Abstract
Breast cancer is the second leading cause of cancer-related mortality in women. Various nutritional compounds possess anti-carcinogenic properties which may be mediated through their effects on the gut microbiota and its production of short-chain fatty acids (SCFAs) for the prevention of breast cancer. We evaluated the impact of broccoli sprouts (BSp), green tea polyphenols (GTPs) and their combination on the gut microbiota and SCFAs metabolism from the microbiota in Her2/neu transgenic mice that spontaneously develop estrogen receptor-negative [ER(-)] mammary tumors. The mice were grouped based on the dietary treatment: control, BSp, GTPs or their combination from beginning in early life (BE) or life-long from conception (LC). We found that the combination group showed the strongest inhibiting effect on tumor growth volume and a significant increase in tumor latency. BSp treatment was integrally more efficacious than the GTPs group when compared to the control group. There was similar clustering of microbiota of BSp-fed mice with combination-fed mice, and GTPs-fed mice with control-fed mice at pre-tumor in the BE group and at pre-tumor and post-tumor in the LC group. The mice on all dietary treatment groups incurred a significant increase of Adlercreutzia, Lactobacillus genus and Lachnospiraceae, S24-7 family in the both BE and LC groups. We found no change in SCFAs levels in the plasma of BSp-fed, GTPs-fed and combination-fed mice of the BE group. Marked changes were observed in the mice of the LC group consisting of significant increases in propionate and isobutyrate in GTPs-fed and combination-fed mice. These studies indicate that nutrients such as BSp and GTPs differentially affect the gut microbial composition in both the BE and LC groups and the key metabolites (SCFAs) levels in the LC group. The findings also suggest that temporal factors related to different time windows of consumption during the life-span can have a promising influence on the gut microbial composition, SCFAs profiles and ER(-) breast cancer prevention.
Introduction
Breast cancer is a significant health concern worldwide as it is the second most common cause of cancer-related mortality among women. In 2019, about 268,600 new cases of breast cancer were diagnosed, which accounted for 30% of all new cancer cases diagnosed in women living in the United States [1]. Breast cancer has been categorized into five main types: luminal A, luminal B, triple negative/basal like, Her2-enriched and normal like, on the basis of hormone receptors (estrogen receptor [ER] and progesterone receptor [PR]), human epidermal growth factor receptor 2 [HER2], and Ki67 [2, 3]. Estrogen receptor-positive [ER (+)] (luminal A/B) breast cancer patients can receive hormone therapy with anti-estrogens and/or aromatase inhibitors [4]. Her2-positive breast cancer patients have shown significant improvement in prognosis and clinical outcome with the incorporation of Her2-target agents such as trastuzumab (a monoclonal antibody) and lapatinib (a tyrosine kinase inhibitor) into the conventional therapy [5, 6]. By contrast, triple-negative breast cancer (TNBC) patients have a poor prognosis and fewer cancer prevention and treatment options due to lack of target-directed approaches and the aggressive nature of this disease [7]. The commonly employed treatment approaches for TNBC patients and metastatic breast cancer patients are surgical, chemotherapy, radiation therapy, and palliative therapy [8]. However, these procedures have an array of short-term or long-term side effects in the patients such as loss of hair, vomiting, skin disorders, fatigue, nausea, anemia, diarrhea, muscle disorder, and nerve diseases [8, 9]. Therefore, there is a need for effective and safe approaches for prevention and treatment of TNBC.
The use of dietary bioactive botanicals is considered as a key alternative approach for prevention, progression and treatment of breast cancer due to their efficacy and safe consumption in humans [10]. For example, broccoli sprouts (BSp) and green tea polyphenols (GTPs) have been reported to reduce the incidence of breast cancer [11, 12]. Sulforaphane (SFN) is an isothiocyanate present in cruciferous vegetables such as BSp, kale, bok choy, cauliflower and cabbage and also has chemopreventive/chemotherapeutic effects against numerous types of cancers via epigenetic mechanisms [13–15]. Studies have shown that SFN is a potent inhibitor of histone deacetylase (HDAC), which is an enzyme that modulates epigenetic machinery by removal of an acetyl group from histone residues. Studies have shown that SFN induced G1/S arrest, led to down-regulation of SEI-1 and cyclin D2, increased levels of p21 and p27 and promoted cellular senescence in breast cancer [16, 17]. (-)-Epigallocatechin-3-gallate (EGCG), a major polyphenol in green tea, induces epigenetic modulations such as inhibition of DNA methytransferases (DNMTs) and has numerous anticarcinogenic properties both in vitro and in vivo against several cancers including breast cancer [18–22]. The anti-tumor mechanisms of GTPs and EGCG involve induction of cell-cycle arrest, mitochondrial-mediated apoptosis, inhibition of IL-6 and induction of tumor necrosis factor-α expression, inhibition of enzymes that regulate the glycolytic process and repression of glucose metabolism [8, 23–25].
Our previous studies have shown that the combination of BSp and GTPs resulted in synergistic inhibition of cellular proliferation, ERα reactivation via regulation of DNMT1 and HDAC1 expression in the ERα (-) breast cancer cell lines MDA-MB-231 and MDA-MB-157, and also resulted in a significant inhibition of tumor development in an ER(-) xenograft mouse model [26]. Additionally, these combined dietary components induced cellular apoptosis and cell cycle arrest in the transformed breast cancer SHR cells (normal human mammary epithelial cells transfected with SV40, hTERT and H-Ras genes), and led to genome-wide epigenetic alterations. This combination treatment administered in a breast cancer xenograft mouse model also resulted in significant inhibition of tumor growth when compared with singly administrated compounds [27]. In addition, our recent study reported that the prenatal or maternal consumption of BSp has more protective effects on tumor development than postnatal or adulthood administration of BSp in SV40 transgenic and Her2/neu transgenic mouse models [28].
The human gastrointestinal tract harbors trillions of microorganisms (≥1014) that are reported to be at an approximate ratio of 1:1 with the human cells [29]. The gut microbiota plays an important role in regulation of human metabolic and physiological functions by production of crucial metabolites such as short chain fatty acids (SCFAs) [30, 31]. SCFAs are produced from the fermentation of non-digestible carbohydrates by gut microbiota and the major SCFAs include butyrate, acetate and propionate [32]. These microbial-produced metabolites actively participate in epigenetic modulations in the host cells, that in turn can have profound effects on the inhibition of cancer [33].
Several studies have attempted to investigate the link between dietary compounds, gut microbiota and breast cancer [34, 35]. However, few studies have explored the impact of BSp or GTPs on the gut microbiota in relation to the breast cancer. Some studies have shown that BSp or GTPs can have a significant impact on gut microbial diversity and metabolite production in humans and animals [36–39]. BSp are rich in glucosinolates, which are metabolized by gut microbiota into isothiocyanates. Further, dietary supplementation with broccoli was reported to lead to alterations in cecal microbiota composition, metabolism and intestinal morphology in an inflammatory disease mouse model [40]. A recent study focused on the impact of broccoli ingestion on gut microbiota of C57BL/6 mice found that increased levels of Clostridiaceae, Lachnospiraceae and Porphyromonadaceae and abundance in gut microbiota diversity was associated with broccoli consumption [36]. EGCG can be degraded by microbial enzymes produced in the digestive tract [41]. Previous studies focused on the consumption of GTPs on intestinal microbiota of healthy humans have found a significant decrease in Clostridium spp. and an increase in Bifidobacterium spp. resulting in significantly high levels of acetate and propionate [42]. Others have demonstrated that supplementation of green tea extract resulted in an increase of Bifidobacterium species in calves and humans. Bifidobacterium is a beneficial bacterial species that possess prebiotic properties and can lead to improvement in colon environment [43, 44]. A deeper understanding of the gut microbial mechanisms underlying the protective effect of BSp and GTPs may allow the design of direct microbial interventions and open new avenues for preventive measures for breast cancer.
Here we investigated the impact of the dietary botanicals, BSp or GTPs and their combination, on the gut microbiota of Her2/neu mice when these compounds were administered lifelong from conception and from the beginning of early life. We evaluated the impact of these dietary treatments on ER(-) mammary cancer prevention in these mice by assessing the tumor volume percentage and average tumor latency. Gut microbial communities are known for direct interaction with the host as well as indirect interactions via production of diverse metabolites in the host [45]. In this study, we performed 16S rRNA gene sequencing to investigate the gut microbial composition of Her2/neu mice before and after tumor onset, and identified key bacterial phylotypes that were significantly altered with dietary treatment. We also studied the impact of BSp, GTPs and the combination diet on the plasma levels of SCFAs, which are gut microbial-produced metabolites.
Materials and methods
Animals
The animal study was reviewed and approved by Institutional Animal Use and Care Committee of the University of Alabama at Birmingham (IACUC; Animal Project Numbers: 10088 and 20653). The Wild type (WT) Her2/neu [FVB-Tg(MMTV-Erbb2)NK1Mul/J] mouse model (Jackson Laboratory, Bar Harbor, ME) was used in this study. We obtained the breeder mice (4 wks) that were bred from 10 wks of age to obtain sufficient colonies for follow-up experiments. We performed a standard PCR analysis with tail DNA of mice (3 wks of age) to identify the Tag genotypes [46]. Mice were housed in the Animal Resource Facility at the University of Alabama at Birmingham and were maintained within 12-hour light/dark cycle, 24 ± 2°C temperatures, and 50 ± 10% humidity. An online Power and Sample Size Calculator (http://powerandsamplesize.com) was used to evaluate the power and sample size by 2-proportion comparison [28]. All animals had free access to food and water.
Mouse diet
Mice on the BSp diet were fed a customized AIN-93G diet from TestDiet (St. Louis, MO) and adjusted for nutrients content as used previously [28]. The modified AIN-93G diet contained 26% (w/w) BSp, which was obtained from Natural Sprout Company (Springfield, MO). Mice on GTPs diet were orally fed 0.5% (w/v) GTPs Sunphenon 90D (SP90D, Taiyo Inc., Minneapolis, MN, USA) in drinking water either alone or in combination with the 26% BSp. The SP90D contained polyphenols (>90%), catechins (>80%), EGCG (>45%) and caffeine (<1%), and is a decaffeinated extract of green tea containing purified polyphenols rich in green tea catechins. Mice on control diet were fed with AIN-93G basal mix diet pellets. Diets were stored in airtight containers and were kept away from light under refrigeration (2°C for up to six months and -20°C for long-term life) to provide maximum protection against possible changes. Mice food was in the form of pellets.
Animal experiment design
Beginning in early life (BE) group
80 Her2/neu female mice were randomly divided into four dietary treatment groups (20 mice/group) as the following: control or BSp or GTPs or combination, upon weaning. The dietary treatment continued from 3 wks of age (prepubescence) through adulthood until termination (Fig 1).
Fig 1. Schematic representation of study design.
Her2/neu transgenic mice were administrated either the control diet or 26% broccoli sprouts (BSp) diet in pellets or 0.5% green tea polyphenol (GTP) in the drinking water or BSp and GTPs in the combination diets at two time points: (i) Lifelong from conception (LC), and (ii) Beginning from early life (BE). In the LC group, dietary treatments were started upon the mating of maternal mice and continued during gestation and lactation. After weaning, offspring female mice (n = 10 mice/group) selected from each group were maintained on the same treatments as their mother throughout their lifespan until termination of the experiment and monitored for tumor growth weekly. In the BE group, female mice (n = 20 mice/group) were fed one of four different dietary regimens upon weaning, which continued throughout the study until termination. Fecal samples were obtained before the onset of tumor (at 16 wks of age) as follows, eight mice per treatment from the BE group and five mice per treatment from the LC group for the analyses of intestinal communities composition with microbiome analyses. Prior to euthanasia, the fecal samples were obtained after the onset of tumor (at 28 wks of age) for investigation of temporal changes in microbial composition by microbiome analyses. On the day of euthanization, blood samples (approximately 500 μl in Eppendorf tubes containing EDTA) were individually collected from the retroorbital sinus and plasma was isolated by centrifugation, and stored at -80°C for SCFAs analysis.
Lifelong from conception (LC) group
40 Her2/neu female mice were randomly divided into four dietary treatments groups (10 mice/group) as the following: control or BSp or GTPs or combination upon pregnancy. The dietary treatments were continued throughout the gestation and lactation periods. After the lactation period, their female offspring mice were weaned at 3 wks of age. Twenty offspring female mice were then randomly selected from each group and fed the same diet as their mother throughout the study until termination.
