Key Points
Question
Is heavy alcohol drinking associated with altered adolescent microstructural brain development, and is that alteration age dependent?
Findings
In this case-control study of 451 adolescents, heavy drinkers exhibited significant reduction of whole-brain fractional anisotropy. The disruption occurred after the onset of drinking and was more pronounced in younger rather than older adolescents.
Meaning
Study results suggest that alcohol consumption is associated with deleterious outcomes on white matter microstructural maturation, supporting the concept of heightened vulnerability associated with alcohol use in early adolescence.
This case-control study uses data from the National Consortium on Alcohol and Neurodevelopment in Adolescence cohort to investigate whether heavy alcohol drinking among adolescents is associated with microstructural brain changes.
Abstract
Importance
Maturation of white matter fiber systems subserves cognitive, behavioral, emotional, and motor development during adolescence. Hazardous drinking during this active neurodevelopmental period may alter the trajectory of white matter microstructural development, potentially increasing risk for developing alcohol-related dysfunction and alcohol use disorder in adulthood.
Objective
To identify disrupted adolescent microstructural brain development linked to drinking onset and to assess whether the disruption is more pronounced in younger rather than older adolescents.
Design, Setting, and Participants
This case-control study, conducted from January 13, 2013, to January 15, 2019, consisted of an analysis of 451 participants from the National Consortium on Alcohol and Neurodevelopment in Adolescence cohort. Participants were aged 12 to 21 years at baseline and had at least 2 usable magnetic resonance diffusion tensor imaging (DTI) scans and up to 5 examination visits spanning 4 years. Participants with a youth-adjusted Cahalan score of 0 were labeled as no-to-low drinkers; those with a score of greater than 1 for at least 2 consecutive visits were labeled as heavy drinkers. Exploratory analysis was conducted between no-to-low and heavy drinkers. A between-group analysis was conducted between age- and sex-matched youths, and a within-participant analysis was performed before and after drinking.
Exposures
Self-reported alcohol consumption in the past year summarized by categorical drinking levels.
Main Outcomes and Measures
Diffusion tensor imaging measurement of fractional anisotropy (FA) in the whole brain and fiber systems quantifying the developmental change of each participant as a slope.
Results
Analysis of whole-brain FA of 451 adolescents included 291 (64.5%) no-to-low drinkers and 160 (35.5%) heavy drinkers who indicated the potential for a deleterious association of alcohol with microstructural development. Among the no-to-low drinkers, 142 (48.4%) were boys with mean (SD) age of 16.5 (2.2) years and 149 (51.2%) were girls with mean (SD) age of 16.5 (2.1) years and 192 (66.0%) were White participants. Among the heavy drinkers, 86 (53.8%) were boys with mean (SD) age of 20.1 (1.5) years and 74 (46.3%) were girls with mean (SD) age of 20.5 (2.0) years and 142 (88.8%) were White participants. A group analysis revealed FA reduction in heavy-drinking youth compared with age- and sex-matched controls (t154 = –2.7, P = .008). The slope of this reduction correlated with log of days of drinking since the baseline visit (r156 = –0.21, 2-tailed P = .008). A within-participant analysis contrasting developmental trajectories of youths before and after they initiated heavy drinking supported the prediction that drinking onset was associated with and potentially preceded disrupted white matter integrity. Age-alcohol interactions (t152 = 3.0, P = .004) observed for the FA slopes indicated that the alcohol-associated disruption was greater in younger than older adolescents and was most pronounced in the genu and body of the corpus callosum, regions known to continue developing throughout adolescence.
Conclusions and Relevance
This case-control study of adolescents found a deleterious association of alcohol use with white matter microstructural integrity. These findings support the concept of heightened vulnerability to environmental agents, including alcohol, associated with attenuated development of major white matter tracts in early adolescence.
Introduction
The development of cortical white matter fiber systems continues both expansion and restructuring after birth and throughout adolescence. The newborn's brain fiber tracts are not myelinated until 1 year after birth,1 and intracranial volume, driven by the growth of white matter in particular,2 continues into early adolescence.3 Fiber systems further extend and remodel to form functional connections, which interact with neural pruning and life experiences4,5 for at least the next 15 years6,7 until peak maturation is attained. These dynamic developmental changes unfold when many psychiatric disorders first emerge,8,9,10 and in some cases may reflect vulnerability to environmental toxins contributing to disrupted neurodevelopmental trajectories. A common such toxin is alcohol, which is used by many adolescents in binge-drinking patterns.11,12
White matter (WM) integrity can be quantified by measuring molecular water diffusion in the brain through magnetic resonance diffusion tensor imaging (DTI) metrics, notably fractional anisotropy (FA). Functional anisotropy reflects cellular density,13 myelination,14 and axonal size14 within WM fiber bundles. Widespread FA alteration has been observed in adolescents who initiated binge drinking.12,15,16 Affected fiber systems include the corpus callosum, superior longitudinal fasciculus, internal and external capsule, brainstem, and cortical projection fibers.17 Disruption of these systems may degrade neural signal transmission and the capacity for certain cognitive functions, resulting in enhanced impulsivity,18 poor inhibitory control,19 and restricted working memory capacity.20
Despite some progress in describing the abnormality of these structural connectivity substrates associated with alcohol consumption during adolescence, prior cross-sectional or even longitudinal studies using DTI have not established a precise relationship between drinking onset or patterns and aberrant fiber microstructural development. Establishing these relationships requires estimating developmental trajectories of heavy-drinking youth, comparing these trajectories with the normal development of control participants, identifying at which stage the abnormality happens, and assessing whether the disruption is age dependent.
Accordingly, we analyzed the first 4 years of longitudinal DTI data of 451 adolescents from the National Consortium on Alcohol and Neurodevelopment in Adolescence (NCANDA) study who were aged 12 to 21 years at baseline. By quantifying the developmental change of WM integrity within each individual as the slope of FA over visits, our study assessed (1) altered microstructural developmental trajectories associated with drinking onset during adolescence and (2) the differential alcohol associations by age with specific regional WM fiber tracts.
