Abstract
BACKGROUND
Coronary artery disease (CAD) is the most common type of cardiovascular disease. Increasing the expression and activity of matrix metalloproteinases (MMPs) facilitates vascular remodeling and cardiovascular complications. Curcumin (the active ingredient of turmeric) is a potent natural anti-inflammatory agent, with cardiovascular protective effects. The present study was a clinical trial for investigating the effects of curcumin on activity and gene expression of MMP-2 and MMP-9 in patients with CAD.
METHODS
In this study, 70 patients with CAD (with 40%-50% stenosis) were randomly divided into two groups of curcumin (80 mg nanomicelle per day) and placebo. The intervention lasted 3 months. The activity levels of MMP-2 and MMP-9 in serum samples of patients were measured using gelatin zymography assay before and after the intervention. MMP-2 and MMP-9 gene expression in peripheral blood mononuclear cells (PBMCs) was also analyzed using real-time polymerase chain reaction (PCR). Statistical significance was set at P < 0.0500.
RESULTS
After 3 months of medication, the expression of MMP-9 produced by PBMCs significantly decreased in the curcumin group (0.811 ± 0.25) in comparison with the placebo group (2.23 ± 0.94) (P < 0.0001). Furthermore, the zymographic analysis showed that the administration of curcumin significantly inhibited the activity levels of MMP-2 (12469.7 ± 5308.64 pixels) and MMP-9 (14007.2 ± 5371.67 pixels) in comparison with that in patients receiving placebo (MMP-2: 17613.8 ± 5250.68 pixels; MMP-9: 20010.1 ± 3259.37 pixels) (P < 0.0500).
CONCLUSION
Our results show that curcumin can significantly reduce the expression and activity of MMP-2 and MMP-9. Because of the anti-inflammatory effects of curcumin, this compound can be considered as a new strategy for the prevention of cardiovascular events.
Keywords: Curcumin, Matrix Metalloproteinases, Coronary Artery Disease
Introduction
Coronary artery disease (CAD) is an epidemic disease that will be the single most important disease in terms of mortality, disability, and cost in the world until 2020.1 Atherosclerosis is currently considered as a chronic and progressive disease in the onset of which the important pathological mechanisms of oxidative stress within the vessel wall and processes are involved.2
The atherosclerotic lesion is formed due to lipoprotein particles accumulation in the intima of the coronary artery wall and gradually develops into the fibrous plaque, which is rich in extracellular matrix (ECM) proteins. It has now been well established that among many proteases, matrix metalloproteinases (MMPs) are the key enzymes in the transformation of the ECM in physiological and pathological conditions that involve inflammatory processes, such as arthritis, cancer, periodontal diseases, and cardiovascular disease (CVD).3
MMPs are a homologous family of calcium-dependent and zinc-containing endopeptidases. Gelatinases (MMP-2, MMP-9) are a subgroup of MMPs which have attracted much attention due to their electrolytic capabilities and elevated levels in cardiovascular lesions.4 The decomposition of ECM components through the activity of these enzymes reduces the size of the plaque and increases its instability. In addition, gelatinases increase the infiltration of immune-inflammatory cells through direct breaking down of the base membrane and provide the conditions for angiogenesis by stimulating the proliferation of endothelial cells. All of these factors are associated with the growth of plaque and its increased sensitivity to rupture.5 During the accumulation of platelets, MMP-2 is transferred from the cytosol to the platelets and is released. Therefore, MMP-2 is involved not only in the formation of plaque, but also in its development through its effect on platelet aggregation and the formation of thrombosis. Interestingly, most risk factors ultimately lead to the activation of MMPs. Cigarette smoking, diabetes, high homocysteine, and high lipid uptake cause oxidative stress in intima and media layers of the arteries and ultimately activate MMPs.6 Given the crucial role of MMPs in atherosclerosis, agents that can suppress MMPs have the potential to be preventive factors in the development of atherosclerosis.
The last decade has witnessed a growing interest in the use of plant-derived products known as phytochemicals. These compounds are used as preventative and therapeutic agents in a wide range of diseases.7
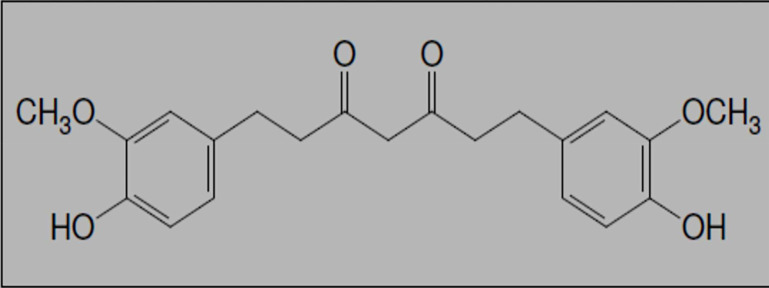
One such agent, curcumin or diferuloylmethane (Figure 1), the main component of turmeric, has been shown to be pharmacologically safe and to be involved in the suppression of MMPs in molecular researches. It also suppresses the effects of various inflammatory stimuli. Today, in order to increase the biocompatibility of this compound, new strategies are being used such as the use of adjuvants like Piperine, which prevent the glucuronidation of curcumin, and use of liposomal curcumin, and curcumin nanoparticles and analogs.8,9 In curcumin nanoparticles, all particles of curcumin have been captured in the hydrophobic section of nanomicelles. These nanomicelles increase the solubility of curcumin in water. After oral administration, soft gel remain intact in the acidic stomach and enter the small intestine. The transfer of curcumin from the water layer at the epithelial cell surface is facilitated by these nanomicelles and the oral absorption of curcumin is increased.10,11
Figure 1.
Chemical structure of curcumin
According to recent reports, curcumin is highly pleiotropic, and thus, it can interact with many contributing molecules in the inflammation pathway. Curcumin regulates transcription factors, cytokines, kinases, sticky molecules, regenerative status, and enzymes associated with inflammation.12 Since irregular inflammatory response plays a major role in the pathogenesis of many CVDs, especially atherosclerosis, the inhibition of inflammatory pathways may be one of the protective mechanisms of curcumin in the cardiovascular system.13
Singh and Aggarwal were the first to show that curcumin suppresses the activation of nuclear factor (NF)-β from various inflammatory stimuli.14 The suppression of this factor suppresses the expression of NF-α-dependent genes that mediate proliferation, invasion, and angiogenesis.15
In this study, we tried to find out whether the anti-inflammatory effect of nanomicelle curcumin also involves the inhibition of these MMPs by studying the effect of nanomicelle curcumin on the production of MMP-2 and MMP-9 by peripheral blood mononuclear cells (PBMCs) and their activity in the serum of atherogenic patients with CAD who receive curcumin (medical follow-up indication) as add-on-therapy.
