Abstract
Purpose
A Phase Ib study in patients with trastuzumab-resistant, human epidermal growth factor receptor-2- (HER2)-positive advanced breast cancer defined the recommended Phase II dose of buparlisib as 100 mg/day in combination with 2 mg/kg weekly trastuzumab, and reported preliminary signs of clinical activity. Here we present results from the Phase II portion.
Methods
Patients with trastuzumab-resistant, HER2-positive advanced breast cancer received buparlisib plus trastuzumab. Study endpoints included safety/tolerability and antitumour activity. The study was extended to include a Phase Ib dose-escalation phase, in which patients with progressive brain metastases also received capecitabine.
Results
In the Phase II portion, of 50 patients treated with buparlisib and trastuzumab, the most common (≥ 30%) all-grade adverse events (AEs) were diarrhoea (54%), nausea (48%), decreased appetite, increased alanine aminotransferase (36% each), increased aspartate aminotransferase (34%), fatigue, rash (32% each), cough and hyperglycemia (30% each). One (2%) patient achieved complete response and four (8%) patients had confirmed partial responses [PR; including two patients with phosphatidylinositol 3-kinase (PI3 K) pathway-activated tumours]. Overall response rate (ORR) was 10%: the primary endpoint (ORR ≥ 25%) was therefore not met. In the Phase Ib portion, all patients with measurable brain lesions at baseline showed tumour shrinkage to some degree; due to low enrollment, maximum tolerated dose of buparlisib in combination with trastuzumab and capecitabine was not determined.
Conclusion
Buparlisib plus trastuzumab, as a chemotherapy-free regimen, demonstrated an acceptable safety profile but limited efficacy in patients with heavily pretreated, trastuzumab-resistant HER2-positive breast cancer, and in patients with progressive brain metastases also receiving capecitabine.
Keywords: Buparlisib, HER2-positive, Trastuzumab pretreated, Advanced/metastatic breast cancer, Brain metastases, Herceptin
Introduction
Human epidermal growth factor receptor-2 (HER2) overexpression, which occurs in up to 20% of all invasive breast cancers, is associated with aggressive disease [1, 2]. The prognosis of patients with HER2-positive (HER2+) breast cancer has been greatly improved in the last 15 years through the introduction of HER2-targeted therapies [3–11]. However, there are few treatment options for the 34% of patients who develop central nervous system (CNS) metastases [12]. As a consequence, approximately 50% of patients with HER2+ CNS metastatic breast cancer die from CNS disease progression [12, 13]. Furthermore, innate or acquired resistance to HER2-targeted agents is a major problem [3, 6, 7].
Activation of the phosphatidylinositol 3-kinase (PI3 K)/AKT/mammalian target of rapamycin (mTOR) pathway, through phosphatase and tensin homolog (PTEN) loss or activating PIK3CA mutations has been linked to the development of resistance to HER2-targeted agents [14–17]. Inhibition of the PI3 K/mTOR pathway in trastuzumab-resistant HER2+ breast cancer can restore sensitivity to trastuzumab [18, 19]. Data from the pivotal Phase III BOLERO-3 trial in a similar patient population demonstrated that the addition of everolimus to trastuzumab and vinorelbine significantly prolonged PFS [20].
Buparlisib (BKM120), an oral pan-PI3 K inhibitor, has demonstrated antitumour activity in preclinical models [21] and the addition of buparlisib to trastuzumab led to tumour shrinkage in xenograft mouse models of trastuzumab-resistant HER2+ breast cancer [19]. In vivo, buparlisib penetrates the blood-brain barrier, inhibiting PI3 K signalling and reducing the incidence of brain metastases [22, 23]. The tolerability of buparlisib and trastuzumab was investigated in a Phase Ib study in patients with HER2+ breast cancer (NCT01132664), which established the recommended Phase II dose (RP2D) as buparlisib (100 mg/day) plus trastuzumab (2 mg/kg weekly) [24].
Here we present efficacy and safety results from the Phase II portion of the study, which investigated buparlisib in combination with trastuzumab in patients with HER2+ locally advanced or metastatic breast cancer whose disease has progressed on prior trastuzumab-based therapy.
Based on the high unmet medical need in patients with brain metastases, promising preliminary clinical data for buparlisib and the ability of buparlisib to penetrate the blood–brain barrier, the study was extended to include an additional Phase Ib cohort of patients with brain metastases who received buparlisib in combination with trastuzumab and capecitabine.
Patients and methods
Study design
This was a Phase Ib/II study of buparlisib in combination with trastuzumab in HER2+ advanced breast cancer progressing on prior trastuzumab therapy. Patients received the RP2D of oral buparlisib (100 mg once daily [QD]) [24] in continuous 28-day cycles.
