Abstract
Doxorubicin (DOX) is commonly used in chemotherapy and biomedical research because of its potent anticancer activity. Although DOX is water soluble, it precipitates when interacting with buffers, such as phosphate-buffered saline, or with drugs such as 5-fluorouracil (5-FU) and heparin. This study reports that DOX precipitates in neutral buffers and 5-FU solution because of the formation of covalently bonded DOX dimers. Additionally, this study proposes a structure for the DOX dimer and a mechanism for dimerization on the basis of mass spectrometry in combination with an experiment to establish the reaction model. The DOX dimer/precipitate formation might be an important phenomenon, considering the frequent use of DOX in chemotherapy and biomedical research.
Introduction
Doxorubicin (DOX) is an anthracycline anticancer drug that is commonly used in chemotherapy and biomedical research. Because of its potent activity against a broad spectrum of cancers, it is used as a standard chemotherapeutic agent.1 DOX has multiple mechanisms of action, including DNA intercalation, inhibition of topoisomerase II, generation of reactive oxygen species (ROS), and direct membrane effects;2−4 however, its use can result in serious adverse side effects including irreversible cardiotoxicity.5,6 Consequently, many studies have focused on the development of DOX delivery carriers to improve their efficacy and safety.7,8 The number of studies focusing on DOX delivery has considerably increased over the past decade (Figure S1). Therefore, understanding the chemical, physical, and biological characteristics of DOX is extremely important for advancing its biomedical application and expanding its clinical research scope.
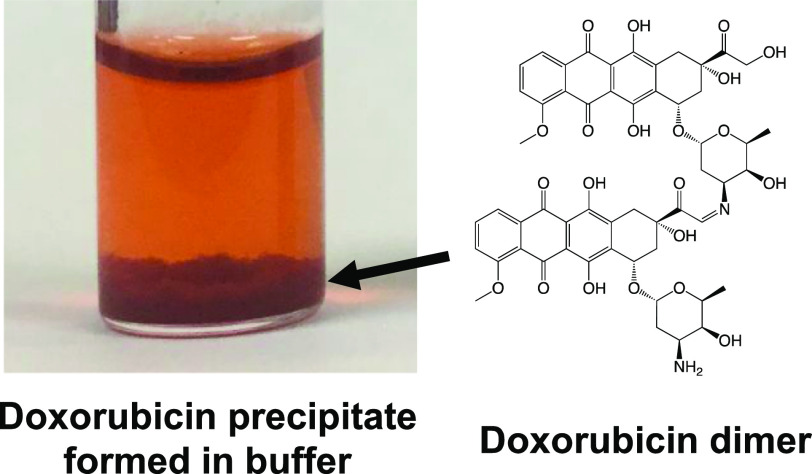
DOX is an amphiphilic compound consisting of a hydrophobic anthraquinone ring and a hydrophilic amino sugar moiety (Figure 1A). DOX is soluble in water and yields a clear orange-red solution. However, DOX is known to form a red precipitate when mixed with buffers, such as phosphate-buffered saline (PBS), or with certain drugs such as fluorouracil (5-FU) and heparin.9,10 Considering the high solubility of DOX at neutral pH (∼20 mg/mL),11 it is unlikely that its precipitation results from its insolubility in a neutral buffer. Furthermore, daunorubicin (DNR), the 14-dehydroxylated version of DOX (Figure 1A), which is more hydrophobic than DOX, has not been reported to precipitate out of solutions with neutral buffers.12 Several studies have demonstrated that an aqueous solution of DOX undergoes self-association by π–π stacking in the presence of NaCl.11,13,14 However, DNR undergoes self-association in solution as well;13 hence, the precipitation of DOX cannot be explained on the basis of the self-association of its molecules. Therefore, it is hypothesized that the precipitation of DOX in buffers occurs through alternative mechanisms.
Figure 1.
