Abstract
Purpose
To determine behavioral and genetic factors associated with incidence and age of progression to advanced age-related macular degeneration (AMD), geographic atrophy (GA), and neovascular disease (NV), and to quantify these effects.
Methods
Longitudinal analyses were conducted among 5421 eyes with nonadvanced AMD at baseline in 2976 participants in the Age-Related Eye Disease Study (mean age of 68.8 (±5.0), 56.1% female). Progression was confirmed based on two consecutive visits on the AMD severity scale. Separate analyses for progression and age of progression were performed. All analyses adjusted for correlation between eyes, demographic and behavioral covariates, baseline severity scale, and genetic variants.
Results
A higher genetic risk score (GRS) including eight genetic variants was associated with a higher rate of progression to advanced AMD within each baseline severity scale, especially for the highest risk intermediate level AMD category, and smoking further increased this risk. When assessing age when progression to advanced disease occurred, smoking reduced age of onset by 3.9 years (P < 0.001), and higher body mass index (BMI) led to earlier onset by 1.7 years (P = 0.003), with similar results for GA and NV. Genetic variants associated with earlier age of progression were CFH R1201C (4.3 years), C3 K155Q (2.15 years), and ARMS2/HTRA1 (0.8 years per allele).
Conclusions
Rare variants in the complement pathway and a common risk allele in ARMS2/HTRA1, smoking, and higher BMI can lead to as much as 11.5 additional years of disease and treatment burden. Closer adherence to healthy lifestyles could reduce years of visual impairment.
Keywords: age-related macular degeneration, age of progression, genetics, lifestyles
Age-related macular degeneration (AMD) has a complex cause and remains a significant public health problem despite recent advances in treatments.1–3 Many patients with neovascular macular degeneration (NV) disease have residual visual impairment after treatment with intravitreal injections, because of varying degrees of chorioretinal atrophy and scarring. Therefore new therapies are being evaluated. The advanced dry form with geographic macular atrophy (GA) has no known treatment, but many clinical trials are underway.
AMD confers a significant individual and societal burden and can lead to loss of independence, increased use of health care resources, and an adverse impact on quality of life. The prevalence of AMD is increasing as the proportion of our elderly population rises, and the number of people with AMD is expected to be 196 million in 2020, increasing to 288 million in 2040.2 AMD is the leading cause of visual disability in the developed world and the third globally.4 The estimated direct health care costs of visual impairment in North America caused by AMD is $98 billion and $255 billion globally.5 The level of reduction in quality of life for severe AMD is comparable to end-stage prostatic cancer or a catastrophic stroke.6 Prevention of AMD is therefore a key public health strategy.
A set of genetic, demographic, and environmental variables can predict with relatively high likelihood which individuals will more likely progress to advanced AMD.7 It has also been shown that individuals with rare genetic variants are more likely to progress.7–9 However, the age at which the transition from nonadvanced to advanced AMD occurs is variable, even among those with the same baseline macular pathology. The independent effect of individual genetic variants and behavioral variables on this age of progression, and quantification of the difference in number of years, have not been evaluated in a longitudinal study. We analyzed data from a large, well-defined cohort to assess the impact of both genetic and lifestyle factors on age when transition to advanced AMD occurs over time, adjusting for other known factors related to AMD.
Analyses included some new methodologic considerations. AMD incidence is not a linear function of age; risk increases nonlinearly after age 70.2,7,10 Thus we used age as the time scale in conducting time-to-event analyses. Severity scales for each eye were analyzed separately to better adjust for baseline macular status. Also, to enhance accuracy in determining the endpoints, we defined a progressing eye on the basis of having a severity scale indicating advanced disease at two consecutive visits. Also, because the effect of the association between severity scale and AMD is not linear, we represented severity scale as a set of indicator variables in the analyses. Finally, we used stepwise regression to identify relevant or predictive genotypes in deriving a genetic risk score on the basis of genotypes related to specific outcomes in longitudinal analyses.
Clinical trials should consider selection criteria that target particular disease subgroups for therapeutic approaches, such as those at higher risk of progression or have earlier age of progression with longer disease burden. To help achieve this goal, the aims of these analyses were to (i) apply new methods for evaluating predictors of developing overall advanced AMD, GA, and NV, and (ii) evaluate the impact of both modifiable and genetic risk factors on age when progression occurs. Herein, we expand upon our previous preliminary analyses and results on this topic. 11,12
Methods
Study Population
The Age-Related Eye Disease Study (AREDS) enrolled a total of 4757 participants in the United States from 1992 to 1998.13 Among these, 2941 individuals (5421 eyes) with follow-up and with all covariate and genetic data were analyzed, and 948 eyes progressed to advanced AMD, 487 eyes progressed to GA, and 495 progressed to NV (some eyes had both endpoints). The mean follow-up time was 9.3 years (range 0.5–13 years), with a median of 10 years. The study adhered to the tenets of the Declaration of Helsinki and was performed under approved institutional review board protocols.
Definition of Progression to Advanced AMD
Incidence of progression to advanced AMD was based on the eye specific severity scale in the AREDS database.14 The scale ranges from 1 to 12, with scale 9 as noncentral GA, 10 as central GA, 11 as NV, and 12 as NV and central GA. Scales 1 to 8 comprise a range from no AMD to early or intermediate stages on the basis of drusen and retinal pigment epithelial irregularities. An eye that progressed was defined as transition from scales 1 to 8 to any GA or evidence of NV or both (levels ≥9) during follow-up. We required that two consecutive visits have scales corresponding to the endpoints GA (9–10,12), NV (11–12), and overall advanced AMD (9–12), because there were several instances of regression to scales 1–8 after a single scale 9–12. Supplementary Figure S1 displays examples of the use of this method for advanced AMD to confirm the endpoints. For progression to GA, two consecutive visits with scale 9, 10, or 12 were needed to confirm the progression, as long as NV was never confirmed before the progression to GA. For progression to NV, two consecutive visits with scale 11 or 12 were needed to confirm progression. If an eye progressed to GA first before NV, it was still included in the analysis of eyes at risk for NV.
Demographic, Behavioral, and Genetic Factors
Demographic factors including age, gender, race (white, non-white), education (high school or below, above high school), and behavioral factors including smoking (never, past, current) and body mass index (BMI) (<25, 25–<30, 30+) were determined from questionnaires administered at baseline visits. All analyses were adjusted for the AREDS treatment assignments including placebo, antioxidants, zinc, and antioxidants plus zinc. Genotyping was performed using array-based genotyping and gene sequencing platforms as previously described.8,9,15–17
Analyses of Progression to Overall Advanced AMD, GA, and NV
Given that the risk of progression is not necessarily a linear function of baseline severity scale and this scale is likely to be the strongest predictor of progression, we represented the severity scale as a set of indicator variables from 2 to 8 with scale =1 as a reference category. Similarly, because the risk of AMD is a nonlinear function of age and increases sharply after age 70, we used age as the time scale in conducting survival analysis. Thus all the risk sets used in survival analyses are based on age in one-year intervals, which was updated throughout the follow-up period. This eliminates all residual confounding by age in subsequent analyses. We conducted survival analysis with the eye as the unit of analysis, using the Cox proportional hazards model, with the counting process style of input using age as the time metric. The age interval (age 1, age 2) was determined for each person, where age1 = baseline age and age2 = minimum (age at the last follow-up visit, age of progression). For each risk factor, we calculated a hazard ratio (HR) that is the ratio of incidence rates at any given point in time between two subjects that differ by one unit on a specific risk factor, holding all other risk factors constant. We included nongenetic variables (sex, race, education, smoking, BMI, baseline severity scale, and AREDS treatment group) and then used stepwise selection to identify additional significant genetic predictors of progression.7 The criteria for selection were P < 0.05 for both entering and staying in the model. Finally, we tested for departures or violations of proportional hazard assumptions by including cross product terms of follow-up time by smoking and BMI, respectively, in our risk models.
