Abstract
Skeletal muscle injuries have bothered doctors and caused great burdens to the public medical insurance system for a long time. Once injured, skeletal muscles usually go through the processes of inflammation, repairing and remodeling. If repairing and remodeling stages are out of balance, scars will be formed to replace injured skeletal muscles. At present, clinicians usually use conventional methods to restore the injured skeletal muscles, such as flap transplantation. However, flap transplantation sometimes needs to sacrifice healthy autologous tissues and will bring extra harm to patients. In recent years, stem cells-based tissue engineering provides us new treatment ideas for skeletal muscle injuries. Stem cells are cells with multiple differentiation potential and have ability to differentiate into adult cells under special condition. Skeletal muscle tissues also have stem cells, called satellite cells, but they are in small amount and new muscle fibers that derived from them may not be enough to replace injured fibers. Bone marrow mesenchymal stem cells (BM-MSCs) could promote musculoskeletal tissue regeneration and activate the myogenic differentiation of satellite cells. Biomaterial is another important factor to promote tissue regeneration and greatly enhance physiological activities of stem cells in vivo. The combined use of stem cells and biomaterials will gradually become a mainstream to restore injured skeletal muscles in the future. This review article mainly focuses on the review of research about the application of BM-MSCs and several major biomaterials in skeletal muscle regeneration over the past decades.
Keywords: BM-MSCs, Skeletal muscle injury, Differentiation, Biomaterial, Tissue regeneration
Abbreviations: ASCs, adipose stem cells; BDNF, brain derived neurotrophic factor; BM-MSCs, bone marrow mesenchymal stem cells; CREB, cAMP- response element binding protein; DPSCs, dental pulp stem cells; ECM, extracellular matrix; ECs, endothelial cells; EGF, epidermal growth factor; FGF, fibroblast growth factor; FGF-2, fibroblast growth factor-2; GCSF, granulocyte colony-stimulating factor; GDNF, glial derived neurotrophic factor; GPT, gelatin-poly(ethylene glycol)- tyramine; HGF, hepatocyte growth factor; IGF-1, insulin-like growth factor-1; IL, interleukin; LIF, leukemia inhibitory factor; MRF, myogenic muscle factor; NSAIDs, non-steroidal drugs; PDGF-BB, platelet derived growth factor-BB; PGE2, prostaglandin E2; PRP, platelet rich plasma; S1P, sphingosine 1-phosphate; SDF-1, stromal cell derived factor-1; TGF-β, transforming growth factor-β; 3D-ECM, three dimensional extracellular matrix; TrkB, tyrosine kinaseB; VML, volumetric muscle loss; VEGF, vascular endothelial growth factor
1. Introduction
Skeletal muscles are important tissues and account for 35~45% of our body weights [1]. The contraction and relaxation of skeletal muscles can be adjusted by self-consciousness, which is different from smooth muscles and myocardium. According to the characteristics of contraction and relaxation, skeletal muscle fibers can be divided into three types: (1) type I fibers contract slow and have anti-fatigue effect; (2) type 2A fibers contract fast and have intermediate anti-fatigue effect; (3) type 2B fibers contract fast and have the worst anti-fatigue effect [2]. Satellite cells are special type of cells in these tissues and reside beneath the basal lamina [[3], [4], [5]]. Normally satellite cells are in resting states and express various specific markers, such as homeobox genes Pax3, Pax7 and myogenic regulatory factor (MRF) gene Myf5 etc. [6,7]. Satellite cells are able to self-renew and replace injured muscle fibers through asymmetric differentiation [[8], [9], [10]].
The incidence of skeletal muscle injury is about 10–55% and it is one of the main types of chronic soft tissue injuries [11]. Various degrees of muscle injuries can be found in patients who suffer from impacts, traffic accidents, stabbings, or overtraining [[12], [13], [14], [15]]. It should be noted that severe types of skeletal muscle injuries, namely volumetric muscle loss (VML), usually occurs in military personnel during war [16,17]. Once injured, skeletal muscle tissues will go through three stages, including inflammation, repairing, and remodeling [2,11,18]. The first stage is the necrosis and degradation of skeletal muscles with inflammatory reaction, which is the main stage of tissue destruction [15]. Inflammatory responses are cascade reactions mediated by neutrophils and macrophages and regulated by various cytokines. Without external interference, this stage usually reaches its peak at 3 days [19]. In the second stage, fibroblasts migrate into injured sites to form scars and local angiogenesis can also be found. In the last stage, new skeletal muscle fibers are gradually matured and connect with each other through connective tissues [13]. The recovery pattern of injured skeletal muscle (regeneration process) is different from that of fractured bone (restoration process). It is worth to mention that skeletal muscle tissues will be totally replaced by scars when the first and second stage are out of balance [20].
It is difficult for skeletal muscle fibers to rebuild themselves when they are seriously injured [21]. Traditionally, the first aid for skeletal muscle injury follows the RICE principle (rest, ice, compression and elevation) and its purpose is to reduce the bleeding of tissues and relieve the pain of patients. The effectiveness of RICE principle has not been proved by clinical trials, but it indeed promotes the recovery of injured tissues according to practical experiences [22]. What's more, immobilization is the general consensus for clinicians to treat skeletal muscle injuries, due to the fact that only in this way can connective tissues fully unite muscle fibers to make it strong enough for bearing strength [23]. Some opposite studies showed that the immobilization should be restricted in a short time so that skeletal muscles have enough time to exercise [23].
Surgical method is still the first choices for severe muscle injuries or long-term chronic pain (>4~6 months). In recent years, some cases about using free flap transplantation (golden standard) from forearms and elbows to repair injured skeletal muscles were reported [13,24]. However, this method needs to sacrifice autologous healthy tissues and is not suitable for large-scale tissue defects. In addition, drug therapy may also be an option in the clinic. Non-steroidal drugs (NSAIDs) are able to inhibit inflammatory responses with no inhibition to tissue regeneration in the early injury stage [25]. But some studies do not recommend the long-term of using NSAIDs due to their negative effects on bone growth [26]. Other treatments for skeletal muscle injuries include hyperbaric oxygen therapy and Chinese medicine therapy etc., but the efficacy of them has not been fully proved [27].
In recent decades, the studies on stem cells provide us with new strategies about the treatment for skeletal muscle injuries. Scientists have isolated kinds of stem cells from various organs in human body, including BM-MSCs from bone marrow and satellite cells from skeletal muscles etc. [27,28]. As stem cells in muscles, satellite cells are able to differentiate into muscle cells in theory. But recent studies have shown that satellite cells have a limited effect on skeletal muscle regeneration in vitro due to their high heterogeneity, small quantity and loss of myogenic differentiation potential [[29], [30], [31]]. Moreover, tissue inflammatory reactions and excessive collagen fibers proliferation can significantly reduce the myogenic differentiation potential of these cells [[32], [33], [34]]. Although some scholars have believed that satellite cells were able to replace the injured skeletal muscle fibers and keep cell pools in stable states [35], whether satellite cells have enough ability to promote skeletal muscle regeneration is not clear. Scientists have pointed out that with the ability of myogenic, neurogenic and angiogenic differentiation, BM-MSCs may contribute to tissue repairing.
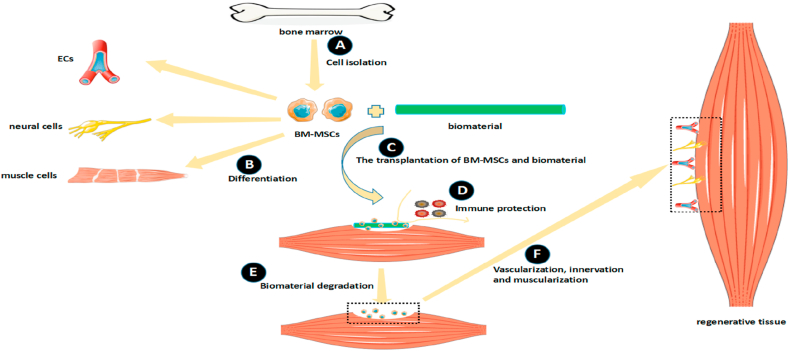
With the complexity of microenvironment and characteristics of stem cells, the existence of stem cells alone is not enough to fully promote tissue regeneration. Therefore, scientists use biomaterials to fill tissue defects and protect stem cells from the immune system. The application of biomaterials has ability to overcome these problems and provide potential therapeutic patterns for tissue regeneration (Fig. 1). In this review article, we retrospect the combined application of BM-MSCs and biomaterials in the field of skeletal muscle regeneration over the past decades.
Fig. 1.
The application of BM-MSCs and biomaterials will be an important tissue engineering strategy in the future. (A) BM-MSCs are isolated from bone marrow. (B) BM-MSCs have ability to differentiate into many kinds of cells, like ECs, neural cells and muscle cells. (C) Scientists have tried to transplant BM-MSCs and biomaterials together into injured muscles. (D) Biomaterials are able to protect BM-MSCs from the immune system. (E) After the degradation of biomaterials, BM-MSCs continue to differentiate at injured sites. (F) The processes of vascularization, innervation and muscularization are activated to form regenerative tissues.
2. BM-MSCs for skeletal muscle regeneration
BM-MSCs were first discovered and isolated 40 years ago. They were derived from bone marrow and mainly exist in marrow cavities, with sometimes being found around blood vessels or other tissues [36,37]. BM-MSCs express CD73, CD90 and CD105, rather than CD11b, CD14, CD34, CD45, CD19, CD79a and HLA-DR surface molecules [38,39]. BM-MSCs can be easily obtained from host in a large quantity and cultured in a short time in vitro. It is worth to mention that BM-MSCs have low expression of HLA class I or II antigens, which make them invisible to immune systems in allogeneic transplantation processes [40]. Several previous studies have shown that BM-MSCs have great potential for multi-directional differentiation, including neurogenic differentiation [41,42], myogenic differentiation [43] and angiogenic differentiation [44], and they are able to stimulate the endogenous regeneration of hosts [[45], [46], [47]]. Furthermore, unlike other kind of stem cells, such as iPSCs and NSCs, BM-MSCs have almost no tumorigenicity after transplantation [48,49]. The multi-directional differentiation potential of BM-MSCs in skeletal muscle regeneration has been summarized in Table 1.
