Abstract
Dietary supplementation of green tea changes the antioxidative capacity of chickens. However, the effect of green tea supplementation in the diet on egg quality and the consequent change in processing capacity is still not well known. The aim of this study was to determine whether green tea powder (GTP) supplementation could affect egg quality, egg antioxidant capacity, and sensory and egg processing characteristics. Huainan partridge chickens (1,080) at 20 wk old were divided into 2 groups, one group fed a basal diet (control) and one group fed a basal diet plus 10 g kgˆ-1 GTP for 12 wk. After the levels of yolk cholesterol had been determined, chickens from the control group were further divided into low- and high-cholesterol groups and were fed a basal diet or a diet with 10 g kgˆ-1 GTP by orthogonal design. After 4 wk, the egg processing characteristics were investigated. Egg specific gravity, shell strength, shell thickness, albumin height, Haugh unit (HU) and cholesterol content were significantly lower in the GTP group than in the control group (P < 0.05). Egg weight, albumin height, yolk color, and HU increased in a time-dependent manner in both the control and GTP groups (P < 0.01). The yolk C16:0, C20:0, C18:1, C18:2, and polyunsaturated fatty acid (PUFA) contents were higher in the GTP group than in the control group (P < 0.05). Egg whites from the GTP group showed increased radical scavenging activity (P < 0.05). Egg appearance and texture from the GTP group were more preferred than those of the control group (P < 0.05). Eggs from the GTP group had lower hardness, chewiness, and water retention capacity than those of eggs from the control group (P < 0.05). Eggs from the GTP group with high yolk cholesterol showed lower chewiness than those from the basal diet group (P < 0.05). The results suggested that GTP supplementation could enrich the PUFA content in egg yolks, improve the overall taste, and change processing characteristics.
Key words: egg quality, green tea powder, consumer taste, fatty acid composition, Huainan partridge chickens
Introduction
Eggs are a kind of animal product that are widely consumed, contain many easily absorbed nutrients, and are easy to digest (Iskender et al., 2017). The nutrients and quality of eggs could be affected by many factors, including genetics, feed composition, age, etc. (Lordelo et al., 2017; Liu et al., 2018). In China, the consumption of eggs from indigenous breeds is preferred by consumers. With intense selection of chickens for egg production, their quality and nutritional properties have become of increasing concern. People's sedentary lifestyles cause them to worry about the risk of cardiovascular disease (Blesso and Fernandez, 2018). Thus, egg producers are trying to develop functional eggs with antioxidant activity, low cholesterol, etc. (Feng et al., 2017). Approximately, 500,000 tons of raw eggs are used for processing each year in China. Therefore, whether functional eggs could affect their processing characteristics is very important for producers.
Green tea is one of the most popular beverages worldwide, and nearly 14,380,000 tons are produced each year in China. Approximately, 5 to 10% green tea powder (GTP) is produced during green tea processing and is generally discarded. The most important components in green tea are polyphenols, including catechins (which constitute approximately 30% of its dry weight), alkaloids, polysaccharides, etc. (Khalesi et al., 2014; Onakpoya et al., 2014). Tea polyphenols are natural antioxidants that can scavenge free radicals and protect cells from damage (Wang et al., 2018). After consuming 2 cups of green tea daily for 42 d, the plasma total antioxidant activity was shown to significantly increased, and plasma peroxides and DNA oxidative damage in lymphocytes significantly decreased (Erba et al., 2005). Green tea can prevent dental caries and reduce cholesterol and lipid absorption in the gastrointestinal tract (Koo and Cho, 2004). Catechins, the major component in tea polyphenols, can decrease the plasma and liver MDA concentrations, as well as serum glucose and total cholesterol levels and have the potential to increase meat quality in fattening quail (Kara et al., 2016a). Xia et al. (2018) suggested that 1% GTP did not affect egg laying and feed conversion, but high amounts of GTP (>2%) decreased egg production performance. Wang et al. (2018) found that tea polyphenol treatment could increase the albumin height and Haugh unit (HU) by maintaining magnum morphology in aged layers. Our previous study also suggested that 1% green tea inclusion could improve meat color and Lactobacillus proliferation in broiler production (Chen et al., 2019).
Although the research mentioned above has reported the effects of green tea on egg-laying hens and broilers, these studies mostly focused on green tea effects on egg or meat production performance. With the increases in processed egg consumption, it is very important to know whether the addition of GTP could change the physical and chemical quality of eggs and whether the changed physical and chemical quality could affect the processing characteristics of eggs. In this experiment, we proposed to test whether green tea incorporation could affect egg quality, yolk fatty acid components, sensory features, and processing characteristics.
Materials and methods
All the experimental protocols involving care, handling, and treatment of broilers were approved by the Institutional Animal Care and Use Committee of Anhui Agricultural University, Hefei, Anhui, China. The permission number is No. SYDW-P2018110702.
Birds and Experimental Design
A total of 1,080 Huainan Partridge hens at their 18 wk of age with similar body weights (1.46 ± 0.13) were assigned to 2 groups. Hens were housed into 30 battery cages (as 30 replicates, 6 tiers, and 6 cages per tier) with one hen per cage, and one row of the battery cages was used as the control group and the other was used as the experimental group. The hens received 13 h light at 20 wk old, which was extended to 16 h light at 32 wk of age. Hens from the control group received a basal diet (without GTP), and hens from the experimental group received a basal diet plus 1% GTP instead of bran. The feed ingredients and their chemical composition are listed in Table 1. The experiment consisted of a 2-wk acclimation period and a 12-wk collection period. Thirty eggs from each group (one egg per replicate) with 2-wk interval were collected to determine the egg quality, egg white antioxidant activity, and yolk fatty acid content. Another 60 eggs from each group (2 eggs per replicate) at 24, 28, and 32 wk of age were used in a panel for a sensory quality test.
Table 1.
