Abstract
Radiotherapy (RT) represents one of the major treatment methods for cancers. However, many studies have observed that in descendant surviving tumor cells, sublethal irradiation can promote metastatic ability, which is closely related to the tumor microenvironment. We therefore investigated the functions and mechanisms of sublethal irradiated liver nonparenchymal cells (NPCs) in hepatocellular carcinoma (HCC). In this study, primary rat NPCs and McA‐RH7777 hepatoma cells were irradiated with 6 Gy X‐ray. Conditioned media (CM) from nonirradiated (SnonR), irradiated (SR), or irradiated plus radiosensitizer celecoxib‐treated (S[R + D]) NPCs were collected and added to sublethal irradiated McA‐RH7777 cells. We showed that CM from sublethal irradiated NPCs significantly promoted the migration and invasion ability of sublethal irradiated McA‐RH7777 cells, which was reversed by celecoxib. The differentially expressed genes in differently treated McA‐RH7777 cells were enriched mostly in the AMP‐activated protein kinase/mammalian target of rapamycin (AMPK/mTOR) signaling pathway. SR increased the migration and invasion ability of HCC cells by inhibiting AMPK/mTOR signaling, which was enhanced by the AMPK inhibitor compound C and blocked by the AMPK activator GSK‐621. Analyses of HCC tissues after neoadjuvant radiotherapy confirmed the effects of radiation on the AMPK/mTOR pathway. Cytokine antibody arrays and further functional investigations showed that matrix metalloproteinase‐8 (MMP‐8) partly mediates the promotion effects of SR on the migration and invasion ability of HCC cells by regulating AMPK/mTOR signaling. In summary, our data indicate that MMP‐8 secreted by irradiated NPCs enhanced the migration and invasion of HCC by regulating AMPK/mTOR signaling, revealing a novel mechanism mediating sublethal irradiation–induced HCC metastasis at the level of the tumor microenvironment.
Keywords: cytokine, hepatocellular carcinoma cells, metastasis, radiotherapy, sublethal irradiation
In this work, we observed that conditioned media from sublethally irradiated liver nonparenchymal cells (NPCs) promoted the migration and invasion ability of sublethally irradiated McA‐RH7777 hepatoma cells. Further mechanistic studies revealed that matrix metalloproteinase‐8 (MMP‐8) secreted by NPCs enhanced the migration and invasion of hepatocellular carcinoma (HCC) by regulating the AMPK/mTOR signaling pathway. These findings might offer a new strategy for improving the therapeutic effect of radiotherapy.
1. INTRODUCTION
The primary goal of cancer treatments is to eradicate all tumor cells, including primary tumors and obvious or hidden metastases, which are key drivers of patient prognosis and outcome. More than half of cancer patients need radiotherapy as a part of their cancer treatment, 1 and this treatment is important for patients with tumors that cannot be operated on or are incompletely removed, as well as patients with recurrent disease. Although radiotherapy has played key roles in many types of cancer, sublethal irradiation has been reported to promote the invasive and metastatic ability of descendant surviving cancer cells. 2 However, little is known about the underlying mechanisms.
Hepatocellular carcinoma (HCC) is the sixth most common malignant tumor and ranks the fourth in terms of cancer mortality worldwide. 3 However, as liver tissue has a low tolerance for radiation, the radiotherapy dose to malignant tumors is usually limited. 4 In the clinic, HCC patients receiving subcurative doses of radiation are likely to develop regional and/or distant metastasis. 5 Several possible mechanisms have been proposed to explain radiation‐induced metastasis. For example, Nagaraja et al showed that radiation‐induced epithelial‐mesenchymal transition (EMT) increases the metastatic potential of lung tumor cells. 6 Radiation‐induced vascular damage may increase the recruitment of migrating tumor cells. 7 An increased fraction of hypoxic cells has been observed after radiotherapy, and hypoxia causes significant upregulation of metastasis‐related genes. 7 , 8 However, most previous reports focused on changes in tumor cells, and the key role of the radiation‐induced tumor microenvironment (TME) in promoting tumor metastasis was underestimated.
In addition to hepatocytes, liver tissue is also composed of nonparenchymal cells (NPCs), which include stellate cells, Kupffer cells, and hepatic sinus endothelial cells. Radiation can induce alterations in the expression of various genes in liver cells and NPCs and generate many substances, leading to changes in the TME and in the biological behaviors of tumor cells. This led us to explore whether the TME is responsible for the increased invasion and metastasis of tumor cells treated with radiotherapy. In a previous study, we observed that many cytokines, such as IL‐6, TGF‐β, and TNF‐a, are released from irradiated NPCs. 9 However, the detailed roles of NPCs in HCC under sublethal irradiation treatment conditions are largely unclear.
Here, we show that conditioned media (CM) from sublethally irradiated NPCs promote the migration and invasion of HCC. Further mechanistic studies revealed that MMP‐8 secreted by NPCs mediates the NPC‐induced migration and invasion of HCC through the AMPK/mTOR signaling pathway. These findings might provide a new strategy for improving the therapeutic effect of radiotherapy.
2. MATERIALS AND METHODS
2.1. Isolation and preparation of rat liver NPCs
Male, 12‐week‐old, inbred Sprague‐Dawley rats were maintained under controlled conditions (24°C ± 2°C temperature, 40%‐70% relative humidity, 12 h light/12 h dark cycle, normal laboratory diet and water). Rat liver NPCs were isolated by collagenase digestion and differential centrifugation. Perfusion buffer was dripped onto the cut portal vein, and the liver was perfused with Ca2+‐free and Mg2+‐free Hanks’ balanced salt solution (HBSS), followed by perfusion with HBSS containing 0.02% collagenase IV (Sigma). After the liver was digested, the suspension was filtered through a strainer and centrifuged at 70 g for 5 min at 4°C. The NPC fraction in the supernatant was washed in phosphate‐buffered saline (PBS) and then pelleted at 650 g for 5 min at 4°C. Cell pellets were mixed with Dulbecco's modified Eagle's medium (DMEM) and centrifuged at 1800 g for 20 min at 4°C. The enriched NPC pellet was resuspended in buffer. The animal experiment was approved by the Clinical Research Ethics Committees of Affiliated Hospital of Jiangnan University (JN. No20190330b0180630).
