Invasive fusariosis has a global increasing incidence and has emerged as a severe infection in nonneutropenic patients.
Keywords: Fusarium, invasive fusariosis, incidence, mortality, neutropenia, fusariosis, Spain, fungi, fungal infections, mycotic diseases
Abstract
Invasive fusariosis (IF) is associated with severe neutropenia in patients with concurrent hematologic conditions. We conducted a retrospective observational study to characterize the epidemiology of IF in 18 Spanish hospitals during 2000–2015. In that time, the frequency of IF in nonneutropenic patients increased from 0.08 cases per 100,000 admissions in 2000–2009 to 0.22 cases per 100,000 admissions in 2010–2015. Nonneutropenic IF patients often had nonhematologic conditions, such as chronic cardiac or lung disease, rheumatoid arthritis, history of solid organ transplantation, or localized fusariosis. The 90-day death rate among nonneutropenic patients (28.6%) and patients with resolved neutropenia (38.1%) was similar. However, the death rate among patients with persistent neutropenia (91.3%) was significantly higher. We used a multivariate Cox regression analysis to characterize risk factors for death: persistent neutropenia was the only risk factor for death, regardless of antifungal therapy.
Invasive fusariosis (IF) is a fungal disease that mostly affects patients with hematologic malignancies or who have received hematopoietic cell transplants. These patients often have prolonged and profound neutropenia, low levels of T cells, or both (1,2). Despite advances in early diagnosis and treatment, IF remains associated with high morbidity and death rates (3,4).
Most studies on this fungal disease have occurred in North and South America (4–9). However, the epidemiology of IF in Europe has not been fully characterized; within Europe, most multicenter studies on IF have occurred in Italy and France (8,10–12). A multicenter study by the European Confederation of Medical Mycology reported 76 cases of IF during 2007–2012 (13). Most (60%) of these IF cases occurred in Italy but none in Spain. Previous single-center studies in Europe have reported regional differences in the distribution of Fusarium species and their susceptibilities to antimicrobial drugs, highlighting the importance of monitoring local epidemiologic data (13–15). We characterized the epidemiology of IF in Spain using a retrospective observational study that examined the effects of clinical and etiologic characteristics on outcomes in a cohort of 58 IF patients at hospitals in Spain.
Methods
We conducted this study in 18 hospitals in Spain, 8 of which belong to the Spanish Network for Research in Infectious Diseases, Instituto de Salud Carlos III, in Madrid, Spain. The study was approved by Reina Sofía University Hospital Institutional Review Board (Córdoba, Spain), which waived the need to obtain written informed consent. During January 2000–December 2015, hospital staff reviewed microbiological and pathologic registries to identify cases of IF. Data were recorded in a password-protected, electronic clinical research file. We monitored the collected data for missing information, inconsistencies, and ambiguities; when necessary, we sent queries to the appropriate hospitals for clarification.
We conducted a blind review of reported cases of IF. In this study, we included only proven cases of IF according to the consensus definitions of invasive fungal diseases established by the European Organization for Research and Treatment of Cancer and the Mycoses Study Group Education and Research Consortium (16,17). IF can be proven in 4 ways: microscopic examination of a specimen obtained by needle aspiration or biopsy that documents hyphae and isolates Fusarium spp. in the same tissue; blood culture yielding Fusarium spp. alongside signs consistent with an infectious disease process; isolation of Fusarium spp. in a normally sterile site (excluding bronchoalveolar lavage fluid, a paranasal or mastoid sinus cavity specimen, and urine) with accompanying signs of infection; or amplification and sequencing of Fusarium DNA in formalin-fixed paraffin-embedded tissue (16,17).
The participating hospitals identified Fusarium isolates using genetic sequencing, matrix-assisted laser desorption/ionization time-of-flight (MALDI-TOF) mass spectrometry, or morphologic characteristics. In addition, all biological samples available at the time of this retrospective study (i.e., Fusarium isolates and specimens for biopsies) were sent to the Mycology Reference Laboratory at the Instituto de Salud Carlos III for confirmatory genetic sequencing. The isolates were freeze-dried on potato agar slants on arrival and stored in distilled water. Molecular identification was based on the translation elongation factor 1α gene (18). Isolates were cultured in GYEP medium (0.3% yeast extract, 1.0% peptone; Francisco Soria Melguizo S.A., http://www.f-soria.es) with 2.0% glucose (Sigma-Aldrich Inc., https://www.sigmaaldrich.com) at 30°C for 24–48 h. Genomic DNA was isolated by using an extraction procedure described previously (19). Internal transcribed spacer region and a portion of the translation elongation factor 1α gene region were amplified as previously described (20). Alignment results were obtained and edited using Lasergene MegAlign Pro software from DNASTAR, Inc. (https://www.dnastar.com). All sequences were compared with reference sequences from GenBank and MycoBank (https://www.mycobank.org) databases by using InfoQuest FP version 4.50 software (Bio-Rad Laboratories, https://www.bio-rad.com). We also used an in-house database of the Mycology Reference Laboratory. When only biopsy specimens were available, we used real-time PCR specific for Fusarium species as previously described (21). When identified cases did not have available isolates or biologic material for PCR, we reported the specific Fusarium species only if obtained by MALDI-TOF mass spectrometry in conjunction with histopathology. We reported the remaining IF cases in patients with compatible clinical signs and symptoms as caused by Fusarium spp.