Feed ingredients and nutrient composition.
| Composition % | Group |
|
|---|---|---|
| Control group (Con) | Experimental group (GTP) | |
| Soybean | 22.40 | 22.40 |
| Corn | 66 | 66 |
| Bran | 4.50 | 3.50 |
| Lime powder | 2 | 2 |
| Premix1 | 5 | 5 |
| Green tea powder | 1 | |
| Nutritional level | ||
| Crude fat % | 4.67 | 4.96 |
| Total energy MJ/kg | 13.07 | 12.99 |
| Crude protein % | 16.49 | 16.48 |
| Ca % | 2.0–3.2 | 2.0–3.2 |
Premix provided per kg of diet: Fe, 65 mg; Cu, 8 mg; Zn, 80 mg; Mn, 105 mg; I, 1 mg; Se, 0.3 mg; vitamin A, 9,800 IU; vitamin D3, 3,100 IU; vitamin E, 26 IU; vitamin B1, 2.5 mg; vitamin B2, 7 mg; vitamin B12, 0.018 mg; vitamin K, 2.2 mg; biotin, 0.09 mg; folic acid, 1 mg; pantothenic acid, 11 mg; niacin, 38 mg.
After the egg yolk cholesterol content was determined in the control group, the hens were then divided into 2 groups, low cholesterol content and high cholesterol content groups. Egg processing characteristics were then investigated by an orthogonal array design. That is, hens producing eggs with low egg yolk cholesterol were fed a diet with 1% GTP or a control diet, and hens producing eggs with high egg yolk cholesterol were also fed a diet with 1% GTP or a control diet. After 4 wk of feeding, eggs were collected and evaluated for egg white hardness, cohesiveness, chewiness, gelling properties, springiness, water retention, foaming, and egg yolk emulsification properties. Each index was tested in 10 times as replicates.
Egg Quality and Yolk Cholesterol Measurement
Egg quality was measured within 24 h after collection. A digital scale (0.01 sensitivity) was used to measure the egg weight. An electronic digital caliper was used to measure the egg longitudinal diameter (LE) and transverse diameter (WE), and the egg shape was calculated as WE/LE. The eggshell strength was measured by using an eggshell strength meter (2nd FI; Robotmation Co., Ltd., Japan). Then, the egg was broken onto a flat surface where the height of the inner thick albumen was measured with an electronic albumen height gauge (EA-01; Orka, Israel). The yolk was separated from the albumen and weighed and then stored at −20°C until cholesterol determination. The shell thickness was measured by using a digital vernier caliper (NFN380; Fujihira Industry Co., Ltd., Tokyo, Japan).
Approximately, 0.1 g yolk was exactly measured and placed in a 1.5-mL tube. Nine times the volume of anhydrous ethanol was added to the tube and then mechanically homogenized (50 Hz) in an ice water bath for 30 s. Then, the tube was centrifuged at 2,500 rpm/min for 10 min. The supernatant (2.5 μL) was transferred to a 96-well plate, and 250 μL working solution (50 mmol/L Good's buffer, 5 mmol/L phenol, 0.3 mmol/L 4-AAP, ≥50 KU/L cholesteryl esterase, ≥25 KU/L cholesterol oxidase, and ≥1.3 KU/L peroxidase). The same volume of ddH2O (2.5 μL) was added to replace the sample supernatant as a blank control, and 2.5 μL correction buffer was also used to replace the sample supernatant as a corrected control. The mixed solutions were allowed to stand for 10 min, and the optical density (OD) was measured at a wavelength of 510 nm. The cholesterol content was determined as follows: cholesterol content (mmol/L) = (sample OD − blank OD)/(corrected OD-blank OD) × times of sample dilution.
Egg White Antioxidant Capacity and Color Detection
The antioxidant activity of egg whites was determined by measuring their 1,1-diphenyl-2-picrylhydrazyl free radical scavenging ability (Sun et al., 2014), 2′-Azinobis-(3-ethylbenzothiazoline-6-sulfonate) free radical scavenging ability (Awe et al., 2013), and hydroxyl radical scavenging activity (Sun et al., 2014).
Yolk Fatty Acid Analysis
The yolk fatty acid profile was determined after the homogenized yolk samples were defrosted. A 2 g sample was extracted by using a chloroform:methanol (2:1, v/v) solution in accordance with Folch's method and esterified with methyl alcohol (93%) containing HCl (3%); samples were then applied to a diethyleneglycol succinate column (DB-WAX, 30 m × 0.25 mm × 0.25 μm) and analyzed with a flame ionization detection by gas chromatography (Agilent GC7890A). Peaks were identified by retention time comparison with those of the corresponding standards (Sigma). The content of each fatty acid was calculated by the ratio of the peak area to the total peak area.
Consumer Sensory Analysis
Each egg was longitudinally divided into 3 equal parts. A panel of 50 evaluators (undergraduates, graduates, and faculty and staff) evaluated the sensory qualities of the hard-boiled eggs from each group. Panelists were asked to evaluate the sample's taste, appearance, flavor, and texture using a 9-point hedonic scale (Wang et al., 2019). Acceptability of appearance was defined as liking the product with respect to the panelists' preferences. Acceptability of texture was defined as liking the product with respect to its juiciness. Acceptability of flavor was defined as liking the product with respect to its taste and smell. Each panelist was allowed to evaluate 4 samples with 2 samples from each group. The sample order was randomized. Water was provided to allow the panelists to rinse their mouths between samples.
Texture Profile Analysis of Egg White Gels
The egg white was carefully separated from the yolk and homogenized by gentle stirring. Once the resistivity was low, 15 mL of egg white was transferred into a 25 mL beaker. After being sealed with a film for freshness, the egg white was heated in a water bath (B-220; Shanghai, China) at a constant temperature of 80°C for 45 min and then cooled to room temperature and stored at 4°C for 24 h to allow for gelling. The gel samples were then allowed to reach room temperature before texture profile measurement. A TA-XTplus2 texture analyzer (Texture Technologies, Scarsdale, NY) was used to evaluate the egg white texture profile by using the method described by Walker et al. (2012). The characteristics measured included hardness, cohesiveness, chewiness, gelling properties, and springiness.