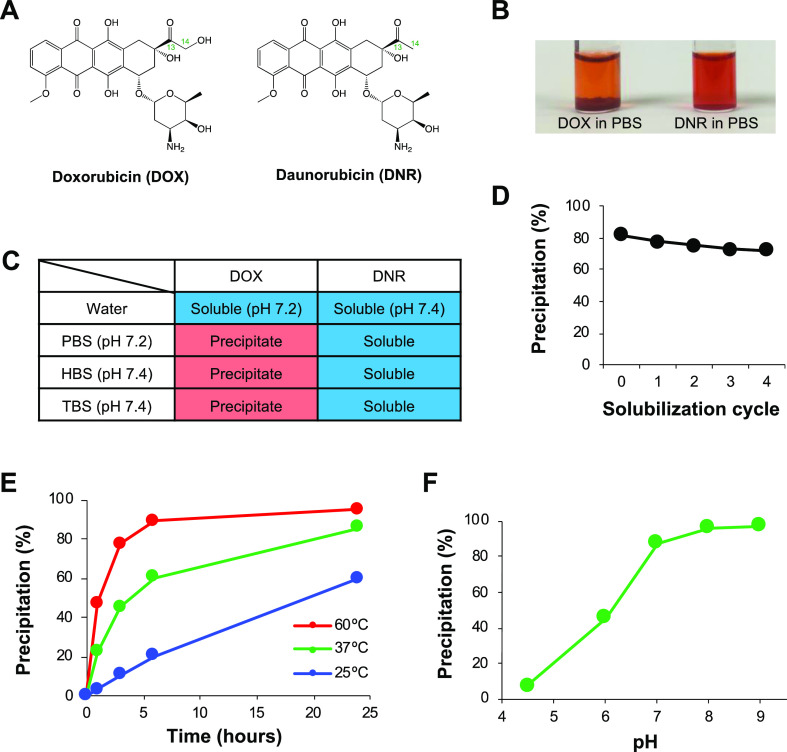
Precipitation of DOX in buffers. DOX and DNR in buffer solutions (1 mg/mL) were incubated for 24 h at 37 °C. (A) Structure of DOX and DNR. (B) Photographs of DOX and DNR in PBS after incubation. (C) The presence or absence of precipitation of DOX and DNR in PBS (10 mM phosphate, 150 mM NaCl, pH 7.2), HBS (25 mM HEPES, 150 mM NaCl, pH 7.4), and TBS (50 mM Tris-HCl, 150 mM NaCl, pH 7.4) after incubation. (D) % precipitation of DOX in PBS after incubation, followed by four solubilization cycles with water. (E) Kinetics of DOX precipitation at different temperatures (25, 37, and 60 °C). (F) DOX precipitation in the 100 mM phosphate buffer with different pH (4.5, 6, 7, 8, and 9).
This study investigates the mechanism of the formation of the DOX precipitate in buffers and 5-FU. These findings differ from the results of previous studies and will considerably impact DOX usage in biomedical research and in clinical practice.
Results and Discussion
DOX Precipitate Formation in Neutral Buffers
DOX and DNR were dissolved in water, yielding transparent orange-red solutions. Upon the addition of PBS, the solutions remained transparent, with no precipitation occurring immediately. However, DOX dissolved in PBS gradually formed a red precipitate upon incubation at 37 °C (Figure 1B). In contrast, DNR did not undergo precipitation, although it is more hydrophobic than DOX.12 The precipitation of DOX occurred in HBS (HEPES-buffered saline) and TBS (Tris-buffered saline) as well (Figure 1C), indicating that phosphate itself was not the direct cause of the precipitation. When incubated in PBS at 37 °C for 24 h, more than 80% of DOX underwent precipitation (Figure 1D). The DOX precipitate was separated by centrifugation and resuspended in water; this solubilization cycle was repeated four times. However, ∼90% of the DOX that was initially precipitated remained as a precipitate following the solubilization cycles, indicating that the precipitation was not caused by the saturation of DOX solubility.
Finally, the temperature and pH dependences of DOX precipitation were examined. Monitoring the progress of the precipitation in PBS at different temperatures (25, 37, and 60 °C) revealed that DOX precipitated more rapidly at higher temperatures (Figure 1E). When DOX was dissolved in phosphate buffers of different pH (4.5, 6, 7, 8, and 9), the solutions initially appeared clear and red. However, in alkaline solutions, there was a gradual increase in the amount of precipitate formed (Figure 1F). These data suggested that the formation of the DOX precipitate was temperature- and pH-dependent.
Characterization of the DOX Precipitate through Mass Spectrometry
The composition of the DOX precipitate was analyzed through flow injection analysis–mass spectroscopy (FIA–MS). The electrospray ionization (ESI) mass spectrum of intact DOX helped to identify ions at m/z 544 as [M + H]+, m/z 1087 as [2M + H]+, and m/z 397 and m/z 321 as fragments produced by the loss of sugar and carbonyl moieties, respectively, as reported previously (Figure 2A).15 The DOX precipitate was dissolved in an aqueous solution containing 50% acetonitrile and 0.1% formic acid and analyzed through FIA–MS (Figure 2B). The mass spectrum of the DOX precipitate exhibited peaks derived from intact DOX (shown in red in Figure 2B) as well as new peaks that were most likely derived from new products (shown in blue in Figure 2B). It is hypothesized that the ion that was detected at m/z 1067 represents [M + H]+ of a covalent dimer of DOX, while the peaks at m/z 2133 and m/z 938 represent [2M + H]+ and a fragment produced by the loss of a sugar moiety of the dimer, respectively. One of the reactions that may occur between two DOX molecules is imine formation by dehydration between the carbonyl and amino groups. However, the imine form of two DOX molecules should have been detected as an ion at m/z 1069 as [M + H]+. Therefore, it is hypothesized that the dimerization of DOX involves the loss of two hydrogen ions in addition to imine formation; therefore, the resulting DOX dimer was two mass units lighter than the imine formed by two DOX molecules.
