Abstract
Background
Anthracycline-induced cardiomyopathy (AIC) may be irreversible with a poor prognosis, disproportionately affecting women and young adults. Administration of allogeneic bone marrow–derived mesenchymal stromal cells (allo-MSCs) is a promising approach to heart failure (HF) treatment.
Objectives
SENECA (Stem Cell Injection in Cancer Survivors) was a phase 1 study of allo-MSCs in AIC.
Methods
Cancer survivors with chronic AIC (mean age 56.6 years; 68% women; NT-proBNP 1,426 pg/ml; 6 enrolled in an open-label, lead-in phase and 31 subjects randomized 1:1) received 1 × 108 allo-MSCs or vehicle transendocardially. Primary objectives were safety and feasibility. Secondary efficacy measures included cardiac function and structure measured by cardiac magnetic resonance imaging (CMR), functional capacity, quality of life (Minnesota Living with Heart Failure Questionnaire), and biomarkers.
Results
A total of 97% of subjects underwent successful study product injections; all allo-MSC–assigned subjects received the target dose of cells. Follow-up visits were well-attended (92%) with successful collection of endpoints in 94% at the 1-year visit. Although 58% of subjects had non-CMR compatible devices, CMR endpoints were successfully collected in 84% of subjects imaged at 1 year. No new tumors were reported. There were no significant differences between allo-MSC and vehicle groups with regard to clinical outcomes. Secondary measures included 6-min walk test (p = 0.056) and Minnesota Living with Heart Failure Questionnaire score (p = 0.048), which tended to favor the allo-MSC group.
Conclusions
In this first-in-human study of cell therapy in patients with AIC, transendocardial administration of allo-MSCs appears safe and feasible, and CMR was successfully performed in the majority of the HF patients with devices. This study lays the groundwork for phase 2 trials aimed at assessing efficacy of cell therapy in patients with AIC. (Stem Cell Injection in Cancer Survivors [SENECA]; NCT02509156)
Key Words: cardiac repair, cardio-oncology, chemotherapy, heart failure, stem cells
Abbreviations and Acronyms: AIC, anthracycline-induced cardiomyopathy; Allo-MSC, allogeneic mesenchymal stromal cells; CMR, cardiac magnetic resonance; HF, heart failure; LVEF, left ventricular ejection fraction; MLHFQ, Minnesota Living with Heart Failure Questionnaire; NT-proBNP, N-terminal pro-brain natriuretic peptide; PRA, panel reactive antibody; 6MWT, 6-min walk test
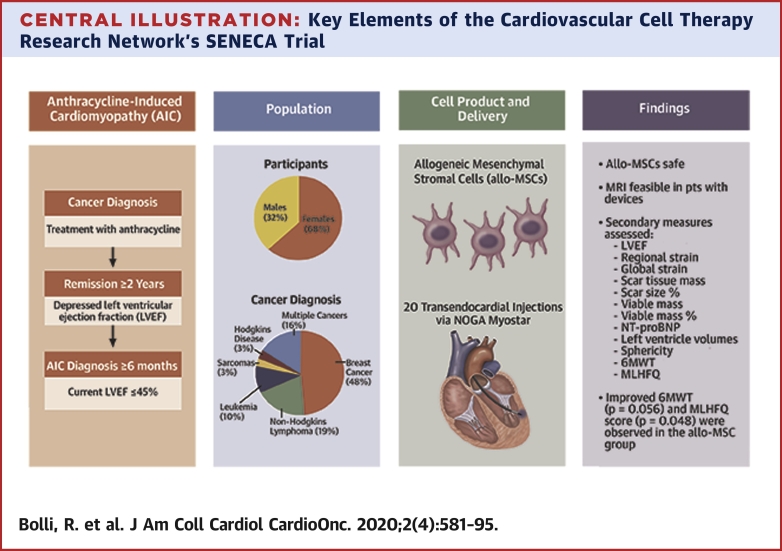
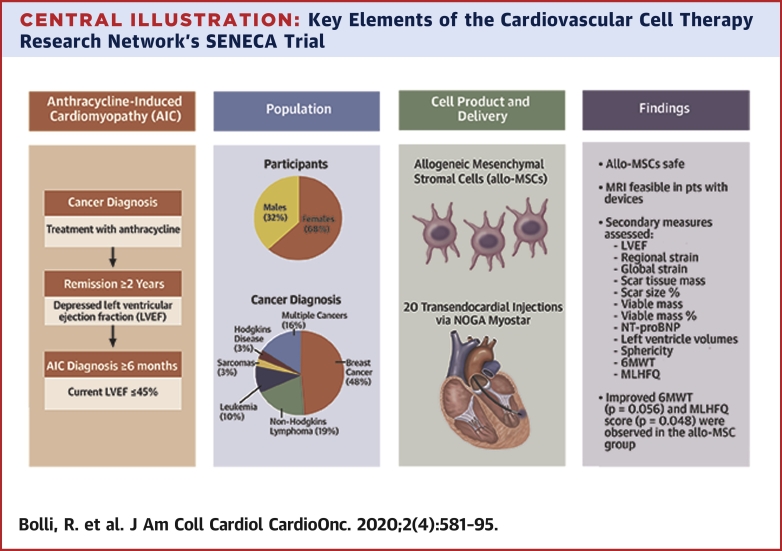
Central Illustration
Although anthracycline-based chemotherapies are effective in treating various forms of cancer, including breast cancer, lymphoma, leukemia, and sarcoma, their use is limited by cardiotoxicity resulting in late-onset heart failure (HF) in 5% to 23% of patients (1, 2, 3). The risk of anthracycline-induced cardiomyopathy (AIC) is primarily related to the lifetime cumulative dose, and an empiric limit of 500 mg/m2 has been suggested to minimize the cardiotoxic effects of doxorubicin, the most common anthracycline (4). However, even at low cumulative doses, such as standard-dose therapy for breast cancer, ∼5% of patients will have evidence of AIC (5, 6, 7). Therefore, every exposure to an anthracycline carries some risk of inducing cardiac dysfunction.
Despite optimal medical therapy for HF, AIC may be irreversible and carry a poor prognosis, worse than that of ischemic or nonischemic cardiomyopathy, with historical data suggesting an ≈3.5-higher relative risk of death within 5 years (8). Moreover, it has been suggested that the percentage of cardiac transplantation being performed secondary to AIC has increased over time (9). The impact of this problem is exacerbated by the fact that patients with AIC are relatively young and ≈70% are women (10). With the possible exception of dexrazoxane, no effective prophylactic treatment for AIC exists. The lack of effective, curative treatments for this disease constitutes an important unmet medical need.
Administration of bone marrow–derived mesenchymal stromal cells (MSCs) is a recent and promising approach to the treatment of HF, which has shown encouraging results at the preclinical level and in phase 1 and 2 clinical trials, both in ischemic and nonischemic cardiomyopathies (11, 12, 13, 14). Although the mechanisms remain unclear, previous studies have suggested that MSCs improve cardiac function by favorable paracrine actions, such as immunomodulatory, antifibrotic, proangiogenic, antiapoptotic, and/or anti-inflammatory effects (11, 12, 13,15). Importantly, MSC-based therapies have proven to be clinically safe (11, 12, 13,16, 17, 18).
Several pathological features of AIC are common to other forms of HF where cell therapy has shown promising results, including loss of viable myocytes, replacement fibrosis, myocyte apoptosis, and low-level cardiac inflammation (19). In a large animal (sheep) model of AIC, allogeneic mesenchymal stromal cell (allo-MSC) delivery via the transendocardial route was shown to improve left ventricular (LV) function, and reduce myocardial fibrosis (20). Based upon these considerations, we postulated that administration of MSCs may be beneficial in patients with AIC.
To test this hypothesis, the National Heart, Lung, and Blood Institute–sponsored Cardiovascular Cell Therapy Research Network conducted the SENECA trial (StEm Cell INjEction in CAncer Survivors), a first-in-human study of allo-MSCs in patients with LV dysfunction secondary to AIC (21). We used allo-MSCs rather than autologous MSCs because of the potential concern that autologous cells may lead to dissemination of the original malignancy for which patients were treated with anthracyclines. MSCs are ideal candidates for allogeneic cellular therapy because they show minimal major histocompatibility complex class II and intercellular adhesion molecule expression, they lack B-7 costimulatory molecules necessary to cause a T-cell mediated immune response (22, 23, 24), and thus they have low immunogenicity (13). The design of SENECA was unique, as pre-existing cancer is usually an exclusion criterion for cell therapy trials.
