SUMMARY
Background
Medical therapy and/or endoscopic balloon dilation with intralesional therapies are options for treatment of small bowel fibrostenotic Crohn’s disease (CD).
Aim
To perform a systematic review summarizing evidence for efficacy of systemic and endoscopic intralesional medical therapy in established small bowel strictures in adult CD patients.
Methods
A systematic search of MEDLINE, EMBASE, CENTRAL, and Scopus was conducted. Primary outcomes were rates of surgical resection and repeat endoscopic dilation. Pooled event rates from random effects models across studies with 95% confidence intervals were reported.
Results
Eleven studies describing systemic medical therapy and eight studies of intralesional injection were included. One randomized controlled trial (RCT) each for systemic therapy and intrastricture injection were identified. Only observational studies were found for systemic biologic therapies, which exclusively included tumor necrosis factor (TNF) antagonists, while intralesional therapies all involved corticosteroids except for one study that evaluated infliximab. Pooled event rates for surgical resection after systemic and intralesional therapy were 27.6% (95% CI: 18.4%−39.3%) and 18.5% (95% CI: 8.3–36.2%), respectively over a median follow up of 23 months (range 5.5–105.8), and 21.8 months (range 5–47). Risk of repeat endoscopic balloon dilation (EBD) in those with intralesional therapy was 58.3% (95% CI: 36.6–77.3%) over a median follow up of 21.8 months (range 5–47).
Conclusions
TNF antagonists are the preferred therapy for patients with stricturing small bowel CD. Data is lacking for ustekinumab and vedolizumab. No endoscopic intralesional medications provided a clear benefit for prevention of repeat EBD or surgery.
Keywords: Stricture, Fibrostenosis, Crohn’s Disease, Biologic therapy, Intralesional therapy
INTRODUCTION
Crohn’s Disease (CD) is a highly heterogeneous form of inflammatory bowel disease (IBD) with distinct phenotypes, including perianal disease, fistulas, and strictures (1). Although these subtypes are challenging to manage, treatment of strictures has been poorly studied and no medical anti-stricture therapies are currently approved (2). Strictures are most common in the terminal ileum, but can also be multifocal, and appear at any gastrointestinal site (1). It is believed that > 50% of CD patients present with clinically apparent strictures over their lifetime (3). Given that no specific anti-fibrotic therapy exists, these patients are often initially treated with the best available medical anti-inflammatory therapy. Strikingly, despite the revolutionary emergence of biologic agents over the past two decades, estimates for surgical resection of fibrostenotic disease either remain unchanged (4), or have shifted from less emergent to more elective procedures (5,6).
Medical therapies for CD strictures may include corticosteroids, purine analogs, methotrexate, and biologic agents. Endoscopic balloon dilation (EBD) is an established modality in CD strictures and acts as a bridge to surgery (7). Intralesional injection of corticosteroids and biologic medications after endoscopic dilatation has also been utilized, but their use is controversial (7).
Although it is generally thought that biologic therapies such as anti-tumor necrosis factor (TNF) agents may provide the greatest benefit in patients with strictures, we sought to analyze and summarize all available studies for all previously tested medical therapies in small bowel CD strictures, while also assessing the number, location, and characteristics of these strictures. In this systematic review, we aim to provide a comprehensive summary of both systemic medical and endoscopic intralesional therapies in already established CD strictures of the small bowel to assess rates of surgical intervention or repeat EBD.
MATERIALS AND METHODS
Search Strategy and Study Selection
This systematic review focused solely on small bowel strictures, since these are the most commonly observed CD lesions (12). Colonic strictures harbor a malignancy risk and accordingly, may not be primarily treated with medical therapy (13).
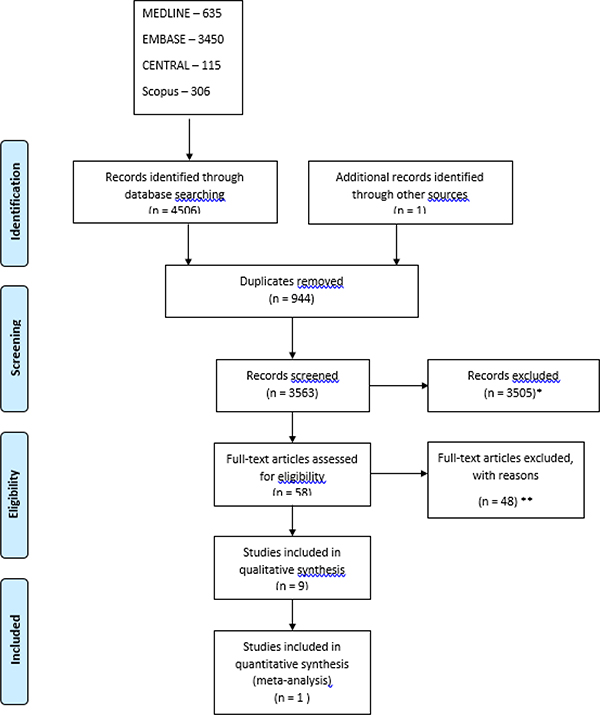
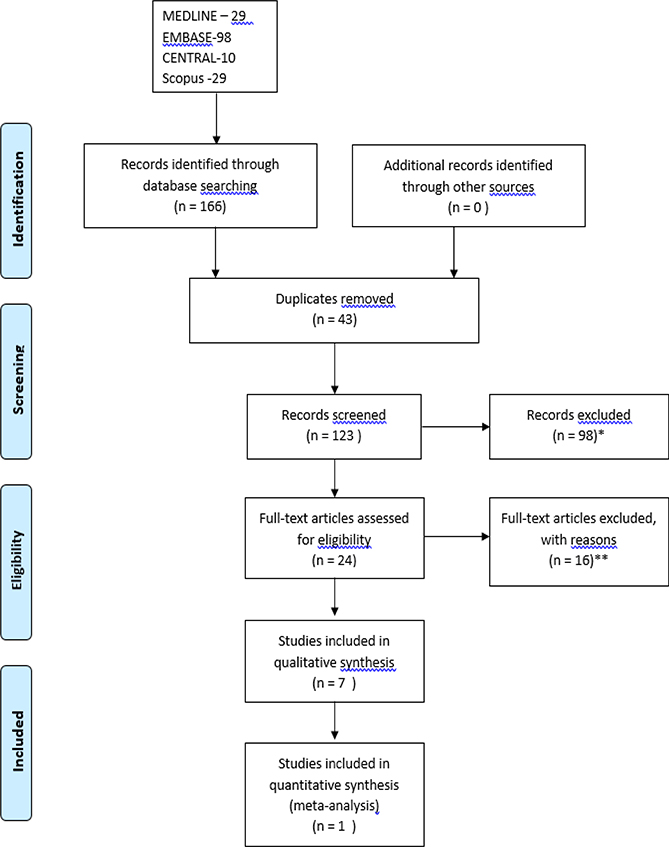
The study methodology, search strategy, and study inclusion criteria are shown in Figures 1 and 2. Separate searches and selection of the retrieved articles were performed to address systemic therapy of already established strictures of the small bowel and endoscopic injection of CD strictures with medications. Citations were identified by searching the following databases from the first date available to May 3, 2019: OVID MEDLINE, EMBASE, and Scopus. Search terms used included the following subject headings plus keywords and synonyms derived from them: Crohn’s Disease, Constriction, Pathologic, Intestinal Obstruction, stricture, inject, intralesion, intrastricture with drugs such as corticosteroid, steroid, infliximab, or adalimumab. The reference lists of studies included for screening (after duplicates removed) were reviewed manually to identify additional relevant publications. Detailed search strings, and search strategies for OVID MEDLINE and EMBASE can be found in Table S1-S4. A non-registered review protocol describing the rationale, hypothesis and planned methods of the review was prepared prior to initiating this systematic review.
Figure 1.
