Abstract
Inhalation of tobacco smoke has been linked to increased risk of viral infection, such as influenza. Inhalation of electronic-cigarette (e-cigarette) aerosol has also recently been linked to immune suppression within the respiratory tract, specifically the nasal mucosa. We propose that changes in the nasal mucosal immune response modify antiviral host-defense responses in e-cigarette users. Nonsmokers, cigarette smokers, and e-cigarette users were inoculated with live-attenuated influenza virus (LAIV) to safely examine the innate immune response to influenza infection. Before and after LAIV inoculation, we collected nasal epithelial-lining fluid, nasal lavage fluid, nasal-scrape biopsy specimens, urine, and blood. Endpoints examined include cytokines and chemokines, influenza-specific IgA, immune-gene expression, and markers of viral load. Statistical analysis included primary comparisons of cigarette and e-cigarette groups with nonsmokers, as well as secondary analysis of demographic factors as potential modifiers. Markers of viral load did not differ among the three groups. Nasal-lavage-fluid anti-LAIV IgA levels increased in nonsmokers after LAIV inoculation but did not increase in e-cigarette users and cigarette smokers. LAIV-induced gene-expression changes in nasal biopsy specimens differed in cigarette smokers and e-cigarette users as compared with nonsmokers, with a greater number of genes changed in e-cigarette users, mostly resulting in decreased expression. The top downregulated genes in cigarette smokers were SMPD3, NOS2A, and KLRB1, and the top downregulated genes in e-cigarette users were MR1, NT5E, and HRAS. Similarly, LAIV-induced cytokine levels in nasal epithelial-lining fluid differed among the three groups, including decreased antiviral host-defense mediators (IFNγ, IL6, and IL12p40). We also detected that sex interacted with tobacco-product exposure to modify LAIV-induced immune-gene expression. Our results demonstrate that e-cigarette use altered nasal LAIV-induced immune responses, including gene expression, cytokine and chemokine release, and LAIV-specific IgA levels. Together, these data suggest that e-cigarette use induces changes in the nasal mucosa that are consistent with the potential for altered respiratory antiviral host-defense function.
Clinical trial registered with www.clinicaltrials.gov (NCT 02019745).
Keywords: e-cigarette, virus, influenza, respiratory, immune
Clinical Relevance
Our results demonstrate that electronic-cigarette use altered nasal live-attenuated influenza virus–induced immune responses, including gene expression, cytokine and chemokine release, and live-attenuated influenza virus–specific IgA levels. The data generated in this study suggest that electronic-cigarette use could increase risk for suppressed host-defense functions in the context of respiratory viral infections. If so, this has important public health implications, especially during influenza season and respiratory-virus pandemics.
The popularity of electronic-cigarettes (e-cigarettes) has grown exponentially since their introduction to the U.S. market in 2007 (1). Their use has become an increasing public health concern because of their addictive nature and popularity with youth and young adults (2). Although proponents of their use suggest that e-cigarettes are a less harmful alternative to cigarettes and can be used as a cigarette-cessation device, we are just beginning to understand the inhalational effects of e-cigarette use (3–6). Case reports and the 2019 outbreak of e-cigarette and vaping–associated lung injury have also linked e-cigarette use with adverse respiratory health outcomes (7–15). Although the number of new cases of e-cigarette and vaping–associated lung injury has decreased significantly, it is not clear what effects e-cigarette use may have on respiratory host-defense functions in otherwise “healthy vapers.” E-cigarette use has been linked to markers of modified respiratory host defense, including suppressed inflammatory gene expression in the airway (16), increased neutrophil activation and altered mucin secretion (4), impaired neutrophil phagocytosis and oxidative burst (17), impaired ciliary motility (18), and acute respiratory effects in clinical and translational studies (5, 19–21). However, whether and to what extent these respiratory immune changes translate into altered host-response functions is unknown.
Susceptibility to and severity of influenza viral infection are dependent on a variety of host factors that can be modified by cigarette smoke. For example, influenza viruses require proteolytic activation by respiratory proteases, which is balanced by antiproteases. Expression and activity of these proteases and antiproteases is affected by cigarette smoke and e-cigarettes (22). In addition, cytokine and chemokine release, which orchestrates the innate and adaptive immune response after influenza infections (23–25), is significantly modified by both cigarette and e-cigarette exposure (16, 26–30). We have previously demonstrated that inoculation with the live-attenuated influenza virus (LAIV) vaccine can be used in controlled clinical studies to assess how nasal mucosal antiviral host-defense functions are altered in cigarette smokers (31). These studies illustrated that innate immune defense responses, marked by cytokine release and viral load, as well as by the presence and activity of immune cells such as natural killer (NK) cells and γδ T cells, are altered in smokers (26, 28, 31). Interestingly, antibody production does not appear to be affected by cigarette smoking, which has been shown in two separate human cohort studies (32–34). However, the effects of e-cigarette use on these host-defense factors present a critical knowledge gap.
It is well established that inhalation of cigarette smoke is linked to an increased risk of viral infection, such as influenza. Inhalation of smoke or aerosol from new and emerging tobacco products, such as e-cigarettes, has also recently been linked to immune suppression within the respiratory tract, within the nasal mucosa (16), and in response to bacterial infection (35). Considering the concurrent threats of increased e-cigarette use and emerging viral infections, such as coronavirus disease (COVID-19), determining whether and how e-cigarette use affects antiviral host-defense functions is of significant public health importance. On the basis of the known immunity-modifying effects of e-cigarettes, we hypothesize that e-cigarette use will be associated with altered nasal host-defense responses to viral infections. Using our well-established model of inoculation with LAIV, the study presented here compares viral load, immune-mediator gene expression and protein levels, and nasal mucosal antibody production among three groups of young adults: nonsmokers, smokers, and e-cigarette users. Some of the results of these studies have been previously reported in conference abstracts (36–38).
Methods
Study Protocol
We inoculated human volunteers with LAIV to examine the innate immune response to influenza infection. Participants recruited were healthy, young adults of 18–40 years of age and were categorized as nonsmokers, cigarette smokers, and e-cigarette users on the basis of self-reported tobacco-product use, smoking or vaping diaries, and biomarkers of exposure to nicotine and tobacco products (Table 1). Exclusion criteria included a history of allergic rhinitis, asthma, and use of immunosuppressive drugs, including corticosteroids, to reduce the possibility of confounders affecting the nasal mucosa. A sample size of ∼16 per group was targeted on the basis of power calculations using previous studies observing differences in smokers and nonsmokers (26, 27, 31). Inclusion criteria for the exposure categories were as follows: 1) nonsmokers were never-smokers, 2) cigarette smokers smoked at least three cigarettes per day on average, 3) e-cigarette users vaped at least 18 puffs per day on average and smoked fewer than five cigarettes per week. Most of the e-cigarette users recruited were former smokers and used mostly second- and third-generation devices. Participants entered into our protocol as shown in Figures 1 and 2.
Table 1.
Subject Demographics and Biomarkers of Nicotine and Tobacco Use
| All (N = 49) | E-Cigarette Users (n = 15) | Cigarette Smokers (n = 14) | Nonsmokers (n = 20) | |
|---|---|---|---|---|
| BMI, mean ± SD | 26.3 ± 5.8 | 26.4 ± 6.3 | 26.5 ± 6.0 | 26.1 ± 5.6 |
| Age, mean ± SD | 27.5 ± 7.6 | 22.8 ± 4.8 | 31.3 ± 6.4 | 28.3 ± 8.4 |
| Sex, n, F/M | 22/27 | 3/12 | 5/9 | 14/6 |
| Race, n, African American/white/other | 9/34/6 | 1/11/3 | 6/7/1 | 2/16/2 |
| Cigarettes/d, mean ± SD (range) | — | 0.0 ± 0.1 (0.0–0.1) | 9.8 ± 5.3 (3.8–20)* | — |
| Cotinine, mean ± SD | — | 99.6 ± 132.0† | 121.7 ± 125.3* | 2.0 ± 7.5 |
| NNAL/creatinine, mean ± SD | — | 4.7 ± 9.5 | 98.1 ± 89.7* | 1.2 ± 3.9 |
Definition of abbreviations: BMI = body mass index; e-cigarette = electronic-cigarette; NNAL = 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol.
P ≤ 0.0001.
P ≤ 0.001, compared with nonsmokers.
Figure 1.
