Summary
Cyclic GMP-AMP synthase (cGAS) is reported essential for detecting intracellular bacteria. However, it remains to be determined whether and how cGAS is involved in extracellular bacterial infection. Here, we report that cGAS is essential for mediating type I interferon (IFN) production in infection by multiple extracellular pathogens, including Pseudomonas aeruginosa, Klebsiella pneumoniae, and Staphylococcus aureus. In addition, the canonical cGAS-stimulator of interferon gene (STING)-IFN axis is required for protecting mice from P. aeruginosa-induced mouse acute pulmonary infection, confirmed in cGAS pathway-specific gene deficiency mouse models. cGAS−/− and STING−/− mice exhibited reduced type I IFNs production, excessive inflammatory response accompanied with decreased resistance to P. aeruginosa challenge. Unfolded protein response was also modulated by cGAS through IRF3 and type I IFNs under P. aeruginosa infection. Collectively, these findings uncover the importance of cGAS in initiating immune responses against extracellular bacterial infection.
Subject Areas: Immune Response, Microbiology, Bacteriology
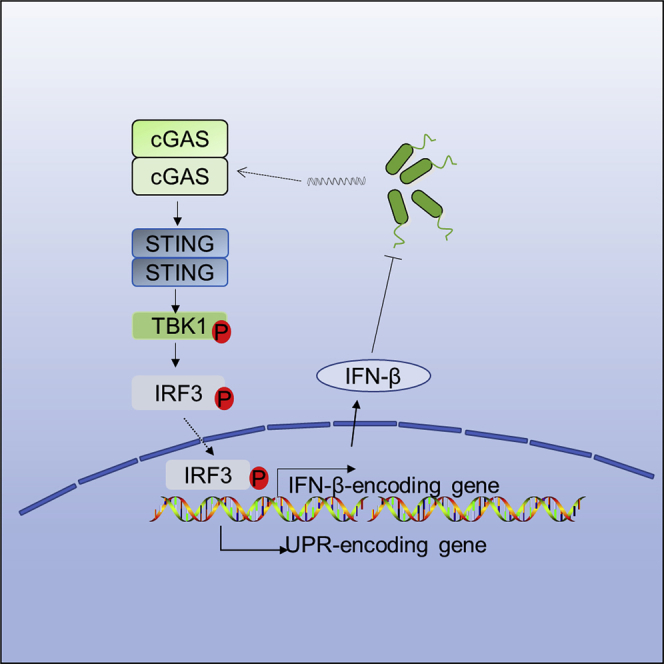
Graphical Abstract
Highlights
-
•
The role of cGAS signaling pathway is expanded in sensing extracellular bacteria
-
•
cGAS/STING/IFNAR axis is necessary host immunity restricting P. aeruginosa
-
•
cGAS signaling pathway is involved in modulating unfolded protein response
-
•
cGAS is an important nucleic acids' sensor in recognizing extracellular bacteria
Immune Response; Microbiology; Bacteriology
Introduction
Nucleic acids are integral parts of pathogens for replication, survival, and invasion and have also been shown as pathogen-associated molecular patterns to trigger innate immune response (Barbalat et al., 2011; Wu and Chen, 2014). To date, various DNA sensors are discovered for the recognition of self- or non-self-nucleic acids, including the endosomal TLR9, and the cytosolic DNA sensors AIM2, cyclic GMP-AMP synthase (cGAS), and IFI16 (Wu and Chen, 2014). Of which, cGAS has recently been identified as a novel DNA sensor responsible for sensing infection of DNA viruses, retroviruses, and intracellular bacteria (Li et al., 2013; Sun et al., 2013; Wu et al., 2013). Upon binding with double-stranded DNA, cGAS is activated and then catalyzes the nucleotide second messenger cyclic AMP-GMP generation, which in turn activates the stimulator of interferon gene (STING) (Gao et al., 2013; Sun et al., 2013). Activated STING is translocated from endoplasmic reticulum (ER) to ER–Golgi intermediate compartment (ERGIC) and Golgi complex triggering TANK-binding kinase 1 (TBK1) and interferon regulatory factor 3 (IRF3) activation and type I IFN production, serving as the important defense mechanism against microbial infection (Wu et al., 2013).
Pseudomonas aeruginosa is an important gram-negative extracellular opportunistic pathogen, which distributes widely in the world. People with immunodeficiency, like cystic fibrosis, burn wounds, chronic obstructive pulmonary disorder, pneumonia, or sepsis, are highly susceptible to P. aeruginosa (Cachia and Hodges, 2003; Curran et al., 2018; Miyoshi-Akiyama et al., 2017). Additionally, growing multi-drug resistance makes P. aeruginosa extremely difficult to treat (Pang et al., 2019), which has become a significant global public health issue. Neutrophils and macrophages are key components of innate immunity against P. aeruginosa attack by promoting inflammatory cytokine production and phagocytosis (Lovewell et al., 2014). It is worth mentioning that that type I IFNs are important for host defense against P. aeruginosa infection by modulating multiple innate immune responses such as phagocytosis and RANTES production (Carrigan et al., 2010; Parker et al., 2012; Parker and Prince, 2011). However, little is known about the interaction between cGAS and extracellular bacterium P. aeruginosa.
ER is a dynamic intracellular organelle with multiple functions, for instance, maintaining cell homeostasis in response to pathogenic infection (Bettigole and Glimcher, 2015; Grootjans et al., 2016). Currently, three transmembrane proteins (ATF6, PERK, and IRE1) located in the ER lumen are known to detect ER stress, activating unfolded protein response (UPR). Once activated, these ER stress initiators are dissociated with BiP, a chaperone and master regulator of the UPR, to elicit the UPR process. Although studies indicate that UPR is involved in innate immunity against P. aeruginosa infection (Richardson et al., 2010; van 't Wout et al., 2015), it remains largely unknown about the underlying mechanisms of UPR activation.
Here, we demonstrate that DNA sensor cGAS serves as an important sensing mechanism for detecting extracellular bacterial infection. P. aeruginosa infection also induced cGAS-STING-IRF3 activation to initiate UPR. To verify that this sensing is a common mechanism, we also tested this phenomenon in other species including Klebsiella pneumoniae and Staphylococcus aureus and confirmed that cGAS indeed senses a variety of extracellular bacteria in infection. Taken together, we delineate a critical role of cGAS signaling in host immunity against extracellular pathogens.