PRISMA flow chart search strategy for systemic medical stricture therapy for small bowel Crohn’s Disease strictures. *Records excluded as review articles, editorials, case reports, Non-Crohn’s disease, book chapters, abstracts, non-English articles. **Records excluded for reasons including: steroid injection (5), pediatric population (4), balloon dilation (7), efficacy of single balloon enteroscopy (1), enteral nutrition (2), tuberculosis medication as intervention (1), absent stricture (post resection) (6), endpoints not specified (2), non-small bowel stricture (19).
Figure 2.
PRISMA flow chart search strategy for endoscopic intralesional medication injection for small bowel Crohn’s Disease strictures. *Records excluded as review articles, case reports, Non-Crohn’s disease, book chapters, abstracts, non-English articles. **Records excluded for reasons including: non-small bowel stricture (pylorus, esophageal, colon, rectum, pouch) (7), pediatric population (2), no use of intralesional therapies after EBD (1), review article (2), peristomal pyoderma gangrenosum (1), ulcerative colitis stricture (1), bowel stent insertion (2).
Inclusion/Exclusion Criteria
Studies were considered eligible if they met the following criteria: (1) study design was to be either an interventional design (randomized or non-randomized), or observational (prospective or retrospective or case-control) studies, (2) adult population (age ≥ 18), (3) sample size of more than 10, (4) established small bowel Crohn’s disease strictures, (5) intralesional therapy in stricture, or systemic therapy. Exclusion criteria were as follows: Strictures outside of the small bowel, non-full text articles, narrative review, studies on postoperative CD, and languages other than English.
Outcomes of Interest
Outcomes of interest were rates of surgical resection following either systemic medical therapy or endoscopic medication injection, and rates of re-dilation of small bowel CD strictures.
Study Selection and Data Extraction
Two reviewers (CL, BB) screened the titles and abstracts that were identified in the search strategy and selected the studies using a predefined strategy according to the eligibility criteria outlined above. Extracted data was entered into an Excel (Microsoft software) database. A third reviewer (FR) was consulted about the eligibility and made the final decision in cases without a consensus. The following variables were extracted from each eligible study: First author name, journal, year of publication, country where study was conducted, study design, number of patients, number of strictures identified, whether definition of stricture was based on endoscopy or radiology, stricture location, intervention, and percentage of primary and secondary endpoints attained. Table 1 and 2 summarizes the extracted data variables.
Table 1.
Summary of Study Details from Literature Review of Medical Therapy of Strictures
| First Author, Year of Publication | Country | Study Design | Number of Patients | Number of Strictures | Stricture Definition (endoscopy/radiology/clinical) | Small Bowel Stricture Location | Follow Up Period | Drug | Surgery | Endoscopic Dilation | Treatment Change |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Amitai, 2018 | Israel | R | 21 | No comment | MRE - wall thickening and luminal narrowing with prestenotic dilatation with a bowel diameter greater than the normal diameter of the bowel. If > 1 stricture, most severe distal ileal stricture was evaluated. | Terminal ileum | 12 months | ADA or IFX | 23.8% (5/21) | 4.7% (1/21) | 14.3% (3/21) |
| Bouhnik, 2018 (CREOLE) | France | P | 97 | ≥ 1 stricture/patient | MRE/endoscopy - constant luminal narrowing with prestenotic dilatation or obstructive signs/symptoms. | duodenum, jejunum or ileum | 24 weeks | ADA | 8.2% (8/97) | 2.0% (2/97) | 36.0% (35/97) |
| Campos, 2017 | France | R | 84 | 13/55 with DI | Obstructive symptoms. CDOS | Ileum, ileocolonic | 60 months | ADA or IFX | 31.0% (26/84) | N/A | N/A |
| Condino, 2013 | Italy | P | 36 (9 fibrostenotic; 2 ileum, 1 ileocolonic) | No comment | SICUS assessment (bowel wall thickness, lumen diameter, bowel dilation and lesion extent) | Ileum, ileocolonic | 23 months | ADA or IFX | 22.2% (2/9) | N/A | N/A |
| De Souza, 2013 | Brazil | RCT | 72 | 1/patient | Obstructive symptoms. X-ray and CT obstruction without abscess. | ileocolonic | 36 months | AZA or mesalamine 3.2 g/day | Mesalamine, 56% (19/34). AZA, 25% (8/32) | N/A | N/A |
| Gibson, 2015 | Ireland | R | 75; 18 fibrostenotic | Not specified | MRE - Small bowel stenosis with or without prestenotic dilatation where stenosis was defined as a reduction of the bowel lumen (diameter ≥80%) lower than that measured in a normal adjacent nonprestenotic loop | Small bowel; not specified | 16.7 months | ADA or IFX | 66.7% (12/18) | N/A | N/A |
| Kim, 2016. | Korea | R | 28 (fibrostenosis on AZA) | Not specified | Imaging or endoscopy | Not specified | 105.8 months | AZA | 24.1% | N/A | N/A |
| Pallotta, 2008 | Italy | P | 15 | 15; 8 patients with 1 stricture, 1 patient with 3 strictures, 1 patient with 4 strictures | Obstructive symptoms, endoscopy - stricture confirmed with inability to pass 11 mm caliber endoscope in terminal and neoterminal ileum, MRI and SICUS. Lumen diameter < 1 cm, measured at the level of maximally distended loop, independent of the presence of prestenotic dilatation; and bowel dilatation - lumen diameter more than 2.5 cm. Regression of stenosis - intestinal lumen with diameter > 1cm on at least 2 follow up SICUS, and confirmed at endoscopy, or MRI for more proximal small bowel stenoses. | 4 Neoterminal ileum, 1 Proximal ileum, 4 Terminal ileum, 1 upper GI tract. | 35 months | IFX | 10% (1/10) | N/A | N/A |
CDOS – Crohn’s disease obstructive score. CT – computed tomography. DI – diagnostic imaging. ADA – adalimumab, AZA – azathioprine, IFX – infliximab. MTX – methotrexate. 6MP- mercaptopurine. R –retrospective. P – prospective. RCT – randomized controlled trial, SASA – sulfasalazine. SICUS – small intestinal contrast ultrasound.
Table 2.
Summary of Study Details from Literature Review of Endoscopic Intralesional Therapy of Small Bowel Crohn’s Disease Strictures
| First Author, Year of Publication | Country | Study Design | Number of Patients | Stricture Definition (endoscopy/radiology/clinical) | CD/UC | IPAA Associated Stricture (Yes/No) | Stricture Length (cm) | Intervention (Medicaiton/Dilation/Surgery, %) | Median Follow Up Period | Complications | Outcomes (Surgery/Repeat EBD) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Atreja, 2014 | USA | R | 128; 44 intralesional steroid | CTE, MRE, endoscopy | 1369 | Upper GI (2.4%), Jejunum (0%), Ileum (77.5%) | 2.7 ± 1.9 | Balloon | 1.8 years | 36% (16/44) | No difference with EBD only patients |
| Brooker, 2003 | UK | R | 14 | Endoscopy | N/A | Ileocolonic (9), ileorectal (1), Ileum (1), left colon (2), one patient had ileocolonic and 3 colonic strictures. | Maximum 7cm | Balloon | 16.4 months (single EBD), 28 months (multiple EBD) | 25.0% (3/12) | 25.0% (3/12) |
| East, 2007 | UK | RCT | 13 (7 steroid, 6 saline placebo) | Barium small bowel follow through | N/A | Ileocolonic anastomosis | 2.0 | Balloon ± guide-wire | 52 weeks | N/A, study prematurely terminated. | 71.4% (5/7; steroid), 16.7% (1/6;placebo) |
| Foster, 2008 | USA | R | 24 (22 adults and 2 pediatric patients) 17 received steroid) | Endoscopy | N/A | 12 anastomotic, 16 de novo (4 colon, 5 rectum, 1 ileocecal valve, 6 small bowel) | < 4 | Balloon | 29 months | 11.8% (2/17) | 54% (13/24) |
| Hendel, 2014 | Belgium | P | 6 | MRI, endoscopy | 1/patient | Jejunum, 16.7% (1/6), Ileum 16.7% (1/6), duodenal bulb 16.7% (1/6), Ileocolic anastomosis 50% (3/6) | < 5 | Balloon | 6 months | 0 | 83.3% (5/6) received ≥ 2 EBD during 6 months. |
| Lian, 2015 | USA | R | 185; 35 intralesional steroid | CT, CTE, MRE, barium | 1/patient | ileocolic | N/A | Balloon | 3.9 years (mean) | 37.1% (13/35) | N/A |
| Ramboer, 1995 | Belgium | P | 13 | Endoscopy | 1/patient | ileocolic anastomosis (9/13) neoterminal ileum (2/13) | N/A | Balloon | 47 months (mean) | 0 | 84.6% (11/13) required repeat EBD. |
| Singh, 2005 | USA | R | 17; 7 fibrostenotic small bowel; 5 patients intralesional steroid | Endoscopy | 20 | Ileal (1/7), 3 (ileocolonic), 3 (duodenal bulb) | 2 | Balloon | 19 months (mean) | 20% (1/5) | 20% (1/5) |
EBD – endoscopic balloon dilation, IFX – infliximab
Risk of Bias in Individual Studies
The quality of randomized studies was analyzed using domain-based risk of bias tables as recommended by the Cochrane Collaboration (14). The studies were assessed across six domains that may be subject to bias, including sequence generation, allocation concealment, blinding, incomplete outcome data, selective outcome reporting, and other sources of bias (Table S5 and S6). The quality of non-randomized studies was assessed by using the Newcastle-Ottawa Scale (15). The quality of the studies were examined for: 1) Selection, 2) Comparability, 3) Outcome (Table S7 and S9). The Grading of Recommendations Assessment, Development and Evaluation (GRADE) system was then used to determine the quality of the evidence as high, moderate, low, or very low (Table S8 and S10) (16).
