Abstract
Objective
This study aimed to compare the efficacy, side effects, and clinical outcomes between parenteral iron sucrose complex (ISC) and low-molecular-weight iron dextran (LMWID) for iron deficiency anemia (IDA) in pregnancy.
Methods
The study was conducted in a Malaysian tertiary hospital for a period of 1 year. Forty pregnant women with IDA between 24 and 38 weeks of gestation were randomized into two groups receiving treatment with either ISC or LMWID.
Results
No significant difference was found between the groups in terms of demographic data, parity, and mean gestational age. A mean total of 835 ± 150 mg doses of ISC and 656 ± 382 mg doses of LMWID were administered (P = 0.0001). Adverse events were reported in five patients who received LMWID and none in those treated with ISC (P = 0.024). The mean hemoglobin (Hb) level increment 2 weeks post treatment was higher among those who received ISC than in those who received LMWID. The ISC group demonstrated an increase of 1.91 ± 1.10 g/dL (from 8.43 ± 1.03 g/dL to 10.29 ± 0.90 g/dL) compared with the LMWID group at 1.39 ± 0.54 g/dL (from 8.61 ± 0.70 g/dL to 9.92 ± 0.88 g/dL, P = 0.023). All participants in both groups delivered at term. The estimated blood loss during delivery was significantly higher in the LMWID group (359 ± 247 mL) than in the ISC group (280 ± 100 mL, P = 0.026). Otherwise, no significant difference was observed in terms of Hb level during delivery and the perinatal outcomes for both groups.
Conclusion
Parenteral ISC is more effective than LMWID in treating maternal IDA, and it is associated with fewer adverse events.
Keywords: iron deficiency anemia treatment, pregnancy, iron dextran, iron sucrose, maternal and perinatal outcomes
Introduction
Iron deficiency anemia (IDA) is an important health condition common among pregnant women.1 Pregnant women with IDA are exposed to increased risk of cardiovascular stress, fatigue, dizziness, exhaustion, pre-eclampsia, renal failure, antepartum hemorrhage, blood transfusion requirement, and even mortality.2–4 IDA is also associated with risk of adverse perinatal outcomes, such as intrauterine growth retardation, stillbirth, prematurity, and low birth weight.5
Treatments for IDA in pregnancy include oral iron, parenteral iron therapy, and blood transfusion. The most common treatment is oral iron therapy.5 However, it is associated with some drawbacks, such as gastrointestinal side effects, poor compliance, and treatment unresponsiveness due to poor iron absorption.3,6 Gastrointestinal adverse effects are reduced with intravenous iron therapy.7
Parenteral iron is an alternative treatment for IDA, indicated when the blood ferritin level drops to below 15 µg/L.1 Parenteral iron is quicker in increasing the hemoglobin (Hb) level and replenishing iron stores than oral iron.8,9 Several types of parenteral iron include intravenous iron sucrose complex (ISC) and low-molecular-weight iron dextran (LMWID). Both are effective, safe, fast, and convenient in treating maternal IDA.9,10 However, ISC requires the patient to stay longer in the hospital due to divided dosage.11 By contrast, LMWID can be administered in a short period as a total dose.12
A retrospective study of outpatients with chronic kidney disease undergoing intravenous ISC (Venofer) versus LMWID (TDI CosmoFer) was conducted by Sinha et al. in 2009.13 It revealed that the safety and efficacy of LMWID is comparable to ISC and not associated with an increase in adverse events.
Evaluation and comparison of the safety and efficacy between ISC and LMWID for the treatment of maternal IDA are rarely reported. Thus, this randomized trial assessed and compared the hematological parameters, clinical outcome, adverse effects, and perinatal outcomes of the two treatments.
Methodology
Study Design and Participants
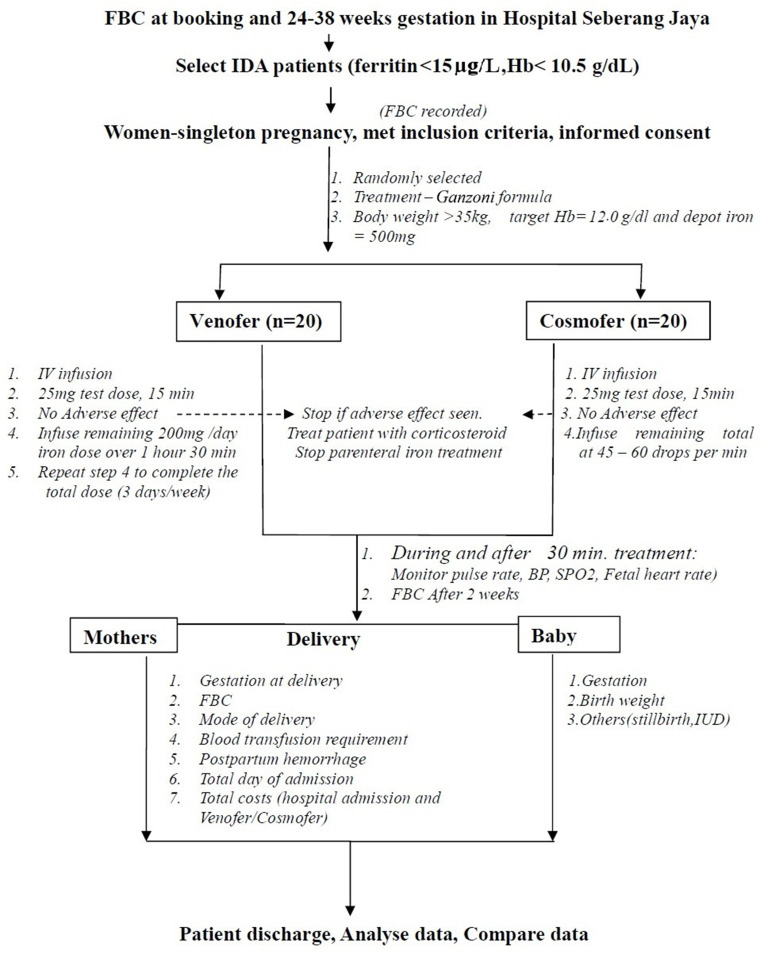
This prospective, open-label, randomized controlled trial involving 40 pregnant women was conducted in Hospital Seberang Jaya, Malaysia from May 5, 2017, to May 4, 2018. Ethical clearance was obtained from the Malaysia Ministry of Health and Ethics Committee (Research Code: NMRR-17-292-34079) and The Medical Research and Ethics Committee, Universiti Kebangsaan Malaysia Medical Centre (Research Code: FF-2017-440). Pregnant women visiting the antenatal clinic with singleton pregnancy between 24–38 weeks and aged 17 and above with IDA (ferritin < 15 µg/L and Hb < 10.5 g/dL) were referred to the Patient Admission Centre where they were screened. The first eligible and consented 40 patients were enrolled in the study (Figure 1). Exclusion criteria included patients with other causes of anemia, such as thalassemia or thalassemia trait, diabetes mellitus, pregnancy-induced hypertension, thyroid disease, liver disease, kidney problems, hemolytic anemia, and known allergy to any intravenous iron infusion. The participants were informed about the purpose of the study and written consent was obtained prior to enrolment. All procedures followed were in accordance with the ethical standards of institutional and national guidelines on human experimentation. The study was conducted in accordance with Good Clinical Practice guidelines and according to the Revised Declaration of Helsinki.14
Figure 1.