In the Phase Ib brain metastases cohort, patients had HER2+ breast cancer whose metastatic brain disease progressed after standard treatments including prior whole-brain radiotherapy and/or stereotactic radiosurgery for brain metastasis, received 80 or 100 mg/day oral buparlisib in combination with trastuzumab and capecitabine. The Phase Ib portion included a dose-escalation part followed by a safety expansion; however, the study was terminated during dose escalation due to the scarcity of patients and consequent challenges in enrolling patients under the existing protocol.
Patients received treatment until disease progression, unacceptable toxicity, withdrawal of consent or investigator decision. The primary objective of the Phase II portion was to assess the activity of buparlisib in combination with trastuzumab; the primary endpoint was met if ORR was ≥ 25%, with a probability of ORR being ≤ 15% of < 0.05. The primary objective of the Phase Ib brain metastases part was to determine the MTD/RP2D. (Additional details are in the supplementary materials.)
Patient population
As described previously [24], patients had histologically confirmed HER2+ locally advanced or metastatic breast cancer, with documented evidence of disease progression as per Response Evaluation Criteria In Solid Tumors (RECIST) v1.0 no more than 16 weeks prior to treatment start, resistance to trastuzumab, World Health Organisation (WHO) performance status ≤ 2 and prior treatment with one to four lines of HER2-targeted therapy. Key exclusion criteria included previous treatment with a PI3 K inhibitor and untreated brain metastases. The Phase Ib brain metastses cohort included patients treated at the RP2D during the Phase Ib portion of the study who met the Phase II eligibility criteria. (Additional specific inclusion criteria are in the supplementary materials.)
Approval was obtained from the ethics committees of participating institutions and regulatory authorities, and all participating patients provided written informed consent and agreed to comply with the protocol. The study was conducted in accordance with the Declaration of Helsinki and guidelines for Good Clinical Practice, as defined by the International Conference on Harmonisation.
Safety
Routine clinical and laboratory assessments were conducted at baseline and at regular intervals throughout the study. Patient self-rated questionnaires for depression (PHQ-9) and anxiety (GAD-7) were administered throughout treatment in order to monitor changes in behaviour and reduce the frequency and severity of psychiatric events [25, 26]. Patients in the brain metastases cohort underwent additional neuropsychiatric assessment via the self-reported Psychiatric Diagnostic Screening Questionnaire. Adverse events (AEs) were recorded continuously from the start of study treatment until 28 days post-treatment discontinuation, and were characterised and graded as per CTCAE v3.0. For the brain metastases cohort, DLTs during Cycle 1 were discussed with investigators at dose-escalation meetings.
Efficacy
Radiologic response was assessed locally by computed tomography or MRI according to RECIST v1.0 at baseline and subsequently every 6 weeks for the first 18 weeks, then every 8 weeks (Phase II cohort) or every 12 weeks (brain metastases cohort), until disease progression or end of treatment. All complete responses (CRs) and partial responses (PRs) were confirmed by a second assessment at least 4 weeks after the initial assessment. Stable disease (SD) and prolonged SD were defined as at least one SD assessment or better ≥ 6 weeks and ≥ 24 weeks, respectively, after the start of treatment, and not qualifying as CR or PR.
Biomarkers
Tumour, skin, hair and blood samples were collected before and during study treatment for the exploratory investigation of biomarkers clinically relevant to PI3 K pathway signalling and antitumour activity. In tumour tissue, PIK3CA and PTEN mutations were detected by Sanger sequencing, and PTEN expression was evaluated by immunohistochemistry.
Blood samples for biomarker analyses were collected throughout the study. Circulating markers of angiogenesis (VEGF, VEGFC, VEGFD, sVEGFRl, sVEGFR2, bFGF, cKIT, TIES2, PIGF) and cellular death (M30, M65) were analysed by enzyme-linked immunosorbent assay (ELISA) at screening, and model-adjusted log2 changes from baseline were used to investigate therapy-induced pharmaco-dynamic changes. ctDNA was extracted from blood samples, and PIK3CA mutations were analysed by Sanger sequencing.
Statistical analyses
All patients who received at least one dose of buparlisib treatment at the RP2D were included in the analysis; this consisted of patients enrolled in the Phase II portion, as well as eligible patients with brain metastases treated at the RP2D in the Phase Ib portion of the study. ORR was defined as the percentage of patients with a best overall response of CR or PR, as per RECIST vl.0, and according to investigator assessment. The true ORR in the Phase II part of the study was calculated using the adaptive Bayesian logistic regression model (BLRM). Minimally informative beta distribution priors with prior medians equal to a 15% clinical threshold for futility were utilised. The posterior distribution of the ORR was derived on completion of the study. Efficacy of the combination was to be declared if the probability of the ORR being ≤ 15% was < 0.05, and the observed ORR was ≥ 25%. With a planned sample size of 41 patients, the minimum number of responders to be observed to declare efficacy was 11, corresponding to an ORR of 26.8%. PFS was determined by Kaplan–Meier analysis.
