Abstract
Previous studies demonstrate profound sex-specific patterns of white matter microstructural neurodevelopment (i.e. fractional anisotropy; FA, and mean diffusivity; MD) during adolescence. While alcohol use has been associated with alterations in FA and MD, no studies have addressed the potential for sex-specific, alcohol-dose-dependent effects, during development. This prospective longitudinal study (2–4 visits, 310 total scans) used voxel-wise multilevel modeling, in 132 (68 female) adolescents (ages 12–21), to assess the sex-specific effects of lifetime alcohol use on FA and MD, during development. Follow-up analyses tested the role of sex hormones, testosterone and estradiol, in explaining the effects of alcohol use on FA and MD. In the splenium of the corpus callosum and posterior thalamic radiata, male adolescents demonstrated lower FA and greater MD as a function of more lifetime alcohol use, while female adolescents demonstrated the opposite. Further, significant associations between sex hormones and FA/MD, partially explained the effect of alcohol use on FA and MD in male adolescents. These results provide evidence for sex-specific and dose-related effects of alcohol use on white matter microstructure, which are partially explained by sex hormones, and highlight the importance of studying sex and hormones when investigating the effects of alcohol use on the adolescent brain.
Keywords: diffusion weighted imaging, sex differences, corpus callosum, testosterone, estradiol
1. Introduction
Adolescent development is often marked by an escalation in risk-taking behavior, which may be reflected as an increased propensity for alcohol use (Chung et al., 2018). Importantly, binge-level alcohol use during adolescence is associated with both acute (e.g. drunk driving, unsafe sex, other substance use) and long-term adverse behavioral consequences (e.g. psychosocial impairment, increased risk for developing an alcohol use disorder or other comorbid psychopathology) (Chung et al., 2018). Although these risks are not unique to adolescents, in addition to these adverse outcomes, engaging in substantial alcohol use during this period of ongoing brain maturation has been shown to impact neurodevelopment, often in a sex-specific manner.
Among the many dynamic changes that occur during adolescent neurodevelopment are non-linear increases and then decreases in gray matter volume and thickness, and a substantial increase in white matter volume, crucial for efficient communication between distal brain regions (e.g. Giedd et al., 1999). Recent longitudinal work suggests that alcohol use during adolescence results in accelerated developmental reductions in cortical gray matter volume (Pfefferbaum et al., 2018; Squeglia et al., 2014; Squeglia et al., 2015) and thickness (Luciana et al., 2013), and attenuated increases in white matter volume (Luciana et al., 2013). However, previous cross-sectional work suggests that these alcohol-related effects may occur in a sex-specific manner. For example, a cross-sectional study in adolescents found significant sex-by-drinking status interactions in four frontal brain regions (frontal pole, pars orbitalis, medial orbital frontal, and rostral anterior cingulate), where male adolescents who binge drank had thinner cortices than alcohol-naïve controls, whereas female adolescents who binge drank had thicker cortices compared to controls (Squeglia et al., 2012). Similarly, male young adults who binge drank demonstrated smaller prefrontal, striatal and medial temporal volumes compared to their alcohol-naive peers, meanwhile, female young adults who binge drank demonstrated larger volumes in these regions compared to alcohol naïve peers (Kvamme et al., 2016).
Beyond these macrostructural brain changes, diffusion weighted imaging (DWI), a non-invasive technique that can be used to examine the microstructural properties of white matter fibers in vivo, has provided insight both into normative white matter development during adolescence, as well as how alcohol use impacts these trajectories (Alexander et al., 2007; Beaulieu, 2002). Among the most common indices derived from DWI are fractional anisotropy (FA) and mean diffusivity (MD). FA describes the overall directionality preference of water diffusion in a given voxel, and conversely, MD quantifies overall water diffusion independent of directionality (Basser, 1995). Although these indices are not specific to any one neurobiological property (e.g. axonal density, myelination, axon caliber), the combination of higher FA values and lower MD is generally thought to reflect greater fiber coherence and/or organization (Alexander et al., 2007; Beaulieu, 2002). During adolescence, there are widespread increases in FA and decrease in MD with age throughout the brain (e.g. Pohl et al., 2016). However, these patterns have been shown to differ by sex, such that adolescent boys tend to demonstrate larger and more protracted increases in FA across adolescence compared to adolescent girls (Asato et al., 2010; Simmonds et al., 2014). Though fewer studies have examined this trend in a longitudinal context, it seems that this sexual dimorphism may be partially explained by pubertal sex hormones (testosterone and estradiol), which are associated with the aforementioned indices of white matter microstructure (Herting et al., 2017; Herting et al., 2012; Sisk and Foster, 2004).
Alcohol use during adolescence has also been associated with alterations in white matter microstructural development. Multiple longitudinal studies have demonstrated diminished normative increases in FA in numerous white matter tracts including the inferior fronto-occipital fasciculus, corpus callosum, prefrontal thalamic fibers and posterior corona radiata, in adolescents who binge-drank compared to controls (Bava et al., 2013; Luciana et al., 2013). However, recent longitudinal work in our lab suggests that some of the observed alterations in FA (and MD) among drinkers may be present prior to initiation of alcohol use (Jones and Nagel, 2019). Although alcohol use has been shown to relate to diminished white matter microstructure in a dose-dependent manner (McQueeny et al., 2009), the extent to which this effect differs by sex and/or relates to sex hormones remains unclear.
The current study sought to investigate the sex-specific influence of alcohol use on white matter microstructure during adolescent development. Although a previous study from our lab demonstrated binge-drinking related alterations in white matter microstructural development (Jones and Nagel, 2019), we did not have the power to detect sex-specific effects at the voxel-wise level. Presently, we aim to expand and improve upon these results by including a larger sample with more within-subject time points and a continuous measure of alcohol use. This approach improves effect sizes, power, and measurement reliability (MacCallum et al., 2002), and allows for assessment of sex-specific effects at the voxel-wise level, all while controlling for other key predictors post-hoc. Based on prior research demonstrating thinner cortices and smaller volumes in male adolescents, and thicker cortices and larger volumes in female adolescents, as a function of alcohol use (Kvamme et al., 2016; Squeglia et al., 2012), we anticipated a similar pattern: that alcohol use would be associated with lower FA (and greater MD) in male adolescent, but greater FA (and lower MD) in female adolescents, particularly in the prefrontal cortex. Further, we hypothesized that this effect might be explained by sex hormones, testosterone and estradiol.