Genetic Risk Score
The Genetic Risk Score (GRSAdvancedAMD) was calculated using the variants associated with progression to overall advanced AMD (Table 1). Based on this model, we calculated a GRS = sum of βi, multiplied by gi, where gi = number of risk alleles present for the ith genetic variant i=1,…,8:
Table 1.
Genetic Variants Associated With Progression to Overall Advanced AMD, GA, and NV
| Advanced AMD (948/5421 Eyes) | GA (487/5421 Eyes) | NV (495/5421 Eyes) | ||||
|---|---|---|---|---|---|---|
| Genetic Variants | HR (95% CI)* | P Value | HR (95% CI)* | P Value | HR (95% CI)* | P Value |
| Complement pathway | ||||||
| CFH Y402H: rs1061170 | 1.31 (1.13–1.51) | <0.001 | ||||
| CFH: rs1410996 | 1.34 (1.18–1.52) | <0.001 | 1.34 (1.11–1.61) | 0.003 | ||
| CFH R1210C: rs121913059 | 2.94 (1.78–4.85) | <0.001 | 2.48 (1.06–5.82) | 0.037 | 2.82 (1.56–5.11) | <0.001 |
| C3 R102G: rs2230199 | 1.16 (1.04–1.30) | 0.007 | 1.18 (1.01–1.38) | 0.033 | ||
| C3 K155Q: rs147859257 | 2.01 (1.45–2.77) | <0.001 | 2.29 (1.56–3.35) | <0.001 | ||
| Angiogenesis pathway | ||||||
| TGFBR1: rs334353 | 0.84 (0.71–1.00) | 0.044 | ||||
| Immune/inflammatory pathway | ||||||
| ARMS2/HTRA1: rs10490924 | 1.44 (1.30–1.59) | <0.001 | 1.24 (1.08–1.43) | 0.002 | 1.65 (1.42–1.91) | <0.001 |
| TNFRSF10A: rs13278062 | 0.88 (0.77–1.01) | 0.066 | ||||
| Extracellular matrix | ||||||
| COL8A1: rs13095226 | 1.19 (1.02–1.38) | 0.023 | 1.28 (1.03–1.59) | 0.028 | ||
| DNA repair/protein binding | ||||||
| RAD51B: rs8017304 | 0.82 (0.73–0.91) | <0.001 | 0.76 (0.65–0.89) | <0.001 | ||
| HSPH1/B3GALTL: rs9542236 | 1.12 (1.02–1.24) | 0.019 | 1.16 (1.01–1.34) | 0.038 | ||
HRs were calculated for time to progression using age as the time scale with the eye as the unit of analysis, adjusted for sex, race, education, smoking, BMI, AMD baseline eye-specific severity scale, and AREDS treatment.
The GRS was then grouped into tertiles, the severity scale was categorized into four groups (1–5/6/7/8) and a second Cox model was run using age as the time metric included GRS tertile and severity scale category and in addition controlling for sex, education, race, BMI, smoking, and AREDS treatment group. We used the baseline statement of SAS with the second survival model to estimate the survival curve for a subject with average value for all covariates.
In the third step of the analysis, we used the baseline survival curve to estimate the five-year survival probability (S) with specific combinations of severity scale and GRS tertile, and average levels of all other covariates:
where GRSi and SEVj for the ith GRS and jth severity scale category, respectively.
We then estimated the five-year incidence, I(5) = 1-S(5), for each combination of GRS category and severity scale category. Similar GRSs were also constructed for GA (GRSGA) and NV (GRSNV). Similar plots were constructed for different GRS tertiles within combinations of smoking status (current/past/never) and representative severity scales (2/5/8), for 12 years.
Area Under the Curve (AUC)
We calculated a risk score for each eye of each subject and used the Mann-Whitney-U statistic to estimate the AUC, the probability that a random progressing eye will have a higher risk score than a random nonprogressing eye, after controlling for age.18 For this purpose, we compared risk scores of eyes that progressed within five years (progressing eyes) to risk scores of eyes that were followed up for at least five years and did not progress (nonprogressing eyes).
Age of Progression
Among eyes that progressed, multivariate analysis was performed to calculate the effect of behavioral covariates and each genetic variant on age of progression to advanced AMD using mixed effects regression models. Thus a subject may contribute either one or two observations to the dataset according to the number of eyes that progressed during the study. A similar stepwise procedure was used forcing in nongenetic variables to identify genetic variables associated with age of progression. The genetic variables were combined into an overall genetic risk score (GRSage) that reflect the associations of several variants with age of progression. For variables associated with earlier age of progression, histograms of the distribution of age of progression were calculated. In addition, boxplots of age of progression for combinations of smoking and BMI were obtained. All analyses were calculated using SAS 9.4. Two-sided P values were calculated, and 0.05 was used as the level of significance.
Population Attributable Fraction
We computed the percentage of eyes from people with specific behavioral characteristics (Pj), for example, smoking (current/past/never) and BMI (<25/25–29/30+) where j = 1,…9 corresponds to combinations of smoking and BMI categories, and the hazard ratio (HRj) corresponding to each combination of characteristics versus the reference group (e.g., never smoker and BMI < 25). We then computed the population percent prevented using the formula below:
A similar calculation was performed to assess the percentage of progression prevented if eyes from people with GRS tertiles 2 and 3 were in GRS tertile 1. The GRS calculation was adjusted for specific combinations of severity scale and age and other demographic characteristics (e.g., sex, race) and behavioral characteristics (e.g., smoking and BMI) assuming proportional hazards for each characteristic and no interactions among combinations of characteristics. The smoking and BMI attributable fraction calculations were adjusted for the same combinations (except behavioral characteristics) plus GRS.
Results
Baseline Characteristics
Baseline characteristics of the study population are shown in Supplementary Table S1. Among 5421 eyes, 948 (17.5%) progressed to advanced AMD over 12 years (mean follow-up time 9.6 years ± SD 2.4 years), 487 (9%) progressed to GA and 495 (9%) progressed to NV. Eighty-one percent of the people were 65 or older at baseline, 44% were male, 96% were white, and 66% had at least some education beyond high school. About 6% were current smokers, 47% were past smokers, whereas more than two thirds of the population were overweight. Fifty-six percent were very low risk for progression at baseline (scales 1–2), 23% were low to moderate risk (scales 3–5), whereas 20% had intermediate- to high-risk AMD (scales 6–8). A total of 31 genes were considered, of which 10 genes were in the complement pathway, two were in the angiogenesis pathway, five were in the lipid pathway, six were in the immune/inflammatory pathway, five were in extracellular matrix, and three were in the DNA repair/protein binding pathway.