Methodology Chart.
Interventions
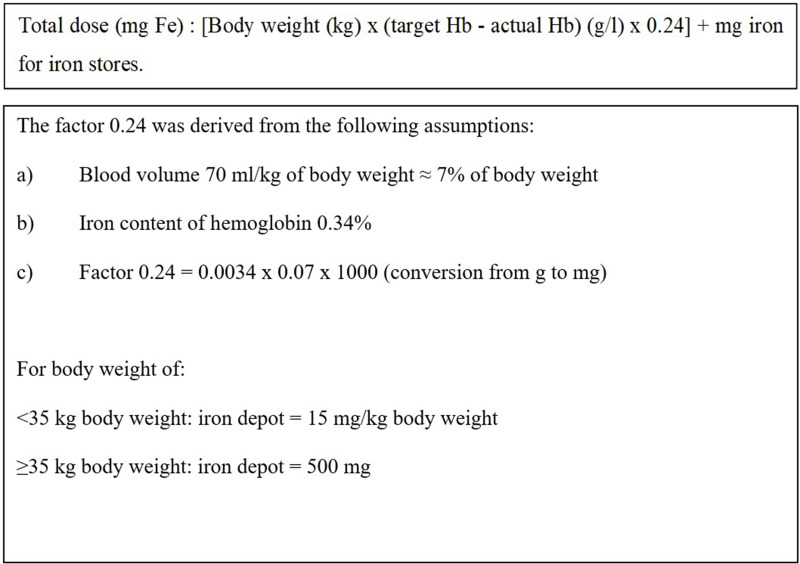
Eligible participants were randomized into two groups in a 1:1 ratio using online software (Sealed Envelope Ltd, 2016). They were administered with either ISC or LMWID. Total iron requirement was calculated using the Ganzoni formula (Figure 2). The participants were admitted to wards for the administration of ISC and LMWID. Any adverse or side effects were observed for at least 24 hours post infusion and recorded in an evaluation form.
Figure 2.
Ganzoni Formula.
The patients in the ISC group were intravenously administered with iron sucrose (Venofer, Vifor, St. Gallen, Switzerland) as a test dose of 25 mg infusion of ISC in 25 mL of 0.9% normal saline for 15 min. The next 175 mg of ISC in 175 mL of normal saline was administered over 90 min at an interval of 1–3 days per week until the dosage was completed, and it did not exceed 600 mg a week.11
For the LMWID group, intravenous LMWID (CosmoFer, Pharmacosmos, Holbaek, Denmark) was infused for 4–6 hours. A maximal dose of 20 mg/kg was diluted into 500 mL of 0.9% normal saline. The first 25 mg of the test dose was infused for over a period of 15 min. If no adverse event was observed, the remaining portion was then infused. The rate of infusion was increased progressively to 45–60 drops per min (or 1.4–2.1 mL/min). The patients were observed carefully during the infusion and for at least 30 min after completion.12
The general condition, blood pressure, and pulse rate of the patients were recorded before infusion and every 5 min during infusion. Fetal heart rate monitoring was also performed before and after infusion.
Infusion for ISC and LMWID were withheld if the patients developed side effects. All patients recruited were followed up in the antenatal clinic after 2 weeks and full blood counts were taken. The results were compared before and after the infusion of ISC or LMWID. The patient’s demography including age, body mass index (BMI), gestation age of pregnancy and total dosage requirement were obtained. BMI (kg/m2) was computed using weight (kg) and height (m) measurements. Other parameter outcomes including hemoglobin increment; adverse effects; timing of delivery; incidence of postpartum hemorrhage (PPH), including blood transfusion requirement postpartum; and length of stay were recorded. The neonatal gestation at delivery and birth weight in the two parenteral iron groups were investigated and compared.
Sample Size Determination
The sample size was estimated using two population mean formulas for power and sample-size calculations. From the pilot study conducted from November 2013 until November 2016, a retrospective data study comparing ISC with oral iron, the number of patients on ISC was 12 (13.8%) compared with 75 patients on oral iron (86.2%). In order to get the response within each subject group distributed with a standard deviation (SD) of 1.2, with an alpha error of 5%, the estimated sample size was 40 per arm for the power of 80% and 20% of the dropout rate. However, at the conclusion of 20 per arm (block randomization size of 4), an interim analysis was conducted. At a sample size of 20 per arm, the result showed a significant difference. Thus, participant recruitment was stopped.
