Abstract
Isoprostanes are the potential biomarkers of endogenous human metabolism and proven clinically to provide the quantitative measure of systematic oxidative injury. An ultra-performance liquid chromatography-tandem mass spectrometric analytical method capable of determining four biomarkers of oxidative stress (8-iso-PGF2α, 2,3-dinor-iPF2α-III, PGE2, and 5-iPF2α-VI) in wastewater was developed and validated. Isoprostanes were quantified in the range of 31.1–1270 ng/L in raw wastewater samples in two communities in western Kentucky and Tennessee during the first four months of the COVID-19 pandemic. Consistent detection of PGE2 and 5-iPF2α-VI in wastewater suggested that PGE2 and 5-iPF2α-VI can be a reliable biomarker of community oxidative anxiety. The higher 4-month average mass load of isoprostanes [(ranged from 22.9 mg/d/1000 people to 807 mg/d/1000 people] may be attributed to the elevated community level oxidative anxiety owing COVID-19 uncertainties. The average mass loads of PGE2 and 5-iPF2α-VI in a community were significantly increased (two-tailed p < 0.001) from the first month of COVID-19 pandemic to the second month; however, significantly decreased (two-tailed p < 0.001) in the third month. Wastewater-based-epidemiological determination of isoprostanes can be a near-real-time and cost-effective approach of a trend in community depression. This is the first report of the quantification of PGE2 and 5-iPF2α-VI in wastewater and estimation of the community level oxidative anxiety.
Keywords: Oxidative stress, Isoprostanes, Wastewater based epidemiology, COVID Pandemic, Ammoniacal nitrogen based population
Graphical abstract
Credit authors statement
Mr. Isaac Bowers: collected wastewater samples, performed sample preparation, instrumental analysis, analytical data interpretation, and prepared the first draft of the manuscript. Dr. Bikram Subedi: conceptualized the project, collected wastewater samples, performed sample preparation, instrumental analysis, analytical data interpretation, prepared and reviewed the manuscript, and supervised overall research project. Both authors reviewed and edited the paper.
1. Introduction
The World Health Organization’s global survey among 130 countries reported the devastating impact of the novel coronavirus disease (COVID-19) including bereavement, isolation, loss of income, and fear that trigerred existing mental health conditions and exacerbating the existing ones (WHO, 2020). The psychological impact of COVID-19 has been reported in Italy (Rossi et al., 2020), Spain (Parrado-Gonzalez and Leon-Jariego, 2020), China (Talevi et al., 2020; Zhang and Ma, 2020), and the U.S. (Fitzpatrick et al., 2020). Typically, the elevated level of reactive oxygen species (ROS) during psychological stress catalyzes the oxidation of arachidonic acid and releases isoprostanes, such as the F2-isoprostanes. The ROS catalyzed oxidation of cellular constituent can eventually result in oxidative damage to DNA (Morrow and Roberts, 1991; Sivonova et al., 2004; Erie et al., 2003). Rats under stress (electrical shock, forced swimming, water immersion, noise, etc.) secrete higher levels of renal oxidative stress inducers (NO and O₂), a marker (malondialdehyde) that increased oxidative DNA damage (measured by DNA fragmentation in the gastral mucosa in rats) (Bagchi et al., 1999; Gidron et al., 2006; Pedreanez et al., 2011). Individuals’ habitual stress (smoking) (Yan et al., 2007), diseases (cardiovascular diseases, select cancers, diabetes) (Barocas et al., 2011; Zhang, 2013; Murai et al., 2000) and depression (high levels of psychological stress) (Blak et al., 2015) were also correlated with elevated levels of isoprostanes. Interestingly, the situational depression such as exam anxiety for students resulted in a significantly higher sensitivity to the lipid peroxidation and DNA damage (Sivonova et al., 2004).
Raw wastewater can be utilized as a pooled urine sample to comprehend the community consumption or exposure to chemicals of concern and the associated community health (Daughton, 2012). There are reports of several F2-Isoprostanes excretion through human urine including 8-iso-prostaglandin F2α (8-iso-PGF2α; 0.25 ± 0.15 μg/g of creatinine), 2,3-dinor-8-iso-prostaglandin F₂α (2,3-dinor-iPF2α-III, 4.6 ± 1.6 μg/g of creatinine), 5-iso Prostaglandin F2α-VI (5-iPF2α-VI, 7.371 ± 0.021 μg/g of creatinine), and prostaglandin E₂ (PGE2, 178 ± 80 ng/day) (Liang et al., 2003; Practico et al., 1998; Cibbattoni et al., 1979). Excreted isoprostanes are typically flushed down the drain and reach the centralized wastewater treatment plants (WWTP). Three isoprostanes including 8-iso-PGF2α, 2,3-dinor-iPF2α-III, and PGE2 were found to be sufficiently stable under typical sewer conditions with half-lives of 130 h, 110 h, and 110 h, respectively (O’Brien et al., 2019). Moreover, 8-iso-PGF2α showed no degradation in raw wastewater even at the ambient temperature for 24 h (Ryu et al., 2015). Two studies in Europe reported 8-iso-PGF2α in wastewater (8.7–23.3 ng/L) collected from 12 European cities in typical weeks which was equivalent to a daily mass load of 4.8–14.3 mg/day/1000 people to the WWTPs (Ryu et al., 2016; Ryu et al., 2019). In the U.S. only one study reported 8-iso-PGF2α in wastewater (∼25 ng/L) in a typical week which was equivalent to ∼16 mg/day/1000 people (Santos et al., 2015). To the authors’ knowledge, there are no reports of 2,3-dinor-iPF2α-III, PGE2, and 5-iPF2α-VI in wastewater.
