Abstract
Background
Frailty syndrome is now becoming a challenge for multidisciplinary teams. Frailty assessment in elderly patients is recommended due to the associated cascade of irreversible alterations that ultimately result in disability.
Aims
The purpose of this article is to identify interventions, which can be implemented and performed by nurses as part of a multidisciplinary plan. Nursing strategies related to nutrition, polypharmacy, adherence to treatment, falls, exercise, and mood and cognitive intervention are described.
Design
Discussion paper.
Data sources
Relevant and up-to-date literature from PubMed, MEDLINE, and Scopus databases regarding the selected issues, such as nutritional status, polypharmacy, falls, physical activity, and cognitive functions.
Conclusion
Frailty is considered preventable or even reversible with the appropriate interventions, which can help maintain or even restore physical abilities, cognitive function, or nutritional status in frail elderly patients. Hence, the nursing interventions are significant in clinical practice and should be implemented for frail patients.
Implications for nursing
Health-care providers, especially nurses, in their clinical practice should recognize not only elderly patients but also elderly patients with concurrent frailty, requiring intensified therapeutic interventions tailored to their individual needs. Frailty syndrome is undoubtedly a challenge for multidisciplinary teams providing health care for geriatric patients.
Keywords: Frailty syndrome, elderly patients, nursing interventions, nutrition in elderly, polypharmacy
Introduction
Aging, an inevitable process, is commonly measured by chronological age and, as a convention, a person aged 65 years or more is often referred to as “elderly.” However, there are no concrete definitions of “elderly” that appropriately characterize this patient population in using the generic terms “elderly” and “older persons” (Singh & Bajorek, 2014). The term frailty is commonly used rather loosely to describe a range of conditions in older people, including general debility and cognitive impairment. However, growing old is not in itself a prerequisite to becoming frail (Lally & Crome, 2007).
Frailty is theoretically defined as a clinically recognizable state of increased vulnerability resulting from an aging-associated decline in reserve and function across multiple physiologic systems, such as the ability to cope with every day or acute stressors is comprised (Xue, 2011). Another definition describes frailty as a biological syndrome of decreased reserve and resistance to stressors, resulting from cumulative declines across multiple physiologic systems, causing vulnerability to adverse outcomes (Xue, 2011).
Frailty syndrome (FS) is considered highly prevalent in elderly patients and to confer high risk of adverse outcomes, including disability, lower quality of life, hospitalization, institutionalization, and mortality (Boyd, Xue, Simpson, Guralnik, & Fried, 2005; R. J. J. Gobbens & van Assen, 2014; Rockwood et al., 2005). However, there is currently still no consensus on how frailty should be defined. In fact, two different conceptual frailty approaches have been adopted. One of these approaches is strongly influenced by medicine and focuses only on how older people function physically, so-called physical frailty.
The other conceptual frailty approach is multidimensional by nature and in addition to physical frailty also includes the psychological and social functioning of older people, indicated with psychological frailty and social frailty, respectively. Definition of frailty by Gobbens, Luijkx, Wijnen-Sponselee, and Schols (2010a) expresses this multidimensional approach:
Frailty is a dynamic state affecting an individual who experiences losses in one or more domains of human functioning (physical, psychological, social), which is caused by the influence of a range of variables and which increases the risk of adverse outcomes (p. 175).
Identification of frail older people is important as evidence suggests that frailty might be reversible using exercise programs or hormone treatment (Faber, Bosscher, Chin A Paw, & van Wieringen, 2006; Srinivas-Shankar et al., 2010). Recognizing frailty could be clinically useful for health-care (e.g., general practitioners, nurses, physical therapists) and welfare professionals in identifying older persons who may benefit from an intervention aimed at reducing frailty, as well as preventing or delaying adverse outcomes. In the last few years, several instruments aimed at identifying frail subjects have been developed.
Data Sources
A relevant and up-to-date literature from PubMed, MEDLINE, and Scopus databases were selected and collected after a computerized search strategy. Research keywords describing nursing interventions for patients with FS, such as nutritional status, polypharmacy, falls, physical activity, and cognitive functions were used. Only papers which have been published in peer-reviewed journals listed in the Journal Citation Reports, available with the full version of the manuscript and written in English only, were included. The literature search was performed in January 2017.
Discussion
Currently, five systematic reviews regarding frailty instruments have been published (Clegg, Rogers, & Young, 2015; de Vries et al., 2011; Drubbel et al., 2014; Sternberg, Wershof Schwartz, Karunananthan, Bergman, & Mark Clarfield, 2011; Sutton et al., 2016). These reviews differ greatly in their objectives. Sternberg et al. (2011) systematically reviewed the literature on the clinical definitions, screening tools, and severity measures of frailty used in community-dwelling older people aged 65 years and above, while Clegg et al. (2015) investigated the diagnostic test accuracy of simple instruments for identifying frailty in community-dwelling older people. Another systematic review aimed to explore whether the Frailty Index is a valid and adequate screening instrument for primary care and de Vries et al. (2011) performed a review on evaluative measures of frailty.
The most recent systematic review regarding frailty instruments was conducted by Sutton et al. (2016). The objective of this study was to identify existing multicomponent instruments to assess frailty in people aged ≥60 years and to systematically and critically evaluate the reliability and validity of these instruments. As a result, they identified 38 frailty instruments, including the Cardiovascular Health Study (CHS) Phenotype Model and the Canadian Study of Health and Ageing Cumulative Deficit Model. The CHS Phenotype Model is developed by Fried et al. and suits the aforementioned definition of physical frailty (Malmstrom, Miller, & Morley, 2014). According to the CHS Phenotype Model, an individual is identified as frail if at least three of five of the following criteria are present: unintentional weight loss, weakness, poor endurance, slowness, and low physical activity. The Canadian Study of Health and Ageing Cumulative model assesses frailty via the Frailty Indicator, an index of age-related deficits, including diseases and disability. However, more and more researchers consider disability as an adverse outcome of frailty (Bergman et al., 2009; Gobbens, Luijkx, Wijnen-Sponselee, & Schols, 2010b). Although these two models are the most widely accepted, evidence for the reliability and validity of the original instruments is limited (Sutton et al., 2016). According to Sutton et al. (2016), the Tilburg Frailty Indicator, a self-report questionnaire developed from an integral approach of frailty, including physical, psychological, and social domains (Gobbens, van Assen, Luijkx, Wijnen-Sponselee, & Schols, 2010), has the most robust evidence of reliability and validity (Sutton et al., 2016).
