Abstract
Background
Limited knowledge exists on the expected long-term effects and cost-effectiveness of initiatives aiming to reduce the burden of obesity.
Aim
To develop a Norwegian obesity-focused disease-simulation model: the MOON model.
Material and Methods
We developed a Markov model and simulated a Norwegian birth cohort’s movement between the health states “normal weight,”“overweight,”“obese 1,”“obese 2,” and “dead” using a lifetime perspective. Model input was estimated using longitudinal data from health surveys and real-world data (RWD) from local and national registers (N = 99,348). The model is deterministic and probabilistic and stratified by gender. Model validity was assessed by estimating the cohort’s expected prevalence, health care costs, and mortality related to overweight and obesity.
Results
Throughout the cohort’s life, the prevalence of overweight increased steadily and stabilized at 45% at 45 y of age. The number of obese 1 and 2 individuals peaked at age 75 y, when 44% of women and 35% of men were obese. The incremental costs per person associated with obesity was highest in older ages and, when accumulated over the lifetime, higher among women (€12,118, €9,495–€15,047) than men (€6,646, €5,252–€10,900). On average, obesity shortened the life expectancy of women/men in the whole cohort by 1.31/1.08 y. The life expectancy for normal-weight women/men at age 30 was 83.31/80.31. The life expectancy was reduced by 1.05/0.65 y if the individual was overweight, obese (2.87/2.71 y), or obese 2 (4.06/4.83 y).
Conclusion
The high expected prevalence of obesity in the future will lead to substantial health care costs and large losses in life-years. This underscores the need to implement interventions to reduce the burden of obesity; the MOON model will enable economic evaluations for a wide range of interventions.
Keywords: cost and cost analysis, economic evaluation, Markov model, obesity
The prevalence of overweight and obesity in children and adults worldwide has increased rapidly during the past 4 decades.1 Obesity is linked to an increased incidence of a number of chronic diseases (e.g., type 2 diabetes, cardiovascular diseases, osteoarthritis, respiratory disease, and certain cancers), which leads to increased morbidity and a marked loss in years of life.2–6
Globally, the awareness of obesity and its consequences is increasing, and efforts to reduce the burden of obesity through prevention and treatment are therefore rising on the political agenda.7 Economic arguments are increasingly used across several countries to inform decision making and to support efficient resource allocation.8
Economic evaluations within obesity call for a long time perspective and comparison of multiple alternatives for the following reasons.9–12 First, the negative outcomes related to obesity tend to be later in life but are also apparent for childhood obesity, in which the increased risk of diseases is primarily driven by the persistence of obesity from childhood to adulthood.13–16 Second, prevention and treatment of obesity can be provided through a wide range of policy initiatives and complex multimodal interventions that—because of time and resource constraints—cannot be compared within a single clinical trial. Analytical frameworks such as disease simulation models (DSMs) can enable the evaluation of a range of interventions, considering both effects and costs while taking a long time perspective.17–21
Several obesity-focused DSMs have been previously created.17,18 One of the earliest examples of model-based economic evaluations is the Assessing Cost-Effectiveness in Obesity (ACE-Obesity) program in Australia (2006),22 in which a broad range of preventive and treatment interventions were evaluated to guide resource allocation using pathway analyses. More recently, as part of the Childhood Obesity Intervention Cost-Effectiveness Study (CHOICES) in the United States, Ward et al.16 used longitudinal data and developed a model in which they simulated individual-level height and weight trajectories for participants aged 2 to 35 y while accounting for secular trends. In Germany, Sonntag et al.23,24 used a more traditional modeling approach whereby they used cross-sectional data to developed a Markov model using the body mass index (BMI) categories “normal weight,”“overweight,” and “obese” as health states to estimate the expected lifetime costs of childhood obesity.
Because of the differences between countries regarding the epidemiology of diseases, health care systems, and cost levels, DSMs cannot be directly applied across countries.25,26 Building on the German approach, we have developed the first Markov model within a Norwegian context using longitudinal health survey data linked to registry data, which provides a unique opportunity to make valid, data-heavy DSMs. In the present study, Modeling Obesity in Norway (MOON), an obesity-focused DSM, was built to enable future economic evaluations in a Norwegian context.
In the present article, we first describe the details of how we developed the MOON model, with a focus on model transparency. We showcase the model and test its validity by first estimating the expected prevalence and incremental health care costs of overweight and obesity throughout the lifetime for a cohort of Norwegian 2-y-olds and, second, by estimating the cohorts’ expected years of life loss (YLL) due to obesity through scenario analyses.
Materials and Methods
Model Structure
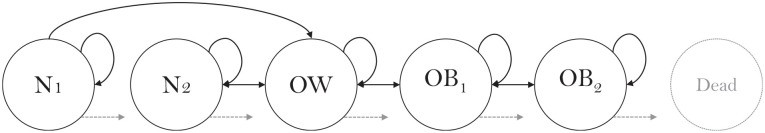
We developed a Markov model that included the following health states: normal weight (NW1 and NW2), overweight (OW), obese grade 1 (OB1), obese grade 2 (OB2), and dead (circles in Figure 1). NW1 represents “always NW,” whereas NW2 represents “NW but previously OW or OB.” In the current article, we assume that the health states N1 and N2 have the same transition probabilities, health care costs, and mortality rates. However, in future analyses, we have the flexibility to separate those who have always been normal weight from those who are normal weight but who have previously been overweight and/or obese.
Figure 1.
Schematic of the Markov model. The light gray arrows indicate how individuals can move to “dead” from each of the other health states.
Health states were defined based on the World Health Organization cutoffs, which are based on the individuals’ BMI (kg/m2).27 In adults, a BMI of 18 to 25, 25 to 30, 30 to 35, or 35+ kg/m2 is classified as NW, OW, OB1, or OB2, respectively. Because these cutoffs are insufficient at classifying children, we used the International Obesity Task Force for children (2–17 y), which classifies children into the same health states, but in which the cutoffs are age and gender specific; for example, a 2-y-old girl is classified as NW, OW, OB1, or OB2 if she has a BMI of 14.96 to 18.09, 18.09 to 19.81, 19.81 to 21.13, or 21.13+kg/m2, respectively.28
A cohort of children entered the model at 2 y of age and were distributed between the health states according to a representative sample of 2-y-olds in Norway in 2002. Accordingly, 89.83%, 8.98%, 0.86%, and 0.33% were NW, OW, OB1, or OB2. The model simulates 1-y cycles, over 98 y (until individuals have died or reach 100 y old). For each cycle, the cohort can move between the health states along transition possibilities (arrows in Figure 1); for example, if a person is OW, she or he can either stay OW, become OB1, NW2, or die. We track the number of individuals in each health state, per year, and estimate the cohorts’ expected annual health care costs and number of deaths. This was done by multiplying the number of persons in each health state by the health care cost and mortality rate of that health state. All input parameters in the model (transition probabilities, mortality rates, and health care costs) varied with age, which coincided with the number of the cycle. We simulated a cohort of 55,120 individuals—the size of the Norwegian birth cohort born in 2018—in which 48% were female and 52% were male.29 The model was built in Excel (Office 2016).
Model Input
Transition probabilities
Transition probabilities were estimated using 4 patient-level longitudinal data sets: 1) Trondheim municipality (T-MU), 2) the Child Growth Study (CGS), 3) Young-HUNT, and 4) Adult HUNT.
T-MU contains all children born in the Trondheim municipality between 1999 and 2017. In Norway, all children are followed up at health stations at predefined time points (12 times during the first 2 y and at approximately 2, 4, 6, 8, and 13 y of age). We extracted data from the children’s electronic medical records, from which data were available from 2003 to 2017. The CGS contains a representative sample of Norwegian children who were aged 8 y in 2010 and information from their electronic medical records from birth. The Nord-Trøndelag Health Study (the HUNT Study) contains several surveys targeting the population in the former county Nord Trøndelag. It is representative of the Norwegian population with regard to geography, economy, industry, sources of income, age distribution, morbidity, and mortality.30 We used information from Young-HUNT 1, 2, and 3 and adult HUNT 1, 2, and 3. In the Young-HUNT 1 survey (1995–1997), 10,000 adolescents in secondary school (aged 13–15 y) and high school (aged 15–19 y) were invited to participate. A 90% participation rate was achieved. The Young-HUNT 2 survey (1999–2000) was a follow-up study of Young-HUNT 1, in which all adolescents in the last 2 y of high school (aged 17–19 y) were invited. A participation rate of 77% was obtained. In Young-HUNT 3 (2006–2008), 10,000 adolecents in secondary and high school were invited, and an 87% response rate was obtained.31 Young-HUNT 1, 2, and 3 can be linked to adult HUNT 3. Adult HUNT 1, 2, and 3 were performed in 3 waves: in 1984–1986, 1995–1997, and 2006–2008. In the adult HUNT surveys, all citizens living in Nord-Trøndelag County aged >20 y were invited. The participation rates in the surveys were 88%, 71%, and 54%, respectively.32 We constructed 2 data sets based on the HUNT surveys: an adolescent data set containing individuals who participated in either Young-HUNT 1, 2, or 3 and could be linked either through the Young-HUNT surveys or to adult HUNT 3 and an adult data set, containing individuals wo participated in adult HUNT 1, 2, and/or 3. From T-MU, CGS, and HUNT, we received data on the individuals’ age, gender, height, and weight. Data on height was measured without shoes, whereas weight was measured with light indoor clothing.