Results
cGAS is required for P. aeruginosa-induced type I IFN response in macrophages
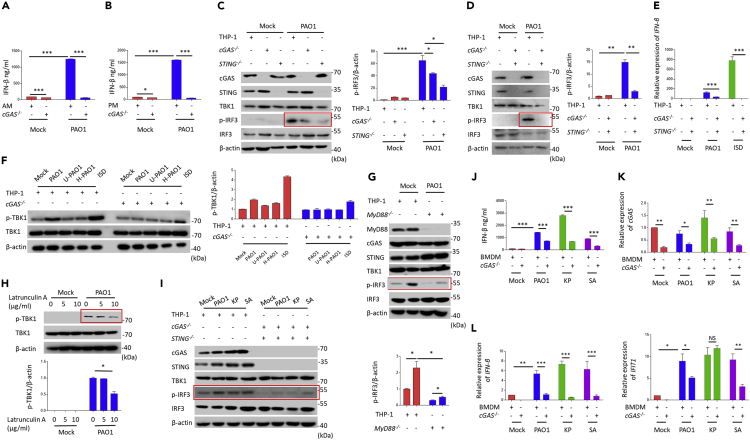
In order to determine the role of cGAS in the infection of the P. aeruginosa strain PAO1, the widely used human macrophage cell line THP-1 was infected with PAO1 at different multiplicity of infection (MOI) (1, 2, and 10) and different time points (1 h, 2 h, and 4 h). It is clearly observed that phosphorylation of TBK1 was significantly induced (Figure S1A and S1B). Importantly, p-TBK1 was also activated under UV radiation or heat-killed PAO1 infection (Figure S1C). To solidify our above observation, a mouse macrophage cell line, RAW264.7, was then transfected with RNAi to knockdown the target cGAS and STING genes (Figures S1D–S1G). We observed that transfection of cGAS siRNA or STING siRNA almost abolished the p-IRF3 in RAW264.7 cells following PAO1 infection (Figures S1F and S1G). Reduced p-TBK1 was further confirmed in STING−/− RAW264.7 cells (Figure S1I). Immunofluorescence assay showed that cGAS siRNA reduced p-IRF3 nuclear translocation (Figure S1H). Meanwhile, transcription of IRF3 targeted genes (IFN-β and IFIT1) and production of IFN-β were suppressed in cGAS siRNA- or STING siRNA-treated RAW264.7 cells after PAO1 infection or interferon-stimulatory DNA (ISD) transfection (Figures S2A–S2D). To seek additional evidence, we next used mouse alveolar macrophage (MH-S) cells and observed similar role of cGAS for host detection PAO1 after transfection with cGAS siRNA (Figure S2E). Since primary cells from genetic deficient mice would demonstrate the unequivocal role of cGAS in sensing pathogens, we isolated primary cGAS−/− mouse bone marrow-derived macrophages (BMDMs) and found reduced p-IRF3 compared to wild-type (WT) counterparts after PAO1 infection (Figure S2F). Likewise, type I IFN response was also blocked in primary cGAS−/− alveolar macrophages and peritoneal macrophages following PAO1 infection (Figures 1A, 1B, S2G, and S2H). To further characterize the roles of cGAS and STING in response to P. aeruginosa infection in human macrophages, we utilized CRISPR-Cas9 technique to construct cGAS−/−, STING −/−, and cGAS/STING−/− THP-1 cells. Consistent with the results observed in mouse macrophages, cGAS or STING deficiency in THP-1 significantly reduced p-IRF3, transcription of IFN-β, or reduced p-TBK1 (Figures 1C–1F). Additionally, we observed that MyD88 deficiency and phagocytosis inhibitor latrunculin A blocked the activation of IFN signal pathway of IRF3 under PAO1 infection (Figures 1G and 1H). Together, these findings reveal the critical roles of cGAS and STING in promoting type I IFN response under P. aeruginosa infection.
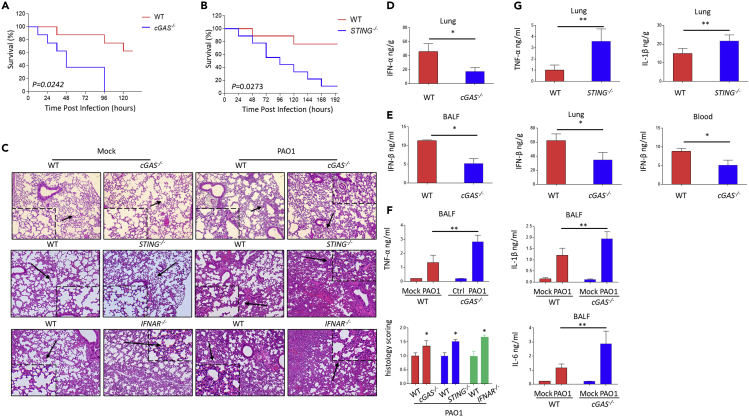
Figure 1.
cGAS is required for Pseudomonas aeruginosa-induced type I IFN response in macrophages
(A) WT and cGAS−/− alveolar macrophages were treated with 10 multiplicity of infection (MOI) PAO1 for 4 h. Cytokine levels of IFN-β in the cell culture supernatant were detected using enzyme-linked immunosorbent assay (ELISA) .
(B) WT and cGAS−/− peritoneal macrophages were treated with 10 MOI PAO1 for 4 h. Cytokine levels of IFN-β in the cell culture supernatant were detected using ELISA.
(C and D) cGAS−/− and STING−/− THP-1 cells were treated with 10 MOI PAO1 for 4 h. Immunoblot analysis of cGAS signaling pathways.
(E) WT and cGAS−/− STING−/− THP-1 cells were treated with 10 MOI PAO1 or ISD for 4 h. qPCR measuring mRNA levels of IFN-β in WT and cGAS−/− STING−/− THP-1 cells.
(F) WT and cGAS−/− THP-1 cells were treated with 10 MOI PAO1, UV-PAO1, and PAO1 DNA for 4 h. qPCR was performed to measure mRNA levels of IFN-β.
(G) WT and MyD88−/− THP-1 cells were treated with 10 MOI PAO1 for 4 h. Immunoblot analysis of cGAS signaling pathways.
(H) WT THP-1 cells were pre-treated with phagocytosis inhibitor latrunculin A and then treated with 10 MOI PAO1, K. pneumoniae, and S. aureus for 4 h. qPCR was performed to measure mRNA levels of IFN-β.
(I) WT and cGAS−/−STING−/− THP-1 cells were treated with 10 MOI PAO1, K. pneumoniae, and S. aureus for 4 h. Immunoblot analysis of cGAS signaling pathways.
(J) WT and cGAS−/− BMDMs were treated with 10 MOI PAO1, K. pneumoniae, and S. aureus for 4 h. Cytokine levels of IFN-β in the cell culture supernatant were detected using ELISA.
(K) WT and cGAS−/− BMDMs were treated with 10 MOI PAO1, K. pneumoniae, and S. aureus for 4 h. qPCR measuring mRNA levels of cGAS in WT and cGAS−/− BMDMs.
(L) WT and cGAS−/− BMDMs were treated with 10 MOI PAO1, K. pneumoniae, and S. aureus for 4 h. qPCR was performed to measure mRNA levels of IFN-β or IFIT1.
ELISA and qPCR data (mean ± standard error of mean (SEM)) are representative of three independent experiments (∗p ≤ 0.05, ∗∗p ≤ 0.01, ∗∗∗p ≤ 0.001 one-way analysis of variance (ANOVA) with Tukey post hoc test).