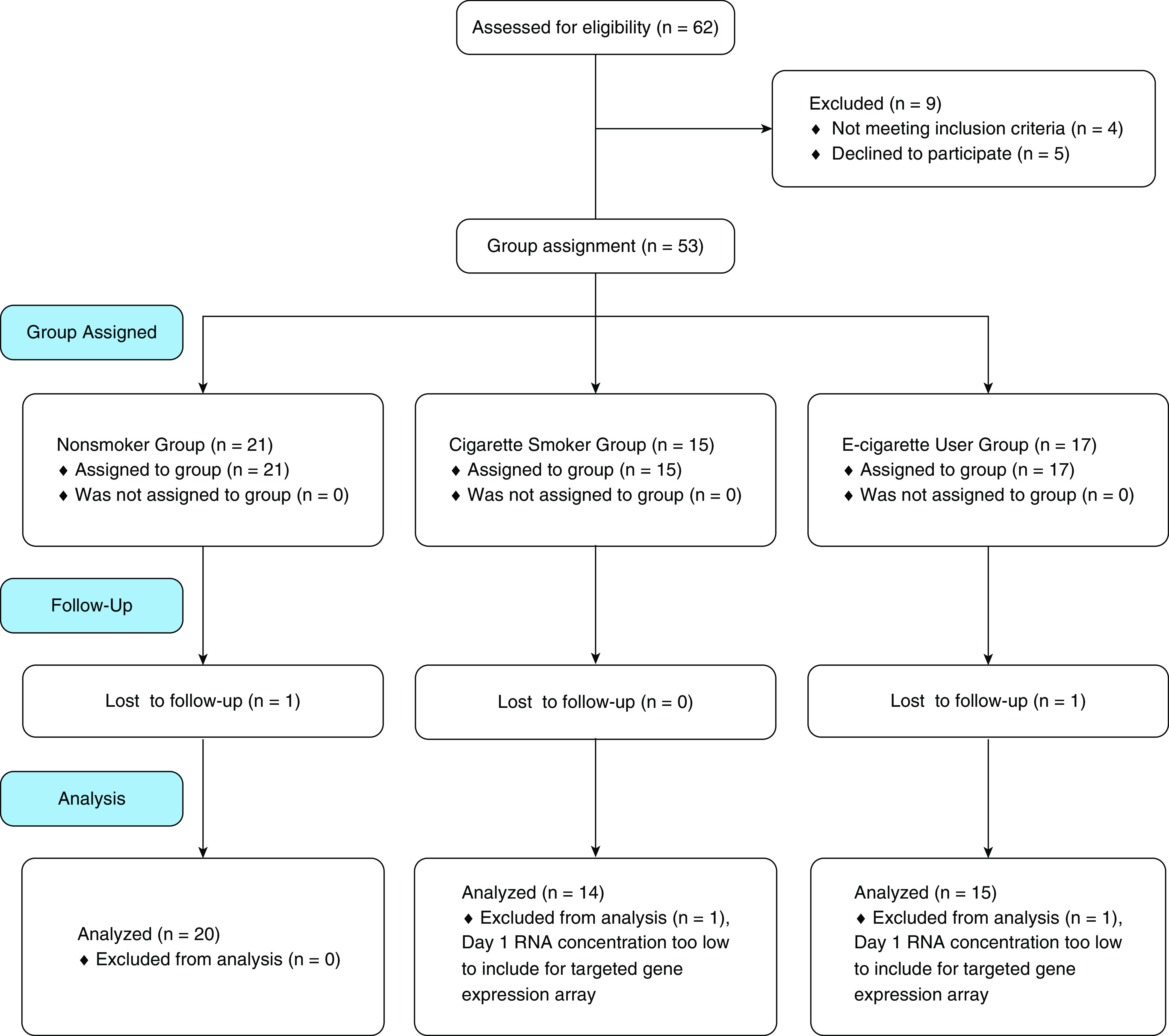
Consolidated Standards of Reporting Trials diagram. Participant recruitment, screening, and group assignment. E-cigarette = electronic-cigarette.
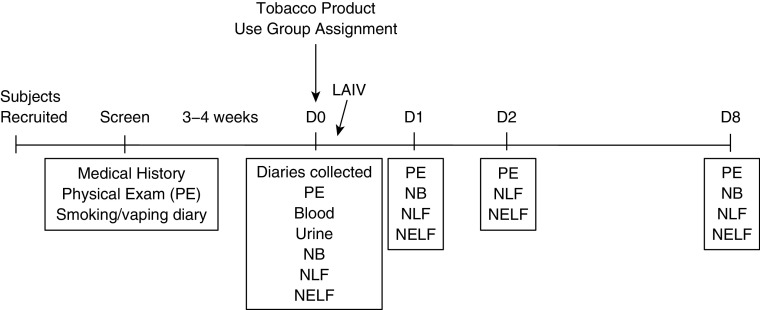
Figure 2.
Study design and sample-collection timeline. D = Day; LAIV = live-attenuated influenza virus; NB = nasal-scrape biopsy; NELF = nasal epithelial-lining fluid; NLF = nasal lavage fluid; PE = physical examination.
During the initial screening visit, participants consented, were examined by a physician, and reported their health history, including tobacco-product use. If participants were cigarette smokers or e-cigarette users, they were given a smoking or vaping diary to complete for 3–4 weeks, after which they returned for their baseline visit, during which diaries were collected. At the baseline visit, participants were first examined by a physician, and then nasal lavage fluid (NLF), nasal epithelial-lining fluid (NELF), a nasal-scrape biopsy specimen obtained via nasal curettage (39), serum, and urine were collected. After baseline sample collection, participants were inoculated with a standard dose of the 2015–2016 or 2016–2017 LAIV vaccine (FluMist; MedImmune, AstraZeneca) within the standard influenza season, as described previously (31, 40, 41). Viral strains in the 2015–2016 vaccine included A/California/7/2009 (H1N1), A/Switzerland/9715293/2013 (H3N2), B/Phuket/3073/2013, and B/Brisbane/60/2008. Virus strains in the 2016–2017 vaccine included A/California/7/2009 (H1N1), A/Hong Kong/4801/2014 (H3N2), B/Brisbane/60/2008 (B/Victoria lineage), and B/Phuket/3073/2013 (B/Yamagata lineage). Participants returned on Days 1, 2, and 8 after inoculation, at which point NLF, NELF, and nasal-scrape biopsy specimens were again collected, as shown in Figure 2. The protocol was approved by the University of North Carolina at Chapel Hill Biomedical Institutional Review Board (13-2246), and all methods were performed in accordance with relevant guidelines and regulations. This study was also registered at clincaltrials.gov (NCT 02019745).
Sample Analysis
Samples including NLF, nasal-scrape biopsy, NELF, blood, and urine samples, were collected and processed as described previously (41, 42) (see data supplement). Nasal-scrape biopsy–specimen RNA was then analyzed for gene expression via the NanoString nCounter PanCancer Immune Profiling code set, with an 8-gene nCounter Panel-Plus add-in to include influenza genes from the 2015–2016 and 2016–2017 seasons of the LAIV FluMist vaccine (Table 2). NanoString data were normalized against the included housekeeping genes that met the criteria for the expression level above the background threshold, as indicated by manufacturer instructions, and stability, with no statistical difference between exposure groups. NELF was analyzed using the V-PLEX Human Cytokine 30-Plex Kit from Meso Scale Diagnostics, and NLF was analyzed using IL-8, IL-6, and IP-10 sandwich ELISAs (Becton Dickinson). Serum and urine were analyzed for cotinine- and tobacco-specific nitrosamine 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol (NNAL), as previously described (16).
Table 2.
Custom Add-In Probe Sequences to NanoString nCounter PanCancer Immunology Code Set
| Gene Name | Accession Number | Target Region | Target Sequence |
|---|---|---|---|
| Infl_A_Cal_HA | FJ966952.1 | 735–834 | CTATTACTGGACACTAGTAGAGCCGGGAGACAAAATAACATTCGAAGCAACTGGAAATCTAGTGGTACCGAGATATGCATTCGCAATGGAAAGAAATGCT |
| Infl_A_Cal_NA | FJ966956.1 | 1,134–1,233 | CGGATGGACTGGGACAGACAATAACTTCTCAATAAAGCAAGATATCGTAGGAATAAATGAGTGGTCAGGATATAGCGGGAGTTTTGTTCAGCATCCAGAA |
| Infl_A_Tex_HA | KC892952.1 | 864–963 | ACCCATTGGCAAATGCAAGTCTGAATGCATCACTCCAAATGGAAGCATTCCCAATGACAAACCATTCCAAAATGTAAACAGGATCACATACGGGGCCTGT |
| Infl_A_Tex_M1 | KC892233.1 | 288–387 | AGTTAAACTGTATAGGAAACTTAAGAGGGAGATAACGTTCCATGGGGCCAAAGAAATAGCTCTCAGTTATTCTGCTGGTGCACTTGCCAGTTGCATGGGC |
| Infl_A_Tex_NA | KC892281.1 | 289–388 | TTTGCACCTTTCTCTAAGGACAATTCGATTAGGCTTTCCGCTGGTGGGGACATCTGGGTGACAAGAGAACCTTATGTGTCATGCGATCCTGACAAGTGTT |
| Infl_B_HA | CY115151.1 | 312–411 | CAGACCTGTTACATCTGGGTGCTTTCCTATAATGCACGACAGAACAAAAATTAGACAGCTGCCTAACCTTCTCCGAGGATACGAACATATCAGGTTATCA |
| Infl_B_M1 | KC866607.1 | 389–488 | CAGCGCTACTATACTGTCTCATGGTCATGTACCTGAATCCTGGAAATTATTCAATGCAAGTAAAACTAGGAACGCTCTGTGCTTTATGCGAGAAACAAGC |
| Infl_B_NA | FJ766839.1 | 482–581 | CAATGGAACAAGAGGAGACAGAAACAAGCTGAGGCATCTAATTTCAGTCAAATTGGGCAAAATCCCAACAGTAGAAAACTCCATTTTCCACATGGCAGCA |
Relative viral gene expression as a marker of influenza viral load in NLF cells was assessed using qRT-PCR and primer and probe pairs specific for the M1 gene of the LAIV influenza B Ann Arbor/1/66 master donor strain: 5′-FAM-CCCTCTTGTTGTTGCCGC-TAMRA-3′ (probe), 5′-GGGTGCAGATGCAACGATT-3′ (sense), and 5′-AATATCAAGTGCAAGATCCCAATG-3′ (antisense). Data were normalized using β-actin mRNA expression, and expression differences were evaluated using the comparative cycle-threshold method (43, 44).