Frailty Syndrome in Clinical Practice
FS is now becoming a challenge for multidisciplinary teams. Frailty assessment in elderly patients is recommended due to the associated cascade of irreversible alterations ultimately resulting in disability. In their clinical practice, health-care providers should recognize not only elderly patients but also elderly patients with concurrent frailty, requiring intensified therapeutic interventions tailored to their individual needs. Future development should, therefore, focus on frailty screening in risk groups, and including frailty in existing or new risk assessment models, especially with regard to perioperative risk. Furthermore, treatment strategies should be implemented to help prevent FS and minimize the adverse health outcomes in frail patients.
FS is undoubtedly a challenge for multidisciplinary teams providing health care for geriatric patients. In clinical practice, special attention should be paid to frail elderly patients, who should receive tailored treatment. Frailty is considered preventable or even reversible with the appropriate interventions, which can help maintain or even restore physical abilities, cognitive function, or nutritional status in frail elderly patients. Often when frail elderly patients come into contact with a whole series of different health-care professionals (e.g., geriatrician, occupational therapist, and physiotherapist), there is a risk that they will not receive an integrated care. Using their integral view of human functioning, nurses can act as a case manager for frail elderly patients and make a multidisciplinary plan in collaboration with involved health-care professionals.
The purpose of this article is to identify interventions, which can be implemented and performed by nurses as part of the multidisciplinary plan. Starting from a broad approach of frailty, including physical, psychological, and social domains, nurses are able to conduct a lot of interventions with the aim to prevent or diminish frailty and prevent or delay its adverse outcomes. In this article, the authors have chosen to focus on nursing strategies related to nutrition, polypharmacy, adherence to treatment, falls, exercise, and mood and cognitive intervention.
Aging and Nutritional Status
Maintenance of a healthy nutritional status is the key to healthy and active aging. However, malnutrition is increasingly diagnosed in the elderly. Elderly patients are classified in the high nutritional risk group due to numerous comorbidities, frequent hospitalizations, and pharmaceutical treatment, among other factors. Malnutrition contributes to functional disorders, increased hospitalization time, and lower quality of life. Research indicates that nearly 40% of geriatric patients undergo significant body weight loss (over 4.5 kg in 3 months), 83.9% have decreased walking speed, 53.8% have a sedentary lifestyle, and 57.7% experience decreased muscle strength. Moreover, screening identifies a nutritional risk in 23.5% of patients and malnutrition in 9%. One can thus conclude that the clinical determinants of frailty, which include weakness, low energy, psychomotor retardation, decreased physical activity, or weight loss, are significantly correlated with nutritional status in the elderly population (Guyonnet, Secher, Ghisolfi, Ritz, & Vellas, 2015).
In daily clinical practice, the routine nutritional assessment may significantly facilitate diagnosis as well as the planning of interventions tailored to the frail patient’s needs. The screening tool recommended by the European Society of Parenteral and Enteral Nutrition, specifically designed for nutritional assessment in elderly patients, is the Mini Nutritional Assessment (MNA) questionnaire; 90% of patients found to be at high nutritional risk or malnourished based on the MNA are either frail or prefrail. The MNA is a sensitive screening tool, and also considers factors which may interfere with maintaining a healthy nutritional status in the elderly, that is, depression, motivation, dementia, fatigue, and mobility. Additionally, the MNA can be useful for planning educational activities and developing prevention programs for frail elderly patients (Bonilla-Palomas et al., 2011; Kagansky et al., 2005).
The causes of nutrient deficiency in elderly patients are multifaceted and include a range of intrinsic and extrinsic factors. One of the intrinsic factors is hormonal imbalance, and specifically, decreased secretion of the “hunger hormone” in the stomach, resulting in reduced appetite; 15% to 30% of elderly patients experience such an appetite decrease, which is strictly correlated with decreased physical activity, weight loss, and disability (Landi et al., 2010; Malafarina, Uriz-Otano, Gil-Guerrero, & Iniesta, 2013; Wilson et al., 2005). In a large European study aimed at identifying nutritional risk factors, malnourished patients ate more easily chewed foods (de Morais et al., 2013). These findings indicate that difficulty chewing is a clinical issue in elderly patients, contributing to decreased appetite and insufficient nutrient intake. Pharmaceutical treatment, dementia, depression, lack of social support, and social isolation have also been named as factors directly affecting appetite loss in elderly patients. There are still no gold standards for interventions contributing to improved appetite in the elderly population, and thus preventing or delaying the issue of poor nutritional status (von Haehling & Anker, 2014).
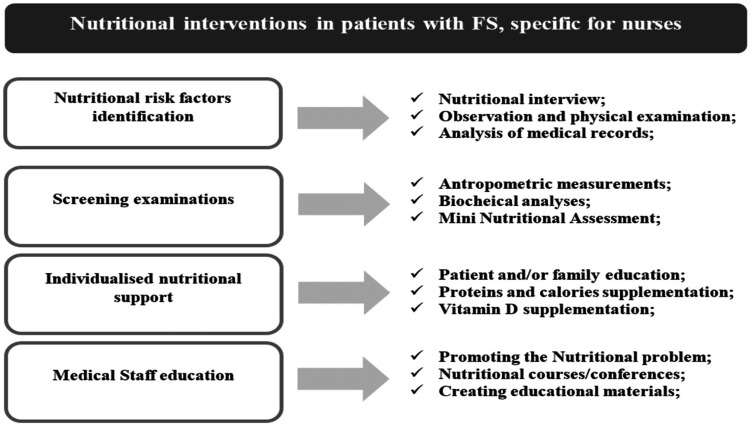
Patients with nutritional disorders are a heterogeneous group, due to the existence of multiple nutritional risk factors. Nutritional interventions should thus begin with the identification of such nutritional risk factors and appropriate screening. On this basis, a tailored treatment plan should be implemented, considering the patient’s preferences, resources, and needs. Research also indicates that nutritional education of medical personnel is correlated with the improved nutritional status of elderly patients (Figure 1).
Figure 1.
Nutritional intervention diagram.
Polypharmacy—General Knowledge
Polypharmacy is defined as the use of 4 or more medications, while severe polypharmacy is defined as the use of 10 or more medications (Zia, Kamaruzzaman, & Tan, 2015). One in 2 elderly patients is affected by polypharmacy and more than 1 in 10 by severe polypharmacy. This can be associated with self-medication and the use of over-the-counter drugs. Polypharmacy and multimorbidity form the so-called illness and treatment spiral, where an increasing number of comorbidities require treatment by multiple specialists, entailing the use of more medications, which in turn contributes to damage to subsequent organs (Agostini, Han, & Tinetti, 2004).