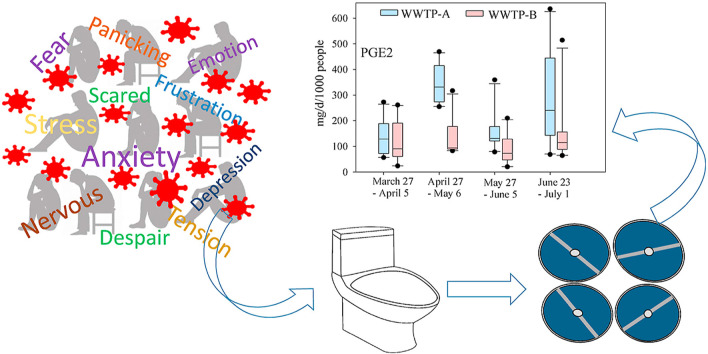
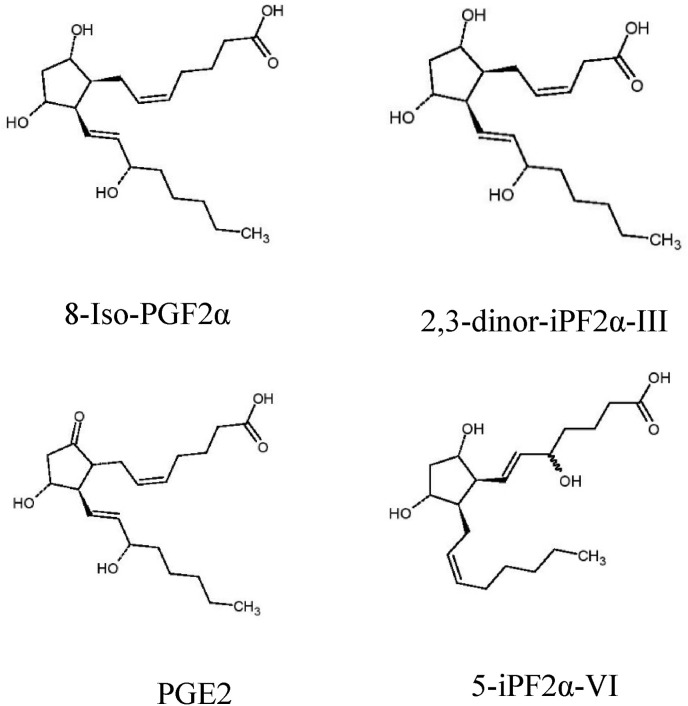
In this study, an analytical method capable of determining four isoprostanes - 8-iso-PGF2α, 2,3-dinor-iPF2α-III (a major metabolite of 8-iso-PGF2α), PGE2, and 5-iPF2α-VI (Fig. 1 ) – in wastewater was developed and validated. Target isoprostanes were monitored in one community in western Kentucky and one community in western Tennessee for 10 consecutive days in typical weeks every month starting from March 27th to July 1st, 2020. The sampling period covers the first four months of COVID-19 outbreak in the U.S.; therefore, the quantitation of isoprostanes potentially portrays the community level oxidative anxiety. The cumulative community anxiety after the unprecedented COVID-19 closure of schools, public gatherings, and non-life-sustaining business in March, fears of civil unrest for prolonged period, and the conditional reopening of select businesses and public gatherings in May potentially vary the community-level stress, and hence the biomarkers of oxidative stress. To the authors’ knowledge, this is the first study that is reporting quantitative measurement of community-level stress during the COVID-19 outbreak.
Fig. 1.
Structure of target isoprostanes. 8-iso-PGF2α: 8-iso-prostaglandin F2α; 2,3-dinor-iPF2α-III: 2,3-dinor-8-iso-prostaglandin F₂α; PGE2: prostaglandin E₂; 5-iPF2α-VI (5-iso Prostaglandin F2α-VI.
2. Materials and methods
2.1. Chemicals and reagents
Standard stock solutions (10 or 100 ppm) of individual isoprostane and their respective deuterated internal standards were purchased at the highest available purity from Cayman Chemical Company (Ann Harbor, Michigan). Target isoprostanes include 8-iso-prostaglandin F2α (8-iso-PGF2α), 2,3-dinor-8-iso-prostaglandin F2α (2,3-dinor-iPF2α-III); a major metabolite of 8-iso-PGF2α), prostaglandin E₂ (PGE2), and 5-iPF2α-VI and the corresponding internal standards include 8-iso-PGF2α-D₄, PGE2-D₄, and 5-iPF2α-VI-D₁₁. HPLC grade methanol and formic acid were purchased from Fisher Scientific (Hampton, NH). β-glucuronidase type H-2 enzyme (≥85,000 units/mL; extracted from Helix pomatia) was purchased from Sigma Aldrich (St. Louis, MO). Ultrapure water was prepared using a Thermo Fisher Scientific MicroPure UV system. All standard solutions of target isoprostanes were stored at −20 °C.
2.2. Sample collection and preparation
Two WWTPs from two communities (A and B), one in western Kentucky and one in western Tennessee, were sampled for ten consecutive days every month starting March 27 to July 1st, 2020. Twenty-four-hour composite samples of raw wastewater (one aliquot every 15 min) were collected using a time-proportional autosampler and maintained at 4 °C during the collection period. WWTP-A treats an average of 4.40 million gallons per day (MGD) of primarily domestic sewage whereas WWTP-B treats an average of 1.45 MGD of sewage. All samples collected in polypropylene bottles were transported on ice to the laboratory and stored at −20 °C until further analysis.