NLF virus-specific IgA
Levels of influenza-specific IgA in the NLF were measured using a direct-sandwich ELISA (see data supplement). Virus-specific IgA levels were determined against the IgA standard curve and normalized to total IgA levels (45). After normalization, the change in the antibody level was determined by using the relative percentage of the baseline level, in which the virus-specific antibody concentration post-LAIV inoculation was divided by pre-LAIV inoculation levels and multiplied by 100.
Statistical Analysis
Effects of LAIV in NLF and NELF
NLF analyses were completed after Shapiro-Wilk normality testing. A mixed-effects analysis with Fisher Least Significant Difference (LSD) was used for NLF cell markers of influenza viral-load analyses, a paired two-way ANOVA with Fisher LSD post hoc test was used for the influenza-specific IgA analysis, and a Kruskal-Wallis test with a Dunn post hoc test was used for the NELF analyses. A Brown-Forsythe and Welsh ANOVA were used for cytokine and chemokine NELF and NLF analyses. Analyses were completed in GraphPad Prism, and significance was determined to be present when P was less than 0.1, which was based on the use of clinical data with a relatively small N (41, 46).
Gene-expression analyses in nasal-scrape biopsy specimens
Baseline effects of tobacco-product exposure were first determined by using linear regression comparing gene expression among tobacco-product exposure groups (e-cigarette users and cigarette users) and control subjects (healthy nonsmokers and nonusers).
Similar to the authors of previous studies measuring the effect of the LAIV and exposure to environmental toxicants (31, 41), we completed an a priori analysis of the dependent variable (LAIV response) calculated from the area under the curve (AUC) by using the pracma package in R (R Foundation for Statistical Computing; https://cran.r-project.org/web/packages/pracma/pracma.pdf) over the days of the study. Linear-regression analysis was then used to determine the relationship between LAIV response and tobacco-product exposure using the baseline-corrected AUC. Baseline correction was used because of significant variability in baseline gene expression among the comparison groups. Demographic covariates were included in a multiple regression as a secondary analysis, in which we identified an interaction of sex and tobacco-product exposure. All analyses were conducted using R (47). For all gene-expression analyses, statistical significance was determined to be present when P was less than 0.05 and the fold change was greater than |1.5| for baseline effects or when the percentage of change was greater than |150%| for effects of the baseline-corrected AUC.
The heatmap was created using the R pheatmap package (47, 48). Interaction networks and pathway-enrichment analyses were performed using STRING (STRING Consortium) (49). Interactions were determined using a confidence score ≥ 0.7. A Markov cluster algorithm was used to cluster genes significant for interaction with an inflation parameter of 1.5.
Results
Demographics
Subject demographics, cigarette use, and tobacco- and nicotine-specific biomarker data are described in Table 1. There were no differences among groups in terms of body mass index or age. As expected on the basis of results from the prescreening recruitment questionnaire, the number of cigarettes smoked per day was greater in cigarette smokers than in e-cigarette users; cigarette smokers also had higher tobacco-specific nitrosamine levels (NNAL/creatinine) in urine than nonsmokers and e-cigarette users, indicating little to no dual use in the e-cigarette group. Both cigarette smokers and e-cigarette users had higher serum cotinine levels than nonsmokers, as expected.
Baseline Effects of Tobacco-Product Use
In an approach similar to that of our previous study (16), we analyzed the effect of tobacco use compared with nonuse on samples at baseline. We found 38 genes to be differentially expressed in the cigarette-smoking group and 3 genes to be differentially expressed in the e-cigarette group, with two genes (USP9Y, CD1A) common to both cigarette smokers and e-cigarette users and changed in the same direction (see Table E1 in the data supplement).
Effect of Tobacco-Product Use and LAIV
Viral load
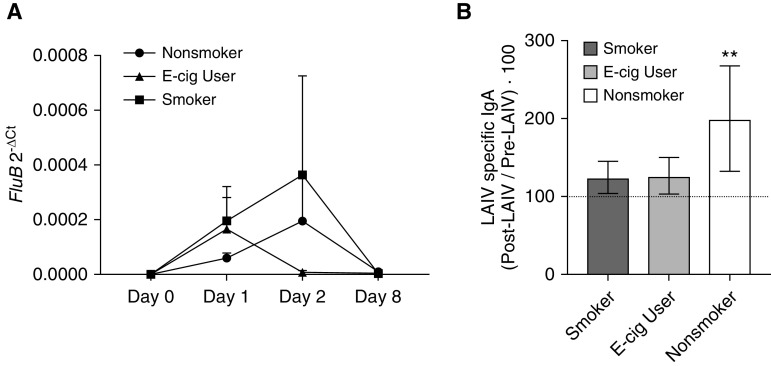
Influenza subunit genes from NLF cells were compared among exposure groups for differential expression but showed no significant effects of the tobacco-exposure group on viral load (Figure 3A). This result differs from those of our previous reports (31), which may be the result of a lower pack-year history or lower numbers of cigarettes smoked per day in our cohort compared with the ones previously studied.
Figure 3.
Viral load and antibody production. (A) Measurement of Influenza B M1 gene by quantitative PCR as a measure of viral load in NLF cells. There were not any significant differences detected between exposure groups, but viral load did increase after infection. (B) Influenza-specific IgA in NLF measured by using an in-house ELISA. Change in LAIV-specific IgA was calculated by using the relative percentage, in which the normalized virus-specific antibody concentration after LAIV inoculation was divided by the prevaccination level and multiplied by 100. Nonsmoker levels of IgA were increased after LAIV inoculation, whereas levels in e-cigarette (e-cig) users and cigarette smokers did not. The dotted line at 100% represents pre-LAIV levels of influenza-specific IgA, **P ≤ 0.05. These data suggest that cigarette smokers and e-cig users may not respond appropriately to the LAIV vaccine. 2-ΔCt = comparative cycle-threshold method; FluB = influenza B.
LAIV nasal mucosal antibody levels
Previous studies have shown that serum antibody levels are a poor measure of LAIV vaccine efficacy (50). In contrast, nasal mucosal secretory IgA, the predominant immunoglobulin produced in response to infection of the nasal mucosa, has been shown to be a much better indicator of LAIV-induced antibody responses (reviewed in Reference 45). We developed an anti–LAIV-IgA ELISA to determine the effects of cigarette smoking and e-cigarette use on antibody production and found that LAIV-specific IgA levels increased as expected in nonsmokers after LAIV inoculation but did not increase in e-cigarette users and cigarette smokers (Figure 3B). These results suggest an impaired humoral response to LAIV-induced IgA secretion in e-cigarette users.
Nasal epithelial gene expression
Using nasal-scrape biopsy specimens obtained at baseline and on Day 1 and Day 8 after LAIV inoculation, we examined the effects of tobacco-product use on LAIV-induced gene-expression changes. Changes in LAIV-induced gene expression were assessed by calculating the AUC over the three analysis days and subtracting the baseline expression to adjust for interindividual variability. There were 191 differentially expressed genes in the e-cigarette–user group as compared with nonsmoker control subjects; 31 genes were upregulated, and 160 genes were downregulated (Figures 4A and 4B and Table E2). The top five upregulated genes in the e-cigarette exposure group by the percentage of change from nonsmokers included CD19, CKLF, BST1, GPI, and AKT3. The top five downregulated genes by the percentage of change from nonsmokers included MR1, NT5E, HRAS, CD55, and IL5RA. Eighty genes were differentially expressed in the cigarette-smoker group as compared with control subjects, 4 were upregulated, and 76 were downregulated. The upregulated genes included GPI, ANP32B, LAMP1, and MAVS. The top five downregulated genes by the percentage of change from nonsmokers included SMPD3, NOS2A, KLRB1, APP, and CXCL1. Fifty-two of the genes in both tobacco-exposure groups overlapped (Figure 4C), with all genes in both exposure groups being differentially expressed in the same direction, 50 genes being downregulated, and 2 genes being upregulated. GPI is one of the overlapping genes that was also one of the top differentially expressed genes in both the e-cigarette and cigarette exposure groups; in both cases, it was upregulated by over 4,000%.
Figure 4.
