Abstract
It is well-known that stroke is one of the leading causes of death and disability all over the world. After a stroke, the blood-brain barrier subsequently breaks down. The BBB consists of endothelial cells surrounded by astrocytes. Microglia, considered the long-living resident immune cells of the brain, play a vital role in BBB function. M1 microglia worsen BBB disruption, while M2 microglia assist in repairing BBB damage. Microglia can also directly interact with endothelial cells and affect BBB permeability. In this review, we are going to discuss the mechanisms responsible for the dual role of microglia in BBB dysfunction after stroke.
Keywords: Blood-brain barrier, stroke, microglia, polarization, inflammation, endothelial cells
1. INTRODUCTION
Stroke is still one of the main causes of long-term disability, leading to a substantial burden to the healthcare system and economy [1]. Currently, the only available treatment for stroke consists of thrombolytic therapy and embolectomy, which have a very limited time window [2]. Previous studies have demonstrated that targeting inflammation and immunomodulation may offer an extended window of application for stroke therapy [3]. Furthermore, numerous studies have established that neuroinflammatory mechanisms greatly contribute to blood-brain barrier (BBB) damage and disruption following ischemic stroke.
The blood-brain barrier is formed by a continuous layer of non-fenestrated endothelial cells (ECs) connected by tight-junctions (TJs). Together with pericytes, astrocytes, microglia and the surrounding basement membrane, the BBB forms a selective physical barrier that separates the bloodstream from the brain parenchyma [4, 5]. The formation of very closed TJs between cerebral ECs comprises the unique barrier properties of the BBB [6]. TJs provide asymmetrical distribution of the apical and basolateral cell membranes of the endothelia and assist in controlling the paracellular permeability across the BBB [7]. Three transmembrane proteins play an important role in maintaining the integrity of TJs: claudins, occludins, and junctional adhesion molecules (JAMs). JAMs interact with intracellular scaffold proteins, such as zonula occludens (ZO)-1, ZO-2, and ZO-3, which are anchored to actin and the cytoskeleton via cingulin dimers [8, 9]. The BBB plays a crucial role in maintaining the homeostatic microenvironment of the CNS and protecting the brain tissue from exposure to potentially toxic substances [10]. During ischemic stroke, the BBB is disrupted, leading to vasogenic edema formation and hemorrhagic transformation [11-13]. Multiple changes at the BBB have been found in stroke, such as inflammation, decreased TJs protein expression, and extended transendothelial permeability [14, 15]. All components of the BBB can be affected by ischemia, including endothelial cells, astrocytes, pericytes, tight-junctions, and the extracellular matrix [9, 16]. Clinical and experimental studies suggest that BBB dysfunction is a common consequence of stroke, and that a disruption in BBB integrity tends to worsen the outcome of stroke patients [16-19]. Extensive animal studies indicate that there are two phases of BBB compromise after stroke onset. Firstly, 4-6 h after ischemia, an immediate early phase of enhanced permeability occurs. Secondly, 2-3 days after stroke, a delayed opening of the BBB is observed [20].
Microglia, which constitute up to 10% of the total cell population of the brain [21, 22], are resident macrophages in the CNS, and function as part of the mononuclear phagocyte system. They originate from the mesoderm/mesenchyme [10], arise in the brain from the early developmental stage and persist into adulthood. In the resting, physiological state, the resident microglia constantly monitor the environment. The microglial surveillance supervises the number of synapses, controls neuronal firings, and removes debris, thus maintaining homeostasis in the CNS [23, 24]. In addition, microglia play a vital role in the immune response and are an important component of the neurovascular unit [25, 26]. Microglia actively communicate with the endothelium, and regulate the BBB, both during development and after stroke [10].
2. MICROGLIA POLARIZATION DIRECTS BBB FUNCTION AFTER STROKE
After migrating into the brain from the mesoderm/mesenchyme during development, microglia acquire a specific ramified morphological phenotype with low phagocytic properties, named ‘resting microglia’ [10, 27]. In response to brain injury and immunological stimuli, microglia are first responders that are activated, and alter their phenotype from resting microglia to activated microglia. The activated phenotype is characterized by an enlarged cell body with short thick processes [27-29].
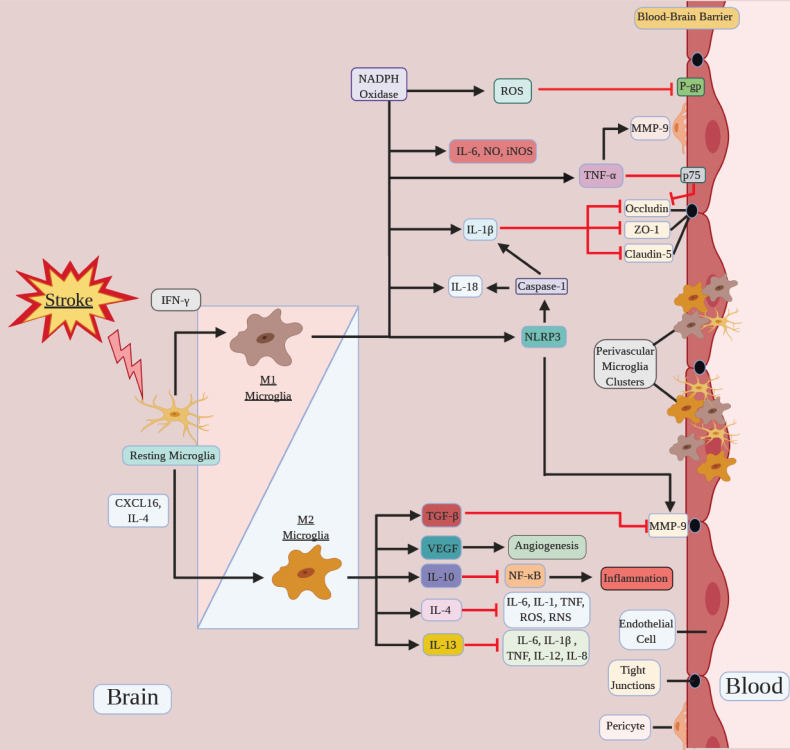
Activated microglial cells modulate neuroinflammation, which is a major source of secondary cell death after cerebral ischemia [30-32]. After the stroke, these cells undergo proliferation, chemotaxis, morphological and genetic alterations, and generate immunomodulatory molecules [22, 27, 33]. Activated microglia play dual roles at the BBB, and consequently, in ischemic stroke. On the one hand, they produce high levels of cytokines and chemokines, which upregulate EC adhesion molecules and accelerate the infiltration of leukocytes. This activation and consequent neuroinflammation contribute to the impairment of the BBB [34-36]. Cytokines promote inflammation and injury to the BBB, and the BBB reacts with further production of chemokines and cytokines, amplifying the pathological response [37]. On the other hand, activated microglia can also phagocytose cellular debris and suppress inflammatory responses, which is beneficial for the recovery and reduction of BBB damage (Fig. 1). When an injury occurs, microglia can develop into a spectrum of different, but overlapping functional phenotypes, including classically-activated (pro-inflammatory) and alternatively-activated (anti-inflammatory) phenotypes. Classically-activated microglia are also known as M1 microglia, while alternatively-activated microglia are also termed M2 microglia [10, 38].
Fig. (1).
Microglia polarization directs BBB function after stroke. Microglia polarize to a spectrum of pro-inflammatory M1/ anti-inflammatory M2 phenotype in response to stroke. M1 microglia promote ROS production, NLRP3 activation and pro-inflammatory cytokines secretion, such as IL-6, NO, iNOS, TNF-α, IL-1β and IL-18, which aggravate BBB disruption. M2 microglia release growth and trophic factors such as VEGF and anti-inflammatory cytokines such as IL-4, IL-13, IL-10, and TGF-β, which facilitate the repair, or inhibit BBB damage. Microglia can also aggregate at the perivascular space to directly interact with the BBB and affect the BBB permeability. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
A variety of molecules have been shown to modulate the direction of microglia polarization, and therefore, they can affect BBB function. Lipopolysaccharide and interferon-γ can induce microglial differentiation toward the M1 phenotype, while CXCL16 and interleukin 4 (IL-4) stimulate M2 polarization [39]. It was also reported that exposure to conditioned media from oxygen-glucose-deprived cerebral endothelial cells or oxygen-glucose-deprived astrocytes can promote microglial polarization. Results show that endothelial-activated microglia were neurotoxic, while astrocyte-activated microglia promoted neuronal dendritogenesis without affecting neuronal viability. These findings prove that endothelial cells and astrocytes activate microglia into different states, and suggest that a gliovascular switch may be involved in the balance between BBB protection and dysfunction [40].
3. PRO-INFLAMMATORY M1 MICROGLIA PROMOTES BBB DISRUPTION
In physiological conditions, M1 microglia are involved in microbial defense and tumor resistance [5]. After the stroke, activated M1 microglia can produce various pro-inflammatory molecules, such as inducible nitric oxide synthase (iNOS) [41], nitric oxide (NO), Tumor Necrosis Factor-α (TNF-α) [42], reactive oxygen species (ROS), IL-1β, IL-6, and IL-18 [43] (Table 1). Those inflammatory factors are key mediators of BBB damage in ischemic stroke [9]. Cytokines can enhance BBB damage and disruption by interacting with the BBB, which includes impairing tight junction activity, increasing paracellular permeability, facilitating leukocyte migration, and inducing adsorptive endocytosis [37].
Table 1.
