Abstract
Youth with autism spectrum disorder (ASD) are at elevated risk for impaired emotion regulation (ER) and clinically impairing anxiety. A prior developmental framework posited that impaired ER leads to co-occurring psychiatric conditions such as anxiety, with outcome determined in part by ASD-specific moderating factors. Using measures developed and validated in ASD, this study evaluated: 1) the association between ER and anxiety in a large, community-based sample of youth with ASD and a wide range of intellectual and verbal abilities; and 2) whether greater core ASD symptoms strengthened the association between impaired ER and anxiety. Parents of 1,107 children with a community diagnosis of ASD (881 boys; age 6–17 years) participated in an online survey assessing their child’s ER, anxiety, and ASD symptoms. ER impairment significantly predicted whether participants had elevated levels of anxiety, after controlling for demographic variables and ASD symptoms; however, there was no interaction of ER and ASD symptoms. This study is the first to support the anxiety-ER association with measures developed and validated specifically for ASD, in a large sample with co-occurring ID and minimally verbal youth with ASD.
Keywords: emotion regulation, anxiety, core ASD symptoms, reactivity
Lay Summary
Many children with autism spectrum disorder (ASD) have problems with managing their emotions (emotion regulation) and anxiety. In this study, over 1,000 parents completed an online survey which showed that emotion regulation and anxiety are closely linked. Although emotion regulation and anxiety are inter-connected, the results also show that autism symptoms play an important role in anxiety in ASD.
Emotion regulation problems may be an important target for the treatment of anxiety in autism.
Co-occurring psychiatric conditions are more common in youth with autism spectrum disorder (ASD) than in the general population (Joshi et al., 2010; Simonoff et al., 2008; van Steensel, Bögels, & de Bruin, 2013). The high rates of co-occurring anxiety disorders in youth and adults with ASD are well-established (van Steensel, Bögels, & Perrin, 2011; White, Oswald, Ollendick, & Scahill, 2009). In a meta-analysis of 83 studies, van Steensel and Heeman (2017) reported that youth with ASD have significantly higher levels of anxiety when measured dimensionally than youth in the general population and youth without ASD ascertained from clinical settings. These findings suggest that ASD may increase the risk of anxiety.
There has been increasing interest in the role of impaired emotion regulation (ER) in the manifestation of anxiety and other psychiatric conditions in ASD (Cai, Richdale, Uljarevic, et al., 2018; White et al., 2014). ER is defined as the ability to modulate arousal and emotional responses in the service of adaptive and socially appropriate behavior (Gross & Thompson, 2006). In ASD, youth report using fewer ER strategies and employing them less flexibly (Cai et al., 2018; Khor, Melvin, Reid, & Gray, 2014). They also tend to rely on potentially maladaptive strategies such as rumination, avoidance, and denial (Khor, Melvin, Reid, & Gray, 2014; Mazefsky et al., 2014). Available data, focused mostly on verbal samples, have shown an association between greater anxiety and impaired ER (Cai et al., 2018). Impairments in ER have also been implicated in disruptive behaviors such as irritability, tantrums, and aggression as well as mood disorders in youth with ASD (Mazefsky & White, 2014; Weiss, 2014).
White and colleagues (2014) proposed a developmental framework in which impaired ER can lead to a host of co-occurring psychopathologies (i.e., multifinality), with the type of psychopathology partially determined by characteristics associated with ASD. For example, biases in attention, sensory sensitivities, alexithymia, repetitive interests, restricted behaviors and intolerance of uncertainty, and insistence on sameness may promote avoidance of certain situations. These biases may accentuate the relationship between ER impairment and anxiety (Rodgers, Glod, Connolly, & McConachie, 2012; Wigham, Rodgers, South, McConachie, & Freeston, 2015). ASD-related social impairments may play a pivotal role in the ER-anxiety association. For example, the association between social communication deficits and social anxiety in the general population of youth is well-established (Pickard, Rijsdijk, Happé, & Mandy, 2017). In ASD, one study found that engaging in characteristically adaptive ER strategies was associated with less social impairment among youth with ASD (Goldsmith & Kelley, 2018), while another observed that higher social impairment was associated with higher ER impairment (Samson et al., 2014). Overall, it may be helpful to consider how impaired ER effects anxiety in ASD, including how ASD core symptoms impact the ER-anxiety relationship.
Previous research in the general pediatric population suggests that impaired ER leads to different forms of psychopathology, including anxiety (Aldao, Nolen-Hoeksema, & Schweizer, 2010). A longitudinal study of a community sample of youth reported that impaired ER predicted later anxiety symptoms (Schneider, Arch, Landy, & Hankin, 2018). Although findings on the association between core ASD symptoms and ER impairment are mixed (White et al., 2014), there is evidence that children with more severe ASD symptoms have increased ER impairment (Samson et al., 2014). Characteristics of ASD such as inflexibility, high demand for daily routine, as well as decreased capacity to read social cues, recognize emotions of others, and difficulties with perspective taking can all contribute to impaired ER (Jahromi, Meek, & Ober-Reynolds, 2012; Konstantareas & Stewart, 2006; Mazefsky & White, 2014; Rieffe et al., 2011).
Several studies have examined the impact of social impairment on the association between anxiety and ER in youth with ASD. Maddox and White (2015) posited that increased desire for social interaction could account for heightened social anxiety in ASD. Swain and colleagues (2015) examined the interaction between social motivation, impaired ER, and social anxiety in young adults with ASD using self- and parent-report measures. Decreased social motivation was associated with greater social anxiety, suggesting that social anxiety may dampen social motivation. Low social motivation can contribute to social avoidance, increased social anxiety and greater social impairment. Social impairment, misreading social cues, and uncertainty in social situations may undermine ER skills. Thus, it is possible that greater social impairment amplifies the relationship between ER and anxiety.
ASD symptoms such as restricted interests, repetitive behaviors and sensory sensitivities, and insistence on sameness are associated with anxiety in youth with ASD (Rodgers et al., 2012; Uljarević & Evans, 2016). In an adult sample comparing ASD and an age- and IQ-matched comparison group, ASD symptoms were significantly associated with greater intolerance of uncertainty, lower emotional acceptance, and increased alexithymia. However, intolerance of uncertainty was not a significant predictor of increased anxiety (Maisel et al., 2016). When core ASD symptoms and ER impairment were examined in a sample of 56 youth with ASD, all core symptom domains were associated with increased ER impairment; restricted and repetitive behaviors were the strongest predictor of ER impairment (Samson et al., 2014). Taken together, available data suggests a complex relationship between ASD severity, anxiety, and ER impairment in ASD.
