Abstract
Pheochromocytomas/paragangliomas (PPGLs) are rare neuroendocrine tumours linked to more than 15 susceptibility genes. PPGLs present with very different genotype/phenotype correlations. Certainly, depending on the mutated gene, and the activated intracellular signalling pathways, as well as their metastatic potential, each tumour is immensely different. One of the major challenges in in vitro research, whatever the study field, is to choose the best cellular model for that study. Unfortunately, most of the time there is not ‘a best’ cell model. Thus, in order to avoid observations that could be related to and/or dependent on a specific cell line, researchers often perform the same experiments using different cell lines simultaneously. The situation is even more complicated when there are only very few cell models obtained in different species for a disease. This is the case for PPGLs. In this review, we will describe the characteristics of the different cell lines and of mouse models, trying to understand if there is one that is more appropriate to use, depending on which aspect of the tumours one is trying to investigate.
Keywords: pheochromocytoma/paraganglioma, cell models, animal models
Introduction
In 2017, the World Health Organization (WHO) of Endocrine Tumours recognised anatomic criteria for PPGL classification (1). Pheochromocytomas (PCCs) are tumours originating from the neural crest-derived chromaffin cells within the adrenal medulla. They commonly produce one or more catecholamines: dopamine (DA), noradrenaline (NA or norepinephrine) or adrenaline (A or epinephrine). The presence of secretory granules at ultrastructure is a diagnostic feature of pheochromocytoma. Paragangliomas (PGLs) derive from extra-adrenal paraganglia cells and can be further classified according to their clinical and biological characteristics. In fact, they can be categorised into two main classes. The first one is Head and Neck (HN)-PGLs. They owe their name to their usual association with branches of the vagus and glossopharyngeal nerves, and typically lack catecholamine secretion (2). They are mostly found at the bifurcation of the common carotid artery, where the carotid body is located, but they may also form in the jugular, tympanic, vagal, or laryngeal paraganglia (3) or in other locations (4, 5). The second class is Sympathetic PGLs that form in sympathetic paraganglia and are biochemically active, since they secrete catecholamines. Despite showing a ubiquitous distribution, from the skull base to the pelvic floor, approximately 85% develop below the diaphragm (3). The standard treatment of PPGLs is the surgical removal of the tumour (6). Since there is no histological system that is currently endorsed for understanding the biological aggressiveness of these tumours, all PPGLs have metastatic potential and their metastatic nature is determined by the presence or lack of distant metastases in sites where normal paraganglia are not found, more specifically bone and histologically confirmed lymph nodes (1). Unfortunately, there is no available cure for metastatic lesions, and understanding the cancer driver events is crucial for the development of targeted therapies.
Pheochromocytomas and paragangliomas (PPGLs)
PCC and PPGLs are rare tumours with variable aggressiveness characterised by a large spectrum of hereditary predisposition. Indeed, over one-third of PPGLs are associated with inherited susceptibility genes, which is the highest rate among all tumour types (7). Mutations have been identified in more than 15 genes and, based on gene profiling, PPGLs can be assigned to three different ‘clusters’ (for recent reviews on the genetic/phenotype correlations of PPGLs see: (8, 9, 10)). PPGLs with mutations in genes encoding the hypoxia-inducible factor (HIF) 2α, von Hippel–Lindau tumour suppressor (VHL), prolyl hydroxylase domain (PHD), fumarate hydratase (FH), and succinate dehydrogenase subunits (SDHx) are included in cluster 1. These tumours are characterised by the activation of pseudohypoxic pathways and by an immature catecholamine-secreting phenotype. Intriguingly, tumours mutated in one of the enzymes involved in the Krebs cycle are more aggressive than others, showing an elevated metastatic potential. Cluster 2 includes PPGLs with mutations in the RE arranged during Transfection (RET) proto-oncogene, Neurofibromatosis type 1 (NF1) tumour suppressor gene, TransMEMbrane protein (TMEM127) gene, Harvey rat sarcoma viral oncogene homolog (HRAS) and MYC Associated Factor X (MAX) gene. Cluster 2 PPGLs show activated MAPK and mTOR signalling pathways, are mostly benign and exhibit a mature catecholamine phenotype with strong expression of phenylethanolamine N-methyltransferase (PNMT), which is the enzyme that converts NA to A. Recently, introducing also a cluster 3 has been suggested, which includes tumours associated with mutations in the Mastermind Like Transcriptional Coactivator 3 (MAML3) gene, which is involved in the Wnt signalling pathway. These PPGLs are characterised by a high Ki67 expression, aggressive behaviour and early metastatic dissemination.
Tumour biology
As reported previously, a common feature of cluster 1 tumours is the activation of HIFs. For this reason, scientists refer to them as pseudohypoxia-driven tumours. Physiologically, in response to low extracellular oxygen levels (i.e. hypoxia), HIF activated pathways are induced. In cluster 1 tumours, HIF pathways are activated regardless of the extracellular oxygen concentration, a condition defined as pseudohypoxia. HIFs are heterodimeric transcription factors and their inducible components (HIF1α and HIF2α) are very closely regulated by proteasomal degradation, which occurs after their hydroxylation by the enzyme PHD1/2. Mutations in some of the susceptibility genes cause an impairment of the Krebs cycle, resulting in an accumulation of the oncometabolites succinate, fumarate, or 2-hydroxyglutarate, that in turn leads to the inhibition of PHD1/2. Inactivation of PHD1/2 results in a decrease of HIF-α hydroxylation that cannot be degraded any longer (11, 12, 13, 14). Therefore, cluster 1 mutations promote angiogenesis, tumour invasion, and metastasis.
Mutations of genes belonging to cluster 2 lead to activation of the phosphatidylinositol-3-kinase (PI3K)/AKT, mammalian target of rapamycin (mTORC1)/p70S6 kinase (p70S6K), and RAS/RAF/ERK signalling pathways, which in turn promote cell proliferation, survival, cancer development and angiogenesis.
The adrenal gland is the body’s only significant source of circulating A. Tumours of cluster 2 are predominantly intra-adrenal and thus secrete A, while the extra-adrenal ones produce NA and/or dopamine. Conversely, cluster 1 tumours, even those occurring in the adrenal gland, which are often associated with VHL mutations, do not produce significant A (15).
