Abstract
Background
Coronavirus disease 2019 (Covid-19) pneumonia is often associated with hyperinflammation. Despite the disproportionate incidence of Covid-19 among underserved and racial and ethnic minority populations, the safety and efficacy of the anti–interleukin-6 receptor antibody tocilizumab in patients from these populations who are hospitalized with Covid-19 pneumonia are unclear.
Methods
We randomly assigned (in a 2:1 ratio) patients hospitalized with Covid-19 pneumonia who were not receiving mechanical ventilation to receive standard care plus one or two doses of either tocilizumab (8 mg per kilogram of body weight intravenously) or placebo. Site selection was focused on the inclusion of sites enrolling high-risk and minority populations. The primary outcome was mechanical ventilation or death by day 28.
Results
A total of 389 patients underwent randomization, and the modified intention-to-treat population included 249 patients in the tocilizumab group and 128 patients in the placebo group; 56.0% were Hispanic or Latino, 14.9% were Black, 12.7% were American Indian or Alaska Native, 12.7% were non-Hispanic White, and 3.7% were of other or unknown race or ethnic group. The cumulative percentage of patients who had received mechanical ventilation or who had died by day 28 was 12.0% (95% confidence interval [CI], 8.5 to 16.9) in the tocilizumab group and 19.3% (95% CI, 13.3 to 27.4) in the placebo group (hazard ratio for mechanical ventilation or death, 0.56; 95% CI, 0.33 to 0.97; P=0.04 by the log-rank test). Clinical failure as assessed in a time-to-event analysis favored tocilizumab over placebo (hazard ratio, 0.55; 95% CI, 0.33 to 0.93). Death from any cause by day 28 occurred in 10.4% of the patients in the tocilizumab group and 8.6% of those in the placebo group (weighted difference, 2.0 percentage points; 95% CI, –5.2 to 7.8). In the safety population, serious adverse events occurred in 38 of 250 patients (15.2%) in the tocilizumab group and 25 of 127 patients (19.7%) in the placebo group.
Conclusions
In hospitalized patients with Covid-19 pneumonia who were not receiving mechanical ventilation, tocilizumab reduced the likelihood of progression to the composite outcome of mechanical ventilation or death, but it did not improve survival. No new safety signals were identified. (Funded by Genentech; EMPACTA ClinicalTrials.gov number, NCT04372186.)
Coronavirus disease 2019 (Covid-19) emerged in China in December 2019 and rapidly led to a public health emergency.1,2 In severe and critical cases of Covid-19, which occur in 14% and 5% of patients, respectively, Covid-19–associated pneumonia can lead to acute respiratory distress syndrome.3,4 Respiratory failure is among the leading causes of death in patients with Covid-19.5,6
Patients hospitalized with Covid-19 pneumonia often receive invasive mechanical ventilation, and mortality is increased among these patients, especially among those older than 65 years of age.7,8 The mainstay of treatment for patients with Covid-19 pneumonia is symptomatic and supportive9; remdesivir is the only approved treatment in the United States, and dexamethasone is the only therapy that has been shown to reduce mortality thus far.10
Therapies for Covid-19 pneumonia are especially needed for underserved and racial and ethnic minority populations, who are disproportionately affected by the pandemic.11-19 According to the Centers for Disease Control and Prevention Covid-19–Associated Hospitalization Surveillance Network, as of November 30, 2020, the rate ratio for hospitalization for Covid-19 in the United States (i.e., the number of residents in a defined area with a positive severe acute respiratory syndrome coronavirus 2 [SARS-CoV-2] laboratory test who were hospitalized divided by the overall population within that area) was 3.7 times as high among non-Hispanic Black persons, 4.1 times as high among Hispanic or Latino persons, and 4.0 times as high among non-Hispanic American Indian or Alaska Native persons as among non-Hispanic White persons.12 This is also a global issue; among 17 million patients in England, all the patients from non-White racial and ethnic groups had a higher risk of Covid-19–related death than White patients.13 Greater inclusion of minorities and underserved populations in clinical trials of Covid-19 therapies is needed; these populations are often not a focus of trial recruitment and have been underrepresented in Covid-19 trials.20
Covid-19 may be associated with a dysregulated immune response and hyperinflammation, which can lead to or exacerbate acute respiratory distress syndrome and multiorgan failure.4,21,22 Higher levels of interleukin-6 have been positively correlated with cases of critical and severe Covid-19, whereas lower levels of interleukin-6 have been correlated with mild disease23,24; in addition, elevated levels of interleukin-6 have been found to be predictive of the likelihood of mechanical ventilation.25 Tocilizumab, an anti–interleukin-6 receptor monoclonal antibody, has been approved for the treatment of multiple inflammatory diseases26,27 and appeared to improve outcomes in patients with Covid-19 pneumonia in observational studies in the United States and globally.28-30 However, randomized trials of tocilizumab have shown mixed results in patients with varying degrees of Covid-19 disease severity as well as in populations with various background standards of care.31-34
In EMPACTA (Evaluating Minority Patients with Actemra), a global, phase 3 clinical trial, we investigated the safety and efficacy of tocilizumab in hospitalized patients with Covid-19 pneumonia who were not receiving mechanical ventilation. The enrollment of patients from high-risk and racial and ethnic minority populations was emphasized.
Methods
Trial Design and Oversight
We conducted this randomized, double-blind, placebo-controlled, phase 3 trial to evaluate the safety and efficacy of tocilizumab in hospitalized patients with Covid-19 pneumonia who were not receiving mechanical ventilation. Global trial sites enrolling high-risk and minority populations were included to enhance the understanding of the clinical profile of tocilizumab in these patients and to allow access to underserved and minority populations, which are not commonly represented in clinical trials. Details on site selection are provided in the Methods section of the Supplementary Appendix, available with the full text of this article at NEJM.org.