The ammoniacal nitrogen (NH4–N) level in raw wastewater was determined as reported elsewhere (O’Rourke and Subedi, 2020; Croft et al., 2020), and used to estimate the size of the population served by the WWTPs. The concentration of ammoniacal nitrogen (ng/L), wastewater inflow (L/d), and the average per capita daily production of NH4–N (6900 mg/d) (Been et al., 2014) were utilized to determine near-accurate population. The average populations found were 16187 ± 5311 for community A and 10941 ± 3297 for community B. The average population for community A and B determined using ammoniacal nitrogen load were 16.3% lower and 3% higher, respectively, than the census-based population (USCB, 2019). The limited non-human sources of ammoniacal nitrogen could not be quantified and corrected; however, it is least affected by non-human sources compared to other conventional hydrological markers used for the estimation of population (Been et al., 2014).
The samples were equilibrated to room temperature, thoroughly mixed, centrifuged (100 mL) at 4500 rpm for 5 min, and vacuum filtered using 0.45 μm nylon membrane (47 mm diameter). The filtered samples were spiked with 500 μL of β-glucuronidase type H-2 enzyme, mixed well, and incubated at 37 °C for 2 h. The incubated samples were then allowed to cool to room temperature, spiked with a mixture of internal standards [250 ng of 8-iso-PGF2α-D₄, 187.5 ng of 5-iPF2α-VI-D₁₁, 250 ng of PGE2-D₄], and mixed thoroughly. The spiked samples were extracted using Oasis® hydrophilic-lipophilic balance (HLB) solid-phase extraction cartridges (6 c.c., 200 mg) preconditioned with 3 mL methanol followed by 3 mL of ultrapure water. Samples were extracted under a gentle vacuum at ∼1 mL/min, dried under a high vacuum for ∼3 min, and eluted with 4 mL of methanol followed by the 3 mL of 5% ammonia in methanol. The combined eluates were concentrated to ∼100 μL using a gentle flow of nitrogen gas at ambient temperature, transferred quantitatively (with multiple wash using methanol) to silanized amber glass LC-vials, adjusted final volume to 500 μL using methanol, and vortex-mixed. The final concentrate was then syringe filtered through 0.2 μm nylon filter (MilliporeSigma, Burlington, MA), and injected 1.0 μL for the analysis of target isoprostanes using ultra-performance liquid chromatography (UPLC) tandem mass spectrometry (MS/MS).
2.3. Isotope dilution liquid chromatography-tandem mass spectrometry
The prepared samples were analyzed for target isoprostanes using UPLC (Agilent 1290 Infinity II LC System) coupled with Triple Quadrupole mass spectrometer (Agilent 6460) (Santa Clara, CA). The gradient flow of HPLC grade methanol and 0.1% aqueous solution of formic acid (Table S1) were used to separate analytes using an ACQUITY UPLC® Oligonucleotide BEH C18 column (150 mm × 2.1 mm i.d. × 1.7 μm particle size). Target analyte’s peak identification was based on the relative retention time (±0.05 min) to their standard solution, two multiple reaction monitoring (MRM) transitions (Table 1 ) in the negative mode of ionization, and the ratio of the abundance of quantitative to qualitative ions (±20%). The gas temperature, sheath gas temperature, and capillary voltage were optimized to 350 °C, 300 °C, and 4000 V, respectively.
Table 1.
Select figure of merits for the developed analytical method.
| Target isoprostanes | MRM transitions (collision energy in eV) | Average absolute % recoveries (n = 3) | Matrix-spike % recoveries (n = 2) | CCV % recoveries (n = 14) | aIntraday repeatability (n = 10) at 25 ng level | aIntraday repeatability (n = 10) at 125 ng level | LOD (ng/L) | LOQ (ng/L) |
|---|---|---|---|---|---|---|---|---|
| 2,3-dinor-iPF2α-III | 325.1 > 237, 136.9 (15 eV) | 64 ± 12 | 128 ± 9.4 | 106 ± 16 | ±20.0 | ±3.8 | 25.6 | 85.5 |
| 8-iso-PGF2α | 353.2 > 193.2, 164.9 (28 eV) | 99 ± 30 | 71.9 ± 1.4 | 106 ± 4.4 | ±3.3 | ±1.4 | 9.30 | 31.1 |
| 5-iPF2α-VI | 353 > 114.9, 299.2 (30 eV) | 126 ± 6.2 | 110 ± 2.4 | 116 ± 4.1 | ±1.1 | ±4.8 | 8.81 | 29.5 |
| PGE2 | 352.1 > 271.8, 333.9 (12 eV) | 58 ± 5.7 | 117 ± 1.1 | 107 ± 10 | ±2.0 | ±1.7 | 13.5 | 45.0 |
Standard errors; CCV: continuing calibration verification; LOD: limit of detection; LOQ: limit of quantitation.
The isotopic dilution mass spectrometry method was applied where a known quantity of deuterated isotopes of each target analyte (internal standard) is spiked directly into the sample prior to extraction and analytes were quantified based on the relative response factors of isotopic-labeled internal standard and the corresponding analyte. This method allows for accurate quantification by correcting for the loss of analytes during the sample preparation and instrumental analysis process. However, 2,3-dinor-iPF2α-III was quantified using the internal standard for PGE2, due to lack of commercial availability of its deuterated standard. The seven-to-ten-point calibration curves (10.0 ng/L to 10,000 ng/L) of each target analyte were prepared by plotting the concentration-dependent response factor against the response-dependent concentration factor. The linear or quadratic (PGE2) regression coefficients (r 2) determined using Agilent MassHunter Workstation for the Quantitative Analysis were 0.999 for all analytes.
