Abstract
Background
Globally, there is increased incidence of Parkinson’s Disease (PD), which is the second most common age-related neurodegenerative disease. The currently available PD-therapeutics provide only symptomatic relief. Thus, there is an urgent need to devise an effective and safe treatment strategy for PD. The holistic approach of Ayurveda can be a potential effective strategy for treating PD. The integration of different medicine systems, such as modern bio-medicine and Ayurveda can be an effective strategy for treatment of complex diseases, including PD.
Objective
This study aimed to evaluate the neuroprotective mechanism of six Ayurvedic nootropics that are commonly used to treat PD.
Material and methods
Six Ayurvedic herbs, namely Mucuna pruriens (MP), Bacopa monnieri (BM), Withania somnifera (WS), Centella asiatica (CA), Sida cordifolia (SC), and Celastrus paniculatus (CP), were selected after consultation with Ayurvedic scholars and physicians. The mode of action of methanolic herbal extracts was evaluated using the Caenorhabditis elegans BZ555 and NL5901 strains, which can be used to model the two main hallmarks of PD, namely degeneration of dopaminergic neurons and aggregation of α-synuclein protein.
Results
All six herbal extracts exhibited neuroprotective effect. The extracts of BM and MP exhibited maximum protection against 1-methyl-4-phenylpyridinium iodide (MPP+ iodide)-induced dopaminergic neurodegeneration in the BZ555 strain. Furthermore, the herbal extracts, except CA extract, inhibited the aggregation of heterologously expressed human α-synuclein in the NL5901 strain.
Conclusion
Ayurvedic herbs used in the treatment of PD exhibited differential neuroprotective and protein aggregation mitigating effects in C. elegans.
Keywords: Parkinson’s disease, Ayurvedic nootropics, Caenorhabditis elegans, Dopaminergic neurons, Alpha-synuclein
1. Introduction
The development of various therapeutic and preventive strategies, such as vaccination, antibiotic therapy, and antiviral therapy has markedly reduced life-threatening infections and increased the average human life span. However, the increased life span has not resulted in concomitant increase in health span [1]. The elderly population is susceptible to age-related non-communicable diseases, such as neurodegenerative disorders, cancer, and cardiac disease. These diseases markedly decrease the quality of life of the elderly population [2].
Parkinson’s disease (PD) is one of the most common age-related neurodegenerative disorders. Globally, PD affects approximately 7–10 million individuals who are mostly aged above 50 years [[3], [4], [5], [6]]. In addition to adversely affecting the quality of life of the patient, PD is associated with economic and social burdens [7].
PD, a complex disease, is associated with several motor and non-motor symptoms. The motor symptoms of patients with PD include tremors, rigidity, bradykinesia, altered gait, and speech difficulty, whereas the non-motor symptoms include constipation, autonomic dysfunction, sleep dysfunction, sensory symptoms, mood disorders, and cognitive abnormalities [8]. The etiological factor for the disease is not well understood. However, age is reported to be a major risk factor for PD [3,5]. The most common pathological changes observed in patients with PD are the degeneration of dopaminergic neurons especially in the Substantia nigra region of the mid-brain, aggregation of α-synuclein in the neurons, and decreased dopamine levels in the brain. Currently, the treatment for PD mainly involves supplementation of dopamine, which enables only the management of the symptoms. Additionally, the long-term supplementation of dopamine is associated with several side effects [9]. The current therapeutic strategies do not delay the progression of PD. Hence, there is a need to develop novel, comprehensive, effective, and safe therapeutic strategies for PD [9]. The integration of different therapies to achieve synergistic effects may aid in the rapid development of an effective therapeutic strategy for PD.
Ayurveda, the traditional Indian medical system, has a unique approach toward prevention, management, and treatment of various age-related neurological diseases. In Ayurveda, the diseases with PD-like symptoms are described as ‘Vatavyadhi’ (health problem caused by vitiated Vata), which includes Kampavata with tremors being the main symptom [10]. Ayurveda prescribes whole-system treatment for PD, which is a personalized, multifaceted treatment regimen involving various external therapies, internal medications, and behavioral alterations. Clinical studies have reported that Ayurvedic interventions can be beneficial for patients with PD [11,12]. The major herbs used in the Ayurvedic treatment of PD are Mucuna pruriens (MP, Kapikachu), Bacopa monnieri (BM, Brahmi), Withania somnifera (WS, Ashwagandha), Centella asiatica (CA, Mandookaparni), Sida cordifolia (SC, Bala), and Celastrus paniculatus (CP, Jyotishmati). Various independent clinical studies [11,12], as well as in vitro and in vivo studies [[13], [14], [15], [16], [17], [18]], have demonstrated the nootropic effect of these herbs. Additionally, these herbs are reported to mitigate neurodegenerative disease phenotypes.
Various model systems, including cell lines, small organisms (Drosophila melanogaster and Caenorhabditis elegans), and rodents, are used to study the disease biology and identifiy potential therapeutic agents. In the model systems, neurodegenerative disease conditions are induced by treatment with neurotoxins, such as 6-hydroxydopamine (6-OHDA), 1-methyl-4-phenylpyridinium (MPP+) iodide, aluminum, rotenone, and paraquat or by modulating the expression of proteins, such α-synuclein, PINK1, and LRRK2 [19]. MP, BM, WS, CA, SC, and CP are reported to promote the nervous system function and mitigate neurodegenerative disease phenotypes. Treatment with BM, MP, and WS rescued the motor defects in PD and amyotrophic lateral sclerosis models [20,21]. Additionally, MP [22], BM [15,23], and SC [24] protects against toxin-induced neurodegeneration in the PD models. The anti-amyloitic effects of BM, CA, and WS were assessed using various transgenic PD and AD models expressing amyloid proteins, such as amyloid beta (Aβ) and α-synuclein [[25], [26], [27]]. Previous studies have reported the therapeutic effects of extracts of these six herbs, such as anti-oxidant activity, neurotransmission regulation, and protection against mitochondrial dysfunction in disease models [16,[28], [29], [30], [31]]. In addition to protection against neurodegenerative diseases, BM is reported to be effective in treating epilepsy [32], depression, and anxiety [33,34]. Some studies have reported the memory- and cognition- enhancing activities of BM, CA, and CP in various amnesia and memory models [[35], [36], [37], [38], [39], [40]]. These findings indicated that these six herbs used in Ayurveda for treating PD exhibit nootropic and neuroprotective effects.