Figure 2.
Characterization of the DOX precipitate by mass spectrometry. The DOX precipitate formed in PBS was dissolved using 0.1% formic acid in 50% acetonitrile and analyzed through FIA–MS (B) and LC/MS (D). (A, B) Mass spectra of (A) DOX and (B) the total DOX precipitate. Chromatograms of (C) DOX, (D) the DOX precipitate, and (E) the DOX dimer isolated from the blue-highlighted peak in (D), monitoring the absorbance at 480 nm.
The composition of the precipitate was further analyzed through liquid chromatography–mass spectrometry (LC/MS). A sharp peak attributed to intact DOX appeared at 22.2 min (Figure 2C). In contrast, the chromatogram of the precipitate exhibited peaks that were attributed to a derivative of the DOX monomer (the leading peak at 22.1 min, m/z 560), intact DOX (the sharp peak at 22.2 min), and the DOX dimer (the broad peak at 27 min) (Figures 2D and S2). When the dimer (peak highlighted in blue in Figure 2D) was isolated using preparative high-performance liquid chromatography (HPLC) and reinjected for LC/MS, the chromatogram retained the peaks attributed to the DOX monomer derivative and intact DOX in addition to the peak attributed to the dimer, indicating that the dimer can be partially hydrolyzed into intact DOX and the DOX monomer derivative (Figure 2E). These results suggested that the main component of the DOX precipitate was the covalent DOX dimer.
Model Reaction Using DOX and Hydroxylamine
Nuclear magnetic resonance (NMR) spectroscopy was performed to determine the structure of the DOX dimer. However, the NMR spectra were unclear, and the structure of the dimer could not be ascertained from the data. Therefore, DOX was reacted with hydroxylamine in an experiment designed to determine the reaction mechanism. The mixture of DOX and hydroxylamine in PBS was incubated at 37 °C for 24 h, and the supernatant was analyzed through LC/MS (Figures 3A and S3). The chromatogram exhibited three major peaks. The first peak, appearing at 21.2 min, was generated by an ion at m/z 559, which is inferred to be the oxime of DOX. The second peak, appearing at 21.8 min, is attributed to intact DOX. The third peak appeared at 23.2 min and was generated by an ion at m/z 557, which is two mass units lighter than the oxime form of DOX, indicating that DOX/hydroxylamine in PBS underwent a reaction similar to the dimerization of DOX. Finally, the eluent that generated the third peak was isolated through HPLC and reinjected for HPLC, revealing that this product was stable in the aqueous solution (Figure 3B). The NMR spectra of the product isolated from the third peak (Figure S4) revealed that the product had an oxime moiety at C14 instead of C13 and had a ketone moiety at C13, as shown in Figure 3C.
Figure 3.
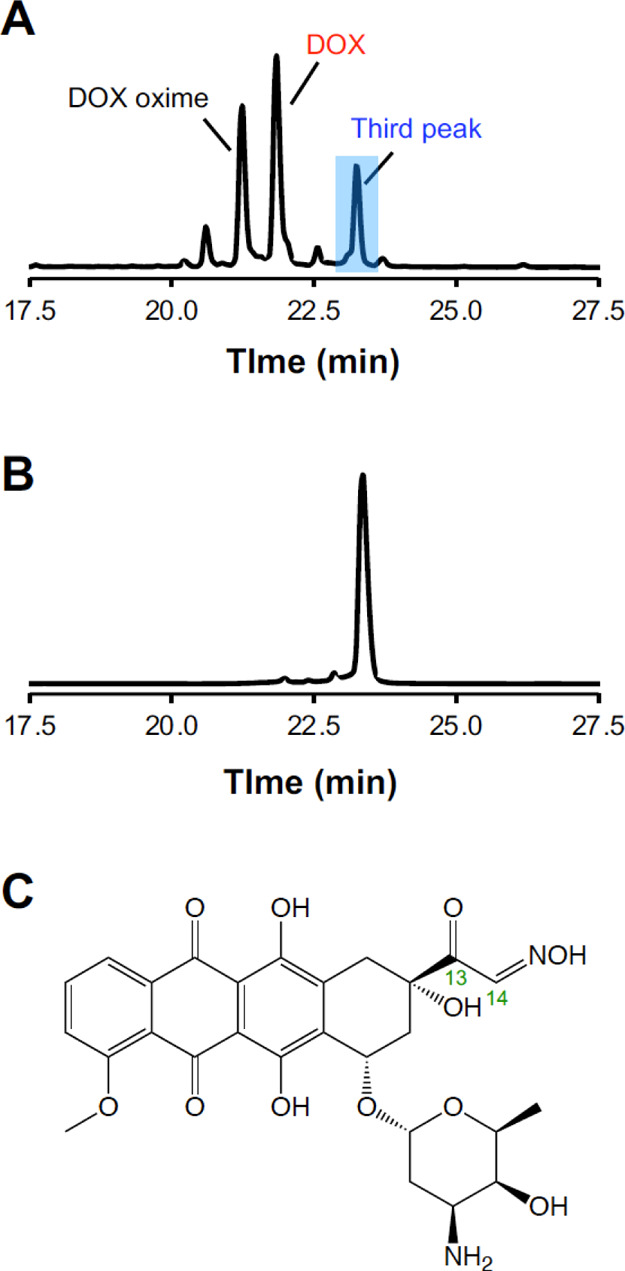