Table 1.
A summary of the multi-directional differentiation potential of BM-MSCs in skeletal muscle regeneration.
| Differentiation direction | Authors | Induction method | Outcome | Ref |
|---|---|---|---|---|
| Myogenic differentiation | Drost et al. | added 5-azacytidine into the medium | Partial BM-MSCs differentiated into muscle cells and expressed corresponding markers. | [68,69] |
| Jung et al. | injected BM-MSCs directly into pericardial space of mice | BM-MSCs could differentiate into cardiomyocytes and replace injured tissues, but the differentiation rate still needed to be improved. | [70] | |
| Muguruma et al. | cultured BM-MSCs in myogenic medium (containing 5% FCS, VEGF, bFGF, and IGF-1) | The efficiency of inducing BM-MSCs to differentiate into cardiomyocytes only by some biological factors or chemical reagents was very low. | [71] | |
| Dreyfus et al. | injected BM-MSCs directly into B6 mice which received radiotherapy before BM-MSCs transplantation | Only a small amount of endogenous BM-MSCs can migrate from bone marrow to injured muscles and it is far from the expectations. | [72] | |
| Egusa et al. | regulated arrangement direction of BM-MSCs in myogenic medium | The myogenic differentiation of BM-MSCs could be greatly enhanced by adjusting the cell arrangement direction. | [74] | |
| Neurogenic differentiation | Fu et al. | seeded BM-MSCs in NSC conditioned medium | About 8% of BM-MSCs had ability to generate neurospheres. | [80] |
| Hermann et al. | plated cells on poly-l-lysine coated glass cover slips in Neurobasal® medium containing several inducing factors. | BM-MSCs were able to effectively converted into neural stem cell-like population with neurosphere-like structures. | [81,82] | |
| Li et al. | seeded BM-MSCs in 50 Hz electromagnetic field | More BM-MSCs could differentiate into neurons in this special environment than that in normal condition. | [83] | |
| Deng et al. | added EGF, bFGF and VEGF into medium | A large amount of BM-MSCs differentiated into neural cells. | [84,85] | |
| Angiogenic differentiation | Wang et al. | added VEGF, bFGF, IGF-1 and EGF into medium | The differentiation efficiency of BM-MSCs into ECs was increased to 60%. | [93] |
| Oswald et al. | added 50 ng/ml VEGF into medium | Tiny amount of BM-MSCs could differentiate into ECs. | [44] | |
| Lu et al. | added S1P and VEGF into medium | Many ECs appeared and new capillary networks could be observed in tissues. | [94] |
2.1. Regulatory mechanism of BM-MSCs in skeletal muscle regeneration
BM-MSCs are able to regulate skeletal muscle regeneration through a complex network, which mainly contains paracrine effect and immunomodulation. Paracrine effect is always considered as an important theoretical basis for stem cells to be used in tissue engineering. BM-MSCs are able to release growth factors, trophic factors and extracellular matrix (ECM) into the microenvironment through paracrine effect [[50], [51], [52]]. Konala et al. have thought that BM-MSCs can fully nourish the damaged skeletal muscle fibers and promote tissue regeneration in vivo [53,54]. In their studies, they found that exosomes derived from BM-MSCs contained some kinds of cytokines, such as fibroblast growth factor-2 (FGF-2), platelet derived growth factor-BB (PDGF-BB) and granulocyte colony-stimulating factor (GCSF). Those factors are the related cytokines of muscle regeneration. The bioactive factors secreted by BM-MSCs can stimulate the formation of vascular networks, remodel ECM and inhibit tissue fibrosis at injured sites [31]. Another important factor that the paracrine effect of BM-MSCs may activate the endogenous repairing mechanism of satellite cells and regulate tissue regeneration processes was identified [55]. Paracrine effect not only promotes the myogenic differentiation of BM-MSCs, but also activates other cells in skeletal muscles.
Other than skeletal muscle fibers, BM-MSCs can also differentiate into neural cells promoted by their neurotrophic factors secretion [56]. In previous studies, some scholars have proved that BM-MSCs are able to promote neural regeneration when they are combined with biological tubules that contain various cytokines (brain derived neurotrophic factor (BDNF) and glial derived neurotrophic factor (GDNF) etc.) [[57], [58], [59]]. In addition to the cytokines in the microenvironment, BM-MSCs can secrete BDNF, insulin-like growth factor-1 (IGF-1), vascular endothelial growth factor (VEGF) to promote their myogenic differentiation processes [60]. VEGF can also stimulate the proliferation of endothelial cells (ECs) and enhance the formation of vascular networks. Murphy et al. have demonstrated that IGF-1, epidermal growth factor (EGF) and VEGF secreted by BM-MSCs can recruit endothelial progenitor cells to injured sites and strengthen the revascularization of tissues [61]. VEGF can also stimulate the proliferation of C2C12 cells which have strong myogenic differentiation abilities [62]. As mentioned above, under the regulation of BM-MSCs, the three major structures (muscle fibers, nerves and blood vessels) can be renewed in the damaged skeletal muscles.
The immunomodulating properties of BM-MSCs is another important regulatory mechanism in tissue regeneration processes [45]. It is well known that inflammation is the first stage after skeletal muscle injuries, so inhibiting or shortening this stage is crucial to tissue restoration. Studies have demonstrated that interleukin-4 (IL-4) and IL-10 secreted by BM-MSCs were able to activate the transformation of M1 macrophages to M2 macrophages [61]. M2 macrophages can eliminate inflammation, clear apoptotic cells, and promote the myogenic differentiation of C2C12 cells [[63], [64], [65]]. In addition, crosstalk between BM-MSCs and immune system is mediated by immunosuppressive factors, such as IL-10, transforming growth factor-β (TGF-β), galactose lectin-1/3, leukemia inhibitory factor (LIF), NO and prostaglandin E2 (PGE2) derived from BM-MSCs [45].
Some signaling pathways may participate in the skeletal muscle regeneration. The cytokines secreted by BM-MSCs, like hepatocyte growth factor (HGF) and fibroblast growth factor (FGF), could up-regulate PI3K/Akt signaling pathway and mediate the paracrine activation of AKT signaling pathway [66]. Liu et al. have proved that BM-MSCs could up-regulate AKT signaling pathway and then regulate satellite cells’ behaviours to enhance skeletal muscle regeneration [67]. Furthermore, BM-MSCs also activate Notch-1 signaling pathway through VEGF secreted by themselves to regulate satellite cells proliferation and differentiation [62]. Pereira et al. have pointed out that if some metabolic substrates existed, like amino acids, several signaling pathways which guided cell physiological activities would be activated [50].
2.2. Myogenic differentiation
5-azacytidine is a potent growth inhibitor, which can cause extensive demethylation of 5-methylcytosine and affect DNA methyltransferase. It is able to induce BM-MSCs to differentiate into skeletal muscle cells and express specific markers in vitro [68,69]. What's more, Jung et al. have proved that injured myocardial fibers, which were considered as being lack of self-renewal capabilities, could be restored by BM-MSCs in animal disease models, although the differentiation rates were very low [70,71]. Some scholars have believed that endogenous BM-MSCs rarely participated in muscle regeneration processes because they thought only a few BM-MSCs would migrate from bone marrow cavities when skeletal muscle damaged [72]. However, Orlic put forward a viewpoint in 2001 and in his opinion, contacting with target cells or damaged organs directly is the most important factor to promote the myogenic differentiation of BM-MSCs [73]. He has believed that different microenvironment led to different differentiation rates in vitro and vivo. With the development of technology, how to mimic the microenvironment in the body has become research hotspots to scientists. For example, Egusa et al. made the same arrangement direction for BM-MSCs with muscle fibers on scaffolds and it can significantly improve the myogenic differentiation efficiency of BM-MSCs [74].
In fact, parts of scholars have believed that BM-MSCs did not directly participate in skeletal muscle regeneration processes, but regulate injured tissue restoration by activating satellite cells for the reason that BM-MSCs would apoptosis in a short time after transplanted into injured sites [31]. Cytokine types and inflammatory reactions in microenvironment determine the bioactivities of satellite cells in vivo [75]. At present, it is well known that BM-MSCs in vivo are able to secrete FGF, HGF and IGF-1 and all of which regulate the myogenic differentiation of satellite cells [76,77]. Immunosuppressive factors (IL-6, IL-8, etc.) derived from BM-MSCs can promote the formation of new muscle fibers and maintain microenvironment stability through inhibiting inflammation [64,78].
Small quantity of satellite cells in tissues may not be enough to completely restore the damaged muscle fibers, so it can be assumed that skeletal muscle regeneration processes may be involved in the myogenic differentiation of both BM-MSCs and satellite cells. Unfortunately, there are not enough evidence to prove this assumption and more research is needed in the future.
2.3. Neurogenic differentiation
There are two main regulatory mechanisms for BM-MSCs to promote neural differentiation. The first one is that they directly differentiate into neural cells to replace damaged nerve fibers [41] and the second one is to promote neurogenic differentiation processes through paracrine effect [79]. According to the study of Fu et al., about 8% BM-MSCs had ability to differentiate into neurosphere-like structures in conditioned medium in vitro [80], while Hermann have thought 60% BM-MSCs could be induced to differentiate into astrocytes, neurons and Schwann cells under the suitable conditions [81,82]. By changing physical and chemical environment, the biological characteristics of BM-MSCs will be improved and the microenvironment can be more and more compatible to BM-MSCs differentiation. For example, Li et al. have showed the ability of BM-MSCs to differentiate into neurons would be significantly enhanced if they were in 50 Hz electromagnetic field [83]. Moreover, if there are some biological factors in the microenvironment, the neurogenic differentiation of BM-MSCs will be promoted. In previous studies, after adding EGF, bFGF and VEGF into medium, BM-MSCs were able to differentiate into neural cells in a large quantity [84,85].