Water Retention Capacity
Approximately, 5 g egg white gel was weighed (M1) and centrifuged (5,000 r/min) for 5 min. The gel was weighed again (M0) after being allowed to absorb the surface water. The gel water retention capacity (WR) was calculated as WR = (1− (M1 − M0)/M1) × 100%.
Foaming Properties Measurement
Foaming properties were determined by using the method described by Gouda et al. (2018).
Egg Yolk Emulsification Properties
Egg yolks were diluted to 0.5% (w/v) with 0.5 mol NaCl solution. A total of 24 mL of the diluted solution was mixed with seed oil (Jinlong first grade oil at approximately 4 ppm) at a constant rate of oil addition (5 mL/min). A speed adjustable homogenizer (FSH-2; Jintan, China) continuously mixed the oil at low speed until an apparent phase inversion was observed. The amount of oil added was used to calculate the emulsification capacity (g oil/g yolk).
The same seed oil (16 mL) was added to 24 mL 0.5% (w/v) yolk and mixed with the same homogenizer at 10,000 r/min for 1 min in a beaker. From the resulting emulsion, 20 μL of the emulsion was collected from the bottom of the beaker and transferred into 10 mL centrifuge tubes for 0 min and 6 min, and then 6 mL 0.1% (w/v) SDS solution was added to the emulsions. The absorbance at 500 nm was measured by using a dual-beam ultraviolet–visible spectrophotometer (TU-1900; PERSEE). An aliquot of the 0.1% SDS solution was utilized as the blank control. The absorbance of the emulsion at 0 min was A1, and the absorbance at 6 min was A2. Emulsion stability (ES) was calculated as ES = A1 × 6/A2.
Statistical Analysis
The results from the egg quality, fatty acid composition, consumer sensory perception, egg white functional properties, water retention capacity, and egg yolk emulsification properties analyses were subjected to 2-way ANOVA by SAS 9.3. The time effect on egg quality and fatty acid composition was analyzed by regression analysis. Egg white antioxidant capacity was compared between control and GTP group by Student t-test.
Results
Egg Quality and Cholesterol Content
The egg quality and cholesterol content over different feeding periods are shown in Table 2. No differences were found in egg weight, egg shape index, yolk weight, and yolk color of the egg between the 2 treatment groups (P > 0.05). The egg specific gravity, shell strength, shell thickness, albumin height, HU, and cholesterol content were significantly higher in eggs from the control group than in those from the GTP inclusion group (P < 0.05). Egg weight, albumin height, yolk color, and HU increased with the feeding time of hens in both the control and GTP inclusion groups (P < 0.01). Egg specific gravity and egg shape decreased with feeding time (P < 0.01, linear and quadratic). Egg yolk cholesterol content decreased after 12 wk of feeding, and a significant decreasing effect of feeding time was observed (quadratic effect, P = 0.005). Egg weight from chickens fed diet containing GTP for 4 wk and 10 wk was significantly lower than that of the control group (P < 0.01). Egg specific gravity was lower in the GTP group fed for 4 to 8 wk than in the control group (P < 0.01). Eggshell strength was significantly lower in the GTP group fed for 4, 6, and 12 wk than in the control group (P = 0.002). Eggshell thickness was significantly lower in the GTP group than that of the control group after 4 wk of feeding (P = 0.005).
Table 2.
Effect of green tea powder and feeding time on egg quality.
| Treatment | Feeding time | Egg weight/g | Egg specific gravity g/cm3 | Egg shape index | Eggshell strength/N | Egg yolk/g | Eggshell thickness/mm | Albumin height/mm | Yolk color | Haugh unit | Cholesterol mg/egg |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Con | 0 | 32.08 | 1.131 | 1.303 | 39.62 | 8.45 | 0.348 | 2.45 | 4.7 | 59.20 | 157.9 |
| 2 | 31.94 | 1.129 | 1.356 | 34.51 | 8.56 | 0.347 | 2.87 | 7.1 | 62.41 | 232.8 | |
| 4 | 38.57 | 1.128 | 1.303 | 43.97 | 10.95 | 0.374 | 3.10 | 7.7 | 61.30 | 191.9 | |
| 6 | 43.18 | 1.129 | 1.297 | 42.16 | 12.13 | 0.372 | 3.57 | 8.2 | 61.89 | 197.3 | |
| 8 | 44.49 | 1.126 | 1.258 | 37.96 | 12.72 | 0.376 | 4.00 | 8.0 | 70.28 | 213.9 | |
| 10 | 47.77 | 1.124 | 1.307 | 44.64 | 13.81 | 0.395 | 4.10 | 8.5 | 67.91 | 169.5 | |
| 12 | 46.89 | 1.132 | 1.297 | 47.14 | 14.32 | 0.405 | 4.28 | 7.4 | 67.50 | 164.1 | |
| GTP | 0 | 30.39 | 1.130 | 1.324 | 38.00 | 7.67 | 0.346 | 3.14 | 5.3 | 66.59 | 167.6 |
| 2 | 33.32 | 1.130 | 1.345 | 35.43 | 10.00 | 0.353 | 2.73 | 7.1 | 59.51 | 189.92 | |
| 4 | 36.811 | 1.1202 | 1.315 | 33.202 | 10.52 | 0.3282 | 3.35 | 7.7 | 64.33 | 146.72 | |
| 6 | 42.39 | 1.1192 | 1.279 | 32.772 | 12.11 | 0.3472 | 3.83 | 7.01 | 65.12 | 161.42 | |
| 8 | 44.74 | 1.1132 | 1.278 | 34.86 | 13.20 | 0.362 | 5.32 | 7.6 | 77.922 | 159.42 | |
| 10 | 43.641 | 1.124 | 1.320 | 42.12 | 12.822 | 0.3742 | 4.891 | 7.81 | 75.351 | 149.41 | |
| 12 | 48.13 | 1.129 | 1.315 | 38.681 | 14.40 | 0.3812 | 4.991 | 7.2 | 73.651 | 141.32 | |
| SEM | 0.952 | 0.001 | 0.015 | 1.425 | 0.197 | 0.004 | 0.179 | 0.241 | 1.672 | 4.576 | |
| P-value | |||||||||||
| Treatment | 0.266 | <0.01 | 0.38 | <0.01 | 0.834 | <0.01 | <0.01 | 0.166 | <0.01 | <0.01 | |
| Feeding time | ANOVA | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 |
| Linear | <0.001 | <0.001 | <0.001 | 0.64 | <0.001 | 0.71 | 0.005 | <0.001 | 0.03 | 0.131 | |
| Quadratic | 0.004 | <0.001 | 0.005 | 0.1 | <0.001 | 0.055 | 0.28 | <0.001 | 0.55 | 0.005 | |
| Treatment × feeding time | <0.01 | <0.01 | 0.775 | 0.002 | 0.001 | 0.005 | 0.131 | 0.401 | 0.316 | 0.005 | |
Abbreviations: Con, control; GTP, green tea powder.