Tumor observation and sampling
The tumor size was measured and calculated weekly. Tumor volume was determined as, tumor volume (cm3) = 0.523 × [length (cm) × width2 (cm2)] [47]. The experiments were terminated when all of the control mice developed tumors and had an average tumor diameter exceeding 1.0 cm. On the day of euthanization, blood samples (approximately 500 μl in Eppendorf tubes containing EDTA) were individually collected from the retroorbital sinus of each mouse, and plasma was isolated by centrifugation before storing at -80°C for SCFAs analysis. All animal studies were carried out in accordance with the guidelines of the IACUC at UAB.
Fecal sample collection
The mammary tumors originate at around 20 wks of age in the Her2/neu transgenic mice model [48]. Therefore, the fecal samples from mice were collected at two time points: before the onset of tumor (at 16 wks) and after the onset of tumor (at 28 wks) for studying the temporal efficacy of dietary botanicals on the microbiota composition and impact on breast cancer prevention. Fecal samples were obtained from eight mice per treatment from the BE group and five mice per treatment from the LC group. Approximately 40–50 mg of fecal specimens were acquired and diluted in modified Cary Blair [49] medium for a total volume of 800 μL with 10% by volume glycerol, mixed uniformly by vortex and stored at -80°C [49].
DNA extraction and PCR amplification
Genomic DNA was isolated from fecal samples by bead-beating with the Fecal DNA Isolation Kit from Zymo Research (Irvine, CA, USA) according to the manufacturer’s instructions. The extracted DNA was immediately used for PCR or stored in standard Tris-EDTA buffer (pH 8) at 4°C. Before PCR, the isolated PCR DNA was quantified using a microspectrophotometer (ThermoFisher, Waltham, MA) [49]. An amplicon library was constructed from isolated DNA samples by PCR to amplify the V4 region of the 16S rRNA gene with the unique barcoded primers [34], and the olignonucleotide primers were as follows (Eurofind Genomics, Inc., Huntsville, AL):
-
Forward V4:
5’AATGATACGGCGACCACCGAGATCTACACTATGGTAATTGTGTGCCAGCMGCCGCGGTAA-3’;
-
Reverse V4:
5’CAAGAGAAGACGGCATACGAGATNNNNNNAGTCAGTCAGCCGGACTACHVGGGTWTCTAAT-3’
The quantification of purified PCR products was carried out by PICO green dsDNA Reagent.
Illumina MiSeq sequencing and bioinformatics analyses
Agarose gel electrophoresis was performed on the individual PCR products and visualized on the UV illuminator. The isolated PCR products were excised from the gel and purified by QIAquick Gel Extraction Kit (Qiagen, Germantown, MD). NextGen sequencing Illumina MiSeq [34, 49] platform was used for sequencing the PCR products of about 250 bp paired-end reads from the V4 region of the 16S rRNA gene. The obtained raw FASTQ files were used for library construction, de-multiplexed, and assessed for quality control using FastQC (FastQ quality control). The respective Phred score values were generated with the average values of about ~30–50 as shown in S1 File, thereby identifying the samples with good quality. Subsequently, the processed library was used for downstream analyses using the Quantitative Insight into Microbial Ecology (QIIME) [50] data analysis package. As a result the samples were grouped using Uclust in-built function, a clustering program and the sequences of 97% similarity were grouped into Operational taxonomic units (OTU) and were used to evaluate changes at phylum level. The multiple sequence alignment of OTUs was created by using PyNAST [51]. Beta diversity was evaluated with Bray Curtis method to quantify continuous dissimilarity between different treatment groups (control-BSp, control-GTPs and control-combination) and compared using [52].
LC-MS analysis of plasma short chain fatty acids
Plasma samples (20 μl) were mixed with ice-cold methanol (60 μl) to precipitate proteins. The methanol contained the internal standard 13C4-butyric acid (0.5 mg/ml). The samples were centrifuged for 10 min at 16,000 x g and supernatants collected. Each supernatant (40 μl) was diluted into 50% methanol (40 μl) in a 1.5 ml microfuge tube. 1-Ethyl-3-(3-dimethylaminopropyl) carbodiimide (10 μl, 0.25 M) and 10 μl of 0.1 M O-benzylhydroxylamine (o-BHA, 10 μl, 0.1 M) were added to samples to chemically modify SCFAs with o-BHA. The resulting mixture was derivatized for 1 h at room temperature. Samples were diluted 20-fold in 50% methanol. The diluted samples (200 μl) were subject to liquid-liquid extraction with dichloromethane (DCM, 600 μl). Samples were vortexed for 1 min and phases were allowed to separate. A portion of the DCM phase (400 μl) was transferred to a glass tube and dried under N2 gas [53]. Samples were reconstituted in 30% methanol (200 μl) and then transferred to loading vials. SCFA standards (MilliporSigma, CRM46957, Burlington, MA) were processed in the same manner as samples. Concentrations from 0.1–5,000 μM were used to create standard curves for each SCFA.
Samples were analyzed by tandem HPLC-MS utilizing a 20A HPLC (Shimadzu, Kyoto, Japan) and an API 4000 triple quadrupole mass spectrophotometer (SCIEX, Framingham, MD). Instrument control and data acquisition utilized Analyst 1.6.2 (SCIEX). Authentic standards and samples were analyzed as previously described [53] with slight alterations. An Accucore C18 reverse-phase column (2.6 μm 100 x 2.1 mm ID, ThermoFisher, Waltham, MA) was employed for gradient separation. Mobile phase B was altered to 20% isopropanol/80 methanol/0.1% formic acid. MultiQuant 1.3.2 (SCIEX) was used for post-acquisition data analysis; peaks in all standards and plasma extracts were normalized to the 13C4-butyric acid internal standard signal. Each standard curve was regressed linearly with 1/x2 weighting.
Statistical analysis
Power calculations for animal experiments were conducted using an online calculator (http://powerandsamplesize.com/). Sample size for animal studies was calculated by one-side 2-propotion comparison. Tumor growth was calculated by using Bonferroni adjustment for multiple comparisons between the dietary treatment groups (80% power, significance level of 0.01, alpha = 0.05 with Bonferroni adjustment for 4 comparisons). Statistical analysis of tumor growth data was performed by SPSS version 24.0. The comparisons between two groups were analyzed by two-tailed Student’s t-test and comparisons between three or more groups were analyzed by one-way independent ANOVA, followed by Tukey’s post-hoc test to determine significance between groups for tumor volume. In addition, tumor-free survival curves were evaluated with the Mantel-Cox proportional model using GraphPad Prism (version 7.04) and significances of difference between dietary treatment groups were tested using the log-rank statistic. Error bars of tumor growth data were standard error of the mean obtained from experiments. Values of SCFAs data are represented as mean ± SD. Statistically significant results were represented as ** (p < 0.01) and * (p < 0.05).
Estimation of beta-diversity and taxonomic abundance
Permutational multivariate analysis of variance (PERMANOVA) was conducted for statistical analyses of associations between microbial communities from the distance matrix of the Bray Curtis test of beta diversity. We used DESeq2 [54] to investigate differential taxonomic abundances between control and dietary treatments (BSp, GTPs and combination) of mice. A p-value < 0.05 was considered as nominal statistical significance. The statistical significance of bacterial abundance at the taxonomic level was adjusted with the false discovery rate (FDR) at 5% and represented as qc value [55]. Furthermore, to visualize the correlation between microbiome data (OTUs) among different samples, heatmaps were constructed using pheatmap package in R (v3.6.0). The heatmaps were generated based on Pearson correlation coefficient (R), wherein the rows represent different treatment groups (control, BSp, GTPs and combination) and columns represent different bacterial taxa. Heatmaps of the bacterial phylum distribution were generated based on the relative abundance of each bacterial phyla in each dietary treatment for both BE and LC groups. All analyses were conducted using R version 3.6.0.
Results
Effects of the BSp, GTPs or combination dietary treatment on ER-negative mammary tumor development
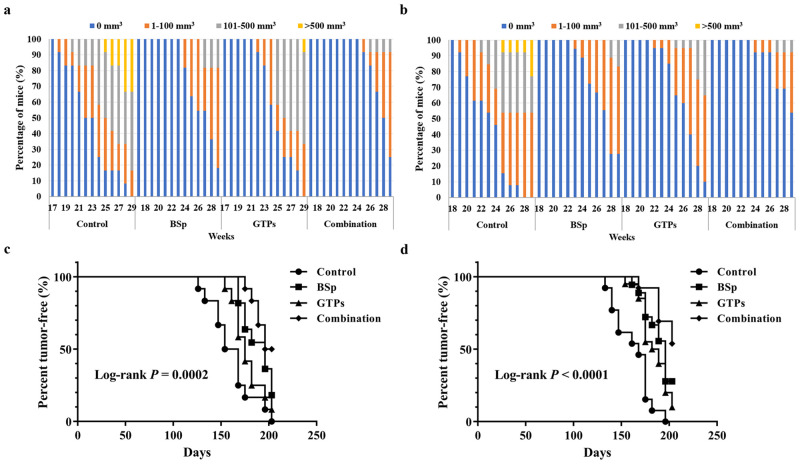
The Her2/neu female transgenic mouse model is an excellent preclinical model for breast cancer prevention studies because the mice develop spontaneous ER-negative mammary cancer that resembles human pathogenesis [56, 57]. The mice develop focal hyperplastic and dysplastic mammary tumors (due to the overexpression of the Her2/neu gene) at an early age (~20 wks) [48]. Fig 2 shows the differences in tumor growth volume and percentage over the whole population, and tumor-free intervals (tumor latency) for survival curves between our dietary treatment groups. The mice on BSp or GTPs dietary treatment showed a delay in tumor growth and the combination dietary treatment rendered the strongest inhibiting effect on tumor growth volume in both BE (Fig 2a) and LC (Fig 2b) groups. As shown in Fig 2c and 2d, for all three dietary treatments, the mean tumor latencies were significantly increased in both BE (Log-rank P = 0.0002, Fig 2c) and LC (Log-rank P < 0.0001, Fig 2d) groups. Therefore, the combination diet group was the most effective in delaying the tumor development and the BSp diet group was integrally more efficacious than the GTPs group when compared to control.
Fig 2. Tumor growth comparisons between control, BSp, GTPs and combination-fed mice dietary groups.
Female Her2/neu mice were monitored for tumor growth weekly. The delay in tumor growth volume and the percentage over the whole population in BSp, GTPs and combination dietary regimens as compared to control in the BE group (a) and in the LC group (b) as shown. Colors in (a) and (b) indicate different tumor volume ranges as shown in the legend above the graph. Tumor-free survival curves for cases classified in four dietary treatments of the BE group (c) and the LC group (d) were calculated using the Mantel-Cox proportional model and differences were tested using the log-rank statistic. n = 16~20 in dietary treatments of the BE group and n = 15~20 in dietary treatments of the LC group. Tumor growth was evaluated with Bonferroni adjustment for multiple comparisons between the dietary treatment groups (80% power, significance level of 0.01, alpha = 0.05 with Bonferroni adjustment for 4 comparisons).