Adverse reactions occur more frequently in elderly patients, and the risk of their occurrence increases in proportion to the number of medications taken (10% per 1 drug). These reactions are typically nonspecific, difficult to diagnose, and viewed as a new clinical problem, which potentially results in a pharmacological cascade. In old age, multimorbidity and polypharmacy are associated with an increased risk of falls and decreased functional capacity (Wilson et al., 2011). Drugs typically associated with risk of falls include diuretics, hypotensives, antiarrhythmics, antidiabetics, antidepressants, and central nervous system agents (Woolcott et al., 2009).
Existing publications discuss the relationship between polypharmacy and fall risk (Weber, White, & McIlvried, 2008; Ziere et al., 2006). Some authors observe the correlation between polypharmacy and falls, which is stronger when elderly patients are prescribed at least one drug associated with fall risk: In such cases, the risk is much higher (Wilson et al., 2011). It seems that a more logical approach to fall risk assessment would take into account the appropriateness of medication rather than the number of drugs. This should include such factors as the type of drug, potential treatment benefits, and potential adverse effects (Brager & Sloand, 2005). The complex treatment protocols in polypharmacy can cause elderly patients to discontinue some medications or to skip doses, which can, in turn, lead to adverse effects (Basger, Chen, & Moles, 2008).
Polypharmacy should be monitored by a multidisciplinary team in cooperation with a hospital when it is necessary (Pulignano et al., 2010). Nurses providing care at the patient’s home and on day wards should be especially involved. Interventions for reducing polypharmacy mainly include geriatric assessment and patient and family education provided by the prescribing physician (Chaudhry, Wang, Gill, & Krumholz, 2010).
Polypharmacy—Adherence Improvement Strategies
Patients’ adherence to treatment is one of the essential criteria of treatment effectiveness. Interventions to improve adherence can be divided into four main categories:
Patient education;
Simplifying dosage regimens;
Facilitating consultations with a physician (extending office hours, especially in the evenings, reducing waiting times);
Improving communication between the patient and the members of the therapeutic team.
Educating the patient and their family is one of the most important areas of adherence-improving interventions (Jasińska, Kurczewska, & Orszulak-Michalak, 2009). Providing appropriately formulated information, monitoring the understanding and acceptance of recommendations on a regular basis, and including the patient in decisions regarding the choice of treatment methods, where possible, are fundamental to good adherence. The patient should be informed not only of the pharmaceutical and nonpharmaceutical recommendations but also on the impact of adherence on pharmaceutical treatment’s effectiveness. One important aspect of intervention is ensuring support from the patient’s family, friends, support groups, or social organizations (Jasińska et al., 2009).
One method significantly increasing adherence to pharmaceutical treatment is minimizing the number of medications administered, especially in elderly patients. This reduces the risk of pharmaceutical treatment discontinuation, as well as the risk of drug interactions and adverse effects. An important element in improving patient cooperation is replacing frequently dosed drugs with long-acting medication. Simplified dosage regimens and the use of combination drugs reduce the pill burden on the patient, simultaneously decreasing treatment costs and improving the safety profile of the pharmaceutical treatment used. Individual daily dose packaging systems with instructions for use and timing improve both patient comfort and treatment effectiveness. In all cases, factors to be considered include the benefits and risks of a given treatment for the particular patient, the patient’s expectations and preferences, treatment safety and cost, and any possible alternatives. Reducing the pill burden may improve the patient’s well-being and perceived health, changing the patient’s attitude toward the chronic treatment (Nieuwenhuis, Jaarsma, van Veldhuisen, & van der Wal, 2012).
Another method for improving adherence is scheduling regular follow-up visits, including the monitoring of the number of pills taken. The analysis of the number of follow-ups and prescriptions is also of importance. Such follow-up visits should routinely involve including the patient as a partner in the decision-making process, providing simple and clear instructions, and highlighting the importance of regular medication-taking. Effective communication between the patient and the multidisciplinary team is the key to improving adherence (Jasińska et al., 2009). Reasons for nonadherence should be identified, and if necessary, alternative treatment plans should be sought together with the patient. Whenever the treatment plan changes, the patient should receive new instructions and schedules in writing.
For chronically ill patients, follow-up appointments should already be scheduled on their being discharged from the hospital. Monitoring and support can also be provided remotely, over the phone, or Internet (van der Wal & Jaarsma, 2008). Mobile applications improving adherence to treatment are increasingly popular. Such applications offer not only self-control journal functions but also enable the monitoring of medication-taking by family members.
Psychosocial support is a significant factor in improving adherence (Table 1). The inclusion of the patient’s friends and family, as well as relating to other patients’ experiences through Internet forums or patient groups, can be perceived better than factual information on the consequences of nontreatment.
Table 1.
Components of the Adherence-Improving Intervention Model (Gociong & Kardas, 2013).
| Adherence-improving intervention - nursing interventions | Adherence-improving intervention - physician interventions |
|---|---|
| Monitoring adherence and correct medication-taking at each visit | Simplifying the treatment plan and Adjusting treatment to patient’s current status |
| Evaluating acceptability of the treatment plan | Optimum treatment for comorbidities and monitoring treatment safety |
| Patient education since first visit and diagnosis | |
| Written treatment and management plan | |
| Discussing patient’s preferences and goals in treatment decisions (number of drugs, frequency, cost) | |
Falls—Assessment, Prevention, and Rehabilitation
The risk of falls increases with age. More than half of patients above 80 years fall once a year, and those who have experienced a fall have a higher risk of subsequent falls. Risk factors for falling are living alone, sedentary lifestyle, polypharmacy, impaired cognitive function, impaired vision, malnutrition, and alcoholism (Sander, 2009). Falls contribute to disability and institutionalization in elderly patients and are the leading cause of mortality among elderly adults with traumatic brain injury (Fu, Fu, Jing, McFaull, & Cusimano, 2017; Taylor, Bell, Breiding, & Xu, 2017). Previous falls, hospitalization due to falls, and visual impairment are considered the most significant risk factors for falling (Clemson, Mackenzie, Ballinger, Close, & Cumming, 2008). Therefore, the best method for assessing fall risk is to gather information on past falls and their circumstances (Al-Aama, 2011).