Statistical Analysis and Summary Measures
Treatment failure was defined as requiring EBD and/or surgical intervention. A generalized linear mixed model (GLMM), which is a random intercept logistic regression model, was utilized for pooling in the meta-analysis of proportions (17), where logit transformations of proportions were implemented to calculate the overall proportion. Clopper-Pearson confidence intervals, also called the exact binomial intervals, were calculated for the single proportions of individual study results.
The rates of surgery or EBD of individual studies were combined into a pooled events ratio using a random-effects meta-analysis model. Due to the variation among study designs, a random-effects model was utilized, rather than a fixed-effects model. Outcomes were reported as pooled event rates (95% confidence interval limits), or as unweighted proportions of the size of the population studied. Measures of consistency were calculated using I2 to assess the within subgroups heterogeneity of effect estimates and t2 to estimate the between-study variance. Forest plots were utilized to assess heterogeneity across studies visually, and numerically (I2 < 25% represents low heterogeneity). Publications that were biased were excluded from data synthesis. Continuous numerical data was reported as means with standard deviations, or as medians with range values. Meta package in the statistical software, R, (Version 3.6.1, made available on 2019–07-05) was used for calculations and plotting.
RESULTS
Search Results for Systemic Medical Therapy
Search strategy results can be found in Tables S1 and S2. A total of 11 studies were included in the qualitative analysis: 7 retrospective, 3 prospective, and one randomized controlled study (RCT).
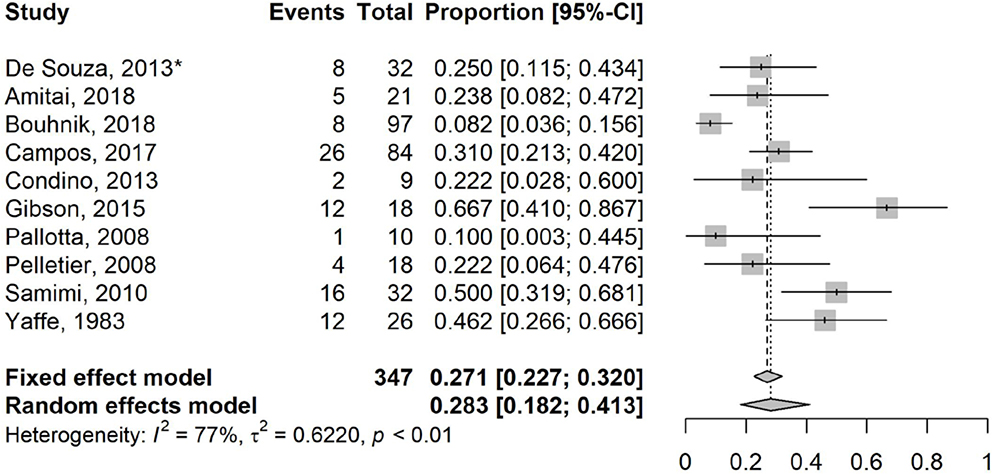
Overall, substantial statistical heterogeneity was identified with a pooled surgical rate of 27.6% (95% CI: 18.4%−39.3%; I2 75%, t2 56.3%, p<0.01) (18–28) over a median follow-up of 23 months (range 5.5 – 105.8; Figure 3). Four studies included patients with ileocolonic strictures (20–22,25), while two studies included upper gastrointestinal strictures (19,25) (Table 1).
Figure 3.
Forest Plot for studies reporting on surgical rate of small bowel Crohn’s disease strictures treated with systemic agents. Random effects model demonstrating a pooled event rate for surgical resection of 27.6% (95% CI: 18.4%−39.3%; I2 75%, t2 56.3%) over a median follow up time of 23 months (range 5.5–105.8). *Randomized controlled trial and only study not involving biologic agents.
Mesalamine:
The only RCT (investigator blinded) of a systemic therapy included 72 patients with partial small bowel obstruction (SBO), who received intravenous hydrocortisone 100mg every 8 hours over a 72 hour period. Patients who responded were randomized to mesalamine (3.2 g/day) or azathioprine (AZA, 2–3mg/kg). Standardized corticosteroid taper was mandated and patients were followed for up to 3 years (22). AZA treated patients had lower surgery rates (25 vs. 56%, respectively; p=0.01), hospital admissions (61% vs. 83.3%; p=0.03) and mean hospital stay duration (3.8±4.7 days vs. 7.7±5.2 days; p=0.002) compared to mesalamine. However, this study was not double blinded, leading to potential for bias.
Corticosteroids:
In one observational study of 26 CD patients over 7 years, corticosteroid use in acute SBO relieved symptoms in all, but one patient. However, 18 patients, who had symptom recurrence within 16 to 106 months, were treated a second time with corticosteroids and all of them experienced symptom resolution. The overall surgery rate in the cohort was high (46%; 12/26) with the main determinant of surgery being the symptom-free interval such that if this was <8 months, the rate was 85.7% (6/7) (28). Thus, while corticosteroid use is effective for short term symptom relief, it conveys a poor prognosis that designates a high risk for obstruction and surgery.
Thiopurines:
In a retrospective South Korean study, 1157 patients were divided into those that received: 1) early immunosuppressive therapy (EIT) within 6 months of CD diagnosis or 2) conventional therapy (24). Within a mean observation duration of 105.8±51.5 months, the EIT group had a lower probability of intestinal surgery than the conventional group (24.1% vs. 36.4%, respectively; p<0.001), suggesting early thiopurine introduction was effective in reducing fibrostenosis progression. However, this study had several limitations. First, lack of randomization indicates that results are susceptible to bias and inability to control for confounders. Second, only 14% of patients had a stricturing phenotype, meaning that the majority had inflammatory behaviour (>70%). It is reasonable to assume that many surgeries were to control inflammatory disease that was refractory to medical therapy as opposed to surgery for fibrostenotic complications. Thus, cautious interpretation regarding the efficacy of thiopurines on already established strictures is in order.