Current Study
Previous research on ER and anxiety in ASD has almost entirely focused on samples without significant language delays and without intellectual disability (ID) (Cai, Richdale, Uljarevic, et al., 2018; van Steensel & Heeman, 2017). Thus, little is known about youth with ASD with ID and those who are minimally verbal (Bal, Katz, Bishop, & Krasileva, 2016). In addition, the instruments used to measure anxiety and ER in ASD were not designed to measure these constructs in youth with ASD and are highly reliant on verbal expression, which may impact their ability to capture the full range of anxiety and ER impairment manifestations in ASD (Bearss et al., 2016; Kerns & Kendall, 2012; Mazefsky et al., 2016; Scahill et al., 2019). Given challenges in the differential diagnosis of anxiety in ASD (Kerns et al., 2015), it may be useful to consider dimensional approaches to anxiety, and explore its relationship to ER using measures validated in ASD (Mazefsky et al. 2018; Scahill et al., 2019).
In the current study, we used measures developed and validated for ASD to examine the association between ER impairment and anxiety as well as the effect of core ASD symptoms on the ER-anxiety relationship in a large sample of youth with ASD. The sample included youth with and without co-occurring ID. First, we tested whether ER impairment was associated with increased anxiety, and whether ER impairment significantly predicted elevated anxiety after controlling for demographic factors, intellectual disability, social impairment, and restricted interests or repetitive behaviors. The second aim was to determine whether more severe core autism symptoms (e.g., social impairment, restricted interests) strengthened the association between ER impairment and anxiety.
Methods
Procedures
The data were collected in partnership with the Interactive Autism Network (IAN). IAN is a national, validated and verified autism registry consisting of a representative sample of children in the U.S. with community diagnoses of ASD (Daniels et al., 2012). Parents of children on the IAN registry, with a score on the Social Communication Questionnaire (Rutter, Bailey, & Lord, 2003) of 12 or higher, between the ages of 6–17 years old, were invited to participate in the study. All IAN registry members who met inclusion criteria (11,648) were invited to participate by email. Of the 1,323 parents who consented to participate, 1,107 completed the study measures (See Table 1 for demographics data). ID diagnoses were based on parental selection: “does not have ID”, “mild ID,” or “severe ID”). Responses were dichotomized to either no ID or ID for this study’s analyses.
Table 1.
Demographics and Clinical Characteristics of Youth in Online Parent Survey (N= 1107)
| Age | Mean= 12.08 (Standard Deviation= 3.18) Range 6– 17.80 |
| Gender | N (%) |
| Male | 881 (79.6) |
| Female | 226 (20.4%) |
| Race | |
| White | 1020 (92.1) |
| Black | 65 (5.9) |
| Asian | 24 (2.2) |
| Native American | 23 (2.1) |
| Other | 45 (4.1) |
| Unknown | 4 (0.4) |
| Ethnicity | |
| Hispanic | 106 (9.6%) |
| Non-Hispanic | 1000 (90.3) |
| Currently prescribed medication | 435 (39.3) |
| Antipsychotic | 198 (17.9) |
| Mood Stabilizer | 91 (8.2) |
| Stimulant | 176 (15.9) |
| Antidepressant | 223 (20.1) |
| Intellectual Disability (ID)* | |
| None | 460 (41.6) |
| Mild ID | 397 (35.9) |
| Severe ID | 188 (17.0) |
| Missing | 62 (5.6) |
| Mean (Standard Deviation) | |
| EDI † Reactivity Short Form theta score | −0.27 (.85) |
| SCQ ‡ total score | 22.93 (6.53) |
| SRS-2 § total t-score | 86.82 (13.54) |
| SRS-2 Social Communication Index t-score | 86.36 (21.59) |
| SRS-2 Restricted Repetitive Behaviors t-score | 20.60 (6.41) |
| PRAS-ASD ¶ total score | 24.47 (13.69) |
Key:
Via parent report;
Emotion Dysregulation Inventory;
Social Communication Questionnaire;
Social Responsiveness Scale-2;
Parent-Rated Anxiety Scale-Autism Spectrum Disorder
Measures
Emotion Dysregulation Inventory (EDI: Mazefsky et al., 2018, 2018).
The EDI is a 30-item, caregiver report designed to measure ER impairment in individuals with ASD. It was developed based on guidelines from the NIH Patient-Reported Outcomes Measurement Information System (PROMIS). The items in the final version of the scale were the product of factor analysis and item response theory (IRT) analyses using data from 1755 youth with ASD. None of the final items had evidence of differential item functioning (e.g., psychometric biases) by gender, age, intellectual ability, or verbal ability, making it suitable for use across heterogeneous populations. Prior research demonstrated test–retest reliability in a treatment stable sample of 901 youth with ASD between the ages of 6 and 18 years (mean difference of 0.05, and effect size of −0.06 for paired t-tests) (Mazefsky, Yu et al., 2018). Items are rated on a 5-point scale based on behavior during the previous seven days (0= Not at all, 1=Mild, 2= Moderate, 3=Severe, and 4= Very Severe). The EDI’s primary scale measures Reactivity (intense, rapid escalating, sustained and poorly regulated emotional reactions), which is available as a 24-item long form or 7-item short form. The long and short forms were correlated .99 in the EDI’s autism psychometric sample. The EDI also includes a secondary 6-item Dysphoria (low positive affect, nervousness and sadness) scale, which was not used for this study due to the overlap with anxiety symptoms. EDI Reactivity short form theta scores, which have a mean of 0 and SD of 1 and provide superior discriminative ability to raw scores (Hamleton, 1991), were used in analyses. EDI Reactivity reliabilities in the original sample were high (α= .97) (Mazefsky, Yu, et al., 2018). For this study, internal consistency of the EDI Reactivity Short Form was also high (α= .94; non-ID subgroup α= .94; ID subgroup α= .94).
Parent-Rated Anxiety Scale-ASD (PRAS-ASD; Scahill et al., 2019).
