Abstract
Objective:
Our prior studies have found that intracerebroventricular injection of blood components can cause hydrocephalus and choroid plexus epiplexus cell activation in rats. To minimize the cross-species reaction, the current study examines whether intraventricular injection of acellular components of cerebrospinal fluid (CSF) from subarachnoid hemorrhage patients can cause hydrocephalus and epiplexus macrophage activation in nude mice which lack a T-cell inflammatory response.
Methods:
Adult male nude mice received intraventricular injections of acellular CSF from subarachnoid hemorrhage patients or a control patient. All mice had preoperative magnetic resonance imaging as baseline and postoperative scans at 24 hours after CSF injection to determine ventricular volume. Brains were harvested at 24 hours for brain histology, immunohistochemistry and electron microscopy.
Results:
Intraventricular injection of CSF from two of five subarachnoid hemorrhage patients obtained <48 hours from ictus resulted in ventricular enlargement at 24 hours. CSF-related hydrocephalus was associated with activation of epiplexus macrophages and ependymal injury.
Conclusions:
Components of the acellular CSF of subarachnoid hemorrhage patients can cause epiplexus macrophage activation, ependymal cell damage, and ventricular enlargement in nude mice. This may serve as a unique model to study mechanisms of hydrocephalus development following subarachnoid hemorrhage.
Keywords: cerebrospinal fluid, epiplexus cells, hydrocephalus, nude mice, subarachnoid hemorrhage
Introduction
Aneurysmal subarachnoid hemorrhage (aSAH) is a significant cause of morbidity and mortality throughout the world. A large proportion of aSAH patients (15–87%) develop hydrocephalus (HCP) often complicating the initial injury after aSAH [1, 2]. Aneurysmal SAH patients with hydrocephalus have higher morbidity and mortality than those without hydrocephalus [3]. Hydrocephalus may lead to an elevated intracranial pressure, thus reducing cerebral perfusion pressure and increasing the potential of further ischemic insults. In addition, even without a rise in intracranial pressure, HCP can lead to confusion and neurological changes. The mainstay therapy for HCP is ventriculostomy followed by shunt placement if necessary, with each being an invasive procedure with inherent risks. To reduce morbidity associated with HCP and its invasive treatment, the mechanism of HCP development following SAH must be elucidated. Decreased CSF absorption, anatomic obstruction of CSF drainage, and increased production of CSF contribute to the development of HCP [2].
Multiple groups have shown that epiplexus cells are activated in rat models of hydrocephalus and that inflammation may lead to CSF hypersecretion[4–6]. In addition, Karimy et al [7] have demonstrated that inflammation leads to hypersecretion of CSF and hydrocephalus formation. Taken together, these findings suggest an important pathophysiologic link between epiplexus cell activation and hydrocephalus formation.
The presence of erythrocytes within the ventricular system may occlude CSF outflow and is a predictor of HCP formation. However, we recently found that other blood components, including thrombin and hemoglobin, can also cause hydrocephalus [8, 9]. The role of acellular CSF components in the development of HCP and subependymal injury post SAH has not been fully elucidated and it represents an opportunity for mechanistic understanding of HCP development and, in turn, an opportunity for therapeutic intervention for HCP prevention. To better understand this question, we have developed a rat intracerebroventricular (ICV) injection model in which acellular CSF derived from SAH patients was injected into rat ventricle, and then MRI and histochemical evaluations were carried out [10]. Previously we showed that ICV injection of acellular SAH patient CSF into rat ventricles led to significant HCP as well as subependymal cellular injury, including cellular necrosis [10]. Nonetheless, the cross-species reaction is certainly a limitation of this model. To address this issue, the current study employed ICV injections of acellular CSF from aSAH patients into nude mice ventricles to understand the degree of HCP development. We also examined whether the induced HCP was associated with epiplexus cell activation and ependymal injury.
Materials and Methods
CSF Collection
CSF samples were collected from aSAH patients or a control patient (prolactinoma patient undergoing treatment of a CSF leak) admitted to the University of Michigan Hospital. The University of Michigan Institutional Review Board approved the study. Informed consent was obtained from the patients whose CSF was sampled. In brief, CSF samples were drawn from SAH patients with ventriculostomy catheter placement within the first 48 hours of admission, as well as at days 7 and 14. CSF samples were collected in sterile tubes and centrifuged at 2000 g for 10 minutes. The supernatant was pipetted into cryo vials and kept in −80 °C freezer until use. The clinical characteristics of the patients whose CSF was utilized for intra-cerebral ventricular injection are summarized in Table 1.
Table 1.
Clinical characteristics of SAH patients (CSF utilized for cerebral-ventricular injection)
| Patient Number | Age (yrs) | Sex | HH | Fisher | Aneurysm | Treatment Modality | EVD | VPS | Discharge mRS |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 40 | F | 4 | 4 | Right PICA | Endovascular | Yes | No | Deceased |
| 2 | 35 | F | 3 | 4 | Right PICA | Endovascular | Yes | No | 2 |
| 3 | 54 | F | 4 | 4 | Right MCA | Microsurgical | Yes | Yes | 3 |
| 4 | 79 | F | 3 | 4 | Right PCOM | Endovascular | Yes | Yes | 2 |
| 5 | 49 | F | 2 | 2 | Right PCOM | Microsurgical | Yes | No | 1 |
EVD, external ventricular drain; HH, Hunt Hess grade; MCA, middle cerebral artery; mRS, modified Rankin Score; PCOM, posterior communicating artery; PICA, posterior inferior cerebellar artery; VPS, ventriculoperitoneal shunt.
Animal Preparation and Model Establishment
Animal use protocols were approved by the University of Michigan Committee on the Use and Care of Animals. A total of 40 male athymic mice (3 months old, Charles River Laboratories, Portage, Michigan) were used in this study.