We created 3 longitudinal data sets (children, adolescents, and adults) by restricting all data sets to contain only individuals who had 2 or more observations including height and weight. Individual observations did not need to be complete (e.g., appear both in Young-HUNT 1, 2, 3 and HUNT 3 to be part of the adolescent data) but could appear in Young-HUNT 1 and then in HUNT 3, or in Young-HUNT 1 and then only in Young-HUNT 2. Eighteen individuals participated in both Young-HUNT 3 and HUNT 3. These were included in the adolescent data set. Similarly, to be part of the adult data set, individuals could appear in HUNT 1, 2, or 3 if they appeared in a minimum of 2 waves. Altogether, we had 2 or more observations on 99,348 individuals (average of 2.84 observations); 35,506 individuals from T-MU and 2909 individuals from CGS (a total of 38,415 individuals in the children data set), 3097 individuals in the adolescent HUNT data set, and 57,836 individuals in the adult HUNT data set. See Supplementary Appendix 1 for a schematic of the data and more information regarding participation in the different data sets.
To exploit the longitudinal structure of the data, transition probabilities were estimated using parametric survival analyses. We conducted separate analyses for 3 different age groups: children (aged 2–13 y), adolescents (aged 13–30 y), and adults (aged 20–102 y), using data from the T-MU and CGS (for children); Young-HUNT 1, 2, and 3 and adult HUNT 3 (for adolescents); and adult HUNT 1, 2, and 3 (for adults). In the MOON model, we used data from the childhood analyses between the ages 2 and 12 y, adolescent analyses between the ages of 13 and 19 y, and adulthood analyses for ages 20+y. We chose to use transition probabilities in the MOON from the starting age of the survival analyses. The age range is therefore higher in the survival analyses than what it was in the MOON model (e.g., for adolescents, we use data from the survival analyses starting at the age of 13 but end at 19 y since the survival data for the adults start at the age of 20 y).
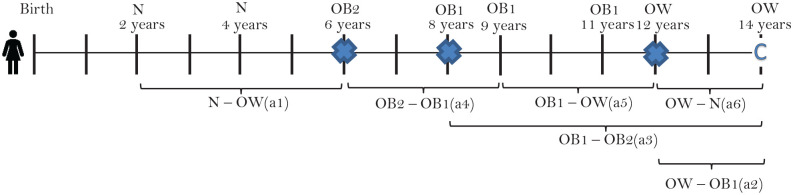
Because data were measured between large time gaps (∼10 y in the HUNT studies, ∼4 y between Young HUNT 1 and 2, and ∼11 y between Young HUNT 1 and adult HUNT 3), we interpolated the data by estimating the height and weight between measurement points. For the children cohort (T-MU and COIS), data were gathered at sufficiently close time gaps, and no interpolation was performed. In the adolescent populations and the adult populations, we interpolated values at a yearly basis between the measurements (i.e., ∼4 y between Young HUNT 1 and Young HUNT 2, ∼7 y between Young HUNT 2 and adult HUNT 3, and ∼10 y between HUNT 1, HUNT 2, and HUNT 3). We did not interpolate between Young-Hunt 3 and HUNT 3, because the average time gap for the 18 individuals who participated in both surveys was 1 y. We assumed a linear increase in height until the age of 18 y, when height was assumed to be constant, and a linear increase in weight between all measurements (both adolescents and adults; see supplementary Appendix 2). For all 3 age groups, we conducted 6 separate survival analyses. Three analyses estimated the probability of progressing (moving to higher BMI classes) 1) from N1/N2→OW, 2) from OW→OB1, and 3) from OB1→OB2), and 3 analyses estimated the probability of regressing (moving to lower BMI classes) 4) from OB2→OB1, 5) from OB1→OW, and 6) OW→N2 (see Figure 1). We restructured the data to enable survival analyses by defining a start time, whether an individual had an event or was censored, and a time-to-event or time-to-censoring. The start time was set when an individual had his or her first observation as either NW (analysis 1), OW (analyses 2 and 6), OB1 (analyses 3 and 5), or OB2 (analysis 4): 1 person could thus inform several of the survival analyses (see Figure 2).
Figure 2.
Schematic of a hypothetical person, built on observations in the data set, and his or her contribution in the different survival analyses (a 1–6). N = NW, OW = OW, OB1 = OB1 OB2 = OB2,  = event,
= event,  = censoring.
= censoring.
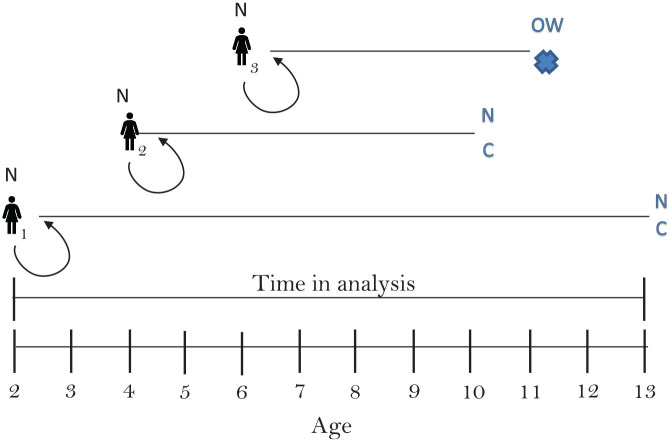
An individual had an event when she or he either moved up a weight class (e.g., from NW to OW) or down a weight class (e.g., from OW to NW), depending on which of the 6 analyses we conducted. Individuals were censored when they no longer had an observation. We used age as the time axis in the analysis and allowed for delayed entry, meaning that individuals entered the survival model when the model reached their age. See Figure 3 for an example.
Figure 3.
Schematic of 3 hypothetical persons in survival analysis 1 (from NW to OW). Person 1 enters the model as NW at the age 2 y and stays NW until the age 13 y, when she is censored (lost to follow-up). Person 2 enters the model as NW at the age of 4 y and stays NW until the age of 10, when she is censored. Person 3 enters the model as NW at the age of 6 y and stays NW until the age of 11, when she becomes OW (has an event),  = event,
= event,  = censoring.
= censoring.
For the 18 survival analyses (6 for each age group), we assessed the goodness-of-fit from 6 distributions (the exponential, Weibull, log-normal, log-logistic, Gompertz, or gamma distribution)33 using the Akaike information criterion and the Bayesian information criterion.34,35 We conducted analyses for both the whole cohort and when stratifying by gender; model selection was performed for the whole cohort, and the same specifications of the survival models were assumed for women and men.
Mortality
We estimated age-, gender-, and BMI-specific mortality rates by using age- and gender-specific national life tables for the Norwegian population in 2017. We multiplied the mortality rates with age- and BMI-specific hazard ratios to estimate the additional mortality from overweight and obesity. The hazard ratios data were estimated from the Global BMI Mortality collaboration.36 See Supplementary Appendix 3.
Health care costs
We used 3 data sets to estimate health care costs: HUNT-3, the Norwegian Patient Registry (NPR), and Control and Payment of Health Reimbursement Register (KUHR).37,38 From HUNT-3, we had access to information on 50,410 respondents and their height, weight, age, gender, smoking status, and marital status. These were linked to NPR and KUHR. In Norway, health care is partly funded based on activity (activity-based funding), and close to 100% of hospital and general practitioners’ (GP) activity is recorded in NPR and KUHR. From NPR, we had access to information on inpatient and outpatient use of secondary care, including somatic and psychiatric care. We used cost weights from the Norwegian activity-based funding system to estimate hospital costs. From KUHR, we had access to information on use of GPs, and because GPs are partly financed through claims, we used these claims to estimate the cost of GPs. Costs were estimated in 2009 EUROs; 1 EURO = 8.7285 NOK = 0.7197 USD.39 For details, see Supplementary Appendix 4.