Tumours mutated for the Cold Shock Domain-containing E1 (CSDE1) and the MAML3 fusion genes belong to cluster 3. MAML3 mutations lead to over-activation of Wnt and Hedgehog signalling and MAML3-mutated PPGLs showed elevated Ki-67 expression, aggressive behaviour and early metastatic spread. CSDE1 mutations lead to over-activation of β-catenin, a target of Wnt signalling, and favour tumour proliferation, invasion, and metastases. The catecholamine phenotype of these tumours is rather unknown (15).
Cell models
Rat pheochromocytoma (PC12)
The adrenal rat pheochromocytoma (PC12) cell line was originally isolated from a PCC developed in an irradiated rat in 1976 (16). This cell line has the characteristic of precursor cells for both sympathetic neurons and chromaffin cells. PC12 can differentiate towards a neuronal phenotype in response to nerve growth factor (NGF) (17), which causes these cells to extend long processes and become electrically excitable (18). In contrast, dexamethasone (DEX) treatment induces a more endocrine-like phenotype and upregulates catecholamine synthesis and storage (19, 20). The responsiveness to NGF and DEX by PC12 cells has allowed them to be used as a model of neuronal differentiation and pluripotency. Chromaffin cells and sympathetic neurons derived from the neural crest and the lineages diverge very early, nevertheless, some transdifferentiation can occur (21, 22, 23). These cells are widely utilised as a model to study synthesis, storage, and secretion of catecholamines and regulation of nervous system development. In addition, PC12 cells are also used for the study of the differentiation effects on exocytotic vesicles and, since PC12 cells present some advantages over other cell models for neurosecretion, including chromaffin cells, it is likely that PC12 will continue to be used as a model to study exocytosis (24). PC12 cells are important also for PPGLs studies. Indeed, they express several of the catecholamine biosynthetic enzymes, including tyrosine hydroxylase (TH) along with the enzymes converting L-DOPA to DA and DA to NA, that is, aromatic l-amino acid decarboxylase (AADC) and DA β-hydroxylase (DBH), respectively. However, the final enzyme in the catecholamine biosynthetic pathway, PNMT, which produces A from NA, is not expressed in these cells. Indeed, PC12 cells are noradrenergic (16). Nevertheless, dexamethasone promotes the expression of the catecholamine biosynthetic enzymes, including PNMT (25). Of note, in these cells, functional MAX protein is not expressed. Hopewell and colleagues have identified a homozygous mutation of MAX gene, which is consistent with the aberrant processing of MAX transcripts in these cells. It has been suggested that the loss of MAX expression may have been a selected event either in the development of the rat PCC or during subsequent cell culturing in vitro (26).
Mouse pheochromocytoma cell line (MPC) and mouse tumour tissue cells (MTT)
In 1994, Jacks et al. constructed a mouse strain with a knock-out (KO) mutation of Nf1, the murine counterpart of NF1 (27). This mutation was associated with the frequent development of PCCs. Tischler et al. characterised these tumours and isolated cells cultured in vitro (28). A few years later, in the same laboratory, five mouse PCC cell lines (MPC) were stabilised from primary cultures of PCCs arising in mice with a Nf1 heterozygous KO mutation. The cell line generally employed for the preclinical studies pertaining to human tumours is MPC4/30/PRR. These cells typically showed extensive spontaneous neuronal differentiation and expressed neurofibromin. Nevertheless, subsequent studies demonstrated that the wild type Nf1 allele was not present, possibly indicating that the protein produced was functionally defective (29).
MPC is a valid tool for studying genes and signalling pathways that regulate cell growth and differentiation in adrenal medullary neoplasms, and are a unique model for studying the regulation of PNMT expression (30). Indeed, a particularly interesting characteristic of the mouse PCCs is positive staining for PNMT and their ability to produce A. Moreover, MPC is also considered as a useful model for studying neurotransmitter release and neuroendocrine secretion (20).
The less differentiated derivative of MPC, designated as MTT (for mouse tumour tissue-derived) cells, was then established from MPC tumour tissue formed after reinjection of the original cell line into nude mice. These cells are characterised by the development of solid organ metastatic lesions, predominantly in the liver, 4 weeks after injection. The MTT cells maintain a PCC phenotype, as confirmed by measuring intracellular catecholamines, by evaluating the expression of TH and PNMT along with monitoring the presence of dense-core secretory granules. Furthermore, gene expression array analysis on MTT revealed genes that may be crucial for the development of an aggressive and more malignant phenotype of PCC (31). The MPC and MTT lines are complementary for drug testing purposes, in that MTT best reflects aggressive metastases, while MPC is better differentiated and more comparable to slow-growing, hormonally active metastases (32). Another interesting aspect of MPC and MTT is that they do not express HIF2α, but only HIF1α (13).
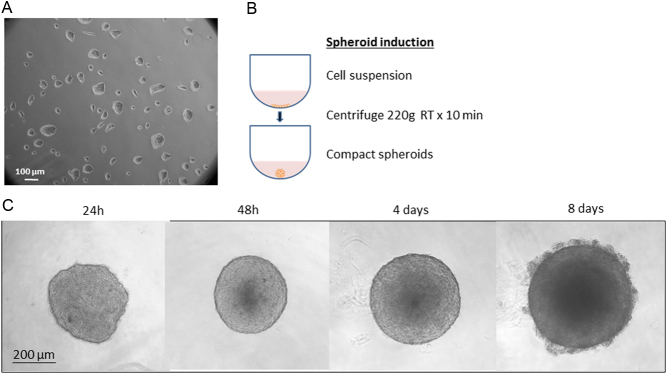
Several cell lines were then derived from MPC and MTT to improve and diversify research studies, with a particular interest in SDHB mutations, because mutations in this gene represent a high-risk factor for malignancy and poor prognosis (33, 34). To assess possible associations between SDHB gene mutations and invasiveness, Richter and colleagues established an MTT SDHB knockdown by viral transduction with lentiviral particles (35). Of note, one of the shortcomings of silencing the SDHB subunit in an Nf1 heterozygous KO mouse is the mixing of the phenotypes of clusters 1 and 2. By exploiting the MPC and MTT characteristic of spontaneously forming clusters in cultures, we generated, for the first time, PCC spheroids with homogeneous size and geometry, as shown in Fig. 1 (36). In contrast to cultures, tumour cell spheroids mimic the tumour mass and better represent the cells’ organisation within the tumour, forming a necrotic core, an external zone with proliferating cells and clear margins. This characteristic allows the operator to easily manipulate the spheroids and use them for performing experiments. Moreover, chromaffin cell features of PCC cell lines remain unchanged during cultivation under spheroid conditions (37). However, multicellular spheroids have some limitations. Although their spherical architecture is quite similar to the ‘Zellballen’ pattern of PCCs, every spheroid has central necrosis, which is absent in most PCCs or present only focally, especially in aggressive tumours. The presence of this core could be a confusing factor for a clear distinction between pseudohypoxic cluster 1 and cluster 2. Nevertheless, it has been shown that three-dimensional tumour cell spheroids provide an excellent in vitro model to study the influence of hypoxia under conditions close to the in vivo situation (37). Indeed, currently not much is known about spheroids maintained in hypoxic conditions and surely some further studies should be performed to take advantage of this research field. Another advantage of using spheroids is the excellent proven suitability of this model for drug screenings (38, 39).