Patients who were 18 years of age or older (with no upper age limit) and who were hospitalized with Covid-19 pneumonia that had been confirmed by a positive polymerase-chain-reaction test and radiographic imaging were eligible for enrollment. Patients had a blood oxygen saturation below 94% while breathing ambient air but were excluded if they were receiving continuous positive airway pressure, bilevel positive airway pressure, or mechanical ventilation. The patients received standard care according to local practice, which could include antiviral treatment, the limited use of systemic glucocorticoids (recommended dose, ≤1 mg per kilogram of body weight of methylprednisolone or equivalent), and supportive care. Patients were excluded if progression of the illness to death was imminent and inevitable within 24 hours, as determined by the treating physician, or if they had active tuberculosis or suspected active bacterial, fungal, or viral infection (other than SARS-CoV-2 infection or well-controlled human immunodeficiency virus infection). Patients with coexisting conditions were not excluded unless the investigator determined that the condition would preclude safe participation in the trial.
Each patient or the patient’s legally authorized representative provided written or witnessed oral informed consent. The trial was conducted in accordance with the International Conference on Harmonisation E6 guidelines for Good Clinical Practice and the Declaration of Helsinki or local regulations, whichever afforded greater patient protection. This trial was approved by all the trial sites through the central Advarra Institutional Review Board, the Western Institutional Review Board, or a local institutional review board; in addition, the trial was approved at some sites by local ethics committees. Institutional review boards or ethics committees approved the protocol (available at NEJM.org) in each participating country. The sponsor designed the trial, conducted it according to the protocol, collected the data, and performed analyses; a contract research organization paid by the sponsor managed and monitored the trial under the direction and supervision of the sponsor. All the authors vouch for the accuracy and completeness of the data and for the fidelity of the trial to the protocol. All drafts of the manuscript were prepared by the authors with editorial and writing assistance funded by the sponsor.
Using permuted-block randomization and an interactive voice- or Web-response system, we randomly assigned the patients, in a 2:1 ratio, to receive standard care plus one or two doses of either intravenous tocilizumab (8 mg per kilogram of body weight, to a maximum of 800 mg per dose) or placebo. The randomization was stratified according to country (the United States, Mexico, Kenya, South Africa, Peru, or Brazil) and age (≤60 or >60 years). Details of trial blinding are provided in the Supplementary Appendix. If a patient’s clinical signs or symptoms worsened or did not improve (i.e., if the patient had a sustained fever or worsening status as assessed with the use of a seven-category ordinal scale), an additional infusion could be administered 8 to 24 hours after the first one.
Efficacy was evaluated by day 28, and patients were followed for a total of 60 days. Patients who were discharged before day 28 were considered to have completed the trial and were followed weekly up to day 28, with a safety follow-up visit conducted by day 60.
Outcome Measures
The primary efficacy outcome was mechanical ventilation (invasive mechanical ventilation or extracorporeal membrane oxygenation) or death by day 28. In addition to the evaluation of the primary efficacy outcome, the results of the primary efficacy analysis were evaluated according to age, race or ethnic group, geographic region, glucocorticoid use, antiviral use, and the number of doses of tocilizumab or placebo received.
The key secondary efficacy outcomes that were evaluated over the 28-day period were the time to hospital discharge or readiness for discharge as assessed with the use of a seven-category ordinal scale (with categories ranging from 1 to 7 and higher categories indicating a worse condition) (Table S1 in the Supplementary Appendix); the time to at least a two-category improvement in clinical status relative to baseline on the seven-category ordinal scale (for patients in category 2 at baseline, those with a clinical status of category 1 were considered to have met the threshold); the time to clinical failure (the time to death, mechanical ventilation, admission to an intensive care unit [ICU] [or, in patients who were already in the ICU at trial enrollment, worsening by two categories from baseline on the seven-category ordinal scale], or withdrawal [whichever occurred first]); and death.
The incidence and severity of adverse events were evaluated. These events were determined according to the National Cancer Institute Common Terminology Criteria for Adverse Events, version 5.0.
Statistical Analysis
The modified intention-to-treat population consisted of all patients who underwent randomization and received either tocilizumab or placebo. We estimated that the assignment of 379 patients with 2:1 randomization would provide at least 80% power to detect a between-group difference of 15 percentage points in the primary outcome with the use of a log-rank test, assuming a cumulative event rate (death or mechanical ventilation) of 25% in the tocilizumab group and 40% in the placebo group. Efficacy analyses were performed in the modified intention-to-treat population, with patients grouped according to treatment assignment. Analyses were stratified according to age group (≤60 or >60 years).
The primary outcome was estimated with the Kaplan–Meier method, and cumulative incidence curves were compared between the two groups with the stratified log-rank test. The stratified Cox proportional-hazards model was used to estimate the hazard ratio (for tocilizumab as compared with placebo) and 95% confidence interval. In this analysis, data on patients who survived and did not receive mechanical ventilation on or before day 28 were censored at the last follow-up date or day 28, whichever occurred first.
The primary and key secondary outcomes were evaluated in a hierarchical manner to control the overall trial-wide type I error rate at the 5% significance level. If the primary outcome reached significance at the two-sided 5% significance level, the key secondary outcomes were tested in the following predefined order: time to hospital discharge or readiness for discharge, time to improvement in clinical status, time to clinical failure, and death.
