Abstract
Several neurodegenerative disorders are characterized by proteasome dysfunctions leading to protein aggregations and pathogenesis. Since we showed that estrogen receptor alpha (ERα) activates the proteasome, drugs able to stimulate ERα in the central nervous system (CNS) could hold potential for therapeutic intervention. However, the transcriptional effects of selective estrogen receptor modulators (SERMs), such as tamoxifen and raloxifene, can be tissue specific. A direct comparison of the effects of different SERMs on gene transcription in the CNS has never been performed. Here, we report an RNA-seq analysis of the spinal cord treated with estrogen, tamoxifen, or raloxifene. We find stark SERM and sex-specific differences in gene expression profiles in the spinal cord. Notably, raloxifene, but not estrogen or tamoxifen, modulates numerous deubiquitinating enzymes, proteasome subunits and assembly factors, and these effects translate into decreased protein aggregates. In the SOD1-G93A mouse model of amyotrophic lateral sclerosis, we found that even a low dose of raloxifene causes a significant decrease in mutant SOD1 aggregates in the spinal cord, accompanied by a delay in the decline of muscle strength in females, but not in males. These results strongly indicate SERM-selective as well as sex-specific effects, and emphasize the importance of sex as a biological variable to be considered for the careful selection of specific SERM for use in clinical trials for neurodegenerative diseases.
Keywords: SERM, estrogen receptor, ALS, raloxifene
Defects in the ubiquitin–proteasome pathway are hallmarks of many neurodegenerative diseases (1). Why the nervous system is more susceptible to these defects than other tissues remains unclear; however, we recently reported that the central nervous system (CNS) is among the tissues with the lowest level of proteasome activity (2). Therefore, a low intrinsic activity of the proteasome in the CNS may contribute to its sensitivity to proteotoxic stress.
Protein aggregates can arise from specific mutations that cause defects in the folding of proteins that are otherwise structured. However, it is estimated that 41% of proteins contain unstructured regions and unstructured proteins are more prone to aggregation (3, 4). Proteasome degradation is the main mechanism to prevent protein aggregations while autophagy can eliminate existing aggregates. However, since inhibition of autophagy in motor neurons specifically does not impact disease progression in a model of amyotrophic lateral sclerosis (ALS), the proteasome appears to be the main mediator of protection against proteotoxic stress in these cells (5).
The 26S proteasome is composed of a catalytic core (20S) and regulatory core (19S) (for a review (6)). The 20S forms a barrel-shaped complex composed of 2 rings of 7 beta-subunits, which encode the 3 proteolytic activities (trypsin like, chymotrypsin like, and caspase like). The beta-rings are sandwiched by 2 rings of 7 alpha-subunits. While the importance of the 20S catalytic core alone in the ubiquitin-independent degradation of unstructured proteins has begun to be recognized (7), the 20S alone has low activity against structured and ubiquitinated proteins, due to the narrow opening of the catalytic core. Access to the proteolytic center is facilitated by the regulatory cores. The 19S regulatory core is composed of a lid and a base. The lid provides the ubiquitin receptor and deubiquitinating enzymes, while the base provides the unfolding activity to unfold structured proteins and push them toward the proteolytic chamber for degradation. In addition to the 19S, other regulatory cores have been described, including the 11S, which unlike the 19S do not provide unfoldase activity and therefore are predicted to be suitable for the degradation of unstructured proteins that do not required unfolding (for a review (6)). Adding to the complexity of proteasome-mediated protein degradation is the large array of transiently proteasome-associated regulatory proteins, the post-translational modifications of several subunits that modulate their activity, and the tissue specific nature of different proteasome species (for a review (2, 6)). In addition, considering that in order to be functional proteasome complexes must be assembled properly, the expression of proteasome assembly factors also impacts the overall activity of the proteasome.
Considering the major impact of the proteasome in neurogenerative diseases, the development of proteasome activators is of great clinical interest. However, in face of the complexity of the proteasome, drugs able to stimulate its activity at multiple levels are predicted to ultimately have the highest clinical impact.
Our group first described that the estrogen receptor alpha (ERα) promotes the activity of the proteasome via the ERα axis of the mitochondrial unfolded protein response (UPRmt) (8). Interestingly, cross-talk between the endoplasmic reticulum mediated unfolded protein response (UPRER) and the ERα has also been reported (9-12). Retrotranslocation of misfolded proteins from the lumen of the endoplasmic reticulum to the cytoplasm for degradation by the proteasome plays a critical role in the UPRER (for a recent review (13)). Therefore, interventions aimed at activating the ERα are predicted to delay the onset and slow the progression of neurodegenerative diseases.
The involvement of the ERα in neurodegenerative diseases is of particular importance in light of the sex differences that characterize these diseases and because the basis of these differences are largely unknown (14). However, an initial enthusiasm for the use of ERα ligands in neurodegeneration has since declined, due to their failure to improve clinical outcomes in several diseases (15-18).
One potential oversight in the interpretation of these trials is the tissue specificity of the mode of action of the ERα (19-22). It is now well established that the epigenetic landscape of individual tissues, in combination with the set of coactivators and corepressors of the ERα expressed, drastically affects the numbers and nature of the binding sites of the ERα in a tissue-specific manner (19-22). The implication of these observations is profound, since it suggests that individual SERMs may have a selective effect in particular tissues and therefore their clinical use should be guided by understanding this selectivity. In agreement with this hypothesis, tamoxifen, but not raloxifene, is associated with increased risk of endometrial cancer (23). Therefore, also in the field of neurodegeneration, it is formally possible that different ERα ligands may have differential effects in specific regions of the CNS and on the activity of the proteasome. The current study was designed to begin addressing this possibility.
Our results indicate that raloxifene uniquely promotes proteostasis by simultaneously modulating the transcription of proteasome subunits, promoting proteasome assembly, altering deubiquitinating enzymes expression, and decreasing insoluble protein aggregation. These effects of raloxifene on proteostasis translate into a delay in disease progression in an animal model of familial ALS; however, these effects are observed only in females. Therefore, our study indicates that raloxifene, a drug widely used clinically, is a female-specific proteostasis therapeutic.
Raloxifene Induces the Upregulation of Proteasome Catalytic Core Genes and Reduces Insoluble Proteins
Unlike the general expectation that by being agonists of the ERα estrogen tamoxifen and raloxifene should have the same effects on transcription, they were reported to have widely different effects on gene expression in an osteoblast cell line (24). Therefore, it is possible that their effects on gene expression, including their effects on the proteasome, are different in the CNS; however, this possibility has never been tested.
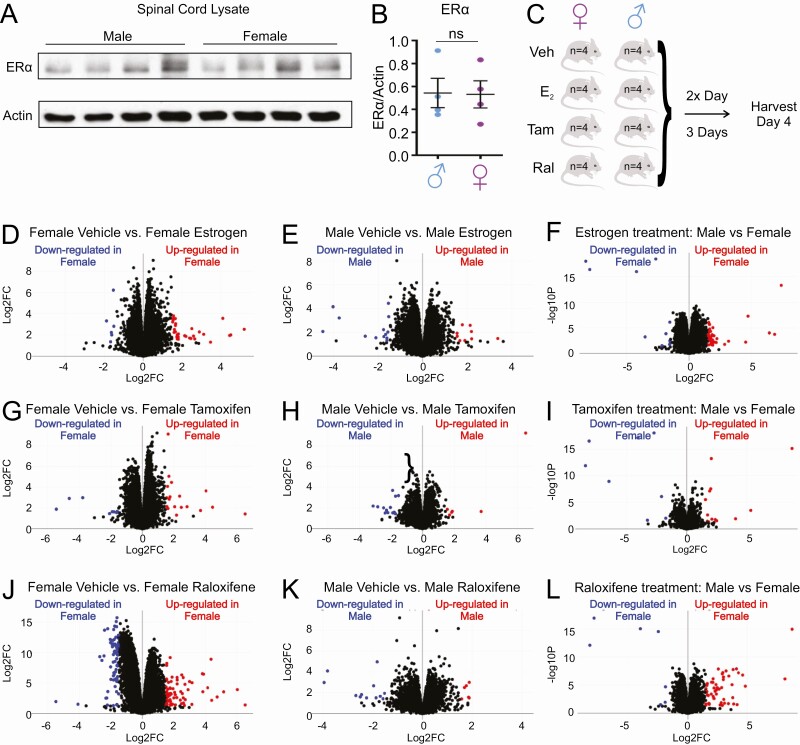
We first established that ERα is expressed in the spinal cord and that no difference in ERα levels is observed between sexes (Fig. 1A and 1B). We then performed an unbiased RNA-seq analysis to compare the effect of the 3 most commonly used SERMs, estrogen, tamoxifen, and raloxifene, on gene expression profiles in the mouse spinal cord. Four C57BL6 female and 4 male mice were treated with subcutaneous injections of either vehicle, estrogen, tamoxifen, or raloxifene (2.5 mg/kg) twice a day over 3 consecutive days (a dosing thereafter referred to as acute treatment). On day 4, the spinal cords were harvested (Fig. 1C).