Figure 1.
Cluster and spheroid formation. (A) Representative image of MTT cells that spontaneously form clusters when cultured in monolayer. (B) Induction of spheroids by centrifugation of 5 × 103 MTT cells/well for 10 min at 220 g in 96-well plate with low adherence round bottom. (C) After 48 h, spheroids assume a compact and rigid shape with clear edges, and they can be easily manipulated. In time, it is possible to observe an increase of spheroids mass, and the formation of a necrotic core surrounded by a proliferating cell crown. All images are representative.
Progenitor cells derived from a human pheochromocytoma (hPheo1)
Previous reports have shown that it is possible to immortalise cells, with minimal alteration of the phenotype, by introducing human telomerase reverse transcriptase (hTERT) into human cells (40, 41). In 2013, Ghayee et al. applied this technology in an attempt to immortalise endocrine tumours and to develop a cell line from a human PCC. In the end, they developed a neuroendocrine progenitor cell line called hPheo1, which should be useful in dissecting the molecular pathways that influence the growth and differentiation leading to PCC (42). The characterisation of these hPheo1 cells showed that genes associated with catecholamine synthesis were highly expressed in the tumour tissue of origin, but most of them were downregulated in hPheo1 cells. Nevertheless, when hPheo1 cells were treated with NGF, they were able to develop neurites and to express chromogranin A and PNMT. Despite the lack of hormone production, these cells might be useful for studying signalling pathways controlling growth and metastasis as well as for studying mechanisms of tumour development (43).
Immortalised chromaffin cells (imCC)
In 2013, Letouzé et al. generated an immortalised mouse chromaffin cell line (imCC) harbouring a complete defect in SDH. They created genetically modified mice in which the endogenous SDHB exon 2 was flanked by LoxP sites. Subsequently, chromaffin cells were isolated from the adrenal medulla, and deleted for SDHB by Cre-mediated recombination (12). This model was created in order to investigate the mechanisms linking SDH deficiency with tumour onset, and it is used to study the metabolic plasticity of SDH-deficient cells. Later on, Morin et al demonstrated that, in these SDHB-deficient cells, hypermethylation and pseudohypoxia act synergistically leading to the acquisition of metastatic treats (44).
Rat SDH-deficient RS0 cells
Recently, Powers et al. (45) developed two cell lines of SDH-deficient chromaffin cells from rats with a heterozygous germline SDHB mutation. Heterozygous Sdhb+/− founder rats were generated with various deletions in the rat SDHB gene: offspring were irradiated and maintained until they were killed. Necropsies were performed and tissue from five PCCs that appeared to be viable was injected subcutaneously into NOD scid gamma (NSG) mice by using a previously described protocol (46). To generate primary cell cultures, minced xenograft tissue was dissociated in collagenase followed by trypsin (30). The utilisation of xenografts to develop cell lines served to expand tumour cell populations, because the usually small primary tumours did not provide a sufficient number of cells for adequate tests of growth conditions. Two cell lines designated RS1/2 (for Sdhb haploinsufficient), and RS0 (for Rat Sdhb null) originated from cultures of xenografts. The first RS1/2 cell line had lost the mutated SDHB allele but retained the wild type allele. These cells show some succinate accumulation consistent with haploinsufficiency, but they cannot be considered fully SDH-deficient. RS1/2 serves either as a control for studies of RS0 or as a potential tool for studying patients’ tumours that may be haploinsufficient for SDH, having undetermined driver mutations. On the contrary, the RS0 cell line is SDH-deficient. The genome, transcriptome and metabolome of RS0 closely resemble those of SDHB-mutated human PPGLs, representing the only real cell model deriving from cluster 1 tumours. The features that RS0 xenografts share with SDH-deficient human PPGLs include identical histology, loss of SDHB protein with retention of SDHA, and expression of the neuroendocrine markers such as TH and chromogranin A. Moreover, in RS0, cytoplasmic vacuoles and sparse, often tiny, secretory granules are present. In contrast, RS1/2 cells have larger secretory granules and lack cytoplasmic vacuoles. Interestingly, RS0 cells grown in a serum-free medium form tumour cell spheroids spontaneously, which are able to grow in suspension.
Mouse models
For research purposes, animal preclinical models are urgently needed, especially because PCC is a rare condition with a limited availability of malignant tissue. The development and proper characterisation of animal models will determine their usefulness as a model for preclinical trials (47). In the past 30 years, many genetically engineered and allografted mouse models have been generated to investigate the mechanisms of PPGL tumourigenesis and test new therapeutic strategies. Among them, only cluster 2-related predisposed models have been successful. In fact, genetically engineered mice with SDHB mutations have failed to develop SDH-deficient PPGLs. Indeed, at present, cluster 1 mouse models, that is, KOs for SDHx, FH, or VHL genes, are not available. The recent progress facilitated by the TALEN, CRISPR/Cas9 or induced pluripotent stem cell (iPSCs) technologies, will promote, most likely, an acceleration of such models in the near future. Regarding SDH-deficient mouse models, the first homozygous KO of SDHD was lethal at an embryonic stage (48). Several years later, a second SDHD KO mouse model was generated. Two conditional SDHD KO were reported, but none of these models resulted in PPGL development (49). Two different teams published negative results regarding engineering a SDHB-deficient mouse model (50, 51). The failure to develop a pure SDHB KO model suggests a cellular lethality of SDHB complete KO in mice. The generation of a predisposed SDH-related model of PPGLs probably requires a second hit that would allow PGL tumourigenesis in rodents.