Inflammatory mediators related to microglia and BBB.
| Phenotype | Cytokines/Molecules | Effects |
|---|---|---|
| M1 microglia (pro-inflammatory) |
IL-1β | increases BBB permeability by inducing the downregulation of ZO-1, occludin and claudin-5 [9, 58, 59]. |
| IL-6 | increases permeability and reduces ZO-1, claudin-5 and occludin expression [44-47]. | |
| ROS | can irreversibly attack cellular lipids, proteins, and DNA and provide a common trigger for many downstream pathways that directly target and compromise the BBB, such as oxidative damage, TJ modification, and MMP activation [48, 49]. | |
| iNOS, NO | iNOS enzyme induction promotes NO production and peroxynitrite formation during reoxygenation, which decreases the expression of ZO-1 and increases BBB leakage [50, 51]. | |
| TNF-α | TNF-α binds to p75 expressed on endothelial cells, leading to the downregulation of occludin and subsequent increase in BBB permeability [60, 61]. promotes MMP-9 release from pericytes, which leads to increased endothelial permeability [62]. |
|
| M2 microglia (anti-inflammatory) |
TGF-β | can increase proliferation and neuroprotection in the ischemic brain and reduce inflammation [28, 75]. inhibits tPA-mediated induction of MMPs to alleviate hemorrhagic transformation after thrombolysis [80]. |
| IL-10 | downregulates NF-κB [78]. directly protects the endothelium from oxidative stress via the downregulation of harmful ROS-producing enzymes, and/or the upregulation of antioxidant pathways [79]. |
|
| IL-4, IL-13 | they share a common receptor and directly promote the M2 microglia phenotype polarization [81]. both downregulate the synthesis of Th1 pro-inflammatory cytokines [81]. IL-4 can inhibit Th1-activated macrophages and suppress the secretion of several potent proinflammatory mediators, including IL-6, IL-1β, TNF-α, ROS and RNS [82]. IL-13 inhibits the production of proinflammatory cytokines, such as IL-6, IL-1β, TNF-α, IL-12, and IL-8 [83]. |
|
| VEGF | responsible for new blood vessel formation after injury, helps to establish collateral circulation to bypass blocked vessels. in later stages of stroke, microglia release VEGF, which promotes angiogenesis and assists in repairing the BBB [86]. |
Proinflammatory cytokines activate or enhance the proinflammatory response, which involves neutrophil recruitment to the ischemic area, further contributing to BBB disruption. However, it was demonstrated that some proinflammatory cytokines can directly affect the structure of the BBB. It was reported that IL-6 treatment of macrovascular endothelial cells caused increased permeability and disruption of ZO-1 cell–cell border immunostaining [44, 45]. A paper by Cohen et al. [46] also proved that IL-6 can reduce levels of claudin-5 and occludin in ovine cerebral microvessels ex vivo [47]. ROS, on the other hand, can irreversibly attack cellular lipids, proteins, and DNA, ultimately causing cell death [48]. ROS provide a common trigger for many downstream pathways that directly target and compromise the BBB, such as oxidative damage, tight junction modification, and matrix metalloproteinases (MMP) activation [49]. Moreover, iNOS enzyme induction promotes NO production [50] and peroxynitrite formation during reoxygenation after a stroke, which decreases the expression of ZO-1 and increases BBB leakage [51]. Peroxynitrite is a powerful oxidant that can lead to extensive cellular damage by oxidizing proteins, lipids, and DNA [52], and it can induce toxicity via nitrosylation of tyrosine residues on proteins [53]. It has been shown that oxygen and glucose deprivation of cultured endothelial cells induces S-nitrosylation of caveolin-1, consequently augmenting MMP-2 and -9 secretion [54]. This demonstrates how ROS and nitrosylation can directly affect the BBB.
IL-1β and TNF-α, released by microglia during the early stage of ischemia [55], can increase the permeability of the BBB [56, 57], thus promoting BBB damage. It was demonstrated in in vitro experiments that IL-1β increases BBB permeability by inducing the downregulation of ZO-1, occludin, and claudin-5 [9, 58, 59]. TNF-α binds to the TNF receptor type 1 (p75) expressed on endothelial cells, also leading to the downregulation of occludin and subsequent increase in BBB permeability [60, 61]. Another TNF-α-related mechanism of BBB damage is MMP-9 released from pericytes, which leads to increased endothelial permeability in an in vitro BBB model [62]. These results indicate a close microglial–endothelium communication in the mechanisms of IL-1/TNF-α-induced cerebrovascular inflammation [9]. It has also been demonstrated that M1 microglia cause P-glycoprotein (P-gp) dysfunction in brain ECs via nicotinamide adenine dinucleotide phosphate (NADPH) oxidase activation, leading to neurotoxic protein accumulation within the brain [10]. Inflammatory factors secreted by microglia may also induce EC necroptosis, and perivascular proinflammatory microglia were shown to contribute to BBB breakdown and disintegration in ischemic stroke [63].
M1 microglia polarization associated with BBB deterioration seems like a counterproductive physiological response after stroke, and one might speculate on the evolutionary purpose of such mechanism. While the ancient evolutionary origin of microglia/macrophages is well established, the evolutionary roots of the specialized polarization into subsets with specific phenotypes are less well defined [64]. However, there are multiple lines of evidence of macrophage polarization in amphibians and fish [65-68]. While their functionality may not be strictly the same as in mammals, it suggests that macrophage polarization and plasticity is an evolutionary ancient trait. In this light, it seems most probable that microglia/macrophage and their polarizations evolved to counter infections and that detrimental response in stroke is a ‘side effect’ of innate immune response. In the M1 state, microglial cells respond to infection by antigen-presenting activity, they produce ROS and iNOS and other pro-inflammatory cytokines/chemokines to induce infiltration of peripheral leukocytes to kill infecting bacteria and clear the insult [69]. The M1 phenotype of microglia is usually associated with protection during acute early infection stages, but it can also be detrimental to host if it persists for a longer time. Although microglia/macrophage adaptability provides a selective advantage in host resistance to pathogens, this same plasticity might be harmful during the stroke. It is unlikely that mammals evolved an M1 microglial phenotype that only serves to exacerbate injury and exerts no positive role. Although the consensus is that proinflammatory microglia response is overall detrimental after stroke, there are some specific beneficial aspects to it. For example, M1 microglia contribute to synaptic remodeling and may also drive the clearance of cellular debris early after acute brain injury. In addition, several factors that are known to be important in CNS repair, such as MMP-9, can be released not only from M2 but also from M1 cells. We also cannot exclude the possibility, that there are still some beneficial mechanisms of M1 microglia activation waiting to be discovered. In this context, both pro-inflammatory and inflammation-resolving cells might be important for effective tissue repair at different time points after stroke. Another explanation for persistence of M1 response is that it might not be a subject to high selection pressure, as stroke usually occurs in older patients and in most cases is not deadly, while innate immune response is at higher importance to organism. Recent discoveries offer new insights into these responses, as they demonstrate that the cGAS/STING pathway, which in physiological conditions is responsible for viral DNA detection, non-specifically activates microglia through endogenous DNA after stroke, which contributes to neuroinflammation. It further supports that these detrimental responses in stroke may be an adverse effect of the pathogen-recognition pathways [70, 71].
4. ANTI-INFLAMMATORY M2 MICROGLIA PRO- MOTE BBB PROTECTION
Contrary, M2-type microglia perform crucial roles in limiting inflammation and damage to BBB. The phagocytic activity of microglia contributes to cleaning up injured tissue and tissue debris, and consequently limits the activation of danger associated molecular pattern (DAMP) receptors, which ultimately dampens the inflammatory response. Activated microglia are the primary phagocytes within the brain. They phagocytose cellular debris and uptake harmful substances to reestablish homeostasis by clearing pathogens or necrotic cells, consequently attenuating inflammation after insults [72, 73]. After the stroke, microglial phagocytosis of neuronal cells begins, even before peripheral macrophages infiltrate into the brain [74]. It was observed that these phagocytotic cells interact with neurons and show neuronal engulfment in the ischemic brain [75].
M2-type microglia can produce a variety of anti-inflammatory cytokines, including IL-10, IL-4, IL-13, and TGF-β [76, 77]. Those anti-inflammatory molecules reduce the inflammatory response and decrease the recruitment of neutrophils and other lymphocytes to the site of injury, which can limit the leakage of the BBB. Besides those indirect mechanisms, there are other mechanisms described in the literature that can protect the BBB. Interleukin-10 was shown to exert its anti-inflammatory effects in part by the downregulation of NF-κB [78]. IL-10 can directly protect the endothelium from oxidative stress via the downregulation of harmful ROS-producing enzymes, and/or the upregulation of antioxidant pathways [79]. TGF-β has been reported to inhibit tPA-mediated induction of MMPs to alleviate hemorrhagic transformation after thrombolysis [80]. TGF-β can additionally increase proliferation and neuroprotection in the ischemic brain and reduce inflammation [28, 75]. IL-4 and IL-13, which share a common receptor, were demonstrated to downregulate the synthesis of T helper type 1 (Th1) pro-inflammatory cytokines. There is also evidence that these interleukins can directly promote the M2 microglia phenotype polarization [81]. IL-4 can inhibit Th1-activated macrophages and suppress the secretion of several potent proinflammatory mediators, including IL-6, IL-1β, TNF-α, ROS and reactive nitrogen species (RNS) [82]. IL-13 is also able to inhibit the production of proinflammatory cytokines, such as IL-6, IL-1β, TNF-α, IL-12, and IL-8 [83].
M2-type microglia were demonstrated to produce growth and trophic factors such as brain-derived neurotrophic factor (BDNF), vascular endothelial growth factor (VEGF), insulin-like growth factor 1 (IGF-1), nerve growth factor (NGF) [5, 43], and scavenger receptors [84, 85]. Moreover, M2 microglia secrete molecules involved in immunoregulation and matrix remodeling [5, 43]. All of those factors affect the function of BBB in the setting of stroke. M2 microglia are also associated with long-term neurovascular remodeling, and can promote angiogenesis, thus improving the recovery of neurological functions after ischemia [10]. VEGF is responsible for new blood vessel formation after injury, and these newly formed arteries can establish collateral circulation to bypass blocked vessels. In later stages of stroke, microglia release VEGF, which promotes angiogenesis and assists in repairing the BBB [86].
A growing body of evidence reveals diversity in M2 phenotype subpopulations, such as M2a, M2b, M2c and Mox, each with unique physiological features and distinct biological functions. Currently, these subpopulations of M2 cells have not yet been fully characterized in CNS injuries and stroke and therefore, our picture of microglia phenotypes is still incomplete.
5. RECEPTORS IMPLICATED IN MICROGLIAL REGULATION OF BBB FUNCTION
Various receptors and downstream pathways can regulate mechanisms, which facilitate microglial regulation of BBB function during the stroke. Next, we will discuss some of the most important ones in more detail.
NOD-, LRR- and pyrin domain-containing protein 3 (NLRP3) is an intracellular sensor that detects a broad range of microbial motifs, endogenous danger signals, and environmental irritants, leading to the formation and activation of the NLRP3 inflammasome. The NLRP3 inflammasome is one of the key components of inflammatory responses, and contributes to neuroinflammation in ischemic stroke [87, 88]. Assembly of the NLRP3 inflammasome causes caspase-1-dependent release of IL-1β and IL-18, as well as cell death [89]. After the ischemic injury of the brain, microglia express NLRP3, and its expression was significantly increased in the ischemic cerebral hemisphere, both in the ischemic core and the penumbra, with a peak expression at 24 hours after reperfusion. Furthermore, more proinflammatory mediators were produced following a high expression of NLRP3, which also activated microglia-mediated neurotoxicity, causing more death of neurons and damage to the BBB. It was shown that NLRP3 contributed to the upregulation of MMP-2 and MMP-9, decreased tight-junction protein expression, and increased endothelial cell permeability, indicating that targeting microglial NLRP3 may be a potential therapeutic strategy for reducing BBB damage in ischemic stroke [87]. NLRP3 inhibition reduced IL-1β production, attenuated neurological deficits and perihematomal brain edema after intracerebral hemorrhage (ICH) induction via injection of either autologous blood or collagenase. In mice with autologous blood-induced ICH, NLRP3 inhibition was associated with reduced leukocyte infiltration into the brain and decreased microglial production of IL-6. NLRP3 inhibition was found to improve the blood-brain barrier integrity and diminish cell death. The protective effect of NLRP3 inhibition was abolished in mice depleted of either microglia or Gr-1+ myeloid cells, which suggests that microglia-expressed NLRP3 is an important contributor to neuroinflammation, and consequently, to BBB breakdown in hemorrhagic stroke [90].
