Abstract
Background:
Long-term risks of cardiovascular disease (CVD) according to levels of cardiovascular health (CVH) have not been characterized in a diverse, representative population.
Methods and Results:
We pooled individual-level data from 30,447 participants (mean [SD] age, 55.0 [13.9] years; 60.6% female; 31.8% black) from 7 US cohort studies. We defined CVH based on levels of 7 American Heart Association health metrics, scored as ideal (2 points), intermediate (1 point), or poor (0 points). The total CVH score was used to quantify overall CVH as high (12–14 points), moderate (9–11 points), or low (0–8 points). We used a modified Kaplan-Meier analysis, accounting for the competing risk of death, to estimate the lifetime risk of CVD (composite of incident myocardial infarction, stroke, heart failure, or CVD death) separately in white and black men and women free of CVD at index ages of <40, 40–59, and ≥60 years. High CVH was more prevalent among women compared with men, white compared with black participants, and in younger compared with older participants. Over 538,477 person-years of follow-up, we observed 6,546 CVD events. In women aged 40 to 59 years, those with high CVH had lower lifetime risk (95% confidence interval) of CVD (white women, 12.6% [2.6%−22.6%]; black women, 0.0%) compared with moderate (white women, 16.6% [13.0%−20.2%]; black women, 12.7% [6.8–18.5%]) and low (white women, 33.8% [30.6%−37.1%]; black women, 34.7% [30.4%−39.0%]) CVH strata. Patterns were similar for men and individuals <40 and ≥60 years of age.
Conclusions:
Higher baseline CVH at all ages in adulthood is associated with substantially lower lifetime risk for CVD compared with moderate and low CVH, in white and black men and women in the US. Public health and healthcare efforts aimed at maintaining and restoring higher CVH throughout the life course could provide substantial benefits for the population burden of CVD.
Cardiovascular disease (CVD) remains the leading cause of death and a major cause of morbidity in the US and globally.1,2 Decades of epidemiologic and clinical trial evidence have established the causal associations of several factors with increased risk of CVD, including hypertension, dyslipidemia, diabetes mellitus, and cigarette smoking.3 In 2010, the American Heart Association (AHA) introduced the concept of cardiovascular health (CVH), a comprehensive construct incorporating the simultaneous presence of 7 favorable health behaviors and factors.4 These behaviors/factors are modifiable and include the following metrics: smoking status, body mass index (BMI), physical activity, diet quality, total cholesterol, blood pressure (BP), and fasting glucose.
Associations of overall CVH with numerous adverse health outcomes have been well-described in previous studies in diverse samples.5–13 However, data are sparse regarding associations of CVH across the life course with longer-term risk of adverse health outcomes, particularly CVD, in broad, representative populations. Furthermore, because the risks of non-cardiovascular causes of death increase over longer time horizons, adjustment for these competing risks is crucial to accurately quantify the lifetime risk of CVD and avoid its overestimation.14,15 A comprehensive analysis of CVH status across adulthood and lifetime risk of CVD, in the context of competing causes of death, can improve estimation of future population disease burden and call attention to the need to investigate strategies for maintaining and restoring high CVH throughout the life course.
The Cardiovascular Disease Lifetime Risk Pooling Project (LRPP) provides the opportunity to estimate the lifetime risk of major CVD events in a collection of US cohort studies comprising more than 30,000 participants with individual-level data on CVH status.15,16 The primary aim of this investigation was to estimate the association of CVH, across the spectrum from low to high CVH, with lifetime risk of CVD among US adults at selected ages across the life course.
Methods
The Cardiovascular Lifetime Risk Pooling Project
The data and materials that support the findings of this study can be requested from the corresponding authors and the National Heart, Lung, and Blood Institute BioLINCC.
The LRPP includes 22 US population-based CVD cohort studies comprising more than 225,000 participants with individual-level data.16 For the present study, we included participants from 7 contemporary cohorts with sufficient data to define CVH by AHA criteria (Supplemental Figure 1): the Atherosclerosis Risk in Communities Study,17 Coronary Artery Risk Development in Young Adults Study,18 Cardiovascular Health Study,19 Multi-Ethnic Study of Atherosclerosis,20 Framingham Heart Study,21 Framingham Offspring Study,22 and the Women’s Health Initiative Observational Study.23 Detailed data ascertainment methods are available in the original design publications from each included study. Although measurement methods differ somewhat across cohorts, data are harmonized for comparability in the LRPP.16,24 Important demographic data included age, sex, and race. Additionally, information regarding education, history of CVD, diabetes mellitus, and use of BP-lowering, lipid-lowering, and glucose-lowering medications was obtained. The baseline examination in each cohort was defined as the first exam in which all 7 CVH metrics were concurrently measured. Data were collected between March 25, 1985, and August 31, 2016. This study was approved by the institutional review board at Northwestern University. Written informed consent was obtained from all participants for initial data collection in each original cohort. For this analysis of deidentified data, specific consent was not required.
The Cardiovascular Health Score
Tobacco smoking was defined as self-reported never, former, and current smoking. Body mass index was calculated as the weight in kilograms divided by height in meters squared. Physical activity was assessed according to cohort-specific quartiles of activity levels, constructed using z-scores estimated from the different physical activity questionnaires used within each cohort, according to ideal (quartile 4), intermediate (quartiles 2 and 3), and poor (quartile 1) categories.13 Healthy diet was assessed according to cohort-specific quintiles of the Alternate Healthy Eating Index 2010 (AHEI-2010; range, 0–110 points),25 based on self-reported diet composition according to ideal (quintile 5), intermediate (quintiles 3 and 4), and poor (quintiles 1 and 2) categories.26 Total cholesterol, BP, and fasting glucose were measured according to standard protocols. The individual CVH metrics are each scored as ideal (2 points), intermediate (1 point), or poor (0 points), according to the definitions promulgated by the AHA4 and adapted to definitions feasible for harmonization within the LRPP (Supplemental Table 1). A composite CVH score was calculated, composed of the sum of individual CVH metric levels, ranging from 0 to 14. We stratified participants a priori into high (12–14), moderate (9–11), and low (0–8) CVH,9,12 which is supported by data on the association of the CVH score integers with the log hazard ratios of CVD events (Supplemental Figure 2).
Outcome Ascertainment
The primary outcome was major CVD events, defined as a composite of incident myocardial infarction, stroke, heart failure, or CVD death. Major CVD events were adjudicated using similar methods in each cohort included in the analysis.16 Participants with a prior CVD event were excluded from the analyses and follow-up time was censored at the time of a first CVD event, death from other cause, or end of follow-up data (i.e. last known date alive). We additionally included information on all-cause death for analyses accounting for the competing risk of death. Cohorts included in the LRPP include adjudication for death or linkage to the National Death Index, and vital status is nearly 100% complete among all cohorts.
Statistical Analysis
All analyses were conducted separately by sex (men and women), race (white and black), and index age group (<40 years, 40–59 years, and ≥60 years); these groupings were chosen to maintain sufficient sample size within stratified groups for reliable lifetime risk estimation. Baseline characteristics of the participants are summarized as the mean (standard deviation [SD]) for continuous variables and percentages for categorical variables. Additionally, we report the proportions of participants characterized as having high, moderate, and low CVH. We used Poisson regression models to calculate crude incidence rates (95% confidence intervals [CIs]) of CVD.
A modified survival analysis approach14,27 was used to estimate the lifetime risks of CVD by categories of high, moderate, and low CVH. In brief, this approach accounts for the competing risk of non-cardiovascular death by treating non-cardiovascular death as a separate event rather than a withdrawal, which avoids overestimation of the lifetime risk for events.14,15 The incidence of CVD and of death free of the end point during follow-up is calculated for each year of age attained, with cumulative incidence calculated similarly to traditional Kaplan-Meier analysis. Lifetime risk estimates reflect the sum of the competing risk-adjusted incidences from study entry to age at oldest observation, up to 40 years after index age. Additionally, we report risk estimates for 5-year increments after index age. Analyses were conducted using SAS version 9.4 (SAS Institute, Cary, NC).
