Abstract
Differential emotional reactivity to social and nonsocial stimuli has been hypothesized but rarely examined empirically in ASD despite its potential importance for development of social motivation, cognition, and comorbid psychopathology. This study examined emotional reactivity, regulation, and attention to social and nonsocial threat in toddlers with ASD (n = 42, Mage: 22 months) and typically developing (TD) toddlers (n = 22, Mage: 23 months), and their mutual associations with autism symptom severity. Participants were exposed to social (stranger), nonsocial (mechanical objects), and ambiguous (masks) threats, and their intensity of distress (iDistress), attention to threat (Attention), and presence of emotion regulation (ER) strategies were measured. Autism symptom severity was quantified using the Autism Diagnostic Observation Schedule-2. In response to social threat, toddlers with ASD exhibited elevated iDistress (P < 0.038) but lower Attention (P < 0.002) and a wider variety of ER strategies (P < 0.040) compared to TD controls, though their ER strategies were less likely to be social. However, nonsocial and ambiguous threat elicited lower iDistress in ASD than in TD toddlers (P = 0.012 and P = 0.034, respectively), but comparable Attention and ER strategy use. Autism severity was not associated with iDistress. The study demonstrates elevated emotional salience but diminished attentional salience of social threat in ASD. A failure to attend adequately to social threats may restrict opportunities to appraise their threat value and engender often observed in ASD negative emotional responses to novel social situations. Early atypical emotional reactivity may independently contribute to the shaping of complex autism phenotypes and may be linked with later emerging affective and behavioral symptoms.
Keywords: social threat, emotional reactivity, attention, emotion regulation
Lay Summary:
Compared to typically developing toddlers, toddlers with ASD show diminished attention yet enhanced distress in response to social threat. Poor attention to potential social threat may limit opportunities to assess its threat value and thus contribute to often observed negative emotional responses to novel social situations. Identifying the precursors of atypical emotional reactivity in infancy and its links with later psychopathology will inform about novel treatment targets and mechanisms of change in the early stages of ASD.
Introduction
Autism spectrum disorder (ASD) is a complex neurodevelopmental disorder characterized by social impairments and presence of restricted interests and repetitive behaviors [American Psychiatric Association, 2013]. A vast majority of young children with ASD also exhibit atypical negative emotional reactivity to everyday challenges [Chandler et al., 2016; Macari, Koller, Campbell, & Chawarska, 2017; Raza et al., 2020]. One of the dimensions of emotional reactivity is the intensity of distress response to potential threat. In the general population, atypical reactivity to threat in infancy predicts later psychopathology, including affective and behavioral symptoms [Colder, Mott, & Berman, 2002; Putnam & Stifter, 2005], both highly prevalent in ASD [Simonoff et al., 2008]. Moreover, there is growing evidence that the type of threat matters, as studies in general population suggest that responses to social and nonsocial threat reflect distinct constructs, follow different developmental trajectories, show limited intraindividual consistency, and differentially predict later psychopathology [Brooker, Kiel, & Buss, 2016; Dyson, Klein, Olino, Dougherty, & Durbin, 2011; Kochanska, 1991].
Despite the potential importance of emotional reactivity to understanding the complex phenotypes and clinical needs of children with ASD, to date, very few studies have focused directly on this topic. Even fewer have assessed whether trigger characteristics (social vs. nonsocial threat) differentially affect children with a social disability. In one study, Macari and colleagues [Macari et al., 2018] employed the Laboratory Temperament Assessment Battery – Locomotor Version (Lab-TAB) [Gagne, Van Hulle, Aksan, Essex, & Goldsmith, 2011; Goldsmith & Rothbart, 1999] and showed attenuated intensity of fear in response to nonsocial threat (approach by novel objects) compared to developmentally delayed (DD) and typical developing (TD) controls. Lower intensity of distress to nonsocial threat in toddlers with ASD was accompanied by significantly lower changes in physiological arousal measured by skin conductance levels compared to TD controls [Vernetti et al., 2020]. In contrast, Scherr and colleagues [Scherr, Hogan, Hatton, & Roberts, 2017] reported that in response to social threat (approach by a stranger), preschool children with ASD exhibited more intense facial expressions of fear compared to TD and Fragile X comparison groups. The extant, albeit limited, evidence suggests that young children with ASD may also respond differentially to social vs. nonsocial threat, though this hypothesis has never been tested directly.
One of the hallmarks of ASD is limited selective attention to people observed in the contexts of virtual or real-life interactions [Chawarska, Macari, & Shic, 2012; Falck-Ytter, Bölte, & Gredebäck, 2013; Guillon, Hadjikhani, Baduel, & Rogé, 2014]. While toddlers with ASD do not avoid looking at social stimuli (e.g. faces), under certain conditions they fail to increase their attention to faces in a manner observed in typically developing (TD) and developmentally delayed toddlers [Moriuchi, Klin, & Jones, 2016; Shic, Wang, Macari, & Chawarska, 2020]. These findings indicate that the intrinsic value of social stimuli for guiding behavioral responses (or salience) [Tatler, Hayhoe, Land, & Ballard, 2011] differs fundamentally between those with and without social disability [Chawarska et al., 2012; Moriuchi et al., 2016]. This evidence is based on social stimuli that are either neutral or positively valenced; however, evidence regarding attention to social stimuli that are potentially threatening is limited. A failure to selectively attend to threatening social stimuli may have a profound impact on emotional reactivity and safety in ASD. If toddlers with ASD direct few attentional resources to novel persons, they may have a limited opportunity to appraise their threat value and consequently either under- or over-react to their presence or approach. To our best knowledge, no study has ever examined links between selective attention and emotional reactivity in response to social and nonsocial threat in ASD.
When faced with emotional challenges, individuals typically engage a range of emotion regulation strategies. Emotion regulation (ER) refers to behaviors that aim to monitor and modulate the duration or intensity of an emotional reaction [Gross, Sheppes, & Urry, 2011]. When upset in a context of mother–child interaction, toddlers with ASD employed a wide range of age-appropriate ER strategies including comfort-seeking, as well as physical and verbal actions [Gulsrud, Jahromi, & Kasari, 2010]. Less-adaptive ER strategies have been observed in preschoolers with ASD in response to frustration-inducing tasks, including more frequent reliance on nonsocial regulatory strategies [Jahromi, Meek, & Ober-Reynolds, 2012] or less mature approaches to emotion regulation including fewer social-communicative strategies [Nuske et al., 2017]. This trend continues as school-age children and adolescents with ASD use fewer normative strategies to cope with their emotions [Jahromi et al., 2012; Mazefsky, Borue, Day, & Minshew, 2014]. Taken together, this work suggests that in contexts designed to elicit negative emotions, children with ASD engage a variety of ER strategies, though they may be less mature or adaptive, especially as the children grow older. No studies, though, have investigated ER strategies specifically in response to threat, either social or nonsocial, in young children with ASD.