Reaction modeling using DOX and hydroxylamine. DOX was incubated in PBS containing a molar equivalent of hydroxylamine for 24 h at 37 °C. Chromatograms of (A) DOX/hydroxylamine in PBS and (B) the product isolated from the blue-highlighted third peak in (A), monitoring the absorbance at 480 nm. (C) Structure of the product isolated from the third peak determined via NMR spectroscopy (NMR spectra are shown in Figure S4).
Proposed Mechanism of Dimerization of DOX
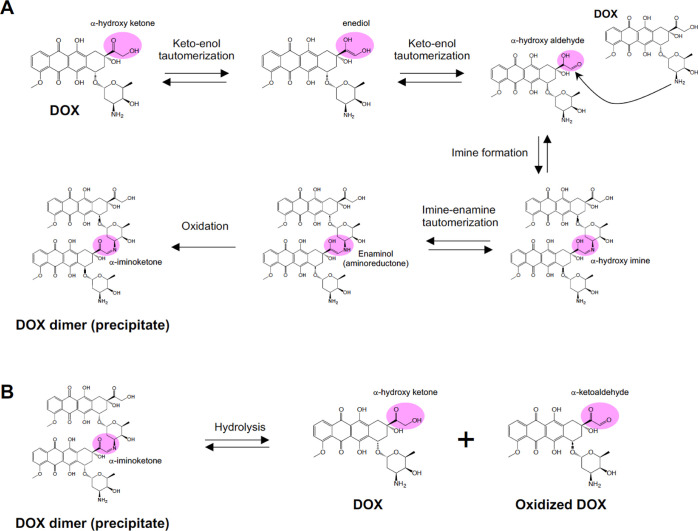
Figure 4 represents the mechanisms of dimerization of DOX and hydrolysis of the dimer, which was deduced from the results of the experiment using DOX + hydroxylamine to model the reaction mechanism. First, the α-hydrogen at the α-hydroxy ketone group (C14) of DOX is deprotonated, undergoes keto–enol tautomerization, and forms an α-hydroxy aldehyde. The α-hydroxy aldehyde reacts with the amine group of another DOX molecule to form an α-hydroxy imine. Subsequent oxidation of the alcohol at C13 forms a DOX dimer having an α-iminoketone structure. The DOX dimer precipitates because of its poor water solubility. These chemical reactions also explain the temperature-dependent nature of precipitate formation (Figure 1E). Following imine formation, the oxidation of the alcohol at C13 is likely to be mediated by the enaminol (aminoreductone) formed by imine–enamine tautomerization. Enaminols are unstable in basic solutions and oxidize to form α-iminoketones.16,17 The increased precipitation of DOX in an alkaline solution (Figure 1F) can be explained by the pH-dependent oxidation of enaminol, the enhancement of the initial keto–enol tautomerization, or the pKa value of the amine of DOX (pKa = 8.2).11Figure S5 represents the nitroblue tetrazolium (NBT) assay, which indicates the presence of reducing substances by forming an insoluble purple formazan. DOX + NBT in PBS formed a purple precipitate, whereas DOX + NBT in water and DNR + NBT in PBS did not. These data support the hypothesis that the solution of DOX in PBS formed an enaminol intermediate during precipitation. The DOX precipitation experiment was performed using PBS in a N2 atmosphere. In a N2 atmosphere, DOX precipitated as readily (57% in 14 h) as it did in air (65% in 14 h), indicating that the oxidation of the DOX dimer was not mainly mediated by air. It is possible that DOX itself acted as an oxidant for the oxidation of its dimer because DOX is known to form a semiquinone by reduction, indicating that it can oxidize reducing substances.18 However, further experiments are required to confirm the mechanism of the oxidation. Theoretically, hydrolysis of the DOX dimer produces intact DOX and an oxidized DOX monomer with an α-ketoaldehyde moiety. In fact, the chromatogram of the DOX precipitate contained a peak attributed to an ion at m/z 560 (Figures 2D and S2B). This ion was most likely derived from the geminal diol form of the oxidized DOX monomer.