Methods
Participants
This case-control study took place from January 13, 2013, to January 15, 2019. The NCANDA cohort21 comprises 831 participants aged 12 to 21 years at baseline who were recruited across 5 collection sites, the University of California at San Diego, SRI International, Duke University Medical Center, the University of Pittsburgh, and Oregon Health & Science University, and assessed yearly on psychobiologic measures, including brain maturation. Adult participants and the parents of minor participants provided written informed consent before participation in the study. Minor participants provided assent before participation. The institutional review boards of each site approved the standardized data collection and use.21
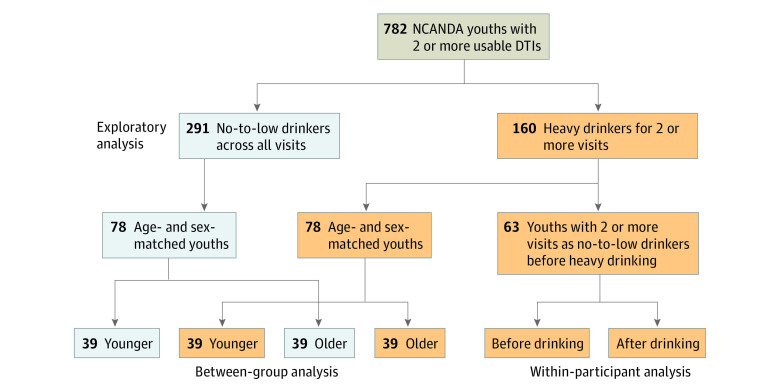
By the fifth annual follow-up in the study, 782 participants had at least 2 usable DTI scans (Figure 1). Participants completed the Customary Drinking and Drug Use Record and Timeline Follow-Back to characterize past and current alcohol and substance use. Drinking groups were defined based on the youth-adjusted Cahalan score on a scale of 1 to 4,22,23 which considered quantity and frequency to classify drinking levels based on past-year patterns. Based on self-reported alcohol-drinking history 291 participants (37.2%) remained no-to-low drinking (youth-adjusted Cahalan score = 0)22,23 throughout the time points examined, and 160 (20.5%) were heavy drinkers for at least 2 consecutive visits (youth-adjusted Cahalan score >1). To study developmental trajectories associated with heavy drinking, which required multiple observations after heavy drinking was initiated, the remaining participants who were moderate drinkers or initiated heavy drinking for only 1 visit were omitted from analysis. The 451 participants (228 boys and 223 girls aged 12-21 years at baseline) included in the analysis had on average 3.7 visits and were characterized by age, sex, pubertal stage using the self-assessment Pubertal Development Scale,24 socioeconomic status as defined by the highest years of education of either parent, self-identified race/ethnicity,24 and the amount of exposure to alcohol, tobacco, and cannabis (eTable in the Supplement). The age of a participant was quantified by both the age at each visit and a participant-age (ie, average age across visits) (eFigure 1 in the Supplement). As the participant-age of the heavy drinkers was older than that of the no-to-low drinkers, 2 age- and sex-matched groups of 78 no-to-low and 78 heavy drinkers (38 girls and 40 boys, mean participant-age [SD], 19.3 [1.8] years in each group) were selected (see the eMethods in the Supplement for matching procedure), each with 39 younger (<19.3 years) and 39 older participants. Furthermore, 63 of the 160 heavy drinkers (39.4%) were classified as transitioners, which indicated that they remained no-to-low drinking for at least 2 visits before initiating heavy drinking. Their mean (SD) participant-age was 17.4 (1.7) years before drinking and 20.0 (1.6) years after drinking. The data were based on a formal, locked data release distributed to the public according to the NCANDA Data Distribution agreement.25 This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline.
Figure 1. Study Design of Identifying Altered Fiber Tract Development Associated With Adolescent Alcohol Use.
Of the 782 National Consortium on Alcohol and Neurodevelopment in Adolescence (NCANDA) participants with at least 2 usable diffusion tensor imaging (DTI) scans, 291 youth were labeled as no-to-low drinkers (youth-adjusted Cahalan = 0) throughout the 4-year study period, and 160 were heavy drinkers (youth-adjusted Cahalan >1) for at least 2 consecutive visits. The analysis examined the association of alcohol with white matter microstructure during adolescence based on (1) a between-group analysis on 78 no-to-low drinkers and 78 age- and sex-matched heavy drinkers and (2) a within-participant analysis on 63 transitioners who were no-to-low drinkers for at least 2 visits before initiating heavy drinking. The differential alcohol outcome with respect to age was examined in the 39 younger and 39 older youth in each matched cohort.
MRI Preprocessing
The structural and diffusion data of all NCANDA participants were preprocessed using the publicly available longitudinal NCANDA pipeline24 (eMethods in the Supplement). The average FA value over the whole-brain tract-based spatial statistics skeleton (Figure 2A) and with respect to the parcellation defined by the Johns Hopkins University DTI atlas26 was computed for each scan. These FA measures were corrected for manufacturer difference based on human-phantom data.24
Figure 2. Between-Group and Within-Participant Analysis on the Whole-Brain FA.
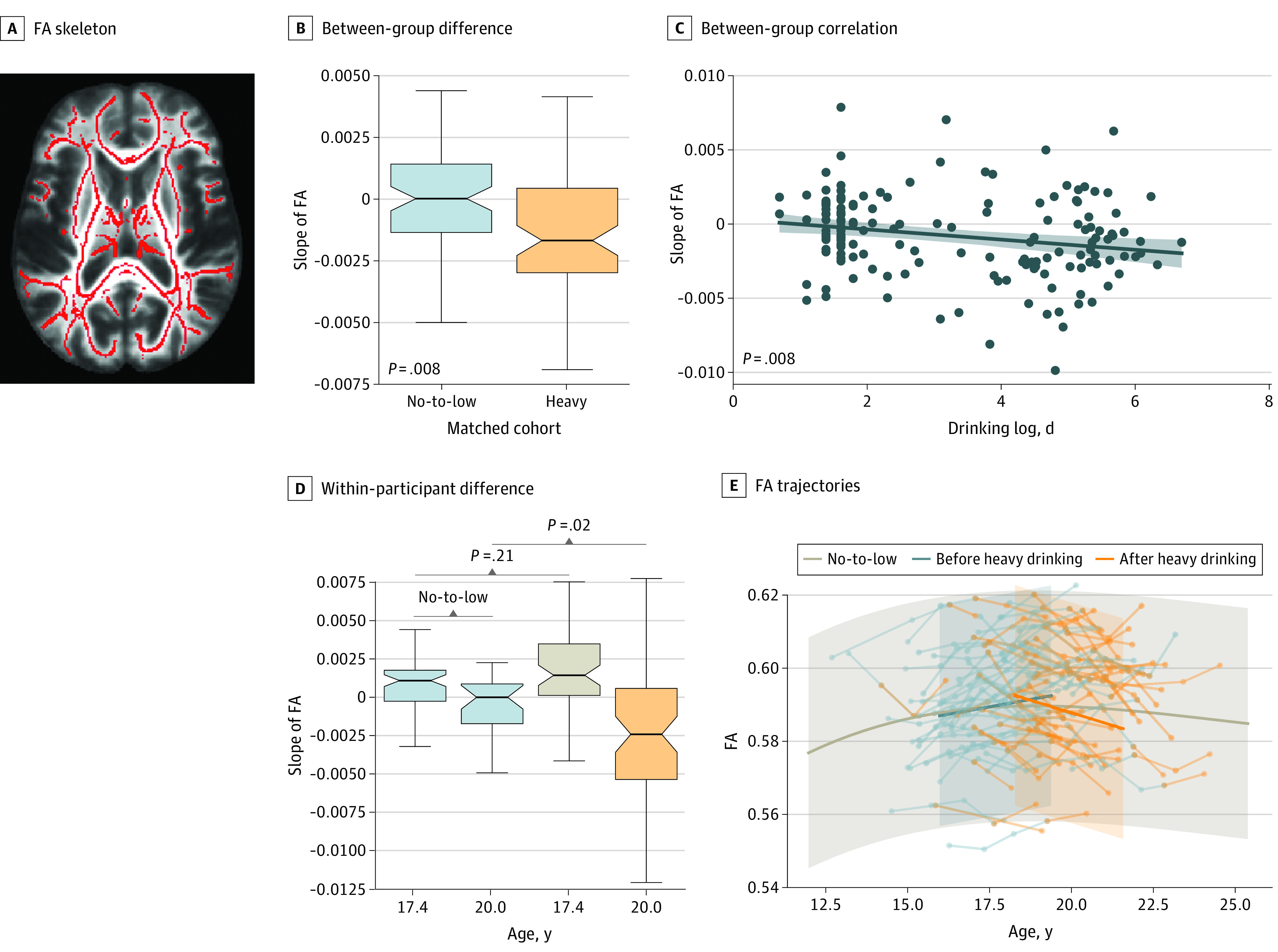