Finally, despite the observations that social and emotional difficulties co-occur at the earliest time that ASD can be reliably diagnosed [Macari et al., 2017; Raza et al., 2020], the mutual links between the social and emotional challenges remain poorly understood. Historically, some have argued that autism symptoms arise from increased negative emotional reactivity [Kanner, 1943/1968; Tinbergen & Tinbergen, 1977], while others suggested that emotional abnormalities are secondary to social perception and cognition deficits inherent to autism [Baron-Cohen et al., 2000; Schultz, 2005]. A more recent conceptualization of the links between social and emotional vulnerabilities in ASD suggests they may represent separable developmental domains with distinct etiologies [Hawks, Marrus, Glowinski, & Constantino, 2019; Micalizzi, Ronald, & Saudino, 2016]. This idea is based on studies in the general population examining links between autistic-like or social traits and precursors of later psychopathology. For instance, a study investigating autistic and affective features in a prospective sample of 2- and 3- year-old twins demonstrated high cross-age stability of the traits but negligible cross-lagged effects, suggesting that early in development, there is a limited contribution of autistic-like traits to affective traits and vice versa [Micalizzi et al., 2016]. Similarly, a study of 18 month olds demonstrated very weak intercorrelations between social characteristics and internalizing and externalizing behaviors and an increasing cross-lagged association by the age of 3 [Hawks et al., 2019]. Uniquely, our study design allowed us to examine the contributions of distress reactivity along with attentional and regulatory behaviors to severity of autism symptoms in toddlers with ASD.
The present study addresses several key gaps in the evidence identified above. It investigates the effects of group (ASD, TD) and threat type (social, nonsocial, ambiguous) on intensity of emotional distress, visual attention, and the ER strategies used. The stimuli aimed to elicit fear were adapted from the Lab-TAB - Locomotor Version [Goldsmith & Rothbart, 1999] and consisted of the Stranger, Objects, and Masks probes. While the Stranger and Objects probes represent, respectively, social and nonsocial threat, the Masks probe has a more ambiguous character as it involves grotesque objects (masks) worn by a person, thus retaining features of both agency and canonical “faceness.” The primary comparisons are between Stranger (social) and Objects (nonsocial) conditions; the ambiguous Masks probe, used widely in studies of the general population, is included in an exploratory manner, with the hope of illuminating its utility for studying emotional reactivity in toddlers with social disabilities. We also examine associations between attention to threat and intensity of distress, as well as regulatory behaviors and the contributions of these variables to the severity of autism symptoms. We focus our investigation on toddlers with ASD (n = 42, MAge = 22.4 months) and chronological age-matched typically developing (TD) (n = 22, MAge = 22.9 months) toddlers without a familial history of ASD. The sample was ascertained at the earliest time when autism can be first reliably diagnosed, and the emotional expression of the toddlers was not likely to be shaped by the acquisition of display rules or the effects of early intervention.
Based on prior work [Macari et al., 2018], in the nonsocial condition, we expected that toddlers with ASD will exhibit attenuated intensity of distress compared to TD controls. In the social condition, however, if social threat is perceived by toddlers with ASD as more emotionally salient [Scherr et al., 2017], intensity of distress in ASD is expected to be higher than in the TD group. Similarly, based on prior work on selective attention [Chawarska et al., 2012; Chawarska, Macari, & Shic, 2013; Pierce et al., 2016; Shic et al., 2020], we hypothesized that toddlers with ASD will display lower attention to social but not to nonsocial threat, compared to the TD group. Further, we investigated whether the groups differ in the range of emotional regulation (ER) strategies employed during the probes, and whether this effect was modulated by probe type as well as whether the groups differ in the proportion of ER strategies that are social in nature. Finally, we examined intercorrelations between distress reactivity, attention to threat, and ER strategies used, and their links with symptom severity.
Methods and Materials
Participants
The study was approved by the Human Investigation Committee of the Yale School of Medicine and informed written consent was obtained from all parents prior to testing. Participants consisted of toddlers with ASD (n = 42; Mage = 22.42 months) and TD controls without a familial history of ASD (n = 22; Mage = 22.97 months). Participants with ASD were referred to a university clinic for a differential diagnosis of ASD by their parents or healthcare providers and TD controls were recruited through advertisements between November 2015 and October 2018. The participants underwent an assessment of developmental skill using the Mullen Scales of Early Learning (MSEL) and severity of autism symptoms using the Autism Diagnostic Observation Schedule – 2 (ADOS-2) Toddler Module. An interdisciplinary team of expert clinicians (clinical psychologist, speech & language pathologist, and social worker) assigned a DSM-5 clinical best estimate (CBE) ASD diagnosis based on the results of direct assessment along with developmental and medical history. Toddlers with known genetic abnormalities or gestational age below 34 weeks were excluded. Females constituted 26% of the ASD sample as compared to 41% in the TD group (χ2(1) = 1.456, P = 0.228) and the groups did not differ in chronological age (P = 0.552). The groups differed in the severity of autism symptoms and the levels of cognitive and adaptive functioning (see Table 1 for sample characteristics). A small subsample of the participants (19 out of 64, 29%) were included in the Macari et al. [2018] study reporting broadly on emotional reactivity in response to fear-, frustration-, and joy-eliciting probes in toddlers with ASD.
Table 1.
Sample Characteristics
| Variable | ASD | TD | |
|---|---|---|---|
|
| |||
| N | 42 | 22 | |
| Age (months) | 22.42 (3.38) | 22.97 (3.84) | P = 0.552 |
| Male (N/%) | 13 (74%) | 13 (59%) | P = 0.232 |
| MSEL Verbal DQ | 51.71 (19.73) | 119.73 (16.28) | P < 0.001 |
| MSEL Nonverbal DQ | 82.31 (12.67) | 112.19 (16.07) | P < 0.001 |
| ADOS-2 Calibrated Severity | 7.00 (2.63) | 1.32 (0.48) | P < 0.001 |
ADOS-2: Autism Diagnostic Observation Schedule-2; DQ: Developmental Quotient; MSEL: Mullen Scales of Early Learning.
Procedure
All toddlers underwent an assessment of emotional reactivity to fear-inducing probes. The induction probes were adapted with minor adjustments from the Lab-TAB - Locomotor Version. The probes were designed to elicit fear through encounters with novel and potentially threatening stimuli: Stranger (social), Objects (nonsocial), and Masks (ambiguous). The Stranger probe involved a female stranger wearing dark clothing, a hat, and sunglasses entering the room, approaching the child, and leaning toward the child for approximately 3 s (one trial). The Objects condition included Spider (large mechanical spider crawling toward the child, three trials) and Dinosaur (mechanical dinosaur with red light-up eyes approaching the child, three trials). Masks involved a female stranger dressed in dark clothes and wearing three grotesque masks in succession (e.g. vampire, Star Wars character) entering the room briefly and maintaining an approximate 1.5-m distance from the child (three trials). Given often atypical responses to social overtures and to touch in toddlers with ASD, the examiners did not speak, touch, or otherwise try to engage the participants during the induction probes. Each probe lasted approximately 60 s with the effective exposure to threat time of approximately 30 s. Breaks were instituted between each probe, with a minimum of 30 s and an average of 75 s (SD = 36 s) needed to ensure that the child’s affect returned to neutral before proceeding to the next probe. The administration of the probes was highly standardized across participants in multiple respects including the examiners’ script, physical arrangement of people, furniture, and stimuli, perceptual appearance of stimuli, and duration of probes. Two video cameras mounted on perpendicular walls afforded ample views of the scene. A parent, seated within reach of the child in the testing room during the probes, was instructed to keep a neutral demeanor and refrain from interacting with the child unless in response to the child’s distress. All children received the identical set of probes in the same order: spider, stranger, masks, and dinosaur.
