Abstract
Introduction
Atrial fibrillation (AF) is the most common form of cardiac arrhythmia and is associated with a number of comorbidities such as coronary artery disease and heart failure. While physical activity is already implemented in current international guidelines for the prevention and treatment of AF, the precise role of different types of exercise in the management of AF remains to be elucidated. The primary aim of the Cologne ExAfib Trial is to assess the feasibility and safety of different exercise modes in patients diagnosed with paroxysmal AF. Secondary outcomes include assessments of physical function, AF burden, quality of life and inflammation, as well as morphological and cardiac adaptations.
Methods and analysis
The study opened for recruitment in September 2019. In the initial pilot phase of this four-armed randomised controlled trial, we aim to enrol 60 patients between 60 years and 80 years of age with paroxysmal AF. After screening and pretesting, patients are randomised into one of the following groups: high-intensity interval training (4×4 min at 75%–85% peak power output (PPO)), moderate-intensity continuous training (25 min at 55%–65% PPO), strength training (whole body, 3 sets of 6–12 repetitions at 70%–90% one repetition maximum [1RM]) or a usual-care control group. Training is performed two times per week for 12 weeks. If the feasibility and safety can be confirmed through the initial pilot phase, the recruitment will be continued and powered for a clinical endpoint.
Feasibility and safety are assessed by measures of recruitment and completion, programme tolerance and adherence as well as reported adverse events, including hospitalisation rates. Secondary endpoints are assessed by measures of peak oxygen consumption and the 1RM of selected muscle groups, questionnaires concerning quality of life and AF burden, serum blood samples for the analysis of C reactive protein, interleukin-6, tumour necrosis factor alpha and N-terminal pro-brain natriuretic peptide concentrations and ultrasound for muscle and heart morphology as well as cardiac function.
Ethics and dissemination
Ethics approval was obtained from the ethics committee of the German Sport University Cologne (No.: 175/2018). All procedures performed in studies involving human participants are in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards. Manuscripts will be written based on international authorship guidelines. No professional writers will be commissioned for manuscript drafting. The findings of this study will be published in peer-reviewed journals and presented at leading exercise and medicine conferences
Trial registration number
The study is registered both at the German and at the WHO trial registers (DRKS00016637); Pre-results.
Keywords: adult cardiology, medical education & training, rehabilitation medicine, sports medicine, echocardiography, atrial fibrillation
Strengths and limitations of this study.
First clinical trial to investigate the effects of high-intensity interval training, moderate-intensity continuous training and strength training in patients with paroxysmal atrial fibrillation.
Due to the single centre design, we do not expect major protocol deviations.
An initial pilot phase allows for refining of the clinical endpoint and/or study arms after safety and feasibility assessment.
Due to the nature of the disease, detection of the disease burden may be limited by a 48-hour Holter ECG recording.
Selection bias is unavoidable in this population, since individuals who are more prone to exercise are more likely to participate in the study.
Introduction
Atrial fibrillation (AF) is the most common form of cardiac arrhythmia, with an estimated European prevalence of ~2%.1 Although this may be significantly lower compared to other cardiovascular diseases such as arterial hypertension, the prevalence of AF is age dependent, reaching highly significant numbers among seniors. Thus, AF has been reported to affect ~8% of adults above 65 years of age, reaching levels of >15% in age groups of over 80 years.1 Due to the demographic transition towards an inverted age pyramid, by 2060 the number of patients will double among the >65-year-old adults and will even triple among the >80-year-old adults, producing ~25 million seniors with AF in the European Union.1
Based on the disease progression, AF may be classified into paroxysmal (ie, self-terminating or cardioverted within 7 days), persistent (ie, AF persisting for >7 days), long-standing persistent (ie, persisting for >1 year) or permanent (ie, arrhythmia is accepted and restoration of sinus rhythm is no longer pursued).2 Symptoms may include dyspnoea, palpitations and angina pectoris, all of which can dramatically decrease overall quality of life (QoL). Moreover, AF is often accompanied by severe comorbidities, such as coronary artery disease, stroke and heart failure, making it a leading cause for hospitalisation.3–6 Thus, the treatment depends on both the severity of symptoms as well as the diagnosed comorbidities and may include stroke prevention via oral anticoagulation, medical rate control and rhythm control via medication, cardioversion or ablation therapy.7 However, despite recent advances in medical and interventional care, the socioeconomic burden remains immense and is expected to increase even further with the demographic change in society.