Data from HUNT-3 were collected between 2006 and 2008, while we used data from NPR and KUHR collected in 2009; we therefore selected people from HUNT-3 who were still alive and living in Norway on 1 January 2010 (N = 49,714). Variables on health care costs were nonnegatively skewed continuous numbers and contained a large quantity of nonusers, and we estimated the incremental health care costs between those who were NW (reference group), OW, OB1, or OB2 using 2-part models with a logistic regression (first part) and a generalized linear model with a log link and a gamma distribution (second part).40,41 In the 2-part model, we included covariates that are commonly used in the obesity literature: age (continuous), smoking status (never-smoker, previous smoker, daily smoker, and occasional smoker), and marital status (married or registered partner, never married, widow/widower, or divorced/separated). We conducted stratified models for women and men. We predicted the average marginal effects at representative values per year from the age of 20 to 80 y and used these as inputs in the MOON model.42 Costs between the age of 80 and 100 y were assumed to be constant at the level predicted in 80-y-olds. For details, see Supplementary Appendix 4.
All statistical analyses were performed in STATA version 15.
Model Output
Base-case analyses
We estimated the expected prevalence, health care costs, and mortality throughout the lifetime for a cohort of Norwegian 2-y-olds. We assumed that trends in obesity prevalence and mortality during the last decades (1980s–2000s) were applicable to future predictions (i.e., mirroring the data). The prevalence was expressed as the annual expected percentage of the cohort—still alive—who were NW, OW, OB1, or OB2. Costs were estimated as the annual expected survival-adjusted incremental health care costs of OW, OB1, and OB2 and as the cumulative expected survival-adjusted incremental health care costs of OW, OB1, and OB2 during the cohort’s lifetime. Annual health care costs were estimated by first multiplying the number of individuals who were in a health state at a certain time (age) with the per-person cost (from the 2-part models) of being in that health state at that time (age). Incremental costs of being OW, OB1, and OB2 were estimated by subtracting the expected costs for those being OW, OB1, or OB2 from the expected costs for those being NW. Cumulative costs were estimated by summarizing the annual expected costs over the cohort’s lifetime. Numbers are primarily displayed as the expected costs per person in the cohort, estimated by dividing the annual or cumulative costs by the start population in the model (n = 26,458 for women and n = 28,662 for men). The model is built to enable discounting; we display results without discounting and when discounting costs at a 4% level.
Sensitivity analyses
The model was built both deterministic and probabilistic. In the deterministic analyses, we used point estimates of the population means as input parameters. In the probabilistic sensitivity analyses (PSA), input parameters were predefined as distributions with predefined variability. The choice of distributions and size of the variability are displayed in Table 1 for transition probabilities (defined from the survival analyses). For mortality rates, we used a log-normal distribution and variability from the Global BMI Mortality collaboration (Supplementary Appendix S1), and for health care costs, we used gamma distributions with variability from the 2-part models (Supplementary Appendix s6–s13).34 When performing the PSA, we allowed for the model to draw randomly from the predefined distributions to make a single realization of the model. We repeated the procedure (random draw and realization) using 1000 iterations. Using results from the PSA, we estimated the 95% confidence bounds (CBs) as the 2.5% and 97.5% observation for relevant variables from the 1000 iterations.
Table 1.
Description of Which Distributions We Used in the Survival Models, Their Corresponding Parameters, and Average Transition Probabilities for the Different Transition Possibilities (Arrows in Figure 1) for Children, Adolescents, and Adultsa
| Womenb | Menb | ||||||
|---|---|---|---|---|---|---|---|
| Age Group | Distribution | n | Parameter | Transition Probabilityc | n | Parameter | Transition Probabilityc |
| N - OW | |||||||
| 2–12 y | Log-normal | 17,312 | (3.16, 1.22) | 2.72% | 18,508 | (3.27, 1.19) | 2.32% |
| 13–19 y | Weibull | 1495 | (−5.75, 1.85) | 1.85% | 1222 | (−6.95, 2.42) | 1.80% |
| 20 + y | Log-logistic | 19,599 | (0.45, 0.04) | 3.25% | 14,499 | (0.50, 0.06) | 3.86% |
| OW – OB1 | |||||||
| 2–12 y | Log-normal | 3562 | (3.32, 1.51) | 2.85% | 3279 | (3.28, 1.31) | 2.56% |
| 13 – 19 y | Weibull | 560 | (−5.22, 1.77) | 2.67% | 483 | (−4.53, 1.54) | 3.28% |
| 20 + y | Log-logistic | 18,062 | (0.61, 0.04) | 2.38% | 18,966 | (0.73, 0.03) | 1.63% |
| OB1– OB2 | |||||||
| 2–12 y | Log-normal | 689 | (3.35, 1.62) | 2.95% | 610 | (3.44, 1.62) | 2.73% |
| 13–19 y | Gompertz | 164 | (0.12, 0.02) | 3.40% | 139 | (0.02, 0.05) | 4.98% |
| 20 + y | Gompertz | 7469 | (−0.02, 0.05) | 2.35% | 5501 | (0.01, 0.02) | 1.38% |
| OW – N | |||||||
| 2–12 y | Log-normal | 3562 | (1.64, 0.95) | 13.17% | 3279 | (1.44, 0.91) | 15.97% |
| 13–19 y | Gompertz | 560 | (−0.30, 0.22) | 7.83% | 483 | (−0.36, 0.22) | 6.88% |
| 20 + y | Gompertz | 18,062 | (0.04, 0.00d) | 1.00% | 18,966 | (0.05, 0.00e) | 1.10% |
| OB1– OW | |||||||
| 2–12 y | Log-normal | 689 | (1.28, 0.92) | 18.11% | 610 | (1.26, 0.89) | 18.88% |
| 13–19 y | Gompertz | 164 | (−0.43, 0.31) | 8.10% | 139 | (−0.32, 0.16) | 5.59% |
| 20 + y | Gompertz | 7469 | (0.03, 0.00f) | 0.96% | 5501 | (0.05, 0.00g) | 1.95% |
| OB2– OB1 | |||||||
| 2–12 y | Log-normal | 222 | (1.27, 0.77) | 20.93% | 194 | (1.37, 0.84) | 18.22% |
| 13–19 y | Gompertz | 41 | (−0.35, 0.48) | 14.43% | 23 | (−0.16, 0.16) | 8.46% |
| 20 + y | Gompertz | 2191 | (0.02, 0.00h) | 2.17% | 846 | (0.04, 0.00i) | 2.79% |
For yearly transition probabilities, see Supplementary Appendix 5.
For the probabilistic sensitivity analysis, we sampled using Cholesky decomposition.31 Covariance matrixes (necessary to perform probabilistic sensitivity analyses) are available upon request from the author.
Transition probabilities given as the average yearly values for the relevant age group. For example, the average yearly transition probability between NW and OW was 2.72% for females between the ages of 2 and 12 y, based on the yearly probabilities 0.49% +1.70% +2.46% +2.88% +3.11% +3.24% +3.31% +3.33% +3.33% +3.31%)/10 ≈ 2.72%. In the model, we used yearly transition probabilities, which varied with age.
Value = 0.00141.
Value = 0.00086.
Value = 0.00227.
Value = 0.00160.
Value = 0.00782.
Value = 0.00414.
Model validation
We assessed the model’s face validity through a continuous and close collaboration with clinicians, internal validity by testing the models’ mathematical accuracy through control cells and by performing a walkthrough of the model with independent researchers, and external validity by comparing results from the model with the input data (dependent comparison).43 For details, see Supplementary Appendix 6.
Scenario analyses
We performed 6 scenario analyses. First, we estimated the expected YLL attributable to OB1 and OB2 in the cohort through 2 scenarios: 1) when eliminating OB2 and 2) when eliminating OB2 and OB1. The scenarios were performed by manipulating transition probabilities from the health state OB1 to OB2 (scenario 1) or from OW to OB1 (scenario 2) to be zero (i.e., no transition to OB2 or OB1, respectively). In the scenarios, we set OB2 individuals as OB1 individuals at age 2 (scenario 1) and OB1 and OB2 individuals as OW at age 2 (scenario 2). The probability of staying in OB1 increased by the reduced probability of going from OB1 to OB2 (scenario 1) and from OW to OB1 (scenario 2) so that the sum of transition probabilities from the health states was always 1. We also ran 4 scenario analyses in which we estimated the expected YLL for individuals aged 30 y conditional on their weight category. Analyses were performed by estimating the life expectancy when all individuals at age 30 was NW (scenario 3), OW (scenario 4), OB1 (scenario 5), or OB2 (scenario 6). YLL was estimated by subtracting the life expectancy for the OW, OB1, or OB2 from that of the NW. In scenarios 3 to 6, individuals could move between health states according to transition probabilities as used in the MOON model after the age of 30; thus, individuals who were NW at age 30 could progress to OW, OB1, or OB2 throughout their lifetime, and likewise, individuals who were OB2 at age 30 could regress to OB1, OW, and NW throughout their lifetime.
Results
Transition between Health States
Transition between health states was frequent in young ages but decreased and stabilized at low levels in older ages (Table 1; Supplementary Appendix 5).