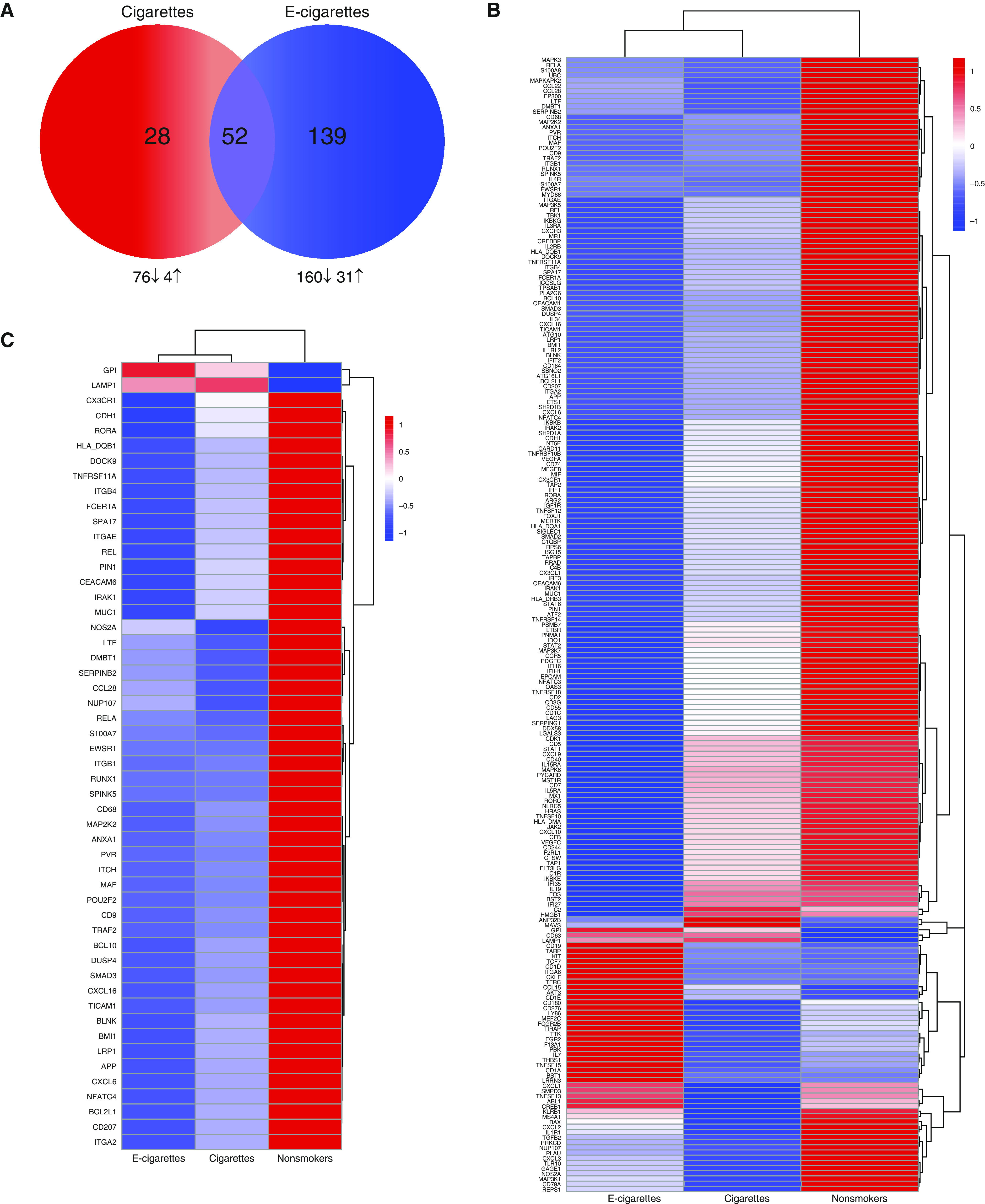
Aggregate effect of tobacco products on response to LAIV inoculation. (A) Venn diagram of differentially expressed (DE) genes in cigarette and e-cigarette groups compared with nonsmokers in response to LAIV inoculation using the baseline-corrected area under the curve (AUC). The total number of DE genes are in the circles labeled cigarettes (red) and e-cigarettes (blue). Numbers and directional arrows below the Venn diagram show numbers of DE genes up- and downregulated for each group. (B) Heatmap of all (219 genes) aggregate baseline differences in each exposure group. (C) Heatmap of aggregate baseline differences that overlap in the e-cigarette and cigarette exposure group (52 genes). Log2 averages for each gene are displayed. Data included are DE genes (P < 0.05 and fold change = |1.5|) in smokers and e-cigarette users compared with nonsmokers.
Nasal mucosal-mediator levels
Using NELF collected by using nasosorption, we measured cytokine levels at baseline and on Days 1, 2, and 8 after LAIV inoculation (Table E3). Similar to changes in gene expression, LAIV-induced changes in mediator levels were determined by calculating the AUC of the 4 analysis days. Chemokines regulating the recruitment and activation of monocytes (MCP-1, MIP-1β) were increased in cigarette smokers as compared with nonsmokers (Figures 5A and 5B). In contrast, cytokines regulating antiviral host-defense responses (IFNγ, IL-6, IL-12p40) were reduced in e-cigarette users but were not reduced in cigarette smokers, as compared with nonsmokers (Figures 5D–5F). Interestingly, IL-2, IL-1α, and VEGF were increased in e-cigarette users as compared with nonsmokers (Figures 5G–5I).
Figure 5.
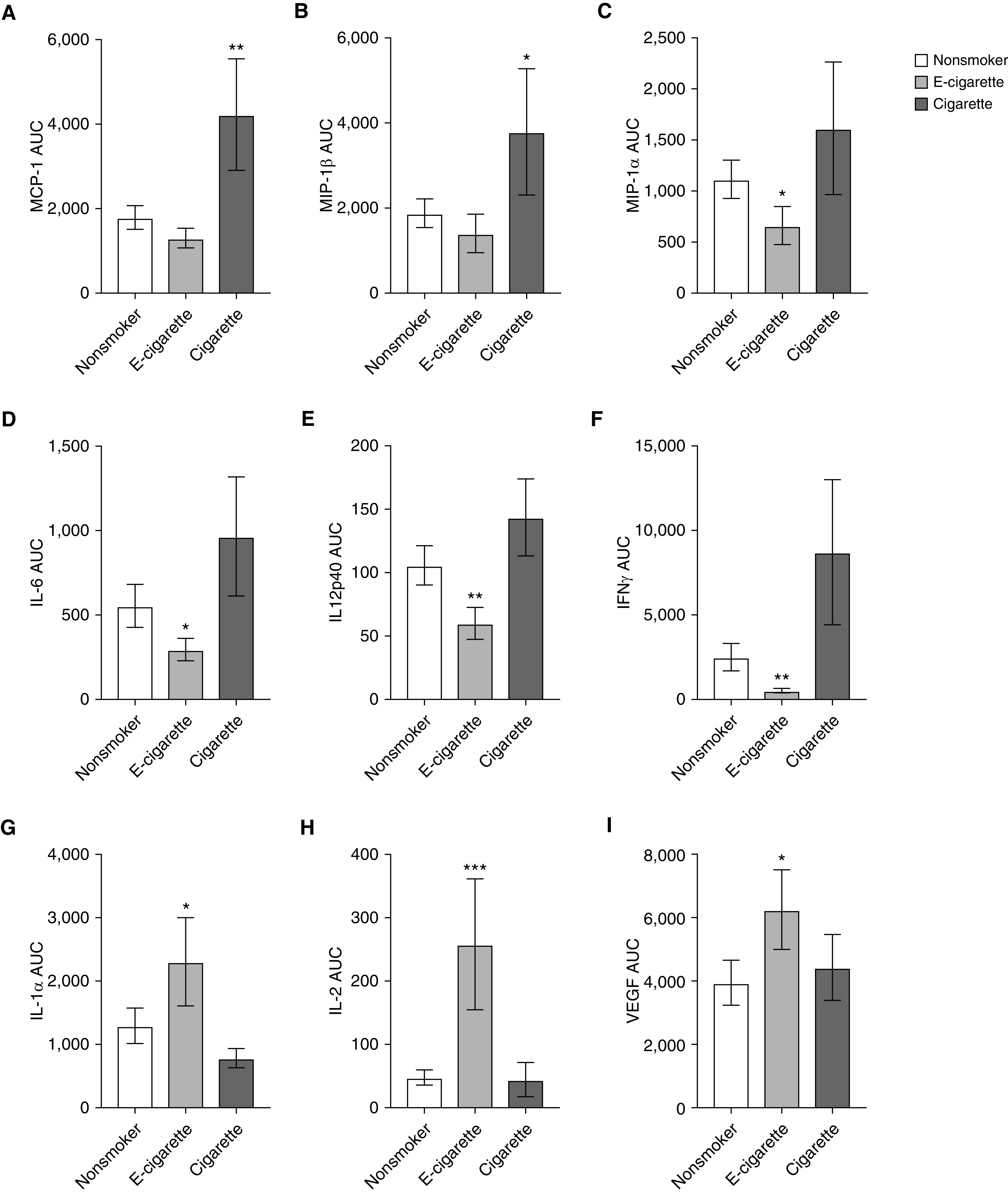
NELF protein-level changes in response to LAIV inoculation. NELF was analyzed for changes in nasal protein levels induced by LAIV by using the AUCs of levels at baseline (D0), D1, D2, and D8. The AUCs for (A) MCP-1, (B) MIP-1β, (C) MIP-1α, (D) IL-6, (E) IL12p40, (F) IFNγ, (G) IL-1α, (H) IL-2, and (I) VEGF are shown. Cigarette smoke–induced increases are shown in A–C, e-cigarette–suppressed responses are shown in D–F, and e-cigarette–induced increases are shown in G–I. Data are shown as the mean ± SEM. *P ≤ 0.1, **P ≤ 0.05, and ***P ≤ 0.01 compared with nonsmokers. MCP-1 = monocyte chemoattractant protein-1; MIP-1 = macrophage inflammatory protein-1; VEGF = vascular endothelial growth factor.