A, Functional anisotropy (FA) skeleton. Whole-brain FA tract-based spatial statistics (TBSS) skeleton of the SRI24 atlas (a magnetic resonance imaging (MRI)–based atlas of normal adult human brain anatomy). B, Between-group difference. Significant group difference in the slope of whole-brain FA between the 78 no-to-low and 78 heavy drinkers. C, Between-group association. Significant association between slope and log of days of drinking since baseline visit in the matched cohorts. D, Within-participant difference. The slopes of the 63 transitioners (defined as no-to-low drinking for at least 2 visits before initiating heavy drinking) were significantly different from age-matched no-to-low drinkers after drinking onset but not before. E, FA trajectories. FA trajectories of the 63 transitioners before and after drinking onset overlaid with the trajectory of the 291 no-to-low drinkers. Boxplots in panels B and D represent the minimum, first quartile, median, third quartile, and maximum FA slope. Shading in C represents the 95% CI for the regression estimate. Shading in E represents +3/-3 SD of FA for the 291 no-to-low drinkers.
Statistical Analysis
Between-Group Analysis
A mixed-effects model27 was fitted to the whole-brain skeleton FA measures from the longitudinal DTIs of the 291 no-to-low drinkers to model normal developmental trajectory during adolescence (eFigure 1 in the Supplement). The model used a cubic function to model the group-level aging trajectory and incorporated linear participant-specific random effects (eMethods in the Supplement). Other covariates included sex, race/ethnicity, supratentorial volume, pubertal development, socioeconomic status, and manufacturer type as fixed effects. For each no-to-low drinking participant, the influence from factors other than age was residualized from the FA measures based on the estimated fixed effects, and a linear model was fitted between age and residualized FA across all visits in order to determine the relative change in FA (ie, slope) (eFigure 1 in the Supplement). For each heavy drinker, the process of removing confounders and fitting a linear model to compute the slope was repeated with respect to the visits during which the participants engaged in heavy drinking.
A one-sample t test examined whether the slopes of a cohort, ie, 78 no-to-low or 78 heavy drinkers, significantly differed from zero. The difference in slopes between the 2 groups was examined by a 2-sample t test, which was then repeated within the younger and older cohort. Across these 156 youths, a general linear model examined the interactions of the participant-age and sex by drinking group on the slope measures with additional covariates of participant-age, sex, and drinking group. This general linear model test was then applied to the entire cohort of 451 youths as an exploratory analysis.
The slope measures were also correlated with the number of visits considered in the analysis and the log of total substance use over those visits. Tested substances included alcohol (total days of drinking), tobacco (number of cigarettes consumed), and cannabis (days of using marijuana). A 2-tailed P value of .05 or below was considered significant for all analyses. Finally, the whole between-group analysis was repeated with respect to the average FA within each WM tract system defined by the Johns Hopkins University atlas (5 major tracts composed of 31 regions). The analysis was repeated in tract regions with significant alcohol outcomes after Bonferroni multiple comparison correction (2-tailed P < .05, corrected for 5 outcomes).28
Within-Participant Analysis
For each of the 63 transitioners, the slope was separately computed before and after the initiation of heavy drinking. The 63 predrinking slopes were compared with the 63 postdrinking slopes by a paired 2-sample t test. Unpaired t tests also compared the predrinking and postdrinking slopes with those of the no-to-low drinkers at the same age range (94 no-to-low drinkers within a mean [SD] age of 17.4 [1.7] years; 28 within a mean [SD] age of 20.0 [1.6] years). A 2-tailed P value of .05 was considered significant. Statistical analyses were conducted from March 29 to September 14, 2020, using Matlab software, version R2016b (MathWorks Inc) and FSL, version 5.0.10 (FSL Software Technologies Inc).
Results
Association of Heavy Drinking With Whole-Brain FA
Analysis of whole-brain FA of 451 adolescents (291 no-to-low drinkers [64.5%]: 142 boys [48.4%] with mean [SD] age of 16.5 [2.2] years and 149 girls [51.2%] with mean [SD] age of 16.5 [2.1] years; 192 White participants [66.0%]; and 160 heavy drinkers [35.5%]: 86 boys [53.8%] with mean [SD] age of 20.1 [1.5] years and 74 girls [46.3%] with mean [SD] age of 20.5 [2.0] years; 142 White participants [88.8%]) indicated the potential for a deleterious outcome of alcohol on microstructural development of WM fibers. The slopes of the 78 heavy drinkers were significantly more negative than those of the no-to-low drinkers (mean [SD], –0.0013 [0.0036] vs 0.0001 [0.0022]; 2-sample t154 = –2.7, P = .008) (Figure 2B). Whereas the no-to-low drinkers had relatively stable FA measures across visits (95% CI of slope, –0.0005 to 0.0004; 1-sample t77 = –0.22, P = .82), heavy drinkers had significant reductions in global FA during the study period (95% CI of slope, –0.002 to –0.0005; 1-sample t77 = –3.4, 2-tailed P = .001). The effect of alcohol on the slope measures was also observed when replacing the t test with Pearson correlation (r156 = –0.21, 2-tailed P = .008) with respect to the alcohol consumption measure (log of days of drinking during the study) (Figure 2C).
