Abstract
Background
The basic helix-loop-helix (bHLH) transcription factor is one of the most important gene families in plants, playing a key role in diverse metabolic, physiological, and developmental processes. Although it has been well characterized in many plants, the significance of the bHLH family in barley is not well understood at present.
Methods
Through a genome-wide search against the updated barley reference genome, the genomic organization, evolution and expression of the bHLH family in barley were systematically analyzed.
Results
We identified 141 bHLHs in the barley genome (HvbHLHs) and further classified them into 24 subfamilies based on phylogenetic analysis. It was found that HvbHLHs in the same subfamily shared a similar conserved motif composition and exon-intron structures. Chromosome distribution and gene duplication analysis revealed that segmental duplication mainly contributed to the expansion of HvbHLHs and the duplicated genes were subjected to strong purifying selection. Furthermore, expression analysis revealed that HvbHLHs were widely expressed in different tissues and also involved in response to diverse abiotic stresses. The co-expression network was further analyzed to underpin the regulatory function of HvbHLHs. Finally, 25 genes were selected for qRT-PCR validation, the expression profiles of HvbHLHs showed diverse patterns, demonstrating their potential roles in relation to stress tolerance regulation.
Conclusion
This study reported the genome organization, evolutionary characteristics and expression profile of the bHLH family in barley, which not only provide the targets for further functional analysis, but also facilitate better understanding of the regulatory network bHLH genes involved in stress tolerance in barley.
Keywords: Barley, bHLH family, expression profile, qRT-PCR, transcription factor, helix-loop-helix
1. Introduction
Transcription factors (TFs) play essential roles in regulating diverse physiological and developmental processes in eukaryotes through interacting with cis-element to activate or repress the expression of downstream functional genes [1, 2]. As the second-largest TF family in plants, the basic helix-loop-helix (bHLH) family is composed of approximately 60 amino acids with two functional domains, namely a basic conserved motif and a HLH motif [3, 4]. The basic motif consists of approximately 17 amino acids and possesses the typical six basic residues at the N-terminal, which functions to bind to the E-box (5’-CANNTG-3’) or the variant G-box (5’-CACGTG-3’) element [5]. The HLH motif is composed of two amphipathic α helices separated by a loop region with variable size, and the loop region could participate in the formation of homodimeric or heterodimeric complexes [6, 7], which contribute to the specific binding of bHLH transcription factors on their target genes [5].
Up to now, the bHLH gene family has been extensively identified in many plant species. It is reported that 167 bHLH genes were identified in Arabidopsis thaliana [2], 167 in rice (Oryza sativa) [7], 146 in Brachypodium distachyon [8], 571 in wheat (Triticum aestivum) [9], 208 in maize (Zea mays) [10] and 602 in Brassica napus [11]. The bHLH genes are widely involved in diverse signal transductions and metabolic pathways. For example, the bHLH transcription factor ROOT HAIR DEFECTIVE SIX-LIKE4 (RSL4) in Arabidopsis stimulates root hair formation [12]; three duplicated transcription factors AtbHLH010, AtbHLH089 and AtbHLH091 mediate anther development in Arabidopsis [13]; the Arabidopsis bHLH transcription factor CFLAP1 (CFL1 associated protein 1) and its homolog CFLAP2 regulate cuticle development [14]; the major quantitative trait locus An-1 in wild rice, which encodes a basic helix-loop-helix protein, regulates awn development, grain size, and grain number in rice [15]. Meanwhile, many bHLH genes respond to various types of stress, such as salt, drought and cold stresses. For instance, AtbHLH112 in Arabidopsis responds to drought and salt signal transduction through proline biosynthesis and reactive oxygen species (ROS) scavenging pathways [16]; OsbHLH006 and OsbHLH148 in rice play a transcriptional role in drought stress through the jasmonic acid signaling pathway [17, 18]; the bHLH transcription factor PHYTOCHROME-INTERACTING FACTOR 4 (PIF4) in Arabidopsis regulates stomatal lineage initiation under high-temperature stress [19]; alternatively, OsbHLH1 in rice, which is independent of ABA, responds to cold stress [20]. In addition, some bHLH genes are also involved in plant photomorphogenesis [21, 22] and phytohormone signaling, such as brassinosteroid [23], abscisic acid [24, 25] and jasmonic acid [26, 27].
Barley (Hordeum vulgare L) is one of the earliest domesticated crops approximately 10,000 years ago, and nowadays, ranks as the fourth most important cereal crop worldwide in terms of cultivated areas and total yield [28]. It has been well-studied in genetics, cytology and genomics and therefore, is considered as a research model for the large-genome small grain temperate cereals. Recently, the completion of its reference genome laid the foundation to identify the gene family at the genome-wide level. Here, we comprehensively identified 141 bHLHs in barley (HvbHLHs) based on a genome-wide search method. Then, the phylogenetic relationships, gene duplication, cis-regulatory elements, conserved motifs and gene structure as well as co-expression networks of these HvbHLHs were also investigated. Finally, the expression patterns of HvbHLHs were detected based on a total of 148 RNA-seq samples, and the expression of 25 genes was further validated by qRT-PCR analysis. This study will not only shed light on the functional study of HvbHLHs, but also contribute to better understanding of the regulatory roles and evolutionary mechanism of the bHLH gene family in barley and beyond.
2. Materials and Methods
2.1. Identification of bHLH Gene Family in Barley
The protein sequences of the updated barley reference genome of genotype Morex [28] were obtained from the IPK database (http://webblast.ipk-gatersleben.de/barley_ibsc/) and used as the local protein database. The bHLH proteins of Arabidopsis and rice were downloaded from the TAIR (https://www.arabidopsis.org/) and RGAP (http://rice.plant biology.msu.edu/) databases respectively, and then used as the query to search against the local protein database by BLASP tool with the identity more than 50% and e-value of 1e-5 as a threshold. Then, the Hidden Markov Model (HMM) of bHLH domain (PF00010) was used as a query to search against the barley local protein database using the HMMER 3.0 tool. The results from these two methods were integrated and explained by manual editing to remove redundancy. The remaining proteins were further verified using Plant Transcription Factor Database (PlantTFDB) v.4.0 (http://planttfdb.cbi.pku.edu.cn/prediction.php), HMMER v3.2.1 (https://www.ebi.ac.uk/Tools/hmmer/search/hmm scan) and PFAM (http://pfam.xfam.org/search/sequence) database. Only the proteins having the complete bHLH domain were considered as putative HvbHLHs.
Finally, the ProtParam tool (https://web.expasy.org/protparam/) was used to estimate the molecular weight (MW), theoretical isoelectric point (pI), instability index (II), aliphatic index and Gravy value. The subcellular localization was predicted based on the Plant-mPLoc online tools (http://www.csbio.sjtu.edu.cn/bioinf/plant-multi/). To confirm the existence of these HvbHLHs, the expressed sequence tags (ESTs) were downloaded from the NCBI database and a BLASTN search was performed with a cut-off e-value of 1e-5 and identity of 80%.
2.2. Multiple Sequence Alignment and Phylogenetic Analysis
Multiple sequence alignment of the HvbHLHs was performed using ClustalX v1.83 with default parameters. To visualize the conserved motifs, the sequence logo of the bHLH domain was generated using WEB LOGO tool (http://weblogo.berkeley.edu/logo.cgi). An unrooted neighbor-joining (NJ) tree was constructed using MEGA v7.0 based on the conserved bHLH domain sequences with the following parameters: 1000 times bootstrap, Poisson model and 95% partial deletion.
2.3. Gene Structure and Conserved Motif Analysis
The intron-exon structure was obtained based on the gene annotation file of the barley genome and visualized using the online Gene Structure Display Server (http://gsds.cbi.pku. edu.cn/). The conserved motifs were identified using MEME software (http://meme-suite.org/tools/meme) with the following parameters: maximum number of motifs with 10, optimum motif width with 8 to 50, other settings with default. The predicted motifs were then submitted to the InterPro database to determine their putative functions (http://www.ebi.ac.uk/interpro/search/sequence/). The upstream 1.5kb sequences from the transcription initiation site of the HvbHLHs were extracted from the barley genome, and then submitted to the PlantCARE database to detect the cis-acting elements. (http://bioinformatics.psb.ugent.be/web tools/plantcare/html/).
2.4. Gene Duplication and Molecular Evolution Analysis
A duplication event was defined based on the criteria described by Chen et al., including (a) the shorter alignment sequence covered > 70% of the longer gene in length; (b) the aligned region had an identity > 70%; and (c) only one duplication event was counted for the tightly linked genes [29]. In order to reveal the evolutionary effect of bHLH genes during barley domestication, the bHLH genes in wild barley were also identified using the same methods as barley domestication with all protein sequences of wild barley (http://db.ncgr.ac.cn/wild_barley/) as local protein database. The orthologous bHLH gene pairs between wild and cultivated barley were identified using inParanoid v4.1 [30]. Moreover, the synteny relationships between barley and other species, including Arabidopsis thaliana, Brachypodium distachyon, Oryza sativa, Solanum lycopersicum, Solanum tuberosum, and Vitis vinifera, were detected using the MCScanX software [31]. The Ka (non-synonymous substitution)/Ks (synonymous substitution) ratio was calculated to estimate the evolutionary effect using the codeml program embedded in the PAML v1.3.1 package [32]. The formula T=Ks/2λ was employed to estimate the divergence time between these gene pairs, where λ refers to the mutation rate showing 6.5 × 10−9 synonymous substitutions per site per year. Circos v0.67 tool was used to visualize the orthologous genes pairs.
2.5. Expression Profiles and Regulatory Network Analysis
A total of 148 RNA-seq samples, of various developmental stages and tissues as well as diverse biotic and abiotic stresses, were downloaded from the NCBI Sequence Read Archive (SRA) database and then were used to investigate the expression patterns of these HvbHLHs. The accession number and sample information of RNA-seq are listed in Table S1 (3.4MB, pdf) . Then, HISAT2 v2.1.0 and StringTie v1.3.5 pipeline were used to calculate the FPKM (fragments per kilobase of transcript per million fragments mapped) value and the differentially expressed genes were identified using the R package Ballgown with the following parameters: fold change ≥ 2, FDR(false discovery rate) ≤ 0.05, and the absolute ratio of log2 ≥ 1 [33]. The log2 transformed FPKM value was displayed using the R package heatmap. In order to identify the candidate miRNA targets, the transcript of HvbHLHs was submitted to psRNATarget (http://plantgrn.noble.org/psRNATarget/) to search against all barley miRNAs in the miRbase. Finally, the WGCNA package in the R software was employed to run the co-expression networks using the 148 RNA-seq samples. The genes linked to the bHLHs with the top 5% weighted parameter obtained from WGCNA were selected for further analysis. The functions of co-expressed genes were determined using BLAST against the Arabidopsis protein database. The network was visualized using the Cytoscape v3.8.0.
2.6. Validation the Expression of HvbHLHs by qRT-PCR Analysis
Seeds of barley cv. Morex were hydroponically cultured in the growth chamber under controlled conditions (23 ± 1 °C, 16-h light/8-h dark cycle). And the seedlings at the three-leaf stage were used for stress treatment. For salt stress, the plants were incubated in 150 mM NaCl solution for 0, 6, 12 and 24 hours. For drought stress, they were incubated into 19.2% (w/v) PEG-6000 solution for 0, 6, 12 and 24 hours. For cold and heat stresses, they were kept at 4 °C for 0, 6, 12 and 24 hours, and at 42 °C for 0, 1, 6 hours, respectively. Seedlings under the normal condition at the same time point were used as the control. Leaves of all samples were collected from three to five plants at each time point with three biological replications. All the plant materials were promptly frozen in liquid nitrogen and stored at -80 °C for RNA extraction. Total RNA isolation, cDNA synthesis and qRT-PCR reaction were performed as described previously [34]. Then, 25 candidates HvbHLHs were selected to design primers for qRT-PCR analysis. HvACTIN2 (GenBank accession no. AY145451.1) was used as the internal reference and all the primers used in this study were listed in Table S2 (3.4MB, pdf) . The TB-Green® Premix Ex Taq™ II kit (Takara, Dalian, China) was used for qRT-PCR amplification using the QuantStudio™ Real-Time PCR system (ThermoFisher, USA). The thermal cycling condition was 95 °C temperature for 30 s followed by 40 cycles of 95 °C for 3s, and at 60 °C for 30 s. Three technological replications were applied and the expression profile was calculated using the 2−ΔΔCT method [35].
