Abstract
Tryptophan metabolites exhibit aryl hydrocarbon receptor (AhR) agonist activity and recent studies show that the phenylalanine metabolites serotonin and carbidopa, a drug used in treating Parkinson’s disease, activated the AhR. In this study, we identified the neuroactive hormone dopamine as an inducer of drug-metabolizing enzymes CYP1A1, CYP1B1, and UGT1A1 in colon and glioblastoma cells and similar results were observed for carbidopa. In contrast, carbidopa but not dopamine exhibited AhR activity in BxPC3 pancreatic cancer cells whereas minimal activity was observed for both compounds in Panc1 pancreatic cancer cells. In contrast with a previous report, the induction responses and cytotoxicity of carbidopa was observed only at high concentrations (100 µM) in BxPC3 cells. Our results show that similar to serotonin and several tryptophan metabolites, dopamine is also an AhR-active compound.
Introduction
Gut microbiota plays an important role in human health and disease, and this is due, in part, to microbial metabolites and their direct influence on the intestine and also distal organs [1–3]. Metabolite formation can be influenced by multiple factors including the diet, and there is evidence that microbial metabolism of diets enriched in fiber generate short chain fatty acids which contribute to intestinal resilience [4–6]. Tryptophan is one of the nine dietary-derived essential amino acids and microbial degradation of tryptophan generates multiple indole-derived compounds that also contribute to intestinal health [7–11]. There is evidence that dietary tryptophan and its metabolites enhance intestinal immunity and inhibit inflammation, and this has been linked, in part, to AhR-active tryptophan metabolites and their AhR-dependent activities [10,12–14]. In addition, dietary tryptophan and AhR-active metabolites of tryptophan such as indole-3-propionic acid and indole-3-aldehyde also exhibit anti-inflammatory activity in the central nervous system (CNS) of mouse models of multiple sclerosis and decreased serum levels of tryptophan metabolites are observed in multiple sclerosis patients [15,16].
Microbial degradation of tryptophan utilizes multiple pathways to produce an array of metabolites in the intestine as well as the CNS where tryptophan is converted (via tryptophan hydroxylase) into serotonin which has also been identified as an AhR active compound [17]. Indoleamine 2,3-dioxygenase in the host catalyzes metabolism of tryptophan into kynurenine and kynurenic acid which in turn are metabolized into both neurotoxic (e.g. quinolinic acid) and neuroprotective (e.g. kynurenic acid) metabolites which are also AhR ligands [18,19]. Carbidopa, a drug used for the treatment of Parkinson’s disease to enhance neuronal levels of L-DOPA, is also an AhR ligand. Carbidopa exhibits AhR activity in multiple cancer cell lines and was shown to inhibit BxPC3 pancreatic cancer cell growth [20].
In this study, we demonstrate that L-DOPA exhibits minimal to non-detectable AhR activity despite structural similarities to carbidopa. We have re-examined the effects of all 3 structurally similar 3,4-dihydroxyphenyl derivatives including carbidopa, L-DOPA, and dopamine. We show that dopamine also exhibited AhR activity similar to carbidopa; however, these effects are cell context and response specific. Thus, the neuroactive hormone dopamine derived from the amino acid phenylalanine is an AhR active compound.
Materials and methods
Cell culture
Panc1, BxPC3, Caco-2 human colon cancer cells and human glioblastoma cell lines U87-MG were obtained from American Type Culture Collection (ATCC, Manassas, VA) and the patient-derived 15-037 cells, 14-015, and 14–104 glioblastoma cells have previously been described [21,22]. Panc1, SW480, and HCT116 cells were maintained in Dulbecco’s modified Eagle medium (DMEM) with nutrient supplemented with 10% fetal bovine serum (FBS) (Gibco, Dublin, Ireland). BxPC3 cells were maintained in RPMI 1640 medium with 10% FBS (Gibco). Caco-2 cells were maintained in DMEM with nutrient mixture supplemented with 20% FBS and 10 m/L 100× MEM non-essential amino acid solution (Gibco). Patient-derived xenografts from human gliomas cell lines were incubated at 37°C in the presence of 5% CO2. Dopamine, L-DOPA, and carbidopa were purchased from Sigma–Aldrich (St. Louis, MO). The 15-037-AhRKO cells were generated by CRISPR/Cas9 as described [22]. A humanAhR CRISPR/Cas9 guide RNA (AGACCGACTTAATACAGAGT) in a pSpCas9 BB-2A-GFP PX458 vector was purchased from GenScript (Piscataway, NJ). Individual clones were isolated and two of these exhibited the targeted frameshift mutations in exon 2 and the resulting AhR cells did not express the AhR and were TCDD non-responsive [22].
Western blot analysis
Cells (3 × 105 cells/well) were plated in six-well plates and grown for 24 h. Cells were treated with different concentrations of the compounds for 24 h. Whole-cell lysates from different cell lines were analyzed by Western blot analysis as described previously [22]. Aliquots of cellular proteins were electrophoresed on 10% SDS–poly-acrylamide gel electrophoresis (PAGE) and transferred to a polyvinylidene difluoride (PVDF) membrane (Bio-Rad Laboratories). The membrane was allowed to react with CYP1A1, CYP1B1 (Santa cruz biotechnology, Santa cruz, CA) and AhR(Enzo Life Sciences, Farmingdale, NY) antibodies, and detection of specific proteins was carried out by enhanced chemiluminescence. Loading differences were normalized by using GAPDH antibody.
Chromatin immunoprecipitation (ChIP) assay
The ChIP assay was performed using ChIP-IT Express Magnetic Chromatin Immunoprecipitation kit (Active Motif, Carlsbad, CA) according to the manufacturer’s protocol. Caco-2 cells were incubated for 24 h and then treated with TCDD, Dopamine and Carbidopa for 2 h prior to cell harvest. Cells were analyzed by ChIP Assay as described previously [21,22]. DNA was extracted from the immunoprecipitate and PCR amplified using CYP1A1 (forward 5′-CCCGTGACCTCAGGGCT-3′, reverse 5′-TTGCACCCACTGGAACGC-3′) primer. PCR products were resolved on a 2% agarose gel in the presence of ethidium bromide.
Quantitative real-time reverse transcriptase PCR
Total RNA was extracted using RNA isolation kit from cells according to the manufacturer’s protocol. cDNA synthesis was performed from the total RNA of cells using High Capacity RNA-to-cDNA Kit (Applied Biosystems, Foster City, CA). Real-Time PCR was carried out in triplicate using Bio-Rad SYSR Universal premix for 1 min at 95°C for initial denaturing, followed by 40 cycles of 95°C for 15 sec and 60°C for 1 min in the Bio-Rad iCycler (MyiQ™2) real-time PCR System. The expression of genes was analyzed with the comparative CT method and normalized to expression levels of TATA-binding protein (TBP). The sequences of the primers used for real-time PCR were as follows: CYP1A1 sense 5′-GAC CAC AAC CAC CAA GAA C-3′, antisense 5′-AGC GAA GAA TAG GGA TGA AG-3′; UGT1A1 sense 5′-GAA TCA ACT GCC TTC ACC AAA AT-3′, antisense 5′-AGA GAA AAC CAC AAT TCC ATG TTC T-3′; TBP sense 5′-GAT CAG AAC AAC AGC CTG CC-3′, antisense 5′-TTC TGA ATA GGC TGT GGG GT-3′.