2.2. Preparation of CM
Isolated NPCs were cultured at 37°C under an atmosphere of 5% CO2 in a 6‐well plate. Cells were cultured for 48 h and then placed in fresh Williams’ E medium containing penicillin, streptomycin, and HEPES. NPCs were divided into a nonirradiated control group, an irradiation group, and an irradiated plus celecoxib group. Cells were cultured to 80% confluence, and then a linear accelerator (Oncor; Siemens) was used to deliver 6 Gy radiation at a rate of 3 Gy per minute. After 48 hours of incubation, the supernatants were collected and then centrifuged at 1000 g for 5 min at 4°C. CM from nonirradiated, irradiated, and irradiated plus celecoxib cultures were termed SnonR, SR and S(R + D), respectively.
2.3. Radiation schedule
McA‐RH7777 cells were cultured to 80% confluence and then received 6 Gy of X‐ray irradiation at a dose rate of 3 Gy per minute using a linear accelerator (Oncor; Siemens). When cells were irradiated, a T25 flask was put on the couch, and a 1.5‐cm‐thick bolus was used to correct the distribution of radiation. Irradiation characteristics were beam energy, 6‐MV photons; source‐surface distance, 100 cm; size of the radiation field, 10 × 10 cm2; gantry, 180°. Dosimetry was measured with a cylindrical ionization chamber before irradiation.
2.4. Reagents and cell lines
Rat McA‐RH7777 cells (from the American Type Culture Collection) were maintained in high‐glucose DMEM containing 10% fetal bovine serum (FBS) and penicillin/streptomycin at 37°C in a humidified atmosphere containing 5% CO2. McA‐RH7777 cells were irradiated in single doses of 0, 2, 4, 6, or 8 Gy, respectively. After subculture, cells were transferred to conditioned SnonR, SR, or S(R + D) medium and then received a single dose of 6 Gy irradiation. Cells from SR, S(R + D), and SnonR cultures were termed RH6Gy‐SR, RH6Gy‐S(R + D), and RH6Gy‐SnonR, respectively. Exogenous recombinant interleukin‐2 (IL‐2), vascular endothelial growth factor (VEGF), transforming growth factor‐beta (TGF‐β), and matrix metalloproteinase‐8 (MMP‐8) were purchased from R&D Systems, and celecoxib was purchased from Dalian Meilun Biology Technology Co., Ltd.
2.5. Colony formation assays
Approximately 500 cancer cells were seeded into each well of six‐well plates and incubated for 6 hours followed by treatment with different doses of IR (0, 1, 2, 4, 6, or 8 Gy) using a linear accelerator. After approximately 14 days, cells were washed with precooled PBS, fixed in precooled methanol, and stained with crystal violet. The cell survival curves were plotted with SigmaPlot 14.0 software using the multi‐target, single‐hit model S = l‐(1‐e−D/D0)N.
2.6. Cytokine antibody arrays
Cytokines were detected in SR, S(R + D), and SnonR conditioned medium with rat antibody arrays (RayBio rat cytokine array L series; RayBiotech) following the manufacturer's instructions, and 90 cytokines related to cell growth, angiogenesis, and inflammation were simultaneously screened.
2.7. Transwell invasion assay
The invasion of RH6Gy‐SR, RH6Gy‐S(R + D), and RH6Gy‐SnonR cells was assessed by transwell invasion assays using medium supplemented with cytokines or CM. Four hours before seeding cancer cells onto the membrane, 50 μl Matrigel (diluted 1:8 with DMEM) (BD Biosciences) was added to each upper transwell chamber and incubated at 37°C for 4 hours. A suspension of McA‐RH7777 cells (at a density of 5 × 104 cells/ml supplemented with cytokines or CM) was prepared. These cells (200 μl) were added to the upper chamber of the transwell chamber, and DMEM containing 10% FBS (1000 μl) was added to the lower chamber. After 48 h, cells reaching the underside of the membrane were stained with crystal violet staining solution (Beyotime) and counted at 10× magnification.
2.8. Wound healing assay
RH6Gy‐SR, RH6Gy‐S(R + D), and RH6Gy‐SnonR cells were grown in six‐well culture plates. When cells reached confluency, we used 200 μl pipette tips to scratch wounds. The cell layer was wounded with a uniform scratch using sterile pipette tips, and the plates were washed three times to remove shedding cells and then incubated in culture medium containing 10% FBS supplemented with cytokines or CM for 24 h. RH6Gy cells were observed at different time points, and cell migration was observed under a light microscope.
2.9. Western blot analyses
Protein extracts were probed with antibodies against rat phospho‐mTOR (1:500, Sangon Biotech), mTOR (1:1000, Proteintech), phospho‐AMPK (1:500, Sangon Biotech), AMPK (1:1000, Sangon Biotech), and GAPDH (1:5000, Sangon Biotech). A peroxidase‐conjugated anti‐rabbit antibody (1:5000, Sangon Biotech) was used as the secondary antibody, and the membranes were visualized with enhanced chemiluminescence (Beyotime).
2.10. Gene chip detection
Total RNA of RH6Gy‐SnonR and RH6Gy‐SR cells (1 × 106 cells) was extracted according to the instruction manual provided with TRIzol reagent (Genewiz Inc), and the concentration and quality of the extracted RNA were measured on a NanoDrop nucleic acid analyzer to ensure reliable RNA quality. Gene chip sequencing was completed by Shanghai Bohao Biotechnology Co., Ltd.
2.11. GO and KEGG enrichment analyses
Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment analyses of the coregulated differentially expressed genes were performed through the online tool Database for Annotation, Visualization, and Integrated Discovery (DAVID) (version 6.8, https://david‐d.ncifcrf.gov/).