We defined survival as the time between the date of diagnosis and death or end of follow-up care (i.e., 90 days). We defined the date of diagnosis as the day of the first Fusarium-positive culture. We defined disseminated fusariosis as the involvement of >1 noncontiguous organ. We did not consider fungemia cases as disseminated disease unless >1 organ was involved (e.g., skin, lung, or sinuses) (3). We defined neutropenia as a blood neutrophil count <500 cells/mm3 temporally related to the onset of fungal disease. We defined persistent neutropenia as cases with continued low neutrophil counts at the end of treatment or death. We defined resolution of neutropenia as a persistent recovery of blood neutrophil count >500/mm3 as determined by available hospital records.
We calculated the incidence of IF using total admissions in the participating hospitals as the denominator; we expressed the incidence as the number of cases per 100,000 admissions. For statistical purposes, we arbitrarily defined 2 time periods: 2000–2009 and 2010–2015. We compared incidences between the different periods by χ2 test using WinPepi version 11.65 (Brixton Health, http://www.brixtonhealth.com). We considered p<0.05 to be significant.
We collected variables describing patient demographic data, concurrent conditions, neutropenia, receipt of corticosteroids, clinical manifestations, diagnostic procedures, treatment, and outcome (i.e., 90-day survival). We compared categorical variables using χ2 or 2-tailed Fisher exact test, and we compared continuous variables using the Mann-Whitney U test. We conducted all statistical tests with SPSS Statistics 16.0 (IBM Inc., https://www.ibm.com) and R version 3.5.0 (R Foundation for Statistical Computing, https://www.r-project.org); we considered 2-sided p values <0.05 to be significant. We constructed unadjusted Kaplan–Meier curves and compared them using the log-rank test. In addition, we evaluated factors associated with 90-day survival. We evaluated the following variables by univariate and multivariable Cox regression analyses: age, sex, age-adjusted Charlson comorbidity index (22), underlying disease, receipt of antifungals and corticosteroids in the previous 30 days, neutropenia at diagnosis of fusariosis and at the end of follow-up, clinical manifestations, and primary treatment (monotherapy with a lipid formulation of amphotericin B, monotherapy with voriconazole, or combination treatment). We entered variables with p<0.1 by univariate analysis into the multivariate analysis; we included variables with p<0.05 by the multivariate analysis in the final model. We evaluated the prediction accuracy of the final Cox model using the area under the receiver operating characteristic curve.
Results
Incidence of IF
We identified 75 patients with Fusarium spp. isolated from clinical samples at 18 hospitals in Spain (Appendix Table 1) during 2000–2015. We excluded 10 patients with superficial infections and 7 with probable cases. In this study, the overall incidence of IF during 2000–2015 was 0.55 cases/100,000 admissions, corresponding to 0.42 neutropenic and 0.13 nonneutropenic patients/100,000 admissions (p<0.01). The overall incidence of IF was 0.40 cases/100,000 admissions during 2000–2009 and 0.79 cases/100,000 admissions during 2010–2015 (p<0.01). Among neutropenic patients, the incidence of IF increased from 0.32 to 0.57 cases/100,000 admissions (p = 0.06). Among nonneutropenic patients, the incidence of IF increased from 0.08 to 0.22 cases/100,000 admissions (p = 0.05). We also determined the annual cumulative incidence curves for the 2 groups of patients (Appendix Figure 1).
Clinical Characteristics
We identified 58 cases of IF: 44 (75.9%) occurred in neutropenic patients and 14 (24.1%) in nonneutropenic patients (Table 1). Most (59%) patients were male. The median age was 67 years (interquartile range [IQR] 38–19 years) in neutropenic patients and 45 years (IQR 28–65 years) in nonneutropenic patients (p = 0.05). In total, 36.4% of neutropenic and 42.9% of nonneutropenic patients had received corticosteroid therapy in the previous 30 days (p = 0.66); 72.7% of neutropenic and 35.7% of nonneutropenic patients had received antifungal therapy in the previous 30 days (p = 0.66). At the end of follow-up (i.e., 90 days after diagnosis), 23 (52.3%) neutropenic patients had persistent neutropenia.
Table 1. Clinical characteristics of patients with invasive fusariosis, Spain, 2000–2015*.