The cGAS signaling pathway is responsible for detecting extracellular bacteria Klebsiella pneumoniae and Staphylococcus aureus
To expand the concept and examine whether cGAS can sense extracellular bacteria in general, we determined whether other extracellular bacteria (Klebsiella pneumoniae and Staphylococcus aureus) also activate cGAS pathway. Compared with PAO1 infection, deficiency in cGAS or STING also inhibited p-IRF3 in THP-1 cells (Figure 1I) and type I IFN response in BMDMs (Figures 1J–1L) under K. pneumoniae or S. aureus infection. Reduced type I IFN response was also noted in STING siRNA-transfected BMDMs (Figures S3A and S3B) under K. pneumoniae or S. aureus infection and IRF3 siRNA-transfected RAW264.7 cells under PAO1 infection or ISD transfection (Figures S3C and S3D). Therefore, these results indicate that extracellular pathogens P. aeruginosa, K. pneumoniae, and S. aureus are capable of activating type I IFN response in macrophages in a cGAS-pathway-dependent manner.
cGAS is required for sensing P. aeruginosa-derived DNA
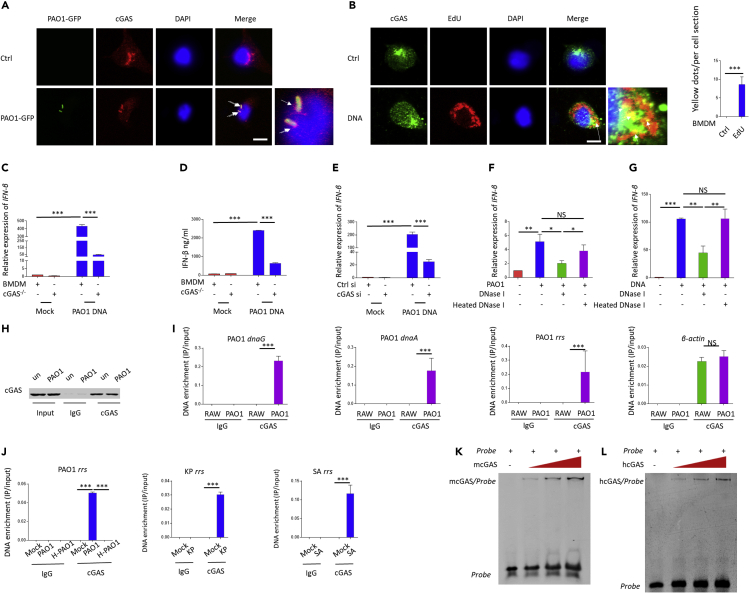
Next, we set out to perform immunofluorescence assay to elucidate whether the DNA sensor cGAS directly senses P. aeruginosa or its genomic DNA. Immunofluorescence assay revealed the colocalization between cGAS and PAO1-GFP in macrophages (Figure 2A). Colocalization between cGAS and PAO1 genomic DNA, which was pre-labeled with a thymidine analog 5-ethynyl-2′-deoxyuridine, was also observed in the BMDM cytoplasm (Figure 2B). To further confirm the role of DNA in activating cGAS under P. aeruginosa infection, P. aeruginosa genomic DNA was isolated, and we found that P. aeruginosa genomic DNA transfection is capable of activating type I IFN response which was abolished in cGAS siRNA-transfected RAW264.7 cells and cGAS−/− BMDMs (Figures 2C–2E). Critically, DNase pretreatments were found to block the type I IFN response induced by PAO1 infection and genomic DNA transfection, respectively (Figures 2F and 2G), indicating that direct contact of bacterial DNA is critical to initiate host defense via cGAS sensing. We next used co-immunoprecipitation (Co-IP) to determine whether cGAS could bind PAO1 genomic DNA directly during infection. RAW264.7 cells infected with PAO1 were immunoprecipitated with cGAS antibody (Figure 2H). The abundance of several PAO1 genes including dnaA, dnaG, and rrs, which are important for bacterial DNA replication, was measured by quantitative polymerase chain reaction (qPCR). Interestingly, we observed that these DNA sequences were significantly enriched by cGAS in infected cells compared to the uninfected controls (Figure 2I). In contrast, no significant change in the abundance of host-derived DNA sequence of β-actin was observed (Figure 2I). Additional data indicated that cGAS is capable of binding PAO1, K. pneumoniae, and S. aureus-derived DNA in THP-1 cells, while cGAS cannot bind PAO1 DNA under heat-killed PAO1 infection (Figure 2J). To further investigate the direct binding affinity of cGAS and PAO1-derived DNA, we then purified His-tagged mcGAS protein using pet-28a expression system. PAO1 rrs probe was obtained by PCR. cGAS was shown to bind the probe as determined by an electrophoretic molecular shift assay (EMSA) (Figure 2K). Similar results were also observed using hcGAS protein (Figure 2L). Taken together, these studies illustrate that cGAS is involved in sensing extracellular bacteria-derived DNA under infection.
Figure 2.
cGAS is required for sensing P. aeruginosa-derived DNA
(A) Colocalization of PAO1-GFP (green) with cGAS (red) in BMDMs. Scale bar, 5 μm.
(B) Colocalization of PAO1 genomic DNA (red) with cGAS (green) in BMDMs. Scale bar, 5 μm.
(C) WT and cGAS−/− BMDMs were transfected with PAO1 genomic DNA for 4 h. qPCR was performed to measure mRNA levels of IFN-β.
(D) WT and cGAS−/− BMDMs were transfected with PAO1 genomic DNA for 4 h. Cytokine levels of IFN-β in the cell culture supernatant were detected using ELISA.
(E) RAW264.7 cells were transfected with control siRNA or cGAS siRNA for 48 h and then transfected with PAO1 genomic DNA for 4 h. mRNA levels of IFN-β were detected.
(F) BMDMs were infected with 10 MOI PAO1 for 4 h with or without DNase I or heated DNase I. qPCR was performed to measure mRNA levels of IFN-β.
(G) BMDMs were transfected with PAO1 DNA with or without DNase I or heated DNase I for 4 h. qPCR was performed to measure mRNA levels of IFN-β.
(H) Immunoblotting of lysates of RAW264.7 cells infected by PAO1 or PBS, then immunoprecipitated with IgG or cGAS antibody, assessed with anti-cGAS. Total cell lysates (TCLs) were applied for immunoblotting analysis as a control.
(I) qPCR of PAO1 genes and mouse β-Actin from DNA samples isolated from cGAS IPs in uninfected or infected RAW264.7 cells. PAO1 gene levels were normalized to inputs.
(J) qPCR of PAO1, K. pneumoniae, or S. aureus genes and human β-actin from DNA samples isolated from cGAS IPs in uninfected or infected THP-1 cells. Gene levels were normalized to inputs.
(K) EMSA for binding of mcGAS to PAO1 rrs probe.
(L) EMSA for binding of hcGAS to PAO1 rrs probe.