Purinergic receptor P2Y, G-protein coupled, 12 (P2RY12), is a type of purinergic receptor expressed on microglia. Microglial P2Y12 receptors cluster at the microglia–neuron interface independent of astrocyte end-feet location [91]. Microglial activation was shown to down-regulate P2Y12, which has been demonstrated to mediate microglial neurotoxicity. It was found that deficiency of P2Y12 impaired microglial polarization, migration, and the ability to extend their processes toward the lesion site in mice model. Moreover, P2Y12 knockout mice had reduced microglial accumulation in the peri-infarct region and decreased neuronal death after cerebral ischemia [92]. On the other hand, there is some data suggesting that the microglial P2RY12 receptor can have a protective function during BBB rupture. It is known that juxtavascular microglia join pericytes and astrocytes as critical contributors to the unique barrier functions of brain endothelial cells. Microglial processes rapidly form a dense plexiform aggregate at the site of injury when the capillary is injured. Congregation of activated juxtavascular microglial processes at sites of capillary injury plays an important role in the closure of BBB disruption after injury. The resealing of BBB leakage was shown to be stopped when microglial cells were photoablated, showing the importance of microglia in BBB integrity. Interestingly, it was demonstrated that inhibition of P2RY12 receptors attenuated microglial process motility and delayed BBB closure [93]. Collectively, these results indicate that the microglial purinergic receptors could be potential targets for controlling microglial activation and limit post-stroke inflammation and BBB damage [94]. P2RY12 receptor serves as a good example to demonstrate the dual effect of certain receptors on microglia function, consequently showing the effect that microglial interaction with the BBB has on BBB function.
Microglial cannabinoid receptor 2 (CB2R) was shown to regulate the microglial effect on the BBB. It is known that microglial activation contributes to the pathogenesis of the BBB in ICH. CB2R plays a key role in neuroprotection after stroke by inactivating central microglia/macrophage, subsequently leading to an anti-inflammatory mechanism by inhibiting the expression of TNF-α, IL-6, IL-12/IL-23p40, monocyte chemoattractant protein-1 (MCP-1), macrophage inflammatory peptide (MIP) -1α, and RANTES (CCL5). It was found that selective activation of CB2R decreased microglial activation in a rat thrombin-induced BBB damage model. It significantly attenuated thrombin-induced brain edema and decreased the number of Iba-1-positive microglia. In mammalian cells, the MAP kinases, including p38, p44/42 and JNK, exert an important role in signaling cascades, which regulate cell responses to external stimuli. p44/42 MAPK, also termed extracellular signal-regulated kinase (ERK), is abundant in the central nervous system, and is activated after ischemic preconditioning and cerebral ischemia [95-99]. CB2R activation was shown to reduce the number of p44/P42(+)/Iba-1(+) microglia, decrease Evans blue extravasation, and inhibit the elevated MMP-9 and MMP-12 activities. Moreover, these effects were reversed by selective CB2R inhibition, further supporting the role of CB2R in the aforementioned mechanisms. CB2R stimulation was also shown to inhibit microglia/macrophage cell migration, which may participate in neuroprotection after intracerebral infusion of thrombin and prevent thrombin-induced BBB damage [22].
CX3CR1 and its only known chemokine ligand, CX3CL1, are implicated in a range of inflammatory diseases [100]. In the CNS, this receptor is exclusively expressed on microglia. It was shown that activated CX3CR1 receptors participate in the process of microglial activation, and in the damage of the BBB. Several studies suggest that CX3CR1-deficient mice have reduced injury after stroke [63]. Selective inactivation of microglial CX3CR1 reduced microglial migration [101], significantly reduced blood extravasation, and protected the BBB [63], thus exerting a neuroprotective function after stroke [86].
6. MICROGLIA-BLOOD VESSEL INTERACTIONS
Vessel-associated microglia initially maintain BBB integrity via expression of the tight-junction protein, claudin-5, and by making physical contact with endothelial cells. The tight junctions are crucial machinery underlying the BBB function. Immunostaining for the tight junction proteins (i.e., ZO-1, claudin-5, and occludin) showed a continuous distribution of these proteins along the cell border in brain microvascular endothelial cells co-cultured with microglia. However, treatment with LPS disrupted this pattern, which was restored to a normal linear shape by an NADPH oxidase inhibitor [35]. It was also shown in in vitro BBB models, that adding LPS to the abluminal side induced a reorganization of tight-junctions, decreased trans-endothelial electrical resistance, and increased the paracellular transport of sodium-fluorescein. Likewise, when the co-cultures were treated with an NADPH oxidase inhibitor, all characteristics of increased permeability of the endothelium were reversed. These findings indicate that activated microglia impair BBB function by producing ROS through NADPH oxidase [5, 35, 102]. After ischemia, microglia form perivascular clusters and phagocytic structures, which show the close interaction between microglia and blood vessel. The accumulation of microglia around the vasculature can subsequently cause disintegration of the vessels and is associated with the invasion of blood-borne molecules during reperfusion [63, 86]. This demonstrates that the interaction of aggregated microglia with endothelial cells can be both protective or detrimental, depending on the context and timing after stroke.
Following ischemia, activated microglia can also express latent matrix metalloproteinase-9 (pro–MMP-9), which contributes to BBB disruption and hemorrhagic transformation [103]. Matrix proteins in the circulation, in turn, promote microglial cell activation and pro-MMP-9 generation during focal cerebral ischemia. These matrix proteins activate microglia through their specific cell surface matrix receptors. This demonstrates a positive feedback-loop mechanism where microglia express MMP-9 contributing to BBB breakdown. MMP-9 can simultaneously activate microglia, which further deteriorates the BBB. Exposure of the ischemic region to plasma matrix proteins (such as plasma fibronectin or vitronectin) can change the local environment of microglial cells, thus stimulating the generation of proteases and attendant pro-inflammatory agonists. Those substances could start or enlarge cerebral damage by activating microglia, generating matrix protease, stimulating endogenous inflammatory responses, or directly modulating astrocytic or axonal behavior [104].
The hypoxic tissue in the penumbra activates the upregulation of vascular endothelial growth factor (VEGF). VEGF can significantly enhance angiogenesis in the ischemic brain and improve neurological function during stroke recovery. However, it was also proved that inhibition of VEGF at the acute stage of stroke might reduce the BBB permeability and the risk of hemorrhagic transformation [105]. VEGF is an important signaling protein involved in both vasculogenesis and angiogenesis. It is a potent angiogenic factor and activates quiescent vessels to sprout [106]. This process causes the partial opening of the BBB and leakage of serum proteins such as fibrinogen and albumin, which transport from the blood into the brain parenchyma. These proteins attract microglia within 24 h after ischemia. Subsequently, perivascular microglia migrate towards the disrupted blood vessels and phagocytize ECs, which contributes to the disintegration of blood vessels and aggravates the BBB damage [63]. In the late phase of BBB opening, microglia-driven disintegration of blood vessels occurs, and is driven by ROS, matrix metalloproteinases (MMPs), pro-inflammatory cytokines, and phagocytosis [5].
7. PROMISING THERAPIES FOR BBB PROTECTION
Certain drug treatments were proven to modulate the microglial effects on the BBB in animal models. Here, we will discuss the most prominent ones.
Minocycline, a member of the tetracycline antibiotic family, is an inhibitor of microglial activation [107]. It attenuates infarct volume, tissue loss, neurological deficits, and markedly reduces BBB disruption and hemorrhage by improving BBB viability and integrity in mice after experimental stroke. It also increases neurogenesis and perfusion in ischemic stroke, and reduces the number of microglia and macrophages, the levels of iron accumulation, and hemorrhage frequency after ICH [33, 108]. Minocycline can prevent excitotoxin-induced microglial proliferation and reduce the release of nitric oxide (NO) metabolites and IL-1β. Minocycline inhibited glutamate-induced transient activation of p38 mitogen-activated protein kinase (p38 MAPK) in microglia, which also indirectly protects the BBB [109]. Moreover, minocycline inhibited the enzymatic activity of gelatin proteases activated by ischemia after experimental stroke, and results suggest that it might selectively inhibit MMP-9 at low doses [110]. During the recovery phase, a single dose of minocycline administered early after stroke induced neurovascular remodeling by promoting the neuroprotective phenotype of microglia alternative activation, which is involved in BBB restoration [111]. Minocycline ameliorated neurological outcome and reduced BBB damage, hemorrhage, and perivascular microglial accumulation. Studies suggest that minocycline treatment could provide long-term protection by attenuating BBB permeability and promoting microglial polarization towards the M2 phenotype after ischemic stroke [94]. Thus, microglial inhibitors, such as Minocycline, may prove to be a potential new therapeutic agent adjunct to fibrinolysis for the acute treatment of ischemic stroke [33, 110].
Adjudin, a small molecular derivative of indazole, performs potent reversible anti-spermatogenic activity by disrupting adhesion of germ cells, most notably spermatids to the Sertoli cells [112]. In the context of stroke, Adjudin exhibits additional function to reduce the production of proinflammatory mediators by the suppression of NF-κB p65 nuclear translocation and DNA binding activity, as well as ERK MAPK phosphorylation in immortalized murine BV2 microglia. It was demonstrated that Adjudin ameliorated brain edema and neurological deficits in mice pMCAO model [113]. Adjudin markedly inhibited MCAO-induced microglial activation in both the cortex and the striatum, accompanied by a reduction in the expression and release of proinflammatory cytokines TNF-α, IL-1β, and IL-6. Concomitantly, Adjudin noticeably prevented BBB disruption after ischemia and reperfusion by reducing MMP-9 activity [113, 114].
MCC950 is a potent, selective, small-molecule NLRP3 inhibitor that blocks NLRP3 activation. MCC950 can improve blood–brain barrier integrity and attenuate brain injury and inflammation after ICH [90]. It was reported that treatment with MCC950 ameliorated the diabetic rats’ hippocampal-dependent memory deficits after ischemic stroke via the resolution of inflammation, as seen through increased resting microglia and lower IL-1β expression, improved BBB integrity, and lower cell death of the neurons in the CA1 and Dentate Gyrus regions of the hippocampus [115]. Hence, the NLRP3 inflammasome inhibitor is a potential treatment for protecting the BBB after stroke.