Materials and Methods
The nanomicelle capsules of curcumin (Exir Nano Sina Company, Tehran, Iran), were developed in the Nanotechnology Research Center of Mashhad University of Medical Sciences, Mashhad, Iran (IRC: 1228225765). Ribonucleic acid (RNA) isolation kit, complementary DNA (cDNA) synthesis kit, and real-time polymerase chain reaction (PCR) kit were purchased from Pars Toos Company, Iran. All other reagents and sterile plastic products were purchased from reliable companies.
The present study was a double-blind randomized clinical trial. All patients and the cardiologist, who followed the patients, were blinded to the treatment until the end of the study.
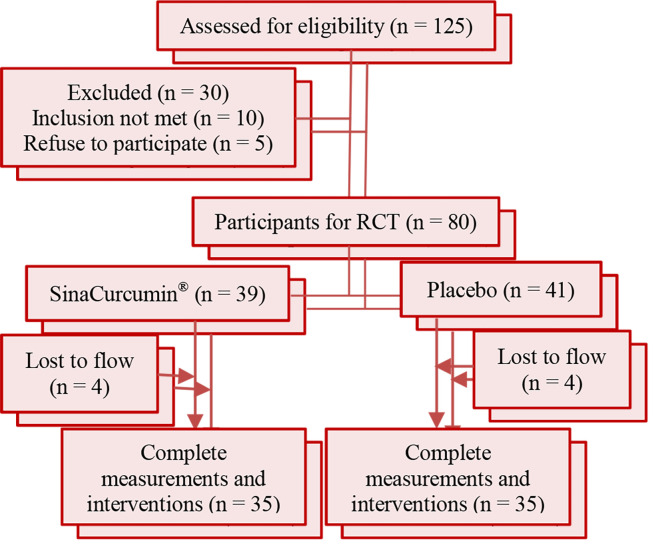
Subjects who were included in this study were men and women over the age of 18 years who were diagnosed with CAD by the selected cardiologist and consented to participate in this study. Based on angiographic findings, 70 patients with CAD (less than 50% obstruction in their coronary arteries) were selected from the department of cardiology of Ghaem Hospital, Mashhad, Iran. All subjects were randomly assigned to either the nanocurcumin (as nanomicelle 80 mg/day) or placebo-treated group (control condition) using a fixed randomization scheme based on random numbers provided by a computer software. After the screening process, randomization procedures, and diet and lifestyle training were done face-to-face. The subjects were informed that two interventions were being evaluated according to figure 2.
Figure 2.
Flow chart of participation and study design
RCT: Randomized controlled trial
The shape and size and all other parameters of the placebo were the same as the other group; the same company provided them for us, without drug as control condition. Sample size was calculated using the frequency data of previous studies,16-18 with 80% power, 5% level of significance, and a SD of 35.3. We enrolled at least 35 subjects in each treatment group.
This study was approved by the Ethics Committee of Mashhad University of Medical Sciences. A double-blind, randomized, placebo-controlled, add-on clinical trial (the curcumin was added to the recommended medical therapies; Registration code: IRCT2013081114330N1) was conducted in Mashhad University of Medical Sciences.
Procedures and variables assessment
Inclusion and exclusion criteria: Subjects who were included in the present study were diagnosed with CAD by the selected cardiologist, men and women of older than 18 years of age, and they understood the study procedures and agreed to participate in the study. The exclusion and inclusion criteria were similar to those described in the study by Rahimi et al.19
Blood samples were obtained an early morning after overnight fasting 3 months after the end of treatment. Blood samples (10 ml) were collected into plain Vacutainer™ tubes, and then, centrifuged (4000 RPM, 4 minutes) for plasma and blood cell separation.
The treatment group received the nanomicelle capsules of curcumin at a dose of 80 mg/day for 3 months. The second group received capsules containing glycerin as a placebo at the same time. At the beginning and end of the treatment period, 10 ml blood samples were collected into plain heparin Vacutainer™ tubes. The samples were evaluated for the activity and expression of MMP-2 and MMP-9. Blood samples were processed using Ficoll to collect the PBMCs; 5 ml of blood sample was added to centrifuge tubes containing 5 ml of Ficoll. After centrifugation for 20 minutes at a rate of 2000 RPM, the plasma layer and PBMC layer could be isolated to evolute the enzyme activity and expression, respectively.
RNA isolation and Real-Time PCR: RNA isolation, cDNA synthesis, and Real-Time PCR were done.
After the total RNA extraction from PBMCs according to the instructions provided on the kit, cDNA synthesis was performed using the cDNA synthesis kit according to the company's instructions. Real-time PCRs were carried out to evaluate the expression of the MMP-2 and MMP-9 genes. Duplicate tests were performed for each gene. The primers used in this experiment are presented in table 1. These primers were designed using the software Primer3 and PubMed database. All results of gene expression were normalized against the β-actin as housekeeping gene. The relative fold change of messenger RNA of MMP2, 9 calculated with 2-ΔΔ CT method. The data used as a relative quantification strategy for quantitative real-time polymerase chain reaction analysis and compared with other groups.
Table 1.