It should be emphasized that BM-MSCs have ability to secrete various neurotrophic factors by paracrine effect when they are transplanted into injured sites. Among the known neurotrophic factors, BDNF is crucial to the development of nervous system [86]. BDNF can activate Ras-MAPK signaling pathway and cAMP-response element binding protein (CREB) by combining with tyrosine kinaseB (TrkB) to promote neurogenic differentiation of stem cells and increase synaptic plasticity [[87], [88], [89]]. With the secretion of BDNF, BM-MSCs not only protect the remaining nerve tissues, but also stimulate their own neurogenic differentiation processes. Besides BDNF, VEGF derived from BM-MSCs can also up-regulate the expression of mTOR through PI3K/AKT or JAK/STAT3 signaling pathways, and mTOR is a kind of positive regulatory protein in neural regeneration [90,91]. The inflammation suppression from BM-MSCs is also conducive to the nerve ending reconstruction and accelerate reinnervation processes in new muscles.
2.4. Angiogenic differentiation
ECs are important components in vessel walls and the natural anticoagulant drugs for tissue bleeding. Under normal circumstances, ECs supplement the cell pool of blood vessels through angiogenic differentiation [92] and some evidence showed that the implantation of ECs in vivo can promote the formation of capillary networks [93]. Vascularization in new muscle tissues in a short time is the key point of successful tissue engineering, or scars will easily replace the functional muscle fibers. In 2000, Oswald et al. firstly induced BM-MSCs to differentiate into ECs with VEGF at a concentration of 50 ng/ml, while the differentiation efficiency was extremely low [44]. Since that time, scientists have always believed that BM-MSCs were likely to be hopeful sources for ECs to reconstruct capillary networks in damaged tissues [94].
Interestingly, if BM-MSCs are cultured in the ordinary medium, almost no capillaries can be found, which may lead to ischemic diseases for new tissues. In order to raise angiogenic differentiation efficiency, Wang et al. added VEGF, bFGF, IGF-1 and EGF into culture medium and they found that the differentiation efficiency was significantly increased to 60% [93]. In other studies, Lu et al. added sphingosine 1-phosphate (S1P) and VEGF into medium to explore new induction methods for angiogenic differentiation of BM-MSCs. They found that the endothelial related gene expressions of BM-MSCs were significantly increased in the conditioned medium and capillary networks were found in new tissues [94]. The possible mechanism is that S1P may up-regulate the secretion of HGF, stromal cell-derived factor-1 (SDF-1) and IGF-1 secreted from BM-MSCs, and these cytokines are considered as the trophic factors to enhance the angiogenic differentiation of BM-MSCs and the maturation of vascular endothelium [95,96].
To summarize, BM-MSCs are able to differentiate into muscle fibers, neural tissues and vascular tissues. Cytokines, such as VEGF, bFGF, IGF and EGF, regulate BM-MSCs differentiation processes, and in turn, BM-MSCs can secrete these cytokines through paracrine effect. This mutual promotion of differentiation pattern is able to significantly enhance the restoration of injured muscles, which lays theoretical foundations for the application of BM-MSCs in the field of tissue regeneration and may provide new strategies for clinicians to treat skeletal muscle injuries.
3. Biomaterials for skeletal muscle regeneration
With the development of research on stem cells, scientists have increasingly believed that these kinds of cells were not the only determinants when repairing the injured tissues. In other words, if there are only stem cells existing, the recovery of skeletal muscles seems to be far less than expected. Therefore, stem cells are not the only research field in tissue engineering. The aim of tissue engineering is to promote tissue regeneration by the integration of biomaterials, cells, and growth factors [97]. Biomaterials are used as scaffolds to guide tissue regeneration, modify the microenvironment, or be used as carriers to continuously release biological factors. Different biomaterials have different physical, biological, and chemical properties, which lead to their obviously different abilities in promoting skeletal muscle regeneration. Among these various properties, the most important is that biomaterials should allow stem cells to adhere and support them to secrete ECM in vivo. Furthermore, some biomaterials have other special functions, such as sustained-releasing drugs [98,99].
As cell carriers, biomaterials can protect cells from immune systems in hosts and continuously release some biological factors to make microenvironment suitable for BM-MSCs survival. If BM-MSCs are directly injected into the injured sites, their programmed apoptosis will be activated within a few hours and only very few BM-MSCs can survive [100]. It is mainly because the colonization areas are usually in the states of inflammatory which is not conducive to cell survival. Although BM-MSCs can secrete anti-inflammatory factors through paracrine effect, once seeded in severe inflammatory environment, the majority of BM-MSCs will die in a short time [101,102]. Nowadays, some clinicians may use immunosuppressive drugs to reduce cell apoptosis in injured skeletal muscles [103], but it is well known that these drugs have serious adverse reactions or side effects on patients [104]. Based on this phenomenon, biomaterials can be used as covering-like substances to wrap BM-MSCs and in a stable microenvironment, BM-MSCs are able to secrete anti-inflammatory factors in a stable microenvironment [45].
The basic function of biomaterials is to improve the microenvironment and form local cell niches [105]. Therefore, different types of injuries need different biomaterials to participate in tissue restoration. The current common biomaterials in tissue engineering are hydrogels, preformed scaffolds and decellularized tissues etc. [105]. Here, we review these three kinds of biomaterials used in skeletal muscle regeneration and their advantages have been summarized in Table 2.
Table 2.
A summary of the advantages of biomaterials
| Types of biomaterials | Subtypes of biomaterials | Authors | Advantages or function of biomaterials | Ref |
|---|---|---|---|---|
| Hydrogels | - | Hoffman et al |
|
[106,107] |
| Kim et al |
|
[[108], [109], [110]] | ||
| alginate hydrogels | Borselli et al |
|
[111,112] | |
| GPT hydrogels | Hwang et al |
|
[113,114] | |
| PRP hydrogels | Amable et al |
|
[[115], [116], [117], [118], [119], [120]] | |
| hyaluronic acid based photopolymerization hydrogels | Rossi et al |
|
[121] | |
| ECM | - | Wolf et al |
|
[122,123] |
| 3D-ECM of skeletal muscle | Panyam et al |
|
[99,[124], [125], [126], [127], [128]] | |
| ECM of porcine small intestinal submucosa | Mase et al |
|
[129] | |
| Preformed scaffolds | Collagen | Liu et al |
|
[[130], [131], [132]] |
| synthetic scaffolds with porous structures | Kim et al |
|
[[133], [134], [135], [136], [137], [138]] | |
| directional aligned electrospun membranes | Choi et al |
|
[139,140] | |
| Aviss et al |
|
[141] | ||
| Bian et al |
|
[[142], [143], [144]] |
3.1. Hydrogels
Hydrogels are polymer materials using water as dispersing medium and can be cut into various shapes. They can absorb large amounts of water and have been widely used in many fields of regenerative medicine. Hydrogels can be harvested from nature or artificial synthesis. Hydrogels can continuously release drugs in designated areas, deliver cells into injured sites, and make stem cells avoid being attacked by immune cells [106,107]. Clinicians have made stem cells, bioactive factors, and hydrogels as a functional whole and injected it into specific area in order to test its efficiency of promoting tissue regeneration [108]. The mixtures are not only able to fill in the tissue defects, but also create excellent ecological niches for stem cells. The previous studies have proved that hydrogels were able to promote skeletal muscle regeneration, because the similar microenvironment was mimicked in vivo [109,110].
Borselli et al. injected alginate gels, which could continuously release myogenic or angiogenesis factors in vivo, into ischemic mice hind limbs. They found that myogenesis, angiogenesis and neurogenesis could be found in the hindlimbs of experimental animals [111]. The alginate gels with drug releasing function can enhance the outward migration of BM-MSCs, stimulate BM-MSCs to differentiate into new muscle fibers and strengthen the contractile force of skeletal muscles [112]. Also, hydrogels that are made from alginate can reduce fibrosis and most importantly, they will self-degrade in vivo as time goes on.
Gelatin-poly (ethylene glycol) -tyramine (GPT) hydrogels were synthesized in recent years and had many unexpected functions in regenerative medicine. With continuously releasing bFGF, GPT hydrogels combined with ASCs could significantly improve the function of injured skeletal muscles [113]. As mentioned above, bFGF plays an important role in promoting wound healing, tissue regeneration and nerve tissue growth. Therefore, although there are few studies on the combination of GPT and BM-MSCs, it can be inferred that BM-MSCs may promote tissue regeneration more effectively when combined with GPT hydrogels. Under certain circumstances, GPT hydrogels are able to enhance the bioactivities of endothelial cells and promote angiogenesis in injured sites [114]. Interestingly. GPT hydrogels can also be used as fillers to fill in tissue defects [114].
Platelet rich plasma (PRP), derived from autologous whole blood, contains lots of growth factors and proteins which are essential for stem cells survival, differentiation. and migration in vitro and vivo [[115], [116], [117]]. In other studies, Sassoli and his colleagues have found that PRP may be the potential nutrient matrix for clinicians [31]. PRP can be made into hydrogels to enhance the myogenic differentiation of BM-MSCs and the reasons for this phenomenon are based on the following two points: (1) PRP hydrogels can continuously release various biological factors contained in serum (2) the function of BM-MSCs may be maintained or stimulated by PRP hydrogels after transplanted into bodies [115,[118], [119], [120]]. Other types of hydrogels also have potential to accelerate skeletal muscle regeneration. For example, Prof. Rossi used hyaluronic acid based photopolymerization hydrogels as cell carriers to promote muscle regeneration and large new muscle fibers could be found in animal disease models [121]. He has also pointed out that these hydrogels are able to enrich niche diversities and stimulate the formation of nerve fibers and capillary networks in the injured muscles.
3.2. ECM
If only cells are removed from tissues, a new type of biomaterials will be obtained, called decellularized tissues. Compared with other biomaterials, the components of decellularized tissues were closest to the microenvironment of hosts. Decellularized tissues contain not only various proteins and cytokines existed in the microenvironment, but also many microstructures to support tissue regeneration, such as capillary networks. Autologous biomaterials can easily build barriers for cells to protect them from immune systems. It ensures the long-term stability of microenvironment and promote the cell proliferation and differentiation [122,123]. ECM belongs to decellularized tissues and are beneficial to the restoration of injured skeletal muscles. For example, three dimensional ECM (3D-ECM) that are harvested from skeletal muscles support the myogenic differentiation of stem cells and accelerate the regeneration processes [124]. As reported in a previous study, BM-MSCs can be induced to differentiation into skeletal muscle cells through the regulation of 3D-ECM [125]. By providing platforms and creating ecological niches, 3D-ECM can strengthen the regeneration of elements in skeletal muscles [99,126,127].