| Variables | Total | Nonneutropenic | Neutropenic | p value† |
|---|---|---|---|---|
| Total |
58 (100.0) |
14 (100.0) |
44 (100.0) |
|
| Sex | ||||
| M | 34 (58.6) | 8 (57.1) | 26 (59.1) | 0.9 |
| F |
24 (41.4) |
6 (42.9) |
18 (40.9) |
0.9 |
| Median age, y (IQR) |
51 (31–67) |
67 (38–79) |
45 (28–65) |
0.05‡ |
| Treatment history (previous 30 d) | ||||
| Antifungal | 37 (63.8) | 5 (35.7) | 32 (72.7) | 0.01 |
| Corticosteroid | 22 (37.9) | 6 (42.9) | 16 (36.4) | 0.66 |
| Persistent neutropenia |
23 (39.7) |
0 |
23 (52.3) |
|
| Concurrent conditions | ||||
| Hematologic malignancy | 46 (79.3) | 5 (35.7) | 41 (93.2) | <0.01§ |
| Acute myeloid leukemia | 20 (34.5) | 1 (7.1) | 19 (43.2) | |
| Acute lymphoid leukemia | 8 (13.8) | 0 | 8 (18.2) | |
| Non-Hodgkin lymphoma | 5 (8.6) | 2 (14.3) | 3 (6.8) | |
| Aplastic anemia | 4 (6.9) | 0 | 4 (9.1) | |
| Multiple myeloma | 3 (5.2) | 2 (14.3) | 1 (2.3) | |
| Myelodysplasia | 2 (3.4) | 0 | 2 (4.5) | |
| Chronic lymphoid leukemia | 2 (3.4) | 0 | 2 (4.5) | |
| Chronic myeloid leukemia | 1 (1.7) | 0 | 1 (2.3) | |
| Hodgkin´s lymphoma | 1 (1.7) | 0 | 1 (2.3) | |
| History of hematopoietic stem cell transplant | 14 (24.1) | 3 (21.4) | 11 (25.0) | 1.00§ |
| Allogenic | 9 (15.5) | 1 (7.1) | 8 (18.2) | |
| Autologous | 4 (6.9) | 2 (14.3) | 2 (4.5) | |
| Cord blood haploidentical | 1 (1.7) | 0 | 1 (2.3) | |
| Graft-versus-host disease | 5 (8.6) | 1 (7.1) | 4 (9.1) | 1.00§ |
| Acute | 3 (5.2) | 0 | 3 (6.8) | |
| Chronic | 2 (3.4) | 1 (7.1) | 1 (2.3) | |
| Solid tumor | 2 (3.4) | 1 (7.1) | 1 (2.3) | 0.43§ |
| History of solid organ transplant | 4 (6.9) | 3 (21.4) | 1 (2.3) | 0.04§ |
| Other¶ |
10 (17.2) |
5 (35.7) |
1 (2.3) |
<0.01§ |
| Clinical manifestations | ||||
| Fever | 48 (82.8) | 8 (57.1) | 40 (90.9) | <0.01§ |
| Skin lesions | 32 (55.2) | 3 (21.4) | 29 (65.9) | <0.01 |
| Lung involvement | 41 (70.7) | 9 (64.3) | 32 (72.7) | 0.74§ |
| Sinusitis | 9 (15.5) | 1 (7.1) | 8 (18.2) | 0.43§ |
| Blindness |
4 (6.9) |
0 |
4 (9.1) |
0.56§ |
| Concurrent infection | 31 (53.4) | 6 (42.9) | 25 (56.8) | 0.36 |
| Bacterial | 17 (29.3) | 2 (14.3) | 15 (34.1) | 0.18§ |
| Fungal | 4 (6.9) | 1 (7.1) | 4 (9.1) | 0.56§ |
| Viral | 6 (10.3) | 3 (21.4) | 3 (6.8) | 0.15§ |
| Polymicrobial |
4 (6.9) |
1 (7.1) |
3 (6.8) |
1.00§ |
| Type of fusariosis | ||||
| Localized | 19 (32.8) | 10 (71.4) | 9 (20.5) | <0.01§ |
| Cutaneous, localized | 3 (5.2) | 1 (7.1) | 2 (4.5) | 1.00§ |
| Pneumonia | 8 (13.8) | 5 (35.7) | 3 (6.8) | 0.02§ |
| Sinusitis | 1 (1.7) | 0 | 1 (2.3) | 1.00§ |
| Fungemia | 7 (12.1) | 4 (28.6) | 3 (6.8) | 0.05§ |
| Disseminated |
39 (67.2) |
4 (28.6) |
35 (79.5) |
<0.01§ |
| Diagnosis | ||||
| Culture | 31 (53.4) | 12 (85.7) | 19 (43.2) | <0.01 |
| Culture and histopathology | 25 (43.1) | 2 (14.3) | 23 (52.3) | 0.01 |
| Histopathology | 2 (3.4) | 0 | 2 (4.5) | 1.00 |
*Values are no. (%), except where indicated. †p values obtained by χ2 test, except where indicated. ‡Determined by Mann-Whitney U test. §Determined by Fisher exact test. ¶Other conditions reported include hemophagocytic lymphohistiocytosis, chronic cardiac disease, T-cell prolymphocytic leukemia, rheumatoid arthritis, chronic obstructive pulmonary disease, and infantile respiratory distress syndrome.
In total, 41 (93.2%) neutropenic and 5 (35.7%) nonneutropenic patients had hematologic malignancies (p<0.01). In the neutropenic patient group, the most common hematologic malignancies were acute myeloid leukemia (43.2%) and acute lymphoid leukemia (18.2%). The proportion of patients who had received hematopoietic stem cell transplants (HSCTs) was 25% (11/44; 8 allogeneic, 2 autologous, and 1 cord blood haploidentical) in neutropenic patients versus 21.4% (3/14; 1 allogeneic and 2 autologous) in nonneutropenic patients (p = 1.00). Four (9.7%) neutropenic patients and 1 (7.1%) nonneutropenic patient had graft-versus-host disease (p = 1.00). Overall, 21.4% of nonneutropenic patients and 2.3% of neutropenic patients had a history of solid organ transplantation (p = 0.04). Finally, 35.7% of nonneutropenic and 2.3% of neutropenic patients had other underlying conditions (p = 0.04). Five nonneutropenic patients had chronic cardiac disease, T-cell prolymphocytic leukemia, rheumatoid arthritis, chronic obstructive pulmonary disease, or infantile respiratory distress syndrome; 1 neutropenic patient had hemophagocytic lymphohistiocytosis.