Genetic and Modifiable Determinants of Incident Age-Related Macular Degeneration
Table 1 displays the genetic variants associated with progression to incident advanced AMD, GA, and NV. Eight genetic variants were associated with progression to advanced AMD, after adjusting for demographic and behavioral factors, AMD baseline eye-specific severity scale and AREDS treatment category. The genetic variants represented several biologic pathways: complement, immune, inflammatory, lipid, extracellular matrix, DNA repair and protein binding. For advanced AMD, four of the variants were in the complement pathway with hazard ratios ranging from 1.16 (C3 R102G) to 2.94 (CFH R1210C) per risk allele. In addition, there was one gene in the immune pathway, one in extracellular matrix, and two in DNA repair pathway (hazard ratio ranging from 0.82 (RAD51B) to 1.44 (ARMS2/HTRA1), related to progression. Five genes were associated with progression to GA, whereas eight were related to progression to NV. For NV, two additional variants in the angiogenesis and immune pathway were associated only with progression to NV. The effects of each of the genetic variants can be visualized in a forest plot in Supplementary Figure S2.
A full risk model with the estimates of each variant and the nongenetic factors are presented in Supplementary Table S2. White subjects showed a borderline significant higher rate of progression to overall AMD and GA compared with nonwhites. Current history of smoking increased the risk for progression to all three outcomes, and past smoking history increased the risk of NV. Higher BMI increased the likelihood of progression to overall AMD and NV, but it was borderline significant for GA. The severity scale was strongly associated with progression to all three outcomes, particularly for progression to GA.
Finally for the outcome advanced AMD, we tested for violations of proportional hazard assumptions with smoking and BMI and found no significant effect modification of either variable when stratified by time in the study either when represented as categorical variables or as ordinal variables (represented as 1/2/3).
Genetic Risk Score and AUC Analyses for Progression to Advanced Age-Related Macular Degeneration
Table 2 displays the GRS for progression to each outcome derived from the selected genetic variants in Table 1. These scores were significantly associated with all three outcomes, adjusting for all covariates including the baseline severity scale (P value <0.001 for all outcomes). For advanced AMD overall, the highest tertile of GRS was associated with a threefold increase in rate of progression compared to the lowest tertile, and almost a threefold increased risk of progression between the ninetieth versus tenth percentile. For GA, the risks were somewhat less pronounced between highest and lowest tertiles than for overall advanced AMD (HR of 1.90), with more than a twofold higher rate of progression for the ninetieth versus tenth percentile. For NV, the risk was 3.7-fold higher for the third GRS tertile compared to the first tertile, and similarly was 3.6 fold higher for ninetieth versus tenth percentile.
Table 2.
GRS for Progression to Advanced AMD, GA, and NV
| Advanced AMD (948/5421 Eyes) | GA (487/5421 Eyes) | NV (495/5421 Eyes) | ||||
|---|---|---|---|---|---|---|
| HR (95% CI) | P Value | HR (95% CI) | P Value | HR (95% CI) | P Value | |
| GRS tertile | ||||||
| 1 | 1 | REF | 1 | REF | 1 | REF |
| 2 | 1.95 (1.48–2.57) | <0.001 | 1.39 (0.97–1.99) | 0.075 | 1.82 (1.24–2.66) | 0.002 |
| 3 | 3.03 (2.31–3.97) | <0.001 | 1.90 (1.35–2.68) | <0.001 | 3.68 (2.58–5.25) | <0.001 |
| GRS* | 2.67 (2.22–3.21) | <0.001 | 2.72 (2.00–3.69) | <0.001 | 2.72 (2.21–3.34) | <0.001 |
| GRS 90th vs. 10th percentile† | 2.97 (1.21–7.27) | 2.18 (1.36–3.50) | 3.64 (1.03–12.78) | |||
Per 1 unit increase in Genetic Risk Score (GRSAdvancedAMD). GRS for each outcome was derived based on the genes identified in Table 1. GRS was adjusted for sex, race, education, smoking, BMI, AMD baseline eye-specific severity scale, and AREDS treatment, where updated age was the time scale and the eye was the unit of analysis.
The difference between the 10th and 90th percentile for GRS was 1.09 for overall AMD, 0.78 for GA, and 1.29 for NV.
For the GRS of progression to advanced AMD, the means are −0.21 (±0.216); 0.29 (±0.125); 0.88 (±0.282) for first, second, and third tertile respectively, and 0.65 (±0.420) for the overall GRS. The range are −0.40 – 0.46; 0.47 – 0.83; 0.83 – 2.29 for first, second, and third tertile respectively.
For the GRS of progression to GA, the means are 0.22 (±0.124); 0.55 (±0.060); 0.91 (±0.208) for first, second, and third tertile respectively, and 0.56 (±0.310) for the overall GRS.
For the GRS of progression to NV, the means are −0.21 (±0.216); 0.30 (±0.125); 0.88 (±0.282) for first, second, and third tertile respectively, and 0.32 (±0.497) for the overall.
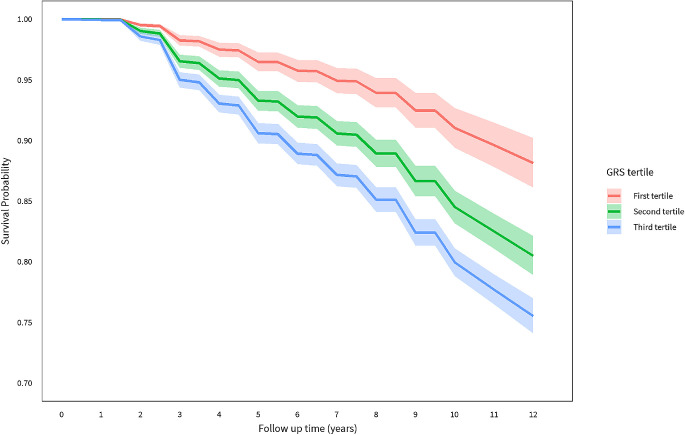
Figure 1 displays Kaplan-Meier (KM) survival curves for progression according to GRS tertiles, adjusting for all covariates, using the baseline command of PROC PHREG of SAS. The estimated 12-year risk of progression was 12%, 20%, and 25% for tertiles 1, 2, 3; indicating about a twofold increased risk for GRS tertile 3 versus 1.
Figure 1.
Survival curves for probability of not progressing to advanced age-related macular degeneration according to GRS tertiles.