Results
A total of 30,447 participants were included from the 7 US cohorts in this analysis (mean [SD] age, 55.0 [13.9] years; 60.6% female; 31.8% black); they contributed 538,477 person-years of follow-up (mean [SD] follow-up, 16.2 [7.2] years; maximum, 31.3 years for individual participants; Supplemental Table 2). The CVH score was approximately normally distributed (Supplemental Figure 3). The distribution of CVH score groups differed by sex, race, and index age group, characterized by a higher prevalence of high CVH in white women across all age groups and worse CVH in older age groups across all sex and race groups (Figure 1).
Figure 1. Percent of Participants in High, Moderate, and Low Cardiovascular Health Groups by Sex, Race, and Index Age Group.
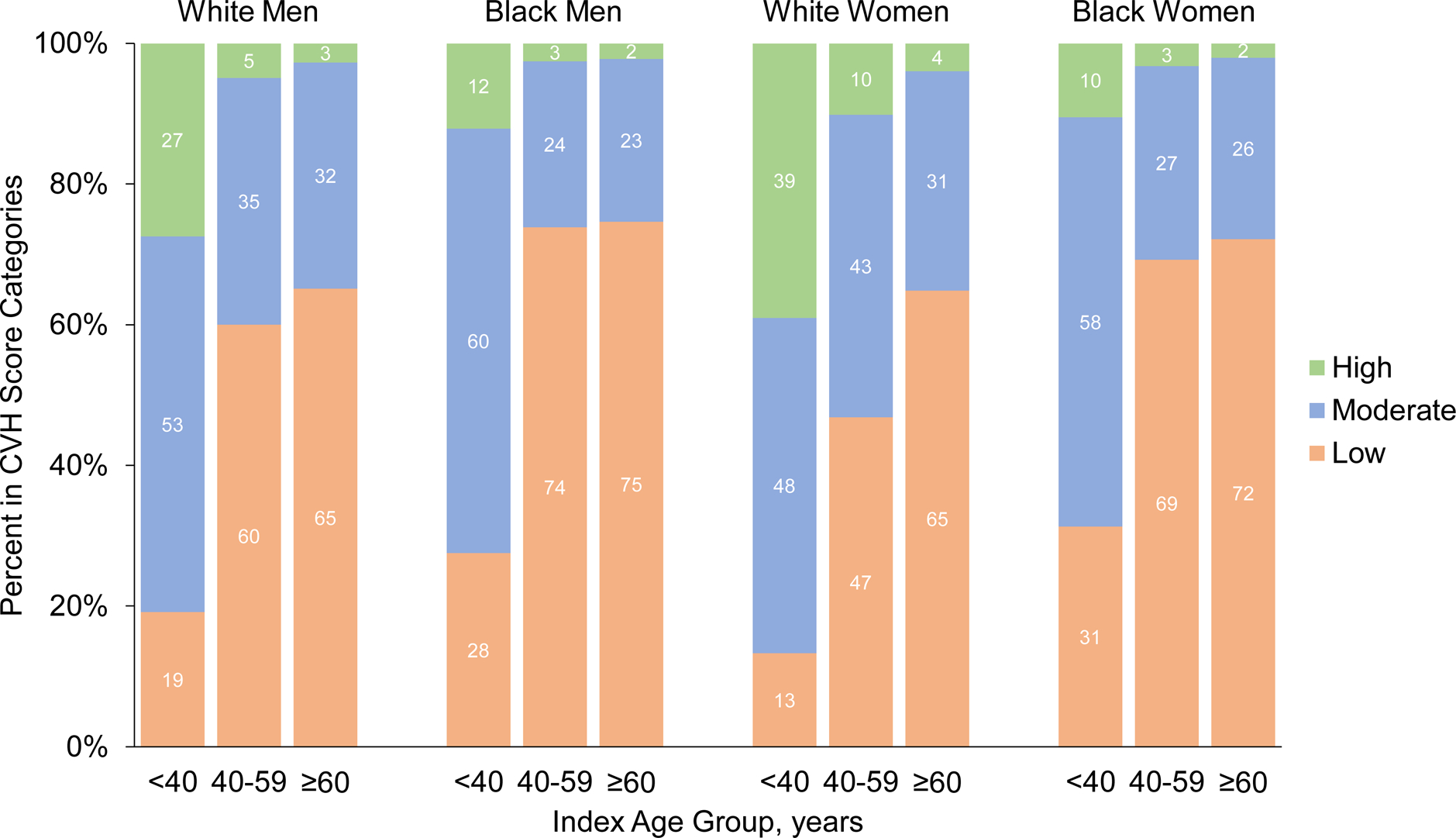
CVH indicates cardiovascular health
In general, men and women in the low CVH group were less likely to have a high school education, be physically active, and consume a healthier diet, and were more likely to use BP-lowering medications, lipid-lowering medications, and have diabetes mellitus, compared with those in moderate and high CVH groups (Table 1 and Supplemental Tables 3 and 4). On average, participants in low CVH groups had higher BMI, total cholesterol, systolic and diastolic BP, and fasting glucose compared with those in moderate and high CVH groups. The prevalence of participants in ideal CVH categories for individual metrics were higher for women compared with men, white compared with black participants, and those younger than 40 years of age (Supplemental Tables 5 and 6).
Table 1.
Characteristics of Participants Aged 40 to 59 Years by Cardiovascular Health Score Groups
| Characteristics | White Participants | Black Participants | ||||
|---|---|---|---|---|---|---|
| High CVH | Moderate CVH | Low CVH | High CVH | Moderate CVH | Low CVH | |
| Men, No. | 227 | 1622 | 2772 | 30 | 278 | 870 |
| Age, mean (SD), y | 50.6 (4.5) | 51.6 (4.5) | 52.2 (4.5) | 49.7 (4.3) | 51.1 (4.3) | 52.0 (4.3) |
| High school education or greater, % | 97.5 | 91.9 | 85.5 | 96.7 | 87.1 | 64.4 |
| Current alcohol drinking, % | 71.4 | 64.7 | 60.4 | 63.3 | 52.9 | 51.5 |
| Antihypertensive medication use, % | 2.2 | 10.1 | 21.3 | 6.7 | 17.6 | 36.2 |
| Lipid-lowering medication use, % | 3.1 | 3.8 | 5.3 | 3.3 | 2.9 | 3.3 |
| History of diabetes, % | 0.5 | 0.7 | 3.8 | 0.0 | 3.8 | 11.1 |
| Body mass index, mean (SD), kg/m2 | 24.0 (2.0) | 26.2 (3.1) | 28.8 (4.1) | 23.8 (2.2) | 26.7 (4.0) | 28.7 (4.9) |
| Physical activity, mean (SD), z-score | 1.04 (1.09) | 0.59 (1.05) | 0.00 (0.97) | 1.69 (2.84) | 0.43 (1.42) | −0.27 (1.03) |
| Healthy diet score, mean (SD), aHEI score | 56.9 (10.8) | 48.4 (11.4) | 41.7 (10.2) | 62.9 (10.5) | 50.1 (11.8) | 41.8 (9.8) |
| Total cholesterol, mean (SD), mg/dL | 179.0 (25.8) | 195.9 (32.5) | 215.9 (38.0) | 169.5 (20.7) | 187.7 (34.7) | 210.7 (45.8) |
| HDL cholesterol, mean (SD), mg/dL | 49.5 (12.3) | 45.1 (11.8) | 41.2 (11.3) | 54.4 (14.0) | 48.8 (12.8) | 49.6 (17.1) |
| Systolic blood pressure, mean (SD), mmHg | 108.7 (10.2) | 114.5 (12.2) | 123.2 (15.5) | 110.1 (9.3) | 117.5 (14.0) | 131.6 (20.3) |
| Diastolic blood pressure, mean (SD), mmHg | 68.4 (7.0) | 71.8 (8.1) | 76.4 (10.1) | 72.3 (7.7) | 76.1 (9.8) | 83.9 (12.2) |
| Fasting glucose, mean (SD), mg/dL | 91.6 (7.1) | 96.0 (10.5) | 107.8 (28.1) | 86.9 (7.2) | 92.5 (12.4) | 112.1 (42.7) |
| Women, No. | 565 | 2396 | 2607 | 100 | 842 | 2123 |
| Age, mean (SD), y | 50.2 (4.4) | 51.0 (4.5) | 52.2 (4.5) | 52.7 (4.0) | 52.7 (4.1) | 52.9 (4.1) |
| High school education or greater, % | 94.9 | 92.5 | 82.2 | 99.0 | 91.8 | 76.4 |
| Current alcohol drinking, % | 61.1 | 49.2 | 37.9 | 53.3 | 45.1 | 34.9 |
| Antihypertensive medication use, % | 3.0 | 11.2 | 28.2 | 10.0 | 23.3 | 46.5 |
| Lipid-lowering medication use, % | 1.1 | 2.5 | 5.0 | 3.0 | 4.8 | 7.4 |
| History of diabetes, % | 0.0 | 0.5 | 4.4 | 0.0 | 1.5 | 13.0 |
| Body mass index, mean (SD), kg/m2 | 22.5 (2.3) | 24.8 (4.0) | 29.0 (6.1) | 24.4 (3.3) | 27.9 (5.3) | 32.4 (6.6) |
| Physical activity, mean (SD), z-score | 0.76 (1.05) | 0.14 (0.91) | −0.40 (0.79) | 0.59 (0.92) | 0.16 (0.99) | −0.36 (0.86) |
| Healthy diet score, mean (SD), aHEI score | 59.1 (8.6) | 51.1 (10.1) | 44.9 (9.6) | 63.1 (9.5) | 55.5 (11.0) | 46.2 (10.6) |
| Total cholesterol, mean (SD), mg/dL | 182.8 (27.5) | 201.1 (33.6) | 226.8 (42.9) | 181.5 (29.8) | 197.7 (36.8) | 221.8 (43.7) |
| HDL cholesterol, mean (SD), mg/dL | 65.6 (15.7) | 60.7 (15.7) | 53.0 (15.6) | 64.8 (15.8) | 60.2 (16.1) | 56.2 (16.1) |
| Systolic blood pressure, mean (SD), mmHg | 104.6 (10.1) | 111.1 (14.0) | 121.4 (18.1) | 112.9 (12.1) | 119.0 (15.0) | 131.1 (19.1) |
| Diastolic blood pressure, mean (SD), mmHg | 65.2 (7.2) | 68.2 (8.9) | 72.3 (10.0) | 71.0 (7.4) | 74.7 (9.2) | 80.0 (10.6) |
| Fasting glucose, mean (SD), mg/dL | 88.8 (7.9) | 92.4 (12.7) | 106.4 (35.0) | 84.6 (7.5) | 89.9 (13.4) | 114.3 (51.7) |
aHEI indicates adjusted Healthy Eating Index 2010; CVH, cardiovascular health; HDL, high-density lipoprotein; and SD, standard deviation