Prevalence
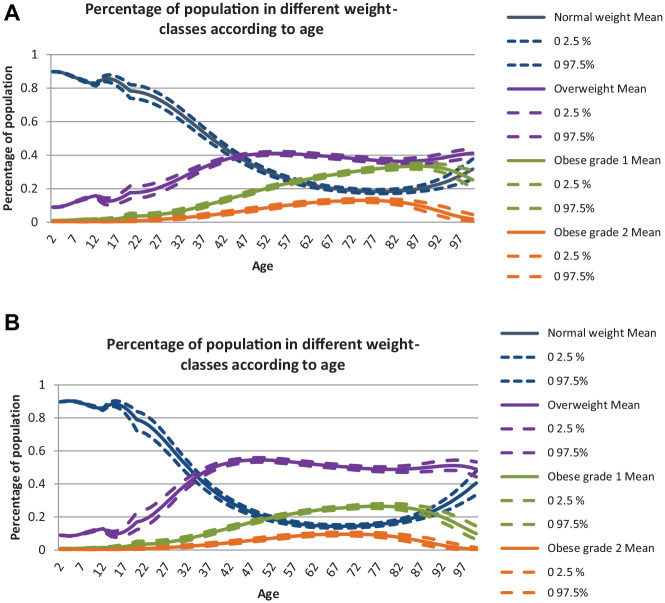
In a Norwegian cohort of 2-y-olds, the proportion of OW individuals is expected to increase to the age of ≈45 y and stabilize thereafter. The number of OB1 and OB2 individuals is expected to increase to the age of ≈70–80 y and thereafter decrease. Compared with men, women are expected to stabilize at a lower rate of OW but have higher rates of OB1 and OB2 (see Figures 4a–b).
Figures 4.
Predicted proportions (and 95% confidence bounds) of the population—still alive—who were NW, OW, OB1, or OB2 at different ages for women (a) and men (b).
Health Care Costs
For women, estimates from the 2-part model showed average marginal effects associated with OW of €160 (P = 0.06) and significant effects at a 5% level for OB1 (€631, P < 0.001) and OB2 (€698, P < 0.001) as compared with NW. Among men, there were nonsignificant average marginal effects associated with OW (−€96, P = 0.31) and OB1 (€182, P = 0.15) but a significant effect for OB2 (€1456, P < 0.001) when compared with NW (see Appendix 4, Table s4 and s5).
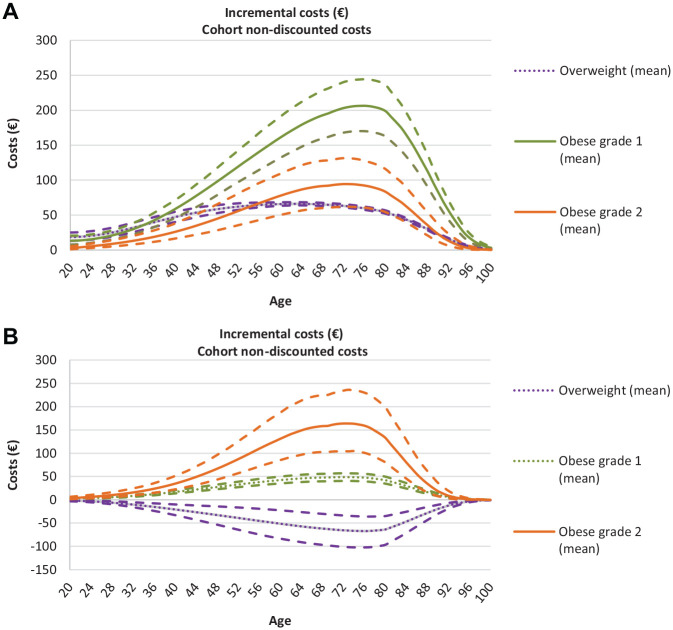
Using these significant and nonsignificant cost estimates as input in the model, we simulated expected annual survival-adjusted incremental health care costs per person in the cohort (Figures 5a and b), which combines the cost of actually being NW, OW, OB1, or OB2 (from the 2-part model) with the probability of becoming NW, OW, OB1, or OB2 (from the expected prevalences; Figures 4a, b). The expected costs of OB increased with age and peaked late in life. For women, the expected costs of OW peaked at age 60 at €66 (€64–€68). The expected costs for OB1 and OB2 peaked at age 75 and were higher for OB1 (€206, €170–€244) than for OB2 (€93, €61–€129) because of the higher probability of becoming OB1 than OB2 (Figure 4a). For men, the expected costs related to OW were negative (−€67, −€102 to −€36) and reached their peak at the age of 75. The expected costs for OB1 and OB2 peaked at age 75 and were higher for OB2 (€164, €104–€234) than for OB1 (€49, €40–€57).
Figures 5.
Expected survival-adjusted incremental health care costs per year with 95% confidence bounds as the cohort ages for women (a) and men (b). NW is the reference value (at €0). Average estimates that are dotted indicate that the point estimate from the 2-part model (cost estimate input) was not significant at a 5% level.
The cumulative expected survival-adjusted incremental health care costs related to obesity (OB1 and OB2) per person in the cohort over the lifetime were €12,118 (€9495–€15,047) for women and €6646 (€5252 – €10,900) for men; consequently, the expected lifetime health care costs related to obesity for the whole cohort accumulate to ≈€320 million (€251 million–€498 million) for women and ≈€191 million (€150 million–€312 million) for men (Table 2).
Table 2.
Lifetime Expected Incremental Survival-Adjusted Health Care Costs Per Person for Women and Men, and, the Percentage of Costs that Accumulated Between the Ages 2−30y, 30−60y and 60+y. Numbers are given in EURO (€) and Percentage (%) and are Displayed Both With and Without Discounting at 4%
| Women (n = 26,458) | Men (n = 28,662) | |
|---|---|---|
| OW | ||
| Nondiscounted (€: Mean (95%CB)) | 3534 (3318; 3764) | −2711 (−4319; −1278) |
| Discounted (€: Mean (95%CB)) | 487 (435; 549) | −274 (−439; −125) |
| OB1 | ||
| Nondiscounted (€: Mean (95%CB)) | 8439 (7018; 9973) | 2124 (1627; 2395) |
| Discounted (€: Mean (95%CB)) | 843 (690; 1011) | 230 (175; 264) |
| OB2 | ||
| Nondiscounted (€: Mean (95%CB)) | 3679 (2477; 5074) | 4522 (3625; 8505) |
| Discounted (€: Mean (95%CB)) | 374 (247; 525) | 448 (360; 832) |
| Total costs | ||
| Nondiscounted (€: Mean (95%CB)) | 15,651 (12,850; 18,835) | 3935 (3852; 6762) |
| Discounted (€: Mean (95%CB)) | 1705 (1378; 2074) | 405 (393; 689) |
| Age 2−30 y | ||
| Nondiscounted (%) | 0.03 | 0.02 |
| Discounted (%) | 0.11 | 0.07 |
| Age 30−60 y | ||
| Nondiscounted (%) | 0.34 | 0.32 |
| Discounted (%) | 0.53 | 0.5 |
| Age 60+y | ||
| Nondiscounted (%) | 0.64 | 0.66 |
| Discounted (%) | 0.36 | 0.43 |
Mortality and Scenario Analyses
On average, obesity shortened the life expectancy of women in the cohort by 1.31 y (1.6%) and men by 1.08 y (1.3%; scenario 1 and 2). The expected impact of OB1 (difference between scenario 1 and 2) was similar between the genders at 0.74 y versus 0.70 y for women and men, respectively. In summary, obesity reduces the life expectancy of the cohort of 55,120 individuals by a total of ≈65,000 life-years.
The average life expectancy for an NW woman/man at age 30 was 83.31/80.31 y and reduced by 1.05/0.65 y, 2.87/2.71 y, and 4.08/4.83 y, if she or he was OW, OB1, or OB2, respectively. See Table 3.
Table 3.
Life Expectancy for the Entire Cohort and When Dividing between Women and Men, Years of Life Loss (YLL) Caused by Obesity I (Scenario 1) and Obesity I and II (Scenario 2), and Expected Life Expectancy for Those Who Are NW, OW, OB1, or OB2 at 30 ya
| Women (n = 26,458) | Men (n = 28,662) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | 95% CB | Δ | Mean | 95% CB | Δ | |||||
| Base case | 82.53 | (81.92–83.14) | r.c. | 79.31 | (78.66–79.77) | r.c. | ||||
| Scenario 1 | 83.10 | (82.69–83.50) | 0.57 | 79.69 | (79.26–80.11) | 0.38 | ||||
| Scenario 2 | 83.84 | (83.54–84.08) | 1.31 | 80.39 | (80.09–80.69) | 1.08 | ||||
| Total life-years loss | ||||||||||
| OB2 (scenario 1)b | 15, 081 | 10, 891 | ||||||||
| OB1 (scenario 2)c | 34, 660 | 30, 954 | ||||||||
| Life expectancy (at 30 y)d | ||||||||||
| Scenario 3 NW | 83.31 | (82.84–83.75) | r.c. | 80.31 | (79.84–80.70) | r.c. | ||||
| Scenario 4 OW | 82.26 | (81.53–82.97) | 1.05 | 79.66 | (78.91–80.15) | 0.65 | ||||
| Scenario 5 OB1 | 80.44 | (79.27–81.47) | 2.87 | 77.60 | (76.14–78.24) | 2.71 | ||||
| Scenario 6 OB2 | 79.23 | (77.68–80.64) | 4.08 | 75.48 | (74.01–76.94) | 4.83 | ||||
Δ Presented as difference from reference category.