Patient assessment after a fall includes an examination of the nervous system and the musculoskeletal system, possible orthostatic hypotension, arrhythmias, infections, dehydration, and visual impairment. The examination must also include an assessment of motor fitness, specifically including an evaluation of balance and mobility in daily activities. The assessment of psychological state, cognitive function, possible depression, or anxiety due to falling risk is also of importance of the multidisciplinary teams (e.g., doctor and nurse; Bauman et al., 2014; Meuleners, Fraser, Bulsara, Chow, & Ng, 2016). The recommended interventions preventing or reducing the number of falls include improving safety in the patient’s environment; identification and treatment of extant acute and chronic diseases and modification of pharmaceutical treatment, especially with psychotropic drugs and in cases of multimorbidity (Gillespie et al., 2003); providing assistive equipment; regular exercise to improve balance, posture, muscle strength, and joint mobility; and patient and family education on safe behaviors. The type of preventive interventions implemented depends on the patient’s capabilities and independence (Bauman et al., 2014).
Nurses should focus on a safe patient environment which includes appropriate lighting in staircases and bathroom facilities adapted to the needs of the mobility-impaired patient. To prevent falls, measures such as raised toilets, shower seats, and grab rails can be used. Another important measure is placing shelves at an easily accessible height and providing chairs, which allow the patient to rest their feet comfortably on the ground while maintaining a 90° angle in knee joints. The elderly patient’s bedroom should be in close proximity to the bathroom; thus, the patient can easily move between the two and should be fitted with a safety alarm or telephone for easy communication. It is also recommended to eliminate all unnecessary rugs, mats, and cables, which can impede mobility. Provision of assistive equipment and technical aids, as well as encouraging their use, also contributes to fewer falls (Clemson et al., 2008; Guo, Tsai, Liao, Tu, & Huang, 2014).
Rehabilitation plans should be comprehensive and should include measures to help maintain balance while walking and in other positions, as well as preparation for coping in case of a fall (El-Khoury, Cassou, Charles, & Dargent-Molina, 2013). One component of rehabilitation is gait improvement, comprising exercises in the proper posture prior to walking, raising and lowering the feet, and using appropriate walking aids. The most commonly used exercises include balance exercises while sitting, standing, and walking. Strength (resistance) exercises are gaining importance in elderly patient activity. Their primary purpose is to prevent the development of sarcopenia and the resulting functional impairment (Twardowska-Rajewska, 2006). Vitamin D supplementation in doses of 800 IU daily or higher has also been reported as a factor reducing the risk of falls, though this remains controversial; some authors report benefits from combined supplementation of vitamin D and calcium (Table 2; Stubbs, Brefka, & Denkinger, 2015).
Table 2.
Components of Patient History Useful in Fall Risk Assessment (Bauman et al., 2014).
| Fall risk assessment | Interventions preventing falls |
|---|---|
| Physical examination, functional and cognitive status | Providing education and information |
| History of past falls | Supplementing vitamin D |
| Balance capabilities and muscle strength | Implementing individual exercise programs |
| Heart function and rhythm | Treating arrhythmias |
| Orthostatic hypotension | Preventing orthostatic hypotension |
| Medication and possible adverse effects | Deprescribing |
| Vision impairment | Treating visual impairment |
| Feet and footwear examination | Preventing walking difficulties |
| Environmental hazards | Adjusting the home environment |
Exercise Interventions
Aging is associated with a decrease in voluntary physical activity, which nonetheless is a significant preventive strategy, as it improves muscle strength, endurance, and maximum aerobic power, and reduces fatigability in elderly patients (Walston et al., 2006). Exercise interventions are considered a key factor to prevent, delay, reverse, or reduce the severity of frailty, or reduce the risk of adverse outcomes in irreversibly frail patients (Theou et al., 2011). In nursing care, the decision to start multicomponent exercise must be based on a careful consideration of all training components to form a consistent program. Thus, the following factors must be considered:
Training frequency. Determining the duration of the exercise intervention program is important for frailty management. A typical recommendation is performing the prescribed exercises three times weekly, for at least 3 months. The frequency recommendations vary between two and five sessions a week. In many studies, the duration of the program ranged from 1 to 18 months (Sihvonen, Sipilä, Taskinen, & Era, 2004); nonetheless, the most common intervention period was 3 months (Rosendahl et al., 2006).
Duration. The optimum duration of a single training session is between 30 and 45 minutes, while some authors recommend sessions of up to 60 minutes. For frail patients, the session duration should be shorter than recommended for healthy populations. This is due to the fact that frail patients may be significantly more fatigable.
Intensity. Intensity can be determined using a perceived exertion scale (Penko, Barkley, Koop, & Alberts, 2017). Nonetheless, interventions incorporating a resistance training program define the intensity as three sets of eight repetitions at approximately 80% of the individual’s one repetition maximum. The ability to maintain higher exercise intensity positively affects the improvement in muscle strength and endurance (Sullivan et al., 2005; Sullivan, Roberson, Smith, Price, & Bopp, 2007).
Type of exercise. Most researchers emphasize the benefits of multicomponent exercise interventions, comprising resistance, balance, aerobic, endurance, and flexibility training (Rejeski et al., 2008; Timonen et al., 2006).
Compliance with exercise sessions. Compliance with the exercise program is an important measure of intervention effectiveness. It was shoved that mean compliance of 74% (McPhate, Simek, & Haines, 2013) is higher than has previously been reported for compliance to home exercise programs for falls prevention (Simek, McPhate, & Haines, 2012). Despite satisfactory outcomes, compliance should be evaluated in all interventions. Older patients are at risk of cognitive impairment, low mood, or even depression, which predisposes to affect the motivation in participating actively in the interventions, and may cause noncompliance by impairing abilities in planning, organizing, and executing tasks (Smith et al., 2017; Valiengo, Stella, & Forlenza, 2016).
Assessment and risk of adverse events. The implementation of the training program should also be based on the assessed risk of any adverse events. In previous studies, no adverse events were observed during the exercise interventions. However, despite the lack of adverse events, attention should be paid to patient-reported back or knee pain or any musculoskeletal injuries, as well as the elderly response to exercise training (e.g., strength training; Latham & Liu, 2010).
Multicomponent training positively affects frail patients, and therefore should be included in the management of frailty (Theou et al., 2011). Moreover, multicomponent exercise interventions have been identified as the only type of intervention, which consistently improves frail patients’ condition in terms of physical and functional fitness, gait, balance, strength, cognitive function, mood, and overall well-being (Landi et al., 2010). No consensus has yet been achieved as to the optimum training program, hence the need for further studies aiming to determine the best exercise intervention model (Table 3).
Table 3.