Table 2 shows the number of CVD events, person-years of follow-up, and incidence rates for CVD events stratified by sex, race, index age, and CVH score groups. A total of 6,546 CVD events were documented (1.79 events per 1,000 person-years in high, 6.79 events per 1,000 person-years in moderate, and 17.94 events per 1,000 person-years in low CVH categories). Men had higher incidence of CVD events compared with women across all race and age groups. Participants 60 years of age or older had higher incidence of CVD events compared with younger participants. Incidence rates were lower for those in high CVH groups compared with moderate and low groups, across all sex, race, and index age groups. For example, white women aged 40 to 59 years with high CVH had lower incidence of CVD (1.28 [95% CI, 0.74–2.21] events per 1,000 person-years) compared with moderate (3.64 [95% CI, 3.12–4.25] events per 1,000 person-years) and low (10.19 [95% CI, 9.32–11.13] events per 1,000 person-years) CVH. Similarly, black women aged 40 to 59 years with high CVH had lower incidence of CVD (0 events per 1,000 person-years) compared with moderate (4.20 [95% CI, 3.21–5.50] events per 1,000 person-years) and low (11.95 [95% CI, 10.83–13.18] events per 1,000 person-years) CVH. Patterns were similar for men and individuals less than 40 and 60 or more years of age. However, black participants 60 years of age or older tended to have lower incidence of CVD compared with white participants. Across sex, race, and CVH groups, heart failure tended to account for a higher proportion of incident events with increasing index age (Supplemental Table 7).
Table 2.
Cardiovascular Disease Event Rates by Age, Sex, Race, and Cardiovascular Health Score Groups
| Subgroups | White Participants | Black Participants | ||||
|---|---|---|---|---|---|---|
| Events, No. | Follow-up, PYs | Incidence Rate, per 1000 PYs | Events, No. | Follow-up, PYs | Incidence Rate, per 1000 PYs | |
| Men | ||||||
| Age <40 years | ||||||
| High CVH | 2 | 8,575 | 0.23 (0.06–0.93) | 2 | 2,608 | 0.77 (0.19–3.07) |
| Moderate CVH | 29 | 16,100 | 1.80 (1.25–2.59) | 28 | 13,170 | 2.13 (1.47–3.08) |
| Low CVH | 26 | 5,419 | 4.80 (3.27–7.05) | 29 | 5,605 | 5.17 (3.60–7.45) |
| Age 40–59 years | ||||||
| High CVH | 12 | 4,052 | 2.96 (1.68–5.21) | 1 | 476 | 2.10 (0.30–14.93) |
| Moderate CVH | 174 | 29,364 | 5.93 (5.11–6.87) | 32 | 4,519 | 7.08 (5.01–10.01) |
| Low CVH | 721 | 49,402 | 14.59 (13.57–15.70) | 293 | 14,639 | 20.02 (17.85–22.44) |
| Age ≥60 years | ||||||
| High CVH | 20 | 1,434 | 13.94 (9.00–21.61) | 3 | 199 | 15.11 (4.87–46.84) |
| Moderate CVH | 382 | 17,173 | 22.24 (20.12–24.59) | 34 | 2,002 | 16.98 (12.13–23.76) |
| Low CVH | 1155 | 32,854 | 35.16 (33.19–37.24) | 183 | 6,821 | 26.83 (23.21–31.01) |
| Women | ||||||
| Age <40 years | ||||||
| High CVH | 7 | 13,813 | 0.51 (0.24–1.06) | 2 | 3,551 | 0.56 (0.14–2.25) |
| Moderate CVH | 13 | 16,712 | 0.78 (0.45–1.34) | 25 | 19,634 | 1.27 (0.86–1.88) |
| Low CVH | 10 | 4,521 | 2.21 (1.19–4.11) | 28 | 10,344 | 2.71 (1.87–3.92) |
| Age 40–59 years | ||||||
| High CVH | 13 | 10,153 | 1.28 (0.74–2.21) | 0 | 1,444 | 0.00 |
| Moderate CVH | 161 | 44,238 | 3.64 (3.12–4.25) | 53 | 12,615 | 4.20 (3.21–5.50) |
| Low CVH | 489 | 48,000 | 10.19 (9.32–11.13) | 398 | 33,310 | 11.95 (10.83–13.18) |
| Age ≥60 years | ||||||
| High CVH | 25 | 2,772 | 9.02 (6.09–13.35) | 2 | 725 | 2.76 (0.69–11.03) |
| Moderate CVH | 390 | 22,857 | 17.06 (15.45–18.84) | 86 | 8,758 | 9.82 (7.95–12.13) |
| Low CVH | 1269 | 45,865 | 27.67 (26.19–29.23) | 449 | 24,754 | 18.14 (16.54–19.90) |
CVH indicates cardiovascular health; and PY, person-year
Incidence rates are reported as events per 1000 PYs (95% confidence intervals); for incidence = 0.00, 95% confidence intervals were not calculated
Tables 3 and 4 and Figures 2 and 3 provide the lifetime risks of CVD events, adjusted for competing mortality risk, for men and women by race and index age groups. Across all sex, race, and index age groups, participants with high CVH had substantially lower lifetime risk of CVD events compared with moderate and low CVH groups. Men had higher lifetime risk of CVD than women across all CVH categories. Lifetime risks of CVD were similar between white and black participants. In women aged 40 to 59 years, those with high CVH had generally lower lifetime risk (95% CI) of CVD (white women, 12.6% [2.6%−22.6%]; black women, 0.0%) compared with moderate (white women, 16.6% [13.0%−20.2%]; black women, 12.7% [6.8–18.5%]) and low (white women, 33.8% [30.6%−37.1%]; black women, 34.7% [30.4%−39.0%]) CVH. Patterns were similar for men and participants less than 40 and 60 or more years of age, but overall lifetime risk estimates were higher, lower, and higher, respectively. Lifetime risks of CVD were comparable for white and black participants less than 60 years of age. However, black participants 60 years of age or older tended to have lower lifetime risk of CVD compared with white participants.