2.12. Immunohistochemical (IHC) staining
Tumor tissues were obtained from stage III‐IV HCC patients treated with or without neoadjuvant radiotherapy (N = 7 for each group) at Zhongshan Hospital Affiliated with Fudan University between 2012 and 2019. The intervals between neoadjuvant radiotherapy and surgery were 6 weeks. All patients were ranging from 52 to 75 years old; the majority of patients have concomitant cirrhosis. All patients’ data were obtained with informed consent, and the Clinical Research Ethics Committee approved the project of the participating institutions (No. 2011‐235). HCC tissue samples were fixed in 4% neutral buffered paraformaldehyde, paraffin embedded and sectioned. The sections were blocked and then incubated with phospho‐mTOR (1:50, Sangon Biotech), mTOR (1:200, Proteintech), phospho‐AMPK (1:50, Sangon Biotech), and AMPK (1:80, Sangon Biotech) antibodies overnight at 4°C. Then, the sections were treated with the corresponding secondary antibody (Gene Tech). Diaminobenzidine (DAB) was dropped onto the sections for color reaction. For the quantitative analysis, the IHC score from three randomly selected microscopic fields (200×) was calculated (SI = staining intensity + staining percentage) and averaged to obtain the mean. Staining intensity scores ranged from 0 to 3 (0 = no staining, 1 = low staining, 2 = medium staining, and 3 = strong staining). The percentage of positively stained cells was also assigned scores of 1‐3, where 1 = <25% positive staining, 2 = 25‐50% positive staining, and 3 = >50% positive staining.
2.13. Statistical analyses
The results were assessed by a paired t‐test and a χ2 test. All statistical analyses were performed using SPSS 23.0 software, and P < .05 was considered to indicate a statistically significant result.
3. RESULTS
3.1. SR promotes HCC cell migration and invasion
As shown in Figure S1, irradiation killed McA‐RH7777 cells in a logarithmic dose‐dependent manner. In the 6 Gy group, about 1% of cells survived, and few cells could survive in the 8 Gy group. So, 6 Gy was selected for subsequent studies. To determine whether sublethal irradiated NPCs can modulate the metastatic ability of HCC, we examined the effects of SR on McA‐RH7777 cell migration and invasion using wound healing and transwell invasion assays. As shown in Figure 1A and B, SR‐treated cells exhibited considerably faster migration and invasion ratio than their control SnonR cells (P < .05). Radiosensitizer celecoxib belongs to the nonsteroidal anti‐inflammatory drugs family and is a selective cyclooxygenase‐2 inhibitor. Celecoxib could inhibit tumor invasion by inhibiting PGE2 production and downregulating MMP‐2 secretion. 10 To evaluate whether the metastasis‐promoting effect of sublethal irradiated NPCs on HCC cells can be reversed by celecoxib, NPCs were pretreated with 30 μmol/L of celecoxib for 24 hours and then irradiated. Our data showed that CM from celecoxib‐pretreated and sublethal irradiated NPCs significantly reduced the promotion effects of SR on cell migration and invasion, further validating that the invasive behaviors of HCC can be enhanced by SR.
Figure 1.
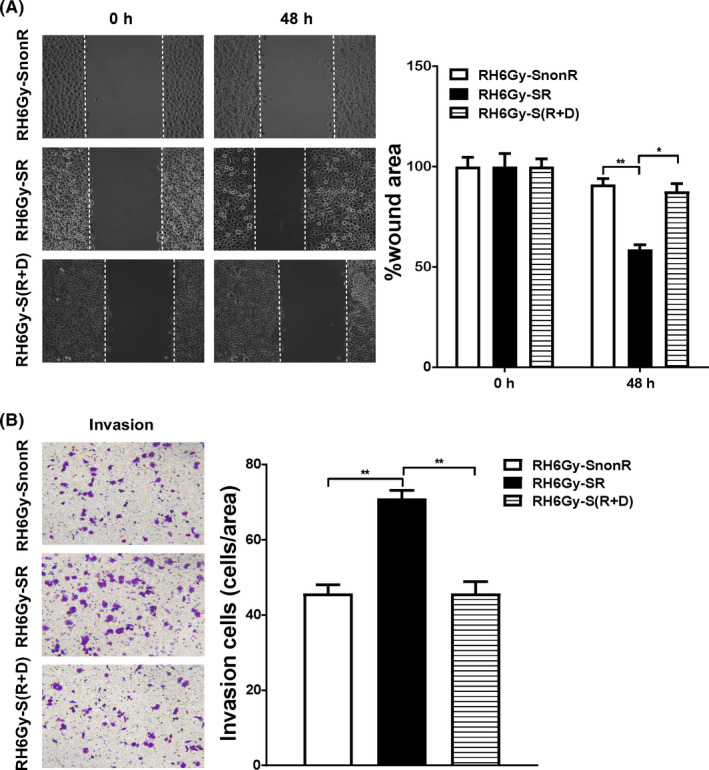
Irradiated supernatant (SR) promotes hepatocellular carcinoma (HCC) cell migration and invasion. A, The effects of nonirradiated (SnonR), irradiated (SR), and irradiated plus drug (celecoxib)‐treated S(R + D) supernatant on RH6Gy cell migration were examined by wound healing assays, and wound healing was quantified as the ratio of the remaining cell‐free area to the area of the initial wound (calculated as the mean percentage) using ImageJ software. B, The effects of SnonR, SR, and S(R + D) on RH6Gy cell invasion were examined by transwell invasion assays (N = 3). Quantitative data are presented as the mean ± standard deviation. *P < .05, **P < .01
3.2. SR may influence the AMPK/mTOR signaling pathway
Next, we investigated whether SR can activate intracellular signaling pathways associated with cell migration and invasion. To this end, mRNA expression profiles in RH6Gy‐SnonR and RH6Gy‐SR cells were analyzed using a microarray to screen differentially expressed mRNAs. The 20 most dysregulated genes and their chromosome positions using the circus plot are represented in Figure 2A and Table 1. These differentially expressed genes were analyzed by GO and KEGG enrichment analyses. KEGG pathway analysis revealed that the most significantly enriched pathway was the “AMPK/mTOR signaling pathway” (Figure 2B), suggesting that the AMPK/mTOR signaling pathway may play key roles in SR‐induced cell migration and invasion. Therefore, we hypothesized that SR may influence the AMPK/mTOR signaling pathway and promote HCC cell migration and invasion. In addition, among the GO terms molecular function (MF), cellular component (CC) and biological process, “MF‐protein binding,” “CC‐cytoplasm,” and “CC‐cytosol” were the three major GO terms (Figure 2C). These results imply that the AMPK/mTOR pathway may play key roles in SR‐induced cell migration and invasion.