Effects of the BSp, GTPs or combination diet on gut bacterial diversity before the onset of tumors
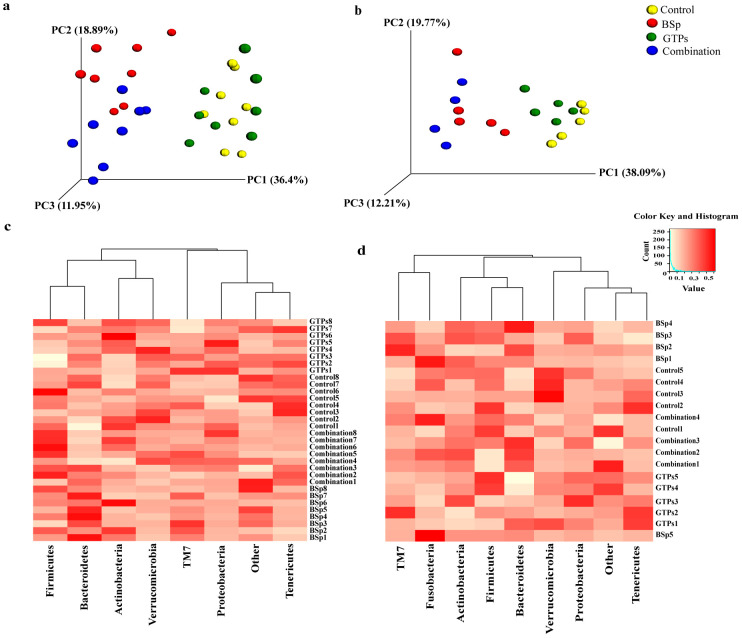
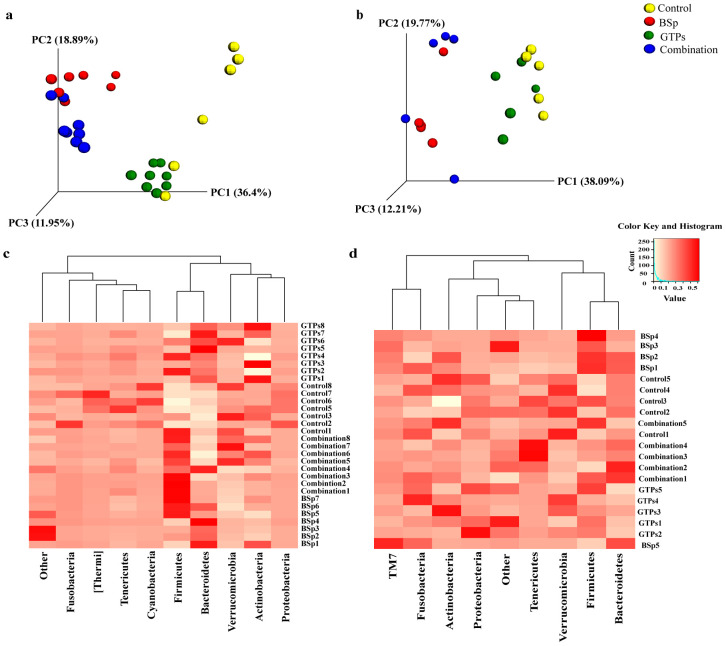
These Her2/neu female transgenic mice begin to develop ER(-) mammary tumor at around 20 wks of age [48]. Therefore, we chose the 16th week of age for collection of fecal samples as an initial time point to investigate the effects induced by BSp, GTPs and combination treatment groups on gut microbiota of Her2/neu female mice before the onset of tumor in both BE and LC groups for temporal analyses. In order to identify the outliers in samples of different treatment groups, a sample dendrogram was generated by performing hierarchical clustering using hclust package in R (v3.6.0) (S1 Fig). As a result, in the BE group, there were no outliers and all the samples were included in further analysis. Subsequently, a 3D Principal Coordinates Analysis (PCoA) plot distance metric (Bray Curtis) was generated. Distinct clustering of the BSp-fed and combination-fed treatment groups as compared to the GTPs-fed and control-fed dietary mice in the BE group was observed (Fig 3a), whereas, the GTPs and control dietary treatments were found to be clustered with each other. The results were validated by PERMANOVA from the distance matrix of the Bray Curtis test of beta diversity in BSp-fed (F = 27.46, p = 0.01), GTPs (F = 1.26, p = 0.25), and combination-fed (F = 27.08, p = 0.01) when compared with control-fed mice. Therefore, these findings indicate the microbiota of BSp-fed mice and combination-fed mice were different from the microbiota of GTPs-fed mice and control-fed mice, which in turn were strongly similar to each other.
Fig 3. Taxonomic distribution of microbial communities in the gut of mice before the onset of tumor.
(a) 3D PCoA plot (Bray Curtis) showing a distinct clustering of the BSp-fed (red) and combination-fed diet groups (blue) as compared to the control-fed (yellow) and GTPs-fed (green) in the BE group. (b) 3D PCoA plot (Bray Curtis) showing a distinct clustering of the control-fed (yellow), BSp-fed (red), GTPs-fed (green) and combination-fed (blue) diet groups in the LC group. PERMANOVA was used for conducting statistical analyses on association between microbial communities from the distance matrix of the Bray Curtis test of beta diversity by using R (v3.6.0). (c) Heatmap representing the pre-tumor phylum level changes in microbial abundance by our dietary treatments in the BE group. (d) Heatmap representing the pre-tumor phylum level changes in microbial composition by our dietary treatments in the LC group. Each row corresponds to differentially expressed microbial communities and each column represents biological replicates in the BE (n = 8) and LC (n = 5) treatment groups. White color denotes lower expression levels and red color denotes higher expression levels.
Similarly, hierarchical clustering using hclust package in R (v3.6.0) in the LC temporal treatment group was performed and no outliers were identified (S2 Fig); thus, we included each sample in this study. We observed an overall similar clustering as seen in the BE group, the microbial composition of BSp-fed mice strongly overlapped with combination-fed mice and the microbial composition of GTPs-fed mice strongly overlapped with control-fed mice (Fig 3b). This observation was validated with PERMANOVA from the distance matrix generated using Bray Curtis method with beta diversity. It was significant in BSp-fed (F = 12.69, p = 0.02) and combination-fed (F = 37.61, p = 0.03) mice when compared with control-fed mice. The microbial composition of GTPs-fed was highly clustered but it failed to show a clear separation from the microbial composition of control-fed (F = 2.78, p = 0.08) mice. Hence, these results suggest that the microbial composition of BSp-fed mice and combination-fed mice were largely similar and the microbial composition of GTPs-fed mice and control-fed mice were lately similar to each other.
Impact of diet on gut bacterial composition before the onset of tumor
When compared at the phylum level, the dietary treatments showed differences in microbial abundances in both the BE and LC groups. In the BE group, the mice that were BSp-fed underwent a significant increase in Bacteroidetes as compared to control-fed mice (35% versus 21%, p = 0.02) (Fig 3c) evaluated by using two-tailed Student’s t-test. The microbiota of mice on the GTPs diet showed a significant decrease in Firmicutes phylum (55% versus 66%, p = 0.031) as compared to control-fed mice. The microbiota of mice on the combination dietary treatment underwent a significant decline in the abundance of Verrucomicrobia as compared to the mice fed with the control diet (2% versus 5%, p = 0.03).
The mice in the LC group showed a similar trend as the mice in the BE group at the phylum level. Mice on the BSp diet (28% versus 16%, p = 0.04) and combination diet (31% versus 16%, p = 0.005) underwent a significant increase in Bacteroidetes levels as compared to mice fed the control diet (Fig 3d) when determined using two-tailed Student’s t-test.
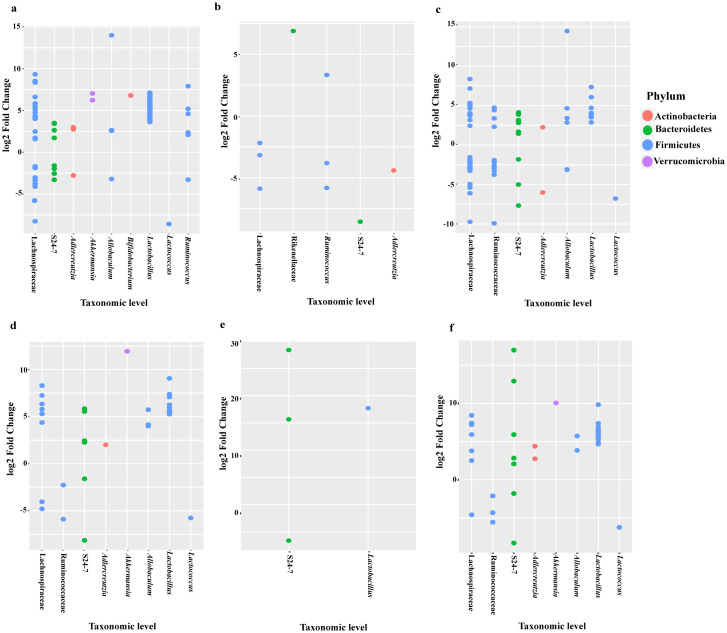
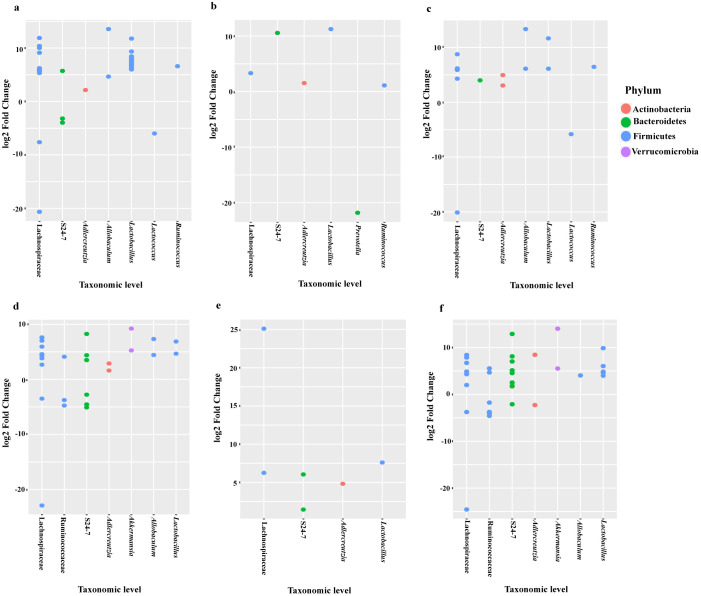
The relative abundance of bacterial taxonomic units of mice from the BE group were analyzed using DESeq2 analyses and depicted in a dotplot (Fig 4a–4c). Compared to the control diet, the relative abundance of Firmicutes (f_Erysipelotrichaceae g_Allobaculum s_unclassified) was higher in the BSp-fed (13.99 log2 fold change), and combination-fed (14.45 log2 fold change) mice. Family Lactobacillaceae showed higher log2 fold change in the BSp-fed and combination-fed diets when compared to control-fed and GTPs-fed diets. After investigation of significance of bacterial communities using false-discovery rate (FDR) correction, numerous significantly different bacterial taxa were identified, BSp-fed mice (n = 167), GTPs (n = 17) and combination-fed mice (n = 111) were found to be significantly different as detailed in S2 File. Table 1 shows the top bacterial taxa that were significantly different between the BSp-fed, GTPs-fed and combination-fed as compared to control-fed mice groups, respectively. The S24-7 and Lachnospiraceae families were increased significantly after consumption of the BSp diet and combination diet in mice. All of the dietary treated mice showed significant increases in the Ruminococcaceae bacterial family. Overall, the BSp-fed and combination-fed groups showed significant changes in the microbial abundance as compared to the control and GTPs diet at the taxonomic level.
Fig 4. Log2 fold change of relative abundance of microbial species in mice before the onset of tumor.
(a) Dotplot of the bacterial abundance between BSp-fed and control-fed mice, (b) GTPs-fed and control-fed mice, (c) combination-fed and control-fed mice before the onset of tumor in the BE group (n = 8), (d) Dotplot of the microbial species abundance between BSp and control, (e) GTPs and control, (f) combination and control dietary treatment before the onset of tumor in the LC group (n = 5).
Table 1. Differentially abundant bacterial species before and after the onset of tumor in BSp-fed, GTPs-fed and combination-fed mice versus control-fed mice of the both BE and LC groups.