The PRAS-ASD is a 25-item parent-report on anxiety symptoms rated on a four-point scale (0 = not present; 1= present sometimes, not a real problem; 2 = often present and a problem; 3 = very frequent and a major problem). Potential PRAS-ASD items were developed via a series of family focus groups and expert panel review. Systematic factor and item analysis in a sample of 990 youth with ASD, also ascertained though the IAN registry, identified 25 items which loaded onto a single factor. Internal consistency was excellent (α= 0.93). The distribution of scores in the sample appeared normal with a mean of 29 (SD of 14.9). A sample of 116 youth (5 to 17 years of age) received a detailed clinical assessment of anxiety, ASD and intellectual functioning to evaluate the validity and test-retest reliability of the PRAS-ASD. There was no difference in PRAS-ASD scores in those with and without ID. The PRAS-ASD demonstrated convergent validity with measures of anxiety and divergent validity with indices of ASD symptom severity and irritability. Test-retest reliability in this sample was high. Across these two samples, the mean score on the PRAS-ASD was approximately 30 (SD of 15). Thus, a score of 45 (i.e., one SD above the mean) was used as a clinical cutoff in this study.
Social Communication Questionnaire, Lifetime Version (SCQ: Rutter et al., 2003).
The SCQ is a 40-item caregiver-report questionnaire of binary questions (Yes/No) that measures social, language and communication, and repetitive and stereotyped behaviors characteristic of ASD over a person’s lifetime. The SCQ has shown strong discriminative validity between those with and without ASD (sensitivity= 0.88, specificity= 0.72) (Chandler et al., 2007). In this study, the SCQ cutoff of ≥ 12 was used to determine eligibility into the study. Reliability of the measure was acceptable in this sample (α= .82; non-ID subgroup α= .96; ID subgroup α= .86).
Social Responsiveness Scale, Second Edition (SRS-2: Constantino & Gruber, 2012).
The SRS-2 is a 65-item parent-report measure of autism symptoms with five expert-derived subscales. Items are rated on a four-point scale (1= Not True, 2= Sometimes True, 3= Often True, 4= Almost Always True). Scales are converted to t-scores, where 60–65 is the Mild Range, 66–75 is the Moderate Range, and ≥ 76 is the Severe Range. Internal consistency of the Total Score of the SRS-2 has been reported to range between .92 and .97 in previous school-aged ASD samples (Constantino & Gruber, 2012). The SRS-2 authors do not recommend the use of the individual social subscales outside of treatment planning (Constantino & Gruber, 2012 p.22). Thus, in this study, we used the Social Communication Index (SCI; α= .92; non-ID subgroup α= .93; ID subgroup α= .91), Restricted and Repetitive Behavior scale (RRB; α= .80; non-ID subgroup α= .84; ID subgroup α= .77), and Total Score (α= .94; non-ID subgroup α= .95; ID subgroup α= .93).
Analysis
To assess the ER-anxiety association (aim 1), we calculated the correlation between the EDI Reactivity and PRAS-ASD total scores. On the PRAS-ASD, the sample was dichotomized at ≥45 as the clinical cutoff for elevated anxiety (Scahill et al. 2019). Independent sample t-tests compared EDI Reactivity scores for youth with elevated anxiety symptoms to those without elevated anxiety. Logistic regression models were used to determine whether EDI Reactivity scores and SRS-2 variables significantly contributed to the probability of participants having elevated anxiety symptoms on the PRAS-ASD, after controlling for gender, age, race, and ID status. We also conducted a supplementary linear regression model with the PRAS-ASD as a continuous measure as the dependent variable, and EDI Reactivity, and SRS-2 total score as independent variables, controlling for gender, age, race, and ID status. Lastly, we examined the role of ID a supplemental analyses within ID and non-ID subgroups separately, using the EDI Reactivity and SRS-2 total score variables in a logistic regression.
Multiple linear regression analyses were run to determine whether SRS-2 Total Score, SCI, or RRB moderated the relationship between EDI Reactivity scores and PRAS-ASD total scores (aim 2). In separate regression models for each SRS-2 scale, age, gender, and ID status were entered as covariates, with EDI Reactivity and either the SRS-2 Total score, SCI, or RRB (and their interaction) entered next, and anxiety as the dependent variable. SRS-2 scores were mean-centered prior to analysis. The PROCESS macro for SPSS (Hayes, 2013) was used to probe significant interactions, including post-hoc tests of simple slopes at 1 SD above and below the moderator’s mean value. We also used the Johnson-Neyman technique (Johnson & Fay, 1950) to test for regions of significance across the range of observed data.
Results
The average age of participants was 12.08 (SD = 3.18) years; 79.6% were male and 92.1% were White (See Table 1). Table 1 also contains descriptive statistics for the EDI Reactivity, PRAS-ASD, and SRS-2. The PRAS-ASD and EDI Reactivity scores were significantly correlated (r= 0.49, p< .001). Participants with PRAS-ASD score ≥ 45 (n= 96) had an EDI Reactivity theta score of 0.52, (SD= 0.73) which was significantly higher than those with PRAS-ASD < 45 (n=1009) (EDI Reactivity theta score −0.35; SD= 0.82) (t (1103) = −10.11, p< .001). Youth with elevated anxiety symptoms on the PRAS-ASD also had significantly higher mean scores on the SCI (99.08, SD= 21.91 versus 85.09, SD= 21.10; t (1103) = −6.19, p< .001), RRB (24.68, SD= 5.76 versus 20.20; SD= 6.33; t (1103) = −6.67, p< .001), and SRS-2 Total Scores (123.76, SD= 26.41versus 105.29, SD= 26.24; t (1103) = −3.88, p< .001).
The logistic regression model to predict PRAS-ASD clinical cut-off groups with EDI Reactivity and SRS-2 Total Score as independent variables (Wald χ2= 458.03, p< .001) explained 41% of the variance in anxiety (Cox & Snell R2= .103; −2Log Likelihood= 495.25). The model correctly identified 91.5% of the participants > 45 on the PRAS-ASD (Table 2). Higher EDI Reactivity (β = 1.22, p < .001) and SRS-2 (β = .03, p = .003) scores significantly predicted of exceeding the clinical cut-off for anxiety on the PRAS-ASD. Bivariate odds ratios (OR) indicated that EDI Reactivity (OR= 3.39; CI: 2.45–4.71) more strongly predicted anxiety than did SRS-2 Total Score (OR=1.03; CI: 1.01–1.05).
Table 2.