Primer sequences used for the quantitative polymerase chain reaction
| Primer sequence | Gene name |
|---|---|
| 5'-AACTACGATGACGACAGCAAGT-3' | Forward primer for MMP-2 |
| 5' -AGGTGTAAATGGGTCCCATCA-3' | Reverse primer for MMP-2 |
| 5' -CCTGCCAGTTTCCATTCATC-3' | Forward primer for MMP-9 |
| 5' -GCCATTCACGTCGTCCTTAT-3' | Reverse primer for MMP-9 |
| 5'-GATCAAGATCATTGCTCCTCCTG-3' | Forward primer for β-actin |
| 5'-CAAGAAAGGGTGTAACGCAACT-3' | Reverse primer for β-actin |
Zymography: Zymography is an electrophoretic technique used for the detection of hydrolytic enzymes. The sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE) was copolymerized with a protein substrate such as gelatin, casein, or fibrin. Gelatin zymography was used to detect enzyme degrading gelatin, MMP-2, and MMP-9. The gelatin embedded in polyacrylamide gel digested by active gelatinases (Refolded enzyme) was run through the gel. After Coomassie Blue staining, the areas of the gel digested by the enzyme are visible as clear bands against a darkly stained background.20
To detect the activity of the enzymes, serum specimens in each group were mixed with no reducing sample buffer containing 10% w/v SDS, and were electrophoretically resolved through 30% w/v polyacrylamide gels copolymerized with 1% w/v gelatin substrate. The samples were subjected to electrophoresis in substrate gel. After electrophoresis, gels were washed 3 times for 15 minutes each in 2.5% Triton X-100 at room temperature to remove the SDS from the gel, incubated for 18 hours in substrate buffer (50 mM Tris-HCl, 1 mM CaCl2, 0.02% NaN3, 1.5 mM NaCl, and 0.02% Triton X-100, pH 7.5) at 37 °C, stained with Coomassie blue R-250, and destained with ethanol and acetic acid solution. Gelatinase activities were visualized as clear bands in a blue background. To detect bands of MMP-2 and MMP-9, the band's position was compared with the protein marker (Figure 3). Intensity was measured using ImageJ software (LOCI, University of Wisconsin, USA). This software provides the quantization of the bands by counting the number of pixels and plotting the curve, and then, calculating the area under the curve.20
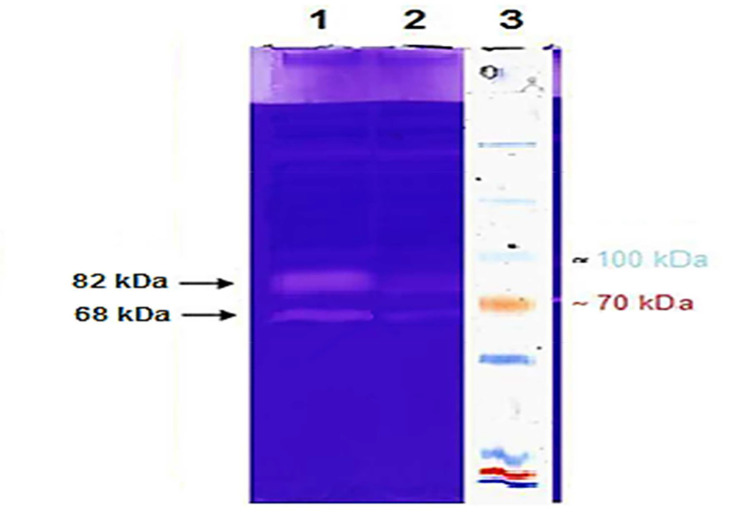
Figure 3.
Gelatin zymography on serum samples of patients with coronary artery disease
Equal concentration of serum samples from patients with coronary artery disease were used in gelatin zymography. Lane 1: Bands from the patient's serum before the intervention; Lane 2: bands from the patient's serum after the intervention; Lane 3: a protein marker
Matrix metalloproteinases (MMP-9) and MMP-2 have a molecular weight of 82 and 68 kDa, respectively. The consumption of curcumin for 3 months significantly reduced the activity of the MMP-2 and MMP-9 enzymes.
All statistical analyses were performed using SPSS software (version 11.5, SPSS Inc., Chicago, IL, USA). Data were expressed as Mean ± SEM [or Median (IQR)]. The normality of data distribution was determined using Kolmogorov-Smirnov test. All quantitative variables were normally distributed. Unpaired t-test was used for comparison of quantitative variables between the two groups. For comparison of these variables before and after the intervention, paired t-test was used. Before the intervention, by adjusting the base value in the two groups, analysis of covariance (ANCOVA) was performed. Moreover, P < 0.0500 was considered statistically significant.
Results
Demographic data: The collected data on the 70 patients with CAD revealed no significant difference in age and sex between the treatment and control groups (P > 0.0500) (Table 2).
Table 2.
Baseline demographic characteristics of the study population and enzyme activity in the two groups before the intervention
| Variable | Groups |
P | ||
|---|---|---|---|---|
| Nanocurcumin (n = 35) | Placebo (n = 35) | |||
| Sex | Male | 17 (48.5) | 14 (40.0) | 0.5180 |
| Female | 18 (51.5) | 21 (60.0) | ||
| BMI (kg/m2) | Normal | 3 (8.5) | 5 (14.3) | > 0.9990 |
| Overweight | 26 (74.2) | 25 (71.4) | ||
| Obesity | 6 (17.3) | 5 (14.3) | ||
| Smoking habit | Current | 4 (11.5) | 3 (8.6) | 0.9490 |
| Former | 10 (28.5) | 9 (25.7) | ||
| Never | 21 (60.0) | 23 (65.7) | ||
| Hypertension [n (%)] | Yes | 18 (51.5) | 21 (60.0) | 0.5180 |
| No | 17 (48.5) | 14 (40.0) | ||
| Age (year) | 56.34 ± 11.17 | 60.95 ± 10.77 | 0.1320 | |
| MMP-2 Enzyme activity (pixels) | 18743.80 ± 5785.70 | 17826.20 ± 6597.70 | 0.6220* | |
| MMP-9 Enzyme activity (pixels) | 23102.40 ± 5582.70 | 20353.50 ± 3327.40 | 0.0700* | |
BMI: Body mass index; MMP-2: Matrix metalloproteinase-2; MMP-9: Matrix metalloproteinase-9
Data are presented as Mean ± standard deviation (SD) or n (%).
No significant difference existed in the activity of enzymes (MMP-2 and MMP-9) before the intervention between the placebo and curcumin groups.