In addition to conventional medical treatment, lifestyle interventions have recently gained scientific interest. These mainly include the management of risk factors, such as overweight, hypertension and hyperglycaemia.8 However, studies have shown that a sedentary lifestyle significantly increases the risk for AF, while already moderate levels of physical activity appear to be a promising countermeasure.9 Surprisingly, studies specifically assessing the effects of distinct exercise interventions with the aim of improving physical fitness and QoL, while at the same time reducing AF burden, are scarce.10–12
In a previous large-scale randomised controlled trial (RCT), Risom et al showed an increase in aerobic capacity following 12 weeks of combined aerobic and strength training (STR) but effects on AF burden or hospitalisation were not investigated.13 In addition, in a study by Malmo et al, a significant reduction in AF burden and concomitant increase in peak oxygen consumption (VO2peak) and QoL were observed following 12 weeks of aerobic high-intensity interval training (HIIT).14 However, in subsequent meta-analyses, it was concluded that the number of high-quality RCTs is insufficient to adequately deduce the effects of exercise-based interventions in the treatment of AF.11 12 This concern was also confirmed in a recent systematic review, including the safety aspects of physical training in AF patients, irrespective of whether patients were classified as paroxysmal, persistent or permanent.15
While physical activity is already reflected in current international guidelines for the prevention and treatment of AF,7 16 specific recommendations concerning different exercise modes (ie, aerobic vs resistance training) and intensities (low-intensity continuous vs HIIT) are lacking. The Cologne ExAfib Trial is a phase I clinical trial that primarily aims at systematically assessing the effects of distinct exercise modes on the feasibility and safety of patients diagnosed with paroxysmal AF. Secondary outcomes include assessments of the physical function, AF burden, QoL, inflammation, muscle morphology as well as cardiac function. The outcomes of this trial will be used to further improve clinical knowledge pertaining to exercise prescription for AF patients. In addition, findings of this study will also be used to design phase II trials, which will help to establish guidelines for exercise prescription.
Materials and methods
The current study is carried out in accordance with the Declaration of Helsinki and received ethical approval by the local ethics committee (175/2018, German Sports University Cologne, Germany). All participants are comprehensively informed about the study procedures and are requested to provide a signed informed consent prior to participation by the authorised study personnel. Personal information about potential and enrolled participants will be collected, shared and maintained, according to European regulations in order to protect confidentiality. The presented protocol is version 2.0. Protocol modifications, if any, will be entered into the trial registry.
Study design
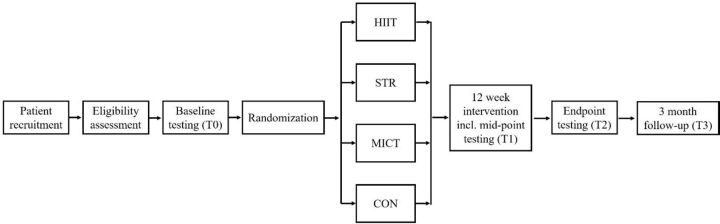
The Cologne ExAfib Trial is a prospective four-armed, randomised controlled single-centre trial (figure 1). The study opened for recruitment in September 2019 and is still ongoing. Testing and interventions take place at the German Sport University Cologne, Germany. The study coordinator is responsible for data handling and storing and is solely able to access the final data set.
Figure 1.
Timeline. CON, Usual care; HIIT, High-intensity interval training; MICT, moderate-intensity continuous training; STR, Strength training.
Following recruitment and pre-screening, patients are randomised into one of the four groups: moderate-intensity continuous training (MICT), HIIT, STR or usual care (CON). After screening, patients that are randomised to the exercise groups perform 12 weeks of supervised exercise training. Patients that are randomised to the usual care group are asked to continue with daily habitual activities but are also offered to participate in supervised exercise training after completion of the study. All patients are followed-up at 3 months after the completion of the intervention period.
Patient recruitment
Patients are recruited by invitation of their treating practitioner. Local hospitals and practitioners provide eligible patients with written information on the study procedures and refer these patients to the study coordinator. The inclusion and exclusion criteria are presented in table 1. For detailed information, see online supplemental appendix 1.
Table 1.
Inclusion and exclusion criteria
| Inclusion criteria | Exclusion criteria |
| Women and men with symptomatic paroxysmal atrial fibrillation (EHRA ≥2) | Participation in regular aerobic or resistance exercise training in the last 6 months (>60 min/week) or former high-performance athletes |
| Age of 60–80 years | Left ventricular ejection fraction <40% during sinus rhythm |
| BMI ≤35 kg/m2 | Significant valve disease |
| Implanted cardiac pacemaker, ICD or resynchronisation therapy | |
| Coronary artery disease with insufficient revascularisation or unstable angina pectoris | |
| Uncontrolled limiting comorbidities (hypertension, diabetes mellitus, hyperthyroidism, etc) | |
| Prior pulmonary vein ablation | |
| Any contraindication to strenuous exercise or testing |
BMI, body mass index; EHRA, European heart rhythm association; ICD, implantable cardioverter defibrillator.
bmjopen-2020-040054supp001.pdf (208.6KB, pdf)
Patient and public involvement
Patients and the public were not involved in any way.
Sample size calculation
Due to the novelty of this phase I clinical trial with the primary endpoint of safety and feasibility, the sample size was assessed based on previous studies investigating the effects of aerobic13 or combined aerobic and STR14 on secondary outcomes, such as physical function. The sample size calculation was performed by G×power (V.3.1.9.2, Heinrich Heine University, Dusseldorf, Germany). For an effect size of 0.5, with a power (1-β) of 0.8 and an alpha level of 0.05 (two-tailed), a total of at least 40 patients would be needed. Since attrition is not known in this population and data on safety and feasibility are lacking for some of the exercise interventions used, we will initially include 60 patients (15 per group). Based on the initial findings, recruitment will then continue with distinct exercise regimens and will be powered based on a clinical endpoint.