2. Methods
2.1. Participants
Adolescents (ages 12 to 16 at baseline) were selected from a larger ongoing longitudinal study on adolescent neurodevelopment (Jones and Nagel, 2019). During initial recruitment, adolescents and their parents provided written consent and assent, respectively, followed by comprehensive screening interviews to determine eligibility. Exclusionary criteria during recruitment were as follows: left handedness, DSM-IV psychiatric diagnosis, serious medical problems (including head trauma), intellectual or learning disability, psychotic illness in a biological parent, prenatal drug or alcohol exposure, MRI contraindications, and baseline drug or alcohol use (>10 lifetime alcohol drinks, >2 alcoholic drinks in a single occasion, >10 lifetime uses of marijuana, >4 cigarettes per day, or any other drug use). As part of our ongoing longitudinal data collection, following their baseline visit, adolescents reported alcohol and drug use every 90 days via telephone and/or online survey (Sobell et al., 1996). If during their 90-day follow-up, adolescents reported a minimum of one binge-drinking episode (≥5 drinks for males, ≥4 drinks for females, in one sitting), they were brought in for their first follow-up visit (including a neuroimaging session). At this time, a “control” subject (matched on age, sex, and time-since-baseline), with little-to-no alcohol use, was also brought in for their first follow-up visit. The average time between baseline and first follow-up visit was 2.6 years. Further, several participants were brought in for 1–2 additional follow-up visits (average of 1.9 years between visits). Participants in the current study are those from the larger study with a minimum of 2 (and up to 4) total within-subject visits (329 visits across 132 subjects; 51.5% female). Data from 109 participants (229 visits) were used in a previous study investigating the effects of binge drinking and family history of alcoholism on white matter maturation (Jones and Nagel, 2019). Study procedures were approved by the Oregon Health & Science University Institutional Review Board.
2.2. Measures
2.2.1. Socioeconomic status
At baseline, parents reported on socioeconomic status using the Hollingshead Index of Social Positioning, based on parents’ highest level of education and professional achievement, with lower scores representing higher socioeconomic status (Hollingshead and Redlich, 2007).
2.2.2. General intelligence (IQ)
General intelligence was assessed in adolescents at all visits using the 2-subtest version of the Weschler Abbreviated Scale of Intelligence (Wechsler D, 1999).
2.2.3. Substance use
During all visits, adolescents completed the Customary Drinking and Drug Use Record (CDDR), which measures lifetime occasions of alcohol and marijuana use (Brown et al., 1998). To improve distributions, lifetime occasions of alcohol and marijuana use were log-transformed prior to all analyses.
2.2.4. Family history of alcoholism
At baseline, parents reported on rates of familial alcoholism using the Family History Assessment Module (Rice et al., 1995). Using these reports, a family history density (FHD) score was calculated based on the number of adolescents’ relatives with an alcohol use disorder; parents contributed 0.5 each, grandparents 0.25 each, and aunts and uncles a weighted ratio of 0.25 divided by their number of siblings.
2.2.5. Pubertal stage
Self-assessment of puberty was obtained using a modified line drawing version of the Tanner’s Sexual Maturation Scale (Taylor et al., 2001), with drawings ranging from stage 1 (pre-adolescent) through stage 5 (adult-like maturation).
2.3. Hormonal assessment
Within seven days of all neuroimaging scans, 4 mL of blood was collected via venipuncture at the Oregon Clinical and Translational Research Institute, between 7:00 am and 10:00 am to limit diurnal effects on hormone levels. Blood collection and neuroimaging scans occurred on the same day for 45% of visits; on all other occasions, blood was collected on average within 2–3 days of neuroimaging scans. To reduce variability and interactions with progesterone (Gillies and McArthur, 2010), all post-menarcheal girls completed lab visits during the follicular phase of their menstrual cycle (days 1–10), as assessed via self-report. Serum testosterone levels were assessed using the DIAsource TESTO-RIA-CT kit (DIAsource Immunoassays, Belgium), with an intra- and inter-assay CV of 3.3% and 4.8% respectively, and a lower level of detection of 0.05 ng/mL. Serum estradiol levels were assessed using the DSL-4800 Ultra-sensitive Estradiol Radioimmunoassay Kit (Beckman Coulter, Fullerton, CA), with an intra- and inter-assay CV of ≤8.9% and 4.1% respectively, and a lower level of detection of 2.2 pg/mL.
2.4. Image acquisition and analysis
During each visit, diffusion-weighted images (DWI) were collected via a whole-brain, high-angular resolution, echo-planar imaging sequence [repetition time (TR) = 9,100 ms, echo time (TE) = 88 ms, field of view (FOV) = 256 mm2, slices = 72, slice thickness = 2 mm]. Due to a scanner upgrade that occurred during longitudinal data collection, 267 visits were collected on a 3T Siemens Magnetom Tim Trio with a 12-channel head coil, and the remaining 62 visits were collected on a 3T Magnetom Prisma with a 20-channel head/neck coil. During acquisition, six images with a b-value of 0 s/mm2 were collected, followed by gradient encoding pulses in 30 directions (b-value = 1,000 s/mm2). Participants received either two (n = 143; scan time = 11:24) or three (n = 186, scan time = 16:52) DWI runs. To correct for eddy current-induced field distortions, a diffusion field map was also acquired (TR = 790 ms, TE1 = 5.19 ms, TE2 = 7.65 ms, flip angle = 60°, FOV = 240 mm2, slices = 72, slice thickness = 2 mm, scan time = 3:13).
Image processing for this sample has been outlined previously (Jones et al., 2019; Schwarz et al., 2014). First, all DWI runs underwent strict visual inspection for motion and scanner-related artifacts. Volumes deemed to contain artifact were censored, and subjects were excluded from analysis if the same diffusion direction was excluded from all DWI runs in a single visit (n = 11), if scanner artifacts affected an entire sequence (e.g. artifacts due to insufficient fat suppression; n = 6), or if there were errors in acquisition (e.g. incomplete whole-brain coverage; n = 2). This resulted in a final sample of 310 visits across 133 subjects. Image processing, carried out in FSL (v.5.0.11) and AFNI (v.19.0.26) consisted of affine registration of the diffusion field map to DWI runs, concatenation of DWI runs within a scan, correction for eddy current distortion, intensity inhomogeneities, and head motion (and subsequent adjustment of the gradient table), as well as estimation of the diffusion tensor for calculation of FA and MD (Andersson and Sotiropoulos, 2016; Saad et al., 2009; Smith et al., 2004). To register images to standard space, FA and MD maps were registered first to an unbiased within-subject space, then to a study-specific template, and finally to Montreal Neurological Institute (MNI) space, in a single-step interpolation using Advanced Normalization Tools (ANTs) linear and non-linear algorithms (Avants et al., 2011; Schwarz et al., 2014). Further, a Gaussian blur (sigma = 1 mm) was applied to all FA and MD maps. Finally, to correct for differences in FA and MD as a function of scanner platform, a voxel-wise correction factor using empirical Bayes methods was applied using the ComBat function in R (v.3.5.3) with age, sex, and lifetime drinking occasions included as biological covariates (Fortin et al., 2017; Johnson et al., 2007). It has been previously demonstrated that ComBat outperforms other correction techniques when applied to DWI and is the best-suited method for longitudinal samples containing confounds between age and scanner platform (Fortin et al., 2017).
2.5. Statistical analyses
Voxel-wise analyses were conducted for FA and MD using Neuropointillist (http://github.com/IBIC/neuropointillist) in conjunction with the nlme package in R, in a white matter mask containing only voxels where mean FA was greater than 0.3 across all scans. A series of multilevel models (Table 1) investigating the effects of age, lifetime drinking occasions, sex, age-by-sex, and lifetime drinking occasions-by-sex on FA and MD were fit using Maximum Likelihood (ML) estimation and included between-individual differences in baseline FA and MD (i.e. random intercepts). Then, for each voxel, the most parsimonious model was identified by comparing deviance statistics between each individual model and all nested (or reduced) models (p < 0.01). As the goal of the current study was to identify white matter regions where the association between alcohol use and white matter microstructure varied by sex, the results of Models 8 & 9 were subsequently corrected for multiple comparisons, via Family Wise Error (FWE) correction, using AFNI’s 3dClustSim (α < 0.05), resulting in a minimum cluster size threshold of 329 voxels and 375 voxels for FA and MD, respectively (Cox et al., 2017).