For ICV injections, mice were anesthetized with ketamine (90 mg/kg, i.p; Abbott Laboratories, Abbott Park, Illinois) and xylazine (5 mg/kg, i.p; Lloyd Laboratories, Manila, Philippines). Core body temperature was maintained at 37.5 °C with a feedback controlled heating pad. Mice were positioned in a stereotaxic frame (Kopf Instruments, Tujunga, California). A cranial burr hole (1 mm) was drilled and a 26-gauge needle was inserted stereotaxically into the right lateral ventricle (coordinates: 0.5 mm posterior, 2.2 mm deep to skull, and 0.9 mm lateral to the bregma). Fifty microliters of acellular CSF from aSAH patients or control CSF (prolactinoma patient without CSF seeding) was injected into the mice lateral ventricle using a micro-infusion pump (World Precision Instruments, Sarasota, Florida). After injection, the needle was removed, the burr hole was filled with bone wax, and the skin incision was sutured closed. The rate of injection into the ventricle was 2.5 μl/min for all animals. After the initial injections of CSF from all patients, we injected the CSF samples from patients 1 and 3 (which had led to hydrocephalus formation in the initial injections) into 6 additional mice per CSF sample at each time point to confirm the effects on hydrocephalus as well as cellular injury.
Magnetic Resonance Imaging and Ventricular Volume Measurement
Nude mice had a preoperative MRI as baseline and postoperative scans at 24 hours. Mice were anesthetized with 2% isoflurane/air mixture throughout MRI examination. MRI scanning was performed in a 7.0-T Varian MR scanner (183-mm horizontal bore; Varian, Palo Alto, California) at the Center for Molecular Imaging of the University of Michigan. The imaging protocol for all mice was a T2 fast spin-echo (repetition time/echo time = 4000/60 ms). The field of view was 20 × 20 mm, and the matrix was 256 × 256 mm. Twenty-five coronal slices (0.5-mm thick) were acquired from the frontal pole to the brain stem, and the images were preserved at 256 × 256 pixels. Ventricle volumes were calculated using Image J (National Institutes of Health). Ventricular enlargement was calculated as follows: (ventricle volume at 24 hours - preoperative ventricle volume)/preoperative ventricle volume × 100% for each animal. All measurements were performed by a blinded observer.
Brain Histology and H&E Staining
Mice were anesthetized (pentobarbital, 60 mg/kg i.p.) and perfused transcardially with 4% paraformaldehyde in 0.1 mol/L phosphate-buffered saline (pH 7.4). Brains were fixed in 4% paraformaldehyde for 6 hours and then immersed in 30% sucrose for dehydration for 3–4 days at 4 °C. Brains were then sectioned on a cryostat (18-μm thick slices). H&E staining was performed following a standard protocol and sections were observed by light microscopy.
Immunohistochemistry
Immunohistochemical studies were performed using the avidin-biotin complex technique as previously described [11]. The primary antibody was rabbit anti-Iba-1 (1:200, Wako, Richmond, Virginia). For Iba-1 positive cell counting, ×20 magnification images containing choroid plexus were selected and counted bilaterally by an observer blinded to the experimental group. The number of Iba-1 positive cells is presented as a percentage of all choroid plexus cells.
Electron Microscopy
Anesthetized mice underwent transcardiac perfusion with 4% paraformaldehyde and 2.5% glutaraldehyde in 0.1 mol/L Sorensen’s buffer (pH 7.4). Brains were removed and 1-mm-thick coronal brain slices were cut with a blade starting approximately 4 mm from the frontal pole. The samples were immersed in the same fixative overnight at 4 °C. Samples were then post-fixed with 1.0% OsO4 and dehydrated in graded ethyl alcohol. After complete dehydration, samples were infiltrated with propylene oxide, embedded in Epon, and sectioned. Ultra-thin sections were stained with uranyl acetate and Reynold’s lead citrate, and evaluated using a Philips CM 100 transmission electron microscope (TEM) and AMRAY 1910 field emission scanning electron microscope.
Statistical Analysis
All data in this study are presented as mean ± standard deviation (SD). Data from different animal groups were analyzed using analysis of variance with a Bonferroni multiple comparisons test or a Student t-test. Differences were considered significant at p < 0.05.
Results
Ventricular Enlargement
No mice died from the ICV injections in this study. Bloodstained acellular CSF from five aSAH patients, collected within 2 days of ictus, or control CSF was injected ICV in nude mice, and MRIs were taken at 24 hours (Fig. 1A). Two of the five SAH CSF-injected mice displayed ventricular enlargement (SAH-patient 1 (Hunt & Hess: grade IV; Fisher: grade IV) and SAH-patient 3 (Hunt & Hess: grade III; Fisher: grade IV); Fig. 1B). In the two mice with hydrocephalus, ventricular volumes were increased by 74% and 78%, respectively. The lateral ventricles were enlarged more significantly than the third ventricle (Fig. 1). Furthermore, we injected the CSF samples from patients 1 and 3 into 6 additional mice per CSF sample to confirm the effects on hydrocephalus, and all mice showed ventricular volume increase (Fig. 2A). The volume increase was 69 ± 17% and 51 ± 5% with the CSF from SAH-patients 1 and 3, respectively (Fig. 2B). Acellular CSF from the same patients but collected at 7 and 14 days after aSAH were also injected ICV. However, those samples did not induce a significant change in ventricular size (5 ± 3% and 1 ± 2% at day 7 and 14, respectively; Fig. 3).
Fig. 1.