In this study, we hypothesized that different herbs, which are used in Ayurvedic whole-system treatment, have differential and additive/complementary effects on PD phenotypes. The anti-PD effects of these six herbs were evaluated using the C. elegans PD models. In particular, the effect of these herbs in mitigating two major hallmarks of PD, namely degeneration of dopaminergic neurons and aggregation of α-synuclein protein was evaluated. The findings of this study revealed that the six herbs exhibit differential effect on neurodegeneration and protein aggregation in C. elegans. This study provides a scientific rationale for implementation of whole-system Ayurvedic treatment for PD.
2. Materials and methods
2.1. Preparation of plant extracts
The following authenticated plant materials were obtained from Nanjangud Pharmacy, Karnataka: MP (seeds), BM (whole plant), WS (roots), SC (roots), CA (whole plant), and CP (seeds). The herbal specimens (approximately 50 g) are stored as reference at our laboratory.
The cold maceration methanolic extracts were prepared using the dried, coarsely ground plant materials of six plants. Each powdered sample was independently soaked in methanol (1:10; w/v) in glass bottles with a tight lid (Schott Duran, Borosil, India). The mixture was incubated in a rotary shaker at 100 rpm and 37 °C for 72 h. The mixture was filtered twice through a blotting paper, followed by filtration through a Whatman filter paper (No. 1). The filtrates were lyophilized under vacuum to obtain the solvent-free extracts. The extracts were stored in glass vials at −80 °C till further use.
2.2. Preparation of standard and working solutions
The following standard compounds were purchased from Natural Remedies Pvt Ltd (Bangalore, India), bacopaside I (>95% purity), vasicinone (>95% purity), withaferin A (>95% purity), asiatic acid (>90% purity), L-3,4-dihydroxyphenylalanine (l-DOPA) (>99% purity), and lupeol (>95%).
The stock solutions of bacopaside I (10 mg/mL), asiatic acid (1 mg/mL), l-DOPA (1 mg/mL), vasicinone (1 mg/mL), and withaferin A (1 mg/mL) were prepared in high-performance liquid chromatography (HPLC) grade methanol. The stock solutions of solvent-free extracts of MP (10 mg/mL), BM (10 mg/mL), WS (50 mg/mL), SC (10 mg/mL), CA (10 mg/mL), and CP (10 mg/mL) were prepared in HPLC grade methanol. The insoluble material was separated by centrifugation.
2.3. High-performance thin-layer chromatography (HPTLC) analysis
The HPTLC analysis was performed using 10 × 10 cm TLC Silica gel 60 F254 (Cat No.1.05554.0007, Merck, Germany) plates. The samples and marker compounds were spotted (8 mm bandwidth, 10 mm apart) onto the plate using a Linomat 5 sample applicator (CAMAG, Switzerland) equipped with a 100-μL syringe (Hamilton, Bonaduz, Switzerland). The development chamber (CAMAG, Switzerland) was saturated with the respective mobile phases for 15 min prior to the development of the chromatogram. The mobile phase was allowed to move to a distance of 8 cm (Table 1).The plates were dried and derivatized using 0.5% anisaldehyde/sulfuric acid solution. The HPTLC conditions were standardized in-house for some extracts, while previously published methods were used for other extracts. The HPTLC conditions are provided in Table 1. Densitometric scanning was performed using CAMAG TLC scanner 3 equipped with WinCATS software (CAMAG). The scanning conditions were as follows: slit dimensions, 6.00 × 0.45 mm; scanning speed, 20 mm/s. The marker compounds in the extracts were quantified using the standard curve of the respective marker compounds [[41], [42], [43]].
Table 1.
Details of HPTLC analysis of the herbal extracts.
| Name of the plant | Marker compound | Mobile phase | Derivitization solution | Scanning wavelength | References |
|---|---|---|---|---|---|
| Mucuna pruriens | L-DOPA (3–16 mg) | n-butanol: acetic acid: water (4:1:5) | 5% anisaldehydesulphuric solution | 280 nm | [41] |
| Bacopa monnieri | Bacopaside I (500–1500 ng) | n-butanol: acetic acid: water (6:3:1) | 5% anisaldehydesulphuric solution | 500 nm | |
| Withania somnifera | Withaferin A (90–500 ng) | toluene: ethyl acetate: formic acid (5:5:1) | 5% anisaldehydesulphuric solution | 500 nm | [43] |
| Sida cordifolia | Vasicinone (100–500 ng) | n-butanol: acetic acid: water (6:3:1) | NA (derivatization not required) | 254 nm | |
| Centella asiatica | Asiatic acid (900–2500 ng) | n-butanol: acetic acid: water (6:3:1) | 5% anisaldehydesulphuric solution | 500 nm | |
| Celastrus paniculatus | Lupeol (200–500 ng) | toluene: chloroform: methanol (4:4.7:1.3) | 5% anisaldehydesulphuric solution | 500 nm | [42] |
2.4. C. elegans culture and maintenance
The following C. elegans transgenic strains were procured from the Caenorhabditis Genetics Center (CGC), University of Minnesota, USA: BZ555 (Pdat-1::GFP; expressing green fluorescent protein (GFP) expressed in dopaminergic neurons) and NL5901 (Punc-54:alpha synuclein::YFP+unc-119; expressing human α-synuclein protein tagged with yellow fluorescent protein (YFP) in the body muscles). The strains were cultured on standard nematode growth medium (NGM) seeded with Escherichia coli OP50 as described previously [44].