Cell viability assay
Cells (1 × 105) were plated in 12-well plates for 24 h and then treated with different concentrations of the compounds in media containing 2.5% FBS. Cells were then trypsinized and counted after 24 h using a Coulter Z1 cell counter (Sykesville, MD). The 100 µM concentration of dopamine, DOPA and carbidopa were the highest concentrations that exhibited a ≤15% decrease in cell viability.
DNA binding assay
DRE binding of AhR was measured using an Episeeker DNA–protein binding assay kit (Abcam) according to the manufacturer’s protocol. A biotinylated 25 bp double-stranded oligonucleotides of DRE promoter region (wild-type; 5-GATCTGGCTCTTCTCACGCAACTCCG-3 and mutant-type; 5-GATCTGGCTCTTCTGTCATC ACTCCG-3) containing AhR binding consensus sequence was used as a capture probe, and 25 bp double-stranded unlabeled oligonucleotide containing the identical consensus sequences was used as a competitor. Whole-cell extracts from Caco-2 cells were used in this experiment.
Clonogenic assay
Clonogenic assay was performed as described recently [23,24]. The cells were seeded at a low density (500 cells/well) and allowed to attach to the substratum. Cultures were then treated with different concentrations of carbidopa (0, 1, 5, 10, 50, and 100 μM), and the colonies were allowed to grow for 10–14 days. Carbidopa was added to cells every 3 days. At the end of the incubation period, the colonies were fixed and stained with crystal violet.
Results
Several AhR-active microbial degradation products of tryptophan [25–27] and dietary AhR active compounds [28,29] induce CYP1A1 in Caco2 cells which are highly responsive to structurally diverse AhR ligands. Results in Figure 1A show that carbidopa induced CYP1A1 in Caco2 cells; this is consistent with previous studies on carbidopa in other cell lines [20]. We also observed that the neurotransmitter dopamine also induced CYP1A1 in Caco2 cells and the induction responses observed for carbidopa, dopamine, and TCDD (positive control) were inhibited after co-treatment with the AhR antagonist CH223191. Both dopamine and carbidopa induced CYP1B1 (Figure 1B) and UGT1A1 (Figure 1C) in Caco2 cells and the magnitude of this response was greater than observed for TCDD. These induced responses (CYP1B1, UGT1A1) were also inhibited after co-treatment with the AhR antagonist CH223191, thus confirming that these were AhR-mediated responses.
Figure 1. Dopamine and carbidopa as AhR ligands in Caco2 and U87 cells.
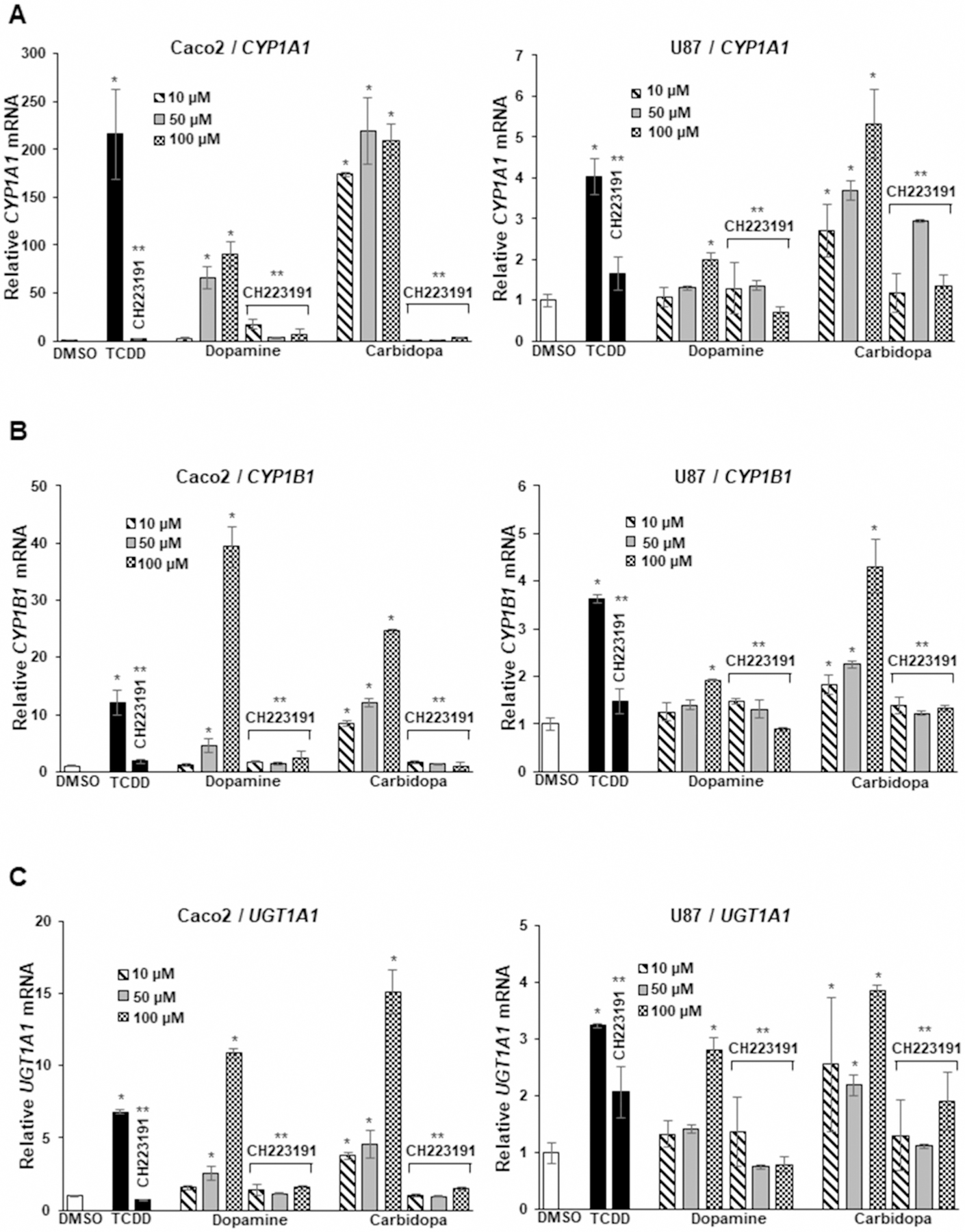
Cells were treated with DMSO (control) 10 nM TCDD, 10–100 µM dopamine, and carbidopa alone in or in combination with 10 µM CH233191, and induction of CYP1A1 (A), CYP1B1 (B), and UGT1A1 (C) was determined by real-time PCR as outlined in the Methods. Results are expressed as means ± SD for at least three replicates for each treatment group and significant (P < 0.05) induction (*) or inhibition by CH223191 (**) is indicated.
A previous report [20] indicated that DOPA was inactive as an AhR agonist in HepG2 cells whereas our results (Supplementary Figure S1A) showed that 50–100 µM DOPA induced CYP1A1 and UGT1A1 in Caco2 cells; however, the magnitude of these responses was low compared with dopamine and carbidopa. Since carbidopa and dopamine have important neuronal functions, we also examined their AhR activity in U87 glioblastoma cell lines. Carbidopa, dopamine, and TCDD induced CYP1A1 (Figure 1A), CYP1B1 (Figure 1B), and UGT1A1 (Figure 1C) in U87 cells and these responses were also inhibited after co-treatment with CH223191. The fold induction of CYP1A1, CYP1B1, and UGT1A1 were significantly lower in U87 cells compared with Caco2 cells, and this was particularly evident for the low (but significant) induction of CYP1A1 and CYP1B1 observed for dopamine in U87 cells. Nevertheless, these results show, for the first time, that dopamine is an AhR-active ligand. We also compared the growth inhibitory effects of carbidopa, dopamine, and L-DOPA in Caco2 cells and observed no effects at concentrations as high as 100 µM (Supplementary Figure S1B).