Neutropenic patients were more likely than nonneutropenic patients to have a fever at the time of diagnosis (90.9% vs. 57.1%; p<0.01). Neutropenic patients also were more likely to have skin lesions (65.9% vs. 21.4%; p<0.01). We did not observe a difference in the proportion of patients with lung involvement in the IF infection (72.7% of neutropenic vs. 64.3% of nonneutropenic patients; p = 0.74). We also did not observe a difference in the rate of concurrent infections (56.8% of neutropenic vs. 42.9% of nonneutropenic patients; p = 0.36) (Appendix Table 2). Disseminated disease occurred more frequently among neutropenic patients (79.5%) than nonneutropenic patients (28.6%; p<0.01). In contrast, localized forms of IF, especially localized pneumonia (6.8% of neutropenic patients vs. 35.7% of nonneutropenic patients; p = 0.02) and fungemia (6.8% of neutropenic patients vs. 28.6% of nonneutropenic patients; p = 0.05), were more common among nonneutropenic patients. However, disseminated fungemia was more common among neutropenic than nonneutropenic patients (45.7% vs. 25%; p = 0.03). Among neutropenic patients, skin lesions were more common in persons with disseminated IF (77.1%) than localized IF (22.2%; p = 0.004); lung involvement also was more common among those with disseminated IF (82.9% vs. 33.3%; p = 0.007).
Nonneutropenic patients were more likely than neutropenic patients to have a diagnosis on the basis of positive culture alone (43.2% of neutropenic patients vs. 85.7% of nonneutropenic patients; p<0.01). Neutropenic patients were more likely to have a diagnosis on the basis of a positive culture and histopathology (52.3%) than nonneutropenic patients (14.3%; p = 0.01).
Isolated Fusarium Species
Thirty-six patients (62.1%) received a species-level etiologic diagnosis using molecular methods: 26 (44.8%) by genetic sequencing and 10 (17.2%) by MALDI-TOF mass spectrometry (Table 2). Among these patients, F. solani species complex (SC) was the most common (18/58; 31.0%), along with Gibberella fujikuroi SC (10/58; 17.2%), and F. oxysporum SC (5/58; 8.6%). The remaining 22 (37.9%) cases were identified as caused by Fusarium spp. Among disseminated infections, most (74.4%) were reported to species level; the most common were F. solani SC (15/39; 38.5%), F. fukikuroi SC (8/39; 20.5%), and F. oxysporum SC (3/39; 7.7%). In the cohort, disseminated IF also was caused by 2 F. brachygibbosum infections and 1 F. dimerum SC infection. Most (63.2%) localized infections were caused by Fusarium spp. F. solani SC (2/39; 5.1%), F. fukikuroi SC (2/39; 5.1%), and F. oxysporum SC (3/39; 7.7%) were the most common etiologic agents.
Table 2. Distribution of isolated Fusarium species, Spain, 2000–2015*.
| Species | Total | Localized | Disseminated | p value† |
|---|---|---|---|---|
| Total |
58 (100.0) |
19 (100.0) |
39 (100.0) |
|
|
F. solani SC |
18 (31.0) |
3 (15.8) |
15 (38.5) |
0.08‡ |
| Gibberella fujikuroi SC | 10 (17.2) | 2 (10.5) | 8 (20.5) | 0.29 |
| F. proliferatum | 6 (10.3) | 0 | 6 (15.4) | 0.08 |
| F. verticillioides | 3 (5.2) | 2 (10.5) | 1 (2.6) | 0.25 |
| F. fujikuroi |
1 (1.7) |
0 |
1 (2.6) |
0.67 |
| F. oxysporum SC | 5 (8.6) | 2 (10.5) | 3 (7.7) | 0.53 |
| F. brachygibbosum | 2 (3.4) | 0 | 2 (5.1) | 0.45 |
| F. dimerum SC | 1 (1.7) | 0 | 1 (2.6) | 0.67 |
| Fusarium spp. | 22 (37.9) | 12 (63.2) | 10 (25.6) | <0.01 |
*Values are no. (%), except where indicated. All identifications (except undefined Fusarium spp.) obtained by genetic sequencing or matrix-assisted laser desorption/ionisation time-of-flight mass spectometry. SC, species complex. †p values obtained by Fisher exact test, except where indicated. ‡Determined by χ2 test.
Therapeutic Regimens
Overall, 62.1% of patients received monotherapy (29.3% azoles, 32.8% amphotericin B), 27.6% of patients received combination therapy (e.g., azoles plus amphotericin B, mostly liposomal amphotericin B plus voriconazole), and 10.4% of patients did not receive an active treatment (3 patients were not treated, 2 received empirical caspofungin, and 1 received empirical micafungin) (Appendix Table 3). Nonneutropenic patients were more likely than neutropenic patients to receive voriconazole monotherapy (54.5% vs. 85.7%; p<0.01). We did not observe a difference in the proportions of patients who were treated with amphotericin B (36.4% of neutropenic patients vs. 21.4% of nonneutropenic patients; p = 0.35). Sixteen (36.4%) neutropenic patients received combination therapy: 14 received a lipid formulation of amphotericin B plus voriconazole and 2 received amphotericin B plus posoconazole. No nonneutropenic patients received combination therapy.
Outcomes
By 90 days after diagnosis, 56.9% of all patients had died: 28.6% of nonneutropenic patients and 65.9% of patients with neutropenia at IF onset (p = 0.01). We analyzed these results using unadjusted Kaplan–Meier survival graphs (Appendix Figure 2; p = 0.03 by log-rank test). The death rate of nonneutropenic patients (28.6%) was similar to that of patients who had recovered from neutropenia (38.1%; p = 0.56) and both rates were significantly lower than among patients with persistent neutropenia (91.3%; p<0.01).
After 90 days, 66.7% of patients with localized and 36.8% with disseminated disease had died (p = 0.03). Overall, 66.7% (2/3) of patients with localized disease of the skin/soft tissue, 50% (4/8) with lung involvement, and 14.3% (1/7) with fungemia died.