Figure 1.
Estrogen, tamoxifen and raloxifene alters gene expression in the spinal cord differently in males and females. (A) Western blot of the ERα in the spinal cord of males (n = 4) and females (n = 4) C57BL mice. (B) Quantification of the Western blot in A. (C) Schematic representation of the experimental design and scheduling of injections. (D) Volcano plot of up- or downregulated genes in females treated with estrogen relative to females treated with vehicle control. (E) Volcano plot of up- or downregulated genes in males treated with estrogen relative to males treated with vehicle control. (F) Volcano plot of up- or downregulated genes in females treated with estrogen relative to males treated with estrogen. (G) Volcano plot of up- or downregulated genes in females treated with tamoxifen relative to females treated with vehicle control. (H) Volcano plot of up- or downregulated genes in males treated with tamoxifen relative to males treated with vehicle control. (I) Volcano plot of up- or downregulated genes in females treated with tamoxifen relative to males treated with tamoxifen. (J) Volcano plot of up- or downregulated genes in females treated with raloxifene relative to females treated with vehicle control. (K) Volcano plot of up- or downregulated genes in males treated with raloxifene relative to males treated with vehicle control. (L) Volcano plot of up- or downregulated genes in females treated with raloxifene relative to males treated with raloxifene.
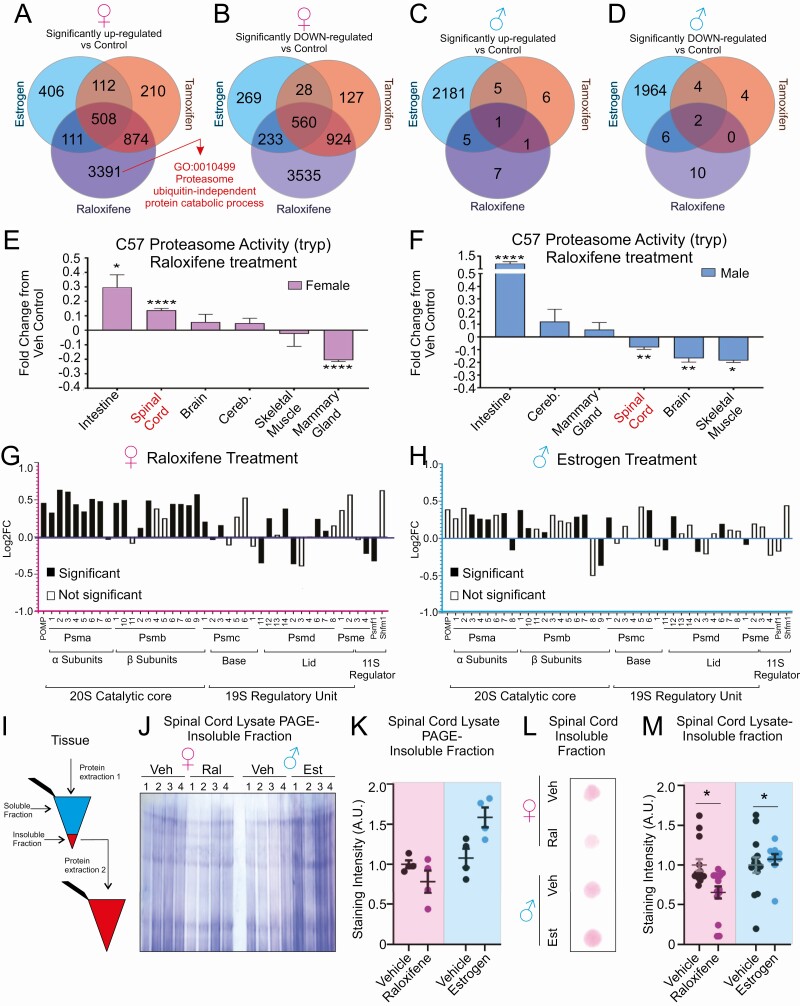
Volcano plots analysis of the effect of each treatment relative to vehicle controls in females (Fig. 1D, 1G, and 1J) or males (Fig. 1E, 1H, and 1K) or between sex (Fig. 1F, 1I, and 1L) revealed unique changes in gene expression. Next, we performed gene set analysis to identify genes uniquely up- or downregulated by each treatment, as well as differentially expressed genes shared among treatments, within each sex. We found that raloxifene is the most potent modulator of gene expression in the female spinal cord, with 3391 and 3535 genes up- or downregulated, respectively (Fig. 2A and 2B). Estrogen and tamoxifen also affected gene expression, but to a much lower extent (Fig. 2A and 2B). In males, we found that estrogen has the most potent effect on gene expression as it upregulates 2181 and downregulates 1964 genes (Fig. 2C and 2D), while tamoxifen and raloxifene have essentially no effect (Fig. 2C and 2D).
Figure 2.
Raloxifene, but not other SERM, activates the proteasome and reduces proteins in the insoluble fraction in females. (A) Venn diagram indicating the number of genes significantly (adjusted P < .05) upregulated by estrogen, tamoxifen, or raloxifene treatment compared to the vehicle control in female c57BL/J6 mice (n = 4). (B) Venn diagram indicating the number of genes significantly (adjusted P < .05) downregulated by estrogen, tamoxifen, or raloxifene treatment compared with the vehicle control in female c57BL/J6 mice (n = 4). (C) Venn diagram indicating the number of genes significantly (adjusted P < .05) upregulated by estrogen, tamoxifen, or raloxifene treatment compared with the vehicle control in male c57BL/J6 mice (n = 4). (D) Venn diagram indicating the number of genes significantly (adjusted P < .05) downregulated by estrogen, tamoxifen, or raloxifene treatment compared with the vehicle control in male c57BL/J6 mice (n = 4). (E) Proteasome activity following raloxifene treatment in the indicated tissues in females (n = 4). (F) Proteasome activity following raloxifene treatment in the indicated tissues in males (n = 4). (G) Differential gene expression (Log2 fold change) of proteasome subunits compared to vehicle control in female c57BL/J6 mice following raloxifene treatment. Solid bars indicated significant (adjusted P < .05) changes vs vehicle control. (H) Expression (log2 fold change) of proteasome subunits compared with vehicle control in male c57BL/J6 mice following estrogen treatment. Solid bars indicated significant (adjusted P < .05) changes vs vehicle control. (I) Schematic of the isolation and processing of insoluble protein fractions. (J) Coomassie stain of the insoluble fraction from spinal cord lysate separated by SDS polyacrylamide gel electrophoresis. (K) Quantification of panel I. *P < .05. (L) Representative trap assay on insoluble fraction from the indicated groups stained by ponceau. (M) Quantification of trap assay in J.