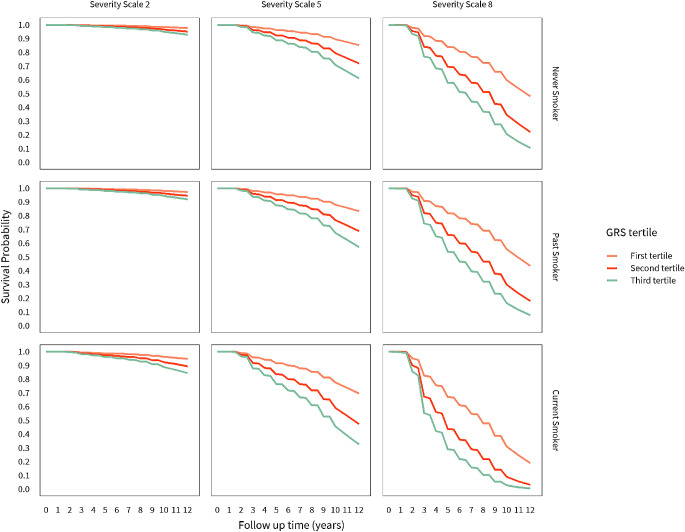
Figure 2 displays KM curves for GRS tertiles stratified by severity scales and smoking status. There is little difference in survival according to GRS for severity scale of 2 (early signs of AMD) and a small proportion of subjects progressed by 12 years. Conversely, there were large differences in survival by GRS categories for severity scale 5 and especially for scale 8, which were magnified further among individuals who were current smokers. For example, among past smokers with baseline severity scale of 8, the 12-year survival probability was 45% for GRS 1 versus 10% for GRS 3. For the current smokers with a severity scale of 8, the 12-year survival probability was 20% for GRS 1 versus less than 5% for GRS 3. Similar findings were apparent for severity scale 5. In summary, GRS differences in survival were only apparent for subjects with at least intermediate AMD, and most discriminating among subjects with the later preadvanced stages of intermediate disease.
Figure 2.
Survival curves for probability of not progressing to advanced AMD over time stratified by GRS tertiles, smoking status, and AMD severity scale.
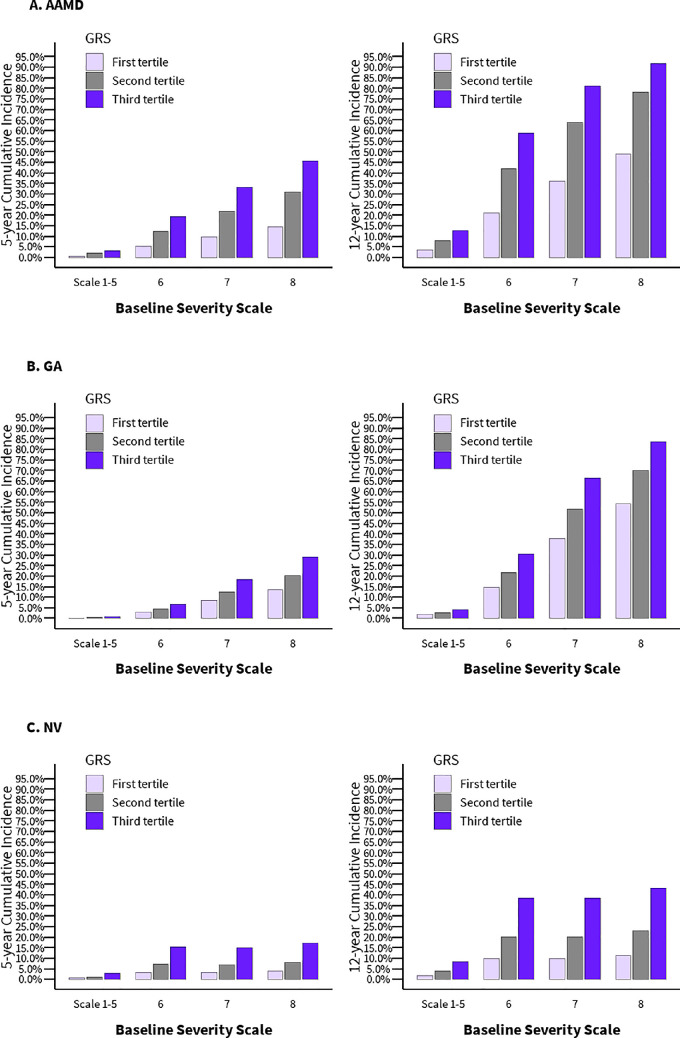
Figure 3 displays the 5 year and 12 year cumulative incidence of progression to advanced AMD, GA, and NV, for various combinations of baseline severity scale and GRS, which adjust for competing mortality risks. For overall progression, there was a strong effect of severity scale on incidence of AMD. Within specific levels of the baseline severity scale, there was an additional gradient of risk when subdividing by tertiles of GRS, particularly in severity scale range 6–8. For example, for severity scale 8, there was approximately a 50% cumulative incidence of advanced AMD over 12 years if the GRS was in the lowest tertile compared with 95% cumulative incidence if the GRS was in the highest tertile. However, if the severity scale was in the lower range 1 to 5, there was a low probability of progression at five or 12 years, regardless of the GRS.
Figure 3.
5 year and 12 year cumulative incidence for progression to advanced AMD, GA, and NV, according to the GRS and baseline AMD severity scale.
Subjects in the highest tertile of GRS and the higher severity scales (6–8) had an increased risk for progression to GA when mutually adjusting for GRS and AMD baseline scale. The incidence rates for progression to NV in eyes with baseline scales 6 to 8 were similar, although within each of these baseline grades, similar to GA, the third tertile of GRS had the highest risk of progression, followed by tertile 2, and the lowest risk was seen in the lowest GRS for each baseline scale 6, 7, and 8.
As shown in Table 3, the AUC for the five-year incidence of advanced AMD was 0.88 among the group with baseline severity scale 1 to 8, adjusting for only demographic and behavioral factors, and was 0.89 after adding the GRS, indicating that there is only a small increase in discrimination when adding the GRS (P = 0.032). Because this AUC calculation is dominated by the large number of eyes in severity scale range 1 to 5, a group with low risk of progression, we also restricted the analysis to eyes within baseline scales 6–8. The AUC was lower but was substantially improved by including tertiles of GRS in addition to the severity scale for overall AMD (ΔAUC = 0.039, P < 0.001). This is illustrated by at least a threefold difference in incidence rate of progression comparing GRS tertile 3 to tertile 1 for individuals within severity scale 6 to 8 (Fig. 3). The effect of the GRS was also more pronounced when restricting eyes to higher risk baseline severity scales 6 to 8 for progression to NV (Δ = 0.062, P < 0.001). In addition, for NV there appears to be a threshold effect of severity scale with a large difference in risk of progression between severity scales 1 to 5 versus 6 to 8, but little change in risk among severity scales 6 to 8. Overall, these results indicate that the GRS provides additional differential information, particularly for eyes at high risk of progression (with baseline scales 6–8).
Table 3.