In the study of Merritt et al., they have found that after injecting BM-MSCs into the ECM that are derived from gastrocnemius muscles and transplanted them into hosts, the function of damaged muscles recovered better than the control group, as well as the expression level of myogenic markers [128]. There is a consensus in biomedical field that autologous tissues can be recognized by immunity, while exogenous or synthetic biomaterials may activate immune systems and cause harm to patients. Nevertheless, Mase et al. transplanted ECM derived from porcine small intestinal submucosa into patients whose quadriceps femoris were traumatic. After operation, new skeletal muscles were formed at injured sites and the motor function of thighs was significantly restored [129]. The reason may be that the vast majority of immune recognition molecules will be eliminated if tissue cells are removed, and the remaining tissues not cause obvious inflammation to hosts.
3.3. Preformed scaffolds
Collagen is widely used in tissue engineering and suitable to support the colonization of various stem cells [130]. Collagen can be found easily in connective tissues and it has lots of advantages, such as low immunogenicity, self-degradability, and good biocompatibility. However, the cell adhesion of synthetic scaffolds is worse than that of natural materials. To solve this problem, scientists have covered biomaterial surface with layers of proteins to significantly improve the adhesion of stem cells, but some slight differences are still existed between synthetic and natural biomaterials [131,132]. How to improve scaffold properties to absorb more cells may become the focus in the future research.
Synthetic scaffolds with porous structures in skeletal muscle regeneration are mostly synthesized by polyester and these scaffolds can be cut into various shapes according to tissue shapes [133]. The porous structures are conducive to cell colonization and adhesion. Interestingly, most synthetic biomaterials can self-degrade in vivo and the space left after self-degradation will be occupied by new tissues [134,135]. For instance, after putting cells and PGA scaffolds into subcutaneous tissues, Kamelger et al. have found the occurrence of angiogenesis and multinucleated myotubes [136]. In addition to polyester materials, elastomer materials have also been proved to be beneficial to the growth of myoblasts [137,138]. Elastomer materials have excellent mechanical properties to greatly enhance, integrate and repair skeletal muscle defects.
With the development of science, electrospinning technology is used to fabricate scaffolds and polymer filaments with nanometer diameters. Electrospinning technology can synthesize scaffolds with mimicking the arrangement of skeletal muscle fibers in vivo, which has a positive effect on the myogenic differentiation of BM-MSCs. In the study of Choi et al., although the proliferation rate of BM-MSCs seeded on non-directional and directional aligned electrospun biomaterials was similar, the myotubes differentiated from BM-MSCs on directional distribution of fibers were significantly longer [139]. Except that it is similar to the tissue structures in vivo, this fiber arrangements can also improve the mechanical properties of biomaterials. Such scaffolds still lack biological recognition and cannot absorb proteins from microenvironment, although protein coatings added on the biomaterial surface [140]. By accident, scholars have found that PLGA fibers synthesized by electrospinning technology are able to adsorb proteins from microenvironment and stimulate the formation of myotubes in recent years [141]. It may change people's view on the properties of these biomaterials.
At present, there are still some disadvantages for electrospinning technology, such as the limited thickness of membranes and low porosity, which are all negative factors for cell proliferation and differentiation. With the maturity of micro/nano technology, some groove structures can be added on scaffold surfaces to improve cell bioactivities [142]. Several research groups have imprinted submicron grooves on the surfaces of some biomaterials to discuss function of these technologies. They found that after optimizing biomaterial surfaces, the myogenic abilities of BM-MSCs are significantly improved and longer myotube structures can be found in regenerative skeletal muscles [143,144].
4. Conclusion
Once tissues are damaged, stem cells will be activated and differentiated into corresponding cells to supplement cell pools. The multi-directional differentiation potential of stem cells leads scholars all over the world to believe that stem cells can promote tissue engineering and provide new strategies for treating skeletal muscle injuries in the clinic. BM-MSCs, one of stem cells derived from bone marrow, have ability to promote the various tissue regeneration processes, including nerves, muscles, and blood vessels. BM-MSCs are capable of myogenic differentiation in vitro and skeletal muscle fibers derived from BM-MSCs have been observed in animal models, although some scholars believe that this process is based on the activation of satellite cells. It is undeniable that BM-MSCs are capable to promote the regeneration of damaged muscles, but how to raise survival and differentiation rate of BM-MSCs in vivo still needs further research.
Biomaterials are another important component in tissue engineering. On the one hand, biomaterials contain various bioactive factors and continuously provide necessary nutrients to stem cells for their proliferation and differentiation. On the other hand, biomaterials can also be used as biological barriers to protect cells from host immune attacks. With the development of synthesis technology, the biological, physical, and chemical properties of biomaterials have been continuously improved. However, we should also realize that biomaterials still have many disadvantages and need us make them more specific. For example, synthetic biomaterials are not as attractive to cells as natural ones and some biomaterials may cause inflammation in the host. The future research should focus on the bio-safety, bio-compatibility and effectiveness of biomaterials. The method based on BM-MSCs and biomaterials is expected to provide a prospective strategy for clinicians to repair injured skeletal muscles though that remains a long way off. Nevertheless, we believe that the combined use of stem cells and biomaterials has a prospective future in the field of regenerative medicine.
Data availability statement
No data were used to support this study.
Declaration of competing interest
The authors declare no conflict of interests.
Acknowledgments
This work was supported by grants from the project of Science & Technology Department of Sichuan Province (2020YFS0182) and the project of West China Hospital of Stomatology (LCYJ2019-1).
Footnotes
Peer review under responsibility of the Japanese Society for Regenerative Medicine.
References
- 1.Sicari B.M., Dearth C.L., Badylak S.F. Tissue engineering and regenerative medicine approaches to enhance the functional response to skeletal muscle injury. Anat Rec (Hoboken) 2014;297(1):51–64. doi: 10.1002/ar.22794. [DOI] [PubMed] [Google Scholar]
- 2.Caseiro A.R., Pereira T., Bártolo P.J., Santos J.D., Luís A.L., Mauricio A. Mesenchymal stem cells and biomaterials systems – perspectives for skeletal muscle tissue repair and regeneration. Procedia Eng. 2015;110:90–97. [Google Scholar]
- 3.Brack A.S., Rando T.A. Tissue-specific stem cells: lessons from the skeletal muscle satellite cell. Cell Stem Cell. 2012;10(5):504–514. doi: 10.1016/j.stem.2012.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang Y.X., Rudnicki M.A. Satellite cells, the engines of muscle repair. Nat Rev Mol Cell Biol. 2011;13(2):127–133. doi: 10.1038/nrm3265. [DOI] [PubMed] [Google Scholar]
- 5.Heredia J.E., Mukundan L., Chen F.M., Mueller A.A., Deo R.C., Locksley R.M. Type 2 innate signals stimulate fibro/adipogenic progenitors to facilitate muscle regeneration. Cell. 2013;153(2):376–388. doi: 10.1016/j.cell.2013.02.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Seale P., Sabourin L.A., Girgis-Gabardo A., Mansouri A., Gruss P., Rudnicki M.A. Pax7 is required for the specification of myogenic satellite cells. Cell. 2000;102(6):777–786. doi: 10.1016/s0092-8674(00)00066-0. [DOI] [PubMed] [Google Scholar]
- 7.Gurevich D.B., Nguyen P.D., Siegel A.L., Ehrlich O.V., Sonntay C., Phan J.M. Asymmetric division of clonal muscle stem cells coordinates muscle regeneration in vivo. Science. 2016;353(6295):aad9969. doi: 10.1126/science.aad9969. [DOI] [PubMed] [Google Scholar]
- 8.Shinin V., Gayraud-Morel B., Gomès D., Tajbakhsh S. Asymmetric division and cosegregation of template DNA strands in adult muscle satellite cells. Nat Cell Biol. 2006;8(7):677–687. doi: 10.1038/ncb1425. [DOI] [PubMed] [Google Scholar]