Results
Phase II portion
Patient characteristics
In total, 53 patients were enrolled and received treatment between May 2010 and March 2014 (Phase II, N = 45; Phase Ib, N = 8). Patients were treated at 19 sites in six countries. In Phase II, three patients received a loading dose of trastuzumab but discontinued prior to receiving buparlisib (due to AE, protocol deviation and abnormal laboratory assessments, respectively), and were therefore not included in safety or efficacy analyses. Here, we present results for the 50 patients who received both buparlisib and trastuzumab. The median age of patients was 52 years (range 28–75 years), and 27 (54%) patients had hormone receptor-positive (HR+) disease (Table 1). The median number of prior antineoplastic therapies (including hormonal therapy), cytotoxic chemotherapies and HER2-targeted therapies was 3 (range 1–7), 1 (range 0–3) and 2 (range 1–5), respectively.
Table 1.
Patient baseline characteristics and disease characteristics
| All patients (N = 50)a | |
|---|---|
| Median age, years (range) | 52 (28–75) |
| Female patients, n (%) | 50 (100) |
| Postmenopausal status | 37 (74) |
| Race, n (%) | |
| Caucasian | 48 (96) |
| Others | 2 (4) |
| WHO performance status, n (%) | |
| 0 | 19 (38) |
| 1 | 31 (62) |
| Prior antineoplastic therapies, median (range) | |
| Prior antineoplastic regimensb | 3 (1–7) |
| Prior cytotoxic chemotherapies | 1 (0–3) |
| Prior HER2-targeted therapiesc | 2 (1–5) |
| Setting at last treatment, n (%) | |
| Therapeutic/palliative | 47 (94) |
| Adjuvant/neoadjuvant | 4 (8) |
| Therapy type at last treatment, n (%) | |
| HER2-targeted therapy | 40 (80) |
| Chemotherapy | 24 (48) |
| Hormonal therapy | 4 (8) |
| Other | 4 (8) |
| Hormonal status, n (%) | |
| ER and/or PgR positive | 27 (54) |
| ER and PgR negative | 23 (46) |
| Tumour histology, n (%) | |
| Invasive ductal carcinoma | 45 (90) |
| Invasive lobular carcinoma | 2 (4) |
| Other | 3 (6) |
| Histologic grade, n (%) | |
| Well differentiated | 0 |
| Moderately differentiated | 18 (36) |
| Poorly differentiated | 27 (54) |
| Unknown | 5 (10) |
| Number of metastatic sites, median (range) | 3 (1–6) |
| Most common site of metastases, n (%) | |
| Lung | 29 (58) |
| Bone | 26 (52) |
| Nodes | 26 (52) |
| Liver | 25 (50) |
| Brain | 6 (12) |
| Skin | 3 (6) |
| Others | 20 (40) |
| PI3 K pathway activation status in tumour tissue | |
| Patients with known status, n (%) | 26 (52) |
| PI3 K pathway-activatedd,e n/N (%) | 10/26 (38) |
| PI3 K pathway-non-activatede n/N (%) | 16/26 (62) |
ER oestrogen receptor, PgR progesterone receptor
Three patients received a loading dose of trastuzumab but not buparlisib, and are not included here
Includes HER2-targeted therapy, chemotherapy and hormonal therapy. All patients had received monoclonal antibodies (primarily trastuzumab) and 86% had received taxanes (primarily docetaxel)
Includes trastuzumab, T-DM1, lapatinib and pertuzumab
PI3 K pathway activation was defined as PIK3CA mutation (eight patients), PTEN mutation (one patient) or PTEN null or low expression by immunohistochemistry (H-score < 50; one patient)
Percentage calculated out of 26 patients with known PI3 K pathway activation status
Patient disposition and treatment exposure
All patients treated with buparlisib discontinued treatment, with the primary reasons for treatment discontinuation being disease progression (70%) and AEs (20%). The median duration of exposure to study treatment was 9.9 weeks (range 2.0–106.6 weeks) and the mean relative dose intensity was 94% (standard deviation 9.3%). Nineteen (38%) patients received study treatment for more than 12 weeks. Four (8%) patients required a dose reduction, of which three (6%) were due to AEs. Twenty-four (48%) patients had at least one dose interruption, of which 23 (46%) were due to AEs.