Within-subject comparison also revealed a reduction in FA. Before drinking onset, the 63 youth showed significantly increased FA over visits (95% CI of slope, 0.0011-0.0024; 1-sample t62 = 5.49, P < .001) and their corresponding slopes were not different from other no-to-low drinkers of the same age range (mean [SD], 0.0017 [0.0025] vs 0.0011 [0.0022]; 2-sample t154 = 0.8, P = .21) (Figure 2D). However, the FA declined significantly over visits in those who transitioned to heavy drinking, resulting in slopes significantly below zero (95% CI of slope, –0.0036 to –0.0014; 1-sample t62 = –4.49, P < .001) and lower than the no-to-low participants of the same age range (mean [SD], –0.0025 [0.0043] vs –0.0003 [0.0026]; 2-sample t89 = –2.4, P = .02) (Figure 2D and E; eResults and eFigure 2 in the Supplement). The association between age of drinking onset and change in slopes was also supported by a piecewise linear regression analysis on the trajectories of transitioners (eFigure 3 in the Supplement). None of the slope measures correlated with the number of visits or the use of tobacco or cannabis (eFigure 4 in the Supplement).
Differential Alcohol Association in Younger and Older Adolescents
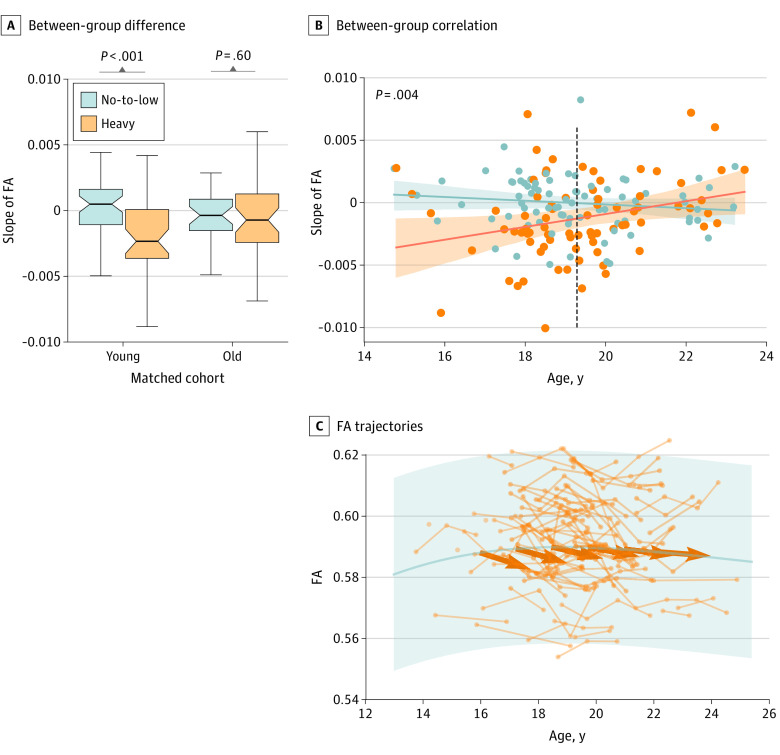
The association of alcohol with the slope measures was observed only in the younger cohort (mean [SD], –0.0019 [0.002] vs –0.0001 [0.002]; 2-sample t76 = –3.28, P = .001) and not in the older cohort (mean [SD], –0.0006 [0.0023] vs –0.0003 [0.0024]; 2-sample t76 = –0.54, P = .60) (Figure 3A). This association was supported by a significant age-alcohol interaction (t152 = 3.0, P = .004) on the slopes (Figure 3B). Although the FA of no-to-low drinkers continued to increase until 19.3 years (95% CI of slope, –0.0004 to 0.0007; Figure 3A and B), the heavy-drinking youth exhibited FA reduction (95% CI of slope, –0.0012 to –0.0006]) (Figure 3A and B) in the younger age range. On the other hand, older heavy drinkers had no significant reduction of FA and thus converged with older no-to-low drinkers whose FA also stopped increasing (Figure 3). The age-alcohol interaction was confirmed in all 451 youth (eFigure 5 in the Supplement).
Figure 3. Alcohol Associations in Younger vs Older Adolescents.
A, Between-group difference. Group difference in slope was only significant in the younger cohort (age <19.3 years) but not in the older cohort. Boxplots represent the minimum, first quartile, median, third quartile, and maximum FA slope. B, Between-group association. Significant age-drinking-group interaction on the slope in the 2 matched cohorts. Shading represents the 95% CI for the regression estimates. C, Functional anisotropy (FA) trajectories. The trajectories of the 78 heavy drinkers overlaid with the trajectory of the 291 no-to-low drinkers. Shading represents the +3/–3 SD of FA for the 291 no-to-low drinkers; bold arrows represent mean FA reduction of heavy drinkers at each age range.
Differential Alcohol Association in Regional FA
Of the 5 major fiber tracts, only the commissural fibers (corpus callosum) showed a significant association with alcohol (t154 = –3.0, P = .003) and an age-alcohol interaction (t152 = 2.6, P = .008) after Bonferroni multiple comparison correction (P threshold, 0.01) (Table) on the matched data set. When further extending the analysis to the 4 subregions of the corpus callosum, only the anterior and middle callosal regions (genu and body) showed significant age-alcohol interactions (t152 = 2.67, P = .009 and t152 = 2.80, P = .006, respectively) (Table and Figure 4). Among the other 4 major fiber tracts, the corticospinal and fasciculi tracts showed trend-level alcohol and age-alcohol associations for the whole matched groups (t152 = 2.58, P = .01 and t152 = 2.50, P = .01, respectively), but the association with alcohol was significant (t76 = –2.76, P = .007 and t76 = –2.83, P = .006, respectively) when confining the t test to the younger matched cohort (Table and eFigure 6 in the Supplement).
Table. Group Differences in FA Slopes Between Age- and Sex-Matched No-to-Low and Heavy Drinkers and Age-Alcohol Interaction in the 2 Matched Cohorts.