Screening and randomisation
This trial spans over a total duration of 14 weeks (including one week for baseline and endpoint testing, respectively) and a 3-month follow-up. After pre-screening and baseline testing, the patients are randomly allocated in a ratio of 1:1:1:1 into the four study arms. Randomisation is performed by a research officer with no patient contact using a stratified minimisation approach by Randomisation In Treatment Arms (RITA Evident, Germany). The stratification factors include patients’ (1) age, (2) gender and (3) BMI. Study investigators and exercise physiologists conducting testing procedures are blinded to group allocation.
Interventions
Patients randomised into one of the training groups perform supervised training two times per week over a total duration of 12 weeks. All training sessions are electronically recorded (heart rate, workload, sets and repetitions). In addition, a one-lead ECG is used in order to continuously monitor cardiac function throughout each training session by means of beat-to-beat recordings with an amplitude frequency of 0.05–125 Hz. Habitual physical activity is assessed objectively in all groups (including CON) by a wrist-worn activity tracker (ActiGraph GT9X, ActiGraph, LLC, Pensacola, Florida, USA). Assessment of habitual physical activity takes place over 7 days at the following timepoints: baseline, after 6 weeks (mid-point testing), after 12 weeks (endpoint testing) as well as at the 3-month follow-up.
All training sessions are time matched (ie, 40 minutes). Training sessions include a standardised warm up (5-minute cycling at 30% of peak power output (PPO)) and cool down (10-minute cycling at 30% of PPO). Each training session is concluded by an additional 10-minute recovery period, including a standardised stretching routine. Perceived exhaustion, pain and dyspnoea are assessed by visual analogue scales ranging from 0 (no symptoms) to 10 (severe symptoms), prior to as well as immediately after each training session.
MICT
Training is performed on a stationary cycle ergometer (Ergoline ergoselect, Bitz, Germany) at a target exercise intensity of 55%–65% of PPO for 25 minutes. Initially, the training intensity commences at 55% of PPO and is progressively increased by 5% every 4 weeks.
HIIT
Training is performed on a stationary cycle ergometer (Ergoline ergoselect, Bitz, Germany) and consists of 4×4 min at 75%–85% of PPO, separated by a 3-minute active rest at 30% of PPO. Initially, the intensity of high-intensity bouts commences at 75% PPO and progressively increases by 5% every 4 weeks.
STR
Training consists of the following upper and lower body exercises, using pneumatic devices (HUR Healthfitness Equipment, Kokkola, Finland): leg press, knee extension, hamstring curls, seated chest press, seated row, lat pull down and shoulder press. Exercise loads are determined individually as percentage of the one-repetition maximum (1RM). The exercises are performed as a circuit with a 2-minute rest between sets. Every session includes leg press, seated row and chest press, while shoulder press or lat pull down as well as knee extension or hamstring curls are performed on alternating days.
The STR intensity is progressively increasing every 2 weeks until week 8 as follows: 3 sets of 12 repetitions at 70% 1RM, 3 sets of 12 repetitions at 75% 1RM, 3 sets of 10 repetitions at 80% 1RM and 3 sets of 10 repetitions at 85% 1RM. The sessions of the remaining 4 weeks consist of 3 sets of 6 repetitions at 90% 1RM.
Measurements
All primary and secondary endpoints are assessed at baseline (T0) as well as at endpoint testing (T2) and the 3-month follow-up (figure 1 and table 2). Venous blood samples are additionally drawn at midpoint testing (T1). Additional measures of feasibility and safety are assessed throughout the study period (table 3).
Table 2.
Measurements
| T0 (baseline testing) | T1 (midpoint testing, after 6 weeks) | T2 (endpoint testing) | T3 (3-month follow-up) |
| Venous blood sampling | Venous blood sampling | Idem T0 |
Idem T0 |
| Bioimpedance analysis |
|
||
| Echocardiography | |||
| Cardiopulmonary exercise test | |||
| Maximal strength test | |||
| Resting ECG (48 hours) | |||
| Resting blood pressure and arteriography | |||
| Panoramic ultrasound of vastus lateralis and rectus femoris | |||
| Questionnaires (SF-36, IBL-VF, PSQI) |
IBL-VF, Questionnaire on the burden and quality of life in patients with atrial fibrilation; PSQI, Pittsburgh Sleep Quality Index; SF-36, 36-item Short Form Health Survey.
Table 3.