- 9.Troy A., Cadwallader A.B., Fedorov Y., Tyner K., Tanaka K.K., Olwin B.B. Coordination of satellite cell activation and self-renewal by Par-complex-dependent asymmetric activation of p38α/β MAPK. Cell Stem Cell. 2012;11(4):541–553. doi: 10.1016/j.stem.2012.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rocheteau P., Gayraud-Morel B., Siegl-Cachedenier I., Blasco M.A., Tajbakhsh S. A subpopulation of adult skeletal muscle stem cells retains all template DNA strands after cell division. Cell. 2012;148(1–2):112–125. doi: 10.1016/j.cell.2011.11.049. [DOI] [PubMed] [Google Scholar]
- 11.Järvinen T.A., Järvinen T.L., Kääriäinen M., Kalimo H., Järvinen M. Muscle injuries: biology and treatment. Am J Sports Med. 2005;33(5):745–764. doi: 10.1177/0363546505274714. [DOI] [PubMed] [Google Scholar]
- 12.Merritt E.K., Hammers D.W., Tierney M., Suggs L.J., Walters T.J., Farrar R.P. Functional assessment of skeletal muscle regeneration utilizing homologous extracellular matrix as scaffolding. Tissue Eng Part A. 2010;16(4):1395–1405. doi: 10.1089/ten.TEA.2009.0226. [DOI] [PubMed] [Google Scholar]
- 13.Järvinen T.A., Järvinen T.L., Kääriäinen M., Aärimaa V., Vaittinen S., Kalimo H. Muscle injuries: optimising recovery. Best Pract Res Clin Rheumatol. 2007;21(2):317–331. doi: 10.1016/j.berh.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 14.Mase V.J., Jr., Hsu J.R., Wolf S.E., Wenke J.C., Baer D.G., Owens J. Clinical application of an acellular biologic scaffold for surgical repair of a large, traumatic quadriceps femoris muscle defect. Orthopedics. 2010;33(7):511. doi: 10.3928/01477447-20100526-24. [DOI] [PubMed] [Google Scholar]
- 15.Gates C., Huard J. Management of skeletal muscle injuries in military personnel. Operat Tech Sports Med. 2005;13(4):247–256. [Google Scholar]
- 16.Bartlett C.S., Helfet D.L., Hausman M.R., Strauss E. Ballistics and gunshot wounds: effects on musculoskeletal tissues. J Am Acad Orthop Surg. 2000;8(1):21–36. doi: 10.5435/00124635-200001000-00003. [DOI] [PubMed] [Google Scholar]
- 17.Zouris J.M., Walker G.J., Dye J., Galarneau M. Wounding patterns for U.S. Marines and sailors during Operation Iraqi Freedom, major combat phase. Mil Med. 2006;171(3):246–252. doi: 10.7205/milmed.171.3.246. [DOI] [PubMed] [Google Scholar]
- 18.Chargé S.B., Rudnicki M.A. Cellular and molecular regulation of muscle regeneration. Physiol Rev. 2004;84(1):209–238. doi: 10.1152/physrev.00019.2003. [DOI] [PubMed] [Google Scholar]
- 19.Tidball J.G. Inflammatory processes in muscle injury and repair. Am J Physiol Regul Integr Comp Physiol. 2005;288(2):R345–R353. doi: 10.1152/ajpregu.00454.2004. [DOI] [PubMed] [Google Scholar]
- 20.Sato K., Li Y., Foster W., Fukushima K., Badlani N., Adachi N. Improvement of muscle healing through enhancement of muscle regeneration and prevention of fibrosis. Muscle Nerve. 2003;28(3):365–372. doi: 10.1002/mus.10436. [DOI] [PubMed] [Google Scholar]
- 21.Ekstrand J., Gillquist J. Soccer injuries and their mechanisms: a prospective study. Med Sci Sports Exerc. 1983;15(3):267–270. doi: 10.1249/00005768-198315030-00014. [DOI] [PubMed] [Google Scholar]
- 22.Bleakley C., McDonough S., MacAuley D. The use of ice in the treatment of acute soft-tissue injury: a systematic review of randomized controlled trials. Am J Sports Med. 2004;32(1):251–261. doi: 10.1177/0363546503260757. [DOI] [PubMed] [Google Scholar]
- 23.Kannus P., Parkkari J., Järvinen T.L., Järvinen T.A., Järvinen M. Basic science and clinical studies coincide: active treatment approach is needed after a sports injury. Scand J Med Sci Sports. 2003;13(3):150–154. doi: 10.1034/j.1600-0838.2003.02225.x. [DOI] [PubMed] [Google Scholar]
- 24.Vekris M.D., Beris A.E., Lykissas M.G., Korompilias A.V., Vekris A.D., Soucacos P.N. Restoration of elbow function in severe brachial plexus paralysis via muscle transfers. Injury. 2008;39(Suppl 3):S15–S22. doi: 10.1016/j.injury.2008.06.008. [DOI] [PubMed] [Google Scholar]
- 25.O’Grady M., Hackney A.C., Schneider K., Bossen E., Steinberg K., Douglas J.M., Jr. Diclofenac sodium (Voltaren) reduced exercise-induced injury in human skeletal muscle. Med Sci Sports Exerc. 2000;32(7):1191–1196. doi: 10.1097/00005768-200007000-00001. [DOI] [PubMed] [Google Scholar]
- 26.Mishra D.K., Fridén J., Schmitz M.C., Lieber R.L. Anti-inflammatory medication after muscle injury. A treatment resulting in short-term improvement but subsequent loss of muscle function. J Bone Joint Surg Am. 1995;77(10):1510–1519. doi: 10.2106/00004623-199510000-00005. [DOI] [PubMed] [Google Scholar]
- 27.Wang Y.H., Pan J., Wang D.R., Liu J.Y. The use of stem cells in neural regeneration: a review of current opinion. Curr Stem Cell Res Ther. 2018;13(7):608–617. doi: 10.2174/1574888X13666180720100738. [DOI] [PubMed] [Google Scholar]
- 28.Sassoli C., Zecchi-Orlandini S., Formigli L. Trophic actions of bone marrow-derived mesenchymal stromal cells for muscle repair/regeneration. Cells. 2012;1(4):832–850. doi: 10.3390/cells1040832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Biressi S., Rando T.A. Heterogeneity in the muscle satellite cell population. Semin Cell Dev Biol. 2010;21(8):845–854. doi: 10.1016/j.semcdb.2010.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Montarras D., Morgan J., Collins C., Relaix F., Zaffran S., Cumano A. Direct isolation of satellite cells for skeletal muscle regeneration. Science. 2005;309(5743):2064–2067. doi: 10.1126/science.1114758. [DOI] [PubMed] [Google Scholar]
- 31.Sassoli C., Vallone L., Tani A., Chellini F., Nosi D., Zecchi-Orlandini S. Combined use of bone marrow-derived mesenchymal stromal cells (BM-MSCs) and platelet rich plasma (PRP) stimulates proliferation and differentiation of myoblasts in vitro: new therapeutic perspectives for skeletal muscle repair/regeneration. Cell Tissue Res. 2018;372(3):549–570. doi: 10.1007/s00441-018-2792-3. [DOI] [PubMed] [Google Scholar]
- 32.Filippin L.I., Moreira A.J., Marroni N.P., Xavier R.M. Nitric oxide and repair of skeletal muscle injury. Nitric Oxide. 2009;21(3–4):157–163. doi: 10.1016/j.niox.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 33.Carosio S., Berardinelli M.G., Aucello M., Musarò A. Impact of ageing on muscle cell regeneration. Ageing Res Rev. 2011;10(1):35–42. doi: 10.1016/j.arr.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 34.Wang W., Pan H., Murray K., Jefferson B.S., Li Y. Matrix metalloproteinase-1 promotes muscle cell migration and differentiation. Am J Pathol. 2009;174(2):541–549. doi: 10.2353/ajpath.2009.080509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tierney M.T., Sacco A. Satellite cell heterogeneity in skeletal muscle homeostasis. Trends Cell Biol. 2016;26(6):434–444. doi: 10.1016/j.tcb.2016.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Méndez-Ferrer S., Michurina T.V., Ferraro F., Mazloom A.R., Macarthur B.D., Lira S.A. Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature. 2010;466(7308):829–834. doi: 10.1038/nature09262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Caplan A.I., Correa D. The MSC: an injury drugstore. Cell Stem Cell. 2011;9(1):11–15. doi: 10.1016/j.stem.2011.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dominici M., Le Blanc K., Mueller I., Slaper-Cortenbach I., Marini F., Krause D. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8(4):315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 39.Boxall S.A., Jones E. Markers for characterization of bone marrow multipotential stromal cells. Stem Cell Int. 2012;2012:975871. doi: 10.1155/2012/975871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Klimczak A., Kozlowska U., Kurpisz M. Muscle stem/progenitor cells and mesenchymal stem cells of bone marrow origin for skeletal muscle regeneration in muscular dystrophies. Arch Immunol Ther Exp. 2018;66(5):341–354. doi: 10.1007/s00005-018-0509-7. (Warsz) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sanchez-Ramos J., Song S., Cardozo-Pelaez F., Hazzi C., Stedeford T., Willing A. Adult bone marrow stromal cells differentiate into neural cells in vitro. Exp Neurol. 2000;164(2):247–256. doi: 10.1006/exnr.2000.7389. [DOI] [PubMed] [Google Scholar]
- 42.Woodbury D., Schwarz E.J., Prockop D.J., Black I.B. Adult rat and human bone marrow stromal cells differentiate into neurons. J Neurosci Res. 2000;61(4):364–370. doi: 10.1002/1097-4547(20000815)61:4<364::AID-JNR2>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 43.Wakitani S., Saito T., Caplan A.I. Myogenic cells derived from rat bone marrow mesenchymal stem cells exposed to 5-azacytidine. Muscle Nerve. 1995;18(12):1417–1426. doi: 10.1002/mus.880181212. [DOI] [PubMed] [Google Scholar]
- 44.Oswald J., Boxberger S., Jørgensen B., Feldmann S., Ehninger G., Bornhäuser M. Mesenchymal stem cells can be differentiated into endothelial cells in vitro. Stem Cell. 2004;22(3):377–384. doi: 10.1634/stemcells.22-3-377. [DOI] [PubMed] [Google Scholar]
- 45.Le Blanc K., Mougiakakos D. Multipotent mesenchymal stromal cells and the innate immune system. Nat Rev Immunol. 2012;12(5):383–396. doi: 10.1038/nri3209. [DOI] [PubMed] [Google Scholar]