Recent studies in our laboratories characterized the Ah-responsiveness of a series of patient-derived glioblastoma cell lines which expressed variable AhR levels and observed that the AhR inhibited cell growth and invasion [22]. Figure 2 summarizes the Ah-responsiveness of patient-derived 15-037 wild-type cells and 15-037 (AhRKO) cells in which the AhR has been silenced by CRISPR/Cas9 [22]. TCDD and carbidopa significantly induced CYP1A1 (Figure 2A) and CYP1B1 (Figure 2B) whereas only minimal induction was observed for dopamine. In contrast, dopamine but not TCDD or carbidopa, induced UGT1A1 in 15-037 glioblastoma cells (Figure 2C) illustrating the gene-specific induction responses by these compounds. No induction responses were observed in 15-037 (AhRKO) cells. We further investigated the induction of CYP1A1 (Figure 3A), CYP1B1 (Figure 3B), and UGT1A1 (Figure 3C) by dopamine and carbidopa in two additional (Ah-responsive 14–104 and Ah-non-responsive 14-015) patient-derived glioblastoma cell lines [22]. Carbidopa and TCDD induced CYP1A1 and CYP1B1, whereas dopamine only induced CYP1B1 (<2-fold) in 14–104 cells whereas UGT1A1 was not inducible by these compounds in 14–104 cells. Higher concentrations of dopamine resulted in lower levels of CYP1A1 and CYP1B1. The 14-015 cells express low AhR levels and inducibility of CYP1A1 and UGT1A1 by TCDD, dopamine, and carbidopa were not observed (Figure 3A–C). CYP1B1, on the other hand, was induced by all three compounds suggesting that this response was AhR-independent.
Figure 2. Dopamine and carbidopa as AhR ligands in 15-037 wild-type and AhRKO cells.
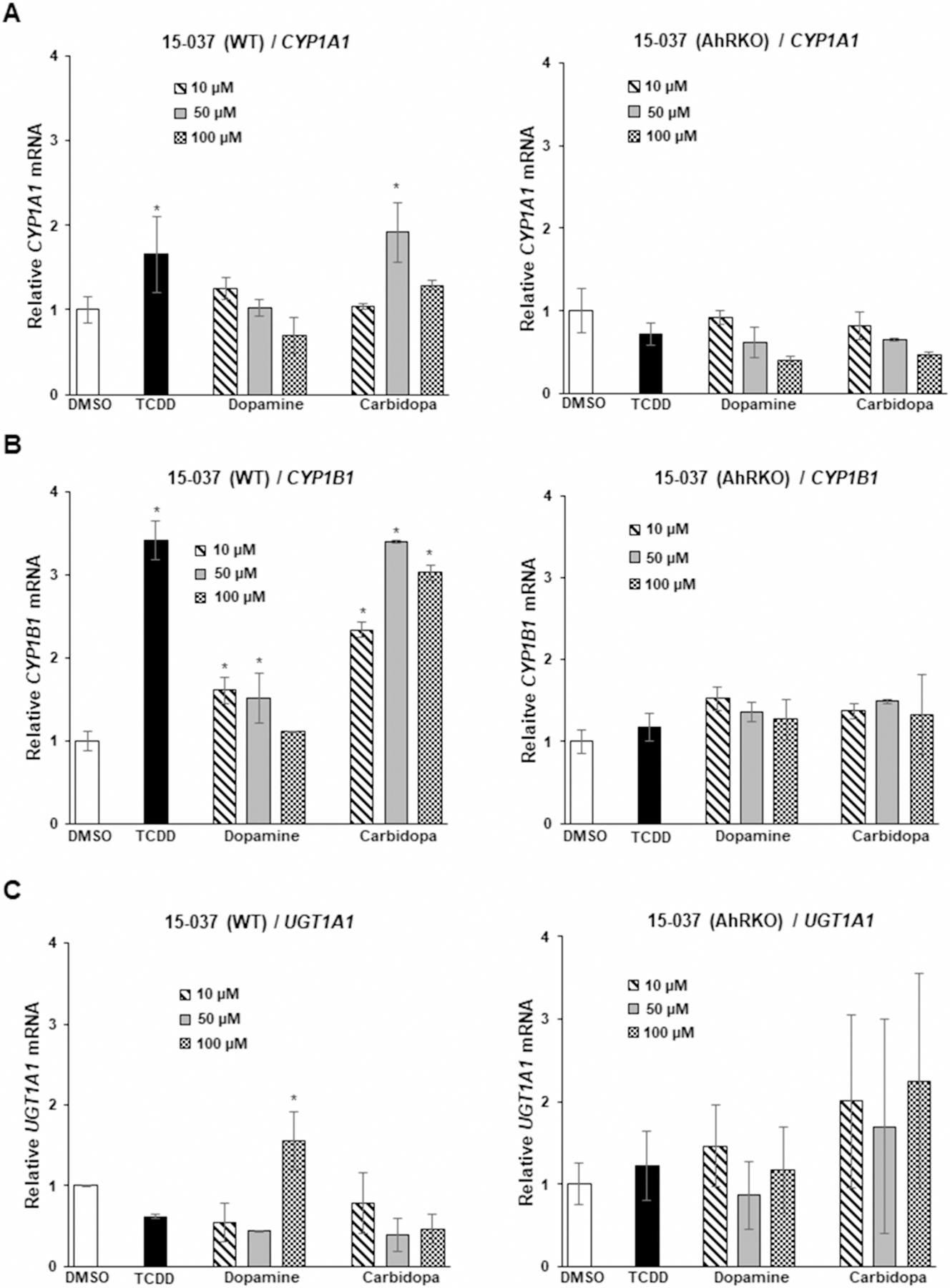
Cells were treated with DMSO, 10 nM TCDD, 10–100 µM dopamine and carbidopa, and induction of CYP1A1 (A), CYP1B1 (B) and UGT1A1 (C) was determined by real-time PCR as outlined in the Methods. Results are expressed as a means ± SD for at least three determinations per treatment group and significant (P < 0.05) induction as indicated (*).
Figure 3. Dopamine and carbidopa as AhR ligands in patient-derived 14-014 and 14-015 glioblastoma cells.