ELISA and qPCR data (mean ± SEM) are representative of three independent experiments (∗p ≤ 0.05, ∗∗p ≤ 0.01, ∗∗∗p ≤ 0.001 one-way ANOVA with Tukey post hoc test).
cGAS deficiency impairs the activation of ER stress after P. aeruginosa infection
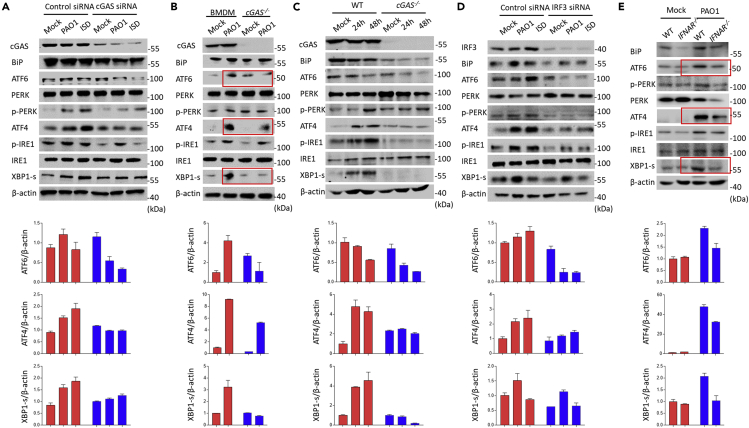
It is known that UPR is involved in innate immunity against P. aeruginosa infection. To unveil the mechanism by which cGAS senses and initiates host immunity against PAO1 infection, we speculate that UPR may be involved in the process as they are linked to the PAO1 infection in previous studies (Richardson et al., 2010; van 't Wout et al., 2015). In agreement with previous studies, PAO1 infection was capable of activating UPR pathway (Figure S4A). Intriguingly, activation of UPR was impaired in cGAS siRNA-transfected RAW264.7 cells in response to infection of PAO1 and transfection of ISD (Figure 3A). cGAS deficiency decreased the transcription of BiP, ATF4, and XBP1-S (Figures S4B–S4D). To further define the biological relevance, we next investigated the role of cGAS in primary BMDMs and lung samples isolated from WT and cGAS−/− mice. cGAS−/− BMDMs and lung samples showed reduced UPR after PAO1 infection (Figures 3B and 3C). Furthermore, a similar immunoblotting pattern was observed in IRF3 siRNA-treated RAW264.7 cells (Figure 3D). Furthermore, we found that activation of UPR following PAO1 infection was significantly blocked in the lung of IFNAR−/− mice (Figure 3E). Together, cGAS impacts ER stress through sensing PAO1.
Figure 3.
cGAS deficiency impairs the activation of ER stress induced by P. aeruginosa infection
(A) RAW264.7 cells were transfected with control siRNA or cGAS siRNA and then treated with 10 MOI PAO1 or ISD for 4 h. Immunoblotting analysis of UPR signaling pathways.
(B) WT and cGAS−/− BMDMs were treated with 10 MOI PAO1 or ISD for 4 h. Immunoblotting analysis of UPR signaling pathways.
(C) WT and cGAS−/− mice were infected with PAO1 for 24 or 48 h. Immunoblotting analysis of UPR signaling pathways in mouse lung samples.
(D) RAW264.7 cells were transfected with control siRNA or IRF3 siRNA and then treated with 10 MOI PAO1 or ISD for 4 h. Immunoblotting analysis of UPR signaling pathways.
(E) WT and IFNAR−/− mice were infected with PAO1 for 48 h. Immunoblotting analysis of UPR signaling pathways in mouse lung samples.
ELISA and qPCR data (mean ± SEM) are representative of three independent experiments (∗p ≤ 0.05, ∗∗p ≤ 0.01, ∗∗∗p ≤ 0.001 one-way ANOVA with Tukey post hoc test).
cGAS-IRF3-IFN axis is required for P. aeruginosa-induced unfolded protein response
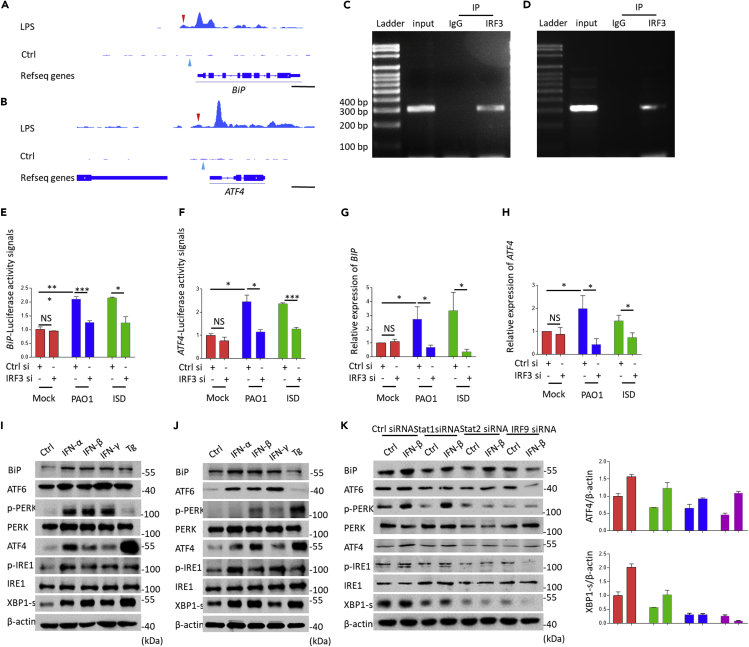
Although cGAS is important for mediating PAO1-induced UPR, the detailed mechanism is still unclear. In view of that cGAS is dependent on IRF3-modulated downstream signals, we hypothesized that IRF3, which is known as the dominant transcription factor for induction of numerous genes (Yanai et al., 2012), may be involved in the process of UPR activation. To elaborate the role of IRF3 in modulating UPR, we performed analysis of the public chromatin immunoprecipitation sequencing (ChIP-seq) data set using bioinformatics approaches. Enriched IRF3 binding sites were found in the promoter region of BiP (−700 to −400) and ATF4 (−590 to −290) (Figures 4A and 4B). To this end, we posit that transcription of BiP and ATF4 may be regulated by IRF3. To assess the binding affinity of IRF3 to BiP and ATF4 promoters, we performed ChIP assay with IRF3-specific antibody and normal rabbit IgG as a control under PAO1 infection. Our ChIP-PCR data showed that the promoter regions of BiP and ATF4 were significantly enriched in the IRF3 antibody group but not in the IgG isotype group, providing direct proof of the recruitment of IRF3 to BiP and ATF4 promoters (Figures 4C and 4D). To further assess the molecule mechanism of IRF3-mediated transcription of BiP and ATF4, we constructed luciferase vectors containing BiP and ATF4 promoters. Our analysis showed that luciferase expression in RAW264.7 cells was inhibited in IRF3 siRNA-treated groups (Figure 4E, 4F and S5A), indicating that mRNA transcription of BiP and ATF4 was regulated by IRF3. We also noted reduced expression of BiP and ATF4 in IRF3 siRNA-transfected RAW264.7 cells compared to siNC-transfected RAW264.7 cells (Figures 4G and 4H). Comparable results were also observed in STING KO RAW264.7 cells (Figures S5B–S5D). Mechanistically, we delineate that transcription of UPR-related molecules BiP and ATF4 was enhanced by IRF3 by directly binding to their promoters.
Figure 4.
cGAS-IRF3-IFN axis is required for P. aeruginosa-induced unfolded protein response
(A and B) Chromatin immunoprecipitation sequencing (ChIP-seq) for IRF3 binding in BiP, ATF4 normalized by input. Red arrows indicate the IRF3 binding region. Blue arrows indicate transcription start site (TSS) of BiP or ATF4. Scale bar, 1000 bp.
(C and D) ChIP-qPCR detecting the BiP or ATF4 promoters in BMDMs.