Figure 2.
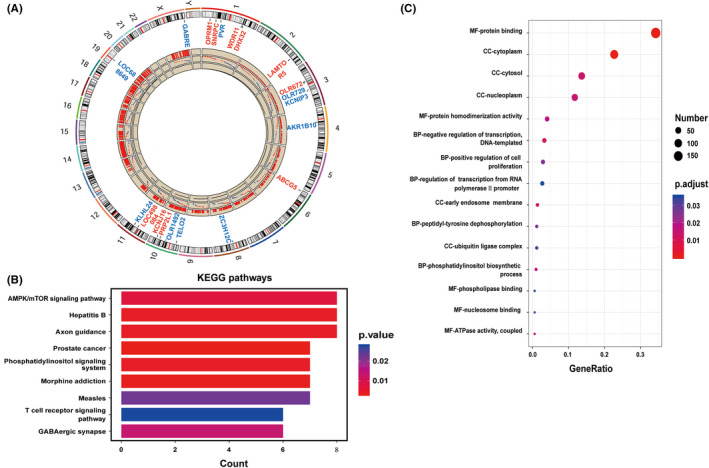
Irradiated supernatant (SR) may influence the AMPK/mTOR signaling pathway. A, Circos plot showing the differentially expressed genes and their chromosome positions. Red and blue indicate up‐ and downregulated genes, respectively. B, Scatter plot of the top nine KEGG pathways. Each color indicates a different P‐value as indicated. KEGG, Kyoto Encyclopedia of Genes and Genomes. C, Scatter plot of the top 15 GO terms. The size of the circle indicates the number of enriched genes in each pathway. Each color indicates a different P‐value as indicated. GO, Gene Ontology. The rich factor represents the ratio of enriched genes in the selected pathway to the total number of genes in the pathway
Table 1.
Top 20 differentially expressed genes in McA‐RH7777 cells receiving sublethal irradiation
| Gene symbol | Fold change (RH6Gy‐SR/RH6Gy‐SnonR) |
|---|---|
| Upregulated | |
| ABCG5 | 191.3249 |
| WDR11 | 183.7237 |
| LOC498084 | 174.045 |
| DHX32 | 165.1955 |
| LAMTOR5 | 161.2523 |
| PRP2L1 | 149.0754 |
| SNRPC | 148.8021 |
| KCNJ16 | 129.4143 |
| OPRM1 | 124.6007 |
| OLR672 | 122.0062 |
| Downregulated | |
| OLR729 | 0.0044 |
| TELO2 | 0.0049 |
| GABRE | 0.0082 |
| LOC688649 | 0.0097 |
| AKR1B10 | 0.0100 |
| KCNIP3 | 0.0104 |
| KLHL24 | 0.0107 |
| OLR1492 | 0.0107 |
| PVR | 0.0111 |
| ZC3H12C | 0.0113 |
Gene expression changes in RH6Gy irradiated supernatant (SR) cells (McA‐RH7777 cells treated with irradiated conditioned media [CM] cultures, then received 6 Gy irradiation) compared with RH6Gy nonirradiated supernatant (SnonR) cells (McA‐RH7777 cells treated with nonirradiated CM cultures, then received 6 Gy irradiation); 726 genes with a fold change greater than 3 were identified. The 20 genes shown in the table exhibited the most dysregulated changes.
3.3. SR enhances the migration and invasion ability of HCC cells by inhibiting the AMPK/mTOR signaling pathway
AMPK is a ubiquitous serine/threonine protein kinase that regulates tumor development, metastasis, and chemoresistance through the negative regulation of mTOR. 11 , 12 AMPK and mTOR have been implicated in cancer progression and metastasis. In human cholangiocarcinoma (CCA), the activated AMPK/mTOR signaling pathway suppresses the invasion and migration of CCA cells. 13 We next explored whether SR promotes RH6Gy cell migration and invasion by regulating AMPK/mTOR signaling. As shown in Figure 3A, the AMPK activator GSK621 significantly inhibited the SR‐induced invasion of RH6Gy cells, while the AMPK inhibitor compound C enhanced invasion ability in all three groups of RH6Gy cells (RH6Gy‐SnonR, RH6Gy‐SR, and RH6Gy‐S[R + D]). The expression of p‐AMPK, AMPK, p‐mTOR, and mTOR in RH6Gy cells was analyzed by Western blot analysis (Figure 3B and Figure S2A). The results showed that the phosphorylated AMPK levels in RH6Gy‐SR cells were significantly lower than those in RH6Gy‐SnonR cells, and S(R + D) treatment significantly inhibited this decrease. In contrast, the expression of phosphorylated mTOR was markedly increased in RH6Gy‐SR cells. Nevertheless, the total AMPK and mTOR levels were not significantly different between these groups. Thus, RH6Gy‐SR can regulate the AMPK/mTOR signaling pathway by inhibiting AMPK phosphorylation and promoting mTOR phosphorylation.
Figure 3.