2.4. Optimization of simultaneous extraction
Raw wastewater samples (n = 3) were centrifuged and filtered as described in section 2.2., spiked with a mixture of target analytes (125 ng each), internal standards, and extracted as described above. One wastewater sample (not spiked with analytes) was included to determine the background concentration of target isoprostanes in wastewater and one ultrapure water sample was included to evaluate the procedural contamination. Moreover, one wastewater sample spiked at 125 ng of each target analyte was extracted using Oasis® MCX mix-mode cation exchange (6 cc, 150 mg) cartridge. All extracts were concentrated and adjusted the final volume to 500 μL using methanol and analyzed.
2.5. Enzymatic hydrolysis
To evaluate the effectiveness of enzyme hydrolysis (deconjugation) of the potential glucuronidated forms of isoprostanes in wastewater, 500 μL of β-glucuronidase type H-2 enzyme was added in each of three 100 mL raw wastewater samples and incubated at 37 °C for 1.5 h prior extraction. In addition, three raw wastewater samples not spiked with enzymes were also incubated at 37 °C for 1.5 h (to evaluate the effect of incubation) and three other raw wastewater samples not spiked with enzymes were placed at ambient lab conditions (to evaluate the effect of enzyme hydrolysis and incubation). After incubation, all samples were allowed to equilibrate to room temperature, spiked with the internal standard mixture, mixed thoroughly, extracted, and analyzed as described in section 2.2.
3. Results and discussion
3.1. Figure of merit
In a triplicate spiking and recovery experiment (section 2.4), the average absolute recoveries of 8-iso-PGF2α, 2,3-dinor-iPF2α-III, PGE2, and 5-iPF2α-VI were 99 ± 30.4%, 64 ± 11.9%, 58 ± 5.7%, and 126 ± 6.2%, respectively (Table 1). However, MCX cartridge extraction of isoprostanes resulted in the extraction recoveries range from 71.2% (PGE2) to 169% (8-iso-PGF2α). Due to the unacceptable recovery of 8-iso-PGF2α, HLB cartridges were considered for further extraction of isoprostanes from raw wastewater.
The intraday repeatability of wastewater extracts spiked at 25 ng and 125 ng levels were determined by analyzing ten consecutive runs. The intraday precision ranged from ±1.1 (PGE2) to ±20.0 (8-iso-PGF2α) at 25 ng level and from ±1.4 (2,3-dinor-iPF2α-III) to ±4.8 (5-iPF2α-VI at 125 ng level (Table 1).
The limit of detection (LOD) and limit of quantitation (LOQ) were calculated in wastewater using the minimum target isoprostane concentration that results in a signal to noise ratio of 3 and 10, respectively. LOD ranged from 8.8 ng/L (5-iPF2α-VI) to 25.6 ng/L (2,3-dinor-iPF2α-III) and LOQ ranged from 29.5 ng/L (5-iPF2α-VI) to 85.5 ng/L (2,3-dinor-iPF2α-III) (Table 1). Data points that fell below LOQ were adjusted by substituting them with their respective ½ LOQ values when 70% of the data from that sampling period were above their LOQ.
As part of quality assurance and quality control, a randomly selected sample was spiked (matrix spike, n = 2) with 125 ng of each target isoprostanes and their corresponding internal standards. The average relative recoveries of isoprostanes ranged from 71.9 ± 1.0% (8-iso-PGF2α) to 128 ± 9% (2,3-dinor-iPF2α-III) (Table 1). A calibration standard mixture was considered as a continuing calibration verification (CCV) solution and analyzed before and after each set of ten samples. The average percent recoveries for all target isoprostanes were within ±20% (Table 1).
3.2. Effect of enzyme hydrolysis
PGE2 and 5-iPF2α-VI were not detected in wastewater samples that were not enzymatically hydrolyzed (and not incubated); however, quantified at the average concentrations of 378 ± 61.2 ng/L and 423 ± 15.1 ng/L, respectively, in enzymatically hydrolyzed samples (Table S2). The average concentration of 8-iso-PGF2α was increased by 71% (444 ± 110 ng/L to 760 ± 98.1 ng/L) after enzymatic hydrolysis. The higher concentration of isoprostanes after enzymatic hydrolysis was potentially due to the deconjugation of glucuronidated forms of isoprostanes. Ryu et al. (2016) also reported a 20-fold increase in 8-iso-PGF2α level in wastewater after enzyme hydrolysis. The glucuronide conjugated forms of 8-isoprostane in human urine samples can vary between 30 and 80% of the total iso-prostane (Holder et al., 2020; Yan et al., 2010). However, 2,3-dinor-iPF2α-III was not detected even after enzyme hydrolysis. On incubation (without adding enzyme), the average concentration of 8-iso-PGF2α was 544 ± 28.1 ng/L compared to 444 ± 110 ng/L at ambient conditions. It is important to note that enzyme hydrolysis was performed only at 37 °C for 1.5 h and the optimum deconjugation of the potential glucuronidated forms of isoprostanes may require to further optimize the incubation time.
3.3. Occurrence of isoprostanes in raw wastewater
Sivonova et al. (2004) reported the significantly increased level of oxidative damage to DNA and sensitivity to lipid peroxidation during daily life stress such as examinations for university students in Slovakia. Oxidative anxiety has also been related to tumor, cardiovascular, neurogenerative, and pulmonary sickness. Severe life stress resulted from several traumatic events such as divorce, child abuse, wars, death of loved ones, loss of a job, etc. induce psychotic symptoms including depression, schizophrenia, and bipolar disorders (Schiavone et al., 2013).