Scenario 1: eliminating obesity II.
Scenario 2: eliminating obesity I and obesity II. r.c. = reference category.
Estimated by multiplying the expected life-years lost of obesity II for an individual by the entire cohort (i.e., 0.57 × 26,458 = 15,081).
Estimated by multiplying the expected life-years lost of obesity I and II for an individual by the entire cohort (i.e., 1.31 × 26,458 = 34,660).
Life expectancy estimated by assuming an individual is NW (scenario 3), OW (scenario 4), OB1 (scenario 5), or OB2 (scenario 6) at age 30 y in the model. In scenarios 3–6, individuals could move between health states according to transition probabilities as used in the MOON model after the age of 30; thus, individuals who were NW at age 30 could progress to OW, OB1, or OB2 throughout their lifetime. Likewise, individuals who were OB2 at age 30 could regress to OB1, OW, and NW throughout their lifetime.
Model Validation
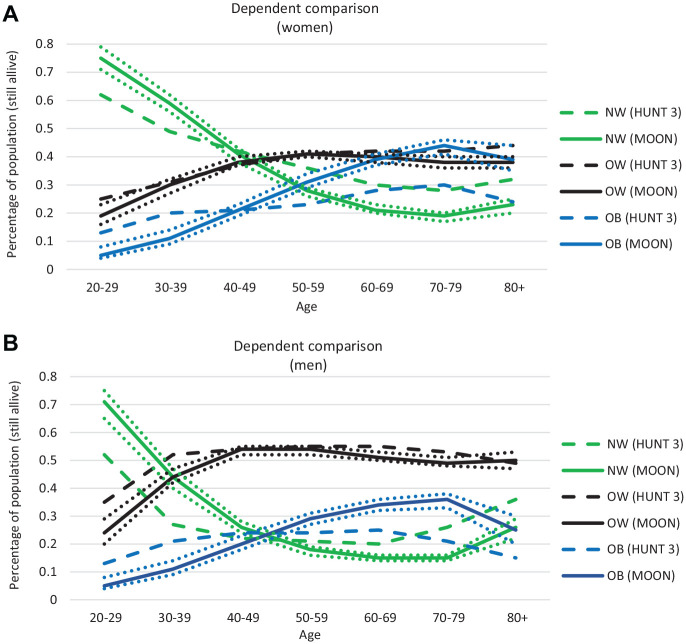
We ensured good face validity through close and continuous cooperation between modelers and clinicians through all steps of the modeling, from conception, statistical analyses and estimation of input parameters, and valuation of the final model. Internal validity was ensured by examining the mathematical calculations used in the model through several steps (for example, by ensuring that the cohorts’ size was constant). We also had a walkthrough of the model with experienced modelers in the field of industrial economics, in which the aim was to search for errors in the logic and mathematical calculations in the model. The external modelers confirmed the models’ internal validity. Figure 6a, b illustrates the external validity (dependent comparison between the model and HUNT 3), which showed that the model predicts a higher prevalence of OB1 and OB2 but equal levels of OW than what is observed in HUNT-3. The reason is that we are predicting the prevalence of overweight and obesity in the future given that the development in overweight and obesity continues as it has been observed to do between 1980s (HUNT 1) and the 2000s (HUNT 3), in which the prevalence of overweight has stabilized while the prevalence of obesity has increased.30 See Supplementary Appendix 6 for more details.
Figure 6.
Prevalence of normal weight, overweight, and obesity (grade 1 and 2) as estimated in the HUNT-3 and in the MOON for women (a) and men (b). The straight line gives the mean from the MOON – with 95% CB indicated by the dotted line.
Discussion
In this article, we describe how we built an obesity-simulation model in the Norwegian context. We validated the model by simulating a birth cohort of 2-y-olds throughout their life. The model predicted that the prevalence of obesity will continue to increase. The expected incremental health care cost per person in the cohort associated with obesity is estimated at €12,118 for women and €6,664 for men, where 65% accumulated at age >60 y. In addition, obesity leads to a YLL of 1.31 y and 1.08 y for woman and men, respectively. Our results indicate that there is a potential for substantial investments to reduce the burden of obesity but that health gains and cost savings might be visible first several decades after the investment.
In Norway, most of the adult population is currently overweight (∼50%) or obese (∼23%).30,44 We predict an equal prevalence of overweight but a higher prevalence of obesity in the future, for example, at 41% (overweight) and 31% (obesity) for women and 54% (overweight) and 29% (obesity) for men aged 50 to 59 y. Because we assumed future projections based on recent secular trends (from the 1980s to 2000s), this pattern is intuitive, given the dramatic increase in the prevalence of obesity observed during the past decades.30,44,45 Recently, some research has indicated that the prevalence of obesity in northern Europe might be leveling off.46 If this is the case, our estimates might be exaggerated. However, our model will be updated continuously—through updated survival analyses or calibration—when new data on the prevalence of obesity in Norway are available (e.g., when HUNT 4 is available).47
To the best of our knowledge, no similar predictions have been made for Norway previously. Kelly et al.48 projected the prevalence of overweight and obesity in 2030 for individuals aged >20 in countries with established market economies at 58% when assuming stable age-specific prevalence and 66% when assuming a continuation of recent secular trends. Our estimates were at 64% (59%–68%) for women and 70% (65%–76%) for men. In Germany, Sonntag et al.,23 who tracked childhood obesity into adulthood, found that 35% of women and 42% of men would be overweight or obese at age 30. Our numbers are similar at 32% (27%–37%) for women and 44% (39%–50%) for men.23,24 In the United States, Ward et al.16 predicted that most (55%) of today’s children will be obese by the age of 35. These estimates are higher than our estimates of 11% (OB1 and OB2).16 Different data (e.g., the use of cross-sectional versus longitudinal data or the nationality of individuals in the data set) and modeling techniques (e.g., handling of secular trends) influence simulation models both in their ability to produce valid epidemiologic output (e.g., prevalence of obesity) and in their ability to be used in future cost-effectiveness analyses. The nationality of the modeled population (i.e., United States, Norway, and Germany) largely influences the difference in the estimated prevalence of obesity between Ward et al.16 Sonntag et al.23,24 and the MOON model, since the obesity epidemic in the United States is more mature than in Norway and Germany.49 Also, whether and how secular trends are incorporated in models influence the estimated prevalence of obesity, as seen in Kelly et al.,48 in which the prevalence of obesity was higher when assuming a continuation of recent secular trends (66%) than when assuming a stable age-specific prevalence (58%).46 Whether models are built using cross-sectional or longitudinal data does not necessarily influence whether they produce valid epidemiologic output. Among the above-mentioned papers, Kelly et al. and Sonntag et al. used cross-sectional data, whereas Ward et al. used longitudinal data.16,23,24,46 Despite this, our estimated prevalence is more similar to Sonntag et al. and to Kelly et al. than to Ward et al. However, models built using longitudinal data more appropriately capture individuals’ true weight fluctuation and, consequently, the true transition between health states. These models will therefore most likely give more precise estimates in future cost-effectiveness analyses. A possible weakness when using longitudinal data is the potential of increasing selection bias, such as selective survival bias (e.g., by including only individuals from HUNT surviving for at least 1 decade) and healthy volunteer bias (e.g., by including only individuals who choose to participate in HUNT 2 times).50 However, this does not seem to influence the prevalence of obesity in the MOON model when comparing it to models using cross-sectional data, and we think that the positive sides of using longitudinal data outweigh the negative sides.
There is wide consensus that obesity leads to increased health care costs,51 and the level of costs we estimate concurs with previous research.40,51–56 In addition, the patterns we identified align with previous research: that costs peak late in life (at 60–70 y old)23,40,54 and are higher among women than men.23,53–55 Generally, cost estimates vary substantially between studies.53 Since different studies use different data sources, it is hard to disentangle how much of this variation is caused by differences in the analytical frameworks applied or the populations under analysis. For example, reversed causality (e.g., the fact that disease decreases body weight) is likely a source of bias within obesity and is handled differently between studies.53 Cawley et al.54 and Dixton et al.,56 who used weight of a biological relative and genetic variation in instrumental variable analyses to estimate the causal inferences between obesity and health care costs, found that cost estimates were substantially higher (>50%) in their analyses than in multivariable regression analyses. Consequently, our cost estimates might be too low, but they are, at the moment, the most accurate cost estimates made for Norway. Because of the simple nature of our model, more precise cost estimates, if made available, can easily be used as input in future versions of the model.