2.5. Evaluation of neuroprotective effect of the extracts
The neurodegeneration assay was performed as described previously [45] with minor modifications. Briefly, the synchronized L1 larvae were obtained by hatching the eggs, which were isolated by treating the gravid adults [46] with bleaching solution (1:1 solution of 4% sodium hypochlorite (Fisher Scientific) and 1 N sodium hydroxide). The assay was performed in 250-μL reaction volume containing approximately 200 larvae (10 μL larval suspension), 5 mM MPP+ iodide (Sigma Aldrich, Cat. No. D048); 1X phosphate buffer saline (pH 7.4), 6 mg/mL (w/v) of fresh overnight grown culture of E. coli OP50, and various concentrations of extracts. The assay was performed in a 24-well plate. The stock solutions of extracts were prepared in dimethyl sulfoxide (DMSO; Spectrochem Pvt Ltd, India; 99.5% purity), which were diluted to the required working concentration in the reaction mixture. The solvent control group included equivalent volume of DMSO. The worms were incubated in the assay mixture at 23 °C for 20 h in a BOD incubator (Servewell Instruments Pvt Ltd, India). The worms were then washed with M9 buffer and transferred to an agar pad (2% agarose in distilled water) prepared on a glass slide. The worms were immobilized on the agar pad using 100 mM sodium azide solution (Sigma Aldrich, Cat. No. S2002-25G). A cover slip was placed and the worms were observed under a fluorescence microscope (Olympus BX41). The effect of MPP+ iodide on dopaminergic neurons of BZ555 worms was examined using the blue filters (excitation wavelengths from 455 nm to 490 nm). The worm was considered to exhibit neurodegeneration if one of the four CEP dendrites exhibited degeneration (Fig. 2A), which was evaluated based on the partial or complete loss of green fluorescence. Neurodegeneration in the randomly chosen experimental groups was evaluated by a blinded assessor to ensure unbiased nature of the findings. The number of worms exhibiting degeneration was counted in each group and the results were expressed in terms of percentage neurodegeneration as follows:
| Percentage of worms with neurodegeneration = (number of worms with degenerated neurons/total number of worms) × 100 |
| Neuroprotection (%) = percentage of worms with neurodegeneration in the DMSO-treated group − percentage of worms with neurodegeneration in the extract-treated group. |
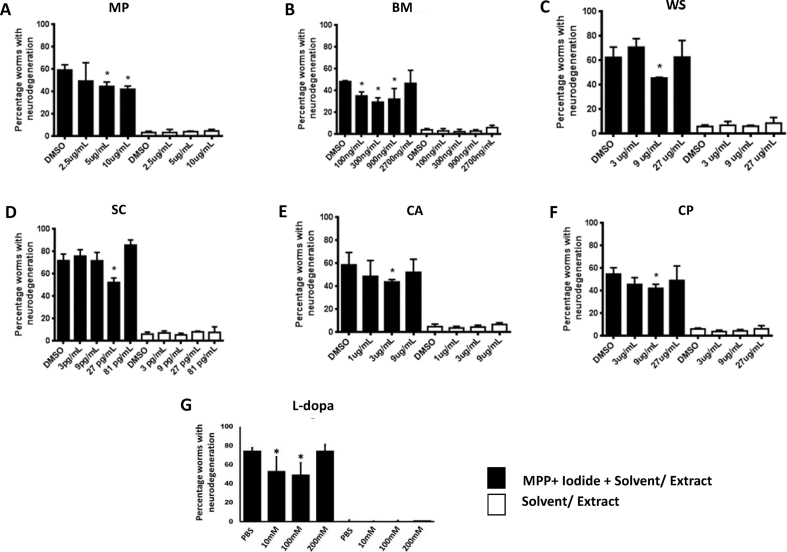
Fig. 2.
Dose response of six herbs andL-3,4-dihydroxyphenylalanine(l-DOPA)against1-methyl-4-phenylpyridinium (MPP+) iodide-inducedneurodegeneration. Effect of different concentrations of the herbal extracts and l-DOPA (positive control) on MPP+ iodide (5 mM)-induced neurodegeneration in the L1 larvae of BZ555 worms after 20 h treatment at 23 °C. Filled bars indicate neurodegeneration in groups treated with MPP+ iodide and herbal extract/l-DOPA, while open bars indicate neurodegeneration in groups treated with extract/l-DOPA alone. Data are represented as mean ± standard deviation. (∗p < 0.05; one-way analysis of variance, followed by Tukey’s post-hoc test).
2.6. Evaluation of effect of the extracts on protein aggregation
The anti- α-synuclein aggregation effect of herbs was evaluated as described previously [15] with minor modifications. The assay was performed on NGM plates spotted with E. coli OP50. The stock solutions of the drugs (10 mg/mL) were prepared in DMSO and diluted to appropriate concentrations in 1X PBS (pH 7.4). The E. coli OP50 lawn was coated with 100 μL of the working concentration of extract. The extract was allowed to dry for 15 min. The synchronized L1 larvae of NL5901 strain were obtained as described in section 2.5. The L1 larvae were allowed to feed on the extract-coated E. coli OP50 lawn till adult stage (72 h at 20 °C). The worms were then washed thrice with M9 buffer to remove the adhering bacteria. The worms were mounted onto a 2% agar pad containing 100 mM sodium azide. A cover slip was placed over the samples and the images of the immobilized worms were captured under a fluorescent microscope (Olympus BX41) equipped with a digital camera (Olympus DP72). The images were captured under a blue filter (excitation wavelengths from 455 nm to 490 nm) using Image-Pro Express (Media Cybernetics Inc). The fluorescent intensity was measured by selecting the region of interest (from mouth to the end of meta corpus) in Image J (National Institutes of Health, Bethesda, MD). In each treatment group, 20 worms were analyzed. The normalized fluorescence intensity was calculated as follows:
| Normalized fluorescence intensity = fluorescence intensity in extract-treated group/fluorescence intensity in DMSO-treated group. |
The fluorescence intensity of randomly chosen experimental groups was evaluated by a blinded assessor to ensure unbiased nature of the findings.
2.7. Statistical analysis
The data from at least three independent trials are expressed as mean ± standard deviation. All statistical analyses were performed using Microsoft Excel 2007. The differences between two groups were analyzed using the Student’s t-test, whereas those between multiple groups were analyzed using one-way analysis of variance (ANOVA), followed by Tukey’s post-hoc test. The differences were considered significant when the p-value was less than 0.05.
3. Results
3.1. Quantification of marker compounds
The methanolic extract yields of the six herbs were in the range of 3–10% (w/w) (Table 2). The respective marker compounds in the extracts were detected and quantified using HPTLC (Fig. 1A–F). Each marker compound was quantified from the respective standard curves (Supplementary Fig. S1A-F). The amount of marker compound per 100 g (dry weight) of the plant is shown in Table 2. The data from three trials are represented.
Table 2.
Amount of marker compounds in the herbal extracts.
| Name of the plant | Marker compound | Amount of extract yield (in g) per 100 g dry weight of plant material | Amount of marker compound (in mg) per 100 g dry weight of plant material |
|---|---|---|---|
| Mucuna pruriens | l-DOPA | 5.756 | 527.997 ± 49.411 |
| Bacopa monnieri | Bacopaside I | 5.357 | 59.588 ± 16.330 |
| Withania somnifera | Withaferin A | 7.969 | 5.15 ± 1.638 |
| Sida cordifolia | Vasicinone | 3.663 | 3.877 ± 0.526 |
| Centella asiatica | Asiatic Acid | 10.141 | 142.441 ± 41.849 |
| Celastrus paniculatus | Lupeol | 33.81 | 85.129 ± 4.262 |
Fig. 1.