AUC for Progression to Advanced AMD, GA, and NV Over Five Years According to Baseline Severity Scale, With and Without Genetic Variables
| SEVERITY SCALE 1–8 | SEVERITY SCALE 6–8 | |||||
|---|---|---|---|---|---|---|
| Model 1: Demographic + Behavioral Factors | Model 2: Demographic + Behavioral + Genetic Factors | Model 3: Demographic + Behavioral Factors | Model 4: Demographic + Behavioral + Genetic Factors | |||
| Outcome | AUC (SE) | AUC (SE) | ΔAUC | AUC (SE) | AUC (SE) | ΔAUC |
| Advanced AMD | 0.880 (0.008) | 0.890 (0.008) | 0.010 (0.005); P = 0.032 | 0.617 (0.018) | 0.656 (0.018) | 0.039 (0.012); P < 0.001 |
| GA | 0.928 (0.009) | 0.929 (0.009) | 0.002 (0.005); P = 0.70 | 0.720 (0.021) | 0.729 (0.021) | 0.009 (0.012); P = 0.41 |
| NV | 0.820 (0.014) | 0.840 (0.013) | 0.0179 (0.008); P = 0.023 | 0.529 (0.024) | 0.593 (0.023) | 0.062 (0.016); P < 0.001 |
Models 1 and 3 adjusted for age, sex, race, education, smoking, BMI, AMD baseline eye-specific severity scale, and AREDS treatment.
Models 2 and 4 adjusted for the same variables as Models 1 and 3, plus genetic variables which were specific for each outcome.
Models 1 and 2 consider the entire range of baseline severity scales 1–8, while models 3 and 4 only consider baseline severity scales of 6–8.
ΔAUC, difference in AUC (model 2 vs. 1 and model 4 vs. 3). Each of the AUC estimates were adjusted for updated age in five-year age groups (≤64; 65–<70; 70–<75; 75–<80; 80+) with weights according to the inverse variance of the age specific AUC. The weighting varies between each of the individual model specific AUC estimates as well as the AUC difference between competing models. In general, strata with larger number of events get more weight.
Age of Progression to Advanced AMD
Table 4 displays the multivariate analysis of the effects of demographic, behavioral, ocular, and genetic factors on age of progression to advanced AMD, GA, and NV. The average age among progressors is shown for a reference group with none of the risk factors: female, nonwhite, higher education, never smoker, normal BMI, baseline severity scale 1, and none of the genetic risk variants.
Table 4.
Multivariate Analysis of Associations Between Demographic, Behavioral, Ocular, and Genetic Factors and Age of Progression to Advanced AMD, GA, and NV Among Eyes that Progressed
| Advanced AMD (948 Eyes) | GA (487 Eyes) | NV (495 Eyes) | ||||
|---|---|---|---|---|---|---|
| Variables | ESTIMATES (±SE)* | P Value | ESTIMATES (±SE)* | P Value | ESTIMATES (±SE)* | P Value |
| Average age† | 81.12 (±2.44) | <0.001 | 80.25 (±6.28) | <0.001 | 79.25 (±2.75) | <0.001 |
| Demographic | ||||||
| Male | 0.77 (±0.44) | 0.084 | 0.11 (±0.64) | 0.86 | 0.87 (±0.59) | 0.14 |
| White | 0.04 (±2.14) | 0.98 | −1.66 (±5.81) | 0.77 | 0.16 (±2.37) | 0.95 |
| >High school | −0.36 (±0.42) | 0.40 | −0.36 (±0.62) | 0.56 | −0.28 (±0.57) | 0.62 |
| Behavioral | ||||||
| Current smoker | −3.90 (±0.74) | <0.001 | −3.49 (±1.17) | 0.003 | −3.24 (±0.97) | <0.001 |
| Past smoker | −0.74 (±0.46) | 0.10 | −0.38 (±0.64) | 0.56 | −0.37 (±0.61) | 0.55 |
| BMI 25–29 | −0.92 (±0.5) | 0.069 | −1.11 (±0.7) | 0.12 | −0.15 (±0.68) | 0.83 |
| BMI ≥30 | −1.66 (±0.55) | 0.003 | −2.06 (±0.79) | 0.009 | −1.17 (±0.74) | 0.11 |
| Baseline Severity Scale | ||||||
| 1 | REF | REF | REF | |||
| 2 | −0.87 (±1.42) | 0.54 | 0.67 (±2.57) | 0.80 | −1.88 (±1.84) | 0.31 |
| 3 | −0.75 (±1.39) | 0.59 | −1.66 (±2.2) | 0.45 | 0.26 (±1.75) | 0.88 |
| 4 | −0.67 (±1.15) | 0.56 | −1.45 (±2.48) | 0.56 | 0.11 (±1.42) | 0.94 |
| 5 | −0.61 (±1.16) | 0.60 | −1.44 (±2.39) | 0.55 | 0.82 (±1.41) | 0.56 |
| 6 | −0.40 (±1.13) | 0.73 | −1.31 (±2.38) | 0.58 | 0.38 (±1.35) | 0.78 |
| 7 | −2.01 (±1.12) | 0.076 | −2.98 (±2.37) | 0.21 | −0.90 (±1.35) | 0.51 |
| 8 | −3.17 (±1.16) | 0.007 | −4.36 (±2.39) | 0.073 | −0.37 (±1.45) | 0.80 |
| P_trend | <0.001 | <0.001 | 0.55 | |||
| Genetic variants | ||||||
| Complement pathway | ||||||
| CFH R1210C: rs121913059 | −4.33 (±1.94) | 0.026 | −5.40 (±2.52) | 0.033 | ||
| C3 K155Q: rs147859257 | −2.15 (±0.97) | 0.027 | ||||
| Immune/inflammatory pathway | ||||||
| ARMS2/HTRA1: rs10490924 (per risk allele) | −0.79 (±0.29) | 0.006 | −0.93 (±0.39) | 0.018 | ||
β estimates for age of progression among eyes which progressed in a multivariate model where all nongenetic variables were included in a starting model and stepwise selection was used to identify additional significant genetic variables. All analyses were based on the eye as the unit of analysis using PROC MIXED of SAS.
The average age among progressors for a reference group with none of the risk factors: sex (female), race (non-white), education (higher than high school), smoking (never smoker), BMI (<25), AMD baseline severity scale (scale 1), AREDS treatment (placebo), and none of the risk variants.
Subjects who were current smokers (P < 0.001) or had higher BMI ≥30 (P = 0.003) had an earlier age at progression to advanced AMD relative to the average age (3.9 years and 1.7 years, respectively), compared with the reference categories of never smoking or having a BMI < 25. Similarly for GA, current smoking and higher BMI were associated with earlier age of progression (3.5 years and 2 years, respectively). Smoking was associated with 3.24 years earlier progression to NV.
Baseline severity scale 8 compared to scale 1 was associated with earlier age of progression to AMD (average of 3.2 years earlier age of progression; P = 0.007). There was a similar trend for GA (average of 4.4 years earlier, P = 0.073). A significant trend for earlier age of progression for increasing severity scale was seen for both advanced AMD and GA.