3. Results and Discussion
3.1. Identification of bHLH Gene Family in Barley
Using the method as described in Materials and Methods, a total of 141 bHLH encoding genes were identified in barley (Table 1), accounting for 0.35% of the total annotated barley genes, which was similar to that of rice (0.44%), while slightly less than Brachypodium (0.55%) and Arabidopsis (0.65%) (Table S3 (3.4MB, pdf) ) [7, 8]. Since there is no standard nomenclature and description, these barley bHLH proteins were named HvbHLH1 to HvbHLH141 according to their physical positions on the chromosomes. As shown in Table 1 and Fig. S1 (3.4MB, pdf) , these HvbHLHs were unevenly distributed across the 7 chromosomes. In detail, 26 HvbHLHs were found on chromosome 5, ranking the most abundant one, followed by chromosome 7 (25) and chromosome 4 (22), whereas only 10 genes were mapped on chromosome 1. The physical and chemical properties of the putative genes were then characterized. The size of them ranged from 36 to 887 amino acids, molecular weight ranged from 4.22 to 95.97 kDa and theoretical isoelectric point ranged from 4.49 to 11.9, which are similar to those of Brachypodium [8]. The value of hydropathicity ranged from -1.247 (HvbHLH62) to 0.057 (HvbHLH55), with an average of -0.49. Except for HvbHLH55, other HvbHLHs displayed the negative gravity values, representing the hydrophilic characteristics of barley bHLH genes. Subcellular location prediction showed that most (90.07%) of the HvbHLHs were localized in the nucleus, which was similar to the previous study on Brassica napus [11]. To further validate the existence of these HvbHLHs, we performed BLAST search against the barley ESTs. Results showed that 116 out of 141 (82.3%) HvbHLHs were supported by EST hits, of which HvbHLH53, possessed the most hits with 27 ESTs. At the same time, 25 HvbHLHs had no EST support, mainly due to their lower expression level and tissue or stage-specific expression.
Table 1.
Characteristics of bHLH transcription factor gene family in barley.
| HvbHLHs | Barley Gene ID | Protein Length (aa) | Isoelectric Point (pI) | Molecular Weight (kDa) |
EST
Validation |
Subcellular Location | Arabidopsis thaliana Ortholog | Oryza sativa Ortholog | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HvbHLH1 | HORVU0Hr1G012790 | 287 | 558 | 3123436 | - | Nucleus | - | - | ||||||||
| HvbHLH2 | HORVU0Hr1G014300 | 330 | 561 | 3567917 | 1 | Nucleus | - | - | ||||||||
| HvbHLH3 | HORVU0Hr1G019640 | 186 | 793 | 2031412 | - | Nucleus | - | OsbHLH122 | ||||||||
| HvbHLH4 | HORVU0Hr1G020200 | 440 | 558 | 4754577 | 5 | Nucleus | AtbHLH049 | OsbHLH086 | ||||||||
| HvbHLH5 | HORVU0Hr1G021220 | 60 | 1067 | 658106 | 6 | Chloroplast | - | - | ||||||||
| HvbHLH6 | HORVU0Hr1G023050 | 164 | 776 | 1823667 | - | Nucleus | - | - | ||||||||
| HvbHLH7 | HORVU0Hr1G025420 | 164 | 649 | 1831167 | - | Nucleus | - | - | ||||||||
| HvbHLH8 | HORVU1Hr1G017900 | 493 | 629 | 5311099 | 14 | Nucleus | AtbHLH008 | OsbHLH106 | ||||||||
| HvbHLH9 | HORVU1Hr1G020370 | 380 | 568 | 4144222 | - | Nucleus | - | - | ||||||||
| HvbHLH10 | HORVU1Hr1G024280 | 212 | 88 | 2308352 | 3 | Nucleus | - | - | ||||||||
| HvbHLH11 | HORVU1Hr1G040810 | 60 | 1012 | 666184 | 8 | Chloroplast | - | - | ||||||||
| HvbHLH12 | HORVU1Hr1G043780 | 60 | 102 | 672488 | 7 | Chloroplast | - | - | ||||||||
| HvbHLH13 | HORVU1Hr1G050560 | 684 | 606 | 7390273 | 17 | Nucleus | AtbHLH006 | OsbHLH009 | ||||||||
| HvbHLH14 | HORVU1Hr1G054260 | 326 | 848 | 3518579 | 9 | Nucleus | AtbHLH016 | OsbHLH113 | ||||||||
| HvbHLH15 | HORVU1Hr1G071330 | 419 | 514 | 4531638 | 2 | Nucleus | AtbHLH097 | OsbHLH051 | ||||||||
| HvbHLH16 | HORVU1Hr1G072810 | 238 | 77 | 2591731 | 18 | Nucleus | - | OsbHLH058 | ||||||||
| HvbHLH17 | HORVU1Hr1G079450 | 400 | 717 | 4133974 | 8 | Nucleus | - | OsbHLH119 | ||||||||
| HvbHLH18 | HORVU2Hr1G013450 | 393 | 79 | 421995 | 7 | Nucleus | AtbHLH047 | OsbHLH063 | ||||||||
| HvbHLH19 | HORVU2Hr1G020990 | 238 | 775 | 2592061 | - | Nucleus | AtbHLH045 | OsbHLH055 | ||||||||
| HvbHLH20 | HORVU2Hr1G034320 | 36 | 998 | 422302 | 7 | - | - | - | ||||||||
| HvbHLH21 | HORVU2Hr1G035190 | 264 | 501 | 286704 | 2 | Nucleus | - | OsbHLH128 | ||||||||
| HvbHLH22 | HORVU2Hr1G044230 | 294 | 861 | 3242503 | 10 | Nucleus | AtbHLH105 | OsbHLH057 | ||||||||
| HvbHLH23 | HORVU2Hr1G060680 | 362 | 599 | 3875149 | 3 | Nucleus | - | OsbHLH104 | ||||||||
| HvbHLH24 | HORVU2Hr1G066000 | 194 | 449 | 2171025 | 1 | Nucleus | - | - | ||||||||
| HvbHLH25 | HORVU2Hr1G066100 | 305 | 501 | 3364493 | 6 | Nucleus | - | OsbHLH006 | ||||||||
| HvbHLH26 | HORVU2Hr1G068960 | 306 | - | - | 1 | Nucleus | AtbHLH029 | OsbHLH156 | ||||||||
| HvbHLH27 | HORVU2Hr1G073240 | 164 | 631 | 1850639 | 6 | Nucleus | - | - | ||||||||
| HvbHLH28 | HORVU2Hr1G096810 | 250 | 10 | 2727246 | - | Nucleus | - | OsbHLH012 | ||||||||
| HvbHLH29 | HORVU2Hr1G104040 | 147 | 91 | 1680628 | 10 | Nucleus | - | OsbHLH101 | ||||||||
| HvbHLH30 | HORVU2Hr1G106030 | 75 | 1139 | 902536 | 1 | Nucleus | - | - | ||||||||
| HvbHLH31 | HORVU2Hr1G114070 | 253 | 599 | 2775054 | 3 | Nucleus | AtbHLH100 | - | ||||||||
| HvbHLH32 | HORVU2Hr1G115240 | 97 | 608 | 1041362 | 1 | Nucleus | AtbHLH135 | OsbHLH154 | ||||||||
| HvbHLH33 | HORVU3Hr1G000170 | 253 | 942 | 27832 | - | Nucleus | - | - | ||||||||
| HvbHLH34 | HORVU3Hr1G000180 | 211 | 946 | 2334342 | 3 | Nucleus | AtbHLH162 | - | ||||||||
| HvbHLH35 | HORVU3Hr1G000280 | 210 | 92 | 2301307 | 4 | Nucleus | AtbHLH162 | - | ||||||||
| HvbHLH36 | HORVU3Hr1G000830 | 275 | 543 | 2930171 | 1 | Nucleus | AtbHLH086 | OsbHLH125 | ||||||||
| HvbHLH37 | HORVU3Hr1G002620 | 230 | 958 | 2551222 | - | Nucleus | - | - | ||||||||
| HvbHLH38 | HORVU3Hr1G018680 | 268 | 89 | 2899176 | 4 | Nucleus | AtbHLH051 | OsbHLH035 | ||||||||
| HvbHLH39 | HORVU3Hr1G027630 | 98 | 8.98 | 10.61165 | - | Nucleus | - | - | ||||||||
| HvbHLH40 | HORVU3Hr1G034540 | 284 | 9.82 | 31.34548 | 12 | Nucleus | AtbHLH093 | - | ||||||||
| HvbHLH41 | HORVU3Hr1G046430 | 59 | 10.16 | 6.65267 | 11 | Nucleus | - | - | ||||||||
| HvbHLH42 | HORVU3Hr1G048770 | 482 | 8.27 | 50.13659 | 2 | Nucleus | - | OsbHLH117 | ||||||||
| HvbHLH43 | HORVU3Hr1G057280 | 767 | 9.51 | 83.53867 | 13 | Nucleus | - | OsbHLH008 | ||||||||
| HvbHLH44 | HORVU3Hr1G057780 | 310 | 6.01 | 33.84592 | 1 | Nucleus | - | - | ||||||||
| HvbHLH45 | HORVU3Hr1G062240 | 60 | 10.37 | 6.67089 | 9 | Chloroplast | - | - | ||||||||
| HvbHLH46 | HORVU3Hr1G066390 | 465 | 7.77 | 49.81545 | 4 | Nucleus | - | OsbHLH010 | ||||||||
| HvbHLH47 | HORVU3Hr1G066600 | 405 | 6.06 | 41.38465 | 6 | Nucleus | - | OsbHLH118 | ||||||||
| HvbHLH48 | HORVU3Hr1G087710 | 210 | 5.88 | 22.86064 | 1 | Nucleus | - | OsbHLH123 | ||||||||
| HvbHLH49 | HORVU3Hr1G089090 | 378 | 7.29 | 39.87731 | 19 | Nucleus | AtbHLH128 | OsbHLH109 | ||||||||
| HvbHLH50 | HORVU3Hr1G096460 | 231 | 5.09 | 23.54851 | 4 | Nucleus | - | - | ||||||||
| HvbHLH51 | HORVU3Hr1G108660 | 232 | 6.63 | 25.95128 | 16 | Nucleus | - | - | ||||||||
| HvbHLH52 | HORVU3Hr1G108670 | 227 | 8.27 | 25.21689 | 25 | Nucleus | - | - | ||||||||
| HvbHLH53 | HORVU3Hr1G108680 | 246 | 6.5 | 27.00253 | 27 | Nucleus | - | OsbHLH056 | ||||||||
| HvbHLH54 | HORVU4Hr1G003210 | 365 | 5.5 | 38.87177 | 6 | Nucleus | - | OsbHLH020 | ||||||||
| HvbHLH55 | HORVU4Hr1G003340 | 269 | 9.28 | 29.29683 | 7 | Nucleus | - | - | ||||||||
| HvbHLH56 | HORVU4Hr1G009440 | 331 | 5.51 | 35.87252 | 3 | Nucleus | AtbHLH025 | OsbHLH018 | ||||||||
| HvbHLH57 | HORVU4Hr1G009970 | 316 | 5.88 | 34.79796 | 2 | Nucleus | - | OsbHLH084 | ||||||||
| HvbHLH58 | HORVU4Hr1G013720 | 313 | 4.64 | 34.03353 | 6 | Nucleus | - | OsbHLH131 | ||||||||
| HvbHLH59 | HORVU4Hr1G020740 | 887 | 5.99 | 95.97193 | 18 | Nucleus | - | OsbHLH151 | ||||||||
| HvbHLH60 | HORVU4Hr1G032690 | 309 | 11.84 | 34.08783 | 8 | Nucleus | - | - | ||||||||
| HvbHLH61 | HORVU4Hr1G039390 | 173 | 9.33 | 19.03418 | - | Nucleus | - | - | ||||||||
| HvbHLH62 | HORVU4Hr1G046830 | 43 | 11.51 | 4.82556 | 7 | - | - | - | ||||||||