Chemical characterization of the herbal extracts. Quantification of the marker compounds. (A–F) Illustrative images of Mucuna pruriens (MP), Bacopa monnieri (BM), Withania somnifera (WS), Sida cordifolia (SC), Centella asiatica (CA), and Celastrus paniculatus (CP). The HPTLC chromatograms of the extracts with the respective marker compounds are shown. Values mentioned in the chromatogram indicate the retention factor (Rf) of the respective marker compound. Lanes 1–5 in all chromatograms indicate increasing concentrations of marker compound standards. Lanes 6–8 indicate different concentration of the methanolic extracts of the herbs.
3.2. Neuroprotective effects of six herbs against MPP+ iodide-mediated neurotoxicity
MPP+ iodide, an active metabolite of a neurotoxin (MPTP), selectively induces the degeneration of dopaminergic neurons [47]. MPP+ iodide (1–7.5 mM) dose-dependently increased the neurodegeneration in the L1 larvae of BZ555 worms (Fig. S2). Treatment with 7.5 mM of MPP+ iodide resulted in the highest percentage of worms exhibiting neurodegeneration (approximately 87%). The percentages of worms exhibiting neurodegeneration after treatment with 1, 2.5, and 5 mM MPP+ iodide were 24, 27, and 67%, respectively. To evaluate the neuroprotective effect of six herbal extracts, the sub-lethal concentration of 5 mM MPP+ iodide was used. The stock solution of MPP+ iodide was prepared in sterile distilled water. The percentage of worms exhibiting neurodegeneration in the negative control and solvent control groups was less than 10% (data not shown).
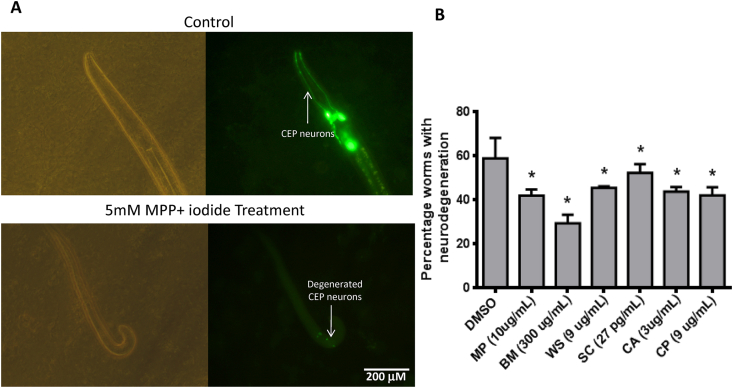
All six extracts exhibited a significant (one-way ANOVA, p < 0.05) neuroprotective effect against MPP+ iodide-induced dopaminergic neurodegeneration (Fig. 2, Fig. 3). The highest percentage neuroprotection (50.06%) was observed upon treatment with BM extract at a dose of 300 ng/mL. At a concentration of 9 μg/mL, the percentage neuroprotection exhibited by MP and CP was 28.69 and 28.56%, respectively. The percentage neuroprotection exhibited by 3 μg/mL CA and 9 μg/mL WS was 25.67 and 22.73%, respectively. The lowest percentage neuroprotection was observed after treatment with 27 pg/mL of SC extract (11.30%). However, the neuroprotective activity of SC extract was observed at a low concentration (27 pg/mL), which was lowest effective concentration among the six herbal extracts (Fig. 2, Fig. 3B).
Fig. 3.
Neuro-protective potential of the herbal extracts. Quantitative analysis of neuroprotective effect of the six herbal extracts (A) Representative bright-field (left panel) and fluorescence (righ panel) images of transgenic BZ555 worms in the control (top panel) and 5 mM 1-methyl-4-phenylpyridinium (MPP+) iodide-treated groups (bottom panel). Arrows indicated CEP dopaminergic neurons, which undergo degeneration upon treatment with MPP+ iodide. The worms in the control group had intact fluorescent neuronal structure, whereas those in the MPP+ iodide-treated groups had loss of fluorescent neuronal structure (B) Treatment with herbal extracts mitigated the MPP+ iodide-induced neurodegeneration in the transgenic BZ555 worms expressing green fluorescent protein in the dopaminergic neurons. Percentage of worms exhibiting neurodegeneration upon MPP+ iodide treatment in the presence of solvent control (DMSO) or herbal extract. Only the most effect concentration of each extract is shown. Data are represented as mean percentage of worms exhibiting neurodegeneration ± standard deviation. (∗p < 0.05; one-way analysis of variance, followed by Tukey’s post-hoc test).
L-DOPA, a common PD drug and a neuroprotective agent, was used as a positive control (Fig. 2G). L-DOPA dose-dependently protected the neurons against MPP+-induced neurodegeneration. For the neurodegeneration assay, 100 μM of L-DOPA, which exhibited 25.23% neuroprotection, was used as a postive control.
3.3. Inhibition of α-synuclein aggregation
One of the characteristic hallmarks of patients with PD is the formation of Lewy bodies in the brain. Aggregated α-synuclein is a major component of the Lewy bodies. The accumulation of Lewy bodies contributes to the progression of neurodegeneration, especially in PD. The NL5901 strain of C. elegans exhibits heterologous expression of YFP-tagged human α-synuclein in the body wall muscles (Fig. 4A). The fluorescence intensity at the anterior end of the worms was considered as a measure of α-synuclein aggregation.
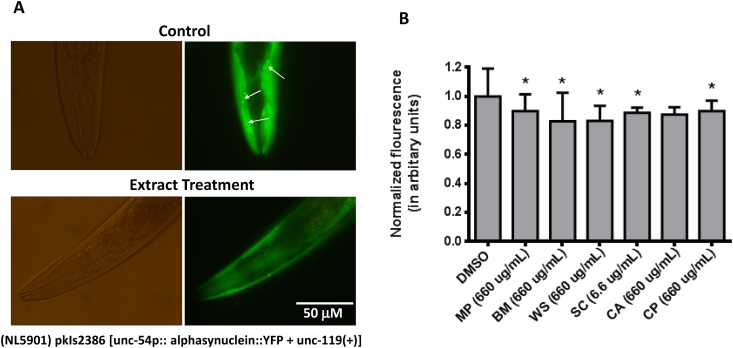
Fig. 4.
Anti-aggregation potential of nootropics. Effect of herbal extracts on α-synuclein aggregation, which was analyzed based on the fluorescence intensity, in the NL5901 worms. (A) Representative bright-field (left panel) and fluorescence (right panel) images of the anterior end of NL5901 worms (expressing yellow flourescent protein (YFP)-tagged α-synuclein protein) in the control (upper panel) and extract-treated (lower panel) groups. (B) Normalized fluorescence intensity of worms in the DMSO-treated and herbal extract-treated groups. Only the most effective concentrations of the extract are shown. Data are represented as normalized mean fluorescence intensity ± standard deviation. (∗p < 0.05; one-way analysis of variance, followed by Tukey’s post-hoc test).