While several biological pathways were found to be modulated (Table 1 and (25)), gene ontology analysis identified proteasome ubiquitin-independent processes as a top biological process upregulated by raloxifene in females (Fig. 2A). However, the proteasome was not identified among the processes upregulated by estrogen or raloxifene in males. We decided to focus on the proteasome for further analysis because a defect in proteostasis is a hallmark of neurodegeneration and more specifically in ALS, where the spinal cord is the most affected tissue. To begin testing the effect of raloxifene on the proteasome and the tissue specificity of this effect, we first measured the activity of the proteasome. This analysis revealed that raloxifene has opposite effects on the proteasome in the spinal cord in males and females, as it promotes proteasome activity in females, but inhibits it in males (Fig. 2E and 2F). Such a statistically significant and opposite effect in males and females was only observed in the spinal cord and not in other tissues tested, including the mammary gland, which is a known target of ERα transcriptional regulation.
Table 1.
List of links to analysis of complete data set including number of reads per group, principal components, gene ontology, and list of genes for each of the indicated comparison
To further investigate the effect of raloxifene on the proteasome in the spinal cord of females, we examined the differential gene expression of proteasome subunit genes. We found that raloxifene significantly upregulates 22 genes in females (Fig. 2G), while estrogen or tamoxifen treatment had no significant effects on the expression of proteasome genes (data not shown). In males, estrogen also significantly increased the expression of some proteasome subunits, although the effect was more modest than in raloxifene-treated females, both in number of subunits affected (13 vs 22 in females) and fold increase (Fig. 2H). Neither raloxifene nor tamoxifen treatment affected expression of these genes in males (data not shown).
Since raloxifene increased the expression of genes of the 20S catalytic core, we reasoned that the elevation in the 20S may increase the degradation of unstructured proteins, which are less soluble and more prone to aggregation. We therefore tested the effect of raloxifene in females and estrogen in males on the level of insoluble proteins. For this analysis, following sonication in lysis buffer, the supernatants containing soluble proteins, were separated from the pellets, containing the insoluble proteins. The pellets were then subjected to a second round of protein extraction (Fig. 2I). Since the insoluble fractions do not contain cytoskeletal proteins, such as actin or tubulin, often used for loading controls, we assessed the protein concentration of the soluble fractions, from which the insoluble fractions were derived, and established a soluble/insoluble protein ratio. We found that spinal cord extracts from females treated with raloxifene show significantly fewer proteins in the insoluble fractions than in extracts from vehicle-treated females (Fig. 2J and 2K). In contrast, spinal cords extracts from males treated with estrogen show more proteins in the insoluble fractions than vehicle-treated males (Fig. 2J and 2K). Since insoluble proteins can lead to the formation of protein aggregates, we used a filter trap assay to assess whether the increase in insoluble proteins correlates with increase in protein aggregates. In this assay, the pore size of the membrane allows passage of small molecular weight protein complexes, while large aggregates remain trapped and can be detected by Ponceau staining. Equal amounts of insoluble proteins were loaded on the membrane. We found that raloxifene decreases the amount of high molecular weight aggregates, while estrogen increases their levels (Fig. 2L and 2M).
Collectively these results suggest that raloxifene promotes the transcription of several proteasome genes of the catalytic core and reduces the amount of proteins in the insoluble fraction in a female-specific manner.
Raloxifene Decreases the Accumulation of Polyubiquitinated Proteins, Activates Proteasome Assembly, and Alters Transcription of Deubiquitinating Enzymes
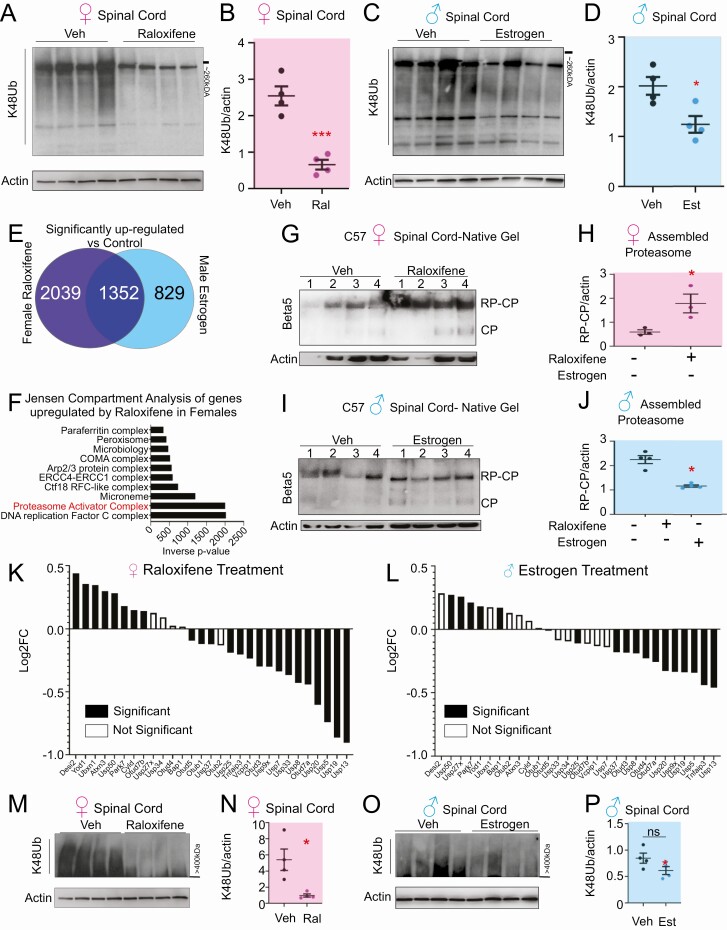
Since the expression of catalytic core genes is only 1 aspect of the complex biology of the proteasome, we next aimed at defining whether raloxifene also impacts the accumulation of proteins linked to lysine 48-ubiquitin (Ub-K48) chains, which are targeted to the 26S proteasome for degradation. We performed immunoblotting using a Ub-K48–specific antibody. We found that raloxifene reduces the level of Ub-K48 chain linked proteins in the spinal cord of females (Fig. 3A and 3B) and that estrogen reduces ubiquitinated proteins in males, although to a lesser extent than raloxifene in females (Fig. 3C and 3D). However, in view of the fact that estrogen increased the level of proteins in the insoluble fraction in males (Fig. 2J-M), we reasoned that the superiority of raloxifene in females over estrogen in males must involve other factors.
Figure 3.
Raloxifene treatment decreases lysine-48 ubiquitinated proteins, activate proteasome assembly, and modulates the expression of DUBs. (A) Western blot analysis for Ub-K48 linked proteins in spinal cord lysate from female c57BL/J6 mice. (B) Quantification of panel A. *P < .05. (C) Western blot analysis for Ub-K48 linked proteins in spinal cord lysate from male c57BL/J6 mice. (D) Quantification of panel C. *P < .05. (E) Venn diagram of genes uniquely upregulated by raloxifene in females or estrogen in males or both. (F) Processes uniquely upregulated by raloxifene in panel E (top 10 hits displayed). (G) Native gel followed by Western blot for the β5 proteasome subunit to distinguish unassembled catalytic core (CP) and assembled with regulatory core (RP) in spinal cord lysates from female c57BL/J6 mice. (H) Quantification of panel G. *P < .05. (I) Native gel followed by Western blot for the β5 proteasome subunit to distinguish unassembled catalytic core (CP) and assembled with regulatory core (RP) in spinal cord lysates from male c57BL/J6 mice. (J) Quantification of panel I. *P < .05. (K) Differential gene expression (log2 fold change) of K48 deubiquitinases (DUBs) compared to vehicle control in female c57BL/J6 mice following raloxifene treatment. Solid bars indicated significant (P < .05) changes vs vehicle control. (L) Differential gene expression (Log2 fold change) of K48 deubiquitinases (DUBs) compared to vehicle control in male c57BL/J6 mice following estrogen treatment. Solid bars indicated significant (P < .05) changes vs vehicle control. (M) Western blot analysis of very high molecular weight (>400 kDa) Ub-K48 linked proteins in spinal cord lysate from female c57BL/J6 mice. (N) Quantification of panel M. *P < .05. (O) Western blot analysis of very high molecular weight (>400 kDa) Ub-K48 linked proteins in spinal cord lysates from male c57BL/J6 mice. (P) Quantification of panel O. *P < .05.