Data Reduction and Outcome Measures
The Lab-TAB sessions were video-recorded and subsequently coded for peak intensity of distress (iDistress) response across facial and vocal channels, for visual attention to the threatening stimuli, and for emotion regulation strategies. Sessions were assigned to coders who were blinded to group membership. Coders had established reliability greater than 0.80 indexed by intraclass correlation coefficient (ICC) with a gold standard coder prior to the beginning of coding. Subsequently interrater reliability was calculated by doublecoding a random selection of the sessions (15%) [Chorney, McMurtry, Chambers, & Bakeman, 2015] utilizing a two-way random ICC model. ICC for the intensity of distress (iDistress) variable was r = 0.89 (range of ICCs for individual codes 0.79–0.95), for ER strategies, r = 0.89, (range of ICCs for individual codes 0.82–0.95), and for attention to threat, r = 0.97.
Distress intensity.
Each video-recorded trial was coded offline for peak intensity of facial and vocal distress. Facial expression coding was based on the AFFEX system [Durbin, Hayden, Klein, & Olino, 2007; Izard, Huebner, Risser, McGinnes, & Dougherty, 1980], which has been used successfully in studies of the stability of emotions [Durbin et al., 2007] and has shown high convergent validity with a microcoding system involving coding in discrete time intervals [Durbin, Klein, Hayden, Buckley, & Moerk, 2005]. Vocal expression codes were adapted from the Lab-TAB-Locomotor Version. Each trial was coded for peak intensity of facial distress (fear, sadness) on a scale of 0–3 and vocal distress (fussing, crying, and other negative vocalizations) on a scale of 0–5. Intensity of distress (iDistress) was computed by averaging scores on intensity of facial and vocal distress across trials within each condition.
Attention to threat.
Recorded sessions were coded offline using Noldus: The Observer behavioral software (version XT 12). Each probe was coded using mutually exclusive duration codes for attention to the threatening stimuli (stranger, spider, masks, or dinosaur) and to other elements in the room (e.g. parent, objects). Coders recorded onset and offset of the children’s focus of attention using Observer. Moments during which gaze direction could not be verified (e.g. because view of the child’s gaze was obstructed) were coded as unknown and excluded from the analysis. For each condition, selective attention to threat (Attention) was computed as the proportion of looking time toward the threat divided by the total looking time toward the threat and other elements in the room.
Emotion regulation (ER).
Sessions were coded offline for the presence of emotion regulation strategies. These strategies were based on those appearing in the Lab-TAB Locomotor manual [Goldsmith & Rothbart, 1999] and related developmental literature [Buss & Goldsmith, 1998; Kopp, 1989; Stansbury & Sigman, 2000]. Each trial was coded for the presence or absence of the following emotion regulation (ER) strategies: social communication (communicative bids with or without eye contact), physical comfort seeking (e.g., reaching toward parent), disengagement from threat (e.g. looking or moving away), seeking sensory input (e.g. thumb sucking), and repetitive movements (e.g. hand flapping). Subsequently, for each trial, we calculated the proportion of ER strategies used (total number of strategies used/total number of strategies coded). In the final step, we averaged the trial ER scores within each condition to compute the condition ER score. Higher scores indicated a wider variety of different strategies used to regulate emotions within a condition. In an exploratory analysis, we also computed a ratio of the number of social strategies (social communication and physical comfort seeking) over the total number of strategies (social communication, physical comfort seeking, disengagement, sensory seeking behaviors, and repetitive movements) used (social ER ratio score) to evaluate whether utilization of social ER strategies varied by group and condition.
Statistical Analysis
The primary dependent variables (iDistress, Attention, ER strategies) were analyzed using linear mixed-effects models with group (ASD, TD) and condition (Stranger, Object, Mask) as fixed effects and compound symmetry covariance structure. The models were fit using restricted maximum likelihood (REML) with significance evaluated using Kenward–Roger degrees of freedom [Kenward & Roger, 1997]. Linear contrasts were estimated and tested using the SAS lsmeans procedure. The hypotheses were tested comparing the Stranger condition with the Objects and Masks conditions using planned contrasts. The intercorrelations between attention to threat and distress intensity were evaluated using Pearson’s r correlation coefficient analysis with Bonferroni correction for multiple comparisons (0.05/6 = 0.008). Predictive models for examining links between severity of autism symptoms and the dependent measures were tested using simple multivariate regression analysis. The analyses were implemented in SAS 9.4 statistical software.
Results
Initial Exclusions
Of the expected 10 trials per participant, toddlers with ASD contributed 93.10% (391 out of 420) trials compared to 87.73% (193 out of 220) trials contributed by TD toddlers [χ2 (1) 5.21, P = 0.024] suggesting that the TD group completed fewer trials than the ASD group. Some trials were terminated due to the child’s negative affect (18/640, 2.81%), and others were excluded from analysis for technical reasons (e.g. toy not working or face not visible during coding) (21/640, 3.28%) or due to parental noncompliance (i.e. parent interfering with probe administration) (17/640, 2.66%). After these initial exclusions, 41 children with ASD contributed valid iDistress data in the Masks condition, and 42 contributed to the remaining two conditions. All 22 TD toddlers contributed data to all three conditions. Probes for which gaze direction could not be coded for more than 50% of the time (i.e. the child’s point of regard could not be discerned reliably) were excluded from the analysis of attention leading to the exclusion of 2.1% of probes (all ASD, one probe per child). Data on ER strategy use were available for all participants.
Distress Reactivity
Effects of diagnosis and condition.
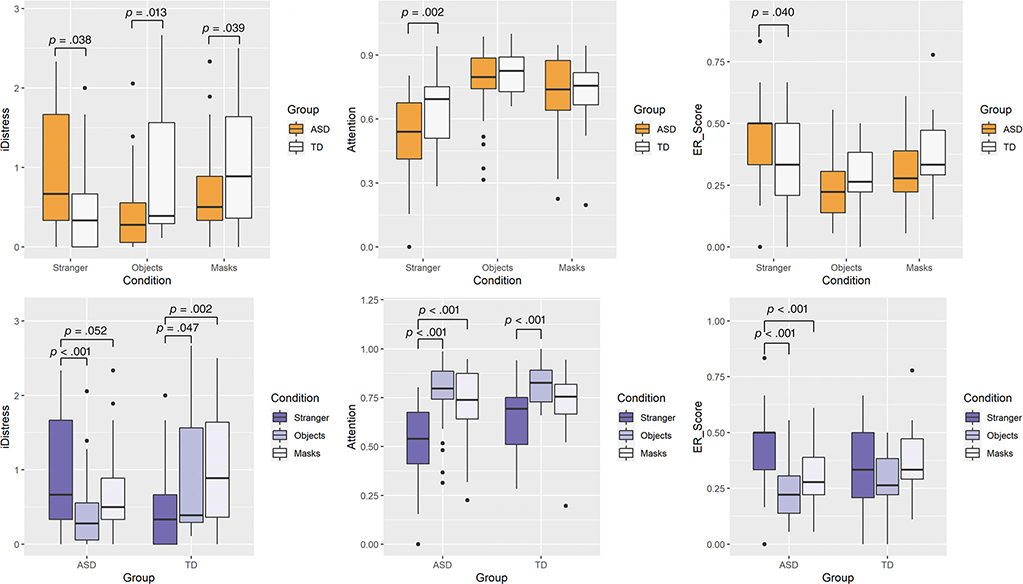
Linear mixed-effects model analysis on mean iDistress indicated no significant effects of group, F(1, 61.3) = 1.21, P = 0.275 or condition, F(2,122) =2.26, P = 0.109 toddlers with ASD exhibited higher iDistress than TD controls (P = 0.038, d = 0.537, ASD – TD difference = 0.398, 95% CI: 0.039–0.758); however, in the Object (P = 0.012, d = 0.697, ASD-TD difference = −0.183, 95% CI: −0.816 to −0.094) and Masks (P = 0.034, d = 0.518, ASD-TD difference = −0.376, 95% CI: −0.736 to −0.015) conditions they exhibited lower iDistress than TD controls (Fig. 1a and Table 2). Within-group comparisons indicated that in the ASD group, iDistress was significantly higher in the Stranger compared to the Object (P < 0.001, Stranger-Object difference = 0.503, 95 CI: 0.263–0.742) and the Masks condition, though the latter was marginally significant (P = 0.052, Stranger-Mask difference = 0.239, 95% CI: 0.001–0.478). In comparison, in the TD group, iDistress in the Stranger condition was lower than in the Object (P = 0.047, Stranger – Object difference = −0.351, 95%CI: −0.679 to −0.023) and Masks (P = 0.002, Stranger – Masks difference = −0.535, 95%CI: −0.863 to −0.208) conditions (Fig. 1b and Table 2).