The day 1 adults of NL5901 worms treated with methanolic extracts of BM, WS, CP, MP, and SC exhibited a significantly lower α-synuclein fluorescence intensity than the untreated day 1 adults of NL5901 worms (Fig. 4B). The lowest average α-synuclein fluorescence intensity was observed in the worms treated with BM (660 μg/mL) and WS (66 μg/mL) extracts. The fluorescence intensity of α-synuclein in the worms treated with CA extract was non-significantly lower than that in the untreated worms (p > 0.05).
4. Discussion
In this study, the effect of six methanolic extracts of herbs, which are commonly used in treatment of PD in Ayurveda, was evaluated using two C. elegans PD models. The six extracts exhibited differential neuroprotective and anti-α-synuclein effects.
PD has a complex etiology with various cellular and tissue-specific events contributing to the development of motor and non-motor symptoms. The major pathological changes observed in the brain of patients with PD are decreased dopamine level, enhanced oxidative stress, mitochondrial damage, and synaptic loss, which result in the degeneration of dopaminergic neurons in Substantia nigra and other neurons. It is important to note that some of these pathological changes, such as aggregation of protein and degeneration of dopaminergic neurons are also associated with aging process. However, these pathological changes in PD are accelerated when compared with those in aging. Thus, in addition to treatment with neuroprotective agents, prevention and management of age-related neuropathological changes are important for PD treatment and management [5].
Ayurveda has a unique approach for treating PD. The therapeutic strategies employed by Ayurveda include treatment with powder of MP (Kapikachhu) seed, a levodopa-containing herb. Additionally, Ayurveda also uses nootropic herbs, such as BM, CA, and CP, as well as Rasayana (anti-aging) herbs, such as WS and SC for treating PD [48]. The clinical efficacy of traditional Ayurveda treatment, which involves the use of different formulations and therapies, has been previously demonstrated [12,49]. The Ayurvedic treatment of PD inspired the isolation of L-DOPA in 1937. L-DOPA, which is effective for controlling tremors, is currently a gold standard in modern medicine for treating PD [50]. However, purified L-DOPA has limited efficacy. Additionally, the long-term usage of l-DOPA results in side-effects, such as bradykinesia [11]. The crude powder of MP seeds (Kapikachhu churna) is reported to have a similar efficacy as l-DOPA with decreased side effects [11]. Thus, different herbs in the multicomponent Ayurvedic treatment are likely to have differential effects on various cellular pathways, which may contribute to the improved alleviation of PD-specific neurodegeneration, oxidative stress, and mitochondria dysfunction. The understanding of mechanism of action of different components of Ayurvedic treatment protocol can aid in devising effective therapeutic strategy for PD.
In this study, the neuroprotective effects of six extracts of herbs used in Ayurveda for treating PD was evaluated using C. elegans, a standard model for neuroscience research. C. elegans has been previously used to understand the nervous system function at the behavioral, cellular, and molecular levels [51]. Prof. Sydney Brenner was the first to use C. elegans in scientific research. The characteristics of C. elegans model include fully sequenced genome (which has high sequence similarity with human genome), cost-effective culture conditions, short life cycle, and transparent body for dissection-less imaging [44]. Recently, C. elegans has been widely used as a model for drug screening and for research related to traditional medicine [[52], [53], [54], [55]]. In this study, we have used the PD models of C. elegans, which mimic two hallmarks of the disease namely, degeneration of dopaminergic neurons and aggregation of α-synuclein.
Four out of the six selected herbs, namely BM, CA, WS, and CP are mentioned as medhya rasayana (nootropics) in Ayurvedic texts. These herbs are also used as nootropics in the clinical practice. Meanwhile, MP and SC are mainly mentioned as ‘Vatahara’ herbs in the Ayurvedic texts and are used in the treatment of neurological disorders, especially motor disorders like PD. Among the six herbal extracts, the highest protection against MPP +-induced neurodegeneration was exhibited by BM (∼50%), MP (∼29%), and CP (∼29%). MPP+ iodide, a neurotoxin, promotes the degeneration of dopaminergic neurons through upregulating the production of reactive oxygen species in the mitochondria, which leads to cellular damage [56,57]. L-DOPA is reported to inhibit MPP+-induced neurodegeneration [58]. Thus, the neuroprotective effect of MP may be due to the presence of L-DOPA. The neuroprotective effects of BM and CP may be due to the presence of anti-oxidant molecules. The extracts of CA, WS, and SC exhibited minimal but significant neuroprotective effects.
The extracts of WS and BM were the most effective in inhibiting the aggregation of α-synuclein. Previously, the WS extract was reported to inhibit the aggregation of Aβ protein through enhanced clearance of protein [27]. BM and CA are also reported to inhibit Aβ aggregation. BM mother tincture extract inhibited α-synuclein aggregation in C. elegans [15]. The mechanism underlying the anti-aggregation effect of BM and CA has not been elucidated.
5. Conclusion
The results of this study suggest that the herbal extracts may act at multiple cellular pathways, including anti-oxidant and protein aggregation clearance pathways. Thus, herbs used in PD treatment in Ayurveda may exhibit differential activities, which may lead to an additive effect on complex disease phenotypes. Future studies at the behavioral (movement, food sensing), cellular (mitochondrial structure-function, ROS levels) and molecular (gene expression) levels would provide useful insights into the therapeutic potential of individual, nootropic (medhya), and vatahara herbs for treating neurodegenerative diseases and enable the development of safe and efficacious formulations and treatment protocols.
Source(s) of funding
None declared.
Conflicts of interest
Dr. Ashwini Godbole is an associate editor in Journal of Ayurveda and Integrative Medicine. However, she was not involved in the peer-review process of this manuscript. The other authors declare no conflict of interest.
Acknowledgements
Anjaneyulu J, Vidyashankar R, and Ashwini Godbole thank ICMR-SRF, JM Financial, and DST-SERB (Young Scientist Scheme), respectively, for generous and timely funding for this research. We also thank DSEF-FRLHT and Pratiksha Trust for timely financial support. We are grateful to Prof Ananthram Vellareddy for his support in the crucial stage of the study. Authors thank support from Dr. Sriranjini J, Dr. Varghese Thomas, and Dr. Subrahmanya Kumar for Ayurvedic knowledge inputs and supply of authentic herbal material. Authors also thank Dr. Noorunnisa Begum, a plant taxonomist, for authenticating the samples. We acknowledge help from Dr. Ravi Kumar, CCAMP for help in preparation of first batch of herbal extract. Authors profusely thank Prof Sandhya Kaushika for her unconditional help and support with C. elegans strains as well as experiments. Authors also thank Caenorhabditis Genetics Center (CGC), University of Minnesota, USA for making required C. elegans strains available at affordable price. Authors acknowledge plant picture credits to ENVIS-FRLHT and Ms. Suma T S.