Table 1.
Models tested
| # | Model | Nested Models |
|---|---|---|
| 1 | ~ 1 | |
| 2 | ~ age | 1 |
| 3 | ~ age + sex | 1,2 |
| 4 | ~ age + drinks | 1,2 |
| 5 | ~ age * sex | 1,2,3 |
| 6 | ~ age + sex + drinks | 1,2,3,4 |
| 7 | ~ age * sex + drinks | 1,2,3,4,5,6 |
| 8 | ~ age + drinks * sex | 1,2,3,4,5,6 |
| 9 | ~ age * sex + drinks * sex | 1,2,3,4,5,6,7,8 |
| 10 | ~ age + drinks * sex + hormones | 8 |
| 11 | ~ age + drinks * sex + hormones * sex | 8,10 |
All models were fit voxel wise predicting either fractional anisotropy (FA) or mean diffusivity (MD); drinks = log-transformed lifetime drinking occasions; hormones = testosterone/estradiol
For all significant regions identified during voxel-wise analysis, mean FA and MD values were extracted and the model was refit in R. Significant fixed effects were then interpreted, with age centered at 14 (approximate median baseline age), and effect sizes were calculated as either Cohen’s d, standardized regression estimates, or the difference between two standardized regression estimates. As previous studies from our lab have demonstrated an association between familial alcoholism and white matter microstructural development (Jones et al., 2019; Jones and Nagel, 2019), FHD scores were tested as covariates in post-hoc analysis. Similarly, given the high degree of comorbid marijuana use in this sample, and previous longitudinal reports of interactive effects of alcohol and marijuana use on white matter microstructural development (Jacobus et al., 2009), number of lifetime occasions of marijuana use was also tested as a covariate post-hoc. Finally, given the broad age range, and previous reports that puberty and age are related, yet distinct processes affecting neurodevelopment (for review, see Herting and Sowell, 2017), pubertal status was also tested as a post-hoc covariate.
To explore the role of sex hormones in the association between alcohol use and white matter microstructure across adolescence, regressors representing sex hormones (testosterone or estradiol, separately) (Model 10, Table 1) and sex hormones-by-sex (Model 11, Table 1) were added to the final model identified in whole brain analysis. These two models (i.e. with and without estradiol/testosterone) were then compared using deviance statistics. Further, Z-tests were used to compare lifetime drinking occasions and sex-by-lifetime drinking occasions fixed-effect estimates between models with and without the sex hormones, to assess change in the alcohol use-by-sex interaction on white matter microstructure accounting for sex hormones (Clogg CC et al., 1995; Paternoster R et al., 1998).
3. Results
3.1. Participant characteristics
Participant demographics and baseline and follow-up sex hormone and substance use characteristics, for male and female adolescents, are presented in Table 2. Follow-up measures represent values for each individuals’ most recent follow-up visit, and substance use measures reflect only those reporting drinking (n = 32 male adolescents, n = 43 female adolescents) or using marijuana (n = 27 male adolescents, n = 31 female adolescents). Male and female adolescents demonstrated no significant differences in demographic composition or lifetime substance use. As expected, compared to female adolescents, male adolescents had significantly greater levels of testosterone at baseline (t108 = 9.958, p < 0.001, d = 1.90) and most recent follow-up (t112 = 16.388, p < 0.001, d = 3.07), and lower levels of estradiol at baseline (t102 = 5.820, p < 0.001, d = 1.14) and most recent follow-up (t109 = 2.955, p < 0.01, d = 0.56). Further, pubertal status across the 310 timepoints in the study follows: 4 pre-pubertal, 11 early-pubertal, 29 mid-pubertal, 69 late-pubertal, 196 post-pubertal, and 1 missing/unreported. The percentage of subjects reaching the post-pubertal stage at most-recent follow-up did not differ between male and female adolescents.
Table 2.
Sample demographics, substance use and hormone levels
| Male (n = 64) mean (SD) |
Female (n = 68) mean (SD) |
Missing data notes | |
|---|---|---|---|
| Baseline | |||
| Age | 14.13 (1.35) | 14.55 (1.41) | |
| IQ | 110.61 (12.93) | 111.26 (9.16) | |
| SES | 30.57 (13.32) | 29.44 (13.74) | Male (n = 1) |
| FHD | 0.33 (0.29) | 0.40 (0.31) | Male (n = 1) |
| Lifetime drinks | 0.14 (0.48) | 0.36 (1.22) | |
| Lifetime marijuana | 0.31 (0.91) | 0.59 (2.25) | |
| Testosterone (ng/dL) *** | 285.53 (178.98) | 34.52 (14.45) | Male (n = 4), Female (n = 4) |
| Estradiol (pg/mL) *** | 20.46 (9.76) | 32.88 (11.94) | Male (n = 7), Female (n = 6)a |
| Follow-up | |||
| Age | 17.70 (1.93) | 18.31 (2.13) | |
| Lifetime drinks | 47.91 (62.62) | 44.84 (63.20) | |
| Lifetime marijuana | 272.04 (439.57) | 101.90 (226.32) | |
| Testosterone (ng/dL) *** | 401.12 (170.78) | 36.08 (12.50) | Male (n = 8), Female (n = 9) |
| Estradiol (pg/mL) ** | 35.18 (13.03) | 52.61 (42.15) | Male (n = 8), Female (n = 9)b |
p < 0.01;
p < 0.001;
one additional female adolescent removed for outlying value;
four additional female adolescents removed for outlying values
3.2. Lifetime alcohol use and white matter microstructure
Voxel-wise analysis revealed no regions where white matter microstructural development with age and the effect of lifetime alcohol use on white matter microstructure both varied as a function of sex (Model 9, Table 1). There was one region of FA and two regions of MD where the effect of alcohol use on white matter varied based on sex (Model 8, Table 1). First, in the splenium of the corpus callosum (CC; 922 voxels) (Figure 1A), male adolescents had lower FA as a function of more lifetime drinking occasions (b = −0.015, p < 0.001, β = −0.343), while female adolescents had greater FA as a function of more lifetime drinking occasions (b = 0.009, p < 0.01, β = 0.205), resulting in a significant sex-by-lifetime drinking occasions interaction effect on FA (b = −0.024, p < 0.001, Δβ = −0.548). Also, in an adjacent and overlapping region of the splenium of the CC (651 voxels; Figure 1B), male adolescents had greater MD as a function of more lifetime drinking occasions (b = 3.58 × 10−5, p < 0.001, β = 0.169), while female adolescents had lower MD as a function of more lifetime drinking occasions (b = −2.44 × 10−5, p < 0.01, β = −0.115), resulting in a significant sex-by-lifetime drinking occasions interaction effect (b = 6.03 × 10−5, p < 0.001, Δβ = 0.285). Lastly, in the posterior thalamic radiations (PTR; 506 voxels) (Figure 1C), male adolescents again had greater MD as a function of lifetime drinks (b = 4.80 × 10−5, p < 0.001, β = 0.361), but female adolescents had no association between MD and lifetime drinking occasions, resulting in a significant sex-by-lifetime drinks interaction (b = 6.00 × 10−5, p < 0.001, Δβ = 0.452). Post-hoc analyses confirmed that all findings remained significant when independently covarying for lifetime marijuana use and pubertal status, and the addition of lifetime marijuana use or pubertal status failed to improve model fit. Further, all findings remained significant when covarying for FHD of alcoholism; however, the addition of FHD-by-age to the model did improve model fit for FA and MD in the CC, with a significant FHD-by-age interaction present, as has been previously demonstrated by our lab in a partially overlapping sample (Jones and Nagel, 2019).