In order to identify the factors that differ between raloxifene treated females from estrogen treated males, we compared the gene set that was upregulated by estrogen in males with the gene set upregulated by raloxifene in females. We found that 2039 genes are upregulated by raloxifene in females but not by estrogen in males (Fig. 3E). Jensen compartment analysis of these 2039 genes revealed that proteasome assembly is the most significant biological process upregulated, along with DNA replication (Fig. 3F). Therefore, to validate the proteasome assembly process identified computationally, we performed native gels, which allows the catalytic particle alone to be distinguished from the assembled regulatory core–catalytic core complex. We found that raloxifene significantly stimulated the assembly of active 26S proteasome in females (Fig. 3G and 3H), while estrogen in males did not (Fig. 3I and 3J).
Further, since a decrease in ubiquitin-linked proteins may also be due to an increased in de-ubiquitinating enzymes (26), we next compared the expression of deubiquitinases (DUBs) between the 2 groups. We found that raloxifene has a wide impact on several DUBs (Fig. 3K), while estrogen in males had limited effect (Fig. 3L). The other treatments had little effect on DUB expression in either sex (data not shown). Deubiquitination can also contribute to the editing of the length of the ubiquitin chains and prevent aggregation. We noticed that the spinal cord is characterized by the presence of Ub-K48 immunoreacting high molecular weight bands migrating above 240 kDa that cannot be resolved under normal electrophoresis conditions (Fig. 3A and 3C). Therefore, to further test the potential impact of the effect of raloxifene on length of ubiquitin chains, we performed Western blots that focused specifically on the very high molecular weight bands. We found that raloxifene also reduces these very high molecular weight protein complexes (Fig. 3M and 3N), while estrogen in males did not (Fig. 3O and 3P).
Taken together, these results indicate that raloxifene strongly activates both proteasome assembly and widely alters transcription of DUBs in females. These effects correlate with a decrease in the accumulation of ubiquitin K48 chains even those migrating at very high molecular weight. The effects of raloxifene in female spinal cord are in contrast to estrogen in males, which shows diminished proteasome assembly and limited effects on DUB transcription.
Raloxifene Delays Disease Progression in a Model of Familial ALS in Females
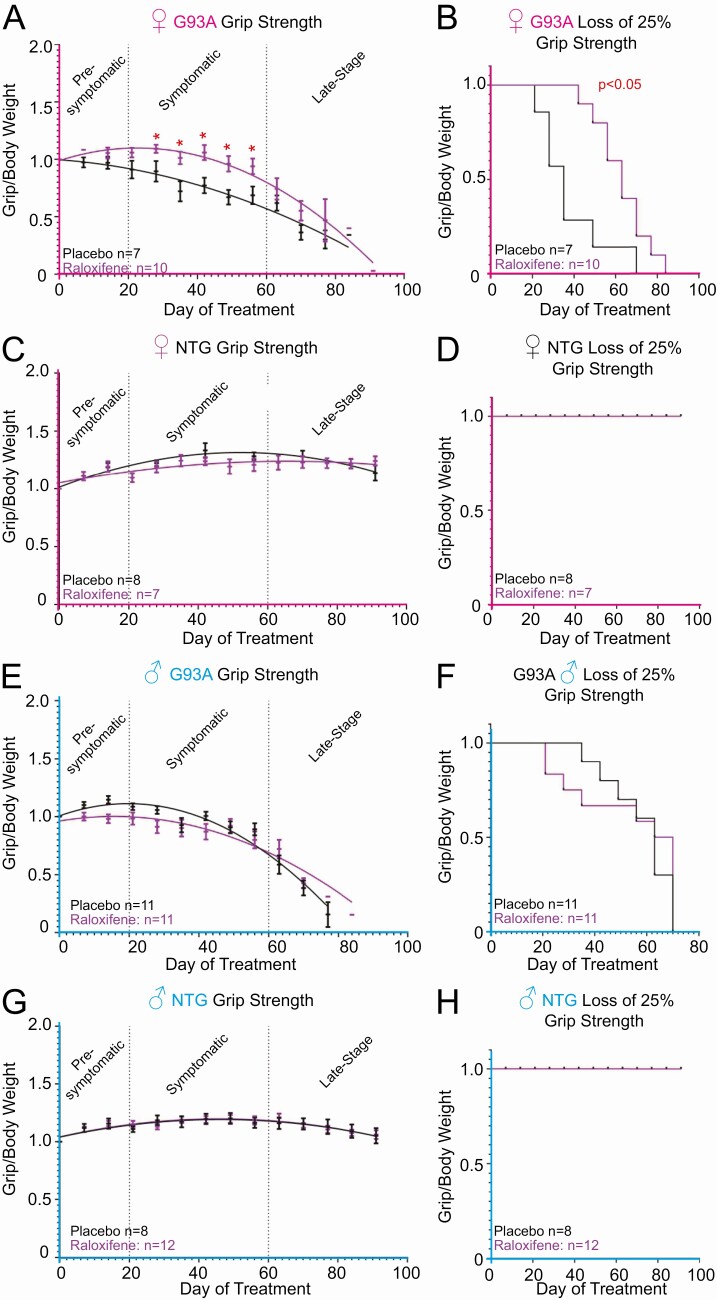
Based on the effect of raloxifene on the proteasome–ubiquitin pathway at multiple levels and the fact the proteasome dysfunction is a pathogenic hallmark of ALS, we initiated a pilot trial with raloxifene in SOD1-G93A mice. Since subcutaneous injections twice daily over months would cause unacceptable distress to the animals, for long-term treatment we used slow release pellets. Male and female SOD1-G93A mice were treated chronically with subcutaneous slow-release raloxifene pellets, which were implanted at 50 days of age, prior to the onset of disease symptoms. We used 10-mg pellets in females and 15-mg pellets in males to account for the larger body weight of males. Mice were randomized in 2 cohorts, placebo treated and raloxifene treated. Grip strength and body weight were measured weekly and the grip/body weight ratio determined. We found that females treated with raloxifene maintained a significantly higher grip/body weight ratio for 4 consecutive weeks during disease progression compared with placebo-treated animals (Fig. 4A and 4B). However, at later time points, no difference in grip/body weight ratio was observed between the 2 groups, and no difference in overall survival was observed. Raloxifene had no effect in nontransgenic (NTG) mice (Fig. 4C and 4D). As expected from the results in wild-type mice, raloxifene had no effect on delaying symptom progression in SOD1-G93A males (Fig. 4E and 4F) and no effect in NTG males (Fig. 4G and 4H).
Figure 4.
Raloxifene delays disease progression in female G93 SOD1 mice. (A) Grip strength/body weight measurements recoded from female G93A mice treated with either 10 mg of raloxifene (n = 10) slow-release pellet, or placebo (n = 7) control. Days indicate days since implantation. Data are shown as mean + standard error of the mean. *P < .05. (B) Kaplan–Meyer curve indicating the time at which each mouse in panel A lost more than 25% of initial grip strength. Days indicate days since implantation. *P < .05. (C) Grip strength/ body weight measurements recoded from female NTG mice treated with either 10 mg of raloxifene (n = 7) or placebo (n = 8). (D) Kaplan–Meyer curve indicating the time at which each mouse in panel C lost more than 25% of initial grip strength. (E) Grip strength/ body weight measurements recoded from male G93A SOD1 mice treated with either 15 mg raloxifene (n = 11) or placebo (n = 11). (F) Kaplan–Meyer curve indicating the time at which each mouse in panel E lost more than 25% of initial grip strength. (G) Grip strength/body weight measurements recoded from male NTG mice treated with either 15 mg raloxifene (n = 12) or placebo (n = 8). (D) Kaplan–Meyer curve indicating the time at which each mouse in panel G lost more than 25% of initial grip strength.