Safety and tolerability
The most common all-grade AEs regardless of study drug relationship were diarrhoea (54%), nausea (48%), decreased appetite (36%) and increased alanine aminotransferase (ALT; 36%) and the most common grade 3/4 AEs regardless of study drug relationship were increased ALT (16%) and increased AST (12%) (Table 2). The most frequent study drug-related psychiatric disorders were anxiety (18%) and depression (14%), and were mostly grade 1/2 in severity; 7 (14%) patients reported grade 3/4 psychiatric events. Among the 34 (68%) patients experiencing an AE which required dose adjustment or treatment interruption, the most frequently reported AEs included increased ALT (24%), increased AST and rash (18% each). The most common study drug-related AEs leading to study drug discontinuation were (all grade 2) depression (6%), increased ALT (4%) and increased AST (4%). Four on-treatment deaths were reported during the Phase II portion of the study due to disease progression, respiratory failure due to disease progression, pleural effusion and liver disorder (one patient each), and no deaths were suspected to be related to study treatment.
Table 2.
Most common (≥ 15% of patients; any grade) adverse events, regardless of study drug relationship
| Adverse event, n (%) | All patients (N = 50)a |
|
|---|---|---|
| Any grade | Grade 3/4 | |
| Total | 50 (100) | 35 (70) |
| Diarrhoea | 27 (54) | 2 (4) |
| Nausea | 24 (48) | 2 (4) |
| Decreased appetite | 18 (36) | 0 |
| Increased ALT | 18 (36) | 8 (16) |
| Increased AST | 17 (34) | 6 (12) |
| Fatigue | 16 (32) | 2 (4) |
| Rash | 16 (32) | 5 (10) |
| Cough | 15 (30) | 0 |
| Hyperglycemia | 15 (30) | 2 (4)b |
| Asthenia | 14 (28) | 4 (8) |
| Vomiting | 14 (28) | 1 (2) |
| Stomatitis | 12 (24) | 2 (4) |
| Headache | 11 (22) | 0 |
| Anxiety | 11 (22) | 2 (4) |
| Depression | 9 (18) | 1 (2) |
Three patients received a loading dose of trastuzumab but not buparlisib, and are not included here
Includes one patient with ‘blood glucose increased’
Clinical activity
Of the 50 patients evaluable for response in the Phase Ib/ II part, one (2%) patient achieved CR; four (8%) patients had confirmed PR and 20 (40%) patients had SD, including two (4%) patients who had SD for ≥ 24 weeks (Table 3; Fig. 1). The ORR (CR + PR) was 10% [90% confidence interval (CI), 4.0–19.9], DCR (CR + PR + SD) was 50% (90% CI 37.6–62.4) and clinical benefit rate (CBR; CR + PR + SD ≥ 24 weeks) was 14% (90% CI, 6.8–24.7). The primary endpoint in Phase II as well as eligible patients with brain metastases treated at the RP2D in the Phase Ib portion of the study (ORR ≥ 25%) (ORR ≥ 25%) was therefore not met.
Table 3.
Summary of best overall response per RECIST v1.0
| All patients (N = 50)a | |
|---|---|
| Best overall response, n (%) | |
| Complete response (CR) | 1 (2) |
| Partial response (PR) | 4 (8) |
| Stable disease (SD) | 20 (40) |
| SD ≥ 24 weeks | 2 (4) |
| Progressive disease | 20 (40) |
| Unknown | 5 (10) |
| Objective response rate (ORR; CR + PR), n (%) [90% CI]b | 5 (10) [4.0–19.9] |
| Disease control rate (DCR; CR + PR + SD), n (%) [90% CI] | 25 (50) [37.6–62.4] |
| Clinical benefit rate (CBR; CR + PR + SD ≥ 24 weeks), n (%) [90% CI] | 7 (14) [6.8–24.7] |
Three patients received a loading dose of trastuzumab but not buparlisib, and are not included here
90% CIs for ORR, DCR and CBR were obtained using the exact binomial 90% CI test
Fig. 1.