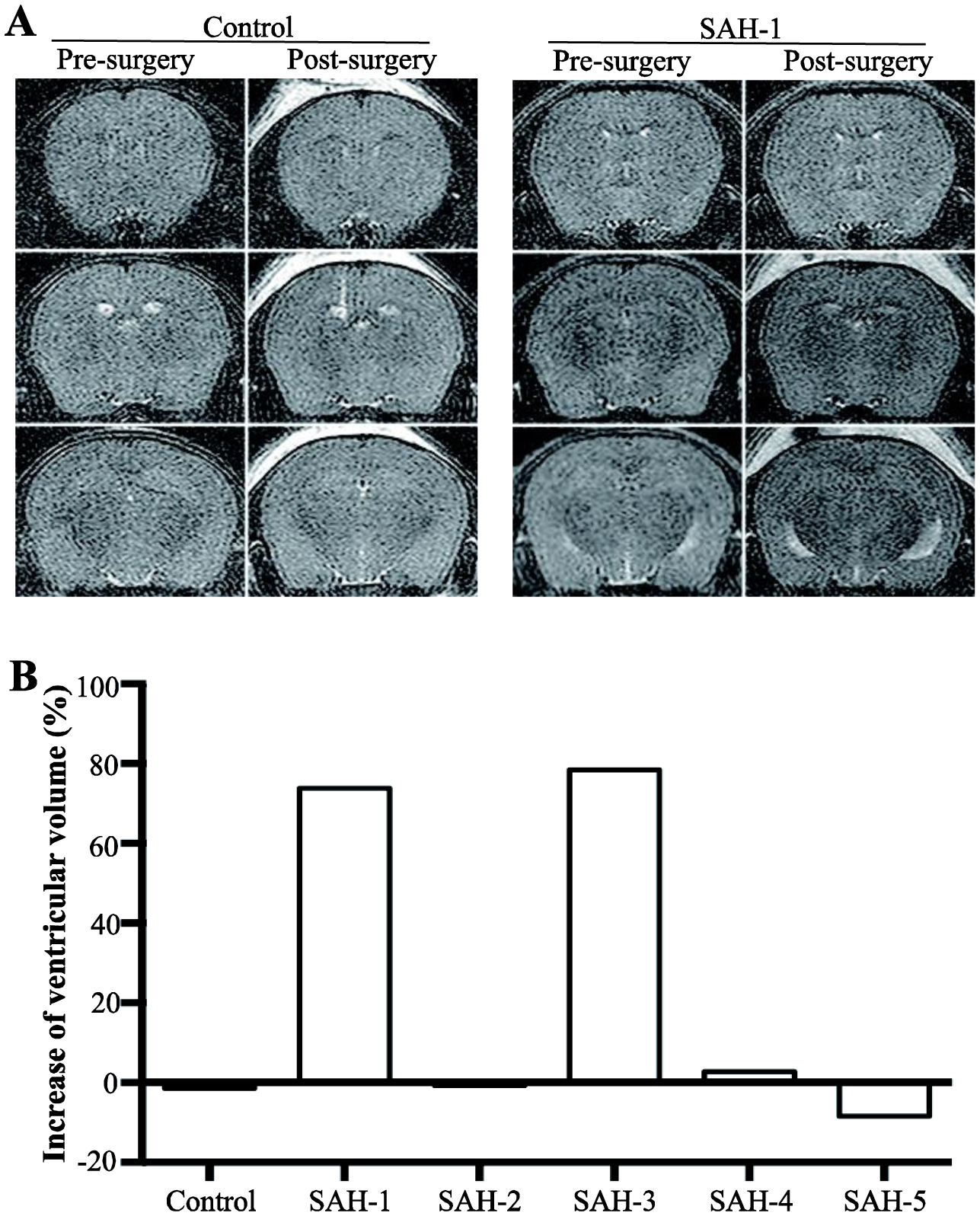
Effects of subarachnoid hemorrhage (SAH) cerebrospinal fluid (CSF) samples on ventricular enlargement. (A) Examples of T2-weighted MRI scans of nude mice before and 24 hours after intraventricular injection of CSF from a control patient and an aneurysmal SAH patient (patient 1). (B) Quantification of the degree of ventricular enlargement (post- vs. pre-surgery) 24 hours after intraventricular injection of CSF from a control and five SAH patients. The CSF was sampled <48 hours after SAH. Note the marked enlargement with the CSF from two patients (1 and 3).
Fig. 2.
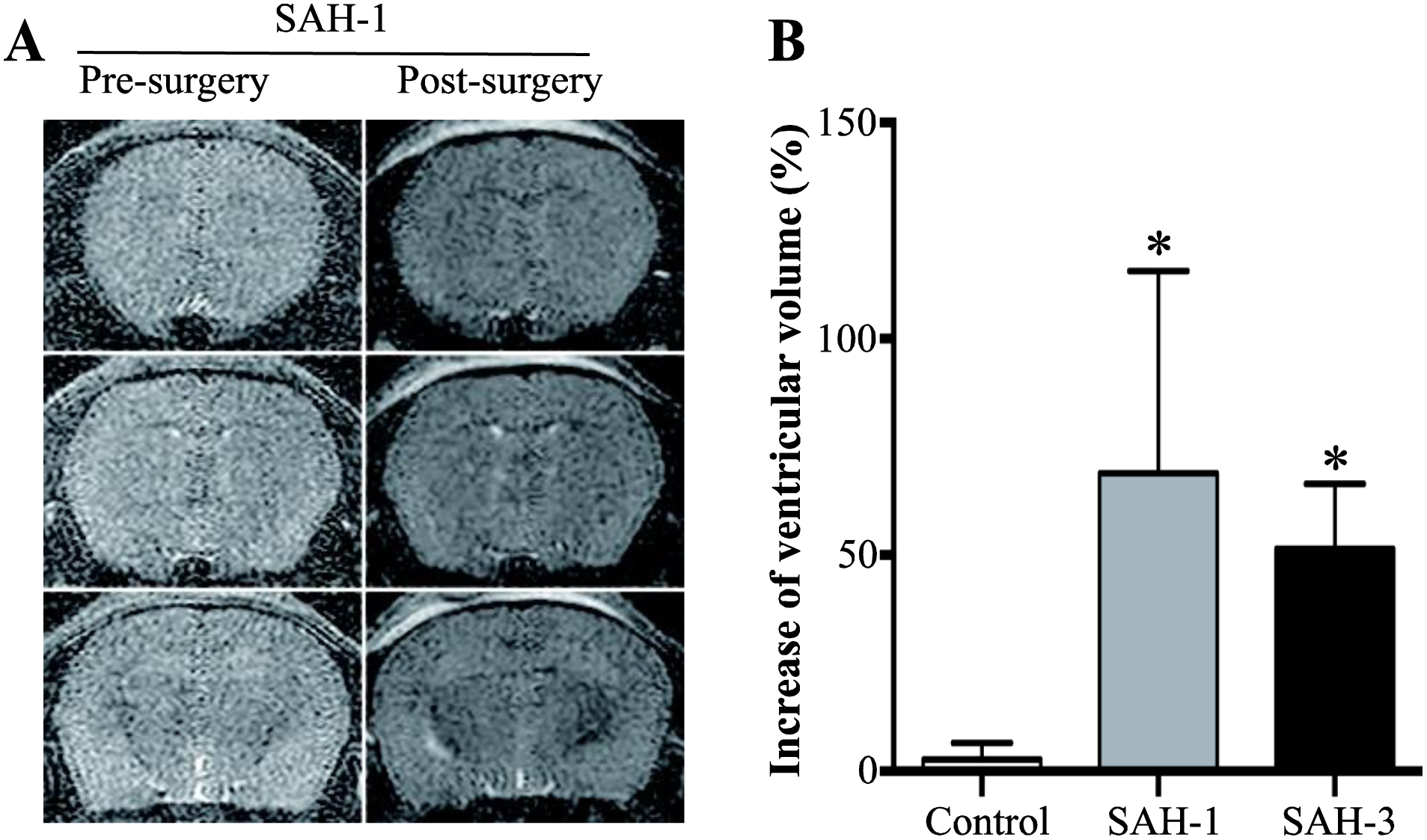
Confirmation of ventricular enlargement caused by SAH acellular CSF (sampled within 48 hours of ictus). (A) Examples of MRIs of nude mice prior to and 24 hours after intracerebroventricular injection of acellular CSF from SAH patient 1. (B) Quantification of the degree of ventricular enlargement. Values are means ± SD, n = 7, *p < 0.05 vs. control.