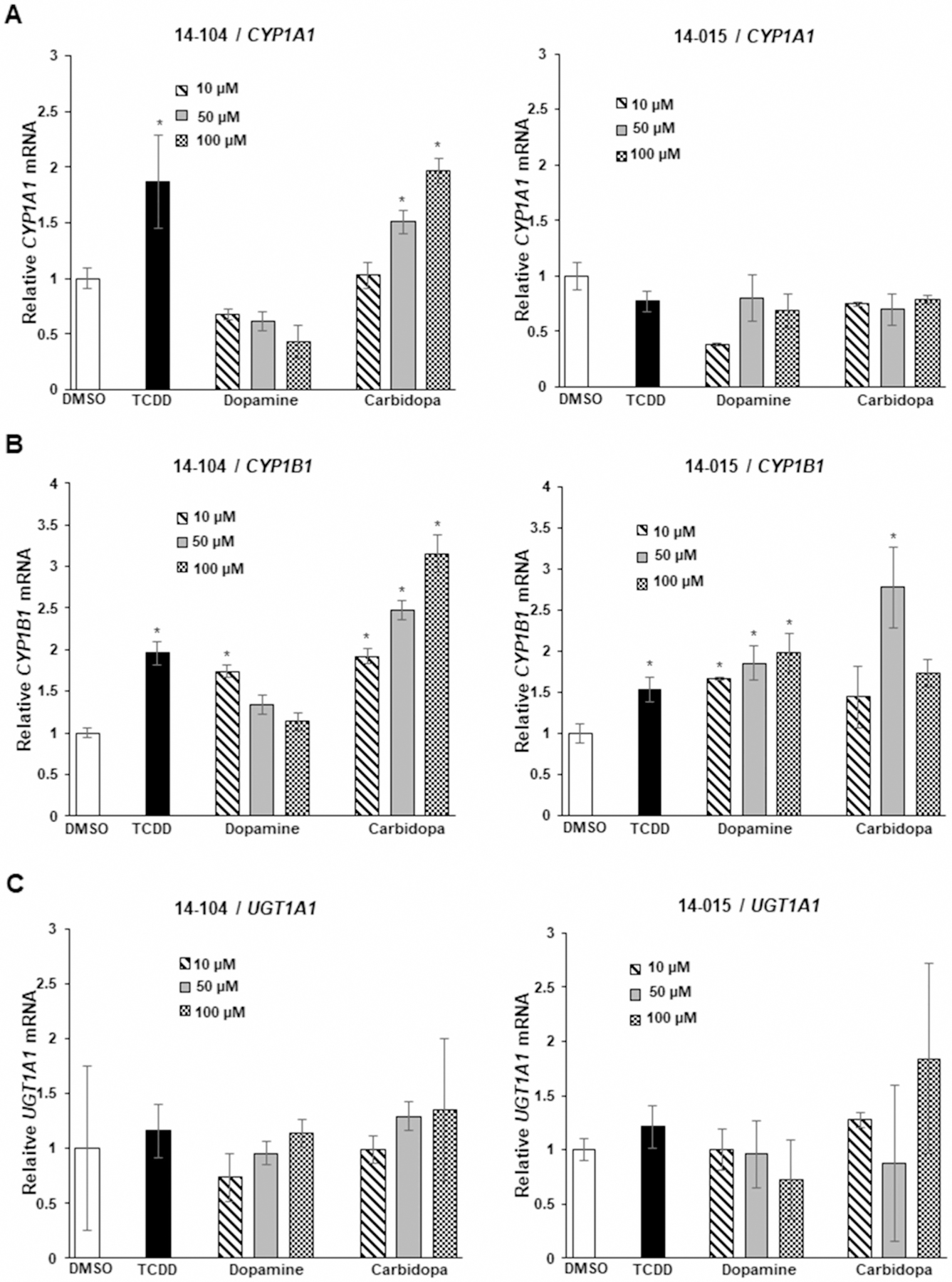
Cells were treated with DMSO, 10 nM TCDD, 10–100 µM dopamine and carbidopa and induction of CYP1A1 (A), CYP1B1 (B) and UGT1A1 (C) were determined by real-time PCR as outlined in the Methods. Results are expressed as means ± SD for at least three determinations for each treatment group and significant (P < 0.05) induction is indicated (*).
We also analyzed effects of TCDD, dopamine and carbidopa on expression of the AhR, CYP1A1 and CYP1B1 in selected cell lines (Caco-2, 14–104s and U87-MG) and showed that the AhR protein is expressed in these cells and TCDD decreases expression of the receptor (Figure 4A). Induction of CYP1A1 and CYP1B1 protein was both ligand and cell context dependent; CYP1A1 was induced by TCDD in Caco2 cells and detectable induction was also observed for dopamine and carbidopa. In contrast, CYP1A1 protein was not induced in 14–104s and U87-MG cells. CYP1B1 protein was induced by all three compounds in U87MG but not in Caco2 or 14–104s cells. A chromatin immunoprecipitation assay (Figure 4B) was used to show that after treatment of Caco2 cells with TCDD, dopamine and carbidopa there was an increase in AhR binding to the DRE region of the CYP1A1 promoter and pol II binding was also increased and these results correlated with the CYP1A1 mRNA and protein induction studies (Figs. 1A and 4A). We used an Episeeker DNA–protein binding kit to determine interactions between TCDD and dopamine with an oligonucleotide containing a dioxin responsive element (DRE) and a second oligonucleotide in which the DRE is mutated (negative control). The free DNA probe contains the DRE and competitively decreases binding to the oligonucleotides (Figure 4C). The results show that 2 and 10 nM TCDD and 25 and 100 µM dopamine bind the wild-type but not the mutant oligonucleotides. In addition, the ‘free DNA’ probe containing the DRE competitively decreased binding to the wild-type (DRE) oligonucleotide. These results show that like TCDD, dopamine binds a consensus DRE which is consistent with the AhR agonist activity observed for this compound.
Figure 4. Induction and binding assay.
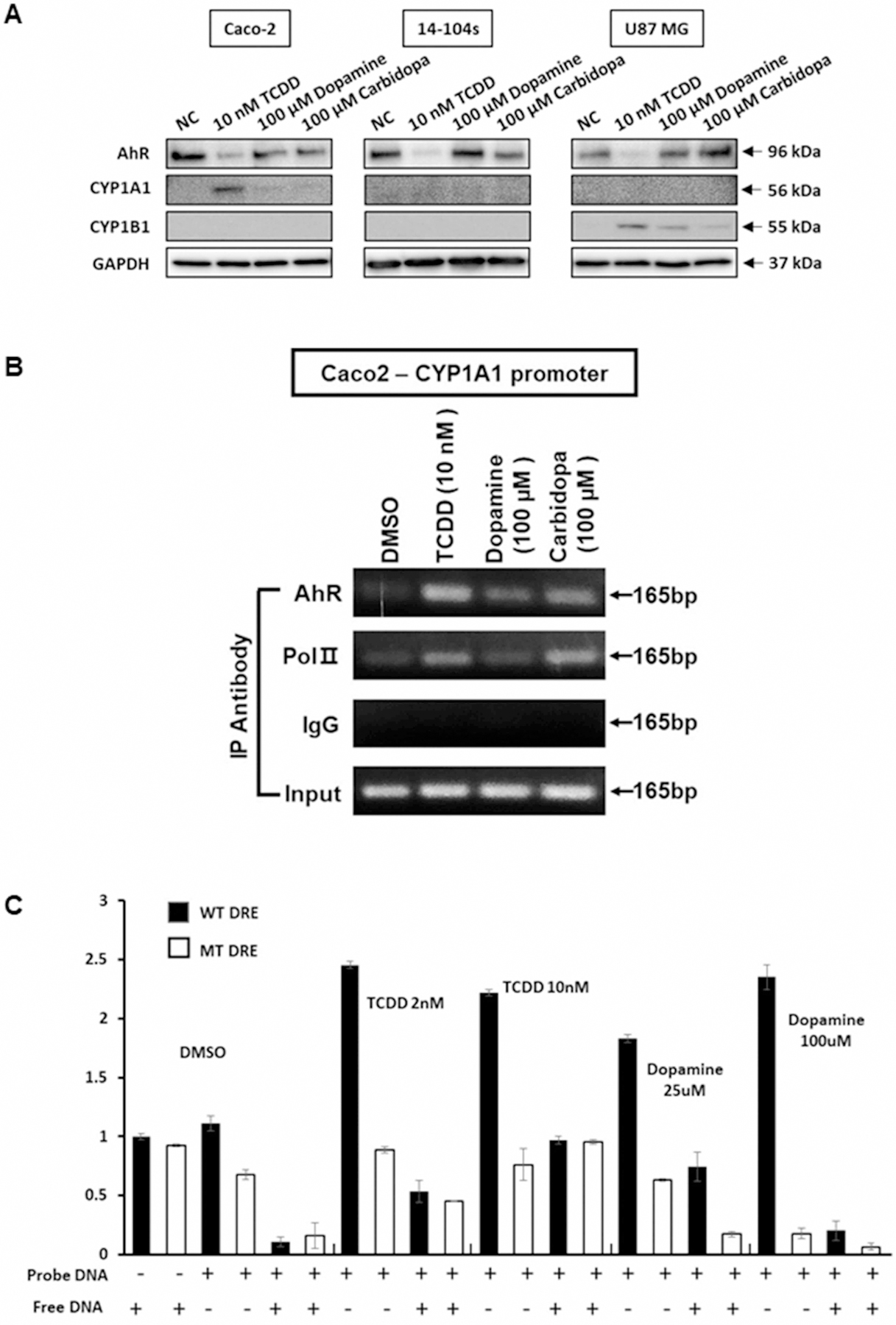
(A) Caco2 cells were treated with DMSO, TCDD, dopamine and carbidopa for 24 h and whole-cell lysates were analyzed by western blots as outlined in the Methods. (B) Using as similar procedure treatment related interactions of the AhR and pol II with the DRE region of the CYP1A1 promoter were determined in a ChIP assay. (C) DRE binding of dopamine wild-type and mutant oligonucleotides derived from the human AhR promoter were incubated with whole-cell lysates from Caco2 cells treated with two different doses of TCDD (2 and 10 nM) and dopamine (25 and 100 µM). Binding was determined in a colorimetric Episeeker DNA–protein assay as outlined in the Methods. Results are expressed as means ± SD for at least three determinations for each treatment group.