Logistic Regression of EDI Reactivity and SRS-2 onto PRAS-ASD Clinical Cut-off Groups (Above/Below)
| Logistic Predictors (n= 1107) | B | Wald χ2 | Odds Ratio | 95% CI |
|---|---|---|---|---|
| With SRS-2 Total Score | ||||
| Age | .01 | .09 | .99 | .92–1.06 |
| Gender | −.24 | .74 | 1.28 | .73–2.22 |
| Race | .47 | .76 | .63 | .22–1.80 |
| Ethnicity | .82 | 5.10* | .44 | .22-.90 |
| Intellectual Disability | −.50 | 3.94* | 1.65 | 1.01–2.70 |
| SRS-2 ‡ Total Score | .03 | 8.64** | 1.03 | 1.01–1.05 |
| EDI † Reactivity Short Form theta score | 1.22 | 53.51*** | 3.39 | 2.45– 4.71 |
| With SRS-2 Index Scores | ||||
| Age | .01 | .05 | .99 | .92–1.07 |
| Gender | −.57 | 4.22* | 1.77 | 1.03–3.05 |
| Race | .45 | .70 | .64 | .22–1.83 |
| Ethnicity | .88 | 5.79* | .42 | .20-.85 |
| Intellectual Disability | −.48 | 3.57 | 1.61 | .98–2.65 |
| SRS-2 ‡ Social Communication Index | .003 | .09 | 1.00 | .99– 1.02 |
| SRS-2 Restricted and Repetitive Behaviors | .07 | 5.52* | 1.07 | 1.01–1.14 |
| EDI † Reactivity Short Form theta score | 1.21 | 52.30*** | 3.36 | 2.42– 4.67 |
Key:
Emotion Dysregulation Inventory;
Social Responsiveness Scale-2;
p<.05;
p<.01;
p<.001
The same model with the SRS-2 subscales (SCI and RRB) as independent variables instead of the SRS-2 Total score (Wald χ2= 495.89, p< .001) explained 41% of the variance in anxiety (Cox & Snell R2= .102; −2Log Likelihood= 495.89). The model correctly identified 91.5% of the participants > 45 on the PRAS-ASD (Table 2). Higher levels of EDI Reactivity (β = 1.21, p < .001) and RRB (β = .07, p = .03), but not SCI (β = .003, p = .63), were significant predictors of elevated anxiety on the PRAS-ASD. Bivariate odds ratios (OR) indicated that EDI Reactivity (OR= 3.36; CI: 2.42–4.67) more strongly predicted anxiety than did RRB (OR=1.07; CI: 1.01–1.14) or SCI (OR= 1.00; CI: .99–1.02).
A supplemental analysis using linear regression with PRAS-ASD scores a continuous dependent variable and the EDI Reactivity and SRS-2 Total Score as independent variables yielded the same pattern of results (see Supplementary Material). Lastly, as a follow-up analysis, the primary model predicting PRAS-ASD scores from EDI Reactivity, SRS-2 Total Score, and their interaction, was re-run in ID and non-ID subgroups separately (see Supplementary Materials). Higher elevated SRS-2 Total Scores were associated with elevated anxiety only in the non-ID group (β = .04, p = .02), and higher EDI Reactivity was significantly associated with elevated anxiety on the PRAS-ASD.
Moderation Analyses
The linear regression with PRAS-ASD continuous score as the dependent variable and EDI Reactivity, SRS-2 Total Score, and their interaction as dependent variables, controlling for age, gender, and ID status accounted for 55% of the variance in PRAS-ASD (See Table 3). Anxiety was significantly associated with younger age (β = −.09, p = .003), non-ID status (β = −.13, p < .001), higher EDI Reactivity (β = .32, p < .001), and higher SRS-2 Total Score (β = .19, p < .001). There was no interaction between EDI Reactivity and SRS-2 Total Score (β = .15, p = .50).
Table 3.
Moderation Analyses
| Dependent Variable | Predictors | B | Beta | T | F Change | R2 |
|---|---|---|---|---|---|---|
| PRAS-ASD total | Age | −.36 | −.09 | −3.20** | ||
| Gender | 1.37 | .04 | 1.51 | |||
| Intellectual Disability | −3.51 | −.13 | −4.75*** | |||
| SRS-2 Total t-score | .19 | .19 | 5.16*** | |||
| EDI Reactivity Short Form theta score | 5.03 | .32 | 3.50*** | |||
| SRS-2 Total Score x EDI Reactivity | .001 | .15 | 1.54 | |||
| Overall Model | 75.64 | .31 | ||||
| PRAS-ASD total | Age | −.37 | −.09 | −3.25** | ||
| Gender | −.44 | −.01 | −.50 | |||
| Intellectual Disability | −3.43 | −.13 | −4.61*** | |||
| SRS-2 Social Communication Index t-score | .10 | .16 | 4.09*** | |||
| EDI Reactivity Short Form theta score | 5.71 | .36 | 4.74*** | |||
| SRS-2 SCI x EDI Reactivity | .001 | .12 | 1.42 | |||
| Overall Model | 73.54 | .30 | ||||
| PRAS-ASD total | Age | −.34 | −.08 | −3.08** | ||
| Gender | −.68 | −.02 | −.76 | |||
| Intellectual Disability | −3.17 | −.12 | −4.36*** | |||
| SRS-2 Restricted Repetitive Behaviors t-score | .38 | .18 | 4.70*** | |||
| EDI Reactivity Short Form theta score | 5.69 | .36 | 5.27*** | |||
| SRS-2 RRB x EDI Reactivity | .003 | .12 | 1.54 | |||
| Overall Model | 76.84 | .31 | ||||
Key:
Parent-Rated Anxiety Scale-Autism Spectrum Disorder;
Social Responsiveness Scale-2;
Emotion Dysregulation Inventory.
p<.05;
p<.01;
p<.001
A linear regression model with EDI Reactivity, SRS-2 SCI, and their interaction, as independent variables, controlling for demographic factors, and PRAS-ASD continuous scores as the dependent variable accounted for 54.5% of variance in anxiety scores. Anxiety scores were significantly and positively associated with younger age (β = −.09, p = .001), not having ID (β = −.13, p < .001), higher EDI Reactivity (β = .36, p < .001), and higher SRS-2 SCI (β = .16, p < .001). There was no interaction between EDI Reactivity and SCI (β = .12, p = .66).