Figure 1. Voxel-wise results for the sex-dependent effect of alcohol use on white matter microstructure.
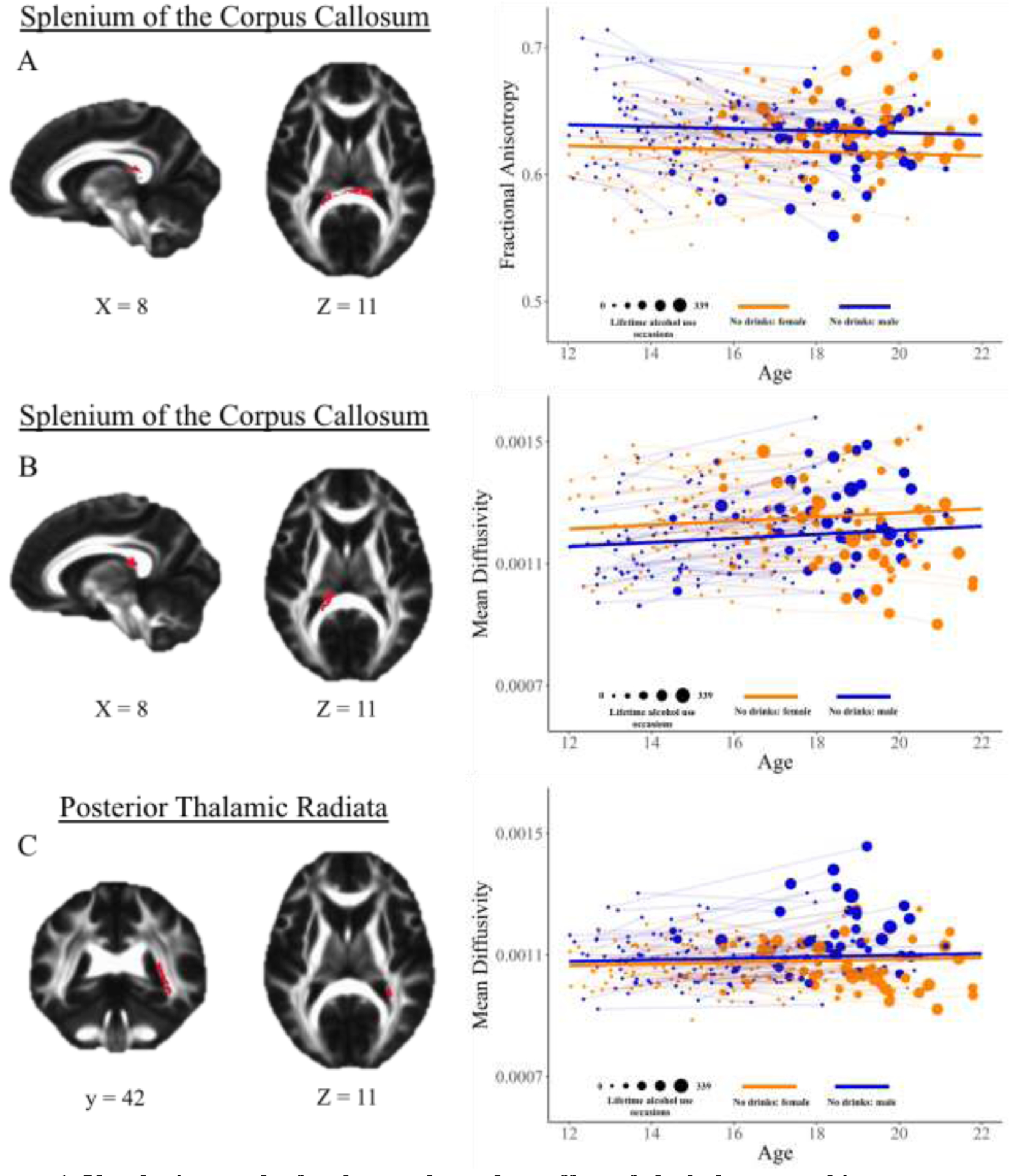
(Left) Significant regions of interest are overlaid on mean fractional anisotropy images in MNI space. (Right) Fractional anisotropy and mean diffusivity are plotted as a function of age. Population level trajectories for those with no lifetime alcohol use are overlaid for male (blue) and female (orange) adolescents. Individual trajectories are underlaid, with the size of individual data points reflective of the number of cumulative lifetime alcohol use occasions at that visit.
3.3. Role of sex hormones
Prior to assessing the role of sex hormones on the sex-dependent effect of lifetime alcohol use on white matter microstructure, several timepoints were excluded for missing data, including 26 visits (12 male, 14 female) from testosterone analyses, and 31 visits (15 male, 16 female) from estradiol analyses. Five additional timepoints from female adolescents were excluded from estradiol analyses due to values falling >3 SD away from the mean for female adolescents. All findings outlined above remained significant in the sub-sample of participants with valid sex hormone data. These results, and full model results looking at the effects of testosterone and estradiol on white matter microstructure, are presented in Tables 3 & 4.
Table 3.
Full model results including the effects of testosterone on white matter microstructure
| Splenium FA | Splenium MD | Posterior Thalamic Radiata MD | ||||
|---|---|---|---|---|---|---|
| Fixed Effects | ||||||
| Intercept | 0.639 *** (3.545 × 10−3) |
0.649 *** (4.999 × 10−3) |
1.168 × 10−3
*** (1.801 × 10−5) |
1.123 × 10−3
*** (2.111 × 10−5) |
1.080 × 10−3
*** (1.019 × 10−5) |
1.060 × 10−3
*** (1.406 × 10−5) |
| Age | −6.694 × 10−4 (7.394 × 10−4) |
−4.781 × 10−5 (7.470 × 10−4) |
6.217 × 10−6
** (2.368 × 10−6) |
2.783 × 10−6 (2.417 × 10−6) |
5.259 × 10−6
* (2.036 × 10−6) |
3.887 × 10−6
(2.134 × 10−6) |
| Female | −0.017 *** (4.909 × 10−3) |
−0.027 *** (5.952 × 10−3) |
5.932 × 10−5
* (2.517 × 10−5) |
1.033 × 10−4
*** (2.732 × 10−5) |
−1.649 × 10−5 (1.413 × 10−5) |
2.841 × 10−6 (1.702 × 10−5) |
| Drinks | −0.016 *** (3.246 × 10−3) |
−0.013 *** (3.333 × 10−3) |
4.007 × 10−5
*** (1.039 × 10−5) |
2.606 × 10−5
* (1.051 × 10−5) |
4.346 × 10−5
*** (8.940 × 10−6) |
3.734 × 10−5
*** (9.266 × 10−6) |
| Female*Drinks | 0.025 *** (3.438 × 10−3) |
0.020 *** (3.756 × 10−3) |
−6.694 × 10−5
*** (1.069 × 10−5) |
−4.636 × 10−5
*** (1.168 × 10−5) |
−6.283 × 10−5
*** (9.430 × 10−6) |
−5.305 × 10−5
*** (1.042 × 10−5) |
| Testosterone | −3.660 × 10−5
** (1.255 × 10−5) |
1.665 × 10−7
*** (4.077 × 10−8) |
7.290 × 10−8
* (3.502 × 10−8) |
|||
| Variance Components | ||||||
| Within-person | 2.333 × 10−4 (0.015) |
2.194 × 10−4 (0.015) |
2.063 × 10−9 (4.542 × 10−5) |
1.867 × 10−9 (4.321 × 10−5) |
1.730 × 10−9 (4.160 × 10−5) |
1.660 × 10−9 (4.074 × 10−5) |
| Intercept | 5.713 × 10−4 (0.024) |
5.844 × 10−4 (0.024) |
1.838 × 10−8 (1.356 × 10−4) |
1.837 × 10−8 (1.355 × 10−4) |
4.892 × 10−9 (6.994 × 10−5) |
5.026 × 10−9 (7.090 × 10−5) |
| Goodness-of-fit | ||||||
| df | 7 | 8 | 7 | 8 | 7 | 8 |
| AIC | −1322 | −1328 | −4477 | −4491 | −4661 | −4663 |
| BIC | −1297 | −1299 | −4451 | −4462 | −4636 | −4634 |
| Deviance | −1336 | −1345 | −4491 | −4507 | −4675 | −4679 |
| Δ Deviance | 8.372** | 16.256 *** | 4.280 * | |||
All values presented for fixed effects and variances components are unstandardized regression coefficients (with standard errors).