| Region name | Alcohol outcome (group difference) | Age-alcohol interaction (GLM), P value (n = 156) | |||||
|---|---|---|---|---|---|---|---|
| Matched all (N = 156) | Matched young (n = 78) | Matched old (n = 78) | |||||
| P value | Cohen d (95% CI) | P value | Cohen d (95% CI) | P value | Cohen d (95% CI) | ||
| Commissural tracts | .003a | 0.47 (0.0004 to 0.0021) | <.001a | 0.76 (0.0009 to 0.0031) | .60 | 0.16 (−0.0008 to 0.0016) | .008b |
| Genu | .01a | 0.40 (0.0004 to 0.0027) | <.001a | 0.75 (0.0013 to 0.0047) | .77 | −0.07 (−0.0018 to 0.0013) | .009b |
| Body | .001a | 0.51 (0.0004 to 0.0017) | .001a | 0.73 (0.0006 to 0.0022) | .15 | 0.35 (−0.0003 to 0.0017) | .006b |
| Tapetum | .79 | −0.04 (−0.0010 to 0.0008) | .74 | 0.07 (−0.0010 to 0.0014) | .39 | −0.21 (−0.0020 to 0.0007) | .17 |
| Splenium | .25 | 0.18 (−0.0005 to 0.0018) | .20 | 0.27 (−0.0006 to 0.0028) | .74 | 0.08 (−0.0016 to 0.0023) | .65 |
| Corticospinal tracts | .02a | 0.37 (0.0002 to 0.0018) | .007a | 0.60 (0.0004 to 0.0026) | .54 | 0.15 (−0.0008 to 0.0016) | .01b |
| Fasciculi | .04a | 0.33 (0.0001 to 0.0018) | .006a | 0.61 (0.0005 to 0.0029) | .96 | 0.01 (−0.0011 to 0.0012) | .01b |
| Brainstem tracts | .15 | 0.23 (−0.0003 to 0.0021) | .06 | 0.44 (0 to 0.0032) | .97 | −0.006 (−0.0020 to 0.0019) | .07 |
| Limbic tracts | .06 | 0.31 (0 to 0.0019) | .12 | 0.34 (−0.0002 to 0.0024) | .22 | 0.29 (−0.0005 to 0.0022) | .32 |
Abbreviations: FA, fractional anisotropy; GLM, general linear model.
Two-tailed 2-sample t test P < .05 (heavy drinkers having more negative slopes than no-to-low drinkers).
Two-tailed age-drinking-group interaction P < .05 (heavy drinkers having more negative slopes than no-to-low drinkers at a younger age).
Figure 4. Alcohol Outcomes in the Genu and Body of the Corpus Callosum.
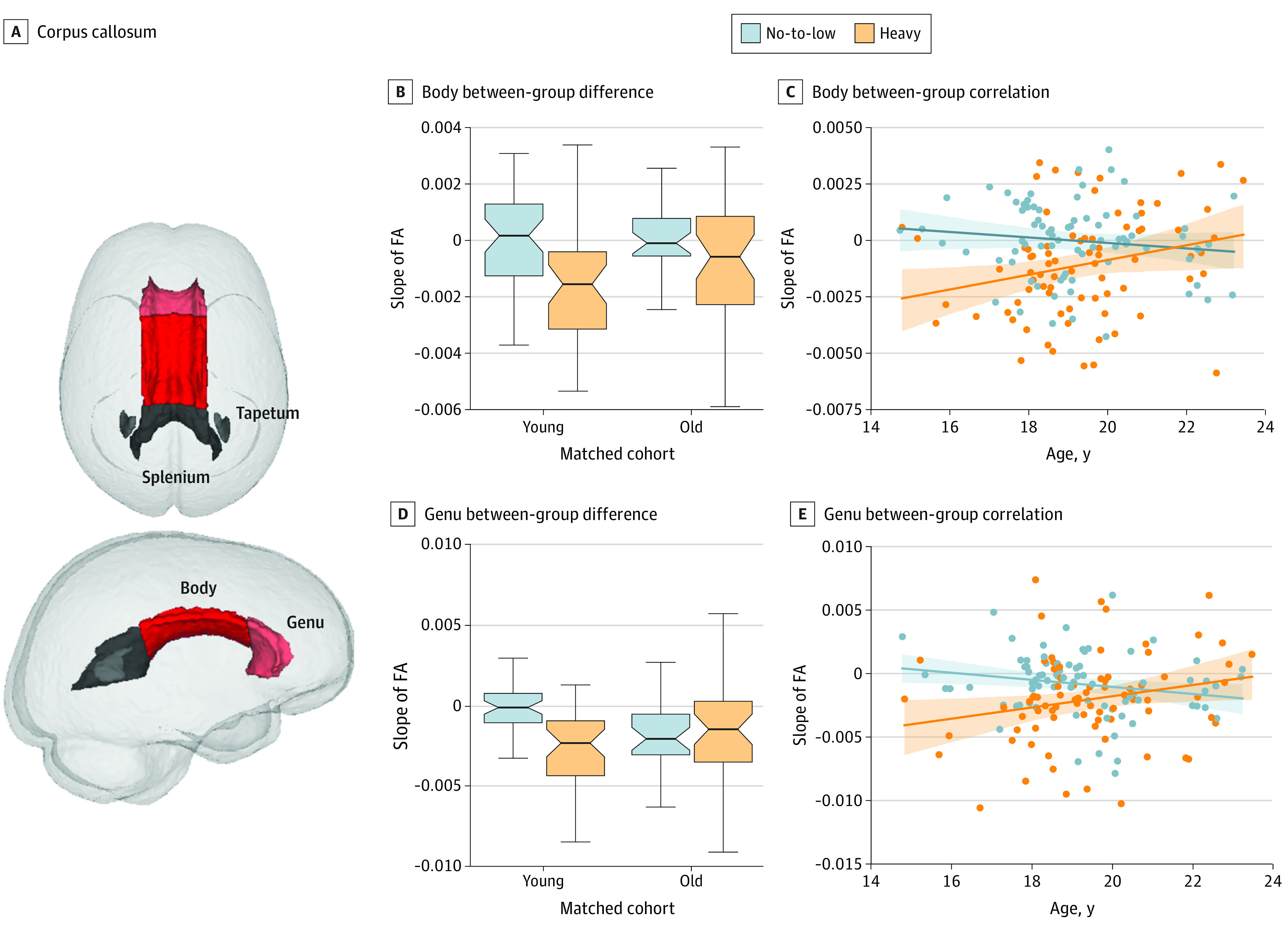
A, Corpus callosum. B-D, Significant alcohol outcome and age-drinking-group interaction were observed in the body (B and C) between-group difference and between-group correlation and in the genu (D and E) between-group difference and between-group correlation of the corpus callosum. Boxplots in B and D represent the minimum, first quartile, median, third quartile, and maximum of slope of FA. Shading in C and E represents the 95% CI for the regression estimates.
Discussion
Adolescence is a critical period of physiological and social maturation accompanied by significant structural, functional, and neurochemical brain changes. The present study found disruptions to the WM fiber microstructural growth trajectories associated with alcohol consumption patterns. Our results further suggest that this alcohol association was more pronounced in younger adolescents and was prominent in the anterior and middle corpus callosum, which serve the interhemispheric integration of frontal networking and communication.