Significant differences among groups (P < 0.05).
Significant differences among groups (P < 0.01).
Fatty Acid Content of the Egg Yolk From Chickens Fed GTP and Control Chickens at Different Times
The fatty acid contents of the egg yolk were compared between chickens fed GTP and the control group (Table 3). The contents of C16:0, C20:0, C18:1, and C18:2, monounsaturated fatty acids (MUFA), polyunsaturated fatty acids (PUFA), and total unsaturated fatty acids (TUFA) increased (P < 0.05), whereas C18:0 decreased in egg yolks from chickens fed a diet with GTP compared with those from chickens fed the control diet (P < 0.05). The content of C14:0 in egg yolks significantly increased when fed for 8 wk and then decreased after 8 wk (linear and quadratic, P < 0.05). There was a significant increasing effect of feeding time on the content of C16:0 and C20:0 (linear and quadratic effect, P < 0.05). There was a significant decreasing effect of feeding time on the content of C18:0 (linear and quadratic effect, P < 0.05). There was a significant decreasing effect of feeding time on C18:3 (linear effect, P < 0.05). There was a significant increasing effect of feeding time on docosapentaenoic acid (quadratic effect, P < 0.05). The content of C20:0 was significantly higher in egg yolks from chickens fed GTP for 12 wk than in the egg yolks from the other groups (P < 0.05). The content of C18:1 was significantly higher in egg yolks from chickens fed GTP for 6 to 12 wk than in egg yolks from the other groups (P < 0.05). The content of C18:3 was significantly lower in egg yolks from chickens fed GTP for 6 and 12 wk than in egg yolks from the other groups. The MUFA and TUFA content was significantly higher in eggs yolks from chickens fed GTP for 6 to 12 wk than in egg yolks from the other groups.
Table 3.
Fatty acid composition of egg yolk from chickens fed a diet with or without GTP.
| Fatty acid | Treatment | Feeding time (wk) |
SEM |
P-value |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 2 | 4 | 6 | 8 | 10 | 12 | Treatment | Feeding time |
Treatment × feeding time | |||||
| ANOVA | Linear | Quadratic | ||||||||||||
| C14:0 | Con | 0.10 | 0.09 | 0.12 | 0.15 | 0.10 | 0.07 | 0.08 | 0.007 | 0.560 | <0.01 | 0.015 | 0.001 | 0.056 |
| GTP | 0.11 | 0.09 | 0.11 | 0.11 | 0.10 | 0.08 | 0.09 | |||||||
| C16:0 | Con | 22.4 | 23.1 | 24.3 | 26.4 | 26.0 | 26.0 | 27.0 | 0.243 | 0.030 | <0.01 | <0.001 | 0.002 | 0.804 |
| GTP | 23.7 | 23.2 | 24.7 | 26.5 | 26.2 | 26.5 | 27.5 | |||||||
| C18:0 | Con | 11.1 | 10.2 | 10.0 | 10.5 | 10.9 | 10.0 | 11.1 | 0.331 | 0.049 | 0.336 | 0.035 | 0.043 | 0.080 |
| GTP | 10.8 | 10.6 | 10.6 | 9.11 | 9.6 | 9.8 | 9.91 | |||||||
| C20:0 | Con | 0.08 | 0.09 | 0.09 | 0.19 | 0.17 | 0.16 | 0.18 | 0.007 | <0.01 | <0.01 | <0.001 | 0.017 | 0.007 |
| GTP | 0.08 | 0.09 | 0.09 | 0.24 | 0.20 | 0.17 | 0.252 | |||||||
| C16:1 | Con | 3.05 | 3.3 | 3.58 | 3.27 | 3.49 | 3.04 | 2.89 | 0.148 | 0.606 | 0.122 | 0.45 | 0.23 | 0.587 |
| GTP | 3.13 | 3.37 | 3.56 | 3.23 | 3.16 | 3.05 | 3.391 | |||||||
| C18:1 | Con | 39.8 | 43.3 | 43.9 | 37.9 | 37.8 | 37.8 | 37.88 | 0.711 | <0.01 | <0.01 | 0.53 | 0.65 | 0.021 |
| GTP | 40.6 | 43.4 | 42.6 | 41.41 | 42.41 | 41.11 | 41.191 | |||||||
| C18:2 | Con | 8.37 | 8.86 | 8.93 | 9.39 | 9.14 | 9.90 | 9.90 | 0.321 | 0.005 | <0.01 | 0.09 | 0.57 | 0.366 |
| GTP | 9.2 | 9.19 | 8.57 | 10.95 | 10.44 | 10.46 | 10.631 | |||||||
| C18:3 | Con | 4.28 | 3.84 | 3.16 | 2.60 | 2.80 | 3.08 | 3.69 | 0.239 | 0.126 | 0.106 | 0.029 | 0.08 | 0.011 |
| GTP | 4.10 | 3.60 | 3.50 | 3.842 | 3.59 | 4.081 | 2.802 | |||||||
| DPA | Con | 1.09 | 0.95 | 0.91 | 1.02 | 0.77 | 1.06 | 1.20 | 0.086 | 0.127 | 0.004 | 0.264 | 0.040 | 0.485 |
| GTP | 1.00 | 0.89 | 0.99 | 1.06 | 1.05 | 1.41 | 1.30 | |||||||
| SFAs | Con | 33.7 | 32.9 | 34.5 | 37.2 | 34.3 | 36.2 | 38.3 | 0.663 | 0.956 | <0.01 | 0.56 | 0.55 | 0.831 |
| GTP | 34.7 | 33.3 | 34.8 | 35.90 | 35.0 | 35.0 | 37.7 | |||||||
| MUFA | Con | 42.9 | 46.7 | 47.5 | 41.2 | 41.0 | 40.8 | 40.8 | 0.748 | <0.01 | <0.01 | 0.61 | 0.54 | 0.026 |
| GTP | 43.7 | 46.8 | 46.2 | 44.61 | 45.62 | 44.01 | 44.61 | |||||||