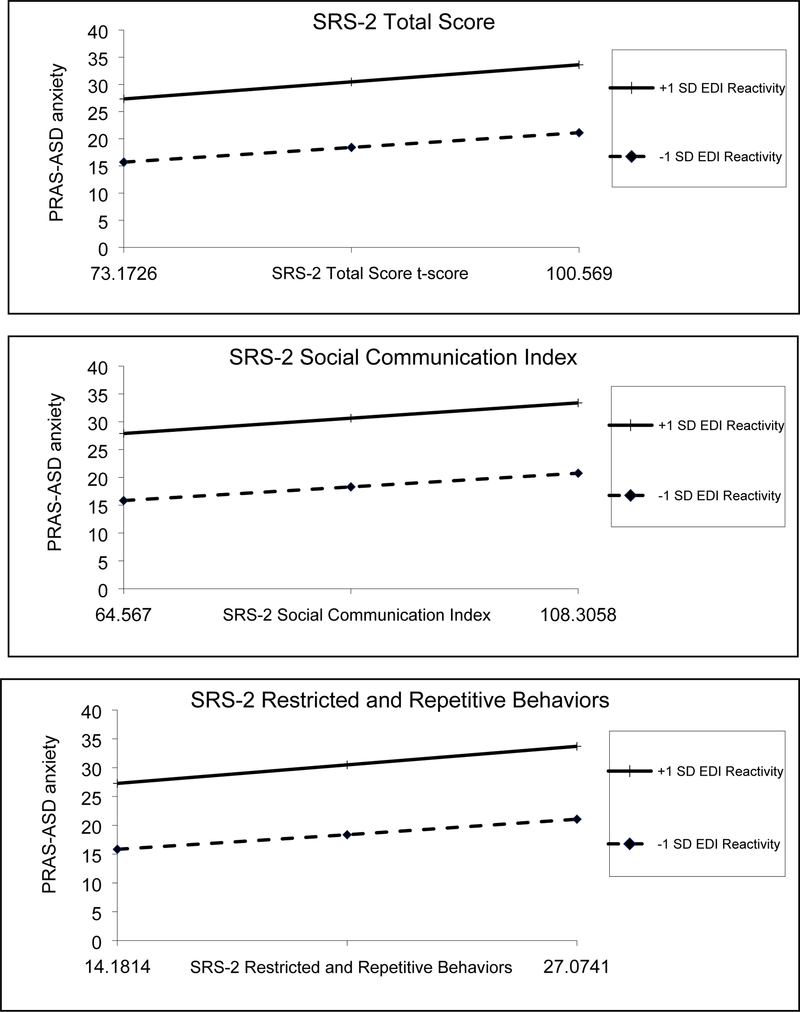
The linear regression model with EDI Reactivity, SRS-2 RRB, and their interaction as independent variables, controlling for demographic factors, explained 55.4% of the variance in PRAS-ASD scores. PRAS-ASD scores were significantly associated with young age (β = −.08, p = .004), not having ID (β = −.12, p < .001), higher EDI Reactivity (β = .36, p < .001), and higher SRS-2 RRB (β = .18, p < .001). There was no significant interaction between EDI Reactivity and SRS-2 RRB (β = .12, p = .42) (See Table 3 and Figure 1).
Figure 1.
Interaction between SRS-2 and Emotion Regulation on Anxiety Symptoms
SD= Standard Deviation; SRS-2= Social Responsiveness Scale, 2nd Edition; PRAS-ASD= Parent-Rated Anxiety Scale-ASD score; EDI= Emotion Dysregulation Inventory
Overall, higher social impairment, RRB symptoms, and ER impairment were significantly associated with elevated PRAS-ASD scores. There were no significant interaction effects between SRS-2 scores and EDI Reactivity on anxiety.
Discussion
The high prevalence of ER impairment and anxiety disorders in youth with ASD is well established (Samson, Hardan, Lee, Phillips, & Gross, 2015; van Steensel, Bögels, & de Bruin, 2013). The current study examined the association between ER and anxiety in a large sample of youth with ASD that included participants with and without ID. As expected, ER impairment predicted clinical levels of anxiety as measured on the PRAS-ASD after controlling for indices of ASD severity, and ER impairment remained strongly associated with anxiety. The medium correlation between ER and PRAS-ASD, however, suggest that these instruments are not measuring the same construct. Although this was a cross-sectional study, we propose that ER impairment may reflect a mechanism, or pathway, for the development of anxiety. If so, ER may be a target for intervention to prevent or remediate anxiety in youth with ASD.
This study also examined whether measures of ASD symptoms such as social impairment, restricted interests, and repetitive behaviors would strengthen the association between ER impairment and anxiety. All SRS-2 scores and ER impairment were significantly associated with higher anxiety scores, suggesting that social impairment, restricted interests and repetitive behaviors, and ER impairment are each associated with anxiety in ASD. These findings are consistent with prior research showing that social impairment (Swain et al., 2015) is associated with increased social anxiety in ASD. These results are also concur with prior reports suggesting that the second cluster of ASD symptoms, including sensory sensitivities, insistence on sameness, restricted interests and repetitive behaviors are associated with anxiety in youth with ASD (Rodgers et al., 2012; Wigham et al., 2015). However, there were no interaction effects between ASD symptom indices on the SRS-2 and ER impairment on anxiety symptoms. Thus, future research should incorporate ASD symptom severity and ER impairment to deepen our understanding on the emergence of anxiety in youth with ASD.
Findings of the current study are consistent with prior work linking core ASD symptoms and ER impairment (Samson et al., 2014). ASD features such as inflexibility, decreased capacity to read social cues, and difficulties with perspective taking appear to increase the risk of ER impairment in ASD (Mazefsky & White, 2014). If biological factors associated with ASD contribute to impaired ER, as suggested by White et al. (2013) and Mazefsky et al. (2014), these biological factors could increase the identification of individuals at elevated risk. A next step could be to develop and test models of how core ASD symptoms, along with impaired ER, inattention, and executive function deficits affect development of co-occurring psychopathology in ASD.