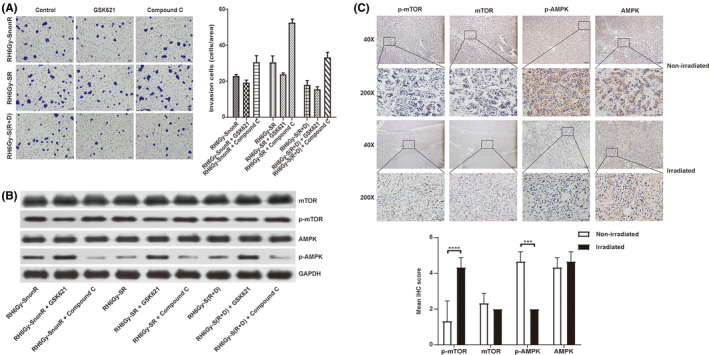
Irradiated supernatant (SR) promotes hepatocellular carcinoma (HCC) cell migration and invasion by inhibiting the AMPK/mTOR signaling pathway. A, The effects of nonirradiated (SnonR), irradiated (SR), and irradiated plus drug (celecoxib)‐treated S(R + D) supernatant on RH6Gy cell invasion were examined by transwell invasion assays. SR enhanced the invasiveness of RH6Gy cells, GSK621 reduced invasion ability, and compound C enhanced invasion ability in all three groups of RH6Gy cells (RH6Gy‐SnonR, RH6Gy‐SR, and RH6Gy‐S[R + D]). B, Representative Western blot showing the levels of p‐AMPK, AMPK, p‐mTOR, and mTOR in RH6Gy‐SnonR, RH6Gy‐SR, and RH6Gy‐S(R + D) cells; GAPDH served as an internal control. C, Immunohistochemical (IHC) analysis of p‐mTOR, mTOR, p‐AMPK, and AMPK and representative images of 14 samples, including HCC patients who received neoadjuvant radiotherapy (N = 7) or did not receive radiotherapy (N = 7). Magnification: 40× (first and third panels), 200× (second and fourth panels). IHC score of p‐mTOR, mTOR, p‐AMPK, and AMPK in HCC tissue. *P < .05, **P < .01, ***P < .001, ****P < .0001
To investigate whether ionizing radiation influences the AMPK/mTOR signaling pathway in human liver cancer tissues following neoadjuvant radiotherapy, we next performed IHC staining to visualize the changes in phospho‐mTOR, mTOR, phospho‐AMPK, and AMPK in HCC tissues following neoadjuvant radiotherapy. As shown in Figure 3C, the expression of p‐mTOR was upregulated, whereas the expression of p‐AMPK was downregulated in the irradiated group compared with the nonirradiated group (P < .001). The total mTOR and AMPK levels were not significantly different between the two groups. Taken together, these results imply that radiation also inhibits the AMPK/mTOR signaling pathway in HCC tissues following neoadjuvant radiotherapy.
3.4. Increased secretion of inflammation‐ and metastasis‐related factors following SR
Because cytokines play key roles in the crosstalk between different cells of the TME, we next investigated whether irradiated NPCs secrete certain cytokines that affect the migration and invasion ability of the offspring after liver cancer irradiation. Therefore, a cytokine array was performed on SnonR, SR, and S(R + D). The results showed that some cytokines, including MMP‐8, IL‐2, TGF‐β1, Tie‐2, and VEGF, were significantly upregulated in the SR group and rescued in the S(R + D) group (Figure 4A and B). Then, we analyzed the expression of and large changes in cytokines of interest, such as IL‐2, VEGF, and MMP‐8. Remarkably, the expression of TGF‐β1 was significantly upregulated after radiation, while celecoxib treatment did not reduce the promotion effects of SR on cell migration and invasion. TGF‐β is well known to facilitate tumor metastasis, so we also evaluated whether TGF‐β is critical for the migration and invasion of HCC.
Figure 4.
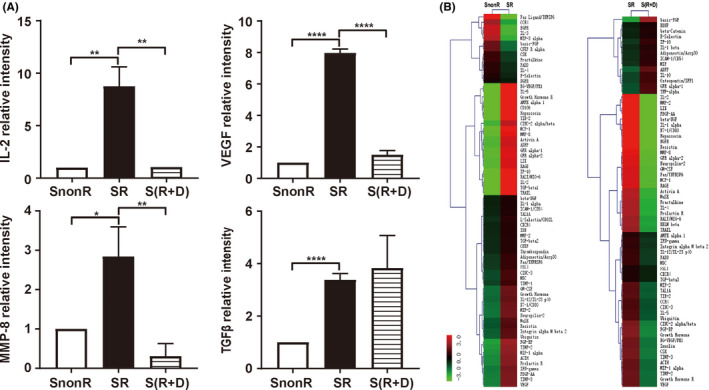
Increase in inflammation‐ and metastasis‐related factors released following SR. A, Relative expression of interleukin‐2 (IL‐2), vascular endothelial growth factor (VEGF), matrix metalloproteinase‐8 (MMP‐8), and transforming growth factor‐beta‐1 (TGF‐β1) in conditioned medium from irradiated (SR), irradiated plus drug (celecoxib)‐treated S(R + D), and nonirradiated (SnonR) supernatant based on rat cytokine arrays. Error bars indicate the mean ± SD. B, Hierarchical clustering analysis of all expressed SnonR, SR, and S(R + D). Maps on the left are based on the expression values in SnonR and SR. Maps on the right correspond to the expression values in SR and S(R + D). The expression values are depicted in line with the color scale. The intensity increases from green to red. Green represents all cytokines expressed in SnonR and SR with a fold change < 0.66, and red indicates all cytokines expressed in SR and S(R + D) with a fold change > 1.5. *P < .05, **P < .01, ***P < .001, ****P < .0001
3.5. MMP‐8 mediates the promoting effects of SR on the migration and invasion of HCC
Exogenous recombinant IL‐2, VEGF, TGF‐β, and MMP‐8 were added to the culture medium of RH6Gy cells at different concentrations for 24 or 48 hours. The results showed that both 2 ng/ml VEGF and 20 ng/ml MMP‐8 promoted cell migration and invasion (Figure 5A,B). Further analyses demonstrated that 20 ng/ml MMP‐8, but not VEGF, significantly decreased the phosphorylated AMPK levels and increased the phosphorylated mTOR levels in RH6Gy cells (Figure 5C and Figure S3A). The expression of total AMPK and mTOR was not significantly changed in either group. Taken together, our results demonstrate that MMP‐8 is the key factor that mediates the promoting effects of SR on the migration and invasion of HCC by regulating the AMPK/mTOR signaling pathway.
