Abstract
Osteoarthritis (OA) is a multifactorial joint disease with pathological changes that affect whole joint tissue. Obesity is acknowledged as the most influential risk factor for both the initiation and progression of OA in weight-bearing and non-weight-bearing joints. Obesity-induced OA is a newly defined phenotypic group in which chronic low-grade inflammation has a central role. Aside from persistent chronic inflammation, abnormal mechanical loading due to increased body weight on weight-bearing joints is accountable for the initiation and progression of obesity-induced OA. The current therapeutic approaches for OA are still evolving. Tissue-engineering-based strategy for cartilage regeneration is one of the most promising treatment breakthroughs in recent years. However, patients with obesity-induced OA are often excluded from cartilage repair attempts due to the abnormal mechanical demands, altered biomechanical and biochemical activities of cells, persistent chronic inflammation, and other obesity-associated factors. With the alarming increase in the number of obese populations globally, the need for an innovative therapeutic approach that could effectively repair and restore the damaged synovial joints is of significant importance for this sub-population of patients. In this review, we discuss the involvement of the systemic and localized inflammatory response in obesity-induced OA and the impact of altered mechanical loading on pathological changes in the synovial joint. Moreover, we examine the current strategies in cartilage tissue engineering and address the critical challenges of cell-based therapies for OA. Besides, we provide examples of innovative ways and potential strategies to overcome the obstacles in the treatment of obesity-induced OA.
The translational potential of this article
Altogether, this review delivers insight into obesity-induced OA and offers future research direction on the creation of tissue engineering-based therapies for obesity-induced OA.
Keywords: Osteoarthritis, Inflammation, Obesity, Biomaterials, Articular cartilage regeneration
Graphical abstract
Introduction
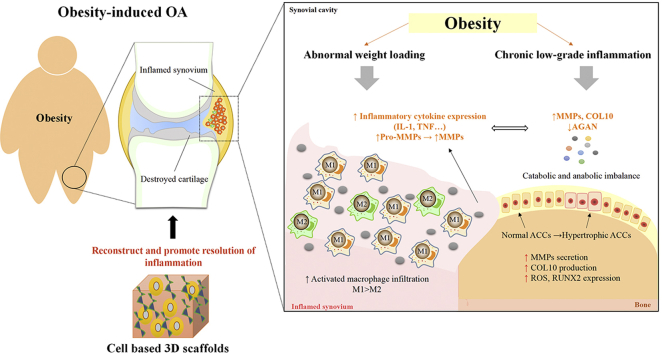
Osteoarthritis (OA) is one of the most common chronic joint diseases affecting all joint structures, ultimately causing pain, prolonged morning stiffness, and muscle weakness, which eventually leads to disability. OA is no longer defined as a “wear and tear” degenerative disease but as an “organ-level failure” of the entire joint with heterogeneous and multifactorial pathogenesis [1]. Increasing evidence suggests that there are multiple subtypes of OA that reflect a complex and multifactorial nature (reviewed by Vina et al. [2]). Obesity is acknowledged as the most influential risk factor for both the initiation and progression of OA in weight-bearing and non-weight-bearing joints. Patients with obesity or OA have a common characteristic—"chronic low-grade inflammation” (also known as meta-inflammation) [3,4]. Obesity-induced OA has been proposed as a new phenotype of OA that displays a unique characteristic. Low-grade chronic inflammation mediated by the innate immune system is a metabolically triggered inflammatory state which is notably characterized by increased macrophage infiltration in adipose tissue and abnormal production of inflammatory cytokines throughout the body [5]. It has been recognized as a critical step in the development of obesity-associated comorbidities, including OA [6]. The local inflammatory events within the joint could reflect systemic changes in obese patients with OA [7]. Swelling, pain, and stiffness are OA’s clinical features attributed to the presence of macrophage-associated synovitis, which has emerged as a novel player in OA pathogenesis. Although the degree of synovitis in OA varies from no symptoms of inflammation to severe inflammation, the high prevalence and increased severity of synovial membrane inflammation are linked to the accelerated progression of obesity-induced OA [8,9].
Aside from persistent inflammation, abnormal mechanical loading as a result of increased body weight on weight-bearing joints is accountable for the initiation and progression of obesity-induced OA. Altered loading is associated with articular cartilage inflammatory state and metabolic imbalances of biosynthesis and degradation that eventually results in cartilage breakdown. The principal function of articular cartilage is to provide a smooth surface with low coefficient friction for articulation and to facilitate the transmission of loads during joint movement [1]. Studies have shown that activation of hypertrophy-like chondrocytes is a consequence of mechanical stress in the extracellular matrix molecules or inflammatory cytokines [10]. Not only cartilage but also subchondral bone is greatly affected by mechanical stress upon weight gain. Horizontal fissuring at the osteochondral interface and cartilage erosion with chronic inflammation and rupture of microcapillaries are now considered one of the key pathological features of OA patients with obesity [11]. Although the underlying mechanism remains elucidated, repetitive mechanical loading and shear forces on the cartilage–bone interface cause the changes of microstructure proposed by Chen et al. [11]. Undoubtedly, the types and frequencies of mechanical stimulation play an essential role in the formation of appropriate tissue type and architecture.
Over the last decades, different strategies have been made worldwide to ameliorate OA progression. However, due to the complicated and unique etiology in each OA patients, OA’s current therapeutic approaches are still evolving. Total knee arthroplasty (TKA) with artificial components is the last-resort effort to treat severe OA when symptoms cannot be controlled by other therapeutic modalities [12]. It has been proven to debilitate pain experience, improve the significant limitation of physical functions and quality of life in patients with OA [13]. However, substantial numbers of patients have suffered from postsurgical functional impairment and complications [14]. Increasing evidence suggests that excessive body weight is a significant risk factor for the success of TKA [15]. Obese TKA patients have significantly higher rates of perioperative and postoperative complications, including failure of wound healing, increased reoperation rate, implant revision, or removal [16]. Therefore, the development of the innovative therapeutic approach and regenerative engineering strategies is a national and international research priority.
Tissue-engineering-based strategy for cartilage regeneration is one of the most promising treatment breakthroughs in recent years. Langer and Vacanti defined tissue engineering in 1993 as “an interdisciplinary field that applies the principles of engineering and the life sciences toward the development of biological substitutes that restore, maintain, or improve tissue function” [17]. Cartilage tissue engineering (CTE) has shown encouraging results with improved signs and symptoms in OA patients and animal models. However, the healing of cartilage defects in OA is currently an intractable problem [18,19]. Many advances have been made in CTE, particularly when considering the increasing number of ongoing preclinical and clinical studies.
Nevertheless, there is a lack of studies that focus on the application of CTE in obesity-induced OA. It is noteworthy that the regeneration of damaged cartilage in obesity-induced OA is more complicated than in traumatic-injured chondral defects due to the persistent inflammatory environment of the disease and altered mechanical stress. Thus, understanding the interaction among mechanical loading, systemic and local immune response, and cellular biochemical activities of articular tissue under obesity-induced OA is an essential step toward developing CTE approaches as therapeutic strategies for cartilage regeneration.
In this review, we discuss the involvement of the systemic and localised inflammatory response in obesity-induced OA and the impact of altered mechanical loading on pathological changes in the synovial joint. Moreover, we examine the current strategies in cartilage tissue engineering and address key challenges of cell-based therapies for OA. We also provide examples of innovative ways and potential strategies to overcome the challenges in the treatment of obesity-induced OA. Altogether, this review delivers insight into obesity-induced OA and offers future research direction on the creation of tissue engineering-based therapies for obesity-induced OA (Abstract graphics).
The challenges of articular cartilage regeneration in obesity-induced OA
Growing evidence indicates that obesity contributes to worse existing OA or induces the development of pathological changes by causing inflammation [20]. Although “inflammation is a beneficial process, designed to contain and eradicate threats to the host organism”, the persistent inflammation-induced alterations in joint homeostasis in obesity-induced OA potentially suppress the resolution of inflammation and lead to failure of tissue regeneration [21]. In addition to modification in physiological conditions, obesity has been associated with abnormal mechanical load distribution in the joint [22]. Abnormal joint loading patterns in obese individuals cause alteration in the molecular composition, organization of the matrix, structural integrity, and mechanical properties of joint tissues, particularly articular cartilage and subchondral bone [11,23]. Moreover, hyper-physiologic magnitudes of loading can lead to increased pro-inflammatory cytokines in articular cartilage chondrocyte and synoviocytes, thus furthering the cycle of pathologic changes in the synovial joint [24,25]. Although the obesity-induced OA mechanism is unclear, the combination of excessive mechanical loading and inflammatory state results in the initiation and acceleration of this disease.
Over the last decades, attempts have been made to develop biological substitutes that can notably restore the function of damaged cartilage. At present, relatively few studies have focused on the investigation of CTE for obesity-induced OA. Progress in tissue engineering and regenerative medicine is hampered by the limited understanding of the etiology and diversity of pathophysiological changes of obesity-induced OA. Because obesity-induced OA pathogenesis is more complicated and destructive compared to non-obese individuals, it is essential to understand how obesity negatively impacts synovial joint and cartilage repair procedures.
Influence of obesity on the development of osteoarthritis
Systemic inflammation in OA
There is mounting evidence suggesting the influence of inflammatory conditions in obesity and OA. Substantial evidence supports that weight loss of 20% in patients with obesity and OA by gastric surgery led to an improvement in pain and physical function and attenuation in systemic inflammation resulting in a structural effect on cartilage [26]. Macrophage-induced white adipose tissue inflammation characterised by the modest increase in circulating pro-inflammatory factors (including tumor necrosis factor-alpha (TNF-α), interleukin-1 beta (IL)-1β and IL-6) has been proposed as the underlying mechanism that links OA with obesity [3]. It is well known that obesity leads to macrophages’ phenotypic switch toward the pro-inflammatory subtype resulting in TNF-α-induced insulin resistance and lipolysis of adipocytes [27]. Macrophages play pivotal roles in innate immunity and can potentially change phenotypes according to different activating stimuli in the microenvironment. The pro-inflammatory M1 macrophages activated by interferon-γ (IFN-γ) and lipopolysaccharide (LPS) or TNF-α are highly phagocytic and particularly active in destroying microorganisms and macromolecular foreign bodies [28]. The anti-inflammatory phenotype, also known as wound-healing macrophages stimulated by several factors such as IL-4, IL-13, and transforming growth factor-beta (TGF-β) exert an immunomodulatory effect on the maintenance of tissue homeostasis and repairing [29]. In contrast to the pro-inflammatory phenotype, the anti-inflammatory M2 macrophages are involved in the resolution of inflammation and anti-fibrotic activity through reduced production of pro-inflammatory cytokines, upregulation of anti-inflammatory mediators, efferocytosis of apoptotic pro-inflammatory M1 macrophages and neutrophils in tissue remodeling and wound healing [29]. Aside from signal transducer and activator of transcription family, macrophage polarisation also can be regulated by regulators of lipid metabolism, transcription factor families, microRNAs and long non-coding RNAs (extensively reviewed by Ref. [30]). Other phenotypes have also been described; however, their function in obesity-induced OA remains unexplored. Thus, this review will focus only on M1-and M2-polarised macrophages and their role in obesity and OA.
Although the inflammatory phase is an essential step for tissue healing, the failure of macrophages to transit from pro-inflammatory to wound-healing phenotypes is detrimental. It may contribute to the initiation and progression of obesity and OA. The previous study has suggested that circulating monocytes/macrophages from obese individuals with OA are activated before they enter into the synovial joints [27]. Obesity stimulates chemokine-mediated macrophage infiltration and pro-inflammatory polarisation in OA. Chemokine (C–C motif) ligand 2 (CCL2) and chemokine (C–C motif) receptor 2 (CCR2) signalling axis have been shown to modulate monocyte/macrophage recruitment and activation in obese adipose tissues [31]. Importantly, multiple studies have reported that elevated CCL2/CCR2 levels in synovial fluid and synovium from patients suffering OA correlated positively with pain, stiffness, and physical function [32,33]. Activated macrophages-associated alarmin S100A8 and S100A9 are high in serum synovial fluid and synovium in both OA patients and animal models, both of which have also been implicated in the development of macrophage-induced adipose tissue inflammation in obesity [[34], [35], [36]]. Schelbergen et al. show that S100A/S100A9 induce over-expression and activation of matrix metalloproteinase, leading to cartilage matrix remodeling and osteophyte formation in experimental OA with high synovial activation [37]. Moreover, in vivo and in vitro studies indicate that the balance between pro- and anti-inflammatory macrophage production has a significant impact on the development of synovial joint inflammation in obesity-induced OA [9,20]. This subject is covered in more detail in the section on “localised joint inflammation."
Adipose tissue is a heterogeneous tissue containing mature adipocytes and immune cells that secrete a wide variety of inflammatory cytokines and adipose tissue-derived adipokines (including leptin, adiponectin, resistin, and others) [38]. A vast number of studies have reported that the abnormal production and secretion of adipokines are believed to play an important role in the association of obesity and systemic inflammation (extensively reviewed by Ref. [39]). For instance, leptin, one of the most important adipokines, regulates the activation of proliferation and phagocytosis of monocytes/macrophages in obese patients [38,40]. Griffin and colleagues have observed that obese mice with leptin impairment showed unchanged systemic inflammatory cytokine levels [41]. Moreover, leptin receptor-deficient (db/db) mice exhibit lower body weight, decreased macrophage infiltration, and less pro-inflammatory gene expression in adipose tissue [42]. Overexpressed leptin level is a crucial regulator linking high-fat diet-induced obesity, chronic inflammation, immune suppression, and tumour progression [43]. These pro-inflammatory cytokines, such as IL-1β, also regulate the expression of leptin [44]. Adiponectin is an anti-inflammatory adipokine that is downregulated in morbidly obese patients and up-regulated with weight loss [45]. It has shown to displays protective action on the development of obesity-induced inflammation by modulating signaling pathways in various types of cells [45]. Previous studies have shown that adiponectin and its receptors, AdipoR1 and AdipoR2, suppress inflammatory activation of macrophages through several ways, including the downregulation of pro-inflammatory cytokines and upregulation of the anti-inflammatory markers in adipose tissue [46]. Thus, the activity of adipose tissue-derived adipokines and its link with pro-inflammatory cytokines to promote or sustain chronic low-grade inflammation that could ultimately favour the development of the obesity-associated disease.