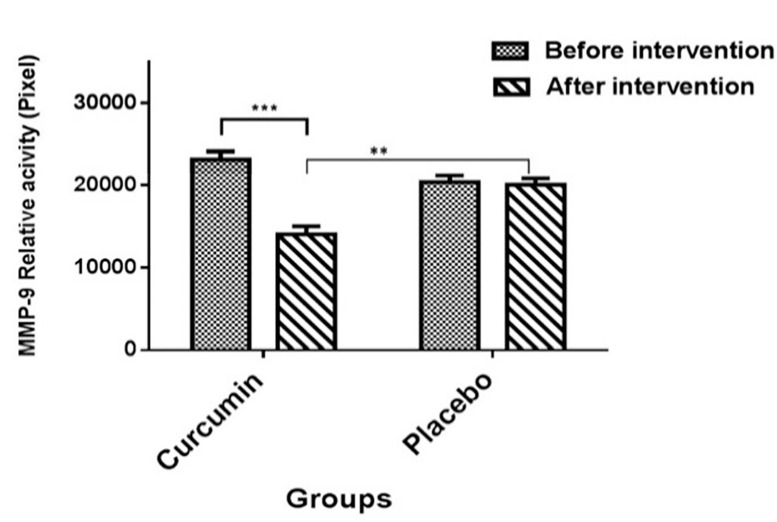
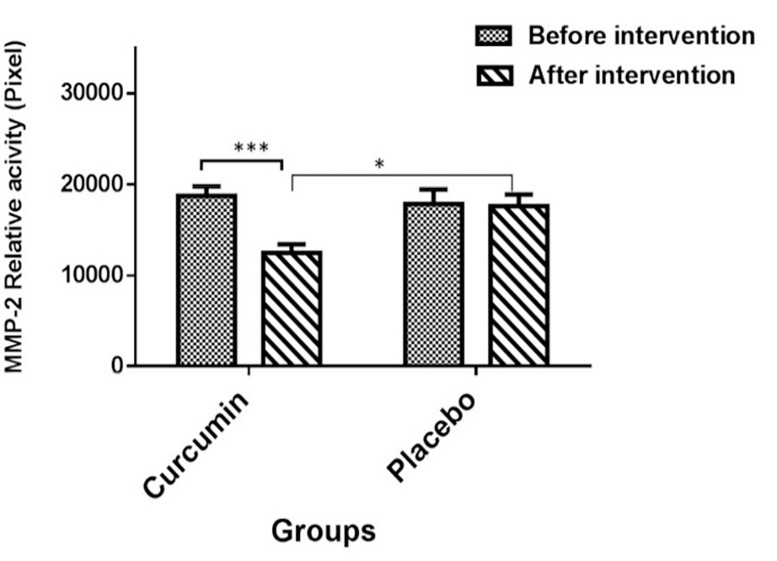
Effect of curcumin on matrix metalloproteinase-9 and matrix metalloproteinas-2 activity: Based on the results of zymography, the mean value of MMP-9 levels in the curcumin and placebo groups was 14007.2 ± 5371.67 and 20010.1 ± 3259.37 pixels, respectively. As shown in figure 4, no significant difference was observed in the mean relative activity of MMP-9 between curcumin and placebo groups before the intervention (P > 0.0500). However, the mean levels of MMP-9 activity were significantly lower in the curcumin treatment group in comparison to the placebo group after the intervention (P < 0.0010).
Figure 4.
Comparison of the mean matrix metalloproteinase-9 relative activity according to pixels in the two groups (Placebo and curcumin) before and after the intervention
Data are expressed as mean ± standard error of the mean (SEM). Paired t-test showed that matrix metalloproteinase-9 (MMP-9) activity was lower in the curcumin group after the intervention (***P < 0.0010); however, no difference was observed in the placebo group after the intervention compared to before the intervention (P > 0.0500). Enzyme activity after the intervention in the curcumin group was lower than the placebo group (**P < 0.0100).
The results of zymography showed that the mean value of MMP-2 level in the curcumin and placebo groups after the intervention was 12469.7 ± 5308.64 and 17613.8 ± 5250.68 pixels, respectively. As illustrated in figure 5, the mean relative activity of MMP-2 showed a significant decline after the intervention. Furthermore, zymographic findings showed a decrease in the values of MMP-2 gelatinase activity in the group receiving curcumin (P < 0.0010).
Figure 5.
Comparison of the mean matrix metalloproteinase-2 relative activity according to pixels in the two groups (Placebo and curcumin) before and after the intervention
Data are expressed as mean ± SEM. Paired t-test showed that matrix metalloproteinase-9 (MMP-9) activity was lower in the curcumin group after the intervention (***P < 0.0010); however, no difference was seen in the placebo group after the intervention compared to before the intervention (P > 0.0500). The activity of this enzyme was lower in the curcumin group compared to the placebo group after the intervention (*P < 0.0500).
The activity of MMP-9 did not differ in the placebo group (P > 0.0500). The activity of this enzyme in the curcumin group was lower than the placebo group after the intervention (P < 0.0500). No significant difference was observed in the activity of enzymes (MMP-2 and MMP-9) between the placebo and nanocurcumin groups before the intervention (Table 2).
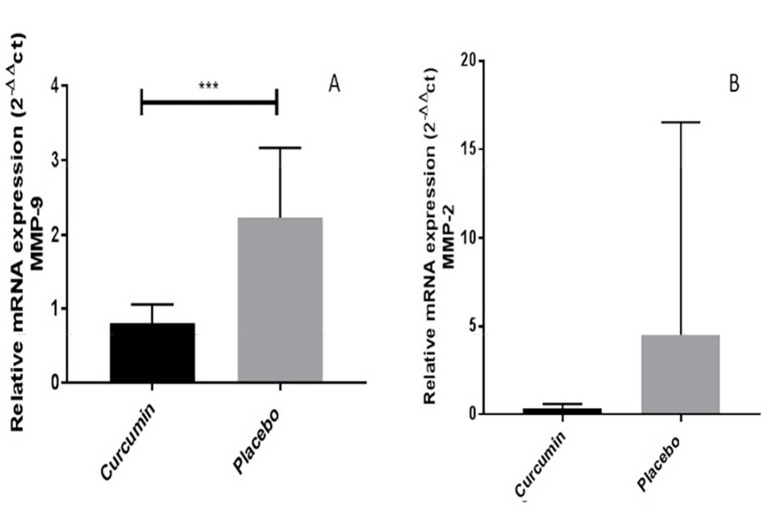
Effects of curcumin on matrix metalloproteinase-9 and matrix metalloproteinase-2 gene expression in human peripheral blood mononuclear cells: In this study, the expression of the MMP-9 gene was compared with the beta-actin gene before and after the intervention. Based on the results of 2-ΔΔCT, the mean value of MMP-9 level in the curcumin group and the placebo group was 0.811 ± 0.25 and 2.23 ± 0.94, respectively. The analysis showed that the expression of MMP-9 was significantly decreased in the group treated with curcumin (P < 0.0010) (Figure 6).