Fig. 3.
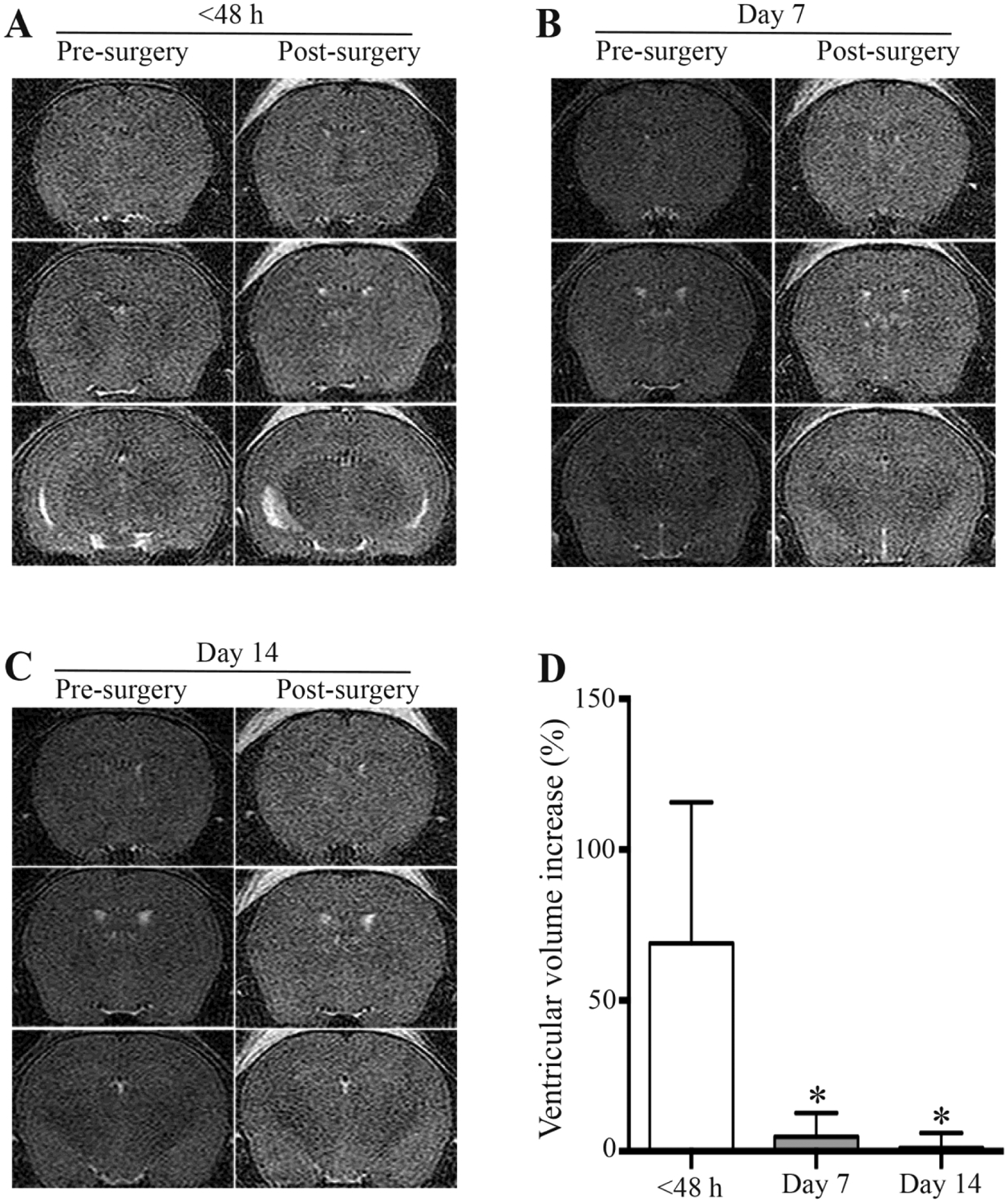
Ventricular enlargement after intracerebroventricular (ICV) injection of acellular CSF from SAH patient 1, where the CSF was sampled at different time points after ictus. Examples of MRIs of nude mice prior to and 24 hours after ICV injection of acellular CSF, where the CSF was sampled at (A) <48 hours, (B) day 7 and (C) day 14. (D) Quantification of the degree of ventricular enlargement. Values are means ± SD, n = 7, *p < 0.05 vs. <48 hours.