Assessment of safety and feasibility.
| Measures | Time of collection |
| Feasibility—recruitment and completion | |
| Referred patients | Trial completion |
| Eligible patients | Trial completion |
| Enrolled patients | Trial completion |
| Trial completion | Trial completion |
| Patient withdrawals | Trial completion |
| Patient dropouts | Trial completion |
| Feasibility—programme tolerance | |
| Pre-sessional and post-sessional exhaustion, pain and dyspnoea | At each exercise session |
| ECG recordings | At each exercise session |
| Feasibility—programme adherence | |
| Number of completed sessions | Trial completion |
| Time/sets completed in each session | At each exercise session |
| Patient safety | |
| Number of adverse events | Once weekly and 3-month follow-up |
| Number of severe adverse events | Once weekly and 3-month follow-up |
| Hospital admissions/days of hospitalisation | Once weekly and 3-months follow-up |
Primary endpoints
Feasibility of the exercise interventions will be quantified by measures of recruitment and completion, programme tolerance and programme adherence (table 3). In order to calculate recruitment rates, the number of referrals will be compared with the number of patients who are deemed eligible after pre-screening. In addition, eligibility rates will be determined by the ratio of eligible patients and those actually enrolled in the trial. Programme tolerance will be assessed by measures of perceived exhaustion, pain and dyspnoea as well as continuous ECG recordings throughout each training session. The number of completed training sessions as well as the time and/or sets completed in each training session will determine programme adherence.
Patient safety will be assessed by means of recorded adverse and severe adverse events as well as hospitalisation rates (table 3, modified from Hart et al17). Adverse events and severe adverse events will be graded as adapted from the NCI Common Terminology Criteria for Adverse Events V.5.0, including also events related to exercise training (such as muscular pain and fatigue).
Secondary endpoints
Physical function
Physical function will be determined by measures of aerobic capacity and maximal strength. Aerobic capacity defined as VO2peak will be determined by a cardiopulmonary exercise test on a cycle ergometer (Ergoline ergoselect, Bitz, Germany). In line with the guidelines provided by the WHO, the test commences at a load of 25 W and is increased by 25 W every 2 minutes until voluntary exhaustion. Patients are requested to maintain a pedalling frequency of 70±5 revolutions per minute. The test is supervised by a cardiologist and ECG is recorded consistently. Early termination due to acute clinical contraindications are subject to further investigation. In addition, capillary blood samples are collected from the earlobe at the end of each stage for the determination of blood lactate concentrations. Breathing gases, heart rate and subjective perceived exertion are monitored throughout the test.
Maximal strength is assessed by the 1RM for the following muscles, using pneumatic devices (HUR Health fitness Equipment, Kokkola, Finland): leg press, seated chest press and seated row.
AF burden and quality of life
The AF burden is assessed by means of ECG recordings at rest and during exercise as well as by questionnaires. Specifically, the total number and overall duration of AF episodes recorded by a 3-lead resting ECG for 48 hours (Holter ECG, Amedtec, Aue, Germany) as well as in the ECG obtained during each exercise and the postexercise recovery period (Ergoline ergoselect, Bitz, Germany) are assessed. In addition, patients are requested to complete the QoL in patients with atrial fibrillation (IBL-VF) questionnaire. Furthermore, the 36-item Short Form Health Survey (SF-36) is used to assess overall QoL, while quality of sleep is assessed by the Pittsburgh Sleep Quality Index.
Inflammatory profile
The following parameters will be analysed from venous blood, as surrogate measures of inflammation: C reactive protein (CRP), interleukin-6 (IL-6), tumour necrosis factor alpha (TNF-α) and collagen turnover markers, such as matrix metalloproteinases 1, 2 and 9, carboxy-terminal telopeptide of collagen type I and propeptide of procollagen type I.
Muscle morphology
Anatomical cross-sectional area of the vastus lateralis and rectus femoris muscles is assessed by the extended field of view mode, using a Vivid iq ultrasound system equipped with a 5-cm 3–9 MHz linear-array probe (Vivid iq, GE Healthcare Systems, USA, 2018).
Cardiovascular morphology and function
Echocardiography is carried out by a Vivid iq with a M5Sc 1.5–4.6 transducer (GE Healthcare Systems, Boston, MA, USA, 2018), with the participant in a semi-supine resting position. Conventional echocardiography includes standard measurements of cardiac dimensions, contractility and diastolic function. Speckle tracking images for calculation of myocardial strain are recorded in apical 3-chamber, 2-chamber and 4-chamber views for longitudinal values and in the parasternal short axis at the level of papillary muscles for circumferential and radial values. The analysis of all images will be performed using the EchoPac Software V.203 (GE Healthcare, Boston, MA, USA). In addition, 48-hour ECG recordings are performed (Holter ECG, Amedtec, Aue, Germany), in order to assess heart rate, number of supraventricular and ventricular extrasystoles and heart rate variability (starting immediately after the testing at T0, T2 and T3). Further measures include systolic and diastolic blood pressures and arterial stiffness (Mobil-O-Graph, IEM, Stolberg, Germany) as well as the serum levels of N-terminal pro-brain natriuretic peptide.
Data analysis
Data are collected digitally and will be cross-checked for accuracy. Within-group and between-group analyses will be performed in order to investigate basal adaptations induced by the training interventions as well as the 3-month follow-up. Data will be checked for normality and log transformed prior to applying parametric statistics. All statistics will be performed using an intention to treat approach. Missing data will be accounted for by multiple imputation. Statistical analysis will include standard descriptive statistics and two-way (group×time) repeated measures analysis of variance (adjusted for baseline, if necessary). Categorical variables will be assessed by χ2 tests. Effect sizes will be calculated as partial eta squared. All included patients will be invited to an informative meeting on the study results following completion of the study.