Three variants were found to be associated with age of progression to advanced AMD: CFH R1210C: rs121913059 with an average of 4.3 years earlier age at progression among carriers of this mutation compared with non-carriers (P = 0.026), C3 K155Q: rs147859257 with an average of 2.15 years earlier age at progression for carriers (P = 0.027), and ARMS2/HTRA1 A69S rs10490924 with an average of 0.79 years earlier age of progression per risk allele (P = 0.006). For carriers of the homozygous genotype for ARMS2/HTRA1, with two risk alleles, the impact would be an average of 1.58 (2 × 0.79) years earlier age of progression. For GA, only CFH R1210C was associated with earlier age of progression (average of 5.4 years earlier among carriers, P = 0.033). For NV, only ARMS2 was found to be associated with earlier age of progression (average of 0.93 years per risk allele, P = 0.018).
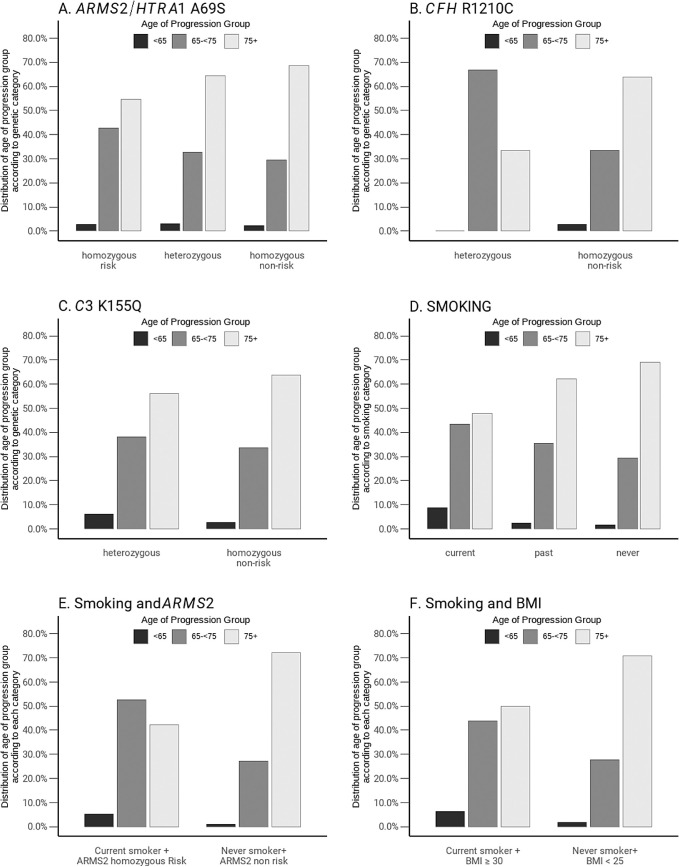
Figure 4 shows the distribution of age of progression to advanced AMD among progressors according to various genetic and non-genetic subgroups. Figures 4A to 4C display the mean age of progression shifted toward younger ages for the common ARMS2/HTRA1 risk allele and the rare CFH and C3 variants. In the homozygous ARMS2/HTRA1 risk group, approximately 45% of eyes progressed at age <75, compared with 30% for the homozygous nonrisk group. In addition, for the rare variant CFH R1210C, approximately 65% of carriers vs 30% of non-carriers had age of progression <75. A similar but weaker trend was seen for carriers vs non-carriers of the C3 K155Q variant. Approximately 50% of current smokers vs 30% of never smokers had an age of progression <75. Similar trends were seen when considering combinations of risk factors (see Figures 4E, 4F). Approximately 50% to 55% of subjects who were current smokers and also homozygous ARMS2 carriers had age of progression <75, compared with 30% of never smokers and ARMS2 nonrisk genotype. Combinations of current smoking and high BMI showed a similar trend of earlier age of progression. Supplementary Figure S3 shows the box plots of the median and distribution of age of progression among subjects grouped by smoking status and BMI status, with the category of current smokers plus highest BMI showing the earliest age of progression.
Figure 4.
Distribution of age of progression to advanced age-related macular degeneration among progressors according to (A) ARMS2/HTRA1, (B) CFH R1210C, (C) C3 K155Q, (D) smoking status, (E) smoking status and ARMS2/HTRA1 alleles, and (F) smoking status and BMI.
Table 5 displays the association between GRSage and age of progression to advanced AMD incorporating three genes related to this outcome. Age of progression was reduced by approximately 1.9 years among subjects with two or more risk alleles; furthermore, age of progression was reduced by 2.5 years per one unit increase in GRS.
Table 5.
Association Between GRS Incorporating CFH R1210C, C3 K155Q, and ARMS2, and Age of Progression to Overall Advanced AMD
| Group | N* | β Estimates (±SE)† | P Value | Range of GRS |
|---|---|---|---|---|
| Having no risk allele | 266 | 0 | REF | 0 |
| Only 1 risk allele in one of the genes | 451 | −0.25 (±0.49) | 0.61 | >0 and <0.755 |
| Having 2 or more risk alleles for any of the 3 genes; or, 1 risk allele for 2 or 3 genes | 244 | −1.88 (±0.56) | <0.001 | 0.755 and above |
| GRS‡ | −2.45 (±0.65) | <0.001 | 0.00–1.44 |
Number of eyes with known genetic variants for GRSage.
β estimates for age of progression among eyes that progressed. One unit of the β estimate refers to one year of age. The average age among progressors for a reference group with none of the risk factors was 80.9 years (±1.3): female, non-white, higher education, never smoker, normal BMI, baseline severity scale 1, and having no risk alleles.
The GRSage was derived based on the genes associated with age of progression to overall AMD shown in Table 4. The effect of GRS was adjusted for sex, race, education, smoking, BMI, AMD baseline eye-specific severity scale, and AREDS treatment. For example, there is an estimated 2.45 years earlier age of progression per unit increase in GRS.
Tables 6A and 6B show population-attributable risks for modifiable behavioral and genetic factors. For never and past smokers, the percentage of progression to advanced AMD prevented by optimizing their BMI to <25 was 11.6% and 12.5%, respectively. Conversely, for current smokers, optimizing their behavior (i.e., changing to past smoker and BMI to <25) would prevent approximately 60% of progression to advanced AMD. Over for the entire study population, 14.7% of disease could be prevented by optimizing modifiable behaviors. Similarly, approximately 37% of disease progression would be prevented if GRS profile changed from second and third tertile to the first tertile; however, this is not modifiable.
Table 6A.
Population Attributable Fraction for Behavioral Factors
| BMI and Smoking | Smoking Status | % of Eyes | BMI Status | Smoking Status of REF Group | BMI Status of REF Group | HR | Percent of Disease Progression Prevented* |
|---|---|---|---|---|---|---|---|
| Never | 47.9 | <25 | Never | <25 | 1.0 | 11.6 | |
| 25-29 | Never | <25 | 1.17 | ||||
| ≥30 | Never | <25 | 1.33 | ||||
| Past | 46.5 | <25 | Past | <25 | 1.0 | 12.5 | |
| 25-29 | Past | <25 | 1.17 | ||||
| ≥30 | Past | <25 | 1.33 | ||||
| Current† | 5.6 | <25 | Past | <25 | 2.27 | 60.6 | |
| 25-29 | Past | <25 | 2.65 | ||||
| ≥30 | Past | <25 | 3.01 | ||||
| Total | 14.7 |
Percent of disease progression prevented—computed from percent of eyes within the group, and the HR of changing from the specific exposure within the group to the optimal exposure in the reference (REF) group.