| HvbHLH63 | HORVU4Hr1G052500 | 205 | 11.12 | 22.85349 | 8 | Nucleus | - | - | ||||||||
| HvbHLH64 | HORVU4Hr1G061760 | 402 | 5.52 | 42.92323 | - | Nucleus | AtbHLH030 | OsbHLH040 | ||||||||
| HvbHLH65 | HORVU4Hr1G065640 | 313 | 5.96 | 34.18329 | - | Nucleus | - | OsbHLH019 | ||||||||
| HvbHLH66 | HORVU4Hr1G069820 | 328 | 5.74 | 35.50931 | 9 | Nucleus | AtbHLH085 | OsbHLH129 | ||||||||
| HvbHLH67 | HORVU4Hr1G075340 | 85 | 6.58 | 9.63389 | 4 | Nucleus | AtbHLH136 | OsbHLH153 | ||||||||
| HvbHLH68 | HORVU4Hr1G078270 | 224 | 9.21 | 24.70528 | 4 | Nucleus | - | - | ||||||||
| HvbHLH69 | HORVU4Hr1G078880 | 346 | 6.17 | 36.89555 | 1 | Nucleus | - | - | ||||||||
| HvbHLH70 | HORVU4Hr1G078910 | 344 | 6.41 | 37.3124 | - | Nucleus | - | - | ||||||||
| HvbHLH71 | HORVU4Hr1G080890 | 355 | 5.17 | 38.55531 | 6 | Nucleus | - | OsbHLH003 | ||||||||
| HvbHLH72 | HORVU4Hr1G084850 | 298 | 8.43 | 31.4009 | - | Nucleus | - | OsbHLH052 | ||||||||
| HvbHLH73 | HORVU4Hr1G087590 | 257 | 5.14 | 27.7684 | 12 | Nucleus | - | - | ||||||||
| HvbHLH74 | HORVU4Hr1G087610 | 251 | 4.98 | 27.97863 | 3 | Nucleus | - | - | ||||||||
| HvbHLH75 | HORVU4Hr1G090880 | 50 | 10.38 | 5.53851 | 7 | Chloroplast | - | - | ||||||||
| HvbHLH76 | HORVU5Hr1G002090 | 341 | 6.42 | 35.58769 | 9 | Nucleus | - | OsbHLH021 | ||||||||
| HvbHLH77 | HORVU5Hr1G011780 | 341 | 5.65 | 37.14074 | 6 | Nucleus | - | OsbHLH103 | ||||||||
| HvbHLH78 | HORVU5Hr1G018100 | 312 | 6.21 | 33.78668 | 15 | Nucleus | - | OsbHLH130 | ||||||||
| HvbHLH79 | HORVU5Hr1G023670 | 202 | - | - | 11 | Nucleus | - | - | ||||||||
| HvbHLH80 | HORVU5Hr1G031400 | 60 | 10.72 | 6.61183 | 7 | Chloroplast | - | - | ||||||||
| HvbHLH81 | HORVU5Hr1G040090 | 760 | 6.94 | 82.40031 | 6 | Nucleus | AtbHLH156 | OsbHLH150 | ||||||||
| HvbHLH82 | HORVU5Hr1G053800 | 36 | 9.52 | 4.25305 | 8 | - | - | - | ||||||||
| HvbHLH83 | HORVU5Hr1G057460 | 541 | 9.36 | 61.68851 | 5 | Chloroplast | - | - | ||||||||
| HvbHLH84 | HORVU5Hr1G059290 | 287 | 5.58 | 31.23436 | - | Nucleus | - | - | ||||||||
| HvbHLH85 | HORVU5Hr1G065450 | 383 | 9.37 | 39.80757 | 7 | Nucleus | - | OsbHLH100 | ||||||||
| HvbHLH86 | HORVU5Hr1G066530 | 367 | 5.38 | 37.86235 | 6 | Nucleus | - | OsbHLH039 | ||||||||
| HvbHLH87 | HORVU5Hr1G068110 | 238 | 5.96 | 25.45977 | 2 | Nucleus | - | OsbHLH120 | ||||||||
| HvbHLH88 | HORVU5Hr1G069580 | 210 | 9.78 | 22.7207 | - | Nucleus | - | OsbHLH043 | ||||||||
| HvbHLH89 | HORVU5Hr1G070000 | 411 | 6.2 | 43.54592 | 1 | Nucleus | AtbHLH096 | OsbHLH046 | ||||||||
| HvbHLH90 | HORVU5Hr1G070510 | 427 | 6.38 | 44.84281 | 5 | Nucleus | AtbHLH049 | OsbHLH085 | ||||||||
| HvbHLH91 | HORVU5Hr1G070800 | 515 | 6.98 | 54.97378 | 15 | Nucleus | - | OsbHLH032 | ||||||||
| HvbHLH92 | HORVU5Hr1G075430 | 258 | 7.63 | 28.29626 | 3 | Nucleus | - | - | ||||||||
| HvbHLH93 | HORVU5Hr1G077390 | 279 | 5.94 | 30.66625 | 7 | Nucleus | AtbHLH044 | OsbHLH081 | ||||||||
| HvbHLH94 | HORVU5Hr1G079900 | 270 | - | - | - | Nucleus | - | - | ||||||||
| HvbHLH95 | HORVU5Hr1G080040 | 268 | 6 | 28.90752 | - | Nucleus | - | OsbHLH167 | ||||||||
| HvbHLH96 | HORVU5Hr1G080080 | 210 | 11.9 | 22.81786 | - | Nucleus | - | - | ||||||||
| HvbHLH97 | HORVU5Hr1G093310 | 233 | 9.44 | 25.75102 | 11 | Nucleus | - | - | ||||||||
| HvbHLH98 | HORVU5Hr1G093960 | 288 | 6.52 | 31.5373 | 4 | Nucleus | AtbHLH092 | OsbHLH148 | ||||||||
| HvbHLH99 | HORVU5Hr1G097520 | 292 | 8.93 | 31.56547 | 9 | Nucleus | AtbHLH139 | OsbHLH134 | ||||||||
| HvbHLH100 | HORVU5Hr1G106310 | 312 | 8.73 | 33.55686 | 9 | Nucleus | AtbHLH007 | OsbHLH098 | ||||||||
| HvbHLH101 | HORVU5Hr1G107520 | 175 | - | - | 3 | Nucleus | - | - | ||||||||
| HvbHLH102 | HORVU6Hr1G002140 | 60 | 10.37 | 6.62881 | 8 | Chloroplast | - | - | ||||||||
| HvbHLH103 | HORVU6Hr1G012730 | 558 | 4.63 | 59.01517 | 3 | Nucleus | AtbHLH021 | OsbHLH005 | ||||||||
| HvbHLH104 | HORVU6Hr1G012760 | 558 | 4.63 | 59.01517 | 3 | Nucleus | AtbHLH021 | OsbHLH005 | ||||||||
| HvbHLH105 | HORVU6Hr1G020520 | 297 | - | - | 5 | Nucleus | AtbHLH095 | OsbHLH146 | ||||||||
| HvbHLH106 | HORVU6Hr1G020710 | 59 | 10.7 | 6.72385 | 7 | Chloroplast | - | - | ||||||||
| HvbHLH107 | HORVU6Hr1G039830 | 631 | 8.11 | 66.77104 | 1 | Nucleus | AtbHLH041 | OsbHLH029 | ||||||||
| HvbHLH108 | HORVU6Hr1G054910 | 306 | 7.21 | 32.17456 | 4 | Nucleus | - | OsbHLH110 | ||||||||
| HvbHLH109 | HORVU6Hr1G067000 | 140 | 9.5 | 15.36348 | 1 | Nucleus | - | - | ||||||||
| HvbHLH110 | HORVU6Hr1G068110 | 98 | 9.77 | 10.80136 | 1 | Chloroplast | - | - | ||||||||
| HvbHLH111 | HORVU6Hr1G068980 | 353 | 8 | 37.25795 | 6 | Nucleus | AtbHLH058 | OsbHLH079 | ||||||||
| HvbHLH112 | HORVU6Hr1G069690 | 114 | 9.72 | 12.2922 | - | Nucleus | - | - | ||||||||
| HvbHLH113 | HORVU6Hr1G072300 | 407 | 8.89 | 44.23082 | 1 | Nucleus | - | OsbHLH034 | ||||||||
| HvbHLH114 | HORVU6Hr1G081120 | 318 | 6.7 | 33.18008 | 2 | Nucleus | - | - | ||||||||
| HvbHLH115 | HORVU6Hr1G085500 | 193 | 5.72 | 19.86918 | 3 | Nucleus | AtbHLH082 | OsbHLH115 | ||||||||
| HvbHLH116 | HORVU6Hr1G088020 | 227 | 7.14 | 25.1175 | 1 | Nucleus | - | - | ||||||||
| HvbHLH117 | HORVU7Hr1G024790 | 251 | - | - | - | Nucleus | - | OsbHLH142 | ||||||||
| HvbHLH118 | HORVU7Hr1G026560 | 338 | 6.54 | 36.56862 | 14 | Nucleus | - | OsbHLH108 | ||||||||
| HvbHLH119 | HORVU7Hr1G026690 | 60 | 10.12 | 6.7149 | 7 | Chloroplast | - | - | ||||||||
| HvbHLH120 | HORVU7Hr1G030250 | 130 | 10.11 | 13.3803 | 1 | Nucleus | - | - | ||||||||
| HvbHLH121 | HORVU7Hr1G032420 | 481 | 4.79 | 51.47423 | 12 | Nucleus | - | OsbHLH096 | ||||||||
| HvbHLH122 | HORVU7Hr1G038060 | 86 | - | - | 2 | Nucleus | - | - | ||||||||
| HvbHLH123 | HORVU7Hr1G046580 | 331 | 5.71 | 35.20252 | - | Nucleus | - | OsbHLH127 | ||||||||
| HvbHLH124 | HORVU7Hr1G047180 | 251 | 10.31 | 28.63651 | 7 | Nucleus | - | - | ||||||||
| HvbHLH125 | HORVU7Hr1G050530 | 269 | - | - | 11 | Nucleus | AtbHLH130 | OsbHLH112 | ||||||||
| HvbHLH126 | HORVU7Hr1G052820 | 182 | 9.98 | 20.4143 | 11 | Nucleus | - | - | ||||||||
| HvbHLH127 | HORVU7Hr1G052870 | 383 | 6.03 | 41.19198 | 4 | Nucleus | AtbHLH078 | OsbHLH091 | ||||||||
| HvbHLH128 | HORVU7Hr1G054880 | 222 | 9.2 | 24.19122 | 2 | Nucleus | AtbHLH037 | OsbHLH121 | ||||||||
| HvbHLH129 | HORVU7Hr1G055180 | 293 | 6.14 | 32.12877 | 2 | Nucleus | AtbHLH137 | OsbHLH080 | ||||||||
| HvbHLH130 | HORVU7Hr1G062400 | 147 | 10.71 | 16.12702 | 8 | Nucleus | - | - | ||||||||
| HvbHLH131 | HORVU7Hr1G073310 | 165 | 7.15 | 18.53204 | 18 | Nucleus | - | OsbHLH060 | ||||||||
| HvbHLH132 | HORVU7Hr1G074490 | 524 | 5.21 | 54.2347 | 5 | Nucleus | AtbHLH116 | OsbHLH002 | ||||||||
| HvbHLH133 | HORVU7Hr1G077270 | 279 | 6.95 | 30.85244 | 2 | Nucleus | AtbHLH043 | OsbHLH124 | ||||||||
| HvbHLH134 | HORVU7Hr1G079250 | 365 | 7.08 | 38.96541 | - | Nucleus | AtbHLH098 | OsbHLH054 | ||||||||
| HvbHLH135 | HORVU7Hr1G083760 | 275 | 5.13 | 29.89411 | 1 | Nucleus | - | - | ||||||||
| HvbHLH136 | HORVU7Hr1G087150 | 525 | 8.35 | 55.65763 | - | Nucleus | - | OsbHLH030 | ||||||||
| HvbHLH137 | HORVU7Hr1G090520 | 60 | 10.12 | 6.6168 | 8 | Chloroplast | - | - | ||||||||
| HvbHLH138 | HORVU7Hr1G094690 | 247 | 11.03 | 27.62075 | 4 | Nucleus | AtbHLH060 | OsbHLH095 | ||||||||
| HvbHLH139 | HORVU7Hr1G118740 | 313 | 5.07 | 33.87134 | 10 | Nucleus | - | OsbHLH155 | ||||||||
| HvbHLH140 | HORVU7Hr1G118860 | 70 | 11.21 | 7.90544 | 7 | Nucleus | - | - | ||||||||
| HvbHLH141 | HORVU7Hr1G120420 | 296 | 7.22 | 32.07814 | 2 | Nucleus | - | - | ||||||||
3.2. Conserved Motif Analysis and DNA Binding Ability of HvbHLHs
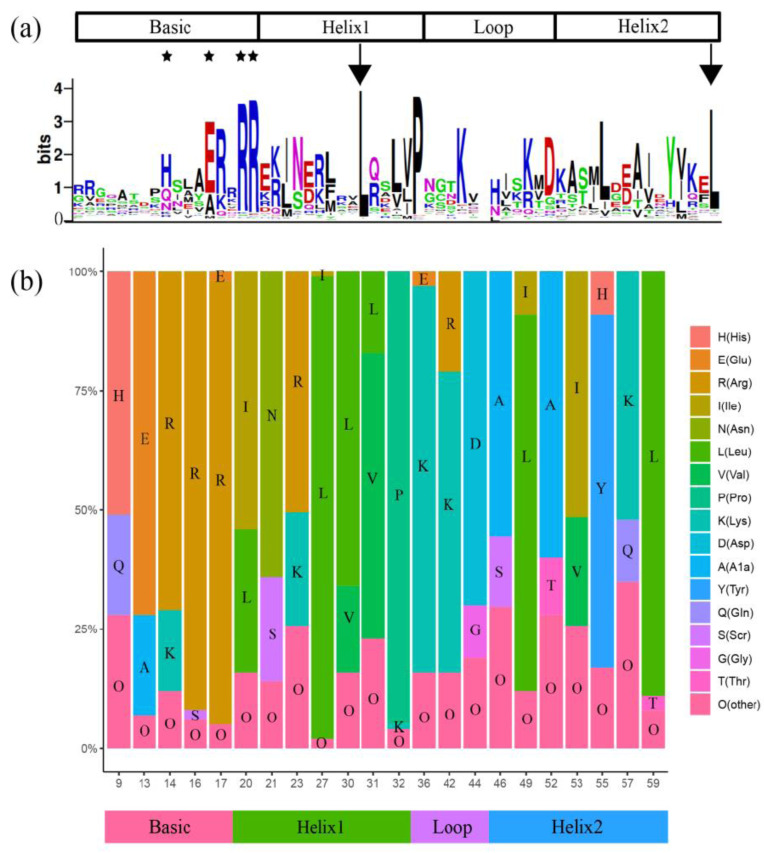
To evaluate the sequence conservation of these HvbHLHs, the conserved bHLH domain sequences were aligned to identify conserved sites. Totally, 5 amino acids in the basic region, 7 in the HLH region, 3 in the loop region and 7 in the second helix region were found to be highly conserved with the consensus sequence ratio more than 50% (Fig. 1, Table S4 (3.4MB, pdf) ). Among them, the residues Arg-16, Arg-17, Leu-27, Pro-32, and Leu-59 showed identical composition in all of the 141 HvbHLHs, which was consistent with
Fig. (1).