Ependymal Cell and Cilia Injury
Pathological changes to the ependymal cell and cilia were observed under light and electron microscopy after ICV injection of acellular CSF from SAH patients 1 and 3 sampled within 48 hours of ictus. H&E staining showed ventricular enlargement 1 day after injection (Fig. 4). Abnormal ependymal cell cilia were observed in mice with acellular CSF injection (Fig. 5). In control mice, the ependymal cells have numerous apical cilia extended into the ventricular lumen as shown by scanning electron microscopy (Fig. 5A). However, mice injected with acellular CSF from aSAH patient 1 showed fewer tufts of cilia, while the ependymal apical surface after injection of CSF from aSAH patient 3 showed areas of ciliary denudation (Fig. 5A). TEM showed the abnormal ependymal cells after injection of acellular CSF from patient 1 with fewer cilia (Fig. 5B, left). Furthermore, when cross-section of normal cilia showing the “9 + 2” microtubule doublet configuration with presence of dynein arms, damaged cilia showed aberrant ciliary profiles (Fig. 5B, right).
Fig. 4.
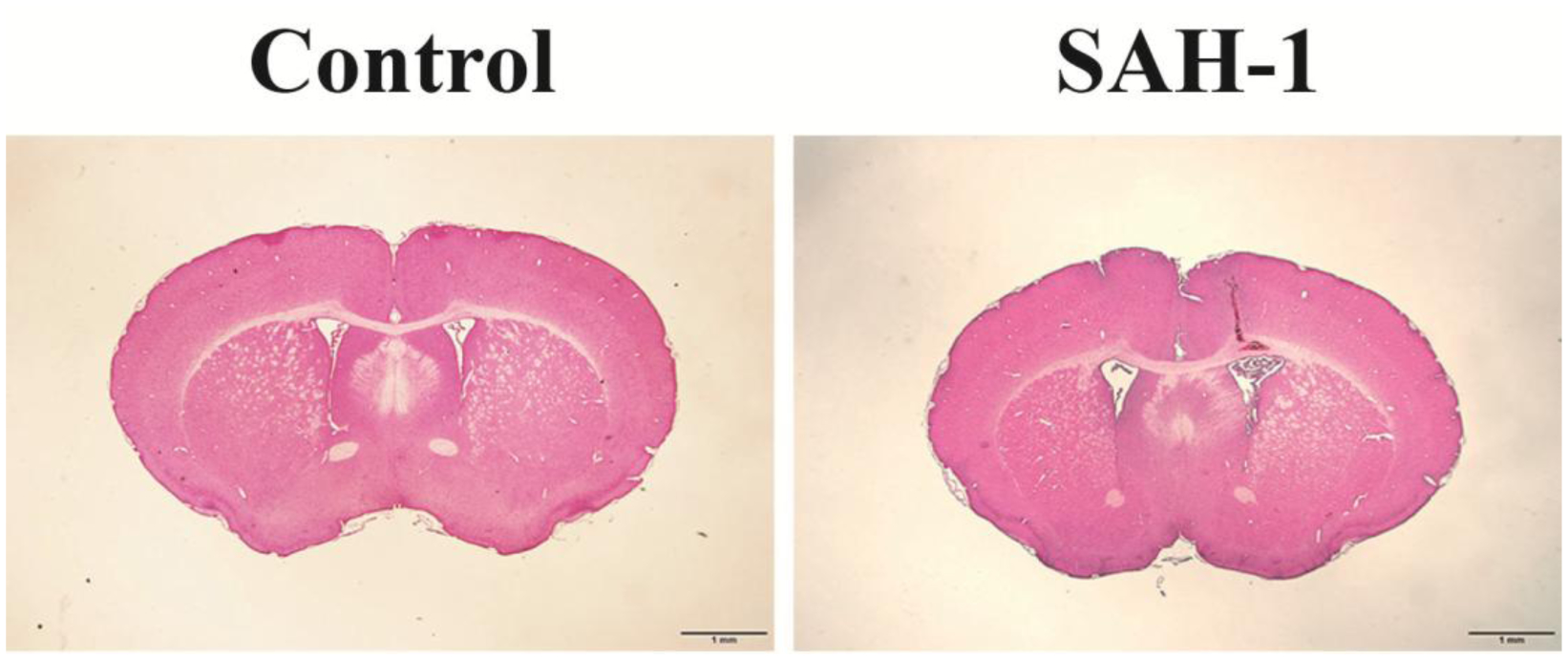
H&E staining showing ventricular enlargement at 24 hours after ICV injection of control acellular CSF or CSF from SAH patient 1. Scale bar = 1 mm.
Fig. 5.