JWH133 is a selective cannabinoid receptor type 2 (CB2R) agonist. It alleviated brain edema, improved neurological deficits, suppressed neuroinflammation, and reduced BBB damage in a rat ICH model. Further studies indicate that JWH133 significantly upregulated the mitogen-activated protein kinase phosphatase-1 (MKP-1) signaling pathway, which suppressed ICH-induced over-activation of MAPKs. JWH133 was also found to inhibit microglial activation and macrophage infiltration at 24 h after ICH. The selective CB2R agonist can restrain the levels of several proinflammatory cytokines, such as IL-1β, IL-6, and TNF-α, and increase the expression of tight junction proteins, such as ZO-1, claudin-5, and occludin following ICH. Moreover, JWH133 significantly reduced the level of tight junction proteins and basal lamina-degrading MMP-9 and MMP-2, which helped to protect the BBB. These observations suggest that CB2R can reverse ICH- induced BBB damage by inhibiting inflammation [116]. It was also demonstrated that JWH133 treatment ameliorated brain injury in a rat ICH model by activating the Ras-related C3 botulinum toxin substrate 1 (Rac1) signaling pathway. Rac1 stabilizes the barrier function of microvascular endothelial cells, which preserves BBB integrity and reduces the development of vasogenic brain edema [117].
Infliximab is a human and mouse chimeric monoclonal IgG-1 antibody that specifically blocks TNF-α, and has been extensively utilized in clinical patients [118]. Infliximab blocks necroptosis of EC co-cultured with microglia in oxygen glucose deprivation/reoxygenation in vitro model and ameliorates BBB disruption in the stroke model. The therapeutic effect of infliximab has been studied in a rat transient MCAO model. The results of MRI post-contrast T1-SE sequencing and Evans blue extravasation demonstrated that infliximab ameliorated BBB disruption after the stroke. In addition, TTC staining and MRI T2-TSE sequencing results revealed a significant reduction of infarction volume in the treatment group compared with the vehicle group. The results of the neurological severity score showed that multiple doses of infliximab improved neurological function on the 3rd day after MCAO. These findings prove that infliximab is efficient and effective in protecting against BBB breakdown after stroke [42].
Tyrosine kinase inhibitors (TKI) are targeted therapy first developed for various types of malignancies. However, many studies have found that TKIs play a beneficial role in several neurological and non-neurological disorders, including stroke [119-121], Alzheimer’s disease (AD) [122], multiple sclerosis (MS) [123, 124], rheumatoid arthritis [125], asthma [126], and mastocytosis [127]. Among TKIs, imatinib and masitinib have the broadest therapeutic spectrum. Both drugs showed effectiveness in ischemic stroke models [119, 128], but only imatinib has a beneficial effect in subarachnoid hemorrhage [121]. Masitinib preserves BBB integrity probably through extracerebral inhibition of mast cell recruitment, which also has a highly significant impact on neuroinflammatory cascade and activity of microglia in the brain [119]. Numerous tyrosine kinases are expressed in microglia. Rodent and human studies have shown an increase in active forms of non-receptor tyrosine kinases, Src and Lyn in reactive microglia [129, 130]. It has been reported that Aβ serves as a specific stimulus for TK-based microglia activation, which causes a pro-inflammatory phenotype of AD [130]. Consistently, masitinib and dasatinib were demonstrated to ameliorate the symptoms of Alzheimer’s disease. Imatinib, also known as Gleevec or ST1-571, is a tyrosine-kinase inhibitor developed as a small molecule therapeutic drug for patients with Bcr-Abl–positive chronic myelogenous leukemia [131, 132]. The Abl family kinases phosphorylate several cytoskeletal effectors that mediate vascular permeability, such as myosin light chain kinase, cortactin, vinculin, and β-catenin. They also regulate cell-cell and cell-matrix junction dynamics and are activated by hyperoxia and contribute to oxidant-induced EC injury. Imatinib has been reported to attenuate vascular leakage and prevent edema formation under permeability-inducing conditions [133]. Abl inhibition protected the endothelium by maintaining vascular endothelial cadherin specific for the endothelial adherens junction [134], as well as by activating anti-inflammatory signals. The inhibition of Abl works against permeability-inducing factors, including thrombin, histamine, VEGF, LPS, and oxidative stress [133, 135-138]. In a rat ischemia/reperfusion injury (IRI) model, imatinib reduced lung injury by playing an anti-permeability and anti-inflammatory role. Therefore, imatinib could be a novel treatment for ischemic injury [139]. Treatment with imatinib was also demonstrated to reduce inflammation in a uric acid crystal-induced acute gouty arthritis mouse model. Moreover, it was also indicated that this potent drug can inhibit IL-1-independent and mast cell-independent pathways [140]. Additionally, it has been shown that imatinib and several other TKIs, including masitinib, dasatinib, sunitinib, sorafenib, and lestaurtinib, had beneficial effects in multiple sclerosis. Imatinib attenuated the severity and delayed the onset of disease in experimental autoimmune encephalomyelitis (EAE), which is an animal model of multiple sclerosis. In vitro, imatinib suppressed cell proliferation, MMP-2 expression and activity, as well as reduced the production of proinflammatory cytokines [141]. It was also proved that imatinib enhances BBB integrity in EAE by decreasing CNS inflammation, T-cell recruitment and demyelination. This was supported by the downregulation of the chemokine receptor 2 in CNS and lymph nodes, and by the modulation of the peripheral immune response towards an anti-inflammatory phenotype. Imatinib can still ameliorate neuroinflammation even when given after the clinical manifestation of the disease [124]. The most frequent target for the TKIs is platelet-derived growth factor receptor (PDGFR), which plays an important role in ischemic stroke and subarachnoid hemorrhage. PDGFR-α is an imatinib-sensitive kinase and a central regulator of BBB integrity during neuroinflammation. It has been shown that imatinib reduces BBB disruption and infarction volume after experimental ischemic stroke by blocking PDGFR-α in the BBB [142]. Taken together, imatinib can be a potentially effective treatment for stroke by protecting BBB integrity and reducing inflammation. Overall, accumulated data suggest that TKIs are very promising candidates for new treatments in neurological diseases [143].
CONCLUSION
Microglia exert dual roles in BBB dysfunction after stroke due to microglial polarization and direct interactions with endothelial cells. Proinflammatory microglia contribute to BBB deterioration, while anti-inflammatory microglia benefit BBB repair by different mechanisms. In response to stroke, microglia secrete proinflammatory cytokines and contribute to damage of the blood vessels in the early stage, but in the later stage, microglial cells can facilitate BBB repair via neovascularization and production of protective factors. Taken together, a literature review on the subject suggests that with fluctuation of various microglial phenotypes after stroke over time, there is a delicate balance in regard to microglial sub-polarizations and their interplay with the BBB, and timing is an important factor to consider when modulating this complex response. Therefore, drugs that can alter microglia polarization, increase microglia proliferation over time, or inhibit microglial death in the late phase of stroke that can prove to be effective in mitigating BBB damage. Reviewed studies show a complicated set of correlations, where microglial cells can be regulated by many pathways and mechanisms, which alter microglia activity, and consequently affect the BBB function and permeability. Further studies are necessary to reveal more connections between microglia and the BBB, and thus introduce more innovative possibilities for clinical therapies. Take home message is that microglia are key cells in the context of BBB function after stroke, and identifying molecules and signaling pathways involved in controlling the opposing microglial responses may lead to strategies that will help maintain the BBB integrity and improve patient outcomes.
ACKNOWLEDGEMENTS
Declared none.
CONSENT FOR PUBLICATION
Not applicable.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
REFERENCES
- 1.Villanueva M.T. Repurposing CCR5 inhibitors for stroke recovery. Nat. Rev. Drug Discov. 2019 doi: 10.1038/d41573-019-00038-3. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 2.Webb R.L., Kaiser E.E., Scoville S.L., Thompson T.A., Fatima S., Pandya C., Sriram K., Swetenburg R.L., Vaibhav K., Arbab A.S., Baban B., Dhandapani K.M., Hess D.C., Hoda M.N., Stice S.L. Human Neural Stem Cell Extracellular Vesicles Improve Tissue and Functional Recovery in the Murine Thromboembolic Stroke Model. Transl. Stroke Res. 2018;9(5):530–539. doi: 10.1007/s12975-017-0599-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Flemming A. Calming inflammation to prevent stroke damage. Nat. Rev. Immunol. 2019;19(8):473. doi: 10.1038/s41577-019-0197-5. [DOI] [PubMed] [Google Scholar]
- 4.Zlokovic B.V. The blood-brain barrier in health and chronic neurodegenerative disorders. Neuron. 2008;57(2):178–201. doi: 10.1016/j.neuron.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 5.Dudvarski Stankovic N., Teodorczyk M., Ploen R., Zipp F., Schmidt M.H.H. Microglia-blood vessel interactions: a double-edged sword in brain pathologies. Acta Neuropathol. 2016;131(3):347–363. doi: 10.1007/s00401-015-1524-y. [DOI] [PubMed] [Google Scholar]