Figure 6.
Messenger ribonucleic acid expression of matrix metalloproteinase-2 and matrix metalloproteinase-9 genes in the comparison of the β-actin gene between the placebo and curcumin groups A: Based on the charts, the mRNA expression of matrix metalloproteinase-9 (MMP-9) showed a significant decrease in the curcumin group compared to the placebo group after 3 months of curcumin administration (***P < 0.0010). B: Based on the charts, the mRNA expression of MMP-2 in the curcumin group did not show a significant decrease compared to the placebo group after 3 months of curcumin administration (P = 0.2800). Data are representative of at least 2 independent experiments with β-actin used as the internal control.
The expression of MMP-2 gene was decreased in response to treatment with curcumin. Based on the results of 2-ΔΔCT, the mean value of MMP-2 level was higher in subjects in the placebo group (4.497 ± 12.037) compared to subjects treated with curcumin (0.341 ± 0.253). However, this difference was not statistically significant (P = 0.2894) (Figure 6).
Discussion
Atherosclerosis is a chronic and progressive disease caused by the development of the focal lesion in the wall of the arteries.21 Despite a decrease in fat with the use of statins, a significant number of cardiovascular events are observed in patients with atherosclerosis. Further advances in the understanding of the pathophysiology of coronary artery syndrome dismissed. Studies have shown that a large number of cardiovascular events originate from arteries that had no significant obstruction in previous angiograms. Vulnerable lesions are unstable lesions with large lipid nuclei and a thin fibrous cap. Plaque instability is closely related to the spreading of inflammation in the intima, and acute coronary syndromes are caused by plaque rupture. Strengthening the plaque by modifying its structure and its content, instead of changing the luminal diameter, has become a potential new therapeutic goal.22
During the process of plaque formation, macrophages penetrate the fibrous cap, and the CD40 ligand on the surface of the T cells binds to their receptor at the surface of macrophages and induces the synthesis of a large number of inflammatory cytokines and proteases, in particular, the MMPs that cause a stable digestion of the matrix. Therefore, these enzymes play a major role in the weakness and rupture of the atherosclerotic plaque.23
Various studies have shown that circulating MMPs increase in patients with heart failure and unstable angina. The increased expression of MMPs in plaque atherosclerosis and that their activation causes the plaque to rupture has also been identified. Protecting the vascular wall in the atherosclerotic lesion is one of the ways to stabilize the plaque and prevent its rupture, which is associated with decreased expression and activity of MMPs. These findings suggest that MMP inhibitors can be useful in drug development and are a way to reduce mortality associated with CVD.24
The use of natural anti-inflammatory compounds is a safe and attractive solution for modulating inflammatory disorders. Curcumin is an anti-inflammatory food product that has been used for centuries in Asian cultures. Many of the activities of curcumin are associated with the ability of this compound to suppress acute and chronic inflammation.25 To date, the anti-oxidant and anti-inflammatory properties of curcumin have been well documented, but it has not been completely determined how this inhibitory response regulated by curcumin.14 Various studies have shown that curcumin targets different molecules, which may include growth factors, growth factor receptors, transcription factors, cytokines, and enzymes. Our findings show that no significant difference existed between the placebo and nanocurcumin groups in terms of the activity of enzymes (MMP-2 and MMP-9) before the intervention (Table 2). Our results indicate that the consumption of curcumin at the dose of 80 mg/day significantly reduced the expression and activity of MMP-9 compared to the placebo group.
These findings confirm the findings of Saja et al., who reported the inhibition of MMP-9 activity in human PBMCs (in vitro) and in PBMCs in rabbits treated with curcumin (in vivo), and that this inhibition mainly occurs at the level of enzyme transcription.26 It seems that the decrease in activity of MMP-9 is not due to the activity of the endogenous inhibitor of the enzyme, because studies have shown that curcumin also reduces tissue inhibitor of metalloproteinase-1 (TIMP-1) activity.26 However, some studies that have investigated the effect of curcumin on the activity of gelatinases in rats' embryonic cardiac cells, suggest that the presence of docking sites for curcumin in the hemopexin domain in gelatinases and prevention of the binding of the substrate to the enzyme are possible mechanisms for reducing activity.27
Analysis of anti-angiogenic activity of demethoxycurcumin (curcumin analog) on cultured endothelial cells derived from the human umbilical cord has shown that this compound can reduce the expression of MMP-9 by up to 5 times. Moreover, the evaluation of the gelatinolytic activity of the enzyme through gelatin zymography has shown that demethoxycurcumin, in addition to reducing the expression of the enzyme, also significantly reduces the gelatinolytic activity of the enzyme.28 Kim et al. also found that the gelatinolytic activity of the MMP-2 enzyme was reduced by the effect of demethoxycurcumin.28 However, this compound did not have direct inhibitory effects on enzyme activity or any effect on post-translational modifications (PTM). This suggests that the inhibitory effect of this compound may be suppressed due its inhibition of the activation of some of the transcription factors that are commonly involved in the expression of MMPs.28
Previous studies have shown that many of the beneficial effects of curcumin are due to its ability to inhibit nuclear factor-kB (NF-kB) activity. Most inflammatory mediators that have been identified, including inflammatory cytokines, chemokines, sticky molecules, enzymes, and kinases, are regulated by NF-kB. Therefore, factors that can reduce the expression of NF-kB and NF-kB regulated gene products have the potential for use as anti-inflammatory agents in inflammatory diseases such as atherosclerosis. Thus, it can be concluded that the inhibition of NF-kB by any compound can reduce the activity of MMPs.25 By inhibiting the activation of NF-kB, curcumin suppresses the expression of proliferation and cell survival genes, especially MMP-9.29 MMP-9 promoter is highly protected and contains locations deemed necessary for NF-kB connection. This indicates the participation of NF-kB in MMP-9 regulation.30
It has been shown that, in addition to NF-kB, AP-1, another transcription factor, is also affected by curcumin.31 Both MMP-2 and MMP-9 promoter encoding genes have an AP-1 binding site. However, this position is located in the proximal section of the MMP-9 and in the distal region of the MMP-2 gene.32 Since the activation of AP-1 and NF-kB transcription factors is required in most cells to maximize MMP-9 expression, it seems that the effect of curcumin on MMP-9 is probably due to a significant reduction in the activity of these factors in endothelial cells.33
Our results further indicate that protection provided by curcumin in atherosclerosis can be due to inhibition of the expression and activity of MMP-2 gene. In this study, the expression of MMP-2 also decreased with the biological activity of the enzyme in the group receiving curcumin. The results of this study suggest that curcumin has a potential effect on reducing the expression of MMP-2 mRNA in peripheral blood lymphocytes (PBL) and its activity in the serum. There is much evidence suggesting that among the MMP family members, MMP-2 plays a key role in promoting the migration and proliferation of vascular smooth muscle cells (VSMCs) and instability of atherosclerotic plaque.34 In addition, the expression of MMP-2 in VSMCs is associated with a wide range of pathological conditions, especially plaque atherosclerosis, and the expression and activity of MMP-2, which significantly increased in vulnerable areas, indicates the pathogenic role of this enzyme in the development of atherosclerosis.35 Based on the results of this study, the consumption of curcumin can be associated with decreased activity and expression of MMP-2 enzyme. The results of this study are in agreement with that of the study by Zhong et al. that showed that curcumin can significantly reduce MMP-2 activity and expression induced by TNF-α from the NF-kB pathway in cultured rat VSMCs.36
One of the limitations of this study was the small number of patients and lack of investigation of gene expression in all groups before the intervention. Although we can refer to previous articles but it is necessary to be done in each study.