Figure 1.
Estimated marginal means (±1 SE) of intensity of distress (iDistress) score, proportion of looking time at threat (Attention) scores, and average number of ER strategies used (ER score) in response to the Stranger, Object, and Masks conditions between the ASD and TD groups (a) and within each group (b).
Table 2.
Average (SD) Facial and Vocal Distress Intensity (iDistress), Proportion of Looking Time at Threat (Attention), and Number of ER Strategies Used (ER score) in Response to Stranger, Objects, and Masks conditions in the ASD and TD groups
| Measure | Condition | ASD | TD | Cohen’s d | P-value |
|---|---|---|---|---|---|
|
| |||||
| iDistress | Stranger | 0.93 (0.80) | 0.55 (0.60) | 0.537 | 0.034 |
| Objects | 0.41 (0.47) | 0.88 (0.83) | 0.697 | 0.015 | |
| Masks | 0.69 (0.66) | 1.07 (0.80) | 0.518 | 0.043 | |
| Attention | Stranger | 0.52 (0.19) | 0.65 (0.17) | 0.721 | 0.003 |
| Objects | 0.77 (0.16) | 0.81 (0.10) | 0.299 | 0.379 | |
| Masks | 0.71 (0.18) | 0.73 (0.16) | 0.117 | 0.745 | |
| ER score | Stranger | 0.44 (0.18) | 0.36 (0.19) | 0.432 | 0.040 |
| Objects | 0.24 (0.12) | 0.29 (0.13) | 0.399 | 0.120 | |
| Masks | 0.31 (0.12) | 0.37 (0.15) | 0.441 | 0.135 | |
Note. The values marked in bold are statistically significant at P < 0.05.
In the ASD group, there were no significant associations between iDistress and verbal and nonverbal DQ scores, respectively, in the Stranger (r(42) = 0.19; r(42) = −0.02), Objects (r(41) = 0.04; r(41) = −0.22), or Masks (r(41) = 0.04, r(41) = 0.06) conditions (all P-values >0.17), suggesting that the observed iDistress responses were not related to overall developmental level.
Attention to Threat
Linear mixed-effects analysis on Attention indicated a nonsignificant effect of group, F(1,61.8) = 3.40, P = 0.069, significant effect of condition, F(1,120) = 37.04, P < 0.001, and group × condition interaction, F(2,120) 3.54, P = 0.032. In the Stranger condition, toddlers with ASD attended less to the stranger than the TD group (P = 0.002, d = 0.721, ASD-TD difference = −0.136, 95% CI: −0.223 to −0.049), but there were no differences between ASD and TD groups in the Masks (P = 0.704) or Objects (P = 0.381) conditions (see Fig. 1a and Table 2). Within-group comparisons indicated that toddlers with ASD looked less at threat in the Stranger than in the Objects (P < 0.001, Stranger – Object difference: −0.254, 95%CI: −0.311 to −0.197) and the Masks (P < 0.001, Stranger – Masks difference = −0.192, 95%CI: −0.249 to −0.135) conditions. The TD group also looked less at threat in the Stranger condition compared to the Object condition (P < 0.001, Stranger – Object difference = −0.157, 95%CI: −0.234 to − 0.080) but not in the Masks condition (P = 0.072, Stranger – Masks difference = −0.071, 95%CI: −0.148 to 0.006) (Fig. 1b and Table 2). Thus, toddlers with ASD attended less to threat in the social condition, but their attention to threat in the nonsocial and ambiguous conditions was comparable to that observed in the TD group.
Subsequently, we evaluated associations between intensity of distress and attention to threat in the three conditions. In the Stranger condition, the association was not statistically significant in either group (ASD: r(41) = −0.122, P = 0.448; TD: r(22) = 0.259, P = 0.244), suggesting that, in the context of the present experiment, looking at threat was not linked with the intensity of distress in response to approach by an unfamiliar adult. In the Object condition, the correlation was significant in the ASD group, r(40) = −0.457, P = 0.003 but not in the TD group, r(22) = 0.295, P = 0.244, suggesting that less distressed toddlers with ASD tended to look more at the spider or dinosaur. Finally, in the Masks condition, there was no significant association between attention to threat and iDistress in either the ASD (r(40) = −0.248, P = 0.129) or the TD (r(22) = −0.155, P = 0.491) group. Associations between attention to threat and intensity of distress were negligible, except for the Object condition where greater distress intensity was associated with lower attention to threat.
Emotion Regulation
Linear mixed-effects analysis on the average ER scores indicated no effect of group, F(1,60.4) = 0.17, P = 0.684, and a significant effect of condition, F(2,121) = 15.27, P < 0.001 and a group × condition interaction, F(2,121) = 5.24, p = 0.006. Planned comparisons indicated that in the Stranger condition, toddlers with ASD used on average, a wider variety of ER strategies than TD controls (P = 0.040, d = 0.432, ASD – TD difference = 0.080, 95% CI: 0.004–0.157). There were, however, no differences between the groups in the average number of strategies used in the Objects (P = 0.120, d = 0.399, ASD-TD difference = −0.055, 95%CI: −0.131 to 0.0223) and Masks (P = 0.135, d = 0.441, ASD-TD difference = −0.059, 95% CI: −0.135 to 0.018) conditions (see Fig. 1a and Table 2). Within-group comparisons indicated that toddlers with ASD employed a wider variety of ER strategies in the Stranger compared to the Objects (P < 0.001, Stranger – Object difference = 0.202, 95%CI: 0.145–0.260) and Masks (P < 0.001, Stranger – Masks difference: 0.129, 95% CI: 0.072–0.186) conditions. The TD group employed a comparable average number of ER strategies in the Stranger and Objects conditions (P = 0.150, Stranger – Objects difference: 0.067, 95%CI: −0.011 to 0.146) and in the Stranger and Masks (P = 0.759, Stranger – Masks difference = −0.010, 95%CI: −0.088 to 0.068) conditions (Fig. 1b and Table 2).
In an exploratory analysis, we investigated what proportion of the strategies was social and whether it varied by group and condition. The analysis of social ER ratio score indicated a significant effect of group (1, 60.7) = 9.96, P = 0.003 and condition, F(2,119) = 10.46, P < 0.001, but no group × condition interaction, F(2,119) = 1.14, P = 0.323. In toddlers with ASD, a lower proportion of the ER strategies were social (M = 0.47, SD = 0.252) compared to the TD group (M = 0.623, SD = 0.263) (ASD – TD difference = −0.152, 95%CL: −0.249 to −0.056). In both groups combined, the proportion of social ER strategies in the Stranger condition (M = 0.457, SD = 0.292) was lower than in the Objects condition (M = 0.610, SD = 0.242) (P < 0.001) (Stranger-Object difference = −0.178, 95%CI: −0.258 to −0.097), but comparable between the Stranger and Masks (M = 0.499, SD = 0.239) (P = 0.262) conditions (Stranger – Masks difference = −0.045, 95%CI: −0.125 to 0.034).