Sequence characteristics and conserved amino acids organization of the HvbHLHs. (a): Sequence logo of the HvbHLH domain by WEBLOGO. The H9, E13 and R16 amino acids in the basic domain are important for DNA binding as indicated by stars. Amino acids are vital for dimerization of the helix-loop-helix domain as indicated by arrows. (b): Distribution of amino acids in the bHLH consensus motif among HvbHLHs. The numbers of horizontal ordinate refer to the positions of the residues in the alignments of the studies. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
the previous results in Brachypodium [8]. It is reported that His-9, Glu-13, Arg-16 and Arg-17 in the basic region of the bHLH domain mainly contribute to DNA binding [36], and Leu-27 and Leu-59 in the first and second helix region are essential for the dimerization [37]. Obviously, the conserved ratio of Lys-36 (81%) within the loop region of barley was significantly higher than that of Brachypodium [8], rice and Arabidopsis [7].
Based on the criteria developed by Massari [6], the HvbHLH proteins were further categorized into two major groups, composing 139 DNA-binding and 2 non-DNA-binding proteins based on 17 N-terminal amino acids within the bHLH domain (Table S5 (3.4MB, pdf) ). In accordance with the presence or absence of amino acid residues Glu-13 and Arg-16 in the basic region, which were essential for binding E-box [37], the HvbHLHs were further divided into 132 putative E-box-binding and 7 non-E-box-binding proteins. The E-box-binding proteins can be further divided into G-box-binding protein (67) and non-G-box-binding protein (65) based on whether His-9, Glu-13 and Arg-17 are found or not in the basic region, which was necessary for recognition of the G-box [38].
3.3. Gene Structure and Conserved Motif Analysis
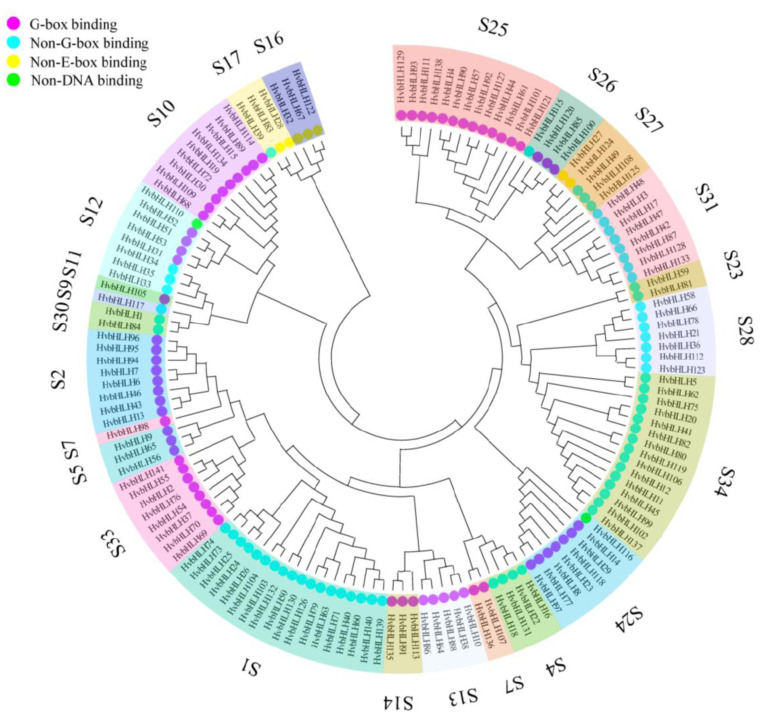
To understand the phylogenetic relationships of these HvbHLHs, an unrooted neighbor-joining tree was generated based on the alignment of 308 bHLH domain sequences, including 141 from barley and 167 from Arabidopsis. According to the criteria for bHLH classifications [2, 11], these bHLH proteins were categorized into 34 subfamilies and the HvbHLHs were divided into 24 subfamilies, of which two subfamilies (S33, S34) were newly identified in our study (Fig. 2 and S2). The number of proteins belonging to different subfamilies varied significantly, of which, the S1 subfamily possessed 18 HvbHLHs, whereas S7 only contained one protein (HvbHLH98), representing the largest and the smallest group, respectively. The G-box binding proteins were mainly grouped within the subfamilies S2, S5, S7, S10, S11, S12, S13, S14, S24, S25, S26 and S33, whereas the non-G-box binding proteins were mostly clustered into subfamilies S1, S4, S9, S12, S17, S23, S27, S28, S30, S31 and S34. Three subfamilies (S16, S17 and S27) were mainly composed of the non-E-box binding proteins and two subfamilies (S12 and S24) were mainly composed of the non-DNA binding proteins.
Fig. (2).
Phylogenetic tree of HvbHLHs based the conserved bHLH domain sequences using the neighbor-joining method. The 24 phylogenetic subfamilies were marked with different colors. The different color on the left side of HvbHLH represents the predicted DNA-binding activity of each protein: G-box in purple, Non-G-box in blue, Non-E-box in yellow, Non-DNA in green. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
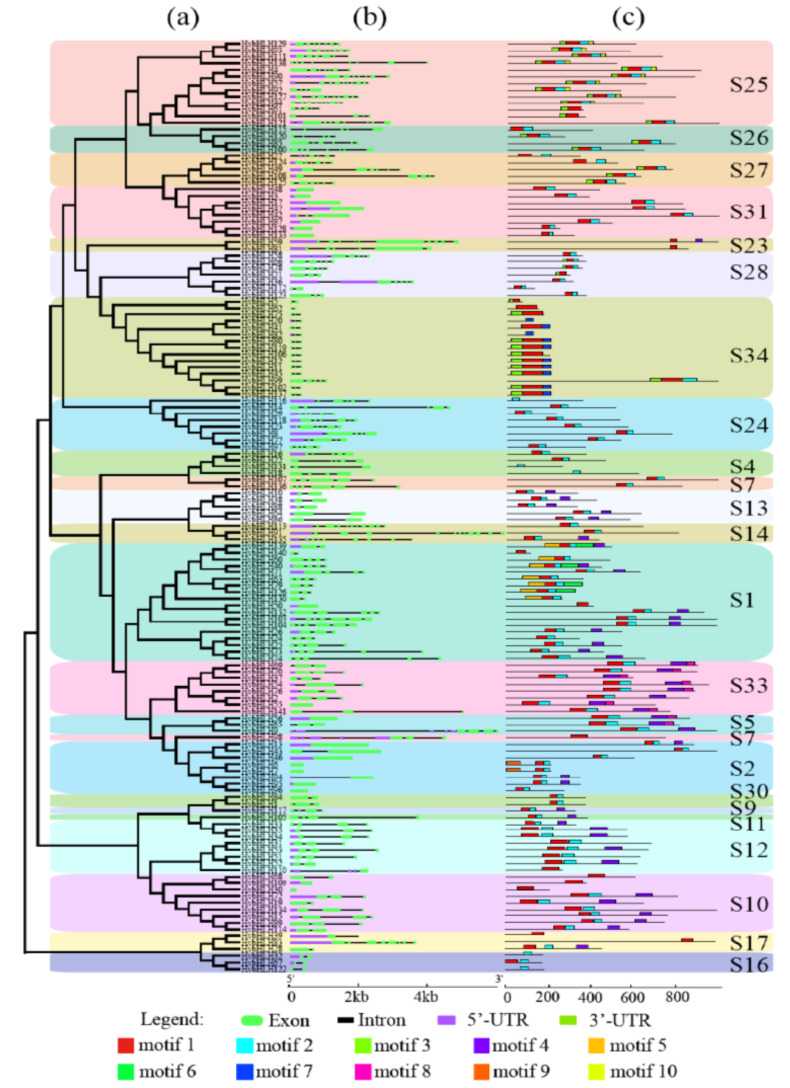
The exon-intron structure variation could provide important evolutionary clues for gene family [39]. The exon-intron organizations of HvbHLHs were also analyzed. The exon numbers ranged from 1 to 12 and exon length varied from 4 bp to 2,053 bp with an average of 219.2 bp. The variations of intron length were significantly higher than those of exon, which ranged from 50bp to 16,093bp with the average of 471.4bp. It is reported that intron gain or loss was influenced by the selection pressure during plant evolution, and intron evolved faster than exon as it can accumulate more mutations with no selective pressure [40]. Notably, the members within the same subfamily in the phylogenetic tree were found to share similar exon-intron structures. Genes within the subfamily S34 showed almost the same gene structure and abundance of intron, which was the most conserved group. We also observed that HvbHLHs within the subfamily S2 tended to be intron-less, while subfamily S14 possessed the most abundant introns with more than 9 introns per gene, which had more complicated gene structure patterns than other groups. The various exon-intron structures indicated that the genetic divergence might have occurred in HvbHLHs during the formation and evolution of a barley genome.