Methods
SENECA was a phase 1, randomized, double-blind, placebo-controlled trial designed to evaluate the use of allo-MSCs in patients with AIC (NCT02509156). The primary objectives of this study were the safety of allo-MSC administration in this population and the feasibility of delivering the cells to the subjects and collecting the outcome measures. The secondary objectives were to determine the effects of allo-MSC administration on LV function and functional status from baseline to 6 months and from baseline to 12 months after treatment. As a small phase 1 trial, SENECA was not powered to assess efficacy; rather, it was designed as a hypothesis-generating study whose results will inform future, larger studies with regard to the selection of appropriate endpoints. A detailed description of the rationale and design of this trial has been published (21).
Enrollment cohorts
Through consultation with the U.S. Food and Drug Administration (FDA) and the National Heart, Lung, and Blood Institute’s Gene and Cell Therapy Data Safety Monitoring Board, this trial was initiated with a 6-subject, open-label lead-in phase. The protocol was reviewed and approved by local institutional review boards at each of the 7 recruiting centers and the data coordinating center. All subjects provided written informed consent prior to study procedures.
The lead-in phase of SENECA afforded the Network an opportunity to review and optimize the safety and feasibility of the procedures for the full study. Between September 2016 and December 2016, 6 candidates consented to participate and received 20 transendocardial, electromechanically-guided injections of allo-MSCs performed with the MyoStar injection catheter (Biologic Delivery Systems, Cordis Corp., Hialeah, Florida). All subjects received the intended dose and completed follow-up according to protocol. A complete safety report of the 6 subjects was provided for review by the data safety monitoring board and FDA. Subsequently, the study was allowed to proceed to the randomization phase. Between March 2017 and October 2018, 31 eligible subjects were randomized 1:1 to receive either allo-MSCs or cell-free placebo (Buminate solution), delivered in a blinded fashion. Randomizations were stratified by clinical center using block sizes of 2.
Screening and eligibility
Male and female cancer survivors age 18 to 79 years, with a diagnosis of AIC, were recruited. Inclusion and exclusion criteria are provided in Supplemental Table 1. Briefly, to be enrolled, subjects had to: 1) have received the initial diagnosis of AIC at least 6 months prior to consent; 2) have a left ventricular ejection fraction (LVEF) ≤45%; 3) be receiving stable, optimally tolerated therapy with beta-blockers, angiotensin-converting enzyme inhibitors/angiotensin II receptor blockers, and/or aldosterone antagonists unless contraindicated; 4) have no evidence of ischemic heart disease; and 5) be cancer-free for at least 2 years with a low likelihood of recurrence (a 5-year risk of recurrence estimated at ≤30%) (21). The risk of recurrence was estimated on the basis of tumor type and stage at diagnosis, response to therapy, and absence of any new metastases at the time of enrollment. A single oncologist, who was not involved in the care of the subjects, estimated recurrence risk for all subjects.
Baseline evaluations to determine eligibility took place within 45 days of subjects providing informed consent. All subjects completed the following assessments: cardiac magnetic resonance imaging (CMR), 6-min walk test (6MWT), Minnesota Living with Heart Failure Questionnaire (MLHFQ), and a comprehensive laboratory panel that included N-terminal pro-brain natriuretic peptide (NT-proBNP).
Study product and intramyocardial delivery
The methods for manufacturing the study product (allo-MSCs), as well as the strategy for targeting the transendocardial injections of study product, have been published elsewhere (21). Briefly, allo-MSCs were manufactured and released by the Center for Cell and Gene Therapy at the Baylor College of Medicine (Houston, Texas). The allo-MSC cell bank was generated from approximately 25 to 50 ml of bone marrow from a single healthy donor (female, age 27 years). The bone marrow was aspirated into pre-heparinized syringes containing preservative-free heparin for a final effective dose of ∼100 U of heparin/ml of bone marrow. The allo-MSCs were isolated and expanded to a final dose of 1 × 108 allo-MSCs according to standard operating procedures of the manufacturer. At passage 3, the cells meeting the following manufacturing criteria were released for distribution to the clinical center: sterility, endotoxin <5.0 EU/ml, negative mycoplasma tests, viability ≥70%, negative bacterial and fungal cultures, negative in vitro virus tests, >95% positivity for CD105/90/73 and <2% positivity for CD45/14/19 and CD34 (flow cytometry), colony formation CFU-F (positive growth), and evidence of trilineage differentiation.
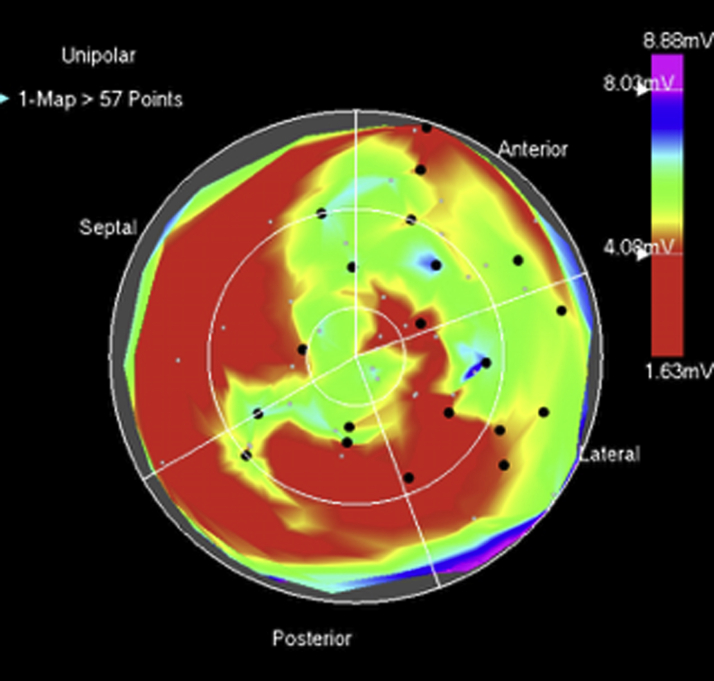
The manufacturer’s group coordinated shipment of frozen allo-MSCs to the appropriate clinical center using a validated liquid nitrogen dry shipper. The cells were thawed on the day of administration and processed according to the manufacturer’s standard operating procedures. The study product was release tested for viability, sterility, and endotoxin and drawn into 10 1-ml syringes (plus a syringe used to flush the product through the catheter) for administration via the NOGA catheter. A total of 1 × 108 allo-MSCs in 8 ml of Buminate 5% solution or placebo (8 ml of cell-free Buminate solution) was delivered via 20 transendocardial injections (0.4 ml each) as described (21). Areas of partial fibrosis (unipolar voltage 4 to 8 mV) were prioritized for injection; however, areas with unipolar voltages up to 12 mV were included in an effort to treat the entire LV. Injections were planned to be distributed over at least 3 to 4 different segments of the LV wall (Figure 1). All subjects were monitored overnight following the procedure.
Figure 1.
NOGA Mapping and Injection Technique
Left ventricular electromechanical 12-segment polar map representing Unipolar voltage. The scale (right) depicts voltage values represented by (visible light spectrum) color. The 20 injection sites of 0.4 ml each are represented by black dots. There is heterogenous distribution of lower voltage values (red) throughout the left ventricular endocardium likely representing areas of fibrosis. Injections were placed in areas of intermediate voltage between 4 and 8 mV and broadly distributed as per the injection strategy guidelines.
Follow-up evaluations
All subjects were followed for 1 year, with evaluations at 0 (day of injection), 1, 7 ± 3, 30 ± 7, 180 ± 30, and 365 ± 30 days.
A physical examination, electrocardiogram, and laboratory evaluations were conducted at each visit, along with the assessment of adverse events and review of medications. In addition, at 6 months and 1 year, subjects performed 6MWTs (2 tests at each time, with the results averaged), completed the MLHFQ, and underwent CMR. For individuals with a pacemaker or implanted cardioverter-defibrillator, the CMR Core Lab (Johns Hopkins University, Baltimore, Maryland) developed an imaging protocol based upon published safety recommendations (25,26). The Core Lab provided training on the CMR procedures and certified technicians at each of the 7 clinical centers. In addition, clinical centers received feedback on the quality of the images acquired in each scan.
Safety monitoring and clinical outcome adjudication
Subjects were examined for adverse events for 12 months after treatment. All adverse events graded 2 or higher in severity using the NCI Common Terminology Criteria for Adverse Events (version 4.0) were submitted by the clinical centers to the Data Coordinating Center safety team at UTHealth (Houston, Texas) for records review and sponsor assessment. Major adverse cardiovascular events (MACE) included death, hospitalization for worsening HF, and other HF exacerbation not requiring hospitalization. Other significant clinical events were also tabulated including: nonfatal stroke, nonfatal myocardial infarction, coronary artery revascularization, ventricular tachycardia/fibrillation, pericardial tamponade, infectious myocarditis, hypersensitivity reaction, neoplasm, and/or any other potential deleterious late effects (27,28). Events deemed to be potential clinical endpoints were assessed by 2 independent physicians not affiliated with any clinical center and masked to treatment assignment.