Table 3.
Lifetime Risks of Cardiovascular Disease Events among White and Black Men Adjusted for Competing Risks of Death
| White Men | Black Men | |||||
|---|---|---|---|---|---|---|
| High CVH | Moderate CVH | Low CVH | High CVH | Moderate CVH | Low CVH | |
| Age <40 years, Risk after Index Age | ||||||
| 5 years | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| 10 years | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.7 (0.0–2.0) |
| 15 years | 0.0 | 0.2 (0.0–0.6) | 0.0 | 0.0 | 0.0 | 1.2 (0.0–2.8) |
| 20 years | 0.0 | 0.4 (0.0–0.9) | 0.0 | 1.1 (0.0–3.1) | 1.1 (0.1–2.0) | 1.2 (0.0–2.8) |
| 25 years | 0.3 (0.0–1.0) | 1.2 (0.3–2.1) | 4.3 (1.6–7.1) | 1.1 (0.0–3.1) | 2.4 (1.0–3.7) | 5.8 (2.6–9.0) |
| 30 years | 0.3 (0.0–1.0) | 2.6 (1.3–3.9) | 7.8 (4.1–11.4) | 2.1 (0.0–5.0) | 4.1 (2.3–5.9) | 10.0 (5.9–14.1) |
| 35 years | 0.7 (0.0–1.7) | 5.0 (3.1–6.9) | 12.6 (7.8–17.4) | 2.1 (0.0–5.0) | 6.1 (3.8–8.3) | 13.6 (8.8–18.5) |
| 40 years | 0.7 (0.0–1.7) | 6.0 (3.8–8.2) | 14.4 (9.1–19.6) | 2.1 (0.0–5.0) | 7.1 (4.4–9.8) | 17.6 (9.9–25.3) |
| Age 40–59 years, Risk after Index Age | ||||||
| 5 years | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| 10 years | 0.0 | 0.7 (0.0–1.6) | 3.4 (1.7–5.0) | 0.0 | 3.8 (0.0–11.2) | 4.2 (1.4–6.9) |
| 15 years | 0.0 | 2.1 (1.0–3.3) | 8.0 (6.1–9.9) | 0.0 | 4.5 (0.0–11.9) | 8.7 (4.5–12.9) |
| 20 years | 1.0 (0.0–2.4) | 3.9 (2.6–5.2) | 13.4 (11.4–15.4) | 0.0 | 8.2 (0.7–15.7) | 13.7 (8.0–19.4) |
| 25 years | 1.5 (0.0–3.2) | 6.1 (4.7–7.6) | 19.8 (17.7–21.9) | 4.2 (0.0–12.2) | 11.8 (4.2–19.4) | 20.1 (12.3–27.9) |
| 30 years | 4.8 (1.5–8.1) | 10.0 (8.2–11.8) | 27.0 (24.9–29.2) | 4.2 (0.0–12.2) | 18.3 (10.3–26.4) | 28.4 (17.7–39.0) |
| 35 years | 8.4 (3.2–13.5) | 15.2 (12.8–17.6) | 34.2 (31.9–36.6) | 4.2 (0.0–12.2) | 19.6 (11.3–27.9) | 35.8 (22.5–49.1) |
| 40 years | 14.9 (1.7–28.1) | 21.5 (17.6–25.5) | 41.2 (38.2–44.1) | 4.2 (0.0–12.2) | 22.3 (12.7–31.9) | 41.8 (26.2–57.4) |
| Age ≥60 years, Risk after Index Age | ||||||
| 5 years | 0.0 | 2.9 (0.2–5.5) | 9.2 (6.4–12.0) | 0.0 | 5.7 (0.0–11.9) | 10.8 (5.7–16.0) |
| 10 years | 0.0 | 7.6 (4.5–10.7) | 18.8 (15.7–21.9) | 0.0 | 8.6 (1.7–15.5) | 18.5 (13.0–24.0) |
| 15 years | 5.6 (0.3–10.9) | 14.2 (10.7–17.6) | 30.3 (27.1–33.5) | 8.3 (0.0–24.0) | 15.2 (7.6–22.9) | 27.4 (21.8–33.1) |
| 20 years | 7.9 (1.8–14.0) | 22.1 (18.5–25.7) | 42.1 (38.9–45.3) | 17.7 (0.0–40.1) | 23.3 (15.1–31.5) | 36.2 (30.5–41.9) |
| 25 years | 18.9 (9.5–28.2) | 34.3 (30.3–38.2) | 52.3 (49.0–55.5) | 36.5 (0.1–72.9) | 28.1 (19.3–36.9) | 42.9 (37.1–48.8) |
| 30 years | 29.1 (16.6–41.6) | 42.0 (37.8–46.3) | 60.9 (57.6–64.2) | 36.5 (0.1–72.9) | 33.7 (22.4–44.9) | 48.4 (41.9–54.9) |
| 35 years | - * | 51.2 (46.3–56.1) | 65.5 (62.1–68.9) | - * | - * | 48.4 (41.9–54.9) |
CVH indicates cardiovascular health
Risk estimates are reported as percentages (95% confidence intervals); for risk = 0.0%, 95% confidence intervals were not calculated
Incidence cannot be calculated due to insufficient follow-up
Table 4.