Statistical Analysis
Data were analyzed on SPSS version 22.0. Descriptive data were expressed as mean ± SD. Normal distribution was recorded, and independent Student’s t-test was used for the analysis. Categorical data were analyzed using Chi-square and Fisher’s Exact tests. A value of P < 0.05 was considered statistically significant. The data collected were analyzed on an intention-to-treat basis.
Results
In accordance with the inclusion and exclusion criteria, the first eligible and consented 40 patients were enrolled in the study and randomized into two groups of 20 each, and they were administered with either ISC or LMWID. The demographic characteristics of the study population were comparable between the two groups (Table 1). No significant difference was found in BMI, gestational age, and mean calculated iron requirement (Table 2).
Table 1.
Demography of the Subjects According to the Treatment Groups Between ISC (n=20) and LMWID (n=20)
| Demography | Measurement | ISC | LMWID | P value |
|---|---|---|---|---|
| Age (years) | Mean ± SD | 26.95 ± 4.37 | 27.10 ± 3.64 | 0.560 |
| 20–24 | N (%) | 6 (30.0) | 4 (20.0) | 0.634 |
| 25–29 | 9 (45.0) | 11 (55.0) | ||
| 30–34 | 4 (20.0) | 5 (25.0) | ||
| 35–39 | 1 (5.0) | 0 (0.0) | ||
| Total | 20 (100.0) | 20 (100.0) | ||
| Ethnicity | N (%) | 0.314 | ||
| Malay | 11 (55.0) | 7 (35.0) | ||
| Indian | 9 (45.0) | 12 (60.0) | ||
| Others | 0 (0.0) | 1 (5.0) | ||
| Total | 20 (100.0) | 20 (100.0) | ||
| Occupation | N (%) | 0.270 | ||
| Housewives | 10 (50.0) | 6 (30.0) | ||
| Government sector | 5 (25.0) | 5 (25.0) | ||
| Private sector | 4 (20.0) | 9 (45.0) | ||
| Post-graduate student | 1 (5.0) | 0 (0.0) | ||
| Total | 20 (100.0) | 20 (100.0) | ||
| Weight | Mean ± SD | 55.76 ± 10.67 | 61.76 ± 15.19 | 0.136 |
| BMI | Mean ± SD | 23.14 ± 4.17 | 24.85 ± 5.60 | 0.275 |
| Underweight | N (%) | 2 (10.0) | 1 (5.0) | 0.611 |
| Ideal weight | 14 (70.0) | 12 (60.0) | ||
| Overweight | 2 (10.0) | 3 (15.0) | ||
| Obese | 2 (10.0) | 2 (10.0) | ||
| Severely obese | 0 (0.0) | 2 (10.0) | ||
| Total | 20 (100.0) | 20 (100.0) | ||
| Gestation (week) | Mean ± SD | 32.45 ± 3.55 | 33.85 ± 3.86 | 0.845 |
| 24+0 till 26+6 | N (%) | 2 (10.0) | 1 (5.0) | |
| 27+0 till 29+6 | 3 (15.0) | 2 (10.0) | ||
| 30+0 till 32+6 | 4 (20.0) | 3 (15.0) | ||
| 33+0 till 35+6 | 7 (35.0) | 7 (35.0) | ||
| 36+0 till 38+6 | 4 (20.0) | 7 (35.0) | ||
| Total | 20 (100.0) | 20 (100.0) | ||
| Gravida | N (%) | 0.370 | ||
| G1P0 | 5 (25.0) | 3 (15.0) | ||
| G2P0+1 to G2P1 | 9 (45.0) | 8 (40.0) | ||
| G3P1+1 to G3P2 | 5 (25.0) | 3 (15.0) | ||
| G4P3 to G4P4 | 1 (5.0) | 2 (10.0) | ||
| G5P2+2 to G5P4 | 0 (0.0) | 3 (15.0) | ||
| G6P3+2 | 0 (0.0) | 1 (5.0) | ||
| Total | 20 (100.0) | 20 (100.0) |
Table 2.
Demography of the Subjects According to the Treatment Groups Between ISC (n=20) and LMWID (n=20), Delivery and Perinatal Outcomes
| Measurement | ISC | LMWID | P value | |
|---|---|---|---|---|
| Demography | ||||
| Age (years) | Mean ± SD | 26.95 ± 4.37 | 27.10 ± 3.64 | 0.560 |
| BMI | Mean ± SD | 23.14 ± 4.17 | 24.85 ± 5.60 | 0.275 |
| Gestation (week) | Mean ± SD | 32.45 ± 3.55 | 33.85 ± 3.86 | 0.845 |
| Dosage (mg) | Mean ± SD | 835.0 ± 149.7 | 656.3 ± 382.2 | 0.0001 |
| Total Hospital Admission (day) | Mean ± SD | 6.7 ± 1.7 | 5.2 ±1.6 | 0.907 |
| Delivery Outcomes | ||||
| Gestation (week) | Mean ± SD | 38.60 ± 0.88 | 39.05 ± 0.76 | 0.116 |
| Perinatal Outcomes | ||||
| Birth weight (kg) | Mean ± SD | 2.949 ± 0.294 | 3.091 ± 0.396 | 0.144 |
The primary outcome was the increment in Hb from baseline 2 weeks post treatment (Table 4), which was significantly higher among those who received ISC at 1.91 ± 1.10 g/dL (from 8.43 ± 1.03 g/dL to 10.29 ± 0.90 g/dL) than in those who received LMWID at 1.39 ± 0.54 g/dL (from 8.61 ± 0.70 g/dL to 9.92 ± 0.88 g/dL, P = 0.023).