At present, it is well known that leptin is an important contributor to the increased risk of obese people to develop OA (reviewed in Ref. [47]). The expression of leptin and its receptors in the synovial fluid have been reported to be higher in obese OA patients than in lean patients [48]. There was a significant association between BMI and whole blood leptin promoter methylation levels in OA patients [49]. Articular chondrocytes isolated from obese OA patients exhibit an altered response pattern to elevated leptin levels compared with lean or overweight patients [50]. Furthermore, a high-fat diet caused a robust increase in leptin that was positively correlated with OA-related cartilage damage, osteophytes, increased infrapatellar fat pad (IPFP) size, and elevated serum OA biomarkers (i.e., tissue inhibitor of metalloproteinases-1, and insulin-like growth factor-I) in obese mice [51]. A recent study by Sachdeva et al. revealed that the leptin level in the synovial fluid of advanced patients with OA was significantly correlated with both local and systemic inflammation [52]. Overexpressed leptin level was also negatively correlated with bone mineral density in patients with knee OA [53]. Accordingly, leptin-impaired (ob/ob and db/db) mice developed extreme obesity phenotypes with alteration in bone thickness and trabecular bone volume. Surprisingly, disruption of leptin signaling had no significant effect on the circulating level of pro-inflammatory cytokines or histological structure of articular cartilage in the knee joint [41]. Increasing evidence suggests the direct pro-inflammatory and catabolic role of leptin in cartilage and other joint tissue. For instance, articular chondrocytes isolated from OA patients with obesity exhibit an altered response pattern to leptin levels compared with lean or overweight patients, indicating the disruption of leptin sensitivity in these cells [50]. Chondrocytes from OA patients showed increased IL-1β, matrix metalloproteinases (MMP)-9, and MMP-13 levels after leptin [54].
Moreover, leptin can directly induce the expression of ADAMTS-4, -5, and -9 in normal human articular chondrocytes via activation of mitogen-activated protein kinases (MAPKs) and NF-ĸB signaling pathways [55]. Additionally, a combined intra-articular injection of MMP13 and ADAMTS 5 small interfering RNA (siRNA) in a mouse OA model has shown a potent protective effect on ECM loss and chondrocyte hypertrophy [56]. The inducing effect of leptin on IL-8 production in synovial fibroblasts from patients with OA suggests that this adipokine is associated with critical pathways of synovial inflammation [57]. Therefore, it is essential to consider leptin effects in obese subjects with OA when developing regenerative approaches for cartilage repair.
Clinical observations have established adiponectin to be an essential mediator for OA. However, its biologic roles in OA pathogenesis are still controversial. The levels of adiponectin measured in plasma and synovial fluid were increased in OA patients compared to controls without radiographic knee OA [58]. Especially adiponectin levels were distinctly higher in female OA patients with obesity, indicating a gender-specific distribution of leptin [59]. Of late, Orellana et al. demonstrated that synovial fluid adiponectin was positively correlated with the radiographic severity and synovial fluid IL-6 in non-obese women with middle and late OA, suggesting the local inflammatory effect of this adipokine [60]. Serum and synovial adiponectin levels were lower in obese women with knee OA than that in non-obese one. In contrast, the inflammatory factor in serum and synovial fluid was up-regulated in response to the increased BMI, which suggested a more complex relationship among obesity, adiponectin, and OA [60]. Duan et al. observed that adiponectin receptor agonist AdipoRon attenuates OA chondrocyte calcification through the AMPK-mTOR signalling pathway. However, some investigators hold the opposite opinion. A recent study, which analysed a cohort of 6671 participants aged 45–65 years, showed that the circulating adiponectin level was not associated with OA [61]. Koskinen et al. have reported that adiponectin treatment enhanced the production of pro-inflammatory mediators and MMPs in OA cartilage [62]. Moreover, adiponectin activated the AMPK/JNK pathways to induce Nitric oxide synthase (iNOS) and MMPs expression in human OA chondrocytes, indicating the catabolic potential of adiponectin in OA progression [63]. The synergistic effect of adipose tissue-derived cytokines and related pro-inflammatory factors on pathological changes of synovial joint has been highlighted in previous studies; however, more studies will be necessary to explore the role of these adipokines in the pathogenesis of obesity-induced OA.
Localised joint inflammation
Inflammation of the synovial membrane was thought to be a secondary collateral phenomenon triggered by the release of degradation products of cartilage. However, there is increasing evidence showing that macrophage-mediated synovitis could play a significant role in the initiation and progression of OA, particularly the obesity-induced OA. Accordingly, a 30-month longitudinal study of patients (BMI, mean (SD) 29.1 ± 4.5) without radiographic OA showed that baseline effusion-synovitis assessed on non-enhanced MRI in the knee joint predicted later cartilage progression [64]. Landsmeer and colleagues investigated the effect of weight gain on the progress of knee OA structural features in middle-aged overweight/obese women without clinical knee OA at baseline using MRI [65]. They found a higher prevalence of synovial inflammation in women with weight gain than those with stable weight. In addition, weight gain in overweight/obese women led to an increased progression of bone marrow lesions and cartilage defects. A similar phenomenon was observed in a previous study showing that rats with diet-induced obesity developed synovial inflammation before cartilage degradation [9]. Kraus et al. provide the first direct in vivo evidence for the involvement of activated synovial macrophage in patients with knee OA using the new 99mTc-EC20 (Etarfolatide, a folate receptor-β–based agent) imaging technique [66]. As observed in patients with knee OA (BMI, mean (SD) 29.2 ± 4.8), etarfolatide labelled activated macrophage in synovial fluid was associated with pain severity and radiographic knee OA severity including joint space narrowing and osteophyte. De Visser et al. demonstrated that a high-fat diet induces an obesity-associated dysmetabolic state resulting in a macrophage-mediated synovitis and osteophytes formation [67]. It has also been shown that obese mice fed high-fat diets rich in pro-inflammatory saturated fatty acid (FAs), and ω-6 FAs showed cartilage degradation, alteration in bone structure, and synovitis as well as increased infiltration of activated macrophages into synovial tissue [68]. In addition, localised depletion of synovial macrophages using clodronate liposome resulted in a reduction of cartilage damage and synovitis in the diet-induced obesity mouse model [20]. These findings suggest that macrophage-mediated synovitis as drivers of OA symptoms and pathology in obesity.
Several lines of evidence implicate an imbalance between M1/M2 macrophages in the pathogenesis of obesity-induced OA. We have demonstrated that synovial macrophages in mice with obesity-induced OA were predominantly polarised to the pro-inflammatory phenotype, while M2 macrophages were observed in inflamed synovium [20]. This skewing to the M1 phenotype in synovium was accompanied by the worsening of OA [69]. The polarised macrophages induce cartilage degradation, synovitis, and osteophyte formation through pro-inflammatory cytokines (e.g. TNF-α, MMPs, IL-1β, IL-6, and IL-12), catabolic mediators and anabolic factors [70]. Chondrocytes in OA articular cartilage display a phenotype of terminally differentiated cells (hypertrophy-like) and actively produce excessive degradation proteases due to inductive stimuli such as extracellular matrix molecules and inflammatory cytokines [10,71]. The phenotypic change of articular chondrocytes results in inappropriate articular cartilage destruction associated with increased gradual loss of proteoglycans followed by type II collagen degradation [72]. In vitro study reported that M1 macrophages significantly increased degradative genes in chondrocytes, while the chondrogenic gene expression level was significantly increased by M2 macrophages [9]. Intra-articular treatment with ω-3 PUFA derivative resolvin D1 reduced synovium thickening and cartilage degradation in high-fat diet-induced obese mice by regulating macrophage polarisation from pro-inflammatory to anti-inflammatory phenotype [20].
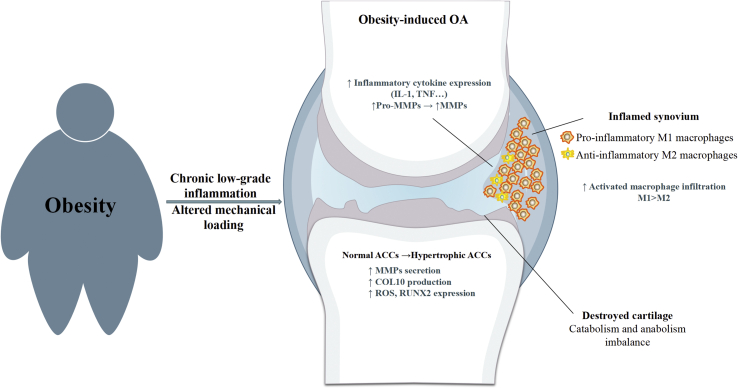
Moreover, type II collagen was shown to shift macrophages from the resting phase to anti-inflammatory M2 phenotype and induce the formation of pro-chondrogenic cytokines. Such interactions between chondrocytes and macrophages constitute a vicious cycle, sustaining chronic inflammation that contributes to pathological changes of synovial joint in obese individuals. In addition to the synovium, the IPFP, as an adipose tissue depot in the synovial joint, attracted considerable research interest in obesity-induced OA during the last decade. The immune cell profile of the synovium and IPFP is similar in obese patients with OA [73]. Pro-inflammatory phenotypes of T cells and macrophages were found to be the most predominant immune cells in the IPFP [73]. Mice with injury-induced OA on a high-fat diet showed increased IPFP inflammation characterised by an increase in crown-like structure and M1 macrophage infiltration and more fibrosis [74]. However, a previous study reported that high-diet alone did not increase the number of immune cells in the IFP or induce a pro-inflammatory phenotypic shift in mice during the early stages of knee OA [75]. These findings suggest that IPFP macrophages play a potential role in the progression of obesity-induced OA, while the role and effect of obesity on the IFP remains to be completely defined. Collectively, increased the propensity for pro-inflammatory M1 macrophage activation as an essential role in the pathogenesis of obesity and OA. The improper coordination of innate and adaptive immune responses has been implicated in perpetuating inflammation and tissue dysfunction (Fig. 1) [76].
Figure 1.
Obesity and OA. Obesity induces systemic chronic low-grade inflammation that is able to increase risk of OA by inducing local inflammation within the synovial joint (i.e. synovitis). The inflamed synovium induces increased infiltration of pro-inflammatory M1 macrophages that causes overexpression of pro-inflammatory cytokines and matrix metalloproteinase. This inflammation contributes to progressive cartilage loss by increasing the production of degradative and proteolytic enzymes. Cartilage degradation fragments induce further degradation of the cartilage by converting the phenotype of chondrocytes and further increases secretion of macrophage-like synoviocytes. Altered mechanical loading gives rise to disturbed tissue homeostasis and cartilage/bone integrity in the articular joints.
Excessive and altered mechanical loading
Moderate mechanical loading is thought to be an important environmental factor for joint homeostasis and integrity during routine daily activity [77]. The impact of biomechanical stimulation on cartilage development, cellular metabolism, and processes in adult tissues has been well documented [78]. Previous studies showed that moderate mechanical joint significantly reduced the expression of degradative activities in articular cartilage, restored abnormal bone remodelling and decreased synovitis by regulating endoplasmic reticulum stress and autophagy [79]. Furthermore, in vitro experiments using bone marrow mesenchymal stem cell-collagen scaffold constructs revealed increased chondrogenic differentiation, cell adhesion, proliferation, and secretion of ECM in response to mechanical loading [80]. In addition, mechanical stimulation exerts potent anti-inflammatory signals by suppressing pro-inflammatory cytokines and catabolic mediators [77]. However, altered mechanical load distribution in the joint due to excess body weight plays an essential role in the pathogenesis of obesity-induced OA. Klets et al. demonstrated that obese patients with OA have significantly larger (by ~2 times) volume of elements exceeding cumulative stresses of 5 MPa in the tibial cartilage compared with normal-weight subjects [81]. Visser and colleagues exploring the contribution of mechanical stress and systemic progress on the relationship between increased body weight and OA demonstrated that excessive mechanical stress on synovial joints is the main risk factor in OA progression [82]. These mechanical failures and overload lead to changes in the configuration, structure, and mechanical properties of articular cartilage, which consecutively causes subchondral bone modifications and narrowed joint space. Messier and colleagues suggested that a higher BMI was associated with greater peak knee compressive and shear forces. Simultaneously, alignment was more closely related to the imbalance of loads across the medial and lateral knee compartments due to characteristic changes in gait kinematics and resultant net kinetics [83]. Another study also supports an association between higher BMI and tibiofemoral compressive and shear contact forces and muscle forces during gait, regardless of the presence and absence of symptomatic knee OA [84]. Additionally, Rai and colleagues explored the impact of obesity-associated mechanical overload on the transcript-level changes in the medial and lateral tibial plateau cartilage. Molecular analyses revealed that numerous transcripts, biological processes, and pathways were significantly different between the medial compartment and lateral compartment from the same knee in obese OA patients, reflecting site-related effects driven by altered mechanical loading [85].
Several studies have found the impact of obesity on the deformation of cartilage in vivo. For instance, Collins et al. showed that obese subjects exhibited significantly increased tibiofemoral cartilage compressive strain after treadmill walking compared with normal BMI subjects [86]. Further analysis found that high BMI was positively correlated with T1rho relaxation time, indicating a decrease in cartilage proteoglycan concentration and the potential of cartilage deformation. Consistently, one previous study showed that weight loss was associated with an increase in proteoglycan content articular cartilage and reduced medial femoral cartilage thickness loss of medial articular cartilage, suggesting the significance of the mechanical factor in changes of cartilage composition [87]. Furthermore, abnormal mechanical compression loaded on cartilage explants highly suppress collagen expression but enhances the production of catalytic enzymes [[88], [89], [90]]. At the cellular level, chondrocytes respond to biomechanical stress, oxidative stress, cell–matrix interaction in the extracellular matrix to preserve the structural stabilisation, and intrinsic and extrinsic growth factors, cytokines and other inflammatory mediators [91]. Biochemical change of chondrocytes in response to altered mechanical loading has been proposed as a cause of OA changes [10,71]. The results of Argote et al. study demonstrated that rapid impact (80%) loading resulted in immediate loss of chondrocyte viability and chondrocyte death and reduced cartilage matrix stiffness [92]. Also, Liu and colleagues have shown that chondrocytes undergo morphological changes in a coordinated manner with load-induced deformation of the articular cartilage. This phenotypic switch of chondrocytes rings about changes in gene expression and matrix composition [89].