In this study, 8-iso-PGF2α, 5-iPF2α-VI, and PGE2 were found at an average from 21.4 to 1270 ng/L in raw wastewater samples from both communities (Table 2 ). The average level of 8-iso-PGF2α quantified from community A in this study was ∼50 folds higher than that reported by Ryu et al. (2016) in European cities and ∼32 folds higher than that reported in Detroit, Michigan (Santos et al., 2015) in typical days. The population served by the WWTP and wastewater inflow can impact the final concentrations of isoprostanes in wastewater (section 3.4); however, wastewater samples in this study were collected during the COVID-19 pandemic that caused many disruptions in people’s lives and the economy and leading to the adverse economic effects of the social-health crisis (Parrado-Gonzalez and Leon-Jariego, 2020). The quarterly GDP growth in the U. S. plunged by 4.8%, the largest decrease since the economic recession in 2008 (Casselman, 2020). However, it is also important to note that the level of isoprostanes could not be compared to that prior COVID-19 due to the unavailability of prior samples.
Table 2.
Concentration of isoprostanes (ng/L) in raw wastewater. Detection frequencies are provided in parentheses.
| Average concentration (n = 10) at WWTP-A |
Average concentration (n = 10) at WWTP-B |
|||||||
|---|---|---|---|---|---|---|---|---|
| 8-iso-PGF2α | 5-iPF2α-VI | PGE2 | 2,3-dinor-iPF2α-III | 8-iso-PGF2α | 5-iPF2α-VI | PGE2 | 2,3-dinor-iPF2α-III | |
| Mar 27-Apr 5 | 404 ± 129 (90%) | 147 ± 49.8 (100%) | 144 ± 75.1 (100%) | nd | 21.4 ± 11.0 (30%) | 110 ± 57.6 (100%) | 206 ± 124 (100%) | nd |
| April 27-May 6 | 734 ± 114 (100%) | 253 ± 57.6 (100%) | 299 ± 71.1 (100%) | nd | 52.4 ± 10.6 (20%) | 156 ± 86.0 (100%) | 252 ± 74.2 (100%) | nd |
| May 27- Jun 5 | 1270 ± 356 (100%) | 197 ± 79.7 (100%) | 201 ± 101 (100%) | nd | 31.1 (10%) | 166 ± 143 (70%) | 194 ± 91.0 (70%) | nd |
| Jun 22-Jul 1 | 815 ± 226 (90%) | 251 ± 65.8 (100%) | 231 ± 122 (100%) | nd | nd | 162 ± 86.8 (100%) | 339 ± 130 (100%) | nd |
nd: non detected.
The peroxidation of arachidonic acid in membrane phospholipids forms 5-iPF2α-VI (5- and 15-series regioisomers) at a significantly higher level than 8-iso-PGF2α (8- and 12-series regioisomers) (Milne et al., 2011). In community B, 8-iso-PGF2α was detected only in 15% of samples at an average concentration of 38.7 ng/L; however, 5-iPF2α-VI was quantified in 93% of the samples at an average concentration of 151 ng/L. The arachidonyl endoperoxide intermediate can isomerize to PGE2 and PGD2. In this study, PGE2 was detected most consistently at average 221 ng/L and 240 ng/L level in communities A and B, respectively. Therefore, determining PGE2 and 5-iPF2α-VI in raw wastewater can be a reliable biomarker of community oxidative anxiety. Future studies are suggested to validate the potential of PGE2 and 5-iPF2α-VI in raw wastewater collected from diverse communities on typical days. To our knowledge, this is the first report of the quantification of 5-iPF2α-VI and PGE2 in wastewater. The target metabolite of 8-iso-PGF2α (2,3-dinor-iPF2α-III) was not detected in any wastewater samples.
3.4. Mass load of isoprostanes to the WWTPs
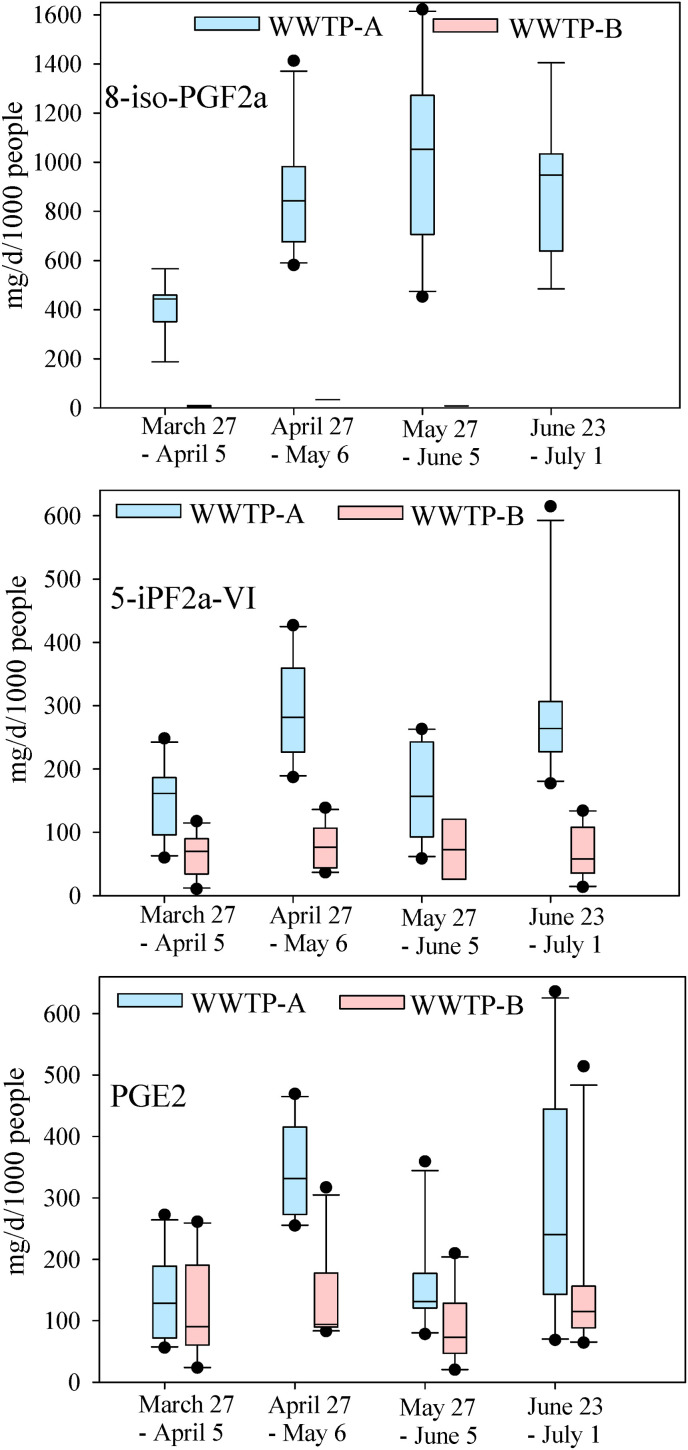
The 4-month average mass load of isoprostanes in both communities ranged from 10.1 mg/d/1000 people (8-iso-PGF2α) to 807 mg/d/1000 people (8-iso-PGF2α) (Fig. 2 ). The average mass load of 8-iso-PGF2α is > 1.4 fold and >50 fold higher in communities A and B, respectively than that reported in typical days in European cities (Ryu et al., 2016; Ryu et al., 2019) and Detroit, Michigan (Santos et al., 2015). Sims et al. (2019) also detected 8-iso-PGF2β, an isomer of 8-iso-PGF2α, in raw wastewater collected from a WWTP serving a community (∼900,000 people) in southwest of England (Sims et al., 2019). Interestingly, the average mass loads of PGE2 and 5-iPF2α-VI in community A were significantly increased (two-tailed p < 0.001) from the first month of COVID-19 pandemic to the second month; however, significantly decreased (two-tailed p < 0.001) in the third month (Fig. 2). In Kentucky and Tennessee, the