Table 4.
The Ferritin (µg/L), Hemoglobin (g/dL) Levels and Increment Rate (g/dL) from Baseline (Pre-Treatment) in ISC and LMWID Treated Groups
| ISC | LMWID |
P value |
|||
|---|---|---|---|---|---|
| n | Mean ± SD | n | Mean ± SD | ||
|
Ferritin (µg/L) Pre-Treatment |
20 | 7.500 ± 2.164 | 20 | 7.050 ± 2.114 | 0.654 |
|
Hemoglobin (g/dL) Booking |
20 | 10.355 ± 2.004 | 20 | 10.605 ± 1.723 | 0.116 |
| Treatment: | |||||
| Pre- | 20 | 8.425 ± 1.026 | 20 | 8.610 ± 0.701 | 0.091 |
| * Post - 2 weeks | 18 | 10.294 ± 0.899 | 13 | 9.923 ± 0.880 | 0.918 |
| **Post - Delivery | 19 | 10.963 ± 0.891 | 20 | 10.660 ± 1.284 | 0.084 |
| P value | < 0.0001 | < 0.0001 | |||
| Hemoglobin (g/dL) increment rate from baseline | |||||
| * Post- 2 weeks | 18 | 1.906 ± 1.101 | 13 | 1.392 ± 0.539 | 0.023 |
| **Post-Delivery | 19 | 2.542 ± 1.290 | 20 | 2.050 ± 1.073 | 0.412 |
Notes: *ISC, default (n = 1), delivered earlier than the 2 weeks follow-up date (n = 1); LMWID, default (n = 2), default due to side effects (n = 2), delivered earlier than the 2 weeks follow-up date (n = 3); **LMWID, delivered at other district hospital (n = 1).
The mean (± SD) days of hospital admission for the ISC group was found to be longer (7 ± 2 days) than the LMWID group (5 ± 2 days). The shorter hospital stay of LMWID was due to one infusion of total dose. However, the different was not significant between the two treated groups (P > 0.05) (Table 2).
The treatments were well-tolerated by all participants in the ISC group, and no adverse effect was observed (Table 3). However, five participants (25.0%) from the LMWID group developed adverse effects (P = 0.024) during the test dose and infusions. These adverse effects included shortness of breath (n = 3), giddiness (n = 2), vomiting (n = 1), nausea (n = 1), chest tightness, and hypotension (n = 1, in each). The participants were assessed for side effects and management instituted accordingly. None of the participants required intensive care monitoring. LMWID was stopped in all participants who developed side effects.
Table 3.
Side Effects, Postpartum Hemorrhage and Blood Transfusion Among Subjects According to the Treatment Groups Between ISC (n=20) and LMWID (n=20)
| Measurement | ISC | LMWID | *P value | |
|---|---|---|---|---|
| Side effects | ||||
| Yes | N (%) | 0 (0.0) | 5 (25.0) | 0.024 |
| No | N (%) | 20 (100.0) | 15 (75.0) | |
| Types of Side effects | ||||
| Short of breathness | N (%) | – | 3 (15.0) | – |
| Giddiness | N (%) | – | 1 (5.0) | – |
| Nausea/Vomiting | N (%) | – | 2 (10.0) | – |
| Chest Tightness | N (%) | – | 1 (5.0) | – |
| Hypotension | N (%) | – | 1 (5.0) | – |
| Postpartum hemorrhage | ||||
| Yes | N (%) | 1 (5.0) | 3 (15.0) | 0.302 |
| No | N (%) | 19 (95.0) | 17 (85.0) | |
| Blood transfusion | N | 0 | 1 | 0.500 |
Note: *P value calculated using Fisher’s Exact test.
The mean (± SD) values of estimated blood loss during delivery in the ISC and LMWID groups were 280.3 ± 99.9 and 359.0 ± 246.5 mL, respectively. The mean difference was significant at −79 mL (95% CI: −199 - 42; P = 0.026). PPH was observed in three participants from the LMWID group and one in the ISC group (P = 0.302). One participant from the LMWID group was transfused with 2 pints of packed red blood cells after delivery. None of the participants from the ISC group required blood transfusion (Table 3).
Blood tests were not performed on two patients from the ISC group. One patient defaulted and another patient delivered before the follow-up date (2 weeks post-treatment). The mean (± SD) of Hb at 2 weeks post-treatment for the ISC group was 10.3 ± 0.9 g/dL. Blood tests were also not performed on seven patients from the LMWID therapy group. Two patients had allergic side effects and did not complete the dose and defaulted on their follow-up. Three patients delivered before their follow-up dates (the gestation during treatment was 37 + 4 and 38 (n=2)). Two other patients defaulted on their follow-up. The mean (± SD) of Hb for the LMWID therapy group was 9.9 ± 0.9 g/dL (Table 6). No significant difference (P > 0.05) was observed between the ISC and LMWID groups according to Hb levels at 2 weeks post-treatment (Table 3).
Table 6.