TNF antagonists:
No RCTs have evaluated the efficacy of TNF antagonists in CD strictures. Overall, two studies analysed use of infliximab (25,29), 1 assessed adalimumab (19), and 5 reported outcomes for both TNF antagonists (18,20,21,23,27). Of these studies, only one assessing infliximab (25), one adalimumab (19), and one including both TNF antagonists (21) were prospective. A description of the retrospective studies can be found in Supplementary information and prospective studies can be found below.
Pallota et al. prospectively studied 15 patients with obstructive symptoms treated with infliximab (25). Small intestinal contrast ultrasound (SICUS) was performed at induction and at 6 month intervals during follow up of 34.7±16.1 months (range 7–58). There was complete regression of 8/15 stenoses (53.3%) after maintenance (6 to 22 infusions) therapy. Furthermore, no strictures progressed and no new strictures occurred. One patient (10%) with small bowel stenosis required surgery. A second prospective study measured the frequency of SBOs in CD strictures after infliximab or adalimumab (21). 25% (9/36) of patients enrolled had a fibrostenotic phenotype, of which two (22.2%) had only ileal involvement and subsequently developed a partial or complete bowel obstruction requiring surgical resection between 16 to 80 weeks post TNF antagonist initiation.
The largest, multicenter, prospective study examining established symptomatic small bowel strictures evaluated 97 patients treated with adalimumab (CREOLE) (19). Strictures were defined as “occurrence of constant luminal narrowing with prestenotic dilation or obstructive signs/symptoms” identified on MRE or endoscopy (19). The primary endpoint was adalimumab continuation without any need for corticosteroids, other TNF antagonists, EBD, or bowel resection at week 24. The primary endpoint was met in 64% of patients with symptomatic strictures. Continued adalimumab treatment was successful in 29% of patients after a median follow up of 3.8±0.1 years. Overall, 50% of patients did not require bowel resection 4 years after study inclusion. Prognostic factors independently related with treatment success were use of immunosuppressives at adalimumab initiation, obstructive symptoms for <5 weeks, a CD obstructive score >4, and MRE small bowel stricture length of <12 cm, a maximal prestenotic diameter of 18–29 mm, marked delayed phase enhancement, and absence of a fistula.
Other Therapies:
No identified studies reported the outcome of methotrexate, ustekinumab or vedolizumab therapy on small bowel CD strictures.
Search Results for Intralesional Medical Therapy
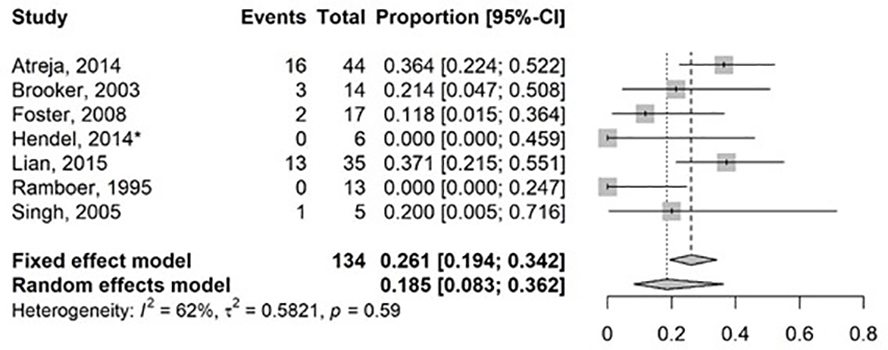
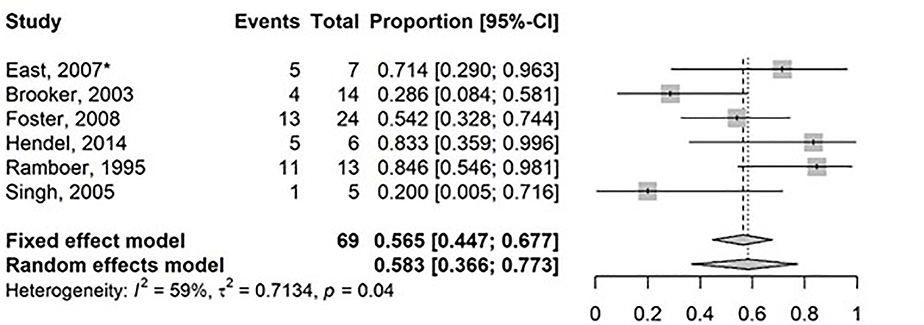
Search results can be found in Figure 2 and Table 2 (30–45). Substantial statistical heterogeneity was demonstrated with a pooled surgical rate of 18.5% (95% CI: 8.3%−36.2%; I2 62%, t2 58.2%, p=0.59) over a median of 21.8 months (range 5–47), for 7 studies (6 intralesional steroid, 1 intralesional infliximab) that reported surgical resection of small bowel CD strictures with injected therapy as an outcome (Figure 4). Similarly, heterogeneity was substantial among 6 studies that reported a repeat EBD rate following prior EBD and intralesional therapy of 58.3% (95% CI: 36.6–77.3%; I2 59%, t2 71.3%, p=0.04) over a median of 21.8 months (range 5–47) (Figure 5). Retrospective studies that evaluated intralesional injection can be found in Supplementary information and prospective studies are described below.
Figure 4.
Forest Plot for studies reporting on surgical rate of small bowel Crohn’s disease strictures treated with intralesional medications post endoscopic balloon dilation. Random effects model demonstrating a pooled event rate for surgical resection 18.5% (95% CI: 8.3–36.2%, I2: 62%, t2 58.2%) over a median follow up time of 21.8 months (range 5–47). *intralesional infliximab, all other studies utilized intralesional corticosteroids.
Figure 5.
Forest Plot for studies reporting on repeat endoscopic balloon dilation of small bowel Crohn’s disease strictures treated intralesional agents. Random effects model demonstrating a pooled event rate for balloon dilation of 58.3% (95% CI: 36.3%−77.3%; I2: 59%, t2 71.3%) over a median follow up of 21.8 months (range 5–47). *Randomized controlled trial.
Corticosteroids:
The only RCT of intralesional steroids in small bowel stenosis (46) evaluated 13 patients with anastomotic ileocolonic stricture ≤ 5 cm in length. Patients were randomized to receive either quadrantic intralesional triamcinolone or saline. Primary endpoint was time to repeat dilation or surgery for obstructive symptoms. Although the study was intended to be double blinded, the endoscopist may have been unblinded with luminal steroid leakage (milky appearance) during injection. No patients received concomitant systemic biologic therapy. In the steroid group, 43% (3/7) of patients and 33% (2/6) of the placebo group were on prednisolone or azathioprine. This trial was prematurely terminated due to a trend toward re-dilation in the steroid group (5/7) versus the placebo group (1/6) in an intention to treat (ITT) analysis (statistically significant in the per-protocol analysis). Study stopping criteria were not pre-specified.
In the only prospective study to evaluate intralesional steroid injection following EBD of ileocolic (9/13) or ileal (2/13) strictures, no patients required surgery over a mean of 47 months, while 84.6% (11/13) required repeat EBD. All patients were concurrently treated with mesalamine and seven were on methylprednisolone (2.5–10mg). Due to lack of a control group, there is no definitive evidence that steroid injection after EBD is superior to EBD alone in preventing surgery (47).
TNF antagonists :
Six patients with a confirmed (MRI and endoscopy) stricture with recurrent obstructive bowel symptoms received intralesional infliximab injections (10 mg week 0, 2 and 6) with 6 month follow-up (48). Three patients had ileocolonic anastomotic strictures, while each of the other 3 patients had a jejunal, ileal, or duodenal stricture length of < 5 cm. All patients had a decrease in mean SES-CD scores by 3 (one to five points). No patients required surgery and all patients described obstructive symptom improvement.