Interactive effect of tobacco-use group and sex on response to LAIV
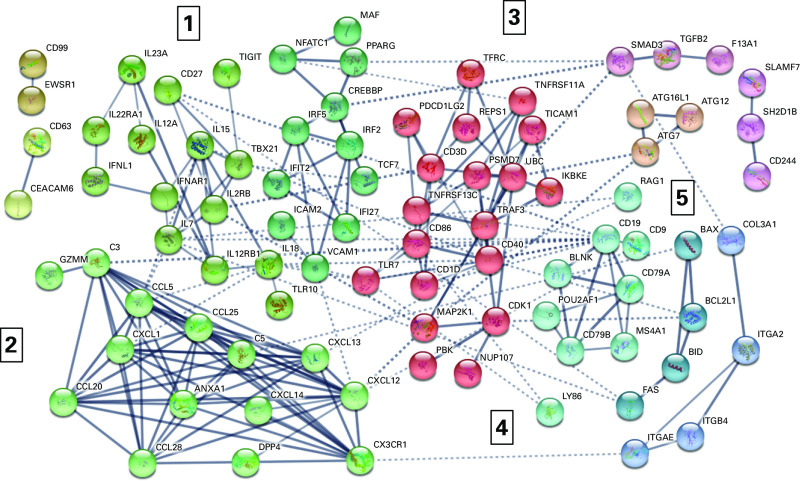
In our covariate analysis, we identified interactions between tobacco-use group and sex. One hundred and nineteen genes displayed an interaction between sex and tobacco-use group in response to LAIV (Table E4). A variety of pathways from the Kyoto Encyclopedia of Genes and Genomes were significantly enriched in this gene set, including cytokine–cytokine-receptor interaction, TLR (Toll-like receptor) signaling, inflammatory and infectious diseases, TNF signaling, and cell-adhesion–molecule pathways (the top 25 enriched pathways are reported in Table E5). Predictive clustering (Figure 6 and Table E6) resulted in five main clusters: IFN regulation–associated genes, including IFNL1, IFNAR1, IFIT2, IFI27, IRF2, and IRF5; chemokines and immune-signaling genes that are involved in chemotaxis and chemoattraction, including CX3CR1, CCL5, CCL20, CXCL12, CXCL1, CCL25, CCL28, and CXCL13; TNF regulation– and adaptive immunity–associated genes (T cell– and B cell–related genes), including TICAM1, TNFRSF11A, TNFRSF13C, TRAF3, TFRC, CD3D, CD86, CD1D, and CDK1; B cell– and B-cell antigen–related genes, including CD19, CD9, CD79A, and CD79B; and cell-death regulation genes, including BAX, BCL2L1, and BID. Because of the small N within sex, we were underpowered to complete sex-stratified analyses.
Figure 6.
Interaction of sex and exposure to tobacco products on response to LAIV inoculation: predictive gene-interaction map. Genes significant (P ≤ 0.05) for a sex–exposure-to-tobacco-product interaction. Predicted interactions were evaluated in STRING and included with a value of 0.7 (high confidence). Clusters were created using a Markov cluster algorithm with an inflation parameter of 1.5. Interactions are shown by lines connecting each node; line thickness indicates the strength of data support for the interaction within STRING. Unconnected network nodes are hidden. Cluster numbers are identified by numbers 1–5 in the figure. Nodes within the cluster and node color are described in more detail in Table E6.
Discussion
The purpose of this study was to test the hypothesis that e-cigarette use alters respiratory antiviral host-defense responses in humans. To test this hypothesis, we used our well-established model of influenza infection, nasal inoculation with LAIV, and compared host-defense responses in groups of e-cigarette users, cigarette smokers, and healthy nonsmoking and nonusing control subjects. Our results demonstrate that e-cigarette use did not appear to affect the markers of viral load tested but was associated with significantly altered LAIV-induced nasal mucosal immune-gene expression, immune-mediator release, and nasal LAIV-specific IgA levels. Similar to findings of our previous studies (16), changes in the expression of nasal immune genes were more abundant and greater in magnitude in e-cigarette users than in cigarette smokers. Furthermore, sex was a significant modifier of LAIV-induced immune-gene expression (Figure 6), suggesting that genes involved in IFN signaling and adaptive immune function (T- and B-cell function), are differentially modified in male and female e-cigarette users. Together, these data suggest that e-cigarette use alters nasal mucosal antibody production, gene expression, and protein production and thereby might alter respiratory antiviral host-defense function and immune memory. Demonstrations of causality would require longer-term studies of immunity and infection rates.
Although NLF for secretory IgA analysis was not collected at an ideal time point (8–9 d after infection rather than 14–21 d after infection), differences in production of LAIV-specific IgA were observed between groups. Nonsmoker LAIV-specific IgA levels increased after LAIV inoculation, whereas e-cigarette–user and cigarette-smoker levels did not, adding potential consequences for long-term memory responses. This identifies a potential effect of e-cigarette use on downstream immunity to infectious diseases and immune memory, which should be further explored. Considering that e-cigarette use is prevalent among teenagers who are recommended for complete immunization schedules, understanding whether and how vaping could modify vaccination-conferred immunity is an unexplored field. Secretory IgA is the principal antibody isotype present in nasal secretions (45) and neutralizes pathogens, like influenza, at the site of infection before they attach, enter, and replicate in the host cell (45). Although conventional intramuscularly administered influenza vaccines generate a serum IgG-antibody response, protection conferred by LAIV vaccination is believed to be derived by its ability to generate a robust and sustained nasal mucosal IgA response (51). Hence, although viral load was not affected by e-cigarette use, suggesting no difference in susceptibility to influenza viral infections, sustained immune-memory response and protection against reinfection may be suppressed by using e-cigarettes. Although it is unclear what levels of pathogen-specific IgA are needed in the nasal mucosa to indicate protection against subsequent infection, these data indicate that e-cigarette use suppresses the nasal mucosal antibody response.
The differential response to LAIV infection as a result of tobacco-product use was substantial, with a total of 219 genes differentially expressed in the nasal epithelium of e-cigarette users and cigarette smokers as compared with nonsmokers. Common in both e-cigarette users and cigarette smokers was the overwhelming downregulation of immune genes in response to LAIV. In addition, e-cigarette use was associated with a greater number of immune-gene expression changes than cigarette smoking when compared with nonsmokers in response to LAIV, suggesting a broader potential for disruption of host-defense functions in e-cigarette users than in cigarette smokers. Interestingly, 52 differentially expressed genes overlapped in both groups, altered in the same direction, indicating some common effects between groups. However, when compared on a gene-by-gene basis, e-cigarette users showed greater suppression of a majority of these genes (Figure 4C). Hence, e-cigarette use was associated with a greater number of immune-gene expression changes, and genes differentially expressed in both e-cigarette users and cigarette smokers showed greater suppression in e-cigarette users. These observations are similar to those of our previous study demonstrating that baseline immune-gene expression in the nasal epithelium of e-cigarette users indicated an overall immunosuppressive phenotype, which was marked by a greater number of differentially expressed genes than that shown in smokers (16).
Among the most downregulated genes in both e-cigarette users and cigarette smokers were APP, NUP107, and ITGB1, which have previously been identified as host factors regulating influenza viral infections (52). For example, APP encodes the Amyloid precursor protein, which can be broken down into smaller fragments, such as β-Amyloid, which in turn has been shown to inhibit influenza viral replication (53). Among the most upregulated genes in e-cigarette users after LAIV inoculation was CKLF, which encodes chemokine-like factor, a potent chemoattractant for neutrophils, monocytes, and lymphocytes, that has been associated with infiltration of inflammatory cells and pulmonary damage (54). One of the most downregulated genes in e-cigarette users after LAIV infection is NT5E, which encodes CD73, a surface ecto-5′-nucleotidase. Knockout mice demonstrate that lack of CD73 does not alter influenza-induced acute lung injury but is necessary for a proper innate antiviral immune response (55). In addition to broad, differential gene-expression changes in response to LAIV, cigarette smokers, e-cigarette users, and nonsmokers also differed significantly in their cytokine responses. Of the 28 cytokines analyzed, 9 were modified by tobacco-product exposure. Interestingly, chemokines regulating the recruitment and activation of monocytes were enhanced in cigarette smokers. In contrast, the cytokines IFNγ, IL-6, and IL-12p40, with known critical function during antiviral host-defense responses (56), including inducing immune memory (57), were decreased in e-cigarette users. Together, these biomarkers of immune response in the nasal epithelium after inoculation with LAIV suggest that e-cigarette use is associated with disrupted normal mucosal host-defense function.