Demography, Delivery and Perinatal Outcomes, Ferritin (µg/L), Hemoglobin, Hb (g/dL) Levels and Increment Rate (g/dL) from Baseline (Pre-Treatment) of the Subjects According to the Treatment Groups Between ISC (n=20) and LMWID (n=20)
| ISC | Before Treatment | After Treatment | Hb Increment Rate (g/dL) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. | Age (Year) | BMI | Gestation (Week) | Total Dose (mg) | Gestation at Delivery (week) | Blood Loss (mL) | Birth Weight (kg) | Booking Hb (g/dL) | Ferritin (µg/L) | Hb (g/dL) | Hb-post 2 Weeks (g/dL) | Hb-at Delivery (g/dL) | Post 2 Weeks | At Delivery |
| 1 | 26 | 28.0 | 36 | 600 | 38 | 350 | 2.87 | 11.5 | 11.0 | 9.9 | 10.1 | 10.1 | 0.2 | 0.2 |
| 2 | 30 | 28.4 | 35 | 700 | 38 | 300 | 2.49 | 8.3 | 7.0 | 9.1 | 11.3 | 10.8 | 2.2 | 1.7 |
| 3 | 28 | 23.4 | 32+3 | 800 | 39+3 | 300 | 2.93 | 12.2 | 5.0 | 9.3 | 11.3 | 11.6 | 2 | 2.3 |
| 4 | 30 | 21.6 | 36+2 | 1000 | 39+5 | 200 | 3.05 | 11.6 | 6.0 | 7.7 | 10.6 | 11.6 | 2.9 | 3.9 |
| 5 | 27 | 32.7 | 36+2 | 1000 | 37+6 | 300 | 3.28 | 8.8 | 10.0 | 8.3 | 9 | 8.8 | 0.7 | 0.5 |
| 6 | 34 | 22.0 | 33+3 | 1000 | 40+2 | 200 | 2.5 | 11.9 | 7.0 | 8.5 | 11.1 | DDH | 2.6 | - |
| 7 | 27 | 21.9 | 35+2 | 1000 | 39 | 500 | 3.22 | 8.3 | 10.0 | 7.5 | 9.3 | 10.6 | 1.8 | 3.1 |
| 8 | 27 | 19.3 | 30+5 | 800 | 38+4 | 300 | 3.13 | 8.3 | 12.0 | 7.8 | 8.6 | 10.2 | 0.8 | 2.4 |
| 9 | 24 | 20.8 | 26+5 | 1000 | 38+5 | 200 | 3.4 | 7.4 | 5.0 | 6.5 | 11.1 | 11.0 | 4.6 | 4.5 |
| 10 | 22 | 31.6 | 35+5 | 800 | 38+1 | 200 | 3.1 | 11.1 | 10.0 | 10.2 | ED | 11.6 | - | 1.4 |
| 11 | 22 | 18.2 | 33 | 900 | 38+4 | 200 | 2.7 | 12.8 | 7.0 | 8.9 | 10.2 | 11.1 | 1.3 | 2.2 |
| 12 | 21 | 18.4 | 34+6 | 800 | 37+6 | 300 | 2.43 | 7.8 | 8.0 | 7.3 | Defaulted | 9.5 | - | 2.2 |
| 13 | 23 | 24.6 | 32+4 | 700 | 38+5 | 475 | 2.76 | 11.3 | 7.0 | 8.4 | 10.0 | 10.3 | 1.6 | 1.9 |
| 14 | 25 | 23.7 | 25+3 | 900 | 39 | 200 | 2.98 | 11.2 | 6.0 | 8.2 | 11.2 | 12 | 3 | 3.8 |
| 15 | 25 | 19.1 | 28+5 | 700 | 40+6 | 200 | 3.42 | 12 | 9.0 | 7.6 | 8.7 | 11.8 | 1.1 | 4.2 |
| 16 | 28 | 20.8 | 34+1 | 500 | 39+5 | 180 | 3.2 | 12.4 | 6.0 | 9.3 | 10.4 | 10.8 | 1.1 | 1.5 |
| 17 | 27 | 22.7 | 32+4 | 900 | 40 | 400 | 2.65 | 12.3 | 7.0 | 8.9 | 11.3 | 11.8 | 2.4 | 2.9 |
| 18 | 34 | 19.2 | 25+6 | 700 | 39+1 | 200 | 3 | 9.2 | 4.0 | 9.4 | 10.7 | 12.2 | 1.3 | 2.8 |
| 19 | 22 | 21.8 | 28+6 | 1000 | 39+1 | 400 | 2.82 | 6.6 | 6.0 | 6.6 | 10 | 11.5 | 3.4 | 4.9 |
| 20 | 37 | 24.6 | 36+3 | 900 | 39+2 | 200 | 3.05 | 12.1 | 7.0 | 9.1 | 10.4 | 11 | 1.3 | 1.9 |
| Mean | 26.95 | 23.14 | 32.45 | 835.00 | 38.60 | 280.25 | 2.95 | 10.36 | 7.50 | 8.43 | 10.29 | 10.96 | 1.91 | 2.54 |
| SDV | 4.37 | 4.17 | 3.55 | 149.65 | 0.88 | 99.93 | 0.29 | 2.00 | 2.16 | 1.03 | 0.90 | 0.89 | 1.10 | 1.29 |
| N | 20 | 20 | 20 | 20 | 20 | 20 | 20 | 20 | 20 | 20 | 18 | 19 | 18 | 19 |
| LMWID | Before Treatment | After Treatment | Hb Increment rate (g/dL) | |||||||||||
| No. | Age (Year) | BMI | Gestation (Week) | Total Dose (mg) | Gestation at Delivery (Week) | Blood Loss (mL) | Birth Weight (kg) | Booking Hb (g/dL) | Ferritin (µg/L) | Hb (g/dL) | Hb-post 2 Weeks (g/dL) | Hb-at Delivery (g/dL) | Post 2 Weeks | At Delivery |
| 1 | 28 | 24.6 | 32 | 1000 | 38+9 | 200 | 3.49 | 12.2 | 10.0 | 7.6 | 9.2 | 11 | 1.6 | 3.4 |
| 2 | 31 | 21.9 | 31+4 | 900 | 40+4 | 300 | 3.78 | 8.4 | 10.0 | 8.8 | 10.8 | 12.2 | 2 | 3.4 |