Lifetime Risks of Cardiovascular Disease among White and Black Women Adjusted for Competing Risks of Death
| White Women | Black Women | |||||
|---|---|---|---|---|---|---|
| High CVH | Moderate CVH | Low CVH | High CVH | Moderate CVH | Low CVH | |
| Age <40 years, Risk after Index Age | ||||||
| 5 years | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| 10 years | 0.0 | 0.0 | 0.0 | 1.0 (0.0–3.1) | 0.0 | 0.7 (0.0–2.0) |
| 15 years | 0.0 | 0.4 (0.0–0.9) | 0.0 | 1.0 (0.0–3.1) | 0.0 | 1.2 (0.0–2.7) |
| 20 years | 0.2 (0.0–0.6) | 0.5 (0.0–1.1) | 0.6 (0.0–1.8) | 1.0 (0.0–3.1) | 0.1 (0.0–0.4) | 2.3 (0.5–4.1) |
| 25 years | 0.4 (0.0–1.0) | 0.7 (0.0–1.4) | 0.6 (0.0–1.8) | 1.0 (0.0–3.1) | 1.5 (0.6–2.4) | 3.1 (1.1–5.2) |
| 30 years | 0.8 (0.0–1.7) | 1.4 (0.4–2.3) | 3.1 (0.4–5.8) | 1.0 (0.0–3.1) | 2.8 (1.6–4.1) | 6.7 (3.9–9.4) |
| 35 years | 1.3 (0.3–2.3) | 2.2 (1.0–3.4) | 5.8 (2.1–9.4) | 2.0 (0.0–4.7) | 3.6 (2.1–5.0) | 8.4 (5.3–11.5) |
| 40 years | 1.7 (0.4–3.0) | 2.2 (1.0–3.4) | 8.6 (2.1–15.2) | 2.0 (0.0–4.7) | 4.4 (2.6–6.3) | 8.4 (5.3–11.5) |
| Age 40–59 years, Risk after Index Age | ||||||
| 5 years | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| 10 years | 0.0 | 0.9 (0.1–1.7) | 1.2 (0.2–2.2) | 0.0 | 0.7 (0.0–2.0) | 2.7 (0.7–4.7) |
| 15 years | 0.0 | 1.4 (0.5–2.2) | 3.5 (2.3–4.8) | 0.0 | 2.7 (0.8–4.6) | 4.8 (2.6–6.9) |
| 20 years | 0.4 (0.0–0.9) | 2.4 (1.5–3.4) | 6.2 (4.8–7.6) | 0.0 | 3.6 (1.6–5.7) | 8.8 (6.6–11.1) |
| 25 years | 1.0 (0.1–1.9) | 4.3 (3.2–5.4) | 10.5 (8.9–12.0) | 0.0 | 5.7 (3.5–8.0) | 14.5 (12.2–16.8) |
| 30 years | 1.2 (0.2–2.2) | 6.6 (5.3–7.9) | 16.5 (14.8–18.3) | 0.0 | 8.9 (6.2–11.5) | 20.9 (18.4–23.4) |
| 35 years | 4.0 (1.1–6.8) | 10.2 (8.4–12.1) | 22.5 (20.4–24.5) | 0.0 | 10.0 (7.1–13.0) | 28.5 (25.4–31.6) |
| 40 years | 12.6 (2.6–22.6) | 16.6 (13.0–20.2) | 33.8 (30.6–37.1) | 0.0 | 12.7 (6.8–18.5) | 34.7 (30.4–39.0) |
| Age ≥60 years, Risk after Index Age | ||||||
| 5 years | 2.2 (0.0–6.5) | 1.9 (0.0–3.8) | 2.1 (0.7–3.6) | 0.0 | 3.1 (0.7–5.5) | 5.3 (3.3–7.2) |
| 10 years | 4.3 (0.0–9.4) | 5.0 (2.7–7.2) | 7.6 (5.8–9.5) | 0.0 | 5.0 (2.3–7.6) | 11.8 (9.4–14.1) |
| 15 years | 4.9 (0.0–10.2) | 9.3 (6.8–11.8) | 15.8 (13.7–17.8) | 2.0 (0.0–5.9) | 8.3 (5.3–11.3) | 17.9 (15.5–20.4) |
| 20 years | 7.5 (1.8–13.1) | 15.2 (12.5–18.0) | 26.5 (24.3–28.6) | 2.0 (0.0–5.9) | 12.7 (9.3–16.2) | 26.3 (23.6–28.9) |
| 25 years | 11.6 (5.2–18.1) | 26.1 (22.9–29.2) | 38.5 (36.3–40.8) | 6.5 (0.0–16.0) | 20.1 (15.6–24.5) | 33.1 (30.1–36.0) |
| 30 years | 24.9 (15.1–34.6) | 37.4 (33.7–41.1) | 50.7 (48.3–53.2) | 6.5 (0.0–16.0) | 26.5 (19.7–33.3) | 41.3 (37.2–45.4) |
| 35 years | 38.6 (22.6–54.7) | 46.5 (41.9–51.1) | 57.1 (54.4–59.7) | - * | - * | 51.9 (43.1–60.8) |
CVH indicates cardiovascular health
Risk estimates are reported as percentages (95% confidence intervals); for risk = 0.0%, 95% confidence intervals were not calculated
Incidence cannot be calculated due to insufficient follow-up
Figure 2. Plots of Lifetime Risks of Cardiovascular Disease Events among White and Black Men by Index Age Group and Adjusted for Competing Risks of Death.
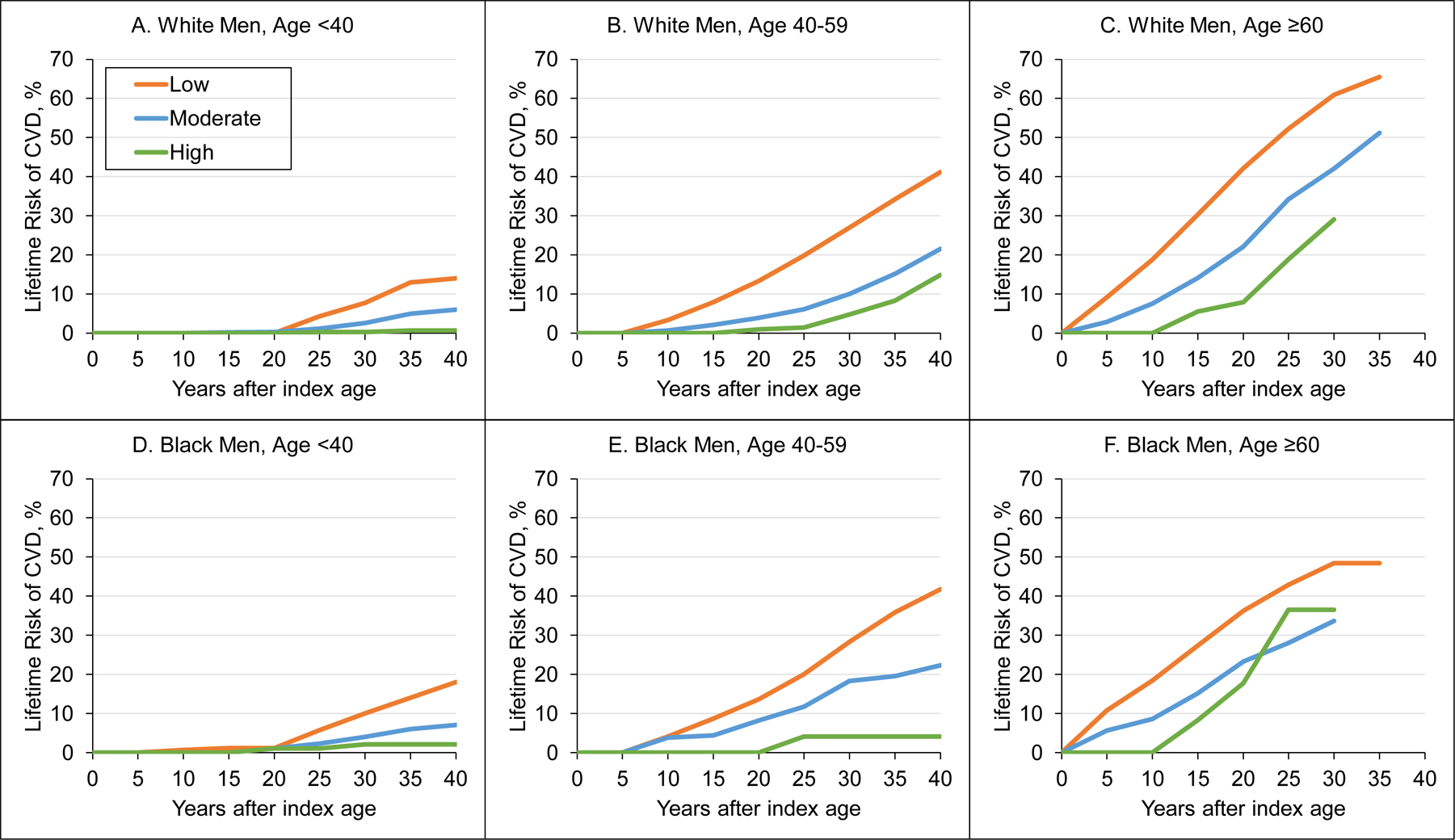
A, Lifetime risk of CVD in CVH score categories for white men aged <40 years. B, Lifetime risk of CVD in CVH score categories for white men aged 40–59 years. C, Lifetime risk of CVD in CVH score categories for white men aged ≥60 years. D, Lifetime risk of CVD in CVH score categories for black men aged <40 years. E, Lifetime risk of CVD in CVH score categories for black men aged 40–59 years. F, Lifetime risk of CVD in CVH score categories for black men aged ≥60 years.