Previous studies showed that carbidopa was also an AhR agonist in pancreatic cancer cells and inhibited BxPC3 cell and tumor growth in a xenograft model [20]. Results illustrated in Figure 5A,B confirm that TCDD and carbidopa induced CYP1A1 and CYP1B1 in BxPC3 cells whereas only minimal induction of UGT1A1 (Figure 5C) was observed. In contrast, dopamine and DOPA were inactive in BxPC3 cells and in the highly invasive Panc1 cells only TCDD slightly induced (<3-fold) drug-metabolizing enzyme gene expression. A key observation reported by Ogura and co-workers was that carbidopa decreased growth of BxPC3 cells [20]. We therefore initially examined effects of carbidopa, dopamine, and DOPA in Panc1 (Figure 6A) and BxPC3 (Figure 6B) cells using a cell viability/counting assay. None of these compounds at concentrations up to 100 µM affected growth of BxPC3 and Panc1 cells and the results for TCDD were comparable to previous reports on the activity of this compound in Panc1 cells [30]. We also observed no cytotoxic effects of dopamine and related compounds in Caco2, SW480, and HCT116 colon cancer cell lines (Supplementary Figure S1B). Similar results were observed in 15-037 patient-derived glioblastoma cells (data not shown). Previous studies showed that 1 mM carbidopa significantly inhibited cell viability in a clonogenic assay in BxPC3 cells [20]; however, our results showed that significant inhibition was observed only at the 100 µM concentration (Figure 6C). In contrast, treatment of the more aggressive Panc1 cells with 1–100 µM carbidopa did not affect cell viability (Figure 6D). Thus, our results show that like carbidopa, dopamine is also an AhR ligand; however, the cytotoxicity of carbidopa in BxPC3 cells was considerably lower than previously described [20] and cytotoxic effects of carbidopa were not observed in Panc1 cells.
Figure 5. Dopamine, carbidopa, and L-DOPA as AhR ligands in BxPC3 and Panc1 cells.
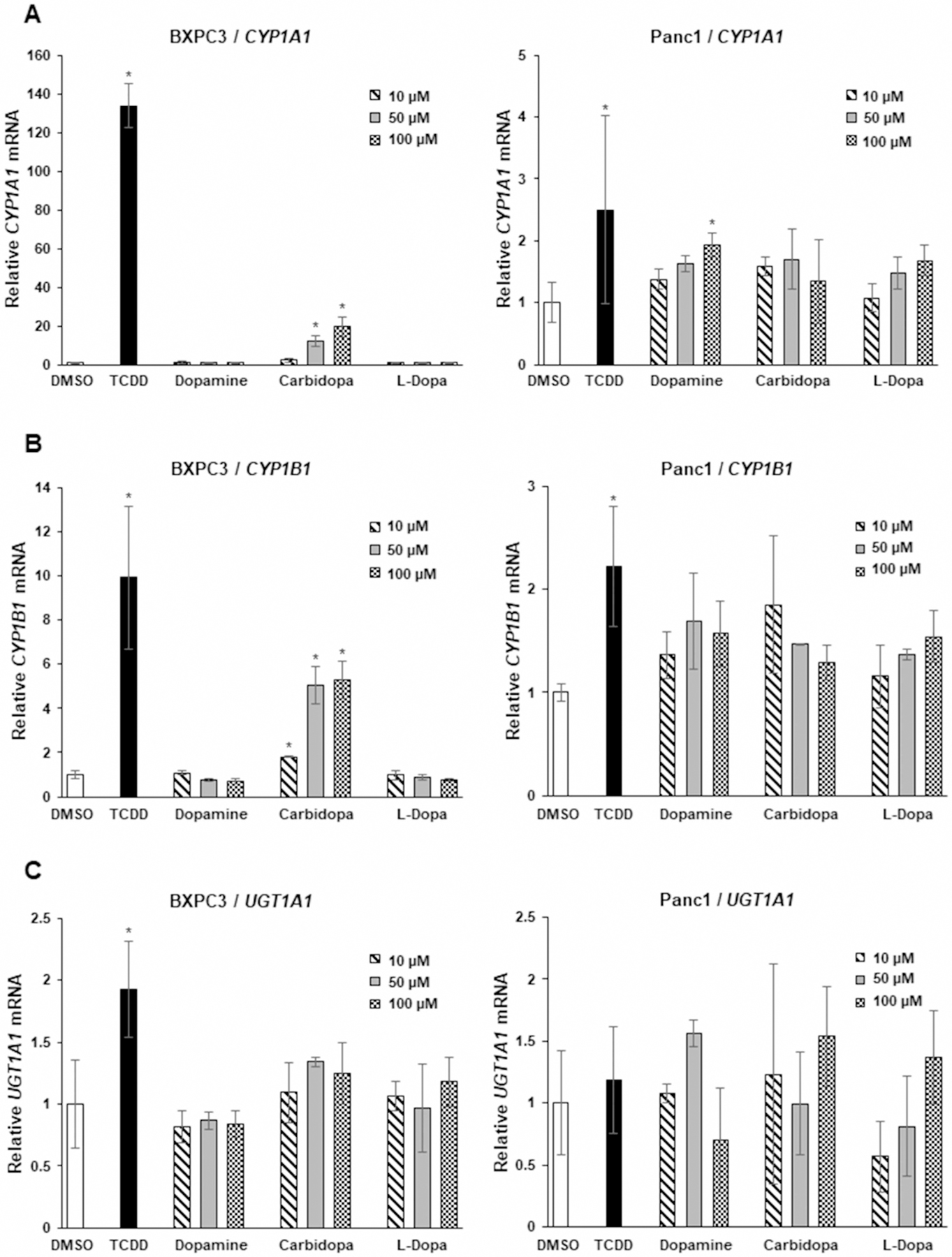
Cells were treated with DMSO, 10 nM TCDD, 10–100 µM dopamine, carbidopa, and L-DOPA. Induction of CYP1A1 (A), CYP1B1 (B) and UGT1A1 (C) was determined by real-time PCR as outlined in the Methods. Results are expressed as means ± SD for at least three determinations for each treatment group and significant (P < 0.05) induction is indicated (*).