DISCUSSION
Fibrostenotic CD is a common and challenging clinical problem. For a significant number of patients, repeat endoscopic dilation or surgery is needed as medical therapy is unlikely to reverse bowel damage and fibrosis. In the absence of anti-fibrotic therapy, management depends on stricture morphometrics such as location, length, concurrent complications, and patient preference (49). Strictures with a dominant inflammatory component are initially treated with medical therapy. This systematic review summarized evidence for the efficacy of systemic medical therapies, as well as endoscopic intralesional medical injections in patients with established small bowel stricturing CD. Pooled event rate for surgery for systemic and intralesional therapy was based upon data from eleven and seven studies, respectively. Notably the majority of these were open label observational studies. The statistical heterogeneity of the studies of systemic therapy was substantial (I2>25) with surgical rates ranging from 10% to 67% for TNF antagonists. The studies that evaluated intralesional therapy post EBD showed that repeat EBD or surgery was required in 58.3% and 18.5%, respectively within a median follow up of 21.8 months.
Our search revealed only one RCT each for systemic and intralesional therapy. The single RCT for systemic therapy did not evaluate the efficacy of biologics, but rather compared AZA to mesalamine. This study showed superiority of AZA over mesalamine with an absolute reduction in rates for surgical intervention of 31% over a 3 year period (22). The study was susceptible to bias due to a lack of blinding. No subsequent studies have validated this finding. Thus, there is insufficient evidence to conclude that thiopurine therapy is effective for treatment of fibrostenosing CD complications. In addition, as mesalamine is not a recommended therapy for small bowel CD as shown in several meta-analyses, this RCT does not impact clinical practice (50–52).
Although our results support the value of systemic corticosteroids only for obstructive symptoms, the long-term use of corticosteroids is limited by both tolerability and serious adverse events. Furthermore, while corticosteroids relieve symptoms through inflammation suppression, there is limited evidence regarding their effects on mucosal healing (53). Granting these data might be considered the basis for a potential benefit of corticosteroids in fibrostenosis, the pathogenesis is poorly understood and it is difficult to tease out specific anti-fibrotic benefits of corticosteroid from the clinical data we reviewed. Moreover, no studies have explicitly studied the role of corticosteroid monotherapy for small bowel CD strictures. Yaffe et al. assessed the role of intermittent prednisone with sulfasalazine following an index episode of ileal obstruction and found that patients without recurrence of obstruction in 8 months, required less resections (28). The lack of a control group renders this finding difficult to interpret with respect to the efficacy of either agent.
In the three prospective studies evaluating TNF antagonists in established small bowel CD, surgical rates vary with the highest being 22.2% over 23 months (21). Surgical rates were the most favorable in CREOLE; the largest study following 97 patients showing that at week 24, 64% of patients continued adalimumab without prohibited treatment (steroids, other TNF antagonists), and did not require EBD or surgery. The other available two studies enrolled less than 40 patients (21,25). Strengths of all of these studies include the use of diagnostic imaging with clear stenosis definitions and relatively long follow up of at least 2 years. However, limitations are that these are uncontrolled observational studies.
The efficacy of TNF antagonists for fibrostenosing disease has been controversial in the past. Earlier concerns that infliximab could increase the risk of complete bowel obstruction in strictures through acceleration of the healing process with subsequent fibrosis and obstruction (54) are now debunked (25,26,55,56). In 20 CD patients with fibrostenotic disease, SICUS found no progression or development of new strictures, and complete regression of stenosis in 80% of patients after 6 to 22 infliximab infusions (25). Likewise, the results of both the TREAT (The Crohn’s Therapy, Resource, Evaluation, and Assessment Tool) registry and ACCENT I (A Crohn’s Disease Clinical Trial Evaluating Infliximab in a New Long-Term Treatment Regimen) did not support the notion of infliximab as a risk for stricture formation or obstruction. Interestingly in TREAT, disease duration, severity, isolated ileal disease, and new corticosteroid use were positively associated with an increased risk of obstruction-related events (55). Finally, in CREOLE, more than half of patients did not have surgery 4 years after adalimumab initiation (19). Again, although the lack of a control group prevents making any definitive conclusions, the results are consistent with an overall benefit of adalimumab and do not support the concept that TNF antagonist administration worsens fibrostenosis.
Although the results from CREOLE are positive, the evidence for the efficacy of TNF antagonist therapy in CD fibrostenosis and impact on surgical intervention remains unclear. In a population-based time trend analysis between 2002–2010, declining surgical rates in CD for intestinal resections along with a shift from elective to emergent operations was reported (5). However, in a recent published population-based interrupted time series study of the effect of introduction of infliximab in Ontario, Canada between 1995 and 2012, population rates of intestinal CD resections have not significantly declined, despite robust market use of biologics (57). The authors hypothesized that misguided use and failure to optimize use of infliximab were contributing factors for these results. A limitation of this study was the absence of detailed clinical data. In other words, the proportion of patients with concurrent strictures and fistulizing disease is unclear. It has been shown that although the rates of intestinal resection have declined with the introduction of infliximab, the rates of small bowel fistula repair have increased significantly (58). In CREOLE, patients without a concurrent fistula as observed in 75% of the study population were associated with success on adalimumab without dilation or surgery.
Other therapies currently approved for CD include methotrexate, ustekinumab and vedolizumab (59–61). However, patients with symptomatic strictures were excluded from pivotal phase III studies. At this time, there is no evidence to support the use of ustekinumab in stricturing CD. Similarly, it is difficult to draw definitive conclusions from the VICTORY consortium study, which assessed vedolizumab use in a real-life setting (61). 118/212 (55.7%) of patients included had strictures or fistulas. After 12 months, resection was required in two patients with small bowel strictures. Although, the available evidence favors the use of systemic TNF antagonists in obstructing CD, its effect of improvement is likely mediated by an anti-inflammatory efficacy. Novel biologics, as long as they show comparable or better anti-inflammatory effects will likely show comparable or better efficacy, but data to date is lacking.
It is generally thought that surgical resection or stricturoplasty are ultimately necessary to resolve symptomatic obstruction in fibrostenotic CD. EBD is a favorable and commonly used modality to treat CD strictures. However, many patients do require a second EBD or surgery following a successful dilation (32). Unfortunately, the addition of intralesional steroids or biologics following EBD has not dramatically changed the natural history. One recent systematic review addressing outcomes of EBD in stricturing CD have shown that after 24 months of follow up from EBD, 73.5% of patients require a re-dilation and 42.9% surgical resection (62). The authors concluded that strictures injected with triamcinolone did not have any difference in short or long term outcomes, or complication rates compared to those without injections. At this time, there is no strong evidence supporting the use of intralesional medications with EBD in small bowel CD strictures. Most available studies that evaluated intralesional medications are limited by small sample size, lack of a control group, and heterogeneous location of de novo and anastomotic strictures. Thus, meaningful recommendations from the current data are difficult to make on the basis of this evidence.
Only two randomized placebo controlled studies (one adult (46), one pediatric (38)) compared intralesional steroids to saline in patients who had failed medical therapy. In adult patients, quadrantic triamcinolone injection after EBD of CD ileocolonic anastomotic strictures was compared to placebo (46). The study was prematurely terminated due to a perceived higher need for re-dilation in the steroid group. However, stopping criteria for this study were not pre-specified. This study was not adequately blinded with the masking of injected syringe contents. The authors cautioned the use of corticosteroid injection until more data are available. In keeping with this recommendation, routine application of intralesional steroids with EBD either is not endorsed by current guidelines (63), or not mentioned for CD management (8). Conversely, in the first and only pediatric RCT on intralesional steroids after EBD, the ITT analysis showed 1/15 patients needed redilation in the steroid group compared to 5/14 receiving placebo. Furthermore, 4/14 receiving placebo required surgery compared to 0/15 receiving steroids. Overall, this study concluded that intralesional steroids are an effective strategy in decreasing redilation and surgery rates.
Only one case series of six patients has evaluated intralesional TNF antagonist injections. An improvement in symptoms and the modified SES-CD was noted in all who received intralesional infliximab injections (48). Study limitations include a short 6 month follow up period, and no prior systemic infliximab exposure, limiting the generalizability of the results. Most importantly, despite the promising results, the lack of randomization, blinding and a control group make definitive conclusions regarding intervention efficacy difficult and no recommendations are possible until well-conducted, controlled studies are available.