We find that overweight and obesity will lead to an average expected YLL of 1.31 and 1.08 y for women and men in the cohort, respectively. To the best of our knowledge, no similar numbers have been previously estimated for obesity, but as a comparison, the average YLL of socioeconomic inequality is estimated at 14.4 mo (1.2 y).57 We also estimated the YLL for 30-y-old women and men conditional on their weight category (Table 3), and our findings are in line with previous research: Fontaine et al.58 estimated that the YLL for 30-y-old white women and men was 0.5 y for a BMI of 30 and increased to 4 y (women) and 5 y (men) if their BMI was 40. Grover et al.59 estimated the YLL for very obese men and women to be 8.4 y and 6.9 y (in those aged 20–39 y), respectively, whereas Finkelstein et al.60 estimated that the YLL loss for 40-y-old white women and men was 1 y for those with OB1 and 4 y (women) and 5 y (men) for those with OB2.
This article, and the MOON model, has several strengths. To the best of our knowledge, this is the first obesity-focused DSM built for a Norway context. Because our model shows valid results, it can be used in future economic evaluations. Obesity is a complex and multifactorial disease with genetic, behavioral, socioeconomic, and environmental origins; changes will most likely happen during the lifetime of a child cohort that we are not be able to simulate.7,61 This should be carefully considered when evaluating interventions in the future, by, in accordance with Squires et al.,62 putting strong effort into understanding the current decision-making process, identifying and involving relevant stakeholders, understanding the relevant problem, and consequently developing and/or justifying the decision model being used to make estimates of the cost-effectiveness. As an example, the ACE-Obesity program in Australia involved a large group of stakeholders who identified relevant interventions. The effect of these interventions was estimated as the expected change on BMI development, preferably directly from clinical trials, and when this was not available, through estimates of the relationship between energy imbalances (from interventions) on change in body weight.22,63 They used simulation models, similar to the MOON, as a basis to extrapolate results in a longer time perspective.
Next, for the purpose of transparency, we aimed at building a simple and transparent model that is easy to understand and use.64,65 Although simplicity is important, the model also needs to be sufficiently complex to represent the complexity of the modeled problem and to exhibit satisfactory validity.64 The quality of data regarding BMI across Europe is generally regarded as poor.66 We are among the first to have access to longitudinal data on large cohorts (n = 99,348) covering the ages 2 to 102 y. The use of real-world data provides us a unique opportunity to contribute with new knowledge regarding obesity, such as the level of transition between health states, and the expected prevalence, health care costs, and mortality of obesity, all depending on gender and age. This knowledge will in itself contribute to the obesity literature. For example, although the prevalence of obesity increases steadily throughout a cohort’s lifetime, there was, in fact, a large proportion of individuals (particularly children) who moved between health states annually. In many children, these movements likely reflect natural fluctuations related to growth spurts, rather than true overweight. This is not possible to detect if using cross-sectional data23,24 but should be considered when introducing new interventions.
The simple but realistic nature of the MOON model can also enable researchers from other countries to duplicate and calibrate the MOON to their settings if they have access to proper targets from cross-sectional data (e.g., epidemiologic data on the prevalence of obesity).47
The MOON also has several shortcomings worth mentioning. We used a health care perspective, which is recommended in Norway.67 However, obesity affects individuals not only through disease but also through factors such as stigma and bullying, thus influencing school and work participation.24,51,52,68 Using a health care perspective might therefore ignore important societal (short-time) effects of preventing obesity. Because of the simple but still flexible framework used in the MOON, our model can easily be extended to include the societal perspective in the future by estimating a variety of consequences (e.g., dropping out of school) depending on BMI category and age.
The development of obesity in the population depends on both socioeconomic status (SES) and living situation (urban or rural living).69 We did not include these factors in our model—primarily because they were not available in the childhood cohort—and therefore cannot differentiate between populations based on SES and living situation. In the future, we can extend the model to differentiate between populations based on SES or living situation, either by using estimates of relative risks of SES and living situation on transition probabilities, or—if data are available—by including these factors in survival analyses when predicting transition probabilities.
In Norway, the life expectancy at birth in 2018 was 84.49 y for women and 81.00 y for men.70 These numbers are higher than the life expectancy estimated for the cohort in the model (82.53 y for women and 79.31 y for men; see Table 1). This discrepancy is primarily caused by the modeled high rates of obesity in the future and thus also higher rates of mortality. The modeled life expectancy is similar to Norwegian estimates in 2009 (83.05 y for women and 78.6 y for men). In the future, the life expectancy is expected to increase even more (i.e., to 88.2 y for women and 85.8 y for men in 2050). An important question is whether the incremental life expectancy between normal-weight individuals and those who are overweight, obese grade 1 or obese grade 2, will change as the life expectancy of the population changes. The life expectancy of a population depends on factors such as health, living conditions, quality of the health care system, medical development, and changes in the population’s lifestyle and quality of life.71 It is difficult to predict how these factors will change and be different in the future among those with different weight status. However, to ensure that the model is as realistic and up to date as possible when it is used in future cost-effectiveness analyses, it will be updated with the most recent numbers from life tables and the most recent (and reliable) numbers on hazard ratios between normal weight, overweight, obese grade 1, and obese grade 2.
Finally, obesity treatment is primarily provided to people with severe obesity.72 For the severely obese, a stabilization or small reduction in weight can be considered a success.73 These patients would not necessarily cross over from OB2 to OB1 in the MOON (see Figure 1); consequently, these treatment effects would not be captured by the model. However, estimating these effects is important, because the risk of adverse health outcomes increases exponentially with weight gain. A stabilization or small reduction in BMI could in fact have a large effect on the individual’s morbidity, mortality, and health care costs.50 A treatment modality of the MOON model, including only the severely obese with a BMI output on a continuous scale (similar to Ward et al.), should be pursued in the future.16
Conclusion
In this article, we describe how we built a data-heavy, valid, and flexible obesity-focused disease-simulation model in the Norwegian context (the MOON model). Through simulation for a Norwegian birth cohort (n = 55,120), the model predicts a high prevalence of obesity, leading to an incremental health care cost of €511 million over the lifetime while causing a loss of ≈65,000 life-years. The results underscore the urgent need to implement preventive and treatment interventions and illustrates potential cost savings associated with effective interventions. In the future, the MOON model can be used as a tool to inform policy makers regarding the expected long-term effect and cost-effectiveness of different sex- and age-specific interventions.
Supplemental Material
Supplemental material, sj-docx-1-mdm-10.1177_0272989X20971589 for Modeling Obesity in Norway (The MOON Study): A Decision-Analytic Approach—Prevalence, Costs, and Years of Life Lost by Gudrun M. W. Bjørnelv, Vidar Halsteinli, Bård E. Kulseng, Diana Sonntag and Rønnaug A. Ødegaard in Medical Decision Making
Supplemental material, sj-docx-2-mdm-10.1177_0272989X20971589 for Modeling Obesity in Norway (The MOON Study): A Decision-Analytic Approach—Prevalence, Costs, and Years of Life Lost by Gudrun M. W. Bjørnelv, Vidar Halsteinli, Bård E. Kulseng, Diana Sonntag and Rønnaug A. Ødegaard in Medical Decision Making
Acknowledgments
The Nord-Trøndelag Health Study (the HUNT Study) is a collaboration between the HUNT Research Centre (Faculty of Medicine and Health Sciences, NTNU, Norwegian University of Science and Technology), Trøndelag County Council, Central Norway Regional Health Authority, and the Norwegian Institute of Public Health.
Footnotes
The study was presented at Norwegian Days of Obesity Research, 2019, Oslo, Norway (oral presentation); the Nordic Health Economic Study Group meeting 2019, Reykjavik, Iceland (oral presentation); and the 26th European Congress on Obesity 2019, Glasgow, Scotland (poster presentation).
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Gudrun M. W. Bjørnelv  https://orcid.org/0000-0003-4997-5426
https://orcid.org/0000-0003-4997-5426
Supplemental Material: Supplementary material for this article is available on the Medical Decision Making Web site at http://journals.sagepub.com/home/mdm.
Contributor Information
Gudrun M. W. Bjørnelv, Regional Centre for Health Care Development, St. Olavs Hospital, Trondheim, Norway; Department of Public Health and Nursing, NTNU, Trondheim, Norway.
Vidar Halsteinli, Regional Centre for Health Care Development, St. Olavs Hospital, Trondheim, Norway; Department of Public Health and Nursing, NTNU, Trondheim, Norway.
Bård E. Kulseng, Regional Center for Obesity Research and Innovation, Department of Surgery, St. Olavs Hospital, Trondheim, Norway Department of Clinical Molecular Medicine, NTNU, Trondheim, Norway.