p < 0.05;
p < 0.01;
p < 0.001
Table 4.
Full model results including the effects of estradiol on white matter microstructure
| Splenium FA | Splenium MD | Posterior Thalamic Radiata MD | ||||
|---|---|---|---|---|---|---|
| Fixed Effects | ||||||
| Intercept | 0.639 *** (3.587 × 10−3) |
0.646 *** (5.166 × 10−3) |
1.171 × 10−3
*** (1.797 × 10−5) |
1.132 × 10−3
*** (2.173 × 10−5) |
1.063 × 10−3
*** (1.048 × 10−5) |
1.067 × 10−3
*** (1.474 × 10−5) |
| Age | −3.584 × 10−4 (7.367 × 10−4) |
−3.268 × 10−4 (8.535 × 10−4) |
5.734 × 10−6
* (2.458 × 10−6) |
2.745 × 10−6
(2.816 × 10−6) |
4.491 × 10−6
* (2.084 × 10−6) |
4.376 × 10−6
(2.391 × 10−6) |
| Female | −0.016 ** (4.933 × 10−3) | −0.031 *** (6.684 × 10−3) |
5.187 × 10−5
* (2.514 × 10−5) |
1.101 × 10−4
*** (2.911 × 10−5) |
−2.220 × 10−5 (1.435 × 10−5) |
1.492 × 10−5 (1.914 × 10−5) |
| Drinks | −0.015 *** (3.200 × 10−3) |
−0.011 ** (3.323 × 10−3) |
3.662 × 10−5
** (1.071 × 10−5) |
1.840 × 10−5
(6.073 × 10−5) |
3.564 × 10−5
*** (9.057 × 10−6) |
2.614 × 10−5
** (9.319 × 10−6) |
| Female*Drinks | 0.024 *** (3.450 × 10−3) |
0.018 ***
(3.985 × 10−3) |
6.261 × 10−5
*** (1.126 × 10−5) |
−3.792 × 10−5
** (1.313 × 10−5) |
−5.418 × 10−5
*** (9.738 × 10−6) |
−4.286 × 10−5
*** (1.116 × 10−5) |
| Estradiol | −4.568 × 10−4
*
(1.817 × 10−4) |
1.840 × 10−6
**
(6.073 × 10−7) |
7.939 × 10−7
(5.101 × 10−7) |
|||
| Estradiol*Female | 5.520 × 10−4
**
(1.820 × 10−4) |
−2.192 × 10−6
*** (6.071 × 10−7) |
−1.228 × 10−6
*
(5.105 × 10−7) |
|||
| Variance Components | ||||||
| Within-person | 2.166 × 10−4
(0.015) |
2.025 × 10−4
(0.014) |
2.102 × 10−9 (4.585 × 10−5) |
1.907 × 10−9 (4.367 × 10−5) |
1.709 × 10−9 (4.134 × 10−5) |
1.555 × 10−9 (3.943 × 10−5) |
| Intercept | 5.749 × 10−4
(0.024) |
5.717 × 10−4
(0.024) |
1.802 × 10−8 (1.342 × 10−4) |
1.780 × 10−8 (1.334 × 10−4) |
4.971 × 10−9 (7.050 × 10−5) |
5.014 × 10−9
(7.081 × 10−5) |
| Goodness-of-fit | ||||||
| df | 7 | 9 | 7 | 9 | 7 | 9 |
| AIC | −1283 | −1291 | −4311 | −4323 | −4493 | −4504 |
| BIC | −1258 | −1259 | −4286 | −4291 | −4468 | −4471 |
| Deviance | −1297 | −1309 | −4325 | −4341 | −4507 | −4522 |
| Δ Deviance | 11.828 ** | 16.348 *** | 14.615 *** | |||
All values presented for fixed effects and variances components are unstandardized regression coefficients (with standard errors).
p < 0.05;
p < 0.01;
p < 0.001
When testosterone was added to the model, greater testosterone levels were associated with lower FA in the CC (b = −3.66 × 10−5, p < 0.01, β = −0.252) and greater MD in the CC (b = 1.67 × 10−7, p < 0.001, β = 0.236) and PTR (b = 7.29 × 10−8, p < 0.05, β = 0.164), with the addition of testosterone providing a significant model improvement in all three regions (all X2(1) ≥ 4.28, p < 0.05). This model improvement was accompanied by a significant reduction in the sex-by-alcohol use interaction in all three regions (all z ≥ 2.93, p < 0.01), which was driven by a significant decrease in the association between lifetime drinking occasions and FA in the CC (z = 2.80, p < 0.01) and MD in the PTR (z = 2.90, p < 0.01) in males. There were no significant changes in the association between lifetime drinking occasions and FA/MD in females. There were also no sex-by-testosterone interaction effects. When pubertal status was added as covariate, the effect of testosterone on MD in the PTR was no longer significant; however, pubertal status itself was not associated with MD in this region, and did not significantly improve model fit. All other effects remained significant while covarying for pubertal status.