Additionally, the conserved protein motifs in HvbHLHs were identified by the MEME search tool. A total of ten conserved motifs were predicted (Fig. 3). Among them, motif 3 and 4 were predicted to be located at the C- and N-terminal, whereas other motifs were found to be located at the functional motif regions. Motif 1 and motif 2, located at the bHLH domains, were shared by 134 and 113 proteins,
Fig. (3).
The gene structure and motif composition of HvbHLHs. (a): Phylogenetic tree. (b): Gene structure. (c): Conserved motif analysis. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
respectively. Motif 7 was detected in 10 proteins within the subfamily S25. Motif 9 and motif 10 were unique to subfamilies S2 and S25, respectively. Additionally, motif 5 and motif 6 were only found in the subfamily S1.Obviously, the bHLH proteins clustered into the same phylogenetic group displayed the same motif organization, indicating that more similar evolutionary history and biological function were shared by the members within the same subfamilies [41].
3.4. Cis-element and miRNA Target Sites Prediction
In order to investigate the potential regulatory functions of the HvbHLHs, the upstream 1.5kb sequences from the transcription initiation sites were extracted to predict the cis-elements. Results showed that a total of 40 functional cis-regulatory elements were identified. The ABRE (ABA-responsive, 122 genes), CGTCA-motif (MeJA-responsive, 122 genes), TGACG-motif (MeJA-responsive, 122 genes) and G-box (light-responsive, 123 genes) were found in most of the HvbHLHs, while MSA-like domain involved in cell cycle regulation, and the AC-II domain, regulating xylem expression and phloem repression, were only found in 3 and 4 genes, respectively. In addition, we also observed a number of organogenesis-related (including cell cycle, xylem, meristem, endosperm and circadian), hormone-responsive (e.g. gibberellin, auxin, salicylic acid and ethylene), light-responsive as well as biotic and abiotic stress-related (e.g. wound, low-temperature, drought and anaerobic) cis-elements in the promoter regions of HvbHLHs (Table S6 (3.4MB, pdf) ), suggesting the potential functions to regulate diverse stress adaption and signal transduction processes in barley.
MicroRNAs (miRNAs) are a class of endogenous, non-coding RNAs with the size of 21 to 25 nucleotides, which play crucial roles in post-transcriptional regulation of gene expression by guiding target mRNAs’ cleavage or translational inhibition [42]. In this study, the putative miRNAs targeted HvbHLHs were also analyzed. Results showed that a total of 19 miRNA-bHLH interaction pairs, referring to 15 HvbHLHs targeted by 12 miRNAs, were detected (Table S7 (3.4MB, pdf) ). Most miRNAs silenced the expression of HvbHLHs through the transcript cleavage, while HvbHLH47 and
HvbHLH26 were predicted to be inhibited by the translation of miRNAs. Furthermore, all the miRNA target sites were found within the CDS region but outside the bHLH domain, of which 10 and 9 pairs were predicted to be targeted upstream and downstream of bHLH domain, respectively. These results indicated that miRNA could be involved in regulating the expression of HvbHLHs and further study on the miRNA-mediated post-transcriptional regulation network of HvbHLHs will help to understand the roles of HvbHLHs in growth and development as well as stress responses of barley.
3.5. Gene Duplication and Synteny Analysis
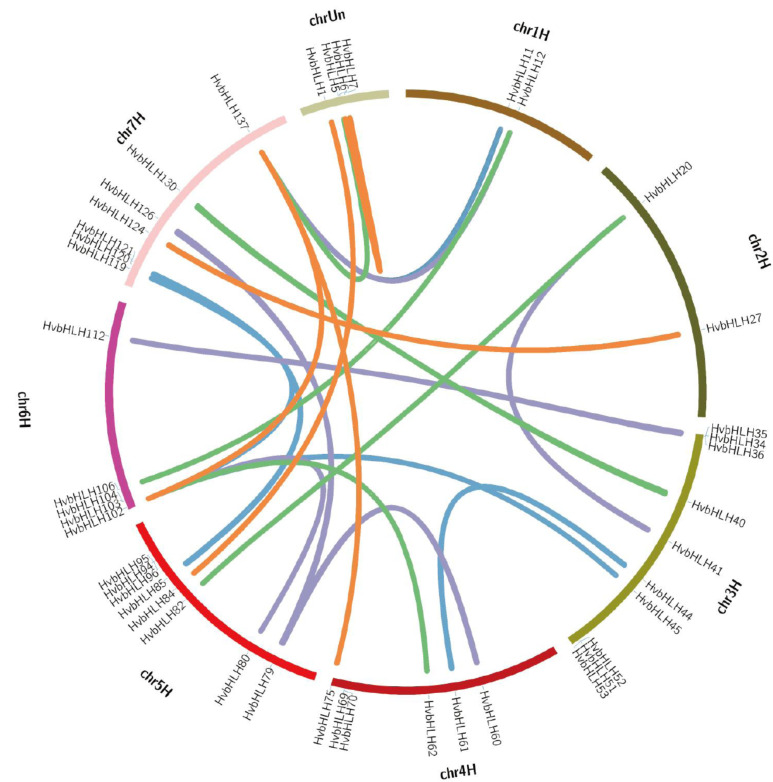
To get insight into the mechanism of the expansion of HvbHLHs, the duplication events were further investigated through a genome-wide synteny analysis. A previous study revealed that some tightly packed bHLH members are most likely to be derived from tandem repeat events in rice and Arabidopsis [7]. In this study, a total of 30 gene pairs composed of 46 genes were identified as segmental duplications, whereas no tandem duplication was found, suggesting that segmental duplication was responsible for the expansion of HvbHLHs (Fig. 4). The segmental duplications were mainly found at chromosome 3, 5 and 7. Intriguingly, all of the 15 genes in the subfamily S34 were subjected to segmental duplications, suggesting that there was specific evolution pressure to drive the segmental duplication of these genes. In order to examine the selection effect, the substitution rate of non-synonymous (Ka) versus synonymous (Ks) was calculated (Table S8 (3.4MB, pdf) ), wherein Ka/Ks < 1 means purifying selection, Ka/Ks = 1 means neutral selection and Ka/Ks > 1 means positive selection [43]. The Ka/Ks ratio for duplicated genes of HvbHLHs ranged from 0 to 0.4049, with an average of 0.09, indicating that they were subjected to strong purifying selection pressure during the expansion process.
Fig. (4).
Genomic distribution of bHLH genes and the gene duplication in barley. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
The Ka/Ks of the orthologous genes between the wild and cultivated barley was also calculated to determine the evolutionary relationships of HvbHLHs. Using the same method, a total of 142 bHLH genes were identified in wild barley (Table S9 (3.4MB, pdf) ) and named as HsbHLH1 to HsbHLH142. Through the orthologous analysis (Fig. S3 (3.4MB, pdf) ), a total of 108 orthologous gene pairs were found (Table S10 (3.4MB, pdf) ). Crops domestication was the result of a positive selective process, leading to wild species that are exposed to new selective environments associated with human demand [44, 45]. Human artificial selection for crops produced a suite of shared phenotypic changes in grain crops referred to as ‘the domestication syndrome’ with various genes [46]. The Ka/Ks ratio between wild and domesticated barley was calculated and the value of 12 HvbHLH-HsbHLH gene pairs was found to be more than 1, which was considered to be positive selected genes. Based on the orthologous analysis of Arabidopsis, these genes were found to be involved in root initiation [47], cell elongation [48], light signal transduction [49, 50], and cold response [51], which reflected the barley domestication process. The Ka/Ks values of the remaining 96 pairs were found to be lower than 1, indicating that these genes have undergone intense purifying selection during domestication.
Finally, the syntenic relationships with the other six plant species were detected. The whole genome-wide orthologous analysis resulted in 44, 87, 78, 41, 46, and 46 syntenic relationships with Arabidopsis thaliana, Brachypodium distachyon, Oryza sativa, Solanum lycopersicum, Solanum tuberosum, and Vitis vinifera, respectively (Table S11 (3.4MB, pdf) and Fig. S4 (3.4MB, pdf) ). The average value of Ka/Ks was 0.2083 between barley and Brachypodium, followed by rice (0.1884), suggesting that these HvbHLHs were subjected to extensive purifying selection. It is noteworthy that most of the HvbHLHs showed biasness on specific chromosomes of the other six species, indicating that chromosomal rearrangement events like duplication and inversion might predominantly shape the composition and organization of bHLH genes in these genomes.
3.6. Expression Profiles of these HvbHLHs
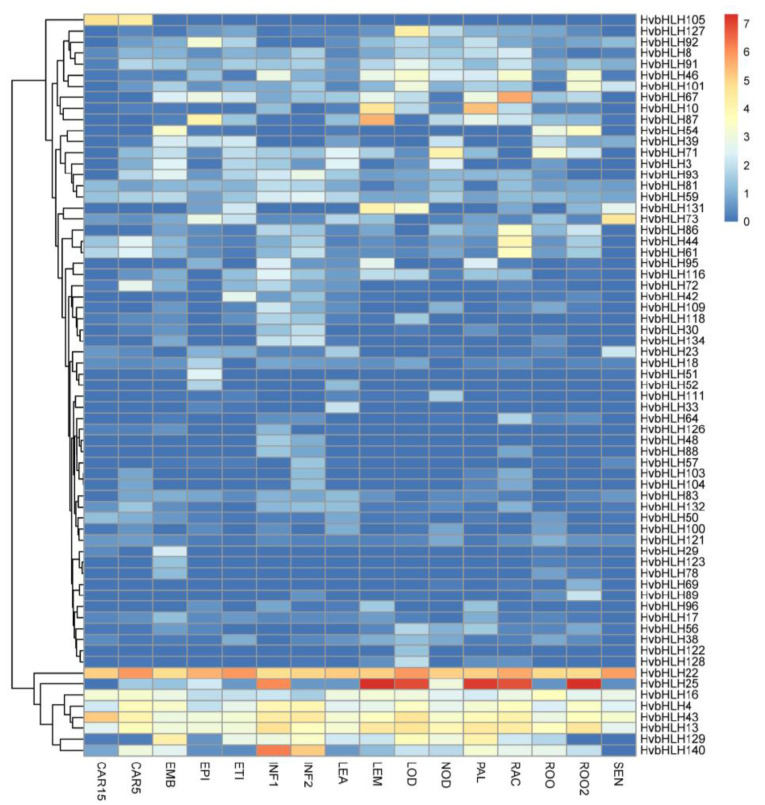
The Spatio-temporal expression profiles of these HvbHLHs were investigated at 16 various developmental stages and tissues using publically available RNA-seq samples (Fig. 5). A total of 67 HvbHLHs expressed in at least one stage or tissue with the FPKM value more than 1.0. Among them, 5 genes (HvbHLH4, HvbHLH13, HvbHLH16, HvbHLH22 and HvbHLH43) showed relatively high expression at all of the tested stages and tissues, suggesting that they played indispensable roles in barley growth and development. Remarkably, HvbHLH22 was stably highly expressed in different organs, suggesting HvbHLH22 may be considered as a housekeeping gene. The tissue- and stage- specific bHLHs were also identified. HvbHLH29 and HvbHLH123 showed preferential expression in embryo while HvbHLH105 and HvbHLH72 were found to be mainly expressed in bracts. The ortholog of HvbHLH105 in Arabidopsis is AtRGE1. A previous study showed that AtRGE1 was highly expressed in the endosperm at the heart stage of embryo development and played an important role in controlling embryo growth [52]. Moreover, HvbHLH48 exhibited the highest expression in the young inflorescences while with extremely low expression levels in other detected samples. Interestingly, its orthologous gene in rice, Lax-panicle (OsLAX) also displayed a specific expression in inflorescence, which determined the inflorescence architecture by controlling rachis-branch and spikelet development in rice. It indicated that HvbHLH48 might share similar functions in barley [53]. Additionally, HvbHLH134 was also found to be specially expressed in inflorescence, while its ortholog in Arabidopsis, SPEECHLESS (AtSPCH), is reported to be indispensable for the asymmetric divisions of stomatal lineage, indicating that HvbHLH134 may have different functions in barley compared to AtSPCH [54]. Most of the HvbHLHs had diverse tissue-specific expression patterns, highlighting the extensive involvement of HvbHLHs in regulating various organs’ development.