In the assessment of prognostic factors, we analyzed 50 IF patients who received active antifungal therapy. In univariate analysis, 90-day death risk was associated with neutropenia at onset of IF and persistent neutropenia. Age, sex, age-adjusted Charlson comorbidity index, corticosteroid therapy in the previous 30 days, administration of antifungal in the previous 30 days, hematologic malignancy, history of HSCT, fungemia, disseminated disease, and the treatment type (i.e., monotherapy with a lipid formulation of amphotericin B, monotherapy with azoles or combined therapy) were not associated with 90-day survival. In multivariate analysis, persistent neutropenia (hazard ratio [HR] 7.08, 95% CI 1.91–26.17; p<0.01) was significantly associated with 90-day death risk (Table 3). We calculated adjusted Kaplan–Meier survival curves for the Cox regression model (Figure; area under the receiver operating characteristic curve = 0.83).
Table 3. Univariate and multivariate Cox regression analyses for 90-day death rate in 50 patients treated for invasive fusariosis, Spain, 2000–2015*.
| Characteristic | Unadjusted hazard ratio (95% CI) | p value | Adjusted hazard ratio (95% CI) | p value |
|---|---|---|---|---|
| Sex | ||||
| M | 1.08 (0.52–2.25) | 0.84 | 0.87 (0.41–1.84) | 0.87 |
| F |
0.93 (0.45-1.93) |
0.84 |
|
|
| Median age, y (IQR) |
1.00 (0.99–1.01) |
0.94 |
|
|
| Charlson index |
1.01 (0.86–1.20) |
0.87 |
1.16 (0.96–1.40) |
0.12 |
| Treatment history (previous 30 d) | ||||
| Antifungal | 1.83 (0.81–4.14) | 0.15 | ||
| Corticosteroid |
0.69 (0.33–1.45) |
0.33 |
|
|
| Neutropenia at onset |
3.16 (0.95–10.44) |
0.06 |
|
|
| Neutropenia at the end of follow-up | ||||
| Nonneutropenia | Referent | Referent | ||
| Recovery from neutropenia | 1.47 (0.38–5.69) | 0.58 | 1.91 (0.48–7.64) | 0.35 |
| Persistent neutropenia |
5.62 (1.65–19.11) |
0.006 |
9.27 (2.43–35.42) |
0.001 |
| Hematologic malignancy | 2.44 (0.74–8.06) | 0.14 | ||
| History of hematopoietic stem cell transplant | 1.39 (0.65–2.99) | 0.4 | ||
| Fungemia | 0.21 (0.03–1.54) | 0.13 | ||
| Disseminated disease |
1.90 (0.77–4.67) |
0.16 |
|
|
| Antifungal therapy | ||||
| Monotherapy with lipid formulation of amphotericin B | 1.95 (0.92–4.12) | 0.08 | ||
| Monotherapy with azoles | 0.47 (0.19–1.15) | 0.10 | ||
| Combined therapy | 1.08 (0.50–2.33) | 0.84 |
*Area under the receiver operating characteristic curve for this model was 0.82.
Figure.
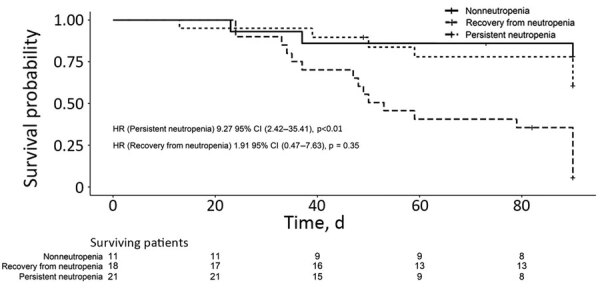
Adjusted Kaplan-Meier curves obtained from the stratified Cox regression model for 90-day survival in 50 patients treated for invasive fusariosis, Spain, 2000–2015. HR, hazard ratio.
Discussion
We described 58 cases of invasive Fusarium infections at 18 hospitals in Spain during a 15-year period (2000–2015). During this time, IF incidence increased from 0.40 (2000–2009) to 0.79 (2010–2015) cases per 100.000 admissions (p<0.01). Incidence of IF in neutropenic patients increased from 0.32 to 0.57 cases per 100,000 admissions (p = 0.06). We observed a 3-fold increase in the incidence of IF in nonneutropenic patients, from 0.08 to 0.22 cases per 100,000 admissions (p = 0.05). This increase might have been caused by an increase in the at-risk population, environmental exposure to Fusarium conidia, the increased use of antifungal prophylaxis, or a combination of these factors.
To date, the highest IF incidence rates worldwide are in healthcare centers in Brazil. A cohort of HSCT recipients at hospitals in Brazil during 1985–2001 had a 0.6% prevalence of IF (1). During 2007–2009, ≈10 years later, a prospective multicenter study by Nucci et al. reported a 3.7% IF prevalence among allogeneic HSCT patients and 3.4% among patients with acute myeloid leukemia (7). That study also found a 1-year cumulative incidence of 5.2% among those who had received an allogeneic HSCT and 3.8% among those with acute myeloid leukemia or myelodysplasia (7). A >10-fold increase, from 86 to 1,023 IF cases per 100,000 admissions, occurred among patients with hematologic malignancies at a healthcare center in Brazil from 2000–2005 to 2006–2010 (5). In the United States, a prospective multicenter study conducted by the Transplant-Associated Infection Surveillance Network during 2001–2006 reported a 1-year cumulative incidence of up to 0.3% of non-Aspergillus mold infections (23). In Italy, a multicenter retrospective study reported a 0.2% incidence of IF among patients who had received an allogenic HSCT during 1999–2003 (24).