- 6.Sandoval K.E., Witt K.A. Blood-brain barrier tight junction permeability and ischemic stroke. Neurobiol. Dis. 2008;32(2):200–219. doi: 10.1016/j.nbd.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 7.Hawkins B.T., Davis T.P. The blood-brain barrier/neurovascular unit in health and disease. Pharmacol. Rev. 2005;57(2):173–185. doi: 10.1124/pr.57.2.4. [DOI] [PubMed] [Google Scholar]
- 8.Ballabh P., Braun A., Nedergaard M. The blood-brain barrier: an overview: structure, regulation, and clinical implications. Neurobiol. Dis. 2004;16(1):1–13. doi: 10.1016/j.nbd.2003.12.016. [DOI] [PubMed] [Google Scholar]
- 9.Thurgur H., Pinteaux E. Microglia in the Neurovascular Unit: Blood-Brain Barrier-microglia Interactions After Central Nervous System Disorders. Neuroscience. 2019;405:55–67. doi: 10.1016/j.neuroscience.2018.06.046. [DOI] [PubMed] [Google Scholar]
- 10.Jiang X., Andjelkovic A.V., Zhu L., Yang T., Bennett M.V.L., Chen J., Keep R.F., Shi Y. Blood-brain barrier dysfunction and recovery after ischemic stroke. Prog. Neurobiol. 2018;163-164:144–171. doi: 10.1016/j.pneurobio.2017.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alluri H., Wiggins-Dohlvik K., Davis M.L., Huang J.H., Tharakan B. Blood-brain barrier dysfunction following traumatic brain injury. Metab. Brain Dis. 2015;30(5):1093–1104. doi: 10.1007/s11011-015-9651-7. [DOI] [PubMed] [Google Scholar]
- 12.Turner R.J., Sharp F.R. Implications of MMP9 for Blood Brain Barrier Disruption and Hemorrhagic Transformation Following Ischemic Stroke. Front. Cell. Neurosci. 2016;10:56. doi: 10.3389/fncel.2016.00056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abdullahi W., Tripathi D., Ronaldson P.T. Blood-brain barrier dysfunction in ischemic stroke: targeting tight junctions and transporters for vascular protection. Am. J. Physiol. Cell Physiol. 2018;315(3):C343–C356. doi: 10.1152/ajpcell.00095.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kleinschnitz C., Blecharz K., Kahles T., Schwarz T., Kraft P., Göbel K., Meuth S.G., Burek M., Thum T., Stoll G., Förster C. Glucocorticoid insensitivity at the hypoxic blood-brain barrier can be reversed by inhibition of the proteasome. Stroke. 2011;42(4):1081–1089. doi: 10.1161/STROKEAHA.110.592238. [DOI] [PubMed] [Google Scholar]
- 15.Burek M., König A., Lang M., Fiedler J., Oerter S., Roewer N., Bohnert M., Thal S.C., Blecharz-Lang K.G., Woitzik J., Thum T., Förster C.Y. Hypoxia-Induced MicroRNA-212/132 Alter Blood-Brain Barrier Integrity Through Inhibition of Tight Junction-Associated Proteins in Human and Mouse Brain Microvascular Endothelial Cells. Transl. Stroke Res. 2019;10(6):672–683. doi: 10.1007/s12975-018-0683-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haley M.J., Lawrence C.B. The blood-brain barrier after stroke: Structural studies and the role of transcytotic vesicles. J. Cereb. Blood Flow Metab. 2017;37(2):456–470. doi: 10.1177/0271678X16629976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lorberboym M., Lampl Y., Sadeh M. Correlation of 99mTc-DTPA SPECT of the blood-brain barrier with neurologic outcome after acute stroke. J. Nucl. Med. 2003;44(12):1898–1904. [PubMed] [Google Scholar]
- 18.Brouns R., Wauters A., De Surgeloose D., Mariën P., De Deyn P.P. Biochemical markers for blood-brain barrier dysfunction in acute ischemic stroke correlate with evolution and outcome. Eur. Neurol. 2011;65(1):23–31. doi: 10.1159/000321965. [DOI] [PubMed] [Google Scholar]
- 19.Zhang C., Jiang M., Wang W.Q., Zhao S.J., Yin Y.X., Mi Q.J., Yang M.F., Song Y.Q., Sun B.L., Zhang Z.Y. Selective mGluR1 negative allosteric modulator reduces blood-brain barrier permeability and cerebral edema after experimental subarachnoid hemorrhage. Transl. Stroke Res. 2019;11(4):799–811. doi: 10.1007/s12975-019-00758-z. [DOI] [PubMed] [Google Scholar]
- 20.Prakash R., Carmichael S.T. Blood-brain barrier breakdown and neovascularization processes after stroke and traumatic brain injury. Curr. Opin. Neurol. 2015;28(6):556–564. doi: 10.1097/WCO.0000000000000248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nimmerjahn A., Kirchhoff F., Helmchen F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science. 2005;308(5726):1314–1318. doi: 10.1126/science.1110647. [DOI] [PubMed] [Google Scholar]
- 22.Li L., Tao Y., Tang J., Chen Q., Yang Y., Feng Z., Chen Y., Yang L., Yang Y., Zhu G., Feng H., Chen Z. A Cannabinoid Receptor 2 Agonist Prevents Thrombin-Induced Blood-Brain Barrier Damage via the Inhibition of Microglial Activation and Matrix Metalloproteinase Expression in Rats. Transl. Stroke Res. 2015;6(6):467–477. doi: 10.1007/s12975-015-0425-7. [DOI] [PubMed] [Google Scholar]
- 23.Kim S.U., de Vellis J. Microglia in health and disease. J. Neurosci. Res. 2005;81(3):302–313. doi: 10.1002/jnr.20562. [DOI] [PubMed] [Google Scholar]
- 24.Salter M.W., Beggs S. Sublime microglia: expanding roles for the guardians of the CNS. Cell. 2014;158(1):15–24. doi: 10.1016/j.cell.2014.06.008. [DOI] [PubMed] [Google Scholar]
- 25.Spindler K.R., Hsu T.H. Viral disruption of the blood-brain barrier. Trends Microbiol. 2012;20(6):282–290. doi: 10.1016/j.tim.2012.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jackson L., Dong G., Althomali W., Sayed M.A., Eldahshan W., Baban B., Johnson M.H., Filosa J., Fagan S.C., Ergul A. Delayed administration of angiotensin II type 2 receptor (AT2R) agonist compound 21 prevents the development of post-stroke cognitive impairment in diabetes through the modulation of microglia polarization. Transl. Stroke Res. 2019;11:762–775. doi: 10.1007/s12975-019-00752-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kettenmann H., Hanisch U.K., Noda M., Verkhratsky A. Physiology of microglia. Physiol. Rev. 2011;91(2):461–553. doi: 10.1152/physrev.00011.2010. [DOI] [PubMed] [Google Scholar]
- 28.Hanisch U.K., Kettenmann H. Microglia: active sensor and versatile effector cells in the normal and pathologic brain. Nat. Neurosci. 2007;10(11):1387–1394. doi: 10.1038/nn1997. [DOI] [PubMed] [Google Scholar]
- 29.Kreutzberg G.W. Microglia: a sensor for pathological events in the CNS. Trends Neurosci. 1996;19(8):312–318. doi: 10.1016/0166-2236(96)10049-7. [DOI] [PubMed] [Google Scholar]
- 30.Borlongan C.V. Cell therapy for stroke: remaining issues to address before embarking on clinical trials. Stroke. 2009;40(3) Suppl.:S146–S148. doi: 10.1161/STROKEAHA.108.533091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Blecharz-Lang K.G., Wagner J., Fries A., Nieminen-Kelhä M., Rösner J., Schneider U.C., Vajkoczy P. Interleukin 6-Mediated Endothelial Barrier Disturbances Can Be Attenuated by Blockade of the IL6 Receptor Expressed in Brain Microvascular Endothelial Cells. Transl. Stroke Res. 2018;9(6):631–642. doi: 10.1007/s12975-018-0614-2. [DOI] [PubMed] [Google Scholar]
- 32.Lee J.Y., Castelli V., Bonsack B., Coats A.B., Navarro-Torres L., Garcia-Sanchez J., Kingsbury C., Nguyen H., Vandenbark A.A., Meza-Romero R., Offner H., Borlongan C.V. Novel partial MHC class II construct, DRmQ, inhibits central and peripheral inflammatory responses to promote neuroprotection in experimental stroke. Transl. Stroke Res. 2019;11:831–836. doi: 10.1007/s12975-019-00756-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yenari M.A., Xu L., Tang X.N., Qiao Y., Giffard R.G. Microglia potentiate damage to blood-brain barrier constituents: improvement by minocycline in vivo and in vitro. Stroke. 2006;37(4):1087–1093. doi: 10.1161/01.STR.0000206281.77178.ac. [DOI] [PubMed] [Google Scholar]
- 34.Zipser B.D., Johanson C.E., Gonzalez L., Berzin T.M., Tavares R., Hulette C.M., Vitek M.P., Hovanesian V., Stopa E.G. Microvascular injury and blood-brain barrier leakage in Alzheimer’s disease. Neurobiol. Aging. 2007;28(7):977–986. doi: 10.1016/j.neurobiolaging.2006.05.016. [DOI] [PubMed] [Google Scholar]
- 35.da Fonseca A.C., Matias D., Garcia C., Amaral R., Geraldo L.H., Freitas C., Lima F.R. The impact of microglial activation on blood-brain barrier in brain diseases. Front. Cell. Neurosci. 2014;8:362. doi: 10.3389/fncel.2014.00362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shao Z., Tu S., Shao A. Pathophysiological Mechanisms and Potential Therapeutic Targets in Intracerebral Hemorrhage. Front. Pharmacol. 2019;10:1079. doi: 10.3389/fphar.2019.01079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pan W., Stone K.P., Hsuchou H., Manda V.K., Zhang Y., Kastin A.J. Cytokine signaling modulates blood-brain barrier function. Curr. Pharm. Des. 2011;17(33):3729–3740. doi: 10.2174/138161211798220918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Franco R., Fernández-Suárez D. Alternatively activated microglia and macrophages in the central nervous system. Prog. Neurobiol. 2015;131:65–86. doi: 10.1016/j.pneurobio.2015.05.003. [DOI] [PubMed] [Google Scholar]
- 39.Zhang S. Microglial activation after ischaemic stroke. Stroke Vasc. Neurol. 2019;4(2):71–74. doi: 10.1136/svn-2018-000196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xing C., Li W., Deng W., Ning M., Lo E.H. A potential gliovascular mechanism for microglial activation: differential phenotypic switching of microglia by endothelium versus astrocytes. J. Neuroinflammation. 2018;15(1):143. doi: 10.1186/s12974-018-1189-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Iadecola C., Zhang F., Casey R., Nagayama M., Ross M.E. Delayed reduction of ischemic brain injury and neurological deficits in mice lacking the inducible nitric oxide synthase gene. J. Neurosci. 1997;17(23):9157–9164. doi: 10.1523/JNEUROSCI.17-23-09157.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen A.Q., Fang Z., Chen X.L., Yang S., Zhou Y.F., Mao L., Xia Y.P., Jin H.J., Li Y.N., You M.F., Wang X.X., Lei H., He Q.W., Hu B. Microglia-derived TNF-α mediates endothelial necroptosis aggravating blood brain-barrier disruption after ischemic stroke. Cell Death Dis. 2019;10(7):487. doi: 10.1038/s41419-019-1716-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Taylor R.A., Sansing L.H. Microglial responses after ischemic stroke and intracerebral hemorrhage. Clin. Dev. Immunol. 2013;2013:746068. doi: 10.1155/2013/746068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Maruo N., Morita I., Shirao M., Murota S. IL-6 increases endothelial permeability in vitro. Endocrinology. 1992;131(2):710–714. doi: 10.1210/endo.131.2.1639018. [DOI] [PubMed] [Google Scholar]