Furthermore, Nakamura et al. subjected bovine cartilage chondrocyte-seeded agarose constructs to cyclic compressive load. Results showed that compressive loading up-regulated the expression of type X collagen and runt-related transcription factor 2, indicating the increased population of hypertrophic differentiated chondrocytes in the cartilage [93]. Activation of phosphorylation of c-Jun N-terminal kinase (JNK), Src, and STAT1 was also observed in chondrocytes subjected to altered functional load [93]. Abnormal mechanical loading alters cellular biochemical activities through stimulation of mechanoreceptors on the surface of chondrocytes that regulate cartilage remodelling and turnover, eventually resulting in cartilage breakdown [94]. Mechanoreceptors, including the stretch-activated channels, the α-5β1 integrin and hyaluronan receptor CD44, are known to be involved in the impaired response of chondrocytes to the obesity-induced overload [95]. Sobue et al. confirmed demonstrated that excessive mechanical stress loading induces the de-differentiation of articular chondrocytes via CD44 cleavage and subsequent CD44- intracytoplasmic domain production, suggesting CD44 as a potential therapeutic target [96]. A more recent study revealed that excessive mechanical stress led to increased activities of integrins αVβ3 and αVβ5 in chondrocytes and up-regulated gene expression of IL-1β, TNF-α, MMP-3, and MMP-13 through phosphorylation of FAK and MAPKs [97]. In addition, Change and co-workers identified gremlin-1 as a mechanical loading-inducible factor in articular cartilage, which showed abundant expression by mechanical overload. The investigators further revealed that upregulation of gremlin-1 by excessive mechanical load activates NF-κB signalling, leading to subsequent induction of catabolic enzymes on articular cartilage [98]. Although it has not been well-studied, these findings highlight the distinct role of mechanical loading on the onset of cartilage degradation under the obese condition.
Changes in the configuration, structure, and mechanical properties of articular cartilage consecutively cause subchondral bone modifications (reviewed by Chen et al. [23]). Conversely, the integrity of the articular cartilage is dependent on the mechanical properties of the underlying bone [99]. Subchondral bone can attenuate about 30% of the dissipated loading stress through the joints, acting as a strong shock absorber [100]. Damage to the subchondral bone can lead to cartilage degeneration through abnormal loading on the tissue [101]. On the other hand, the deformation of cartilage can alter the load transfer mechanism to the beneath stiff bone tissue under high compression. It is becoming apparent that OA affects the entire osteochondral unit, rather than cartilage or bone separately. The cartilage–bone interface is a sophisticated functional unit. It consists of a deeper layer of non-calcified cartilage, the tidemark, calcified cartilage, the cement line (between the calcified cartilage and the subchondral bone) and subchondral bone [102]. This osteochondral junction is a biomechanical and biochemical cross-talk region that plays a vital role in maintaining joint health and integrity (extensively reviewed by Goldring et al. [103]). Increased attention is being directed at investigating the relationship between abnormalities in bone and articular degradation in OA [104].
Using modern 3D image analysis, Bowes et al. have demonstrated that bone marrow lesions were strongly co-located with areas of cartilage denudation in OA patients (BMI, mean (SD) 31.4 ± 4.6). Alteration in the areas of bone underlying denuded cartilage was suggested to be a consequence of abnormal mechanical loading, indicating an underlying causative relationship between histological abnormalities of cartilage and bone [105]. In a compelling preclinical study by Chen and co-workers, the authors demonstrated that obesity-induced overload caused horizontal fissures at the predominant compartment of the osteochondral interface in patients with OA. This novel pathological feature was characterised by cartilage erosion, fibro-granulation tissue infiltration, cartilage/bone debris and rupture of microcapillaries at the osteochondral interface [11]. Horizontal fissuring at the osteochondral interface is one of the key pathological features of OA patients with obesity.
Moreover, mechanical loading enhanced pro-inflammatory factor expression and glycosaminoglycans synthesis and decreased section of ECM components in synovial fibroblasts isolated from non-OA patients, but not in synovial fibroblasts derived from OA [24]. In obese OA patients, synovial fibroblasts secrete more significant IL-6 compared to those from normal-weight patients induced by chondrocyte-derived IL-6 via the raised level of obesity-related adipokine leptin [106], leading to inhibition of type II collagen production [107]. The structural alteration induces catabolic enzymes that react with an inflammatory reaction of the synovial tissue and further accelerates joint deterioration in patients with obesity-induced OA. There is currently limited information about these pathological changes in the synovial joint and the underlying mechanism in the initiation and progression of obesity-induced OA.
Obesity-induced OA and cartilage tissue engineering: challenges
As discussed in Influence of obesity on the development of osteoarthritis section above, the persistence of systemic and local inflammation in obesity-induced OA is strongly associated with catabolic and anabolic metabolism of tissues in synovial joints. The inflammatory cytokines and proteinases present in the joints degrade cartilage and would also be able to degrade scaffold biomaterials [108]. The early collapse of the biomaterials results in the loss of structural integrity and mechanical properties before the presence of newly formed tissue. Moreover, these materials’ degradation products can cause an inflammatory response, thus furthering the cycle of pathologic changes in the synovial joint and accelerating joint deterioration [109]. CTE approach is the potential solution for the repair and regeneration of damaged cartilage. It provides a physical environment to support cellular activities and exploits the tissue regeneration ability of macrophages [110]. However, the dramatic infiltration of inflammatory cells with a release of the high level of pro-/anti-inflammatory cytokines was observed at the implant site [111]. The M1/M2 macrophage paradigm plays a dynamic role in the outcome of biomaterial implantation and subsequent tissue homeostasis (extensively reviewed elsewhere [112]). Foreign biomaterials inevitably elicit an excess inflammatory response when implanted in vivo, and macrophages play a central role in this foreign body reaction (FBR) [113]. The hallmark feature of the FBR is the presence of macrophage-derived multinucleated foreign body giant cells (FBGCs) on the biomaterial surface, which is formed by macrophage membrane fusion during frustrated phagocytosis. These intermediate macrophages (i.e. FBGCs) secrete large quantities of ROS and degradative enzymes and exhibit both M1 and M2 markers, which contribute to biomaterial degradation and chronic inflammation [114]. The prolonged presence of phagocytic macrophages and FBGCs on the biomaterial surface induces fibroblast activation and leads to the implant’s fibrous encapsulation instead of complete resorption of the scaffold and full replacement by native-like tissue [115]. The obese condition further promotes inflammatory response in the system and localised synovial joint, exacerbating the effects of biomaterial-induced adverse immune reaction in the joints and ultimately resulting in additional tissue damage and implant failure.
Mesenchymal stem cells (MSCs)-based tissue engineering therapeutic strategies have provided novel prospects for destroyed cartilage healing in OA. MSCs chondrogenic differentiation is a critical step in MSC therapy for OA. However, chondrogenesis is required to occur in the presence of inflammatory cytokines and activated macrophages within osteoarthritic joint after transplantation. Han et al. stated that CD14(+) synovial macrophages inhibited the expression of chondrogenic-related genes in osteoarthritic synovium-derived stem cells [116]. The biochemical stimuli play an important role in cell differentiation and phenotype maintenance. Given previous indications that the presence of inflammatory cytokines such as IL-1β induces cartilage degradation by obesity-induced OA, several studies have confirmed the inhibition of the chondrogenic capacity of MSC in response to inflammatory mediators. Wehling et al. subjected aggregate cultures of MSCs recovered from the femoral intramedullary canal to the chondrogenic differentiation medium. The addition of IL-1β/TNF- α reduced the chondrogenic differentiation of human MSCs by activating NF-ĸB pathway [117]. Liu and co-workers isolated synovial fluid-derived MSCs obtained from patients with temporomandibular joint disorder and placed them with a variety of inflammatory cytokines. SRY-box 9 (SOX9) was downregulated, and MMP-13 was up-regulated upon chondrogenic differentiation induced in the cells exposed to IL-1β [118]. Under inflammatory signals, cartilaginous pellets of differentiated bone marrow-derived MSCs demonstrated significant upregulation of gene expression of ADAMTS1, 4, and 5 and rapid proteoglycan depletion [119]. Adipose tissue-derived adipokines, such as leptin, have been found to affect the chondrogenic differentiation fate of MSCs. With a high dose of leptin treatment, rat chondrogenic progenitor cells showed the decreased ability of migration, inhibited chondrogenic potential and increased osteogenic potential, indicating that leptin changes differentiation fates in chondrogenic progenitor cells. The investigators revealed that leptin-induced chondrogenic progenitor cells senescence by activating the p53/p21 pathway and inhibiting the Sirt1 pathway [120]. These mediators could prevent cells from differentiating into chondrocytes following OA-induced erosion in obese subjects.
Functional suitability and phenotypic stability of the differentiated MSCs are crucial traits for cartilage tissue engineering. Following implantation, maintaining phenotypic stability in vivo remains the key challenge. Chondrogenesis of MSCs in vitro is deeply affected by a variety of factors. To date, chondrogenic differentiation strategies have focused almost on the stimulation of anabolism using chondro-inductive growth factors, including TGF-β family and bone morphogenetic proteins (BMPs). There was a strong association between osteophytes-defined radiographic knee OA and BMI [121]. The effect on pathologic bone formation (i.e., osteophytes) in OA was attributed to M2 anti-inflammatory macrophages derived growth factors. Depleting synovial lining macrophages in the mouse model by clodronate liposome treatment results in a significant reduction of osteophyte formation by inhibiting the expression and secretion of TGF-β, BMP-2 and BMP-4 [122]. Moreover, TGF-β-dependent chondrogenic differentiation of MSCs in cartilage tissue engineering fields often accompanied by hypertrophic differentiation with increased expression of type X collagen after implantation [90]. In addition to MSC differentiation state, cell source is a key consideration in tissue engineering. Many studies indicated the adverse effects of pathological conditions on function and senescence of MSCs, which may be compromised by low-grade systemic inflammation [123,124]. Adipose tissue-derived MSCs from abdominal subcutaneous fat of morbidly obese patients exhibited impaired cellular proliferative capacities, greater senescence and altered functional characteristics [123]. In addition to abnormal functionalities, adipose tissue-derived MSCs from obese patients displayed reduced efficient chondrogenic differentiation. Further analysis found that impaired differentiation capacity in these MSCs was associated with up-regulation of miR-23b and miR-27b, which are transcriptional repressors of the Smad signalling pathway [125]. Furthermore, there was a significant reduction in proliferative capacity and in vitro chondrogenic activities in cultures of bone-marrow-derived MSCs from OA patients. Given the complex micro-environment of obesity-induced OA, utilization of the MSC-based tissue engineering approach for cartilage regeneration remains a challenge.
In addition, mechanical overload is a significant concern in cartilage tissue engineering due to its harmful effects on cellular activities. It is generally accepted that mechanical loading at “normal” physiological levels has a beneficial impact on the development of cartilage and maintenance of chondrocyte phenotype [78]. For instance, Huang et al. showed that cyclic compressive loading alone promoted the chondrogenesis of rabbit bone-marrow MSCs in agarose cultures by inducing the synthesis of TGF-β1 [126]. The application of 10 MPa of intermittent hydrostatic pressure (IHP) at a frequency of 1 Hz for 4 h/day for 14 days has been reported to stimulate Sox9, collagen type II, and aggrecan gene expression levels and collagen levels in human bone marrow-derived MSCs in the presence of TGF-β3 [127]. Additionally, Leong and colleagues observed that moderate (2.5 MPa, 1 Hz) levels of IHP suppresses basal MMP-1 expression and up-regulates ED-rich tail 2 (a mechanosensitive transcriptional coregulator), whereases high IHP (10 MPa) caused a profound increase in CITED2 and a decrease in MMP-1 mRNA expression in rat articular cartilage explants [128]. In obese individuals, mechanical overloading significantly decreased the mRNA expression of SOX9, aggrecan, and COL2A but increased COL1 mRNA expression in bovine articular chondrocyte by enhancing CD44 cleavage, thus inhibiting chondrogenic differentiation [96]. Together, these findings illustrate that understanding how stem cells respond to the hostile disease microenvironment will be central to realising the potential of stem cell-based therapies for articular cartilage repair.
Tissue engineering: biomaterials based articular cartilage repair
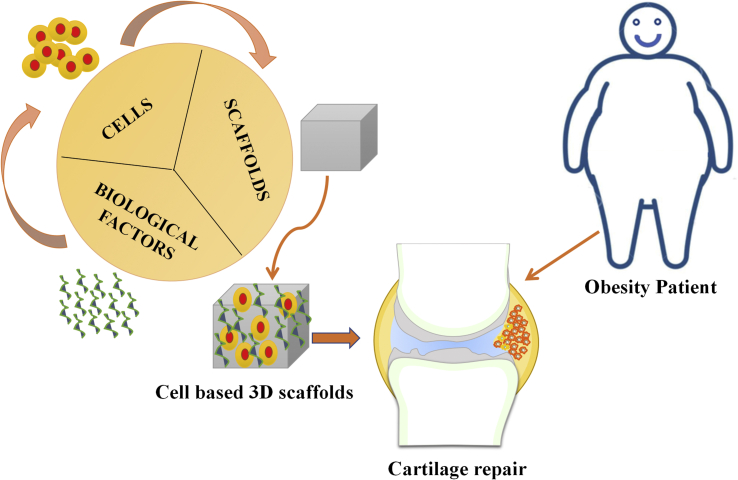
Tissue engineering is a promising field with a combination of biological science and engineering to create the potential biological-based substitutes for restore, remodel the impaired tissue functions. The association of cells, biological factors and biomaterials have been involved in this great discipline [129]. Cartilage tissue engineering is an emerging field and highly promoted from material engineering advances, stems cell and growth factors in tissue regeneration [130].