(E and F) Control siRNA or IRF3 siRNA-treated RAW264.7 cells were pre-transfected with BiP or ATF4 luciferase vectors for 24 h and then treated with 10 MOI PAO1 or ISD for 4 h. Luciferase activity was detected using Dual-Luciferase Reporter Assay System.
(G and H) RAW264.7 cells were transfected with control siRNA or IRF3 siRNA and then treated with 10 MOI PAO1 or ISD for 4 h. qPCR measured mRNA levels of BiP or ATF4.
(I) RAW264.7 cells were treated with IFN-α, IFN-β, IFN-γ, and Tg for 6 h. Immunoblotting analysis of UPR signaling pathways.
(J) BMDMs were treated with IFN-α, IFN-β, IFN-γ, and Tg for 6 h. Immunoblotting analysis of UPR signaling pathways.
(K) RAW264.7 cells were transfected with control siRNA, STAT1 siRNA, STAT2 siRNA, or IRF9 siRNA and then treated with IFN-β for 6 h. Immunoblotting analysis of UPR signaling pathways.
ELISA and qPCR data (mean ± SEM) are representative of three independent experiments (∗p ≤ 0.05, ∗∗p ≤ 0.01, ∗∗∗p ≤ 0.001 one-way ANOVA with Tukey post hoc test).
Now that type I IFNs are downstream of cGAS-STING, we next investigated whether type I IFNs, especially IFN-α and IFN-β, influence the activation of UPR. We found that the ER chaperone protein BiP was strongly induced in response to type I IFNs treatment in both BMDMs and RAW264.7 cells (Figures 4I, 4J, and S6A–S6D). Critically, immunoblotting also showed that type I IFN treatment not only activated ATF4 pathway but also induced additional signaling pathways in a time-dependent manner (Figures S6D, 4I, and 4J). Moreover, we showed that IFN-γ also activated all 3 UPR pathways similar to type I IFNs (Figures S6E, S6F, 4I, and 4J). Our data also suggest that ATF6, ATF4, and XBP1 were activated and translocated into the nucleus after IFN treatment, which was comparable to Tg treatment (Figure S6G). We then used siRNA to knock down STAT1, STAT2, and IRF9, respectively (Figure S6H). Immunoblotting analysis showed that silencing of STAT1, STAT2, and IRF9 significantly blocked IFN-induced UPR (Figure 4K). To further elucidate whether interferon-stimulated gene factor 3 (ISGF3) may function in modulating the UPR directly, we analyzed STAT1-ChIP-seq data and noticed that there was no binding affinity between STAT1 to BiP and ATF4 promoters (Figures S6I and S6J), indicating that ISGF3 may not directly regulate the transcription of BiP and ATF4. Although the detailed mechanism remains to be studied, these data confirm that type I IFNs play important roles in modulating UPR.
cGAS protects against P. aeruginosa in pulmonary infection
Increasing evidence supports that cGAS is involved in intracellular bacterial infection both in vitro and in vivo (Hansen et al., 2014; Wassermann et al., 2015; Watson et al., 2015). The cGAS signaling pathway is also important for restricting infection of multiple intracellular pathogens to maintain host homeostasis. To elaborate the protective role of cGAS in extracellular bacterial infection in vivo, an acute pulmonary infection model was established through intranasal instillation of 0.5 × 107 CFU PAO1 in C57BL/6J WT, cGAS−/− and STING−/− mice. We noticed that PAO1-infected cGAS−/− and STING−/− mice exhibited increased lethality (Figures 5A and 5B) and increased bacterial burdens compared to WT mice (Figures S7A and S7B). To further define how cGAS functions in limiting PAO1 pathogenesis, lung pathological changes were detected in both cGAS−/− and STING−/− mice. Lungs of cGAS−/− STING−/− mice showed severe tissue injury compared to WT mice (Figure 5C). Additionally, infection of PAO1 in cGAS−/− mice led to decreased type I IFN levels compared to WT mice, indicating that IFN pathways were partially blocked (Figures 5D and 5E). cGAS−/− and STING−/− mice also showed higher production of non-type I IFN inflammatory cytokines than those in WT mice (Figures 5F and 5G). Moreover, IFNAR−/− mice also exhibited severe lung injury compared to WT mice (Figure 5C). Collectively, these findings affirm that cGAS has an essential role in defending against extracellular pathogens such as P. aeruginosa in the acute pulmonary infection model.
Figure 5.
cGAS protects mice against P. aeruginosa in pulmonary infection
(A) C57BL/6J WT and cGAS−/− mice were challenged with 0.5 × 107 CFU PAO1. Survival of mice was monitored up to 7 days. Kaplan-Meier survival curves were obtained using GraphPad. p = 0.0242.
(B) C57BL/6J WT and STING−/− mice were challenged with 0.5 × 107 CFU PAO1. Survival of mice was monitored up to 8 days. Kaplan-Meier survival curves were obtained using GraphPad. p = 0.0273.
(C) Pathology of WT, cGAS−/−, STING−/−, and IFNAR−/− mice in response to PAO1 infection. H&E staining of lung sections from mice. Original magnification ×200.
(D) ELISA analyzes cytokine levels of IFN-α in WT and cGAS−/− mice lung.
(E) ELISA analyzes cytokine levels of TNF-α, IL-1β, and IL-6 in in WT and cGAS−/− mice lung BALF.
(F) ELISA analyzes cytokine levels of IFN-β in WT and cGAS−/− mice BALF, lung, and blood.
(G) ELISA analyzes cytokine levels of TNF-α and IL-1β in WT and STING−/− mice lung.
ELISA and qPCR data (mean ± SEM) are representative of three independent experiments (∗p ≤ 0.05, ∗∗p ≤ 0.01, ∗∗∗p ≤ 0.001 one-way ANOVA with Tukey post hoc test).
Discussion
Viral and intracellular bacterial pathogens are well established to be sensed by cGAS-mediated immune defense (Hansen et al., 2014; Lahaye et al., 2013; Ma et al., 2015; Wassermann et al., 2015; Watson et al., 2015). Although a previous study has confirmed that group B streptococcus promoted the activation of cGAS (Andrade et al., 2016), the entire cGAS sensing pathways and molecular mechanisms have not been postulated and confirmed systematically in sensing extracellular bacteria. Here, our data demonstrate systemically the key role of cGAS signaling pathway in sensing extracellular bacterium P. aeruginosa invasion.
While P. aeruginosa is early considered an extracellular bacterium (Sadikot et al., 2005), multiple studies confirmed the penetration of P. aeruginosa into host cells, which has been maintained over the course of P. aeruginosa evolution. P. aeruginosa may be able to enter nonphagocytic cells, which relies on the interaction between T6SS effector VgrG2b and γTuRC (Chi et al., 1991; Sana et al., 2012, 2015). After injection into cells, invading P. aeruginosa may be sensed by DNA sensor cGAS directly via targeting P. aeruginosa genomic DNA, which was supported by our cGAS Co-IP assay. Additionally, our immunofluorescence data also indicated the significant colocalization between cGAS and P. aeruginosa genomic DNA, despite not overwhelming, suggesting that the process of signaling is dynamic and spatial temporarily imbalanced. Additionally, phagocytosis inhibitors blocked the activation of TBK1, which indicates that P. aeruginosa internalization may be essential for mediating type I IFN response. However, the detailed mechanism is not clear and warrants further investigation whether P. aeruginosa internalization effectors and T6SS are involved in cGAS activation. Based on these studies, we suggest that P. aeruginosa be considered a unique quasi extracellular bacterium. It is worth mentioning that outer membrane vesicles (OMVs) derived from multiple gram-negative bacteria are important in host-pathogen interactions and also contain nucleic acids (Bitto et al., 2017). Hence, it is interesting to further investigate the interaction of OMVs and cGAS signal pathway. Additionally, release of mtDNA was confirmed capable of activating cGAS pathway under several microbe infection models (Aguirre et al., 2017; McArthur et al., 2018; Sun et al., 2017; Zheng et al., 2018). Though our evidence suggests that cGAS binds to bacterial DNA during P. aeruginosa infection, we cannot rule out the involvement of other host DNA agonists, like mtDNA.