Associations Between Symptom Severity and iDistress, Attention to Threat, and ER Strategies
Correlations between autism severity scores and iDistress, attention to threat, and average number of ER strategies are presented in Table 3. Multiple regression analysis was employed to test if intensity of distress, attention to threat, and average number of ER strategies predicted participants’ severity of autism symptoms as measured by ADOS-2 total calibrated severity scores (Table 4). The only significant predictor of autism severity in the Stranger condition was Attention (β = −0.467, P = 0.002), explaining 20% of the variance (Adjusted R2 = 0.20, F(1,39) = 10.89, P = 0.002). Two significant predictors of severity in the Objects condition were identified: Attention (β = −0.60, P < 0.001) and average number of ER strategies (β = −0.58, p = 0.002), accounting jointly for 25.9% of the variance in the autism severity score (Adjusted R2 = 0.26, F(1,39) = 7.80, p = 0.002). Finally, in the Masks condition, the analysis indicated only a marginal contribution of Attention (β = −0.31, P = 0.058, Adjusted R2 = 0.07, F(1,39) = 3.82, P = 0.058. Taken together, the results suggest that severity of autism symptoms was predicted most consistently by low attention to the threatening stimuli, but not by intensity of the emotional response to the stimuli.
Table 3.
Correlations Between Autism Symptom Severity Measured by ADOS-T Total Calibrated Severity Score and the iDistress, ER strategies, and Attention Measures in the Stranger, Objects, and Masks conditions in the ASD Group
| Condition | Statistic | iDistress | ER Strategies | Attention |
|---|---|---|---|---|
|
| ||||
| Stranger | r | 0.033 | −0.018 | −0.561 |
| P-value | 0.840 | 0.912 | <0.001 | |
| n | 41 | 41 | 40 | |
| Objects | r | 0.075 | −0.211 | −0.331 |
| P-value | 0.643 | 0.186 | 0.037 | |
| n | 41 | 41 | 40 | |
| Masks | r | 0.089 | −0.053 | −0.371 |
| P-value | 0.579 | 0.741 | 0.020 | |
| n | 41 | 41 | 39 | |
Note. The correlations marked in bold are statistically significant at P < 0.05.
Table 4.
Summary of Simple Regression Analysis for Variables Predicting ADOS-2 Calibrated Severity Scores in Toddlers With ASD Based on Distress Reactivity, Attention to Threat, and Average Number of Emotion Regulation Strategies used in the Stranger, Objects, and Masks conditions
| Condition | Predictor | B | SE B | beta | t-value | P-value |
|---|---|---|---|---|---|---|
|
| ||||||
| Stranger | iDistress | −0.54 | 0.90 | −0.08 | −0.59 | 0.556 |
| Attention | −16.57 | 3.92 | −0.58 | −4.23 | <0.001 | |
| ER | −3.21 | 4.09 | −0.11 | −0.79 | 0.437 | |
| Objects | iDistress | −0.06 | 1.79 | −0.01 | −0.03 | 0.973 |
| Attention | −22.48 | 6.14 | −0.65 | −3.66 | <0.001 | |
| ER | −24.88 | 7.51 | −0.56 | −3.31 | 0.002 | |
| Masks | iDistress | −0.19 | 1.40 | −0.02 | −0.14 | 0.891 |
| Attention | −11.57 | 4.98 | −0.38 | −2.32 | 0.026 | |
| ER | −2.31 | 7.55 | −0.05 | −0.31 | 0.761 | |
Note. The correlations marked in bold are statistically significant at P < 0.05.
Discussion
To the best of our knowledge, this is the first study examining whether in the early stages of ASD, the affected children exhibit atypical responses to social vs. nonsocial threat on emotional, attentional, and regulatory levels. The study suggests that social threat elicits less visual attention, more intense distress, and triggers deployment of a wider variety of regulatory behaviors in toddlers with ASD compared to TD controls. In contrast, nonsocial threat elicits a dampened distress response in the ASD group compared to TD toddlers, but comparable attention and ER strategy use. Even though toddlers with ASD employ a comparable or higher average number of ER strategies as TD controls, the strategies are less likely to be social. Intensity of distress to social threat does not track with either attention to threat or severity of autism symptoms. We discuss clinical and theoretical implications of these findings in turn.
In the TD toddlers, the approaching stranger was the least distressing stimulus compared to potentially threatening objects and masks, which is consistent with prior studies showing a decrease in stranger fear response from infancy into toddlerhood [Brooker et al., 2016; LoBue & Adolph, 2019]. This phenomenon has been attributed to the development of the ability to seek out and to read contextual social cues (e.g. from parents) as well as by the prior history of interacting with unfamiliar people, which together may mitigate the distress response to strangers in toddlers [LoBue & Adolph, 2019]. As in the TD group, threat type modulated emotional reactivity in the ASD group; however, the pattern was reversed. Specifically, consistent with prior work [Scherr et al., 2017] toddlers with ASD exhibited a higher distress to the approaching stranger relative to TD controls. Toddlers with ASD attempted to regulate their responses to emotional challenges using a variety of emotional regulation strategies. However, the strategies, in general, were less likely to involve seeking proximity of parents, making eye contact, or otherwise communicating their distress to others. This means, that even though the toddlers attempt to regulate their emotions, they are less likely to use powerful and effective strategies that involve leveraging parental capacity to calm and comfort. This may put already emotionally vulnerable toddlers at further risk for developing affective and behavioral problems later on. Thus, therapeutic efforts at this age may need to be directed specifically at fostering a more frequent utilization of social ER strategies in distressing contexts.
The study also examined attention to threat and its links with distress intensity. Compared to the TD group, toddlers with ASD exhibited lower attention to the stranger despite elevated distress intensity; their attention to the stranger was also lower than their attention to the objects and masks. No group differences were found in attention to nonsocial or ambiguous threat. While it might be tempting to conclude that shorter dwell time on social threat in toddlers with ASD is a strategy to downregulate distress caused by an approaching stranger [Scherr et al., 2017], support for this conclusion is limited. Specifically, the magnitude of the association between the intensity of distress and attention to social threat was negligible in both groups; thus, there was no evidence that looking at or away from social threat was associated with distress, at least at the level of measurement employed in the present study. The observed effects are consistent with prior work, which suggests that while gaze aversion is used as a regulatory mechanism by 6-month-old infants, by 18 months this strategy is no longer predominant and is often replaced by other ER strategies [Mangelsdorf, Shapiro, & Marzolf, 1995]. Notably, toddlers with ASD attend less to people not only when they are potentially threatening (present study), but also when they display neutral or positive affect in the context of real-world encounters [Ozonoff et al., 2010; Swettenham et al., 1998] and experimental eye-tracking studies [Chawarska et al., 2012, 2013; Pierce et al., 2016; Shic et al., 2020]. Thus, poor selective attention to social stimuli (threatening or not) appears to represent a trait related to the social disability dimension rather than a trait involved in emotional reactivity to threat. Consistent with this notion and with prior work [Chawarska et al., 2012; Frazier et al., 2016; Shic et al., 2020], in our study, attention to threat was strongly associated with severity of autism symptoms. Importantly, in very young children with ASD, poor visual attention to novel persons may limit the opportunities to appraise their threat value and consequently lead to negative over-reaction to their presence or approach, with this effect being the strongest in the most severely affected children with ASD.