Immunological monitoring
Serum samples were collected at baseline and 1, 6, and 12 months after treatment to determine whether there were any significant changes in panel reactive antibody (PRA).
Efficacy endpoints
To explore whether allo-MSCs produced a trend toward improved LV function and functional status when compared with placebo, several variables were evaluated. Measures of cardiac function (change in LVEF and global and regional strain [Harmonic Phase CMR]), cardiac structure (change in LV end-diastolic volume index, LV end-systolic volume index, LV sphericity index), and cardiac morphology (change in area of injury) were obtained by CMR (see the Supplemental Appendix for additional details). Functional capacity was evaluated by changes in exercise tolerance (6MWT). Change in quality of life was measured via MLHFQ score. Clinical outcome was assessed by measurement of MACE (death, hospitalization for worsening HF, and exacerbation of HF not requiring hospitalization) and days alive and out of the hospital (DAOH) (21). Biomarker assessment included changes in NT-proBNP.
Statistical analysis
All statistical analyses were conducted using SAS version 9.4 (SAS institute Inc., Cary, North Carolina) and R version 3.2.2 (2015, R Core Team, Vienna, Austria). The principal analysis was based on intention-to-treat. All analyses were conducted on 2 cohorts: 1) the randomized cohort; and 2) the total cohort (randomized plus the 6 subjects who received open-label therapy). Descriptive statistics for baseline characteristics are provided using mean ± SD for continuous variables and number (percentage) for categorical variables. Continuous variables were evaluated for normality using Shapiro-Wilks and histograms. Non-normally distributed variables were transformed. Fisher’s exact test for categorical variables and Student’s t-tests for continuous variables were used to evaluate differences in baseline and follow-up between treatment groups. Safety data were analyzed by therapy group using Fisher’s exact test between baseline and: 1) 6 months; and 2) 12 months. Feasibility of study procedures was evaluated as the number and percentage of subjects who failed to receive the intervention according to protocol, did not have an interpretable CMR scan, or failed to complete follow-up.
For each of the 2 prospectively declared follow-up durations (6 and 12 months), the change in each efficacy measure of interest was compared using analysis of covariance analyses adjusting for baseline values. Repeated-measures regression models were used to address trajectories (upward or downward trends) over time within each of the treatment groups in the randomized and total cohorts. Also, a treatment by time interaction was assessed in these cohorts. No adjustments for multiple testing were made in this phase 1 trial.
Results
Study population
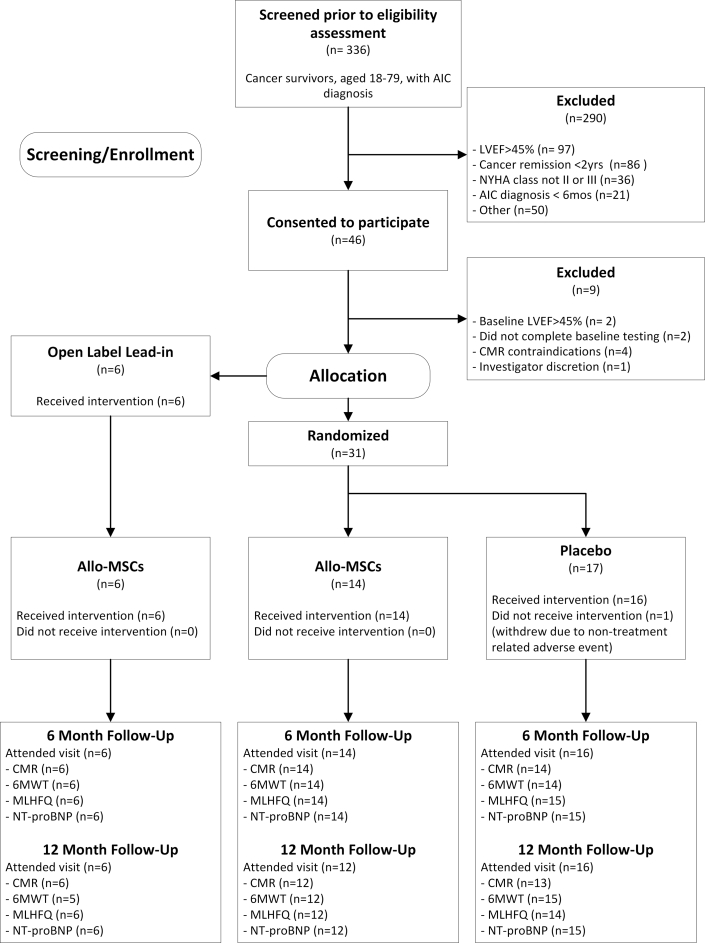
Between September 2016 and October 2018, 336 cancer survivors with a diagnosis of AIC were screened. Of these, most were excluded because of (in descending order of prevalence) LVEF >45%, duration of cancer-free state <2 years, New York Heart Association functional class I or IV, and AIC diagnosis made <6 months earlier (Figure 2). In total, 46 individuals provided consent for trial participation and underwent baseline evaluations; of these, 37 met the eligibility criteria. The first 6 individuals were treated in the open-label lead-in phase of the trial. The remaining 31 subjects were randomized (1:1) to allo-MSCs or placebo. One randomized placebo subject was involved in a vehicle accident prior to study product delivery and withdrew consent to focus on recovery.
Figure 2.
The SENECA CONSORT Diagram
CONSORT diagram shows patients screened, enrolled, and treated in the SENECA trial, as well as number of patients that completed follow-up for each endpoint, along with reasons for noncompletion. 6MWT = 6-min walk test; AIC = anthracycline-induced cardiomyopathy; CMR = cardiac magnetic resonance; Allo-MSC = allogeneic mesenchymal stromal cells; HF = heart failure; LVEF = left ventricular ejection fraction; MLHFQ = Minnesota Living with Heart Failure Questionnaire; NT-proBNP = N-terminal pro-brain natriuretic peptide; NYHA = New York Heart Association.
Baseline characteristics of randomized subjects are summarized in Table 1. The randomized study cohort included 14 subjects in the allo-MSC group and 17 in the placebo group. There were no statistically significant differences in baseline variables between the 2 treatment groups. The mean age was 56.6 ± 11.8 years. Women constituted 68% of the randomized cohort; 32% were non-White, and 14% were Hispanic. The average LVEF at baseline was 33 ± 5.3%, and 84% of subjects were in New York Heart Association functional class II. The average NT-proBNP value was 1,426 pg/ml. All subjects were receiving maximally tolerated medical therapy for HF at the time of consent. Over one-half (58%) of randomized subjects had a cardiac device (implantable cardioverter-defibrillator or pacemaker). Oncological disease history included breast cancer (48%), non-Hodgkin’s lymphoma (19%), leukemia (10%), Hodgkin’s disease (3%), and sarcomas (3%). A total of 5 subjects (16%) had more than 1 diagnosis of cancer in their lifetime; in all cases, the first instance of cancer involved treatment with an anthracycline. The average interval since cancer diagnosis was 17.7 ± 8.9 years and since last cancer treatment was 15.2 ± 8.4 years. In 90% of the subjects, the antineoplastic therapy involved doxorubicin; the remaining 10% received either epirubicin or daunorubicin. Because many cancer treatment records were from >15 years prior, information on anthracycline dosage could be collected only in a subset of the randomized cohort (n = 20). The average time since AIC diagnosis was 7.5 ± 5.5 years.
Table 1.