| PUFA | Con | 13.7 | 13.4 | 13.0 | 13.0 | 12.5 | 13.9 | 14.1 | 0.483 | 0.038 | 0.411 | 0.98 | 0.74 | 0.114 |
| GTP | 14.3 | 13.2 | 12.9 | 15.52 | 15.02 | 14.2 | 14.0 | |||||||
| TUFA | Con | 56.6 | 60.0 | 60.5 | 54.2 | 52.2 | 54.8 | 54.8 | 0.724 | <0.01 | <0.01 | 0.34 | 0.97 | <0.01 |
| GTP | 58.0 | 60.0 | 59.1 | 60.12 | 60.32 | 58.11 | 58.52 | |||||||
Abbreviations: C14:0, myristic acid; C16:0, palmitic acid; C18:0, stearic acid; C20:0, arachidic acid; C16:1, palmitic acid; C18:1, oleic acid; C18:2, g-linolenic acid; C18:3, linolenic acid; Con, control; DPA, docosahexaenoic acid; GTP, green tea powder; MUFA, monounsaturated fatty acids; PUFA, polyunsaturated fatty acids; SFAs, saturated fatty acids; TUFA, total unsaturated fatty acids.
Significant differences among groups (P < 0.05).
Significant differences among groups (P < 0.01).
GTP Dependence of Antioxidant Capacity of Egg Whites From Hens
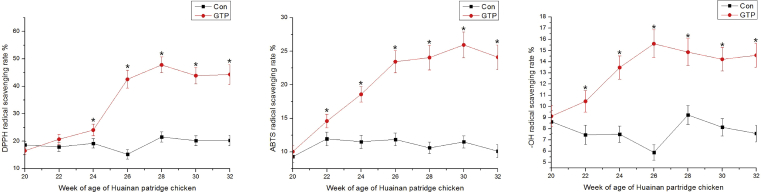
Egg whites from hens fed a diet containing GTP showed an increased radical scavenging activity compared with that of egg whites from hens in the control group after a 2-wk feeding period. The radical scavenging activity of the egg white increased to a plateau after 6 wk of feeding with GTP (Figure 1).
Figure 1.
The radical scavenging activity of egg whites from chickens fed diets with or without green tea powder (GTP).
Consumer Acceptability of Eggs From Chickens Fed GTP
Consumers rated the eggs between like extremely or dislike extremely with respect to appearance, aroma, flavor, texture, and overall acceptability (Table 4). The appearance, texture, and overall acceptability of the eggs of chickens subjected to dietary supplementation with 1% GTP for 4 wk were preferred by panelists than the control eggs (P < 0.05). Only egg appearance and texture were preferred after 8 wk of treatment of chickens with 1% GTP inclusion (P < 0.05). After 12 wk of treatment, egg appearance, texture, and overall acceptability were more liked by panelists for eggs from chickens fed a diet with GTP (P < 0.05). However, aroma and flavor showed no differences between the 2 treatment groups (P > 0.05).
Table 4.
Mean scores for overall consumer acceptability of eggs from chickens fed a diet with or without GTP.
| Item | Con |
GTP |
SEM |
P-value |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 4 wk | 8 wk | 12 wk | 4 wk | 8 wk | 12 wk | Treatment | Feeding time | Interaction | ||
| Appearance | 7.325b | 7.418b | 7.418b | 7.476a,b | 7.512a | 7.512a | 0.426 | 0.049 | 0.414 | 0.289 |
| Aroma | 7.215 | 7.259 | 7.32 | 7.205 | 7.259 | 7.315 | 0.696 | 0.932 | 0.548 | 0.762 |
| Flavor | 7.45 | 7.465 | 7.45 | 7.425 | 7.444 | 7.425 | 0.821 | 0.608 | 0.995 | 0.812 |
| Texture | 7.325b | 7.259b | 7.355b | 7.425a | 7.371a,b | 7.435a | 0.323 | 0.043 | 0.739 | 0.175 |
| Overall acceptability | 7.475b | 7.407b | 7.415b | 7.575a | 7.482a,b | 7.550a | 0.341 | 0.050 | 0.389 | 0.431 |
a,bMeans with no common superscripts are different (P < 0.05).
Abbreviations: Con, control; GTP, green tea powder.
Egg White and Yolk Functional Properties
Eggs from chickens fed a diet with 1% GTP had significantly lower hardness, chewiness, gelling property, and water retention capacity than those of eggs from chickens fed the control diet (P < 0.05, Table 5). Diet supplementation with GTP did not significantly affect egg white cohesiveness or springiness (P > 0.05). Eggs with a high yolk cholesterol content showed significantly higher cohesiveness than that of eggs with a low yolk cholesterol content. Yolk cholesterol content did not affect egg white hardness, chewiness, gelling properties, springiness, or water retention ability (P > 0.05). Eggs with a high yolk cholesterol content from chickens fed a diet with GTP showed lower chewiness than that of eggs with high or low yolk cholesterol contents from chickens fed a basal diet (P < 0.05). The interaction between treatment and yolk cholesterol did not affect egg white hardness, cohesiveness, gelling properties, springiness, or water retention ability (P > 0.05).