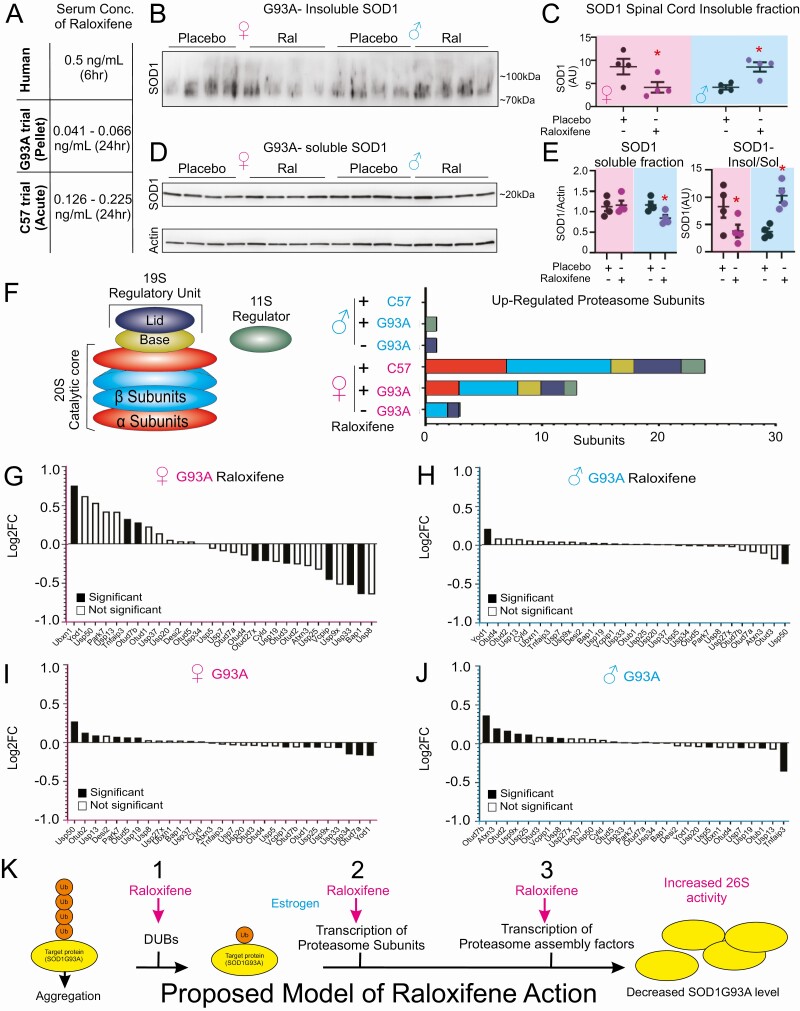
Since raloxifene has potent effects on proteostasis, the limited beneficial effect observed in the trial in SOD1-G93A mice was surprising. We reasoned that 1 major difference is the mode of drug delivery between the acute treatment administered by twice daily subcutaneous injection to the wild-type mice, in which we performed RNA-seq analysis, versus slow release pellets in the trial using SOD1-G93A mice. To test this hypothesis, we measured raloxifene serum concentration in both conditions. We found that the serum concentration ranged between 0.042 and 0.066 ng/mL in mice treated with the slow-release pellets while it was 0.126 to 0.225 ng/mL in the acute treatment (Fig. 5A). Importantly, the serum concentrations in the acute treatment were performed 24 hours after the last injections and were within the concentration range measured in humans, 0.5 mg/mL 6 hours after oral administration with a half-life of 27 hours, which supports the estimation of approximately 0.24 ng/mL after 24 hours in humans (27). Therefore, since a delay in disease progression is observed in the SOD1-G93A female mice, despite a dose 10-fold lower than what is typically achieved in humans treated with raloxifene, this result raises the possibility that the beneficial effect could be much greater in humans. However, a “U” shape dose response cannot be ruled out by these experiments and more work will be needed to define a dose response curve of raloxifene in these mice.
Figure 5.
Raloxifene increases proteasome subunit and DUB expression in female mice. (A) Serum concentration of raloxifene in humans (measured 6 hours after 60 mg oral dosage), G93A SOD1 mice treated with raloxifene subcutaneous pellets (measured 24 hours after pellet removal), or C57 mice treated with 2.5 mg/kg subcutaneous injection 2×/day for 3 days (measured 24 hours after the final injection). (B) Immunoblot for SOD1 in the insoluble fraction of spinal cord lysates collected from G93A SOD1 mice sacrificed after 60 days of treatment. (C) Densitometry analysis of panel I. *P < .05. (D) Immunoblot of SOD1 from the soluble fractions in the SOD1-G93A mice in the indicated groups. Actin was used as loading control. (E) Quantification of the SOD1 relative to actin from panel D (left panel) or of the insoluble to soluble ratio (right panel). (F) Diagram of proteasome subunits that are significantly (P < .05) different in G93A mice compared with NTG controls, raloxifene treated G93A mice compared with placebo treated controls, or raloxifene treated (acute treatment) C57 mice treated compared with vehicle treated control mice. (G) Differential gene expression (log2 fold change) of deubiquitinases (DUBs) in raloxifene-treated female SOD1-G93A mice compared with placebo controls. (H) Differential gene expression of DUBs in female SOD1-G93A mice compared to female nontransgenic mice. (I) Differential gene expression (log2 fold change) of DUBs in raloxifene treated male SOD1-G93A mice compared with placebo controls. (J) Differential gene expression of DUBs in male SOD1-G93A mice compared with female nontransgenic mice. (K) Model of the mode of action of raloxifene in females (pink) and estrogen in males (blue). Raloxifene stimulates the deubiquitination of proteins to facilitate their processing by the proteasome, promotes the transcription of several proteasome subunits and proteasome assembly. Collectively these effects lead to increased proteasome activity, which decreases SOD1-G93A aggregates and results in delayed disease progression in the SOD1-G93A model of ALS.
In the trial, we also included mice that were sacrificed after 60 days of treatment to determine if raloxifene reached its target. Since ultimately the pathogenesis in this ALS model involves the formation of large insoluble aggregates of SOD1-G93A protein ranging from 70 to 100 kDa in size, we monitored the level of SOD1-G93A in the insoluble fraction. We found that raloxifene significantly decreased the aggregates in the females (Fig. 5B and 5C). However, the aggregates were increased in males (Fig. 5B and 5C), an observation that may contribute to explain the finding that the grip/body weight ratio was lower in raloxifene-treated males than in the placebo group (Fig. 4E and 4F), although this difference did not reach statistical significance. As a control, we monitored the level of SOD1 in the soluble fraction for each of the samples and found that raloxifene did not affect the total level of soluble SOD1 in females; however, the soluble fraction was reduced in males (Fig. 5D and 5E).
To further our understanding of the effects of chronic raloxifene treatment in the SOD1-G93A mice, we analyzed the proteasome subunit gene set and found that while only 3 proteasome genes were upregulated in the untreated SOD1-G93A mice compared with NTG untreated mice, 13 proteasome genes were upregulated in the SOD1-G93A mice upon treatment with raloxifene (Fig. 5F). However, in agreement with the observation that the dosing in the chronic trial was 10-fold lower than in the acute treatment, 24 proteasome genes were upregulated by raloxifene in the acutely treated mice (Fig. 5F). As expected, raloxifene had no effect on proteasome genes in SOD1-G93A male mice (Fig. 5F). Further, raloxifene was found to upregulate some DUB genes in females (Fig. 5G) compared with untreated females (Fig. 5H), while no effect on DUBs was observed in males (Fig. 5I and 5J).