Fig. (5).
Hierarchical clustering of expression profiles of HvbHLHs across different stages. CAR15: bracts removed grains at 5DPA; CAR5: bracts removed grains at 5DPA; EMB: embryos dissected from 4d-old germinating grains; EPI: epidermis with 4 weeks old; ETI: etiolated from 10-day old seedling; INF1: young inflorescences with 5 mm; INF2: young inflorescences with 1–1.5 cm; LEA: shoot with the size of 10 cm from the seedlings; LEM: lemma with 6 weeks after anthesis; LOD: lodicule with 6 weeks after anthesis; NOD: developing tillers at six-leaf stage; PAL: 6-week old palea; RAC: rachis with 5 weeks after anthesis; ROO2: root from 4-week old seedlings; ROO: Roots from the seedlings at 10 cm shoot stage; SEN: senescing leaf. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
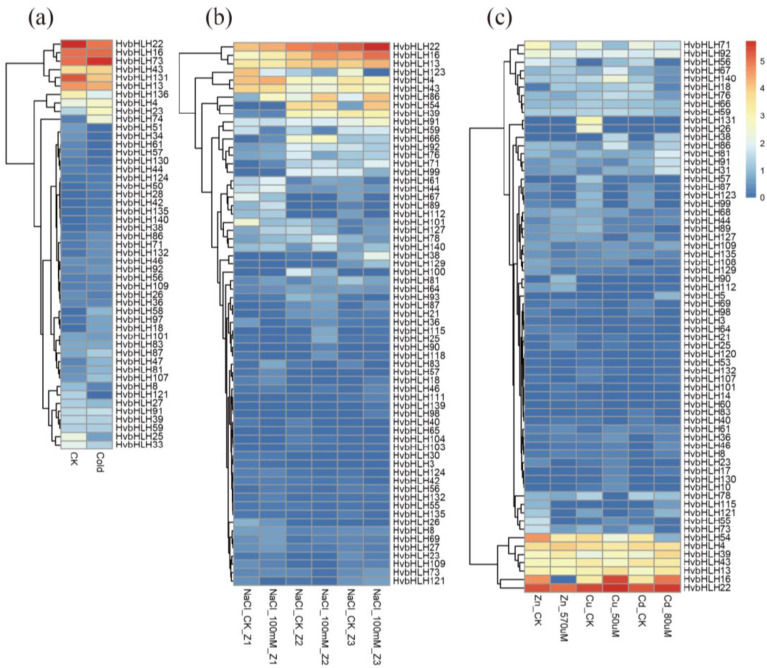
In order to get preliminary information about the roles of HvbHLHs in response to abiotic stresses, the expression patterns of HvbHLHs under cold, salt and metal poisoning conditions were also analyzed. Results showed that 49 genes were expressed under cold stress (Fig. 6a), of which 13 and 7 genes were significantly up-regulated and down-regulated, respectively. HvbHLH74 showed about 19.7 fold higher expressions under cold stress compared to control. Its orthologous gene in rice, OsPIL12 had the ability to interact with the biological clock component OsPRR1 to control circadian rhythms, suggesting this gene may have different functions in barley [55].
Fig. (6).
Hierarchical clustering of expression profiles of barley HvbHLHs under five stressed conditions. (a): Cold stress; (b): Salt stress; (c): Zinc, copper and cadmium stress. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
The expression profile of bHLH genes under salt stress was further investigated (Fig. 6b). In response to the high concentration of salt, 12, 10 and 12 genes were identified as up-regulated genes in meristematic root, elongation zone and meristematic zone, respectively, of which, the expression level of HvbHLH46 was more than 25.22 times higher at the elongation zone and HvbHLH54 showed 25.07-fold higher expression level at the meristematic zone under salt stress. It is noteworthy that HvbHLH86 was up-regulated at all tested samples, including meristematic root (6.62 times), elongation zone (3.79 times) and meristematic zones (5.57 times). Previous studies indicated that the knockout lines of AtbHLH106 (orthologous to HvbHLH86) showed more sensitivity to NaCl and over-expression of AtbHLH106 could enhance the NaCl tolerance in Arabidopsis [56]. In addition, we identified 10, 14 and 9 genes to be down-regulated at the meristematic zone, elongation zone and root of meristem. Among them, HvbHLH59 showed 8.62 and 6.55 times lower expressions at the meristematic zone and elongation zone under salt treatment compared to that of control, suggesting that these genes may act as the negative regulators in response to stress salt stress in barley. Increasing evidence highlights the potential roles of bHLH transcription factors that could be functional as the negative regulators in various biological processes. For example, bHLH transcription factor Paclobutrazol Resistance 6 (PRE6) is a transcription repressor that negatively regulates auxin responses in Arabidopsis through the direct interaction with Auxin Response Factors 5 (ARF5) and ARF8 [57]. Tanabe et al., also reported the transcription factor bHLH11, a negative regulator of Fe homeostasis in plants [58]. Additionally, the bHLH transcription factor BEE was found to be a redundant negative regulator involving in Arabidopsis drought and salinity tolerance [59]. In summary, HvbHLH59 may also play a role of a negative regulator to regulate salt stress response in barley.
Finally, the expression patterns of these genes under metal poisoning stress were determined (Fig. 6c). Results showed a total of 11, 14 and 20 up-regulated HvbHLHs that were identified under zinc, copper and cadmium ion stresses, and 17, 22 and 18 down-regulated genes were also found, respectively. It is noteworthy that HvbHLH26 was differentially expressed under all the 3 stress conditions. Homology analysis revealed that this gene is required for iron deficiency to regulate iron uptake in rice (OsFIT1) [60]. Besides, a previous study showed that OsIRO2 (orthologous to
HvbHLH53) was an essential regulator involved in iron uptake under Fe-deficient conditions and it was responsible for maintaining cellular zinc availability in rice [61-63]. In our study, the expression patterns of HvbHLH53 under zinc and cadmium were 14.11 and 19.17 times significantly higher than that of control.
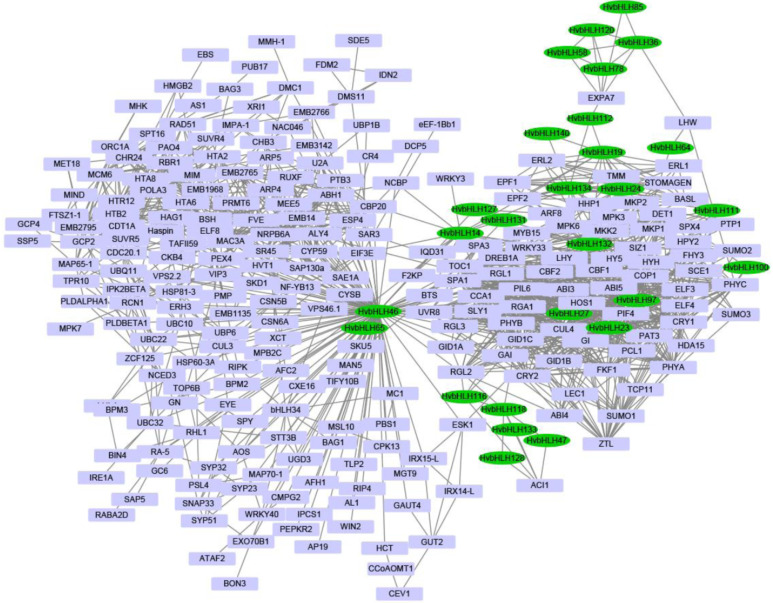
3.7. Co-expression Network Analysis between bHLHs and Other Genes in Barley
To reveal the regulatory function of bHLHs in relation to the other barley genes, the co-expression network was constructed based on the available 148 RNA-seq samples (Fig. 7 and Table S12 (3.4MB, pdf) ). The top 5% related gene nodes were filtered based on the WGCNA weighted values and only the correlations among the selected nodes were observed. The co-expression network resulted in a total of 1,324 branches composed of 28 HvbHLHs and 279 other genes. Among them, 89 (31.90%) genes were co-expressed with HvbHLH46, suggesting the central regulatory role of HvbHLH46 in the co-expression network. Six HvbHLHs (HvbHLH36, HvbHLH58, HvbHLH78, HvbHLH85, HvbHLH112 and HvbHLH120) were co-expressed with EXP7. Previous studies reported that EXPA7 played crucial roles in the regulation of root hair elongation and growth in plants [64]. Other five HvbHLHs (HvbHLH16, HvbHLH23, HvbHLH46, HvbHLH97, HvbHLH116 and HvbHLH118) were predicted to be co-expressed with RGL2, which has a clear function in the regulation of flower development, ovule number and fertility in Arabidopsis [65]. It is noteworthy that HvbHLH127 was co-expressed with ARF8. In Arabidopsis, the module AtPRE6 (ortholog of HvbHLH27)-AtARF8
Fig. (7).
The co-expression network analysis of HvbHLHs with other barley genes. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
has been validated to regulate auxin responses [57], suggesting that HvbHLH127 could function on HvARF8 to regulate auxin responses in barley. Remarkably, the gibberellin-insensitive gene GAI was predicted to be co-expressed with HvbHLH14, HvbHLH23, HvbHLH46, HvbHLH97 and HvbHLH116. In Arabidopsis, a light-labile basic helix-loop-helix protein PIL5 could increase the expression of GAI through directly binding to the promoter region of GAI while it did not regulate gibberellin metabolic-related genes in darkness [66]. Moreover, several light responsive motifs such as G-box, GATA-motif, GT1-motif, Sp1, TCCC-motif and TCT-motif were abundantly found within the promoter regions of these genes. Thus, we postulated that these HvbHLHs might be functional as the candidate genes in relation to photosynthesis signaling and circadian rhythm management in barley.
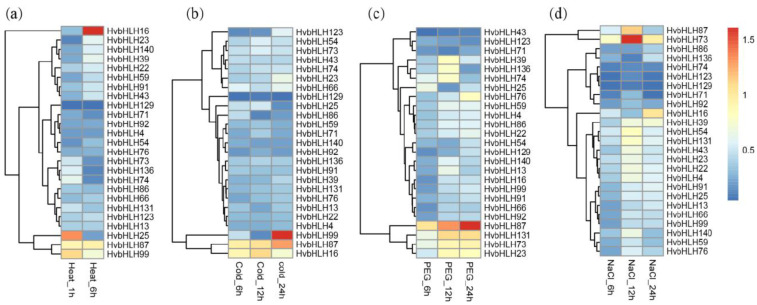
3.8. Validation of the Expression of HvbHLHs in Response to Abiotic Stresses by qRT-PCR Analysis
To validate the expression of these HvbHLHs, 25 stress-responsive candidates revealed by RNA-seq analysis were selected to investigate their expression levels under diverse abiotic stresses by qRT-PCR approach (Fig. 8). Results showed 11 HvbHLHs were up-regulated and 2 HvbHLHs were down-regulated under drought stress at all of the 3-time points (6, 12 and 24h). HvbHLH71 and HvbHLH129 were up-regulated at 24h, whereas HvbHLH136 down-regulated at 24h. In addition, the expression patterns seem to link with the cis-elements in the promoter sequences. HvbHLH39, HvbHLH86 and HvbHLH87 were significantly highly expressed under drought stress, and several MYS elements (MYB binding site involved in drought-induced) were found in the promoter regions of these genes.
Fig. (8).
The expression levels of the 25 HvbHLHs under diverse abiotic stresses were investigated using qRT-PCR method. The relative expression levels under: (a): Heat treatment; (b): Cold treatment; (c): Drought treatment and (d): Salt treatment. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
Under cold treatment, a total of 7 HvbHLHs were up-regulated and 11 HvbHLHs were down-regulated at 6, 12 and 24h treatment. The LTR cis-acting element that is involved in low-temperature responsiveness within the promoter regions was also predicted among these differentially expressed genes, suggesting that the cis-elements may have potential regulatory roles in gene-specific expressions. There were 2 HvbHLHs (HvbHLH23 and HvbHLH136) that were found to be down-regulated at 6h, but up-regulated at 12h and 24h. Remarkably, the expression levels of HvbHLH99 and HvbHLH87 were significantly increased with 20.24 and 10.57 times than control at 24h.