Our study is the first systematic review to evaluate the rates of surgical resection or repeat EBD in both systemic medical or endoscopic intralesional therapies in established small bowel CD strictures. However there were several important limitations, primarily due to the quality and quantity of available data. The primary weakness is the paucity of high quality RCTs. Comparisons across studies were particularly challenging with stricture characteristics, follow up length, and medication therapy duration being highly variable and/or not fully reported. Population size was utilized to calculate primary outcomes for this study. As the studies were heterogeneous and there was a lack of RCTs, a meta-analysis could not be performed and caution in the interpretation of the data is warranted. Another important limitation is that most studies analyzed in this systematic review combined de-novo strictures and anastomotic ileocolic strictures. Post-surgical strictures may have a component of ischemia that may affect their responsiveness to systemic and local therapies. Anastomotic CD strictures have been described to have better long-term outcomes in EBD than de novo strictures (64). Furthermore, few studies reported dietary changes, which may be a confounding factor in the rapidity with which surgery or EBD was required.
In conclusion, this systematic review has detected a significant need for controlled studies of both systemic medical and intralesional therapies for small bowel CD strictures. Despite not tested in a randomized controlled fashion, the strongest support exists for systemic TNF antagonists. Intralesional steroid therapies do not provide long term benefit and may be harmful (46). Therefore, the need for controlled studies with biologic therapies, and specifically the need for anti-fibrotic therapies in stricturing CD are substantial. The Stenosis Therapy and Anti-fibrotic Stricture Consortium created definitions and targets for stricture therapy (49) and is currently developing validated clinical trial endpoints to test them.
Supplementary Material
Acknowledgments
iv. Kerri Novak has received honoraria for consultation/ speaker’s bureau with Janssen, Abbvie, Takeda, Shire, Pendopharm, and Ferring. She has received research support from Abbvie. Educational support has been received from Abbvie, Janssen, and Takeda.
vi. Remo Panaccione has receiving consulting fees from Abbvie Abbott, Amgen, Aptalis, AstraZeneca, Baxter, BMS, Celgene, Cubist, Eisai, Ferring, Gilead, Janssen, Merck, Robarts, Salix, Samsung,, Shire, Centocor, Elan, Glaxo-Smith Kline, UCB, Pfizer, Takeda. RP has been on the speaker’s bureau for Abbvie, Abbott, Aptalis, AstraZeneca, Ferring, Janssen, Merck, Prometheus, Shire, Takeda. Advisory Board participation for Abbvie, Abbott, Amgen, Aptalis, AstraZeneca, Baxter, Eisai, Ferring, Genentech, Jansen, Merck, Schering-Plough, Shire, Centocor, Elan, Glaxo-Smith Kline, UCB, Pfizer, Bristol-Myers Squibb, Takeda, Cubist, Celgene, Salix. RP has received Research/Educational Support from Abbvie, Abbott, Ferring, Janssen, Takeda
vii. David Bruining has received research support and consulting agreement with Medtronics.
ix. Brian Feagan has received grant/research support from AbbVie Inc., Amgen Inc., AstraZeneca/MedImmune Ltd., Atlantic Pharmaceuticals Ltd., Boehringer-Ingelheim, Celgene Corporation, Celltech, Genentech Inc/Hoffmann-La Roche Ltd., Gilead Sciences Inc., GlaxoSmithKline (GSK), Janssen Research & Development LLC., Pfizer Inc., Receptos Inc./Celgene International, Sanofi, Santarus Inc., Takeda Development Center Americas Inc., Tillotts Pharma AG and UCB; consulting fees from Abbott/AbbVie, Akebia Therapeutics, Allergan, Amgen, Applied Molecular Transport Inc., Aptevo Therapeutics, Astra Zeneca, Atlantic Pharma, Avir Pharma, Biogen Idec, BioMx Israel, Boehringer-Ingelheim, Bristol-Myers Squibb, Calypso Biotech, Celgene, Elan/Biogen, EnGene, Ferring Pharma, Roche/Genentech, Galapagos, GiCare Pharma, Gilead, Gossamer Pharma, GSK, Inception IBD Inc, JnJ/Janssen, Kyowa Kakko Kirin Co Ltd., Lexicon, Lilly, Lycera BioTech, Merck, Mesoblast Pharma, Millennium, Nestle, Nextbiotix, Novonordisk, Pfizer, Prometheus Therapeutics and Diagnostics, Progenity, Protagonist, Receptos, Salix Pharma, Shire, Sienna Biologics, Sigmoid Pharma, Sterna Biologicals, Synergy Pharma Inc., Takeda, Teva Pharma, TiGenix, Tillotts, UCB Pharma, Vertex Pharma, Vivelix Pharma, VHsquared Ltd. and Zyngenia; speakers bureau fees from Abbott/AbbVie, JnJ/Janssen, Lilly, Takeda, Tillotts and UCB Pharma; is a scientific advisory board member for Abbott/AbbVie, Allergan, Amgen, Astra Zeneca, Atlantic Pharma, Avaxia Biologics Inc., Boehringer-Ingelheim, Bristol-Myers Squibb, Celgene, Centocor Inc., Elan/Biogen, Galapagos, Genentech/Roche, JnJ/Janssen, Merck, Nestle, Novartis, Novonordisk, Pfizer, Prometheus Laboratories, Protagonist, Salix Pharma, Sterna Biologicals, Takeda, Teva, TiGenix, Tillotts Pharma AG and UCB Pharma; and is the Senior Scientific Officer of Robarts Clinical Trials Inc.; all of these activities are outside the submitted work.
Declaration of funding interests: This work was supported by the Helmsley Charitable Trust through the Stenosis Therapy and Anti-Fibrotic Research (STAR) Consortium.
Abbreviations:
- ADA
Adalimumab
- AZA
Azathioprine
- cm
Centimetres
- CD
Crohn’s Disease
- CI
Confidence interval
- CDAI
Crohn’s Disease Activity Index
- EBD
Endoscopic balloon dilation
- IBD
Inflammatory bowel disease
- IFX
Infliximab
- ITT
Intention to treat
- MRE
Magnetic Resonance Enterography
- MRI
Magnetic Resonance Imaging
- RCT
Randomized Controlled Trial
- SBO
Small bowel obstruction
- SICUS
Small intestinal contrast ultrasound
- SES
Simple endoscopic score
- TNF
Tumor necrosis factor
Footnotes
STATEMENT OF INTERESTS
Authors’ declaration of personal interests:
Cathy Lu has received consulting fees from AbbVie, Janssen, Takeda; speaker’s fees from AbbVie, Janssen.
ii. Brandon Baraty, Helen Lee Robertson, Alexis Filyk, Tak Fung, and Jean-Paul Achkar have no conflicts of interests to declare.
Hua Shen is supported by the Discovery Grant (RGPIN-2016-04594) from the National Sciences and Engineering Research Council of Canada.
Christopher Ma has received consulting fees from AbbVie, Janssen, Pfizer, and Robarts Clinical Trials, Inc; speaker’s fees from AbbVie, Janssen, Pfizer, Takeda.
Vipul Jairath has received has received consulting fees from AbbVie, Eli Lilly, GlaxoSmithKline, Arena pharmaceuticals, Genetech, Pendopharm, Sandoz, Merck, Takeda, Janssen, Robarts Clinical Trials, Topivert, Celltrion; speaker’s fees from Takeda, Janssen, Shire, Ferring, Abbvie, Pfizer
x. Florian Rieder is a consultant to Allergan, AbbVie, Boehringer-Ingelheim, Celgene, Cowen, Gilead, Gossamer, Helmsley, Janssen, Koutif, Metacrine, Morphic, Pliant, Pfizer, Receptos, RedX, Roche, Samsung, Takeda, Thetis, UCB.