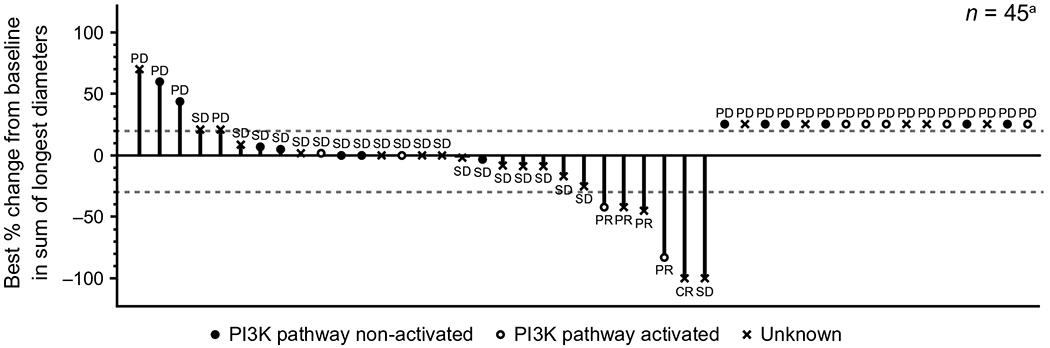
Waterfall plot of best percentage change from baseline and best overall response per RECIST v1.0. PD, progressive disease. aFive patients with missing best percentage change from baseline and unknown overall response are not presented. Patients with missing best percentage change from baseline are shown on the right
Biomarker analyses
PI3 K pathway activation was identified in 10/26 (38%) patients with known pathway status: eight (31%) patients had PIK3CA mutations, and two (4%) patients each had a PTEN mutation or loss of PTEN expression. PI3 K pathway status was unknown (missing PIK3CA and/or PTEN status) in the remaining 24 patients in Phase II (Supplementary Table S1). Best overall response was PR in 2/10 patients with PI3 K pathway-activated tumours (Supplementary Table S2). In contrast, there were no CRs or PRs reported among the 16 patients with PI3 K pathway-non-activated tumours. Median PFS for patients with wild-type and mutated PIK3CA was 1.7 months (95% CI 1.5–5.4) and 1.8 months (95% CI 1.3–3.4), respectively. A meaningful interpretation of PIK3CA mutation status in ctDNA was not possible due to the low number of samples collected, in addition to the low sensitivity observed with Sanger sequencing method.
Baseline levels of biomarkers of angiogenesis and cellular death were analysed in 47 patients. Patients with above-median VEGF expression had longer median PFS [5.3 (95% CI 1.8-not evaluable) months] than patients with below-median VEGF expression [1.9 (95% CI 1.6—3.4) months], with hazard ratio 0.39 (95% CI 0.17–0.88) (Supplementary Fig. S1).
Plasma biomarkers were also analysed for dynamic changes from baseline with respect to tumour response. Only VEGF expression was associated with tumour response: patients with progressive disease showed an increase in VEGF levels throughout treatment, while no changes in VEGF expression were observed in patients with PR or SD (data not shown). No other biomarkers were found to be significantly associated with treatment response. Biomarkers for tumour burden (circulating tumour cells and soluble HER2 extracellular domain) appeared to be decreased on Cycle 3 Day 1, suggesting a decrease in tumour burden with study treatment (data not shown). Pharmaco-dynamic analyses were consistent with previously published results from the Phase Ib dose-escalation portion of the study, which reported a decrease in pS6 expression in 4/6 patients treated with combination buparlisib and trastuzumab; the largest reduction in pS6 was observed at the highest dose of buparlisib (100 mg/day) and associated with the best clinical response (PR) [24].
Phase Ib brain metastases cohort
Nine patients (three receiving 80 mg/day and six receiving 100 mg/day buparlisib, in combination with capecitabine, plus trastuzumab) were treated in the brain metastases cohort between May 2010 and August 2014. Median age in the brain metastases cohort was 52 years (range 37–63 years), 78% of patients were Caucasian and all patients had a WHO performance status of 1. Five (56%) patients had HR+ tumours. Patients had received a median of 2 (range 1–4 weeks) prior lines of HER2-targeted therapy. Eight patients were evaluable for MTD assessment (one patient did not meet the minimum exposure criterion for buparlisib and capecitabine, defined as treatment with buparlisib for ≥ 16 days and capecitabine for ≥ 11 days at the planned dose, and receiving all three scheduled trastuzumab doses during the first treatment cycle). The median duration of exposure to buparlisib was 12.9 weeks (range 3–52 weeks). One patient receiving buparlisib 100 mg/day experienced a DLT (grade 3 stomatitis and diarrhoea) during Cycle 1.
Best overall response was PR in one patient; 7/9 (78%) patients achieved SD, including two patients who were stable for ≥ 24 weeks. In total, 7/9 (78%) patients progressed during the study: five in the brain and two extracranially. Of the 4/9 (44%) patients with measurable lesions (per RECIST v1.0) in the brain at baseline, all showed some degree of tumour shrinkage: one patient had confirmed PR, one patient had unconfirmed PR in the brain and two patients had tumour shrinkage that did not qualify for PR (i.e. SD). Three of these four patients subsequently progressed in the brain (after 85, 127 and 165 days of treatment, respectively), and the other progressed in the bone after 54 days.
Discussion
In this Phase II study in patients with HER2+ advanced breast cancer that had progressed on or after trastuzumab-based therapy, buparlisib 100 mg/day in combination with trastuzumab was generally well tolerated, with a similar safety profile to that reported in the Phase Ib portion of this trial [24] and in an earlier study of single-agent buparlisib in advanced solid tumours [27].