These findings indicate that, despite the low dose achieved in this trial, raloxifene delayed disease progression, decreased SOD1-G93A aggregates, and increased transcription of several proteasome genes as well as some genes encoding DUBs. Therefore, collectively these observations support the exciting possibility that raloxifene treatment in humans, where chronic treatment can achieve the same dose as the acute treatment observed in mice, may have significant clinical impact in delaying the progression of neurodegenerative disorders.
Discussion
The historical assumption was that, since they bind to the same receptor, all ligands of the ERα are expected to induce largely overlapping gene transcription profiles. To our knowledge, the findings of the current study are the first to demonstrate that, in vivo, estrogen, tamoxifen, and raloxifene differ widely on their effects on gene transcription in the CNS, where we report a fundamental sexual dimorphic response to ERα ligands in spinal cord. Since tamoxifen and raloxifene have been shown to cross the brain–blood barrier and no differences in the penetrance between males and females were observed (28-32), our data indicate that the differences in gene expression in males and females are not due to differences in their ability to cross the brain–blood barrier and are the results of the transcriptional activity of the ERα. The implications of these findings could be profound for neurodegeneration, since, while the neuroprotective effect of SERMs originally generated great enthusiasm, the mixed effect of SERMs in clinical trials rapidly led to a lack of interest in these drugs, at least toward neurodegenerative diseases. Our data suggest that the potential of SERMs as a therapeutic intervention against neurodegenerative diseases should remain high and consider specific SERMs for specific diseases as well as sex as a critical biological variable in the design of future trials.
A summary of a hypothetical model of action of raloxifene and estrogen in females and males, respectively, which emerges from this study, is shown in Fig. 5K, where the pink writing indicates the effect in females, and blue writing the effect in males. Disease-linked proteins, such as mutant SOD1, are known to form high molecular weight ubiquitinated aggregates in the cytoplasm; these aggregates are resistant to degradation by the proteasome. In females, we hypothesize that the modulation of DUBs by raloxifene promotes Ub-K48 chain editing, leading to a reduction in their lengths and limiting their aggregation, thereby enhancing their accessibility to proteasome degradation (Fig. 5K, arrow 1). Importantly, raloxifene stimulates the transcription of a large number of proteasome genes (Fig. 5K, arrow 2) and promotes the assembly of the proteasome (Fig. 5K, arrow 3). Collectively, these effects of raloxifene synergize to enhance proteasome function. In males, estrogen activates transcription of proteasome genes, but to a lesser extent than raloxifene in females (Fig. 5K, arrow 2). However, estrogen does not modulate DUB or the assembly of the proteasome and therefore the proteasome in males treated with estrogen is not as active as in raloxifene-treated females. In fact, estrogen may be detrimental in males since the level of proteins in the insoluble fraction and the SOD1-G93A aggregates increased following treatment. The reasons for this increase are unclear, but it argues against the use of estrogen in males as a therapeutic for neurodegeneration.
The mechanism of the differential effect of SERM remains to be elucidated, but is likely to involve 2 aspects; first, the tissue-specific expression of ERα coactivators and corepressors and, second, the sex-specific epigenetic landscape of individual tissues. Notably, a recent study revealed that the epigenetic landscape of the male and female brain is largely different due the highly sexual dimorphic effect of gonadal steroids in the preoptic area that inhibit the expression of DNA methylase (Dnmt) enzymes (33). Decreased expression of Dnmt enzymes reduced DNA methylation and released the repression of masculinization genes (34). It will be of interest to understand how these epigenetic aspects affect the binding of the pioneer factor FoxA1, a major player in defining the binding sites of the ERα across the genome.
In the current study, our analysis focused on the spinal cord and therefore utilized a model of ALS, where the spinal cord is the major tissue affected. Nevertheless, our findings in the CNS do not rule out a potential contribution of a systemic effect of raloxifene in other organs and tissues, which may play a role in the pathogenesis of ALS. The unique ability of raloxifene to induce the expression of genes leading to increased proteostasis is beyond expectation and raises great hope for future investigations, since not only defects in proteostasis are critical to the pathogenesis of this disease, but significant delay in disease progression was observed, despite the fact that the dose achieved in the trial was 10-fold lower than typically achieved in humans. Interestingly, in the course of our study, another group identified raloxifene in a screen for compounds able to reduce tau aggregation in an in vitro cell model (35). Therefore, combined this in vitro study and our in vivo study highlight the notion that raloxifene is unique as a proteostasis therapeutic.
Further, a recent study reported that hormones and physiological states that increase 3′,5′-cyclic adenosine monophosphate lead to enhanced proteasome activity through a post-translational mechanism (36). Although their effects were not tested in the CNS, nevertheless this observation raises the possibility of combining raloxifene with some of these hormones or physiologic states to further enhance proteasome activity.
Finally, our findings open multiple new lines of future investigation, including cell type specificity of the transcriptional effects of SERM, which could be studied by single cell transcriptomics of the CNS, as well as the effects of additional SERM. Notably, acolbifene, turemifene, clomifene, and endoxifene, to name only a few, are all SERM that have never been tested for their neuroprotective effect. The data presented in the current study may launch an entirely new search for SERM with better efficacy, specifically in the CNS. Therefore, it is expected that in the future the knowledge gained will allow the development of more rational use of SERM in disease affecting the CNS.
Materials and Methods
Acute treatment and tissue collection in wild-type mice
All animal experiments were conducted according to IACUC guidelines. Two-month-old (20 females 20 males) C57B6 (subtype) were purchased from Jackson laboratories and housed in Mt. Sinai’s vivarium under standard conditions. Animals (females n = 4, males n = 4) received twice daily subcutaneous injections on the upper back (2.5 mg/kg) of tamoxifen (Sigma part number T5648-1G), raloxifene hydrochloride (Sigma part number R1402-1G), β-estradiol (Sigma part number E8875-250MG), or vehicle (5% dimethylsulfoxide in vegetable oil) alone twice daily for 3 consecutive days. Animals were sacrificed on the fourth day when tissue was harvested and flash frozen.
Spinal cords were isolated by hydraulic extraction, minced with forceps, and flash frozen for later analysis. Tissue was lysed in NP40 lysis buffer (50 mM Tris, 250 mM NaCl, 5 mM EDTA, 0.5% NP-40, 50 mM NaF, 1 mM dithiothreitol plus protease inhibitors) on ice with roughly a 1:3 volume to volume ratio of tissue powder to lysis buffer. All samples were subjected to probe sonication (Fisher Scientific FB505) with 2 to 3 rounds of 1-second intervals at 20% amplitude on ice. Protein was then cleared by spinning at 20 800g at 4°C for 20 minutes. The soluble fraction was then moved to a new tube and frozen at –20°C for later analysis.
RNA sequencing and analyses
RNA seq was performed on all 4 males and 4 females from each group (vehicle, estrogen, tamoxifen and raloxifene) for a total of 32 mice in technical duplicates. Flash frozen spinal cord tissue was sent to Genewiz (South Plainfield, NJ) for next generation RNA sequencing. Raw.fastq files were supplied and are available for analysis on the BioJupies cloud (https://amp.pharm.mssm.edu/biojupies/analyze/tools?uid=ETXiK0nxxgj) (25)
Analysis of RNA-seq fastq files was performed using the BioJupies suite of tools for alignment, annotation, and differential expression analysis of our treatment groups. Differentially expressed genes that were significantly (adjusted P < .05) up- or downregulated compared with vehicle control or estrogen treatment were used for further gene ontology enrichment analysis using the ontology enrichment analysis tool for biological processes provided by the Gene Ontology Consortium or the suite of analysis tools provided through Enrichr. The complete set of links to data generated in Biojupies can be found in Table 1 and (25). Differential gene expression was determined in Biojupies with built-in scripts currently used in limma (37).