- 45.Desai T.R., Leeper N.J., Hynes K.L., Gewertz B.L. Interleukin-6 causes endothelial barrier dysfunction via the protein kinase C pathway. J. Surg. Res. 2002;104(2):118–123. doi: 10.1006/jsre.2002.6415. [DOI] [PubMed] [Google Scholar]
- 46.Cohen S.S., Min M., Cummings E.E., Chen X., Sadowska G.B., Sharma S., Stonestreet B.S. Effects of interleukin-6 on the expression of tight junction proteins in isolated cerebral microvessels from yearling and adult sheep. Neuroimmunomodulation. 2013;20(5):264–273. doi: 10.1159/000350470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rochfort K.D., Cummins P.M. The blood-brain barrier endothelium: a target for pro-inflammatory cytokines. Biochem. Soc. Trans. 2015;43(4):702–706. doi: 10.1042/BST20140319. [DOI] [PubMed] [Google Scholar]
- 48.Chen S.D., Yang D.I., Lin T.K., Shaw F.Z., Liou C.W., Chuang Y.C. Roles of oxidative stress, apoptosis, PGC-1α and mitochondrial biogenesis in cerebral ischemia. Int. J. Mol. Sci. 2011;12(10):7199–7215. doi: 10.3390/ijms12107199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Thiel V.E., Audus K.L. Nitric oxide and blood-brain barrier integrity. Antioxid. Redox Signal. 2001;3(2):273–278. doi: 10.1089/152308601300185223. [DOI] [PubMed] [Google Scholar]
- 50.Christopoulos A., El-Fakahany E.E. The generation of nitric oxide by G protein-coupled receptors. Life Sci. 1999;64(1):1–15. doi: 10.1016/S0024-3205(98)00348-8. [DOI] [PubMed] [Google Scholar]
- 51.Khan M., Dhammu T.S., Sakakima H., Shunmugavel A., Gilg A.G., Singh A.K., Singh I. The inhibitory effect of S-nitrosoglutathione on blood-brain barrier disruption and peroxynitrite formation in a rat model of experimental stroke. J. Neurochem. 2012;123(Suppl. 2):86–97. doi: 10.1111/j.1471-4159.2012.07947.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Beckman J.S., Koppenol W.H. Nitric oxide, superoxide, and peroxynitrite: the good, the bad, and ugly. Am. J. Physiol. 1996;271(5 Pt 1):C1424–C1437. doi: 10.1152/ajpcell.1996.271.5.C1424. [DOI] [PubMed] [Google Scholar]
- 53.Hobbs A.J., Higgs A., Moncada S. Inhibition of nitric oxide synthase as a potential therapeutic target. Annu. Rev. Pharmacol. Toxicol. 1999;39:191–220. doi: 10.1146/annurev.pharmtox.39.1.191. [DOI] [PubMed] [Google Scholar]
- 54.Song H., Cheng Y., Bi G., Zhu Y., Jun W., Ma W., Wu H. Release of Matrix Metalloproteinases-2 and 9 by S-Nitrosylated Caveolin-1 Contributes to Degradation of Extracellular Matrix in tPA-Treated Hypoxic Endothelial Cells. PLoS One. 2016;11(2):e0149269. doi: 10.1371/journal.pone.0149269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lambertsen K.L., Biber K., Finsen B. Inflammatory cytokines in experimental and human stroke. J. Cereb. Blood Flow Metab. 2012;32(9):1677–1698. doi: 10.1038/jcbfm.2012.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mayhan W.G. Cellular mechanisms by which tumor necrosis factor-alpha produces disruption of the blood-brain barrier. Brain Res. 2002;927(2):144–152. doi: 10.1016/S0006-8993(01)03348-0. [DOI] [PubMed] [Google Scholar]
- 57.Sibson N.R., Blamire A.M., Perry V.H., Gauldie J., Styles P., Anthony D.C. TNF-alpha reduces cerebral blood volume and disrupts tissue homeostasis via an endothelin- and TNFR2-dependent pathway. Brain. 2002;125(Pt 11):2446–2459. doi: 10.1093/brain/awf256. [DOI] [PubMed] [Google Scholar]
- 58.Kangwantas K., Pinteaux E., Penny J. The extracellular matrix protein laminin-10 promotes blood-brain barrier repair after hypoxia and inflammation in vitro. J. Neuroinflammation. 2016;13:25. doi: 10.1186/s12974-016-0495-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Labus J., Wöltje K., Stolte K.N., Häckel S., Kim K.S., Hildmann A., Danker K. IL-1β promotes transendothelial migration of PBMCs by upregulation of the FN/α5β1 signalling pathway in immortalised human brain microvascular endothelial cells. Exp. Cell Res. 2018;373(1-2):99–111. doi: 10.1016/j.yexcr.2018.10.002. [DOI] [PubMed] [Google Scholar]
- 60.Fiala M., Looney D.J., Stins M., Way D.D., Zhang L., Gan X., Chiappelli F., Schweitzer E.S., Shapshak P., Weinand M., Graves M.C., Witte M., Kim K.S. TNF-alpha opens a paracellular route for HIV-1 invasion across the blood-brain barrier. Mol. Med. 1997;3(8):553–564. doi: 10.1007/BF03401701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nishioku T., Matsumoto J., Dohgu S., Sumi N., Miyao K., Takata F., Shuto H., Yamauchi A., Kataoka Y. Tumor necrosis factor-alpha mediates the blood-brain barrier dysfunction induced by activated microglia in mouse brain microvascular endothelial cells. J. Pharmacol. Sci. 2010;112(2):251–254. doi: 10.1254/jphs.09292SC. [DOI] [PubMed] [Google Scholar]
- 62.Yang C., Hawkins K.E., Doré S., Candelario-Jalil E. Neuroinflammatory mechanisms of blood-brain barrier damage in ischemic stroke. Am. J. Physiol. Cell Physiol. 2019;316(2):C135–C153. doi: 10.1152/ajpcell.00136.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jolivel V., Bicker F., Binamé F., Ploen R., Keller S., Gollan R., Jurek B., Birkenstock J., Poisa-Beiro L., Bruttger J., Opitz V., Thal S.C., Waisman A., Bäuerle T., Schäfer M.K., Zipp F., Schmidt M.H.H. Perivascular microglia promote blood vessel disintegration in the ischemic penumbra. Acta Neuropathol. 2015;129(2):279–295. doi: 10.1007/s00401-014-1372-1. [DOI] [PubMed] [Google Scholar]
- 64.Edholm E.S., Rhoo K.H., Robert J. Evolutionary Aspects of Macrophages Polarization. Results Probl. Cell Differ. 2017;62:3–22. doi: 10.1007/978-3-319-54090-0_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.McKinney E.C., Haynes L., Droese A.L. Macrophage-like effector of spontaneous cytotoxicity from the shark. Dev. Comp. Immunol. 1986;10(4):497–508. doi: 10.1016/0145-305X(86)90171-0. [DOI] [PubMed] [Google Scholar]
- 66.Rieger A.M., Hall B.E., Barreda D.R. Macrophage activation differentially modulates particle binding, phagocytosis and downstream antimicrobial mechanisms. Dev. Comp. Immunol. 2010;34(11):1144–1159. doi: 10.1016/j.dci.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 67.Grayfer L., Robert J. Divergent antiviral roles of amphibian (Xenopus laevis) macrophages elicited by colony-stimulating factor-1 and interleukin-34. J. Leukoc. Biol. 2014;96(6):1143–1153. doi: 10.1189/jlb.4A0614-295R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mazzolini J., Le Clerc S., Morisse G., Coulonges C., Kuil L.E., van Ham T.J., Zagury J.F., Sieger D. Gene expression profiling reveals a conserved microglia signature in larval zebrafish. Glia. 2020;68(2):298–315. doi: 10.1002/glia.23717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Qin Y., Sun X., Shao X., Cheng C., Feng J., Sun W., Gu D., Liu W., Xu F., Duan Y. Macrophage-Microglia Networks Drive M1 Microglia Polarization After Mycobacterium Infection. Inflammation. 2015;38(4):1609–1616. doi: 10.1007/s10753-015-0136-y. [DOI] [PubMed] [Google Scholar]
- 70.Li Q., Cao Y., Dang C., Han B., Han R., Ma H., Hao J., Wang L. Inhibition of double-strand DNA-sensing cGAS ameliorates brain injury after ischemic stroke. EMBO Mol. Med. 2020;12(4):e11002. doi: 10.15252/emmm.201911002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gamdzyk M., Doycheva D.M., Araujo C., Ocak U., Luo Y., Tang J., Zhang J.H. cGAS/STING Pathway Activation Contributes to Delayed Neurodegeneration in Neonatal Hypoxia-Ischemia Rat Model: Possible Involvement of LINE-1. Mol. Neurobiol. 2020;57(6):2600–2619. doi: 10.1007/s12035-020-01904-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Neumann H., Kotter M.R., Franklin R.J. Debris clearance by microglia: an essential link between degeneration and regeneration. Brain. 2009;132(Pt 2):288–295. doi: 10.1093/brain/awn109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jin R., Yang G., Li G. Inflammatory mechanisms in ischemic stroke: role of inflammatory cells. J. Leukoc. Biol. 2010;87(5):779–789. doi: 10.1189/jlb.1109766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Colton C.A., Mott R.T., Sharpe H., Xu Q., Van Nostrand W.E., Vitek M.P. Expression profiles for macrophage alternative activation genes in AD and in mouse models of AD. J. Neuroinflammation. 2006;3:27. doi: 10.1186/1742-2094-3-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Patel A.R., Ritzel R., McCullough L.D., Liu F. Microglia and ischemic stroke: a double-edged sword. Int. J. Physiol. Pathophysiol. Pharmacol. 2013;5(2):73–90. [PMC free article] [PubMed] [Google Scholar]
- 76.Hu X., Leak R.K., Shi Y., Suenaga J., Gao Y., Zheng P., Chen J. Microglial and macrophage polarization—new prospects for brain repair. Nat. Rev. Neurol. 2015;11(1):56–64. doi: 10.1038/nrneurol.2014.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zheng Z.V., Lyu H., Lam S.Y.E., Lam P.K., Poon W.S., Wong G.K.C. The Dynamics of Microglial Polarization Reveal the Resident Neuroinflammatory Responses After Subarachnoid Hemorrhage. Transl. Stroke Res. 2019;11:433–449. doi: 10.1007/s12975-019-00728-5. [DOI] [PubMed] [Google Scholar]