However, grafted models are available to study cluster 1-related tumours. In a grafted model, human (xenograft) or animal (xeno-/allograft) cancer cells are transplanted either under the skin (ectopic) or into the organ of tumour origin (orthotopic) using immunodeficient rodents, such as athymic nude mice or severely compromised immunodeficient (SCID) mice. Regarding the engrafted mice, Giubellino et al. developed a MTT derivative, known as MTT-luc and an additional cell line expressing green fluorescent protein (GFP) and firefly luciferase obtained by transducing the cells with a pre-packaged lentiviral construct. It is intended to be used for in vivo bioluminescence imaging of tumour deposits. In vivo, implantation of tumour cells transfected or transduced with the reported gene such as luciferase or GFP allows sequential monitoring of tumour growth within the viscera by measuring these photon signals. This technology is reshaping efficacy evaluations and drug-targ et al.orithms in drug animal testing for several tumour types (52). Indeed, allografts with murine cell lines remain the only feasible option to study, in vivo, the behaviour of such tumours.
As described previosuly, recently Powers and co-workers have developed xenograft models from PCCs that arose in rats with a heterozygous germline mutation in SDHB. Two distinct, serially transplantable, PCC xenograft models, which have been designated as RS0 and RS1/2, were derived at 84 and 74 weeks, respectively, from macro PCCs that arose in irradiated rats. RS0 xenografts histologically show sharply defined ‘Zellballen’ architecture, slightly clear cells and prominent blood vessels closely resembling human PGLs, while RS1/2 shows more diffuse growth. RS0 closely recapitulates the genotype and phenotype of hereditary SDHB-mutated human PPGLs and appears to be a promising model for preclinical studies of these tumours (45).
Lussey-Lepoutre reported a detailed overview of existing rodent models carrying the susceptibility genes involved in human PPGLs and their contribution to the improvement of PCC experimental research (53). Briefly, in both the c-mos and MEN2B models, multiple PCCs are associated with thyroid C-cell proliferations, as in human MEN2A and MEN2B. However, some lines of c-mos mice exclusively develop PCCs or exclusively C-cell tumours, possibly due to differences in the transgene integration site. Interestingly, Nf1 heterozygous KO mice, which frequently develop PCCs (28), express high levels of wild type RET (54), suggesting that an increase of the downstream RET activated signalling pathways predispose mice to developing PCC. Indeed, patients harbouring RET gain of function mutations develop MEN 2 syndrome.
PCCs in Pten KO mice show consistent deletions in mouse chromosome 4 (55), which is homologous to human chromosome 1p, the most frequent deletion in human PCCs. ErbB2 and B-Raf proto-oncogenes mutated mice are also able to develop PCCs (56, 57), as well as those mutated in Rb, Trp53, VHL, and InK4a tumour suppressor genes (58, 59).
Which model for which tumour?
For preclinical models, it is important to represent the selected tumour cluster as closely as possible, because each cluster is associated with distinctive signalling pathways and metabolic characteristics. These specific features represent potential drug targets (60, 61, 62) or might be responsible for different responses to drug treatments (63).
The generation of an increasing number of animal and cellular models of PPGL has been seen in the past 30 years. Based on cluster classification, cluster 1-cell models only recently became available, with the development of the RS0 cell line. Before that, only cluster 2 cell and mouse models were available. RS0 is, in fact, a new xenograft and cell line model of SDH-deficient PCC from rats with a heterozygous germline SDHB mutation. The genome, transcriptome and metabolome of this model closely resemble those of SDHB-mutated human PPGLs. Nevertheless, further experimental data using this model are needed to understand its importance in preclinical PPGL research. Even if Tischler and colleagues have always done their utmost to characterise their models fully and openly, it should be noted and acknowledged that MPC, MTT and RS0 cell lines all required irradiation to induce tumours in the original animals. This approach introduces unknown elements regarding the translation of any results to the human situation.
About tumour cell spheroids, since necrosis is an adverse factor correlated with risk of metastasis (64), a 3D cell model is a good model for the study of PCC metastasis because of the necrotic core in the middle of the spheroids. On the other hand, the necrotic core can represent a limitation for the study of PCC because it creates an artificial hypoxic environment on the central cells within the spheroid, thereby potentially muddying the distinction between pseudohypoxic cluster 1 and cluster 2. For this reason, monolayers may be equivalent or superior for some types of studies.
Given the fact that PC12 and MPC cells are both chromaffin cells, although they belong to different species, these models are somehow comparable. Morphologically, PC12 and MPC are different in size, shape and show distinct adherence and clumping behaviours, which also vary in response to different culture conditions. In fact, they respond dissimilarly to growth substrates and to nerve growth factor. MPC cells genetically and biochemically resemble human PPGLs. For example, they express substantial levels of the A synthesising enzyme, PNMT, and they do not significantly respond to NGF. In contrast, PC12 are noradrenergic and become excitable following NGF treatment. A surprising finding is that MPC cell lines express high levels of the receptor tyrosine kinase RET, which is involved in the pathogenesis of human PCCs, in hereditary MEN2. They also respond to the RET-activating ligand by exhibiting RET phosphorylation, neurite outgrowth, decreased proliferation, and altered expression of catecholamine biosynthetic enzyme. Consequently, cells undergo neuronal differentiation similar to what has been observed in NGF-treated PC12. Usually, RET is minimally expressed by normal mouse chromaffin cells and its high expression in MPC cells suggests possible relationships between two previously unrelated tumour syndromes, neurofibromatosis and MEN2 (65). Moreover, MPC and their metastatic derivative MTT cells naturally lack HIF2α. On the contrary, the rat PC12 cell lines express both HIF1α and HIF2α (13). These data indicate that PC12 cell lines could be utilised to study chromaffin cell or sympathetic neuron biology, while MPC cell lines are a useful model for the study of adrenergic tumours. The major cell line characteristics are summarised in Table 1.
Table 1.
Schematic representation of the different cell line characteristics.
| Cell | Origin | Genotype and cluster | Catecholamine secretion | Forming clusters/spheroids |
|---|---|---|---|---|
| PC12 | Rat pheochromocytoma | MAX chromosomal rearrangement or translocation, cluster 2 | Dopamine, noradrenaline | − |
| MPC | Mouse pheochromocytoma | Heterozygous NF1 knock out, cluster 2 | Noradrenaline, adrenaline | + |
| MTT | Metastatic mouse pheochromocytoma | Heterozygous NF1 knock out, cluster 2 | Noradrenaline, adrenaline | + |
| hPheo1 | Human pheochromocytoma | hTERT immortalised, cluster 2 | Not secreting | − |
| imCC | Mouse | SDHB knock out, cluster 1 | Not known | − |
| RS 1/2 | Rat pheochromocytoma | Heterozygous SDHB knock out, cluster 1 | As xenograft: dopamine, noradrenaline | +/− |
| RS0 | Rat pheochromocytoma | SDHB knock out, cluster 1 | As xenograft: dopamine, adrenaline | + |
In addition to the fact that the in vitro conditions are very different from those found in vivo, making correlations among data obtained by different research groups, even if acquired using the same cell lines, is not easy for several reasons. Indeed, the same cell types in different laboratories are often maintained in dissimilar culture conditions, including medium composition, O2 or CO2 levels and nutrient concentration (such as diverse percentages of serum or factors added to the medium). Another complication is the passage number. In fact, it is good laboratory practice to work with early cell passages in order to obtain reproducible effects under treatments and avoid the accumulation of possible additional mutations occurring over time. Cryopreservation of early passages, whenever possible, is recommended. As an example, PC12 cells were developed almost 50 years ago and have been maintained in different culture media by some laboratories. Therefore, the ability to induce PNMT may vary according to passage number and the way cultures have been maintained.