Baseline Characteristics of Randomized Subjects
| Allo-MSCs (n = 14) | Placebo (n = 17) | |
|---|---|---|
| Demographics | ||
| Age | 54.7 ± 12.8 | 58.2 ± 11.2 |
| Female | 8 (57) | 13 (76) |
| Race | ||
| White | 8 (57) | 13 (76) |
| Black | 4 (29) | 3 (18) |
| Other | 2 (14) | 1 (6) |
| Hispanic | 1 (8) | 3 (19) |
| Physical findings and risk factors | ||
| Body mass index, kg/m2 | 30.2 ± 9.0 | 30.4 ± 6.5 |
| Heart rate, beats/min | 74.4 ± 9.0 | 76.1 ± 11.2 |
| SBP, mm Hg | 118.6 ± 21.6 | 115.4 ± 11.0 |
| DBP, mm Hg | 68.9 ± 18.6 | 67.1 ± 11.2 |
| Diabetes | 3 (21) | 5 (29) |
| Hypertension | 6 (43) | 10 (59) |
| Smoking (lifetime) | 5 (36) | 3 (18) |
| Cardiovascular history | ||
| Left ventricular ejection fraction | 33.7 ± 3.4 | 32.5 ± 6.5 |
| Previous hospitalization for heart failure | 7 (50) | 8 (47) |
| Previous emergency department visit for heart failure | 4 (29) | 3 (18) |
| Time since AIC diagnosis, yrs | 6.1 ± 5.9 | 8.7 ± 5.0 |
| New York Heart Association functional class II | 13 (93) | 13 (76) |
| New York Heart Association functional class III | 1 (7) | 4 (24) |
| Device present | 6 (43) | 12 (71) |
| Pacemaker | 0 (0) | 0 (0) |
| Implantable cardioverter-defibrillator | 5 (36) | 8 (47) |
| Biventricular pacing alone | 0 (0) | 0 (0) |
| Biventricular pacing and ICD | 1 (7) | 4 (24) |
| Angina | 1 (7) | 1 (6) |
| Percutaneous coronary intervention/coronary artery bypass graft | 0 (0) | 0 (0) |
| Atrial fibrillation | 3 (21) | 5 (29) |
| Sustained ventricular arrhythmia | 5 (36) | 7 (41) |
| Oncological history | ||
| Leukemia | 3 (21) | 0 (0) |
| Breast cancer | 6 (43) | 9 (53) |
| Hodgkin's disease | 0 (0) | 1 (6) |
| Non-Hodgkin's lymphoma | 1 (7) | 5 (29) |
| Sarcomas | 1 (7) | 0 (0) |
| Multiple cancers | 3 (21) | 2 (12) |
| Antineoplastic Treatment | ||
| Anthracyclines | ||
| Doxorubicin | 11 (79) | 17 (100) |
| Epirubicin | 1 (7) | 0 (0) |
| Daunorubicin | 2 (14) | 0 (0) |
| AIC exposure, mg/m2∗ | 353.0 ± 351.3 | 339.5 ± 113.6 |
| Other potential cardiotoxic agents | ||
| Her2-directed therapy | 1 (7) | 1 (6) |
| Tyrosine kinase inhibitors | 0 (0) | 0 (0) |
| Other chemotherapies | ||
| Cyclophosphamide | 4 (29) | 3 (18) |
| Cytoxan | 4 (29) | 6 (35) |
| Paclitaxel | 3 (21) | 3 (18) |
| Docetaxel | 1 (7) | 2 (12) |
| Other | 6 (43) | 8 (47) |
| Time from cancer diagnosis, yrs† | 16.4 ± 9.5 | 18.8 ± 8.5 |
| Time from last anthracycline treatment, yrs | 13.3 ± 9.3 | 16.8 ± 7.6 |
| Radiation history | ||
| Total who had radiation | 9 (64) | 12 (71) |
| Whole breast | 3 (21) | 0 (0) |
| Partial breast | 1 (7) | 1 (6) |
| Post-mastectomy chest wall | 2 (14) | 2 (12) |
| Internal mammary field | 0 (0) | 0 (0) |
| Axillary | 1 (7) | 2 (12) |
| Mantle | 0 (0) | 1 (6) |
| Radiation field included left chest | 2 (14) | 2 (12) |
| Medications | ||
| Aspirin | 7 (50) | 5 (29) |
| Beta-blockers | 13 (93) | 16 (94) |
| Angiotensin-converting enzyme inhibitors/angiotensin II blockers/angiotensin receptor-neprilysin inhibitors | 12 (86) | 15 (88) |
| Aldosterone antagonists | 9 (64) | 10 (59) |
| Calcium-channel blockers | 1 (7) | 0 (0) |
| Hydralazine | 0 (0) | 1 (6) |
| Nitrates | 0 (0) | 2 (12) |
| Statins | 5 (36) | 8 (47) |
| Diuretics | 9 (64) | 14 (82) |
| Anticoagulants | 2 (14) | 4 (24) |
Values are mean ± SD or n (%).
AIC = anthracycline-induced cardiomyopathy; Allo-MSC = allogeneic mesenchymal stromal cells.
Anthracycline dose information only available for a subset of cohort.
Time from earliest diagnosis requiring anthracycline treatment (n = 20)
Allo-MSC characteristics
Including the 6 open-label treated subjects, a total of 20 subjects received allo-MSCs (1 × 108 cells) and 16 received placebo. All products met the protocol-specified release criteria for administration. In 1 case, the culture results obtained 14 days after injection showed growth of gram-negative aerobic organisms (Sphingomonas paucimobilis) and fungal organisms (Anthrobacter species); the subject was monitored for clinical signs of infection (twice daily temperature readings), remained asymptomatic and there were no clinical sequelae. Mean viability for the cell product was 93.9%.
Transendocardial delivery
All 6 open-label and 30 randomized subjects received 20 transendocardial injections of study product as described (21). One patient received 11 injections secondary to technical issues at the site. There were no complications associated with product delivery. Only 6% of subjects received injections outside of the target unipolar voltage parameters (2% <4 mV; 4% >12 mV), and in 97% of injection procedures at least 4 LV segments were injected.
Safety and clinical outcomes
All safety evaluations included the 6 open-label, lead-in subjects from the period of consent to 12 months post-treatment regardless of relationship to study intervention. A total of 93 adverse events were reported across 27 of the participants. In total, 42 of these events met the definition of serious adverse events. All reported events were either expected or deemed unlikely/unrelated to therapy; thus, none met protocol-specified reporting requirements for the FDA.
Events grade 2 or higher in severity are shown in Table 2, displayed by MedDRA system organ classification. If a subject experienced more than 1 adverse event within a classification, they are only included once in the category. Examples of events within each category are provided in the footnote to Table 2. A breakdown of cardiac adverse events by treatment group is included in the Supplemental Appendix. Similarly, Table 3 depicts the number of subjects experiencing a serious adverse event within each category. In total, 8 subjects (21.6%) experienced serious adverse events that were cardiac in nature, 4 in the allo-MSC group and 4 in the placebo group. Noncardiac events did not differ significantly between the 2 groups. There were no serious, unexpected, and treatment-related events reported during the trial. Based on serial PRA measurements, no acute or chronic immune reaction to the study product was observed. Importantly, no new neoplasms were identified or reported during the 12-month follow-up period, a conclusion also supported by the ongoing oncological assessments of subjects by their care providers (all but 2 patients were assessed for neoplasms at 12 months).
Table 2.
Participants Experiencing Adverse Events by Treatment Group
| System Organ Classification∗ | Pre-Assign | Post-Assign (n = 37) | Allo-MSC (n = 20) | Placebo (n = 17) |
|---|---|---|---|---|
| Cardiac disorders | 0 | 15 (40.5) | 7 (35.0) | 8 (47.1) |
| Endocrine disorders | 1 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Gastrointestinal disorders | 0 | 3 (8.1) | 1 (5.0) | 2 (11.7) |
| General disorders and administration site conditions | 0 | 5 (13.5) | 2 (10.0) | 3 (17.6) |
| Hepatobiliary disorders | 0 | 1 (2.7) | 0 (0.0) | 1 (5.8) |
| Infections and infestations | 1 | 6 (16.2) | 3 (15.0) | 3 (17.6) |
| Injury, poisoning and procedural complications | 1 | 4 (10.8) | 1 (5.0) | 3 (17.6) |
| Investigations | 0 | 5 (13.5) | 4 (20.0) | 1 (5.8) |
| Metabolism and nutrition disorders | 0 | 4 (10.8) | 2 (10.0) | 2 (11.7) |
| Musculoskeletal and connective tissue disorders | 1 | 5 (13.5) | 4 (20.0) | 1 (5.8) |
| Neoplasms benign, malignant, and unspecified (including cysts and polyps) | 1 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Nervous system disorders | 0 | 7 (18.9) | 5 (25.0) | 2 (11.7) |
| Product issues | 0 | 1 (2.7) | 0 (0.0) | 1 (5.8) |
| Renal and urinary disorders | 0 | 4 (10.8) | 3 (15.0) | 1 (5.8) |
| Reproductive system and breast disorders | 0 | 1 (2.7) | 0 (0.0) | 1 (5.8) |
| Respiratory, thoracic, and mediastinal disorders | 0 | 4 (10.8) | 3 (15.0) | 1 (5.8) |
| Surgical and medical procedures | 0 | 1 (2.7) | 0 (0.0) | 1 (5.8) |
| Vascular disorders | 0 | 2 (5.4) | 1 (5.0) | 1 (5.8) |
Values are n (%). Example events for SOCs: cardiac disorders = heart failure, arrhythmias (ventricular tachycardia, supraventricular tachycardia, palpitations, atrial fibrillation), and pericardial effusions (not related to treatment); general disorders = pyrexia, noncardiac chest pain, and injury associated with implanted device; infections = tooth abscess, cystitis, post-operative wound infection; investigations = glomerular filtration rate decrease, hepatic enzyme increase, platelet count decrease; nervous disorders = syncope, migraines, Parkinson’s; vascular disorders = hypotension.
allo-MSC = allogeneic mesenchymal stromal cell; post-assign = after allocation to treatment assignment; pre-assign = prior to allocation to treatment assignment.