This study was cross-sectional, which precludes any conclusions about causation. Although our model posits that the level of anxiety may be moderated (i.e., amplified) by social impairment, restricted interests and repetitive behaviors, it is also possible that anxiety can contribute to social impairment, restricted interests and repetitive behaviors. Duvekot and colleagues followed 79 youth with ASD over a two-year period. Participants with high levels of anxiety at Time 1 had greater social communication impairment at Time 2 (Duvekot, van der Ende, Verhulst, & Greaves-Lord, 2017). A larger study in the general population showed that social communication deficits predicted increased social anxiety over time between ages 7 and 13 (Pickard et al., 2017). These findings support a dynamic interaction between impaired social communication and social anxiety – but temporal ordering remains uncertain. For example, anxiety in youth with ASD may promote social avoidance, reduce opportunities for social interaction, and hinder the development of social skills. Alternatively, social communication deficits may contribute to failures in social situations followed by social avoidance (Kerns et al., 2015; Scahill et al., 2019; White, Maddox, & Panneton, 2015). Longitudinal studies could move beyond association to confirmation of predisposing and perpetuating factors.
This study also suggested that some other demographic and patient characteristics may play a role in anxiety in ASD, namely younger age and lack of ID were associated with higher parent-reported anxiety. Interestingly, this finding contrasts both anxiety prevalence in the general population as well as findings suggesting that risk for anxiety in ASD may be exacerbated by social impairment (Swain et al., 2015; White et al., 2014). We note that the current study relied on parent report of ID status, which may have affected the observed results. In a sample of 116 youth with ASD assessed with the PRAS-ASD and standardized cognitive testing, there was no difference in anxiety level in those with IQ above or below 70 (Scahill et al., 2019). However, prior research has suggested that anxiety is more common among children with autism who have higher intellectual ability (Lecavalier et al., 2019).
There are several limitations of the current study. A community diagnosis of ASD as self-declared in the IAN sample and an SCQ score above clinical cutoff were used to define cases. Although this method has been verified by medical records in a series of IAN participants (Daniels et al., 2012), we could not confirm the ASD diagnosis in our participants. Furthermore, all surveys were parent reported, so biases arising from a single informant cannot be ruled out. Although the sample size was relatively large, respondents were self-selected, predominantly white and non-Hispanic/Latino. Future studies could use more fine-grained cognitive and language assessment to compare how differences cognitive functioning and age affect the interaction between social disability, ER impairment, and anxiety. Treatment studies could use these characteristics to guide treatment of ER impairment and anxiety.
In summary, this study established the association between ER impairment and anxiety in a large sample that included youth with ASD across a wide range of cognitive function. Higher ASD symptoms were also associated with increased anxiety, in accordance with prior research (Swain et al., 2015; Uljarević & Evans, 2016). Impaired ER, in particular, may be a vital underlying construct for anxiety. The emergence of heightened anxiety may also undermine capacity for emotion regulation. Recent research has indeed suggested that improving ER leads to decreased anxiety in ASD (Conner et al., 2019). ER impairment should be considered in our pursuit for mechanistically driven and transdiagnostic, personalized treatments.
Supplementary Material
Acknowledgements:
Data for this project was supported by R01 HD079512-04 (PI: Mazefsky). Subjects were recruited and data obtained in partnership with the Interactive Autism Network (IAN) Research Database at Kennedy Krieger Institute.
Dr. Scahill has served as a consultant Roche, Janssen, Shire, Supernus, Neurocrine, Yamo and has received royalties from Guilford and Oxford. Dr. White has received royalties from Guilford, Springer, and Oxford.
Footnotes
Conflict of Interest Statement: Dr. Conner and Dr. Mazefsky report no conflicts.
References
- Aldao A, Nolen-Hoeksema S, & Schweizer S (2010). Emotion-regulation strategies across psychopathology: A meta-analytic review. Clinical Psychology Review, 30(2), 217–237. doi: 10.1016/j.cpr.2009.11.004 [DOI] [PubMed] [Google Scholar]
- Bal VH, Katz T, Bishop SL, & Krasileva K (2016). Understanding definitions of minimally verbal across instruments: evidence for subgroups within minimally verbal children and adolescents with autism spectrum disorder. Journal of Child Psychology and Psychiatry and Allied Disciplines, 57(12), 1424–1433. doi: 10.1111/jcpp.12609 [DOI] [PubMed] [Google Scholar]
- Bearss K, Taylor CA, Aman MG, Whittemore R, Lecavalier L, Miller J, … Scahill L (2016). Using qualitative methods to guide scale development for anxiety in youth with autism spectrum disorder. Autism, 20(6), 663–672. doi: 10.1177/1362361315601012 [DOI] [PubMed] [Google Scholar]
- Cai RY, Richdale A, Uljarevic M, Dissanayake C, & Samson AC (2018). Emotion regulation in autism spectrum disorder: Where we are and where we need to go. Autism Research, 11(7), 962–978. doi: 10.1002/aur.1968 [DOI] [PubMed] [Google Scholar]
- Chandler S, Charman T, Baird G, Simonoff E, Loucas T, Meldrum D, … Pickles A (2007). Validation of the Social Communication Questionnaire in a population cohort of children with autism spectrum disorders. Journal of the American Academy of Child and Adolescent Psychiatry, 46(10), 1324–1332. doi: 10.1097/chi.0b013e31812f7d8d [DOI] [PubMed] [Google Scholar]
- Chevallier C, Kohls G, Troiani V, Brodkin ES, & Schultz RT (2013). The social motivation theory of autism. Trends in Cognitive Neuroscience, 16(4), 231–239. doi: 10.1016/j.tics.2012.02.007.The [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conner CM, White SW, Beck KB, Golt J, Smith IC, & Mazefsky CA (2019). Improving emotion regulation ability in autism: The Emotional Awareness and Skills Enhancement (EASE) program. Autism, 23(5), 1273–1287. doi: 10.1177/1362361318810709 [DOI] [PubMed] [Google Scholar]