| Pre-tumor BE group | |||||||||
| Phylum; Class; Order; Family; Genus; Species | BSp | GTPs | Combination | ||||||
| Log2FC | p-value | qc | Log2FC | p-value | qc | Log2FC | p-value | qc | |
| Firmicutes; Clostridia; Clostridiales; Lachnospiraceae | 8.35 | 4.73E-24 | 6.39E-22 | -3.17 | 1.33E-07 | 1.07E-05 | 7.18 | 9.87E-19 | 2.00E-16 |
| Bacteroidetes; Bacteroidia; Bacteroidales; S24-7 | 2.63 | 2.15E-10 | 3.78E-09 | -8.56 | 2.59E-13 | 1.05E-10 | 3.05 | 1.00E-13 | 4.05E-12 |
| Firmicutes; Clostridia; Clostridiales; Ruminococcaceae | 3.92 | 1.14E-19 | 7.73E-18 | 3.32 | 2.91E-09 | 3.93E-07 | 4.75 | 1.66E-08 | 2.31E-14 |
| Post-tumor BE group | |||||||||
| Phylum; Class; Order; Family; Genus; Species | BSp | GTPs | Combination | ||||||
| Log2FC | p-value | qc | Log2FC | p-value | qc | Log2FC | p-value | qc | |
| Bacteroidetes; Bacteroidia; Bacteroidales; S24-7 | 5.72 | 4.64E-06 | 0.00011 | 10.56 | 0.000028 | 0.0043 | 3.99 | 0.00025 | 0.0052 |
| Actinobacteria; Coriobacteriia; Coriobacteriales; Coriobacteriaceae; Adlercreutzia | 2.15 | 0.00049 | 0.00458 | 1.53 | 0.00062 | 0.0078 | 4.94 | 2.38E-06 | 0.0076 |
| Firmicutes; Bacilli; Lactobacillales; Lactobacillaceae; Lactobacillus | 11.77 | 6.75E-26 | 1.58E-23 | 11.25 | 6.33E-25 | 2.96E-22 | 11.65 | 1.97E-22 | 5.81E-20 |
| Firmicutes; Clostridia; Clostridiales; Lachnospiraceae | 9.12 | 2.59E-12 | 4.05E-10 | 3.32 | 0.00006 | 0.0074 | 6.17 | 4.28E-08 | 6.31E-06 |
| Firmicutes; Clostridia; Clostridiales; Ruminococcaceae; Ruminococcus | 6.60 | 0.00035 | 0.0035 | 1.11 | 0.0042 | 0.0015 | 6.45 | 0.00048 | 0.0084 |
| Pre-tumor LC group | |||||||||
| Phylum; Class; Order; Family; Genus; Species | BSp | GTPs | Combination | ||||||
| Log2FC | p-value | qc | Log2FC | p-value | qc | Log2FC | p-value | qc | |
| Bacteroidetes; Bacteroidia; Bacteroidales; S24-7 | 5.54 | 1.28E-25 | 4.40E-23 | 28.39 | 9.43E-46 | 2.05E-43 | 12.92 | 2.90E-25 | 9.96E-23 |
| Firmicutes; Bacilli; Lactobacillales; Lactobacillaceae; Lactobacillus; reuteri | 9.06 | 5.97E-24 | 1.02E-21 | 17.71 | 1.65E-15 | 1.19E-13 | 9.84 | 3.85E-24 | 6.61E-22 |
| Post-tumor LC group | |||||||||
| Phylum; Class; Order; Family; Genus; Species | BSp | GTPs | Combination | ||||||
| Log2FC | p-value | qc | Log2FC | p-value | qc | Log2FC | p-value | qc | |
| Bacteroidetes; Bacteroidia; Bacteroidales; S24-7 | 8.26 | 2.37E-32 | 8.66E-30 | 6.04 | 5.39E-18 | 6.36E-16 | 8.12 | 2.49E-31 | 9.08E-29 |
| Firmicutes; Clostridia; Clostridiales; Lachnospiraceae | 5.94 | 1.13E-09 | 4.59E-08 | 25.10 | 1.36E-05 | 0.00053 | 7.88 | 4.88E-10 | 1.48E-08 |
| Firmicutes; Bacilli; Lactobacillales; Lactobacillaceae; Lactobacillus; reuteri | 6.88 | 3.16E-14 | 2.30E-12 | 7.60 | 7.14E-05 | 0.0016 | 9.87 | 6.54E-21 | 5.97E-19 |
| Actinobacteria; Coriobacteriia; Coriobacteriales; Coriobacteriaceae; Adlercreutzia | 1.61 | 3.25E-06 | 5.65E-05 | 4.82 | 3.41E-05 | 0.001 | 9.50 | 7.94E-14 | 1.7E-11 |
qc represents FDR adjusted p-value.
The relative abundance of the bacterial taxonomic units of mice from LC groups are depicted in the dotplot (Fig 4d and 4e). Similar to the BE group, the relative abundance of Firmicutes (f_Erysipelotrichaceae g_Allobaculum s_unclassified) was higher in the BSp-fed (5.73 log2 fold change) and the combination-fed (5.73 log2 fold change) mice. After the correction for multiple comparisons (FDR < 0.05), we found significant difference in microbial communities induced by our dietary treatment groups as follows: BSp-fed mice (n = 45), GTPs-fed mice (n = 4) and combination-fed mice (n = 56) as listed in S2 File. The top bacterial species that were significantly different in the mice on BSp, GTPs and combination dietary treatments and control are shown in Table 1. The S24-7 family and Lactobacillus reuteri bacteria were increased significantly after the consumption of the BSp or GTPs or combination diet. Moreover, the mice on BSp and combination dietary treatment displayed significant increase in Adlercreutzia genus Akkermansia muciniphila and Lachnospiraceae family, and significant decrease in Lactococcus. Overall, the mice in all of the dietary treatment groups displayed significant differences in bacterial taxa as compared with the control group of mice.
Effects of the BSp, GTPs or combination diet on gut bacterial diversity after the onset of tumor
We chose another time point (28th wks) for collection of fecal samples to investigate whether the gut microbiota was altered after the onset of tumor by our dietary treatments in mice. We found a distinct clustering of microbial communities with the treatment of BSp, GTPs or combination as compared to the control diet in the BE group (Fig 5a). PERMANOVA test on the Bray Curtis clustering supported our observation: BSp (F = 28.88, p = 0.01), GTPs (F = 11.16, p = 0.01) and combination (F = 35.10, p = 0.01) dietary treatments demonstrated significant clustering against the control group. In addition, the microbiota of BSp-fed and combination-fed mice were clustered together again, which implied a similar distribution of bacterial taxa even after the tumor onset.
Fig 5. Changes in microbial composition by the dietary treatments after the onset of tumor in mice.
(a) After tumor onset, the 3D PCoA plot (Bray Curtis) showed a distinct clustering of the BSp-fed (red), GTPs-fed (green) and combination-fed diet groups (blue) as compared to the control-fed (yellow) in the BE group. (b) 3D PCoA plot (Bray Curtis) showed a distinct clustering of dietary groups after the tumor onset in the LC group. (c) Heatmap depicting the phylum level changes in microbial abundance by our dietary treatments in the BE group. (d) Heatmap depicting the post-tumor phylum level changes in microbial abundance by our dietary treatments in the LC group. The phylum levels of bacterial communities are represented in rows and columns correspond to biological replicates in the BE (n = 8) and LC (n = 5) groups. Higher expression levels are represented in red color and lower expression levels are represented in white color. Statistical analyses for association between bacterial communities of beta diversity was determined by using PERMANOVA.
In the LC group, we observed distinct clustering of the mice on BSp diet or GTPs diet or combination diet or control diet (Fig 5b). PERMANOVA test on the Bray Curtis clustering validated our findings- BSp (F = 11.43, p = 0.01), GTPs (F = 3.25, p = 0.03) and combination (F = 11.43, p = 0.01). The same microbial clustering pattern of BSp with combination, and GTPs with control is observed as was reported in pre-tumor evaluation.
Impact of diet on gut bacterial composition after onset of tumor
The changes in phylum levels of microbial composition were also observed after tumor onset for all dietary treatments of both groups. In the BE group, the mice on the BSp diet showed a significant increase in Bacteroidetes (33% versus 18%, p < 0.01) and a significant decrease in Actinobacteria (6% versus 11%, p = 0.02) and Proteobacteria (0.14% versus 12%, p < 0.01) as compared to mice on the control diet (Fig 5c). The mice on GTPs diet had a significant increase in the Bacteroidetes levels (31% versus 18%, p < 0.001) and a significant decrease in Proteobacteria (0.28% versus 12%, p < 0.01) as compared to mice on the control diet. The mice on the combination dietary treatment revealed a significant increase in Firmicutes (73% versus 51%, p < 0.001), and a significant decrease in Proteobacteria (0.11% versus 12%, p < 0.01) abundance.
The alterations in microbial compositions were also observed in the LC group after the onset of tumors (Fig 5d). BSp treatment led to a significant decrease in levels of Proteobacteria (0.07% versus 0.61%, p = 0.001). GTPs treatment led to significantly higher levels of Firmicutes (65% versus 51%, p = 0.026) and also significantly lower levels of Bacteroidetes (21% versus 31%, p < 0.001) in comparison to the control-fed mice. Furthermore, the combination treatment led to a significant decrease in Proteobacteria (0.07% versus 0.61%, p = 0.001) when compared with the control-fed mice.
The dotplot (Fig 6a–6c) depicts the abundance of family Erysipelotrichaceae (f_Erysipelotrichaceae g_Allobaculum s_unclassified) to be high in BSp (13.55 log2 fold change), and combination (13.34 log2 fold change) dietary treatments. We found several significantly different bacterial taxonomic abundance following correction with FDR (<0.05) (S2 File) in BSp-fed mice (n = 63), GTPs-fed mice (n = 6) and combination-fed mice (n = 17). Table 1 lists the top taxa that had different abundance between the dietary treatments’ post-tumor temporal period. The S24-7, Lachnospiraceae family of bacteria, and Adlercreutzia, Lactobacillus and Ruminococcus genera were increased significantly in mice of all dietary treatments when compared with the control treatment. Specifically, Lactococcus genus levels were significantly lowered in BSp (-5.98 log2 fold change) and combination (-5.82 log2 fold change) when compared to control dietary treatment administered to mice. Collectively, an overlap of numerous bacterial communities was observed due to the dietary treatments that were significantly different from microbial communities of the control group mice.
Fig 6. Dotplots showing the difference between relative abundance of microbial species after the onset of tumor in mice.
(a) Dotplot of the bacterial abundance between BSp-fed and control-fed, (b) GTPs-fed and control-fed, (c) combination-fed and control-fed mice after tumor onset in the BE group (n = 8). (d) Dotplot of the microbial species abundance between mice on BSp and control, (e) GTPs and control, (f) combination and control dietary treatments after tumor onset in LC group (n = 5).
The dotplots (Fig 6d and 6e) indicated the augmentation of bacteria of genus Allobaculum in BSp-fed (7.33 log2 fold change) and combination-fed (4.05 log2 fold change) diet groups when compared to the control group. There were several significantly different bacterial taxa between dietary treatments after FDR correction (<0.05) (S2 File) as follows: BSp-fed mice (n = 21), GTPs-fed mice (n = 17) and combination-fed mice (n = 23). Table 1 displays the top microbial species that had significantly different abundance between the dietary groups of mice after the onset of tumor. Some of the same bacterial communities were found to be changed such as families S24-7 and Lachnospiraceae, and genera Adlercreutzia and Lactobacillus that were significantly increased when compared with the control group. The mice on BSp and combination dietary treatment showed significant decrease in Lactococcus genus and significant increase in Akkermansia muciniphila. Overall, these results show that dietary treatments led to significant changes in microbial species after the onset of tumors.
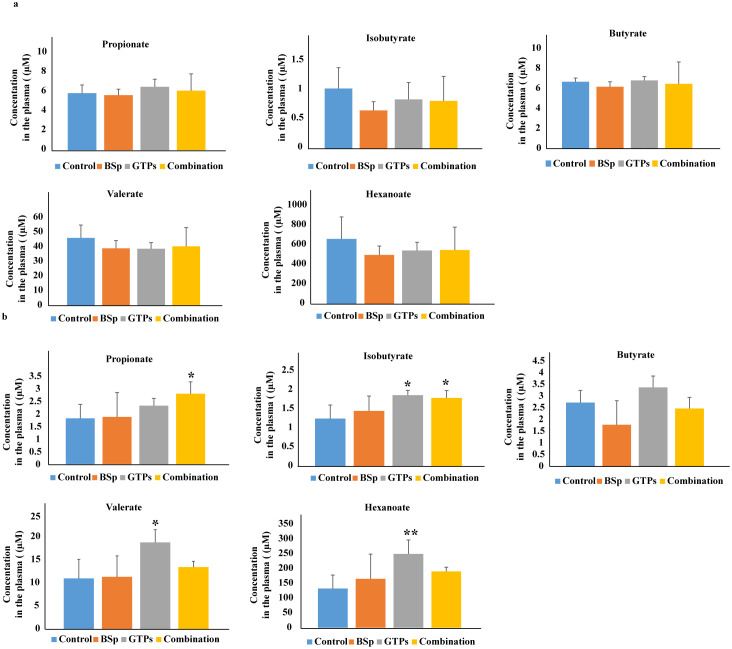
Analyses of the quantity and type of SCFAs in mice on the BSp, GTPs, combination or control diet
Short-chain fatty acids are the crucial metabolites produced from fermentation of dietary fiber by intestinal communities, and act as signaling molecules in the complex crosstalk network of the gut with distal organs [58]. Therefore, we investigated whether the plasma SCFA profiles changed with our dietary treatments. As shown in Fig 7a, we detected the levels of propionate, butyrate, isobutyrate, valerate and hexanoate in the plasma samples of mice fed with BSp diet, GTPs diet, combination diet or control diet. In the BE group, the plasma concentrations of all SCFAs were found to be unchanged in BE-fed, GTPs-fed and combination-fed mice when compared with the plasma levels of control-diet mice.
Fig 7. Analyses of plasma SCFAs levels in BSp-fed, GTPs-fed, combination-fed or control-fed mice.
(a) Concentrations of SCFAs propionate, isobutyrate, butyrate, valerate and hexanoate in our dietary treatment mice versus control treatment mice in BE group (b) Concentrations of the SCFAs in BSp-fed, GTPs-fed or combination-fed mice when compared with control-fed mice in LC group. Data are presented as mean ± standard deviation (SD) (n = 6). Significance was determined by using one-way independent ANOVA, followed by Tukey’s post-hoc test. * p < 0.05, ** p < 0.01, ***p <0.001.