REFERENCES
- 1.Satsangi J, Silverberg MS, Vermeire S, et al. The Montreal classification of inflammatory bowel disease: controversies, consensus, and implications. Gut 2006;55:749–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rieder F, Latella G, Magro F, et al. European Crohn’s and Colitis Organisation Topical Review on Prediction, Diagnosis and Management of Fibrostenosing Crohn’s Disease. J. Crohn’s Colitis 2016;10:873–885. [DOI] [PubMed] [Google Scholar]
- 3.Thia KT, Sandborn WJ, Harmsen WS, et al. Risk factors associated with progression to intestinal complications of Crohn’s disease in a population-based cohort. Gastroenterology 2010;139:1147–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lazarev M, Uliman T, Schraut WH, et al. Small bowel resection rates in Crohn’s disease and the indication for surgery over time: Experience from a large tertiary care center. Inflamm. Bowel Dis. 2010;16:830–835. [DOI] [PubMed] [Google Scholar]
- 5.Ma C, Moran GW, Benchimol EI, et al. Surgical Rates for Crohn’s Disease are Decreasing: A Population-Based Time Trend Analysis and Validation Study. Am. J. Gastroenterol. 2017;112:1840–1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fu YTN, Hong T, Round A, et al. Impact of medical therapy on patients with Crohn’s disease requiring surgical resection. World J. Gastroenterol. 2014;20:11808–11814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bessissow T, Reinglas J, Aruljothy A, et al. Endoscopic management of Crohn’s strictures. World J. Gastroenterol. 2018;24:1859–1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gomollón F, Dignass A, Annese V, et al. 3rd European Evidence-based Consensus on the Diagnosis and Management of Crohn’s Disease 2016: Part 1: Diagnosis and Medical Management. J. Crohn’s Colitis 2017;11:3–25. [DOI] [PubMed] [Google Scholar]
- 9.Rieder F, Zimmermann EM, Remzi FH, et al. Crohn’s disease complicated by strictures: a systematic review. Gut 2013;62:1072–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Klag T, Wehkamp J, Goetz M. Endoscopic balloon dilation for crohn’s disease-associated strictures. Clin. Endosc. 2017;50:429–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morar PS, Faiz O, Warusavitarne J, et al. Systematic review with meta-analysis: endoscopic balloon dilatation for Crohn’s disease strictures. Aliment. Pharmacol. Ther. 2015;42:1137–1148. [DOI] [PubMed] [Google Scholar]
- 12.Peyrin-Biroulet L, Loftus EV, Colombel J-F, et al. The Natural History of Adult Crohn’s Disease in Population-Based Cohorts. Am. J. Gastroenterol. 2009;105:289–297. [DOI] [PubMed] [Google Scholar]
- 13.Sonnenberg A, Genta RM. Epithelial Dysplasia and Cancer in IBD Strictures. J. Crohn’s Colitis 2015;9:769–775. [DOI] [PubMed] [Google Scholar]
- 14.Collaboration. TC. No Title [Internet]. Cochrane Handb. Syst. Rev. Interv. 2011;[cited 2019 Jun 15] Available from: https://training.cochrane.org/handbook [Google Scholar]
- 15.Wells G, Shea BOD et al. No Title [Internet]. Newcastle-Ottawa Scale Assess. Qual. nonrandomised Stud. meta-analyses 2011;Available from: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp
- 16.Guyatt GH, Oxman AD, Vist GE, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336:924–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stijnen T, Hamza TH, Ozdemir P. Random effects meta-analysis of event outcome in the framework of the generalized linear mixed model with applications in sparse data. Stat. Med. 2010;29:3046–67. [DOI] [PubMed] [Google Scholar]
- 18.Amitai MM, Klang E, Levartovsky A, et al. Diffusion-weighted magnetic resonance enterography for prediction of response to tumor necrosis factor inhibitors in stricturing Crohn’s disease. Abdom. Radiol. 2018;43:3207–3212. [DOI] [PubMed] [Google Scholar]
- 19.Bouhnik Y, Carbonnel F, Laharie D, et al. Efficacy of adalimumab in patients with Crohn’s disease and symptomatic small bowel stricture: a multicentre, prospective, observational cohort (CREOLE) study. Gut 2018;67:53–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Campos C, Perrey A, Lambert C, et al. Medical Therapies for Stricturing Crohn’s Disease: Efficacy and Cross-Sectional Imaging Predictors of Therapeutic Failure. Dig. Dis. Sci. 2017;62:1628–1636. [DOI] [PubMed] [Google Scholar]
- 21.Condino G, Calabrese E, Zorzi F, et al. Anti-TNF-alpha treatments and obstructive symptoms in Crohn’s disease: a prospective study. Dig. Liver Dis. 2013;45:258–62. [DOI] [PubMed] [Google Scholar]
- 22.de Souza GS, Vidigal FM, Chebli LA, et al. Effect of azathioprine or mesalazine therapy on incidence of re-hospitalization in sub-occlusive ileocecal Crohn’s disease patients. Med. Sci. Monit. 2013;19:716–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gibson DJ, Murphy DJ, Smyth AE, et al. Magnetic resonance enterography findings as predictors of clinical outcome following antitumor necrosis factor treatment in small bowel Crohn’s disease. Eur. J. Gastroenterol. Hepatol. 2015;27:956–62. [DOI] [PubMed] [Google Scholar]
- 24.Kim B, Cheon JH, Moon HJ, et al. Crohn’s disease prognosis and early immunomodulator therapy: Results from the CONNECT study. J. Gastroenterol. Hepatol. 2016;31:126–132. [DOI] [PubMed] [Google Scholar]
- 25.Pallotta N, Barberani F, Hassan NA, et al. Effect of infliximab on small bowel stenoses in patients with Crohn’s disease. World J. Gastroenterol. 2008;14:1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.PELLETIER A-L, KALISAZAN B, WIENCKIEWIC ZJ, et al. Infliximab treatment for symptomatic Crohn’s disease strictures. Aliment. Pharmacol. Ther. 2009;29:279–285. [DOI] [PubMed] [Google Scholar]
- 27.Samimi R, Flasar MH, Kavic S, et al. Outcome of medical treatment of stricturing and penetrating Crohnʼs disease. Inflamm. Bowel Dis. 2010;16:1187–1194. [DOI] [PubMed] [Google Scholar]
- 28.Yaffe BH, Korelitz BI. Prognosis for Nonoperative Management of Small-Bowel Obstruction in Crohnʼs Disease. J. Clin. Gastroenterol. 1983;5:211–216. [DOI] [PubMed] [Google Scholar]
- 29.Pelletier A-L, Kalisazan B, Wienckiewicz J, et al. Infliximab treatment for symptomatic Crohn’s disease strictures. Aliment. Pharmacol. Ther. 2009;29:279–85. [DOI] [PubMed] [Google Scholar]
- 30.Frist Michael, Scott J, Kim R, Borum M. Balloon dilation with corticosteroid injections a durable endoscopic option for Crohn’s-related pyloric stenosis. Inflamm. Bowel Dis. 2011;17:S53. [Google Scholar]
- 31.Irani S, Jalaj S, Ross A, et al. Use of a lumen-apposing metal stent to treat GI strictures (with videos). Gastrointest. Endosc. 2017;85:1285–1289. [DOI] [PubMed] [Google Scholar]
- 32.Bettenworth D, Gustavsson A, Atreja A, et al. A Pooled Analysis of Efficacy, Safety, and Long-term Outcome of Endoscopic Balloon Dilation Therapy for Patients with Stricturing Crohn’s Disease. Inflamm. Bowel Dis. 2017;23:133–142. [DOI] [PubMed] [Google Scholar]
- 33.De Felice KM, Katzka DA, Raffals LE. Crohn’s Disease of the Esophagus: Clinical Features and Treatment Outcomes in the Biologic Era. Inflamm. Bowel Dis. 2015;21:2106–13. [DOI] [PubMed] [Google Scholar]