Biomaterials in terms of their physical and chemical properties, such as architecture, composition, and interface with growth factors and cells, have been monitored and developed for the better repair of cartilage [131]. However, the main requirements of biomaterials scaffolds to use them in tissue engineering applications are (i) biocompatible response with the host (ii) biodegradation ability (iii) porosity/permeability to nutrients, oxygen, and exchange, diffusion of waste and (iv) adequate mechanical property [132]. In cartilage tissue engineering, the perfect biomaterial scaffolds should possess a regeneration of cartilage with similar connective tissue composition of solid and liquid phases; it can also mimic the region and zonal organization. It should be an engineer with anisotropic mechanical property and assist easily with new tissue integration with side tissue [133]. The various types of scaffolds have been widely used in cartilage tissue engineering due to their superior advantages such as extracellular matrix mimicking, minimal obstacles at donor sites, easier control of shape and size to implant in injured sites, less complication during surgery and fast rate recovery. Also, its three-dimensional structure highly supports the production of chondrocytes to hyaline-like cartilage [134]. However, it is imperative to find suitable biomaterials that can highly encourage and advance the regeneration of the cartilage defect. Different types of natural and synthetic polymer-based scaffolds have been created by various methods and employed in cartilage tissue engineering [135]. Therefore, based on ideal applications of scaffolds towards cartilage tissue engineering, we have discussed in brief as follows.
Mimura et al. studied the concentration based collagen scaffolds and designed well to engage the mesenchymal stem cells in the central region of cartilage full-thickness defects through haptotaxis [136]. YoungWon et al. developed the rabbit articular chondrocytes seeded collagen scaffolds by 3D bioprinting and in vivo studies confirmed the formation of hyaline cartilage, which integrated well with surrounding cartilage tissue [137]. Almeida et al. investigated the ECM microparticles decorated injectable fibrin hydrogel with the inclusion of stromal cells and in vivo results proved that these injectable hydrogels could induce the repair and regeneration of cartilage [138]. Moshaverinia et al. developed an alginate hydrogel with coupled of RGD and TGF- β1 and seeded with dental MSCs. In vivo studies, they reported that the regeneration of ectopic cartilage was observed and periodontal ligament stem cells showed significant chondrogenesis than others [139]. Agheb et al. fabricated the gelatin nanofibers by electrospinning technique. They examined in vitro studies with chondrocytes, which shown excellent adhesion, spreading and proliferation for potential applications in cartilage tissue engineering [140]. Man et al. fabricated the hybrid scaffolds of chitosan hydrogel and demineralized bone matrix and encapsulated with allogenic chondrocytes for treating the injury of rabbit cartilage tissue through one-step surgery [141]. Yin et al. worked on the bioactive hydrogel scaffolds with a combination of agarose and platelet-rich plasma and found that which has excellent ability in the proliferation of chondrocytes and accumulation of matrix for cartilage repair [142]. Chen et al. employed selective laser sintering to fabricate the polycaprolactone scaffolds. Its surface was modified with a coating of gelatin or collagen to improve the hydrophilic and mechanical properties. They suggested that collagen-coated scaffolds were encouraged the formation of cartilage tissue in craniofacial reconstruction [143]. Hung et al. used water-based 3D printing technology to develop the scaffold with a combination of hyaluronan, biodegradable polyurethane, and bioactive ingredients such as TGF-β3 or Y27632 drug and were seeded with MSCs. They reported that the implantation of scaffolds in rabbit chondral defects could improve cartilage regeneration [144]. Cui et al. investigated the articular cartilage repair in non-weight comportment areas using the moulding process produced polyglycolic acid scaffold with the stabilizing agent of polylactic acid. These scaffolds were seeded with adipose-derived stem cells and proved that scaffolds with cells were achieved good chondrogenesis and found to have an excellent repair of articular cartilage defects [145].
Chang et al. developed a cell-free porous poly(lactide-co-glycolide) scaffold using solvent casting and particulate leaching. They proved that these scaffolds could regenerate hyaline cartilage and promote the regeneration of osteochondral full-thickness defects by early loading exercise in knee joints of rabbits [146]. In past years, few commercially available products were discovered and used in the repair and regeneration of articular cartilage tissues. The protein-based products are MACI®, Maix®, Atelocollagen®, MaioRegen®, and Tissucol kit®. The polysaccharides and synthetic polymer-based products are HYAFF-11®, BST-CarGel®, and Bio-Seed®-C, respectively [147]. However, as per our literature knowledge, there are no specific research findings on biomaterials for the obesity-induced articular cartilage defects treatments. Therefore, the creation of new biomaterials must use them in the repair of cartilage tissue for obesity patients (Fig. 2).
Figure 2.
Potential approach of tissue engineering-based treatment for obesity-induced cartilage degradation.
Prospects in obesity-induced OA treatment
Unlike other tissue, articular cartilage has a very limited capacity for healing and repairing results because of the unique characteristics of chondrocytes and the absence of vascularisation [148]. Tissue engineering strategies provide new prospects in overcoming the self-repair limitation of articular cartilage and preventing their progression to late-stage OA. Attempts have been made to develop biological substitutes that can notably restore the function of damaged cartilage. Current cartilage regeneration treatments have shown satisfactory results in OA patients with near-ideal surgical condition. However, patients with obesity-induced OA are often excluded from cartilage repair attempts due to the abnormal mechanical demands, altered biomechanical and biochemical activities of cells, persistent chronic inflammation, and other obesity-associated factors [149]. Yet, there is a lack of publications report on the development of engineered cartilage tissue for obesity-induced OA. With the alarming increase in the number of obese populations in the world, the need for an innovative therapeutic approach that could effectively repair and restore the damaged synovial joints is of significant importance for this sub-population of patients.
In the previous sections, chronic inflammation was highlighted as the main contributor to the development of OA in obese patients. In this respect, the potential impact of the persistent inflammatory environment on the cell-based CTE was also discussed above. Inflammation is the first stage of tissue repair that aims to harness the potential of inflammatory cells actively and re-establish tissue homeostasis. Proper host immune response to engineered tissue following implantation is also necessary for successful outcomes in CTE [111]. Efforts focused on modulating inflammatory response and promoting tissue repair are required to treat obesity-induced OA from a tissue-engineering perspective effectively. Among the potential strategies to achieve this aim, MSC lineage cells appear to be a promising option owing to their various bioactive factors that have potent immunomodulatory, antiapoptotic, antifibrotic, and anti-inflammatory effects [150]. MSCs play a central role in maintaining immune homeostasis by secreting and producing several mediators which interact with cells of both the adaptive and innate immune system [151]. MSCs and their derivatives suppress macrophage polarisation to pro-inflammatory phenotype and promote immunosuppressive M2 subset via various mechanisms. MSCs-derived Prostaglandin E2 (PGE2) was able to reduce the expression of pro-inflammatory cytokines in activated M1 macrophages [152]. Human MSCs-derived tumour necrosis factor-α stimulated protein 6 significantly inhibited activation of M1 macrophages through the modulation of nuclear factor (NF)-κB signalling, resulting in aborting early inflammatory responses [153]. The immunomodulatory effects of MSCs have been observed in obesity. As described by Zhang et al., adipose-derived stem cells (ADSCs) suppress pro-inflammatory cytokines TNF-α, IL-6, and IL-1β in the obese mice through inhibition of the ITGAM/NF-κB pathway and polarisation of M2 macrophages [154]. Another recent study found that lean mouse-derived ADSCs treatment successfully reduced obesity-associated white adipose tissue inflammation and substantially improved insulin action in obese mice by re-modulating the phenotypes of adipose-resident macrophages from pro-inflammatory M1 toward anti-inflammatory M2 subtypes [155].
The findings suggest a potential stem cell-based approach for the treatment of obesity-related diseases. In experimental mouse OA, intraarticular injection of ADSCs has shown to inhibit synovial thickening by directly interacting with activated macrophages within the intimal layer, leading to a significant reduction of joint destruction [156]. Co-culture studies released that human MSC-derived exosomes and microparticles prevented OA-like chondrocytes apoptosis and inhibited macrophage differentiation [157]. Orozco et al. have shown in a pilot study that the treatment of knee OA in patients with autologous expanded BMSCs led to the significant progressive improvement of algofunctional indices [158]. Another study performed by Vega et al. also indicated that intra-articular injection of 40 × 106 BMSCs in 30 patients led to significant decreases in pain level and cartilage degradative area [19]. These facts highlighted the therapeutic potential of MSCs in OA patients with obesity. However, despite extensive research in this field, it should be clearly stated that long-term efficacy and safety for MSC-based tissue engineering will require further investigation due to several major limitations, including uncontrollable massive cell death and cells leaking out of the synovial joint space after injection.
Modification of the inflammatory response is possible in obesity-induced OA through the use of biomaterials incorporated with anti-inflammatory therapeutics [159]. The incorporation of anti-inflammatory molecules IL-10 into liposomes containing phosphatidylserine as a tool to manipulate macrophage function, dramatically improved the obesity-associated metabolic alterations, systemic inflammatory condition, and organ injure [160]. Intra-articular injection of kartogenin significantly decreased pain severity and joint destruction in the OA model through Induction of IL-10 [161]. Overexpression of IL-10 may be considered as the potential avenue for anti-inflammatory therapeutics. A porous poly (lactide-co-glycolide) scaffold was loaded with anti-inflammatory drug resveratrol to decrease activated monocyte and lymphocyte numbers and increase anti-inflammatory expression cytokines in dysfunctional adipose tissue. It was shown that poly(lactide-co-glycolide) scaffold designed to integrate with adipose tissue could reduce the inflammatory tissue response at the implant site, adjunctive resveratrol administration augmented this anti-inflammatory environment, suggesting an important role of biomaterial in immunomodulation [162]. The anti-inflammatory drug resveratrol also exerts a protective effect on obesity-induced OA by inhibiting TLR4 via the activation of PI3K/Akt signalling pathways in animal models [163]. In addition, new emerging agent, interleukin-1 receptor antagonist (IL-1ra) has been suggested as a potential therapy to treat OA via local delivery. However, neither of these approaches showed long-term effect due to rapid clearance [149]. Given the complex microenvironment of the individuals with obesity-induced OA, the choice of biomaterials and delivery mechanism is an important design consideration to prolong the retention time of drugs in the synovial cavity. Several strategies have been explored to improve the efficacy of drugs. For instance, dexamethasone conjugation to the hyaluronic acid hydrogel had better chondroprotective and anti-inflammatory effects in OA models than hyaluronic acid alone. However, the injection of hyaluronic acid in OA joints positively impacted cartilage regeneration [164]. The IL-1ra tethered poly(2-hydroxyethyl methacrylate)-pyridine nanoparticles were stable and cytocompatible in serum-containing solutions for several days. As a result, these engineered nanoparticles effectively blocked IL-1β signalling in an NF-κB inducible reporter cell line [165]. In addition, Goodman and co-workers developed two novel approaches to immunomodulation by altering MSCs to improve tissue healing in inflammatory clinical scenarios: (1) preconditioning the MSCs in the inflammatory microenvironment, and (2) genetically modifying MSCs to over-express the immune-modulating pro-regenerative cytokine IL-4 [166]. An emerging approach is to design biomaterials/scaffolds incorporated with anti-inflammatory therapeutics and to test tissue-engineered constructs in an inflammatory environment.
As discussed above, the capacity for differentiation and phenotypic stability of differentiated cells decreases with altered microenvironment in obesity-induced OA. Facilitating chondrogenesis of MSC lineage cells should be addressed in regenerative strategies. Post-transcriptional mechanisms play an important role in cell differentiation, proliferation, apoptosis and tissue development and homeostasis. MicroRNA (miRNA) is a short non-coding RNA that acts in a complex functional network in which each miRNA regulates multiple genes through interaction with target messenger RNA within the ‘3′untranslated region of the mRNA [167]. Circulating miRNAs have been proposed as a biomarker for obesity and its associated comorbidities, including OA. Xie et al. demonstrated that the expression of miR-26a was negatively correlated to chronic inflammation in the chondrocytes and circulation in the obese mouse model. Further analysis revealed that saturated free fatty acid-induced activation of NF-κB (p65) and the production of pro-inflammatory cytokines directly suppressed miR-26a production in obesity-related chondrocytes [168]. Moreover, there is growing evidence for the involvement of miRNA in the regulation of chondrogenic differentiation. For example, pro-chondrogenic miR-140 plays an important role in cartilage development and homeostasis by targeting histone deacetylase 4 [169]. During chondrogenic differentiation, miR-140 expression in human MSCs increased in parallel with the expression of SOX9 and COL2A, while the human OA articular cartilage chondrocytes showed significantly lower expression of miR-140 [170]. Moreover, the expression of miR-140 is mainly controlled by SOX9, a major transcription factor in maintaining cellular phenotype and preventing hypertrophy [171]. Overexpression of miR-483 in human BMSCs resulted in the downregulation of chondrogenic gene expression and suppressing chondrogenic differentiation by inhibiting the TGF-β pathway member SMAD4 [172]. Hwang et al. demonstrated that overexpression of miR-365 significantly suppressed L-1β-induced up-regulation of catabolic factors and prevented extracellular matrix loss in pellet culture of primary chondrocytes [173]. Dysregulated expression of miR-140, miR-483 and miR-365 were also found in obesity [[174], [175], [176]]. Overall, these studies highlight the importance of miRNA in controlling the fate of chondrogenesis and maintaining the chondrocyte phenotype. Thus, miRNA modified MSCs pose the basis for future use in tissue engineering for OA treatment. Understanding the chondrogenic potential of MSCs and optimising chondrogenic differentiation is important for achieving the clinical translation of engineered cartilage into in obesity-induced OA.
Conclusion
Cartilage tissue engineering holds great promise for regenerative medicine. Yet, there is a lack of publications report on the development of engineered cartilage tissue for obesity-induced OA. The persistent inflammatory response in circulation and localised synovial joints contributes to the development of obesity-induced OA. Altered mechanical loading gives rise to disturbing tissue homeostasis and cartilage/bone integrity in the articular joints. In relation to our topic, cartilage tissue engineering strategies for obesity-induced OA must aim to create neo-tissue that withstands a complex plethora of inflammatory stimuli in the arthritic joint, maintains the chondrocyte phenotype, readily integrates into surrounding native tissue and provide mechanical support during the remodelling stage of cartilage recovery. Interdisciplinary research utilising tissue engineering could be a potential approach to develop innovative therapeutics that could effectively repair and restore the damaged synovial joints for patients with obesity-induced OA.