System Organ Classification by MedDRA version 19.1.
Table 3.
Participants Experiencing Serious Adverse Events by Treatment Group
| System Organ Classification∗ | Pre-Assign | Post-Assign (n = 37) | Allo-MSC (n = 20) | Placebo (n = 17) |
|---|---|---|---|---|
| Cardiac disorders | 0 | 8 (21.6) | 4 (20.0) | 4 (23.5) |
| Gastrointestinal disorders | 0 | 1 (2.7) | 0 (0.0) | 1 (5.8) |
| General disorders and administration site conditions | 0 | 1 (2.7) | 1 (5.0) | 0 (0.0) |
| Hepatobiliary disorders | 0 | 1 (2.7) | 0 (0.0) | 1 (5.8) |
| Infections and infestations | 1 | 2 (5.4) | 0 (0.0) | 2 (11.7) |
| Injury, poisoning, and procedural complications | 1 | 2 (5.4) | 1 (5.0) | 1 (5.8) |
| Metabolism and nutrition disorders | 0 | 2 (5.4) | 1 (5.0) | 1 (5.8) |
| Musculoskeletal and connective tissue disorders | 1 | 1 (2.7) | 1 (5.0) | 0 (0.0) |
| Neoplasms benign, malignant, and unspecified (including cysts and polyps) | 1 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Nervous system disorders | 0 | 2 (5.4) | 1 (5.0) | 1 (5.8) |
| Product issues | 0 | 1 (2.7) | 0 (0.0) | 1 (5.8) |
| Renal and urinary disorders | 0 | 2 (5.4) | 1 (5.0) | 1 (5.8) |
| Reproductive system and breast disorders | 0 | 1 (2.7) | 0 (0.0) | 1 (5.8) |
| Vascular disorders | 0 | 2 (5.4) | 1 (5.0) | 1 (5.8) |
MACE and other important clinical outcomes
The distribution and timeline of adjudicated clinical and safety outcomes (27,28) are displayed in Table 4. Ten clinical endpoints were adjudicated in 6 subjects, including 1 death (due to progressive HF), 7 hospitalizations for worsening HF, 1 nonhospitalized HF exacerbation, and 1 instance of hypersensitivity reaction to CMR contrast agent. In addition, there was 1 heart transplant; however, this was not a pre-specified clinical endpoint.
Table 4.
Adjudicated Clinical and Safety Outcomes
| Lead-In (n = 6) |
Allo-MSC (n = 14) |
Placebo (n = 17) |
||||
|---|---|---|---|---|---|---|
| SPI to 6 Months | 6 Months to 12 Months | SPI to 6 Months | 6 Months to 12 Months | SPI to 6 Months | 6 Months to 12 Months | |
| Major adverse cardiac events | ||||||
| Deaths (cardiovascular) | 0 | 0 | 0 | 1 | 0 | 0 |
| Hospitalization for worsening HF | 0 | 1 | 1 | 0 | 2∗ | 3∗ |
| Other exacerbation of HF (nonhospitalization) | 0 | 0 | 0 | 0 | 0 | 1 |
| Other significant clinical events | ||||||
| Nonfatal stroke | 0 | 0 | 0 | 0 | 0 | 0 |
| Nonfatal myocardial infarction | 0 | 0 | 0 | 0 | 0 | 0 |
| Coronary artery revascularization | 0 | 0 | 0 | 0 | 0 | 0 |
| Ventricular tachycardia/fibrillation | 0 | 0 | 0 | 0 | 0 | 0 |
| Pericardial tamponade | 0 | 0 | 0 | 0 | 0 | 0 |
| Infectious myocarditis | 0 | 0 | 0 | 0 | 0 | 0 |
| Hypersensitivity reaction | 0 | 0 | 0 | 0 | 0 | 1 |
| Neoplasm | 0 | 0 | 0 | 0 | 0 | 0 |
| Total participants | 0 | 1 | 1 | 1 | 1∗ | 2∗ |
Values are n.
Allo-MSC = allogeneic mesenchymal stromal cells; HF = heart failure; SPI = study product injection.
Includes multiple events in a participant during this time period.
Days alive and out of the hospital
Subjects were allotted a visit window extending 30 days past their anticipated 12-month visit (i.e., 395 days). In total, 14 patients were hospitalized >24 h (any cause); 7 hospitalizations involving 5 patients were for worsening HF. The average number of DAOH (any cause) was 367 ± 25.9 days (allo-MSC) versus 360 ± 31.3 days (placebo); the average DAOH for HF was 368 ± 25.8 days versus 363 ± 31.9 days, respectively.
Feasibility
Receipt of study product
As mentioned in the previous text, 1 randomized subject failed to receive the study product (due to withdrawal of consent). One randomized subject received less than the protocol-specified 20 injections due to operator error. Nevertheless, all subjects randomized to the allo-MSC group (including the 6 open-label-treated subjects) received the protocol-specified dose of cells (1 × 108).
Performance of CMR studies
During screening, a small number of individuals were found to be ineligible because of their body habitus. Although several techniques were used to alleviate artifacts caused by cardiac devices (21), useable images could not be obtained in 2 individuals because of cardiac devices; these individuals were therefore excluded.
CMR scans were collected on 32 (94%) of the 34 participants who attended the 12-month follow-up. Images could not be obtained in 1 participant because of device lead issues, and no images were collected in the participant who received a cardiac transplant. Measurements of LVEF, LV end-diastolic volume index, LV end-systolic volume index, sphericity index, and regional longitudinal strain were obtained in 97% of the scans (1 participant experienced claustrophobia and the relevant sequences could not be collected before the scan was aborted). Global circumferential strain was collected in 94% of the scans; it could not be calculated in 1 participant because of artifacts at 1 of the 3 timepoints (to accurately calculate strain measurements, matching segments across the assessment timepoints must be unencumbered by device-related artifacts). Measurements of scar size and viable mass were obtained in 84% of the subjects at 12 months. Main reasons for the inability to assess these variables were device artifacts (creating low signal-to-noise ratio) or low glomerular filtration rate (resulting in the inability to use contrast dye).
Follow-up attendance
In total, 36 of the 37 subjects (97%) attended the 6-month follow-up visit and 34 (92%) attended the 12-month follow-up visit.
Efficacy endpoints
The secondary efficacy endpoints for the randomized cohort at 12 months are presented in Table 5. In total, 10 of 13 efficacy measures favored allo-MSCs. However, this small phase 1 trial was not powered for any of these outcomes, and thus, the wide confidence intervals and nonsignificant p values reflect uncertainty in the estimated value. The only statistically significant difference was observed in the MLHFQ score, which decreased from 46.63 to 21.18 in the allo-MSC group and from 53.14 to 40.51 in the placebo group; the difference in the changes between the 2 treatment groups was −12.82 (95% CI: −30.49 to 4.84; p = 0.048 by analysis of covariance adjusting for baseline values). The efficacy endpoints for the randomized cohort at 6 months are presented in Supplemental Table 2; those for the total cohorts (lead-in patients included) at 6 and 12 months are presented in Supplemental Tables 3 and 4. In general, the results in the entire cohort (Supplemental Tables 3 and 4) are consistent with the 12-month data in the randomized cohort (Table 5).
Table 5.