Discussion
Exercise interventions have previously been shown to be safe and effective for patients diagnosed with a variety of cardiovascular diseases, such as coronary heart disease, cerebral apoplexy, hypertension, heart failure and intermittent claudication.18 Interestingly, the evidence for exercise interventions in the treatment of AF is scarce. To the best of our knowledge, previously only three RCTs have assessed the effects of exercise interventions in patients with paroxysmal AF, mainly focusing on physical capacity (e.g. VO2peak) and AF burden.13 14 19 However, feasibility as defined by recruitment and completion rates, programme tolerance and programme adherence was not thoroughly assessed in these studies. Moreover, the existing data are limited to aerobic training programs or combined aerobic and STR regimens, not allowing to directly assess and compare the feasibility and safety but also the efficacy of distinct types of exercise.
The importance of this phase I clinical trial is further underlined by recent investigations that have provided evidence for an increased prevalence of AF in athletes, leaving it unclear as to whether regular strenuous exercise may actually be adverse for AF patients.20–22 This may possibly depend on the overall dose of exercise performed. On the one hand, a lifetime training volume at high-intensity levels of >2000 hours was previously associated with an increase in the incidence of AF.23 Regular high-intensity exercise at a recreational or preventive volume (<2000 hours per lifetime), on the other hand, was strongly associated with a reduced AF risk, as well as with a reduction in cardiovascular, metabolic and neoplastic disease and overall mortality.24 Validating this observational data in a randomised and controlled setting is among the primary aims of this trial.
In addition to feasibility and safety, the efficacy of distinct types of exercise interventions will be assessed in this study. We do expect significant adaptations in physical function, such as VO2peak and maximal strength. VO2peak as a marker of cardiorespiratory fitness (CRF) is a well-established predictor of mortality in healthy adults,25 as well as in cardiovascular patients, including those diagnosed with heart failure26 27 and coronary heart disease.28 29 In fact, reduced exercise tolerance has also been associated with increased mortality in patients with AF.30 Importantly, previous large-scale prospective longitudinal cohort studies observed changes in CRF concomitantly with mortality outcomes, suggesting that improving VO2peak through exercise may well reduce mortality.31 In addition, in healthy elderly men (>65 years of age), muscle strength appears to be directly related to all-cause mortality,32 even irrespective of muscle mass.33 We hypothesise that also in AF patients the magnitude of the changes in VO2peak and maximal strength will be dependent on the training mode. Thus, major improvements in cardiorespiratory function are expected in the aerobic training groups, while neuromuscular function is expected to increase to a larger extent in the STR group.
In this trial, we will also be able to document arrhythmia occurrences during exercise. Furthermore, we have included questionnaires that will allow us to determine possible effects of the exercise interventions on AF symptoms and frequencies. However, while we also include 48-hour Holter ECG recordings, it has to be acknowledged that the chances to detect objective changes in AF burden of patients with paroxysmal AF are slim. This is especially true in light of a previous study reporting an absolute reduction of AF duration following an aerobic HIIT intervention of ~3% (from 8% to 5%) using implanted loop recorders.14 However, after assessing safety and feasibility in the initial phase of this study, AF burden may well be a relevant primary endpoint of the second phase of this trial. For this purpose, implantable loop recording or constant rhythm assessment via wearables is currently being considered.
One of the strengths of the current trial is the in depth mechanistic analysis of potential cardiac factors underlying changes in AF burden and QoL. Thus, echocardiographic data, including atrial strain analysis, may reveal mechanistic components of beneficial effects of exercise. While defining ‘the cause’ of AF seems problematic, a host of evidence revealed etiological factors (ie, hypertension, heart failure, diabetes mellitus, etc) as well as structural aspects involved in the development of AF.7 Thus, inflammatory processes associated with fibrotic restructuring as well as pathological changes in contractility and size of the left atrium seem to play key roles in atrial remodelling and the development of AF.34–42 Exercise-induced reductions in inflammation and fibrosis may lead to therapeutic reverse remodelling.43–46 The data of the present trial may well document improved systemic inflammation (ie, reductions in serum levels of CRP, IL-6 and TNF-α), potentially associated with measurable signs of atrial reverse remodelling on a structural (ie, reduced fibrotic restructuring) and functional (ie, improved atrial diastolic and systolic function) level.
The improvement of cardiac function may also be a key component in potentially beneficial effects of distinct exercise interventions for patients with AF. While the mechanisms of aerobic exercise leading to volume-dependent, pre-load associated cardiac adaptations are well-understood and are consistent throughout previous studies,47 the cardiac processes induced by STR remain unclear.48 The initial hypothesis that the increased vascular resistance and associated after-load elevation observed in strength-trained athletes will lead to concentric hypertrophy as seen in hypertensive patients was not confirmed consistently in previous trials.49 Contrarily, STR may actually reduce systolic and diastolic blood pressures in hypertensive patients.48 However, the available literature is scarce and an in-depth analysis of underlying cardiac processes and mechanisms is often lacking. Hypothetically, the intermittent pressure elevation in the cardiovascular system observed during STR may actually induce similar structural cardiac benefits as intermittent increases of cardiac output in aerobic exercise. However, this remains conjecture due to the paucity of studies analysing the underlying mechanisms of STR on the cardiovascular system.