Apart from cGAS, many pattern recognition receptors (PRRs) were reported to be involved in type I IFN response, such as membrane and endosome Toll-like receptors (TLRs), cytoplasmic RNA sensor RIG-I and MDA5, and novel nuclear DNA sensor hnRNPA2B1 (Anthoney et al., 2018; Wang et al., 2019; Wu and Chen, 2014). Of the various PRRs, TLRs were the most characterized to be involved in detecting bacterial infection. It is known that membrane TLR2 mainly functions by sensing gram-positive bacterial diacylated lipopeptides, while TLR4 recognizes the gram-negative bacterial LPS, and TLR5 senses bacterial flagellin. Endosomal TLR9 serves as cellular PRRs by detecting bacterial unmethylated CpG-containing DNA, whereas TLR3 and TLR8 are involved in responding to double-stranded RNA and single-stranded RNA, respectively. These studies support that the sensing pathways for TLRs may be extremely heterogenic and varied with pathogen strains and species, as well as their particular components. Hence, we propose that the recognition of pathogens may be not solely dependent on one pathway, complementary and somewhat redundant mechanisms may serve to strengthen the innate immunity to achieve robust and dynamic response. Here, we also found that type I IFN response induced by P. aeruginosa infection is significantly but only partially blocked in cGAS−/− mice and cells, supporting that cGAS and TLR (TLR4 or TLR9)/MYD88 pathways may also be involved in host defense together against P. aeruginosa by inducing type I IFN production (Huang et al., 2005; Parker et al., 2012).
P. aeruginosa is shown capable of activating UPR, which is reported to be essential for host defense against P. aeruginosa infection (Richardson et al., 2010; van 't Wout et al., 2015). To date, different studies demonstrate that activation of ER stress promotes the type I IFN response mediated by STING (Liu et al., 2012; Petrasek et al., 2013). Here, we observed that P. aeruginosa infection-induced UPR is associated with the cGAS signaling pathway, supporting the data that STING is shown to influence UPR (Guimaraes et al., 2019; Moretti et al., 2017; Wu et al., 2019). Additionally, we reveal that cGAS was necessary for inducing UPR in an IRF3-dependent fashion as a potentially upstream regulator of UPR. IRF3 was shown to interact with BiP and ATF4 promoters using ChIP-seq analysis. We then reasoned that type I IFNs may mediate UPR activation under P. aeruginosa infection. We noticed that type I IFNs were able to activate UPR. Furthermore, IFNAR−/− deficiency blocked the activation of UPR in response to P. aeruginosa infection in mice.
It is well accepted that type I IFN is key in antiviral replication. However, the interaction of type I IFNs with bacteria seems somewhat pleiotropic and unpredictable (Boxx and Cheng, 2016; Manzanillo et al., 2012; Yamashiro et al., 2020). Here, by using multiple KO mice lacking cGAS or its related signals, we clearly identified an important role of cGAS signaling pathway against P. aeruginosa infection. Critically, we revealed that cGAS−/− and STING−/− mice exhibited heightened mortality, severe lung injury, impaired type I IFNs, and increased inflammatory cytokines in lungs compared to WT mice. In particular, IFNAR−/−associated lung injury, as well as cGAS and STING KO phenotypes, also reflects that the cGAS-STING-IFN axis is critical for host defense in extracellular pathogens, supporting the role of type I IFNs in restricting the attack of P. aeruginosa infection (Carrigan et al., 2010; Parker et al., 2012; Parker and Prince, 2011).
The cGAS signaling pathway was shown to positively modulate inflammatory response through NF-κB. However, STING has been previously shown as an anti-inflammatory molecule by inhibiting p38, JNK, ERK, and NF-κB activity in a keratitis model (Chen et al., 2018). Here, our studies further investigated the role of the cGAS-STING axis in inflammatory responses and confirmed that cGAS−/− and STING−/− mice exacerbated enhanced inflammatory response under P. aeruginosa infection, indicating that the cGAS signaling axis acted as a negative regulator of inflammatory function in this particular P. aeruginosa infection mouse model.
In summary, we identified cGAS as a DNA sensor for detecting and restricting extracellular pathogen P. aeruginosa by promoting the production of type I IFNs, which may be a general mechanism in a variety of bacterial infections. IRF3 and type I IFNs are shown to trigger the activation of UPR pathways. Our study expands the list of pathogens to be sensed by cGAS and identified the related mechanism involving UPR in protective processes occurring in both cell and mouse infection models.
Limitations of the study
In this study, we identify the cGAS as an important DNA sensor for recognizing and restricting P. aeruginosa infection. However, we also made a number of interesting observations remaining further investigation. How does P. aeruginosa release or inject its genomic DNA into cell plasma for cGAS recognition? Is it dependent on T6SS? Furthermore, is endosomal DNA receptor TLR9 involved in sensing P. aeruginosa infection? Whether mtDNA is recognized by cGAS under P. aeruginosa infection? Answering those questions is important for understanding DNA sensor and P. aeruginosa interaction, providing a basis for future research.
Resource availability
Lead contact
Min Wu, Department of Biomedical Sciences, University of North Dakota, Grand Forks, North Dakota 58203-9037, USA; email: min.wu@med.und.edu.
Materials availability
All related information or materials generated in this study are available upon request.
Data and code availability
This study did not involve code generation.
Methods
All methods can be found in the accompanying Transparent methods supplemental file.
Acknowledgments
This project was supported by National Institutes of Health of USA 364 (R01 AI109317-04 and R01 AI138203-01 to M.W.). We thank UND imaging core for confocal imaging supported by NIH grants INBRE P20GM103442 and P20GM113123. We also thank Dr. H.W. Virgin and Dr. Charles Rice for kindly providing cGAS−/− mice.
Author contributions
C.M.Z. performed the experiment, data analysis, and wrote the manuscript; B.W., P.L., Q.W., S.G.Q., and Q.Q.P. assisted in data analysis and some experiments; M.W. and C.M.Z. conceived of the study and revised the manuscript; X.J.Y. revised the manuscript.
Declaration of interests
The authors declare no competing interests.
Published: January 22, 2021
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.isci.2020.101928.
Contributor Information
Xue-jie Yu, Email: yuxuejie@whu.edu.cn.
Min Wu, Email: min.wu@und.edu.