state announced executive orders to close schools, public gatherings, and non-life-sustaining business in March after COVID-19 state of emergency declared in the first week of March. In the second week of May, the state announced the conditional reopening of select businesses and public gatherings. The significant drop in the mass load of PGE2 and 5-iPF2α-VI in the third month (wastewater sampled from May 27 to June 5) can have resulted from the partial reopening of businesses. Despite the partial reopening of businesses, surge in COVID-19 cases in late June in the U.S. including Kentucky and Tennessee can have significantly contributed to the elevated level of isoprostanes again, and hence community anxiety.
Fig. 2.
Mass load of 8-iso-PGF2α (8-iso-prostaglandin F2α), 5-iPF2α-VI (5-iso Prostaglandin F2α-VI, and PGE2 (prostaglandin E₂) in communities A and B. Boxplots showing the median line, interquartile range (25–75 percentiles), whiskers (10 and 90 percentiles), and outliers.
Moreover, ∼25% of college students in China reported mild to severe anxiety and depression associated with the COVID-19 pandemic (Cao et al., 2020). College students comprise a significant proportion (∼45%) of both of these communities. The combined COVID-19 anxiety and examination stress in April–May could have contributed to the high level of isoprostanes in wastewater.
4. Implications and summary
Overall, an analytical method capable of determining four biomarkers of oxidative stress (8-iso-PGF2α, 2,3-dinor-iPF2α-III, PGE2, and 5-iPF2α-VI) in wastewater was developed and validated. The enzyme hydrolysis of wastewater significantly increased the free isoprostanes in wastewater. The higher level of isoprostanes determined in wastewater in this study can have resulted from the potential oxidative anxiety induced by the COVID-19 uncertainties. For the first time, PGE2 and 5-iPF2α-VI were quantified consistently in both communities. We conclude that the determination of PGE2 and in raw wastewater may provide a more reliable biomarker of community oxidative anxiety than 8-iso-PGF2α. The higher mass load of isoprostanes in this study, compared to the reported values, may be attributed to the community level oxidative anxiety owing to COVID-19 uncertainties. It is important to note that the stabilities of target isoprostanes in wastewater were not evaluated in this study as previous studies (Ryu et al., 2015; O’Brien et al., 2019) reported the prominent stability of isoprostanes even at room temperature; however, the stability of target isoprostanes in wastewater would minimize the uncertainties on mass loads of target isoprostanes into the WWTPs. Further research is warranted to establish isoprostanes including PGE2 and 5-iPF2α-VI as the reliable biomarker of community-level oxidative injuries.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Authors are thankful to The Jones/Ross Research Center at the Department of Chemistry, Murray State University for providing access to the UPLC-MS/MS. Wastewater treatment plants are acknowledged for providing access to the 24 h composite wastewater samples. Authors also would like to thank Mr. Alexander B. Montgomery for the assistnace with wastewater sampling. Funding: This study was funded by grants from the Kentucky Biomedical Research Infrastructure Network (Grant# NIGMS - P20GM103436) and the Committee on Institutional Studies and Research, Murray State University. The content is solely the responsibility of the authors and does not necessarily represent the official views of the sponsors. Authors Contributions: Isaac Bowers collected wastewater samples, performed sample preparation, instrumental analysis, analytical data interpretation, and prepared the first draft of the manuscript. Bikram Subedi conceptualized the project, collected wastewater samples, performed sample preparation, instrumental analysis, analytical data interpretation, prepared and reviewed the manuscript, and supervised overall research project. Both authors reviewed and edited the paper. Competing financial interests: The authors declare no conflict of interests.