Moreover, the appropriate cell of origin is of primary importance (66). Another variable to be considered when working with cell lines is that modifying the gene expression pattern, for example with the silencing of MTT cells with shRNA anti-SDHB, leads to combining the characteristics of the different clusters muddying the distinction between cluster 1 and cluster 2. However, at the moment the cell models described are the only ones available and they allow us to study metabolic functions of PCC/PGL. Nevertheless, interesting future perspectives consist in reintroducing in RS0 cells, by transfection, the wt SDHB subunit, in order to clarify the metabolic features and functions of intracellular pathways in the tumourigenesis processes.
Conclusions
Tumours do not exist as a homogeneous population of malignant cells. Rather a tumour is characterised by a changing microenvironment, resulting from an interplay between a heterogeneous population of malignant cells and their assorted support of various tumour-associated cells, including macrophages, fibroblasts, pericytes, endothelial and immune cells. The multitude reciprocal interactions between tumour cells and the tumour microenvironment (TME) allow minor populations of tumour cells to evade apoptosis and to develop resistance. Moreover, the TME is rich in cytokines and growth factors, which are secreted by either tumour cells or stromal cells and contribute to aberrant growth, angiogenesis, metastasis and drug resistance.
Given these considerations, the importance of studying and targeting the microenvironment as a possible anti-tumour therapy is increasingly clear. Notably, MPC or MTT spheroid stability provides an opportunity for a future generation of multicellular spheroids consisting of PCC cells, endothelial cells and/or fibroblasts that provide a closer model to the in vivo situation within the tumour microenvironment. Despite several attempts, in contrast with MPC and MTT, PC12 cells are unable to form spheroids, even using different methods for spheroid generation (37). In addition, the new isolated RS0 cells, in serum-free medium, spontaneously form spheroid-like masses. However, the possibility of handling these spheres and their stability in culture must be further evaluated. Besides spheroids, a future perspective for PCC studies includes the possibility of developing organoids, which better resemble the human adrenal gland, and that could provide the opportunity to understand the role of the adrenal cortex in modulating PCC onset.
With regard to imCC and hPheo1 cells, although they seem to be promising models, all the data present in the literature are limited to those coming from the research groups that have isolated the two cell lines. In this case, further studies are necessary to make a proper comparison with the other cell lines.
About the in vivo models, knock-in and knock-out mice are useful models to investigate the pathogenesis of human tumours including their metastatic potential, as well as subcutaneous or tail vain injection of tumour cells (allograft or xenograft) (67). The development of these models is particularly challenging given the diverse causes and manifestations of tumours.
Considering the dissimilarities among the different models, it is still necessary to be very careful in drawing general conclusions from the results obtained from any single cell line.
Declaration of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of this review.
Funding
This research did not receive any specific grant from any funding agency in the public, commercial or not-for-profit sector.
References
- 1.Lam AK.Update on adrenal tumours in 2017 World Health Organization (WHO) of endocrine tumours. Endocrine Pathology 2017. 28 213–227. ( 10.1007/s12022-017-9484-5) [DOI] [PubMed] [Google Scholar]
- 2.Smith JD, Harvey RN, Darr OA, Prince ME, Bradford CR, Wolf GT, Else T, Basura GJ.Head and neck paragangliomas: a two-decade institutional experience and algorithm for management. Laryngoscope Investigative Otolaryngology 2017. 2 380–389. ( 10.1002/lio2.122) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.DeLellis R, Lloyd R, Heitz P, Eng C.Pathology and Genetics of Tumours of Endocrine Organs (IARC/World Health Organization Classification of Tumours). Lyon: IARC Press, 2004. [Google Scholar]
- 4.Lloyd R, Osamura R, Klöppel G, Rosai J.WHO Classification of Tumours of Endocrine Organs, 4th ed. IARC, 2017. [Google Scholar]
- 5.Asa SL, Ezzat S, Mete O.The diagnosis and clinical significance of paragangliomas in unusual locations. Journal of Clinical Medicine 2018. 7 280. ( 10.3390/jcm7090280) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Petri BJ, Van Eijck CH, De Herder WW, Wagner A, De Krijger RR.Phaeochromocytomas and sympathetic paragangliomas. British Journal of Surgery 2009. 96 1381–1392. ( 10.1002/bjs.6821) [DOI] [PubMed] [Google Scholar]
- 7.Dahia PL.Pheochromocytoma and paraganglioma pathogenesis: learning from genetic heterogeneity. Nature Reviews: Cancer 2014. 14 108–119. ( 10.1038/nrc3648) [DOI] [PubMed] [Google Scholar]
- 8.Fishbein L, Leshchiner I, Walter V, Danilova L, Robertson AG, Johnson AR, Lichtenberg TM, Murray BA, Ghayee HK, Else Tet al. Comprehensive molecular characterization of pheochromocytoma and paraganglioma. Cancer Cell 2017. 31 181–193. ( 10.1016/j.ccell.2017.01.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Crona J, Lamarca A, Ghosal S, Welin S, Skogseid B, Pacak K.Genotype phenotype correlations in pheochromocytoma and paraganglioma. Endocrine-Related Cancer 2019. 26 539–550. ( 10.1530/ERC-19-0024) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Buffet A, Burnichon N, Favier J, Gimenez-Roqueplo AP.An overview of 20 years of genetic studies in pheochromocytoma and paraganglioma. Best Practice and Research in Clinical Endocrinology and Metabolism 2020. 10 101416 ( 10.1016/j.beem.2020.101416) [DOI] [PubMed] [Google Scholar]