The Benefits of Exercise Interventions (de Labra, Guimaraes-Pinheiro, Maseda, Lorenzo, & Millán-Calenti, 2015).
| Health benefits of exercise interventions |
|---|
| Increased mobility and flexibility |
| Improved gait |
| Improved motor control and coordination |
| Improved balance |
| Increased bone mineral density |
| Lower fall risk |
| Maintenance of functional independence in activities of daily living |
| Improved general well-being |
| Increased mobility and flexibility |
| Improved gait |
Mood and Cognitive Function
Cognitive functions include a number of intellectual processes, such as short-term memory, long-term memory, language processes (writing, reading, speech), visual and spatial processes, abstract thinking, and perceiving external stimuli. Overall, full cognitive function enables normal everyday bio-psychosocial functioning. Physiologically, aging processes involve age-associated memory impairment or age-related cognitive decline (Ishizaki et al., 2005). Prevention of cognitive decline is essential as well as nursing interventions for recovering or slowing cognitive deterioration in people who become frail. The International Consensus Group on “Cognitive Frailty” provides the first definition of a Cognitive Frailty condition in older adults. Cognition has already been considered as a component of frailty. Worse cognitive performance is dependently associated with higher mortality, even more than twice (Kelaiditi et al., 2013).
It is explained by problems in recognizing symptoms of diseases, worse adherence to therapeutically interventions, and lack of the healthy lifestyle behaviors (van der Wardt et al., 2017). The interventions, which may be taken by nurses are (Gluhm et al., 2013) as follows:
Screening for cognitive decline using available tests: for example, Mini-Mental State Examination;
Stimulation exercises that result in an improvement in memory (memory training programs), for example, activities including learning strategies used to recall verbal and visual information, tasks such as categorical naming, “spot the differences,” and coding used to enhance attention and processing speed and also matrix reasoning exercises, and tangram-like games aimed at enhancing reasoning and problem-solving abilities;
Utilization of external memory aids such as notes, calendars, or other resources;
Strategies to improve episodic memory (list recall, face-name association, and text memory);
Training in self-assertiveness;
Creating an educational and training plan;
Creating linkages between the health-care system and the communities (Reichman, Fiocco, & Rose, 2010; Willis et al., 2006).
Conclusion
FS is a serious problem in the elderly population; therefore, managing frailty involves maintaining the balance between assets and deficits. It should be noted that FS is not synonymous with either comorbidity or disability, but comorbidity is an etiologic risk factor for FS and disability is an outcome of FS. Frailty is also a complex problem and it has a multidimensional nature; therefore, multiple interventions can be necessary to preserve this balance. Because of this nature, complex interventions integrating several components (e.g., nutrition, rehabilitation, and exercises) are more likely to be effective than simple, individual interventions.
Implications for Nursing
This article shows that nurses can play an important role in frailty management. Nursing teams may implement their own strategies to take care of elderly frail patients. Care strategies should be aimed at maintaining this homeostatic balance. To be able to meet the needs of frail elderly patients collaboration among health-care professionals is essential; and interventions should be coordinated. This requires collaborative skills of the health-care professionals and also demands a willingness of professionals to look beyond the borders of their own disciplines.
Author's note
Robbert Gobbens is also affiliated to “Zonnehuisgroep Amstelland, Amstelveen, the Netherlands” and “Department of General Practice, University of Antwerp, Antwerp, Belgium”.
Author Contributions
All the authors made a substantial contribution to the concept or design of the work or acquisition, analysis, or interpretation of data; drafted the article or revised it critically for important intellectual content; and approved the version to be published. Each author has participated sufficiently in the work to take public responsibility for appropriate portions of the content.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD
Izabella Uchmanowicz http://orcid.org/0000-0001-5452-0210
References
- Agostini J. V., Han L., Tinetti M. E. (2004) The relationship between number of medications and weight loss or impaired balance in older adults. Journal of the American Geriatrics Society 52(10): 1719–1723. doi:10.1111/j.1532-5415.2004.52467.x. [DOI] [PubMed] [Google Scholar]
- Al-Aama T. (2011) Falls in the elderly: Spectrum and prevention. Canadian Family Physician Medecin De Famille Canadien 57(7): 771–776. [PMC free article] [PubMed] [Google Scholar]
- Basger B. J., Chen T. F., Moles R. J. (2008) Inappropriate medication use and prescribing indicators in elderly Australians: Development of a prescribing indicators tool. Drugs & Aging 25(9): 777–793. [DOI] [PubMed] [Google Scholar]
- Bauman C. A., Milligan J. D., Patel T., Pritchard S., Labreche T., Dillon-Martin S., Riva J. J. (2014) Community-based falls prevention: Lessons from an interprofessional mobility clinic. The Journal of the Canadian Chiropractic Association 58(3): 300–311. [PMC free article] [PubMed] [Google Scholar]
- Bergman H., Béland F., Karunananthan S., Hummel S., Hogan D., Wolfson C. (2009) Développement d’un cadre de travail pour comprendre et étudier la fragilité [Development of a framework to understand and study fragility]. Gérontologie et Société 109: 15–29. [Google Scholar]
- Bonilla-Palomas J. L., Gámez-López A. L., Anguita-Sánchez M. P., Castillo-Domínguez J. C., García-Fuertes D., Crespin-Crespin M., Suárez de Lezo J. (2011) Impact of malnutrition on long-term mortality in hospitalized patients with heart failure. Revista Espanola De Cardiologia 64(9): 752–758. doi:10.1016/j.recesp.2011.03.009. [DOI] [PubMed] [Google Scholar]