Due to the role of the PI3 K/mTOR pathway in insulin resistance, the hyperglycemia observed in 28% of patients is likely to be an on-target effect of PI3 K/mTOR signalling inhibition [28]. Hyperglycemia was effectively managed through the use of concomitant antidiabetic medications such as metformin, and/or buparlisib dose interruption or reduction, resulting in few patients requiring permanent discontinuation due to this AE. Liver toxicities (ALT/AST increases) were also frequent (24% each), consisting mainly of isolated transaminitis. Transaminitis was typically asymptomatic, reversible and rarely associated with impaired liver function or liver failure, and was among the most frequent AEs leading to permanent treatment discontinuation. Mood disorders (mostly grade 1/2 in severity) consisted mainly of depression and anxiety, as well as several other psychiatric disorders. Mood disorders have not been reported with other PI3 K inhibitors; however, the ability of buparlisib to cross the blood-brain barrier and inhibit PI3 K/mTOR signalling in the brain may contribute to the prevalence of this AE [22, 23]. Although patients with pre-existing major psychiatric disorders were excluded from this study (and other studies of buparlisib), the proactive evaluation of potential psychiatric disorders through the use of the PHQ-9 and GAD-8 self-rated questionnaires may also have led to higher reporting. Psychiatric AEs were generally detected early and were well managed by buparlisib dose adjustments or administration of concomitant medications, although three (6%) patients discontinued study treatment due to grade 2 depression. Rash was adequately managed by concomitant medications and/ or dose reduction/interruption.
Preliminary signs of clinical activity were observed in this study; however, the ORR was lower than in the initial Phase Ib part [24], and the study did not meet its pre-specified primary endpoint of ORR ≥ 25%. The clinical activity of combined buparlisib and trastuzumab was moderate compared to that achieved with chemotherapy-based combinations in the first-line setting [3]. Despite previous studies suggesting that patients with HER2+ tumours and PI3 K pathway activation may be more sensitive to PI3 K inhibition (especially in the context of trastuzumab resistance) [20, 29], the results of this study suggest that a doublet regimen of PI3 K inhibition and trastuzumab may not be sufficient, and a backbone of chemotherapy may be needed in order to achieve maximal clinical benefit. Due to the low number of events for overall survival (OS) at the cut-off date, follow-up for OS analysis is ongoing.
Retrospective biomarker analyses have revealed associations between PIK3CA mutation and treatment response in HER2+ advanced breast cancer [29, 30]. In the current study, 2/10 patients with activated PI3 K pathway had PR, compared to 0/18 patients with wild-type PIK3CA. However, there was no difference in median PFS between these two groups, and the limited number of patients makes a robust analysis challenging.
Among the nine patients enrolled in the brain metastases cohort, preliminary signs of clinical activity in the brain were observed: all four patients with target lesions in the brain at baseline showed some degree of tumour shrinkage (including one patient with a confirmed PR); however, three of these four patients subsequently progressed in the brain, with a duration of response of approximately 3–5 months. Moreover, of the 7/9 (78%) patients who achieved SD as best overall response, five patients subsequently progressed in the brain. The rarity of eligible patients resulted in slow enrollment under the current study protocol; due to the limited sample size and the lack of a control arm in this part of the study, a robust analysis of the antitumour activity of buparlisib in this patient population was not possible. The MTD/ RP2D was not declared for this cohort, and the planned safety expansion was not conducted. In summary, the combination of buparlisib and trastuzumab in a chemotherapy-free regimen for the treatment of patients with HER2+ locally advanced or metastatic breast cancer, whose disease has progressed on trastuzumab-based therapy, demonstrated limited clinical activity. Therefore, combinations of isoform-specific PI3 K inhibitors with chemotherapy and/or endocrine therapy are currently explored in ongoing clinical trials.
Supplementary Material
Acknowledgements
The authors would like to thank the patients who took part in this study, and their families. We thank Shaun Su, Shanthy Nuti and Tong Zhu (Novartis Pharmaceuticals Corporation) for their significant contributions towards the conduct of the study and data analyses. Editorial assistance was provided by Sarah Amir PhD and Maria Alfaradhi PhD of Articulate Science, and was funded by Novartis Pharmaceuticals Corporation.