Figure 5.
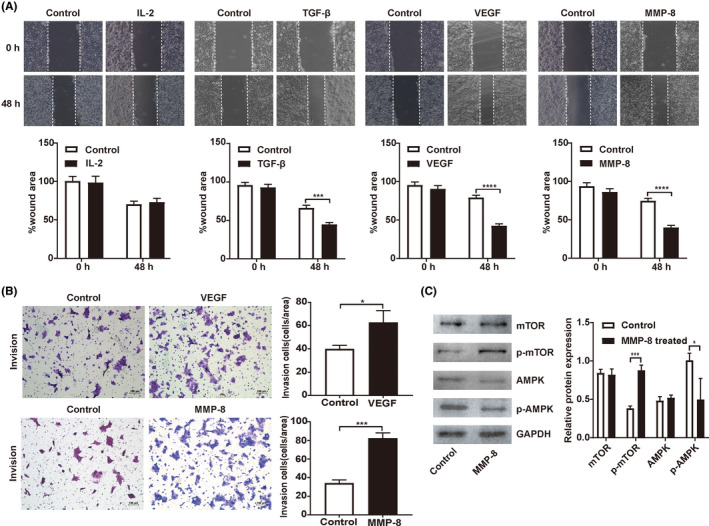
Matrix metalloproteinase‐8 (MMP‐8) enhances the migration and invasion of hepatocellular carcinoma (HCC) through the AMPK/mTOR signaling pathway. A, Cell migration was measured by wound healing assays. B, Cell invasion was detected by transwell invasion assays (N = 3 in each group). C, Representative Western blot showing the levels of p‐AMPK, AMPK, p‐mTOR, and mTOR in RH6Gy cells supplemented with exogenous recombinant MMP‐8; GAPDH served as an internal control. *P < .05, **P < .01, ***P < .001, ****P < .0001
4. DISCUSSION
In this study, we established stable radioresistant HCC cell lines and investigated the roles of NPCs in enhanced cell migration and invasion. Our data demonstrated that the sublethal irradiation of NPCs promotes HCC’s metastatic potential. Our finding is consistent with the clinical observation that failure of HCC radiotherapy is usually related to metastasis. 14 We also showed that the increased metastatic potential of residual cells after irradiation is mediated by the secretion of cytokines such as MMP‐8 by NPCs into the TME and subsequent inhibition of the AMPK/mTOR signaling pathway. Our results suggest that the use of inhibitors that block AMPK/mTOR signaling or MMP‐8 secretion may decrease the risk of sublethal irradiation–associated metastases in HCC.
Malignant tumors are characterized by their uncontrolled growth, often accompanied by invasion and metastasis, the latter being the leading cause of death in patients. Among the factors that affect tumor metastasis, the migration and invasion of tumor cells are prerequisites and are the most important driving forces that promote tumor progression by increasing the depth of tumor cell infiltration and facilitating both local and distant metastases. 15 Several classes of proteins are associated with tumor invasion and metastasis: integrins, calcium‐dependent cadherins, lymphangiogenesis factors, angiogenetic factors, and extracellular proteases. 16 Among these factors, MMPs play significant roles in promoting tumor invasion and metastasis by degrading the extracellular matrix, disabling cellular adhesion, enhancing EMT, and promoting tumor angiogenesis. 17 The abnormally high expression of MMPs is closely related to tumor metastasis and poor patient survival. 18 , 19 Qian showed that MMP inhibitors can block the radiation‐induced metastasis of human pancreatic cancer cells, and another study showed that radiotherapy for lung cancer and breast cancer increased plasma MMP‐9 levels. 20 Nirmala found that radiation significantly increased MMP‐9 levels and their mRNA expression in cocultures of retinal endothelial cells and glioblastoma cells. 21 Murawaki demonstrated that MMPs synthesized by HCC cells play an important role in angiogenesis, cell invasion, and metastasis. 22 Similarly, in this study, sublethal irradiation enhanced the secretion of cytokines such as MMP‐8 in NPCs, promoting the migration and invasion of RH6Gy cells. Therefore, our data demonstrate that MMP‐8 may be a radiation‐responsive mediator that degrades the extracellular matrix and promotes cancer invasion and metastasis.
Noncurative tumor irradiation can enhance the incidence of tumor metastasis. Through preclinical investigations, many mechanisms involved in this change have been identified, including EMT, vascular damage, and the increased flux of tumor cells into the circulation. 23 , 24 , 25 , 26 However, no systematic study has focused on sublethal irradiation–induced changes in the TME. The TME is the environment in which tumor cells are located and is closely related to tumorigenesis and metastasis. The TME provides cancer cells with cues and a physical environment to progress and eventually metastasize, representing a welcoming site for these migrating cells. 1 , 27 The effects of radiotherapy on the TME include the induction of hypoxia and the increase in cytokines and growth factors secreted by stromal cells and immune cells, 28 , 29 , 30 , 31 , 32 which may contribute to increased metastasis. However, the correlation between radiation‐induced metastasis and clinical experience and the mechanisms involved are still unclear. There remains a need to explain the interaction between the TME and cancer cells after radiotherapy. It is important to elucidate the phenotypic and functional heterogeneity of the TME so that these strategies can serve as molecular leaders in cancer treatment while optimizing tumor control.