- 46.Baiguera S., Jungebluth P., Mazzanti B., Macchiarini P. Mesenchymal stromal cells for tissue-engineered tissue and organ replacements. Transpl Int. 2012;25(4):369–382. doi: 10.1111/j.1432-2277.2011.01426.x. [DOI] [PubMed] [Google Scholar]
- 47.Yi T., Song S.U. Immunomodulatory properties of mesenchymal stem cells and their therapeutic applications. Arch Pharm Res. 2012;35(2):213–221. doi: 10.1007/s12272-012-0202-z. [DOI] [PubMed] [Google Scholar]
- 48.Wang Y., Han Z.B., Song Y.P., Han Z.C. Safety of mesenchymal stem cells for clinical application. Stem Cell Int. 2012;2012:652034. doi: 10.1155/2012/652034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cashman T.J., Gouon-Evans V., Costa K.D. Mesenchymal stem cells for cardiac therapy: practical challenges and potential mechanisms. Stem Cell Rev Rep. 2013;9(3):254–265. doi: 10.1007/s12015-012-9375-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pereira T., Ivanova G., Caseiro A.R., Barbosa P., Bártolo P.J., Santos J.D. MSCs conditioned media and umbilical cord blood plasma metabolomics and composition. PloS One. 2014;9(11) doi: 10.1371/journal.pone.0113769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Skalnikova H., Motlik J., Gadher S.J., Kovarova H. Mapping of the secretome of primary isolates of mammalian cells, stem cells and derived cell lines. Proteomics. 2011;11(4):691–708. doi: 10.1002/pmic.201000402. [DOI] [PubMed] [Google Scholar]
- 52.Doorn J., Moll G., Le Blanc K., van Blitterswijk C., de Boer J. Therapeutic applications of mesenchymal stromal cells: paracrine effects and potential improvements. Tissue Eng B Rev. 2012;18(2):101–115. doi: 10.1089/ten.TEB.2011.0488. [DOI] [PubMed] [Google Scholar]
- 53.Konala V.B., Mamidi M.K., Bhonde R., Das A.K., Pochampally R., Pal R. The current landscape of the mesenchymal stromal cell secretome: a new paradigm for cell-free regeneration. Cytotherapy. 2016;18(1):13–24. doi: 10.1016/j.jcyt.2015.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nakamura Y., Miyaki S., Ishitobi H., Matsuyama S., Nakasa T., Kamei N. Mesenchymal-stem-cell-derived exosomes accelerate skeletal muscle regeneration. FEBS Lett. 2015;589(11):1257–1265. doi: 10.1016/j.febslet.2015.03.031. [DOI] [PubMed] [Google Scholar]
- 55.Anderson J.E. Hepatocyte growth factor and satellite cell activation. Adv Exp Med Biol. 2016;900:1–25. doi: 10.1007/978-3-319-27511-6_1. [DOI] [PubMed] [Google Scholar]
- 56.Chau M.J., Deveau T.C., Gu X., Kim Y.S., Xu Y., Yu S. Delayed and repeated intranasal delivery of bone marrow stromal cells increases regeneration and functional recovery after ischemic stroke in mice. BMC Neurosci. 2018;19(1):20. doi: 10.1186/s12868-018-0418-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nijhuis T.H., Bodar C.W., van Neck J.W., Walbeehm E.T., Siemionow M., Madajka M. Natural conduits for bridging a 15-mm nerve defect: comparison of the vein supported by muscle and bone marrow stromal cells with a nerve autograft. J Plast Reconstr Aesthetic Surg. 2013;66(2):251–259. doi: 10.1016/j.bjps.2012.09.011. [DOI] [PubMed] [Google Scholar]
- 58.Nijhuis T.H., Brzezicki G., Klimczak A., Siemionow M. Isogenic venous graft supported with bone marrow stromal cells as a natural conduit for bridging a 20 mm nerve gap. Microsurgery. 2010;30(8):639–645. doi: 10.1002/micr.20818. [DOI] [PubMed] [Google Scholar]
- 59.Wang Y., Jia H., Li W.Y., Tong X.J., Liu G.B., Kang S.W. Synergistic effects of bone mesenchymal stem cells and chondroitinase ABC on nerve regeneration after acellular nerve allograft in rats. Cell Mol Neurobiol. 2012;32(3):361–371. doi: 10.1007/s10571-011-9764-4. [DOI] [PubMed] [Google Scholar]
- 60.Teixeira F.G., Carvalho M.M., Sousa N., Salgado A.J. Mesenchymal stem cells secretome: a new paradigm for central nervous system regeneration? Cell Mol Life Sci. 2013;70(20):3871–3882. doi: 10.1007/s00018-013-1290-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Murphy M.B., Moncivais K., Caplan A.I. Mesenchymal stem cells: environmentally responsive therapeutics for regenerative medicine. Exp Mol Med. 2013;45(11):e54. doi: 10.1038/emm.2013.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sassoli C., Pini A., Chellini F., Mazzanti B., Nistri S., Nosi D. Bone marrow mesenchymal stromal cells stimulate skeletal myoblast proliferation through the paracrine release of VEGF. PloS One. 2012;7(7) doi: 10.1371/journal.pone.0037512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Németh K., Leelahavanichkul A., Yuen P.S., Mayer B., Parmelee A., Doi K. Bone marrow stromal cells attenuate sepsis via prostaglandin E(2)-dependent reprogramming of host macrophages to increase their interleukin-10 production. Nat Med. 2009;15(1):42–49. doi: 10.1038/nm.1905. [published correction appears in Nat Med. 2009 Apr;15(4):462] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chen L., Tredget E.E., Wu P.Y., Wu Y. Paracrine factors of mesenchymal stem cells recruit macrophages and endothelial lineage cells and enhance wound healing. PloS One. 2008;3(4) doi: 10.1371/journal.pone.0001886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bencze M., Negroni E., Vallese D., Yacoub-Youssef H., Chaouch S., Wolff A. Proinflammatory macrophages enhance the regenerative capacity of human myoblasts by modifying their kinetics of proliferation and differentiation. Mol Ther. 2012;20(11):2168–2179. doi: 10.1038/mt.2012.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chen J., Crawford R., Chen C., Xiao Y. The key regulatory roles of the PI3K/Akt signaling pathway in the functionalities of mesenchymal stem cells and applications in tissue regeneration. Tissue Eng B Rev. 2013;19(6):516–528. doi: 10.1089/ten.TEB.2012.0672. [DOI] [PubMed] [Google Scholar]
- 67.Liu S., Gao F., Wen L., Ouyang M., Wang Y., Wang Q. Osteocalcin induces proliferation via positive activation of the PI3K/Akt, P38 MAPK pathways and promotes differentiation through activation of the GPRC6A-ERK1/2 pathway in C2C12 myoblast cells. Cell Physiol Biochem. 2017;43(3):1100–1112. doi: 10.1159/000481752. [DOI] [PubMed] [Google Scholar]
- 68.Drost A.C., Weng S., Feil G., Schäfer J., Baumann S., Kanz L. In vitro myogenic differentiation of human bone marrow-derived mesenchymal stem cells as a potential treatment for urethral sphincter muscle repair. Ann N Y Acad Sci. 2009;1176:135–143. doi: 10.1111/j.1749-6632.2009.04610.x. [DOI] [PubMed] [Google Scholar]
- 69.Maeda Y., Yonemochi Y., Nakajyo Y., Hidaka H., Ikeda T., Ando Y. CXCL12 and osteopontin from bone marrow-derived mesenchymal stromal cells improve muscle regeneration. Sci Rep. 2017;7(1):3305. doi: 10.1038/s41598-017-02928-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jung N., Rupp H., Koczulla A.R., Vogelmeier C.F., Alter P. Myocardial homing of mesenchymal stem cells following intrapericardial application and amplification by inflammation - an experimental pilot study. Can J Physiol Pharmacol. 2017;95(9):1064–1066. doi: 10.1139/cjpp-2016-0373. [DOI] [PubMed] [Google Scholar]
- 71.Muguruma Y., Reyes M., Nakamura Y., Sato T., Matsuzawa H., Miyatake H. In vivo and in vitro differentiation of myocytes from human bone marrow-derived multipotent progenitor cells [published correction appears in Exp Hematol. 2008 Aug;36(8):1055] Exp Hematol. 2003;31(12):1323–1330. doi: 10.1016/j.exphem.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 72.Dreyfus P.A., Chretien F., Chazaud B., Kirova Y., Caramelle P., Garcia L. Adult bone marrow-derived stem cells in muscle connective tissue and satellite cell niches. Am J Pathol. 2004;164(3):773–779. doi: 10.1016/S0002-9440(10)63165-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Orlic D., Kajstura J., Chimenti S., Jakoniuk I., Anderson S.M., Li B. Bone marrow cells regenerate infarcted myocardium. Nature. 2001;410(6829):701–705. doi: 10.1038/35070587. [DOI] [PubMed] [Google Scholar]
- 74.Egusa H., Kobayashi M., Matsumoto T., Sasaki J., Uraguchi S., Yatani H. Application of cyclic strain for accelerated skeletal myogenic differentiation of mouse bone marrow-derived mesenchymal stromal cells with cell alignment. Tissue Eng Part A. 2013;19(5–6):770–782. doi: 10.1089/ten.TEA.2012.0164. [DOI] [PubMed] [Google Scholar]
- 75.Dumont N.A., Wang Y.X., Rudnicki M.A. Intrinsic and extrinsic mechanisms regulating satellite cell function. Development. 2015;142(9):1572–1581. doi: 10.1242/dev.114223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Thomas K., Engler A.J., Meyer G.A. Extracellular matrix regulation in the muscle satellite cell niche. Connect Tissue Res. 2015;56(1):1–8. doi: 10.3109/03008207.2014.947369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tonkin J., Temmerman L., Sampson R.D., Gallego-Colon E., Barberi L., Bilbao D. Monocyte/Macrophage-derived IGF-1 orchestrates murine skeletal muscle regeneration and modulates autocrine polarization. Mol Ther. 2015;23(7):1189–1200. doi: 10.1038/mt.2015.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mirabella T., Cilli M., Carlone S., Cancedda R., Gentili C. Amniotic liquid derived stem cells as reservoir of secreted angiogenic factors capable of stimulating neo-arteriogenesis in an ischemic model. Biomaterials. 2012;33(20):5175. doi: 10.1016/j.biomaterials.2011.01.071. [published correction appears in. [DOI] [PubMed] [Google Scholar]