By quantifying developmental change as a slope spanning 4 years (and up to 5 visits), our findings suggest an alcohol-related detrimental association with the developing WM microstructure of the adolescent brain. Despite FA deficits that are consistently observed in adults with alcohol use disorder,17,29,30 adolescent studies often come to mixed conclusions in the assessment of alcohol drinking on FA status. Although cross-sectional31,32,33 and longitudinal studies34,35,36 have interpreted abnormally low FA as attributable to alcohol’s neurotoxic effects, greater FA has also been reported in adolescents who binge drink compared with youth with limited drinking experience.33,37,38 One potential reason for such inconsistency is that investigating disruption related to alcohol drinking is complicated by the co-occurring microstructural neurodevelopmental changes of adolescence. Characterized by an inverted U-shape24,39 (eFigure 1 in the Supplement), FA continues to increase throughout early-to-middle adolescence but starts to decline in later adolescence. Prior longitudinal studies34,35,36,40 have too few observations to disentangle this normal neurodevelopment from subtle disruption following heavy yet non–dependent-level drinking. By tracking participants for a more extended period, our within-participant analysis (Figure 2E) suggests that initiation of alcohol use during adolescence was most likely associated with a reduction in FA. Our results also comported with the causality established in animal studies, where a rodent model of binge alcohol exposure induced FA deficits in the rat brain followed by FA recovery after 1 week without alcohol.41,42
Although the untoward outcomes of alcohol on the adolescent brain have been widely reported,6,7,8 our study, to our knowledge, is the first to suggest in vivo differential vulnerability in WM microstructure with respect to age (Figure 3). The findings are consistent with greater macrostructural and functional disruptions being associated with younger drinking onset during adolescence.40,43 Given that damage in WM tracts was associated with heightened neural reactivity to alcohol cues in adults with alcohol use disorder,44 the greater WM degradation at younger relative to older ages might help explain why adolescents who initiate early drinking are more likely to develop addiction later in life. The age-alcohol interaction, however, was observed only in the slopes and not evident from the FA measures themselves (Figure 3D). A difficulty in seeking aging outcomes during adolescence directly from FA is related to differences in age of onset of alcohol drinking, which influences drinking history factors, notably, the amounts consumed over time. Thus, the FA measures were unlikely to follow a coherent group-level trajectory with respect to age as has been observed in adult alcohol use disorder (such as accelerated aging45). This observation is also supported by Jones and Nagel,38 who found persistent alcohol-associated but not age-dependent deviation in FA in adolescents.
Both alcohol-associated FA reduction and the age-alcohol interaction were pronounced in the corpus callosum, the great interhemispheric commissure. White matter volume shrinkage and callosal demyelination are 2 of the most prominent markers in adult alcoholism46,47,48 and are potential markers in adolescent alcohol abuse.37 The more prominent disruption in the anterior and middle regions (the genu and body) relative to posterior callosal regions (the splenium and tapetum) has been observed across the life span,30,45,49 with the anterior and middle callosum demonstrating more macrostructural thinning and compromised fiber integrity attributing to poorer working memory, visuospatial ability, and gait and balance.50 For adolescents, the differential vulnerability may also relate to the distinct regional maturation pattern across callosal subregions.51,52,53 An early MRI study suggested that although the callosal area increased linearly across adolescence, the posterior callosum underwent nonlinear growth with the greatest increases in the younger years.52 Consistent with that growth pattern, callosal thickness increased across all subregions at early pubertal stages (age <15 years), but only the body and genu regions showed growth at later stages.53 Therefore, the ongoing maturation of these regions during the age range studied herein might render them more vulnerable to alcohol consumption compared with the earlier matured splenium.
Limitations
This study has some limitations. The study revealed age-alcohol interactions in mid to late adolescence. This age span was more inclusive than the traditionally defined end point of adolescence as 19 years of age and consistent with the recently suggested end point of 24 years54 to align more closely to the contemporary patterns of brain development during this life phase. One way of improving modeling of early adolescence is to include youth from the NCANDA cohort who initiated moderate drinking (youth-adjusted Cahalan score = 1). However, alcohol outcomes of moderate drinkers are expected to be subtle and therefore more challenging to detect than the ones reported herein.
Another limitation is use of a linear model (slope) to examine development of global and regional FA, which may be restricted in ability to detect outcomes in localized areas. In our analysis, no alcohol outcomes were observed in the brainstem or limbic tracts, and only trend-level outcomes were detected in the corticospinal tracts and fasciculi. A finer-grained nonlinear voxel-wise analysis, potentially accompanied by a multimodal analysis of macrostructural and functional MRI, would provide a more comprehensive and localized pattern of hazardous alcohol use in adolescence. Nonetheless, an escape of brainstem fiber systems has been documented in adults with alcohol use disorder and may offer an avenue of functional compensation for damaged systems.17
Conclusions
By analyzing 4-year longitudinal DTI data of 451 youth from the NCANDA study, our results suggest that alcohol was associated with detrimental changes in microstructural neurodevelopment during adolescence. Our novel within-participant analysis, based on analyzing neurodevelopmental trajectories of youth before and after they initiated heavy drinking, suggests that the disrupted WM integrity was associated with drinking onset. The analysis also suggests that the younger heavy drinkers had greater deviation from normal developmental trajectory compared with the older drinkers, and the associations were more pronounced in the anterior and middle corpus callosum. Taken together, our results bolster the emerging evidence for the period of early adolescence and its rapid neurodevelopment as a possible magnifier of vulnerability of the brain's WM communication systems to the risks of heavy alcohol consumption.
eMethods. Supplementary Methods
eResults. Supplementary Results
eFigure 1. Subject-Level and Group-Level Trajectory of FA
eFigure 2. Extension of Figure 2d in the Main Text
eFigure 3. Piecewise Linear Regression to Estimate Drinking Onset
eFigure 4. Correlation Between Confounding Variables and Slope of FA
eFigure 5. Extension of Figure 3b in the Main Text
eFigure 6. Extension of Figure 4 in the Main Text
eTable. Demographics of the NCANDA Participants
eReferences
References
- 1.Barkovich MJ, Barkovich AJ. MR imaging of normal brain development. Neuroimaging Clin N Am. 2019;29(3):325-337. doi: 10.1016/j.nic.2019.03.007 [DOI] [PubMed] [Google Scholar]
- 2.Pfefferbaum A, Mathalon DH, Sullivan EV, Rawles JM, Zipursky RB, Lim KO. A quantitative magnetic resonance imaging study of changes in brain morphology from infancy to late adulthood. Arch Neurol. 1994;51(9):874-887. doi: 10.1001/archneur.1994.00540210046012 [DOI] [PubMed] [Google Scholar]
- 3.Giedd JN, Blumenthal J, Jeffries NO, et al. Brain development during childhood and adolescence: a longitudinal MRI study. Nat Neurosci. 1999;2(10):861-863. doi: 10.1038/13158 [DOI] [PubMed] [Google Scholar]
- 4.Feinberg I. Cortical pruning and the development of schizophrenia. Schizophr Bull. 1990;16(4):567-570. doi: 10.1093/schbul/16.4.567 [DOI] [PubMed] [Google Scholar]
- 5.Campbell IG, Grimm KJ, de Bie E, Feinberg I. Sex, puberty, and the timing of sleep EEG measured adolescent brain maturation. Proc Natl Acad Sci U S A. 2012;109(15):5740-5743. doi: 10.1073/pnas.1120860109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yakovlev PI, Lecours AR. The myelogenetic cycles of regional maturation of the brain. In: Minkowski A, ed. Regional Development of the Brain in Early Life. Blackwell Scientific Publications Inc; 1967:3-70.