Estradiol was also significantly associated with white matter microstructure, but in a sex-dependent manner. Greater estradiol levels were associated with lower FA in the CC in male adolescents (b = −4.57 × 10−4, p < 0.05, β = −0.436), and greater FA in female adolescents (b = 9.52 × 10−5, p < 0.05, β = 0.091), resulting in a significant estradiol-by-sex interaction (b = 5.52 × 10−4, p < 0.01, Δβ = 0.527). Meanwhile, greater estradiol was associated with greater MD in the CC (b = 1.84 × 10−6, p < 0.01, β = 0.361) in male adolescents, and lower in MD in the CC (b = −3.52 × 10−7, p < 0.05, β = −0.069) and PTR (b = −4.34 × 10−7, p < 0.01, β = −0.135) in female adolescents, resulting in a significant estradiol-by-sex interaction in the CC (b = 2.19 × 10−6, p < 0.001, Δβ = 0.430) and PTR (b = 1.22 × 10−6, p < 0.05, Δβ = 0.383). The addition of estradiol to the lifetime alcohol use model revealed a significant increase in model improvement in all three regions (all X2(2) ≥ 11.82, p < 0.01). This was accompanied by a significant reduction in the sex-by-lifetime drinking occasions interaction in all three regions (all z ≥ 2.19, p < 0.05), and a significant decrease in the association between alcohol and FA in the CC (z = 2.39, p < 0.05) and MD in the PTR (z = 2.01, p < 0.05) in male adolescents. There were no significant changes in the association between lifetime drinking occasions and FA/MD in female adolescents. When pubertal status was added to the model, all effects of estradiol remained significant, pubertal status was not associated with FA/MD, and its addition did not result in any significant model improvements.
4. Discussion
While no effects of alcohol use were observed in hypothesized prefrontal white matter tracts, this study revealed two white matter regions of the brain, the splenium of the corpus callosum (CC) and the posterior thalamic radiations (PTR), where lifetime alcohol use was associated with alterations in white matter microstructure in our hypothesized sex-dependent manner during development. In male adolescents, those with a greater number of lifetime drinking occasions had lower FA in the CC and greater MD in the CC and PTR, whereas in female adolescents, those with more lifetime drinking occasions showed greater FA and lower MD in the CC. Follow-up analyses demonstrated that including either testosterone or estradiol in the model partially explained the effect of lifetime alcohol use on white matter microstructure in male adolescents, but not female adolescents.
These findings, at least with respect to male adolescents, mirror prior literature demonstrating that alcohol use during adolescence is generally associated with alterations in white matter microstructure, possibly as a result of neurotoxic effects of alcohol or attenuated white matter development (Bava et al., 2013; Luciana et al., 2013; McQueeny et al., 2009). More specifically, lower FA and higher MD values in portions of the CC and PTR have been previously reported in adolescents using both alcohol and marijuana (Bava et al., 2013). In congruence with prior studies comparing light and heavy drinkers, we also show a dose-response relationship between alcohol use and FA (McQueeny et al., 2009). Interestingly, though, we found the directionality of the relationship between lifetime drinking occasions and white matter microstructure to vary by sex, such that female adolescents with more lifetime drinking occasions tended to have higher FA and lower MD values in the CC. These results corroborate previous reports of reduced FA, in the splenium of the CC, in men with an alcohol use disorder, but not women (Pfefferbaum and Sullivan, 2002), and provide evidence for a dose-related effect of alcohol use in adolescents, prior to the emergence of an alcohol use disorder. Further, these results demonstrate a pattern of sex-specific effects of alcohol use in adolescents that is similar to prior findings in gray matter. That is, alcohol use by male adolescents appears to be associated with the potential deleterious effects of alcohol, such as thinner cortices (Squeglia et al., 2012), smaller gray matter volumes (Kvamme et al., 2016), and the lower FA and greater MD noted in our current study; whereas, the opposite effect is demonstrated in female adolescents. However, the differential neurobiological processes occurring in male and female adolescents that results in these differential effects is remains unclear.
Nearly all major white matter tracts in the brain demonstrate significant microstructural changes with age (Pohl et al., 2016), but there is significant regional specificity in in the timing of this development (Lebel et al., 2019). The splenium of the CC in particular, shows marked growth early in adolescence, and demonstrates peaks in FA and MD between 11–16 years of age (Lebel et al., 2019). As such, the relatively moderate decline in FA and increase in MD in the CC in the current study may be more reflective of the trajectory expected in mid to late adolescence/early-adulthood. Similarly, there is evidence to suggest that development of the PTR occurs largely in childhood (Simmonds et al., 2014), in line with the lack of significant developmental changes in MD in this region. While the current study found no sex-by-age effects in FA or MD development in the CC and PTR, previous literature suggests that males male adolescents show larger and more protracted increases in FA compared to female adolescents (Asato et al., 2010; Simmonds et al., 2014). Further, while we did not have the power nor the necessary number of within-subject timepoints on all subjects to appropriately model sex differences in non-linear trajectories of white matter microstructure at the voxel-wise level, previous studies suggest white matter microstructure may develop in a non-linear fashion (Lebel et al., 2019). As such, it is possible that these differential effects of alcohol use on CC and PTR microstructure are due to subtle variations in the typical developmental trajectories of male and female adolescents, and may also vary depending on when in this developmental process alcohol use is initiated (a limitation of the current study, discussed below). Future studies will be necessary to further tease apart the interactive effect of age, sex, and onset of alcohol use on white matter microstructural development in adolescents.
A relevant caveat to the interpretation of these findings is that measures of FA and MD are not specific to underlying neurobiological processes. While greater FA is often thought to reflect greater axonal integrity (i.e. greater fiber density, axonal diameter, and/or myelination), structural changes at the neuronal level cannot be directly observed using the diffusion metrics from tradition DWI analyses. It has been suggested that diminished coherence of major white matter tracts (indicted by lower FA and higher MD) may be a result of neurotoxicity sustained from prolonged substance exposure during a particularly sensitive neurodevelopmental period. For example, rodent models suggest that alcohol self-administration during adolescence results in significant myelin degradation (e.g. Vargas et al., 2014). However, altered FA and MD could also suggest that alcohol use impedes a developmentally typical process, such as the elimination of transient or exuberant axonal projections (Riccomagno and Kolodkin, 2015), or long-distance dopaminergic axonal growth (Hoops and Flores, 2017). Most importantly, the mechanism by which alcohol alters white matter microstructural properties may be sex-dependent, and may help explain the differing effects of alcohol use on diffusion metrics observed in this study. Future mechanistic work, likely in preclinical models, will be necessary to address these hypothesized explanations for the effects demonstrated in our study.
In addition to sex-differences in the association between alcohol use and white matter microstructure, we found that in all regions, testosterone and estradiol levels partially explain the effect of alcohol on white matter microstructure, but only in male adolescents. That is, sex hormones helped explain a portion of the sex-specific effect of alcohol use on white matter microstructure, via a reduction in the association between alcohol use and white matter microstructure in male adolescents. Previous studies on the association between sex hormones and white matter microstructure are mixed. While we found testosterone to be associated with reduced FA and greater MD (in both male and female adolescents), previous studies have demonstrated greater testosterone is associated with greater FA in male adolescents (Herting et al., 2012), and greater MD in both male and female adolescents (Peper et al., 2015) and reduced MD in male adolescents (Menzies et al., 2015). Further, we found a sex-specific effect of estradiol, with greater estradiol associated with lower FA and greater MD in male adolescents, and greater FA and lower MD in female adolescents. Meanwhile, previous literature has found greater estradiol to be associated with reduced FA in both males and female adolescents (Herting et al., 2012; Stoica et al., 2019). These disparate findings could be explained by many things, including regional specificity of effects, temporal specificity during various stages of development, or the role of sex hormones in white matter microstructure could even be moderated by the effects of other hormones such as dehydroepiandrosterone (DHEA) (Barendse et al., 2018). Additional research will be necessary to explore exact role of sex hormones in the development of white matter microstructure and how they related to the impact alcohol has on this development.