| 3 | 29 | 36.7 | 33 | 100 (D=900) | 39+2 | 1000 | 3.05 | 13 | 7.0 | 8.6 | 9.7 | 9.9 | 1.1 | 1.3 |
| 4 | 28 | 18.9 | 35+5 | 25 (D= 800) | 37+5 | 300 | 2.58 | 8.7 | 6.0 | 7.7 | 8.1 | 8.1 | 0.4 | 0.4 |
| 5 | 28 | 21.9 | 27+3 | 900 | 39+6 | 180 | 2.6 | 8.1 | 10.0 | 8.4 | 10.9 | 10.8 | 2.5 | 2.4 |
| 6 | 22 | 23.9 | 34+5 | 25 (D= 500) | 38+5 | 200 | 2.56 | 7 | 3.0 | 7.8 | Defaulted | 9.7 | - | 1.9 |
| 7 | 33 | 19.1 | 27+4 | 500 | 40+2 | 200 | 3.4 | 10.8 | 8.0 | 9.0 | 10.2 | 11.0 | 1.2 | 2 |
| 8 | 27 | 26.8 | 36+3 | 900 | 39+3 | 300 | 3.17 | 10.4 | 5.0 | 8.6 | 10.3 | 11.2 | 1.7 | 2.6 |
| 9 | 25 | 23.1 | 34 | 800 | 40 | 200 | 2.5 | 11.4 | 8.0 | 9.4 | 11.2 | 13 | 1.8 | 3.6 |
| 10 | 30 | 22.2 | 35+1 | 900 | 40+3 | 500 | 2.57 | 11.3 | 7.0 | 9.1 | 10.2 | 12.0 | 1.1 | 2.9 |
| 11 | 22 | 25.7 | 36+1 | 25 (D= 1000) | 39+6 | 200 | 3.49 | 9.6 | 6.0 | 8.2 | 9.1 | 10.3 | 0.9 | 2.1 |
| 12 | 20 | 30.3 | 35+5 | 1000 | 39+3 | 200 | 2.98 | 12.2 | 3.0 | 7.4 | Defaulted | 9.4 | - | 2 |
| 13 | 28 | 23.5 | 24 | 1000 | 39+2 | 400 | 3.66 | 8.7 | 7.0 | 7.6 | 9 | 10.5 | 1.4 | 2.9 |
| 14 | 27 | 34.1 | 37+4 | 1000 | 39+2 | 300 | 3.36 | 11.1 | 6.0 | 8.5 | ED | 9.3 | - | 0.8 |
| 15 | 20 | 19.9 | 37+2 | 800 | 40+2 | 200 | 2.97 | 13 | 7.0 | 9.5 | Defaulted | 11.7 | - | 2.2 |
| 16 | 26 | 21.3 | 38 | 700 | 39+5 | 200 | 3.31 | 10.7 | 5.0 | 9.8 | ED | 12.4 | - | 2.6 |
| 17 | 31 | 23.2 | 30+6 | 900 | 38+5 | 600 | 2.78 | 10.3 | 7.0 | 8.9 | 9.9 | 11.8 | 1 | 2.9 |
| 18 | 28 | 16.9 | 38 | 700 | 39+3 | 1000 | 3.04 | 12.6 | 7.0 | 9 | ED | 9.1 | - | 0.1 |
| 19 | 29 | 26.7 | 36 | 900 | 39+2 | 400 | 3.1 | 12.0 | 9.0 | 9.0 | 10.4 | 10.4 | 1.4 | 1.4 |
| 20 | 30 | 36.3 | 34+4 | 25 (D= 900) | 39 | 300 | 3.42 | 10.6 | 10.0 | 9.3 | Defaulted | 9.4 | - | 0.1 |
| Mean | 27.10 | 24.85 | 33.85 | 656.25 | 39.05 | 359.00 | 3.09 | 10.61 | 7.05 | 8.61 | 9.92 | 10.66 | 1.39 | 2.05 |
| SDV | 3.64 | 5.60 | 3.86 | 382.16 | 0.76 | 246.53 | 0.40 | 1.72 | 2.11 | 0.70 | 0.88 | 1.28 | 0.54 | 1.07 |
| N | 20 | 20 | 20 | 20 | 20 | 20 | 20 | 20 | 20 | 20 | 13 | 20 | 13 | 20 |
| P value | 0.560 | 0.275 | 0.845 | 0.0001 | 0.116 | 0.026 | 0.144 | 0.116 | 0.654 | 0.091 | 0.918 | 0.084 | 0.023 | 0.412 |
Abbreviations: D, Planned dose; ED, Delivered before the appointment date; DDH, Delivered at different hospital.
It was found that 44.4% of the subjects from the ISC treated group were successfully treated and achieved the targeted Hb level at ≥ 10.5 g/dL (Table 5). However, only 23.1% of the LMWID group had achieved targeted levels (P > 0.22).
Table 5.
Hemoglobin Level and Number of Subjects at Post-Treatment Using ISC and LMWID
| Hb (g/dL) |
Post-Treatment - 2 Weeks | Post Treatment - Admission for Delivery | ||
|---|---|---|---|---|
| ISC (n) | LMWID (n) | ISC (n) | LMWID (n) | |
| < 10.5 | 10 (55.6%) | 10 (76.9%) | 5 (26.3%) | 9 (45.0%) |
| ≥ 10.5 | 8 (44.4%) | 3 (23.1%) | 14 (73.7%) | 11 (55.0%) |
| Total | 18 (100.0%) | 13 (100.0%) | 19 (100.0%) | 20 (100.0%) |
| P value | 0.220 | 0.224 | ||
Compliance Rate
Given the adverse effects and incomplete total dose infusion, the compliance rate among participants in the LMWID group was only 75.0% (n = 15). Those (n = 5) who suffered from adverse effects were less than 30 years old, with gestation age of above 33 weeks. The compliance rate in the ISC group was 100%.