Table 5.
Effect of cholesterol and green tea powder on the gel properties of egg whites.
| Treatment |
Con |
GTP |
SEM |
P-value |
||||
|---|---|---|---|---|---|---|---|---|
| Cholesterol | High | Low | High | Low | Treatment | Cholesterol | Interaction | |
| Hardness g | 799.3a | 710.1a | 361.9b | 468.9b | 23.58 | <0.001 | 0.861 | 0.071 |
| Cohesiveness | 0.59a | 0.55a,b | 0.57a,b | 0.55b | 0.005 | 0.401 | 0.022 | 0.852 |
| Chewiness | 421.14a | 328.19a,b | 179.30c | 247.77b,c | 16.42 | <0.001 | 0.703 | 0.021 |
| Gelling property | 467.7a | 394.1a | 207.9b | 256.5b | 10.18 | <0.001 | 0.68 | 0.06 |
| Springiness/% | 0.21 | 0.19 | 0.2 | 0.18 | 0.003 | 0.66 | 0.204 | 0.97 |
| Water retention/% | 88.1a | 81.9a,b | 81.2a,b | 76.2b | 1.36 | 0.029 | 0.051 | 0.817 |
a–cMeans with no common superscripts are different (P < 0.05).
Abbreviations: Con, control; GTP, green tea powder.
Eggs from chickens fed a diet with GTP or the control diet showed no significant differences in egg white foaming properties or egg yolk emulsification properties (P > 0.05, Table 6). Egg yolks with a higher cholesterol content showed a lower foaming capacity of the egg white than that of eggs with low-cholesterol yolks (P < 0.05), but the yolk cholesterol content did not significantly affect egg white foaming stability or yolk emulsification properties (P > 0.05). Moreover, the interaction between treatment and yolk cholesterol did not affect egg white foaming properties or yolk emulsification properties (P > 0.05).
Table 6.
Effect of cholesterol and green tea powder on the foaming properties of egg whites and the emulsification properties of egg yolks.
| Treatment |
Con |
GTP |
SEM |
P-value |
|||||
|---|---|---|---|---|---|---|---|---|---|
| Cholesterol | High | Low | High | Low | Treatment | Cholesterol | Interaction | ||
| Egg white | Foaming | 118.1b | 162.1a | 150.8a | 155.1a | 2.98 | 0.21 | 0.02 | 0.063 |
| Foaming stability | 95.1 | 90.3 | 93.8 | 90.5 | 0.92 | 0.78 | 0.061 | 0.712 | |
| Egg yolk | Emulsification | 0.684 | 0.644 | 0.66 | 0.687 | 0.035 | 0.594 | 0.729 | 0.071 |
| Emulsion stability | 8.03 | 8.13 | 8.16 | 7.93 | 0.28 | 0.847 | 0.697 | 0.356 | |
a,bMeans with no common superscripts are different (P < 0.05).
Abbreviations: Con, control; GTP, green tea powder.
Discussion
In our previous research, we demonstrated that 1% GTP as a feed additive could promote intestinal health and meat quality but not affect the body weight of broilers (Chen et al., 2019). Xia et al. (2018) also suggested that 1% GTP has beneficial effects on egg quality from Chinese local chicken breeds, but a high amount of GTP (>2%) inclusion in the diet could decrease the egg weight and increase the feed-to-egg ratio. Thus, 1% GTP inclusion was selected to analyze its effect on egg quality, yolk cholesterol content, chicken blood parameters, albumin antioxidants, and consumer acceptability.
Some plant extracts contain specific functional peptides possessing a strong ability to chelate calcium (Walters et al., 2018). The inclusion of GTP might cause calcium chelation and thus decrease eggshell thickness and strength. Similar results were also found by Xia et al. (2018). Several studies have stated that green tea addition could increase the albumen height and HU value (Ariana et al., 2011; Kara et al., 2016b). Ovomucin is one of the most important proteins determining the height of albumin. Ovomucin contains 2 subunits, α and β, in which the β subunit is responsible for albumin viscosity (Omana et al., 2010). It has been suggested that polyphenols from green tea can form complexes with proteins and polysaccharides and then increase albumin gelling (Xia et al., 2018). Chickens fed a diet with green tea produce eggs with an increased content of β-ovomucin, thus promoting the albumen height and HU value (Xia et al., 2018).
Catechins in green tea can improve plasma antioxidant status through increases in plasma total antioxidant activity levels and can decrease peroxide levels (Hashimoto et al., 2000; Coimbra et al., 2006). Pietta and Simonetti (1998) suggested that catechins and their metabolites might exert their antioxidant protection in vivo by inhibiting reactive-oxygen-species generation and downregulating activated polyADP-ribose polymerase and caspase-3 (Pietta and Simonetti, 1998). As small molecules, catechin and tea polyphenols can easily transfer from the blood to organs, including the ovary and magnum, which results in an increased antioxidant capacity in egg white from chickens fed a diet with GTP. Furthermore, the improved oxidation stability of the egg white and yolk may indirectly stabilize unsaturated fatty acids in the yolk and promote a higher content of MUFA and polyunsaturated fatty acids (PUFA), which was observed in this experiment. Moreover, chickens usually produce reactive oxygen species while aging, which results in damage to the proteins (Ariana et al., 2011), causing a decrease in the HU value. Higashi-Okai et al. (2001) identified 6 pigments, chlorophylls a and b, pheophytins a and b, and carotenoids (β-carotene and lutein), from the nonpolyphenolic fraction of residual green tea. Hens can transfer these carotenoids to the yolk, which makes the yolk color deeper in chickens fed a diet with GTP.