Supplemental information
References
- Aguirre S., Luthra P., Sanchez-Aparicio M.T., Maestre A.M., Patel J., Lamothe F., Fredericks A.C., Tripathi S., Zhu T., Pintado-Silva J. Dengue virus NS2B protein targets cGAS for degradation and prevents mitochondrial DNA sensing during infection. Nat. Microbiol. 2017;2:17037. doi: 10.1038/nmicrobiol.2017.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrade W.A., Firon A., Schmidt T., Hornung V., Fitzgerald K.A., Kurt-Jones E.A., Trieu-Cuot P., Golenbock D.T., Kaminski P.A. Group B Streptococcus degrades cyclic-di-AMP to modulate STING-dependent type I interferon production. Cell Host Microbe. 2016;20:49–59. doi: 10.1016/j.chom.2016.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anthoney N., Foldi I., Hidalgo A. Toll and Toll-like receptor signalling in development. Development. 2018;145:dev156018. doi: 10.1242/dev.156018. [DOI] [PubMed] [Google Scholar]
- Barbalat R., Ewald S.E., Mouchess M.L., Barton G.M. Nucleic acid recognition by the innate immune system. Annu. Rev. Immunol. 2011;29:185–214. doi: 10.1146/annurev-immunol-031210-101340. [DOI] [PubMed] [Google Scholar]
- Bettigole S.E., Glimcher L.H. Endoplasmic reticulum stress in immunity. Annu. Rev. Immunol. 2015;33:107–138. doi: 10.1146/annurev-immunol-032414-112116. [DOI] [PubMed] [Google Scholar]
- Bitto N.J., Chapman R., Pidot S., Costin A., Lo C., Choi J., D'Cruze T., Reynolds E.C., Dashper S.G., Turnbull L. Bacterial membrane vesicles transport their DNA cargo into host cells. Sci. Rep. 2017;7:7072. doi: 10.1038/s41598-017-07288-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boxx G.M., Cheng G. The roles of type I interferon in bacterial infection. Cell Host Microbe. 2016;19:760–769. doi: 10.1016/j.chom.2016.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cachia P.J., Hodges R.S. Synthetic peptide vaccine and antibody therapeutic development: prevention and treatment of Pseudomonas aeruginosa. Biopolymers. 2003;71:141–168. doi: 10.1002/bip.10395. [DOI] [PubMed] [Google Scholar]
- Carrigan S.O., Junkins R., Yang Y.J., Macneil A., Richardson C., Johnston B., Lin T.J. IFN regulatory factor 3 contributes to the host response during Pseudomonas aeruginosa lung infection in mice. J. Immunol. 2010;185:3602–3609. doi: 10.4049/jimmunol.0903429. [DOI] [PubMed] [Google Scholar]
- Chen K., Fu Q., Liang S., Liu Y., Qu W., Wu Y., Wu X., Wei L., Wang Y., Xiong Y. Stimulator of interferon genes promotes host resistance against Pseudomonas aeruginosa keratitis. Front. Immunol. 2018;9:1225. doi: 10.3389/fimmu.2018.01225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi E., Mehl T., Nunn D., Lory S. Interaction of Pseudomonas aeruginosa with A549 pneumocyte cells. Infect. Immun. 1991;59:822–828. doi: 10.1128/iai.59.3.822-828.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curran C.S., Bolig T., Torabi-Parizi P. Mechanisms and targeted therapies for Pseudomonas aeruginosa lung infection. Am. J. Respir. Crit. Care Med. 2018;197:708–727. doi: 10.1164/rccm.201705-1043SO. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao D., Wu J., Wu Y.T., Du F., Aroh C., Yan N., Sun L., Chen Z.J. Cyclic GMP-AMP synthase is an innate immune sensor of HIV and other retroviruses. Science. 2013;341:903–906. doi: 10.1126/science.1240933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grootjans J., Kaser A., Kaufman R.J., Blumberg R.S. The unfolded protein response in immunity and inflammation. Nat. Rev. Immunol. 2016;16:469–484. doi: 10.1038/nri.2016.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guimaraes E.S., Gomes M.T.R., Campos P.C., Mansur D.S., Dos Santos A.A., Harms J., Splitter G., Smith J.A., Barber G.N., Oliveira S.C. Brucella abortus cyclic dinucleotides trigger STING-dependent unfolded protein response that favors bacterial replication. J. Immunol. 2019;202:2671–2681. doi: 10.4049/jimmunol.1801233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen K., Prabakaran T., Laustsen A., Jorgensen S.E., Rahbaek S.H., Jensen S.B., Nielsen R., Leber J.H., Decker T., Horan K.A. Listeria monocytogenes induces IFNbeta expression through an IFI16-, cGAS- and STING-dependent pathway. EMBO J. 2014;33:1654–1666. doi: 10.15252/embj.201488029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X., Barrett R.P., McClellan S.A., Hazlett L.D. Silencing Toll-like receptor-9 in Pseudomonas aeruginosa keratitis. Invest. Ophthalmol. Vis. Sci. 2005;46:4209–4216. doi: 10.1167/iovs.05-0185. [DOI] [PubMed] [Google Scholar]
- Lahaye X., Satoh T., Gentili M., Cerboni S., Conrad C., Hurbain I., El Marjou A., Lacabaratz C., Lelievre J.D., Manel N. The capsids of HIV-1 and HIV-2 determine immune detection of the viral cDNA by the innate sensor cGAS in dendritic cells. Immunity. 2013;39:1132–1142. doi: 10.1016/j.immuni.2013.11.002. [DOI] [PubMed] [Google Scholar]
- Li X., Shu C., Yi G., Chaton C.T., Shelton C.L., Diao J., Zuo X., Kao C.C., Herr A.B., Li P. Cyclic GMP-AMP synthase is activated by double-stranded DNA-induced oligomerization. Immunity. 2013;39:1019–1031. doi: 10.1016/j.immuni.2013.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y.P., Zeng L., Tian A., Bomkamp A., Rivera D., Gutman D., Barber G.N., Olson J.K., Smith J.A. Endoplasmic reticulum stress regulates the innate immunity critical transcription factor IRF3. J. Immunol. 2012;189:4630–4639. doi: 10.4049/jimmunol.1102737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovewell R.R., Patankar Y.R., Berwin B. Mechanisms of phagocytosis and host clearance of Pseudomonas aeruginosa. Am. J. Physiol. Lung Cell. Mol. Physiol. 2014;306:L591–L603. doi: 10.1152/ajplung.00335.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Z., Jacobs S.R., West J.A., Stopford C., Zhang Z., Davis Z., Barber G.N., Glaunsinger B.A., Dittmer D.P., Damania B. Modulation of the cGAS-STING DNA sensing pathway by gammaherpesviruses. Proc. Natl. Acad. Sci. U S A. 2015;112:E4306–E4315. doi: 10.1073/pnas.1503831112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manzanillo P.S., Shiloh M.U., Portnoy D.A., Cox J.S. Mycobacterium tuberculosis activates the DNA-dependent cytosolic surveillance pathway within macrophages. Cell Host Microbe. 2012;11:469–480. doi: 10.1016/j.chom.2012.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McArthur K., Whitehead L.W., Heddleston J.M., Li L., Padman B.S., Oorschot V., Geoghegan N.D., Chappaz S., Davidson S., San Chin H. BAK/BAX macropores facilitate mitochondrial herniation and mtDNA efflux during apoptosis. Science. 2018;359:eaao6047. doi: 10.1126/science.aao6047. [DOI] [PubMed] [Google Scholar]