Diana Sonntag, Mannheim Institute of Public Health, Social and Preventive Medicine, Mannheim Medical Faculty of the Heidelberg University, Mannheim, Germany; Department of Health Sciences, University of York, UK.
Rønnaug A. Ødegaard, Regional Center for Obesity Research and Innovation, Department of Surgery, St. Olavs Hospital, Trondheim, Norway Department of Clinical Molecular Medicine, NTNU, Trondheim, Norway.
References
- 1. World Health Organization. Obesity: Preventing and Managing the Global Epidemic. WHO tech.report. Series No. 894. Geneva (Switzerland): World Health Organization; 2000. [PubMed] [Google Scholar]
- 2. Prospective Studies Collaboration; Whitlock G, Lewington S, et al. Body-mass index and cause-specific mortality in 900 000 adults: collaborative analyses of 57 prospective studies. Lancet. 2009;373(9669):1083–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Global BMI Mortality Collaboration, Di Angelantonio E, Bhupathiraju S, et al. Body-mass index and all-cause mortality: individual-participant-data meta-analysis of 239 prospective studies in four continents. Lancet. 2016;388(10046):776–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Guh DP, Zhang W, Bansback N, et al. The incidence of co-morbidities related to obesity and OW: a systematic review and meta-analysis. BMC Public Health. 2009;9:88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fontaine KR, Redden DT, Wang C, et al. Years of life lost due to obesity. JAMA. 2003;289(2):187–93. [DOI] [PubMed] [Google Scholar]
- 6. Grover SA, Kaouache M, Rempel P, et al. Years of life lost and healthy life-years lost from diabetes and cardiovascular disease in overweight and obese people: a modelling study. Lancet Diabetes Endocrinol. 2015;3(2):114–22. [DOI] [PubMed] [Google Scholar]
- 7. World Health Organization. Report of the Commission on Ending Childhood Obesity: implementation plan: executive summary. 2017. Contract No. WHO/NMH/PND/ECHO/17.1. [Google Scholar]
- 8. Bertram MY, Lauer JA, Joncheere KD, et al. Cost-effectiveness thresholds: pros and cons. Bull World Health Organ. 2016;94(12):925–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Robertson W, Fleming J, Kamal A, et al. Randomised controlled trial evaluating the effectiveness and cost-effectiveness of ‘Families for Health’, a family-based childhood obesity treatment intervention delivered in a community setting for ages 6 to 11 years. Health Technol Assess. 2017;21(1):1–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Canaway A, Frew E, Lancashire E, et al. Economic evaluation of a childhood obesity prevention programme for children: results from the WAVES cluster randomised controlled trial conducted in schools. PLoS One. 2019;14(7):e0219500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sassi F, Cecchini M, Laurer J, Chisholm D. Improving Lifestyles, Tackling Obesity: The Health and Economic Impact of Prevention Strategies. OECD Health Working Papers, No. 48. Paris: OECD Publishing; 2009. Available from: 10.1787/220087432153 [DOI] [Google Scholar]
- 12. Ramsey SD, Willke RJ, Glick H, et al. Cost-effectiveness analysis alongside clinical trials II: an ISPOR Good Research Practices Task Force report. Value Health. 2015;18(2):161–72. [DOI] [PubMed] [Google Scholar]
- 13. Bhupathiraju SN, Hu FB. Epidemiology of obesity and diabetes and their cardiovascular complications. Circ Res. 2016;118(11):1723–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bridger T. Childhood obesity and cardiovascular disease. Paediatr Child Health. 2009;14(3):177–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Engeland A, Bjørge T, Tverdal A, Søgaard AJ. Obesity in adolescence and adulthood and the risk of adult mortality. Epidemiology. 2004;15(1):79–85. [DOI] [PubMed] [Google Scholar]
- 16. Ward ZJ, Long MW, Resch SC, Giles CM, Cradock AL, Gortmaker SL. Simulation of growth trajectories of childhood obesity into adulthood. N Engl J Med. 2017;377(22):2145–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Levy DT, Mabry PL, Wang YC, et al. Simulation models of obesity: a review of the literature and implications for research and policy. Obes Rev. 2011;12(5):378–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Schwander B, Hiligsmann M, Nuijten M, Evers S. Systematic review and overview of health economic evaluation models in obesity prevention and therapy. Expert Rev Pharmacoecon Outcomes Res. 2016;16(5):561–70. [DOI] [PubMed] [Google Scholar]
- 19. Gortmaker SL, Swinburn BA, Levy D, et al. Changing the future of obesity: science, policy, and action. Lancet. 2011;378:838–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Caro JJ, Briggs AH, Siebert U. Modeling good research practices—overview: a report of the ISPOR-SMDM Modeling Good Research Practices Task Force-1. Med Decis Making. 2012;32(5):667–77. [DOI] [PubMed] [Google Scholar]
- 21. Cooper N, Coyle D, Abrams K, Mugford M, Sutton A. Use of evidence in decision models: an appraisal of health technology assessments in the UK since 1997. J Health Serv Res Policy. 2005;10(4):245–50. [DOI] [PubMed] [Google Scholar]
- 22. Carter R1, Moodie M, Markwick A, et al. Assessing cost-effectiveness in obesity (ACE-obesity): an overview of the ACE approach, economic methods and cost results. BMC Public Health. 2009;9:419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sonntag D, Ali S, Lehnert T, Konnopka A, Riedel-Heller S, König HH. Estimating the lifetime cost of childhood obesity in Germany: results of a Markov Model. Pediatr Obes. 2015;10(6):416–22. [DOI] [PubMed] [Google Scholar]
- 24. Sonntag D, Ali S, De Bock F. Lifetime indirect cost of childhood OW and obesity: a decision analytic model. Obesity (Silver Spring). 2016;24(1):200–6. [DOI] [PubMed] [Google Scholar]
- 25. Roberts M, Russell LB, Paltiel AD. Conceptualizing a model: a report of the ISPOR-SMDM Modeling Good Research Practices Task Force-2. Med Decis Making. 2012;32(5):678–89. [DOI] [PubMed] [Google Scholar]
- 26. Sculpher MJ, Pang FS, Manca A, et al. Generalisability in economic evaluation studies in healthcare: a review and case studies. Health Technol Assess. 2004;8(49):iii–iv, 1–192. [DOI] [PubMed] [Google Scholar]
- 27. World Health Organization. Obesity: Preventing and Managing the Global Epidemic. Geneva (Switzerland): World Health Organization; 1998. [PubMed] [Google Scholar]
- 28. Cole TJ, Lobstein T. Extended international (IOTF) body mass index cut-offs for thinness, OW and obesity. Pediatr Obes. 2012;7(4):284–94. [DOI] [PubMed] [Google Scholar]
- 29. Statistics Norway. Fødte [Born]. Available from: https://www.ssb.no/befolkning/statistikker/fodte. Accessed 30 January 2020.
- 30. Midthjell K, Lee CMY, Langhammer A, et al. Trends in OW and obesity over 22 years in a large adult population: the HUNT Study, Norway. Clin Obes. 2013;3(1–2):12–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Norwegian University of Science and Technology. The Young-HUNT Study. Available from: https://www.ntnu.edu/hunt/young-hunt. Accessed 4 December 2019.
- 32. Langhammer A, Krokstad S, Romundstad P, Heggland J, Holmen J. The HUNT study: participation is associated with survival and depends on socioeconomic status, diseases and symptoms. BMC Med Res Methodol. 2012;12:143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Briggs A, Claxton C, Sculpher M. Decision Modelling for Health Economic Evaluation. Oxford (UK): Oxford University Press; 2008. [Google Scholar]
- 34. Akaike H. Statistical predictor identification. In: Parzen E, Tanabe K, Kitagawa G, eds. Selected Papers of Hirotugu Akaike. New York: Springer; 1998. p 137–51. [Google Scholar]
- 35. Schwarz G. Estimating the dimension of a model. Ann Stat. 1978;6(2):461–4. [Google Scholar]
- 36. Global BMI Mortality Collaboration, Di Angelantonio E, Bhupathiraju SN, Wormser D, Gao P, Kaptoge S. Body-mass index and all-cause mortality: individual-participant-data meta-analysis of 239 prospective studies in four continents. Lancet. 2016;388(10046):776–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Norwegian Directorate of Health. Innsatsstyrt finansiering 2009 [Activity based funding 2009]. 2009. Available from: https://www.helsedirektoratet.no/tema/finansiering
- 38. Norwegian Directorate of Health. KUHR-databasen [the KUHR database]. 2017. Available from: https://helsedirektoratet.no/kuhr-databasen
- 39. Norges bank. Valutakurser [Exchangerates] Available from: https://www.norges-bank.no/tema/Statistikk/Valutakurser/. Accessed 30 January 2020.