In the LC group (Fig 7b), the BSp-fed mice showed a no effect on the levels of SCFAs in comparison to the control-fed mice. Strikingly, however GTPs-fed mice showed a significant increase in isobutyrate (1.5 fold, p = 0.017), valerate (1.48 fold, p = 0.02) and hexanoate (1.88 fold, p < 0.01) as compared to the control-fed mice. The mice on the combination diet had significantly higher levels of propionate (1.5 fold, p = 0.037) and isobutyrate (1.44 fold, p = 0.036) in contrast to mice on the control diet. Therefore, the mice on GTPs and combination diet groups had significantly increased levels of SCFAs in the LC group.
Discussion
The use of dietary bioactive compounds as an adjuvant therapy for chemoprevention of breast cancer has been of great interest. The gut microbiome is an important determinant of human health [59] and alterations in composition and diversity of colonizing microbial communities have been associated with pathogenesis of a vast number of disorders over the past decade [60–63]. We investigated the impact of early life consumption of these dietary compounds on the inhibition of breast cancer, intestinal communities and their derived metabolites in Her2/neu mice by analyzing the gut microbiota and plasma SCFAs profiles.
Our study involved administration of several diets—the BSp diet, the GTPs diet and their combination diet at two exposure periods, beginning in early life (BE) and lifelong from conception (LC) in the Her2/neu transgenic spontaneous ER(-) mammary cancer mouse model. We found in both treatment plans the combination group achieved maximum efficacy in delaying mammary tumor volume and significantly delay in the tumor latency, followed by the BSp dietary treatment that was more efficacious than the GTPs group when compared to the control group.
The pre-tumor beta diversity analyses revealed that microbial communities of BSp-fed mice and combination-fed mice clustered with each other, whereas microbial communities of GTPs-fed mice and control-fed mice clustered with each other in both BE and LC groups. This may imply BSp treatment contributes more towards the combination treatment as compared to the GTPs group. To investigate changes induced in the gut microbiota by our dietary treatments, we observed the phylum and taxonomic abundance of bacterial communities residing in the gut of mice. At the phylum level, we found an increase of Bacteroidetes in BSp-fed and combination-fed group in both BE and LC groups.
At the taxonomic level, an increase of genera Allobaculum, Lactobacillus, S24-7 and Lachnospiraceae family were observed in BSp-fed and combination-fed mice, and an increase of Ruminococcaceae family was observed in all dietary treatments of BE group. Pre-tumor analyses of gut microbiota in the LC group showed significant increases in bacteria of genera Lactobacillus and family S24-7 in all dietary treatments of mice. Moreover, the BSp-fed and combination-fed displayed an increase in Allobaculum Adlercreutzia genus and Lachnospiraceae family. These findings also support the promising impact of BSp on the combination treatment in transgenic mice and also may imply a strong association of bacterial abundance with our dietary treatments in both BE and LC groups.
Adlercreutzia is a gram-positive, strict anaerobic bacterium, which has been considered to exhibit beneficial effects and possess immunoregulatory properties [64]. In addition, Adlercreutzia is an equol-producing bacterium, which has been isolated from the gut of animals and humans [65]. This bacterium’s levels were reported to be decreased in mice fed with a high fat diet, resulting in lowered S-equol concentration in a prostate cancer transgenic mice model [66]. Lactobacillus are gram-positive, bacilli-shaped and lactic acid producing probiotic bacteria. They reside in the gastrointestinal tract of healthy individuals and are generally considered as non-pathogenic organisms [67]. A recent study by Zhu et al. investigated gut diversity of women suffering from breast cancer [68]. Their findings revealed a significant decrease in several bacterial communities including Lactobacillus vaginalis in postmenopausal breast cancer patients as compared with postmenopausal controls.
After the onset of tumor in mice, we sought to investigate the temporal impact induced by our dietary treatment on gut microbiota and beta diversity analysis revealed that BSp-fed mice microbial composition was clustered with combination-fed mice in both BE and LC groups. The microbial diversity of GTPs-fed mice was distinctly clustered from control-fed mice in the BE group, whereas clustered with each other in the LC group. This observation implies a strong association of the BSp treatment group with the combination group, as compared with the GTPs group. We also found modifications at phylum levels induced by our dietary botanicals. For instance, we observed a significant decrease in Proteobacteria levels in BSp-fed and combination-fed mice of both the BE and the LC groups. Proteobacteria phyla consists of various human pathogens such as Escherichia, Helicobacter, Neisseria, Salmonella and Shigella, which has been implicated in human diseases [69–71].
We also compared the gut microbial composition before and after the establishment of tumor burden to investigate the effect of tumor on the gut microbiota composition. In the BE group, control-fed mice underwent a significant increase in Proteobacteria after the onset of tumor as compared to the control-fed mice before the onset of tumor (12.11% versus 0.16%, p = 0.001). In addition, we observed a significant decrease in Firmicutes levels after the onset of tumor (50.79% versus 65.94%, p = 0.011). The GTPs-fed mice underwent a significant decrease in Verrucomicrobia after onset of tumor as compared to before onset of tumor (5.22% versus 1.01%, p = 0.013). The BSp-fed and combination-fed mice did not show any significant changes in the microbial abundance when compared with before onset of tumor.
Similarly, in the LC group, control-fed mice displayed a significant decrease in levels of Firmicutes (50.66% versus 73.87%, p < 0.001) and Actinobacteria (2.37% versus 7.15%, p < 0.01) when compared with control-fed mice before the onset of tumor at phylum level. These control-fed mice also showed a significant increase in Bacteroidetes (31.51% versus 15.87%, p < 0.001) and Verrucomicrobia (13.7% versus 2.43%, p < 0.01) composition after the onset of tumor as compared to before onset of tumor. We found a significant decline in Actinobacteria (1.99% versus 7.32%, p < 0.01) levels of BSp-fed mice and combination-fed mice (3.1% versus 8.99%, p = 0.001) after the onset of tumor when compared with before onset of tumor. Mice on GTPs diet did not show any significant changes in the gut microbial composition at the phylum level. These results indicate significant differences arose in gut microbial composition of control-fed mice after the onset of tumor at the phylum level in both BE and LC groups. These results also demonstrate the consumption of BSp or GTPs or combination diet may prevent major alterations in gut microbiota composition induced by tumors.
The investigation of taxonomic microbial abundance in the BE group revealed a significant increase in Allobaculum genus levels in mice on the BSp and combination diets. Furthermore, a significant rise of Adlercreutzia, Lactobacillus, S24-7, Ruminococcaceae and Lachnospiraceae families was observed in all dietary treatment groups versus the control group. Ruminococcaceae are primary butyrate-producing bacterial family members that inhabit a healthy colon [72]. The increase of these bacterial taxa due to our dietary treatments illustrates the profound impact of dietary compounds on the establishment of gut microbiota in transgenic mice.
The bacteria from S24-7 family are known for butyrate production and exert beneficial effects on the digestive system [73]. Specifically, an increased abundance of the S24-7 family was notable in mice administrated a low-fat diet and, in association with increased exercise, prevented weight gain in C57BL/6 mice [73]. Moreover, studies have found decreases in the abundance of S24-7 family in various inflammatory disorders such as Crohn’s disease, colitis and type I diabetes [74–76]. A recent study by Acharya et al. investigated the impact of estradiol treatment and obesity on the body weight, energy intake and gut microbiota of mice. They found that estradiol provided protection against HFD-induced weight gain with increased levels of the S24-7 bacterial family [77]. Lachnospiraceae family of bacteria have the ability to generate energy in the host by degradation of polysaccharides in the plants [78].
In the LC group after the onset of tumor, we found a significant increase in S24-7, Lachnospiraceae family, Lactobacillus and Adlercreutzia genus at taxonomic levels induced by our dietary treatments. In addition, the mice in the LC group showed a significant increase in Allobaculum genus levels when on the BSp and combination diet. Studies have reported that Allobaculum is a beneficial bacteria that produces SCFAs in the intestine of mice [79]. The Allobaculum genus also exhibits diverse functions such as anti-inflammatory processes, protection of intestinal barrier, regulation of immune system and host metabolism [80]. A recent study investigated the impact of probiotics supplement on the gut microbiome in a colitis-associated colon cancer mice model and found a significant abundance in the levels of Allobaculum in the probiotic group as compared to the control group of mice [81]. Furthermore, an investigation by Zagato et al. revealed under-representation of an associated bacteria belonging to the Erysipelotrichaceae family, Faecalibaculum rodentium in both ApcMin/+ mice and C57BL/6 wild-type mice, which was found to have anti-tumorigenic effects towards colorectal cancer [82].
Therefore, our findings may implicit potent impact of our dietary compounds on establishment and composition of gut microbiota pre-tumor and post-tumor of mice, and identified bacterial candidates that may serve as predictive biomarkers for breast cancer prevention including Adlercreutzia genus and the S24-7 family. These findings also suggest that consumption of BSp, GTPs and their combination in either the beginning to early life (BE) group or life-long from conception (LC) group results in establishment of almost the same microbial consumption such as an increase of Allobaculum, Lactobacillus, Lachnospiraceae and S24-7 family in BSp-fed and combination-fed mice before the onset of tumor. Similarly, there was an increase in levels of Adlercreutzia, Lactobacillus genus, Lachnospiraceae and S24-7 family in all dietary treatments after the onset of tumor, which implies the strong influence of these dietary compounds and that may be potentially beneficial for health and in cancer prevention. The fecal samples (n = 8 in BE group and n = 5 in LC group) were randomly collected from three different cages; however, this study had a limitation that about 6–7 mice of the same treatment group were co-housed with each other. A recent study by Liu et al. investigated the role of gut microbiota in NZB/W F1 mice, which is a mouse model of lupus and patients suffering from systemic lupus erythematosus (an autoimmune disease) [83]. Their findings revealed considerable changes in the composition of gut microbiota of mice before and after the onset of lupus and in group of patients suffering from disease. However, this study also had the limitation that cage-effect was not considered and a refined study design would be recommended for future studies.
The relationship between commensal communities residing in the gut and host physiology have been well studied [84]. Certain bacterial communities do not show direct oncogenic effects on the tumor, as they can indirectly lead to tumor inhibition by production of gut-derived metabolites. For instance, studies have indicated that gut microbiota can metabolize lignans present in the edible plants into enterolactone, which can regulate estrogen signaling and may lead to protective effects against breast cancer [85, 86]. We sought to investigate if the potential impact of gut microbiota from our dietary treatments on mammary tumorgenesis is modulated via microbial-produced metabolites. SCFAs, such as acetate, propionate and butyrate are major metabolites produced by gut microbiota [87]. Butyrate is a known HDAC inhibitor [88] and has shown antineoplastic properties in various cancers [89]. Sodium butyrate has shown to induce apoptosis in breast cancer cell lines via production of reactive oxygen species and mitochondrial impairment [90]. Studies have found that propionate and valerate are also HDAC inhibitors [91]. Administration of sodium propionate resulted in inhibition of MCF-7 cellular proliferation in a dose-dependent manner and cell-cycle arrest [92]. Additionally, isobutyrate (a minor SCFA) can exhibit anticarcinogenic effects in colon carcinoma [93]. Hexanoate is a medium chain fatty acid that can contribute to cellular inhibition in colorectal cancer, breast cancer and skin cancer cell lines by down-regulation of cell cycle genes and induction of apoptosis [94]. Moreover, the microbial produced metabolites, such as lithocholic acid (a bile acid) can induce oxidative and nitrosative stress resulting in breast cancer inhibition [95].
Our SCFAs analyses in the BE group revealed that the mice on the BSp diet, GTPs diet and combination diet showed no change in the SCFAs levels. In the LC group, we found that mice on the BSp diet had unchanged plasma levels of SCFAs. The mice on the GTPs diet showed significant increases in isobutyrate, valerate and hexanoate and the mice on the combination diet had significantly higher levels of propionate and isobutyrate. This may be attributed to the treatment window, as maternal gestation and lactational periods are crucial in fundamental developmental processes of offspring. The dietary exposure in the maternal environment may have altered the epigenetic reprogramming during early embryogenesis, which could result in increased SCFAs profiles via transplacental effects in the female offspring [28, 96].
A study focused on daily intake of freeze-dried BSp in mice showed significant reduction only in E. coli abundance and had no significant change in the levels of SCFAs such as acetate, propionate, butyrate, iso-butyrate and lactate in the cecum when compared with the mice on control diet [97]. Another study investigated the impact of SFN treatment on the intestinal injury induced by 5-Fluorouracil (a chemotherapy drug) in mice. The administration of SFN attenuated the severity of intestinal injury by showing significant improvement in weight, intestinal inflammation and intestinal permeability in mice. On the other hand, the SFN intake did not show any significant changes in SCFAs levels that were reduced previously due to 5-Fluorouracil treatment [98]. These studies may imply administration of BSp has a minor effect on the SCFAs profile, although that might be attributed to the characteristics of the experimental mice and could presumably reflect the complexity of interactions between the several bacterial species residing in the gut of these mice. Further studies are warranted to investigate the potential influence on gut microbiota profile and levels of key metabolites in breast cancer patients by dietary consumption of BSp that may provide deeper understanding on the correlation between host and metabolic pathways governed by these commensal communities.