- Miyoshi-Akiyama T., Tada T., Ohmagari N., Viet Hung N., Tharavichitkul P., Pokhrel B.M., Gniadkowski M., Shimojima M., Kirikae T. Emergence and spread of epidemic multidrug-resistant Pseudomonas aeruginosa. Genome Biol. Evol. 2017;9:3238–3245. doi: 10.1093/gbe/evx243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moretti J., Roy S., Bozec D., Martinez J., Chapman J.R., Ueberheide B., Lamming D.W., Chen Z.J., Horng T., Yeretssian G. STING senses microbial viability to orchestrate stress-mediated autophagy of the endoplasmic reticulum. Cell. 2017;171:809–823.e3. doi: 10.1016/j.cell.2017.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang Z., Raudonis R., Glick B.R., Lin T.J., Cheng Z. Antibiotic resistance in Pseudomonas aeruginosa: mechanisms and alternative therapeutic strategies. Biotechnol. Adv. 2019;37:177–192. doi: 10.1016/j.biotechadv.2018.11.013. [DOI] [PubMed] [Google Scholar]
- Parker D., Cohen T.S., Alhede M., Harfenist B.S., Martin F.J., Prince A. Induction of type I interferon signaling by Pseudomonas aeruginosa is diminished in cystic fibrosis epithelial cells. Am. J. Respir. Cell Mol. Biol. 2012;46:6–13. doi: 10.1165/rcmb.2011-0080OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker D., Prince A. Type I interferon response to extracellular bacteria in the airway epithelium. Trends Immunol. 2011;32:582–588. doi: 10.1016/j.it.2011.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrasek J., Iracheta-Vellve A., Csak T., Satishchandran A., Kodys K., Kurt-Jones E.A., Fitzgerald K.A., Szabo G. STING-IRF3 pathway links endoplasmic reticulum stress with hepatocyte apoptosis in early alcoholic liver disease. Proc. Natl. Acad. Sci. U S A. 2013;110:16544–16549. doi: 10.1073/pnas.1308331110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson C.E., Kooistra T., Kim D.H. An essential role for XBP-1 in host protection against immune activation in C. elegans. Nature. 2010;463:1092–1095. doi: 10.1038/nature08762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadikot R.T., Blackwell T.S., Christman J.W., Prince A.S. Pathogen-host interactions in Pseudomonas aeruginosa pneumonia. Am. J. Respir. Crit. Care Med. 2005;171:1209–1223. doi: 10.1164/rccm.200408-1044SO. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sana T.G., Baumann C., Merdes A., Soscia C., Rattei T., Hachani A., Jones C., Bennett K.L., Filloux A., Superti-Furga G. Internalization of Pseudomonas aeruginosa strain PAO1 into epithelial cells is promoted by interaction of a T6SS effector with the microtubule network. mBio. 2015;6:e00712. doi: 10.1128/mBio.00712-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sana T.G., Hachani A., Bucior I., Soscia C., Garvis S., Termine E., Engel J., Filloux A., Bleves S. The second type VI secretion system of Pseudomonas aeruginosa strain PAO1 is regulated by quorum sensing and Fur and modulates internalization in epithelial cells. J. Biol. Chem. 2012;287:27095–27105. doi: 10.1074/jbc.M112.376368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun B., Sundstrom K.B., Chew J.J., Bist P., Gan E.S., Tan H.C., Goh K.C., Chawla T., Tang C.K., Ooi E.E. Dengue virus activates cGAS through the release of mitochondrial DNA. Sci. Rep. 2017;7:3594. doi: 10.1038/s41598-017-03932-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun L., Wu J., Du F., Chen X., Chen Z.J. Cyclic GMP-AMP synthase is a cytosolic DNA sensor that activates the type I interferon pathway. Science. 2013;339:786–791. doi: 10.1126/science.1232458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van 't Wout E.F., van Schadewijk A., van Boxtel R., Dalton L.E., Clarke H.J., Tommassen J., Marciniak S.J., Hiemstra P.S. Virulence factors of Pseudomonas aeruginosa induce both the unfolded protein and integrated stress responses in airway epithelial cells. PLoS Pathog. 2015;11:e1004946. doi: 10.1371/journal.ppat.1004946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L., Wen M., Cao X. Nuclear hnRNPA2B1 initiates and amplifies the innate immune response to DNA viruses. Science. 2019;365:eaav0758. doi: 10.1126/science.aav0758. [DOI] [PubMed] [Google Scholar]
- Wassermann R., Gulen M.F., Sala C., Perin S.G., Lou Y., Rybniker J., Schmid-Burgk J.L., Schmidt T., Hornung V., Cole S.T. Mycobacterium tuberculosis differentially activates cGAS- and inflammasome-dependent intracellular immune responses through ESX-1. Cell Host Microbe. 2015;17:799–810. doi: 10.1016/j.chom.2015.05.003. [DOI] [PubMed] [Google Scholar]
- Watson R.O., Bell S.L., MacDuff D.A., Kimmey J.M., Diner E.J., Olivas J., Vance R.E., Stallings C.L., Virgin H.W., Cox J.S. The cytosolic sensor cGAS detects Mycobacterium tuberculosis DNA to induce type I interferons and activate autophagy. Cell Host Microbe. 2015;17:811–819. doi: 10.1016/j.chom.2015.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J., Chen Y.J., Dobbs N., Sakai T., Liou J., Miner J.J., Yan N. STING-mediated disruption of calcium homeostasis chronically activates ER stress and primes T cell death. J. Exp. Med. 2019;216:867–883. doi: 10.1084/jem.20182192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J., Chen Z.J. Innate immune sensing and signaling of cytosolic nucleic acids. Annu. Rev. Immunol. 2014;32:461–488. doi: 10.1146/annurev-immunol-032713-120156. [DOI] [PubMed] [Google Scholar]
- Wu J., Sun L., Chen X., Du F., Shi H., Chen C., Chen Z.J. Cyclic GMP-AMP is an endogenous second messenger in innate immune signaling by cytosolic DNA. Science. 2013;339:826–830. doi: 10.1126/science.1229963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashiro L.H., Wilson S.C., Morrison H.M., Karalis V., Chung J.J., Chen K.J., Bateup H.S., Szpara M.L., Lee A.Y., Cox J.S. Interferon-independent STING signaling promotes resistance to HSV-1 in vivo. Nat. Commun. 2020;11:3382. doi: 10.1038/s41467-020-17156-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanai H., Negishi H., Taniguchi T. The IRF family of transcription factors: inception, impact and implications in oncogenesis. Oncoimmunology. 2012;1:1376–1386. doi: 10.4161/onci.22475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y., Liu Q., Wu Y., Ma L., Zhang Z., Liu T., Jin S., She Y., Li Y.P., Cui J. Zika virus elicits inflammation to evade antiviral response by cleaving cGAS via NS1-caspase-1 axis. EMBO J. 2018;37:e99347. doi: 10.15252/embj.201899347. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
This study did not involve code generation.