- Boyd C. M., Xue Q.-L., Simpson C. F., Guralnik J. M., Fried L. P. (2005) Frailty, hospitalization, and progression of disability in a cohort of disabled older women. The American Journal of Medicine 118(11): 1225–1231. doi:10.1016/j.amjmed.2005.01.062. [DOI] [PubMed] [Google Scholar]
- Brager R., Sloand E. (2005) The spectrum of polypharmacy. The Nurse Practitioner 30(6): 44–50. [DOI] [PubMed] [Google Scholar]
- Chaudhry S. I., Wang Y., Gill T. M., Krumholz H. M. (2010) Geriatric conditions and subsequent mortality in older patients with heart failure. Journal of the American College of Cardiology 55(4): 309–316. doi:10.1016/j.jacc.2009.07.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clegg A., Rogers L., Young J. (2015) Diagnostic test accuracy of simple instruments for identifying frailty in community-dwelling older people: A systematic review. Age and Ageing 44(1): 148–152. doi:10.1093/ageing/afu157. [DOI] [PubMed] [Google Scholar]
- Clemson L., Mackenzie L., Ballinger C., Close J. C. T., Cumming R. G. (2008) Environmental interventions to prevent falls in community-dwelling older people: A meta-analysis of randomized trials. Journal of Aging and Health 20(8): 954–971. doi:10.1177/0898264308324672. [DOI] [PubMed] [Google Scholar]
- de Labra C., Guimaraes-Pinheiro C., Maseda A., Lorenzo T., Millán-Calenti J. C. (2015) Effects of physical exercise interventions in frail older adults: A systematic review of randomized controlled trials. BMC Geriatrics 15: 154 doi:10.1186/s12877-015-0155-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Morais C., Oliveira B., Afonso C., Lumbers M., Raats M., de Almeida M. D. V. (2013) Nutritional risk of European elderly. European Journal of Clinical Nutrition 67(11): 1215–1219. doi:10.1038/ejcn.2013.175. [DOI] [PubMed] [Google Scholar]
- de Vries N. M., Staal J. B., van Ravensberg C. D., Hobbelen J. S. M., Olde Rikkert M. G. M., Nijhuis-van der Sanden M. W. G. (2011) Outcome instruments to measure frailty: A systematic review. Ageing Research Reviews 10(1): 104–114. doi:10.1016/j.arr.2010.09.001. [DOI] [PubMed] [Google Scholar]
- Drubbel I., Numans M. E., Kranenburg G., Bleijenberg N., de Wit N. J., Schuurmans M. J. (2014) Screening for frailty in primary care: A systematic review of the psychometric properties of the frailty index in community-dwelling older people. BMC Geriatrics 14: 27 doi:10.1186/1471-2318-14-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Khoury F., Cassou B., Charles M.-A., Dargent-Molina P. (2013) The effect of fall prevention exercise programmes on fall induced injuries in community dwelling older adults: Systematic review and meta-analysis of randomised controlled trials. BMJ (Clinical Research Ed.) 347: f6234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faber M. J., Bosscher R. J., Chin A., Paw M. J., van Wieringen P. C. (2006) Effects of exercise programs on falls and mobility in frail and pre-frail older adults: A multicenter randomized controlled trial. Archives of Physical Medicine and Rehabilitation 87(7): 885–896. doi:10.1016/j.apmr.2006.04.005. [DOI] [PubMed] [Google Scholar]
- Fu W. W., Fu T. S., Jing R., McFaull S. R., Cusimano M. D. (2017) Predictors of falls and mortality among elderly adults with traumatic brain injury: A nationwide, population-based study. PLoS One 12(4): e0175868 doi:10.1371/journal.pone.0175868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie L. D., Gillespie W. J., Robertson M. C., Lamb S. E., Cumming R. G., Rowe B. H. (2003) Interventions for preventing falls in elderly people. Cochrane Database of Systematic Reviews 4: CD000340 doi:10.1002/14651858.CD000340. [DOI] [PubMed] [Google Scholar]
- Gluhm S., Goldstein J., Loc K., Colt A., Van Liew C., Corey-Bloom J. (2013) Cognitive performance on the mini-mental state examination and the Montreal cognitive assessment across the healthy adult lifespan. Cognitive and Behavioral Neurology: Official Journal of the Society for Behavioral and Cognitive Neurology 26(1): 1–5. doi:10.1097/WNN.0b013e31828b7d26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gobbens R. J. J., Luijkx K. G., Wijnen-Sponselee M. T., Schols J. M. G. A. (2010. a) Towards an integral conceptual model of frailty. The Journal of Nutrition, Health & Aging 14(3): 175–181. [DOI] [PubMed] [Google Scholar]
- Gobbens R. J. J., Luijkx K. G., Wijnen-Sponselee M. T., Schols J. M. G. A. (2010. b) Toward a conceptual definition of frail community dwelling older people. Nursing Outlook 58(2): 76–86. doi:10.1016/j.outlook.2009.09.005. [DOI] [PubMed] [Google Scholar]
- Gobbens R. J. J., van Assen M. A. L. M. (2014) The prediction of quality of life by physical, psychological and social components of frailty in community-dwelling older people. Quality of Life Research: An International Journal of Quality of Life Aspects of Treatment, Care and Rehabilitation 23(8): 2289–2300. doi:10.1007/s11136-014-0672-1. [DOI] [PubMed] [Google Scholar]
- Gobbens R. J. J., van Assen M. A. L. M., Luijkx K. G., Wijnen-Sponselee M. T., Schols J. M. G. A. (2010) The Tilburg Frailty Indicator: Psychometric properties. Journal of the American Medical Directors Association 11(5): 344–355. doi:10.1016/j.jamda.2009.11.003. [DOI] [PubMed] [Google Scholar]
- Gociong Z., Kardas P. (2013) Non-adherence to medical recommendations. From reasons to practical solutions, Warsaw, Poland: Index Copernicus Publisher. [Google Scholar]
- Guo J.-L., Tsai Y.-Y., Liao J.-Y., Tu H.-M., Huang C.-M. (2014) Interventions to reduce the number of falls among older adults with/without cognitive impairment: An exploratory meta-analysis. International Journal of Geriatric Psychiatry 29(7): 661–669. doi:10.1002/gps.4056. [DOI] [PubMed] [Google Scholar]
- Guyonnet S., Secher M., Ghisolfi A., Ritz P., Vellas B. (2015) Nutrition, frailty and prevention of disabilities with aging. The Journal of Frailty & Aging 4(1): 13–25. doi:10.14283/jfa.2015.36. [DOI] [PubMed] [Google Scholar]
- Ishizaki T., Yoshida H., Suzuki T., Watanabe S., Niino N., Ihara K., Imanaka Y. (2005) Effects of cognitive function on functional decline among community-dwelling non-disabled older Japanese. Archives of Gerontology and Geriatrics 42(1): 47–58. doi:10.1016/j.archger.2005.06.001. [DOI] [PubMed] [Google Scholar]
- Jasińska M., Kurczewska U., Orszulak-Michalak D. (2009) Medication non-adherence in pharmaceutical care process. Polish Pharmacology 65(11): 765–771. [Google Scholar]