Under heat treatment, only 5 up-regulated and 7 down-regulated genes were detected at 1h and 6h. Notably, the expression of HvbHLH16 was significantly 21.04 times higher than that of control. Under salt treatment, only four genes (HvbHLH23, HvbHLH54, HvbHLH73 and HvbHLH131) were up-regulated at all the points, whereas five genes (HvbHLH71, HvbHLH74, HvbHLH86, HvbHLH92 and HvbHLH129) were found to be down-regulated. In addition, HvbHLH87 showed a relatively higher expression at 6, 12 and 24h. In addition, a series of HvbHLHs were found to be related to multiple stresses, including HvbHLH99 induced by cold and heat, HvbHLH73 by salt and drought, HvbHLH16 by cold, salt and drought, as well as HvbHLH87 by cold, heat, salt and drought, which could be used as invaluable gene resources for enhancement of barley stress tolerance in future.
Finally, most of the qRT-PCR results were highly consistent with that of RNA-seq. For example, HvbHLH23, HvbHLH73, HvbHLH74 and HvbHLH87 were up-regulated while HvbHLH131 and HvbHLH136 were down-regulated in both RNA-seq and qRT-PCR when exposed to low temperature. Additionally, both RNA-seq and qRT-PCR showed that HvbHLH23 and HvbHLH66 were up-regulated and HvbHLH123 and HvbHLH129 were down-regulated under salt stress. However, some inconsistent results were also observed. RNA-seq data revealed that HvbHLH86 was significantly up-regulated at three zones of barley root under salt stress, while qRT-PCR showed HvbHLH86 to be down-regulated at 6, 12 and 24h. We proposed that the inconsistent results were probably caused by different genotypes used, suggesting that some HvbHLHs have a tissue-specific expression. The HvbHLHs mediated a complicated mechanism to regulate stress response in barley.
Conclusion
In this study, we identified a total of 141 HvbHLHs and categorized them into 24 subfamilies, as supported by the phylogenetic analysis. Members within the same subfamily shared the similar protein composition and gene structure. Segmental duplications were found to contribute significantly to the expansion of these HvbHLHs. Expression analysis revealed that HvbHLHs were widely expressed in 16 different tissues and also involved in response to diverse abiotic stresses. Furthermore, 28 HvbHLHs were found to be co-expressed with 279 functional genes, which underpinned the regulatory roles of HvbHLHs. Finally, the expression of 25 stress-responsive HvbHLHs was validated by the qRT-PCR method and some candidates were obtained for further functional study. Taken together, this study provides a solid foundation for further evolutionary and functional investigations of HvbHLHs in the future.
Acknowledgements
We are grateful to the three anonymous reviewers for their insightful comments. We also thank Dr. Ruimin Li for his constructive comments on data analysis, and we thank the High-Performance Computing Center of Northwest A&F University for providing computational resources in this work.
Ethics Approval and Consent to Participate
Not applicable.
Human and Animal Rights
No Animals/Humans were used for studies that are the basis of this research.
Consent for Publication
Not applicable.
Availability of Data and Materials
The data supporting the findings of the article is available within the article and its supplementary material.
Funding
This research was funded by the Jiangxi Natural Science Foundation (Grant No. 20202BAB215002), the Science and Technology Research Project of Jiangxi Provincial Department of Education (Grant No. GJJ180241) and the Open Project Program of the State Key Laboratory of Crop Stress Biology in Arid Areas, Northwest A&F University (Grant No. CSBAA2019001). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
SUPPLEMENTARY MATERIAL
Supplementary material is available on the publisher’s website along with the published article.
References
- 1.Ledent V., Vervoort M. The basic helix-loop-helix protein family: comparative genomics and phylogenetic analysis. Genome Res. 2001;11(5):754–770. doi: 10.1101/gr.177001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carretero-Paulet L., Galstyan A., Roig-Villanova I., Martínez-García J.F., Bilbao-Castro J.R., Robertson D.L. Genome-wide classification and evolutionary analysis of the bHLH family of transcription factors in Arabidopsis, poplar, rice, moss, and algae. Plant Physiol. 2010;153(3):1398–1412. doi: 10.1104/pp.110.153593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Feller A., Machemer K., Braun E.L., Grotewold E. Evolutionary and comparative analysis of MYB and bHLH plant transcription factors. Plant J. 2011;66(1):94–116. doi: 10.1111/j.1365-313X.2010.04459.x. [DOI] [PubMed] [Google Scholar]
- 4.Murre C., McCaw P.S., Baltimore D. A new DNA binding and dimerization motif in immunoglobulin enhancer binding, daughterless, MyoD, and myc proteins. Cell. 1989;56(5):777–783. doi: 10.1016/0092-8674(89)90682-x. [DOI] [PubMed] [Google Scholar]
- 5.Atchley W.R., Terhalle W., Dress A. Positional dependence, cliques, and predictive motifs in the bHLH protein domain. J. Mol. Evol. 1999;48(5):501–516. doi: 10.1007/pl00006494. [DOI] [PubMed] [Google Scholar]
- 6.Massari M.E., Murre C. Helix-loop-helix proteins: regulators of transcription in eucaryotic organisms. Mol. Cell. Biol. 2000;20(2):429–440. doi: 10.1128/mcb.20.2.429-440.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li X., Duan X., Jiang H., Sun Y., Tang Y., Yuan Z., Guo J., Liang W., Chen L., Yin J. Genome-wide analysis of basic/helix-loop-helix transcription factor family in rice and Arabidopsis. Plant Physiol. 2006;141(4):1167–1184. doi: 10.1104/pp.106.080580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Niu X., Guan Y., Chen S., Li H. Genome-wide analysis of basic helix-loop-helix (bHLH) transcription factors in Brachypodium distachyon. BMC Genomics. 2017;18(1):619. doi: 10.1186/s12864-017-4044-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wei K., Chen H. Comparative functional genomics analysis of bHLH gene family in rice, maize and wheat. BMC Plant Biol. 2018;18(1):309. doi: 10.1186/s12870-018-1529-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang T., Lv W., Zhang H., Ma L., Li P., Ge L., Li G. Genome-wide analysis of the basic Helix-Loop-Helix (bHLH) transcription factor family in maize. BMC Plant Biol. 2018;18(1):235. doi: 10.1186/s12870-018-1441-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ke Y.Z., Wu Y.W., Zhou H.J., Chen P., Wang M.M., Liu M.M., Li P.F., Yang J., Li J.N., Du H. Genome-wide survey of the bHLH super gene family in Brassica napus. BMC Plant Biol. 2020;20(1):115. doi: 10.1186/s12870-020-2315-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hwang Y., Choi H.S. Tracheophytes contain conserved orthologs of a basic helix-loop-helix transcription factor that modulate ROOT HAIR SPECIFIC genes. Plant Cell. 2017;29(1):39–53. doi: 10.1105/tpc.16.00732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhu E., You C., Wang S., Cui J., Niu B., Wang Y., Qi J., Ma H., Chang F. The DYT1-interacting proteins bHLH010, bHLH089 and bHLH091 are redundantly required for Arabidopsis anther development and transcriptome. Plant J. 2015;83(6):976–990. doi: 10.1111/tpj.12942. [DOI] [PubMed] [Google Scholar]
- 14.Li S., Wang X., He S., Li J., Huang Q., Imaizumi T., Qu L., Qin G., Qu L.J., Gu H. CFLAP1 and CFLAP2 are two bHLH transcription factors participating in synergistic regulation of AtCFL1-mediated cuticle development in arabidopsis. PLoS Genet. 2016;12(1):e1005744. doi: 10.1371/journal.pgen.1005744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Luo J., Liu H., Zhou T., Gu B., Huang X., Shangguan Y., Zhu J., Li Y., Zhao Y., Wang Y. An-1 encodes a basic helix-loop-helix protein that regulates awn development, grain size, and grain number in rice. Plant Cell. 2013;25(9):3360–3376. doi: 10.1105/tpc.113.113589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu Y., Ji X., Nie X., Qu M., Zheng L., Tan Z., Zhao H., Huo L., Liu S., Zhang B. Arabidopsis AtbHLH112 regulates the expression of genes involved in abiotic stress tolerance by binding to their E-box and GCG-box motifs. New Phytol. 2015;207(3):692–709. doi: 10.1111/nph.13387. [DOI] [PubMed] [Google Scholar]
- 17.Seo J.S., Joo J., Kim M.J., Kim Y.K., Nahm B.H., Song S.I., Cheong J.J., Lee J.S., Kim J.K., Choi Y.D. OsbHLH148, a basic helix-loop-helix protein, interacts with OsJAZ proteins in a jasmonate signaling pathway leading to drought tolerance in rice. Plant J. 2011;65(6):907–921. doi: 10.1111/j.1365-313X.2010.04477.x. [DOI] [PubMed] [Google Scholar]
- 18.Kiribuchi K., Sugimori M., Takeda M., Otani T., Okada K., Onodera H., Ugaki M., Tanaka Y., Tomiyama-Akimoto C., Yamaguchi T. RERJ1, a jasmonic acid-responsive gene from rice, encodes a basic helix-loop-helix protein. Biochem. Biophys. Res. Commun. 2004;325(3):857–863. doi: 10.1016/j.bbrc.2004.10.126. [DOI] [PubMed] [Google Scholar]
- 19.Lau O.S., Song Z., Zhou Z., Davies K.A., Chang J., Yang X., Wang S., Lucyshyn D., Tay I.H.Z., Wigge P.A. Direct control of SPEECHLESS by PIF4 in the high-temperature response of stomatal development. Curr. Biol. 2018;28(8):1273–1280.e1273. doi: 10.1016/j.cub.2018.02.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang Y.J., Zhang Z.G., He X.J., Zhou H.L., Wen Y.X., Dai J.X., Zhang J.S., Chen S.Y. A rice transcription factor OsbHLH1 is involved in cold stress response. Theor. Appl. Genet. 2003;107(8):1402–1409. doi: 10.1007/s00122-003-1378-x. [DOI] [PubMed] [Google Scholar]
- 21.Martín G., Rovira A., Veciana N., Soy J., Toledo-Ortiz G., Gommers C.M.M., Boix M., Henriques R., Minguet E.G., Alabadí D. Circadian waves of transcriptional repression shape PIF-regulated photoperiod-responsive growth in Arabidopsis. Curr. Biol. 2018;28(2):311–318.e315. doi: 10.1016/j.cub.2017.12.021. [DOI] [PubMed] [Google Scholar]
- 22.Li K., Yu R., Fan L.M., Wei N., Chen H. DELLA-mediated PIF degradation contributes to coordination of light and gibberellin signalling in Arabidopsis. Nat. Commun. 2016;7:11868. doi: 10.1038/ncomms11868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chandler J.W., Cole M., Flier A., Werr W. BIM1, a bHLH protein involved in brassinosteroid signalling, controls Arabidopsis embryonic patterning via interaction with DORNROSCHEN and DORNROSCHEN-LIKE. Plant Mol. Biol. 2009;69(1-2):57–68. doi: 10.1007/s11103-008-9405-6. [DOI] [PubMed] [Google Scholar]
- 24.Tian H., Guo H., Dai X., Cheng Y., Zheng K., Wang X., Wang S. An ABA down-regulated bHLH transcription repressor gene, bHLH129 regulates root elongation and ABA response when overexpressed in Arabidopsis. Sci. Rep. 2015;5:17587. doi: 10.1038/srep17587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Takahashi Y., Kinoshita T., Matsumoto M., Shimazaki K. Inhibition of the Arabidopsis bHLH transcription factor by monomerization through abscisic acid-induced phosphorylation. Plant J. 2016;87(6):559–567. doi: 10.1111/tpj.13217. [DOI] [PubMed] [Google Scholar]