SOD1-G93A mouse raloxifene trial
Transgenic B6SJL-TG_SOD1*G93A)1Gur/J (SOD1-G93A) mice were obtained from the Jackson Laboratory. SOD1-G93A males were bred with B6SJL NTG females also from the Jackson Laboratories and maintained according to IACUC approved methods. SOD1-G93A and NTG mice (aged 50 days) were implanted with time release pellets (Innovative Research of America) containing either a vehicle control placebo (placebo), 10 mg of raloxifene (for female mice), or 15 mg of raloxifene (for male mice). Body weight was assessed weekly starting the day prior to pellet implantation. Forepaw muscle strength was measured using a digital grip strength meter (Columbus Instruments). Animals were trained to grasp a horizontal grasping ring. During the testing phase, the average of 3 trials recorded at 5-minute intervals was recorded per mouse. The age of death or the age at which institutional guidelines required euthanasia were recorded for each animal and used to construct a Kaplan–Meier survival curve. An independent cohort of SOD1-G93A and NTG mice was sacrificed at day 60 of raloxifene treatment (ie, 110 days of age), after symptom onset, for biochemical measurements.
Western blots
Western blot analysis was performed using the Bio-Rad Criterion Cell Midi blot system. Protein was collected as described above and quantified using the Bio-Rad Protein Assay Dye method (cat. No. 500-0006) before being separated on a 4% to 20% gradient TGX™ Precast Midi (Bio-Rad, cat. No. 5671094) polyacrylamide gel and transferred to a nitrocellulose blotting membrane (GE Healthcare Life Sciences; 1 M tris-HCl, 25% glycerol, 2% sodium dodecyl sulfate [SDS], 5% 2-mercaptoethanol, 0.1% bromophenol blue) and heated to 95°C for 5 minutes before being resolved on the gel. Following transfer, membranes were blocked in 5% nonfat dry milk suspended in Tris-buffered saline plus 0.1% Tween 20 (TBST) for 45 to 60 minutes at room temperature and probed with primary antibodies against K48Ub (38) (EMD-Millilore, cat. No. 05-1307), actin (39) (Santa Cruz Biotechnology, cat. No. sc-47778), β5 proteasome subunit (40) (Enzo life sciences cat No. BML-PW8895-0025), or SOD1 (41) (Santa Cruz Biotechnology Cat# sc-11407, RRID:AB_2193779) overnight at 4°C. All primary antibodies were suspended in 2.5% milk TBST. After a wash series in TBST, blots were then probed with horseradish peroxidase conjugated antimouse (42) (Kindle Biosciences Cat# R1005) or antirabbit (43) (Kindle Biosciences Cat# R1006) antibodies and detected using enhanced chemiluminescence (Millipore or Kindle Biosciences). Images of developed blots were captured either using film and then digitized with a flatbed scanner or captured using the KwikQuant Imager system (Kindle Biosciences, cat. No. D1001). Band quantification was performed in ImageJ using the built-in densitometry tools.
Proteasome activity and assembly assays
Proteasome activity was assessed as has been described (2). Ten micrograms of protein lysate was added to proteasome activity assay buffer (50 mM Tris-HCl, pH 7.5) along with (Ac-RLR-AMC) (Boston Biochem, cat. no. s290) fluorogenic proteasome substrate. Reactions were allowed to incubate at 37°C protected from light for 3 hours. Following incubation, samples were transferred to black walled 96-well plates (Greiner Bio-One, cat No. 655097). Release of free 7-amino-4-methyl-coumarin was determined using a SpectraMax M5e microplate reader (Molecular Devices) with excitation at 380 nm and emission recorded at 460 nm. Reported values are the fold increase over a no-protein control that was incubated along with every experiment.
Native gels were performed similarly to as has previously described (44). Tissues were snap frozen and ground into powder with a pestle and mortar. Tissue powders (~50 µL) were then triturated using a 25G syringe on ice in native tissue lysis buffer (50 mM Tris-HCl (ph8.0), 5 mM MgCl2, 0.5 mM EDTA, and 1 mM ATP) with 3 1-second vortex pulses. Lysates were then clarified by centrifugation at 4°C for 25 minutes at 20 000g. Clarified supernatants were transferred to a new tube and quantified as described above. Twenty micrograms of protein was loaded per well along with 6× native protein loading buffer (Biorad cat # 1610738) into a 4% to 20% polyacrylamide gel electrophoresis gel (Biorad cat # 5671094) in native running buffer (90 mM Tris base, 90 mM boric acid, 5 mM MgCl2, 0.5 mM EDTA, 1 mM ATP-MgCl2), and resolved for 3.5 hours at 100 V on ice. The gel was then soaked in transfer buffer (25 mM tris base, 192 mM glycine) containing 1% SDS for 5 minutes, then soaked for 10 minutes in transfer buffer without SDS. Proteins were then transferred to a nitrocellulose membrane in transfer buffer at 250 mA for 90 minutes on ice. The membrane was then probed for the β5 proteasome subunit (Enzo life sciences cat. no. BML-PW8895-0025) as described above for Western blot.
Serum collection and raloxifene measurements
Whole blood was collected from animals treated with raloxifene by either subcutaneous time release pellet implantation (G93A trial), or subcutaneous injection (C57B6 acute treatment) 24 hours after the completion of the experiment. Blood was collected in a Microvette CB300 300 uL capacity EDTA coated (Fisher Scientific NC9141704) and centrifuged at 2000g for 5 minutes at 4°C. Serum was then transferred to a new tube and snap frozen on dry ice. Samples were stored at –20°C until they could be analyzed by mass spectroscopy. Mass spectroscopy and raloxifene quantification were performed at the Stony Brook University Biological Mass Spectrometry Shared Resource facility.
Filter trap assay
Insoluble fractions of spinal cord lysates were suspended in TEN buffer (10 mM Tris-HcCl, 1 mM EDTA, 100 mM NaCl plus protease inhibitors) and quantified using the Bio-Rad protein Assay Dye system (cat. no. 500-0006). A 25-µg bolus from each sample was brought to an equal volume in TEN buffer. Five percent Trition-X100 in tris-buffered saline was then added to each sample to a final concentration of 0.5% and incubated on ice for 10 minutes. Samples were then added to the filter trap assembly (Bio-Rad) and passed through a nitrocellulose membrane with a pore size of 0.2 µm (Optitran). After transfer, the membrane was removed, washed in TBST, and stained with Ponceau S (Sigma P7170-1L). Densitometry was performed in ImageJ.
Statistical methods
Data on graphs are represented as the mean ± standard error of the mean. Student’s t-test was used to ascertain a significant difference in mean when comparing 2 groups. P values of less than .05 were considered statistically significant.
Acknowledgments
Financial Support: This work was funded by NIH R01NS084486 and NIH 1R21NS109913 awards to D. Germain and G. Manfredi and by NIH R01NS062055 award to G. Manfredi.
Glossary
Abbreviations
- ALS
amyotrophic lateral sclerosis
- CNS
central nervous system
- DUB
deubiquitinase
- ER
estrogen receptor
- NTG
nontransgenic
- SDS
sodium dodecyl sulfate
- SERM
selective estrogen receptor modulators
- TBST
tris-buffered saline plus 0.1% Tween 20
- Ub-K48
lysine 48-ubiquitin
- UPRmt
mitochondrial unfolded protein response
Additional Information
Disclosures: The authors have nothing to disclose
Data Availability
Some or all data generated or analyzed during this study are included in this published article or in the data repositories listed in References.