- 78.Driessler F., Venstrom K., Sabat R., Asadullah K., Schottelius A.J. Molecular mechanisms of interleukin-10-mediated inhibition of NF-kappaB activity: a role for p50. Clin. Exp. Immunol. 2004;135(1):64–73. doi: 10.1111/j.1365-2249.2004.02342.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Garcia J.M., Stillings S.A., Leclerc J.L., Phillips H., Edwards N.J., Robicsek S.A., Hoh B.L., Blackburn S., Doré S. Role of Interleukin-10 in Acute Brain Injuries. Front. Neurol. 2017;8:244. doi: 10.3389/fneur.2017.00244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cai Y., Liu X., Chen W., Wang Z., Xu G., Zeng Y., Ma Y. TGF-β1 prevents blood-brain barrier damage and hemorrhagic transformation after thrombolysis in rats. Exp. Neurol. 2015;266:120–126. doi: 10.1016/j.expneurol.2015.02.013. [DOI] [PubMed] [Google Scholar]
- 81.Mori S., Maher P., Conti B. Neuroimmunology of the Interleukins 13 and 4. Brain Sci. 2016;6(2):E18. doi: 10.3390/brainsci6020018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Röcken M., Racke M., Shevach E.M. IL-4-induced immune deviation as antigen-specific therapy for inflammatory autoimmune disease. Immunol. Today. 1996;17(5):225–231. doi: 10.1016/0167-5699(96)80556-1. [DOI] [PubMed] [Google Scholar]
- 83.Raphael I., Nalawade S., Eagar T.N., Forsthuber T.G. T cell subsets and their signature cytokines in autoimmune and inflammatory diseases. Cytokine. 2015;74(1):5–17. doi: 10.1016/j.cyto.2014.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Veremeyko T., Siddiqui S., Sotnikov I., Yung A., Ponomarev E.D. IL-4/IL-13-dependent and independent expression of miR-124 and its contribution to M2 phenotype of monocytic cells in normal conditions and during allergic inflammation. PLoS One. 2013;8(12):e81774. doi: 10.1371/journal.pone.0081774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Elkabes S., DiCicco-Bloom E.M., Black I.B. Brain microglia/macrophages express neurotrophins that selectively regulate microglial proliferation and function. J. Neurosci. 1996;16(8):2508–2521. doi: 10.1523/JNEUROSCI.16-08-02508.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhao X., Eyo U.B., Murugan M., Wu L.J. Microglial interactions with the neurovascular system in physiology and pathology. Dev. Neurobiol. 2018;78(6):604–617. doi: 10.1002/dneu.22576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yang F., Wang Z., Wei X., Han H., Meng X., Zhang Y., Shi W., Li F., Xin T., Pang Q., Yi F. NLRP3 deficiency ameliorates neurovascular damage in experimental ischemic stroke. J. Cereb. Blood Flow Metab. 2014;34(4):660–667. doi: 10.1038/jcbfm.2013.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Shao A., Wu H., Hong Y., Tu S., Sun X., Wu Q., Zhao Q., Zhang J., Sheng J. Hydrogen-Rich Saline Attenuated Subarachnoid Hemorrhage-Induced Early Brain Injury in Rats by Suppressing Inflammatory Response: Possible Involvement of NF-κB Pathway and NLRP3 Inflammasome. Mol. Neurobiol. 2016;53(5):3462–3476. doi: 10.1007/s12035-015-9242-y. [DOI] [PubMed] [Google Scholar]
- 89.Swanson K.V., Deng M., Ting J.P. The NLRP3 inflammasome: molecular activation and regulation to therapeutics. Nat. Rev. Immunol. 2019;19(8):477–489. doi: 10.1038/s41577-019-0165-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ren H., Kong Y., Liu Z., Zang D., Yang X., Wood K., Li M., Liu Q. Selective NLRP3 (Pyrin Domain-Containing Protein 3) Inflammasome Inhibitor Reduces Brain Injury After Intracerebral Hemorrhage. Stroke. 2018;49(1):184–192. doi: 10.1161/STROKEAHA.117.018904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Szalay G., Martinecz B., Lénárt N., Környei Z., Orsolits B., Judák L., Császár E., Fekete R., West B.L., Katona G., Rózsa B., Dénes Á. Microglia protect against brain injury and their selective elimination dysregulates neuronal network activity after stroke. Nat. Commun. 2016;7:11499. doi: 10.1038/ncomms11499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Webster C.M., Hokari M., McManus A., Tang X.N., Ma H., Kacimi R., Yenari M.A. Microglial P2Y12 deficiency/inhibition protects against brain ischemia. PLoS One. 2013;8(8):e70927. doi: 10.1371/journal.pone.0070927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lou N., Takano T., Pei Y., Xavier A.L., Goldman S.A., Nedergaard M. Purinergic receptor P2RY12-dependent microglial closure of the injured blood-brain barrier. Proc. Natl. Acad. Sci. USA. 2016;113(4):1074–1079. doi: 10.1073/pnas.1520398113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ma Y., Wang J., Wang Y., Yang G.Y. The biphasic function of microglia in ischemic stroke. Prog. Neurobiol. 2017;157:247–272. doi: 10.1016/j.pneurobio.2016.01.005. [DOI] [PubMed] [Google Scholar]
- 95.Alessandrini A., Namura S., Moskowitz M.A., Bonventre J.V. MEK1 protein kinase inhibition protects against damage resulting from focal cerebral ischemia. Proc. Natl. Acad. Sci. USA. 1999;96(22):12866–12869. doi: 10.1073/pnas.96.22.12866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Gonzalez-Zulueta M., Feldman A.B., Klesse L.J., Kalb R.G., Dillman J.F., Parada L.F., Dawson T.M., Dawson V.L. Requirement for nitric oxide activation of p21(ras)/extracellular regulated kinase in neuronal ischemic preconditioning. Proc. Natl. Acad. Sci. USA. 2000;97(1):436–441. doi: 10.1073/pnas.97.1.436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hu B.R., Wieloch T. Tyrosine phosphorylation and activation of mitogen-activated protein kinase in the rat brain following transient cerebral ischemia. J. Neurochem. 1994;62(4):1357–1367. doi: 10.1046/j.1471-4159.1994.62041357.x. [DOI] [PubMed] [Google Scholar]
- 98.Kozawa O., Tokuda H., Miwa M., Ito H., Matsuno H., Niwa M., Kato K., Uematsu T. Involvement of p42/p44 mitogen-activated protein kinase in prostaglandin f(2alpha)-stimulated induction of heat shock protein 27 in osteoblasts. J. Cell. Biochem. 1999;75(4):610–619. doi: 10.1002/(SICI)1097-4644(19991215)75:4<610:AID-JCB7>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 99.Xi G., Hua Y., Keep R.F., Duong H.K., Hoff J.T. Activation of p44/42 mitogen activated protein kinases in thrombin-induced brain tolerance. Brain Res. 2001;895(1-2):153–159. doi: 10.1016/S0006-8993(01)02064-9. [DOI] [PubMed] [Google Scholar]
- 100.D’Haese J.G., Friess H., Ceyhan G.O. Therapeutic potential of the chemokine-receptor duo fractalkine/CX3CR1: an update. Expert Opin. Ther. Targets. 2012;16(6):613–618. doi: 10.1517/14728222.2012.682574. [DOI] [PubMed] [Google Scholar]
- 101.Cardona A.E., Huang D., Sasse M.E., Ransohoff R.M. Isolation of murine microglial cells for RNA analysis or flow cytometry. Nat. Protoc. 2006;1(4):1947–1951. doi: 10.1038/nprot.2006.327. [DOI] [PubMed] [Google Scholar]
- 102.Sumi N., Nishioku T., Takata F., Matsumoto J., Watanabe T., Shuto H., Yamauchi A., Dohgu S., Kataoka Y. Lipopolysaccharide-activated microglia induce dysfunction of the blood-brain barrier in rat microvascular endothelial cells co-cultured with microglia. Cell. Mol. Neurobiol. 2010;30(2):247–253. doi: 10.1007/s10571-009-9446-7. [DOI] [PubMed] [Google Scholar]
- 103.Chen H., Guan B., Wang B., Pu H., Bai X., Chen X., Liu J., Li C., Qiu J., Yang D., Liu K., Wang Q., Qi S., Shen J. Glycyrrhizin Prevents Hemorrhagic Transformation and Improves Neurological Outcome in Ischemic Stroke with Delayed Thrombolysis Through Targeting Peroxynitrite-Mediated HMGB1 Signaling. Transl. Stroke Res. 2019;11:967–982. doi: 10.1007/s12975-019-00772-1. [DOI] [PubMed] [Google Scholar]
- 104.del Zoppo G.J., Milner R., Mabuchi T., Hung S., Wang X., Berg G.I., Koziol J.A. Microglial activation and matrix protease generation during focal cerebral ischemia. Stroke. 2007;38(2) Suppl.:646–651. doi: 10.1161/01.STR.0000254477.34231.cb. [DOI] [PubMed] [Google Scholar]
- 105.Zhang Z.G., Zhang L., Jiang Q., Zhang R., Davies K., Powers C., Bruggen Nv., Chopp M. VEGF enhances angiogenesis and promotes blood-brain barrier leakage in the ischemic brain. J. Clin. Invest. 2000;106(7):829–838. doi: 10.1172/JCI9369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Potente M., Gerhardt H., Carmeliet P. Basic and therapeutic aspects of angiogenesis. Cell. 2011;146(6):873–887. doi: 10.1016/j.cell.2011.08.039. [DOI] [PubMed] [Google Scholar]
- 107.Drieu A., Buendia I., Levard D., Helie P., Brodin C., Vivien D., Rubio M. Immune responses and anti-inflammatory strategies in a clinically relevant model of thromboembolic ischemic stroke with reperfusion. Transl. Stroke Res. 2019;11:481–495. doi: 10.1007/s12975-019-00733-8. [DOI] [PubMed] [Google Scholar]
- 108.Xiong X.Y., Liu L., Yang Q.W. Functions and mechanisms of microglia/macrophages in neuroinflammation and neurogenesis after stroke. Prog. Neurobiol. 2016;142:23–44. doi: 10.1016/j.pneurobio.2016.05.001. [DOI] [PubMed] [Google Scholar]
- 109.Tikka T., Fiebich B.L., Goldsteins G., Keinanen R., Koistinaho J. Minocycline, a tetracycline derivative, is neuroprotective against excitotoxicity by inhibiting activation and proliferation of microglia. J. Neurosci. 2001;21(8):2580–2588. doi: 10.1523/JNEUROSCI.21-08-02580.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Machado L.S., Kozak A., Ergul A., Hess D.C., Borlongan C.V., Fagan S.C. Delayed minocycline inhibits ischemia-activated matrix metalloproteinases 2 and 9 after experimental stroke. BMC Neurosci. 2006;7:56. doi: 10.1186/1471-2202-7-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Yang Y., Salayandia V.M., Thompson J.F., Yang L.Y., Estrada E.Y., Yang Y. Attenuation of acute stroke injury in rat brain by minocycline promotes blood-brain barrier remodeling and alternative microglia/macrophage activation during recovery. J. Neuroinflammation. 2015;12:26. doi: 10.1186/s12974-015-0245-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Mok K.W., Mruk D.D., Lie P.P., Lui W.Y., Cheng C.Y. Adjudin, a potential male contraceptive, exerts its effects locally in the seminiferous epithelium of mammalian testes. Reproduction. 2011;141(5):571–580. doi: 10.1530/REP-10-0464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Shao J., Liu T., Xie Q.R., Zhang T., Yu H., Wang B., Ying W., Mruk D.D., Silvestrini B., Cheng C.Y., Xia W. Adjudin attenuates lipopolysaccharide (LPS)- and ischemia-induced microglial activation. J. Neuroimmunol. 2013;254(1-2):83–90. doi: 10.1016/j.jneuroim.2012.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Liu T., Zhang T., Yu H., Shen H., Xia W. Adjudin protects against cerebral ischemia reperfusion injury by inhibition of neuroinflammation and blood-brain barrier disruption. J. Neuroinflammation. 2014;11:107. doi: 10.1186/1742-2094-11-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ward R., Li W., Abdul Y., Jackson L., Dong G., Jamil S., Filosa J., Fagan S.C., Ergul A. NLRP3 inflammasome inhibition with MCC950 improves diabetes-mediated cognitive impairment and vasoneuronal remodeling after ischemia. Pharmacol. Res. 2019;142:237–250. doi: 10.1016/j.phrs.2019.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Li L., Yun D., Zhang Y., Tao Y., Tan Q., Qiao F., Luo B., Liu Y., Fan R., Xian J., Yu A. A cannabinoid receptor 2 agonist reduces blood-brain barrier damage via induction of MKP-1 after intracerebral hemorrhage in rats. Brain Res. 2018;1697:113–123. doi: 10.1016/j.brainres.2018.06.006. [DOI] [PubMed] [Google Scholar]