Discussion
Maternal anemia, which is defined as Hb concentration < 11.0 g/dL, is one of the most common and widespread public health problems affecting more than 56 million women globally, two-thirds of whom are in Asia.15 According to the World Health Organization, the highest prevalence of anemia is in Africa and Southeast Asia. The report by WHO also highlighted India as the country with the highest prevalence of maternal anemia (49.7%) against the global prevalence of 41.8%. In Malaysia, 38% of pregnant women have anemia.16 The main factors that contribute to this disorder are young age, multiparity, and iron deficiency.
The demography of subjects in this study (age, ethnicity, occupation, BMI, gestation age, gravida, parity, and total dosage) was not significantly different between ISC and LMWID before initiation of the treatment (P > 0.05). According to da Costa et al. (2016), maternal age was identified as a risk factor for iron depletion during pregnancy.17 The majority (85.0%) of the IDA patients in this study were in the age range of 20–30 years old, which was consistent with Khaskheli et al.’s (2016) findings that the majority of IDA patients were 20–30 years old.18 The Hb levels (mean ± SD) among patients (n = 40) before undergoing any parenteral iron treatments were found to be significantly different (P = 0.014) between those aged ≤ 30 years (8.44 ± 0.92 g/dL) and those who were > 30 years old (8.95 ± 0.30 g/dL).
Parenteral iron increases Hb levels rapidly and replenishes iron stores more effectively than oral iron.16,19 In the present study, the mean Hb increment after treatment was significantly higher among those who received ISC than those who received LMWID, with a mean difference of 0.5 g/dL (95% CI: −0.17–1.2; P = 0.023). This finding was in agreement with that of Waziri et al. (2016), who saw a similar outcome among pre-dialysis patients with chronic kidney disease. They found that the Hb concentration increase from baseline was higher in ISC than in LMWID, with a mean difference of 0.2 g/dL (95% CI: −0.26–0.61; P = 0.028).20 The rate of Hb level increment (per day) 2 weeks post treatment was significantly faster in ISC (0.14 ± 0.08 g/dL per day) than in LMWID (0.10 ± 0.04 g/dL per day), with a mean difference of 0.04 g/dL per day (95% CI: −0.01–0.08; P = 0.023).
Even though the Hb levels increased after 2 weeks of parenteral iron treatments, the success rate of achieving the target Hb level (≥ 10.5 g/dL in third trimester) was low. A study by Gupta et al. (2014) showed a moderately similar finding, where the Hb level at pretreatment, 2 weeks post treatment, and delivery were 7.8 ± 0.4, 8.4 ± 0.4, and 11.5 ± 0.8 g/dL, respectively.21 As shown in Table 4, the present study also revealed that the Hb levels of the ISC group were 8.4 ± 1.0 (pre-treatment), 10.3 ± 0.9 (2 weeks post treatment), and 11.0 ± 0.9 (delivery) g/dL. However, the success rate of achieving the target Hb level in the ISC group was two times higher than that in the LMWID at 2 weeks post treatment. After parenteral iron therapy, the Hb levels during delivery (average of 6 weeks post treatment) showed that a higher number of patients achieved an acceptable maternal Hb level in the ISC group (73.7%) than in the LMWID group (55.0%) (Table 4).
IDA in pregnancy is associated with serious risks, such as renal failure, antepartum hemorrhage and mortality, cardiovascular stress, fatigue, dizziness, exhaustion, pre-eclampsia, bleeding, prolonged hospitalization, and an increase in blood transfusion requirement.2,4,16
The most commonly reported adverse reactions (≥ 2%) following the administration of ISC are diarrhea, nausea, vomiting, headache, dizziness, hypotension, pruritus, pain in extremity, fever, arthralgia, back pain, muscle cramp, injection site reactions, chest pain, and peripheral edema.13,16,22 However, in the present study, none of the 20 participants from the ISC group experienced any side effects.
The reported severe adverse reaction in LMWID included acute anaphylactic reactions, which usually happen within the first few minutes of infusion and are generally characterized by the sudden onset of respiratory difficulty and/or cardiovascular collapse. The less severe hypersensitivity reactions are urticaria, rashes, itching, nausea, and shivering.20 Five patients from the LMWID group experienced some of these reactions, with one patient developing shortness of breath. The side effects or adverse reactions were significantly higher in the LMWID group than in the ISC group (P = 0.024). Waziri et al. (2016) and Sinha et al. (2009) also found that patients in the LMWID group experienced an almost two times higher rate of adverse reactions than those in the ISC group.13,20
Luis J. (2016) reported that pregnant women with IDA experience a higher rate of PPH than pregnant women with normal Hb levels. He reported that pregnant women with severe IDA are associated with higher blood loss at delivery than pregnant women with normal Hb levels. In the present study, the LMWID group lost significantly more blood than the ISC group, with a mean difference of −79 mL (95% CI: −199–42; P = 0.026).
Adverse perinatal outcomes associated with IDA, including intrauterine growth retardation, stillbirth, prematurity, and low birth weight were previously reported.2,22 However, in the present study, all the fetuses were well and delivered at term, with a mean birth weight of > 2.5 kg, and no congenital abnormality detected in all participants.
Study Limitations
For clear understanding of the maternal outcomes, the IDA status based on ferritin levels post-treatment may need to be monitored, and the mode of delivery may need to be standardized. Perinatal Hb and ferritin levels should be considered when obtaining more information on the perinatal outcomes between ISC and LMWID therapies. An increase in the sample size consisting of various major ethnic groups is also needed to provide more substantial results.
Conclusions
In the treatment of maternal IDA, the increment in Hb level was higher in parenteral ISC than in LMWID and parenteral ISC had a higher rate of achieving the target Hb level. Besides, ISC was associated with less blood loss and lower incidence of adverse events than LMWID.
Acknowledgments
The authors would like to thank the Director General of Health Malaysia for his permission to publish this article. This work was presented at Malaysian International Scientific Congress O&G (MISCOG 2018).
Disclosure
The authors declare no competing interest.