- Kagansky N., Berner Y., Koren-Morag N., Perelman L., Knobler H., Levy S. (2005) Poor nutritional habits are predictors of poor outcome in very old hospitalized patients. The American Journal of Clinical Nutrition 82(4): 784–791–914. [DOI] [PubMed] [Google Scholar]
- Kelaiditi E., Cesari M., Canevelli M., van Kan G. A., Ousset P.-J., Gillette-Guyonnet S. (2013) IANA/IAGG. Cognitive frailty: Rational and definition from an (I.A.N.A./I.A.G.G.) international consensus group. The Journal of Nutrition, Health & Aging 17(9): 726–734. doi:10.1007/s12603-013-0367-2. [DOI] [PubMed] [Google Scholar]
- Lally F., Crome P. (2007) Understanding frailty. Postgraduate Medical Journal 83(975): 16–20. doi:10.1136/pgmj.2006.048587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landi F., Russo A., Liperoti R., Tosato M., Barillaro C., Pahor M., Onder G. (2010) Anorexia, physical function, and incident disability among the frail elderly population: Results from the ilSIRENTE study. Journal of the American Medical Directors Association 11(4): 268–274. doi:10.1016/j.jamda.2009.12.088. [DOI] [PubMed] [Google Scholar]
- Latham N., Liu C. (2010) Strength training in older adults: The benefits for osteoarthritis. Clinics in Geriatric Medicine 26(3): 445–459. doi:10.1016/j.cger.2010.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malafarina V., Uriz-Otano F., Gil-Guerrero L., Iniesta R. (2013) The anorexia of ageing: Physiopathology, prevalence, associated comorbidity and mortality. A systematic review. Maturitas 74(4): 293–302. doi:10.1016/j.maturitas.2013.01.016. [DOI] [PubMed] [Google Scholar]
- Malmstrom T. K., Miller D. K., Morley J. E. (2014) A comparison of four frailty models. Journal of the American Geriatrics Society 62(4): 721–726. doi:10.1111/jgs.12735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McPhate L., Simek E. M., Haines T. P. (2013) Program-related factors are associated with adherence to group exercise interventions for the prevention of falls: A systematic review. Journal of Physiotherapy 59(2): 81–92. doi:10.1016/S1836-9553(13)70160-7. [DOI] [PubMed] [Google Scholar]
- Meuleners L. B., Fraser M. L., Bulsara M. K., Chow K., Ng J. Q. (2016) Risk factors for recurrent injurious falls that require hospitalization for older adults with dementia: A population based study. BMC Neurology 16: 188 doi:10.1186/s12883-016-0711-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieuwenhuis M. M. W., Jaarsma T., van Veldhuisen D. J., van der Wal M. H. L. (2012) Self-reported versus “true” adherence in heart failure patients: A study using the Medication Event Monitoring System. Netherlands Heart Journal: Monthly Journal of the Netherlands Society of Cardiology and the Netherlands Heart Foundation 20(7–8): 313–319. doi:10.1007/s12471-012-0283-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penko A. L., Barkley J. E., Koop M. M., Alberts J. L. (2017) Borg scale is valid for ratings of perceived exertion for individuals with Parkinson’s disease. International Journal of Exercise Science 10(1): 76–86. [PMC free article] [PubMed] [Google Scholar]
- Pulignano G., Del Sindaco D., Di Lenarda A., Tarantini L., Cioffi G., Gregori D., Minardi G. (2010) Usefulness of frailty profile for targeting older heart failure patients in disease management programs: A cost-effectiveness, pilot study. Journal of Cardiovascular Medicine (Hagerstown, Md.) 11(10): 739–747. doi:10.2459/JCM.0b013e328339d981. [DOI] [PubMed] [Google Scholar]
- Reichman W., Fiocco A., Rose N. (2010) Exercising the brain to avoid cognitive decline: Examining the evidence. Aging Health 6(5): 565–584. [Google Scholar]
- Rejeski W. J., King A. C., Katula J. A., Kritchevsky S., Miller M. E., Walkup M. P., LIFE Investigators. (2008) Physical activity in prefrail older adults: Confidence and satisfaction related to physical function. The Journals of Gerontology. Series B, Psychological Sciences and Social Sciences 63(1): P19–P26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rockwood K., Song X., MacKnight C., Bergman H., Hogan D. B., McDowell I., Mitnitski A. (2005) A global clinical measure of fitness and frailty in elderly people. CMAJ: Canadian Medical Association Journal 173(5): 489–495. doi:10.1503/cmaj.050051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosendahl E., Lindelöf N., Littbrand H., Yifter-Lindgren E., Lundin-Olsson L., Håglin L., Nyberg L. (2006) High-intensity functional exercise program and protein-enriched energy supplement for older persons dependent in activities of daily living: A randomised controlled trial. The Australian Journal of Physiotherapy 52(2): 105–113. [DOI] [PubMed] [Google Scholar]
- Sander R. (2009) Risk factors for falls. Nursing Older People 21(8): 15 doi:10.7748/nop.21.8.15.s21. [DOI] [PubMed] [Google Scholar]
- Sihvonen S., Sipilä S., Taskinen S., Era P. (2004) Fall incidence in frail older women after individualized visual feedback-based balance training. Gerontology 50(6): 411–416. doi:10.1159/000080180. [DOI] [PubMed] [Google Scholar]
- Simek E. M., McPhate L., Haines T. P. (2012) Adherence to and efficacy of home exercise programs to prevent falls: a systematic review and meta-analysis of the impact of exercise program characteristics. Preventive Medicine 55(4): 262–275. doi:10.1016/j.ypmed.2012.07.007. [DOI] [PubMed] [Google Scholar]
- Singh S., Bajorek B. (2014) Defining “elderly” in clinical practice guidelines for pharmacotherapy. Pharmacy Practice 12(4): Retrieved from http://www.ncbi.nlm.nih.gov/pmc/articles/PMC4282767/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D., Lovell J., Weller C., Kennedy B., Winbolt M., Young C., Ibrahim J. (2017) A systematic review of medication non-adherence in persons with dementia or cognitive impairment. PLoS One 12(2): e0170651 doi:10.1371/journal.pone.0170651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivas-Shankar U., Roberts S. A., Connolly M. J., O’Connell M. D. L., Adams J. E., Oldham J. A., Wu F. C. W. (2010) Effects of testosterone on muscle strength, physical function, body composition, and quality of life in intermediate-frail and frail elderly men: A randomized, double-blind, placebo-controlled study. The Journal of Clinical Endocrinology and Metabolism 95(2): 639–650. doi:10.1210/jc.2009-1251. [DOI] [PubMed] [Google Scholar]