- 11.Jochmanova I, Yang C, Zhuang Z, Pacak K.Hypoxia-inducible factor signaling in pheochromocytoma: turning the rudder in the right direction. Journal of the National Cancer Institute 2013. 105 1270–1283. ( 10.1093/jnci/djt201) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Letouzé E, Martinelli C, Loriot C, Burnichon N, Abermil N, Ottolenghi C, Janin M, Menara M, Nguyen AT, Benit Pet al. SDH mutations establish a hypermethylator phenotype in paraganglioma. Cancer Cell 2013. 23 739–752. ( 10.1016/j.ccr.2013.04.018) [DOI] [PubMed] [Google Scholar]
- 13.Qin N, de Cubas AA, Garcia-Martin R, Richter S, Peitzsch M, Menschikowski M, Lenders JW, Timmers HJLM, Mannelli M, Opocher Get al. Opposing effects of HIF1a and HIF2a on chromaffin cell phenotypic features and tumor cell proliferation: insights from MYC-associated factor X. International Journal of Cancer 2014. 135 2054–2064. ( 10.1002/ijc.28868) [DOI] [PubMed] [Google Scholar]
- 14.Kluckova K, Tennant DA.Metabolic implications of hypoxia and pseudohypoxia in pheochromocytoma and paraganglioma. Cell and Tissue Research 2018. 372 367–378. ( 10.1007/s00441-018-2801-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nölting S, Ullrich M, Pietzsch J, Ziegler CG, Eisenhofer G, Grossman A, Pacak K.Current management of pheochromocytoma/paraganglioma: a guide for the practicing clinician in the era of precision medicine. Cancers 2019. 11 1505. ( 10.3390/cancers11101505) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Greene LA, Tischler AS.Establishment of a noradrenergic clonal line of rat adrenal pheochromocytomacells which respond to nerve growth factor. PNAS 1976. 73 2424–2428. ( 10.1073/pnas.73.7.2424) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tischler AS, Greene LA.Nerve growth factor-induced process formation by cultured rat pheochromocytoma cells. Nature 1975. 258 341–342. ( 10.1038/258341a0) [DOI] [PubMed] [Google Scholar]
- 18.Greene LA, Rein G.Release, storage and uptake of catecholamines by a clonal cell line of nerve growth factor (NGF) responsive pheochromocytoma cells. Brain Research 1977. 129 247–263. ( 10.1016/0006-8993(7790005-1) [DOI] [PubMed] [Google Scholar]
- 19.Tischler AS, Perlman RL, Morse GM, Sheard BE.Glucocorticoids increase catecholamine synthesis and storage in PC12 pheochromocytoma cell cultures. Journal of Neurochemistry 1983. 40 364–370. ( 10.1111/j.1471-4159.1983.tb11291.x) [DOI] [PubMed] [Google Scholar]
- 20.Harkins AB, Cahill AL, Powers JF, Tischler AS, Fox AP.Expression of recombinant calcium channels support secretion in a mouse pheochromocytoma cell line. Journal of Neurophysiology 2003. 90 2325–2333. ( 10.1152/jn.00425.2003) [DOI] [PubMed] [Google Scholar]
- 21.Furlan A, Dyachuk V, Kastriti ME, Calvo-Enrique L, Abdo H, Hadjab S, Chontorotzea T, Akkuratova N, Usoskin D, Kamenev Det al. Multipotent peripheral glial cells generate neuroendocrine cells of the adrenal medulla. Science 2017. 357 eaal3753. ( 10.1126/science.aal3753) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Furlan A, Adameyko I.Schwann cell precursor: a neural crest cell in disguise? Developmental Biology 2018. 444 (Supplement 1) S25–S35. ( 10.1016/j.ydbio.2018.02.008) [DOI] [PubMed] [Google Scholar]
- 23.Kastriti ME, Kameneva P, Kamenev D, Dyachuk V, Furlan A, Hampl M, Memic F, Marklund U, Lallemend F, Hadjab Set al. Schwann cell precursors generate the majority of chromaffin cells in Zuckerkandl organ and some sympathetic neurons in paraganglia. Frontiers in Molecular Neuroscience 2019. 12 6. ( 10.3389/fnmol.2019.00006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Westerink RH, Ewing AG.The PC12 cell as model for neurosecretion. Acta Physiologica 2008. 192 273–285. ( 10.1111/j.1748-1716.2007.01805.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Unsworth BR, Hayman GT, Carroll A, Lelkes PI.Tissue-specific alternative mRNA splicing of phenylethanolamine N-methyltransferase (PNMT) during development by intron retention. International Journal of Developmental Neuroscience 1999. 17 45–55. ( 10.1016/s0736-5748(9800058-6) [DOI] [PubMed] [Google Scholar]
- 26.Hopewell R, Ziff EB.The nerve growth factor-responsive PC12 cell line does not express the Myc dimerization partner Max. Molecular and Cellular Biology 1995. 15 3470–3478. ( 10.1128/mcb.15.7.3470) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jacks T, Shih TS, Schmitt EM, Bronson RT, Bernards A, Weinberg RA.Tumour predisposition in mice heterozygous for a targeted mutation in Nf1. Nature Genetics 1994. 7 353–361. ( 10.1038/ng0794-353) [DOI] [PubMed] [Google Scholar]
- 28.Tischler AS, Shih TS, Williams BO, Jacks T.Characterization of pheochromocytomas in a mouse strain with a targeted disruptive mutation of the neurofibromatosis gene Nf1. Endocrine Pathology 1995. 6 323–335. ( 10.1007/BF02738732) [DOI] [PubMed] [Google Scholar]
- 29.Tischler AS, Powers JF, Alroy J.Animal models of pheochromocytoma. Histology and Histopathology 2004. 19 883–895. ( 10.14670/HH-19.883) [DOI] [PubMed] [Google Scholar]
- 30.Powers JF, Evinger MJ, Tsokas P, Bedri S, Alroy J, Shahsavari M, Tischler AS.Pheochromocytoma cell lines from heterozygous neurofibromatosis knockout mice. Cell and Tissue Research 2000. 302 309–320. ( 10.1007/s004410000290) [DOI] [PubMed] [Google Scholar]