While this study was the first of its kind to utilize longitudinal DWI data to examine sex differences in white matter microstructure among alcohol using adolescents, there are several limitations to be addressed. First, we obtained blood samples for hormonal assessments during the follicular phase for post-menarcheal girls - a time when estradiol levels are routinely low. This may explain why we did not find an effect of estradiol on the relationship between lifetime alcohol use and white matter microstructure in female adolescents. It is possible that by measuring sex hormones during the luteal phase of the menstrual cycle, when there is greater variability in estradiol levels, we may have detected an effect. Similarly, as many participants in the study had lab visits and neuroimaging scans on separate days, the hormone effects presented in this study more likely represent trait-level associations (as opposed to state-level effects) of sex hormones and white matter microstructure. Further, this analysis did not account for differences in the age of alcohol use initiation or pattern of use (binge drinking vs. more frequent but lower quantity), both of which may differentially affect white matter microstructural development (Cservenka and Brumback, 2017). To appropriately assess the effect of timing of alcohol use initiation on microstructural development, it would require multiple time points both before and after initiation of alcohol use, to appropriately assess changes in developmental trajectories. Unfortunately, the current dataset does not support such an analysis. Finally, these results may not be generalizable to the broader population due to a demographic bias toward predominantly Caucasian participants of high socioeconomic status and IQ. Future work should endeavor to expand upon these findings with larger and more demographically diverse samples, as well as more within-subjects time points to examine the possibility of non-linear developmental trajectories. It may also be worthwhile to incorporate measures of adrenal hormones, as gonadarche and adrenarche are separate developmental processes and findings are mixed as to whether they exert unique influences on white matter microstructure (Herting et al., 2017; Menzies et al., 2015).
In conclusion, the present study expands on our previous work (Jones and Nagel, 2019) by incorporating a larger sample size and directly examining sex-specific effects of alcohol use on white matter microstructure. We demonstrated that 1) for male adolescents, a greater number of lifetime drinking occasions is associated with lower FA in the CC and greater MD in the CC and PTR, but the opposite pattern is observed for female adolescent girls in the CC, and 2) sex-hormones, testosterone and estradiol, partially explain the effect of alcohol use on white matter microstructure in male adolescents. Together, these findings highlight the importance of studying sex-specific effects of alcohol use during adolescence, and contribute to a larger body of research demonstrating alcohol-related effects on adolescent brain development.
Highlights.
Effects of adolescent alcohol use on white matter microstructure are dose-dependent
The association between white matter microstructure and alcohol use varies by sex
Sex hormones partially explain effect of alcohol use on white matter microstructure
Acknowledgements
Past and present members of the Developmental Brain Imaging Lab are thanked for assisting in participant recruitment and data collection. This work was supported by the National Institute on Alcohol Abuse and Alcoholism [Nagel R01 AA017664; Kliamovich T32 AA07468], as well as the National Institute of Health (S10OD021701 and S10OD018224) for the 3T Siemens Prisma MRI instrument and High-Performance Computing Cluster, housed in OHSU’s Advanced Imaging Research Center, and supported by the Oregon Opportunity Partnership for advancing biomedical research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest
All authors declare no conflicts of interest.
References
- Alexander AL, Lee JE, Lazar M, Field AS, 2007. Diffusion tensor imaging of the brain. Neurotherapeutics 4, 316–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson JLR, Sotiropoulos SN, 2016. An integrated approach to correction for off-resonance effects and subject movement in diffusion MR imaging. NeuroImage 125, 1063–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asato MR, Terwilliger R, Woo J, Luna B, 2010. White matter development in adolescence: a DTI study. Cerebral cortex 20, 2122–2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avants BB, Tustison NJ, Song G, Cook PA, Klein A, Gee JC, 2011. A reproducible evaluation of ANTs similarity metric performance in brain image registration. NeuroImage 54, 2033–2044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barendse MEA, Simmons JG, Byrne ML, Seal ML, Patton G, Mundy L, Wood SJ, Olsson CA, Allen NB, Whittle S, 2018. Brain structural connectivity during adrenarche: Associations between hormone levels and white matter microstructure. Psychoneuroendocrinology 88, 70–77. [DOI] [PubMed] [Google Scholar]
- Basser PJ, 1995. Inferring microstructural features and the physiological state of tissues from diffusion-weighted images. NMR Biomed 8, 333–344. [DOI] [PubMed] [Google Scholar]
- Bava S, Jacobus J, Thayer RE, Tapert SF, 2013. Longitudinal changes in white matter integrity among adolescent substance users. Alcoholism, clinical and experimental research 37 Suppl 1, E181–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaulieu C, 2002. The basis of anisotropic water diffusion in the nervous system - a technical review. NMR Biomed 15, 435–455. [DOI] [PubMed] [Google Scholar]
- Brown SA, Myers MG, Lippke L, Tapert SF, Stewart DG, Vik PW, 1998. Psychometric evaluation of the Customary Drinking and Drug Use Record (CDDR): a measure of adolescent alcohol and drug involvement. Journal of studies on alcohol 59, 427–438. [DOI] [PubMed] [Google Scholar]
- Chung T, Creswell KG, Bachrach R, Clark DB, Martin CS, 2018. Adolescent Binge Drinking. Alcohol Res 39, 5–15. [PMC free article] [PubMed] [Google Scholar]
- Clogg CC, Petkova E, Haritou A, 1995. Statistical methods for comparing regression coefficients between models. American Journal of Sociology 100, 1261–1293. [Google Scholar]
- Cox RW, Chen G, Glen DR, Reynolds RC, Taylor PA, 2017. FMRI Clustering in AFNI: False-Positive Rates Redux. Brain connectivity 7, 152–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cservenka A, Brumback T, 2017. The Burden of Binge and Heavy Drinking on the Brain: Effects on Adolescent and Young Adult Neural Structure and Function. Front Psychol 8, 1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortin JP, Parker D, Tunc B, Watanabe T, Elliott MA, Ruparel K, Roalf DR, Satterthwaite TD, Gur RC, Gur RE, Schultz RT, Verma R, Shinohara RT, 2017. Harmonization of multi-site diffusion tensor imaging data. Neuroimage 161, 149–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenbos A, Paus T, Evans AC, Rapoport JL, 1999. Brain development during childhood and adolescence: a longitudinal MRI study. Nature neuroscience 2, 861–863. [DOI] [PubMed] [Google Scholar]