A unique finding of this study is that sex and tobacco-product exposure interact to influence the host-defense response to LAIV, impacting 119 genes in our data set. Sex has been demonstrated as a significant modifier of viral infection and tobacco-product use individually but had yet to be shown as having an interactive effect. Sex is a known and substantial modifier of the response to viral infection because of physiological and anatomical differences between males and females (58). These differences affect antibody responses, viral clearance, vaccine efficacy, and levels of virus-induced inflammation, generally resulting in a more protective response to viral infection in females than in males (58–62). Interestingly, a similar interactive effect was observed in our previous study on wood-smoke exposure, emphasizing that sex and inhaled pollutants can more broadly interact to influence host-defense responses against viral infections (41). The five clusters significantly enriched in the sex–tobacco-exposure interactome include IFN regulation–associated genes (IFNL1, IL18, IFNAR1, IRF2, IRF5, IFI27, IFIT2), chemokine genes involved in attraction and activation of cells involved in immune memory (CXCL12, CXCL13, CCL28, CCL20), and genes involved in responses to pathogens (CD40, TICAM1, TLR7). The altered IFN-related genes are critical to the response to viral infection. Alteration of many of these innate immune genes likely affect recruitment and activation of pathways critical for mounting an adaptive response to a virus, especially by altering type 1 IFN, shown to induce B-cell activation and appropriate antibody responses (63). Similarly, alteration of the expression of genes actively involved in response to pathogens has been shown to impair outcomes of influenza and other viruses. For example, TLR7, which is a key pathogen-recognition receptor, has been shown to mediate natural-killer–cell activation and necessary IFNγ production after influenza infection (64). Altered expression of these critical host-defense genes were dependent on both sex and tobacco-exposure group, indicating that susceptibility to influenza differs by both factors. Although our N was not sufficient to complete sex-stratified analyses, this should be a strong focus of future work, especially because of recent male-biased susceptibility to emerging viral infections and further links to lifestyle factors such as smoking and vaping (65–67).
Previous research into the effects of e-cigarettes on immune function may give us insights into the role of specific e-cigarette components on the impaired host-defense response to LAIV infection observed here. We have previously demonstrated that e-cigarettes can suppress immune-gene expression and, particularly, that flavoring compounds can alter critical host-defense responses such as macrophage, neutrophil, and natural-killer–cell function; airway epithelial-cell ciliary motility; and mucin secretion (4, 5, 16–18, 68). Specifically, reactive flavoring chemicals, such as aldehydic flavoring chemicals, were shown to alter immune-cell functions critical to the response to pathogenic infection, such as phagocytosis and natural-killer–cell function (5, 17), and to compromise induction of adaptive immune responses via suppression of key innate immune-cell functions (69–71). Additional studies of chronic nicotine exposure and exposure to the e-cigarette components propylene glycol and vegetable glycerin have shown that these exposures affect the immune system and response to viral infections (72, 73). Although the number of participants in this study limited our ability to stratify by popular flavoring profiles, nicotine content, or other e-cigarette components to investigate their role in viral infection, uncovering how specific e-cigarette components modify antiviral host-defense function should be a target of future investigation.
This study, although novel and informative, does include limitations. This study was conducted from 2015 to 2017 and is thus most informative about the response to LAIV inoculation when use of second- and third-generation e-cigarette devices were most prominent and may not fully inform potential responses to newer devices or e-cigarette formulations (74–76). How the differences in devices, e-cigarette liquid formulations, and changes in aerosol deliveries affect the antiviral host-defense responses described here needs to be examined in future studies. Similarly, because of the time period in which this study was conducted, our e-cigarette–user participants were also mostly former smokers; thus, changes seen in this group may differ from the effects in younger e-cigarette users who are predominantly nonsmokers. However, based on our inclusion of both biomarkers of nicotine use (cotinine) and combusted tobacco (NNAL), our recruited e-cigarette users are not likely to have been substantial dual users. Specifically, in our study population, e-cigarette users had elevated levels of cotinine but not of NNAL, and smokers had elevated levels of both metabolites, indicating that e-cigarette users were not likely to have been dual users of e-cigarettes and conventional cigarettes. These results are consistent with previous findings in cohorts of e-cigarette users and cigarette smokers around the same timeframe (4, 16). Our sample-collection timeframe also limited our ability to analyze the ideal time for virus-specific secretory IgA, missing the class-switching peak by several days. Despite this limitation, we were able to detect increased LAIV-specific IgA levels in the NLF of nonsmokers, which was absent in both the e-cigarette–user and cigarette-smoker groups. Finally, although our analysis demonstrated a significant interaction between sex and tobacco-user group (Figure 6), our study was not sufficiently powered to stratify the different user groups by sex.
Overall, this study demonstrates that e-cigarette use is associated with significant suppression of host-defense responses in the context of experimental respiratory viral infections. Similar to data from previous studies published by us and others, our data further support the notion that e-cigarette use is associated with different effects on markers of mucosal immune responses as compared with smoking cigarettes and that e-cigarette use is not without harm (34, 77). These data also build on rodent work that demonstrated a connection between e-cigarette exposure and influenza-induced inflammation and tissue injury (73). However, population-based studies are needed to determine whether and to what extent the observations shown here are applicable to community-acquired respiratory infections. The data generated in this study suggest that e-cigarette use could increase the risk of suppressed host-defense functions in the context of respiratory viral infections. If so, this has important public health implications, especially during influenza season and respiratory-virus pandemics.
Supplementary Material
Acknowledgments
Acknowledgment
The authors thank the study participants for their willingness to participate in this research, their study coordinators for their role in executing the study, and their study recruiter. They also thank Dr. Neal Benowitz of the University of California at San Francisco for analyzing serum cotinine and urine NNAL measures for this study.
Footnotes
Supported by the National Heart, Lung, and Blood Institute, and the National Institute of Environmental Health Sciences grants P50HL12010004 and T32ES00712634 (I.J.), and a Leon and Bertha Goldberg Postdoctoral Fellowship. Research reported in this publication was in part supported by the U.S. National Institutes of Health and the U.S. Food and Drug Administration Center for Tobacco Products. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the U.S. Food and Drug Administration.
Author Contributions: T.L.N. and I.J. conceptualized and designed the study. M.E.R., E.G.-B., C.R., A.M.S., and E.A.P. acquired the samples. M.E.R., J.R.H., P.F.D., A.M.S., and R.D. analyzed the samples and data. M.E.R., P.F.D., A.M.S., R.D., and I.J. interpreted the findings. M.E.R. drafted the manuscript. M.E.R., E.G.-B., J.R.H., P.F.D., C.R., A.M.S., E.A.P., R.D., T.L.N., and I.J. revised the manuscript critically for intellectual content, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
This article has a data supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1165/rcmb.2020-0164OC on October 23, 2020
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.National Center for Chronic Disease Prevention and Health Promotion (U.S.) Office on Smoking and Health; Publications and Reports of the Surgeon General. E-cigarette use among youth and young adults: a report of the Surgeon General. Atlanta, GA: U.S. Centers for Disease Control and Prevention; 2016. [Google Scholar]
- 2.Harrell MB, Weaver SR, Loukas A, Creamer M, Marti CN, Jackson CD, et al. Flavored e-cigarette use: characterizing youth, young adult, and adult users. Prev Med Rep. 2016;5:33–40. doi: 10.1016/j.pmedr.2016.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chun LF, Moazed F, Calfee CS, Matthay MA, Gotts JE. Pulmonary toxicity of e-cigarettes. Am J Physiol Lung Cell Mol Physiol. 2017;313:L193–L206. doi: 10.1152/ajplung.00071.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reidel B, Radicioni G, Clapp PW, Ford AA, Abdelwahab S, Rebuli ME, et al. E-cigarette use causes a unique innate immune response in the lung, involving increased neutrophilic activation and altered mucin secretion. Am J Respir Crit Care Med. 2018;197:492–501. doi: 10.1164/rccm.201708-1590OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clapp PW, Pawlak EA, Lackey JT, Keating JE, Reeber SL, Glish GL, et al. Flavored e-cigarette liquids and cinnamaldehyde impair respiratory innate immune cell function. Am J Physiol Lung Cell Mol Physiol. 2017;313:L278–L292. doi: 10.1152/ajplung.00452.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rowell TR, Reeber SL, Lee SL, Harris RA, Nethery RC, Herring AH, et al. Flavored e-cigarette liquids reduce proliferation and viability in the CALU3 airway epithelial cell line. Am J Physiol Lung Cell Mol Physiol. 2017;313:L52–L66. doi: 10.1152/ajplung.00392.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hua M, Talbot P. Potential health effects of electronic cigarettes: a systematic review of case reports. Prev Med Rep. 2016;4:169–178. doi: 10.1016/j.pmedr.2016.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Khan MS, Khateeb F, Akhtar J, Khan Z, Lal A, Kholodovych V, et al. Organizing pneumonia related to electronic cigarette use: a case report and review of literature. Clin Respir J. 2018;12:1295–1299. doi: 10.1111/crj.12775. [DOI] [PubMed] [Google Scholar]
- 9.Sommerfeld CG, Weiner DJ, Nowalk A, Larkin A. Hypersensitivity pneumonitis and acute respiratory distress syndrome from e-cigarette use. Pediatrics. 2018;141:e20163927. doi: 10.1542/peds.2016-3927. [DOI] [PubMed] [Google Scholar]
- 10.Macedonia TV, Krefft SD, Rose CS. Severe fixed obstructive lung disease in a former smoker P1 with heavy e-cigarette use [abstract] Am J Respir Crit Care Med. 2018;197:A3572. [Google Scholar]