In this cohort in Spain, IF occurred frequently in patients with hematologic conditions and in HSCT recipients. IF occurs almost entirely in markedly immunosuppressed patients who usually are neutropenic; we identified IF in nonneutropenic patients, including patients with a history of solid organ transplants, chronic cardiac or lung disease, or rheumatoid arthritis. These findings are in agreement with Park et al. (9), who studied 37 patients with IF and noted that 54.1% of IF patients were nonneutropenic, 83.8% had hematologic malignancies, and 16.2% had history of solid organ transplantation (9). Thus, nonneutropenia and certain nonhematologic conditions might not be uncommon among IF patients.
Lungs, the bloodstream, and the skin were the organs most frequently affected by IF. Fusarium species produce aleuroconidia, yeastlike structures that can invade the bloodstream and might cause fungemia and metastatic skin lesions (25).
IF is associated with high death rates. This cohort had a 90-day death rate of 56.9%, similar to rates noted in other studies (3,4,12,26). The death rates of nonneutropenic and neutropenic patients who recovered from neutropenia were similar (28.6% of nonneutropenic patients vs. 38.1% of neutropenic patients; p = 0.56), consistent with previous reports that 70% of IF cases resolve when patients recover from neutropenia (27). In this cohort, patients with persistent neutropenia had a 91.3% death rate despite antifungal therapy. Persistent neutropenia was the single most predictive prognostic factor in IF, consistent with previous reports (2).
Researchers must identify effective therapeutic strategies to improve the prognosis of IF patients with persistent neutropenia. The treatment practices observed in our study align with guidelines from the European Society of Clinical Microbiology and Infectious Diseases Fungal Infection Study Group and European Confederation of Medical Mycology (13). We examined the effects of different therapeutic regimens on 90-day death rate. We found no differences in the outcome of patients treated with voriconazole and those receiving a lipid formulation of amphotericin B. Combination therapy also was not associated with outcome, consistent with results from the largest study series conducted so far (3,12). However, these results must be interpreted with caution. Nucci et al. (3) found that patients receiving combination therapy had more severe disease (3), possibly influencing the clinician’s decision to administer 2 drugs. In our study, no nonneutropenic patients received combination therapy; further studies should examine the potential benefits of combination therapy in nonneutropenic patients. Finally, because we observed similar clinical responses in patients treated with a lipid formulation of amphotericin B or voriconazole as monotherapies, clinicians might not need to rely on antifungal susceptibility tests to guide treatment. Most clinically relevant Fusarium isolates exhibit high minimal inhibitory concentrations to most antifungals, including azoles, echinocandins, and polyenes (28–30).
Our study is subject to the limitations of retrospective observational studies. For example, the sample size was limited to the cases with available data. Despite being a multicenter study during a 15-year period, the sample size might be insufficient to detect significant differences in some groups. Furthermore, clinicians might prescribe a more potent treatment regimen for patients with severe disease, possibly skewing our analysis of treatment outcomes. Although all IF cases in this retrospective study met standard criteria (16,17), we could not determine the causative species in every case. Thus, the Fusarium species was only reported when determined by genomic sequencing or MALDI-TOF mass spectrometry, because these techniques have high agreement rates (89.8%–97.0%) (31–33). All remaining IF cases in our series were reported as Fusarium spp. We also were not able to culture all Fusarium isolates because of a lack of specimens, especially for cases occurring before 2010 in hospitals in which Fusarium isolates were not collected as part of routine procedures
In conclusion, our data show that IF is an emerging infection in Spain. We report an increase in the incidence of IF among nonneutropenic patients, including those with hematologic conditions and other concurrent conditions, such as chronic cardiac or lung diseases, rheumatoid arthritis, or history of solid organ transplants. Our results support previous studies reporting that IF survival is critically dependent on resolution of neutropenia.
Additional information on invasive fusariosis in nonneutropenic patients, Spain, 2000–2015.
Acknowledgments
This work was supported by Plan Nacional de I+D+I 2013–2016 and Instituto de Salud Carlos III, Subdirección General de Redes y Centros de Investigación Cooperativa, Ministerio de Ciencia, Innovación y Universidades, Spanish Network for Research in Infectious Diseases (REIPI RD16/0016/0008; RD16/CIII/0004/0003), cofinanced by European Development Regional Fund “A way to achieve Europe,” Operative Program Intelligent Growth 2014–2020. M.F.R. holds a “Miguel Servet” research contract (CP18/00073) from Instituto de Salud Carlos III, Madrid.
Biography
Dr. Pérez-Nadales is a research fellow at the Infectious Diseases Group at Maimonides Biomedical Research Institute of Córdoba, Spain. Her research interests include clinical and molecular epidemiology of invasive fungal infections and infections caused by multidrug-resistant bacteria.
Footnotes
Suggested citation for this article: Pérez-Nadales E, Alastruey-Izquierdo A, Linares-Sicilia MJ, Soto-Debrán JC, Abdala E, García-Rodríguez J, et al.; the Spanish Fusariosis Study Group. Invasive fusariosis in nonneutropenic patients, Spain, 2000–2015. Emerg Infect Dis. 2021 Jan [date cited]. https://doi.org/10.3201/eid2701.190782
These senior authors contributed equally to this article.
Members are listed at the end of this article.