- 79.Razavi S., Razavi M.R., Kheirollahi-Kouhestani M., Mardani M., Mostafavi F.S. Co-culture with neurotrophic factor secreting cells induced from adipose-derived stem cells: promotes neurogenic differentiation. Biochem Biophys Res Commun. 2013;440(3):381–387. doi: 10.1016/j.bbrc.2013.09.069. [DOI] [PubMed] [Google Scholar]
- 80.Fu L., Zhu L., Huang Y., Lee T.D., Forman S.J., Shih C.C. Derivation of neural stem cells from mesenchymal stemcells: evidence for a bipotential stem cell population. Stem Cell Dev. 2008;17(6):1109–1121. doi: 10.1089/scd.2008.0068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hermann A., Gastl R., Liebau S., Popa M.O., Fiedler J., Boehm B.O. Efficient generation of neural stem cell-like cells from adult human bone marrow stromal cells. J Cell Sci. 2004;117(Pt 19):4411–4422. doi: 10.1242/jcs.01307. [DOI] [PubMed] [Google Scholar]
- 82.Tohill M., Terenghi G. Stem-cell plasticity and therapy for injuries of the peripheral nervous system. Biotechnol Appl Biochem. 2004;40(Pt 1):17–24. doi: 10.1042/BA20030173. [DOI] [PubMed] [Google Scholar]
- 83.Li W.K., Bai W.F., Xu W.C. 50Hz electromagnetic fields facilitate bone marrow stroma cells-derived neural progenitor cells to differentiate into neurons. Chin J Rehabil Med. 2016;31(7):723–728. [Google Scholar]
- 84.Deng J., Petersen B.E., Steindler D.A., Jorgensen M.L., Laywell E.D. Mesenchymal stem cells spontaneously express neural proteins in culture and are neurogenic after transplantation. Stem Cell. 2006;24(4):1054–1064. doi: 10.1634/stemcells.2005-0370. [DOI] [PubMed] [Google Scholar]
- 85.Zurita M., Vaquero J., Oya S., Miguel M. Schwann cells induce neuronal differentiation of bone marrow stromal cells. Neuroreport. 2005;16(5):505–508. doi: 10.1097/00001756-200504040-00017. [DOI] [PubMed] [Google Scholar]
- 86.Wei X., Zhao L., Zhong J., Gu H., Feng D., Johnstone B.H. Adipose stromal cells-secreted neuroprotective media against neuronal apoptosis. Neurosci Lett. 2009;462(1):76–79. doi: 10.1016/j.neulet.2009.06.054. [DOI] [PubMed] [Google Scholar]
- 87.Franchi S., Valsecchi A.E., Borsani E., Procacci P., Ferrari D., Zalfa C. Intravenous neural stem cells abolish nociceptive hypersensitivity and trigger nerve regeneration in experimental neuropathy. Pain. 2012;153(4):850–861. doi: 10.1016/j.pain.2012.01.008. [published correction appears in Pain. 2012 Aug;153(8):1775. Zaffa, Cristina [corrected to Zalfa, Cristina]] [DOI] [PubMed] [Google Scholar]
- 88.Kingham P.J., Kolar M.K., Novikova L.N., Novikov L.N., Wiberg M. Stimulating the neurotrophic and angiogenic properties of human adipose-derived stem cells enhances nerve repair. Stem Cell Dev. 2014;23(7):741–754. doi: 10.1089/scd.2013.0396. [DOI] [PubMed] [Google Scholar]
- 89.Hsu M.N., Liao H.T., Li K.C., Chen H.H., Yen T.C., Makarevich P. Adipose-derived stem cell sheets functionalized by hybrid baculovirus for prolonged GDNF expression and improved nerve regeneration. Biomaterials. 2017;140:189–200. doi: 10.1016/j.biomaterials.2017.05.004. [DOI] [PubMed] [Google Scholar]
- 90.Eisel F., Boosen M., Beck M., Heide H., Wittig I., Beck K.F. Platelet-derived growth factor triggers PKA-mediated signalling by a redox-dependent mechanism in rat renal mesangial cells. Biochem Pharmacol. 2013;85(1):101–108. doi: 10.1016/j.bcp.2012.10.017. [DOI] [PubMed] [Google Scholar]
- 91.Unachukwu U.J., Sauane M., Vazquez M., Redenti S. Microfluidic generated EGF-gradients induce chemokinesis of transplantable retinal progenitor cells via the JAK/STAT and PI3kinase signaling pathways. PloS One. 2014;8(12) doi: 10.1371/journal.pone.0083906. [published correction appears in. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ko S.H., Bandyk D.F. Therapeutic angiogenesis for critical limb ischemia. Semin Vasc Surg. 2014;27(1):23–31. doi: 10.1053/j.semvascsurg.2014.10.001. [DOI] [PubMed] [Google Scholar]
- 93.Wang C., Li Y., Yang M., Zou Y., Liu H., Liang Z. Efficient differentiation of bone marrow mesenchymal stem cells into endothelial cells in vitro. Eur J Vasc Endovasc Surg. 2018;55(2):257–265. doi: 10.1016/j.ejvs.2017.10.012. [DOI] [PubMed] [Google Scholar]
- 94.Lu W., Xiu X., Zhao Y., Gui M. Improved proliferation and differentiation of bone marrow mesenchymal stem cells into vascular endothelial cells with sphingosine 1-phosphate. Transplant Proc. 2015;47(6):2035–2040. doi: 10.1016/j.transproceed.2015.05.032. [DOI] [PubMed] [Google Scholar]
- 95.Tao Z.W., Li L.G., Geng Z.H., Dang T., Zhu S.J. Growth factors induce the improved cardiac remodeling in autologous mesenchymal stem cell-implanted failing rat hearts. J Zhejiang Univ Sci B. 2010;11(4):238–248. doi: 10.1631/jzus.B0900244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Van Overstraeten-Schlögel N., Beguin Y., Gothot A. Role of stromal-derived factor-1 in the hematopoietic-supporting activity of human mesenchymal stem cells. Eur J Haematol. 2006;76(6):488–493. doi: 10.1111/j.1600-0609.2006.00633.x. [DOI] [PubMed] [Google Scholar]
- 97.Langer R., Vacanti J.P. Tissue engineering. Science. 1993;260(5110):920–926. doi: 10.1126/science.8493529. [DOI] [PubMed] [Google Scholar]
- 98.Curtis A., Wilkinson C. Topographical control of cells. Biomaterials. 1997;18(24):1573–1583. doi: 10.1016/s0142-9612(97)00144-0. [DOI] [PubMed] [Google Scholar]
- 99.Panyam J., Labhasetwar V. Biodegradable nanoparticles for drug and gene delivery to cells and tissue. Adv Drug Deliv Rev. 2003;55(3):329–347. doi: 10.1016/s0169-409x(02)00228-4. [DOI] [PubMed] [Google Scholar]
- 100.Smythe G.M., Hodgetts S.I., Grounds M.D. Immunobiology and the future of myoblast transfer therapy. Mol Ther. 2000;1(4):304–313. doi: 10.1006/mthe.2000.0049. [DOI] [PubMed] [Google Scholar]
- 101.Fan Y., Maley M., Beilharz M., Grounds M. Rapid death of injected myoblasts in myoblast transfer therapy. Muscle Nerve. 1996;19(7):853–860. doi: 10.1002/(SICI)1097-4598(199607)19:7<853::AID-MUS7>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 102.Guérette B., Asselin I., Vilquin J.T., Roy R., Tremblay J.P. Lymphocyte infiltration following allo- and xenomyoblast transplantation in mdx mice. Muscle Nerve. 1995;18(1):39–51. doi: 10.1002/mus.880180107. [DOI] [PubMed] [Google Scholar]
- 103.Guérette B., Asselin I., Skuk D., Entman M., Tremblay J.P. Control of inflammatory damage by anti-LFA-1: increase success of myoblast transplantation. Cell Transplant. 1997;6(2):101–107. doi: 10.1177/096368979700600203. [DOI] [PubMed] [Google Scholar]
- 104.Hernández R.M., Orive G., Murua A., Pedraz J.L. Microcapsules and microcarriers for in situ cell delivery. Adv Drug Deliv Rev. 2010;62(7–8):711–730. doi: 10.1016/j.addr.2010.02.004. [DOI] [PubMed] [Google Scholar]
- 105.Qazi T.H., Mooney D.J., Pumberger M., Geissler S., Duda G.N. Biomaterials based strategies for skeletal muscle tissue engineering: existing technologies and future trends. Biomaterials. 2015;53:502–521. doi: 10.1016/j.biomaterials.2015.02.110. [DOI] [PubMed] [Google Scholar]
- 106.Hoffman A.S. Hydrogels for biomedical applications. Adv Drug Deliv Rev. 2002;54(1):3–12. doi: 10.1016/s0169-409x(01)00239-3. [DOI] [PubMed] [Google Scholar]
- 107.Lee K.Y., Mooney D.J. Hydrogels for tissue engineering. Chem Rev. 2001;101(7):1869–1879. doi: 10.1021/cr000108x. [DOI] [PubMed] [Google Scholar]
- 108.Kim J., Hadlock T., Cheney M., Varvares M., Marler J. Muscle tissue engineering for partial glossectomy defects. Arch Facial Plast Surg. 2003;5(5):403–407. doi: 10.1001/archfaci.5.5.403. [DOI] [PubMed] [Google Scholar]
- 109.Hinds S., Bian W., Dennis R.G., Bursac N. The role of extracellular matrix composition in structure and function of bioengineered skeletal muscle. Biomaterials. 2011;32(14):3575–3583. doi: 10.1016/j.biomaterials.2011.01.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Salimath A.S., García A.J. Biofunctional hydrogels for skeletal muscle constructs. J Tissue Eng Regen Med. 2016;10(11):967–976. doi: 10.1002/term.1881. [DOI] [PubMed] [Google Scholar]
- 111.Borselli C., Storrie H., Benesch-Lee F., Shvartsman D., Cezar C., Lichtman J.W. Functional muscle regeneration with combined delivery of angiogenesis and myogenesis factors. Proc Natl Acad Sci U S A. 2010;107(8):3287–3292. doi: 10.1073/pnas.0903875106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Borselli C., Cezar C.A., Shvartsman D., Vandenburgh H.H., Mooney D.J. The role of multifunctional delivery scaffold in the ability of cultured myoblasts to promote muscle regeneration. Biomaterials. 2011;32(34):8905–8914. doi: 10.1016/j.biomaterials.2011.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Hwang J.H., Kim I.G., Piao S., Jung A.R., Lee J.Y., Park K.D. Combination therapy of human adipose-derived stem cells and basic fibroblast growth factor hydrogel in muscle regeneration. Biomaterials. 2013;34(25):6037–6045. doi: 10.1016/j.biomaterials.2013.04.049. [DOI] [PubMed] [Google Scholar]