Considers the change from current to past smoker, and BMI ≥25 to <25.
Table 6B.
Population Attributable Fraction for GRS Factors*
| Tertile | % of Eyes | HR | Percent Prevented† |
| 1 | 33.3 | 1.0 | 37.3 |
| 2 | 33.4 | 1.87 | |
| 3 | 33.3 | 2.90 |
GRS was a composite of 8 genetic variants associated with the progression to advanced age-related macular degeneration, going from tertile 2 or 3, to 1.
Percent of disease progression prevented—computed from percent of eyes within the group.
Discussion
Main Findings
We determined that behavioral factors modify risk of progression to advanced AMD, GA, and NV, and genetic variation at multiple AMD risk alleles is associated with increased rate of progression after adjusting for baseline severity scale and all covariates. In other words, for each level of baseline severity scale, especially for the higher risk scales 6–8, time to conversion to late AMD decreased with increasing genetic risk and with unhealthy behaviors.
Age when progression to advanced AMD occurred was also affected by genes and environment. Smoking and higher BMI were associated with earlier age of progression and thus more years of disease and treatment burden. Genetic susceptibility also played a role, and higher genetic burden due to carrying high risk rare variants in CFH or C3 in the complement pathway, or the common risk allele in the ARMS2/HTRA1 gene, were associated with earlier age of transitioning from non-advanced to advanced AMD. Smoking together with a higher BMI were associated with 5.6 years earlier age of progression to advanced disease, and genetic burden determined by a common variant, e.g. ARMS2/HTRA1, was associated with lower age of progression by 1.6 years for homozygous carriers (combined total of up to 7.2 years). If an additional rare variant, such as CFH R1210C, was present, then disease onset would be shortened by an additional 4.3 years, or as much as 11.5 years in total earlier onset of advanced disease and longer disease burden, adjusting for all other covariates.
The greatest impact of genetic factors on enhancing prognostic ability to predict who will progress to advanced AMD, especially for NV, was seen among eyes with baseline scales 6–8, which represent increasing levels of intermediate disease with larger drusen. This is important because these are the eyes at highest risk for developing advanced stages causing visual loss. It appears therefore that there are three distinct subgroups: eyes with severity scale 1–5 with low risk regardless of their genetic burden, eyes with intermediate risk, which have severity scales 6–8 but a low genetic burden, and another group of eyes with high risk, which have severity scales of 6 to 8 and a high genetic burden. For eyes with severity scales 6 to 8 and high genetic burden (highest tertile of GRS), about 35% will progress to advanced AMD over five years, whereas about 10% of eyes with baseline scales 6 to 8 with low genetic risk (lowest tertile of GRS) and <5% of eyes with baseline scales of 1 to 5 developed advanced AMD over 5 years.
Proposed Mechanisms
The behavioral lifestyle factors found to play a role in disease progression and earlier age of being affected with advanced stages are associated with biologic mechanisms that could heighten risk. Cigarette smoking is associated with increased oxidative stress, lipid peroxidation, platelet aggregation and increased fibrinogen levels.19 Nicotine has been reported to increase size and severity of experimental choroidal neovascularization and may increase VEGF levels.20 Smoking, abdominal and overall obesity are associated with higher levels of inflammatory cytokines, including high sensitivity C-reactive protein.21–23
The complement pathway is a major player in the pathogenesis of AMD, and the rare CFH R1210C variant has the strongest effect on AMD among those who carry this variant compared to common and other variants.9 The rare C3 variant also confers higher impact than the common variants.17 These rare variants in the complement pathway cause dysregulation and overactivation of the complement pathway. The ARMS2/HTRA1 variant has a lower effect size but is much more common in the population, and therefore also has an important impact.24,25 Proposed mechanisms involve inflammation and complement activation, mitochondria, effects on photoreceptors, and VEGF expression.26
Comparison with Literature
Previous studies explored behavioral and genetic factors related to progression of disease using different methodologies than what we used in these analyses.1,7,8,10,27 The literature regarding age of progression is more sparse. A European cross-sectional study evaluated only the neovascular form of AMD in a cohort of 275 subjects in Europe, among which 214 had complete information. They found that smoking history, CFH Y402H and the A69S variant in ARMS2 were associated with the earlier age of diagnosis of neovascular AMD, based on a retrospective review of medical records.28 We have previously shown that carriers of the rare variant CFH R1210C tend to have younger age of onset of AMD in a case-control study9 and have higher risk of progression.8
The study reported herein differs in several ways. Most notably, this was a longitudinal analysis of age of transition to advanced AMD as well as GA and NV in a large cohort, and both rare and common genetic variants, as well as behavioral factors were considered in the analyses. We adjusted the statistical model to account for correlation between fellow eyes and all covariates in the same model, to determine which variables were independently related. As noted earlier, we also adjusted fully for age and required confirmed endpoints at two consecutive visits based on a detailed severity scale to reduce misclassification.
Strengths and Limitations
Strengths of the study include the new analyses summarized above, the standardized data collection, and longitudinal follow-up with exams and imaging at regular intervals. Grades were assigned without knowledge of genetic and nongenetic predictive factors. Limitations include the use of baseline data as predictors of subsequent progression, although many studies have reliably assessed baseline data and associations with subsequent outcomes. Furthermore, predictive models rely on baseline data to predict risk of disease in later years in an elderly population. Fundus photography was used to determine the severity scale and time of progression to advanced AMD, and use of optical coherence tomography or machine learning methods could improve precision of these outcomes.29
Conclusions
In summary, in this first longitudinal analysis of the impact of both genetic and non-genetic factors on age of progression to GA and NV, results underscore the impact of nature and nurture on both developing advanced disease leading to visual loss, and also increasing the likelihood of having this adverse outcome at an earlier age. A combined risk of smoking, higher BMI and genetic factors could lower age of developing advanced stages of AMD by 7 to 11.5 years, leading to a longer burden of disease and more treatments. Thus, adhering to healthy habits and changing unhealthy habits could reduce societal and economic costs.
Knowledge of factors underlying age of transition to advanced AMD can impact clinical care by underscoring the importance of adhering to healthy habits and provides biologic mechanisms to explore to shorten disease burden. Accounting for individual differences in lifestyles, as well as genetic susceptibility, may lead to avenues for personalized medicine. We will ultimately be able to target different disease subgroups in clinical trials and offer more precise therapeutic approaches.
Supplementary Material
Acknowledgments
Supported by NIH R01-EY011309, R01-EY028602, R01-EY022445, American Macular Degeneration Foundation, Northampton, MA, The Macular Degeneration Center of Excellence, University of Massachusetts Medical School, Department of Ophthalmology and Visual Sciences, Worcester, MA.