Studies have shown that growth factors, cytokines, and hormones can mediate the expression of MMP-2 by activating NF-kB and AP-1 from Ras/MAPK pathways because MMP-2 promoter has binding sites for various transcription factors such as NF-kB, AP-1, and SP-1.37 The expression of MMP-2 is regulated by extracellular signal-regulated kinase (ERK) activity, which is a component of the MAPK signaling pathway. Moreover, blocking this pathway with ERK inhibitors can suppress the expression of MMP-2. Accordingly, the possible mechanism of curcumin in inhibiting the expression of MMP-2 is the inhibition of the MAPK pathway components, and in particular its ERK component.38 Contrary to MMP-9, it seems that MMP-2 regulation in endothelial cells (ECs) is more dependent on the ERK path than the P38 MAPK route.39 Curcumin can suppress AP-1 activity by preventing an increase in phosphorus-ERK and nuclear ERK accumulation.40
It has been confirmed that curcumin can reduce endothelial cell damage, which is linked to inhibiting MMPs from the CD40-CD40L pathway.41 This pathway plays a major role in the inflammatory aspect of atherosclerotic plaque formation, progression, and rupture by suppressing the activity of MMPs.42
In addition to T cells, activated endothelial cells, SMCs, and macrophages can also produce CD40 and its ligand. In in-vitro conditions, the binding of CD40 to CD40L in cells present in atherosclerotic lesions triggers atherosclerotic changes such as induction of sticky molecules, the expression of pro-inflammatory cytokines, and MMPs that are present in atherosclerotic plaques.43
A study on CD40-CD40L pathway inhibitors has shown that inhibition of this route leads to decreased activity and expression of MMP-2; however, the inhibition of this pathway did not have any effect on MMP-9. The difference in the effect of this inhibitor on MMP-2 and MMP-9 may be due to differences in the cells producing these enzymes. MMP-2 is mainly secreted from macrophages, which are highly capable of producing CD40, whereas MMP-9 is produced in neutrophils.44 However, in some studies, the simultaneous inhibition of CD40, MMP-9, and TNF-α by curcumin has been reported in mouse coronary artery tissue.45
The novelty of this study investigation of the actual results of nanomicelle curcumin on both the expression and enzyme activity of MMP-2 and MMP-9 on PBMC.
Conclusion
In conclusion, our results indicate that curcumin attenuates MMP-2 and MMP-9 expression and activity. Based on the results of this study and previous studies that show the inhibitory effect of curcumin on a range of inflammatory markers, curcumin can be suggested as a new intervention in secondary prevention of cardiovascular events.
Low cost, pharmacological safety, efficacy, and multiple molecular targets have turned curcumin into a promising product for the prevention and treatment of various diseases. However, more studies are required to validate all the benefits of curcumin therapy.
Acknowledgments
The authors would like to thank Mashhad University of Medical Sciences Research Council for supporting this research, Dar Al-Shifa Imam Reza Medical laboratory staff, Mrs. Sareh Ejlali, and Dr. Taghiabadi drugstore. Data presented here were from a master’s thesis and this study was approved by Mashhad University of Medical Sciences with the No. 931058.
Footnotes
Conflicts of Interest
Authors have no conflict of interests.