The antioxidant activity increased with increased lightness of the egg white, and the carotenoids from the GTP made the egg yolk color deeper than that of the control egg yolks. These color changes resulted in the improved appearance and overall acceptability of eggs from chickens fed a diet with GTP compared with that of eggs from the control group. It has been reported that the inclusion of conjugated linoleic acid can increase the content of PUFA and reduce the flavor of egg yolks because of reduced flavor components and the presence of conjugated linoleic acid in the egg yolk (Liu et al., 2017). Increased PUFA might decrease the hardness and thus promote improvements in the texture of the egg yolk. Although there have been no previous reports on egg texture changes by PUFA, research into meat quality has suggested that higher levels of PUFA increase its chewiness and meat texture (Hcini et al., 2018).
Paraskevopoulou and Kosseoglou (1997) tested the texture profiles of gels from low-cholesterol yolks and found that these gels exhibited higher hardness and springiness and lower cohesiveness than those of control yolks. In this experiment, a lower cohesiveness was also observed in egg whites from the low-cholesterol-content group. Shafer et al. (1998) found that increased albumen did not affect the hardness or springiness of albumen and yolk gel. However, several studies have demonstrated that egg quality could significantly affect the gelling properties of albumin (Katekhong and Charoenrein, 2016). A higher HU and albumin height could decrease the gelling properties of albumin. A diet containing GTP increased these properties of the egg white, which lowered the egg white texture properties in this experiment. In addition, a higher content of mucin lysozyme made the egg white more water-like and reduced its water retention capacity, which caused lower water retention by eggs from chickens fed a diet with GTP. Fauziah et al. (2016) compared the texture properties between cholesterol-reduced eggs and normal eggs and found that the foaming capacity decreased from 4% in the normal group to 1.96% in the cholesterol-reduced group. A high content of lipids and proteins were noncovalently bound to form large lipoprotein complexes, which conferred a high foaming capacity to the egg yolk (Moros et al., 2002). A similar decreased foaming capacity was observed in this experiment, which might be due to the decreased cholesterol content of the egg yolk.
This study has demonstrated that green tea supplementation could promote the content of MUFA, PUFA, and TUFA in egg yolks. Egg appearance and texture were more preferred in the GTP group than in the control group. Green tea powder supplementation and the cholesterol content changed egg processing characteristics.
Acknowledgments
This work was financially supported by China Agriculture Research System (No. CARS-19), and Major Scientific and Technological Special Project in Anhui Province (18030701174). The authors thank Dr. K Cai from the Hefei University of Technology for helping us measure yolk fatty acids.
Disclosures
All authors declare that there is no conflict of interest.
References
- Ariana M., Samie A., Edriss M.A., Jahanian R. Effects of powder and extract form of green tea and marigold, and α-tocopheryl acetate on performance, egg quality and egg yolk cholesterol levels of laying hens in late phase of production. J. Med. Plants Res. 2011;5:2710–2716. [Google Scholar]
- Awe F.B., Fagbemi T.N., Ifesan B.O.T., Badejo A.A. Antioxidant properties of cold and hot water extracts of cocoa, hibiscus flower extract, and ginger beverage blends. Food Res. Int. 2013;52:490–495. [Google Scholar]
- Blesso C.N., Fernandez M.L. Dietary cholesterol, serum lipids, and heart disease: are eggs working for or against you? Nutrients. 2018;10:426. doi: 10.3390/nu10040426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X., Zhu W., Liu X., Li T., Geng Z., Wan X. The growth performance, meat quality, and gut bacteria of broilers raised with or without antibiotics and green tea powder. J. Appl. Poult. Res. 2019;28:712–721. [Google Scholar]
- Coimbra S., Santos-Silva A., Rocha-Pereira P., Rocha S., Castro E. Green tea consumption improves plasma lipid profiles in adults. Nutr. Res. 2006;26:604–607. [Google Scholar]
- Erba D., Riso P., Bordoni A., Foti P., Biagi P.L., Testolin G. Effectiveness of moderate green tea consumption on antioxidative status and plasma lipid profile in humans. J. Nutr. Biochem. 2005;16:144–149. doi: 10.1016/j.jnutbio.2004.11.006. [DOI] [PubMed] [Google Scholar]
- Fauziah C.I., Zaibunnisa A.H., Osman H., Wan Aida W.M. Physicochemical analysis of cholesterol-reduced egg yolk powder and its application in mayonnaise. Int. Food Res. J. 2016;23:575–582. [Google Scholar]
- Feng Z.H., Gong J.G., Zhao G.X., Lin X., Liu Y.C., Ma K.W. Effects of dietary 343 supplementation of resveratrol on performance, egg quality, yolk cholesterol and antioxidant enzyme activity of laying hens. Br. Poult. Sci. 2017;58:544–549. doi: 10.1080/00071668.2017.1349295. [DOI] [PubMed] [Google Scholar]
- Gouda M., Zu L., Ma S., Sheng L., Ma M. Influence of bio-active terpenes on the characteristics and functional properties of egg yolk. Food Hydrocolloids. 2018;80:222–230. [Google Scholar]
- Hashimoto R., Yaita M., Tanaka K., Hara Y., Kojo S. Inhibition of radical reaction of apolipoprotein B-100 and α-tocopherol in human plasma by green tea catechins. J. Agric. Food Chem. 2000;48:6380–6383. doi: 10.1021/jf000973i. [DOI] [PubMed] [Google Scholar]