Figure 6.
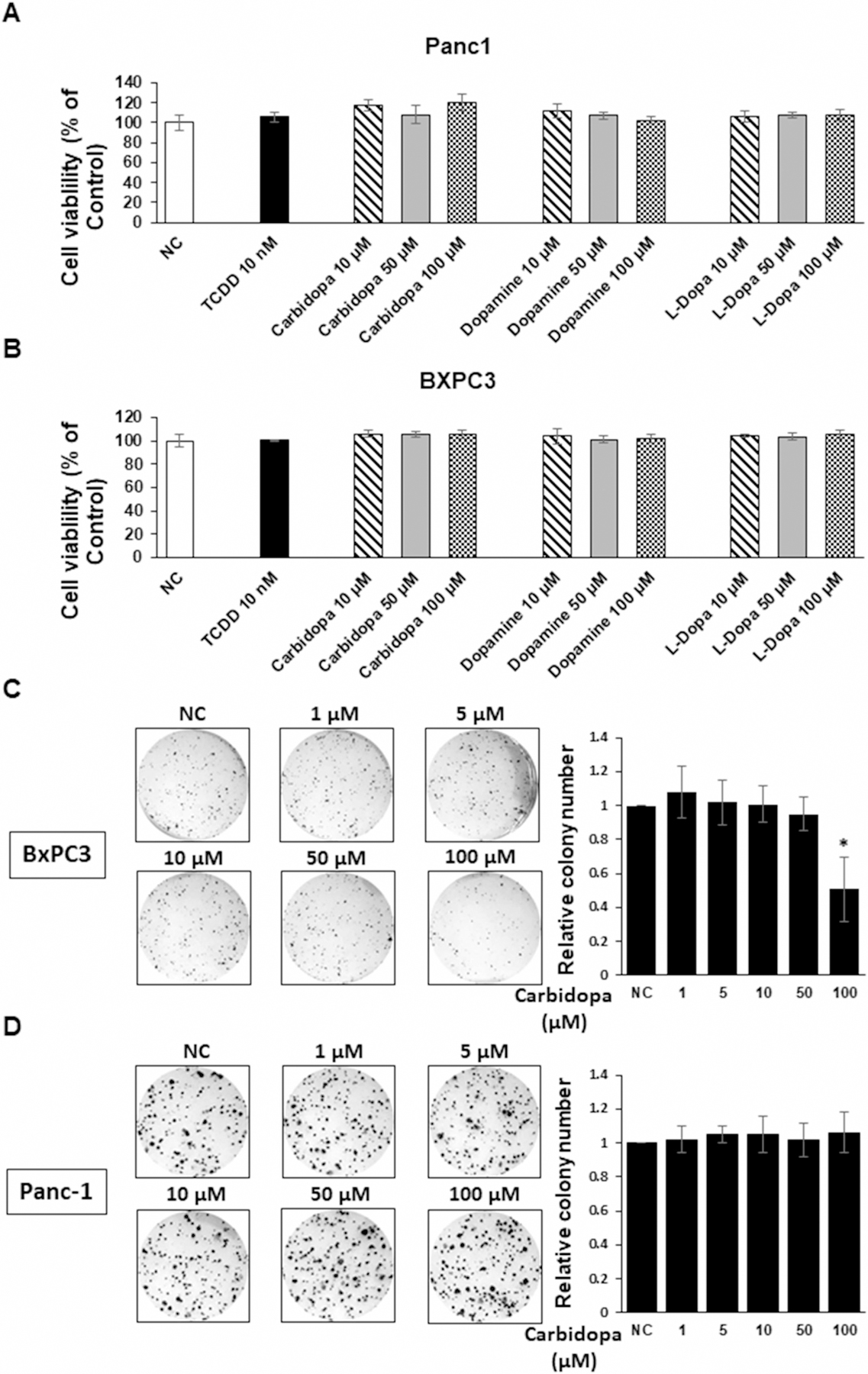
Cytotoxicity of dopamine, carbidopa, and L-DOPA in Panc1 (A) and BxPC3 (B) cells treated with DMSO, 10 nM TCDD, and 10–100 µM dopamine, carbidopa and L-DOPA for 24 h. Cell viability were determined by cell counting as outlined in the Methods. Cell viability was also determined in BxPC3 (C) and Panc1 (D) cells in a clonogenic assay as described [20] and as outlined in the Methods. Results are expressed as a means ± SD for at least three determinations for each treatment group and significant (P < 0.05) inhibition is indicated (*).
Discussion
The AhR was first discovered as the intracellular protein that mediated the biochemical and toxic responses of TCDD and related halogenated aromatics [31]. Subsequent studies have identified structurally diverse chemicals as AhR ligands and they include other industrial compounds, pharmaceuticals, phytochemicals including flavonoids, alkaloids, other polyhydroxy compounds, and other phytochemicals found in plant extracts, microbial metabolites, and endogenous biochemicals [32–35]. In contrast with TCDD, many of these compounds are health-promoting and this is consistent with the identification of multiple functions of the AhR in maintaining cellular homeostasis and in many diseases including cancer [36–38]. There is considerable evidence that many AhR ligands act as tissue type-specific AhR agonists and antagonists and are selective AhR modulators (SAhRMs) [39–43]. For example, the first AhR antagonist developed in this laboratory [6-methyl-1,3,8-trichlor-odibenzofuran (MCDF)] antagonized TCDD-induced CYP1A1 induction and some TCDD-induced responses but acted as an AhR agonist (like TCDD) and an inhibitor of estrogen signaling [39,44].
Several studies show that tryptophan and tyrosine metabolites such as tryptamine, indole-3-aldehyde, several indole acids, serotonin, and carbidopa (an L-DOPA derivative) exhibit AhR agonist activity [20,25–27,12]. Moreover, carbidopa not only induced CYP1A1 in some cancer cell lines but this compound also inhibited BxPC3 and Capan-2 cell proliferation and tumor growth in an athymic nude mouse xenograft model [20]. Research in our laboratories has used Caco2 cells as a sensitive model for screening phytochemicals and tryptophan metabolites as inducers of drug-metabolizing enzyme gene expression. We confirmed that like TCDD, carbidopa induced CYP1A1, CYP1B1, and UGT1A1 in Caco2 cells and similar results were also observed for dopamine in this cell line (Figure 1) whereas only minimal (but significant) induction was observed for L-DOPA (Supplementary Figure S1). These responses were inhibited by the AhR antagonists CH223191 and induction responses were minimal in CRISPR/Cas9 generated 15-037-AhRKO cells (Figure 2) and in 14-015 GBM cells (Figure 3) which exhibit minimal AhR-responsiveness [22]. We also observed variable responses for TCDD, carbidopa and dopamine among the colon and GBM cells with Caco2 cells being the most responsive (Figures 1–3). In Caco2 cells, TCDD dopamine and carbidopa induced CYP1A1 and enhanced DNA Binding in a ChIP and modified gel shift assay (Figure 4).
