocompatible, mechanically stable and osteoinductive biological scaffold for use in bone tissue engineering. J Tissue Eng Regen Med. 2015;9(5):595–604. doi: 10.1002/term.1934. [DOI] [PubMed] [Google Scholar]
- 22.Pearle A.D., Warren R.F., Rodeo S.A. Basic science of articular cartilage and osteoarthritis. Clin Sports Med. 2005;24(1):1–12. doi: 10.1016/j.csm.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 23.Gao Y., Liu S., Huang J., Guo W., Chen J., Zhang L. The ECM-cell interaction of cartilage extracellular matrix on chondrocytes. BioMed Res Int. 2014;2014:648459. doi: 10.1155/2014/648459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maldonado M., Nam J. The role of changes in extracellular matrix of cartilage in the presence of inflammation on the pathology of osteoarthritis. BioMed Res Int. 2013;2013:284873. doi: 10.1155/2013/284873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goldring M.B., Tsuchimochi K., Ijiri K. The control of chondrogenesis. J Cell Biochem. 2006;97(1):33–44. doi: 10.1002/jcb.20652. [DOI] [PubMed] [Google Scholar]
- 26.Lefebvre V., Smits P. Transcriptional control of chondrocyte fate and differentiation. Birth Defects Res C Embryo Today. 2005;75(3):200–212. doi: 10.1002/bdrc.20048. [DOI] [PubMed] [Google Scholar]
- 27.Shoulders M.D., Raines R.T. Collagen structure and stability. Annu Rev Biochem. 2009;78:929–958. doi: 10.1146/annurev.biochem.77.032207.120833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Prockop D.J., Kivirikko K.I. Collagens: molecular biology, diseases, and potentials for therapy. Annu Rev Biochem. 1995;64:403–434. doi: 10.1146/annurev.bi.64.070195.002155. [DOI] [PubMed] [Google Scholar]
- 29.Marlovits S., Hombauer M., Tamandl D., Vecsei V., Schlegel W. Quantitative analysis of gene expression in human articular chondrocytes in monolayer culture. Int J Mol Med. 2004;13(2):281–287. [PubMed] [Google Scholar]
- 30.Cheng A., Hardingham T.E., Kimber S.J. Generating cartilage repair from pluripotent stem cells. Tissue Eng B Rev. 2014;20(4):257–266. doi: 10.1089/ten.teb.2012.0757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tatari H. The structure, physiology, and biomechanics of articular cartilage: injury and repair. Acta Orthop Traumatol Turcica. 2007;41(Suppl 2):1–5. [PubMed] [Google Scholar]
- 32.Erggelet C., Vavken P. Microfracture for the treatment of cartilage defects in the knee joint - a golden standard? J Clin Orthop Trauma. 2016;7(3):145–152. doi: 10.1016/j.jcot.2016.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gracitelli G.C., Moraes V.Y., Franciozi C.E., Luzo M.V., Belloti J.C. Surgical interventions (microfracture, drilling, mosaicplasty, and allograft transplantation) for treating isolated cartilage defects of the knee in adults. Cochrane Database Syst Rev. 2016;9:CD010675. doi: 10.1002/14651858.CD010675.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Richter D.L., Schenck R.C., Jr., Wascher D.C., Treme G. Knee articular cartilage repair and restoration techniques: a review of the literature. Sport Health. 2016;8(2):153–160. doi: 10.1177/1941738115611350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Richter D.L., Tanksley J.A., Miller M.D. Osteochondral autograft transplantation: a review of the surgical technique and outcomes. Sports Med Arthrosc. 2016;24(2):74–78. doi: 10.1097/JSA.0000000000000099. [DOI] [PubMed] [Google Scholar]
- 36.Ikawa T., Yano K., Watanabe N., Masamune K., Yamato M. Non-clinical assessment design of autologous chondrocyte implantation products. Regen Ther. 2015;1:98–108. doi: 10.1016/j.reth.2015.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Niemeyer P., Pestka J.M., Kreuz P.C., Erggelet C., Schmal H., Suedkamp N.P. Characteristic complications after autologous chondrocyte implantation for cartilage defects of the knee joint. Am J Sports Med. 2008;36(11):2091–2099. doi: 10.1177/0363546508322131. [DOI] [PubMed] [Google Scholar]
- 38.Mainil-Varlet P., Aigner T., Brittberg M., Bullough P., Hollander A., Hunziker E. Histological assessment of cartilage repair: a report by the histology endpoint committee of the international cartilage repair society (ICRS) J Bone Joint Surg Am. 2003;85-A(Suppl 2):45–57. [PubMed] [Google Scholar]
- 39.Lu P., Takai K., Weaver V.M., Werb Z. Extracellular matrix degradation and remodeling in development and disease. Cold Spring Harb Perspect Biol. 2011;3(12):a005058. doi: 10.1101/cshperspect.a005058. [DOI] [PMC free article] [PubMed] [Google Scholar]