- 31.Martiniova L, Lai EW, Elkahloun AG, Abu-Asab M, Wickremasinghe A, Solis DC, Perera SM, Huynh TT, Lubensky IA, Tischler ASet al. Characterization of an animal model of aggressive metastatic pheochromocytoma linked to a specific gene signature. Clinical and Experimental Metastasis 2009. 26 239–250. ( 10.1007/s10585-009-9236-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Powers JF, Korgaonkar PG, Fliedner S, Giubellino A, Pacak K, Sahagian GG, Tischler AS.Cytocidal activities of topoisomerase 1 inhibitors and 5 azacytidine against pheochromocytoma/paraganglioma cells in primary human tumor cultures and mouse cell lines. PLoS ONE 2014. 9 e87807. ( 10.1371/journal.pone.0087807) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gimenez-Roqueplo AP, Favier J, Rustin P, Rieubland C, Crespin M, Nau V, Khau Van Kien P, Corvol P, Plouin PF, Jeunemaitre Xet al. Mutations in the SDHB gene are associated with extra-adrenal and/or malignant phaeochromocytomas. Cancer Research 2003. 63 5615–5621. [PubMed] [Google Scholar]
- 34.Amar L, Baudin E, Burnichon N, Peyrard S, Silvera S, Bertherat J, Bertagna X, Schlumberger M, Jeunemaitre X, Gimenez-Roqueplo APet al. Succinate dehydrogenase B gene mutations predict survival in patients with malignant pheochromocytomas or paragangliomas. Journal of Clinical Endocrinology and Metabolism 2007. 92 3822–3828. ( 10.1210/jc.2007-0709) [DOI] [PubMed] [Google Scholar]
- 35.Richter S, D’Antongiovanni V, Martinelli S, Bechmann N, Riverso M, Poitz DM, Pacak K, Eisenhofer G, Mannelli M, Rapizzi E.Primary fibroblast co-culture stimulates growth and metabolism in Sdhb-impaired mouse pheochromocytoma MTT cells. Cell and Tissue Research 2018. 374 473–485. ( 10.1007/s00441-018-2907-x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.D’Antongiovanni V, Martinelli S, Richter S, Canu L, Guasti D, Mello T, Romagnoli P, Pacak K, Eisenhofer G, Mannelli Met al. The microenvironment induces collective migration in SDHB-silenced mouse pheochromocytoma spheroids. Endocrine-Related Cancer 2017. 24 555–564. ( 10.1530/ERC-17-0212) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bechmann N, Poser I, Seifert V, Greunke C, Ullrich M, Qin N, Walch A, Peitzsch M, Robledo M, Pacak Ket al. Impact of extrinsic and intrinsic hypoxia on catecholamine biosynthesis in absence or presence of Hif2α in pheochromocytoma cells. Cancers 2019. 11 594. ( 10.3390/cancers11050594) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chatzinikolaidou M.Cell spheroids: the new frontiers in in vitro models for cancer drug validation. Drug Discovery Today 2016. 21 1553–1560. ( 10.1016/j.drudis.2016.06.024) [DOI] [PubMed] [Google Scholar]
- 39.Fankhauser M, Bechmann N, Lauseker M, Goncalves J, Favier J, Klink B, William D, Gieldon L, Maurer J, Spöttl Get al. Synergistic highly potent targeted drug combinations in different pheochromocytoma models including human tumor cultures. Endocrinology 2019. 160 2600–2617. ( 10.1210/en.2019-00410) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bodnar AG, Ouellette M, Frolkis M, Holt SE, Chiu CP, Morin GB, Harley CB, Shay JW, Lichtsteiner S, Wright WE.Extension of life-span by introduction of telomerase into normal human cells. Science 1998. 279 349–352. ( 10.1126/science.279.5349.349) [DOI] [PubMed] [Google Scholar]
- 41.Leão R, Apolónio JD, Lee D, Figueiredo A, Tabori U, Castelo-Branco P.Mechanisms of human telomerase reverse transcriptase (hTERT) regulation: clinical impacts in cancer. Journal of Biomedical Science 2018. 25 22. ( 10.1186/s12929-018-0422-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ghayee HK, Bhagwandin VJ, Stastny V, Click A, Ding LH, Mizrachi D, Zou YS, Chari R, Lam WL, Bachoo RMet al. Progenitor cell line (hPheo1) derived from a human pheochromocytoma tumor. PLoS ONE 2013. 8 e65624. ( 10.1371/journal.pone.0065624) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu Y, Pang Y, Caisova V, Ding J, Yu D, Zhou Y, Huynh TT, Ghayee H, Pacak K, Yang C.Targeting NRF2-governed glutathione synthesis for SDHB-mutated pheochromocytoma and paraganglioma. Cancers 2020. 12 280. ( 10.3390/cancers12020280) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Morin A, Goncalves J, Moog S, Casto-Vega LJ, Job S, Buffet A, Fontenille MJ, Woszczyk J, Gimenez-Roqueplo AP, Letouzé Eet al. TET-mediated hypermethylation primes SDH-Deficient cells for HIF2α-driven mesenchymal transition. Cell Reports 2020. 30 4551, .e7–4566.e7. ( 10.1016/j.celrep.2020.03.022) [DOI] [PubMed] [Google Scholar]
- 45.Powers JF, Cochran B, Baleja JD, Sikes HD, Pattison AD, Zhang X, Lomakin I, Barry AS, Pacak K, Moon SJet al. A xenograft and cell line model of SDH-deficient pheochromocytoma derived from 2 Sdhb+/- rats. Endocrine-Related Cancer 2020. 27 337–354. ( 10.1530/ERC-19-0474) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Powers JF, Pacak K, Tichler AS.Pathology of human pheochromocytoma and paraganglioma xenografts in NSG mice. Endocrine Pathology 2017. 28 2–6. ( 10.1007/s12022-016-9452-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ohta S, Lai EW, Morris JC, Pang ALY, Watanabe M, Yazawa H, Zhang R, Green JE, Chan WY, Sirajuddin Pet al. Metastasis-associated gene expression profile of liver and subcutaneous lesions derived from mouse pheochromocytoma cells. Molecular Carcinogenesis 2008. 47 245–251. ( 10.1002/mc.20388) [DOI] [PubMed] [Google Scholar]
- 48.Piruat JI, Pintado CO, Ortega-Saenz P, Roche M, Lopez-Barneo J.The mitochondrial SDHD gene is required for early embryogenesis, and its partial deficiency results in persistent carotid body glomus cell activation with full responsiveness to hypoxia. Molecular and Cellular Biology 2004. l24 10933–10940. ( 10.1128/MCB.24.24.10933-10940.2004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Diaz-Castro B, Pintado CO, Garcia-Flores P, Lopez-Barneo J, Piruat JI.Differential impairment of catecholaminergic cell maturation and survival by genetic mitochondrial complex II dysfunction. Molecular and Cellular Biology 2012. 32 3347–3357. ( 10.1128/MCB.00128-12) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Maher LJ, III, Smith EH, Rueter EM, Becker NA, Bida JP, Nelson-Holte M, Piruat Palomo JI, García-Flores P, López-Barneo Oet al. Mouse models of human familial paraganglioma. In Pheochromocytoma – A New View of the Old Problem. Ed Martin JF. InTechweb, 2011. [Google Scholar]