Handling Editor: Klaus Kümmerer
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.chemosphere.2020.129489.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- Bagchi D., Carryl O.R., Tran M.X., Bagchi M., Garg A., Milnes M.M., Williams C.B., Balmoori J., Bagchi D.J., Mitra S., Stohs S.J. Acute and chronic stress-induced oxidative gastrointestinal mucosal injury in rats and protection by bismuth subsalicylate. Mol. Cell. Biochem. 1999;196:109–119. [PubMed] [Google Scholar]
- Barocas D.A., Motley S., Cookson M.S., Chang S.S., Penson D.F., Dai Q., Milne G., Roberts L.J., Morrow J., Concepcion R.S., Smith J.A., Fowke J.H. Oxidative stress measured by urine F2-isoprostane level is associated with prostate cancer. J. Urol. 2011;185:2102–2107. doi: 10.1016/j.juro.2011.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Been F., Rossi L., Ort C., Rudaz S., Delémont O., Esseiva P. Population normalization with ammonium in wastewater-based epidemiology: application to illicit drug monitoring. Environ. Sci. Technol. 2014;48:8162–8169. doi: 10.1021/es5008388. [DOI] [PubMed] [Google Scholar]
- Blak C.N., Bot M., Scheffer P.G., Cuijpers P., Penninx B.W.J.H. Is depression associated with increased oxidative stress? A systematic review and meta-analysis. Psychoneuroendocrinology. 2015;51:164–175. doi: 10.1016/j.psyneuen.2014.09.025. [DOI] [PubMed] [Google Scholar]
- Cao W., Fang Z., Hou G., Han M., Xu X., Dong J. The psychological impact of the COVID-19 epidemic on college students in China. Psychiatr. Res. 2020;287:112934–112939. doi: 10.1016/j.psychres.2020.112934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casselman B. N. Y. Times; 2020. Worst economy in the decade. What’s next? “Worst in our lifetime”.https://www.nytimes.com/2020/04/29/business/economy/us-gdp.html [Google Scholar]
- Cibbattoni G., Pugliese F., Spaldi M., Cinotti G.A., Patrono C. Radioimmunoassay measurement of prostaglandins E2 and F2α in human urine. J. Endocrinol. Invest. 1979;2:173–183. doi: 10.1007/BF03349310. [DOI] [PubMed] [Google Scholar]
- Croft T.L., Huffines R.A., Pathak M., Subedi B. Prevalence of illicit and prescribed neuropsychiatric drugs in three communities in Kentucky using wastewater-based epidemiology and Monte Carlo simulation for the estimation of associated uncertainties. J. Hazard Mater. 2020;384:121–360. doi: 10.1016/j.jhazmat.2019.121306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daughton C. Using biomarkers in sewage to monitor community-wide human health: isoprostanes as conceptual prototype. Sci. Total Environ. 2012;424:16–38. doi: 10.1016/j.scitotenv.2012.02.038. [DOI] [PubMed] [Google Scholar]
- Erie M., Asami S., Ikeda M., Kasai H. Depressive state relates to female oxidative DNA damage via neutrophil activation. Bichem. Biophys. Res. Commun. 2003;311:1014–1018. doi: 10.1016/j.bbrc.2003.10.105. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick K.M., Harris C., Drawve G. Fear of COVID-19 and the mental health consequences in America. Psychological Trauma: Theory, Research, Practice, and Policy. 2020;12S1:S17–S21. doi: 10.1037/tra0000924. [DOI] [PubMed] [Google Scholar]
- Gidron Y., Russ K., Tissarchondou H., Warner J. The relation between psychological factors and DNA-damage: a critical review. Biol. Psychol. 2006;72:291–304. doi: 10.1016/j.biopsycho.2005.11.011. [DOI] [PubMed] [Google Scholar]
- Holder C., Adams A., McGahee E., Xia B., Blount B.C., Wang L. High-throughput and sensitive analysis of free and total 8-isoprostane in urine with isotope-dilution liquid chromatography-tandem mass spectrometry. ACS Omega. 2020;5:10919–10926. doi: 10.1021/acsomega.0c00661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang Y., Wei P., Duke R.W., Reaven P.D., Harman M., Cutler R.G., Heward C.B. Quantification of 8-iso prostaglandin-F2α and 2,3-dinor-8-isoprostaglandin-F2α in human urine using liquid chromatography-tandem mass spectrometry. Free Radical Biol. Med. 2003;34:409–418. doi: 10.1016/s0891-5849(02)01018-3. [DOI] [PubMed] [Google Scholar]
- Milne G.L., Yin H., Hardy K.D., Davies S.S., Roberts L.J. Isoprostane generation and function. Chem. Rev. 2011;111:5973–5996. doi: 10.1021/cr200160h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrow J.D., Roberts L.J. Quantification of noncyclooxygenase derived prostanoids as a marker of oxidative stress. Free Radical Biol. Med. 1991;10:195–200. doi: 10.1016/0891-5849(91)90076-f. [DOI] [PubMed] [Google Scholar]
- Murai Y., Hishinuma T., Suzuki N., Satoh J., Toyota T., Mizugaki M. Determination of urinary 8-epi-prostaglandin F(2α) using liquid chromatography-tandem mass spectrometry: increased excretion in diabetics. Prostag. Other Lipid Mediat. 2000;62:173–181. doi: 10.1016/s0090-6980(00)00061-7. [DOI] [PubMed] [Google Scholar]
- O’Brien J.W., Choi P.M., Li J., Thai P.K., Jiang G., Tscharke B.J., Mueller J.F., Thomas K.V. Evaluating the stability of three oxidative stress biomarkers under sewer conditions and potential impact for use in wastewater-based epidemiology. Water Res. 2019;166:115068–115075. doi: 10.1016/j.watres.2019.115068. [DOI] [PubMed] [Google Scholar]