Our results demonstrate that irradiation affects cancer cells and surrounding normal cells. Radiotherapy should consider the effect of irradiation on the TME, and this phenomenon may play a role in the microenvironment of sublethally irradiated cancer cells. Interestingly, we observed that the metastasis‐associated cytokine MMP‐8 secreted by irradiated NPCs enhanced the migration and invasion of HCC by regulating the AMPK/mTOR signaling pathway, as shown in Figure 6. The clinical implication of this study is a key requirement for inhibiting sublethal radiation–enhanced cancer cell metastasis. Therapeutic improvement is based not only on the eradication of local disease but also on the control of the systemic metastasis of cancer cells. By clarifying the signal transduction mediators involved in this process, it is possible to develop specific inhibitors to modulate harmful metastatic signals and retain the therapeutic benefits of radiation therapy for local disease.
Figure 6.
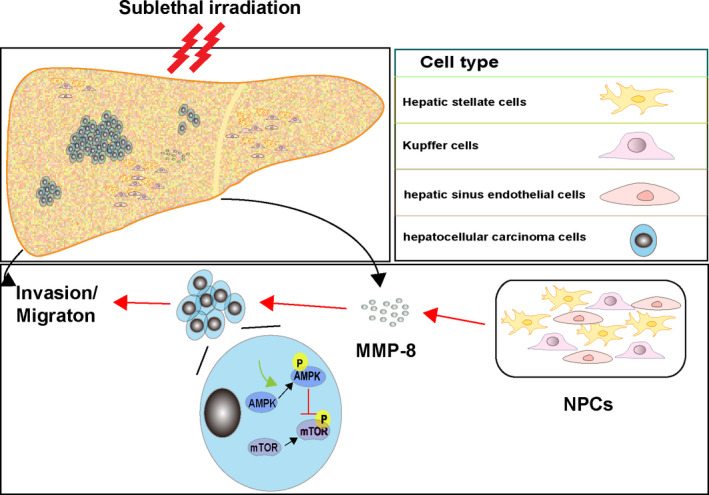
Illustration of how irradiated nonparenchymal cells (NPCs) enhance the migration and invasion of hepatocellular carcinoma (HCC). Matrix metalloproteinase‐8 (MMP‐8) secreted by irradiated NPCs enhances the migration and invasion of HCC by regulating the AMPK/mTOR signaling pathway. The red line indicates activation; the green line indicates inhibition
DISCLOSURE
The authors declare that there are no conflicts of interest.
Supporting information
Figure S1‐S3
ACKNOWLEDGEMENTS
This study was partially supported by grants from the National Natural Science Foundation of China (81672328, 81972220, 81772636, and 81301920), Social Development Project of Jiangsu Province (BE2019632), Double Hundred Medical Professionals Program of Wuxi (BJ2020053 and BJ2020058), Medical Key Professionals Program of Jiangsu Province (AF052141), Medical Youth Professionals Program of Jiangsu Province (QNRC2016162), Medical Innovation Team Program of Wuxi (CXTP003), Six Talent Peaks Projects of Jiangsu Province (WSW‐196 and WSW‐155), Medical Key Professionals Program of Wuxi (ZDRC013), and the High‐Level Health Talent Project of Jiangsu Province (LGY2019017 and LGY2018012).
Cao Y, Yin Y, Wang X, et al. Sublethal irradiation promotes the metastatic potential of hepatocellular carcinoma cells. Cancer Sci. 2021;112:265–274. 10.1111/cas.14724
Yulin Cao and Yuan Yin contributed equally to this work.
Contributor Information
Zhaohui Huang, Email: zhaohuihuang@jiangnan.edu.cn.
Leyuan Zhou, Email: zhouleyuan99@126.com.
REFERENCES
- 1. Vilalta M, Rafat M, Graves EE. Effects of radiation on metastasis and tumor cell migration. Cell Mol Life Sci. 2016;73(16):2999‐3007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zhou Y‐C, Liu J‐Y, Li J, et al. Ionizing radiation promotes migration and invasion of cancer cells through transforming growth factor‐beta‐mediated epithelial‐mesenchymal transition. Int J Radiat Oncol Biol Phys. 2011;81(5):1530‐1537. [DOI] [PubMed] [Google Scholar]
- 3. World Health Organization (WHO) . Cancer incidence and mortality statistics worldwide and by region [Internet]. Globocan 2018 [cited 2019 March]. Available from: https://gco.iarc.fr/today/data/factsheets/cancers/11‐Liver‐fact‐sheet.pdf
- 4. Chen Y, Wu Z, Yuan B, et al. Microrna‐146a‐5p attenuates irradiation‐induced and LPS‐induced hepatic stellate cell activation and hepatocyte apoptosis through inhibition of TLR4 pathway. Cell Death Dis. 2018;9(2):22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zhou L‐Y, Wang Z‐M, Gao Y‐B, et al. Stimulation of hepatoma cell invasiveness and metastatic potential by proteins secreted from irradiated nonparenchymal cells. Int J Radiat Oncol Biol Phys. 2012;84(3):822‐828. [DOI] [PubMed] [Google Scholar]
- 6. Nagaraja SS, Nagarajan D. Radiation‐induced pulmonary epithelial‐mesenchymal transition: A review on targeting molecular pathways and mediators. Curr Drug Targets. 2018;19(10):1191‐1204. [DOI] [PubMed] [Google Scholar]
- 7. Kadhim MA, Mayah A, Brooks SA. Does direct and indirect exposure to ionising radiation influence the metastatic potential of breast cancer cells. Cancers (Basel). 2020;12(1):236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Liu Y, Liu Y, Bu W, et al. Radiation‐/hypoxia‐induced solid tumor metastasis and regrowth inhibited by hypoxia‐specific upconversion nanoradiosensitizer. Biomaterials. 2015;49:1‐8. [DOI] [PubMed] [Google Scholar]
- 9. Du S‐S, Qiang M, Zeng Z‐C, et al. Inactivation of kupffer cells by gadolinium chloride protects murine liver from radiation‐induced apoptosis. Int J Radiat Oncol Biol Phys. 2010;76(4):1225‐1234. [DOI] [PubMed] [Google Scholar]
- 10. Li WZ, Huo QJ, Wang XY, et al. Inhibitive effect of celecoxib on the adhesion and invasion of human tongue squamous carcinoma cells to extracellular matrix via down regulation of MMP‐2 expression. Prostaglandins Other Lipid Mediat. 2010;93(3–4):113‐119. [DOI] [PubMed] [Google Scholar]