CVD indicates cardiovascular disease; CVH, cardiovascular health
Figure 3. Plots of Lifetime Risks of Cardiovascular Disease Events among White and Black Women by Index Age Group and Adjusted for Competing Risks of Death.
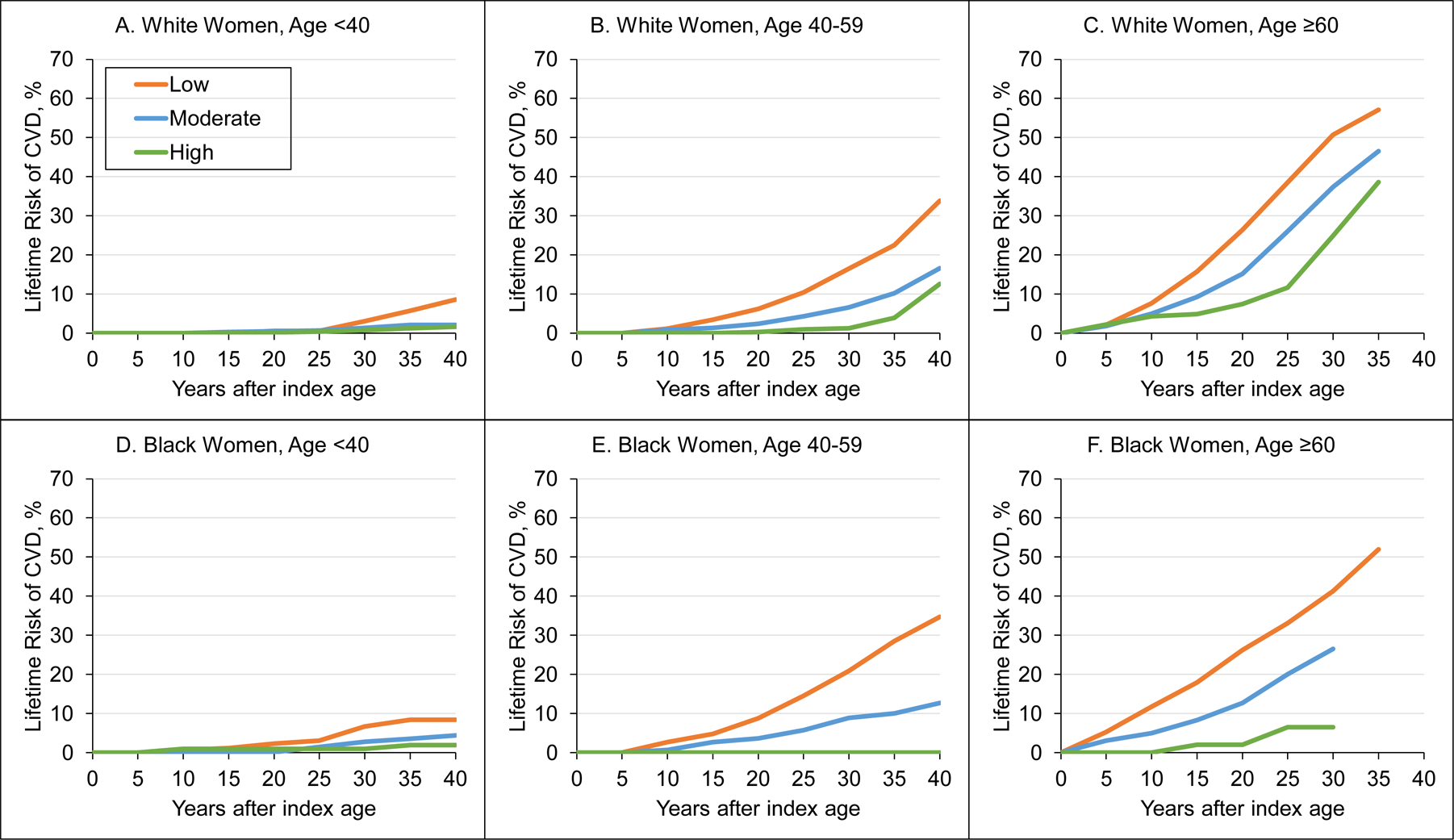
A, Lifetime risk of CVD in CVH score categories for white women aged <40 years. B, Lifetime risk of CVD in CVH score categories for white women aged 40–59 years. C, Lifetime risk of CVD in CVH score categories for white women aged ≥60 years. D, Lifetime risk of CVD in CVH score categories for black women aged <40 years. E, Lifetime risk of CVD in CVH score categories for black women aged 40–59 years. F, Lifetime risk of CVD in CVH score categories for black women aged ≥60 years.
CVD indicates cardiovascular disease; CVH, cardiovascular health
Discussion
In this analysis of more than 30,000 US adults, including more than 500,000 person-years of follow-up, those with high CVH had markedly lower lifetime risks for CVD events compared with individuals with moderate and low CVH. However, high CVH levels were uncommon in the included participants, especially among black participants and older index age groups. On average, and across index age groups, women tended to have higher prevalence of high CVH compared with men, and lower rates of CVD. Additionally, men in moderate and low CVH groups tended to have steeper slopes of increasing CVD event rates over time compared with women, indicating that individuals with low CVH accumulated events at younger ages. These results help to quantify the potential long-term implications of meeting high CVH recommendations across the age spectrum. Furthermore, our findings speak to the benefits of maintaining high CVH as long as possible across the life course and call attention to the need for comprehensive strategies to preserve, and restore when possible, high CVH to prevent associated morbidity and mortality.
Cardiovascular health is a construct developed by the AHA to define the ideal state of the cardiovascular system and includes 7 of the most important modifiable risk factors and health behaviors for the prevention of CVD.4 Our results complement several previous analyses of CVH demonstrating the value of this construct in defining risk of CVD.5–13 A meta-analysis combining results from 13 prospective studies among 193,126 individuals, with follow-up duration ranging from 4.0 to 18.7 years, identified linear associations between CVH score and risk of CVD and all-cause mortality, finding that risk of CVD mortality was 19% (95% CI, 14%−24%) lower for each 1 point higher CVH score.11 Our findings build upon these previous results by estimating the lifetime risk for CVD in a large, pooled population with individual-level data and in the context of competing risks for non-CVD mortality. Our data suggest that those with high CVH maintain a low risk of CVD several decades after all index ages. Conversely, those with moderate and low CVH scores have substantially higher risk of CVD, especially in those greater than 40 years of age. Recently, Enserro et al. described temporal trends in CVH and associations with CVD among 3,460 participants from the Framingham Heart Study.13 Their results suggest a decreasing prevalence of high CVH over the past 20 years, resulting in higher risk of CVD and death, which is corroborated by our findings over a longer follow-up duration and among a larger, more diverse sample.
As expected, the prevalence of high CVH was lower at older ages. Aging is one of the most important risk factors in the development of CVD.28 However, our results reinforce the idea that aging, alone, does not explain the increasing risk of CVD over the life course. While physiologic risk factors like BP, cholesterol, and glucose were higher, on average, in older compared with younger age groups, ideal levels of behavioral factors like physical activity and diet were also less prevalent. It is attractive to consider the possibility that maintenance of high CVH in young and middle-age adults, especially in the presence of increasing age, may result in markedly lower rates of CVD in the population. Our analysis suggests that high CVH among those less than 40 years of age confers low risk of CVD (≤2.1% in all subgroups) up to 40 years after index age. Conversely, periods of very low risk among those with high CVH were apparent only for approximately 20 years after index age for those aged 40–59 years and approximately 10 years after index age for those 60 years or older. Furthermore, among older participants with high CVH, the lifetime risk of CVD was higher compared with younger participants who had worse CVH, suggesting that long-term benefits of maintaining high CVH may not overcome the aging process. This finding may be related to older participants having a higher risk specifically for heart failure, which constituted a larger proportion of CVD events among these participants. Future research to support lifestyle interventions in the aging population should be considered to maintain high CVH throughout adulthood.