Disclosure: J.M. Seddon, Scientific Co-Founder, Gemini Therapeutics, Inc.; R. Widjajahakim, None; B. Rosner, None
References
- 1. Seddon JM. Macular degeneration epidemiology: nature-nurture, lifestyle factors, genetic risk, and gene-environment interactions – The Weisenfeld Award Lecture. Invest Ophthalmol Vis Sci. 2017; 58(14): 6513–6528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wong WL, Su X, Li X, et al.. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: a systematic review and meta-analysis. The Lancet Global Health. 2014; 2(2): e106–e116. [DOI] [PubMed] [Google Scholar]
- 3. Brown GC, Brown MM, Sharma S, et al.. The burden of age-related macular degeneration: A value-based medicine analysis. Trans Am Ophthalmol Soc. 2005; 103: 173–186. [PMC free article] [PubMed] [Google Scholar]
- 4. World Health Organization, https://www.who.int/health-topics/blindness-and-vision-loss#tab=tab_1. Accessed August 17, 2020.
- 5. Age-Related Macular Degeneration: Facts & Figures. BrightFocus Foundation, https://www.brightfocus.org/sources-macular-degeneration-facts-figures. Accessed August 17, 2020.
- 6. Mangione CM, Gutierrez PR, Lowe G, Orav EJ, Seddon JM.. Influence of age-related maculopathy on visual functioning and health-related quality of life. Am J Ophthalmol. 1999; 128(1): 45–53. [DOI] [PubMed] [Google Scholar]
- 7. Seddon JM, Rosner B.. Validated prediction models for macular degeneration progression and predictors of visual acuity loss identify high-risk individuals. Am J Ophthalmol. 2019; 198: 223–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Seddon JM, Reynolds R, Yu Y, Rosner B.. Three new genetic loci (R1210C in CFH, variants in COL8A1 and RAD51B) are independently related to progression to advanced macular degeneration. PLoS ONE. 2014; 9(1): e87047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Raychaudhuri S, Iartchouk O, Chin K, et al.. A rare penetrant mutation in CFH confers high risk of age-related macular degeneration. Nat Genet. 2011; 43(12): 1232–1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Seddon JM, Reynolds R, Maller J, Fagerness JA, Daly MJ, Rosner B.. Prediction model for prevalence and incidence of advanced age-related macular degeneration based on genetic, demographic, and environmental variables. Invest Ophthalmol Vis Sci. 2009; 50(5): 2044–2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Widjajahakim R, Rosner B, Seddon JM.. Rare and common genetic variants and behavioral modifiable factors are associated with earlier age of progression to advanced AMD. Invest Ophthalmol Vis Sci. 2020; 61(7):ARVO E-Abstract 2436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Seddon J, Widjajahakim R, Rosner B.. Rare and common genetic variants, smoking and higher body mass index are associated with earlier age of progression to geographic atrophy and neovascular advanced stages of macular degeneration in a prospective analysis. medRxiv August 2020:2020.08.13.20174383.
- 13. Age-Related Eye Disease Study Research Group. A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E, beta carotene, and zinc for age-related macular degeneration and vision loss: AREDS report no. 8. Arch Ophthalmol. 2001; 119(10): 1417–1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Davis MD, Gangnon RE, Lee L-Y, et al.. The Age-Related Eye Disease Study severity scale for age-related macular degeneration: AREDS Report No. 17. Arch Ophthalmol. 2005; 123(11): 1484–1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Maller JB, Fagerness JA, Reynolds RC, Neale BM, Daly MJ, Seddon JM.. Variation in complement factor 3 is associated with risk of age-related macular degeneration. Nat Genet. 2007; 39(10): 1200–1201. [DOI] [PubMed] [Google Scholar]
- 16. Yu Y, Wagner EK, Souied EH, et al.. Protective coding variants in CFH and PELI3 and a variant near CTRB1 are associated with age-related macular degeneration†. Hum Mol Genet. 2016; 25(23): 5276–5285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Seddon JM, Yu Y, Miller EC, et al.. Rare variants in CFI, C3 and C9 are associated with high risk of advanced age-related macular degeneration. Nat Genet. 2013; 45(11): 1366–1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rosner B, Glynn RJ.. Power and sample size estimation for the Wilcoxon rank sum test with application to comparisons of C statistics from alternative prediction models. Biometrics. 2009; 65(1): 188–197. [DOI] [PubMed] [Google Scholar]
- 19. Seddon JM, Willett WC, Speizer FE, Hankinson SE.. A prospective study of cigarette smoking and age-related macular degeneration in women. JAMA. 1996; 276(14): 1141–1146. [PubMed] [Google Scholar]
- 20. Suñer IJ, Espinosa-Heidmann DG, Marin-Castano ME, Hernandez EP, Pereira-Simon S, Cousins SW.. Nicotine increases size and severity of experimental choroidal neovascularization. Invest Ophthalmol Vis Sci. 2004; 45(1): 311–317. [DOI] [PubMed] [Google Scholar]
- 21. Seddon JM, Cote J, Davis N, Rosner B.. Progression of age-related macular degeneration: association with body mass index, waist circumference, and waist-hip ratio. Arch Ophthalmol. 2003; 121(6): 785–792. [DOI] [PubMed] [Google Scholar]
- 22. Seddon JM, Gensler G, Milton RC, Klein ML, Rifai N.. Association between C-reactive protein and age-related macular degeneration. JAMA. 2004; 291(6): 704–710. [DOI] [PubMed] [Google Scholar]
- 23. Seddon JM, Gensler G, Klein ML, Milton RC.. C-reactive protein and homocysteine are associated with dietary and behavioral risk factors for age-related macular degeneration. Nutrition. 2006; 22(4): 441–443. [DOI] [PubMed] [Google Scholar]
- 24. Rivera A, Fisher SA, Fritsche LG, et al.. Hypothetical LOC387715 is a second major susceptibility gene for age-related macular degeneration, contributing independently of complement factor H to disease risk. Hum Mol Genet. 2005; 14(21): 3227–3236. [DOI] [PubMed] [Google Scholar]
- 25. Dewan A, Liu M, Hartman S, et al.. HTRA1 promoter polymorphism in wet age-related macular degeneration. Science. 2006; 314(5801): 989–992. [DOI] [PubMed] [Google Scholar]
- 26. Lu Z, Lin V, May A, et al.. HTRA1 synergizes with oxidized phospholipids in promoting inflammation and macrophage infiltration essential for ocular VEGF expression. PLoS ONE. 2019; 14(5): e0216808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Saunier V, Merle BMJ, Delyfer M-N, et al.. Incidence of and risk factors associated with age-related macular degeneration: four-year follow-up from the ALIENOR Study. JAMA Ophthalmol. 2018; 136(5): 473–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lechanteur YTE, van de Camp PL, Smailhodzic D, et al.. Association of smoking and CFH and ARMS2 risk variants with younger age at onset of neovascular age-related macular degeneration. JAMA Ophthalmol. 2015; 133(5): 533–541. [DOI] [PubMed] [Google Scholar]
- 29. Ferrara D, Silver RE, Louzada RN, Novais EA, Collins GK, Seddon JM.. Optical coherence tomography features preceding the onset of advanced age-related macular degeneration. Invest Ophthalmol Vis Sci. 2017; 58(9): 3519–3529. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.