- 7.Lebel C, Walker L, Leemans A, Phillips L, Beaulieu C. Microstructural maturation of the human brain from childhood to adulthood. Neuroimage. 2008;40(3):1044-1055. doi: 10.1016/j.neuroimage.2007.12.053 [DOI] [PubMed] [Google Scholar]
- 8.Giedd JN, Raznahan A, Alexander-Bloch A, Schmitt E, Gogtay N, Rapoport JL. Child psychiatry branch of the National Institute of Mental Health longitudinal structural magnetic resonance imaging study of human brain development. Neuropsychopharmacology. 2015;40(1):43-49. doi: 10.1038/npp.2014.236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bashyam VM, Erus G, Doshi J, et al. MRI signatures of brain age and disease over the lifespan based on a deep brain network and 14 468 individuals worldwide. Brain. 2020;143(7):2312-2324. doi: 10.1093/brain/awaa160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lim KO, Harris D, Beal M, et al. Gray matter deficits in young onset schizophrenia are independent of age of onset. Biol Psychiatry. 1996;40(1):4-13. doi: 10.1016/0006-3223(95)00356-8 [DOI] [PubMed] [Google Scholar]
- 11.US Department of Health and Human Services. Results from the 2012 national survey on drug use and health: summary of national findings. Accessed June 1, 2020. https://www.samhsa.gov/data/sites/default/files/NSDUHresults2012/NSDUHresults2012.pdf
- 12.Bava S, Tapert SF. Adolescent brain development and the risk for alcohol and other drug problems. Neuropsychol Rev. 2010;20(4):398-413. doi: 10.1007/s11065-010-9146-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alexander AL, Lee JE, Lazar M, Field AS. Diffusion tensor imaging of the brain. Neurotherapeutics. 2007;4(3):316-329. doi: 10.1016/j.nurt.2007.05.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chang EH, Argyelan M, Aggarwal M, et al. The role of myelination in measures of white matter integrity: Combination of diffusion tensor imaging and two-photon microscopy of CLARITY intact brains. Neuroimage. 2017;147:253-261. doi: 10.1016/j.neuroimage.2016.11.068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Silveri MM, Dager AD, Cohen-Gilbert JE, Sneider JT. Neurobiological signatures associated with alcohol and drug use in the human adolescent brain. Neurosci Biobehav Rev. 2016;70:244-259. doi: 10.1016/j.neubiorev.2016.06.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jacobus J, Tapert SF. Neurotoxic effects of alcohol in adolescence. Annu Rev Clin Psychol. 2013;9:703-721. doi: 10.1146/annurev-clinpsy-050212-185610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pfefferbaum A, Rosenbloom M, Rohlfing T, Sullivan EV. Degradation of association and projection white matter systems in alcoholism detected with quantitative fiber tracking. Biol Psychiatry. 2009;65(8):680-690. doi: 10.1016/j.biopsych.2008.10.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Achterberg M, Peper JS, van Duijvenvoorde AC, Mandl RC, Crone EA. Frontostriatal white matter integrity predicts development of delay of gratification: a longitudinal study. J Neurosci. 2016;36(6):1954-1961. doi: 10.1523/JNEUROSCI.3459-15.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Seghete KL, Herting MM, Nagel BJ. White matter microstructure correlates of inhibition and task-switching in adolescents. Brain Res. 2013;1527:15-28. doi: 10.1016/j.brainres.2013.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Charlton RA, Barrick TR, Lawes IN, Markus HS, Morris RG. White matter pathways associated with working memory in normal aging. Cortex. 2010;46(4):474-489. doi: 10.1016/j.cortex.2009.07.005 [DOI] [PubMed] [Google Scholar]
- 21.Brown SA, Brumback T, Tomlinson K, et al. The National Consortium on Alcohol and Neurodevelopment in Adolescence (NCANDA): a multisite study of adolescent development and substance use. J Stud Alcohol Drugs. 2015;76(6):895-908. doi: 10.15288/jsad.2015.76.895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pfefferbaum A, Kwon D, Brumback T, et al. Altered brain developmental trajectories in adolescents after initiating drinking. Am J Psychiatry. 2018;175(4):370-380. doi: 10.1176/appi.ajp.2017.17040469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cahalan D, Cisin IH, Crossley HM. American Drinking Practices: A National Study of Drinking Behavior and Attitudes. Rutgers Center of Alcohol Studies; 1969.