- O’Rourke C.E., Subedi B. Occurrence and mass loading of synthetic opioids, synthetic cathinones, and synthetic cannabinoids in wastewater treatment plants in four U.S. communities. Environ. Sci. Technol. 2020;54:6661–6670. doi: 10.1021/acs.est.0c00250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parrado-Gonzalez A., Leon-Jariego J.C. COVID-19: factors associated with emotional distress and psychological morbidity in Spanish population. Rev. Esp. Salud Publica. 2020;94 e1–16. [PubMed] [Google Scholar]
- Pedreanez A., Arcaya J.L., Carrizo E., Rincon J., Viera N., Pena C., Vargas R., Mosquera J. Experimental depression induces renal oxidative stress in rats. Physiol. Behav. 2011;104:1002–1009. doi: 10.1016/j.physbeh.2011.06.021. [DOI] [PubMed] [Google Scholar]
- Pratico D., Barry O.P., Lawson J.A., Adiyaman F., Hwang S.W., Khanapure S.P., Iuliano L., Rokach J., Fitzgerald G.A. IPF2α-I: an index of lipid peroxidation in humans. Proc. Natl. Acad. Sci. U.S.A. 1998;95:3449–3454. doi: 10.1073/pnas.95.7.3449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi R., Socci V., Talevi D., Mensi S., Niolu C., Paccitti F., Marco A.D., Rossi A., Siracusano A., Lorenzo D.G. COVID-19 pandemic and lockdown 1 measures impact on mental health among the general population in Italy. An N=18147 web-based survey. Front. Psychol. 2020;11:1–6. doi: 10.3389/fpsyt.2020.00790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryu Y., Reid M.J., Thomas K.V. Liquid chromatography–high resolution mass spectrometry with immunoaffinity clean-up for the determination of the oxidative stress biomarker 8-iso-prostaglandin F2α in wastewater. J. Chromatgr. A. 2015;1409:146–151. doi: 10.1016/j.chroma.2015.07.060. [DOI] [PubMed] [Google Scholar]
- Ryu Y., Gracia-Lor E., Bade R., Baz-Lomba J.A., Bramness J.G., Castiglioni S., Castrignano E., Causanilles A., Covaci A., Voogt P., Hernamdez F., Kasprzyk-Hordern B., Kinuya J., McCall A.K., Ort C., Plosz B.G., Ramin P., Rousis N.I., Reid M.J., Thomas K.V. Increased levels of the oxidative stress biomarker 8-isoprostaglandin F2α in wastewater associated with tobacco use. Sci. Rep. 2016;6:39055–39072. doi: 10.1038/srep39055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos J.M., Jurban M., Kim H. Could sewage epidemiology be a strategy to assess lifestyle and wellness of a large scale population? Med. Hypotheses. 2015;85:408–411. doi: 10.1016/j.mehy.2015.06.020. [DOI] [PubMed] [Google Scholar]
- Schiavone S., Jaquet V., Trabace L., Krause K.H. Severe life stress and oxidative stress in the brain: from animal models to human pathology. Antioxd. Redox Signaling. 2013;18:1475–1490. doi: 10.1089/ars.2012.4720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sims N., Rice J., Kasprzyk-Hordern B. An ultra-performance liquid chromatography tandem mass spectrometry method for oxidative stress biomarker analysis in wastewater. Anal. Bioanal. Chem. 2019;411:2261–2271. doi: 10.1007/s00216-019-01667-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivonova M., Zitnanova I., Hlincikova L., Skodacek I., Trebaticka J., Durackova Z. Oxidative stress in university students during examinations. Stress. 2004;7:183–188. doi: 10.1080/10253890400012685. [DOI] [PubMed] [Google Scholar]
- Talevi D., Socci V., Carai M., Carnaghi G., Faleri S., Trebbi E., Bernardo A.D., Capelli F., Pacitti F. Mental health outcomes of the CoViD-19 pandemic. Riv. Psichiatr. 2020;55:137–144. doi: 10.1708/3382.33569. [DOI] [PubMed] [Google Scholar]
- USCB (United States Census Bureau) 2019. Population and Housing Unit Estimates.https://www.census.gov/programs-surveys/popest.html?intcmp=serp [Google Scholar]
- WHO World Health Organization . WHO survey; 2020. COVID-19 Disrupting Mental Health Services in Most Countries.https://www.who.int/news/item/05-10-2020-covid-19-disrupting-mental-health-services-in-most-countries-who-survey [Google Scholar]
- Yan W., Byrd G.D., Ogden M.W. Quantitation of isoprostane isomers in human urine from smokers and nonsmokers by LC-MS/MS. J. Lipid Res. 2007;48:1607–1617. doi: 10.1194/jlr.M700097-JLR200. [DOI] [PubMed] [Google Scholar]
- Yan Z., Mas E., Mori T.A., Croft K.D., Barden A.E. A significant proportion of F2-isoprostanes in human urine are excreted as glucuronide conjugates. Anal. Biochem. 2010;403:126–128. doi: 10.1016/j.ab.2010.04.016. [DOI] [PubMed] [Google Scholar]
- Zhang Z. Systematic review on the association between F2-isoprostanes and cardiovascular disease. Ann. Clin. Biochem. 2013;50:108–114. doi: 10.1258/acb.2012.011263. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Ma Z.F. Impact of the COVID-19 pandemic on mental health and quality of life among local residents in Liaoning province, China: a cross-sectional study. Int. J. Environ. Res. Publ. Health. 2020;17:2381–2393. doi: 10.3390/ijerph17072381. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.