Time-to-event secondary outcomes were compared between the two groups with the use of the Kaplan–Meier approach. Deaths were censored at day 28 in the analysis of time to hospital discharge or readiness for charge and time to improvement in clinical status. Data on patients who discontinued the trial before hospital discharge or readiness for discharge or before improvement in clinical status were censored on the date of the last ordinal-scale assessment. In the analysis of the time to clinical failure, data on patients who did not have clinical failure on or before day 28 were censored at the last contact date or day 28, whichever occurred first. The Cochran–Mantel–Haenszel test, with adjustment for age, was used to assess the between-group difference in mortality by day 28. The reported 95% confidence intervals were not adjusted for multiplicity and cannot be used to assess effects. Sensitivity analyses of the time to hospital discharge and time to improvement in clinical status, with death treated as a competing risk, were performed. Information regarding source data verification and subgroup analyses is provided in the Supplementary Appendix.
Safety was assessed in all the patients who received either tocilizumab or placebo; patients were grouped according to the actual agent received. An interim safety review was performed by an internal monitoring committee.
Results
Patients
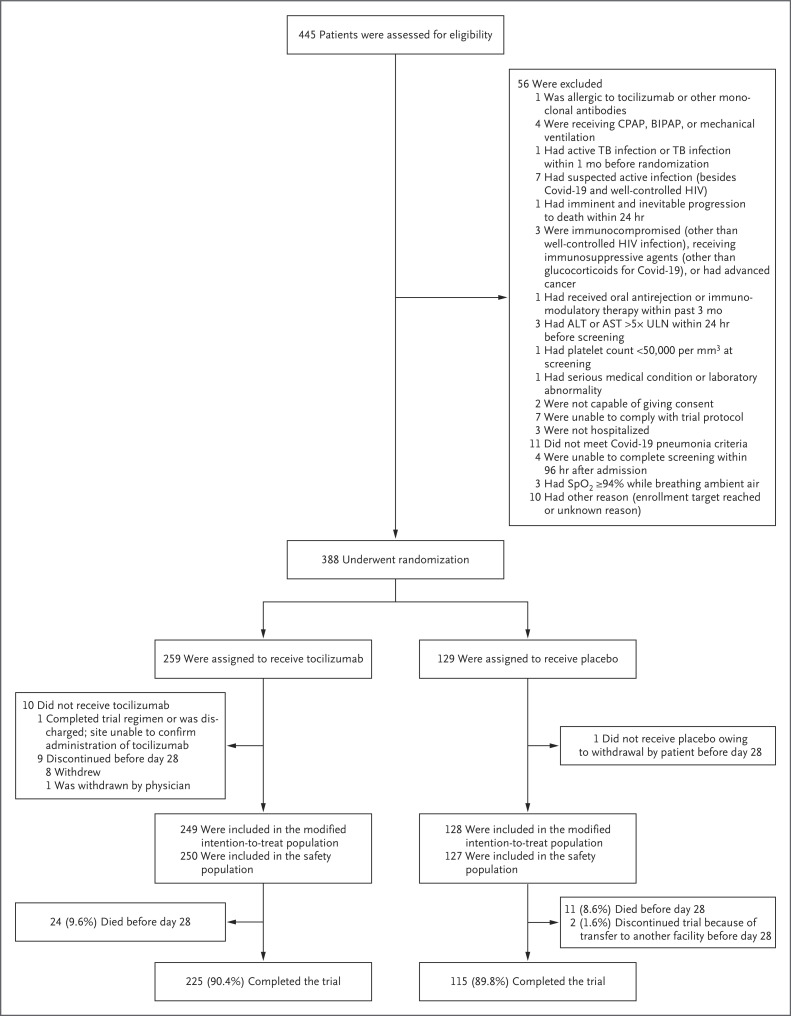
Overall, 389 patients from six countries underwent randomization; 1 patient underwent randomization in error, and 377 patients received either tocilizumab or placebo (Figure 1 and Table S2). In the modified intention-to-treat population, 249 patients were assigned to receive tocilizumab plus standard care and 128 were assigned to receive placebo plus standard care; the safety population included 250 and 127 patients, respectively, because 1 patient who was assigned to receive placebo received tocilizumab and 11 patients did not receive tocilizumab or placebo. Information on exposure to tocilizumab or placebo is provided in Table S3. Overall, 225 patients (90.4%) completed the trial in the tocilizumab group and 115 (89.8%) completed the trial in the placebo group; excluding those who died, no patients in the tocilizumab group and 2 patients in the placebo group (1.6%) discontinued the trial before day 28. The median follow-up time was 60 days in both groups.
Figure 1. Enrollment, Randomization, and Follow-up.
The modified intention-to-treat population included all the patients who underwent randomization and received tocilizumab or placebo. Patients may have been excluded for more than one reason. A total of 389 patients underwent randomization, and 388 patients had data that could be evaluated. (One patient underwent randomization before local institutional review board approval of the trial site. This patient did not receive tocilizumab or placebo, and no further data were collected.) One patient who was randomly assigned to the placebo group received tocilizumab and was included in the tocilizumab group in the safety population. The 2 patients who had another reason for discontinuation of placebo were transferred to other facilities. Patients who completed day 28 of the trial before discharge or were discharged before day 28 were considered to have completed the trial. Percentages shown are for the modified intention-to-treat population. ALT denotes alanine aminotransferase, AST aspartate aminotransferase, BIPAP bilevel positive airway pressure, CPAP continuous positive airway pressure, HIV human immunodeficiency virus, SpO2 oxygen saturation as measured by pulse oximetry, TB tuberculosis, and ULN the upper limit of the normal range.