- 51.Lussey-Lepoutre C, Thibault C, Buffet A, Morin A, Badoual C, Benit P, Rustin P, Ottolenghi C, Janin M, Castro-Vega LJet al. From Nf1 to Sdhb knockout: successes and failures in the quest for animal models of pheochromocytoma. Molecular and Cellular Endocrinology 2015. 421 40–48. ( 10.1016/j.mce.2015.06.027) [DOI] [PubMed] [Google Scholar]
- 52.Giubellino A, Woldemichael GM, Sourbier C, Lizak MJ, Powers JF, Tischler AS, Pacak K.Characterization of two mouse models of metastatic pheochromocytoma using bioluminescence imaging. Cancer Letters 2012. 316 46–52. ( 10.1016/j.canlet.2011.10.019) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lussey-Lepoutre C, Buffet A, Morin A, Goncalves J, Favier J.Rodent models of pheochromocytoma, parallels in rodent and human tumorigenesis. Cell and Tissue Research 2018. 372 379–392. ( 10.1007/s00441-018-2797-y) [DOI] [PubMed] [Google Scholar]
- 54.Powers JF, Schelling K, Brachold JM, Tsokas P, Schayek H, Friedman E, Tischler AS.High-level expression of receptor tyrosine kinase Ret and responsiveness to retactivating ligands in pheochromocytoma cell lines from neurofibromatosis knockout mice. Molecular and Cellular Neurosciences 2002. 20 382–389. ( 10.1006/mcne.2002.1139) [DOI] [PubMed] [Google Scholar]
- 55.Korpershoek E, Loonen AJ, Corvers S, van Nederveen FH, Jonkers J, Ma X, Ziel-van der Made A, Korsten H, Trapman J, Dinjens WNet al. Conditional Pten knock-out mice: a model for metastatic phaeochromocytoma. Journal of Pathology 2009. 217 597–604. ( 10.1002/path.2492) [DOI] [PubMed] [Google Scholar]
- 56.Lai EW, Rodriguez OC, Aventian M, Cromelin C, Fricke ST, Martiniova L, Lubensky IA, Lisanti MP, Picard KL, Powers JFet al. ErbB-2induces bilateral adrenal pheochromocytoma formation in mice. Cell Cycle 2007. 6 1946–1950. ( 10.4161/cc.6.15.4521) [DOI] [PubMed] [Google Scholar]
- 57.Urosevic J, Sauzeau V, Soto-Montenegro ML, Reig S, Desco M, Wright EM, Canamero M, Mulero F, Ortega S, Bustelo XRet al. Constitutive activation of B-Raf in the mouse germ line provides a model for human cardio-facio-cutaneous syndrome. PNAS 2011. 108 5015–5020. ( 10.1073/pnas.1016933108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nikitin AY, Juarez-Perez MI, Li S, Huang L, Lee WH.RB mediated suppression of spontaneous multiple neuroendocrine neoplasia and lung metastases in Rb+/− mice. PNAS 1999. 96 3916–3921. ( 10.1073/pnas.96.7.3916) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.You MJ, Castrillon DH, Bastian BC, O'Hagan RC, Bosenberg MW, Parsons R, Chin L, DePinho RA.Genetic analysis of Pten and Ink4a/Arf interactions in the suppression of tumorigenesis in mice. PNAS 2002. 99 1455–1460. ( 10.1073/pnas.022632099) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Richter S, Peitzsch M, Rapizzi E, Lenders JW, Qin N, de Cubas AA, Schiavi F, Rao JU, Beuschlein F, Quinkler Met al. Krebs cycle metabolite profiling for identification and stratification of pheochromocytomas/paragangliomasdue to succinate dehydrogenase deficiency. Journal of Clinical Endocrinology and Metabolism 2014. 99 3903–3911. ( 10.1210/jc.2014-2151) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lussey-Lepoutre C, Hollinshead KE, Ludwig C, Menara M, Morin A, Castro-Vega LJ, Parker SJ, Janin M, Martinelli C, Ottolenghi Cet al. Loss of succinate dehydrogenase activity results in dependency on pyruvate carboxylation for cellular anabolism. Nature Communications 2015. 6 8784. ( 10.1038/ncomms9784) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tella SH, Taieb D, Pacak K.HIF-2alpha: Achilles’ heel of pseudohypoxic subtype paraganglioma and other related conditions. European Journal of Cancer 2017. 86 1–4. ( 10.1016/j.ejca.2017.08.023) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Muir A, Vander Heiden MG.The nutrient environment affects therapy. Science 2018. 360 962–963. ( 10.1126/science.aar5986) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Thompson LDR, Gill AJ, Asa SL, Clifton-Bligh RJ, de Krijger RR, Kimura N, Komminoth P, Lack EE, Lenders JWM, Lloyd RVet al. Dataset for the reporting of pheochromocytoma and paraganglioma: explanations and recommendations of the guidelines from the International Collaboration on Cancer Reporting (ICCR ). Human Pathology 2020. [epub]. ( 10.1016/j.humpath.2020.04.012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tischler AS.Chromaffin cells as models of endocrine cells and neurons. Annals of the New York Academy of Sciences 2002. 971 366–370. ( 10.1111/j.1749-6632.2002.tb04498.x) [DOI] [PubMed] [Google Scholar]
- 66.Kluckova K, Thakker A, Vettore L, Escribano-Gonzalez C, Hindshaw RL, Tearle JLE, Goncalves J, Kaul B, Lavery GG, Favier Jet al. Succinate dehydrogenase deficiency in a chromaffin cell model retains metabolic fitness through the maintenance of mitochondrial NADH oxidoreductase function. FASEB Journal 2020. 34 303–315. ( 10.1096/fj.201901456R) [DOI] [PubMed] [Google Scholar]
- 67.Korpershoek E, Pacak K, Martiniova L.Murine models and cell lines for the investigation of pheochromocytoma: applications for future therapies? Endocrine Pathology 2012. 23 43–54. ( 10.1007/s12022-012-9194-y) [DOI] [PMC free article] [PubMed] [Google Scholar]



 This work is licensed under a
This work is licensed under a