Disclosure Timothy Pluard reports personal fees from Novartis outside the submitted work. Thomas Bachelot reports grants, personal fees and non-financial support from Roche and Novartis and grant and personal fees from Astra Zeneca and Pfizer, outside the submitted work. Hyo Han reports research funding support from Novartis during the conduct of the study. Guy Jerusalem reports grants, personal fees and non-financial support from Novartis, Roche and BMS; grant and personal fees from Amgen; personal fees and non-financial support from Lilly; grant from MSD and personal fees from Celgene, Pfizer, Puma and Daiichi Sankyo, outside the submitted work. Emmanuelle di Tomaso reports employment with Novartis during the conduct of the study. Cristina Saura has received personal fees from Puma Biotechnology, Pfizer and Roche, outside the submitted work. Douglas Robinson and Patrick Urban report employment with Novartis.
Funding This work was supported by Novartis Pharmaceuticals. Anthony Kong was supported by a Breakthrough Breast Cancer Clinician Scientist Fellowship (CSF 07/08) through the Holbeck Charitable Trust when this study was conducted. Stephen Chan is supported by the Nottingham Charitable Research Fund. No financial support was declared by the other authors.
Footnotes
Electronic supplementary material The online version of this article (https://doi.org/10.1007/s10549-017-4596-7) contains supplementary material, which is available to authorized users.
References
- 1.Shah S, Chen B (2011) Testing for HER2 in breast cancer: a continuing evolution. Patholog Res Int. 2011:903202 10.4061/2011/903202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL (1987) Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science 235:177–182 [DOI] [PubMed] [Google Scholar]
- 3.Slamon DJ, Leyland-Jones B, Shak S et al. (2001) Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med 344:783–792. 10.1056/NEJM200103153441101 [DOI] [PubMed] [Google Scholar]
- 4.Vogel CL, Cobleigh MA, Tripathy D et al. (2002) Efficacy and safety of trastuzumab as a single agent in first-line treatment of HER2-overexpressing metastatic breast cancer. J Clin Oncol 20:719–726. 10.1200/JCO.2002.20.3.719 [DOI] [PubMed] [Google Scholar]
- 5.Geyer CE, Forster J, Lindquist D et al. (2006) Lapatinib plus capecitabine for HER2-positive advanced breast cancer. N Engl J Med 355:2733–2743. 10.1056/NEJMoa064320 [DOI] [PubMed] [Google Scholar]
- 6.Johnston S, Pippen J Jr, Pivot X et al. (2009) Lapatinib combined with letrozole versus letrozole and placebo as first-line therapy for postmenopausal hormone receptor-positive metastatic breast cancer. J Clin Oncol 27:5538–5546. 10.1200/JCO.2009.23.3734 [DOI] [PubMed] [Google Scholar]
- 7.Baselga J, Cortés J, Kim SB et al. (2012) Pertuzumab plus trastuzumab plus docetaxel for metastatic breast cancer. N Engl J Med 366:109–119. 10.1056/NEJMoa1113216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Swain SM, Baselga J, Kim S-B et al. (2015) Pertuzumab, trastuzumab, and docetaxel in HER2-positive metastatic breast cancer. N Engl J Med 372:724–734. 10.1056/NEJMoa1413513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Verma S, Miles D, Gianni L et al. (2012) Trastuzumab emtansine for HER2-positive advanced breast cancer. N Engl J Med 367:1783–1791. 10.1056/NEJMoa1209124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Krop IE, Kim SB, Gonzalez-Martin A et al. (2014) Trastuzumab emtansine versus treatment of physician’s choice for pretreated HER2-positive advanced breast cancer (TH3RESA): a randomised, open-label, phase 3 trial. Lancet Oncol 15:689–699. 10.1016/S1470-2045(14)70178-0 [DOI] [PubMed] [Google Scholar]
- 11.Baselga J, Gelmon KA, Verma S et al. (2010) Phase II trial of pertuzumab and trastuzumab in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer that progressed during prior trastuzumab therapy. J Clin Oncol 28:1138–1144. 10.1200/JCO.2009.24.2024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bendell JC, Domchek SM, Burstein HJ et al. (2003) Central nervous system metastases in women who receive trastuzumab-based therapy for metastatic breast carcinoma. Cancer 97:2972–2977. 10.1002/cncr.11436 [DOI] [PubMed] [Google Scholar]
- 13.Eichler AF, Kuter I, Ryan P, Schapira L, Younger J, Henson JW (2008) Survival in patients with brain metastases from breast cancer: the importance of HER-2 status. Cancer 112:2359–2367. 10.1002/cncr.23468 [DOI] [PubMed] [Google Scholar]