Footnotes
Peer review under responsibility of Transdisciplinary University, Bangalore.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jaim.2020.07.006.
Appendix A. Supplementary data
The following are the supplementary data to this article:
Calibration curve of marker compounds: (A–F) Calibration curve (left panel) and chromatogram (right panel) used for high-performance thin-layer chromatography (HPTLC)-based quantification of the marker compounds in the herbal extracts. The linear equation and r2 are denoted in the calibration curve. The identification of marker compound on HPTLC chromatogram is provided along with the calibration curve.
Dose response of1-methyl-4-phenylpyridinium (MPP+)iodide: Effect of different concentrations of MPP+ iodide on the dopaminergic neurons the BZ555 L1 larvae treated for 20 h at 23 °C. Data are represented as mean ± standard deviation (SD). (∗p < 0.05; one-way analysis of variance, followed by Tukey’s post-hoc test).
Dose response of six herbs against alpha-synuclein aggregation: Effect of different concentrations of the herbal extracts on the aggregation of human α-synuclein in the NL5901 worms. Treatment was performed from egg stage to day one adult stage at 20 °C. Data are represented as mean ± standard deviation. (∗p < 0.05; one-way analysis of variance, followed by Tukey’s post-hoc test).
References
- 1.WHO world report on ageing and health. World Health Organisation; 2015. [Google Scholar]
- 2.WHO global health and ageing. World Health Organisation; 2011. [Google Scholar]
- 3.Hirsch L., Jette N., Frolkis A., Steeves T., Pringsheim T. The incidence of Parkinson’s disease: a systematic review and meta-analysis. Neuroepidemiology. 2016;46(4):292–300. doi: 10.1159/000445751. [DOI] [PubMed] [Google Scholar]
- 4.Muangpaisan W., Mathews A., Hori H., Seidel D. A systematic review of the worldwide prevalence and incidence of Parkinson’s disease. J Med Assoc Thai. 2011;94(6):749–755. [PubMed] [Google Scholar]
- 5.Rodriguez M., Rodriguez-Sabate C., Morales I., Sanchez A., Sabate M. Parkinson’s disease as a result of aging. Aging Cell. 2015;14(3):293–308. doi: 10.1111/acel.12312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tysnes O.B., Storstein A. Epidemiology of Parkinson’s disease. J Neural Transm. 2017;124(8):901–905. doi: 10.1007/s00702-017-1686-y. [DOI] [PubMed] [Google Scholar]
- 7.Boland D.F., Stacy M. The economic and quality of life burden associated with Parkinson’s disease: a focus on symptoms. Am J Manag Care. 2012;18(7 Suppl):S168–S175. [PubMed] [Google Scholar]
- 8.Zesiewicz T.A., Sullivan K.L., Arnulf I., Chaudhuri K.R., Morgan J.C., Gronseth G.S. Practice parameter: treatment of nonmotor symptoms of Parkinson disease: report of the quality standards subcommittee of the American Academy of Neurology. Neurology. 2010;74(11):924–931. doi: 10.1212/WNL.0b013e3181d55f24. [DOI] [PubMed] [Google Scholar]
- 9.Rascol O., Payoux P., Ory F., Ferreira J.J., Brefel-Courbon C., Montastruc J.L. Limitations of current Parkinson’s disease therapy. Ann Neurol. 2003;53(Suppl. 3) doi: 10.1002/ana.10513. S3-12 discussion S12-15. [DOI] [PubMed] [Google Scholar]
- 10.Sengupta N.N. Neeraj Publishing House; Delhi: 1984. The Ayurveda system of medicine. [Google Scholar]
- 11.Cilia R., Laguna J., Cassani E., Cereda E., Pozzi N.G., Isaias I.U. Mucuna pruriens in Parkinson disease: a double-blind, randomized, controlled, crossover study. Neurology. 2017;89(5):432–438. doi: 10.1212/WNL.0000000000004175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nagashayana N., Sankarankutty P., Nampoothiri M.R., Mohan P.K., Mohanakumar K.P. Association of L-DOPA with recovery following Ayurveda medication in Parkinson’s disease. J Neurol Sci. 2000;176(2):124–127. doi: 10.1016/s0022-510x(00)00329-4. [DOI] [PubMed] [Google Scholar]
- 13.Berrocal R., Vasudevaraju P., Indi S.S., Sambasiva Rao K.R., Rao K.S. In vitro evidence that an aqueous extract of Centella asiatica modulates alpha-synuclein aggregation dynamics. J Alzheimers Dis. 2014;39(2):457–465. doi: 10.3233/JAD-131187. [DOI] [PubMed] [Google Scholar]
- 14.Haleagrahara N., Ponnusamy K. Neuroprotective effect of Centella asiatica extract (CAE) on experimentally induced parkinsonism in aged Sprague-Dawley rats. J Toxicol Sci. 2010;35(1):41–47. doi: 10.2131/jts.35.41. [DOI] [PubMed] [Google Scholar]
- 15.Jadiya P., Khan A., Sammi S.R., Kaur S., Mir S.S., Nazir A. Anti-Parkinsonian effects of Bacopa monnieri: insights from transgenic and pharmacological Caenorhabditis elegans models of Parkinson’s disease. Biochem Biophys Res Commun. 2011;413(4):605–610. doi: 10.1016/j.bbrc.2011.09.010. [DOI] [PubMed] [Google Scholar]
- 16.Kumar M.H., Gupta Y.K. Antioxidant property of Celastrus paniculatus willd.: a possible mechanism in enhancing cognition. Phytomedicine. 2002;9(4):302–311. doi: 10.1078/0944-7113-00136. [DOI] [PubMed] [Google Scholar]
- 17.Kumar S., Seal C.J., Howes M.J., Kite G.C., Okello E.J. In vitro protective effects of Withania somnifera (L.) dunal root extract against hydrogen peroxide and beta-amyloid(1-42)-induced cytotoxicity in differentiated PC12 cells. Phytother Res. 2010;24(10):1567–1574. doi: 10.1002/ptr.3261. [DOI] [PubMed] [Google Scholar]
- 18.Shinomol G.K., Bharath M.M., Muralidhara Neuromodulatory propensity of Bacopa monnieri leaf extract against 3-nitropropionic acid-induced oxidative stress: in vitro and in vivo evidences. Neurotox Res. 2012;22(2):102–114. doi: 10.1007/s12640-011-9303-6. [DOI] [PubMed] [Google Scholar]