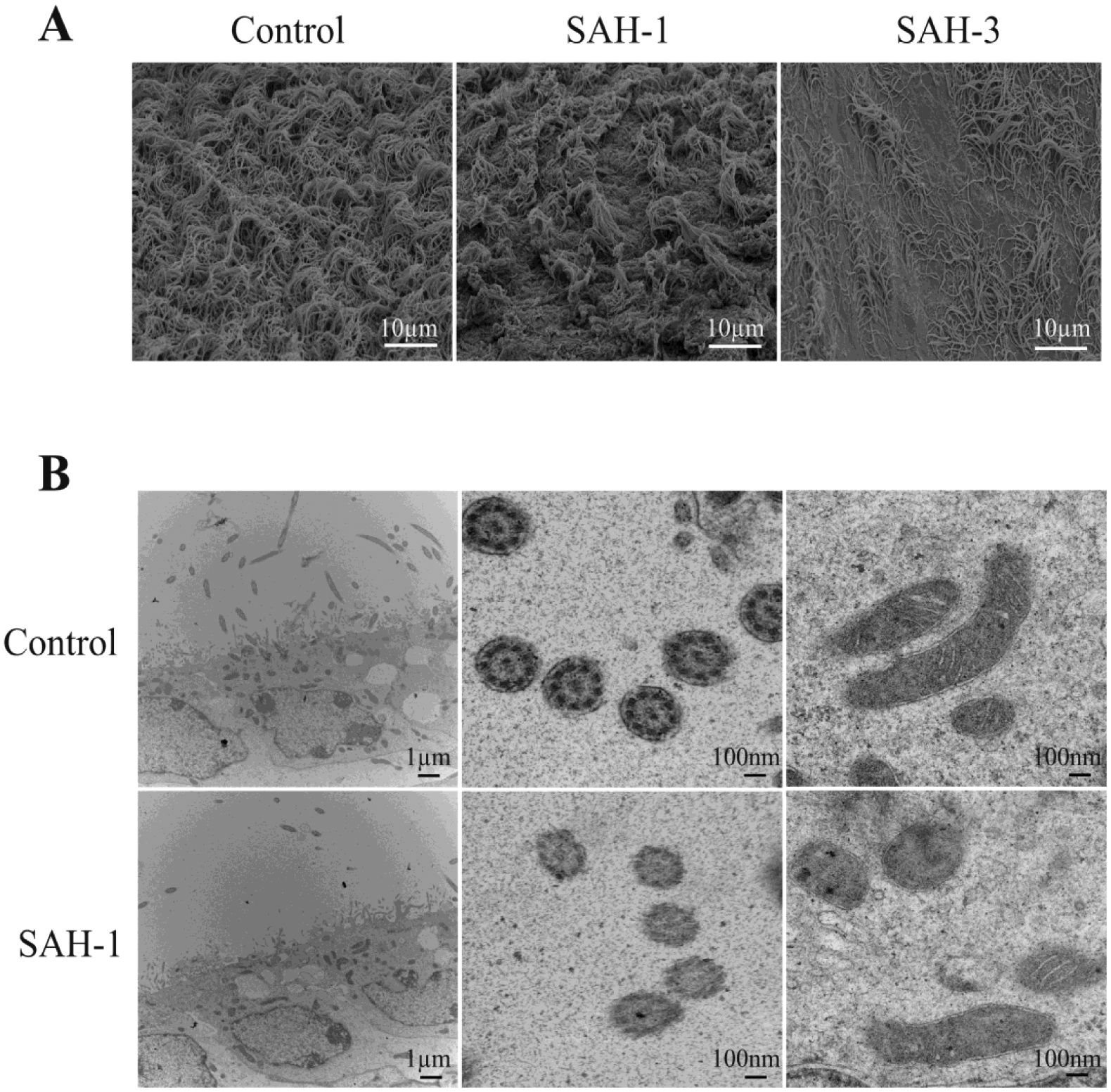
Scanning and transmission electron microscope images showing changes of ependymal cells and cilia 24 hours following ICV injection of acellular CSF from a control patient and from SAH patients 1 and 3. (A) Examples of scanning electron microscopy of the ependymal wall. (B) Examples of transmission electron microscopy of the ependyma and ependymal cilia. Note the relative absence of cilia and the aberrant cilia profiles in the animal injected with CSF from SAH patient 1.
Epiplexus Cell Activation
Epiplexus cells are positive for the macrophage marker Iba-1. At 24 hours after injection, an approximately 3-fold increase of Iba-1 positive macrophages was observed in the choroid plexus after injection of CSF from SAH patients 1 and 3 (Fig. 6).
Fig. 6.
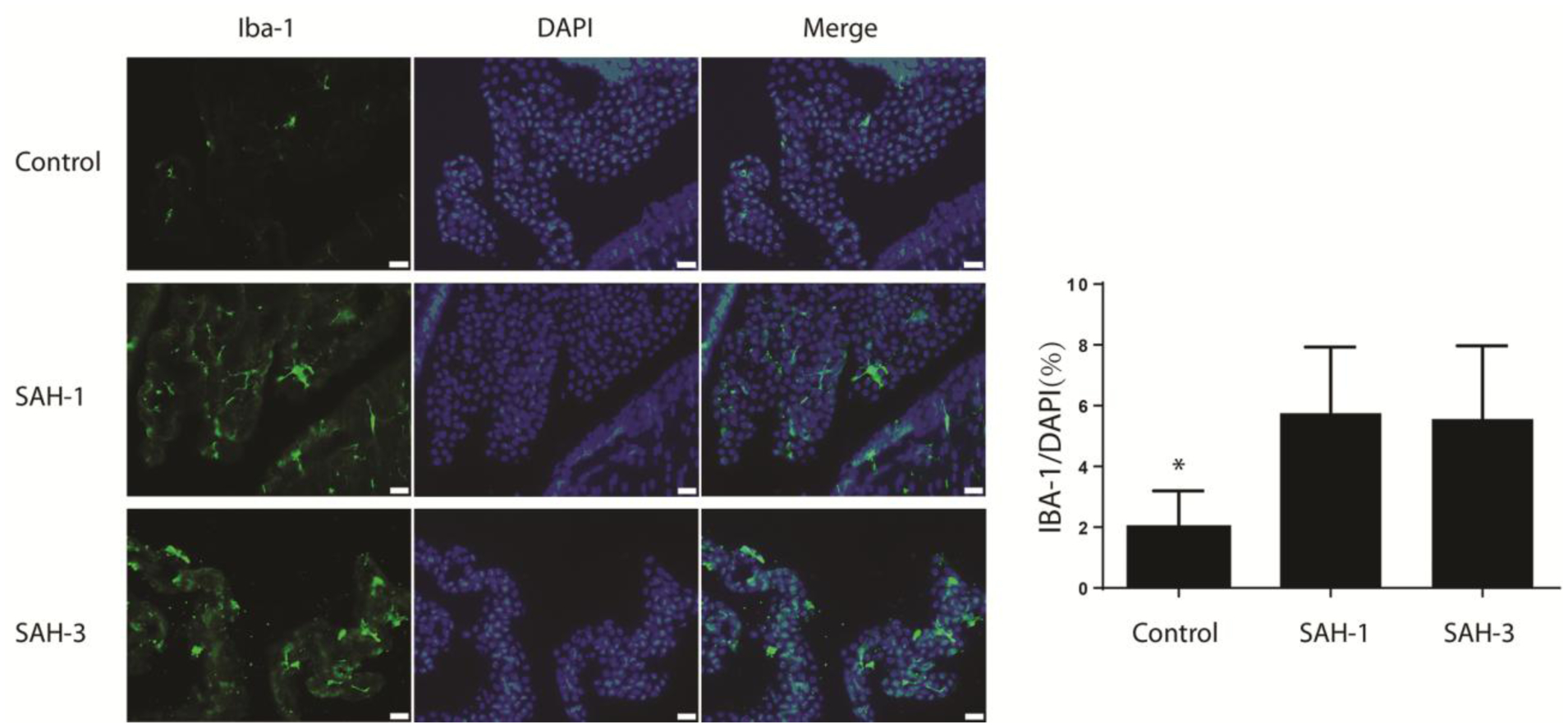
Epiplexus cell activation at 24 hours following ICV injection of acellular CSF from a control patient and from SAH patients 1 and 3. Immunohistochemistry for Iba-1 in the choroid plexus. Iba-1 is a macrophage marker that detects epiplexus cells. The same sections were counterstained with DAPI, a nuclear marker, and the merged images are shown. The number of Iba-1 cells was quantified as a percent of all cells as determined by DAPI. Values are means ± SD, n = 7, *p < 0.05 vs. the other groups. Scale bar = 20 μm.