Figure 4.
Proposed reaction mechanisms of (A) DOX dimer (precipitate) formation and (B) hydrolysis of the DOX dimer.
Although this study is the first to describe the precipitation of DOX as a result of its dimerization, Yokoyama et al. have previously observed the formation of this DOX dimer as a side product during the preparation of DOX-loaded polymeric micelles.19 The structure of the DOX dimer proposed therein is identical to the one proposed in this work. Fukushima et al. evaluated the role of the DOX dimer in the antitumor activity of the DOX-loaded micelles; however, they did not describe the relationship between the precipitation of DOX and the formation of its dimer.20 Their findings, taken together with the findings of the present study, indicate that the dimerization of DOX needs to be considered when using the drug for material research, regardless of the occurrence of precipitation.
The reaction of the α-hydroxy ketone with an amine can not only occur between two DOX molecules but also between DOX and other molecules containing amine groups. For example, Zunino et al. demonstrated that DOX binds covalently to proteins, although DNR does not.21 They proposed that DOX formed an α-hydroxy aldehyde by keto–enol tautomerization and, subsequently, the aldehyde formed imines by reacting with the amino groups of proteins. Furthermore, they speculated that the imines were stabilized by some conformational and/or hydrophobic effects. The stable binding of DOX to proteins was presumed to be mediated by a mechanism similar to that of the dimerization of DOX. The covalent binding of DOX to serum proteins may influence its pharmacokinetics and pharmacological effects in vivo and in vitro. These data suggest that both the dimerization of DOX and its binding to proteins or other amine-containing molecules via the reactive α-hydroxy ketone need to be considered when using DOX in basic and clinical research.
Characterization of the DOX Precipitate Formed by 5-FU and Heparin
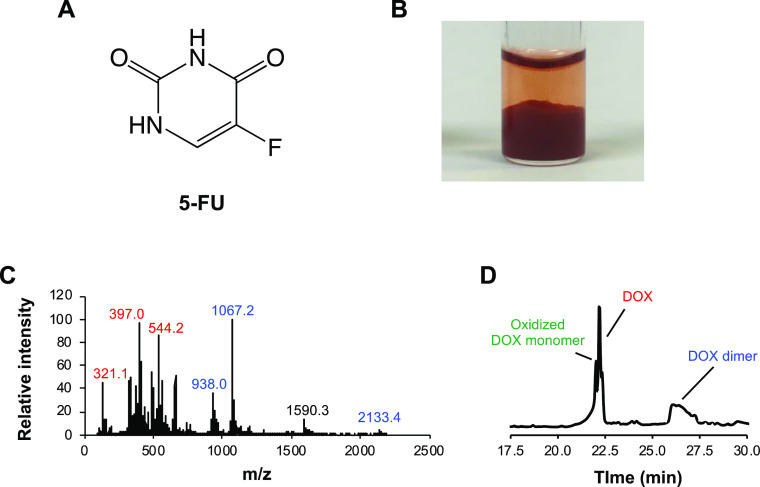
DOX is known to precipitate when mixed with 5-FU.10 Therefore, combination chemotherapy using these two drugs necessitates separate administration to avoid precipitation. This work examined whether the DOX dimer was present in DOX precipitated by 5-FU (Figure 5). DOX + 5-FU dissolved in water (pH = ∼9) formed a red precipitate, similar to that formed by DOX in neutral buffers (Figure 5B). ESI-MS and LC/MS revealed that this precipitate did not contain 5-FU and was identical to the DOX precipitate in the buffer, indicating that 5-FU also induced the dimerization of DOX. It is likely that 5-FU acts as a proton acceptor and initiates dimerization. Heparin has also been reported to induce DOX precipitation;10 however, LC/MS results suggested that the DOX precipitate formed upon the interaction with heparin contained only intact DOX (Figure S6). This precipitate was most likely caused by electrostatic interactions between DOX and heparin. These data indicate that while some drugs can mediate DOX dimerization, not all DOX precipitation is caused by dimerization.
Figure 5.
Precipitation of DOX in the 5-FU solution. (A) Structure of 5-FU. (B) Photograph of the DOX precipitate in the 5-FU solution after incubation for 24 h at 37 °C. The DOX precipitate formed by 5-FU when dissolved with 0.1% formic acid in 50% acetonitrile and analyzed using (C) FIA–MS and (D) LC/MS. (C) Mass spectrum of the total DOX precipitate in 5-FU. (D) Chromatogram of the DOX precipitate in 5-FU, monitoring the absorbance at 480 nm.