REFERENCES
- 1.Sanderson JE, Mayosi B, Yusuf S, Reddy S, Hu S, Chen Z, et al. Global burden of cardiovascular disease. Heart. 2007;93(10):1175. doi: 10.1136/hrt.2007.131060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Haarala A. Inflammation and early atherosclerosis [Thesis]; Tampere, Finland: University of Tampere; 2012. [Google Scholar]
- 3.Katrib A, Tak PP, Bertouch JV, Cuello C, McNeil HP, Smeets TJ, et al. Expression of chemokines and matrix metalloproteinases in early rheumatoid arthritis. Rheumatology (Oxford) 2001;40(9):988–94. doi: 10.1093/rheumatology/40.9.988. [DOI] [PubMed] [Google Scholar]
- 4.Huang J. Role of matrix metalloproteinase-2 in therosclerosis and abdominal aortic aneurysms in apolipoprotein e deficient mice [Thesis]; Lexington, KY: University of Kentucky; 2015. [Google Scholar]
- 5.Bendeck MP, Zempo N, Clowes AW, Galardy RE, Reidy MA. Smooth muscle cell migration and matrix metalloproteinase expression after arterial injury in the rat. Circ Res. 1994;75(3):539–45. doi: 10.1161/01.res.75.3.539. [DOI] [PubMed] [Google Scholar]
- 6.Momi S, Falcinelli E, Giannini S, Ruggeri L, Cecchetti L, Corazzi T, et al. Loss of matrix metalloproteinase 2 in platelets reduces arterial thrombosis in vivo. J Exp Med. 2009;206(11):2365–79. doi: 10.1084/jem.20090687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kapakos G, Youreva V, Srivastava AK. Cardiovascular protection by curcumin: Molecular aspects. Indian J Biochem Biophys. 2012;49(5):306–15. [PubMed] [Google Scholar]
- 8.Rahimi H, Jaafari M, Mohammadpour A, Abnous K, Ghayour Mobarhan M, Ramezanzadeh E, et al. Curcumin: Reintroduced Therapeutic Agent from Traditional Medicine for Alcoholic Liver Disease. Asia Pac J Med Toxicol. 2015;4(1):25–30. [Google Scholar]
- 9.Rahimi HR, Nedaeinia R, Sepehri SA, Nikdoust S, Kazemi OR. Novel delivery system for natural products: Nano-curcumin formulations. Avicenna J Phytomed. 2016;6(4):383–98. [PMC free article] [PubMed] [Google Scholar]
- 10.Anand P, Kunnumakkara AB, Newman RA, Aggarwal BB. Bioavailability of curcumin: Problems and promises. Mol Pharm. 2007;4(6):807–18. doi: 10.1021/mp700113r. [DOI] [PubMed] [Google Scholar]
- 11.Hatamipour M, Sahebkar A, Alavizadeh SH, Dorri M, Jaafari MR. Novel nanomicelle formulation to enhance bioavailability and stability of curcuminoids. Iran J Basic Med Sci. 2019;22(3):282–9. doi: 10.22038/ijbms.2019.32873.7852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kosalova D, Bezakova L, Raekovac L, Mosovska S, Sturdik E. Therapeutic potential of curcumin in medicinal chemistry. Acta Chimica Slovaca. 2013;6(1):89–99. [Google Scholar]
- 13.Wongcharoen W, Phrommintikul A. The protective role of curcumin in cardiovascular diseases. Int J Cardiol. 2009;133(2):145–51. doi: 10.1016/j.ijcard.2009.01.073. [DOI] [PubMed] [Google Scholar]
- 14.Singh S, Aggarwal BB. Activation of transcription factor NF-kappa B is suppressed by curcumin (diferuloylmethane) [corrected]. J Biol Chem. 1995;270(42):24995–5000. doi: 10.1074/jbc.270.42.24995. [DOI] [PubMed] [Google Scholar]
- 15.Hatcher H, Planalp R, Cho J, Torti FM, Torti SV. Curcumin: From ancient medicine to current clinical trials. Cell Mol Life Sci. 2008;65(11):1631–52. doi: 10.1007/s00018-008-7452-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chuengsamarn S, Rattanamongkolgul S, Phonrat B, Tungtrongchitr R, Jirawatnotai S. Reduction of atherogenic risk in patients with type 2 diabetes by curcuminoid extract: A randomized controlled trial. J Nutr Biochem. 2014;25(2):144–50. doi: 10.1016/j.jnutbio.2013.09.013. [DOI] [PubMed] [Google Scholar]
- 17.Ghorbani Z, Hekmatdoost A, Mirmiran P. Anti-hyperglycemic and insulin sensitizer effects of turmeric and its principle constituent curcumin. Int J Endocrinol Metab. 2014;12(4):e18081. doi: 10.5812/ijem.18081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nauck MA, Meininger G, Sheng D, Terranella L, Stein PP. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor, sitagliptin, compared with the sulfonylurea, glipizide, in patients with type 2 diabetes inadequately controlled on metformin alone: A randomized, double-blind, non-inferiority trial. Diabetes Obes Metab. 2007;9(2):194–205. doi: 10.1111/j.1463-1326.2006.00704.x. [DOI] [PubMed] [Google Scholar]
- 19.Rahimi HR, Mohammadpour AH, Dastani M, Jaafari MR, Abnous K, Ghayour Mobarhan M, et al. The effect of nano-curcumin on HbA1c, fasting blood glucose, and lipid profile in diabetic subjects: A randomized clinical trial. Avicenna J Phytomed. 2016;6(5):567–77. [PMC free article] [PubMed] [Google Scholar]
- 20.Toth M, Fridman R. Assessment of Gelatinases (MMP-2 and MMP-9) by Gelatin Zymography. Methods Mol Med. 2001;57:163–74. doi: 10.1385/1-59259-136-1:163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Libby P, Aikawa M. Mechanisms of plaque stabilization with statins. Am J Cardiol. 2003;91(4A):4B–8B. doi: 10.1016/s0002-9149(02)03267-8. [DOI] [PubMed] [Google Scholar]
- 22.Dupuis J. Mechanisms of acute coronary syndromes and the potential role of statins. Atheroscler Suppl. 2001;2(1):9–14. doi: 10.1016/s1567-5688(00)00004-0. [DOI] [PubMed] [Google Scholar]
- 23.Simionescu M, Sima A. Morphology of atherosclerotic lesions. In: Wick G, Grundtman C, editors. Inflammation and atherosclerosis. Berlin, Germany: Springer Science & Business Media; 2011. pp. 19–37. [Google Scholar]
- 24.Jones CB, Sane DC, Herrington DM. Matrix metalloproteinases: A review of their structure and role in acute coronary syndrome. Cardiovasc Res. 2003;59(4):812–23. doi: 10.1016/s0008-6363(03)00516-9. [DOI] [PubMed] [Google Scholar]
- 25.Shishodia S, Sethi G, Aggarwal BB. Curcumin: Getting back to the roots. Ann N Y Acad Sci. 2005;1056:206–17. doi: 10.1196/annals.1352.010. [DOI] [PubMed] [Google Scholar]