- 40. Finkelstein EA, Trogdon JG, Brown DS, Allaire BT, Dellea PS, Kamal-Bahl SJ. The lifetime medical cost burden of overweight and obesity: implications for obesity prevention. Obesity (Silver Spring). 2008;16(8):1843–8. [DOI] [PubMed] [Google Scholar]
- 41. Deb P, Norton EC, Manning WG. Health Econometrics Using Stata. 1st ed College Station (TX): Stata Press; 2017. [Google Scholar]
- 42. Williams R. Using the margins command to estimate and interpret adjusted predictions and marginal effects. Stata J. 2012;12:26. [Google Scholar]
- 43. Eddy DM, Hollingworth W, Caro JJ, Tsevat J, McDonald KM, Wong JB; ISPOR-SMDM Modeling Good Research Practices Task Force. Model transparency and validation: a report of the ISPOR-SMDM Modeling Good Research Practices Task Force-7. Med Decis Making. 2012;32(5):733–43. [DOI] [PubMed] [Google Scholar]
- 44. Jacobsen BK, Aars NA. Changes in body mass index and the prevalence of obesity during 1994-2008: repeated cross-sectional surveys and longitudinal analyses. The Tromsø Study. BMJ Open. 2015;5(6):e007859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. NCD Risk Factor Collaboration (NCD-RisC). Trends in adult body-mass index in 200 countries from 1975 to 2014: a pooled analysis of 1698 population-based measurement studies with 19·2 million participants. Lancet. 2016;387(10026):1377–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Rokholm B, Baker JL, Sørensen TI. The levelling off of the obesity epidemic since the year 1999—a review of evidence and perspectives. Obes Rev. 2010;11(12):835–46. [DOI] [PubMed] [Google Scholar]
- 47. Vanni T, Karnon J, Madan J, et al. Calibrating models in economic evaluation: a seven-step approach. Pharmacoeconomics. 2011;29(1):35–49. [DOI] [PubMed] [Google Scholar]
- 48. Kelly T, Yang W, Chen CS, Reynolds K, He J. Global burden of obesity in 2005 and projections to 2030. Int J Obes (Lond). 2008;32(9):1431–7. [DOI] [PubMed] [Google Scholar]
- 49. Ng M, Fleming T, Robinson M. Global, regional, and national prevalence of OW and obesity in children and adults during 1980-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2014;384(9945):766–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Delgado-Rodríguez M, Llorca J. Bias. J Epidemiol Community Health. 2004;58(8):635–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Cawley J. An economy of scales: a selective review of obesity’s economic causes, consequences, and solutions. J Health Econ. 2015;43:244–68. [DOI] [PubMed] [Google Scholar]
- 52. Tremmel M, Gerdtham UG, Nilsson PM, et al. 2017. Economic burden of obesity: a systematic literature review. Int J Environ Res Public Health. 2017;14(4):435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Kent S, Fusco F, Gray A, Jebb SA, Cairns BJ, Mihaylova B. Body mass index and healthcare costs: a systematic literature review of individual participant data studies. Obes Rev. 201718(8):869–79. [DOI] [PubMed] [Google Scholar]
- 54. Cawley J, Meyerhoefer C. The medical care costs of obesity: an instrumental variables approach. J Health Econ. 2012;31(1):219–30. [DOI] [PubMed] [Google Scholar]
- 55. Edwards CH, Aas E, Kinge JM. Body mass index and lifetime healthcare utilization. BMC Health Serv Res. 2019;19(1):696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Dixon P, Hollingworth W, Harrison S, Davies NM, Smith GD. The causal effect of adiposity on hospital costs: Mendelian randomization analysis of over 300,000 individuals from the UK Biobank. Available from: 10.1101/589820 [DOI] [PMC free article] [PubMed]
- 57. Lewer D, Jayatunga W, Aldridge RW, et al. Premature mortality attributable to socioeconomic inequality in England between 2003 and 2018: an observational study. Lancet Public Health. 2020;5(1):e33–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Fontaine KR, Redden DT, Wang C, Westfall AO, Allison DB. Years of life lost due to obesity. JAMA. 2003;289(2):187–93. [DOI] [PubMed] [Google Scholar]
- 59. Grover S, Kaouache M, Rempel P, et al. Years of life lost and healthy life-years lost from diabetes and cardiovascular disease in OW and obese people: a modelling study. Lancet Diabetes Endocrinol. 2015;3(2):114–22. [DOI] [PubMed] [Google Scholar]
- 60. Finkelstein EA, Brown DS, Wrage LA, Allaire BT, Hoerger TJ. Individual and aggregate years-of-life-lost associated with overweight and obesity. Obesity (Silver Spring). 2010;18(2):333–9. [DOI] [PubMed] [Google Scholar]
- 61. Hruby A, Hu FB. The epidemiology of obesity: a big picture. Pharmacoeconomics. 2015;33(7):673–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Squires H, Chilcott J, Akehurst R, Burr J, Kelly MP. A framework for developing the structure of public health economic models. Value Health. 2016;19(5):588–601. [DOI] [PubMed] [Google Scholar]
- 63. Swinburn BA, Jolley D, Kremer PJ, Salbe AD, Ravussin E. Estimating the effects of energy imbalance on changes in body weight in children. Am J Clin Nutr. 2006;83(4):859–63. [DOI] [PubMed] [Google Scholar]
- 64. Caro JJ, Briggs AH, Siebert U, Kuntz KM. Modeling good research practices—overview: a report of the ISPOR-SMDM Modeling Good Research Practices Task Force-1. Med Decis Making. 2012;32(5):667–77. [DOI] [PubMed] [Google Scholar]
- 65. Roberts M, Russell LB, Paltiel AD. Conceptualizing a model: a report of the ISPOR-SMDM Modeling Good Research Practices Task Force-2. Med Decis Making. 2012;32(5):678–89. [DOI] [PubMed] [Google Scholar]
- 66. Pineda E, Sanchez-Romero LM, Brown M, et al. Forecasting future trends in obesity across Europe: the value of improving surveillance. Obes Facts. 2018;11(5):360–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Norwegian Ministry of Health and Social Affairs. Meld.St.34 (2015-2016). Verdier i pasientens helsetjeneste – Melding om prioritering [The patients healthcare - prioritising]. 2016. Available from: https://www.regjeringen.no/no/dokumenter/meld.-st.-34-20152016/id2502758/
- 68. Puhl RM, King KM. Weight discrimination and bullying. Best Pract Res Clin Endocrinol Metab. 2013;27(2):117–27. [DOI] [PubMed] [Google Scholar]
- 69. Bixby H, Bentham J, Zhou B, et al. Rising rural body-mass index is the main driver of the global obesity epidemic in adults. Nature. 2019;569:260–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Statistics Norway. Døde [Dead]. Available from: https://www.ssb.no/statbank/table/05375/tableViewLayout1/. Accessed 18 August 2020.
- 71. Statistics Norway. Forventet levealder ved fødselen [Life expectancy at birth]. Available from: https://www.ssb.no/natur-og-miljo/barekraft/forventet-levealder-ved-fodselen. Accessed 18 August 2020.
- 72. Norwegian Directorate of Health. Forebygging, utredning og behandling av overvekt og fedme hos voksne [prevention, diagnosis and treatment of overweight and obesity in adults]. Available from: https://www.helsedirektoratet.no/retningslinjer/overvekt-og-fedme-hos-voksne/Overvekt%20og%20fedme%20hos%20voksne%20%E2%80%93%20Nasjonal%20faglig%20retningslinje%20for%20forebygging,%20utredning%20og%20behandling.pdf/_/attachment/inline/24ec824b-646d-4248-951f-db6b867ce6cb:4e0740b933ffd5bc03c8f0fdcab00b4135fe4ae9/Overvekt%20og%20fedme%20hos%20voksne%20%E2%80%93%20Nasjonal%20faglig%20retningslinje%20for%20forebygging,%20utredning%20og%20behandling.pdf. Accessed 31 January 2020.
- 73. Skjåkødegård HF, Danielsen YS, Morken M, et al. Study protocol: the FABO-study. BMC Public Health. 2016;16(1):1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-mdm-10.1177_0272989X20971589 for Modeling Obesity in Norway (The MOON Study): A Decision-Analytic Approach—Prevalence, Costs, and Years of Life Lost by Gudrun M. W. Bjørnelv, Vidar Halsteinli, Bård E. Kulseng, Diana Sonntag and Rønnaug A. Ødegaard in Medical Decision Making
Supplemental material, sj-docx-2-mdm-10.1177_0272989X20971589 for Modeling Obesity in Norway (The MOON Study): A Decision-Analytic Approach—Prevalence, Costs, and Years of Life Lost by Gudrun M. W. Bjørnelv, Vidar Halsteinli, Bård E. Kulseng, Diana Sonntag and Rønnaug A. Ødegaard in Medical Decision Making