Subsequent studies in established and patient-derived glioblastoma cells and pancreatic cancer cells demonstrate tumor-type and gene-specific induction responses for dopamine and carbidopa. Both compounds induced drug-metabolizing enzyme gene expression in Caco2 cells and to a lesser extent in glioblastoma cells; whereas, only carbidopa was AhR-active in BxPC3 cells and both compounds were inactive in Panc1 cells. These differences in Ah-responsiveness between BxPC3 and Panc1 cells are not surprising since ligand-induced nuclear uptake of the AhR is observed in BxPC3 [20] but not Panc1 cells [30]. Despite the differences in Ah-responsiveness (e.g. CYP1A1 induction) of carbidopa and dopamine in BxPC3 cells, neither compound affected proliferation of BxPC3 or Panc1 cells (Figure 6A,B) or colon cancer cells (Supplementary Figure S1B). We further confirmed that carbidopa inhibited cell viability in a clonogenic assay in BxPC3 cells (Figure 6C) but this was observed only after treatment with 100 µM carbidopa; whereas, 1 µM carbidopa was previously reported to be inhibitory in this same cell line [20]. In contrast, inhibition of cell viability by carbidopa in the more aggressive Panc1 cells was not observed (Figure 6D).
Results of this study demonstrate that dopamine is an AhR agonist and activation of Ah-responsive genes by dopamine and carbidopa is gene- and cell- context specific which is consistent with their activity as SAhRMs. The cytotoxicity of dopamine and carbidopa is relatively low in pancreatic and colon cancer cells and they are unlikely to affect tumorigenicity at current physiological (dopamine) levels or pharmacological (carbidopa) doses. We also observed that norepinephrine did not exhiobit AhR activity (data not shown) and are currently investigating other catecholamines and drugs used for treating Parkinson’s disease for their activity as AhR ligands.
Supplementary Material
Funding
The National Institutes of Health [R01-AT010282, R01-CA202697, R35-CA197707, and P30-ES029607], the Syd Kyle Chair, Allen Endowed Chair in Nutrition & Chronic Disease Prevention, Ray Nesbitt Endowed Chair, and Texas Agrilife is gratefully acknowledged.
Abbreviations
- AhR
aryl hydrocarbon receptor
- CYP
cytochrome P450
- DOPA
3,4-dihydroxy-L-phenylalanine
- DRE
dioxin responsive element
- FBS
fetal bovine serum
- MCDF
6-methyl-1,3,8-trichlorodibenzofuran
- PCR
polymerase chain reaction
- SAhRM
selective AhR modulator
- UGT UDP
glucuronosyl transferase
Footnotes
Competing Interests
The authors declare that there are no competing interests associated with the manuscript.
References
- 1.Donia MS and Fischbach MA (2015) HUMAN MICROBIOTA. Small molecules from the human microbiota. Science 349, 1254766 10.1126/science.1254766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Russell WR, Hoyles L, Flint HJ and Dumas ME (2013) Colonic bacterial metabolites and human health. Curr. Opin. Microbiol 16, 246–254 10.1016/j.mib.2013.07.002 [DOI] [PubMed] [Google Scholar]
- 3.Martinez KB, Leone V and Chang EB (2017) Microbial metabolites in health and disease: Navigating the unknown in search of function. J. Biol. Chem 292, 8553–8559 10.1074/jbc.R116.752899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Trompette A, Gollwitzer ES, Yadava K, Sichelstiel AK, Sprenger N, Ngom-Bru C, et al. (2014) Gut microbiota metabolism of dietary fiber influences allergic airway disease and hematopoiesis. Nat. Med 20, 159–166 10.1038/nm.3444 [DOI] [PubMed] [Google Scholar]
- 5.Riviere A, Selak M, Lantin D, Leroy F and De Vuyst L (2016) Bifidobacteria and butyrate-producing colon bacteria: importance and strategies for their stimulation in the human gut. Front. Microbiol 7, 979 10.3389/fmicb.2016.00979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baxter NT, Schmidt AW, Venkataraman A, Kim KS, Waldron C and Schmidt TM (2019) Dynamics of human gut microbiota and short-chain fatty acids in response to dietary interventions with three fermentable fibers. mBio 10, e02566–18 10.1128/mBio.02566-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Le Floc’h N, Otten W and Merlot E (2011) Tryptophan metabolism, from nutrition to potential therapeutic applications. Amino Acids 41, 1195–1205 10.1007/s00726-010-0752-7 [DOI] [PubMed] [Google Scholar]
- 8.Agus A, Planchais J and Sokol H (2018) Gut microbiota regulation of tryptophan metabolism in health and disease. Cell Host Microbe 23, 716–724 10.1016/j.chom.2018.05.003 [DOI] [PubMed] [Google Scholar]
- 9.Sonner JK, Keil M, Falk-Paulsen M, Mishra N, Rehman A, Kramer M, et al. (2019) Dietary tryptophan links encephalogenicity of autoreactive T cells with gut microbial ecology. Nat. Commun 10, 4877 10.1038/s41467-019-12776-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Platten M, Nollen EAA, Rohrig UF, Fallarino F and Opitz CA (2019) Tryptophan metabolism as a common therapeutic target in cancer, neurodegeneration and beyond. Nat. Rev. Drug Discov 18, 379–401 10.1038/s41573-019-0016-5 [DOI] [PubMed] [Google Scholar]
- 11.Roager HM and Licht TR (2018) Microbial tryptophan catabolites in health and disease. Nat. Commun 9, 3294 10.1038/s41467-018-05470-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zelante T, Iannitti RG, Cunha C, De Luca A, Giovannini G, Pieraccini G, et al. (2013) Tryptophan catabolites from microbiota engage aryl hydrocarbon receptor and balance mucosal reactivity via interleukin-22. Immunity 39, 372–385 10.1016/j.immuni.2013.08.003 [DOI] [PubMed] [Google Scholar]
- 13.Alexeev EE, Lanis JM, Kao DJ, Campbell EL, Kelly CJ, Battista KD, et al. (2018) Microbiota-derived indole metabolites promote human and murine intestinal homeostasis through regulation of interleukin-10 receptor. Am. J. Pathol 188, 1183–1194 10.1016/j.ajpath.2018.01.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aoki R, Aoki-Yoshida A, Suzuki C and Takayama Y (2018) Indole-3-pyruvic acid, an aryl hydrocarbon receptor activator, suppresses experimental colitis in mice. J. Immunol 201, 3683–3693 10.4049/jimmunol.1701734 [DOI] [PubMed] [Google Scholar]
- 15.Rothhammer V, Mascanfroni ID, Bunse L, Takenaka MC, Kenison JE, Mayo L, et al. (2016) Type I interferons and microbial metabolites of tryptophan modulate astrocyte activity and central nervous system inflammation via the aryl hydrocarbon receptor. Nat. Med 22, 586–597 10.1038/nm.4106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rothhammer V, Borucki DM, Tjon EC, Takenaka MC, Chao CC, Ardura-Fabregat A, et al. (2018) Microglial control of astrocytes in response to microbial metabolites. Nature 557, 724–728 10.1038/s41586-018-0119-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Manzella C, Singhal M, Alrefai WA, Saksena S, Dudeja PK and Gill RK (2018) Serotonin is an endogenous regulator of intestinal CYP1A1 via AhR. Sci. Rep 8, 6103 10.1038/s41598-018-24213-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.DiNatale BC, Murray IA, Schroeder JC, Flaveny CA, Lahoti TS, Laurenzana EM et al. (2010) Kynurenic acid is a potent endogenous aryl hydrocarbon receptor ligand that synergistically induces interleukin-6 in the presence of inflammatory signaling. Toxicol. Sci 115, 89–97 10.1093/toxsci/kfq024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Seok SH, Ma ZX, Feltenberger JB, Chen H, Chen H, Scarlett C, et al. (2018) Trace derivatives of kynurenine potently activate the aryl hydrocarbon receptor (AHR). J. Biol. Chem 293, 1994–2005 10.1074/jbc.RA117.000631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ogura J, Miyauchi S, Shimono K, Yang S, Gonchigar S, Ganapathy V et al. (2017) Carbidopa is an activator of aryl hydrocarbon receptor with potential for cancer therapy. Biochem. J 474, 3391–3402 10.1042/BCJ20170583 [DOI] [PubMed] [Google Scholar]