- 19.Blesa J., Przedborski S. Parkinson’s disease: animal models and dopaminergic cell vulnerability. Front Neuroanat. 2014;8:155. doi: 10.3389/fnana.2014.00155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.De Rose F., Marotta R., Talani G., Catelani T., Solari P., Poddighe S. Differential effects of phytotherapic preparations in the hSOD1 Drosophila melanogaster model of ALS. Sci Rep. 2017;7:41059. doi: 10.1038/srep41059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jansen R.L., Brogan B., Whitworth A.J., Okello E.J. Effects of five Ayurvedic herbs on locomotor behaviour in a Drosophila melanogaster Parkinson’s disease model. Phytother Res. 2014;28(12):1789–1795. doi: 10.1002/ptr.5199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yadav S.K., Prakash J., Chouhan S., Singh S.P. Mucuna pruriens seed extract reduces oxidative stress in nigrostriatal tissue and improves neurobehavioral activity in paraquat-induced Parkinsonian mouse model. Neurochem Int. 2013;62(8):1039–1047. doi: 10.1016/j.neuint.2013.03.015. [DOI] [PubMed] [Google Scholar]
- 23.Nellore J., Pauline C., Amarnath K. Bacopa monnieri phytochemicals mediated synthesis of platinum nanoparticles and its neurorescue effect on 1-methyl 4-phenyl 1,2,3,6 tetrahydropyridine-induced experimental parkinsonism in Zebrafish. J Neurodegener Dis. 2013;2013:972391. doi: 10.1155/2013/972391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Khurana N., Gajbhiye A. Ameliorative effect of Sida cordifolia in rotenone induced oxidative stress model of Parkinson’s disease. Neurotoxicology. 2013;39:57–64. doi: 10.1016/j.neuro.2013.08.005. [DOI] [PubMed] [Google Scholar]
- 25.Dhanasekaran M., Holcomb L.A., Hitt A.R., Tharakan B., Porter J.W., Young K.A. Centella asiatica extract selectively decreases amyloid beta levels in hippocampus of Alzheimer’s disease animal model. Phytother Res. 2009;23(1):14–19. doi: 10.1002/ptr.2405. [DOI] [PubMed] [Google Scholar]
- 26.Holcomb L.A., Dhanasekaran M., Hitt A.R., Young K.A., Riggs M., Manyam B.V. Bacopa monniera extract reduces amyloid levels in PSAPP mice. J Alzheimers Dis. 2006;9(3):243–251. doi: 10.3233/jad-2006-9303. [DOI] [PubMed] [Google Scholar]
- 27.Sehgal N., Gupta A., Valli R.K., Joshi S.D., Mills J.T., Hamel E. Withania somnifera reverses Alzheimer’s disease pathology by enhancing low-density lipoprotein receptor-related protein in liver. Proc Natl Acad Sci U S A. 2012;109(9):3510–3515. doi: 10.1073/pnas.1112209109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chakrabarty M., Bhat P., Kumari S., D’Souza A., Bairy K.L., Chaturvedi A. Cortico-hippocampal salvage in chronic aluminium induced neurodegeneration by Celastrus paniculatus seed oil: neurobehavioural, biochemical, histological study. J Pharmacol Pharmacother. 2012;3(2):161–171. doi: 10.4103/0976-500X.95520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Godkar P.B., Gordon R.K., Ravindran A., Doctor B.P. Celastrus paniculatus seed oil and organic extracts attenuate hydrogen peroxide- and glutamate-induced injury in embryonic rat forebrain neuronal cells. Phytomedicine. 2006;13(1–2):29–36. doi: 10.1016/j.phymed.2003.11.011. [DOI] [PubMed] [Google Scholar]
- 30.Kumar P., Kumar A. Possible neuroprotective effect of Withania somnifera root extract against 3-nitropropionic acid-induced behavioral, biochemical, and mitochondrial dysfunction in an animal model of Huntington’s disease. J Med Food. 2009;12(3):591–600. doi: 10.1089/jmf.2008.0028. [DOI] [PubMed] [Google Scholar]
- 31.Rai S.N., Birla H., Singh S.S., Zahra W., Patil R.R., Jadhav J.P. Mucuna pruriens protects against MPTP intoxicated neuroinflammation in Parkinson’s disease through NF-kappaB/pAKT signaling pathways. Front Aging Neurosci. 2017;9:421. doi: 10.3389/fnagi.2017.00421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Krishnakumar A., Anju T.R., Abraham P.M., Paulose C.S. Alteration in 5-HT(2)C, NMDA receptor and IP3 in cerebral cortex of epileptic rats: restorative role of Bacopa monnieri. Neurochem Res. 2015;40(1):216–225. doi: 10.1007/s11064-014-1472-2. [DOI] [PubMed] [Google Scholar]
- 33.Chatterjee M., Verma P., Palit G. Comparative evaluation of Bacopa monniera and Panax quniquefolium in experimental anxiety and depressive models in mice. Indian J Exp Biol. 2010;48(3):306–313. [PubMed] [Google Scholar]
- 34.Sairam K., Dorababu M., Goel R.K., Bhattacharya S.K. Antidepressant activity of standardized extract of Bacopa monniera in experimental models of depression in rats. Phytomedicine. 2002;9(3):207–211. doi: 10.1078/0944-7113-00116. [DOI] [PubMed] [Google Scholar]
- 35.Arora R., Kumar R., Agarwal A., Reeta K.H., Gupta Y.K. Comparison of three different extracts of Centella asiatica for anti-amnesic, antioxidant and anticholinergic activities: in vitro and in vivo study. Biomed Pharmacother. 2018;105:1344–1352. doi: 10.1016/j.biopha.2018.05.156. [DOI] [PubMed] [Google Scholar]
- 36.Bhagya V., Christofer T., Shankaranarayana Rao B.S. Neuroprotective effect of Celastrus paniculatus on chronic stress-induced cognitive impairment. Indian J Pharmacol. 2016;48(6):687–693. doi: 10.4103/0253-7613.194853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Doknark S., Mingmalairak S., Vattanajun A., Tantisira B., Tantisira M.H. Study of ameliorating effects of ethanolic extract of Centella asiatica on learning and memory deficit in animal models. J Med Assoc Thai. 2014;97(Suppl. 2):S68–S76. [PubMed] [Google Scholar]
- 38.Pandareesh M.D., Anand T., Khanum F. Cognition enhancing and neuromodulatory propensity of Bacopa monniera extract against scopolamine induced cognitive impairments in Rat Hippocampus. Neurochem Res. 2016;41(5):985–999. doi: 10.1007/s11064-015-1780-1. [DOI] [PubMed] [Google Scholar]