Discussion
There are four major findings in this study evaluating the effects of injecting human SAH acellular CSF into nude mice ventricles. (1) Some, but not all, SAH CSF samples induced ventricular dilation within 24 hours. (2) This dilation depended upon the timing of CSF sampling, being present in samples taken at <48 hours after ictus, but not samples from 7 and 14 days post SAH. (3) There was evidence of ependymal cell and cilia damage. (4) Choroid plexus epiplexus (Kolmer) cells were activated on the choroid plexus in animals developing HCP post-injection.
Hydrocephalus often occurs after SAH in both humans and animals. Blood entering CSF is a harmful factor that may lead to post-hemorrhagic hydrocephalus [12]. In this study, we injected acellular CSF from aSAH patients ICV into nude mice, which mimicked the clinical condition of the presence of biochemical toxins within the CSF space post SAH. Consistent with our previous study of SAH patient CSF injection into rat ventricles [10], the current study demonstrates that injection of CSF from patients with aSAH can result in hydrocephalus in immunocompromised mice, indicating these findings are not solely the result of immune activation.
We found that the CSF from the acute stage (within 2 days) of aSAH caused ventricular dilation but not the CSF from the sub-acute stages (7 or 14 days after aSAH). It is still unclear why the CSF at 7 and 14 days after aSAH failed to cause ventricular dilation, but we hypothesize that it is associated with component changes in CSF. Once entering the CSF, crenation of red blood cells occurs very soon and erythrolysis can take place within several hours [13, 14]. The concentration of oxyhemoglobin and bilirubin in CSF increases during first week and decreases thereafter [15]. HO-1, the enzyme for degrading heme to iron, CO, and biliverdin, was also upregulated in CSF from SAH patients. Iron overload is a major contributor for neuronal injury after hemorrhage, and it also contributes to hydrocephalus after intraventricular hemorrhage and SAH [16–18]. A previous study showed an increase of unbound CSF iron and ferritin (an iron-storage protein) within 5 days after SAH, and another suggested the concentration of iron (unbound and bound) was increased at 5 days and maintained to 14 days after SAH, while ferritin was increased at 5 to 10 days and then decreased [19, 20]. Our observation reveals that the acute SAH CSF samples led to HCP, whereas samples collected at 1 and 2 weeks post SAH did not lead to HCP. Thus it is feasible that iron and inflammatory cells which have previously been shown to be present up to 14 days post SAH may not be the factors leading to HCP given that our samples at 7 and 14 days did not lead to HCP formation.
Ependymal cells lining the ventricles are polarized with multiple cilia extending into the ventricle that are thought to direct CSF flow by their planar-polarized beating [21]. Absent or dysfunctional cilia could lead to altered ependymal function and further result in the formation of hydrocephalus [22]. In this study, aberrant ependymal cells and cilia were observed at 24 hours after injection of acellular CSF taken from patients within 2 days of acute SAH. Morphological abnormalities of ependymal cilia were displayed clearly both under light and electron microscope. The severely damaged cilia may be implicated in this human bloodstained CSF-induced hydrocephalus.
In addition, the current study found a significantly increased number of activated epiplexus (Kolmer) cells on the choroid plexus of animals injected with human SAH acellular CSF as compared to control CSF (Fig. 6). Epiplexus cells are thought to function as immune cells and may be contributing to the blood-CSF barrier. These cells communicate with the epithelial cells of the choroid plexus via the pannexin-1 channels, thus allowing them to move along the epithelium [6]. The inflammatory nature of these cells was further clarified by Kaur and Ling [23], who reported that the administration of dexamethasone significantly reduced the number of epiplexus cells. Epiplexus cells express major histocompatibility complex (MHC I and II), complement receptor (CR-3) and leukocyte antigen. The intraperitoneal injection of interferon gamma leads to an enhancement of epiplexus cell functionality as it relates to phagocytosis and antigen presentation [24]. While the immune nature of epiplexus cells is well documented, the role of these cells in hydrocephalus formation has not been previously documented. Further work needs to be dedicated to understanding their mechanism of activation after ICV injection of acellular CSF and whether the activation has a causal relationship to hydrocephalus development.
There are limitations to this study: (1) Only 5 human CSF samples were used to determine the incidence of ventricular dilation after human CSF injection; (2) the exact blood components contributing to that dilation were not deciphered; (3) the persistence of the ventricular dilation was not examined (i.e., does it result in long-term hydrocephalus?); (4) sex differences were not tested. These research opportunities are currently being pursued as well as a mechanistic understanding of hydrocephalus formation post SAH towards the goal of medical therapies for the treatment of HCP.
Conclusions
Acellular components in the CSF of aSAH patients can induce ventricular dilation, ependymal damage, and epiplexus cell activation in nude mice. Only acute SAH acellular CSF samples (within 48 hours of ictus) led to HCP. The results from this study suggest that ICV injection of CSF from aSAH patients into nude mice may be a useful model for studying the underlying mechanisms of hydrocephalus formation.