Declaration of competing interest
The authors have no conflicts of interest to disclose in relation to this article.
Acknowledgment
The authors are thankful for the funding from This research was supported by the National Key R&D Program of China (2018YFC1705205); Foreign cooperation project of Chinese Academy of Sciences (GJHZ2063); National Natural Science Foundation of China: NSFC81672224, NSFC81473709,NSFC81830041 & NSFC31671562; Science and Technology Innovation Fund of Shenzhen (JCYJ20170818153602439, JCYJ20180302150101316 & JCYJ20170818163724754), Sanming Project of Medicine in Shenzhen (SZSM201808072); Development and Reform Commission of Shenzhen Municipality (XMHT20190106001) and The Outstanding Youth Innovation Research Fund of the Shenzhen Institutes of Advanced Technology, Chinese Academy of Sciences (Y9G031). Shenzhen Double Chain Project for Innovation and Development Industry supported by Bureau of Industry and Information Technology of Shenzhen (201908141541).
Contributor Information
Antonia RuJia Sun, Email: antonia@siat.ac.cn.
Anjaneyulu Udduttula, Email: anjaneyulu@siat.ac.cn.
Jian Li, Email: li.jian@siat.ac.cn.
Yanzhi Liu, Email: liuyanzhi02@163.com.
Pei-Gen Ren, Email: pg.ren@siat.ac.cn.
Peng Zhang, Email: peng.zhang@siat.ac.cn.
References
- 1.Loeser R.F., Goldring S.R., Scanzello C.R., Goldring M.B. Osteoarthritis: a disease of the joint as an organ. Arthritis Rheum. 2012;64(6):1697–1707. doi: 10.1002/art.34453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vina E.R., Kwoh C.K. Epidemiology of osteoarthritis: literature update. Curr Opin Rheumatol. 2018;30(2):160–167. doi: 10.1097/BOR.0000000000000479. [eng] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang X., Hunter D., Xu J., Ding C. Metabolic triggered inflammation in osteoarthritis. Osteoarthritis Cartilage. 2015;23(1):22–30. doi: 10.1016/j.joca.2014.10.002. [eng] [DOI] [PubMed] [Google Scholar]
- 4.Hellevik A.I., Johnsen M.B., Langhammer A., Baste V., Furnes O., Storheim K. Metabolic syndrome as a risk factor for total hip or knee replacement due to primary osteoarthritis: a prospective cohort study (the HUNT study and the Norwegian Arthroplasty Register) Clin Epidemiol. 2018;10:83–96. doi: 10.2147/CLEP.S145823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heilbronn L.K., Campbell L.V. Adipose tissue macrophages, low grade inflammation and insulin resistance in human obesity. Curr Pharmaceut Des. 2008;14(12):1225–1230. doi: 10.2174/138161208784246153. [eng] [DOI] [PubMed] [Google Scholar]
- 6.Scanzello C.R. Role of low-grade inflammation in osteoarthritis. Curr Opin Rheumatol. 2017;29(1):79–85. doi: 10.1097/BOR.0000000000000353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berenbaum F. Osteoarthritis as an inflammatory disease (osteoarthritis is not osteoarthrosis!) Osteoarthritis Cartilage. 2013;21(1):16–21. doi: 10.1016/j.joca.2012.11.012. [DOI] [PubMed] [Google Scholar]
- 8.Benito M.J., Veale D.J., FitzGerald O., van den Berg W.B., Bresnihan B. Synovial tissue inflammation in early and late osteoarthritis. Ann Rheum Dis. 2005;64(9):1263–1267. doi: 10.1136/ard.2004.025270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sun A.R., Panchal S.K., Friis T., Sekar S., Crawford R., Brown L. Obesity-associated metabolic syndrome spontaneously induces infiltration of pro-inflammatory macrophage in synovium and promotes osteoarthritis. PloS One. 2017;12(8) doi: 10.1371/journal.pone.0183693. [eng] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goldring M.B., Otero M., Plumb D.A., Dragomir C., Favero M., El Hachem K. Roles of inflammatory and anabolic cytokines in cartilage metabolism: signals and multiple effectors converge upon MMP-13 regulation in osteoarthritis. Eur Cell Mater. 2011;21:202–220. doi: 10.22203/ecm.v021a16. [eng] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen L., Yao F., Wang T., Li G., Chen P., Bulsara M. Horizontal fissuring at the osteochondral interface: a novel and unique pathological feature in patients with obesity-related osteoarthritis. Ann Rheum Dis. 2020;79:811–818. doi: 10.1136/annrheumdis-2020-216942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hunter D.J., Felson D.T. Osteoarthritis. BMJ. 2006;332(7542):639–642. doi: 10.1136/bmj.332.7542.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shan L., Shan B., Graham D., Saxena A. Total hip replacement: a systematic review and meta-analysis on mid-term quality of life. Osteoarthritis Cartilage. 2014;22(3):389–406. doi: 10.1016/j.joca.2013.12.006. [eng] [DOI] [PubMed] [Google Scholar]
- 14.Wise B.L., Niu J., Felson D.T., Hietpas J., Sadosky A., Torner J. Functional impairment is a risk factor for knee replacement in the multicenter osteoarthritis study. Clin Orthop Relat Res. 2015;473(8):2505–2513. doi: 10.1007/s11999-015-4211-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Amin A.K., Clayton R.A., Patton J.T., Gaston M., Cook R.E., Brenkel I.J. Total knee replacement in morbidly obese patients. Results of a prospective, matched study. J Bone Jt Surg Br Vol. 2006;88(10):1321–1326. doi: 10.1302/0301-620X.88B10.17697. [eng] [DOI] [PubMed] [Google Scholar]
- 16.Foran J.R., Mont M.A., Etienne G., Jones L.C., Hungerford D.S. The outcome of total knee arthroplasty in obese patients. J Bone Jt Surg. 2004;86(8):1609–1615. doi: 10.2106/00004623-200408000-00002. American volume [eng] [DOI] [PubMed] [Google Scholar]
- 17.Langer R., Vacanti J.P. Tissue engineering. Science. 1993;260(5110):920–926. doi: 10.1126/science.8493529. (New York, NY) [eng] [DOI] [PubMed] [Google Scholar]
- 18.Deng Y., Lin X.S., Zheng Z., Deng J.G., Chen J.C., Ma H. Poly(hydroxybutyrate-co-hydroxyhexanoate) promoted production of extracellular matrix of articular cartilage chondrocytes in vitro. Biomaterials. 2003;24(23):4273–4281. doi: 10.1016/s0142-9612(03)00367-3. [eng] [DOI] [PubMed] [Google Scholar]
- 19.Vega A., Martin-Ferrero M.A., Del Canto F., Alberca M., Garcia V., Munar A. Treatment of knee osteoarthritis with allogeneic bone marrow mesenchymal stem cells: a randomized controlled trial. Transplantation. 2015;99(8):1681–1690. doi: 10.1097/TP.0000000000000678. [eng] [DOI] [PubMed] [Google Scholar]
- 20.Sun A.R., Wu X., Liu B., Chen Y., Armitage C.W., Kollipara A. Pro-resolving lipid mediator ameliorates obesity induced osteoarthritis by regulating synovial macrophage polarisation. Sci Rep. 2019;9(1) doi: 10.1038/s41598-018-36909-9. 426-26. [eng] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fullerton J.N., Gilroy D.W. Resolution of inflammation: a new therapeutic frontier. Nat Rev Drug Discov. 2016;15:551. doi: 10.1038/nrd.2016.39. [DOI] [PubMed] [Google Scholar]
- 22.Andriacchi T.P., Mundermann A. The role of ambulatory mechanics in the initiation and progression of knee osteoarthritis. Curr Opin Rheumatol. 2006;18(5):514–518. doi: 10.1097/01.bor.0000240365.16842.4e. [eng] [DOI] [PubMed] [Google Scholar]
- 23.Chen L., Yi Zheng J.J., Li G., Yuan J., Ebert J.R., Li H. Pathogenesis and clinical management of obesity-related knee osteoarthritis: impact of mechanical loading. J Orthop Transl. 2020;24(66):75. doi: 10.1016/j.jot.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schröder A., Nazet U., Muschter D., Grässel S., Proff P., Kirschneck C. Impact of mechanical load on the expression profile of synovial fibroblasts from patients with and without osteoarthritis. Int J Mol Sci. 2019;20(3):585. doi: 10.3390/ijms20030585. [eng] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Honda K., Ohno S., Tanimoto K., Ijuin C., Tanaka N., Doi T. The effects of high magnitude cyclic tensile load on cartilage matrix metabolism in cultured chondrocytes. Eur J Cell Biol. 2000;79(9):601–609. doi: 10.1078/0171-9335-00089. [eng] [DOI] [PubMed] [Google Scholar]
- 26.Richette P., Poitou C., Garnero P., Vicaut E., Bouillot J.-L., Lacorte J.-M. Benefits of massive weight loss on symptoms, systemic inflammation and cartilage turnover in obese patients with knee osteoarthritis. Ann Rheum Dis. 2011;70(1):139–144. doi: 10.1136/ard.2010.134015. [DOI] [PubMed] [Google Scholar]
- 27.Loukov D., Karampatos S., Maly M.R., Bowdish D.M.E. Monocyte activation is elevated in women with knee-osteoarthritis and associated with inflammation, BMI and pain. Osteoarthritis Cartilage. 2018;26(2):255–263. doi: 10.1016/j.joca.2017.10.018. [DOI] [PubMed] [Google Scholar]
- 28.Mosser D.M., Edwards J.P. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008;8(12):958–969. doi: 10.1038/nri2448. [eng] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wynn T.A., Vannella K.M. Macrophages in tissue repair, regeneration, and fibrosis. Immunity. 2016;44(3):450–462. doi: 10.1016/j.immuni.2016.02.015. [eng] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li C., Xu M.M., Wang K., Adler A.J., Vella A.T., Zhou B. Macrophage polarization and meta-inflammation. Transl Res. 2018;191:29–44. doi: 10.1016/j.trsl.2017.10.004. [eng] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weisberg S.P., Hunter D., Huber R., Lemieux J., Slaymaker S., Vaddi K. CCR2 modulates inflammatory and metabolic effects of high-fat feeding. J Clin Invest. 2006;116(1):115–124. doi: 10.1172/JCI24335. [eng] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stankovic A., Slavic V., Stamenkovic B., Kamenov B., Bojanovic M., Mitrovic D.R. Serum and synovial fluid concentrations of CCL2 (MCP-1) chemokine in patients suffering rheumatoid arthritis and osteoarthritis reflect disease activity. Bratisl Lek Listy. 2009;110(10):641–646. [eng] [PubMed] [Google Scholar]
- 33.Li L., Jiang B.E. Serum and synovial fluid chemokine ligand 2/monocyte chemoattractant protein 1 concentrations correlates with symptomatic severity in patients with knee osteoarthritis. Ann Clin Biochem. 2015;52(Pt 2):276–282. doi: 10.1177/0004563214545117. [eng] [DOI] [PubMed] [Google Scholar]
- 34.Xia C., Braunstein Z., Toomey A.C., Zhong J., Rao X. S100 proteins as an important regulator of macrophage inflammation. Front Immunol. 2018;8 doi: 10.3389/fimmu.2017.01908. 1908-08. [eng] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.van Lent P.L., Blom A.B., Schelbergen R.F., Slöetjes A., Lafeber F.P., Lems W.F. Active involvement of alarmins S100A8 and S100A9 in the regulation of synovial activation and joint destruction during mouse and human osteoarthritis. Arthritis Rheum. 2012;64(5):1466–1476. doi: 10.1002/art.34315. [eng] [DOI] [PubMed] [Google Scholar]
- 36.Sekimoto R., Fukuda S., Maeda N., Tsushima Y., Matsuda K., Mori T. Visualized macrophage dynamics and significance of S100A8 in obese fat. Proc Natl Acad Sci USA. 2015;112(16):E2058–E2066. doi: 10.1073/pnas.1409480112. [eng] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schelbergen R.F.P., de Munter W., van den Bosch M.H.J., Lafeber F.P.J.G., Sloetjes A., Vogl T. Alarmins S100A8/S100A9 aggravate osteophyte formation in experimental osteoarthritis and predict osteophyte progression in early human symptomatic osteoarthritis. Ann Rheum Dis. 2016;75(1):218–225. doi: 10.1136/annrheumdis-2014-205480. [DOI] [PubMed] [Google Scholar]
- 38.Kang Y.E., Kim J.M., Joung K.H., Lee J.H., You B.R., Choi M.J. The roles of adipokines, proinflammatory cytokines, and adipose tissue macrophages in obesity-associated insulin resistance in modest obesity and early metabolic dysfunction. PloS One. 2016;11(4) doi: 10.1371/journal.pone.0154003. e03. [eng] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ouchi N., Parker J.L., Lugus J.J., Walsh K. Adipokines in inflammation and metabolic disease. Nat Rev Immunol. 2011;11(2):85–97. doi: 10.1038/nri2921. [eng] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Monteiro L., Pereira J., Palhinha L., Moraes-Vieira P.M.M. Leptin in the regulation of the immunometabolism of adipose tissue-macrophages. J Leukoc Biol. 2019;106(3):703–716. doi: 10.1002/JLB.MR1218-478R. [eng] [DOI] [PubMed] [Google Scholar]
- 41.Griffin T.M., Huebner J.L., Kraus V.B., Guilak F. Extreme obesity due to impaired leptin signaling in mice does not cause knee osteoarthritis. Arthritis Rheum. 2009;60(10):2935–2944. doi: 10.1002/art.24854. [eng] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dib L.H., Ortega M.T., Fleming S.D., Chapes S.K., Melgarejo T. Bone marrow leptin signaling mediates obesity-associated adipose tissue inflammation in male mice. Endocrinology. 2014;155(1):40–46. doi: 10.1210/en.2013-1607. [eng] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Clements V.K., Long T., Long R., Figley C., Smith D.M.C., Ostrand-Rosenberg S. High fat diet and leptin promote tumor progression by inducing myeloid-derived suppressor cells. J Leukoc Biol. 2018;103(3):395–407. doi: 10.1002/JLB.4HI0517-210R. [eng] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Otero M., Ro Lago, Lago F., Casanueva F.F., Dieguez C., Gómez-Reino J.J. Leptin, from fat to inflammation: old questions and new insights. 2005;579(2):295–301. doi: 10.1016/j.febslet.2004.11.024. [DOI] [PubMed] [Google Scholar]