- 39.Saraf M.K., Prabhakar S., Khanduja K.L., Anand A. Bacopa monniera attenuates scopolamine-induced impairment of spatial memory in mice. Evid Based Complement Alternat Med. 2011;2011:236186. doi: 10.1093/ecam/neq038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vollala V.R., Upadhya S., Nayak S. Learning and memory-enhancing effect of Bacopa monniera in neonatal rats. Bratisl Lek Listy. 2011;112(12):663–669. [PubMed] [Google Scholar]
- 41.The ayurvedic pharmacopoeia of India, Part 1. Government of India, Ministry of Health and Family Welfare, Department of AYUSH; 2014. [Google Scholar]
- 42.Sharma P., Shrivastava N.M., Shrivastava S. Identification of Lupeol in ethathanolic extract of Celastrus paniculatus by HPTLC method. Int J Pharma Sci Res. 2014;5(9):3876–3878. [Google Scholar]
- 43.Sharma V., Gupta A.P., P B, Gupta R.C., Singh B. A validated and densitometric HPTLC method for the quantification of Withaferin-A and Withanolide-A in different plant parts of two morphotypes of Withania somnifera. Chromatographia. 2007;66(9):801–804. [Google Scholar]
- 44.Brenner S. The genetics of Caenorhabditis elegans. Genetics. 1974;77(1):71–94. doi: 10.1093/genetics/77.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.De Jesus-Cortes H., Xu P., Drawbridge J., Estill S.J., Huntington P., Tran S. Neuroprotective efficacy of aminopropyl carbazoles in a mouse model of Parkinson disease. Proc Natl Acad Sci U S A. 2012;109(42):17010–17015. doi: 10.1073/pnas.1213956109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stiernagle T. Maintenance of C. elegans. WormBook. 2006:1–11. doi: 10.1895/wormbook.1.101.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Langston J.W., Irwin I., Langston E.B., Forno L.S. 1-Methyl-4-phenylpyridinium ion (MPP+): identification of a metabolite of MPTP, a toxin selective to the substantia nigra. Neurosci Lett. 1984;48(1):87–92. doi: 10.1016/0304-3940(84)90293-3. [DOI] [PubMed] [Google Scholar]
- 48.Bezard E., Gross C.E., Qin L., Gurevich V.V., Benovic J.L., Gurevich E.V. L-DOPA reverses the MPTP-induced elevation of the arrestin2 and GRK6 expression and enhanced ERK activation in monkey brain. Neurobiol Dis. 2005;18(2):323–335. doi: 10.1016/j.nbd.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 49.Rao R.V., Descamps O., John V., Bredesen D.E. Ayurvedic medicinal plants for Alzheimer’s disease: a review. Alzheimer’s Res Ther. 2012;4(3):22. doi: 10.1186/alzrt125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pathak-Gandhi N., Vaidya A.D. Management of Parkinson’s disease in Ayurveda: medicinal plants and adjuvant measures. J Ethnopharmacol. 2017;197:46–51. doi: 10.1016/j.jep.2016.08.020. [DOI] [PubMed] [Google Scholar]
- 51.Damodaran M., Ramaswamy R. Isolation of l-3: 4-dihydroxyphenylalanine from the seeds of Mucuna pruriens. Biochem J. 1937;31(12):2149–2152. doi: 10.1042/bj0312149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sengupta P., Samuel A.D. Caenorhabditis elegans: a model system for systems neuroscience. Curr Opin Neurobiol. 2009;19(6):637–643. doi: 10.1016/j.conb.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kim W., Hendricks G.L., Lee K., Mylonakis E. An update on the use of C. elegans for preclinical drug discovery: screening and identifying anti-infective drugs. Expert Opin Drug Discov. 2017;12(6):625–633. doi: 10.1080/17460441.2017.1319358. [DOI] [PubMed] [Google Scholar]
- 54.Luo Y. Alzheimer’s disease, the nematode Caenorhabditis elegans, and ginkgo biloba leaf extract. Life Sci. 2006;78(18):2066–2072. doi: 10.1016/j.lfs.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 55.O’Reilly L.P., Luke C.J., Perlmutter D.H., Silverman G.A., Pak S.C. C. elegans in high-throughput drug discovery. Adv Drug Deliv Rev. 2014;69–70:247–253. doi: 10.1016/j.addr.2013.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yu Y.B., Dosanjh L., Lao L., Tan M., Shim B.S., Luo Y. Cinnamomum cassia bark in two herbal formulas increases life span in Caenorhabditis elegans via insulin signaling and stress response pathways. PloS One. 2010;5(2):e9339. doi: 10.1371/journal.pone.0009339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jeong K.H., Jeon M.T., Kim H.D., Jung U.J., Jang M.C., Chu J.W. Nobiletin protects dopaminergic neurons in the 1-methyl-4-phenylpyridinium-treated rat model of Parkinson’s disease. J Med Food. 2015;18(4):409–414. doi: 10.1089/jmf.2014.3241. [DOI] [PubMed] [Google Scholar]
- 58.Kopin I.J. MPTP: an industrial chemical and contaminant of illicit narcotics stimulates a new era in research on Parkinson’s disease. Environ Health Perspect. 1987;75:45–51. doi: 10.1289/ehp.877545. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Calibration curve of marker compounds: (A–F) Calibration curve (left panel) and chromatogram (right panel) used for high-performance thin-layer chromatography (HPTLC)-based quantification of the marker compounds in the herbal extracts. The linear equation and r2 are denoted in the calibration curve. The identification of marker compound on HPTLC chromatogram is provided along with the calibration curve.
Dose response of1-methyl-4-phenylpyridinium (MPP+)iodide: Effect of different concentrations of MPP+ iodide on the dopaminergic neurons the BZ555 L1 larvae treated for 20 h at 23 °C. Data are represented as mean ± standard deviation (SD). (∗p < 0.05; one-way analysis of variance, followed by Tukey’s post-hoc test).
Dose response of six herbs against alpha-synuclein aggregation: Effect of different concentrations of the herbal extracts on the aggregation of human α-synuclein in the NL5901 worms. Treatment was performed from egg stage to day one adult stage at 20 °C. Data are represented as mean ± standard deviation. (∗p < 0.05; one-way analysis of variance, followed by Tukey’s post-hoc test).