- 21.Karki K, Li X, Jin UH, Mohankumar K, Zarei M, Michelhaugh SK et al. (2020) Nuclear receptor 4A2 (NR4A2) is a druggable target for glioblastomas. J. Neurooncol 146, 25–39 10.1007/s11060-019-03349-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jin UH, Karki K, Cheng Y, Michelhaugh SK, Mittal S and Safe S (2019) The aryl hydrocarbon receptor is a tumor suppressor-like gene in glioblastoma. J. Biol. Chem 294, 11342–11353 10.1074/jbc.RA119.008882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Coothankandaswamy V, Cao S, Xu Y, Prasad PD, Singh PK, Reynolds CP et al. (2016) Amino acid transporter SLC6A14 is a novel and effective drug target for pancreatic cancer. Br. J. Pharmacol 173, 3292–3306 10.1111/bph.13616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Franken NA, Rodermond HM, Stap J, Haveman J and van Bree C (2006) Clonogenic assay of cells in vitro. Nat. Protoc 1, 2315–2319 10.1038/nprot.2006.339 [DOI] [PubMed] [Google Scholar]
- 25.Jin UH, Lee SO, Sridharan G, Lee K, Davidson LA, Jayaraman A et al. (2014) Microbiome-derived tryptophan metabolites and their aryl hydrocarbon receptor-dependent agonist and antagonist activities. Mol. Pharmacol 85, 777–788 10.1124/mol.113.091165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cheng Y, Jin UH, Allred CD, Jayaraman A, Chapkin RS and Safe S (2015) Aryl hydrocarbon receptor activity of tryptophan metabolites in young adult mouse colonocytes. Drug Metab. Dispos 43, 1536–1543 10.1124/dmd.115.063677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hubbard TD, Murray IA and Perdew GH (2015) Indole and tryptophan metabolism: endogenous and dietary routes to Ah receptor activation. Drug Metab. Dispos 43, 1522–1535 10.1124/dmd.115.064246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jin UH, Park H, Li X, Davidson LA, Allred C, Patil B, et al. (2018) Structure-dependent modulation of aryl hydrocarbon receptor-mediated activities by flavonoids. Toxicol. Sci 164, 205–217 10.1093/toxsci/kfy075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Park H, Jin UH, Orr AA, Echegaray SP, Davidson LA, Allred CD, et al. (2019) Isoflavones as Ah receptor agonists in colon-Derived cell lines: structure-activity relationships. Chem. Res. Toxicol 32, 2353–2364 10.1021/acs.chemrestox.9b00352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jin UH, Kim SB and Safe S (2015) Omeprazole inhibits pancreatic cancer cell invasion through a nongenomic aryl hydrocarbon receptor pathway. Chem. Res. Toxicol 28, 907–918 10.1021/tx5005198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Poland A, Glover E and Kende AS (1976) Stereospecific, high affinity binding of 2,3,7,8-tetrachlorodibenzo-p-dioxin by hepatic cytosol: evidence that the binding species is receptor for induction of aryl hydrocarbon hydroxylase. J. Biol. Chem 251, 4936–4946 [PubMed] [Google Scholar]
- 32.Denison MS and Nagy SR (2003) Activation of the aryl hydrocarbon receptor by structurally diverse exogenous and endogenous chemicals. Annu. Rev. Pharmacol. Toxicol 43, 309–334 10.1146/annurev.pharmtox.43.100901.135828 [DOI] [PubMed] [Google Scholar]
- 33.Denison MS and Faber SC (2017) And now for something completely different: diversity in ligand-dependent activation of Ah receptor responses. Curr. Opin. Toxicol 2, 124–131 10.1016/j.cotox.2017.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Denison MS, Seidel SD, Rogers WJ, Ziccardi MH, Winter GM and Heath-Pagliuso S (1998) Natural and synthetic ligands for the Ah receptor In Molecular Bioloy Approaches to Toxicology (Puga A and Kendall KB, eds), pp 3–33, Taylor and Francis, London [Google Scholar]
- 35.Soshilov AA and Denison MS (2014) Ligand promiscuity of aryl hydrocarbon receptor agonists and antagonists revealed by site-directed mutagenesis. Mol. Cell. Biol 34, 1707–1719 10.1128/MCB.01183-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Esser C and Rannug A (2015) The aryl hydrocarbon receptor in barrier organ physiology, immunology, and toxicology. Pharmacol. Rev 67, 259–279 10.1124/pr.114.009001 [DOI] [PubMed] [Google Scholar]
- 37.Stockinger B, Di Meglio P, Gialitakis M and Duarte JH (2014) The aryl hydrocarbon receptor: multitasking in the immune system. Annu. Rev. Immunol 32, 403–432 10.1146/annurev-immunol-032713-120245 [DOI] [PubMed] [Google Scholar]
- 38.Safe S, Lee SO and Jin UH (2013) Role of the aryl hydrocarbon receptor in carcinogenesis and potential as a drug target. Toxicol. Sci 135, 1–16 10.1093/toxsci/kft128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McDougal A, Wormke M, Calvin J and Safe S (2001) Tamoxifen-induced antitumorigenic/antiestrogenic action synergized by a selective Ah receptor modulator. Cancer Res 61, 3901–3907 [PubMed] [Google Scholar]
- 40.Murray IA, Morales JL, Flaveny CA, Dinatale BC, Chiaro C, Gowdahalli K et al. (2010) Evidence for ligand-mediated selective modulation of aryl hydrocarbon receptor activity. Mol. Pharmacol 77, 247–254 10.1124/mol.109.061788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhao B, Degroot DE, Hayashi A, He G and Denison MS (2010) CH223191 is a ligand-selective antagonist of the Ah (Dioxin) receptor. Toxicol. Sci 117, 393–403 10.1093/toxsci/kfq217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Flaveny CA, Murray IA, Chiaro CR and Perdew GH (2009) Ligand selectivity and gene regulation by the human aryl hydrocarbon receptor in transgenic mice. Mol. Pharmacol 75, 1412–1420 10.1124/mol.109.054825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Murray IA, Krishnegowda G, DiNatale BC, Flaveny C, Chiaro C, Lin JM et al. (2010) Development of a selective modulator of aryl hydrocarbon (Ah) receptor activity that exhibits anti-inflammatory properties. Chem. Res. Toxicol 23, 955–966 10.1021/tx100045h [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Harris M, Zacharewski T, Astroff B and Safe S (1989) Partial antagonism of 2,3,7,8-tetrachlorodibenzo-p-dioxin-mediated induction of aryl hydrocarbon hydroxylase by 6-methyl-1,3,8-trichlorodibenzofuran: mechanistic studies. Mol. Pharmacol 35, 729–735 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