The baseline demographic and disease characteristics were generally balanced in the two groups (Table 1 and Table S4). In the tocilizumab and placebo groups, 60.2% and 57.0% of the patients were men, respectively, and the mean (±SD) age was 56.0±14.3 years and 55.6±14.9 years, respectively. In the tocilizumab and placebo groups, 143 patients (57.4%) and 68 patients (53.1%), respectively, were Hispanic or Latino, 35 (14.1%) and 21 (16.4%) were Black, and 33 (13.3%) and 15 (11.7%) were American Indian or Alaska Native. In the 7 days before the trial or during the trial, 200 patients in the tocilizumab group (80.3%) and 112 patients in the placebo group (87.5%) received systemic glucocorticoids and 196 (78.7%) and 101 (78.9%), respectively, received antiviral treatment (Table S5); 55.4% and 67.2% of the patients received dexamethasone, and 52.6% and 58.6% received remdesivir (Table S6).
Table 1. Characteristics of the Patients at Baseline in the Modified Intention-to-Treat Population.*.
| Characteristic | Tocilizumab (N=249) |
Placebo (N=128) |
All Patients (N=377) |
|---|---|---|---|
| Male sex — no. (%) | 150 (60.2) | 73 (57.0) | 223 (59.2) |
| Age | |||
| Mean — yr | 56.0±14.3 | 55.6±14.9 | 55.9±14.4 |
| Distribution — no. (%) | |||
| ≤60 yr | 151 (60.6) | 76 (59.4) | 227 (60.2) |
| >60 yr | 98 (39.4) | 52 (40.6) | 150 (39.8) |
| BMI† | 32.0±7.9 | 33.1±7.2 | 32.4±7.6 |
| Race or ethnic group — no. (%)‡ | |||
| Hispanic or Latino | 143 (57.4) | 68 (53.1) | 211 (56.0) |
| American Indian or Alaska Native | 33 (13.3) | 15 (11.7) | 48 (12.7) |
| Black | 35 (14.1) | 21 (16.4) | 56 (14.9) |
| Non-Hispanic White | 28 (11.2) | 20 (15.6) | 48 (12.7) |
| Unknown or other | 10 (4.0) | 4 (3.1) | 14 (3.7) |
| Country group — no. (%) | |||
| United States | 201 (80.7) | 103 (80.5) | 304 (80.6) |
| Mexico, Kenya, South Africa, Peru, and Brazil | 48 (19.3) | 25 (19.5) | 73 (19.4) |
| Category on seven-category ordinal scale for clinical status — no. (%)§ | |||
| 2 | 24 (9.6) | 11 (8.6) | 35 (9.3) |
| 3 | 161 (64.7) | 81 (63.3) | 242 (64.2) |
| 4 | 64 (25.7) | 36 (28.1) | 100 (26.5) |
| Median laboratory values (range) | |||
| C-reactive protein level — mg/liter | 124.50 (2.5–2099.0) |
143.40 (9.0–3776.0) |
136.10 (2.5–3776.0) |
| d-Dimer level — μg/ml FEU | 1.60 (0.2–8873.0) |
1.21 (0.2–10,384.0) |
1.50 (0.2–10,384.0) |
| Ferritin level — pmol/liter | 1401.34 (29.2–38,482.1) |
1353.14 (110.1–122,328.9) |
1395.39 (29.2–122,328.9) |
Plus–minus values are means ±SD. Percentages may not total 100 because of rounding. FEU denotes fibrinogen equivalent units.
The body-mass index (BMI) is the weight in kilograms divided by the square of the height in meters.
Race or ethnic group was reported by the patients.
Categories on the seven-category ordinal scale range from 1 to 7, with higher categories indicating a worse condition. Category 1 indicates that the patient was discharged (or ready for discharge as evidenced by normal body temperature and respiratory rate, as well as stable oxygen saturation while breathing ambient air or ≤2 liters of supplemental oxygen); 2, hospitalized in a non–intensive care unit (ICU) hospital ward (or ready for a hospital ward) and not receiving supplemental oxygen; 3, hospitalized in a non–ICU hospital ward (or ready for a hospital ward) and receiving supplemental oxygen; 4, hospitalized in an ICU or a non–ICU hospital ward and receiving noninvasive ventilation or high-flow oxygen; 5, hospitalized in an ICU and receiving intubation and mechanical ventilation; 6, hospitalized in an ICU and receiving extracorporeal membrane oxygenation or mechanical ventilation and additional organ support; and 7, died.
Primary Efficacy Outcome
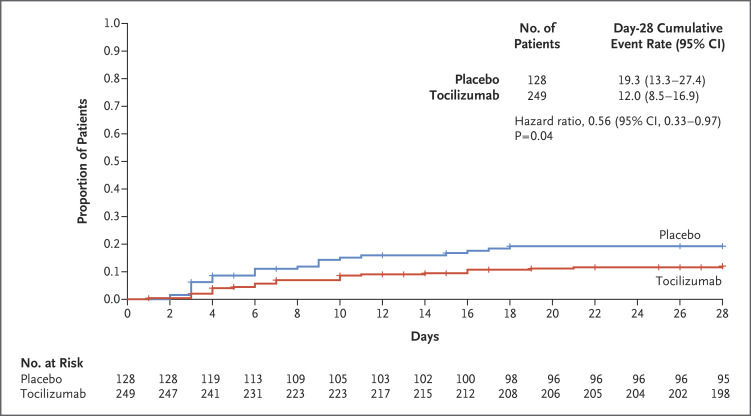
The cumulative percentage of patients who had received mechanical ventilation or who had died by day 28 was significantly lower in the tocilizumab group (12.0%; 95% confidence interval [CI], 8.5 to 16.9) than in the placebo group (19.3%; 95% CI, 13.3 to 27.4) (hazard ratio, 0.56; 95% CI, 0.33 to 0.97; P=0.04 by the log-rank test). Results are shown in Table 2 and Figure 2.