- Constantino JN, & Gruber CP (2012). Social responsiveness scale-2nd Edition (SRS-2). Torrance, CA: Western Psychological Services. [Google Scholar]
- Daniels AM, Rosenberg RE, Anderson C, Law JK, Marvin AR, & Law PA (2012). Verification of parent-report of child autism spectrum disorder diagnosis to a web-based autism registry. Journal of Autism and Developmental Disorders, 42(2), 257–265. doi: 10.1007/s10803-011-1236-7 [DOI] [PubMed] [Google Scholar]
- Duvekot J, van der Ende J, Verhulst FC, & Greaves-Lord K (2017). Examining bidirectional effects between the autism spectrum disorder (ASD) core symptom domains and anxiety in children with ASD. Journal of Child Psychology and Psychiatry. doi: 10.1111/jcpp.12829 [DOI] [PubMed] [Google Scholar]
- Gantman A, Kapp SK, Orenski K, & Laugeson EA (2012). Social skills training for young adults with high-functioning autism spectrum disorders: a randomized controlled pilot study. Journal of Autism and Developmental Disorders, 42(6), 1094–1103. doi: 10.1007/s10803-011-1350-6 [DOI] [PubMed] [Google Scholar]
- Goldsmith SF, & Kelley E (2018). Associations Between Emotion Regulation and Social Impairment in Children and Adolescents with Autism Spectrum Disorder. Journal of Autism and Developmental Disorders, 48(6), 2164–2173. doi: 10.1007/s10803-018-3483-3 [DOI] [PubMed] [Google Scholar]
- Gross JJ, & Thompson RA (2006). Emotion regulation: Conceptual foundations In J. J. G. (Ed.) (Ed.), Handbook of Emotion Regulation (pp. 3–26). New York: Guilford Press. [Google Scholar]
- Happé F, Cook JL, & Bird G (2017). The Structure of Social Cognition: In(ter)dependence of Sociocognitive Processes. Annual Review of Psychology, 68(1), 243–267. doi: 10.1146/annurevpsych-010416-044046 [DOI] [PubMed] [Google Scholar]
- Hayes AF (2013). Introduction to mediation, moderation, and conditional process analysis: A regression-based approach. New York: The Guilford Press. [Google Scholar]
- Hus V, Bishop S, Gotham K, Huerta M, & Lord C (2013). Factors influencing scores on the social responsiveness scale. Journal of Child Psychology and Psychiatry and Allied Disciplines, 54(2), 216–224. doi: 10.1111/j.1469-7610.2012.02589.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahromi LB, Meek SE, & Ober-Reynolds S (2012). Emotion regulation in the context of frustration in children with high functioning autism and their typical peers. Journal of Child Psychology and Psychiatry, and Allied Disciplines, 53(12), 1250–1258. doi: 10.1111/j.1469-7610.2012.02560.x [DOI] [PubMed] [Google Scholar]
- Johnson PO, & Fay LC (1950). The Johnson-Neyman Technique, its theory and application. Psychometrika, 15(4), 349–367. [DOI] [PubMed] [Google Scholar]
- Joshi G, Petty C, Wozniak J, Henin A, Fried R, Galdo M, … Biederman J (2010). The heavy burden of psychiatric comorbidity in youth with autism spectrum disorders: a large comparative study of a psychiatrically referred population. Journal of Autism and Developmental Disorders, 40(11), 1361–1370. doi: 10.1007/s10803-010-0996-9 [DOI] [PubMed] [Google Scholar]
- Kerns CM, & Kendall PC (2012). The Presentation and Classification of Anxiety in Autism Spectrum Disorder. Clinical Psychology: Science and Practice, 19(4), 323–347. doi: 10.1111/cpsp.12009 [DOI] [Google Scholar]
- Kerns CM, Rump K, Worley J, Kratz H, McVey A, Herrington J, & Miller J (2015). The Differential Diagnosis of Anxiety Disorders in Cognitively-Able Youth With Autism. Cognitive and Behavioral Practice. doi: 10.1016/j.cbpra.2015.11.004 [DOI] [Google Scholar]
- Khor AS, Melvin GA, Reid SC, & Gray KM (2014). Coping, daily hassles and behavior and emotional problems in adolescents with high-functioning autism / Asperger’s disorder. Journal of Autism and Developmental Disorders, 44, 593–608. doi: 10.1007/s10803-013-1912-x [DOI] [PubMed] [Google Scholar]
- Konstantareas MM, & Stewart K (2006). Affect regulation and temperament in children with Autism Spectrum Disorder. Journal of Autism and Developmental Disorders, 36(2), 143–154. doi: 10.1007/s10803-005-0051-4 [DOI] [PubMed] [Google Scholar]
- Lecavalier L, McCracken CE, Aman MG, McDougle CJ, McCracken JT, Tierney E, … Scahill L (2019). An exploration of concomitant psychiatric disorders in children with autism spectrum disorder. Comprehensive Psychiatry, 88, 57–64. doi: 10.1016/j.comppsych.2018.10.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerner MD, Mazefsky CA, Weber RJ, Transue E, Siegel M, & Gadow KD (2017). Verbal Ability and Psychiatric Symptoms in Clinically Referred Inpatient and Outpatient Youth with ASD. Journal of Autism and Developmental Disorders, 0(0), 0. doi: 10.1007/s10803-017-3344-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddox BB, & White SW (2015). Comorbid Social Anxiety Disorder in Adults with Autism Spectrum Disorder. Journal of Autism and Developmental Disorders, 45(12), 3949–3960. doi: 10.1007/s10803-015-2531-5 [DOI] [PubMed] [Google Scholar]
- Maisel ME, Stephenson KG, South M, Rodgers J, Freeston MH, & Gaigg SB (2016). Modeling the cognitive mechanisms Linking autism symptoms and Anxiety in adults. Journal of Abnormal Psychology, 125(5), 692–703. doi: 10.1037/abn0000168 [DOI] [PubMed] [Google Scholar]