- 11.Poponea N, Shehada E, Freeman N. The dark side of vaping; acute dyspnea and granulomatous lung disease associated with electronic cigarettes [abstract] Am J Respir Crit Care Med. 2018;197:A3565. [Google Scholar]
- 12.Ring Madsen L, Vinther Krarup NH, Bergmann TK, Bærentzen S, Neghabat S, Duval L, et al. A cancer that went up in smoke: pulmonary reaction to e-cigarettes imitating metastatic cancer. Chest. 2016;149:e65–e67. doi: 10.1016/j.chest.2015.09.003. [DOI] [PubMed] [Google Scholar]
- 13.Thota D, Latham E. Case report of electronic cigarettes possibly associated with eosinophilic pneumonitis in a previously healthy active-duty sailor. J Emerg Med. 2014;47:15–17. doi: 10.1016/j.jemermed.2013.09.034. [DOI] [PubMed] [Google Scholar]
- 14.Bradford LE, Rebuli ME, Ring BJ, Jaspers I, Clement KE, Loughlin CE. Danger in the vapor? ECMO for adolescents with status asthmaticus after vaping. J Asthma. 2020;57:1168–1172. doi: 10.1080/02770903.2019.1643361. [DOI] [PubMed] [Google Scholar]
- 15.King BA, Jones CM, Baldwin GT, Briss PA. The EVALI and youth vaping epidemics: implications for public health. N Engl J Med. 2020;382:689–691. doi: 10.1056/NEJMp1916171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Martin EM, Clapp PW, Rebuli ME, Pawlak EA, Glista-Baker E, Benowitz NL, et al. E-cigarette use results in suppression of immune and inflammatory-response genes in nasal epithelial cells similar to cigarette smoke. Am J Physiol Lung Cell Mol Physiol. 2016;311:L135–L144. doi: 10.1152/ajplung.00170.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hickman E, Herrera CA, Jaspers I. Common e-cigarette flavoring chemicals impair neutrophil phagocytosis and oxidative burst. Chem Res Toxicol. 2019;32:982–985. doi: 10.1021/acs.chemrestox.9b00171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Clapp PW, Lavrich KS, van Heusden CA, Lazarowski ER, Carson JL, Jaspers I. Cinnamaldehyde in flavored e-cigarette liquids temporarily suppresses bronchial epithelial cell ciliary motility by dysregulation of mitochondrial function. Am J Physiol Lung Cell Mol Physiol. 2019;316:L470–L486. doi: 10.1152/ajplung.00304.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brożek GM, Jankowski M, Zejda JE. Acute respiratory responses to the use of e-cigarette: an intervention study. Sci Rep. 2019;9:6844. doi: 10.1038/s41598-019-43324-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wills TA, Pagano I, Williams RJ, Tam EK. E-cigarette use and respiratory disorder in an adult sample. Drug Alcohol Depend. 2019;194:363–370. doi: 10.1016/j.drugalcdep.2018.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rankin GD, Wingfors H, Uski O, Hedman L, Ekstrand-Hammarström B, Bosson J, et al. The toxic potential of a fourth-generation e-cigarette on human lung cell lines and tissue explants. J Appl Toxicol. 2019;39:1143–1154. doi: 10.1002/jat.3799. [DOI] [PubMed] [Google Scholar]
- 22.Ghosh A, Coakley RD, Ghio AJ, Muhlebach MS, Esther CR, Jr, Alexis NE, et al. Chronic e-cigarette use increases neutrophil elastase and matrix metalloprotease levels in the lung. Am J Respir Crit Care Med. 2019;200:1392–1401. doi: 10.1164/rccm.201903-0615OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miura TA. Respiratory epithelial cells as master communicators during viral infections. Curr Clin Microbiol Rep. 2019;6:10–17. doi: 10.1007/s40588-019-0111-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ye L, Schnepf D, Becker J, Ebert K, Tanriver Y, Bernasconi V, et al. Interferon-λ enhances adaptive mucosal immunity by boosting release of thymic stromal lymphopoietin. Nat Immunol. 2019;20:593–601. doi: 10.1038/s41590-019-0345-x. [DOI] [PubMed] [Google Scholar]
- 25.Peiró T, Patel DF, Akthar S, Gregory LG, Pyle CJ, Harker JA, et al. Neutrophils drive alveolar macrophage IL-1β release during respiratory viral infection. Thorax. 2018;73:546–556. doi: 10.1136/thoraxjnl-2017-210010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Horvath KM, Brighton LE, Herbst M, Noah TL, Jaspers I. Live attenuated influenza virus (LAIV) induces different mucosal T cell function in nonsmokers and smokers. Clin Immunol. 2012;142:232–236. doi: 10.1016/j.clim.2011.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Horvath KM, Herbst M, Zhou H, Zhang H, Noah TL, Jaspers I. Nasal lavage natural killer cell function is suppressed in smokers after live attenuated influenza virus. Respir Res. 2011;12:102. doi: 10.1186/1465-9921-12-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rebuli ME, Pawlak EA, Walsh D, Martin EM, Jaspers I. Distinguishing human peripheral blood NK cells from CD56dimCD16dimCD69+CD103+ resident nasal mucosal lavage fluid cells. Sci Rep. 2018;8:3394. doi: 10.1038/s41598-018-21443-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Holownia A, Wielgat P, Rysiak E, Braszko JJ. Intracellular and extracellular cytokines in A549 cells and THP1 cells exposed to cigarette smoke. Adv Exp Med Biol. 2016;910:39–45. doi: 10.1007/5584_2016_214. [DOI] [PubMed] [Google Scholar]
- 30.Scott A, Lugg ST, Aldridge K, Lewis KE, Bowden A, Mahida RY, et al. Pro-inflammatory effects of e-cigarette vapour condensate on human alveolar macrophages. Thorax. 2018;73:1161–1169. doi: 10.1136/thoraxjnl-2018-211663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Noah TL, Zhou H, Monaco J, Horvath K, Herbst M, Jaspers I. Tobacco smoke exposure and altered nasal responses to live attenuated influenza virus. Environ Health Perspect. 2011;119:78–83. doi: 10.1289/ehp.1002258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lebiush M, Rannon L, Kark JD. An outbreak of A/USSR/90/77 (H1N1) influenza in army recruits: clinical and laboratory observations. Mil Med. 1982;147:43–48. [PubMed] [Google Scholar]
- 33.Kark JD, Lebiush M. Smoking and epidemic influenza-like illness in female military recruits: a brief survey. Am J Public Health. 1981;71:530–532. doi: 10.2105/ajph.71.5.530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kark JD, Lebiush M, Rannon L. Cigarette smoking as a risk factor for epidemic a(h1n1) influenza in young men. N Engl J Med. 1982;307:1042–1046. doi: 10.1056/NEJM198210213071702. [DOI] [PubMed] [Google Scholar]
- 35.Corriden R, Moshensky A, Bojanowski CM, Meier A, Chien J, Nelson RK, et al. E-cigarette use increases susceptibility to bacterial infection by impairment of human neutrophil chemotaxis, phagocytosis, and NET formation. Am J Physiol Cell Physiol. 2020;318:C205–C214. doi: 10.1152/ajpcell.00045.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rebuli M, Glista-Baker E, Pawlak E, Speen A, Hoffman J, Dang H, et al. E-cigarettes and cigarettes differentially modify nasal mucosal immune response to infection with live-attenuated influenza virus (LAIV) [abstract] Am J Respir Crit Care Med. 2020;201:A4454. [Google Scholar]
- 37.Rebuli ME, Glista-Baker E, Pawlak E, Speen AM, Clapp P, Noah T, et al. Infection with live-attenuated influenza virus (LAIV) causes altered immune responses in the nasal mucosa of e-cigarette users [abstract] Am J Respir Crit Care Med. 2017;195:A3064. [Google Scholar]
- 38.Rebuli ME, Glista-Baker E, Speen AM, Hoffman JR, Duffney PF, Pawlak E, et al. Nasal mucosal immune response to infection with live-attenuated influenza virus (LAIV) is altered with exposure to e-cigarettes and cigarettes [abstract] Am J Respir Crit Care Med. 2019;199:A4170. [Google Scholar]
- 39.Müller L, Brighton LE, Carson JL, Fischer WA, II, Jaspers I. Culturing of human nasal epithelial cells at the air liquid interface. J Vis Exp. 2013;(80):e50646. doi: 10.3791/50646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Noah TL, Zhang H, Zhou H, Glista-Baker E, Müller L, Bauer RN, et al. Effect of broccoli sprouts on nasal response to live attenuated influenza virus in smokers: a randomized, double-blind study. PLoS One. 2014;9:e98671. doi: 10.1371/journal.pone.0098671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rebuli ME, Speen AM, Martin EM, Addo KA, Pawlak EA, Glista-Baker E, et al. Wood smoke exposure alters human inflammatory responses to viral infection in a sex-specific manner: a randomized, placebo-controlled study. Am J Respir Crit Care Med. 2019;199:996–1007. doi: 10.1164/rccm.201807-1287OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rebuli ME, Speen AM, Clapp PW, Jaspers I. Novel applications for a noninvasive sampling method of the nasal mucosa. Am J Physiol Lung Cell Mol Physiol. 2017;312:L288–L296. doi: 10.1152/ajplung.00476.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jaspers I, Ciencewicki JM, Zhang W, Brighton LE, Carson JL, Beck MA, et al. Diesel exhaust enhances influenza virus infections in respiratory epithelial cells. Toxicol Sci. 2005;85:990–1002. doi: 10.1093/toxsci/kfi141. [DOI] [PubMed] [Google Scholar]
- 44.Jaspers I, Zhang W, Fraser A, Samet JM, Reed W. Hydrogen peroxide has opposing effects on IKK activity and IκBα breakdown in airway epithelial cells. Am J Respir Cell Mol Biol. 2001;24:769–777. doi: 10.1165/ajrcmb.24.6.4344. [DOI] [PubMed] [Google Scholar]
- 45.Gianchecchi E, Manenti A, Kistner O, Trombetta C, Manini I, Montomoli E. How to assess the effectiveness of nasal influenza vaccines? Role and measurement of sIgA in mucosal secretions. Influenza Other Respir Viruses. 2019;13:429–437. doi: 10.1111/irv.12664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Carraro G, Mulay A, Yao C, Mizuno T, Konda B, Petrov M, et al. reconstruction of human basal cell diversity in normal and idiopathic pulmonary fibrosis lungs. Am J Respir Crit Care Med. 2020;202:1540–1550. doi: 10.1164/rccm.201904-0792OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.R Development Core Team. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2008. [Google Scholar]
- 48.Kolde R. pheatmap: pretty heatmaps—implementation of heatmaps that offers more control over dimensions and appearance. Vienna, Austria: R Foundation for Statistical Computing; 2018. [accessed 2019 Apr 25]. Available from: https://cran.r-project.org/web/packages/pheatmap/index.html. [Google Scholar]
- 49.Szklarczyk D, Franceschini A, Wyder S, Forslund K, Heller D, Huerta-Cepas J, et al. STRING v10: protein-protein interaction networks, integrated over the tree of life. Nucleic Acids Res. 2015;43:D447–D452. doi: 10.1093/nar/gku1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bandell A, Woo J, Coelingh K. Protective efficacy of live-attenuated influenza vaccine (multivalent, Ann Arbor strain): a literature review addressing interference. Expert Rev Vaccines. 2011;10:1131–1141. doi: 10.1586/erv.11.73. [DOI] [PubMed] [Google Scholar]
- 51.Beyer WE, Palache AM, de Jong JC, Osterhaus AD. Cold-adapted live influenza vaccine versus inactivated vaccine: systemic vaccine reactions, local and systemic antibody response, and vaccine efficacy. A meta-analysis. Vaccine. 2002;20:1340–1353. doi: 10.1016/s0264-410x(01)00471-6. [DOI] [PubMed] [Google Scholar]
- 52.Capitanio JS, Wozniak RW. Host cell factors necessary for influenza A infection: meta-analysis of genome wide studies [preprint] arXiv; 2012 [accessed 2012 Nov 30]. Available from: https://arxiv.org/abs/1211.3690.