Secondary Efficacy Endpoints for the Randomized Cohort at 12 Months
| Allo-MSCs |
Placebo |
Effect Estimate∗ |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| N | Mean | SD | N | Mean | SD | Diff | 95% CI | p Value | |
| Function | |||||||||
| LVEF, % | |||||||||
| Baseline | 12 | 34.51 | 2.86 | 13 | 33.31 | 6.21 | |||
| 12 months | 12 | 37.98 | 7.84 | 13 | 35.99 | 7.98 | |||
| Delta | 12 | 3.47 | 6.00 | 13 | 2.68 | 6.85 | 0.80 | -4.52 to 6.11 | 0.746 |
| Within group p value | 0.070 | 0.184 | |||||||
| Global circumferential strain, % | |||||||||
| Baseline | 11 | −10.21 | 1.83 | 13 | -9.76 | 2.43 | |||
| 12 months | 11 | −11.04 | 2.39 | 13 | -9.88 | 2.53 | |||
| Delta | 11 | −0.83 | 1.70 | 13 | -0.12 | 2.86 | -0.71 | -2.68 to 1.26 | 0.328 |
| Within group p value | 0.136 | 0.883 | |||||||
| Regional longitudinal strain, %‡ | |||||||||
| Baseline | 12 | −12.45 | 2.58 | 13 | -11.17 | 1.95 | |||
| 12 months | 12 | −13.65 | 3.01 | 13 | -10.79 | 3.32 | |||
| Delta | 12 | −1.20 | 3.34 | 13 | 0.38 | 3.73 | -1.58 | -4.51 to 1.35 | 0.071 |
| Within group p value | 0.239 | 0.720 | |||||||
| Structure | |||||||||
| LV end-diastolic volume index | |||||||||
| Baseline | 12 | 103.47 | 27.88 | 13 | 108.64 | 21.57 | |||
| 12 months | 12 | 101.17 | 28.74 | 13 | 105.28 | 26.03 | |||
| Delta | 12 | −2.30 | 16.97 | 13 | -3.36 | 15.18 | 1.06 | -12.33 to 14.45 | 0.935 |
| Within group p value | 0.647 | 0.440 | |||||||
| LV end-systolic volume index | |||||||||
| Baseline | 12 | 68.22 | 20.33 | 13 | 73.35 | 20.83 | |||
| 12 months | 12 | 64.60 | 26.25 | 13 | 69.07 | 24.43 | |||
| Delta | 12 | −3.62 | 15.66 | 13 | -4.28 | 14.18 | 0.66 | -11.75 to 13.08 | 0.919 |
| Within group p value | 0.440 | 0.298 | |||||||
| LV sphericity index | |||||||||
| Baseline | 12 | 0.50 | 0.12 | 13 | 0.56 | 0.09 | |||
| 12 months | 12 | 0.51 | 0.12 | 13 | 0.49 | 0.09 | |||
| Delta | 12 | 0.01 | 0.08 | 13 | -0.07 | 0.09 | 0.077 | 0.003 to 0.15 | 0.124 |
| Within group p value | 0.685 | 0.024 | |||||||
| Morphology | |||||||||
| Scar size percent | |||||||||
| Baseline | 11 | 13.78 | 8.29 | 10 | 8.83 | 5.49 | |||
| 12 months | 11 | 12.72 | 7.41 | 10 | 8.42 | 5.57 | |||
| Delta | 11 | −1.06 | 2.44 | 10 | -0.41 | 2.76 | -0.66 | -3.05 to 1.74 | 0.993 |
| Within group p value | 0.180 | 0.652 | |||||||
| Scar tissue mass, g | |||||||||
| Baseline | 11 | 15.29 | 10.43 | 10 | 10.45 | 7.38 | |||
| 12 months | 11 | 15.05 | 10.75 | 10 | 10.30 | 8.23 | |||
| Delta | 11 | −0.24 | 3.15 | 10 | -0.15 | 3.12 | -0.090 | -2.96 to 2.78 | 0.949 |
| Within group p value | 0.808 | 0.885 | |||||||
| Viable mass, g | |||||||||
| Baseline | 11 | 106.75 | 37.32 | 10 | 112.40 | 24.55 | |||
| 12 months | 11 | 109.73 | 41.63 | 10 | 111.93 | 21.64 | |||
| Delta | 11 | 2.98 | 11.96 | 10 | -0.47 | 13.39 | 3.45 | -8.23 to 15.12 | 0.567 |
| Within group p value | 0.428 | 0.914 | |||||||
| Viable mass percent (transformation)† | |||||||||
| Baseline | 11 | 92.82 | 6.72 | 10 | 95.83 | 3.68 | |||
| 12 months | 11 | 92.92 | 6.67 | 10 | 95.65 | 3.78 | |||
| Delta | 11 | 0.10 | 2.73 | 10 | -0.18 | 2.08 | 0.28 | -1.93 to 2.49 | 0.704 |
| Within group p value | 0.946 | 0.919 | |||||||
| Functional capacity | |||||||||
| 6MWT, m | |||||||||
| Baseline | 12 | 384.38 | 77.88 | 15 | 359.57 | 48.60 | |||
| 12 months | 12 | 419.33 | 93.83 | 15 | 356.50 | 55.46 | |||
| Delta | 12 | 34.96 | 61.62 | 15 | -3.07 | 42.90 | 38.03 | -5.84 to 81.89 | 0.056 |
| Within group p value | 0.075 | 0.786 | |||||||
| MLHFQ score | |||||||||
| Baseline | 12 | 46.63 | 25.92 | 14 | 53.14 | 30.01 | |||
| 12 months | 12 | 21.18 | 17.22 | 14 | 40.51 | 30.98 | |||
| Delta | 12 | −25.45 | 25.65 | 14 | -12.63 | 14.59 | -12.82 | -30.49 to 4.84 | 0.048 |
| Within group p value | 0.006 | 0.006 | |||||||
| Biomarkers | |||||||||
| NT-proBNP, pg/ml (transformation)† | |||||||||
| Baseline | 12 | 1046.58 | 1064.32 | 15 | 695.07 | 624.02 | |||
| 12 months | 12 | 654.00 | 619.46 | 15 | 618.31 | 491.23 | |||
| Delta | 12 | −392.58 | 953.71 | 15 | -76.76 | 410.48 | -315.80 | −947.50 to 315.80 | 0.199 |
| Within group p value | 0.129 | 0.871 | |||||||
6MWT = 6-min walk test; AIC = anthracycline-induced cardiomyopathy; Allo-MSC = allogeneic mesenchymal stromal cells; LV = left ventricular; LVEF = left ventricular ejection fraction; MLHFQ = Minnesota Living with Heart Failure Questionnaire; NT-proBNP = N-terminal pro-brain natriuretic peptide.
Least-squares mean (LS-mean) difference from the analysis of covariance model. Analysis of covariance adjusted for baseline.
Transformation- arc sine square root transformation for viable mass percent; Log transformation for NT-proBNP; p-values were obtained from transformed data.
Longitudinal strain was assessed across 7 segments each from tagged images of one 4-chamber (basal lateral, midventricular lateral, apical lateral, apex, apical septal, midventricular septal, and basal septal regions) and one 2-chamber view (basal anterior, midventricular anterior, apical anterior, apex, apical inferior, midventricular inferior, and basal inferior segments).
Graphical depictions of the change over time for each of the 13 efficacy measures in the randomized cohort are presented in Supplemental Figure 1. The results of repeated-measures regression modeling are presented in Supplemental Table 5 for the randomized cohort and in Supplemental Table 6 for the total cohort. Only the LV sphericity index showed a significant interaction over time between treatment groups, with a decrease of 0.042 per 6 months in the placebo group compared with the allo-MSC group (p = 0.024), which favored the control group. The MLHFQ score decreased by 14.8 per 6 months in the allo-MSC versus placebo groups, but the treatment effect was not significant (p = 0.140). There was also a significant difference in regional longitudinal strain favoring the allo-MSC group compared with the placebo group (−1.76 per 6 months; p = 0.038). The results for the total cohort were generally consistent with those for the randomized cohort.
Discussion
SENECA is the first clinical trial of cell therapy for patients with AIC. This phase 1 study met its primary objectives (safety and feasibility) by demonstrating that allo-MSCs are well-tolerated, that they could be delivered as planned, and that the outcome measures (particularly CMR endpoints in subjects with cardiac devices) could be collected successfully (Central Illustration). Our exploratory evaluation of efficacy endpoints will be important to design phase 2/3 studies. Taken together, these results provide the necessary groundwork for future larger studies focused on efficacy of cell therapy in AIC patients.
Central Illustration.
Key Elements of the Cardiovascular Cell Therapy Research Network’s SENECA Trial
Key elements highlighted in the Cardiovascular Cell Therapy Research Network’s SENECA trial include the etiology of anthracycline-induced cardiomyopathy, in survivors of diverse cancers, and the notable findings demonstrating safety and feasibility of transendocardial administration allogeneic mesenchymal stromal cells.