Ultimately, the present study aims to be the first RCT to underline the feasibility and safety of distinct exercise modes, including highly strenuous aerobic and strength exercise, while at the same time analysing functional and mechanistic intervention effects with the goal of conducting follow-up studies powered for clinically relevant end-points.
Ethics and dissemination
Ethics approval was obtained from the ethics committee of the German Sports University Cologne (No.: 175/2018). All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards. Manuscripts will be written based on international authorship guidelines. No professional writers will be commissioned for manuscript drafting. The findings of this study will be published in peer-reviewed journals and presented at leading exercise and medicine conferences.
Supplementary Material
Footnotes
JZ and KD contributed equally.
H-GP and MS contributed equally.
Contributors: JZ, KD, NF, TK, BB, WB, H-GP and MS contributed to planning, conduct and reporting of the study, and were involved in data analysis and interpretation. JZ, KD, NF, TK, BB and MS conceptualised and designed the study. JZ, KD, NF and MS have drafted the manuscript. TK, BB, WB and H-GP have edited the manuscript. All authors have approved the final version of the manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent for publication: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
References
- 1.Di Carlo A, Bellino L, Consoli D, et al. Prevalence of atrial fibrillation in the Italian elderly population and projections from 2020 to 2060 for Italy and the European Union: the FAI project. Europace 2019;21:1468–75. 10.1093/europace/euz141 [DOI] [PubMed] [Google Scholar]
- 2.Chiang C-E, Naditch-Brûlé L, Murin J, et al. Distribution and risk profile of paroxysmal, persistent, and permanent atrial fibrillation in routine clinical practice: insight from the real-life global survey evaluating patients with atrial fibrillation international registry. Circ Arrhythm Electrophysiol 2012;5:632–9. 10.1161/CIRCEP.112.970749 [DOI] [PubMed] [Google Scholar]
- 3.Oldgren J, Healey JS, Ezekowitz M, et al. Variations in cause and management of atrial fibrillation in a prospective registry of 15,400 emergency department patients in 46 countries: the RE-LY atrial fibrillation registry. Circulation 2014;129:1568–76. 10.1161/CIRCULATIONAHA.113.005451 [DOI] [PubMed] [Google Scholar]
- 4.Kannel WB, Wolf PA, Benjamin EJ, et al. Prevalence, incidence, prognosis, and predisposing conditions for atrial fibrillation: population-based estimates. Am J Cardiol 1998;82:2N–9. 10.1016/S0002-9149(98)00583-9 [DOI] [PubMed] [Google Scholar]
- 5.Ball J, Carrington MJ, McMurray JJV, et al. Atrial fibrillation: profile and burden of an evolving epidemic in the 21st century. Int J Cardiol 2013;167:1807–24. 10.1016/j.ijcard.2012.12.093 [DOI] [PubMed] [Google Scholar]
- 6.Zoni-Berisso M, Lercari F, Carazza T, et al. Epidemiology of atrial fibrillation: European perspective. Clin Epidemiol 2014;6:213–20. 10.2147/CLEP.S47385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kirchhof P, Benussi S, Kotecha D, et al. 2016 ESC guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur J Cardiothorac Surg 2016;50:e1–88. 10.1093/ejcts/ezw313 [DOI] [PubMed] [Google Scholar]
- 8.Pathak RK, Mahajan R, Lau DH, et al. The implications of obesity for cardiac arrhythmia mechanisms and management. Can J Cardiol 2015;31:203–10. 10.1016/j.cjca.2014.10.027 [DOI] [PubMed] [Google Scholar]
- 9.Mohanty S, Mohanty P, Tamaki M, et al. Differential association of exercise intensity with risk of atrial fibrillation in men and women: evidence from a meta-analysis. J Cardiovasc Electrophysiol 2016;27:1021–9. 10.1111/jce.13023 [DOI] [PubMed] [Google Scholar]
- 10.Plisiene J, Blumberg A, Haager G, et al. Moderate physical exercise: a simplified approach for ventricular rate control in older patients with atrial fibrillation. Clin Res Cardiol 2008;97:820–6. 10.1007/s00392-008-0692-3 [DOI] [PubMed] [Google Scholar]
- 11.Reed JL, Terada T, Chirico D, et al. The effects of cardiac rehabilitation in patients with atrial fibrillation: a systematic review. Can J Cardiol 2018;34:S284–95. 10.1016/j.cjca.2018.07.014 [DOI] [PubMed] [Google Scholar]
- 12.Risom SS, Zwisler A-D, Johansen PP, et al. Exercise-based cardiac rehabilitation for adults with atrial fibrillation. Cochrane Database Syst Rev 2017;2:CD011197. 10.1002/14651858.CD011197.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Risom SS, Zwisler A-D, Rasmussen TB, et al. Cardiac rehabilitation versus usual care for patients treated with catheter ablation for atrial fibrillation: Results of the randomized CopenHeartRFA trial. Am Heart J 2016;181:120–9. 10.1016/j.ahj.2016.08.013 [DOI] [PubMed] [Google Scholar]