The BSp and GTPs diets used in this study are equivalent to a daily intake of ~234 g BSp and ~5.7 g GTPs for an adult (60 kg) human, respectively, which are considered pharmacologically achievable and has translational potential [26, 99, 100]. The significance of our findings is that breast cancer patients may benefit from regular consumption of broccoli sprouts and green tea polyphenols in their diet as it might induce crucial alterations of their microbial and key metabolic profile. Moreover, due to the induced changes in the gut microbiota, the metabolic profile of breast cancer patients in turn may enhance survival from breast cancer.
Conclusion
The current study highlights the impact of BSp, GTPs and combination diet on the gut microbiome and SCFAs levels of Her2/neu transgenic mice at two specific time periods, which may help reveal a potential mechanism by which diet can regulate breast carcinogenesis. Overall our results demonstrated that combination dietary treatment showed the strongest delaying effect on tumor volume and led to a significant increase in tumor latency and the BSp diet group was integrally more efficacious than the GTPs group when compared to control treatments in both the BE and LC groups. Additionally, administration of these dietary treatments had efficacious effects on the beta diversity as the gut microbiota of BSp-fed mice had similar clustering with combination-fed mice and the gut microbiota of GTPs-fed mice had similar clustering with control-fed mice at pre-tumor and post-tumor onset in LC group and pre-tumor onset in BE group. This finding indicates a similar microbial composition induced by consumption of BSp and the combination diet in both BE and LC groups. Our study has shown that after consumption of BSp and combination diet, there was an increase in abundance of the Allobaculum genus in both exposure periods. Furthermore, the mice from all dietary treatment groups showed a significant rise of Adlercreutzia, Lactobacillus genus, bacterial families such as S24-7, Ruminococcaceae and Lachnospiraceae in the BE group. Moreover, our diet consumption led to a significant increase in S24-7, Lachnospiraceae family and Lactobacillus, Adlercreutzia genus in the LC group. Therefore, a significant increase of Adlercreutzia genus and S24-7 family associated with all dietary treatments may be exploited as a predictive biomarker for prevention of breast cancer. However, the SCFAs analyses revealed no change in SCFAs levels by consumption of BSp, GTPs and combination diet in mice of BE group. In the LC group, we found unchanged levels of SCFAs in the BSp-fed mice when compared with control-fed mice. The consumption of GTPs led to a significant increase in levels of isobutyrate, valerate and hexanoate, and the combination diet led to a significant increase in levels of propionate and isobutyrate. Therefore, we hypothesize that the increased levels of SCFAs in GTPs and the combination diet may have induced epigenetic modifications resulting in the decrease in tumor volume and the increase in tumor latency in the LC group. As both the gut microbial communities and blood factors contribute to tumor prevention, it appears that microbial factors may have contributed significantly in the LC group as compared to the BE group. The combination of these nutrients appear to have optimal effects. These findings also reveal that temporal factors associated with different time windows of dietary treatments during the life-span can have a profound effect on the gut microbiota establishment, SCFAs profiles and could have translational potential and might represent a potential regimen for ER(-) breast cancer prevention in women. Future studies are warranted to investigate the direct impact of altered bacterial species due to our dietary treatment on the tumor development.
Supporting information
This clustering dendrogram shows no outliers and therefore all samples from dietary treatments were used in the fecal samples analyses of BE group.
(TIF)
This hierarchical clustering dendrogram verifies that no outliers were present and we have therefore included every sample for microbiome analyses of the LC group.
(TIF)
This excel sheet provides detail of quality check (FASTQC) results including number of samples, sequence per sample, read length and GC content.
(XLSX)
This sheet enlists the changes in relative abundance (base mean, log2 fold change, p-values and adjusted p-values) according to each group of bacterial communities of all treatment groups: BSp-fed, GTPs-fed, combination-fed and control-fed mice, in BE and LC group at both before and after onset of the tumor.
(XLSX)
Acknowledgments
The authors would like to thank Dr. Peter Eipers of the Department of Cell, Developmental and Integrative Biology at UAB for assisting with the microbiome analyses. We would also like to thank William Van Der Pol in the Center for Clinical & Translational Science at UAB for assistance with bioinformatics analyses. Appreciation is expressed to Landon S. Wilson from the Targeted Metabolomics and Proteomics Laboratory at UAB for technical assistance.
Abbreviations
- BE
Beginning in early life
- BSp
Broccoli sprouts
- DCM
Dichloromethane
- DNMTs
DNA methytransferases
- EGCG
Epigallocatechin-3-gallate
- ER
Estrogen receptor
- FastQC
FastQ quality control
- FC
Fold chang
- FDR
False-discovery rate
- GTPs
Green tea polyphenols
- HDAC
Histone deacetylase
- HER2
Human epidermal growth factor receptor
- LC
Life-long from conception
- o-BHA
O-benzylhydroxylamine
- OTU
Operational taxonomic unit
- PCoA
Principal Coordinates Analysis
- PERMANOVA
Permutation Multivariate Analysis of Variance
- PR
Progesterone receptor
- QIIME
Quantitative Insight into Microbial Ecology
- SCFA
Short-chain fatty acid
- SFN
Sulforaphane
- TNBC
Triple negative breast cancer
- WT
Wild type
Data Availability
All relevant data are within the manuscript and its Supporting information files.
Funding Statement
This work was supported in part by grants from the National Institutes of Health (https://www.nih.gov/) (NCI R01CA178441 to TOT, NCI R01CA204346 to TOT, NCCIH K01AT009373 to YL, and NIDDK P30DK056336 to YL). Funds for the purchase of the mass spectrometer used for the analysis of the SCFAs came from an award from the UAB Health Services Foundation General Endowment (https://www.uab.edu/medicine/peds/casg/casgforms/159-research/836-university-of-alabama-health-services-foundation-uahsf-general-endowment-fund-gef-grants) to SB. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. There was no additional external funding received for this study.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69(1):7–34. Epub 2019/01/09. 10.3322/caac.21551 . [DOI] [PubMed] [Google Scholar]
- 2.Taneja P, Maglic D, Kai F, Zhu S, Kendig RD, Fry EA, et al. Classical and Novel Prognostic Markers for Breast Cancer and their Clinical Significance. Clin Med Insights Oncol. 2010;4:15–34. 10.4137/cmo.s4773 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kittaneh M, Montero AJ, Gluck S. Molecular profiling for breast cancer: a comprehensive review. Biomarkers in cancer. 2013;5:61–70. Epub 2013/11/20. 10.4137/BIC.S9455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ross JS, Ross JS, Hortobagyi GN. Molecular Oncology of Breast Cancer: Jones and Bartlett Publishers; 2005. [Google Scholar]
- 5.Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. The New England journal of medicine. 2001;344(11):783–92. Epub 2001/03/15. 10.1056/NEJM200103153441101 . [DOI] [PubMed] [Google Scholar]
- 6.Blackwell KL, Burstein HJ, Storniolo AM, Rugo H, Sledge G, Koehler M, et al. Randomized study of Lapatinib alone or in combination with trastuzumab in women with ErbB2-positive, trastuzumab-refractory metastatic breast cancer. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2010;28(7):1124–30. Epub 2010/02/04. 10.1200/JCO.2008.21.4437 . [DOI] [PubMed] [Google Scholar]
- 7.Putti TC, El-Rehim DMA, Rakha EA, Paish CE, Lee AHS, Pinder SE, et al. Estrogen receptor-negative breast carcinomas: a review of morphology and immunophenotypical analysis. Modern Pathology. 2005;18(1):26–35. 10.1038/modpathol.3800255 [DOI] [PubMed] [Google Scholar]
- 8.Liu SM, Ou SY, Huang HH. Green tea polyphenols induce cell death in breast cancer MCF-7 cells through induction of cell cycle arrest and mitochondrial-mediated apoptosis. Journal of Zhejiang University Science B. 2017;18(2):89–98. Epub 2017/01/27. 10.1631/jzus.B1600022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gho SA, Steele JR, Jones SC, Munro BJ. Self-reported side effects of breast cancer treatment: a cross-sectional study of incidence, associations, and the influence of exercise. Cancer causes & control: CCC. 2013;24(3):517–28. Epub 2013/01/09. 10.1007/s10552-012-0142-4 . [DOI] [PubMed] [Google Scholar]
- 10.Shaikh AA, Braakhuis AJ, Bishop KS. The Mediterranean Diet and Breast Cancer: A Personalised Approach. Healthcare (Basel). 2019;7(3):104 10.3390/healthcare7030104 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang G, Wang Y, Zhang Y, Wan X, Li J, Liu K, et al. Anti-cancer activities of tea epigallocatechin-3-gallate in breast cancer patients under radiotherapy. Current molecular medicine. 2012;12(2):163–76. Epub 2012/01/28. 10.2174/156652412798889063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ambrosone CB, McCann SE, Freudenheim JL, Marshall JR, Zhang Y, Shields PG. Breast cancer risk in premenopausal women is inversely associated with consumption of broccoli, a source of isothiocyanates, but is not modified by GST genotype. The Journal of nutrition. 2004;134(5):1134–8. Epub 2004/04/29. 10.1093/jn/134.5.1134 . [DOI] [PubMed] [Google Scholar]
- 13.Leone A, Diorio G, Sexton W, Schell M, Alexandrow M, Fahey JW, et al. Sulforaphane for the chemoprevention of bladder cancer: molecular mechanism targeted approach. Oncotarget. 2017;8(21):35412–24. Epub 2017/04/21. 10.18632/oncotarget.16015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gasparello J, Gambari L, Papi C, Rozzi A, Manicardi A, Corradini R, et al. High Levels of Apoptosis Are Induced in the Human Colon Cancer HT-29 Cell Line by Co-Administration of Sulforaphane and a Peptide Nucleic Acid Targeting miR-15b-5p. Nucleic acid therapeutics. 2020. Epub 2020/02/19. 10.1089/nat.2019.0825 . [DOI] [PubMed] [Google Scholar]
- 15.Dos Santos P, Machado ART, De Grandis RA, Ribeiro DL, Tuttis K, Morselli M, et al. Transcriptome and DNA methylation changes modulated by sulforaphane induce cell cycle arrest, apoptosis, DNA damage, and suppression of proliferation in human liver cancer cells. Food and chemical toxicology: an international journal published for the British Industrial Biological Research Association. 2020;136:111047 Epub 2019/12/16. 10.1016/j.fct.2019.111047 . [DOI] [PubMed] [Google Scholar]
- 16.Cheng AC, Shen CJ, Hung CM, Hsu YC. Sulforaphane Decrease of SERTAD1 Expression Triggers G1/S Arrest in Breast Cancer Cells. Journal of medicinal food. 2019;22(5):444–50. Epub 2019/05/16. 10.1089/jmf.2018.4195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lewinska A, Adamczyk-Grochala J, Deregowska A, Wnuk M. Sulforaphane-Induced Cell Cycle Arrest and Senescence are accompanied by DNA Hypomethylation and Changes in microRNA Profile in Breast Cancer Cells. Theranostics. 2017;7(14):3461–77. Epub 2017/09/16. 10.7150/thno.20657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Namiki K, Wongsirisin P, Yokoyama S, Sato M, Rawangkan A, Sakai R, et al. (-)-Epigallocatechin gallate inhibits stemness and tumourigenicity stimulated by AXL receptor tyrosine kinase in human lung cancer cells. Scientific reports. 2020;10(1):2444 Epub 2020/02/14. 10.1038/s41598-020-59281-z . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lewis KA, Jordan HR, Tollefsbol TO. Effects of SAHA and EGCG on Growth Potentiation of Triple-Negative Breast Cancer Cells. Cancers. 2018;11(1). Epub 2018/12/29. 10.3390/cancers11010023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yeo C, Han DS, Lee HJ, Lee EO. Epigallocatechin-3-Gallate Suppresses Vasculogenic Mimicry through Inhibiting the Twist/VE-Cadherin/AKT Pathway in Human Prostate Cancer PC-3 Cells. International journal of molecular sciences. 2020;21(2). Epub 2020/01/16. 10.3390/ijms21020439 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen J, Chen L, Lu T, Xie Y, Li C, Jia Z, et al. ERalpha36 is an effective target of epigallocatechin-3-gallate in hepatocellular carcinoma. International journal of clinical and experimental pathology. 2019;12(9):3222–34. Epub 2020/01/15. [PMC free article] [PubMed] [Google Scholar]