As illustrated in Tables 2, 3, and 4, no differences in safety signals were detected between the 2 groups. Consistent with previous studies of allo-MSCs (16, 17, 18), there was no immune reaction to the allogeneic cell product, as shown by repeated PRA analyses, confirming that allo-MSCs can be safely administered without triggering activation of the immune system. Finally, neither examination at follow-up visits nor the patients’ oncologist/follow-up care provider detected any new neoplasms following allo-MSC administration. Taken together, these data indicate that administration of allo-MSCs is safe in patients with AIC, consistent with the safety record observed in other patient populations (16, 17, 18). SENECA also shows that transendocardial injection of allo-MSCs is feasible in patients with AIC.
The demonstration that cardiac CMR can be performed safely and effectively even in patients with devices is an important outcome of this trial. We were able to collect CMR scans in 32 of 34 (94%) participants who attended the 12-month follow-up visit, despite the fact that 20 of these 34 participants (59%) had a cardiac device. CMR scans not obtained were due to cardiac transplantation and a device lead issue. These results demonstrate the effectiveness of the measures that were taken to enable imaging of patients with devices, including use of specific software (QMass version 7.6, Medis Medical Imaging Systems, Inc., Leiden, the Netherlands) (29,30), appropriate positioning of the subject and their device in the magnet, and training of clinical centers in the performance of cardiac CMR in these subjects. CMR often is regarded as the gold standard for assessing LV function and structure (29,31), but its use in HF patients has been limited by the frequent presence of pacemakers or implanted defibrillators (25,26). Our results demonstrating the safety of cardiac CMR in the vast majority of device patients have important implications for the design of future HF trials, not only in the field of cell therapy but in other fields as well.
Previous phase 2 studies have suggested beneficial effects of MSCs on LV function, scar size, functional capacity, and quality of life in patients with ischemic and nonischemic cardiomyopathy (11, 12, 13, 14,16,17). As noted previously, SENECA was not powered for efficacy evaluation; however, a subset of participants (11 to 12 treated and 10 to 15 control) was available for secondary endpoint analysis (Table 5). Given the exploratory nature of the study and the fact that the effects of cell therapy in this population have never been examined, we assessed a broad array of endpoints, including changes in LV volumes, LV function, myocardial structure (e.g., fibrosis), functional capacity (6MWT and MLHFQ), clinical outcomes (MACE and cumulative days alive and out of the hospital), and biomarkers of HF (NT-proBNP). Despite the small group sizes, allo-MSC therapy was associated with a significant improvement in quality of life, as measured by the MLHFQ score, which is consistent with previous studies of MSC therapy in ischemic HF (12,16,17,32). When considering efficacy, it should be noted that allo-MSCs were given as a supplement to maximal tolerated medical therapy.
Of the variables related to efficacy, although the absolute values of some of these effect estimates tended to favor the allo-MSC group, the confidence intervals were wide and the differences were largely not statistically significant (Table 5). The only statistically significant difference was observed for the MLHFQ score, suggesting improved quality of life in allo-MSC-treated patients, as mentioned in the previous text. There was also a borderline significant difference in exercise tolerance measured by the 6MWT, favoring the allo-MSC group. Determining whether these differences reflect a genuine therapeutic effect of allo-MSCs will require larger studies. Importantly, the present results will inform the design of future phase 2 trials, particularly with respect to feasibility, sample size estimations, and endpoint(s). For example, based on the variability observed in SENECA, a total of 54 patients achieved at least 80% power to detect an absolute difference of 5% in LVEF with a significance level of 0.05 and assuming a change from baseline to 12 months of 2.7 ± 6.7 within the placebo and 7.7 ± 6.0 within the active treatment groups.
Study limitations
Because of the nature of the AIC population, SENECA is the first cardiovascular trial of cell therapy with a majority of women. A number of important lessons were learned. The experience gained in SENECA highlights the fact that the AIC patient population is heterogeneous with regard to several variables, including type of cancer, number of cancers, chemotherapeutic regimen, and exposure to radiation, all of which could influence the outcome of a trial. It should also be noted that the interval elapsed since the end of anthracycline treatment was rather long (averaging 13 to 17 years), resulting in long-standing myocardial changes that could hinder the beneficial actions of cell therapy. The long interval since treatment made it difficult to acquire medical records and, in some cases, to determine the exact dose of anthracycline given. To address these issues, in future trials of cell therapy in AIC patients, it may be advantageous to limit enrollment to patients with recent diagnoses and/or stratify subjects according to the time from AIC diagnosis. Finally, the natural history of AIC was found to be better than expected on the basis of the published data, with 1 death and 1 cardiac transplant observed in 36 patients over 12 months of follow-up. This may make it challenging to conduct Phase 2 studies where MACE is the primary endpoint.
Conclusions
This first-in-human trial indicates that transendocardial injection of allo-MSCs is safe and feasible in patients with AIC. Importantly, it demonstrates that cardiac CMR can be effectively used in HF patients despite the high prevalence of devices. Only 1 of the 13 measures of efficacy (i.e., the MLHFQ score) was significantly improved by allo-MSC therapy; however, this phase 1 study was small and not powered or designed to assess efficacy. The results lay an important foundation for designing future, larger phase 2 and 3 trials aimed at assessing efficacy.
Perspectives.
COMPETENCY IN MEDICAL KNOWLEDGE: The SENECA trial provides recent data on the demographics, morbidity, and mortality of AIC. In this first human clinical trial of cell therapy in patients with AIC, transendocardial administration of allo-MSCs was safe and feasible.
TRANSLATIONAL OUTLOOK: As a phase 1 trial, SENECA supports the potential safety and feasibility of allogeneic mesenchymal stromal cell administration in patients with anthracycline-induced cardiomyopathy. It lays the groundwork for larger studies aimed at assessing efficacy.
Author Disclosures
This work is supported by the National Institutes of Health (5 UM1 HL087318). All investigators received funding from the NIH National Heart, Lung, and Blood Institute (NHLBI) for conduct of the SENECA trial through the Cardiovascular Cell Therapy Research Network (CCTRN). Dr. Hare has held stock in Longeveron; has held stock and intellectual property in Vestion; and has a grant with Biologics Delivery Systems. Dr. Henry has served as a consultant for Biosense Webster. Dr. Pepine has served as a consultant for XyloCor, Caladrius, Imbria, and Biocardia; and has grants with Adelphi Values, Brigham and Women’s Hospital, Department of Defense, Gilead Sciences, Inc., McJunkin Foundation, Mesoblast, and Sanofi US. Dr. Perin has served as a consultant for Mesoblast. Dr. Taylor is co-founder of Stem Cell Security. Dr. Yang has served as a consultant for Terumo. Dr. Ebert has served as a staff member of the National Heart, Lung, and Blood Institute, the source of funding for the SENECA trial. The views expressed in this article are those of the authors and do not necessarily represent the views of the NHLBI, National Institutes of Health, or the United States Department of Health and Human Services. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Acknowledgments
The Cardiovascular Cell Therapy Research Network would like to extend its appreciation to the coordinators who made this trial possible: Stephanie Wagner, Nichole Piece, Jennifer Chambers, Nicole Bostick, Sarah Long, Jane Fox, Shari Williams, Heidi Wilson, Cindy Delgado, Lina Caceres, Fouzia Khan, Divya Rajmohan, and Alicia Limbach; as well as Carrie Lenneman, MD, for her invaluable assistance in the development and promotion of the SENECA protocol.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the JACC: CardioOncologyauthor instructions page.
Appendix
For an expanded Methods section as well as supplemental tables and a figure, please see the online version of this paper.