- 45.Gariballa S., Alkaabi J., Yasin J., Al Essa A. Total adiponectin in overweight and obese subjects and its response to visceral fat loss. BMC Endocr Disord. 2019;19(1):55. doi: 10.1186/s12902-019-0386-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hui X., Gu P., Zhang J., Nie T., Pan Y., Wu D. Adiponectin enhances cold-induced browning of subcutaneous adipose tissue via promoting M2 macrophage proliferation. Cell Metabol. 2015;22(2):279–290. doi: 10.1016/j.cmet.2015.06.004. [DOI] [PubMed] [Google Scholar]
- 47.Gao Y.-H., Zhao C.-W., Liu B., Dong N., Ding L., Li Y.-R. An update on the association between metabolic syndrome and osteoarthritis and on the potential role of leptin in osteoarthritis. Cytokine. 2020;129:155043. doi: 10.1016/j.cyto.2020.155043. [DOI] [PubMed] [Google Scholar]
- 48.Vuolteenaho K., Koskinen A., Moilanen T., Moilanen E. Leptin levels are increased and its negative regulators, SOCS-3 and sOb-R are decreased in obese patients with osteoarthritis: a link between obesity and osteoarthritis. 2012;71(11):1912–1913. doi: 10.1136/annrheumdis-2011-201242. [DOI] [PubMed] [Google Scholar]
- 49.Yang T.-P., Chen H.-M., Hu C.-C., Chen L.-Y., Shih F.-F., Tantoh D.M. Interaction of osteoarthritis and BMI on leptin promoter methylation in Taiwanese adults. Int J Mol Sci. 2019;21(1):123. doi: 10.3390/ijms21010123. [eng] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pallu S., Francin P.-J., Guillaume C., Gegout-Pottie P., Netter P., Mainard D. Obesity affects the chondrocyte responsiveness to leptin in patients with osteoarthritis. Arthritis Res Ther. 2010;12(3):R112. doi: 10.1186/ar3048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Griffin T.M., Batushansky A., Hudson J., Lopes E.B.P. Correlation network analysis shows divergent effects of a long-term, high-fat diet and exercise on early stage osteoarthritis phenotypes in mice. J Sport Health Sci. 2020;9(2):119–131. doi: 10.1016/j.jshs.2019.05.008. [eng] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sachdeva M., Aggarwal A., Sharma R., Randhawa A., Sahni D., Jacob J. Chronic inflammation during osteoarthritis is associated with an increased expression of CD161 during advanced stage. 2019;90(1) doi: 10.1111/sji.12770. [DOI] [PubMed] [Google Scholar]
- 53.Wu J., Xu J., Wang K., Zhu Q., Cai J., Ren J. Associations between circulating adipokines and bone mineral density in patients with knee osteoarthritis: a cross-sectional study. BMC Muscoskel Disord. 2018;19(1):16. doi: 10.1186/s12891-018-1936-7. [eng] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Simopoulou T., Malizos K.N., Iliopoulos D., Stefanou N., Papatheodorou L., Ioannou M. Differential expression of leptin and leptin’s receptor isoform (Ob-Rb) mRNA between advanced and minimally affected osteoarthritic cartilage; effect on cartilage metabolism. Osteoarthritis Cartilage. 2007;15(8):872–883. doi: 10.1016/j.joca.2007.01.018. [DOI] [PubMed] [Google Scholar]
- 55.Yaykasli K.O., Hatipoglu O.F., Yaykasli E., Yildirim K., Kaya E., Ozsahin M. Leptin induces ADAMTS-4, ADAMTS-5, and ADAMTS-9 genes expression by mitogen-activated protein kinases and NF-ĸB signaling pathways in human chondrocytes. Cell Biol Int. 2015;39(1):104–112. doi: 10.1002/cbin.10336. [eng] [DOI] [PubMed] [Google Scholar]
- 56.Hoshi H., Akagi R., Yamaguchi S., Muramatsu Y., Akatsu Y., Yamamoto Y. Effect of inhibiting MMP13 and ADAMTS5 by intra-articular injection of small interfering RNA in a surgically induced osteoarthritis model of mice. Cell Tissue Res. 2017;368(2):379–387. doi: 10.1007/s00441-016-2563-y. [eng] [DOI] [PubMed] [Google Scholar]
- 57.Tong K.-M., Shieh D.-C., Chen C.-P., Tzeng C.-Y., Wang S.-P., Huang K.-C. Leptin induces IL-8 expression via leptin receptor, IRS-1, PI3K, Akt cascade and promotion of NF-κB/p300 binding in human synovial fibroblasts. Cell Signal. 2008;20(8):1478–1488. doi: 10.1016/j.cellsig.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 58.de Boer T.N., van Spil W.E., Huisman A.M., Polak A.A., Bijlsma J.W., Lafeber F.P. Serum adipokines in osteoarthritis; comparison with controls and relationship with local parameters of synovial inflammation and cartilage damage. Osteoarthritis Cartilage. 2012;20(8):846–853. doi: 10.1016/j.joca.2012.05.002. [eng] [DOI] [PubMed] [Google Scholar]
- 59.Koskinen-Kolasa A., Vuolteenaho K., Moilanen T., Moilanen E. Adipokines leptin, adiponectin and resistin and their associations to MMPS, IL-6, COMP and radiographic severity of OA. Osteoarthritis Cartilage. 2016;24:S78. [Google Scholar]
- 60.Orellana C., Calvet J., Berenguer-Llergo A., Albiñana N., García Manrique M., Galisteo Lencastre C. Synovial adiponectin was more associated with clinical severity than synovial leptin in women with knee osteoarthritis. Cartilage. 2020;1947603520904776 doi: 10.1177/1947603520904776. [eng] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kroon F.P.B., Veenbrink A.I., de Mutsert R., Visser A.W., van Dijk K.W., le Cessie S. The role of leptin and adiponectin as mediators in the relationship between adiposity and hand and knee osteoarthritis. Osteoarthritis Cartilage. 2019;27(12):1761–1767. doi: 10.1016/j.joca.2019.08.003. [eng] [DOI] [PubMed] [Google Scholar]
- 62.Koskinen A., Juslin S., Nieminen R., Moilanen T., Vuolteenaho K., Moilanen E. Adiponectin associates with markers of cartilage degradation in osteoarthritis and induces production of proinflammatory and catabolic factors through mitogen-activated protein kinase pathways. Arthritis Res Ther. 2011;13(6):R184. doi: 10.1186/ar3512. [eng] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kang E.H., Lee Y.J., Kim T.K., Chang C.B., Chung J.H., Shin K. Adiponectin is a potential catabolic mediator in osteoarthritis cartilage. Arthritis Res Ther. 2010;12(6):R231. doi: 10.1186/ar3218. [eng] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Roemer F.W., Guermazi A., Felson D.T., Niu J., Nevitt M.C., Crema M.D. Presence of MRI-detected joint effusion and synovitis increases the risk of cartilage loss in knees without osteoarthritis at 30-month follow-up: the MOST study. Ann Rheum Dis. 2011;70(10):1804–1809. doi: 10.1136/ard.2011.150243. [eng] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Landsmeer M.L.A., de Vos B.C., van der Plas P., van Middelkoop M., Vroegindeweij D., Bindels P.J.E. Effect of weight change on progression of knee OA structural features assessed by MRI in overweight and obese women. Osteoarthritis Cartilage. 2018;26(12):1666–1674. doi: 10.1016/j.joca.2018.08.006. [DOI] [PubMed] [Google Scholar]
- 66.Kraus V.B., McDaniel G., Huebner J.L., Stabler T.V., Pieper C.F., Shipes S.W. Direct in vivo evidence of activated macrophages in human osteoarthritis. Osteoarthritis Cartilage. 2016;24(9):1613–1621. doi: 10.1016/j.joca.2016.04.010. [eng] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.de Visser H.M., Mastbergen S.C., Kozijn A.E., Coeleveld K., Pouran B., van Rijen M.H. Metabolic dysregulation accelerates injury-induced joint degeneration, driven by local inflammation; an in vivo rat study. J Orthop Res. 2018;36(3):881–890. doi: 10.1002/jor.23712. [DOI] [PubMed] [Google Scholar]
- 68.Wu C.-L., Jain D., McNeill J.N., Little D., Anderson J.A., Huebner J.L. Dietary fatty acid content regulates wound repair and the pathogenesis of osteoarthritis following joint injury. Ann Rheum Dis. 2015;74(11):2076–2083. doi: 10.1136/annrheumdis-2014-205601. [eng] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.O’Brien K., Tailor P., Leonard C., DiFrancesco L.M., Hart D.A., Matyas J.R. Enumeration and localization of mesenchymal progenitor cells and macrophages in synovium from normal individuals and patients with pre-osteoarthritis or clinically diagnosed osteoarthritis. Int J Mol Sci. 2017;18(4) doi: 10.3390/ijms18040774. [eng] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Utomo L., Bastiaansen-Jenniskens Y.M., Verhaar J.A.N., van Osch G.J.V.M. Cartilage inflammation and degeneration is enhanced by pro-inflammatory (M1) macrophages in vitro, but not inhibited directly by anti-inflammatory (M2) macrophages. Osteoarthritis Cartilage. 2016;24(12):2162–2170. doi: 10.1016/j.joca.2016.07.018. [DOI] [PubMed] [Google Scholar]
- 71.Tang Q., Zheng G., Feng Z., Tong M., Xu J., Hu Z. Wogonoside inhibits IL-1beta induced catabolism and hypertrophy in mouse chondrocyte and ameliorates murine osteoarthritis. Oncotarget. 2017;8(37):61440–61456. doi: 10.18632/oncotarget.18374. [eng] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Li H., Wang D., Yuan Y.J., Min J.K. New insights on the MMP-13 regulatory network in the pathogenesis of early osteoarthritis. Arthritis Res Ther. 2017;19 doi: 10.1186/s13075-017-1454-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Klein-Wieringa I.R., de Lange-Brokaar B.J., Yusuf E., Andersen S.N., Kwekkeboom J.C., Kroon H.M. Inflammatory cells in patients with endstage knee osteoarthritis: a comparison between the synovium and the infrapatellar fat pad. J Rheumatol. 2016;43(4):771–778. doi: 10.3899/jrheum.151068. [eng] [DOI] [PubMed] [Google Scholar]
- 74.Warmink K., Kozijn A.E., Bobeldijk I., Stoop R., Weinans H., Korthagen N.M. High-fat feeding primes the mouse knee joint to develop osteoarthritis and pathologic infrapatellar fat pad changes after surgically induced injury. Osteoarthritis Cartilage. 2020;28(5):593–602. doi: 10.1016/j.joca.2020.03.008. [DOI] [PubMed] [Google Scholar]
- 75.Barboza E., Hudson J., Chang W.-P., Kovats S., Towner R.A., Silasi-Mansat R. Profibrotic infrapatellar fat pad remodeling without M1 macrophage polarization precedes knee osteoarthritis in mice with diet-induced obesity. Arthritis Rheumatol. 2017;69(6):1221–1232. doi: 10.1002/art.40056. (Hoboken, NJ) [eng] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Andersen C.J., Murphy K.E., Fernandez M.L. Impact of obesity and metabolic syndrome on immunity. Adv Nutrition. 2016;7(1):66–75. doi: 10.3945/an.115.010207. (Bethesda, Md) [eng] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sun H.B. Mechanical loading, cartilage degradation, and arthritis. Ann N Y Acad Sci. 2010;1211:37–50. doi: 10.1111/j.1749-6632.2010.05808.x. [eng] [DOI] [PubMed] [Google Scholar]
- 78.Sun H.B., Cardoso L., Yokota H. Mechanical intervention for maintenance of cartilage and bone. Clin Med Insights Arthritis Musculoskelet Disord. 2011;4:65–70. doi: 10.4137/CMAMD.S6982. [eng] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zheng W., Li X., Liu D., Li J., Yang S., Gao Z. Mechanical loading mitigates osteoarthritis symptoms by regulating endoplasmic reticulum stress and autophagy. Faseb J : Off Pub Feder Am Soc Experiment Biol. 2019;33(3):4077–4088. doi: 10.1096/fj.201801851R. [eng] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cao W., Lin W., Cai H., Chen Y., Man Y., Liang J. Dynamic mechanical loading facilitated chondrogenic differentiation of rabbit BMSCs in collagen scaffolds. Regen Biomater. 2019;6(2):99–106. doi: 10.1093/rb/rbz005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Klets O., Mononen M.E., Liukkonen M.K., Nevalainen M.T., Nieminen M.T., Saarakkala S. Estimation of the effect of body weight on the development of osteoarthritis based on cumulative stresses in cartilage: data from the osteoarthritis initiative. Ann Biomed Eng. 2018;46(2):334–344. doi: 10.1007/s10439-017-1974-6. [eng] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Visser A.W., de Mutsert R., le Cessie S., den Heijer M., Rosendaal F.R., Kloppenburg M. The relative contribution of mechanical stress and systemic processes in different types of osteoarthritis: the NEO study. Ann Rheum Dis. 2015;74(10):1842–1847. doi: 10.1136/annrheumdis-2013-205012. [eng] [DOI] [PubMed] [Google Scholar]
- 83.Messier S.P., Pater M., Beavers D.P., Legault C., Loeser R.F., Hunter D.J. Influences of alignment and obesity on knee joint loading in osteoarthritic gait. Osteoarthritis Cartilage. 2014;22(7):912–917. doi: 10.1016/j.joca.2014.05.013. [eng] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Harding G.T., Dunbar M.J., Hubley-Kozey C.L., Stanish W.D., Astephen Wilson J.L. Obesity is associated with higher absolute tibiofemoral contact and muscle forces during gait with and without knee osteoarthritis. Clin BioMech. 2016;31:79–86. doi: 10.1016/j.clinbiomech.2015.09.017. [DOI] [PubMed] [Google Scholar]