Cytotoxicity and Cytolocalization of the DOX Dimer
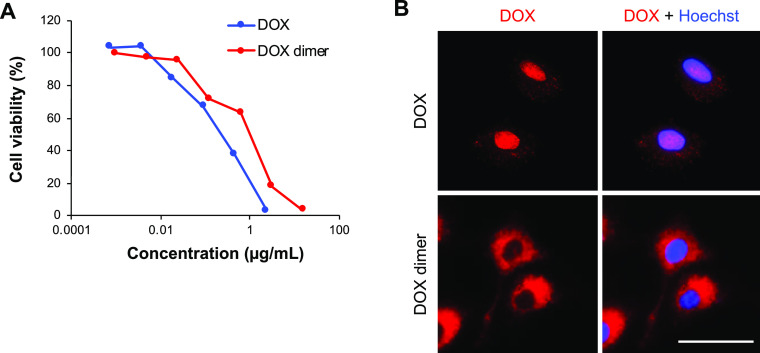
Finally, the cytotoxicity of the DOX dimer (precipitate) was compared with that of intact DOX in A549 cells (Figure 6A). To generate the DOX dimer solution, the DOX precipitate was dissolved in dimethyl sulfoxide (DMSO); in addition to the precipitate, this solution might also have contained intact DOX and oxidized DOX formed by hydrolysis (Figures 2E and 4B). The DOX dimer exhibited a lower cytotoxicity (IC50 = 1.17 μg/mL) than intact DOX (IC50 = 0.25 μg/mL). Additionally, the intercellular localization of the DOX dimer was visualized using fluorescence microscopy (Figure 6B). Intact DOX was observed primarily in the nucleus and cytoplasm. In contrast, the DOX dimer was observed only in the cytoplasm, indicating that dimerization altered the intercellular localization of DOX. DOX cytotoxicity is mediated by DNA intercalation, inhibition of topoisomerase II, and ROS generation; however, DNA intercalation and topoisomerase II inhibition require translocation of DOX to the nucleus. Therefore, the relatively weak cytotoxicity of the DOX dimer may be attributed to the lack of nuclear localization.
Figure 6.
Cytotoxicity and cytolocalization of intact DOX and the DOX dimer. A549 cells were cultured with DOX and the DOX dimer. (A) Cell viability was measured using CCK-8 on day 3. (B) DOX (red) and nuclei stained with Hoechst 33342 (blue) were visualized 1 h after incubation. Scale bar = 50 μm.
Conclusions
This study revealed that the dimerization of DOX is responsible for its precipitation in the neutral buffers and 5-FU solution. The structure of the DOX dimer and the mechanism of its formation were determined using mass spectrometry of the DOX dimer and of all the products formed when DOX reacted with hydroxylamine in PBS. The proposed mechanism of dimerization of DOX includes the formation of an α-hydroxy aldehyde by keto–enol tautomerization of the α-hydroxy ketone, imine formation between two DOX molecules, and oxidation of the alcohol group at C13. This covalent dimer formation occurs under physiological conditions and, therefore, needs consideration in biomedical research using DOX, such as drug delivery studies and DOX-based therapies.
Experimental Section
DOX Precipitate Formation
DOX hydrochloride (AvaChem Scientific, San Antonio, TX, USA) and DNR hydrochloride (AvaChem Scientific) were dissolved in water to yield concentrations of 2 mg/mL. Subsequently, 2× PBS (20 mM phosphate, 300 mM NaCl, pH 7.2), 2× HEPES-buffered saline (2× HBS, 50 mM HEPES, 300 mM NaCl, pH 7.4), 2× Tris-buffered saline (2× TBS, 100 mM Tris-HCl, 300 mM NaCl, pH 7.4), 50 mg/mL 5-FU (Adrucil, Teva Parenteral Medicines, Irvine, CA, USA), or 2 mg/mL heparin (MilliporeSigma, St. Louis, MO, USA) was added to the DOX or DNR solution at a volume ratio of 1:1. Each solution was incubated for 24 h at 37 °C and allowed to form a precipitate. The temperature dependence of DOX precipitation was investigated by incubating 1 mg/mL DOX in PBS for 1, 3, 6, and 24 h at 25, 37, and 60 °C. The pH dependence of DOX precipitation was investigated by incubating 1 mg/mL DOX in 100 mM phosphate at pH 4.5, 6, 7, 8, and 9 for 24 h at 37 °C. The resulting precipitates were centrifuged (10,000g for 3 min), and the percentage of DOX precipitated was calculated on the basis of the absorbance of the supernatants at 480 nm.