Taken together, toddlers with ASD display limited attention but elevated distress to social threat and they employ fewer social emotional regulation strategies at the time when ASD can be first reliably diagnosed. It may be that due to poor social attention [Chawarska et al., 2012; Shic et al., 2020] and impaired value learning in the social domain [Wang, Chang, & Chawarska, 2020], toddlers with ASD have few resources at their disposal to evaluate the threat value of potential social partners to mitigate naturally occurring wariness of unfamiliar adults common in infancy. The elevated distress accompanying these encounters may in turn result in diminished motivation to engage in novel interactions, compromising development of social and emotional interaction and coping skills downstream. The present study design did not allow us to test the causal relationships between the attentional and emotional vulnerabilities. To disambiguate the relationships between the two dimensions in ASD, it would be best to model these dimensions prospectively from infancy into toddlerhood. There is already evidence that infants later diagnosed with ASD exhibit lower attention to novel interactive partners [Macari et al., 2020; Ozonoff et al., 2010] and may display greater physiological dysregulation when interacting with strangers [McCormick et al., 2018], though their developmental dynamics and interactions remain to be examined. Improving our understanding of how these vulnerabilities interact in infancy and contribute to the phenotypes observed in newly diagnosed toddlers may inform about novel treatment targets and strategies that could be implemented during the presymptomatic phase of the disorder in populations at heightened risk for ASD [Chawarska & Volkmar, 2020].
Importantly, in the ASD group, the association between distress intensity and autism severity was negligible, regardless of the type of threat. The latter finding is consistent with studies suggesting that fear responses and social deficits are not mediated by the same circuitry in the amygdala [Emery et al., 2001; Emery & Amaral, 2000; Prather et al., 2001] and with evidence from genetic twin studies highlighting etiologic independence of autistic-like traits and precursors of later affective and behavioral psychopathology [Hawks et al., 2019; Micalizzi et al., 2016]. The findings are also consistent with prior parent-report and experimental studies, which indicate that negative (fear and frustration) affectivity does not track with severity of autism symptoms in infants [Paterson et al., 2019], toddlers [Macari et al., 2017; Macari et al., 2018], and older children with ASD [Herrington et al., 2017; Konstantareas & Stewart, 2006]. This may suggest a “dual hit” where affective and social vulnerabilities coalesce to produce complex sets of challenges for the affected toddlers. In this context, it may be that the enhanced distress response to the stranger along with the attenuated emotional response to novel/threatening objects represent early childhood predictors of later comorbid psychopathology common among school-aged children with ASD. Indeed, in the general population, attenuated distress reactivity to threat in early childhood has been linked with both internalizing and externalizing symptoms later on, whereas elevated reactivity to threat is predictive of internalizing symptoms [Colder et al., 2002; Putnam & Stifter, 2005]. With regard to threat type, an elevated distress response to strangers in infancy is associated with later behavioral inhibition [Brooker et al., 2013; De Rosnay, Cooper, Tsigaras, & Murray, 2006; Kagan, Reznick, & Snidman, 1987], a precursor to social anxiety [Brooker et al., 2016; Rosenbaum et al., 1991]. In this context, it is plausible that the atypical response to unfamiliar adults may forecast later emerging internalizing or social anxiety symptoms often comorbid with ASD, whereas attenuated distress to nonsocial threat may indicate the potential for safety concerns and later emerging behavioral symptoms. Prospective studies following the toddlers into school age would help to discover how the combined social, attentional, and affective vulnerabilities affect long-term outcomes in young children with ASD with an aim of identifying novel treatment targets and mechanisms of change.
While TD toddlers perceived the threat level emanating from a person wearing a mask as significantly higher than the threat level associated with an approaching stranger, toddlers with ASD made less of a distinction between the two types of threat. That is, in the ASD group, masks elicited a distress intensity that fell between social and nonsocial threat on the emotional level; on the ER and attentional levels however, responses to Masks were more aligned with the responses to the nonsocial threat. This performance feature may inform future study designs aiming to capture emotional reactivity to threat in socially disabled children.
Limitations and Future Directions
Due to the absence of a developmentally delayed control group, it is not clear to what extent the observed results are specific to ASD or common in other developmental disorders. However, the lack of significant associations between intensity of distress and verbal and nonverbal skills in our ASD sample mitigates, to some extent, this concern. Although the study represents an advancement over those based solely on parent report, in order to capture the full extent of the emotional reactivity to perceived threat in children with ASD, future studies should attempt to investigate their emotional reactivity in real-world contexts (daycare, preschool, home environment) using a combination of behavioral and physiological measures. Lastly, given the sex ratio observed in ASD, our sample did not allow us to examine contribution of sex to emotional reactivity; this potentially important moderator will need to be investigated in a context of larger studies focused more specifically on effects of sex on emotional development in ASD.
Conclusions
This, to the best of our knowledge, is the first direct demonstration that the social-nonsocial dimension of threat modulates the fear response in toddlers with ASD in an atypical manner and motivates an investigation into the roots of this phenomenon in infancy and its consequences in later childhood. The study also demonstrates that toddlers with ASD exhibit limited attentional salience but elevated emotional salience of social threat. Poor attention to novel people may limit opportunities to appraise their threat value and lead to more generalized distress in novel social situations. Atypical emotional reactivity to social and nonsocial threat is not related to severity of autism symptoms and thus, may independently contribute to the development of the heterogenous clinical phenotypes in young children with ASD. The significance of this work is twofold. First, emotional reactivity constitutes an important contributor of social and emotional development [Emde, Gaensbauer, & Harmon, 1981; Izard, 2002]. Identification of atypical facets of emotional reactivity at the earliest time when ASD can be diagnosed reliably informs about factors shaping complex phenotypes and developmental outcomes of children with ASD and may assist in identification of novel treatment targets. Second, atypical emotional reactivity in early childhood predicts later onset of internalizing and externalizing symptoms [Colder et al., 2002; Putnam & Stifter, 2005] common in older children with ASD [Simonoff et al., 2008]. Considering the high prevalence of such symptoms in ASD, identifying early patterns of emotional reactivity in ASD may lead to better diagnostic precision in terms of early detection of comorbid psychopathology in ASD and greater insight into emotional features that confer risk for affective and problem behaviors in this population.
Acknowledgments
The study was supported by the National Institutes of Mental Health R01 MH100182, R01 MH111652 grants awarded to Katarzyna Chawarska. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The authors thank the children and their families participating in the study. The authors acknowledge Wendy Silverman, PhD, of Yale for her comments on earlier drafts of the manuscript, and the clinical team of the Yale Toddler Developmental Disabilities Clinic for their contribution to sample characterization.
Footnotes
Conflict of Interest
The authors report no conflict of interest.
Data Availability
The data are available from the corresponding author upon reasonable request.