- 85.Rai M.F., Sandell L.J., Barrack T.N., Cai L., Tycksen E.D., Tang S.Y. A microarray study of articular cartilage in relation to obesity and severity of knee osteoarthritis. Cartilage. 2018;1947603518796122 doi: 10.1177/1947603518796122. [eng] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Collins A.T., Kulvaranon M.L., Cutcliffe H.C., Utturkar G.M., Smith W.A.R., Spritzer C.E. Obesity alters the in vivo mechanical response and biochemical properties of cartilage as measured by MRI. Arthritis Res Ther. 2018;20(1) doi: 10.1186/s13075-018-1727-4. 232-32. [eng] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Anandacoomarasamy A., Leibman S., Smith G., Caterson I., Giuffre B., Fransen M. Weight loss in obese people has structure-modifying effects on medial but not on lateral knee articular cartilage. 2012;71(1):26–32. doi: 10.1136/ard.2010.144725. [DOI] [PubMed] [Google Scholar]
- 88.Fehrenbacher A., Steck E., Rickert M., Roth W., Richter W. Rapid regulation of collagen but not metalloproteinase 1, 3, 13, 14 and tissue inhibitor of metalloproteinase 1, 2, 3 expression in response to mechanical loading of cartilage explants in vitro. Arch Biochem Biophys. 2003;410(1):39–47. doi: 10.1016/s0003-9861(02)00658-6. [eng] [DOI] [PubMed] [Google Scholar]
- 89.Liu Q., Hu X., Zhang X., Duan X., Yang P., Zhao F. Effects of mechanical stress on chondrocyte phenotype and chondrocyte extracellular matrix expression. Sci Rep. 2016;6(1):37268. doi: 10.1038/srep37268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Vinardell T., Sheehy E.J., Buckley C.T., Kelly D.J. A comparison of the functionality and in vivo phenotypic stability of cartilaginous tissues engineered from different stem cell sources. Tissue engineering Part A. 2012;18(11-12):1161–1170. doi: 10.1089/ten.tea.2011.0544. [eng] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Goldring M.B., Marcu K.B. Cartilage homeostasis in health and rheumatic diseases. Arthritis Res Ther. 2009;11(3):224. doi: 10.1186/ar2592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Argote P.F., Kaplan J.T., Poon A., Xu X., Cai L., Emery N.C. Chondrocyte viability is lost during high-rate impact loading by transfer of amplified strain, but not stress, to pericellular and cellular regions. Osteoarthritis Cartilage. 2019;27(12):1822–1830. doi: 10.1016/j.joca.2019.07.018. [eng] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Nakamura F., Tsukamoto I., Inoue S., Hashimoto K., Akagi M. Cyclic compressive loading activates angiotensin II type 1 receptor in articular chondrocytes and stimulates hypertrophic differentiation through a G-protein-dependent pathway. FEBS Open Bio. 2018;8(6):962–973. doi: 10.1002/2211-5463.12438. [eng] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ramage L., Nuki G., Salter D.M. Signalling cascades in mechanotransduction: cell-matrix interactions and mechanical loading. Scand J Med Sci Sports. 2009;19(4):457–469. doi: 10.1111/j.1600-0838.2009.00912.x. [eng] [DOI] [PubMed] [Google Scholar]
- 95.Pottie P., Presle N., Terlain B., Netter P., Mainard D., Berenbaum F. Obesity and osteoarthritis: more complex than predicted! Ann Rheum Dis. 2006;65(11):1403–1405. doi: 10.1136/ard.2006.061994. [eng] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sobue Y., Takahashi N., Ohashi Y., Suzuki M., Nishiume T., Kobayakawa T. Inhibition of CD44 intracellular domain production suppresses bovine articular chondrocyte de-differentiation induced by excessive mechanical stress loading. Sci Rep. 2019;9(1):14901. doi: 10.1038/s41598-019-50166-4. [eng] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hirose N., Okamoto Y., Yanoshita M., Asakawa Y., Sumi C., Takano M. Protective effects of cilengitide on inflammation in chondrocytes under excessive mechanical stress. Cell Biol Int. 2020;44(4):966–974. doi: 10.1002/cbin.11293. [eng] [DOI] [PubMed] [Google Scholar]
- 98.Chang S.H., Mori D., Kobayashi H., Mori Y., Nakamoto H., Okada K. Excessive mechanical loading promotes osteoarthritis through the gremlin-1–NF-κB pathway. Nat Commun. 2019;10(1):1442. doi: 10.1038/s41467-019-09491-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Radin E.L., Rose R.M. Role of subchondral bone in the initiation and progression of cartilage damage. Clin Orthop Relat Res. 1986;(213):34–40. [eng] [PubMed] [Google Scholar]
- 100.Imhof H., Sulzbacher I., Grampp S., Czerny C., Youssefzadeh S., Kainberger F. Subchondral bone and cartilage disease: a rediscovered functional unit. Invest Radiol. 2000;35(10):581–588. doi: 10.1097/00004424-200010000-00004. [DOI] [PubMed] [Google Scholar]
- 101.Lajeunesse D. The role of bone in the treatment of osteoarthritis11Supported by Procter & Gamble Pharmaceuticals, Mason, OH. Osteoarthritis Cartilage. 2004;12:34–38. doi: 10.1016/j.joca.2003.09.013. [DOI] [PubMed] [Google Scholar]
- 102.Lyons T.J., McClure S.F., Stoddart R.W., McClure J. The normal human chondro-osseous junctional region: evidence for contact of uncalcified cartilage with subchondral bone and marrow spaces. BMC Muscoskel Disord. 2006;7 doi: 10.1186/1471-2474-7-52. 52-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Goldring S.R., Goldring M.B. Changes in the osteochondral unit during osteoarthritis: structure, function and cartilage-bone crosstalk. Nat Rev Rheumatol. 2016;12(11):632–644. doi: 10.1038/nrrheum.2016.148. [eng] [DOI] [PubMed] [Google Scholar]
- 104.Muratovic D., Cicuttini F., Wluka A., Findlay D., Wang Y., Otto S. Bone marrow lesions detected by specific combination of MRI sequences are associated with severity of osteochondral degeneration. Arthritis Res Ther. 2016;18(1):54. doi: 10.1186/s13075-016-0953-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Bowes M.A., McLure S.W., Wolstenholme C.B., Vincent G.R., Williams S., Grainger A. Osteoarthritic bone marrow lesions almost exclusively colocate with denuded cartilage: a 3D study using data from the Osteoarthritis Initiative. Ann Rheum Dis. 2016;75(10):1852–1857. doi: 10.1136/annrheumdis-2015-208407. [eng] [DOI] [PubMed] [Google Scholar]
- 106.Pearson M.J., Herndler-Brandstetter D., Tariq M.A., Nicholson T.A., Philp A.M., Smith H.L. IL-6 secretion in osteoarthritis patients is mediated by chondrocyte-synovial fibroblast cross-talk and is enhanced by obesity. Sci Rep. 2017;7(1):3451. doi: 10.1038/s41598-017-03759-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Poree B., Kypriotou M., Chadjichristos C., Beauchef G., Renard E., Legendre F. Interleukin-6 (IL-6) and/or soluble IL-6 receptor down-regulation of human type II collagen gene expression in articular chondrocytes requires a decrease of Sp1.Sp3 ratio and of the binding activity of both factors to the COL2A1 promoter. J Biol Chem. 2008;283(8):4850–4865. doi: 10.1074/jbc.M706387200. [eng] [DOI] [PubMed] [Google Scholar]
- 108.Wissing T.B., Bonito V., van Haaften E.E., van Doeselaar M., Brugmans M.M.C.P., Janssen H.M. Macrophage-driven biomaterial degradation depends on scaffold microarchitecture. 2019;7(87) doi: 10.3389/fbioe.2019.00087. [English] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ceonzo K., Gaynor A., Shaffer L., Kojima K., Vacanti C.A., Stahl G.L. Polyglycolic acid-induced inflammation: role of hydrolysis and resulting complement activation. Tissue Eng. 2006;12(2):301–308. doi: 10.1089/ten.2006.12.301. [eng] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Rayahin J.E., Gemeinhart R.A. Activation of macrophages in response to biomaterials. In: Kloc M., editor. Macrophages: origin, functions and biointervention. Springer International Publishing; Cham: 2017. pp. 317–351. [Google Scholar]
- 111.Sarkar K., Xue Y., Sant S. Host response to synthetic versus natural biomaterials. In: Corradetti B., editor. The immune response to implanted materials and devices: the impact of the immune system on the success of an implant. Springer International Publishing; Cham: 2017. pp. 81–105. [Google Scholar]
- 112.Chung L., Maestas D.R., Housseau F., Elisseeff J.H. Key players in the immune response to biomaterial scaffolds for regenerative medicine. Adv Drug Deliv Rev. 2017;114:184–192. doi: 10.1016/j.addr.2017.07.006. [DOI] [PubMed] [Google Scholar]
- 113.Klopfleisch R. Macrophage reaction against biomaterials in the mouse model – phenotypes, functions and markers. Acta Biomater. 2016;43:3–13. doi: 10.1016/j.actbio.2016.07.003. [DOI] [PubMed] [Google Scholar]
- 114.Sheikh Z., Brooks P.J., Barzilay O., Fine N., Glogauer M. Macrophages, foreign body giant cells and their response to implantable biomaterials. Materials. 2015;8(9):5671–5701. doi: 10.3390/ma8095269. (Basel) [eng] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Witherel C.E., Abebayehu D., Barker T.H., Spiller K.L. Macrophage and fibroblast interactions in biomaterial-mediated fibrosis. Adv Healthc Mater. 2019;8(4) doi: 10.1002/adhm.201801451. [eng] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Han S.A., Lee S., Seong S.C., Lee M.C. Effects of CD14 macrophages and proinflammatory cytokines on chondrogenesis in osteoarthritic synovium-derived stem cells. Tissue Eng Part A. 2014;20(19–20):2680–2691. doi: 10.1089/ten.tea.2013.0656. [eng] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Wehling N., Palmer G.D., Pilapil C., Liu F., Wells J.W., Müller P.E. Interleukin-1beta and tumor necrosis factor alpha inhibit chondrogenesis by human mesenchymal stem cells through NF-kappaB-dependent pathways. Arthritis Rheum. 2009;60(3):801–812. doi: 10.1002/art.24352. [eng] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Liu W., Sun Y., He Y., Zhang H., Zheng Y., Yao Y. IL-1β impedes the chondrogenic differentiation of synovial fluid mesenchymal stem cells in the human temporomandibular joint. Int J Mol Med. 2017;39(2):317–326. doi: 10.3892/ijmm.2016.2832. [eng] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Boeuf S., Graf F., Fischer J., Moradi B., Little C.B., Richter W. Regulation of aggrecanases from the ADAMTS family and aggrecan neoepitope formation during in vitro chondrogenesis of human mesenchymal stem cells. Eur Cell Mater. 2012;23:320–332. doi: 10.22203/ecm.v023a25. [eng] [DOI] [PubMed] [Google Scholar]
- 120.Zhao X., Dong Y., Zhang J., Li D., Hu G., Yao J. Leptin changes differentiation fate and induces senescence in chondrogenic progenitor cells. Cell Death Dis. 2016;7(4) doi: 10.1038/cddis.2016.68. [eng] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Karvonen-Gutierrez C.A., Sowers M.R., Heeringa S.G. Sex dimorphism in the association of cardiometabolic characteristics and osteophytes-defined radiographic knee osteoarthritis among obese and non-obese adults: NHANES III. Osteoarthritis Cartilage. 2012;20(7):614–621. doi: 10.1016/j.joca.2012.02.644. [eng] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Blom A.B., van Lent P.L., Holthuysen A.E., van der Kraan P.M., Roth J., van Rooijen N. Synovial lining macrophages mediate osteophyte formation during experimental osteoarthritis. Osteoarthritis Cartilage. 2004;12(8):627–635. doi: 10.1016/j.joca.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 123.Conley S.M., Hickson L.J., Kellogg T.A., McKenzie T., Heimbach J.K., Taner T. Human obesity induces dysfunction and early senescence in adipose tissue-derived mesenchymal stromal/stem cells. Front Cell Dev Biol. 2020;8 doi: 10.3389/fcell.2020.00197. 197-97. [eng] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kornicka K., Houston J., Marycz K. Dysfunction of mesenchymal stem cells isolated from metabolic syndrome and type 2 diabetic patients as result of oxidative stress and autophagy may limit their potential therapeutic use. Stem Cell Rev Rep. 2018;14(3):337–345. doi: 10.1007/s12015-018-9809-x. [eng] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Murphy J.M., Dixon K., Beck S., Fabian D., Feldman A., Barry F. Reduced chondrogenic and adipogenic activity of mesenchymal stem cells from patients with advanced osteoarthritis. 2002;46(3):704–713. doi: 10.1002/art.10118. [DOI] [PubMed] [Google Scholar]
- 126.Huang C.Y., Hagar K.L., Frost L.E., Sun Y., Cheung H.S. Effects of cyclic compressive loading on chondrogenesis of rabbit bone-marrow derived mesenchymal stem cells. Stem cells. 2004;22(3):313–323. doi: 10.1634/stemcells.22-3-313. (Dayton, Ohio) [eng] [DOI] [PubMed] [Google Scholar]
- 127.Miyanishi K., Trindade M.C., Lindsey D.P., Beaupré G.S., Carter D.R., Goodman S.B. Dose- and time-dependent effects of cyclic hydrostatic pressure on transforming growth factor-beta3-induced chondrogenesis by adult human mesenchymal stem cells in vitro. Tissue Eng. 2006;12(8):2253–2262. doi: 10.1089/ten.2006.12.2253. [eng] [DOI] [PubMed] [Google Scholar]
- 128.Leong D.J., Li Y.H., Gu X.I., Sun L., Zhou Z., Nasser P. Physiological loading of joints prevents cartilage degradation through CITED2. Faseb J : Off Pub Feder Am Soc Experiment Biol. 2011;25(1):182–191. doi: 10.1096/fj.10-164277. [eng] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Chen F.M., Liu X. Advancing biomaterials of human origin for tissue engineering. Prog Polym Sci. 2016;53:86–168. doi: 10.1016/j.progpolymsci.2015.02.004. [eng] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Risbud M.V., Sittinger M. Tissue engineering: advances in in vitro cartilage generation. Trends Biotechnol. 2002;20(8):351–356. doi: 10.1016/s0167-7799(02)02016-4. [eng] [DOI] [PubMed] [Google Scholar]