- 14.Malmo V, Nes BM, Amundsen BH, et al. Aerobic interval training reduces the burden of atrial fibrillation in the short term: a randomized trial. Circulation 2016;133:466–73. 10.1161/CIRCULATIONAHA.115.018220 [DOI] [PubMed] [Google Scholar]
- 15.Myrstad M, Malmo V, Ulimoen SR, et al. Exercise in individuals with atrial fibrillation. Clin Res Cardiol 2019;108:347–54. 10.1007/s00392-018-1361-9 [DOI] [PubMed] [Google Scholar]
- 16.Gorenek B, Pelliccia A, Benjamin EJ, et al. European heart rhythm association (EHRA)/European association of cardiovascular prevention and rehabilitation (EACPR) position paper on how to prevent atrial fibrillation endorsed by the heart rhythm society (HRS) and Asia pacific heart rhythm society (APHRS). Eur J Prev Cardiol 2017;24:4–40. 10.1177/2047487316676037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hart NH, Galvão DA, Saunders C, et al. Mechanical suppression of osteolytic bone metastases in advanced breast cancer patients: a randomised controlled study protocol evaluating safety, feasibility and preliminary efficacy of exercise as a targeted medicine. Trials 2018;19:695. 10.1186/s13063-018-3091-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pedersen BK, Saltin B. Exercise as medicine - evidence for prescribing exercise as therapy in 26 different chronic diseases. Scand J Med Sci Sports 2015;25:1–72. 10.1111/sms.12581 [DOI] [PubMed] [Google Scholar]
- 19.Skielboe AK, Bandholm TQ, Hakmann S, et al. Cardiovascular exercise and burden of arrhythmia in patients with atrial fibrillation - A randomized controlled trial. PLoS One 2017;12:e0170060. 10.1371/journal.pone.0170060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Myrstad M, Nystad W, Graff-Iversen S, et al. Effect of years of endurance exercise on risk of atrial fibrillation and atrial flutter. Am J Cardiol 2014;114:1229–33. 10.1016/j.amjcard.2014.07.047 [DOI] [PubMed] [Google Scholar]
- 21.Myrstad M, Aarønæs M, Graff-Iversen S, et al. Does endurance exercise cause atrial fibrillation in women? Int J Cardiol 2015;184:431–2. 10.1016/j.ijcard.2015.03.018 [DOI] [PubMed] [Google Scholar]
- 22.Myrstad M, Løchen M-L, Graff-Iversen S, et al. Increased risk of atrial fibrillation among elderly Norwegian men with a history of long-term endurance sport practice. Scand J Med Sci Sports 2014;24:e238–44. 10.1111/sms.12150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Calvo N, Ramos P, Montserrat S, et al. Emerging risk factors and the dose-response relationship between physical activity and lone atrial fibrillation: a prospective case-control study. Europace 2016;18:57–63. 10.1093/europace/euv216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wen CP, Wai JPM, Tsai MK, et al. Minimum amount of physical activity for reduced mortality and extended life expectancy: a prospective cohort study. Lancet 2011;378:1244–53. 10.1016/S0140-6736(11)60749-6 [DOI] [PubMed] [Google Scholar]
- 25.Kodama S, Saito K, Tanaka S, et al. Cardiorespiratory fitness as a quantitative predictor of all-cause mortality and cardiovascular events in healthy men and women: a meta-analysis. JAMA 2009;301:2024–35. 10.1001/jama.2009.681 [DOI] [PubMed] [Google Scholar]
- 26.O'Neill JO, Young JB, Pothier CE, et al. Peak oxygen consumption as a predictor of death in patients with heart failure receiving beta-blockers. Circulation 2005;111:2313–8. 10.1161/01.CIR.0000164270.72123.18 [DOI] [PubMed] [Google Scholar]
- 27.Reibis RK, Treszl A, Wegscheider K, et al. Exercise capacity is the most powerful predictor of 2-year mortality in patients with left ventricular systolic dysfunction. Herz 2010;35:104–10. 10.1007/s00059-010-3226-5 [DOI] [PubMed] [Google Scholar]
- 28.Keteyian SJ, Brawner CA, Savage PD, et al. Peak aerobic capacity predicts prognosis in patients with coronary heart disease. Am Heart J 2008;156:292–300. 10.1016/j.ahj.2008.03.017 [DOI] [PubMed] [Google Scholar]
- 29.Myers J, Prakash M, Froelicher V, et al. Exercise capacity and mortality among men referred for exercise testing. N Engl J Med 2002;346:793–801. 10.1056/NEJMoa011858 [DOI] [PubMed] [Google Scholar]
- 30.Elshazly MB, Senn T, Wu Y, et al. Impact of atrial fibrillation on exercise capacity and mortality in heart failure with preserved ejection fraction: insights from cardiopulmonary stress testing. J Am Heart Assoc 2017;6 10.1161/JAHA.117.006662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Blair SN, Kohl HW, Paffenbarger RS, et al. Physical fitness and all-cause mortality. A prospective study of healthy men and women. JAMA 1989;262:2395–401. 10.1001/jama.262.17.2395 [DOI] [PubMed] [Google Scholar]