References
- 1.Nucci M, Marr KA, Queiroz-Telles F, Martins CA, Trabasso P, Costa S, et al. Fusarium infection in hematopoietic stem cell transplant recipients. Clin Infect Dis. 2004;38:1237–42. 10.1086/383319 [DOI] [PubMed] [Google Scholar]
- 2.Nucci M, Anaissie EJ, Queiroz-Telles F, Martins CA, Trabasso P, Solza C, et al. Outcome predictors of 84 patients with hematologic malignancies and Fusarium infection. Cancer. 2003;98:315–9. 10.1002/cncr.11510 [DOI] [PubMed] [Google Scholar]
- 3.Nucci M, Marr KA, Vehreschild MJGT, de Souza CA, Velasco E, Cappellano P, et al. Improvement in the outcome of invasive fusariosis in the last decade. Clin Microbiol Infect. 2014;20:580–5. 10.1111/1469-0691.12409 [DOI] [PubMed] [Google Scholar]
- 4.Horn DL, Freifeld AG, Schuster MG, Azie NE, Franks B, Kauffman CA. Treatment and outcomes of invasive fusariosis: review of 65 cases from the PATH Alliance(®) registry. Mycoses. 2014;57:652–8. 10.1111/myc.12212 [DOI] [PubMed] [Google Scholar]
- 5.Nucci M, Varon AG, Garnica M, Akiti T, Barreiros G, Trope BM, et al. Increased incidence of invasive fusariosis with cutaneous portal of entry, Brazil. Emerg Infect Dis. 2013;19:1567–72. 10.3201/eid1910.120847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nucci M, Anaissie E. Cutaneous infection by Fusarium species in healthy and immunocompromised hosts: implications for diagnosis and management. Clin Infect Dis. 2002;35:909–20. 10.1086/342328 [DOI] [PubMed] [Google Scholar]
- 7.Nucci M, Garnica M, Gloria AB, Lehugeur DS, Dias VCH, Palma LC, et al. Invasive fungal diseases in haematopoietic cell transplant recipients and in patients with acute myeloid leukaemia or myelodysplasia in Brazil. Clin Microbiol Infect. 2013;19:745–51. 10.1111/1469-0691.12002 [DOI] [PubMed] [Google Scholar]
- 8.Girmenia C, Pagano L, Corvatta L, Mele L, del Favero A, Martino P. The epidemiology of fusariosis in patients with haematological diseases. Gimema Infection Programme. Br J Haematol. 2000;111:272–6. [DOI] [PubMed] [Google Scholar]
- 9.Park BJ, Pappas PG, Wannemuehler KA, Alexander BD, Anaissie EJ, Andes DR, et al. Invasive non-Aspergillus mold infections in transplant recipients, United States, 2001-2006. Emerg Infect Dis. 2011;17:1855–64. 10.3201/eid1710.110087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hennequin C, Lavarde V, Poirot JL, Rabodonirina M, Datry A, Aractingi S, et al. Invasive Fusarium infections: a retrospective survey of 31 cases. The French ‘Groupe d’Etudes des Mycoses Opportunistes’ GEMO. J Med Vet Mycol. 1997;35:107–14. 10.1080/02681219780000991 [DOI] [PubMed] [Google Scholar]
- 11.Pagano L, Caira M, Candoni A, Offidani M, Fianchi L, Martino B, et al. The epidemiology of fungal infections in patients with hematologic malignancies: the SEIFEM-2004 study. Haematologica. 2006;91:1068–75. [PubMed] [Google Scholar]
- 12.Lortholary O, Obenga G, Biswas P, Caillot D, Chachaty E, Bienvenu A-L, et al. ; French Mycoses Study Group. International retrospective analysis of 73 cases of invasive fusariosis treated with voriconazole. Antimicrob Agents Chemother. 2010;54:4446–50. 10.1128/AAC.00286-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tortorano AM, Prigitano A, Esposto MC, Arsic Arsenijevic V, Kolarovic J, Ivanovic D, et al. ; ECMM Working Group. European Confederation of Medical Mycology (ECMM) epidemiological survey on invasive infections due to Fusarium species in Europe. Eur J Clin Microbiol Infect Dis. 2014;33:1623–30. 10.1007/s10096-014-2111-1 [DOI] [PubMed] [Google Scholar]
- 14.Tortorano AM, Prigitano A, Dho G, Esposto MC, Gianni C, Grancini A, et al. Species distribution and in vitro antifungal susceptibility patterns of 75 clinical isolates of Fusarium spp. from northern Italy. Antimicrob Agents Chemother. 2008;52:2683–5. 10.1128/AAC.00272-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dalyan Cilo B, Al-Hatmi AMS, Seyedmousavi S, Rijs AJMM, Verweij PE, Ener B, et al. Emergence of fusarioses in a university hospital in Turkey during a 20-year period. Eur J Clin Microbiol Infect Dis. 2015;34:1683–91. 10.1007/s10096-015-2405-y [DOI] [PubMed] [Google Scholar]
- 16.Donnelly JP, Chen SC, Kauffman CA, Steinbach WJ, Baddley JW, Verweij PE, et al. ; Revision and Update of the Consensus Definitions of Invasive Fungal Disease From the European Organization for Research and Treatment of Cancer and the Mycoses Study Group Education and Research Consortium. Revision and Update of the Consensus Definitions of Invasive Fungal Disease From the European Organization for Research and Treatment of Cancer and the Mycoses Study Group Education and Research Consortium. Clin Infect Dis. 2020;71:1367–76. 10.1093/cid/ciz1008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.De Pauw B, Walsh TJ, Donnelly JP, Stevens DA, Edwards JE, Calandra T, et al. ; European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group; National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clin Infect Dis. 2008;46:1813–21. 10.1086/588660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Geiser DM, del Mar Jiménez-Gasco M, Kang S, Makalowska I, Veeraraghavan N, Ward TJ, et al. FUSARIUM-ID v. 1.0: A DNA sequence database for identifying Fusarium. Eur J Plant Pathol. 2004;110:473–9. 10.1023/B:EJPP.0000032386.75915.a0 [DOI] [Google Scholar]