- 26.Saja K, Babu MS, Karunagaran D, Sudhakaran PR. Anti-inflammatory effect of curcumin involves downregulation of MMP-9 in blood mononuclear cells. Int Immunopharmacol. 2007;7(13):1659–67. doi: 10.1016/j.intimp.2007.08.018. [DOI] [PubMed] [Google Scholar]
- 27.Kohli S, Chhabra A, Jaiswal A, Rustagi Y, Sharma M, Rani V. Curcumin suppresses gelatinase B mediated norepinephrine induced stress in H9c2 cardiomyocytes. PLoS One. 2013;8(10):e76519. doi: 10.1371/journal.pone.0076519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim JH, Shim JS, Lee SK, Kim KW, Rha SY, Chung HC, et al. Microarray-based analysis of anti-angiogenic activity of demethoxycurcumin on human umbilical vein endothelial cells: Crucial involvement of the down-regulation of matrix metalloproteinase. Jpn J Cancer Res. 2002;93(12):1378–85. doi: 10.1111/j.1349-7006.2002.tb01247.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhou H, Beevers CS, Huang S. The targets of curcumin. Curr Drug Targets. 2011;12(3):332–47. doi: 10.2174/138945011794815356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bond M, Chase AJ, Baker AH, Newby AC. Inhibition of transcription factor NF-kappaB reduces matrix metalloproteinase-1, -3 and -9 production by vascular smooth muscle cells. Cardiovasc Res. 2001;50(3):556–65. doi: 10.1016/s0008-6363(01)00220-6. [DOI] [PubMed] [Google Scholar]
- 31.Divya CS, Pillai MR. Antitumor action of curcumin in human papillomavirus associated cells involves downregulation of viral oncogenes, prevention of NFkB and AP-1 translocation, and modulation of apoptosis. Mol Carcinog. 2006;45(5):320–32. doi: 10.1002/mc.20170. [DOI] [PubMed] [Google Scholar]
- 32.Vincenti MP. The matrix metalloproteinase (MMP) and tissue inhibitor of metalloproteinase (TIMP) genes. Transcriptional and posttranscriptional regulation, signal transduction and cell-type-specific expression. Methods Mol Biol. 2001;151:121–48. doi: 10.1385/1-59259-046-2:121. [DOI] [PubMed] [Google Scholar]
- 33.Parodi FE, Mao D, Ennis TL, Pagano MB, Thompson RW. Oral administration of diferuloylmethane (curcumin) suppresses proinflammatory cytokines and destructive connective tissue remodeling in experimental abdominal aortic aneurysms. Ann Vasc Surg. 2006;20(3):360–8. doi: 10.1007/s10016-006-9054-7. [DOI] [PubMed] [Google Scholar]
- 34.Aoyagi M, Yamamoto M, Azuma H, Nagashima G, Niimi Y, Tamaki M, et al. Immunolocalization of matrix metalloproteinases in rabbit carotid arteries after balloon denudation. Histochem Cell Biol. 1998;109(2):97–102. doi: 10.1007/s004180050206. [DOI] [PubMed] [Google Scholar]
- 35.Newby AC, Zaltsman AB. Fibrous cap formation or destruction-the critical importance of vascular smooth muscle cell proliferation, migration and matrix formation. Cardiovasc Res. 1999;41(2):345–60. [PubMed] [Google Scholar]
- 36.Zhong Y, Yu W, Feng J, Fan Z, Li J. Curcumin suppresses tumor necrosis factor-alpha-induced matrix metalloproteinase-2 expression and activity in rat vascular smooth muscle cells via the NF-kappaB pathway. Exp Ther Med. 2014;7(6):1653–8. doi: 10.3892/etm.2014.1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lin ML, Lu YC, Chung JG, Wang SG, Lin HT, Kang SE, et al. Down-regulation of MMP-2 through the p38 MAPK-NF-kappaB-dependent pathway by aloe-emodin leads to inhibition of nasopharyngeal carcinoma cell invasion. Mol Carcinog. 2010;49(9):783–97. doi: 10.1002/mc.20652. [DOI] [PubMed] [Google Scholar]
- 38.Stoica G, Lungu G. Role of MMP2 in Brain Metastasis. In: Hayat MA, editor. Tumors of the central nervous system, volume 13: Types of tumors, diagnosis, ultrasonography, surgery, brain metastasis, and general CNS diseases. Berlin, Germany: Springer Science & Business Media; 2014. pp. 195–205. [Google Scholar]
- 39.Boyd PJ, Doyle J, Gee E, Pallan S, Haas TL. MAPK signaling regulates endothelial cell assembly into networks and expression of MT1-MMP and MMP-2. Am J Physiol Cell Physiol. 2005;288(3):C659–C668. doi: 10.1152/ajpcell.00211.2004. [DOI] [PubMed] [Google Scholar]
- 40.Qin L, Yang YB, Tuo QH, Zhu BY, Chen LX, Zhang L, et al. Effects and underlying mechanisms of curcumin on the proliferation of vascular smooth muscle cells induced by Chol: MbetaCD. Biochem Biophys Res Commun. 2009;379(2):277–82. doi: 10.1016/j.bbrc.2008.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jana S, Paul S, Swarnakar S. Curcumin as anti-endometriotic agent: Implication of MMP-3 and intrinsic apoptotic pathway. Biochem Pharmacol. 2012;83(6):797–804. doi: 10.1016/j.bcp.2011.12.030. [DOI] [PubMed] [Google Scholar]
- 42.Schonbeck U, Libby P. CD40 signaling and plaque instability. Circ Res. 2001;89(12):1092–103. doi: 10.1161/hh2401.101272. [DOI] [PubMed] [Google Scholar]
- 43.Hakkinen T, Karkola K, Yla-Herttuala S. Macrophages, smooth muscle cells, endothelial cells, and T-cells express CD40 and CD40L in fatty streaks and more advanced human atherosclerotic lesions. Colocalization with epitopes of oxidized low-density lipoprotein, scavenger receptor, and CD16 (Fc gammaRIII). Virchows Arch. 2000;437(4):396–405. doi: 10.1007/s004280000239. [DOI] [PubMed] [Google Scholar]
- 44.Nagashima H, Aoka Y, Sakomura Y, Uto K, Sakuta A, Aomi S, et al. Matrix metalloproteinase 2 is suppressed by trapidil, a CD40-CD40 ligand pathway inhibitor, in human abdominal aortic aneurysm wall. J Vasc Surg. 2004;39(2):447–53. doi: 10.1016/j.jvs.2003.07.005. [DOI] [PubMed] [Google Scholar]
- 45.Li X, Lu Y, Sun Y, Zhang Q. Effect of curcumin on permeability of coronary artery and expression of related proteins in rat coronary atherosclerosis heart disease model. Int J Clin Exp Pathol. 2015;8(6):7247–53. [PMC free article] [PubMed] [Google Scholar]