- Mazefsky CA, Borue X, Day TN, & Minshew NJ (2014). Emotion regulation patterns in adolescents with high functioning autism spectrum disorder: Comparison to typically developing adolescents and association with psychiatric symptoms. Autism Research, 7(3), 344–354. doi: 10.1002/aur.1366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazefsky CA, Day TN, Siegel M, White SW, Yu L, & Pilkonis PA (2018). Development of the Emotion Dysregulation Inventory: A PROMIS®ing Method for Creating Sensitive and Unbiased Questionnaires for Autism Spectrum Disorder. Journal of Autism and Developmental Disorders, 48(11), 3736–3746. doi: 10.1007/s10803-016-2907-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazefsky CA, & White SW (2014). Emotion Regulation. Child and Adolescent Psychiatric Clinics of North America, 23(1), 15–24. doi: 10.1016/j.chc.2013.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazefsky CA, Yu L, White SW, Siegel M, & Pilkonis PA (2018). The Emotion Dysregulation Inventory: Psychometric properties and Item Response Theory calibration in an autism spectrum disorder sample. Autism Research, 11(6), 928–941. doi: 10.1002/aur.1947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pallathra AA, Calkins ME, Parish-Morris J, Maddox BB, Perez LS, Miller J, … Brodkin ES (2018). Defining behavioral components of social functioning in adults with autism spectrum disorder as targets for treatment. Autism Research, 11(3), 488–502. doi: 10.1002/aur.1910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickard H, Rijsdijk F, Happé F, & Mandy W (2017). Are Social and Communication Difficulties a Risk Factor for the Development of Social Anxiety? Journal of the American Academy of Child and Adolescent Psychiatry, 56(4), 344–351.e3. doi: 10.1016/j.jaac.2017.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieffe C, Oosterveld P, Terwogt MM, Mootz S, van Leeuwen E, & Stockmann L (2011). Emotion regulation and internalizing symptoms in children with autism spectrum disorders. Autism, 15(6), 655–670. doi: 10.1177/1362361310366571 [DOI] [PubMed] [Google Scholar]
- Rodgers J, Glod M, Connolly B, & McConachie H (2012). The Relationship Between Anxiety and Repetitive Behaviours in Autism Spectrum Disorder. Journal of Autism and Developmental Disorders, 42(11), 2404–2409. doi: 10.1007/s10803-012-1531-y [DOI] [PubMed] [Google Scholar]
- Rutter M, Bailey A, & Lord C (2003). The Social Communication Questionnaire. Los Angeles, CA: Western Psychological Services. [Google Scholar]
- Samson AC, Hardan AY, Lee IA, Phillips JM, & Gross JJ (2015). Maladaptive Behavior in Autism Spectrum Disorder: The Role of Emotion Experience and Emotion Regulation. Journal of Autism and Developmental Disorders, 45(11), 3424–3432. doi: 10.1007/s10803-015-2388-7 [DOI] [PubMed] [Google Scholar]
- Samson AC, Phillips JM, Parker KJ, Shah S, Gross JJ, & Hardan AY (2014). Emotion Dysregulation and the Core Features of Autism Spectrum Disorder. Journal of Autism and Developmental Disorders, 44(7), 1766–1772. doi: 10.1007/s10803-013-2022-5 [DOI] [PubMed] [Google Scholar]
- Scahill L, Lecavalier L, Schultz RT, Evans AN, Maddox B, Pritchett J, … Edwards MC (2019). Development of the parent-rated anxiety scale for youth with autism spectrum disorder. Journal of the American Academy of Child and Adolescent Psychiatry, 1–34. doi: 10.1016/j.jaac.2018.10.016 [DOI] [PubMed] [Google Scholar]
- Schneider RL, Arch JJ, Landy LN, & Hankin BL (2018). The Longitudinal Effect of Emotion Regulation Strategies on Anxiety Levels in Children and Adolescents. Journal of Clinical Child and Adolescent Psychology, 47(6), 978–991. doi: 10.1080/15374416.2016.1157757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonoff E, Pickles A, Charman T, Chandler S, Loucas T, & Baird G (2008). Psychiatric disorders in children with autism spectrum disorders: prevalence, comorbidity, and associated factors in a population-derived sample. Journal of the American Academy of Child and Adolescent Psychiatry, 47(8), 921–929. doi: 10.1097/CHI.0b013e318179964f [DOI] [PubMed] [Google Scholar]
- Swain D, Scarpa A, White S, & Laugeson E (2015). Emotion dysregulation and anxiety in adults with ASD: Does social motivation play a role? Journal of Autism and Developmental Disorders, 45(12), 3971–3977. doi: 10.1007/s10803-015-2567-6 [DOI] [PubMed] [Google Scholar]
- Uljarević M, & Evans DW (2016). Relationship between repetitive behaviour and fear across normative development, autism spectrum disorder, and down syndrome. Autism Research, 1–6. doi: 10.1002/aur.1674 [DOI] [PubMed] [Google Scholar]
- van Steensel FJA, Bögels SM, & de Bruin EI (2013). Psychiatric Comorbidity in Children with Autism Spectrum Disorders: A Comparison with Children with ADHD. Journal of Child and Family Studies, 22(3), 368–376. doi: 10.1007/s10826-012-9587-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Steensel FJA, Bögels SM, & Perrin S (2011). Anxiety disorders in children and adolescents with autistic spectrum disorders: a meta-analysis. Clinical Child and Family Psychology Review, 14(3), 302–317. doi: 10.1007/s10567-011-0097-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Steensel FJA, & Heeman EJ (2017). Anxiety Levels in Children with Autism Spectrum Disorder: A Meta-Analysis. Journal of Child and Family Studies, 26(7), 1753–1767. doi: 10.1007/s10826-017-0687-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss JA (2014). Transdiagnostic case conceptualization of emotional problems in youth with ASD: An emotion regulation approach. Clinical Psychology: Science and Practice, 21, 331–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White SW, Mazefsky CA, Dichter GS, Chiu PH, Richey JA, & Ollendick TH (2014). Social-cognitive, physiological, and neural mechanisms underlying emotion regulation impairments: understanding anxiety in autism spectrum disorder. International Journal of Developmental Neuroscience, 39, 22–36. doi: 10.1016/j.ijdevneu.2014.05.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- White SW, Oswald D, Ollendick T, & Scahill L (2009). Anxiety in children and adolescents with autism spectrum disorders. Clinical Psychology Review, 29(3), 216–229. doi: 10.1016/j.cpr.2009.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- White SW, Maddox BB, & Panneton RK (2015). Fear of Negative Evaluation Influences Eye Gaze in Adolescents with Autism Spectrum Disorder: A Pilot Study. Journal of Autism and Developmental Disorders, 3446–3457. doi: 10.1007/s10803-014-2349-6 [DOI] [PubMed] [Google Scholar]
- White SW, Keonig K, & Scahill L (2007). Social skills development in children with autism spectrum disorders: a review of the intervention research. Journal of Autism and Developmental Disorders, 37(10), 1858–1868. doi: 10.1007/s10803-006-0320-x [DOI] [PubMed] [Google Scholar]
- Wigham S, Rodgers J, South M, McConachie H, & Freeston M (2015). The interplay between sensory processing abnormalities, intolerance of uncertainty, anxiety and restricted and repetitive behaviours in autism spectrum disorder. Journal of Autism and Developmental Disorders, 45(4), 943–952. doi: 10.1007/s10803-014-2248-x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.