References
- 1. Kawamata H, Manfredi G. Proteinopathies and OXPHOS dysfunction in neurodegenerative diseases. J Cell Biol. 2017;216(12):3917-3929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Jenkins EC, Shah N, Gomez M, et al. Proteasome mapping reveals sexual dimorphism in tissue-specific sensitivity to protein aggregations. EMBO Rep. 2020;21(4):e48978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Baugh JM, Viktorova EG, Pilipenko EV. Proteasomes can degrade a significant proportion of cellular proteins independent of ubiquitination. J Mol Biol. 2009;386(3):814-827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dunker AK, Obradovic Z, Romero P, Garner EC, Brown CJ. Intrinsic protein disorder in complete genomes. Genome Inform Ser Workshop Genome Inform. 2000;11:161-171. [PubMed] [Google Scholar]
- 5. Tashiro Y, Urushitani M, Inoue H, et al. Motor neuron-specific disruption of proteasomes, but not autophagy, replicates amyotrophic lateral sclerosis. J Biol Chem. 2012;287(51):42984-42994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Eakle WS, Braly BV. Fracture resistance of human teeth with mesial-occlusal-distal cavities prepared with sharp and round internal line forms. J Prosthet Dent. 1985;53(5):646-649. [DOI] [PubMed] [Google Scholar]
- 7. Kumar Deshmukh F, Yaffe D, Olshina MA, Ben-Nissan G, Sharon M. The contribution of the 20S proteasome to proteostasis. Biomolecules. 2019;9(5):190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Papa L, Germain D. Estrogen receptor mediates a distinct mitochondrial unfolded protein response. J Cell Sci. 2011;124(Pt 9):1396-1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Andruska ND, Zheng X, Yang X, et al. Estrogen receptor α inhibitor activates the unfolded protein response, blocks protein synthesis, and induces tumor regression. Proc Natl Acad Sci U S A. 2015;112(15):4737-4742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Andruska N, Zheng X, Yang X, Helferich WG, Shapiro DJ. Anticipatory estrogen activation of the unfolded protein response is linked to cell proliferation and poor survival in estrogen receptor α-positive breast cancer. Oncogene. 2015;34(29):3760-3769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cook KL, Clarke R. Estrogen receptor-alpha signaling and localization regulates autophagy and unfolded protein response activation in ER+ breast cancer. Receptors Clin Investig. 2014;1(6):e316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chapple RH, Hu T, Tseng YJ, et al. ERalpha promotes murine hematopoietic regeneration through the Ire1alpha-mediated unfolded protein response. Elife. 2018;7:e31158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wu X, Rapoport TA. Mechanistic insights into ER-associated protein degradation. Curr Opin Cell Biol. 2018;53:22-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zagni E, Simoni L, Colombo D. Sex and gender differences in central nervous system-related disorders. Neurosci J. 2016;2016:2827090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rapp SR, Espeland MA, Shumaker SA, et al. Effect of estrogen plus progestin on global cognitive function in postmenopausal women: the Women’s Health Initiative Memory Study: a randomized controlled trial. JAMA. 2003;289(20):2663-2672. [DOI] [PubMed] [Google Scholar]
- 16. Espeland MA, Rapp SR, Shumaker SA, et al. Conjugated equine estrogens and global cognitive function in postmenopausal women: Women’s Health Initiative Memory Study. JAMA. 2004;291(24):2959-2968. [DOI] [PubMed] [Google Scholar]
- 17. Gleason CE, Dowling NM, Wharton W, et al. Effects of hormone therapy on cognition and mood in recently postmenopausal women: findings from the randomized, controlled KEEPS-cognitive and affective study. PLoS Med. 2015;12(6):e1001833; discussion e1001833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Henderson VW, Ala T, Sainani KL, et al. Raloxifene for women with Alzheimer disease: a randomized controlled pilot trial. Neurology. 2015;85(22):1937-1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Martinkovich S, Shah D, Planey SL, Arnott JA. Selective estrogen receptor modulators: tissue specificity and clinical utility. Clin Interv Aging. 2014;9:1437-1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Eeckhoute J, Carroll JS, Geistlinger TR, Torres-Arzayus MI, Brown M. A cell-type-specific transcriptional network required for estrogen regulation of cyclin D1 and cell cycle progression in breast cancer. Genes Dev. 2006;20(18):2513-2526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jeselsohn R, Bergholz JS, Pun M, et al. Allele-specific chromatin recruitment and therapeutic vulnerabilities of ESR1 activating mutations. Cancer Cell. 2018;33(2):173-186.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lupien M, Eeckhoute J, Meyer CA, et al. FoxA1 translates epigenetic signatures into enhancer-driven lineage-specific transcription. Cell. 2008;132(6):958-970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. DeMichele A, Troxel AB, Berlin JA, et al. Impact of raloxifene or tamoxifen use on endometrial cancer risk: a population-based case-control study. J Clin Oncol. 2008;26(25):4151-4159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Miki Y, Suzuki T, Nagasaki S, Hata S, Akahira J-i, Sasano H. Comparative effects of raloxifene, tamoxifen and estradiol on human osteoblasts in vitro: estrogen receptor dependent or independent pathways of raloxifene. J Steroid Biochem Mol Biol. 2009;113(3–5):281-289. [DOI] [PubMed] [Google Scholar]
- 25. Biojupies Analysis Tools for RNAseq. ProMED-mail website. https://amp.pharm.mssm.edu/biojupies/analyze/tools?uid=ETXiK0nxxgj. Accessed March 2, 2020.
- 26. Eletr ZM, Wilkinson KD. Regulation of proteolysis by human deubiquitinating enzymes. Biochim Biophys Acta. 2014;1843(1):114-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Heringa M. Review on raloxifene: profile of a selective estrogen receptor modulator. Int J Clin Pharmacol Ther. 2003;41(8):331-345. [DOI] [PubMed] [Google Scholar]
- 28. Valny M, Honsa P, Kirdajova D, Kamenik Z, Anderova M. Tamoxifen in the mouse brain: implications for fate-mapping studies using the tamoxifen-inducible Cre-loxP system. Front Cell Neurosci. 2016;10:243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lien EA, Wester K, Lønning PE, Solheim E, Ueland PM. Distribution of tamoxifen and metabolites into brain tissue and brain metastases in breast cancer patients. Br J Cancer. 1991;63(4):641-645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wu X, Glinn MA, Ostrowski NL, et al. Raloxifene and estradiol benzoate both fully restore hippocampal choline acetyltransferase activity in ovariectomized rats. Brain Res. 1999;847(1):98-104. [DOI] [PubMed] [Google Scholar]
- 31. Goekoop R, Duschek EJ, Knol DL, et al. Raloxifene exposure enhances brain activation during memory performance in healthy elderly males; its possible relevance to behavior. Neuroimage. 2005;25(1):63-75. [DOI] [PubMed] [Google Scholar]
- 32. Goekoop R, Barkhof F, Duschek EJ, et al. Raloxifene treatment enhances brain activation during recognition of familiar items: a pharmacological fMRI study in healthy elderly males. Neuropsychopharmacology. 2006;31(7):1508-1518. [DOI] [PubMed] [Google Scholar]
- 33. Forger NG. Epigenetic mechanisms in sexual differentiation of the brain and behaviour. Philos Trans R Soc Lond B Biol Sci. 2016;371(1688):20150114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Nugent BM, Wright CL, Shetty AC, et al. Brain feminization requires active repression of masculinization via DNA methylation. Nat Neurosci. 2015;18(5):690-697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Liu M, Dexheimer T, Sui D, et al. Hyperphosphorylated tau aggregation and cytotoxicity modulators screen identified prescription drugs linked to Alzheimer's disease and cognitive functions. Sci Rep. 2020;10(1):16551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. VerPlank JJS, Lokireddy S, Zhao J, Goldberg AL. 26S Proteasomes are rapidly activated by diverse hormones and physiological states that raise cAMP and cause Rpn6 phosphorylation. Proc Natl Acad Sci USA. 2019;116(10):4228-4237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Torre D, Lachmann A, Ma’ayan A. BioJupies: automated generation of interactive notebooks for RNA-Seq data analysis in the cloud. Cell Syst. 2018;7(5):556-561.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. RRID:AB_1587578.
- 39. RRID:AB_2714189.
- 40. RRID:AB_2052392.
- 41. RRID:AB_2193779.
- 42. RRID:AB_2800463.
- 43. RRID:AB_2800464.
- 44. Elsasser S, Schmidt M, Finley D. Characterization of the proteasome using native gel electrophoresis. Methods Enzymol. 2005;398:353-363. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Some or all data generated or analyzed during this study are included in this published article or in the data repositories listed in References.