- 22.Md Nesran ZN, Shafie NH, Ishak AH, Mohd Esa N, Ismail A, Md Tohid SF. Induction of Endoplasmic Reticulum Stress Pathway by Green Tea Epigallocatechin-3-Gallate (EGCG) in Colorectal Cancer Cells: Activation of PERK/p-eIF2alpha/ATF4 and IRE1alpha. Biomed Res Int. 2019;2019:3480569 Epub 2020/01/14. 10.1155/2019/3480569 publication of this paper. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wei R, Mao L, Xu P, Zheng X, Hackman RM, Mackenzie GG, et al. Suppressing glucose metabolism with epigallocatechin-3-gallate (EGCG) reduces breast cancer cell growth in preclinical models. Food & function. 2018;9(11):5682–96. Epub 2018/10/13. 10.1039/c8fo01397g . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jang JY, Lee JK, Jeon YK, Kim CW. Exosome derived from epigallocatechin gallate treated breast cancer cells suppresses tumor growth by inhibiting tumor-associated macrophage infiltration and M2 polarization. BMC cancer. 2013;13:421 Epub 2013/09/21. 10.1186/1471-2407-13-421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Arora I, Sharma M, Tollefsbol TO. Combinatorial Epigenetics Impact of Polyphenols and Phytochemicals in Cancer Prevention and Therapy. International journal of molecular sciences. 2019;20(18). Epub 2019/09/22. 10.3390/ijms20184567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li Y, Meeran SM, Tollefsbol TO. Combinatorial bioactive botanicals re-sensitize tamoxifen treatment in ER-negative breast cancer via epigenetic reactivation of ERα expression. Scientific reports. 2017;7(1):9345 10.1038/s41598-017-09764-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li Y, Buckhaults P, Cui X, Tollefsbol TO. Combinatorial epigenetic mechanisms and efficacy of early breast cancer inhibition by nutritive botanicals. Epigenomics. 2016;8(8):1019–37. Epub 2016/08/02. 10.2217/epi-2016-0024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li Y, Buckhaults P, Li S, Tollefsbol T. Temporal Efficacy of a Sulforaphane-Based Broccoli Sprout Diet in Prevention of Breast Cancer through Modulation of Epigenetic Mechanisms. Cancer prevention research (Philadelphia, Pa). 2018;11(8):451–64. Epub 2018/05/17. 10.1158/1940-6207.CAPR-17-0423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sender R, Fuchs S, Milo R. Revised Estimates for the Number of Human and Bacteria Cells in the Body. PLoS biology. 2016;14(8):e1002533 Epub 2016/08/20. 10.1371/journal.pbio.1002533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wilson AS, Koller KR, Ramaboli MC, Nesengani LT, Ocvirk S, Chen C, et al. Diet and the Human Gut Microbiome: An International Review. Digestive diseases and sciences. 2020. 10.1007/s10620-020-06112-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sharma M, Li Y, Stoll ML, Tollefsbol TO. The Epigenetic Connection Between the Gut Microbiome in Obesity and Diabetes. Frontiers in genetics. 2019;10:1329 Epub 2020/02/06. 10.3389/fgene.2019.01329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Waldecker M, Kautenburger T, Daumann H, Busch C, Schrenk D. Inhibition of histone-deacetylase activity by short-chain fatty acids and some polyphenol metabolites formed in the colon. The Journal of nutritional biochemistry. 2008;19(9):587–93. Epub 2007/12/07. 10.1016/j.jnutbio.2007.08.002 . [DOI] [PubMed] [Google Scholar]
- 33.Bhat MI, Kapila R. Dietary metabolites derived from gut microbiota: critical modulators of epigenetic changes in mammals. Nutrition reviews. 2017;75(5):374–89. 10.1093/nutrit/nux001 [DOI] [PubMed] [Google Scholar]
- 34.Paul B, Royston KJ, Li Y, Stoll ML, Skibola CF, Wilson LS, et al. Impact of genistein on the gut microbiome of humanized mice and its role in breast tumor inhibition. PloS one. 2017;12(12):e0189756 Epub 2017/12/22. 10.1371/journal.pone.0189756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Newman TM, Vitolins MZ, Cook KL. From the Table to the Tumor: The Role of Mediterranean and Western Dietary Patterns in Shifting Microbial-Mediated Signaling to Impact Breast Cancer Risk. Nutrients. 2019;11(11). Epub 2019/10/28. 10.3390/nu11112565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wu Y, Shen Y, Zhu Y, Mupunga J, Zou L, Liu C, et al. Broccoli ingestion increases the glucosinolate hydrolysis activity of microbiota in the mouse gut. International journal of food sciences and nutrition. 2019;70(5):585–94. Epub 2019/02/19. 10.1080/09637486.2018.1554624 . [DOI] [PubMed] [Google Scholar]
- 37.Yuan X, Long Y, Ji Z, Gao J, Fu T, Yan M, et al. Green Tea Liquid Consumption Alters the Human Intestinal and Oral Microbiome. Mol Nutr Food Res. 2018;62(12):e1800178–e. Epub 2018/06/10. 10.1002/mnfr.201800178 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vetrani C, Maukonen J, Bozzetto L, Della Pepa G, Vitale M, Costabile G, et al. Diets naturally rich in polyphenols and/or long-chain n-3 polyunsaturated fatty acids differently affect microbiota composition in high-cardiometabolic-risk individuals. Acta diabetologica. 2020. Epub 2020/03/03. 10.1007/s00592-020-01494-9 . [DOI] [PubMed] [Google Scholar]
- 39.Wang Y, Tang C, Tang Y, Yin H, Liu X. Capsaicin has an anti-obesity effect through alterations in gut microbiota populations and short-chain fatty acid concentrations. Food & nutrition research. 2020;64 Epub 2020/03/18. 10.29219/fnr.v64.3525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Paturi G, Mandimika T, Butts CA, Zhu S, Roy NC, McNabb WC, et al. Influence of dietary blueberry and broccoli on cecal microbiota activity and colon morphology in mdr1a−/− mice, a model of inflammatory bowel diseases. Nutrition (Burbank, Los Angeles County, Calif). 2012;28(3):324–30. 10.1016/j.nut.2011.07.018 [DOI] [PubMed] [Google Scholar]
- 41.Lee HC, Jenner AM, Low CS, Lee YK. Effect of tea phenolics and their aromatic fecal bacterial metabolites on intestinal microbiota. Research in Microbiology. 2006;157(9):876–84. 10.1016/j.resmic.2006.07.004 [DOI] [PubMed] [Google Scholar]
- 42.Okubo T, Ishihara N, Oura A, Serit M, Kim M, Yamamoto T, et al. In Vivo Effects of Tea Polyphenol Intake on Human Intestinal Microflora and Metabolism. Bioscience, Biotechnology, and Biochemistry. 1992;56(4):588–91. 10.1271/bbb.56.588 [DOI] [PubMed] [Google Scholar]
- 43.Ishihara N, Chu DC, Akachi S, Juneja LR. Improvement of intestinal microflora balance and prevention of digestive and respiratory organ diseases in calves by green tea extracts. Livestock Production Science. 2001;68(2):217–29. 10.1016/S0301-6226(00)00233-5 [DOI] [Google Scholar]
- 44.Jin J-S, Touyama M, Hisada T, Benno Y. Effects of green tea consumption on human fecal microbiota with special reference to Bifidobacterium species. Microbiology and Immunology. 2012;56(11):729–39. 10.1111/j.1348-0421.2012.00502.x [DOI] [PubMed] [Google Scholar]
- 45.Rooks MG, Garrett WS. Gut microbiota, metabolites and host immunity. Nat Rev Immunol. 2016;16(6):341–52. 10.1038/nri.2016.42 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Konigshoff M, Wilhelm J, Bohle RM, Pingoud A, Hahn M. HER-2/neu gene copy number quantified by real-time PCR: comparison of gene amplification, heterozygosity, and immunohistochemical status in breast cancer tissue. Clinical chemistry. 2003;49(2):219–29. Epub 2003/02/01. 10.1373/49.2.219 . [DOI] [PubMed] [Google Scholar]
- 47.Zhou J-R, Gugger ET, Tanaka T, Guo Y, Blackburn GL, Clinton SK. Soybean phytochemicals inhibit the growth of transplantable human prostate carcinoma and tumor angiogenesis in mice. The Journal of nutrition. 1999;129(9):1628–35. 10.1093/jn/129.9.1628 [DOI] [PubMed] [Google Scholar]
- 48.Guy CT, Webster MA, Schaller M, Parsons TJ, Cardiff RD, Muller WJ. Expression of the neu protooncogene in the mammary epithelium of transgenic mice induces metastatic disease. Proceedings of the National Academy of Sciences. 1992;89(22):10578–82. 10.1073/pnas.89.22.10578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kumar R, Eipers P, Little RB, Crowley M, Crossman DK, Lefkowitz EJ, et al. Getting started with microbiome analysis: sample acquisition to bioinformatics. Current protocols in human genetics. 2014;82:18.8.1–29. Epub 2014/07/22. 10.1002/0471142905.hg1808s82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Caporaso JG, Lauber CL, Walters WA, Berg-Lyons D, Lozupone CA, Turnbaugh PJ, et al. Global patterns of 16S rRNA diversity at a depth of millions of sequences per sample. Proceedings of the National Academy of Sciences. 2011;108(Supplement 1):4516–22. 10.1073/pnas.1000080107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Caporaso JG, Bittinger K, Bushman FD, DeSantis TZ, Andersen GL, Knight R. PyNAST: a flexible tool for aligning sequences to a template alignment. Bioinformatics. 2009;26(2):266–7. 10.1093/bioinformatics/btp636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Beals EW. Bray-Curtis Ordination: An Effective Strategy for Analysis of Multivariate Ecological Data Advances in Ecological Research: Elsevier; 1984. p. 1–55. [Google Scholar]
- 53.Zeng M, Cao H. Fast quantification of short chain fatty acids and ketone bodies by liquid chromatography-tandem mass spectrometry after facile derivatization coupled with liquid-liquid extraction. Journal of chromatography B, Analytical technologies in the biomedical and life sciences. 2018;1083:137–45. Epub 2018/03/17. 10.1016/j.jchromb.2018.02.040 . [DOI] [PubMed] [Google Scholar]
- 54.Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15(12):550 10.1186/s13059-014-0550-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Benjamini Y, Hochberg Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. Journal of the Royal Statistical Society: Series B (Methodological). 1995;57(1):289–300. 10.1111/j.2517-6161.1995.tb02031.x [DOI] [Google Scholar]
- 56.Thakur ML, Devadhas D, Zhang K, Pestell RG, Wang C, McCue P, et al. Imaging spontaneous MMTVneu transgenic murine mammary tumors: targeting metabolic activity versus genetic products. Journal of nuclear medicine: official publication, Society of Nuclear Medicine. 2010;51(1):106–11. Epub 2009/12/17. 10.2967/jnumed.109.069542 . [DOI] [PubMed] [Google Scholar]
- 57.Jolicoeur P, Bouchard L, Guimond A, Ste-Marie M, Hanna Z, Dievart A. Use of mouse mammary tumour virus (MMTV)/neu transgenic mice to identify genes collaborating with the c-erbB-2 oncogene in mammary tumour development. Biochemical Society symposium. 1998;63:159–65. Epub 1998/03/26. . [PubMed] [Google Scholar]
- 58.Baxter NT, Schmidt AW, Venkataraman A, Kim KS, Waldron C, Schmidt TM. Dynamics of Human Gut Microbiota and Short-Chain Fatty Acids in Response to Dietary Interventions with Three Fermentable Fibers. mBio. 2019;10(1):e02566–18. 10.1128/mBio.02566-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gyles C. The microbiome—a major determinant of health? Can Vet J. 2015;56(6):537–40. [PMC free article] [PubMed] [Google Scholar]
- 60.Sun ZZ, Li XY, Wang S, Shen L, Ji HF. Bidirectional interactions between curcumin and gut microbiota in transgenic mice with Alzheimer’s disease. Applied microbiology and biotechnology. 2020. Epub 2020/02/26. 10.1007/s00253-020-10461-x . [DOI] [PubMed] [Google Scholar]