Table 2. Primary and Key Secondary Efficacy Outcomes by Day 28 in the Modified Intention-to-Treat Population.*.
| Outcome | Tocilizumab (N=249) |
Placebo (N=128) |
Hazard Ratio (95% CI) |
Weighted Difference (95% CI) |
P Value† |
|---|---|---|---|---|---|
| Primary outcome: mechanical ventilation or death — % (95% CI)‡ | 12.0 (8.5 to 16.9) | 19.3 (13.3 to 27.4) | 0.56 (0.33 to 0.97) | NA | 0.04 |
| Secondary outcomes | |||||
| Median time to hospital discharge or readiness for discharge (95% CI) — days§ | 6.0 (6.0 to 7.0) | 7.5 (7.0 to 9.0) | 1.16 (0.91 to 1.48) | NA | |
| Median time to improvement in clinical status (95% CI) — days§¶ | 6.0 (6.0 to 7.0) | 7.0 (6.0 to 9.0) | 1.15 (0.90 to 1.48) | NA | |
| Median time to clinical failure (95% CI) — days§ | NE | NE | 0.55 (0.33 to 0.93) | NA | |
| Death — no. (% [95% CI])‖ | 26 (10.4 [7.2 to 14.9]) | 11 (8.6 [4.9 to 14.7]) | NA | 2.0 (−5.2 to 7.8)** |
NA denotes not applicable, and NE could not be estimated.
The P value was calculated with the log-rank test. Significance testing was performed hierarchically to control the trial-wide type I error rate at a 5% significance level.
The cumulative percentages of patients were estimated with the Kaplan–Meier method and compared with the use of the stratified log-rank test with age group (≤60 or >60 years) as a stratification factor. The stratified Cox proportional-hazards model with age group (≤60 or >60 years) as a stratification factor was used to estimate the hazard ratio and 95% confidence interval.
The median time to a secondary outcome event was estimated with the Kaplan–Meier approach.
Improvement in clinical status was determined with the use of the seven-category ordinal scale.
The Wilson method was used to estimate the 95% confidence interval for the observed proportion. The Cochran–Mantel–Haenszel weighting approach with age group (≤60 years or >60 years) as the stratification factor was used to calculate the weighted difference in percentages. The Newcombe method was used to estimate the 95% confidence interval for the weighted difference. Deaths by day 28 included all deaths reported from ordinal-scale scoring, adverse events reporting, and public death records during the hospital stay and after hospital discharge.
The weighted difference is expressed as percentage points.
Figure 2. Time to Mechanical Ventilation or Death by Day 28 in the Modified Intention-to-Treat Population.
The cumulative proportion of patients was estimated with the Kaplan–Meier method and compared in the two groups with the use of the stratified log-rank test. The stratified Cox proportional-hazards model was used to estimate the hazard ratio and 95% confidence interval. Data on patients who did not receive mechanical ventilation or who died on or before day 28 were censored at day 28 or the date of the last available follow-up, whichever occurred first.
Secondary Outcomes
The median time to hospital discharge or readiness for discharge over the 28-day period was 6.0 days (95% CI, 6.0 to 7.0) in the tocilizumab group and 7.5 days (95% CI, 7.0 to 9.0) in the placebo group (hazard ratio, 1.16; 95% CI, 0.91 to 1.48) (Table 2 and Fig. S1). The results were similar when death was treated as a competing risk (hazard ratio, 1.14; 95% CI, 0.92 to 1.42) (Table S7 and Fig. S2). The median time to improvement in clinical status as assessed with the seven-category ordinal scale over the 28-day period was 6.0 days (95% CI, 6.0 to 7.0) with tocilizumab and 7.0 days (95% CI, 6.0 to 9.0) with placebo (hazard ratio, 1.15; 95% CI, 0.90 to 1.48) (Table 2 and Fig. S3). The results were similar when death was treated as a competing risk (hazard ratio, 1.14; 95% CI, 0.92 to 1.41) (Table S8 and Fig. S4). The median time to clinical failure over the 28-day period could not be estimated in either group (hazard ratio, 0.55; 95% CI, 0.33 to 0.93) (Table 2 and Fig. S5). Mortality by day 28 was 10.4% (95% CI, 7.2 to 14.9) in the tocilizumab group and 8.6% (95% CI, 4.9 to 14.7) in the placebo group (weighted difference, 2.0 percentage points; 95% CI, –5.2 to 7.8) (Table 2).
Exploratory Outcomes
The results of the primary efficacy analysis according to race or ethnic group were consistent with the results in the modified intention-to-treat population. The results of additional subgroup analyses are provided in Figure S6.
Safety
The data cutoff date was September 30, 2020. Overall, adverse events through day 60 were reported in 50.8% of 250 patients in the tocilizumab group and 52.8% of 127 patients in the placebo group, and serious adverse events were reported in 15.2% and 19.7%, respectively (Table 3 and Table S9). Death occurred in 29 patients (11.6%) in the tocilizumab group and 15 patients (11.8%) in the placebo group (Table 3 and Table S10); no systematic pattern in deaths that occurred before as compared with after mechanical ventilation was noted (Fig S7). Through day 60, a total of 16 serious infections were observed in 13 patients who received tocilizumab (5.2%) and 11 serious infections were observed in 9 patients who received placebo (7.1%). Through day 28, a total of 8 of 250 patients (3.2%) in the tocilizumab group and 6 of 127 patients (4.7%) in the placebo group had progression of illness to category 6 on the seven-category ordinal scale.