- Gillies GE, McArthur S, 2010. Estrogen actions in the brain and the basis for differential action in men and women: a case for sex-specific medicines. Pharmacol Rev 62, 155–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herting MM, Kim R, Uban KA, Kan E, Binley A, Sowell ER, 2017. Longitudinal changes in pubertal maturation and white matter microstructure. Psychoneuroendocrinology 81, 70–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herting MM, Maxwell EC, Irvine C, Nagel BJ, 2012. The impact of sex, puberty, and hormones on white matter microstructure in adolescents. Cereb Cortex 22, 1979–1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herting MM, Sowell ER, 2017. Puberty and structural brain development in humans. Frontiers in neuroendocrinology 44, 122–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollingshead AB, Redlich FC, 2007. Social class and mental illness: a community study. 1958. Am J Public Health 97, 1756–1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoops D, Flores C, 2017. Making Dopamine Connections in Adolescence. Trends in Neurosciences 40, 709–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobus J, McQueeny T, Bava S, Schweinsburg BC, Frank LR, Yang TT, Tapert SF, 2009. White matter integrity in adolescents with histories of marijuana use and binge drinking. Neurotoxicology and teratology 31, 349–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson WE, Li C, Rabinovic A, 2007. Adjusting batch effects in microarray expression data using empirical Bayes methods. Biostatistics (Oxford, England) 8, 118–127. [DOI] [PubMed] [Google Scholar]
- Jones SA, Morales AM, Nagel BJ, 2019. Resilience to Risk for Psychopathology: The Role of White Matter Microstructural Development in Adolescence. Biol Psychiatry Cogn Neurosci Neuroimaging 4, 180–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones SA, Nagel BJ, 2019. Altered frontostriatal white matter microstructure is associated with familial alcoholism and future binge drinking in adolescence. Neuropsychopharmacology 44, 1076–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kvamme TL, Schmidt C, Strelchuk D, Chang-Webb YC, Baek K, Voon V, 2016. Sexually dimorphic brain volume interaction in college-aged binge drinkers. NeuroImage. Clinical 10, 310–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebel C, Treit S, Beaulieu C, 2019. A review of diffusion MRI of typical white matter development from early childhood to young adulthood. NMR in biomedicine 32, e3778. [DOI] [PubMed] [Google Scholar]
- Luciana M, Collins PF, Muetzel RL, Lim KO, 2013. Effects of alcohol use initiation on brain structure in typically developing adolescents. The American journal of drug and alcohol abuse 39, 345–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacCallum RC, Zhang S, Preacher KJ, Rucker DD, 2002. On the practice of dichotomization of quantitative variables. Psychol Methods 7, 19–40. [DOI] [PubMed] [Google Scholar]
- McQueeny T, Schweinsburg BC, Schweinsburg AD, Jacobus J, Bava S, Frank LR, Tapert SF, 2009. Altered white matter integrity in adolescent binge drinkers. Alcoholism, clinical and experimental research 33, 1278–1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menzies L, Goddings AL, Whitaker KJ, Blakemore SJ, Viner RM, 2015. The effects of puberty on white matter development in boys. Developmental cognitive neuroscience 11, 116–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paternoster R, Brame R, Mazerolle P, Piquero A, 1998. Using the correct statistical test for the equality of regression coefficients. Criminology 36, 859–866. [Google Scholar]
- Peper JS, de Reus MA, van den Heuvel MP, Schutter DJ, 2015. Short fused? associations between white matter connections, sex steroids, and aggression across adolescence. Human brain mapping 36, 1043–1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfefferbaum A, Kwon D, Brumback T, Thompson WK, Cummins K, Tapert SF, Brown SA, Colrain IM, Baker FC, Prouty D, 2018. Altered brain developmental trajectories in adolescents after initiating drinking. American journal of psychiatry 175, 370–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfefferbaum A, Sullivan EV, 2002. Microstructural but not macrostructural disruption of white matter in women with chronic alcoholism. NeuroImage 15, 708–718. [DOI] [PubMed] [Google Scholar]
- Pohl KM, Sullivan EV, Rohlfing T, Chu W, Kwon D, Nichols BN, Zhang Y, Brown SA, Tapert SF, Cummins K, Thompson WK, Brumback T, Colrain IM, Baker FC, Prouty D, De Bellis MD, Voyvodic JT, Clark DB, Schirda C, Nagel BJ, Pfefferbaum A, 2016. Harmonizing DTI measurements across scanners to examine the development of white matter microstructure in 803 adolescents of the NCANDA study. NeuroImage 130, 194–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riccomagno MM, Kolodkin AL, 2015. Sculpting neural circuits by axon and dendrite pruning. Annual review of cell and developmental biology 31, 779–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice JP, Reich T, Bucholz KK, Neuman RJ, Fishman R, Rochberg N, Hesselbrock VM, Nurnberger JI Jr., Schuckit MA, Begleiter H, 1995. Comparison of direct interview and family history diagnoses of alcohol dependence. Alcoholism, clinical and experimental research 19, 1018–1023. [DOI] [PubMed] [Google Scholar]
- Saad ZS, Glen DR, Chen G, Beauchamp MS, Desai R, Cox RW, 2009. A new method for improving functional-to-structural MRI alignment using local Pearson correlation. Neuroimage 44, 839–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz CG, Reid RI, Gunter JL, Senjem ML, Przybelski SA, Zuk SM, Whitwell JL, Vemuri P, Josephs KA, Kantarci K, Thompson PM, Petersen RC, Jack CR Jr., Alzheimer’s Disease Neuroimaging I, 2014. Improved DTI registration allows voxel-based analysis that outperforms tract-based spatial statistics. Neuroimage 94, 65–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmonds DJ, Hallquist MN, Asato M, Luna B, 2014. Developmental stages and sex differences of white matter and behavioral development through adolescence: A longitudinal diffusion tensor imaging (DTI) study. NeuroImage 92, 356–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sisk CL, Foster DL, 2004. The neural basis of puberty and adolescence. Nat Neurosci 7, 1040–1047. [DOI] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE, Niazy RK, Saunders J, Vickers J, Zhang Y, De Stefano N, Brady JM, Matthews PM, 2004. Advances in functional and structural MR image analysis and implementation as FSL. NeuroImage 23 Suppl 1, S208–219. [DOI] [PubMed] [Google Scholar]
- Sobell LC, Brown J, Leo GI, Sobell MB, 1996. The reliability of the Alcohol Timeline Followback when administered by telephone and by computer. Drug and alcohol dependence 42, 49–54. [DOI] [PubMed] [Google Scholar]
- Squeglia LM, Rinker DA, Bartsch H, Castro N, Chung Y, Dale AM, Jernigan TL, Tapert SF, 2014. Brain volume reductions in adolescent heavy drinkers. Developmental cognitive neuroscience 9, 117–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squeglia LM, Sorg SF, Schweinsburg AD, Wetherill RR, Pulido C, Tapert SF, 2012. Binge drinking differentially affects adolescent male and female brain morphometry. Psychopharmacology 220, 529–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squeglia LM, Tapert SF, Sullivan EV, Jacobus J, Meloy MJ, Rohlfing T, Pfefferbaum A, 2015. Brain development in heavy-drinking adolescents. The American journal of psychiatry 172, 531–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoica T, Knight LK, Naaz F, Ramic M, Depue BE, 2019. Cortical morphometry and structural connectivity relate to executive function and estradiol level in healthy adolescents. Brain and behavior 9, e01413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor SJ, Whincup PH, Hindmarsh PC, Lampe F, Odoki K, Cook DG, 2001. Performance of a new pubertal self-assessment questionnaire: a preliminary study. Paediatric and perinatal epidemiology 15, 88–94. [DOI] [PubMed] [Google Scholar]
- Vargas WM, Bengston L, Gilpin NW, Whitcomb BW, Richardson HN, 2014. Alcohol binge drinking during adolescence or dependence during adulthood reduces prefrontal myelin in male rats. Journal of Neuroscience 34, 14777–14782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D, 1999. Wechsler abbreviated scale of intelligence. Psychological Corporation, San Antonio, TX. [Google Scholar]