References
- American Psychiatric Association. (2013). Diagnostic and statistical manual of mental disorders: DSM-5. Washington, DC: American Psychiatric Association. [Google Scholar]
- Baron-Cohen S, Ring HA, Bullmore ET, Wheelwright S, Ashwin C, & Williams S (2000). The amygdala theory of autism. Neuroscience & Biobehavioral Reviews, 24(3), 355–364. [DOI] [PubMed] [Google Scholar]
- Brooker RJ, Buss KA, Lemery-Chalfant K, Aksan N, Davidson RJ, & Goldsmith HH (2013). The development of stranger fear in infancy and toddlerhood: normative development, individual differences, antecedents, and outcomes. Developmental Science, 16(6), 864–878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooker RJ, Kiel EJ, & Buss KA (2016). Early social fear predicts kindergarteners’ socially anxious behaviors: Direct associations, moderation by inhibitory control, and differences from nonsocial fear. Emotion, 16(7), 997–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buss KA, & Goldsmith HH (1998). Fear and anger regulation in infancy: Effects on the temporal dynamics of affective expression. Child Development, 69(2), 359–374. [PubMed] [Google Scholar]
- Chandler S, Howlin P, Simonoff E, O’sullivan T, Tseng E, Kennedy J, … Baird G (2016). Emotional and behavioural problems in young children with autism spectrum disorder. Developmental Medicine & Child Neurology, 58(2), 202–208. [DOI] [PubMed] [Google Scholar]
- Chawarska K, Macari S, & Shic F (2012). Context modulates attention to social scenes in toddlers with autism. Journal of Child Psychology and Psychiatry, 53(8), 903–913. 10.1111/j.1469-7610.2012.02538.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chawarska K, Macari S, & Shic F (2013). Decreased spontaneous attention to social scenes in 6-month-old infants later diagnosed with autism spectrum disorders. Biological Psychiatry, 74(3), 195–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chawarska K, & Volkmar FR (Eds.). (2020). Autism Spectrum Disorder in the First Years of Life. New York, NY: Guilford Publications. [Google Scholar]
- Chorney JM, McMurtry CM, Chambers CT, & Bakeman R (2015). Developing and modifying behavioral coding schemes in pediatric psychology: A practical guide. Journal of Pediatric Psychology, 40(1), 154–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colder CR, Mott JA, & Berman AS (2002). The interactive effects of infant activity level and fear on growth trajectories of early childhood behavior problems. Development and Psychopathology, 14(1), 1–23. [DOI] [PubMed] [Google Scholar]
- De Rosnay M, Cooper PJ, Tsigaras N, & Murray L (2006). Transmission of social anxiety from mother to infant: An experimental study using a social referencing paradigm. Behaviour Research and Therapy, 44(8), 1165–1175. [DOI] [PubMed] [Google Scholar]
- Durbin C, Hayden EP, Klein DN, & Olino TM (2007). Stability of laboratory-assessed temperamental emotionality traits from ages 3 to 7. Emotion, 7(2), 388–399. [DOI] [PubMed] [Google Scholar]
- Durbin C, Klein DN, Hayden EP, Buckley ME, & Moerk KC (2005). Temperamental emotionality in preschoolers and parental mood disorders. Journal of Abnormal Psychology, 114(1), 28–37. [DOI] [PubMed] [Google Scholar]
- Dyson MW, Klein DN, Olino TM, Dougherty LR, & Durbin C (2011). Social and non-social behavioral inhibition in preschool-age children: Differential associations with parent-reports of temperament and anxiety. Child Psychiatry and Human Development, 42(4), 390–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emde RN, Gaensbauer T, & Harmon RJ (1981). Using our emotions: Some principles for appraising emotional development and intervention. In Lewis M & Taft LT (Eds.), Developmental Disabilities (pp. 409–424). Dordrecht: Springer. 10.1007/978-94-011-6314-9_25. [DOI] [Google Scholar]
- Emery NJ, & Amaral DG (2000). The role of the amygdala in primate social cognition. In Lane RD & Nadel L (Eds.), Cognitive neuroscience of emotion (pp. 156–191). New York, NY: Oxford University Press. [Google Scholar]
- Emery NJ, Capitanio JP, Mason WA, Machado CJ, Mendoza SP, & Amaral DG (2001). The effects of bilateral lesions of the amygdala on dyadic social interactions in rhesus monkeys (Macaca mulatta). Behavioral Neuroscience, 115(3), 515–544. [PubMed] [Google Scholar]
- Falck-Ytter T, Bölte S, & Gredebäck G (2013). Eye tracking in early autism research. Journal of Neurodevelopmental Disorders, 5(1), 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frazier TW, Klingemier EW, Beukemann M, Speer L, Markowitz L, Parikh S, … Strauss MS (2016). Development of an objective autism risk index using remote eye tracking. Journal of the American Academy of Child & Adolescent Psychiatry, 55(4), 301–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gagne JR, Van Hulle CA, Aksan N, Essex MJ, & Goldsmith H (2011). Deriving childhood temperament measures from emotion-eliciting behavioral episodes: Scale construction and initial validation. Psychological Assessment, 23 (2), 337–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldsmith H, & Rothbart MK (1999). The Laboratory Temperament Assessment Battery: Locomotor Version. [Google Scholar]
- Gross JJ, Sheppes G, & Urry HL (2011). Emotion generation and emotion regulation: A distinction we should make (carefully). Cognition and Emotion (Print), 25(5), 765–781. [DOI] [PubMed] [Google Scholar]
- Guillon Q, Hadjikhani N, Baduel S, & Rogé B (2014). Visual social attention in autism spectrum disorder: Insights from eye tracking studies. Neuroscience & Biobehavioral Reviews, 42, 279–297. [DOI] [PubMed] [Google Scholar]
- Gulsrud AC, Jahromi LB, & Kasari C (2010). The co-regulation of emotions between mothers and their children with autism. Journal of Autism and Developmental Disorders, 40(2), 227–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawks ZW, Marrus N, Glowinski AL, & Constantino JN (2019). Early origins of autism comorbidity: neuropsychiatric traits correlated in childhood are independent in infancy. Journal of Abnormal Child Psychology, 47(2), 369–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrington JD, Maddox BB, McVey AJ, Franklin ME, Yerys BE, Miller JS, & Schultz RT (2017). Negative valence in autism spectrum disorder: The relationship between amygdala activity, selective attention, and co-occurring anxiety. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging, 2(6), 510–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izard CE (2002). Translating emotion theory and research into preventive interventions. Psychological Bulletin, 128(5), 796–824. [DOI] [PubMed] [Google Scholar]
- Izard CE, Huebner RR, Risser D, McGinnes GC, & Dougherty LM (1980). The young infant’s ability to produce discrete emotion expressions. Developmental Psychology, 16(132), 140. [Google Scholar]
- Jahromi LB, Meek SE, & Ober-Reynolds S (2012). Emotion regulation in the context of frustration in children with high functioning autism and their typical peers. Journal of Child Psychology and Psychiatry, 53(12), 1250–1258. [DOI] [PubMed] [Google Scholar]
- Kagan J, Reznick J, & Snidman N (1987). The physiology and psychology of behavioral inhibition in children. Child Development, 58, 1459–1473. [PubMed] [Google Scholar]
- Kanner L (1943/1968). Autistic disturbances of affective contact. Acta Paedopsychiatrica: International Journal of Child & Adolescent Psychiatry, 35(4–8), 98–136. [PubMed] [Google Scholar]
- Kenward MG, & Roger JH (1997). Small sample inference for fixed effects from restricted maximum likelihood. Biometrics, 53, 983–997. [PubMed] [Google Scholar]