- 19.Holden D. DNA mini prep method for Aspergillus fumigatus (and other filamentous fungi). In: Maresca B, Kobayashi G, editors. Molecular biology of pathogenic fungi, a laboratory manual. New York: Telos Press; 1994. p. 3–4. [Google Scholar]
- 20.Alastruey-Izquierdo A, Alcazar-Fuoli L, Rivero-Menéndez O, Ayats J, Castro C, García-Rodríguez J, et al. Molecular identification and susceptibility testing of molds isolated in a prospective surveillance of triazole resistance in Spain (FILPOP2 Study). Antimicrob Agents Chemother. 2018;62.AAC.00358-18. [DOI] [PMC free article] [PubMed]
- 21.Bernal-Martínez L, Buitrago MJ, Castelli MV, Rodríguez-Tudela JL, Cuenca-Estrella M. Detection of invasive infection caused by Fusarium solani and non-Fusarium solani species using a duplex quantitative PCR-based assay in a murine model of fusariosis. Med Mycol. 2012;50:270–5. 10.3109/13693786.2011.604047 [DOI] [PubMed] [Google Scholar]
- 22.Charlson M, Szatrowski TP, Peterson J, Gold J. Validation of a combined comorbidity index. J Clin Epidemiol. 1994;47:1245–51. 10.1016/0895-4356(94)90129-5 [DOI] [PubMed] [Google Scholar]
- 23.Kontoyiannis DP, Marr KA, Park BJ, Alexander BD, Anaissie EJ, Walsh TJ, et al. Prospective surveillance for invasive fungal infections in hematopoietic stem cell transplant recipients, 2001-2006: overview of the Transplant-Associated Infection Surveillance Network (TRANSNET) Database. Clin Infect Dis. 2010;50:1091–100. 10.1086/651263 [DOI] [PubMed] [Google Scholar]
- 24.Pagano L, Caira M, Nosari A, Van Lint MT, Candoni A, Offidani M, et al. Fungal infections in recipients of hematopoietic stem cell transplants: results of the SEIFEM B-2004 study—Sorveglianza Epidemiologica Infezioni Fungine Nelle Emopatie Maligne. Clin Infect Dis. 2007;45:1161–70. 10.1086/522189 [DOI] [PubMed] [Google Scholar]
- 25.Anaissie E, Grazziutti M, Nucci M. Invasive fungal infections in cancer patients. In: Anaissie E, McGinnis M, Pfaller M, editors. Clinical mycology. 2nd ed. Amsterdam: Elsevier; 2009. p. 431–71. [Google Scholar]
- 26.Campo M, Lewis RE, Kontoyiannis DP. Invasive fusariosis in patients with hematologic malignancies at a cancer center: 1998-2009. J Infect. 2010;60:331–7. 10.1016/j.jinf.2010.01.010 [DOI] [PubMed] [Google Scholar]
- 27.Lionakis MS, Kontoyiannis DP. Fusarium infections in critically ill patients. Semin Respir Crit Care Med. 2004;25:159–69. 10.1055/s-2004-824900 [DOI] [PubMed] [Google Scholar]
- 28.Al-Hatmi AMS, van Diepeningen AD, Curfs-Breuker I, de Hoog GS, Meis JF. Specific antifungal susceptibility profiles of opportunists in the Fusarium fujikuroi complex. J Antimicrob Chemother. 2015;70:1068–71. [DOI] [PubMed] [Google Scholar]
- 29.Alastruey-Izquierdo A, Cuenca-Estrella M, Monzón A, Mellado E, Rodríguez-Tudela JL. Antifungal susceptibility profile of clinical Fusarium spp. isolates identified by molecular methods. J Antimicrob Chemother. 2008;61:805–9. 10.1093/jac/dkn022 [DOI] [PubMed] [Google Scholar]
- 30.Al-Hatmi AMS, Normand A-C, Ranque S, Piarroux R, de Hoog GS, Meletiadis J, et al. Comparative evaluation of Etest, EUCAST, and CLSI Methods for amphotericin B, voriconazole, and posaconazole against clinically relevant Fusarium species. Antimicrob Agents Chemother. 2016;61:e01671–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marinach-Patrice C, Lethuillier A, Marly A, Brossas JY, Gené J, Symoens F, et al. Use of mass spectrometry to identify clinical Fusarium isolates. Clin Microbiol Infect. 2009;15:634–42. 10.1111/j.1469-0691.2009.02758.x [DOI] [PubMed] [Google Scholar]
- 32.Triest D, Stubbe D, De Cremer K, Piérard D, Normand AC, Piarroux R, et al. Use of matrix-assisted laser desorption ionization-time of flight mass spectrometry for identification of molds of the Fusarium genus. J Clin Microbiol. 2015;53:465–76. 10.1128/JCM.02213-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Paziani MH, Tonani Carvalho L, Carvalho Melhem MS, Gottardo de Almeida MT, Nadaletto Bonifácio da Silva ME, Martinez R, et al. First comprehensive report of clinical Fusarium strains isolated in the state of Sao Paulo (Brazil) and identified by MALDI-TOF MS and molecular biology. Microorganisms. 2019;8:66. 10.3390/microorganisms8010066 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional information on invasive fusariosis in nonneutropenic patients, Spain, 2000–2015.