Table 3. Adverse Events through Day 60 in the Safety Population.*.
| Variable | Tocilizumab (N=250) |
Placebo (N=127) |
All Patients (N=377) |
|---|---|---|---|
| Total adverse events — no. | 357 | 187 | 544 |
| Patients with ≥1 adverse event — no. (%) | 127 (50.8) | 67 (52.8) | 194 (51.5) |
| Total deaths — no. (%) | 29 (11.6) | 15 (11.8) | 44 (11.7) |
| Withdrawal from trial because of adverse event — no. (%) | 0 | 0 | 0 |
| Patients with ≥1 adverse event — no. (%) | |||
| Event with fatal outcome | 28 (11.2) | 13 (10.2) | 41 (10.9) |
| Serious event | 38 (15.2) | 25 (19.7) | 63 (16.7) |
| Event leading to withdrawal from trial, excluding serious events leading to death | 0 | 0 | 0 |
| Event leading to dose modification or interruption | 0 | 0 | 0 |
| Event related to tocilizumab or placebo, as determined by the investigator | 3 (1.2) | 0 | 3 (0.8) |
| Event leading to withdrawal from trial, excluding events leading to death | 0 | 0 | 0 |
| Event leading to dose modification or interruption | 1 (0.4) | 0 | 1 (0.3) |
| Event related to tocilizumab or placebo, as determined by the investigator | 32 (12.8) | 5 (3.9) | 37 (9.8) |
| Event leading to discontinuation of trial regimen | 0 | 0 | 0 |
| Event leading to dose modification or interruption | 0 | 0 | 0 |
| Grade 3 to 5 event, at greatest intensity | 46 (18.4) | 31 (24.4) | 77 (20.4) |
| Infection | 25 (10.0) | 16 (12.6) | 41 (10.9) |
| Serious infection | 13 (5.2) | 9 (7.1) | 22 (5.8) |
| Total no. of events | 16 | 11 | 27 |
| Events with incidence of >1% in either group — no. (%) | |||
| Septic shock | 5 (2.0) | 3 (2.4) | 8 (2.1) |
| Covid-19 pneumonia | 2 (0.8) | 3 (2.4) | 5 (1.3) |
| Pneumonia, not otherwise specified | 0 | 3 (2.4) | 3 (0.8) |
| Bacterial pneumonia | 0 | 2 (1.6) | 2 (0.5) |
The incidence and severity of adverse events was determined according to the National Cancer Institute Common Terminology Criteria for Adverse Events, version 5.0.
Discussion
Our phase 3 trial of tocilizumab in hospitalized patients with Covid-19 pneumonia who were not receiving mechanical ventilation emphasized the enrollment of patients from high-risk and racial and ethnic minority groups. These patients are disproportionately affected by Covid-19 and are often underrepresented in clinical trials.11-20 Overall, more than 25% of the patients were older than 65 years of age, more than 75% had at least one coexisting condition, and more than 80% were in a minority racial or ethnic group.
This trial showed that the likelihood of progression to mechanical ventilation or death by day 28 was significantly lower among patients who received tocilizumab plus standard care than among those who received placebo plus standard care. When death from any cause alone was evaluated as a secondary outcome, no benefit with respect to mortality was observed. One hypothesis is that patients who had progression to mechanical ventilation after receiving tocilizumab may compose a subgroup of patients with more severe disease and therefore a higher risk of death; this hypothesis is supported by the fact that a larger percentage of patients in the placebo group than in the tocilizumab group died without receiving mechanical ventilation. Studies are under way to address this question further. The median time to hospital discharge up to day 28 was 1.5 days shorter in the tocilizumab group than in the placebo group, but there was an overlapping range.
Health care disparities among patients with Covid-19 are a critical issue because studies continue to show that racial and ethnic minority populations are disproportionately affected by the Covid-19 pandemic.11-19 The role of race and ethnic group in the clinical course of Covid-19 is complex and not fully understood; social determinants of health, socioeconomic factors, and historical and structural inequities may play a role but do not completely explain the observations, and further research is needed.14,16,19,35 To directly address the discrepancy between the overrepresentation of racial and ethnic minorities with Covid-19 disease and the underrepresentation of these minorities in Covid-19 trials,20 we gave priority to sites that provided care to underserved and minority populations while we continued to enroll all eligible patients. As a result, 84% of the patients in the trial were Hispanic or Latino, Black, or American Indian or Alaska Native. Although the analysis was exploratory, the primary efficacy outcomes assessed according to race or ethnic group were consistent with the outcomes in the overall patient population.