- 61.Gorkiewicz G, Moschen A. Gut microbiome: a new player in gastrointestinal disease. Virchows Archiv: an international journal of pathology. 2018;472(1):159–72. Epub 2017/12/16. 10.1007/s00428-017-2277-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Elfil M, Kamel S, Kandil M, Koo BB, Schaefer SM. Implications of the Gut Microbiome in Parkinson’s Disease. Movement disorders: official journal of the Movement Disorder Society. 2020. Epub 2020/02/25. 10.1002/mds.28004 . [DOI] [PubMed] [Google Scholar]
- 63.Younis N, Zarif R, Mahfouz R. Inflammatory bowel disease: between genetics and microbiota. Molecular biology reports. 2020. Epub 2020/02/23. 10.1007/s11033-020-05318-5 . [DOI] [PubMed] [Google Scholar]
- 64.Maruo T, Sakamoto M, Ito C, Toda T, Benno Y. Adlercreutzia equolifaciens gen. nov., sp. nov., an equol-producing bacterium isolated from human faeces, and emended description of the genus Eggerthella. International Journal of Systematic and Evolutionary Microbiology. 2008;58(5):1221–7. 10.1099/ijs.0.65404-0 [DOI] [PubMed] [Google Scholar]
- 65.Tamura M, Tsushida T, Shinohara K. Isolation of an isoflavone-metabolizing, Clostridium-like bacterium, strain TM-40, from human faeces. Anaerobe. 2007;13(1):32–5. Epub 2006/11/23. 10.1016/j.anaerobe.2006.10.001 . [DOI] [PubMed] [Google Scholar]
- 66.Liu Y, Wu X, Jiang H. High dietary fat intake lowers serum equol concentration and promotes prostate carcinogenesis in a transgenic mouse prostate model. Nutrition & metabolism. 2019;16:24 Epub 2019/04/24. 10.1186/s12986-019-0351-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Shi LH, Balakrishnan K, Thiagarajah K, Mohd Ismail NI, Yin OS. Beneficial Properties of Probiotics. Trop Life Sci Res. 2016;27(2):73–90. 10.21315/tlsr2016.27.2.6 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhu J, Liao M, Yao Z, Liang W, Li Q, Liu J, et al. Breast cancer in postmenopausal women is associated with an altered gut metagenome. Microbiome. 2018;6(1):136 Epub 2018/08/08. 10.1186/s40168-018-0515-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhu L, Baker SS, Gill C, Liu W, Alkhouri R, Baker RD, et al. Characterization of gut microbiomes in nonalcoholic steatohepatitis (NASH) patients: A connection between endogenous alcohol and NASH. Hepatology. 2013;57(2):601–9. 10.1002/hep.26093 [DOI] [PubMed] [Google Scholar]
- 70.Amar J, Lange C, Payros G, Garret C, Chabo C, Lantieri O, et al. Blood microbiota dysbiosis is associated with the onset of cardiovascular events in a large general population: the D.E.S.I.R. study. PloS one. 2013;8(1):e54461–e. Epub 2013/01/25. 10.1371/journal.pone.0054461 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Carvalho Frederic A, Koren O, Goodrich Julia K, Johansson Malin EV, Nalbantoglu I, Aitken Jesse D, et al. Transient Inability to Manage Proteobacteria Promotes Chronic Gut Inflammation in TLR5-Deficient Mice. Cell host & microbe. 2012;12(2):139–52. 10.1016/j.chom.2012.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Vital M, Howe AC, Tiedje JM. Revealing the bacterial butyrate synthesis pathways by analyzing (meta)genomic data. mBio. 2014;5(2):e00889 Epub 2014/04/24. 10.1128/mBio.00889-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Evans CC, LePard KJ, Kwak JW, Stancukas MC, Laskowski S, Dougherty J, et al. Exercise Prevents Weight Gain and Alters the Gut Microbiota in a Mouse Model of High Fat Diet-Induced Obesity. PloS one. 2014;9(3):e92193 10.1371/journal.pone.0092193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Krych Ł, Nielsen DS, Hansen AK, Hansen CHF. Gut microbial markers are associated with diabetes onset, regulatory imbalance, and IFN-γ level in NOD mice. Gut microbes. 2015;6(2):101–9. Epub 2015/02/03. 10.1080/19490976.2015.1011876 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rooks MG, Veiga P, Wardwell-Scott LH, Tickle T, Segata N, Michaud M, et al. Gut microbiome composition and function in experimental colitis during active disease and treatment-induced remission. Isme j. 2014;8(7):1403–17. Epub 2014/02/07. 10.1038/ismej.2014.3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Morgan XC, Tickle TL, Sokol H, Gevers D, Devaney KL, Ward DV, et al. Dysfunction of the intestinal microbiome in inflammatory bowel disease and treatment. Genome Biol. 2012;13(9):R79–R. 10.1186/gb-2012-13-9-r79 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Acharya KD, Gao X, Bless EP, Chen J, Tetel MJ. Estradiol and high fat diet associate with changes in gut microbiota in female ob/ob mice. Scientific reports. 2019;9(1):20192-. 10.1038/s41598-019-56723-1 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ma Q, Li Y, Wang J, Li P, Duan Y, Dai H, et al. Investigation of gut microbiome changes in type 1 diabetic mellitus rats based on high-throughput sequencing. Biomedicine & pharmacotherapy = Biomedecine & pharmacotherapie. 2020;124:109873 Epub 2020/01/28. 10.1016/j.biopha.2020.109873 . [DOI] [PubMed] [Google Scholar]
- 79.Greetham HL, Gibson GR, Giffard C, Hippe H, Merkhoffer B, Steiner U, et al. Allobaculum stercoricanis gen. nov., sp. nov., isolated from canine feces. Anaerobe. 2004;10(5):301–7. 10.1016/j.anaerobe.2004.06.004 [DOI] [PubMed] [Google Scholar]
- 80.Cox Laura M, Yamanishi S, Sohn J, Alekseyenko Alexander V, Leung Jacqueline M, Cho I, et al. Altering the Intestinal Microbiota during a Critical Developmental Window Has Lasting Metabolic Consequences. Cell. 2014;158(4):705–21. 10.1016/j.cell.2014.05.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mendes MCS, Paulino DS, Brambilla SR, Camargo JA, Persinoti GF, Carvalheira JBC. Microbiota modification by probiotic supplementation reduces colitis associated colon cancer in mice. World journal of gastroenterology. 2018;24(18):1995–2008. 10.3748/wjg.v24.i18.1995 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zagato E, Pozzi C, Bertocchi A, Schioppa T, Saccheri F, Guglietta S, et al. Endogenous murine microbiota member Faecalibaculum rodentium and its human homologue protect from intestinal tumour growth. Nature microbiology. 2020;5(3):511–24. 10.1038/s41564-019-0649-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Luo XM, Edwards MR, Mu Q, Yu Y, Vieson MD, Reilly CM, et al. Gut Microbiota in Human Systemic Lupus Erythematosus and a Mouse Model of Lupus. Applied and environmental microbiology. 2018;84(4). 10.1128/AEM.02288-17 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gong L, Wen T, Wang J. Role of the Microbiome in Mediating Health Effects of Dietary Components. Journal of agricultural and food chemistry. 2020. Epub 2020/03/07. 10.1021/acs.jafc.9b08231 . [DOI] [PubMed] [Google Scholar]
- 85.Webb AL, McCullough ML. Dietary lignans: potential role in cancer prevention. Nutrition and cancer. 2005;51(2):117–31. Epub 2005/04/30. 10.1207/s15327914nc5102_1 . [DOI] [PubMed] [Google Scholar]
- 86.Saarinen NM, Warri A, Airio M, Smeds A, Makela S. Role of dietary lignans in the reduction of breast cancer risk. Mol Nutr Food Res. 2007;51(7):857–66. Epub 2007/06/20. 10.1002/mnfr.200600240 . [DOI] [PubMed] [Google Scholar]
- 87.Louis P, Hold GL, Flint HJ. The gut microbiota, bacterial metabolites and colorectal cancer. Nature reviews Microbiology. 2014;12(10):661–72. Epub 2014/09/10. 10.1038/nrmicro3344 . [DOI] [PubMed] [Google Scholar]
- 88.Davie JR. Inhibition of histone deacetylase activity by butyrate. The Journal of nutrition. 2003;133(7 Suppl):2485s–93s. Epub 2003/07/04. 10.1093/jn/133.7.2485S . [DOI] [PubMed] [Google Scholar]
- 89.Guilloteau P, Martin L, Eeckhaut V, Ducatelle R, Zabielski R, Van Immerseel F. From the gut to the peripheral tissues: the multiple effects of butyrate. Nutrition research reviews. 2010;23(2):366–84. Epub 2010/10/12. 10.1017/S0954422410000247 [DOI] [PubMed] [Google Scholar]
- 90.Salimi V, Shahsavari Z, Safizadeh B, Hosseini A, Khademian N, Tavakoli-Yaraki M. Sodium butyrate promotes apoptosis in breast cancer cells through reactive oxygen species (ROS) formation and mitochondrial impairment. Lipids in health and disease. 2017;16(1):208 Epub 2017/11/04. 10.1186/s12944-017-0593-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hinnebusch BF, Meng S, Wu JT, Archer SY, Hodin RA. The effects of short-chain fatty acids on human colon cancer cell phenotype are associated with histone hyperacetylation. The Journal of nutrition. 2002;132(5):1012–7. Epub 2002/05/02. 10.1093/jn/132.5.1012 . [DOI] [PubMed] [Google Scholar]
- 92.Semaan J, El-Hakim S, Ibrahim J-N, Safi R, Elnar AA, El Boustany C. Comparative effect of sodium butyrate and sodium propionate on proliferation, cell cycle and apoptosis in human breast cancer cells MCF-7. Breast Cancer. 2020. 10.1007/s12282-020-01063-6 [DOI] [PubMed] [Google Scholar]
- 93.Ohara T, Suzutani T. Intake of Bifidobacterium longum and Fructo-oligosaccharides prevents Colorectal Carcinogenesis. Euroasian journal of hepato-gastroenterology. 2018;8(1):11–7. Epub 2018/07/03. 10.5005/jp-journals-10018-1251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Narayanan A, Baskaran SA, Amalaradjou MA, Venkitanarayanan K. Anticarcinogenic properties of medium chain fatty acids on human colorectal, skin and breast cancer cells in vitro. International journal of molecular sciences. 2015;16(3):5014–27. Epub 2015/03/10. 10.3390/ijms16035014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Miko E, Vida A, Kovacs T, Ujlaki G, Trencsenyi G, Marton J, et al. Lithocholic acid, a bacterial metabolite reduces breast cancer cell proliferation and aggressiveness. Biochimica et biophysica acta Bioenergetics. 2018;1859(9):958–74. Epub 2018/04/16. 10.1016/j.bbabio.2018.04.002 . [DOI] [PubMed] [Google Scholar]
- 96.Hilakivi-Clarke L, Clarke R, Lippman M. The influence of maternal diet on breast cancer risk among female offspring. Nutrition (Burbank, Los Angeles County, Calif). 1999;15(5):392–401. Epub 1999/06/04. 10.1016/s0899-9007(99)00029-5 . [DOI] [PubMed] [Google Scholar]
- 97.Rosendale D, Butts C, de Guzman E, Maddox I, Martell S, McIntyre L, et al. The contribution of dietary broccoli sprouts towards the microbial metabolite profile in the hind gut of mice. International Journal of Food Science & Technology. 2012;47(6):1328–32. 10.1111/j.1365-2621.2012.02947.x [DOI] [Google Scholar]
- 98.Wei L, Wang J, Yan L, Shui S, Wang L, Zheng W, et al. Sulforaphane attenuates 5-fluorouracil induced intestinal injury in mice. Journal of Functional Foods. 2020;69:103965 10.1016/j.jff.2020.103965 [DOI] [Google Scholar]
- 99.Shen G, Khor TO, Hu R, Yu S, Nair S, Ho CT, et al. Chemoprevention of familial adenomatous polyposis by natural dietary compounds sulforaphane and dibenzoylmethane alone and in combination in ApcMin/+ mouse. Cancer research. 2007;67(20):9937–44. Epub 2007/10/19. 10.1158/0008-5472.CAN-07-1112 . [DOI] [PubMed] [Google Scholar]
- 100.Lee MJ, Maliakal P, Chen L, Meng X, Bondoc FY, Prabhu S, et al. Pharmacokinetics of tea catechins after ingestion of green tea and (-)-epigallocatechin-3-gallate by humans: formation of different metabolites and individual variability. Cancer epidemiology, biomarkers & prevention: a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2002;11(10 Pt 1):1025–32. Epub 2002/10/12. . [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
This clustering dendrogram shows no outliers and therefore all samples from dietary treatments were used in the fecal samples analyses of BE group.
(TIF)
This hierarchical clustering dendrogram verifies that no outliers were present and we have therefore included every sample for microbiome analyses of the LC group.
(TIF)
This excel sheet provides detail of quality check (FASTQC) results including number of samples, sequence per sample, read length and GC content.
(XLSX)
This sheet enlists the changes in relative abundance (base mean, log2 fold change, p-values and adjusted p-values) according to each group of bacterial communities of all treatment groups: BSp-fed, GTPs-fed, combination-fed and control-fed mice, in BE and LC group at both before and after onset of the tumor.
(XLSX)
Data Availability Statement
All relevant data are within the manuscript and its Supporting information files.