- 114.Park K.M., Lee Y., Son J.Y., Bae J.W., Park K.D. In situ SVVYGLR peptide conjugation into injectable gelatin-poly(ethylene glycol)-tyramine hydrogel via enzyme-mediated reaction for enhancement of endothelial cell activity and neo-vascularization. Bioconjugate Chem. 2012;23(10):2042–2050. doi: 10.1021/bc300110b. [DOI] [PubMed] [Google Scholar]
- 115.Amable P.R., Teixeira M.V., Carias R.B., Granjeiro J.M., Borojevic R. Mesenchymal stromal cell proliferation, gene expression and protein production in human platelet-rich plasma-supplemented media. PloS One. 2014;9(8) doi: 10.1371/journal.pone.0104662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kelc R., Trapecar M., Gradisnik L., Rupnik M.S., Vogrin M. Platelet-rich plasma, especially when combined with a TGF-β inhibitor promotes proliferation, viability and myogenic differentiation of myoblasts in vitro. PloS One. 2015;10(2) doi: 10.1371/journal.pone.0117302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Tsai W.C., Yu T.Y., Lin L.P., Lin M.S., Tsai T.T., Pang J.S. Platelet rich plasma promotes skeletal muscle cell migration in association with up-regulation of FAK, paxillin, and F-Actin formation. J Orthop Res. 2017;35(11):2506–2512. doi: 10.1002/jor.23547. [DOI] [PubMed] [Google Scholar]
- 118.Formigli L., Paternostro F., Tani A., Mirabella C., Quattrini Li A., Nosi D. MSCs seeded on bioengineered scaffolds improve skin wound healing in rats. Wound Repair Regen. 2015;23(1):115–123. doi: 10.1111/wrr.12251. [DOI] [PubMed] [Google Scholar]
- 119.Rubio-Azpeitia E., Andia I. Partnership between platelet-rich plasma and mesenchymal stem cells: in vitro experience. Muscles Ligaments Tendons J. 2014;4(1):52–62. [PMC free article] [PubMed] [Google Scholar]
- 120.Jalowiec J.M., D’Este M., Bara J.J., Denom J., Menzel U., Alini M. An in vitro investigation of platelet-rich plasma-gel as a cell and growth factor delivery vehicle for tissue engineering. Tissue Eng C Methods. 2016;22(1):49–58. doi: 10.1089/ten.tec.2015.0223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Rossi C.A., Flaibani M., Blaauw B., Pozzobon M., Figallo E., Reggiani C. In Vivo tissue engineering of functional skeletal muscle by freshly isolated satellite cells embedded in a photopolymerizable hydrogel. Faseb J. 2011;25(7):2296–2304. doi: 10.1096/fj.10-174755. [DOI] [PubMed] [Google Scholar]
- 122.Wolf M.T., Daly K.A., Reing J.E., Badylak S.F. Biologic scaffold composed of skeletal muscle extracellular matrix. Biomaterials. 2012;33(10):2916–2925. doi: 10.1016/j.biomaterials.2011.12.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.De Coppi P., Bellini S., Conconi M.T., Sabatti M., Simonato E., Gamba P.G. Myoblast-acellular skeletal muscle matrix constructs guarantee a long-term repair of experimental full-thickness abdominal wall defects. Tissue Eng. 2006;12(7):1929–1936. doi: 10.1089/ten.2006.12.1929. [DOI] [PubMed] [Google Scholar]
- 124.Osses N., Brandan E. ECM is required for skeletal muscle differentiation independently of muscle regulatory factor expression. Am J Physiol Cell Physiol. 2002;282(2):C383–C394. doi: 10.1152/ajpcell.00322.2001. [DOI] [PubMed] [Google Scholar]
- 125.Machingal M.A., Corona B.T., Walters T.J., Kesireddy V., Koval C.N., Dannahower A. A tissue-engineered muscle repair construct for functional restoration of an irrecoverable muscle injury in a murine model. Tissue Eng Part A. 2011;17(17–18):2291–2303. doi: 10.1089/ten.tea.2010.0682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Kannan R.Y., Salacinski H.J., Sales K., Butler P., Seifalian A.M. The roles of tissue engineering and vascularisation in the development of micro-vascular networks: a review. Biomaterials. 2005;26(14):1857–1875. doi: 10.1016/j.biomaterials.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 127.Stern-Straeter J., Riedel F., Bran G., Hörmann K., Goessler U.R. Advances in skeletal muscle tissue engineering. In Vivo. 2007;21(3):435–444. [PubMed] [Google Scholar]
- 128.Merritt E.K., Cannon M.V., Hammers D.W., Le L.N., Gokhale R., Sarathy A. Repair of traumatic skeletal muscle injury with bone-marrow-derived mesenchymal stem cells seeded on extracellular matrix. Tissue Eng Part A. 2010;16(9):2871–2881. doi: 10.1089/ten.TEA.2009.0826. [DOI] [PubMed] [Google Scholar]
- 129.Mase V.J., Jr., Hsu J.R., Wolf S.E., Wenke J.C., Baer D.J., Owens J. Clinical application of an acellular biologic scaffold for surgical repair of a large, traumatic quadriceps femoris muscle defect. Orthopedics. 2010;33(7):511. doi: 10.3928/01477447-20100526-24. Published 2010 Jul 13. [DOI] [PubMed] [Google Scholar]
- 130.Liu G., Wang X., Sun X., Deng C., Atala A., Zhang Y. The effect of urine-derived stem cells expressing VEGF loaded in collagen hydrogels on myogenesis and innervation following after subcutaneous implantation in nude mice. Biomaterials. 2013;34(34):8617–8629. doi: 10.1016/j.biomaterials.2013.07.077. [DOI] [PubMed] [Google Scholar]
- 131.Cronin E.M., Thurmond F.A., Bassel-Duby R., Williams R.S., Wright W.E., Nelson K.D. Protein-coated poly(L-lactic acid) fibers provide a substrate for differentiation of human skeletal muscle cells. J Biomed Mater Res. 2004;69(3):373–381. doi: 10.1002/jbm.a.30009. [DOI] [PubMed] [Google Scholar]
- 132.Thorrez L., Shansky J., Wang L., Fast L., VandenDriessche T., Chuah M. Growth, differentiation, transplantation and survival of human skeletal skeletal muscle fibers on biodegradable scaffolds. Biomaterials. 2008;29(1):75–84. doi: 10.1016/j.biomaterials.2007.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Kim M., Choi Y.S., Yang S.H., Hong H.N., Cho S.W., Cha S.M. Muscle regeneration by adipose tissue-derived adult stem cells attached to injectable PLGA spheres. Biochem Biophys Res Commun. 2006;348(2):386–392. doi: 10.1016/j.bbrc.2006.07.063. [published correction appears in Biochem Biophys Res Commun. 2006 Nov 17;350(2):499] [DOI] [PubMed] [Google Scholar]
- 134.Saxena A.K., Willital G.H., Vacanti J.P. Vascularized three-dimensional skeletal muscle tissue-engineering. Bio Med Mater Eng. 2001;11(4):275–281. [PubMed] [Google Scholar]
- 135.Saxena A.K., Marler J., Benvenuto M., Willital G.H., Vacanti J.P. Skeletal muscle tissue engineering using isolated myoblasts on synthetic biodegradable polymers: preliminary studies. Tissue Eng. 1999;5(6):525–532. doi: 10.1089/ten.1999.5.525. [DOI] [PubMed] [Google Scholar]
- 136.Kamelger F.S., Marksteiner R., Margreiter E., Klima G., Wechselberger G., Hering S. A comparative study of three different biomaterials in the engineering of skeletal muscle using a rat animal model. Biomaterials. 2004;25(9):1649–1655. doi: 10.1016/s0142-9612(03)00520-9. [DOI] [PubMed] [Google Scholar]
- 137.Vandenburgh H., Shansky J., Benesch-Lee F., Barbata V., Reid J., Thorrez L. Drug-screening platform based on the contractility of tissue-engineered muscle. Muscle Nerve. 2008;37(4):438–447. doi: 10.1002/mus.20931. [DOI] [PubMed] [Google Scholar]
- 138.Mulder M.M., Hitchcock R.W., Tresco P.A. Skeletal myogenesis on elastomeric substrates: implications for tissue engineering. J Biomater Sci Polym Ed. 1998;9(7):731–748. doi: 10.1163/156856298x00118. [DOI] [PubMed] [Google Scholar]
- 139.Choi J.S., Lee S.J., Christ G.J., Atala A., Yoo J.J. The influence of electrospun aligned poly(epsilon-caprolactone)/collagen nanofiber meshes on the formation of self-aligned skeletal muscle myotubes. Biomaterials. 2008;29(19):2899–2906. doi: 10.1016/j.biomaterials.2008.03.031. [DOI] [PubMed] [Google Scholar]
- 140.Riboldi S.A., Sampaolesi M., Neuenschwander P., Cossu G., Mantero S. Electrospun degradable polyesterurethane membranes: potential scaffolds for skeletal muscle tissue engineering. Biomaterials. 2005;26(22):4606–4615. doi: 10.1016/j.biomaterials.2004.11.035. [DOI] [PubMed] [Google Scholar]
- 141.Aviss K.J., Gough J.E., Downes S. Aligned electrospun polymer fibres for skeletal muscle regeneration. Eur Cell Mater. 2010;19:193–204. doi: 10.22203/ecm.v019a19. [DOI] [PubMed] [Google Scholar]
- 142.Bian W., Liau B., Badie N., Bursac N. Mesoscopic hydrogel molding to control the 3D geometry of bioartificial muscle tissues. Nat Protoc. 2009;4(10):1522–1534. doi: 10.1038/nprot.2009.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Patz T.M., Doraiswamy A., Narayan R.J., Modi R., Chrisey D.B. Two-dimensional differential adherence and alignment of C2C12 myoblasts. Mater Sci Eng B. 2005;123(3):242–247. [Google Scholar]
- 144.Yuan C.C., Ma K.J., Li K.C., Chien H.H., Lu H.E., Tseng C.P. Submicron-grooved culture surface extends myotube length by forming parallel and elongated motif. Micro Nano Lett. 2013;8(8):440–444. Iet. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data were used to support this study.