- Hcini E., Slima A.B., Kallel I., Zormati S., Traore A.I., Gdoura R. Does supplemental zeolite (clinoptilolite) affect growth performance, meat texture, oxidative stress and production of polyunsaturated fatty acid of turkey poults? Lipids Health Dis. 2018;17:177. doi: 10.1186/s12944-018-0820-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higashi-Okai K., Yamazaki M., Nagamori H., Okai Y. Identification and antioxidant activity of several pigments from the residual green tea (Camellia sinensis) after hot water extraction. J. UOEH. 2001;23:335–344. doi: 10.7888/juoeh.23.335. [DOI] [PubMed] [Google Scholar]
- Iskender H., Yenice G., Dokumacioglu E., Kaynar O., Hayirli A., Kaya A. Comparison of the effects of dietary supplementation of flavonoids on laying hen performance, egg quality and egg nutrient profile. Br. Poult. Sci. 2017;58:550–556. doi: 10.1080/00071668.2017.1349297. [DOI] [PubMed] [Google Scholar]
- Kara K., Güçlü B.K., Şentürk M., Konca Y. Influence of catechin (flavan-3-ol) addition to breeder quail (Coturnix coturnix japonica) diets on productivity, reproductive performance, egg quality and yolk oxidative stability. J. Appl. Anim. Res. 2016;44:436–441. [Google Scholar]
- Kara K., Şentürk M., Guclu B.K., Sariözkan S., Eren M. Effect of catechins on fattening performance, meat quality, some antioxidant and blood parameters and fattening costs in Japanese quail (Coturnix coturnix japonica) Br. Poult. Sci. 2016;57:522–530. doi: 10.1080/00071668.2016.1174977. [DOI] [PubMed] [Google Scholar]
- Katekhong W., Charoenrein S. Changes in physical and gelling properties of freeze-dried egg white as a result of temperature and relative humidity. J. Sci. Food Agric. 2016;96:4423–4431. doi: 10.1002/jsfa.7653. [DOI] [PubMed] [Google Scholar]
- Khalesi S., Sun J., Buys N., Jamshidi A., Nikbakht-Nasrabadi E., Khosravi-Boroujeni H. Green tea catechins and blood pressure: a systematic review and meta-analysis of randomized controlled trials. Eur. J. Nutr. 2014;53:1299–1311. doi: 10.1007/s00394-014-0720-1. [DOI] [PubMed] [Google Scholar]
- Koo M.W.L., Cho C.Ho. Pharmacological effects of green tea on the gastrointestinal system. Eur. J. Pharmacol. 2004;500:177–185. doi: 10.1016/j.ejphar.2004.07.023. [DOI] [PubMed] [Google Scholar]
- Liu Z., Sun C., Yan Y., Li G., Wu G., Liu A., Yang N. Genome-wide association analysis of age-dependent egg weights in chickens. Front. Genet. 2018;9:128. doi: 10.3389/fgene.2018.00128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X., Zhang Y., Yan P., Shi T., Wei X. Effects of conjugated linoleic acid on the performance of laying hens, lipid composition of egg yolk, egg flavor, and serum components. Asian-Australas J. Anim. 2017;30:417–423. doi: 10.5713/ajas.15.1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lordelo M., Fernandes E., Bessa R.J.B., Alves S.P. Quality of eggs from different laying hen production systems, from indigenous breeds and specialty eggs. Poult. Sci. 2017;96:1485–1491. doi: 10.3382/ps/pew409. [DOI] [PubMed] [Google Scholar]
- Moros J.E., Franco J.M., Gallegos C. Rheology of spray-dried egg yolk-stabilized emulsions. Int. J. Food Sci. Tech. 2002;37:297–307. [Google Scholar]
- Omana D.A., Wang J.P., Wu J.P. Ovomucin-a glycoprotein with promising potential. Trends Food Sci. Tech. 2010;21:455–463. doi: 10.1016/j.tifs.2010.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onakpoya I., Spencer E., Heneghan C., Thompson M. The effect of green tea on blood pressure and lipid profile: a systematic review and meta-analysis of randomized clinical trials. Nutr. Metab. Cardiovasc. Dis. 2014;24:823–836. doi: 10.1016/j.numecd.2014.01.016. [DOI] [PubMed] [Google Scholar]
- Paraskevopoulou A., Kosseoglou V. Texture profile analysis of heat-formed gels and cakes prepared with low cholesterol egg yolk concentrates. J. Food Sci. 1997;62:208–211. [Google Scholar]
- Pietta P., Simonetti P. Dietary flavonoids and interaction with endogenous antioxidant. Biochem. Mol. Biol. Int. 1998;44:1069–1074. [Google Scholar]
- Shafer D.J., Carey J.B., Prochaska J.F., Sams A.R. Dietary methionine intake effects on egg component yield, composition, functionality, and texture profile analysis. Poult. Sci. 1998;77:1056–1062. doi: 10.1093/ps/77.7.1056. [DOI] [PubMed] [Google Scholar]
- Sun S., Niu H., Yang T., Lin Q., Luo F., Ma M. Antioxidant and anti-fatigue activities of egg white peptides prepared by pepsin digestion. J. Sci. Food Agric. 2014;94:3195–3200. doi: 10.1002/jsfa.6671. [DOI] [PubMed] [Google Scholar]
- Walker L.A., Wang T., Xin H., Dolde D. Supplementation of laying-hen feed with palm tocos and algae astaxanthin for egg yolk nutrient enrichment. J. Agric. Food Chem. 2012;60:1989–1999. doi: 10.1021/jf204763f. [DOI] [PubMed] [Google Scholar]
- Walters M.E., Esfandi R., Tsopmo A. Potential of food hydrolyzed proteins and peptides to chelate iron or calcium and enhance their absorption. Foods. 2018;7:172. doi: 10.3390/foods7100172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B., Du Y., Liu X., Xie C., Geng Z., Chen X. Comparative study of growth performance and meat quality of three-line crossbred commercial group from Shanzhongxian and W-line chicken. Ital. J. Anim. Sci. 2019;18:63–69. [Google Scholar]
- Wang X., Wang X., Wang J., Wang H., Zhang S Wu H., Qi G. Dietary tea polyphenol supplementation improved egg production performance, albumen quality, and magnum morphology of hy-line brown hens during the late laying period. J. Anim. Sci. 2018;96:225–235. doi: 10.1093/jas/skx007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia B., Liu Y., Sun D., Liu J., Zhu Y., Lu L. Effects of green tea powder supplementation on egg production and egg quality in laying hens. J. Appl. Anim. Res. 2018;46:927–931. [Google Scholar]