- 24.Pohl KM, Sullivan EV, Rohlfing T, et al. Harmonizing DTI measurements across scanners to examine the development of white matter microstructure in 803 adolescents of the NCANDA study. Neuroimage. 2016;130:194-213. doi: 10.1016/j.neuroimage.2016.01.061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.National Consortium on Alcohol and Neurodevelopment in Adolescence . NCANDA data distribution agreement. Accessed June 1, 2020. https://www.niaaa.nih.gov/research/major-initiatives/national-consortium-alcohol-and-neurodevelopment-adolescence/ncanda-data
- 26.Mori S, Wakana S, Nagae-Poetscher LM, van Zijl PCM. MRI atlas of human white matter. Am J Neuroradiol. 2006;27(6):1384-1385. [Google Scholar]
- 27.Laird NM, Ware JH. Random-effects models for longitudinal data. Biometrics. 1982;38(4):963-974. doi: 10.2307/2529876 [DOI] [PubMed] [Google Scholar]
- 28.Shaffer JP. Multiple hypothesis testing. Annu Rev Psychol. 1995;46:561–584. doi: 10.1146/annurev.ps.46.020195.003021 [DOI] [Google Scholar]
- 29.Monnig MA, Caprihan A, Yeo RA, et al. Diffusion tensor imaging of white matter networks in individuals with current and remitted alcohol use disorders and comorbid conditions. Psychol Addict Behav. 2013;27(2):455-465. doi: 10.1037/a0027168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pfefferbaum A, Sullivan EV. Disruption of brain white matter microstructure by excessive intracellular and extracellular fluid in alcoholism: evidence from diffusion tensor imaging. Neuropsychopharmacology. 2005;30(2):423-432. doi: 10.1038/sj.npp.1300623 [DOI] [PubMed] [Google Scholar]
- 31.McQueeny T, Schweinsburg BC, Schweinsburg AD, et al. Altered white matter integrity in adolescent binge drinkers. Alcohol Clin Exp Res. 2009;33(7):1278-1285. doi: 10.1111/j.1530-0277.2009.00953.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jacobus J, McQueeny T, Bava S, et al. White matter integrity in adolescents with histories of marijuana use and binge drinking. Neurotoxicol Teratol. 2009;31(6):349-355. doi: 10.1016/j.ntt.2009.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bava S, Frank LR, McQueeny T, Schweinsburg BC, Schweinsburg AD, Tapert SF. Altered white matter microstructure in adolescent substance users. Psychiatry Res. 2009;173(3):228-237. doi: 10.1016/j.pscychresns.2009.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bava S, Jacobus J, Thayer RE, Tapert SF. Longitudinal changes in white matter integrity among adolescent substance users. Alcohol Clin Exp Res. 2013;37(suppl 1):E181-E189. doi: 10.1111/j.1530-0277.2012.01920.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jacobus J, Squeglia LM, Infante MA, Bava S, Tapert SF. White matter integrity pre- and post marijuana and alcohol initiation in adolescence. Brain Sci. 2013;3(1):396-414. doi: 10.3390/brainsci3010396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Luciana M, Collins PF, Muetzel RL, Lim KO. Effects of alcohol use initiation on brain structure in typically developing adolescents. Am J Drug Alcohol Abuse. 2013;39(6):345-355. doi: 10.3109/00952990.2013.837057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.De Bellis MD, Van Voorhees E, Hooper SR, et al. Diffusion tensor measures of the corpus callosum in adolescents with adolescent onset alcohol use disorders. Alcohol Clin Exp Res. 2008;32(3):395-404. doi: 10.1111/j.1530-0277.2007.00603.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jones SA, Nagel BJ. Altered frontostriatal white matter microstructure is associated with familial alcoholism and future binge drinking in adolescence. Neuropsychopharmacology. 2019;44(6):1076-1083. doi: 10.1038/s41386-019-0315-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hasan KM, Kamali A, Iftikhar A, et al. Diffusion tensor tractography quantification of the human corpus callosum fiber pathways across the lifespan. Brain Res. 2009;1249:91-100. doi: 10.1016/j.brainres.2008.10.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nguyen-Louie TT, Simmons AN, Squeglia LM, Alejandra Infante M, Schacht JP, Tapert SF. Earlier alcohol use onset prospectively predicts changes in functional connectivity. Psychopharmacology (Berl). 2018;235(4):1041-1054. doi: 10.1007/s00213-017-4821-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zahr NM, Lenart AM, Karpf JA, et al. Multi-modal imaging reveals differential brain volumetric, biochemical, and white matter fiber responsivity to repeated intermittent ethanol vapor exposure in male and female rats. Neuropharmacology. 2020;170:108066. doi: 10.1016/j.neuropharm.2020.108066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pfefferbaum A, Zahr NM, Mayer D, Rohlfing T, Sullivan EV. Dynamic responses of selective brain white matter fiber tracts to binge alcohol and recovery in the rat. PLoS One. 2015;10(4):e0124885. doi: 10.1371/journal.pone.0124885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.De Bellis MD, Clark DB, Beers SR, et al. Hippocampal volume in adolescent-onset alcohol use disorders. Am J Psychiatry. 2000;157(5):737-744. doi: 10.1176/appi.ajp.157.5.737 [DOI] [PubMed] [Google Scholar]
- 44.Monnig MA, Thayer RE, Caprihan A, et al. White matter integrity is associated with alcohol cue reactivity in heavy drinkers. Brain Behav. 2014;4(2):158-170. doi: 10.1002/brb3.204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pfefferbaum A, Rosenbloom MJ, Chu W, et al. White matter microstructural recovery with abstinence and decline with relapse in alcohol dependence interacts with normal ageing: a controlled longitudinal DTI study. Lancet Psychiatry. 2014;1(3):202-212. doi: 10.1016/S2215-0366(14)70301-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zahr NM, Pfefferbaum A. Alcohol’s effects on the brain: neuroimaging results in humans and animal models. Alcohol Res. 2017;38(2):183-206. [PMC free article] [PubMed] [Google Scholar]
- 47.Sullivan EV, Harris RA, Pfefferbaum A. Alcohol’s effects on brain and behavior. Alcohol Res Health. 2010;33(1-2):127-143. [PMC free article] [PubMed] [Google Scholar]
- 48.de la Monte SM, Kril JJ. Human alcohol-related neuropathology. Acta Neuropathol. 2014;127(1):71-90. doi: 10.1007/s00401-013-1233-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.De Santis S, Bach P, Pérez-Cervera L, et al. Microstructural white matter alterations in men with alcohol use disorder and rats with excessive alcohol consumption during early abstinence. JAMA Psychiatry. 2019;76(7):749-758. doi: 10.1001/jamapsychiatry.2019.0318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pfefferbaum A, Adalsteinsson E, Sullivan EV. Dysmorphology and microstructural degradation of the corpus callosum: Interaction of age and alcoholism. Neurobiol Aging. 2006;27(7):994-1009. doi: 10.1016/j.neurobiolaging.2005.05.007 [DOI] [PubMed] [Google Scholar]
- 51.Luders E, Thompson PM, Toga AW. The development of the corpus callosum in the healthy human brain. J Neurosci. 2010;30(33):10985-10990. doi: 10.1523/JNEUROSCI.5122-09.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Giedd JN, Blumenthal J, Jeffries NO, et al. Development of the human corpus callosum during childhood and adolescence: a longitudinal MRI study. Prog Neuropsychopharmacol Biol Psychiatry. 1999;23(4):571-588. doi: 10.1016/S0278-5846(99)00017-2 [DOI] [PubMed] [Google Scholar]
- 53.Chavarria MC, Sánchez FJ, Chou YY, Thompson PM, Luders E. Puberty in the corpus callosum. Neuroscience. 2014;265:1-8. doi: 10.1016/j.neuroscience.2014.01.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sawyer SM, Azzopardi PS, Wickremarathne D, Patton GC. The age of adolescence. Lancet Child Adolesc Health. 2018;2(3):223-228. doi: 10.1016/S2352-4642(18)30022-1 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods. Supplementary Methods
eResults. Supplementary Results
eFigure 1. Subject-Level and Group-Level Trajectory of FA
eFigure 2. Extension of Figure 2d in the Main Text
eFigure 3. Piecewise Linear Regression to Estimate Drinking Onset
eFigure 4. Correlation Between Confounding Variables and Slope of FA
eFigure 5. Extension of Figure 3b in the Main Text
eFigure 6. Extension of Figure 4 in the Main Text
eTable. Demographics of the NCANDA Participants
eReferences