- 34.Teich N, Wallstabe I, Schiefke I. Topic infliximab injection for refractory rectal stenosis in Crohn’s disease: long-term follow-up in two patients. Int. J. Colorectal Dis. 2017;32:1289–1294. [DOI] [PubMed] [Google Scholar]
- 35.Fumery M, Patel NS, Boland BS, et al. Efficacy and Safety of Endoscopic Balloon Dilatation of Ileoanal Pouch Strictures. Inflamm. Bowel Dis. 2018;24:1316–1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shen B, Lian L, Kiran RP, et al. Efficacy and safety of endoscopic treatment of ileal pouch strictures. Inflamm. Bowel Dis. 2011;17:2527–35. [DOI] [PubMed] [Google Scholar]
- 37.Shen B, Fazio VW, Remzi FH, et al. Endoscopic balloon dilation of ileal pouch strictures. Am. J. Gastroenterol. 2004;99:2340–2347. [DOI] [PubMed] [Google Scholar]
- 38.Di Nardo G, Oliva S, Passariello M, et al. Intralesional steroid injection after endoscopic balloon dilation in pediatric Crohn’s disease with stricture: a prospective, randomized, double-blind, controlled trial. Gastrointest. Endosc. 2010;72:1201–8. [DOI] [PubMed] [Google Scholar]
- 39.Foster EN, Quiros JA, Prindiville TP. Long-term Follow-up of the Endoscopic Treatment of Strictures in Pediatric and Adult Patients With Inflammatory Bowel Disease. J. Clin. Gastroenterol. 2008;42:880–885. [DOI] [PubMed] [Google Scholar]
- 40.Arebi N, Hart AL, Thomas-Gibson S. A review of endoscopic balloon dilatation techniques for treating Crohn’s strictures: time to standardise therapy. Expert Rev. Gastroenterol. Hepatol. 2016;10:1101–1107. [DOI] [PubMed] [Google Scholar]
- 41.Teich N Failure of sublesional infliximab injection for refractory parastomal pyoderma gangrenosum in a patient with Crohn’s disease. Tech. Coloproctol. 2014;18:965–6. [DOI] [PubMed] [Google Scholar]
- 42.Spiceland CM, Lodhia N. Endoscopy in inflammatory bowel disease: Role in diagnosis, management, and treatment. World J. Gastroenterol. 2018;24:4014–4020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shivashankar R, Edakkanambeth Varayil J, Scott Harmsen W, et al. Outcomes of Endoscopic Therapy for Luminal Strictures in Crohn’s Disease. Inflamm. Bowel Dis. 2018;24:1575–1581. [DOI] [PubMed] [Google Scholar]
- 44.Loras C, Pérez-Roldan F, Gornals JB, et al. Endoscopic treatment with self-expanding metal stents for Crohn’s disease strictures. Aliment. Pharmacol. Ther. 2012;36:833–9. [DOI] [PubMed] [Google Scholar]
- 45.Taida T, Nakagawa T, Ohta Y, et al. Long-Term Outcome of Endoscopic Balloon Dilatation for Strictures in Patients with Crohn’s Disease. Digestion 2018;98:26–32. [DOI] [PubMed] [Google Scholar]
- 46.East JE, Brooker JC, Rutter MD, et al. A Pilot Study of Intrastricture Steroid Versus Placebo Injection After Balloon Dilatation of Crohn’s Strictures. Clin. Gastroenterol. Hepatol. 2007;5:1065–1069. [DOI] [PubMed] [Google Scholar]
- 47.Ramboer C, Verhamme M, Dhondt E, et al. Endoscopic treatment of stenosis in recurrent Crohn’s disease with balloon dilation combined with local corticosteroid injection. Gastrointest. Endosc. 1995;42:252–255. [DOI] [PubMed] [Google Scholar]
- 48.Hendel J, Karstensen JG, Vilmann P. Serial intralesional injections of infliximab in small bowel Crohn’s strictures are feasible and might lower inflammation. United Eur. Gastroenterol. J. 2014;2:406–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rieder F, Bettenworth D, Ma C, et al. An expert consensus to standardise definitions, diagnosis and treatment targets for anti-fibrotic stricture therapies in Crohn’s disease. Aliment. Pharmacol. Ther. 2018;48:347–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lim W-C, Hanauer S. Aminosalicylates for induction of remission or response in Crohn’s disease. Cochrane database Syst. Rev. 2010;CD008870. [DOI] [PubMed] [Google Scholar]
- 51.Lim W-C, Wang Y, MacDonald JK, et al. Aminosalicylates for induction of remission or response in Crohn’s disease. Cochrane database Syst. Rev. 2016;7:CD008870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ford AC, Kane SV, Khan KJ, et al. Efficacy of 5-aminosalicylates in Crohn’s disease: systematic review and meta-analysis. Am. J. Gastroenterol. 2011;106:617–29. [DOI] [PubMed] [Google Scholar]
- 53.Olaison G, Sjodahl R, Tagesson C. Glucocorticoid treatment in ileal Crohn’s disease: relief of symptoms but not of endoscopically viewed inflammation. Gut 1990;31:325–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vasilopoulos Sotirios, Subra Kugathasan KS. Intestinal strictures complicating initially successful infliximab treatment for luminal Crohn’s disease. Am. J. Gastroenterol. 2000;95:2503. [Google Scholar]
- 55.Lichtenstein GR, Olson A, Travers S, et al. Factors Associated with the Development of Intestinal Strictures or Obstructions in Patients with Crohn’s Disease. Am. J. Gastroenterol. 2006;101:1030–1038. [DOI] [PubMed] [Google Scholar]
- 56.BOUGUEN G, TROUILLOUD I, SIPROUDHIS L, et al. Long-term outcome of non-fistulizing (ulcers, stricture) perianal Crohn’s disease in patients treated with infliximab. Aliment. Pharmacol. Ther. 2009;30:749–756. [DOI] [PubMed] [Google Scholar]
- 57.Murthy SK, Begum J, Benchimol EI, et al. Introduction of anti-TNF therapy has not yielded expected declines in hospitalisation and intestinal resection rates in inflammatory bowel diseases: A population-based interrupted time series study. Gut 2020;69:274–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jones DW, Finlayson SRG. Trends in surgery for crohn’s disease in the era of infliximab. Ann. Surg. 2010;252:307–312. [DOI] [PubMed] [Google Scholar]
- 59.Rouiller-Braunschweig C, Fournier N, Pittet V, et al. Efficacy, Safety and Mucosal Healing of Methotrexate in a Large Longitudinal Cohort of Inflammatory Bowel Disease Patients. Digestion 2017;96:220–227. [DOI] [PubMed] [Google Scholar]
- 60.Feagan BG, Sandborn WJ, Gasink C, et al. Ustekinumab as Induction and Maintenance Therapy for Crohn’s Disease. N. Engl. J. Med. 2016;375:1946–1960. [DOI] [PubMed] [Google Scholar]
- 61.Dulai PS, Singh S, Jiang X, et al. The Real-World Effectiveness and Safety of Vedolizumab for Moderate–Severe Crohn’s Disease: Results From the US VICTORY Consortium. Am. J. Gastroenterol. 2016;111:1147–1155. [DOI] [PubMed] [Google Scholar]
- 62.Bettenworth D, Gustavsson A, Atreja A, et al. A Pooled Analysis of Efficacy, Safety, and Long-term Outcome of Endoscopic Balloon Dilation Therapy for Patients with Stricturing Crohn’s Disease. Inflamm. Bowel Dis. 2017;23:133–142. [DOI] [PubMed] [Google Scholar]
- 63.Shergill AK, Lightdale JR, Bruining DH, et al. The role of endoscopy in inflammatory bowel disease. Gastrointest. Endosc. 2015;81:1101–1121.e13. [DOI] [PubMed] [Google Scholar]
- 64.Endo K, Takahashi S, Shiga H, et al. Short and long-term outcomes of endoscopic balloon dilatation for Crohn’s disease strictures. World J. Gastroenterol. 2013;19:86–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.