- 32.Ruiz JR, Sui X, Lobelo F, et al. Association between muscular strength and mortality in men: prospective cohort study. BMJ 2008;337:a439–5. 10.1136/bmj.a439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li R, Xia J, Zhang XI, et al. Associations of muscle mass and strength with all-cause mortality among US older adults. Med Sci Sports Exerc 2018;50:458–67. 10.1249/MSS.0000000000001448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nguyen BL, Fishbein MC, Chen LS, et al. Histopathological substrate for chronic atrial fibrillation in humans. Heart Rhythm 2009;6:454–60. 10.1016/j.hrthm.2009.01.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Spach MS, Heidlage JF, Barr RC, et al. Cell size and communication: role in structural and electrical development and remodeling of the heart. Heart Rhythm 2004;1:500–15. 10.1016/j.hrthm.2004.06.010 [DOI] [PubMed] [Google Scholar]
- 36.Schotten U, Ausma J, Stellbrink C, et al. Cellular mechanisms of depressed atrial contractility in patients with chronic atrial fibrillation. Circulation 2001;103:691–8. 10.1161/01.CIR.103.5.691 [DOI] [PubMed] [Google Scholar]
- 37.Frustaci A, Chimenti C, Bellocci F, et al. Histological substrate of atrial biopsies in patients with lone atrial fibrillation. Circulation 1997;96:1180–4. 10.1161/01.CIR.96.4.1180 [DOI] [PubMed] [Google Scholar]
- 38.Ravassa S, Ballesteros G, López B, et al. Combination of Circulating Type I Collagen-Related Biomarkers Is Associated With Atrial Fibrillation. J Am Coll Cardiol 2019;73:1398–410. 10.1016/j.jacc.2018.12.074 [DOI] [PubMed] [Google Scholar]
- 39.Ravassa S, López B, Querejeta R, et al. Phenotyping of myocardial fibrosis in hypertensive patients with heart failure. Influence on clinical outcome. J Hypertens 2017;35:853–61. 10.1097/HJH.0000000000001258 [DOI] [PubMed] [Google Scholar]
- 40.López B, Ravassa S, González A, et al. Myocardial collagen cross-linking is associated with heart failure hospitalization in patients with hypertensive heart failure. J Am Coll Cardiol 2016;67:251–60. 10.1016/j.jacc.2015.10.063 [DOI] [PubMed] [Google Scholar]
- 41.Molvin J, Jujic A, Melander O, et al. Exploration of pathophysiological pathways for incident atrial fibrillation using a multiplex proteomic CHIP. Open Heart 2020;7:e001190. 10.1136/openhrt-2019-001190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Okumura Y, Watanabe I, Nakai T, et al. Impact of biomarkers of inflammation and extracellular matrix turnover on the outcome of atrial fibrillation ablation: importance of matrix metalloproteinase-2 as a predictor of atrial fibrillation recurrence. J Cardiovasc Electrophysiol 2011;22:987–93. 10.1111/j.1540-8167.2011.02059.x [DOI] [PubMed] [Google Scholar]
- 43.Tops LF, Bax JJ, Zeppenfeld K, et al. Effect of radiofrequency catheter ablation for atrial fibrillation on left atrial cavity size. Am J Cardiol 2006;97:1220–2. 10.1016/j.amjcard.2005.11.043 [DOI] [PubMed] [Google Scholar]
- 44.Thomas L, Abhayaratna WP. Left atrial reverse remodeling: mechanisms, evaluation, and clinical significance. JACC Cardiovasc Imaging 2017;10:65–77. 10.1016/j.jcmg.2016.11.003 [DOI] [PubMed] [Google Scholar]
- 45.Reant P, Lafitte S, Jaïs P, et al. Reverse remodeling of the left cardiac chambers after catheter ablation after 1 year in a series of patients with isolated atrial fibrillation. Circulation 2005;112:2896–903. 10.1161/CIRCULATIONAHA.104.523928 [DOI] [PubMed] [Google Scholar]
- 46.Hagens VE, Van Veldhuisen DJ, Kamp O, et al. Effect of rate and rhythm control on left ventricular function and cardiac dimensions in patients with persistent atrial fibrillation: results from the rate control versus electrical cardioversion for persistent atrial fibrillation (race) study. Heart Rhythm 2005;2:19–24. 10.1016/j.hrthm.2004.09.028 [DOI] [PubMed] [Google Scholar]
- 47.Baggish AL, Wang F, Weiner RB, et al. Training-specific changes in cardiac structure and function: a prospective and longitudinal assessment of competitive athletes. J Appl Physiol 2008;104:1121–8. 10.1152/japplphysiol.01170.2007 [DOI] [PubMed] [Google Scholar]
- 48.Hegde SM, Solomon SD. Influence of physical activity on hypertension and cardiac structure and function. Curr Hypertens Rep 2015;17:1–8. 10.1007/s11906-015-0588-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Utomi V, Oxborough D, Whyte GP, et al. Systematic review and meta-analysis of training mode, imaging modality and body size influences on the morphology and function of the male athlete’s heart. Heart 2013;99:1727–33. 10.1136/heartjnl-2012-303465 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2020-040054supp001.pdf (208.6KB, pdf)