- 131.Vial X., Andreopoulos F.M. Novel biomaterials for cartilage tissue engineering. Curr Rheumatol Rev. 2009;5(1):51–57. [Google Scholar]
- 132.Parisi L., Toffoli A., Ghiacci G., Macaluso G.M. Tailoring the interface of biomaterials to design effective scaffolds. J Funct Biomater. 2018;9(3) doi: 10.3390/jfb9030050. [eng] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Armiento A.R., Stoddart M.J., Alini M., Eglin D. Biomaterials for articular cartilage tissue engineering: learning from biology. Acta Biomater. 2018;65:1–20. doi: 10.1016/j.actbio.2017.11.021. [eng] [DOI] [PubMed] [Google Scholar]
- 134.Haleem A.M., Chu C.R. Advances in tissue engineering techniques for articular cartilage repair. Operat Tech Orthop. 2010;20(2):76–89. doi: 10.1053/j.oto.2009.10.004. [eng] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Duarte Campos D.F., Drescher W., Rath B., Tingart M., Fischer H. Supporting biomaterials for articular cartilage repair. Cartilage. 2012;3(3):205–221. doi: 10.1177/1947603512444722. [eng] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Mimura T., Imai S., Kubo M., Isoya E., Ando K., Okumura N. A novel exogenous concentration-gradient collagen scaffold augments full-thickness articular cartilage repair. Osteoarthritis Cartilage. 2008;16(9):1083–1091. doi: 10.1016/j.joca.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 137.Koo Y., Choi E-J., Lee J., Kim H-J., Kim G., Do S.H. 3D printed cell-laden collagen and hybrid scaffolds for in vivo articular cartilage tissue regeneration. J Ind Eng Chem. 2018;66:343–355. [eng] [Google Scholar]
- 138.Almeida H.V., Eswaramoorthy R., Cunniffe G.M., Buckley C.T., O’Brien F.J., Kelly D.J. Fibrin hydrogels functionalized with cartilage extracellular matrix and incorporating freshly isolated stromal cells as an injectable for cartilage regeneration. Acta Biomater. 2016;36:55–62. doi: 10.1016/j.actbio.2016.03.008. [eng] [DOI] [PubMed] [Google Scholar]
- 139.Moshaverinia A., Xu X., Chen C., Akiyama K., Snead M.L., Shi S. Dental mesenchymal stem cells encapsulated in an alginate hydrogel co-delivery microencapsulation system for cartilage regeneration. Acta Biomater. 2013;9(12):9343–9350. doi: 10.1016/j.actbio.2013.07.023. [eng] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Agheb M., Dinari M., Rafienia M., Salehi H. Novel electrospun nanofibers of modified gelatin-tyrosine in cartilage tissue engineering. Mater Sci Eng C Mater Biol Appl. 2017;71:240–251. doi: 10.1016/j.msec.2016.10.003. [eng] [DOI] [PubMed] [Google Scholar]
- 141.Man Z., Hu X., Liu Z., Huang H., Meng Q., Zhang X. Transplantation of allogenic chondrocytes with chitosan hydrogel-demineralized bone matrix hybrid scaffold to repair rabbit cartilage injury. Biomaterials. 2016;108:157–167. doi: 10.1016/j.biomaterials.2016.09.002. [eng] [DOI] [PubMed] [Google Scholar]
- 142.Yin Z., Yang X., Jiang Y., Xing L., Xu Y., Lu Y. Platelet-rich plasma combined with agarose as a bioactive scaffold to enhance cartilage repair: an in vitro study. J Biomater Appl. 2014;28(7):1039–1050. doi: 10.1177/0885328213492573. [eng] [DOI] [PubMed] [Google Scholar]
- 143.Chen C.H., Lee M.Y., Shyu V.B., Chen Y.C., Chen C.T., Chen J.P. Surface modification of polycaprolactone scaffolds fabricated via selective laser sintering for cartilage tissue engineering. Mater Sci Eng C Mater Biol Appl. 2014;40:389–397. doi: 10.1016/j.msec.2014.04.029. [eng] [DOI] [PubMed] [Google Scholar]
- 144.Hung K.C., Tseng C.S., Dai L.G., Hsu S.H. Water-based polyurethane 3D printed scaffolds with controlled release function for customized cartilage tissue engineering. Biomaterials. 2016;83:156–168. doi: 10.1016/j.biomaterials.2016.01.019. [eng] [DOI] [PubMed] [Google Scholar]
- 145.Cui L., Wu Y., Cen L., Zhou H., Yin S., Liu G. Repair of articular cartilage defect in non-weight bearing areas using adipose derived stem cells loaded polyglycolic acid mesh. Biomaterials. 2009;30(14):2683–2693. doi: 10.1016/j.biomaterials.2009.01.045. [eng] [DOI] [PubMed] [Google Scholar]
- 146.Chang N.J., Lin C.C., Shie M.Y., Yeh M.L., Li C.F., Liang P.I. Positive effects of cell-free porous PLGA implants and early loading exercise on hyaline cartilage regeneration in rabbits. Acta Biomater. 2015;28:128–137. doi: 10.1016/j.actbio.2015.09.026. [eng] [DOI] [PubMed] [Google Scholar]
- 147.Vinatier C., Guicheux J. Cartilage tissue engineering: from biomaterials and stem cells to osteoarthritis treatments. Ann Phys Rehabil Med. 2016;59(3):139–144. doi: 10.1016/j.rehab.2016.03.002. [DOI] [PubMed] [Google Scholar]
- 148.Chen F.S., Frenkel S.R., Di Cesare P.E. Repair of articular cartilage defects: part I. Basic Science of cartilage healing. Am J Orthoped. 1999;28(1):31–33. (Belle Mead, NJ) [eng] [PubMed] [Google Scholar]
- 149.Martín A.R., Patel J.M., Zlotnick H.M., Carey J.L., Mauck R.L. Emerging therapies for cartilage regeneration in currently excluded ‘red knee’ populations. npj Regen Med. 2019;4(1):12. doi: 10.1038/s41536-019-0074-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Diekman B.O., Guilak F. Stem cell-based therapies for osteoarthritis: challenges and opportunities. Curr Opin Rheumatol. 2013;25(1):119–126. doi: 10.1097/BOR.0b013e32835aa28d. [eng] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Han Y., Li X., Zhang Y., Han Y., Chang F., Ding J. Mesenchymal stem cells for regenerative medicine. Cells. 2019;8(8):886. doi: 10.3390/cells8080886. [eng] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Manferdini C., Paolella F., Gabusi E., Gambari L., Piacentini A., Filardo G. Adipose stromal cells mediated switching of the pro-inflammatory profile of M1-like macrophages is facilitated by PGE2: in vitro evaluation. Osteoarthritis Cartilage. 2017;25(7):1161–1171. doi: 10.1016/j.joca.2017.01.011. [eng] [DOI] [PubMed] [Google Scholar]
- 153.Oh J.Y., Lee R.H., Yu J.M., Ko J.H., Lee H.J., Ko A.Y. Intravenous mesenchymal stem cells prevented rejection of allogeneic corneal transplants by aborting the early inflammatory response. Mol Ther. 2012;20(11):2143–2152. doi: 10.1038/mt.2012.165. [eng] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Zhang W.-C., Qin F., Wang X.-J., Liu Z.-F., Zhu L., Zeng A. Adipose-derived stromal cells attenuate adipose inflammation in obesity through adipocyte browning and polarization of M2 macrophages. Mediat Inflamm. 2019;2019 doi: 10.1155/2019/1731540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Shang Q., Bai Y., Wang G., Song Q., Guo C., Zhang L. Delivery of adipose-derived stem cells attenuates adipose tissue inflammation and insulin resistance in obese mice through remodeling macrophage phenotypes. Stem Cell Dev. 2015;24(17):2052–2064. doi: 10.1089/scd.2014.0557. [eng] [DOI] [PubMed] [Google Scholar]
- 156.ter Huurne M., Schelbergen R., Blattes R., Blom A., de Munter W., Grevers L.C. Antiinflammatory and chondroprotective effects of intraarticular injection of adipose-derived stem cells in experimental osteoarthritis. 2012;64(11):3604–3613. doi: 10.1002/art.34626. [DOI] [PubMed] [Google Scholar]
- 157.Cosenza S., Ruiz M., Toupet K., Jorgensen C., Noël D. Mesenchymal stem cells derived exosomes and microparticles protect cartilage and bone from degradation in osteoarthritis. Sci Rep. 2017;7(1) doi: 10.1038/s41598-017-15376-8. 16214-14. [eng] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Orozco L., Munar A., Soler R., Alberca M., Soler F., Huguet M. Treatment of knee osteoarthritis with autologous mesenchymal stem cells: a pilot study. Transplantation. 2013;95(12):1535–1541. doi: 10.1097/TP.0b013e318291a2da. [eng] [DOI] [PubMed] [Google Scholar]
- 159.Browne S., Pandit A. Biomaterial-mediated modification of the local inflammatory environment. 2015;3(67) doi: 10.3389/fbioe.2015.00067. [English] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Toita R., Kawano T., Murata M., Kang J.H. Anti-obesity and anti-inflammatory effects of macrophage-targeted interleukin-10-conjugated liposomes in obese mice. Biomaterials. 2016;110:81–88. doi: 10.1016/j.biomaterials.2016.09.018. [eng] [DOI] [PubMed] [Google Scholar]
- 161.Kwon J.Y., Lee S.H., Na H.S., Jung K., Choi J., Cho K.H. Kartogenin inhibits pain behavior, chondrocyte inflammation, and attenuates osteoarthritis progression in mice through induction of IL-10. Sci Rep. 2018;8(1):13832. doi: 10.1038/s41598-018-32206-7. [eng] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Murphy K.P., Hendley M.A., Isely C., Annamalai P., Peña E., Gower R.M. Resveratrol delivery from porous poly(lactide- co-glycolide) scaffolds promotes an anti-inflammatory environment within visceral adipose tissue. ACS Appl Mater Interfaces. 2018;10(50):43363–43374. doi: 10.1021/acsami.8b13421. [eng] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Xu X., Liu X., Yang Y., He J., Gu H., Jiang M. Resveratrol inhibits the development of obesity-related osteoarthritis via the TLR4 and PI3K/Akt signaling pathways. Connect Tissue Res. 2019;60(6):571–582. doi: 10.1080/03008207.2019.1601187. [eng] [DOI] [PubMed] [Google Scholar]
- 164.Zhang Z., Wei X., Gao J., Zhao Y., Zhao Y., Guo L. Intra-articular injection of cross-linked hyaluronic acid-dexamethasone hydrogel attenuates osteoarthritis: an experimental study in a rat model of osteoarthritis. Int J Mol Sci. 2016;17(4):411. doi: 10.3390/ijms17040411. [eng] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Agarwal R., Volkmer T.M., Wang P., Lee L.A., Wang Q., García A.J. Synthesis of self-assembled IL-1Ra-presenting nanoparticles for the treatment of osteoarthritis. J Biomed Mater Res A. 2016;104(3):595–599. doi: 10.1002/jbm.a.35601. [eng] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Goodman S.B., Pajarinen J., Yao Z., Lin T. Inflammation and bone repair: from particle disease to tissue regeneration. Front Bioeng Biotechnol. 2019;7:230. doi: 10.3389/fbioe.2019.00230. [eng] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Bartel D.P. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136(2):215–233. doi: 10.1016/j.cell.2009.01.002. [eng] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Xie Q., Wei M., Kang X., Liu D., Quan Y., Pan X. Reciprocal inhibition between miR-26a and NF-κB regulates obesity-related chronic inflammation in chondrocytes. Biosci Rep. 2015;35(3) doi: 10.1042/BSR20150071. [eng] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Tuddenham L., Wheeler G., Ntounia-Fousara S., Waters J., Hajihosseini M.K., Clark I. The cartilage specific microRNA-140 targets histone deacetylase 4 in mouse cells. FEBS Lett. 2006;580(17):4214–4217. doi: 10.1016/j.febslet.2006.06.080. [eng] [DOI] [PubMed] [Google Scholar]
- 170.Miyaki S., Nakasa T., Otsuki S., Grogan S.P., Higashiyama R., Inoue A. MicroRNA-140 is expressed in differentiated human articular chondrocytes and modulates interleukin-1 responses. Arthritis Rheum. 2009;60(9):2723–2730. doi: 10.1002/art.24745. [eng] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Le L.T.T., Swingler T.E., Clark I.M. Review: the role of MicroRNAs in osteoarthritis and chondrogenesis. 2013;65(8):1963–1974. doi: 10.1002/art.37990. [DOI] [PubMed] [Google Scholar]
- 172.Anderson B.A., McAlinden A. miR-483 targets SMAD4 to suppress chondrogenic differentiation of human mesenchymal stem cells. 2017;35(11):2369–2377. doi: 10.1002/jor.23552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Hwang H.S., Park S.J., Lee M.H., Kim H.A. MicroRNA-365 regulates IL-1β-induced catabolic factor expression by targeting HIF-2α in primary chondrocytes. Sci Rep. 2017;7(1):17889. doi: 10.1038/s41598-017-18059-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Gallo W., Esguerra J.L.S., Eliasson L., Melander O. miR-483-5p associates with obesity and insulin resistance and independently associates with new onset diabetes mellitus and cardiovascular disease. PloS One. 2018;13(11) doi: 10.1371/journal.pone.0206974. [eng] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Zhang X., Chang A., Li Y., Gao Y., Wang H., Ma Z. miR-140-5p regulates adipocyte differentiation by targeting transforming growth factor-β signaling. Sci Rep. 2015;5:18118. doi: 10.1038/srep18118. [eng] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Belarbi Y., Mejhert N., Gao H., Arner P., Rydén M., Kulyté A. MicroRNAs-361-5p and miR-574-5p associate with human adipose morphology and regulate EBF1 expression in white adipose tissue. Mol Cell Endocrinol. 2018;472:50–56. doi: 10.1016/j.mce.2017.11.018. [eng] [DOI] [PubMed] [Google Scholar]