- Sternberg S. A., Wershof Schwartz A., Karunananthan S., Bergman H., Mark Clarfield A. (2011) The identification of frailty: A systematic literature review. Journal of the American Geriatrics Society 59(11): 2129–2138. doi:10.1111/j.1532-5415.2011.03597.x. [DOI] [PubMed] [Google Scholar]
- Stubbs B., Brefka S., Denkinger M. D. (2015) What works to prevent falls in community-dwelling older adults? Umbrella review of meta-analyses of randomized controlled trials. Physical Therapy 95(8): 1095–1110. doi:10.2522/ptj.20140461. [DOI] [PubMed] [Google Scholar]
- Sullivan D. H., Roberson P. K., Johnson L. E., Bishara O., Evans W. J., Smith E. S., Price J. A. (2005) Effects of muscle strength training and testosterone in frail elderly males. Medicine and Science in Sports and Exercise 37(10): 1664–1672. [DOI] [PubMed] [Google Scholar]
- Sullivan D. H., Roberson P. K., Smith E. S., Price J. A., Bopp M. M. (2007) Effects of muscle strength training and megestrol acetate on strength, muscle mass, and function in frail older people. Journal of the American Geriatrics Society 55(1): 20–28. doi:10.1111/j.1532-5415.2006.01010.x. [DOI] [PubMed] [Google Scholar]
- Sutton J. L., Gould R. L., Daley S., Coulson M. C., Ward E. V., Butler A. M., Howard R. J. (2016) Psychometric properties of multicomponent tools designed to assess frailty in older adults: A systematic review. BMC Geriatrics 16: 55 doi:10.1186/s12877-016-0225-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor C. A., Bell J. M., Breiding M. J., Xu L. (2017) Traumatic brain injury-related emergency department visits, hospitalizations, and deaths—United States, 2007 and 2013. Morbidity and Mortality Weekly Report. Surveillance Summaries (Washington, DC: 2002) 66(9): 1–16. doi:10.15585/mmwr.ss6609a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theou O., Stathokostas L., Roland K. P., Jakobi J. M., Patterson C., Vandervoort A. A., Jones G. R. (2011) The effectiveness of exercise interventions for the management of frailty: A systematic review. Journal of Aging Research 2011: 569194 doi:10.4061/2011/569194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timonen L., Rantanen T., Mäkinen E., Timonen T. E., Törmäkangas T., Sulkava R. (2006) Effects of a group-based exercise program on functional abilities in frail older women after hospital discharge. Aging Clinical and Experimental Research 18(1): 50–56. doi:10.1007/BF03324640. [DOI] [PubMed] [Google Scholar]
- Twardowska-Rajewska J. (2006) Short programme of the rehabilitation of seniors for minimizing balance disturbances. Preliminary report. Polish Gerontology 14(1): 41–45. [Google Scholar]
- Valiengo L., Stella F., Forlenza O. V. (2016) Mood disorders in the elderly: prevalence, functional impact, and management challenges. Neuropsychiatric Disease and Treatment 12: 2105–2114. doi:10.2147/NDT.S94643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Wal M. H. L., Jaarsma T. (2008) Adherence in heart failure in the elderly: Problem and possible solutions. International Journal of Cardiology 125(2): 203–208. doi:10.1016/j.ijcard.2007.10.011. [DOI] [PubMed] [Google Scholar]
- van der Wardt V., Hancox J., Gondek D., Logan P., Das Nair R., Pollock K., Harwood R. (2017) Adherence support strategies for exercise interventions in people with mild cognitive impairment and dementia: A systematic review. Preventive Medicine Reports 7: 38–45. doi:10.1016/j.pmedr.2017.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Haehling S., Anker S. D. (2014) Treatment of cachexia: An overview of recent developments. Journal of the American Medical Directors Association 15(12): 866–872. doi:10.1016/j.jamda.2014.09.007. [DOI] [PubMed] [Google Scholar]
- Walston J., Hadley E. C., Ferrucci L., Guralnik J. M., Newman A. B., Studenski S. A., Fried L. P. (2006) Research agenda for frailty in older adults: Toward a better understanding of physiology and etiology: Summary from the American Geriatrics Society/National Institute on Aging Research Conference on Frailty in Older Adults. Journal of the American Geriatrics Society 54(6): 991–1001. doi:10.1111/j.1532-5415.2006.00745.x. [DOI] [PubMed] [Google Scholar]
- Weber V., White A., McIlvried R. (2008) An electronic medical record (EMR)-based intervention to reduce polypharmacy and falls in an ambulatory rural elderly population. Journal of General Internal Medicine 23(4): 399–404. doi:10.1007/s11606-007-0482-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis S. L., Tennstedt S. L., Marsiske M., Ball K., Elias J., Koepke K. M., ACTIVE Study Group (2006) Long-term effects of cognitive training on everyday functional outcomes in older adults. JAMA 296(23): 2805–2814. doi:10.1001/jama.296.23.2805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson M.-M. G., Thomas D. R., Rubenstein L. Z., Chibnall J. T., Anderson S., Baxi A., Morley J. E. (2005) Appetite assessment: Simple appetite questionnaire predicts weight loss in community-dwelling adults and nursing home residents. The American Journal of Clinical Nutrition 82(5): 1074–1081. [DOI] [PubMed] [Google Scholar]
- Wilson N. M., Hilmer S. N., March L. M., Cameron I. D., Lord S. R., Seibel M. J., Sambrook P. N. (2011) Associations between drug burden index and falls in older people in residential aged care. Journal of the American Geriatrics Society 59(5): 875–880. doi:10.1111/j.1532-5415.2011.03386.x. [DOI] [PubMed] [Google Scholar]
- Woolcott J. C., Richardson K. J., Wiens M. O., Patel B., Marin J., Khan K. M., Marra C. A. (2009) Meta-analysis of the impact of 9 medication classes on falls in elderly persons. Archives of Internal Medicine 169(21): 1952–1960. doi:10.1001/archinternmed.2009.357. [DOI] [PubMed] [Google Scholar]
- Xue Q.-L. (2011) The frailty syndrome: Definition and natural history. Clinics in Geriatric Medicine 27(1): 1–15. doi:10.1016/j.cger.2010.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zia A., Kamaruzzaman S. B., Tan M. P. (2015) Polypharmacy and falls in older people: Balancing evidence-based medicine against falls risk. Postgraduate Medicine 127(3): 330–337. doi:10.1080/00325481.2014.996112. [DOI] [PubMed] [Google Scholar]
- Ziere G., Dieleman J. P., Hofman A., Pols H. A. P., van der Cammen T. J. M., Stricker B. H. C. (2006) Polypharmacy and falls in the middle age and elderly population. British Journal of Clinical Pharmacology 61(2): 218–223. doi:10.1111/j.1365-2125.2005.02543.x. [DOI] [PMC free article] [PubMed] [Google Scholar]