Sources of Support
YH, RFK, GX and ASP were supported by grants NS-090925, NS-096917, NS-106746, NS-108042 and NS-112394 from the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Conflicts of Interest
The authors report no conflicts of interest.
Ethical Approval
The study was approved by the University of Michigan Institutional Review Board. Animal use protocols were approved by the University of Michigan Committee on the Use and Care of Animals.
Informed Consent
Informed consent was obtained from the patients whose cerebrospinal fluid was sampled.
References
- 1.Connolly ES Jr., Rabinstein AA, Carhuapoma JR, et al. Guidelines for the management of aneurysmal subarachnoid hemorrhage: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2012;43(6):1711–37. [DOI] [PubMed] [Google Scholar]
- 2.Germanwala AV, Huang J, Tamargo RJ. Hydrocephalus after aneurysmal subarachnoid hemorrhage. Neurosurg Clin N Am. 2010;21(2):263–70. [DOI] [PubMed] [Google Scholar]
- 3.Suarez-Rivera O Acute hydrocephalus after subarachnoid hemorrhage. Surg Neurol. 1998;49(5):563–5. [DOI] [PubMed] [Google Scholar]
- 4.Gu C, Hao X, Li J, et al. Effects of minocycline on epiplexus macrophage activation, choroid plexus injury and hydrocephalus development in spontaneous hypertensive rats. J Cereb Blood Flow Metab. 2019;39:1936–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ling EA, Tseng CY, Wong WC. An electron microscopical study of epiplexus and supraependymal cells in the prenatal rat brain following a maternal injection of 6-aminonicotinamide. J Anat. 1985;140 (Pt 1):119–29. [PMC free article] [PubMed] [Google Scholar]
- 6.Maslieieva V, Thompson RJ. A critical role for pannexin-1 in activation of innate immune cells of the choroid plexus. Channels (Austin). 2014;8(2):131–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Karimy JK, Zhang J, Kurland DB, et al. Inflammation-dependent cerebrospinal fluid hypersecretion by the choroid plexus epithelium in posthemorrhagic hydrocephalus. Nat Med. 2017;23(8):997–1003. [DOI] [PubMed] [Google Scholar]
- 8.Gao F, Liu F, Chen Z, et al. Hydrocephalus after intraventricular hemorrhage: the role of thrombin. J Cereb Blood Flow Metab. 2014;34(3):489–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Strahle JM, Garton T, Bazzi AA, et al. Role of hemoglobin and iron in hydrocephalus after neonatal intraventricular hemorrhage. Neurosurgery. 2014;75(6):696–705; discussion 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li P, Chaudhary N, Gemmete JJ, et al. Intraventricular injection of noncellular cerebrospinal fluid from subarachnoid hemorrhage patient into rat ventricles leads to ventricular enlargement and periventricular injury. Acta Neurochir Suppl. 2016;121:331–4. [DOI] [PubMed] [Google Scholar]
- 11.Wu J, Hua Y, Keep RF, et al. Iron and iron-handling proteins in the brain after intracerebral hemorrhage. Stroke. 2003;34(12):2964–9. [DOI] [PubMed] [Google Scholar]
- 12.Okubo S, Strahle J, Keep RF, Hua Y, Xi G. Subarachnoid hemorrhage-induced hydrocephalus in rats. Stroke. 2013;44(2):547–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vermeulen M, van Gijn J. The diagnosis of subarachnoid haemorrhage. J Neurol Neurosurg Psychiatry. 1990;53(5):365–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van Gijn J, Rinkel GJ. Subarachnoid haemorrhage: diagnosis, causes and management. Brain. 2001;124(Pt 2):249–78. [DOI] [PubMed] [Google Scholar]
- 15.Vermeulen M, Hasan D, Blijenberg BG, Hijdra A, van Gijn J. Xanthochromia after subarachnoid haemorrhage needs no revisitation. J Neurol Neurosurg Psychiatry. 1989;52(7):826–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen Z, Gao C, Hua Y, et al. Role of iron in brain injury after intraventricular hemorrhage. Stroke. 2011;42(2):465–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee JY, Keep RF, He Y, et al. Hemoglobin and iron handling in brain after subarachnoid hemorrhage and the effect of deferoxamine on early brain injury. J Cereb Blood Flow Metab. 2010;30(11):1793–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Loftspring MC. Iron and early brain injury after subarachnoid hemorrhage. J Cereb Blood Flow Metab. 2010;30(11):1791–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gomes JA, Selim M, Cotleur A, et al. Brain iron metabolism and brain injury following subarachnoid hemorrhage: iCeFISH-Pilot (CSF Iron in SAH). Neurocrit Care. 2014;21(2):285–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Suzuki H, Muramatsu M, Kojima T, Taki W. Intracranial heme metabolism and cerebral vasospasm after aneurysmal subarachnoid hemorrhage. Stroke. 2003;34(12):2796–800. [DOI] [PubMed] [Google Scholar]
- 21.Mirzadeh Z, Han YG, Soriano-Navarro M, Garcia-Verdugo JM, Alvarez-Buylla A. Cilia organize ependymal planar polarity. J Neurosci. 2010;30(7):2600–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Banizs B, Pike MM, Millican CL, et al. Dysfunctional cilia lead to altered ependyma and choroid plexus function, and result in the formation of hydrocephalus. Development. 2005;132(23):5329–39. [DOI] [PubMed] [Google Scholar]
- 23.Kaur C, Ling EA. Effects of dexamethasone on the epiplexus cells in postnatal rats. Neurosci Lett. 1995;196(3):165–8. [DOI] [PubMed] [Google Scholar]
- 24.Lu J, Kaur C, Ling EA. Immunophenotypic features of epiplexus cells and their response to interferon gamma injected intraperitoneally in postnatal rats. J Anat. 1994;185 (Pt 1):75–84. [PMC free article] [PubMed] [Google Scholar]