- Kochanska G (1991). Patterns of inhibition to the unfamiliar in children of normal and affectively ill mothers. Child Development, 62(2), 250–263. [DOI] [PubMed] [Google Scholar]
- Konstantareas M, & Stewart K (2006). Affect regulation and temperament in children with autism spectrum disorder. Journal of Autism and Developmental Disorders, 36, 143–154. [DOI] [PubMed] [Google Scholar]
- Kopp CB (1989). Regulation of distress and negative emotions: A developmental view. Developmental Psychology, 25(3), 343–354. [Google Scholar]
- LoBue V, & Adolph KE (2019). Fear in infancy: Lessons from snakes, spiders, heights, and strangers. Developmental Psychology, 55(9), 1889–1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macari S, DiNicola L, Kane-Grade F, Prince E, Vernetti A, Powell K, … Chawarska K (2018). Emotional expressivity in toddlers with autism spectrum disorder. Journal of the American Academy of Child & Adolescent Psychiatry, 57(11), 828–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macari S, Koller J, Campbell DJ, & Chawarska K (2017). Temperamental markers in toddlers with autism spectrum disorder. Journal of Child Psychology and Psychiatry, 58(7), 819–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macari S, Milgramm A, Reed J, Shic F, Powell KK, Macris D, & Chawarska K (2020). Context-specific dyadic attention vulnerabilities during the first year in infants later developing autism spectrum disorder. Journal of the American Academy of Child & Adolescent Psychiatry. 10.1016/j.jaac.2019.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangelsdorf SC, Shapiro JR, & Marzolf D (1995). Developmental and temperamental differences in emotion regulation in infancy. Child Development, 66(6), 1817–1828. [PubMed] [Google Scholar]
- Mazefsky CA, Borue X, Day TN, & Minshew NJ (2014). Emotion regulation patterns in adolescents with high-functioning autism spectrum disorder: Comparison to typically developing adolescents and association with psychiatric symptoms. Autism Research, 7(3), 344–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick CE, Sheinkopf SJ, Levine TP, LaGasse LL, Tronick E, & Lester BL (2018). Diminished respiratory sinus arrhythmia response in infants later diagnosed with autism spectrum disorder. Autism Research, 11(5), 726–731. [DOI] [PubMed] [Google Scholar]
- Micalizzi L, Ronald A, & Saudino KJ (2016). A genetically informed cross-lagged analysis of autistic-like traits and affective problems in early childhood. Journal of Abnormal Child Psychology, 44(5), 937–947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriuchi JM, Klin A, & Jones W (2016). Mechanisms of diminished attention to eyes in autism. American Journal of Psychiatry, 174(1), 26–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuske HJ, Hedley D, Woollacott A, Thomson P, Macari S, & Dissanayake C (2017). Developmental delays in emotion regulation strategies in preschoolers with autism. Autism Research, 10(11), 1808–1822. [DOI] [PubMed] [Google Scholar]
- Ozonoff S, Iosif A, Baguio F, Cook ID, Hill MM, Hutman T, … Young GS (2010). A prospective study of the emergence of early behavioral signs of autism. Journal of the American Academy of Child & Adolescent Psychiatry, 49 (3), 258–268. [PMC free article] [PubMed] [Google Scholar]
- Paterson SJ, Wolff JJ, Elison JT, Winder-Patel B, Zwaigenbaum L, Estes A, … Hazlett HC (2019). The importance of temperament for understanding early manifestations of autism spectrum disorder in high-risk infants. Journal of Autism and Developmental Disorders, 49(7), 2849–2863. [DOI] [PubMed] [Google Scholar]
- Pierce K, Marinero S, Hazin R, McKenna B, Barnes CC, & Malige A (2016). Eye tracking reveals abnormal visual preference for geometric images as an early biomarker of an autism spectrum disorder subtype associated with increased symptom severity. Biological Psychiatry, 79(8), 657–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prather MD, Lavenex P, Mauldin-Jourdain ML, Mason WA, Capitanio JP, Mendoza SP, & Amaral DG (2001). Increased social fear and decreased fear of objects in monkeys with neonatal amygdala lesions. Neuroscience, 106(4), 653–658. [DOI] [PubMed] [Google Scholar]
- Putnam SP, & Stifter CA (2005). Behavioral approach–inhibition in toddlers: Prediction from infancy, positive and negative affective components, and relations with behavior problems. Child Development, 76(1), 212–226. [DOI] [PubMed] [Google Scholar]
- Raza S, Sacrey L-AR, Zwaigenbaum L, Bryson S, Brian J, Smith IM, … Garon N (2020). Relationship between early social-emotional behavior and autism spectrum disorder: A high-risk sibling study. Journal of Autism and Developmental Disorders, 50(7), 2527–2539. [DOI] [PubMed] [Google Scholar]
- Rosenbaum JF, Biederman J, Hirshfeld DR, Bolduc EA, Faraone SV, Kagan J, … Reznick JS (1991). Further evidence of an association between behavioral inhibition and anxiety disorders: Results from a family study of children from a non-clinical sample. Journal of Psychiatric Research, 25(1–2), 49–65. [DOI] [PubMed] [Google Scholar]
- Scherr JF, Hogan AL, Hatton D, & Roberts JE (2017). Stranger fear and early risk for social anxiety in preschoolers with Fragile X syndrome contrasted to autism spectrum disorder. Journal of Autism and Developmental Disorders, 47(12), 3741–3755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz RT (2005). Developmental deficits in social perception in autism: The role of the amygdala and fusiform face area. International Journal of Developmental Neuroscience, 23 (2–3), 125–141. [DOI] [PubMed] [Google Scholar]
- Shic F, Wang Q, Macari SL, & Chawarska K (2020). The role of limited salience of speech in selective attention to faces in toddlers with autism spectrum disorders. Journal of Child Psychology and Psychiatry, 61(4), 459–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonoff E, Pickles A, Charman T, Chandler S, Loucas T, & Baird G (2008). Psychiatric disorders in children with autism spectrum disorders: Prevalence, comorbidity, and associated factors in a population-derived sample. Journal of the American Academy of Child & Adolescent Psychiatry, 47(8), 921–929. [DOI] [PubMed] [Google Scholar]
- Stansbury K, & Sigman M (2000). Responses of preschoolers in two frustrating episodes: Emergence of complex strategies for emotion regulation. The Journal of Genetic Psychology, 161 (2), 182–202. [DOI] [PubMed] [Google Scholar]
- Swettenham J, Baron-Cohen S, Charman T, Cox A, Baird G, Drew A, … Wheelwright S (1998). The frequency and distribution of spontaneous attention shifts between social and nonsocial stimuli in autistic, typically developing, and nonautistic developmentally delayed infants. Journal of Child Psychology & Psychiatry & Allied Disciplines, 39(5), 747–753. [PubMed] [Google Scholar]
- Tatler BW, Hayhoe MM, Land MF, & Ballard DH (2011). Eye guidance in natural vision: Reinterpreting salience. Journal of Vision, 11(5), 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tinbergen E, & Tinbergen N (1977). The aetiology of childhood autism: a criticism of the Tinbergens’ theory: a rejoinder. Psychological Medicine, 6(4), 545–549. [DOI] [PubMed] [Google Scholar]
- Vernetti A, Shic F, Boccanfuso L, Macari S, Kane-Grade F, Millgram A, … Chawarka K (2020). Atypical emotional electrodermal activity in toddlers with autism spectrum disorder. Autism Research, 13, 1476–1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Chang J, & Chawarska K (2020). Atypical valuedriven selective attention in young children with autism spectrum disorder. JAMA Network Open, 3(5), e204928. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data are available from the corresponding author upon reasonable request.