- 26.Wang H., Li Y., Pan J., Lou D., Hu Y., Yu D. The bHLH transcription factors MYC2, MYC3, and MYC4 are required for jasmonate-mediated inhibition of flowering in arabidopsis. Mol. Plant. 2017;10(11):1461–1464. doi: 10.1016/j.molp.2017.08.007. [DOI] [PubMed] [Google Scholar]
- 27.Cui Y., Chen C.L., Cui M., Zhou W.J., Wu H.L., Ling H.Q. Four IVa bHLH transcription factors are novel interactors of FIT and mediate JA inhibition of iron uptake in arabidopsis. Mol. Plant. 2018;11(9):1166–1183. doi: 10.1016/j.molp.2018.06.005. [DOI] [PubMed] [Google Scholar]
- 28.Mascher M., Gundlach H., Himmelbach A., Beier S., Twardziok S.O., Wicker T., Radchuk V., Dockter C., Hedley P.E., Russell J. A chromosome conformation capture ordered sequence of the barley genome. Nature. 2017;544(7651):427–433. doi: 10.1038/nature22043. [DOI] [PubMed] [Google Scholar]
- 29.Chen L., Hu W., Tan S., Wang M., Ma Z., Zhou S., Deng X., Zhang Y., Huang C., Yang G. Genome-wide identification and analysis of MAPK and MAPKK gene families in Brachypodium distachyon. PLoS One. 2012;7(10):e46744. doi: 10.1371/journal.pone.0046744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ostlund G., Schmitt T., Forslund K., Köstler T., Messina D.N., Roopra S., Frings O., Sonnhammer E.L. InParanoid 7: new algorithms and tools for eukaryotic orthology analysis. Nucleic Acids Res. 2010;38(Database issue):D196–D203. doi: 10.1093/nar/gkp931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang Y., Tang H., Debarry J.D., Tan X., Li J., Wang X., Lee T.H., Jin H., Marler B., Guo H. MCScanX: a toolkit for detection and evolutionary analysis of gene synteny and collinearity. Nucleic Acids Res. 2012;40(7):e49. doi: 10.1093/nar/gkr1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang Z. PAML 4: phylogenetic analysis by maximum likelihood. Mol. Biol. Evol. 2007;24(8):1586–1591. doi: 10.1093/molbev/msm088. [DOI] [PubMed] [Google Scholar]
- 33.Pertea M., Kim D., Pertea G.M., Leek J.T., Salzberg S.L. Transcript-level expression analysis of RNA-seq experiments with HISAT. StringTie and Ballgown. 2016;11(9):1650–1667. doi: 10.1038/nprot.2016.095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yue W., Nie X., Cui L., Zhi Y., Zhang T., Du X., Song W. Genome-wide sequence and expressional analysis of autophagy Gene family in bread wheat (Triticum aestivum L.). J. Plant Physiol. 2018;229:7–21. doi: 10.1016/j.jplph.2018.06.012. [DOI] [PubMed] [Google Scholar]
- 35.Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25(4):402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 36.Atchley W.R., Fitch W.M. A natural classification of the basic helix-loop-helix class of transcription factors. Proc. Natl. Acad. Sci. USA. 1997;94(10):5172–5176. doi: 10.1073/pnas.94.10.5172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ferré-D’Amaré A.R., Pognonec P., Roeder R.G., Burley S.K. Structure and function of the b/HLH/Z domain of USF. EMBO J. 1994;13(1):180–189. doi: 10.1002/j.1460-2075.1994.tb06247.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shimizu T., Toumoto A., Ihara K., Shimizu M., Kyogoku Y., Ogawa N., Oshima Y., Hakoshima T. Crystal structure of PHO4 bHLH domain-DNA complex: flanking base recognition. EMBO J. 1997;16(15):4689–4697. doi: 10.1093/emboj/16.15.4689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xu G., Guo C., Shan H., Kong H. Divergence of duplicate genes in exon-intron structure. Proc. Natl. Acad. Sci. USA. 2012;109(4):1187–1192. doi: 10.1073/pnas.1109047109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pyle A.M. The tertiary structure of group II introns: implications for biological function and evolution. Crit. Rev. Biochem. Mol. Biol. 2010;45(3):215–232. doi: 10.3109/10409231003796523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Toledo-Ortiz G., Huq E., Quail P.H. The Arabidopsis basic/helix-loop-helix transcription factor family. Plant Cell. 2003;15(8):1749–1770. doi: 10.1105/tpc.013839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bartel D.P. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116(2):281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 43.Lynch M., Conery J.S. The evolutionary fate and consequences of duplicate genes. Science. 2000;290(5494):1151–1155. doi: 10.1126/science.290.5494.1151. [DOI] [PubMed] [Google Scholar]
- 44.Purugganan M.D. Evolutionary insights into the nature of plant domestication. Curr. Biol. 2019;29(14):R705–R714. doi: 10.1016/j.cub.2019.05.053. [DOI] [PubMed] [Google Scholar]
- 45.Purugganan M.D., Fuller D.Q. The nature of selection during plant domestication. Nature. 2009;457(7231):843–848. doi: 10.1038/nature07895. [DOI] [PubMed] [Google Scholar]
- 46.Lai X., Yan L., Lu Y., Schnable J.C. Largely unlinked gene sets targeted by selection for domestication syndrome phenotypes in maize and sorghum. Plant J. 2018;93(5):843–855. doi: 10.1111/tpj.13806. [DOI] [PubMed] [Google Scholar]
- 47.Schlereth A., Möller B., Liu W., Kientz M., Flipse J., Rademacher E.H., Schmid M., Jürgens G., Weijers D. MONOPTEROS controls embryonic root initiation by regulating a mobile transcription factor. Nature. 2010;464(7290):913–916. doi: 10.1038/nature08836. [DOI] [PubMed] [Google Scholar]
- 48.Zhang L.Y., Bai M.Y., Wu J., Zhu J.Y., Wang H., Zhang Z., Wang W., Sun Y., Zhao J., Sun X. Antagonistic HLH/bHLH transcription factors mediate brassinosteroid regulation of cell elongation and plant development in rice and Arabidopsis. Plant Cell. 2009;21(12):3767–3780. doi: 10.1105/tpc.109.070441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liu Y., Li X., Li K., Liu H., Lin C. Multiple bHLH proteins form heterodimers to mediate CRY2-dependent regulation of flowering-time in Arabidopsis. PLoS Genet. 2013;9(10):e1003861. doi: 10.1371/journal.pgen.1003861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Castelain M., Le Hir R., Bellini C. The non-DNA-binding bHLH transcription factor PRE3/bHLH135/ATBS1/TMO7 is involved in the regulation of light signaling pathway in Arabidopsis. Physiol. Plant. 2012;145(3):450–460. doi: 10.1111/j.1399-3054.2012.01600.x. [DOI] [PubMed] [Google Scholar]
- 51.Petridis A., Döll S., Nichelmann L., Bilger W., Mock H.P. Arabidopsis thaliana G2-LIKE FLAVONOID REGULATOR and BRASSINOSTEROID ENHANCED EXPRESSION1 are low-temperature regulators of flavonoid accumulation. New Phytol. 2016;211(3):912–925. doi: 10.1111/nph.13986. [DOI] [PubMed] [Google Scholar]
- 52.Kondou Y., Nakazawa M., Kawashima M., Ichikawa T., Yoshizumi T., Suzuki K., Ishikawa A., Koshi T., Matsui R., Muto S. RETARDED GROWTH OF EMBRYO1, a new basic helix-loop-helix protein, expresses in endosperm to control embryo growth. Plant Physiol. 2008;147(4):1924–1935. doi: 10.1104/pp.108.118364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Komatsu M., Maekawa M., Shimamoto K., Kyozuka J. The LAX1 and FRIZZY PANICLE 2 genes determine the inflorescence architecture of rice by controlling rachis-branch and spikelet development. Dev. Biol. 2001;231(2):364–373. doi: 10.1006/dbio.2000.9988. [DOI] [PubMed] [Google Scholar]
- 54.de Marcos A., Houbaert A. A mutation in the bHLH domain of the SPCH transcription factor uncovers a BR-dependent mechanism for stomatal development. 2017. [DOI] [PMC free article] [PubMed]
- 55.Nakamura Y., Kato T., Yamashino T., Murakami M., Mizuno T. Characterization of a set of phytochrome-interacting factor-like bHLH proteins in Oryza sativa. Biosci. Biotechnol. Biochem. 2007;71(5):1183–1191. doi: 10.1271/bbb.60643. [DOI] [PubMed] [Google Scholar]
- 56.Ahmad A., Niwa Y., Goto S., Ogawa T., Shimizu M., Suzuki A., Kobayashi K., Kobayashi H. bHLH106 integrates functions of multiple genes through their g-box to confer salt tolerance on arabidopsis. PLoS One. 2015;10(5):e0126872. doi: 10.1371/journal.pone.0126872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zheng K., Wang Y., Zhang N., Jia Q., Wang X., Hou C., Chen J.G., Wang S. Involvement of PACLOBUTRAZOL RESISTANCE6/KIDARI, an atypical bHLH transcription factor, in auxin responses in Arabidopsis. Front. Plant Sci. 2017;8:1813. doi: 10.3389/fpls.2017.01813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tanabe N., Noshi M., Mori D., Nozawa K., Tamoi M., Shigeoka S. The basic helix-loop-helix transcription factor, bHLH11 functions in the iron-uptake system in Arabidopsis thaliana. J. Plant Res. 2019;132(1):93–105. doi: 10.1007/s10265-018-1068-z. [DOI] [PubMed] [Google Scholar]
- 59.Moreno J.E., Moreno-Piovano G., Chan R.L. The antagonistic basic helix-loop-helix partners BEE and IBH1 contribute to control plant tolerance to abiotic stress. Plant Sci. 2018;271:143–150. doi: 10.1016/j.plantsci.2018.03.024. [DOI] [PubMed] [Google Scholar]
- 60.Colangelo E.P., Guerinot M.L. The essential basic helix-loop-helix protein FIT1 is required for the iron deficiency response. Plant Cell. 2004;16(12):3400–3412. doi: 10.1105/tpc.104.024315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Masuda H., Aung M.S., Kobayashi T., Hamada T., Nishizawa N.K. Enhancement of iron acquisition in rice by the mugineic acid synthase gene with ferric iron reductase gene and OsIRO2 confers tolerance in submerged and nonsubmerged calcareous soils. Front. Plant Sci. 2019;10:1179. doi: 10.3389/fpls.2019.01179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ogo Y., Itai R.N., Kobayashi T., Aung M.S., Nakanishi H., Nishizawa N.K. OsIRO2 is responsible for iron utilization in rice and improves growth and yield in calcareous soil. Plant Mol. Biol. 2011;75(6):593–605. doi: 10.1007/s11103-011-9752-6. [DOI] [PubMed] [Google Scholar]
- 63.Ogo Y., Itai R.N., Nakanishi H., Kobayashi T., Takahashi M., Mori S., Nishizawa N.K. The rice bHLH protein OsIRO2 is an essential regulator of the genes involved in Fe uptake under Fe-deficient conditions. Plant J. 2007;51(3):366–377. doi: 10.1111/j.1365-313X.2007.03149.x. [DOI] [PubMed] [Google Scholar]
- 64.ZhiMing Y.; Bo, K.; XiaoWei, H.; ShaoLei, L.; YouHuang, B.; WoNa, D.; Ming, C.; Hyung-Taeg, C.; Ping, W. Root hair-specific expansins modulate root hair elongation in rice. Plant J. 2011;66(5):725–734. doi: 10.1111/j.1365-313X.2011.04533.x. [DOI] [PubMed] [Google Scholar]
- 65.Gómez M.D., Fuster-Almunia C., Ocaña-Cuesta J., Alonso J.M., Pérez-Amador M.A. RGL2 controls flower development, ovule number and fertility in Arabidopsis. Plant Science: Int. J. Exp. Plant Biol. 2019;281:82–92. doi: 10.1016/j.plantsci.2019.01.014. [DOI] [PubMed] [Google Scholar]
- 66.Oh E., Yamaguchi S., Hu J., Yusuke J., Jung B., Paik I., Lee H.S., Sun T.P., Kamiya Y., Choi G. PIL5, a phytochrome-interacting bHLH protein, regulates gibberellin responsiveness by binding directly to the GAI and RGA promoters in Arabidopsis seeds. Plant Cell. 2007;19(4):1192–1208. doi: 10.1105/tpc.107.050153. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material is available on the publisher’s website along with the published article.
Data Availability Statement
The data supporting the findings of the article is available within the article and its supplementary material.