- 117.Wang Z., Li Y., Cai S., Li R., Cao G. Cannabinoid receptor 2 agonist attenuates blood-brain barrier damage in a rat model of intracerebral hemorrhage by activating the Rac1 pathway. Int. J. Mol. Med. 2018;42(5):2914–2922. doi: 10.3892/ijmm.2018.3834. [DOI] [PubMed] [Google Scholar]
- 118.Van den Bosch F., Kruithof E., De Vos M., De Keyser F., Mielants H. Crohn’s disease associated with spondyloarthropathy: effect of TNF-alpha blockade with infliximab on articular symptoms. Lancet. 2000;356(9244):1821–1822. doi: 10.1016/S0140-6736(00)03239-6. [DOI] [PubMed] [Google Scholar]
- 119.Kocic I., Kowianski P., Rusiecka I., Lietzau G., Mansfield C., Moussy A., Hermine O., Dubreuil P. Neuroprotective effect of masitinib in rats with postischemic stroke. Naunyn Schmiedebergs Arch. Pharmacol. 2015;388(1):79–86. doi: 10.1007/s00210-014-1061-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Shiba M., Suzuki H., Fujimoto M., Shimojo N., Imanaka-Yoshida K., Yoshida T., Kanamaru K., Matsushima S., Taki W. Role of platelet-derived growth factor in cerebral vasospasm after subarachnoid hemorrhage in rats. Acta Neurochir. Suppl. (Wien) 2013;115:219–223. doi: 10.1007/978-3-7091-1192-5_40. [DOI] [PubMed] [Google Scholar]
- 121.Zhan Y., Krafft P.R., Lekic T., Ma Q., Souvenir R., Zhang J.H., Tang J. Imatinib preserves blood-brain barrier integrity following experimental subarachnoid hemorrhage in rats. J. Neurosci. Res. 2015;93(1):94–103. doi: 10.1002/jnr.23475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Piette F., Belmin J., Vincent H., Schmidt N., Pariel S., Verny M., Marquis C., Mely J., Hugonot-Diener L., Kinet J.P., Dubreuil P., Moussy A., Hermine O. Masitinib as an adjunct therapy for mild-to-moderate Alzheimer’s disease: a randomised, placebo-controlled phase 2 trial. Alzheimers Res. Ther. 2011;3(2):16. doi: 10.1186/alzrt75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Vermersch P., Benrabah R., Schmidt N., Zéphir H., Clavelou P., Vongsouthi C., Dubreuil P., Moussy A., Hermine O. Masitinib treatment in patients with progressive multiple sclerosis: a randomized pilot study. BMC Neurol. 2012;12:36. doi: 10.1186/1471-2377-12-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Adzemovic M.V., Zeitelhofer M., Eriksson U., Olsson T., Nilsson I. Imatinib ameliorates neuroinflammation in a rat model of multiple sclerosis by enhancing blood-brain barrier integrity and by modulating the peripheral immune response. PLoS One. 2013;8(2):e56586. doi: 10.1371/journal.pone.0056586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Tebib J., Mariette X., Bourgeois P., Flipo R.M., Gaudin P., Le Loët X., Gineste P., Guy L., Mansfield C.D., Moussy A., Dubreuil P., Hermine O., Sibilia J. Masitinib in the treatment of active rheumatoid arthritis: results of a multicentre, open-label, dose-ranging, phase 2a study. Arthritis Res. Ther. 2009;11(3):R95. doi: 10.1186/ar2740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Humbert M., de Blay F., Garcia G., Prud’homme A., Leroyer C., Magnan A., Tunon-de-Lara J.M., Pison C., Aubier M., Charpin D., Vachier I., Purohit A., Gineste P., Bader T., Moussy A., Hermine O., Chanez P. Masitinib, a c-kit/PDGF receptor tyrosine kinase inhibitor, improves disease control in severe corticosteroid-dependent asthmatics. Allergy. 2009;64(8):1194–1201. doi: 10.1111/j.1398-9995.2009.02122.x. [DOI] [PubMed] [Google Scholar]
- 127.Paul C., Sans B., Suarez F., Casassus P., Barete S., Lanternier F., Grandpeix-Guyodo C., Dubreuil P., Palmérini F., Mansfield C.D., Gineste P., Moussy A., Hermine O., Lortholary O. Masitinib for the treatment of systemic and cutaneous mastocytosis with handicap: a phase 2a study. Am. J. Hematol. 2010;85(12):921–925. doi: 10.1002/ajh.21894. [DOI] [PubMed] [Google Scholar]
- 128.Rieckmann P. Imatinib buys time for brain after stroke. Nat. Med. 2008;14(7):712–713. doi: 10.1038/nm0708-712. [DOI] [PubMed] [Google Scholar]
- 129.Dhawan G., Floden A.M., Combs C.K. Amyloid-β oligomers stimulate microglia through a tyrosine kinase dependent mechanism. Neurobiol. Aging. 2012;33(10):2247–2261. doi: 10.1016/j.neurobiolaging.2011.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Dhawan G., Combs C.K. Inhibition of Src kinase activity attenuates amyloid associated microgliosis in a murine model of Alzheimer’s disease. J. Neuroinflammation. 2012;9:117. doi: 10.1186/1742-2094-9-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Romeo U., Palaia G., Fantozzi P.J., Tenore G., Bosco D. A Rare Case of Melanosis of the Hard Palate Mucosa in a Patient with Chronic Myeloid Leukemia. Case Rep. Dent. 2015;2015:817094. doi: 10.1155/2015/817094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Capdeville R., Buchdunger E., Zimmermann J., Matter A. Glivec (STI571, imatinib), a rationally developed, targeted anticancer drug. Nat. Rev. Drug Discov. 2002;1(7):493–502. doi: 10.1038/nrd839. [DOI] [PubMed] [Google Scholar]
- 133.Rizzo A.N., Aman J., van Nieuw Amerongen G.P., Dudek S.M. Targeting Abl kinases to regulate vascular leak during sepsis and acute respiratory distress syndrome. Arterioscler. Thromb. Vasc. Biol. 2015;35(5):1071–1079. doi: 10.1161/ATVBAHA.115.305085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Corada M., Mariotti M., Thurston G., Smith K., Kunkel R., Brockhaus M., Lampugnani M.G., Martin-Padura I., Stoppacciaro A., Ruco L., McDonald D.M., Ward P.A., Dejana E. Vascular endothelial-cadherin is an important determinant of microvascular integrity in vivo. Proc. Natl. Acad. Sci. USA. 1999;96(17):9815–9820. doi: 10.1073/pnas.96.17.9815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Aman J., van Bezu J., Damanafshan A., Huveneers S., Eringa E.C., Vogel S.M., Groeneveld A.B., Vonk Noordegraaf A., van Hinsbergh V.W., van Nieuw Amerongen G.P. Effective treatment of edema and endothelial barrier dysfunction with imatinib. Circulation. 2012;126(23):2728–2738. doi: 10.1161/CIRCULATIONAHA.112.134304. [DOI] [PubMed] [Google Scholar]
- 136.Chislock E.M., Pendergast A.M. Abl family kinases regulate endothelial barrier function in vitro and in mice. PLoS One. 2013;8(12):e85231. doi: 10.1371/journal.pone.0085231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Rizzo A.N., Sammani S., Esquinca A.E., Jacobson J.R., Garcia J.G., Letsiou E., Dudek S.M. Imatinib attenuates inflammation and vascular leak in a clinically relevant two-hit model of acute lung injury. Am. J. Physiol. Lung Cell. Mol. Physiol. 2015;309(11):L1294–L1304. doi: 10.1152/ajplung.00031.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Stephens R.S., Servinsky L.E., Rentsendorj O., Kolb T.M., Pfeifer A., Pearse D.B. Protein kinase G increases antioxidant function in lung microvascular endothelial cells by inhibiting the c-Abl tyrosine kinase. Am. J. Physiol. Cell Physiol. 2014;306(6):C559–C569. doi: 10.1152/ajpcell.00375.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Tanaka S., Chen-Yoshikawa T.F., Kajiwara M., Menju T., Ohata K., Takahashi M., Kondo T., Hijiya K., Motoyama H., Aoyama A., Masuda S., Date H. Protective Effects of Imatinib on Ischemia/Reperfusion Injury in Rat Lung. Ann. Thorac. Surg. 2016;102(5):1717–1724. doi: 10.1016/j.athoracsur.2016.05.037. [DOI] [PubMed] [Google Scholar]
- 140.Reber L.L., Starkl P., Balbino B., Sibilano R., Gaudenzio N., Rogalla S., Sensarn S., Kang D., Raghu H., Sokolove J., Robinson W.H., Contag C.H., Tsai M., Galli S.J. The tyrosine kinase inhibitor imatinib mesylate suppresses uric acid crystal-induced acute gouty arthritis in mice. PLoS One. 2017;12(10):e0185704. doi: 10.1371/journal.pone.0185704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Azizi G., Haidari M.R., Khorramizadeh M., Naddafi F., Sadria R., Javanbakht M.H., Sedaghat R., Tofighi Zavareh F., Mirshafiey A. Effects of imatinib mesylate in mouse models of multiple sclerosis and in vitro determinants. Iran. J. Allergy Asthma Immunol. 2014;13(3):198–206. [PubMed] [Google Scholar]
- 142.Su E.J., Fredriksson L., Geyer M., Folestad E., Cale J., Andrae J., Gao Y., Pietras K., Mann K., Yepes M., Strickland D.K., Betsholtz C., Eriksson U., Lawrence D.A. Activation of PDGF-CC by tissue plasminogen activator impairs blood-brain barrier integrity during ischemic stroke. Nat. Med. 2008;14(7):731–737. doi: 10.1038/nm1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Gągało I., Rusiecka I., Kocić I. Tyrosine Kinase Inhibitor as a new Therapy for Ischemic Stroke and other Neurologic Diseases: is there any Hope for a Better Outcome? Curr. Neuropharmacol. 2015;13(6):836–844. doi: 10.2174/1570159X13666150518235504. [DOI] [PMC free article] [PubMed] [Google Scholar]