Flow Injection Analysis–Mass Spectrometry and Liquid Chromatography–Mass Spectrometry
The DOX precipitate formed in PBS was centrifuged (10,000g for 3 min) and resuspended in fresh water. This washing step was performed three times. The precipitate was then solubilized with 0.1% formic acid in a 50% aqueous solution of acetonitrile. ESI mass spectra of intact DOX and the total DOX precipitate were obtained by FIA–MS by using an Agilent LC/MSD system (Santa Clara, CA, USA). The DOX precipitate was separated and analyzed using a Shimadzu LC/MS system (Kyoto, Japan) equipped with a Vydac C18 column. The absorbance was monitored at 480 nm, and the ESI mass spectra of the components present in the DOX precipitate were acquired. Solvent A (0.1% formic acid in water) and solvent B (0.1% formic acid in acetonitrile) were used, and a linear gradient of 1.5%/min of solvent B was maintained.
Reaction Modeling Using DOX and Hydroxylamine
DOX (1 mg/mL) was incubated for 24 h at 37 °C in PBS containing a molar equivalent of hydroxylamine. The products were separated and analyzed using a Shimadzu LC/MS system. DOX oxime, which was detected at ∼23 min, was purified using an Agilent 1200 series analytical HPLC system, and its 1H NMR, 13C NMR, and 1H–13C HSQC spectra were obtained using a Bruker 400 MHz spectrometer.
In Vitro Cytotoxicity and Cytolocalization Assays
The DOX precipitate formed in PBS was washed with water three times and lyophilized. The lyophilized powder was completely dissolved in DMSO. The resulting solution was used as a DOX dimer solution to determine in vitro cytotoxicity and for cytolocalization assays. Human lung adenocarcinoma A549 cells (ATCC, Manassas, VA, USA) were maintained in Dulbecco’s modified Eagle medium containing 10% fetal bovine serum, 100 U/mL penicillin, and 100 μg/mL streptomycin. A549 cells were seeded in 96-well plates (5 × 103 cells/well) and incubated for 24 h. Thereafter, the cells were treated with various concentrations of DOX or the DOX dimer. Cell viability was measured using the Cell Counting Kit-8 (Dojindo, Kumamoto, Japan) on day 3. Cytolocalization of DOX (5 μg/mL) and the DOX dimer (5 μg/mL) was observed after 1 h using a BZ-X810 microscope (Keyence, Osaka, Japan). Nuclei were stained using 5 μg/mL Hoechst 33342 (Thermo Fisher Scientific, Waltham, MA, USA) for 10 min.
Acknowledgments
The author would like to thank Dr. Joel P. Schneider (National Cancer Institute-Frederick, National Institutes of Health, Frederick, MD, USA) for critical discussions. The author would also like to thank Editage (www.editage.com) for English language editing.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.0c04925.
Number of publications of DOX delivery study, mass spectrum of DOX, DOX monomer derivative, DOX dimer, mass spectrum of the DOX oxime and oxidized DOX oxime, NMR spectra of the oxidized DOX oxime, NBT test, and mass spectrum and the chromatogram of the DOX precipitate formed by heparin (PDF)
The author declares no competing financial interest.
Supplementary Material
References
- Arcamone F.; Franceschi G.; Penco S.; Selva A. Adriamycin (14-hydroxydaunomycin), a novel antitumor antibiotic. Tetrahedron Lett. 1969, 10, 1007–1010. 10.1016/s0040-4039(01)97723-8. [DOI] [PubMed] [Google Scholar]
- Gewirtz D. A critical evaluation of the mechanisms of action proposed for the antitumor effects of the anthracycline antibiotics adriamycin and daunorubicin. Biochem. Pharmacol. 1999, 57, 727–741. 10.1016/s0006-2952(98)00307-4. [DOI] [PubMed] [Google Scholar]
- Tacar O.; Sriamornsak P.; Dass C. R. Doxorubicin: an update on anticancer molecular action, toxicity and novel drug delivery systems. J. Pharm. Pharmacol. 2013, 65, 157–170. 10.1111/j.2042-7158.2012.01567.x. [DOI] [PubMed] [Google Scholar]
- Thorn C. F.; Oshiro C.; Marsh S.; Hernandez-Boussard T.; McLeod H.; Klein T. E.; Altman R. B. Doxorubicin pathways: pharmacodynamics and adverse effects. Pharmacogenet. Genomics 2011, 21, 440–446. 10.1097/fpc.0b013e32833ffb56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singal P. K.; Iliskovic N. Doxorubicin-induced cardiomyopathy. N. Engl. J. Med. 1998, 339, 900–905. 10.1056/nejm199809243391307. [DOI] [PubMed] [Google Scholar]