Appendix
References
- 1.Mulrooney D.A., Yeazel M.W., Kawashima T. Cardiac outcomes in a cohort of adult survivors of childhood and adolescent cancer: retrospective analysis of the Childhood Cancer Survivor Study cohort. BMJ. 2009;339:b4606. doi: 10.1136/bmj.b4606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Steinherz L.J., Steinherz P.G., Tan C.T., Heller G., Murphy M.L. Cardiac toxicity 4 to 20 years after completing anthracycline therapy. JAMA. 1991;266:1672–1677. [PubMed] [Google Scholar]
- 3.Cardinale D., Colombo A., Lamantia G. Anthracycline-induced cardiomyopathy: clinical relevance and response to pharmacologic therapy. J Am Coll Cardiol. 2010;55:213–220. doi: 10.1016/j.jacc.2009.03.095. [DOI] [PubMed] [Google Scholar]
- 4.Shan K., Lincoff A.M., Young J.B. Anthracycline-induced cardiotoxicity. Ann Intern Med. 1996;125:47–58. doi: 10.7326/0003-4819-125-1-199607010-00008. [DOI] [PubMed] [Google Scholar]
- 5.Billingham M.E., Mason J.W., Bristow M.R., Daniels J.R. Anthracycline cardiomyopathy monitored by morphologic changes. Cancer Treat Rep. 1978;62:865–872. [PubMed] [Google Scholar]
- 6.Swain S.M., Whaley F.S., Ewer M.S. Congestive heart failure in patients treated with doxorubicin: a retrospective analysis of three trials. Cancer. 2003;97:2869–2879. doi: 10.1002/cncr.11407. [DOI] [PubMed] [Google Scholar]
- 7.Perez E.A. Cardiac toxicity of ErbB2-targeted therapies: what do we know? Clin Breast Cancer. 2008;8(Suppl 3):S114–S120. doi: 10.3816/cbc.2008.s.007. [DOI] [PubMed] [Google Scholar]
- 8.Felker G.M., Thompson R.E., Hare J.M. Underlying causes and long-term survival in patients with initially unexplained cardiomyopathy. N Engl J Med. 2000;342:1077–1084. doi: 10.1056/NEJM200004133421502. [DOI] [PubMed] [Google Scholar]
- 9.Lenneman A.J., Wang L., Wigger M. Heart transplant survival outcomes for adriamycin-dilated cardiomyopathy. Am J Cardiol. 2013;111:609–612. doi: 10.1016/j.amjcard.2012.10.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lipshultz S.E., Lipsitz S.R., Mone S.M. Female sex and drug dose as risk factors for late cardiotoxic effects of doxorubicin therapy for childhood cancer. N Engl J Med. 1995;332:1738–1743. doi: 10.1056/NEJM199506293322602. [DOI] [PubMed] [Google Scholar]
- 11.Sanganalmath S.K., Bolli R. Cell therapy for heart failure: a comprehensive overview of experimental and clinical studies, current challenges, and future directions. Circ Res. 2013;113:810–834. doi: 10.1161/CIRCRESAHA.113.300219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Banerjee M.N., Bolli R., Hare J.M. Clinical Studies of Cell Therapy in Cardiovascular Medicine: Recent Developments and Future Directions. Circ Res. 2018;123:266–287. doi: 10.1161/CIRCRESAHA.118.311217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Karantalis V., Hare J.M. Use of mesenchymal stem cells for therapy of cardiac disease. Circ Res. 2015;116:1413–1430. doi: 10.1161/CIRCRESAHA.116.303614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mathiasen A.B., Qayyum A.A., Jorgensen E. Bone marrow-derived mesenchymal stromal cell treatment in patients with severe ischaemic heart failure: a randomized placebo-controlled trial (MSC-HF trial) Eur Heart J. 2015;36:1744–1753. doi: 10.1093/eurheartj/ehv136. [DOI] [PubMed] [Google Scholar]
- 15.Wysoczynski M., Khan A., Bolli R. new paradigms in cell therapy: repeated dosing, intravenous delivery, immunomodulatory actions, and new cell types. Circ Res. 2018;123:138–158. doi: 10.1161/CIRCRESAHA.118.313251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hare J.M., Fishman J.E., Gerstenblith G. Comparison of allogeneic vs autologous bone marrow-derived mesenchymal stem cells delivered by transendocardial injection in patients with ischemic cardiomyopathy: the POSEIDON Randomized Trial. JAMA. 2012;308:2369–2379. doi: 10.1001/jama.2012.25321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hare J.M., DiFede D.L., Rieger A.C. Randomized comparison of allogeneic versus autologous mesenchymal stem cells for nonischemic dilated cardiomyopathy: POSEIDON-DCM Trial. J Am Coll Cardiol. 2017;69:526–537. doi: 10.1016/j.jacc.2016.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Perin E.C., Borow K.M., Silva G.V. A phase II dose-escalation study of allogeneic mesenchymal precursor cells in patients with ischemic or nonischemic heart failure. Circ Res. 2015;117:576–584. doi: 10.1161/CIRCRESAHA.115.306332. [DOI] [PubMed] [Google Scholar]
- 19.Lenneman C.G., Sawyer D.B. Cardio-oncology: an update on cardiotoxicity of cancer-related treatment. Circ Res. 2016;118:1008–1020. doi: 10.1161/CIRCRESAHA.115.303633. [DOI] [PubMed] [Google Scholar]
- 20.Psaltis P.J., Carbone A., Nelson A.J. Reparative effects of allogeneic mesenchymal precursor cells delivered transendocardially in experimental nonischemic cardiomyopathy. J Am Coll Cardiol Intv. 2010;3:974–983. doi: 10.1016/j.jcin.2010.05.016. [DOI] [PubMed] [Google Scholar]
- 21.Bolli R., Hare J.M., Henry T.D. Rationale and design of the SENECA (StEm cell iNjECtion in cAncer survivors) trial. Am Heart J. 2018;201:54–62. doi: 10.1016/j.ahj.2018.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Klyushnenkova E., Shustova V., Mosca J., Moseley A., McIntosh K. Human mesenchymal stem cells induce unresponsiveness in preactivated but not naive alloantigen specific T cells. Exp Hematol. 1999;27:122. [Google Scholar]
- 23.Klyushnenkova E., Mosca J.D., Zernetkina V. T cell responses to allogeneic human mesenchymal stem cells: immunogenicity, tolerance, and suppression. J Biomed Sci. 2005;12:47–57. doi: 10.1007/s11373-004-8183-7. [DOI] [PubMed] [Google Scholar]
- 24.Le Blanc K., Tammik L., Sundberg B., Haynesworth S.E., Ringden O. Mesenchymal stem cells inhibit and stimulate mixed lymphocyte cultures and mitogenic responses independently of the major histocompatibility complex. Scand J Immunol. 2003;57:11–20. doi: 10.1046/j.1365-3083.2003.01176.x. [DOI] [PubMed] [Google Scholar]
- 25.Levine G.N., Gomes A.S., Arai A.E. Safety of magnetic resonance imaging in patients with cardiovascular devices: an American Heart Association scientific statement from the Committee on Diagnostic and Interventional Cardiac Catheterization, Council on Clinical Cardiology, and the Council on Cardiovascular Radiology and Intervention: endorsed by the American College of Cardiology Foundation, the North American Society for Cardiac Imaging, and the Society for Cardiovascular Magnetic Resonance. Circulation. 2007;116:2878–2891. doi: 10.1161/CIRCULATIONAHA.107.187256. [DOI] [PubMed] [Google Scholar]
- 26.Junttila M.J., Fishman J.E., Lopera G.A. Safety of serial CMR in patients with implantable cardioverter defibrillators. Heart. 2011;97:1852–1856. doi: 10.1136/heartjnl-2011-300153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hicks K.A., Tcheng J.E., Bozkurt B. 2014 ACC/AHA key data elements and definitions for cardiovascular endpoint events in clinical trials: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Data Standards (Writing Committee to Develop Cardiovascular Endpoints Data Standards) J Am Coll Cardiol. 2015;66:403–469. doi: 10.1016/j.jacc.2014.12.018. [DOI] [PubMed] [Google Scholar]
- 28.Hicks K.A., Mahaffey K.W., Mehran R. 2017 cardiovascular and stroke endpoint definitions for clinical trials. J Am Coll Cardiol. 2018;71:1021–1034. doi: 10.1016/j.jacc.2017.12.048. [DOI] [PubMed] [Google Scholar]
- 29.Schulz-Menger J., Bluemke D.A., Bremerich J. Standardized image interpretation and post processing in cardiovascular magnetic resonance: Society for Cardiovascular Magnetic Resonance (SCMR) Board of Trustees Task Force on Standardized Post Processing. J Cardiovasc Magn Reson. 2013;15:35. doi: 10.1186/1532-429X-15-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rizzi PB, Nacif, Marcelo, Volpe GJ, et al. Quantification of myocardial scar assessed by late gadolinium enhancement CMR in the multi-ethnics study of atherosclerosis: comparisons of 7 different methods. J Cardiovasc Magn Reson 2013;15 Suppl 1:O49.
- 31.Grothues F., Smith G.C., Moon J.C. Comparison of interstudy reproducibility of cardiovascular magnetic resonance with two-dimensional echocardiography in normal subjects and in patients with heart failure or left ventricular hypertrophy. Am J Cardiol. 2002;90:29–34. doi: 10.1016/s0002-9149(02)02381-0. [DOI] [PubMed] [Google Scholar]
- 32.Florea V., Rieger A.C., Natsumeda M. The impact of patient sex on the response to intramyocardial mesenchymal stem cell administration in patients with non-ischemic dilated cardiomyopathy. Cardiovasc Res. 2020;116:2131–2141. doi: 10.1093/cvr/cvaa004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.