Beyond age, our results highlight sex and race differences in CVD risk and shed light on potential explanations. Sex differences in CVD risk have been well-described, consistently showing higher CVD mortality among men compared with women, until old age.29 Women tended to have higher prevalence of high CVH across all index age groups compared with men, but especially in middle- and older-age groups, and tended to have lower rates of CVD. Our results also corroborate previous findings detailing persistent racial disparities in the prevalence of high CVH,30 which likely contribute to associated disparities in lifetime risks of CVD. White adults tended to have higher prevalence of high CVH and lower rates of CVD compared with black adults. However, among black adults in older age groups, high CVH conferred a comparatively lower lifetime risk of CVD compared with white adults. While we cannot rule out the possibility of survival bias among older black vs. white participants owing to unmeasured factors, our results suggest that maintenance of high CVH may eliminate, or even reverse, race-associated disparities in CVD risk, which should be explored in future studies. There are several barriers to addressing these disparities, relating not only to the healthcare environment, but the social and cultural environment.31 Discussions of the mechanisms underpinning sex and race differences in CVD risk should consider differences in risk factor profiles across the life course, and multilevel interventions that also address the social determinants of health should be developed to support maintenance of high CVH as early as possible, while identifying best practices for disseminating such interventions to all adults.32
Limitations
Our analysis has several limitations. First, while efforts were made to harmonize data, different measurement methods used across cohorts may contribute to imprecision in quantification of individual CVH metrics. Second, by including data from a time period spanning more than 30 years, the potential for secular trends is unavoidable. However, the relative associations between these risk factors and CVD have been mostly consistent over time15 and our estimates are similar to data from the US National Health and Nutrition Examination Survey.33 Third, sample size was small in some analysis subgroups, particularly older black men and women with high CVH. Thus, estimates of absolute lifetime risk in these groups should be interpreted with caution, and we were unable to calculate estimates for additional, smaller age categories within strata. Fourth, we used CVH measures from only one time point. Thus, we cannot determine how changes in CVH over time may contribute to CVD risk. However, prior analyses suggest that improvement in CVH over time is associated with more favorable subclinical and clinical CVD outcomes in the shorter term.13,34 Finally, cohorts included in the LRPP are solely from the US, and we were able to analyze only white and black adults owing to insufficient data in other race/ethnicity groups. Thus, results may not be generalizable to international and global populations or other race/ethnicity groups.
Our results have potential clinical and public health importance. The attractiveness of the CVH framework resides in its simplicity, its positive framing around health rather than disease, and its comprehensive inclusion of levels of 7 of the most important risk factors for CVD, which is the leading cause of death and disability in the US. For the clinician, discussions with individual patients about the CVH score may be helpful. It is reasonable to expect that maintenance of high CVH throughout adulthood translates into decreased public health burden of CVD. Additionally, as a composite construct, CVH is a potential target for comprehensive interventions targeting dietary and lifestyle practices in addition to major risk factors like dyslipidemia, hypertension, diabetes mellitus, and cigarette smoking.35 Furthermore, future research should continue to evaluate strategies for maintaining high CVH during critical periods in the life course, such as young age, before major divergences in CVH occur.34
In conclusion, high CVH is associated with substantially lower lifetime risk for CVD in US men and women across the life course. However, the prevalence of high CVH is low, particularly in those 40 years of age or older. Further research is warranted to investigate strategies for maintaining and restoring high CVH throughout the life course to prevent associated risk of CVD and death.
Supplementary Material
WHAT IS KNOWN.
The cardiovascular health (CVH) score integrates 7 of the most important risk factors for preventing cardiovascular disease (CVD), and previous studies show higher CVH is associated with lower risk of CVD.
However, data are sparse regarding associations of CVH across the life course with longer-term risk of adverse health outcomes, particularly CVD, in broad, representative populations.
WHAT THE STUDY ADDS.
In this pooled cohort analysis of 30,447 US adults, those with high CVH had markedly lower lifetime risks for CVD events compared with individuals with moderate and low CVH.
The trend of lower lifetime risk for CVD among those with higher CVH was consistent across all age, sex, and race groups.
Persistent sex and race disparities in lifetime risks of CVD were observed and may be explained, in part, by corresponding disparities in levels of CVH metrics.
Funding sources:
The Cardiovascular Lifetime Risk Pooling Project (LRPP) was supported in its inception by the National Institutes of Health/National Heart, Lung, and Blood Institute grant R21HL085375 and is currently supported by funds from the Northwestern University Feinberg School of Medicine. Dr. Bundy was supported by the National Institutes of Health/National Heart, Lung, and Blood Institute Cardiovascular Epidemiology training grant T32HL069771 and the National Institutes of Health/Eunice Kennedy Shriver National Institute of Child Health and Human Development career development grant K12HD043451. Dr. Zhong was supported by a postdoctoral fellowship from the American Heart Association Strategically Focused Research Networks (14SFRN20480260). Dr. Paluch was supported by a postdoctoral fellowship from the American Heart Association Strategically Focused Research Networks (15SFDRN25080331).
Footnotes
Disclosures: None
References
- 1.The US Burden of Disease Collaborators. The State of US Health, 1990–2016: Burden of Diseases, Injuries, and Risk Factors Among US States. JAMA. 2018;319:1444–1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.GBD 2017 Causes of Death Collaborators. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392:1736–1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goff DC, Lloyd-Jones DM, Bennett G, Coady S, D’Agostino RB, Gibbons R, Greenland P, Lackland DT, Levy D, O’Donnell CJ, Robinson JG, Schwartz JS, Shero ST, Smith SC, Sorlie P, Stone NJ, Wilson PWF. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: A report of the American College of Cardiology/American Heart Association task force on practice guidelines. Circulation. 2013;63:2935–2959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lloyd-Jones DM, Hong Y, Labarthe D, Mozaffarian D, Appel LJ, Van Horn L, Greenlund K, Daniels S, Nichol G, Tomaselli GF, Arnett DK, Fonarow GC, Ho PM, Lauer MS, Masoudi FA, Robertson RM, Roger V, Schwamm LH, Sorlie P, Yancy CW, Rosamond WD. Defining and setting national goals for cardiovascular health promotion and disease reduction: The American Heart Association’s strategic impact goal through 2020 and beyond. Circulation. 2010;121:586–613. [DOI] [PubMed] [Google Scholar]