- Carvalho C.; Santos R.; Cardoso S.; Correia S.; Oliveira P.; Santos M.; Moreira P. Doxorubicin: the good, the bad and the ugly effect. Curr. Med. Chem. 2009, 16, 3267–3285. 10.2174/092986709788803312. [DOI] [PubMed] [Google Scholar]
- Kanwal U.; Irfan Bukhari N.; Ovais M.; Abass N.; Hussain K.; Raza A. Advances in nano-delivery systems for doxorubicin: an updated insight. J. Drug Targeting 2018, 26, 296–310. 10.1080/1061186x.2017.1380655. [DOI] [PubMed] [Google Scholar]
- Zhao N.; Woodle M. C.; Mixson A. J. Advances in delivery systems for doxorubicin. J. Nanomed. Nanotechnol. 2018, 9, 519. 10.4172/2157-7439.1000519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue Q.; Ren H.; Xu C.; Wang G.; Ren C.; Hao J.; Ding D. Nanospheres of doxorubicin as cross-linkers for a supramolecular hydrogelation. Sci. Rep. 2015, 5, 8764. 10.1038/srep08764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson-Arbor K.; Dubey R.. Doxorubicin; StatPearls: Treasure Island (FL), 2020. [PubMed] [Google Scholar]
- Fülöp Z.; Gref R.; Loftsson T. A permeation method for detection of self-aggregation of doxorubicin in aqueous environment. Int. J. Pharm. 2013, 454, 559–561. 10.1016/j.ijpharm.2013.06.058. [DOI] [PubMed] [Google Scholar]
- Gallois L.; Fiallo M.; Garnier-Suillerot A. Comparison of the interaction of doxorubicin, daunorubicin, idarubicin and idarubicinol with large unilamellar vesicles. Circular dichroism study. Biochim. Biophys. Acta 1998, 1370, 31–40. 10.1016/s0005-2736(97)00241-1. [DOI] [PubMed] [Google Scholar]
- Menozzi M.; Valentini L.; Vannini E.; Arcamone F. Self-association of doxorubicin and related compounds in aqueous solution. J. Pharm. Sci. 1984, 73, 766–770. 10.1002/jps.2600730615. [DOI] [PubMed] [Google Scholar]
- Hayakawa E.; Furuya K.; Kuroda T.; Moriyama M.; Kondo A. Viscosity Study on the Self-Association of Doxorubicin in Aqueous Solution. Chem. Pharm. Bull. 1991, 39, 1282–1286. 10.1248/cpb.39.1282. [DOI] [Google Scholar]
- Kaushik D.; Bansal G. Four new degradation products of doxorubicin: An application of forced degradation study and hyphenated chromatographic techniques. J. Pharm. Anal. 2015, 5, 285–295. 10.1016/j.jpha.2015.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurata T.; Otsuka Y. Amino-reductones. Formation mechanisms and structural characteristics. Adv. Exp. Med. Biol. 1998, 434, 269–276. 10.1007/978-1-4899-1925-0_22. [DOI] [PubMed] [Google Scholar]
- Hofmann T.; Bors W.; Stettmaier K. Studies on radical intermediates in the early stage of the nonenzymatic browning reaction of carbohydrates and amino acids. J. Agric. Food Chem. 1999, 47, 379–390. 10.1021/jf980626x. [DOI] [PubMed] [Google Scholar]
- Guin P. S.; Das S. Exploration of Electrochemical Intermediates of the Anticancer Drug Doxorubicin Hydrochloride Using Cyclic Voltammetry and Simulation Studies with an Evaluation for Its Interaction with DNA. Int. J. Electrochem. 2014, 2014, 517371. 10.1155/2014/517371. [DOI] [Google Scholar]
- Yokoyama M.; Fukushima S.; Uehara R.; Okamoto K.; Kataoka K.; Sakurai Y.; Okano T. Characterization of physical entrapment and chemical conjugation of adriamycin in polymeric micelles and their design for in vivo delivery to a solid tumor. J. Controlled Release 1998, 50, 79–92. 10.1016/s0168-3659(97)00115-6. [DOI] [PubMed] [Google Scholar]
- Fukushima S.; Machida M.; Akutsu T.; Shimizu K.; Tanaka S.; Okamoto K.; Mashiba H.; Yokoyama M.; Okano T.; Sakurai Y.; Kataoka K. Roles of adriamycin and adriamycin dimer in antitumor activity of the polymeric micelle carrier system. Colloids Surf., B 1999, 16, 227–236. 10.1016/s0927-7765(99)00073-9. [DOI] [Google Scholar]
- Zunino F.; Gambetta R. A.; Zaccara A.; Carsana R. A differential interaction of doxorubicin and daunorubicin with human serum proteins. Tumori 1981, 67, 399–403. 10.1177/030089168106700502. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.