- 53.White MR, Kandel R, Tripathi S, Condon D, Qi L, Taubenberger J, et al. Alzheimer’s associated β-amyloid protein inhibits influenza A virus and modulates viral interactions with phagocytes. PLoS One. 2014;9:e101364. doi: 10.1371/journal.pone.0101364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tan YX, Han WL, Chen YY, Ouyang NT, Tang Y, Li F, et al. Chemokine-like factor 1, a novel cytokine, contributes to airway damage, remodeling and pulmonary fibrosis. Chin Med J (Engl) 2004;117:1123–1129. [PubMed] [Google Scholar]
- 55.Aeffner F, Woods PS, Davis IC. Ecto-5′-nucleotidase CD73 modulates the innate immune response to influenza infection but is not required for development of influenza-induced acute lung injury. Am J Physiol Lung Cell Mol Physiol. 2015;309:L1313–L1322. doi: 10.1152/ajplung.00130.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lauder SN, Jones E, Smart K, Bloom A, Williams AS, Hindley JP, et al. Interleukin-6 limits influenza-induced inflammation and protects against fatal lung pathology. Eur J Immunol. 2013;43:2613–2625. doi: 10.1002/eji.201243018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Longhi MP, Wright K, Lauder SN, Nowell MA, Jones GW, Godkin AJ, et al. Interleukin-6 is crucial for recall of influenza-specific memory CD4 T cells. PLoS Pathog. 2008;4:e1000006. doi: 10.1371/journal.ppat.1000006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Klein SL, Flanagan KL. Sex differences in immune responses. Nat Rev Immunol. 2016;16:626–638. doi: 10.1038/nri.2016.90. [DOI] [PubMed] [Google Scholar]
- 59.Potluri T, Fink AL, Sylvia KE, Dhakal S, Vermillion MS, Vom Steeg L, et al. Age-associated changes in the impact of sex steroids on influenza vaccine responses in males and females. NPJ Vaccines. 2019;4:29. doi: 10.1038/s41541-019-0124-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.vom Steeg LG, Klein SL. SeXX matters in infectious disease pathogenesis. PLoS Pathog. 2016;12:e1005374. doi: 10.1371/journal.ppat.1005374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Peretz J, Pekosz A, Lane AP, Klein SL. Estrogenic compounds reduce influenza A virus replication in primary human nasal epithelial cells derived from female, but not male, donors. Am J Physiol Lung Cell Mol Physiol. 2016;310:L415–L425. doi: 10.1152/ajplung.00398.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Robinson DP, Lorenzo ME, Jian W, Klein SL. Elevated 17β-estradiol protects females from influenza A virus pathogenesis by suppressing inflammatory responses. PLoS Pathog. 2011;7:e1002149. doi: 10.1371/journal.ppat.1002149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lam JH, Baumgarth N. The multifaceted B cell response to influenza virus. J Immunol. 2019;202:351–359. doi: 10.4049/jimmunol.1801208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Stegemann-Koniszewski S, Behrens S, Boehme JD, Hochnadel I, Riese P, Guzmán CA, et al. Respiratory influenza A virus infection triggers local and systemic natural killer cell activation via toll-like receptor 7. Front Immunol. 2018;9:245. doi: 10.3389/fimmu.2018.00245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Intensive Care National Audit & Research Centre. London, UK: Case Mix Programme in England, Wales, and Northern Ireland, Intensive Care National Audit & Research Centre; 2020. ICNARC report on COVID-19 in critical care: 19 March 2020. [Google Scholar]
- 66.Norwegian Surveillance System for Communicable Diseases (MSIS), Norwegian Institute of Public Health. Oslo, Norway: Norwegian Surveillance System for Communicable Diseases (MSIS), Norwegian Institute of Public Health; 2020. Daily reports about coronavirus disease (COVID-19): March 29, 2020 [in Norwegian] [accessed 2020 Mar 29]. Available from: https://www.fhi.no/contentassets/e110607a67df46cbba8e30a443264a73/vedlegg/2020-03-20---dagsrapport---covid-19_oppdatert.pdf. [Google Scholar]
- 67.Remuzzi A, Remuzzi G. COVID-19 and Italy: what next? Lancet. 2020;395:1225–1228. doi: 10.1016/S0140-6736(20)30627-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chung S, Baumlin N, Dennis JS, Moore R, Salathe SF, Whitney PL, et al. Electronic cigarette vapor with nicotine causes airway mucociliary dysfunction preferentially via TRPA1 receptors. Am J Respir Crit Care Med. 2019;200:1134–1145. doi: 10.1164/rccm.201811-2087OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Liew PX, Kubes P. The neutrophil’s role during health and disease. Physiol Rev. 2019;99:1223–1248. doi: 10.1152/physrev.00012.2018. [DOI] [PubMed] [Google Scholar]
- 70.Kubes P. The enigmatic neutrophil: what we do not know. Cell Tissue Res. 2018;371:399–406. doi: 10.1007/s00441-018-2790-5. [DOI] [PubMed] [Google Scholar]
- 71.Gianchecchi E, Delfino DV, Fierabracci A. NK cells in autoimmune diseases: linking innate and adaptive immune responses. Autoimmun Rev. 2018;17:142–154. doi: 10.1016/j.autrev.2017.11.018. [DOI] [PubMed] [Google Scholar]
- 72.Razani-Boroujerdi S, Singh SP, Knall C, Hahn FF, Peña-Philippides JC, Kalra R, et al. Chronic nicotine inhibits inflammation and promotes influenza infection. Cell Immunol. 2004;230:1–9. doi: 10.1016/j.cellimm.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 73.Madison MC, Landers CT, Gu BH, Chang CY, Tung HY, You R, et al. Electronic cigarettes disrupt lung lipid homeostasis and innate immunity independent of nicotine. J Clin Invest. 2019;129:4290–4304. doi: 10.1172/JCI128531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Huang J, Duan Z, Kwok J, Binns S, Vera LE, Kim Y, et al. Vaping versus JUULing: how the extraordinary growth and marketing of JUUL transformed the US retail e-cigarette market. Tob Control. 2019;28:146–151. doi: 10.1136/tobaccocontrol-2018-054382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Jackler RK, Ramamurthi D. Nicotine arms race: JUUL and the high-nicotine product market. Tob Control. 2019;28:623–628. doi: 10.1136/tobaccocontrol-2018-054796. [DOI] [PubMed] [Google Scholar]
- 76.Galstyan E, Galimov A, Sussman S. Commentary: the emergence of pod mods at vape shops. Eval Health Prof. 2019;42:118–124. doi: 10.1177/0163278718812976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Arcavi L, Benowitz NL. Cigarette smoking and infection. Arch Intern Med. 2004;164:2206–2216. doi: 10.1001/archinte.164.20.2206. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.