Prevention of progression to mechanical ventilation, which may greatly alter patient outcomes and the availability of health care resources, is critical for potential therapies for Covid-19. In an observational study involving 10,021 patients who were hospitalized with Covid-19, 17% received mechanical ventilation and in-hospital mortality was higher among these patients than among those who did not receive mechanical ventilation (53% vs. 16%).8 In addition to clinical decision making, a key factor that determines whether patients will receive mechanical ventilation is resource availability. The critical shortage of ventilators that has occurred globally during the pandemic has underscored the need for therapies that conserve this limited resource36; this is a particular concern in Africa.37 Decreasing the proportion of patients who receive mechanical ventilation could help to reduce the burden on critical care services, reduce direct health care costs, and ensure the availability of ventilators for the most critically ill patients.38
The efficacy of tocilizumab in patients with Covid-19 has been examined in other randomized trials. The randomized, placebo-controlled COVACTA31 and Boston Area COVID-19 Consortium (BACC) Bay Tocilizumab34 trials included patients with a different disease severity at baseline than that in the EMPACTA trial. The COVACTA trial included patients with disease that ranged from moderate hypoxia to invasive mechanical ventilation at baseline, whereas the EMPACTA trial enrolled patients who were not receiving mechanical ventilation at baseline and were at an earlier disease stage.31 The BACC Bay Tocilizumab Trial also included patients who were not receiving mechanical ventilation at baseline; however, 96% of the patients had baseline categories of 2 or 3 on the seven-category ordinal scale, whereas in the EMPACTA trial, 26.5% of the patients had a baseline category of 4 on this scale.34
As compared with patients in the COVACTA, BACC Bay Tocilizumab, RCT-TCZ-COVID-19, and CORIMUNO-TOCI-1 trials,31-34 more than 50% of the patients in the EMPACTA trial received concomitant glucocorticoid or antiviral agents; these agents have become the mainstay of standard care for patients with Covid-19.39 Although these treatments were not used uniformly and patients may not have received a full treatment course, our results reflect the most up-to-date standard of care, especially considering the approval of remdesivir in the United States. Glucocorticoid and antiviral use was generally balanced in the two groups, and our trial showed the added clinical benefit of tocilizumab with respect to a lower incidence of progression to mechanical ventilation. In the COVACTA trial, the median time to hospital discharge was 20 days in the tocilizumab group and 28 days in the placebo group, whereas in the EMPACTA trial, the median time was 6.0 and 7.5 days, respectively.31 Patients had more severe illness or had different coexisting conditions at baseline in the COVACTA trial than in the EMPACTA trial, different proportions of patients received concomitant therapy in the two trials, and standards of care have improved since the COVACTA trial.
The criteria for one of two efficacy outcomes — survival without invasive or noninvasive mechanical ventilation by day 14 — were met in the CORIMUNO-TOCI-1 trial, but mortality at day 28 was not different between the tocilizumab and placebo groups.33 The COVACTA, BACC Bay Tocilizumab, and RCT-TCZ-COVID-19 trials did not meet the criteria for their efficacy outcomes, and tocilizumab was not associated with a mortality benefit at day 28 in either the COVACTA trial or the BACC Bay Tocilizumab trial.31,32,34 Our trial also did not show a significant between-group difference in mortality.
The results of our trial suggest that patients who are most likely to benefit from tocilizumab have moderate or severe disease (i.e., they have hypoxia but are not yet receiving mechanical ventilation) and that tocilizumab may add to the potential benefit of antiviral treatment and glucocorticoids. In this trial, 55.4% of the patients in the tocilizumab group and 67.2% of those in the placebo group received concomitant dexamethasone, and a greater benefit was observed with tocilizumab than with placebo with respect to the primary outcome. Ongoing trials are under way to provide clarity on the patient subgroups that are most likely to benefit from specific immunomodulatory therapies.
Our trial showed that tocilizumab plus standard care was more efficacious than placebo plus standard care in reducing the likelihood of the composite outcome of progression to mechanical ventilation or death among hospitalized patients with Covid-19 pneumonia who were not receiving mechanical ventilation; however, there was no difference in the incidence of death from any cause. This reduction in risk was observed in a group of patients that included underserved populations that are often underrepresented in clinical research. The results of the primary analysis according to race or ethnic group were consistent with the results in the modified intention-to-treat population.
Acknowledgments
We thank the patients who participated in this trial, the clinical site investigators, and Claire Stedden, Ph.D., and Nicola Gillespie, D.V.M., of Health Interactions for medical writing assistance with an earlier version of the manuscript.
Protocol
Supplementary Appendix
Disclosure Forms
Data Sharing Statement
This article was published on December 17, 2020, at NEJM.org.
A data sharing statement provided by the authors is available with the full text of this article at NEJM.org.
Footnotes
Supported by Genentech.
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
References
- 1.Phelan AL, Katz R, Gostin LO. The novel coronavirus originating in Wuhan, China: challenges for global health governance. JAMA 2020;323:709-710. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization. COVID-19 Public Health Emergency of International Concern (PHEIC) global research and innovation forum. 2020. (https://www.who.int/publications/m/item/covid-19-public-health-emergency-of-international-concern-(pheic)-global-research-and-innovation-forum).
- 3.Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA 2020;323:1239-1242. [DOI] [PubMed] [Google Scholar]
- 4.Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet 2020;395:507-513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gupta S, Hayek SS, Wang W, et al. Factors associated with death in critically ill patients with coronavirus disease 2019 in the US. JAMA Intern Med 2020;180:1-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ruan Q, Yang K, Wang W, Jiang L, Song J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med 2020;46:846-848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Richardson S, Hirsch JS, Narasimhan M, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA 2020;323:2052-2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Karagiannidis C, Mostert C, Hentschker C, et al. Case characteristics, resource use, and outcomes of 10 021 patients with COVID-19 admitted to 920 German hospitals: an observational study. Lancet Respir Med 2020;8:853-862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fan E, Beitler JR, Brochard L, et al. COVID-19-associated acute respiratory distress syndrome: is a different approach to management warranted? Lancet Respir Med 2020;8:816-821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.The RECOVERY Collaborative Group. Dexamethasone in hospitalized patients with Covid-19 — preliminary report. N Engl J Med DOI: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pan D, Sze S, Minhas JS, et al. The impact of ethnicity on clinical outcomes in COVID-19: a systematic review. EClinicalMedicine 2020;23:100404-100404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Centers for Disease Control and Prevention. Cases, data and surveillance — COVID-19 hospitalization and death by race/ethnicity, November 30, 2020. (https://www.cdc.gov/coronavirus/2019-ncov/covid-data/investigations-discovery/hospitalization-death-by-race-ethnicity.html).