- 14.Berns K, Horlings HM, Hennessy BT et al. (2007) A functional genetic approach identifies the PI3 K pathway as a major determinant of trastuzumab resistance in breast cancer. Cancer Cell 12:395–402. 10.1016/j.ccr.2007.08.030 [DOI] [PubMed] [Google Scholar]
- 15.Nagata Y, Lan KH, Zhou X et al. (2004) PTEN activation contributes to tumor inhibition by trastuzumab, and loss of PTEN predicts trastuzumab resistance in patients. Cancer Cell 6:117–127. 10.1016/j.ccr.2004.06.022 [DOI] [PubMed] [Google Scholar]
- 16.O’Brien NA, Browne BC, Chow L et al. (2010) Activated phosphoinositide 3-kinase/AKT signaling confers resistance to trastuzumab but not lapatinib. Mol Cancer Ther 9:1489–1502. 10.1158/1535-7163.MCT-09-1171 [DOI] [PubMed] [Google Scholar]
- 17.Wang L, Zhang Q, Zhang J et al. (2011) PI3 K pathway activation results in low efficacy of both trastuzumab and lapatinib. BMC Cancer 11:248 10.1186/1471-2407-11-248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chakrabarty A, Bhola NE, Sutton CR et al. (2012) Trastuzumabresistant cells rely on a HER2-PI3 K-FoxO-survivin axis and are sensitive to PI3 K inhibitors. Cancer Res 73:1190–1200. 10.1158/0008-5472.CAN-12-2440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.O’Brien NA, McDonald K, Tong L et al. (2014) Targeting PI3 K/ mTOR overcomes resistance to HER2-targeted therapy independent of feedback activation of AKT. Clin Cancer Res 20:3507–3520. 10.1158/1078-0432.CCR-13-2769 [DOI] [PubMed] [Google Scholar]
- 20.Andre F, O’Regan R, Ozguroglu M et al. (2014) Everolimus for women with trastuzumab-resistant, HER2-positive, advanced breast cancer (BOLERO-3): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet Oncol 15:580–591. 10.1016/S1470-2045(14)70138-X [DOI] [PubMed] [Google Scholar]
- 21.Maira SM, Pecchi S, Huang A et al. (2012) Identification and characterization of NVP-BKM120, an orally available pan-class I PI3-kinase inhibitor. Mol Cancer Ther 11:317–328. 10.1158/1535-7163.MCT-11-0474 [DOI] [PubMed] [Google Scholar]
- 22.Maira M, Schnell C, Lollini P et al. (2012) Preclinical and preliminary clinical activity of NVP-BKM120, an oral pan-class I PI3 K inhibitor, in the brain. ESMO meeting abstracts; (Suppl 15): 1675 (abstract) [Google Scholar]
- 23.Nanni P, Nicoletti G, Palladini A et al. (2012) Multiorgan metastasis of human HER-2(+) breast cancer in Rag2(−/−);Il2rg(−/−) mice and treatment with PI3 K inhibitor. PLoS ONE 7:e39626 10.1371/journal.pone.0039626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Saura C, Bendell J, Jerusalem G et al. (2014) Phase Ib study of buparlisib plus trastuzumab in patients with HER2-positive advanced or metastatic breast cancer that has progressed on trastuzumab-based therapy. Clin Cancer Res 20:1935–1945. 10.1158/1078-0432.CCR-13-1070 [DOI] [PubMed] [Google Scholar]
- 25.Kroenke K, Spitzer RL, Williams JB (2001) The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med 16:606–613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Spitzer RL, Kroenke K, Williams JB, Löwe B (2006) A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med 166:1092–1097. 10.1001/archinte.166.10.1092 [DOI] [PubMed] [Google Scholar]
- 27.Rodon J, Brana I, Siu LL et al. (2014) Phase I dose-escalation and -expansion study of buparlisib (BKM120), an oral pan-class I PI3 K inhibitor, in patients with advanced solid tumors. Invest New Drugs 32:670–681. 10.1007/s10637-014-0082-9 [DOI] [PubMed] [Google Scholar]
- 28.Foukas LC, Claret M, Pearce W et al. (2006) Critical role for the p110alpha phosphoinositide-3-OH kinase in growth and metabolic regulation. Nature 441:366–370. 10.1038/nature04694 [DOI] [PubMed] [Google Scholar]
- 29.Baselga J, Cortes J, Im SA, Clark E, Ross G, Kiermaier A, Swain SM et al. (2014) Biomarker analyses in CLEOPATRA: a phase III, placebo-controlled study of pertuzumab in human epidermal growth factor receptor 2-positive, first-line metastatic breast cancer. J Clin Oncol 32:3753–3761. 10.1200/JCO.2013.54.5384 [DOI] [PubMed] [Google Scholar]
- 30.Andre F, Hurvitz S, Fasolo A et al. (2016) Molecular alterations and everolimus efficacy in human epidermal growth factor receptor 2-overexpressing metastatic breast cancers: combined exploratory biomarker analysis from BOLERO-1 and BOLERO-3. J Clin Oncol 34:2115–2124. 10.1200/JCO.2015.63.9161 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