- 5.Folsom AR, Yatsuya H, Nettleton JA, Lutsey PL, Cushman M, Rosamond WD. Community prevalence of ideal cardiovascular health, by the American Heart Association definition, and relationship with cardiovascular disease incidence. J Am Coll Cardiol. 2011;57:1690–1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang Q, Cogswell ME, Dana Flanders W, Hong Y, Zhang Z, Loustalot F, Gillespie C, Merritt R, Hu FB. Trends in cardiovascular health metrics and associations with all-cause and CVD mortality among US adults. JAMA. 2012;307:1273–1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ford ES, Greenlund KJ, Hong Y. Ideal cardiovascular health and mortality from all causes and diseases of the circulatory system among adults in the United States. Circulation. 2012;125:987–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kulshreshtha A, Vaccarino V, Judd SE, Howard VJ, Mcclellan WM, Muntner P, Hong Y, Safford MM, Goyal A, Cushman M. Life’s Simple 7 and Risk of Incident Stroke: The Reasons for Geographic and Racial Differences in Stroke Study. Stroke. 2013;44:1909–1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Desai CS, Ning H, Liu K, Reis JP, Gidding SS, Armstrong A, Lima JAC, Lloyd-Jones DM. Cardiovascular Health in Young Adulthood and Association with Left Ventricular Structure and Function Later in Life: The Coronary Artery Risk Development in Young Adults Study. J Am Soc Echocardiogr. 2015;28:1452–1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ommerborn MJ, Blackshear CT, Hickson DA, Griswold ME, Kwatra J, Djoussé L, Clark CR. Ideal Cardiovascular Health and Incident Cardiovascular Events. Am J Prev Med. 2016;51:502–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guo L, Zhang S. Association between ideal cardiovascular health metrics and risk of cardiovascular events or mortality: A meta-analysis of prospective studies. Clin Cardiol. 2017;40:1339–1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Polonsky TS, Ning H, Daviglus ML, Liu K, Burke GL, Cushman M, Eng J, Folsom AR, Lutsey PL, Nettleton JA, Post WS, Sacco RL, Szklo M, Lloyd-Jones DM. Association of Cardiovascular Health With Subclinical Disease and Incident Events: The Multi-Ethnic Study of Atherosclerosis. J Am Heart Assoc. 2017;6:e004894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Enserro DM, Vasan RS, Xanthakis V. Twenty-Year Trends in the American Heart Association Cardiovascular Health Score and Impact on Subclinical and Clinical Cardiovascular Disease: The Framingham Offspring Study. J Am Heart Assoc. 2018;7:e008741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gaynor JJ, Feuer EJ, Tan CC, Wu DH, Little CR, Straus DJ, Clarkson BD, Brennan MF. On the use of cause-specific failure and conditional failure probabilities: Examples from clinical oncology data. J Am Stat Assoc. 1993;88:400–409. [Google Scholar]
- 15.Berry JD, Dyer A, Cai X, Garside DB, Ning H, Thomas A, Greenland P, Van Horn L, Tracy RP, Lloyd-Jones DM. Lifetime Risks of Cardiovascular Disease. N Engl J Med. 2012;366:321–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wilkins JT, Karmali KN, Huffman MD, Allen NB, Ning H, Berry JD, Garside DB, Dyer A, Lloyd-Jones DM. Data Resource Profile: The Cardiovascular Disease Lifetime Risk Pooling Project. Int J Epidemiol. 2015;44:1557–1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.The Atherosclerosis Risk in Communities (ARIC) study, design and objectives: the ARIC investigators. Am J Epidemiol. 1989;129:687–702. [PubMed] [Google Scholar]
- 18.Hughes GH, Cutter G, Donahue R, Friedman GD, Hulley S, Hunkeler E Jr. DRJ, Liu K, Orden S, Pirie P, Tucker B, Wagenknecht L. Recruitment in the Coronary Artery Disease Risk Development in Young Adults (CARDIA) study. Control Clin Trials. 1987;8:68–73. [DOI] [PubMed] [Google Scholar]
- 19.Fried LP, Borhani N, Enright P, Furberg CD, Gardin JM, Kronmal RA, Kuller LH, Manolio TA, Mittelmark MB, Newman A, Daniel HO, Psaty B, Rautaharju P, Tracy RP. The Cardiovascular Health Study: Design and Rationale. Ann Epidemiol. 1991;1:263–76. [DOI] [PubMed] [Google Scholar]
- 20.Bild DE, Bluemke DA, Burke GL, Detrano R, Diez Roux AV, Folsom AR, Greenland P, Jacobs DR, Kronmal R, Liu K, Nelson JC, O’Leary D, Saad MF, Shea S, Szklo M, Tracy RP. Multi-Ethnic Study of Atherosclerosis: Objectives and Design. Am J Epidemiol. 2002;156:871–881. [DOI] [PubMed] [Google Scholar]
- 21.Dawber TR, Kannel WB, Lye LP. An approach to longitudinal studies in a community: the Framingham Study. Ann N Y Acad Sci. 1963;107:539–556. [DOI] [PubMed] [Google Scholar]
- 22.Feinleib M, Kannel W, Garrison R, McNamara P, Castelli W. The Framingham Offspring Study. Design and preliminary data. Prev Med. 1975;4:518–525. [DOI] [PubMed] [Google Scholar]
- 23.Design of the Women’s Health Initiative Clinical Trial and Observational Study: The Women’s Health Initiative Study Group. Control Clin Trials. 1998;19:61–109. [DOI] [PubMed] [Google Scholar]
- 24.Zhong VW, Van Horn L, Cornelis MC, Wilkins JT, Ning H, Carnethon MR, Greenland P, Mentz RJ, Tucker KL, Zhao L, Norwood AF, Lloyd-Jones DM, Allen NB. Associations of Dietary Cholesterol or Egg Consumption With Incident Cardiovascular Disease and Mortality. JAMA. 2019;321:1081–1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chiuve SE, Fung TT, Rimm EB, Hu FB, McCullough ML, Wang M, Stampfer MJ, Willett WC. Alternative Dietary Indices Both Strongly Predict Risk of Chronic Disease. J Nutr. 2012;142:1009–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chomistek AK, Chiuve SE, Eliassen AH, Mukamal KJ, Willett WC, Rimm EB. Healthy lifestyle in the primordial prevention of cardiovascular disease among young women. J Am Coll Cardiol. 2015;65:43–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Beiser A, D’Agostino RBS, Seshadri S, Sullivan LM, Wolf PA. Computing estimates of incidence, including lifetime risk: Alzheimer’s disease in the Framingham Study. The Practical Incidence Estimators (PIE) macro. Stat Med. 2000;19:1495–1522. [DOI] [PubMed] [Google Scholar]
- 28.North BJ, Sinclair DA. The intersection between aging and cardiovascular disease. Circ Res. 2012;110:1097–1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bots SH, Peters SAE, Woodward M. Sex differences in coronary heart disease and stroke mortality: a global assessment of the effect of ageing between 1980 and 2010. BMJ Glob Heal. 2017;2:e000298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brown AF, Liang LJ, Vassar SD, Escarce JJ, Merkin SS, Cheng E, Richards A, Seeman T, Longstreth WT. Trends in racial/ethnic and nativity disparities in cardiovascular health among adults without prevalent cardiovascular disease in the United States, 1988 to 2014. Ann Intern Med. 2018;168:541–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Carnethon MR, Pu J, Howard G, Albert MA, Anderson CAM, Bertoni AG, Mujahid MS, Palaniappan L, Taylor HA Jr, Willis M, Yancy CW, Council AHAC on E and PC on CD in the YC on C and SNC on CCC on FG and TB and. Cardiovascular Health in African Americans: A Scientific Statement From the American Heart Association. Circulation. 2017;136:e393–e423. [DOI] [PubMed] [Google Scholar]
- 32.Mensah GA, Cooper RS, Siega-Riz AM, Cooper LA, Smith JD, Hendricks Brown C, Westfall JM, Ofili EO, Price LN, Arteaga S, Green Parker MC, Nelson CR, Newsome BJ, Redmond N, Roper RA, Beech BM, Brooks JL, Furr-Holden D, Gebreab SY, Giles WH, James RS, Lewis TT, Mokdad AH, Moore KD, Ravenell JE, Richmond A, Schoenberg NE, Sims M, Singh GK, Sumner AE, Treviño RP, Watson KS, Larissa Avilés-Santa M, Reis JP, Pratt CA, Engelgau MM, Goff DC, Pérez-Stable EJ. Reducing cardiovascular disparities through community-engaged implementation research: A National Heart, Lung, and Blood Institute workshop report. Circ Res. 2018;122:213–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huffman MD, Capewell S, Ning H, Shay CM, Ford ES, Lloyd-Jones DM. Cardiovascular Health Behavior and Health Factor Changes (1988–2008) and Projections to 2020. Circulation. 2012;125:2595–2602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Spring B, Moller AC, Colangelo LA, Siddique J, Roehrig M, Daviglus ML, Polak JF, Reis JP, Sidney S, Liu K. Healthy lifestyle change and subclinical atherosclerosis in young adults: Coronary Artery Risk Development in Young Adults (CARDIA) study. Circulation. 2014;130:10–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stamler J, Neaton JD, Cohen JD, Cutler J, Eberly L, Grandits G, Kuller LH, Ockene J, Prineas R, MRFIT Research Group. Multiple risk factor intervention trial revisited: a new perspective based on nonfatal and fatal composite endpoints, coronary and cardiovascular, during the trial. J Am Heart Assoc. 2012;1:e003640. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