- 13.Williamson EJ, Walker AJ, Bhaskaran K, et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature 2020;584:430-436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Egede LE, Walker RJ. Structural racism, social risk factors, and Covid-19 — a dangerous convergence for Black Americans. N Engl J Med 2020;383(12):e77-e77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Karaca-Mandic P, Georgiou A, Sen S. Assessment of COVID-19 hospitalizations by race/ethnicity in 12 states. JAMA Intern Med 2020. August 17 (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Evans MK. Covid’s color line — infectious disease, inequity, and racial justice. N Engl J Med 2020;383:408-410. [DOI] [PubMed] [Google Scholar]
- 17.Price-Haywood EG, Burton J, Fort D, Seoane L. Hospitalization and mortality among Black patients and White patients with Covid-19. N Engl J Med 2020;382:2534-2543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chowkwanyun M, Reed AL Jr. Racial health disparities and Covid-19 — caution and context. N Engl J Med 2020;383:201-203. [DOI] [PubMed] [Google Scholar]
- 19.Golestaneh L, Neugarten J, Fisher M, et al. The association of race and COVID-19 mortality. EClinicalMedicine 2020;25:100455-100455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chastain DB, Osae SP, Henao-Martínez AF, Franco-Paredes C, Chastain JS, Young HN. Racial disproportionality in Covid clinical trials. N Engl J Med 2020;383(9):e59-e59. [DOI] [PubMed] [Google Scholar]
- 21.Mehta P, McAuley DF, Brown M, et al. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet 2020;395:1033-1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Giamarellos-Bourboulis EJ, Netea MG, Rovina N, et al. Complex immune dysregulation in COVID-19 patients with severe respiratory failure. Cell Host Microbe 2020;27(6):992-1000.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aziz M, Fatima R, Assaly R. Elevated interleukin-6 and severe COVID-19: a meta-analysis. J Med Virol 2020;92:2283-2285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhu J, Pang J, Ji P, et al. Elevated interleukin-6 is associated with severity of COVID-19: a meta-analysis. J Med Virol 2020. May 29 (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Herold T, Jurinovic V, Arnreich C, et al. Elevated levels of IL-6 and CRP predict the need for mechanical ventilation in COVID-19. J Allergy Clin Immunol 2020;146(1):128-136.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Actemra prescribing information. South San Francisco, CA: Genentech, 2019. (package insert). [Google Scholar]
- 27.RoActemra summary of product characteristics. Grenzach-Wyhlen, Germany: Roche, 2020. [Google Scholar]
- 28.Xu X, Han M, Li T, et al. Effective treatment of severe COVID-19 patients with tocilizumab. Proc Natl Acad Sci U S A 2020;117:10970-10975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Morrison AR, Johnson JM, Griebe KM, et al. Clinical characteristics and predictors of survival in adults with coronavirus disease 2019 receiving tocilizumab. J Autoimmun 2020;114:102512-102512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gupta S, Wang W, Hayek SS, et al. Association between early treatment with tocilizumab and mortality among critically ill patients with COVID-19. JAMA Intern Med 2020. October 20 (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rosas I, Bräu N, Waters M, et al. Tocilizumab in hospitalized patients with COVID-19 pneumonia. September 12, 2020. (https://www.medrxiv.org/content/10.1101/2020.08.27.20183442v2). preprint. [Google Scholar]
- 32.Salvarani C, Dolci G, Massari M, et al. Effect of tocilizumab vs standard care on clinical worsening in patients hospitalized with COVID-19 pneumonia: a randomized clinical trial. JAMA Intern Med 2020. October 20 (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hermine O, Mariette X, Tharaux P-L, et al. Effect of tocilizumab vs usual care in adults hospitalized with COVID-19 and moderate or severe pneumonia: a randomized clinical trial. JAMA Intern Med 2020. October 20 (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stone JH, Frigault MJ, Serling-Boyd NJ, et al. Efficacy of tocilizumab in patients hospitalized with Covid-19. N Engl J Med. 10.1056/NEJMoa2028836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Department of Health and Human Services. Social determinants of health. 2020. (https://www.healthypeople.gov/2020/topics-objectives/topic/social-determinants-of-health).
- 36.Truog RD, Mitchell C, Daley GQ. The toughest triage — allocating ventilators in a pandemic. N Engl J Med 2020;382:1973-1975. [DOI] [PubMed] [Google Scholar]
- 37.El-Sadr WM, Justman J. Africa in the path of Covid-19. N Engl J Med 2020;383(3):e11-e11. [DOI] [PubMed] [Google Scholar]
- 38.Centers for Medicare & Medicaid Services. COVID-19 frequently asked questions (FAQs) on Medicare fee-for-service (FFS) billing. 2019. (https://www.cms.gov/files/document/03092020-covid-19-faqs-508.pdf).
- 39.National Institutes of Health. Coronavirus disease 2019 (COVID-19) treatment guidelines. 2020. (https://www.covid19treatmentguidelines.nih.gov/). [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.