- 11. Zhang Y, Fan Y, Huang S. Thymoquinone inhibits the metastasis of renal cell cancer cells by inducing autophagy via AMPK/mTOR signaling pathway. Cancer Sci. 2018;109(12):3865‐3873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lu T, Sun L, Wang Z, et al. Fatty acid synthase enhances colorectal cancer cell proliferation and metastasis via regulating AMPK/mTOR pathway. Onco Targets Ther. 2019;12:3339‐3347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lyu SC, Han DD, Li XL, et al. Fyn knockdown inhibits migration and invasion in cholangiocarcinoma through the activated ampk/mtor signaling pathway. Oncol Lett. 2018;15(2):2085‐2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zhao J‐D, Liu J, Ren Z‐G, et al. Maintenance of sorafenib following combined therapy of three‐dimensional conformal radiation therapy/intensity‐modulated radiation therapy and transcatheter arterial chemoembolization in patients with locally advanced hepatocellular carcinoma: A phase I/II study. Radiat Oncol. 2010;5:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Entschladen F, Drell TL, Lang K, et al. Tumour‐cell migration, invasion, and metastasis: Navigation by neurotransmitters. Lancet Oncol. 2004;5(4):254‐258. [DOI] [PubMed] [Google Scholar]
- 16. Cheng JC, Chou CH, Kuo ML, et al. Radiation‐enhanced hepatocellular carcinoma cell invasion with MMP‐9 expression through PI3K/Akt/NF‐kappaB. Oncogene. 2006;25(53):7009‐7018. [DOI] [PubMed] [Google Scholar]
- 17. Groblewska M, Siewko M, Mroczko B, et al. The role of matrix metalloproteinases (MMPs) and their inhibitors (TIMPs) in the development of esophageal cancer. Folia Histochem Cytobiol. 2012;50(1):12‐19. [DOI] [PubMed] [Google Scholar]
- 18. Kasurinen A, Tervahartiala T, Laitinen A, et al. High serum MMP‐14 predicts worse survival in gastric cancer. PLoS One. 2018;13(12):e0208800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chavali PL, Saini RKR, Zhai Q, et al. TLX activates MMP‐2, promotes self‐renewal of tumor spheres in neuroblastoma and correlates with poor patient survival. Cell Death Dis. 2014;5(10):e1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Qian LW, Mizumoto K, Urashima T, et al. Radiation‐induced increase in invasive potential of human pancreatic cancer cells and its blockade by a matrix metalloproteinase inhibitor, CGS27023. Clin Cancer Res. 2002;8(4):1223‐1227. [PubMed] [Google Scholar]
- 21. Nirmala C, Jasti SL, Sawaya R, et al. Effects of radiation on the levels of MMP‐2, MMP‐9 and TIMP‐1 during morphogenic glial‐endothelial cell interactions. Int J Cancer. 2000;88(5):766‐771. [DOI] [PubMed] [Google Scholar]
- 22. Murawaki Y, Ikuta Y, Okamoto K, et al. Plasma matrix metalloproteinase‐9 (gelatinase B) in patients with hepatocellular carcinoma. Res Commun Mol Pathol Pharmacol. 2000;108(5–6):351‐357. [PubMed] [Google Scholar]
- 23. Vilalta M, Rafat M, Giaccia A, et al. Recruitment of circulating breast cancer cells is stimulated by radiotherapy. Cell Rep. 2014;8(2):402‐409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Park J, Jang SU, Kang S, et al. Establishment of animal model for the analysis of cancer cell metastasis during radiotherapy. Radiat Oncol. 2012;7:153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Martin OA, Anderson RL, Russell PA, et al. Mobilization of viable tumor cells into the circulation during radiation therapy. Int J Radiat Oncol Biol Phys. 2014;88(2):395‐403. [DOI] [PubMed] [Google Scholar]
- 26. Park HJ, Griffin RJ, Hui S, et al. Radiation‐induced vascular damage in tumors: Implications of vascular damage in ablative hypofractionated radiotherapy (SBRT and SRS). Radiat Res. 2012;177(3):311‐327. [DOI] [PubMed] [Google Scholar]
- 27. Zhang Y, Tanno T, Kanellopoulou C. Cancer therapeutic implications of microRNAs in the regulation of immune checkpoint blockade. ExRNA. 2019;1. [Google Scholar]
- 28. Boss MK, Bristow R, Dewhirst MW. Linking the history of radiation biology to the hallmarks of cancer. Radiat Res. 2014;181(6):561‐577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Burroughs SK, Kaluz S, Wang D, et al. Hypoxia inducible factor pathway inhibitors as anticancer therapeutics. Future Med Chem. 2013;5(5):553‐572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bouchard G, Bouvette G, Therriault H, et al. Pre‐irradiation of mouse mammary gland stimulates cancer cell migration and development of lung metastases. Br J Cancer. 2013;109(7):1829‐1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Desmarais G, Fortin D, Bujold R, et al. Infiltration of glioma cells in brain parenchyma stimulated by radiation in the F98/Fischer rat model. Int J Radiat Biol. 2012;88(8):565‐574. [DOI] [PubMed] [Google Scholar]
- 32. Russell JS, Brown JM. The irradiated tumor microenvironment: role of tumor‐associated macrophages in vascular recovery. Front Physiol. 2013;4:157. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1‐S3