References
- 1.Breymann C. Iron deficiency anemia in pregnancy. Semin Hematol. 2015;52(4):339–347. doi: 10.1053/j.seminhematol.2015.07.003 [DOI] [PubMed] [Google Scholar]
- 2.Milman N. Iron deficiency and anaemia in pregnant women in Malaysia? Still a significant and challenging health problem. J. Pregnancy Child Heal. 2015;02(03):168–176. doi: 10.4172/2376-127x.1000168 [DOI] [Google Scholar]
- 3.Breymann C, Honegger C, Holzgreve W, Surbek D. Diagnosis and treatment of iron-deficiency anaemia during pregnancy and postpartum. Arch Gynecol Obstet. 2010;282(5):577–580. doi: 10.1007/s00404-010-1532-z [DOI] [PubMed] [Google Scholar]
- 4.Scholl TO. Iron status during pregnancy: setting the stage for mother and infant. Am J Clin Nutr. 2005;81:1218S–1222S. [DOI] [PubMed] [Google Scholar]
- 5.Tiwari AKM, Mahdi AA, Mishra S. Assessment of liver function in pregnant anemic women upon oral iron and folic acid supplementation. J Gynecol Obstet Hum Reprod. 2018;47:45–49. doi: 10.1016/j.jogoh.2017.11.010 [DOI] [PubMed] [Google Scholar]
- 6.Breymann C; Anaemia Working Group. [Current aspects of diagnosis and therapy of iron deficiency anemia in pregnancy]. Praxis (Bern 1994). 2001;90:1283–1291. [PubMed] [Google Scholar]
- 7.Avni T, Bieber A, Grossman A, et al. The safety of intravenous iron preparations: systematic review and meta-analysis In: Mayo Clinic Proceedings. Elsevier; 2015. doi: 10.1016/j.mayocp.2014.10.007 [DOI] [PubMed] [Google Scholar]
- 8.Esen UI. Iron deficiency anaemia in pregnancy: the role of parenteral iron. J Obstet Gynaecol (Lahore). 2017;37:15–18. doi: 10.1080/01443615.2016.1180505 [DOI] [PubMed] [Google Scholar]
- 9.Bayoumeu F, Subiran-Buisset C, Baka N-E, et al. Iron therapy in iron deficiency anemia in pregnancy: intravenous route versus oral route. Eur J Obstet Gyneco Reprod Biol. 2005;123:S15–S19. doi: 10.1016/s0301-2115(05)80402-6 [DOI] [PubMed] [Google Scholar]
- 10.Jamaiyah Haniff MP, Anita Das MP, Onn LT, et al. Anemia in pregnancy in Malaysia: a cross-sectional survey. Asia Pac J Clin Nutr. 2007;16:527. doi: 10.6133/apjcn.2007.16.3.18 [DOI] [PubMed] [Google Scholar]
- 11.Practitioner T. Intravenous iron sucrose (Venofer). 2008:1–11.
- 12.Practitioner T Protocol for the use of intravenous iron dextran (CosmoFer ®). 2008:1–11.
- 13.Sinha S, Chiu DYY, Peebles G, et al. Comparison of intravenous iron sucrose versus low-molecular-weight iron dextran in chronic kidney disease. J Ren Care. 2009;35(2):67–73. doi: 10.1111/j.1755-6686.2009.00099.x [DOI] [PubMed] [Google Scholar]
- 14.WMA Declaration of Helsinki. Ethical principles for medical research involving human subjects. WMA – The World Medical Association; Available from: https://www.wma.net/policies-post/wma-declaration-of-helsinki-ethical-principles-for-medical-research-involving-human-subjects/. [PubMed] [Google Scholar]
- 15.McMahon LP. Iron deficiency in pregnancy. Obstet Med. 2010;3(1):17–24. doi: 10.1258/om.2010.100004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Luis J, Fadel MG, Lau GY, et al. The effects of severe iron-deficiency anaemia on maternal and neonatal outcomes: a case–control study in an inner-city London hospital. J Obstet Gynaecol (Lahore). 2016;36(4):473–475. doi: 10.3109/01443615.2015.1085848 [DOI] [PubMed] [Google Scholar]
- 17.da Costa AG, Vargas S, Clode N, Graça LM. Prevalence and risk factors for iron deficiency anemia and iron depletion during pregnancy: a prospective study. Acta Med Port. 2016. doi: 10.20344/amp.6808 [DOI] [PubMed] [Google Scholar]
- 18.Khaskheli MN, Baloch S, Sheeba A, Baloch S, Khaskheli FK. Iron deficiency anaemia is still a major killer of pregnant women. Pakistan J Med Sci. 2016;32(3):630. doi: 10.12669/pjms.323.9557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abhilashini GD, Sagili H, Rani R. Intravenous iron sucrose and oral iron for the treatment of iron deficiency Anaemia in pregnancy. J Clin Diagnostic Res. 2014. doi: 10.7860/JCDR/2014/6568.4382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Waziri B, Mabayoje M, Bello B. Comparison of intravenous low molecular weight iron dextran and intravenous iron sucrose for the correction of anaemia in pre-dialysis chronic kidney disease patients: a randomized single-centre study in Nigeria. Clin Kidney J. 2016;9(6):817–822. doi: 10.1093/ckj/sfw064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gupta A, Manaktala U, Rathore AM. A randomised controlled trial to compare intravenous iron sucrose and oral iron in treatment of iron deficiency anemia in pregnancy. Indian J Hematol Blood Transfus. 2014;30(2):120–125. doi: 10.1007/s12288-012-0224-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Khalafallah AA, Dennis AE. Iron deficiency anaemia in pregnancy and postpartum: pathophysiology and effect of oral versus intravenous iron therapy. J Pregnancy. 2012. doi: 10.1155/2012/630519 [DOI] [PMC free article] [PubMed] [Google Scholar]