Summary
There is a general consensus that overconsumption of sugar sweetened beverages contributes to the prevalence of obesity and related comorbidities such as type 2 diabetes (T2D). Whether a similar relationship exists for no, or low-calorie “diet” drinks is a subject of intensive debate and controversy. Here, we demonstrate that consuming seven sucralose sweetened beverages with, but not without a carbohydrate over 10 days, decreases insulin sensitivity in healthy human participants; an effect that correlates with reductions in midbrain, insular and cingulate responses to sweet, but not sour, salty or savory taste as assessed with fMRI. Taste perception was unaltered and consuming the carbohydrate alone had no effect. These findings indicate that consumption of sucralose in the presence of a carbohydrate rapidly impairs glucose metabolism and results in longer-term decreases in brain, but not perceptual sensitivity to sweet taste, suggesting dysregulation of gut-brain control of glucose metabolism.
Keywords: low-calorie sweetener, glucose tolerance, fMRI, midbrain, insula, mice, diabetes, obesity, indirect calorimetry, taste perception, substrate utilization
Introduction
Significant controversy exists over the effects of consuming no, or low-calorie sweeteners (LCS) on health. Human studies have reported that consumption of LCS is positively associated with weight gain and/or diabetes (Fowler, 2016; Fowler et al., 2008; Imamura et al., 2015; Nettleton et al., 2009), positively associated with lower BMI and weight loss (Greenwood et al., 2014; Miller and Perez, 2014; Wiebe et al., 2011), or unrelated to metabolic and body weight measures (Grotz et al., 2017a; Rogers et al., 2016), possibly due to methodological limitations (Toews et al., 2019). A similar inconsistency exists in the animal literature, with three recent reviews reaching three different and mutually exclusive conclusions (Fowler, 2016; Glendinning, 2016; Rogers et al., 2016). Given the growing use of LCS (Sylvetsky et al., 2012), especially in relation to the obesity and diabetes pandemics, it is of pressing importance to resolve the controversy surrounding LCS consumption.
Central to resolving this debate is defining and testing biologically plausible mechanisms by which LCS could lead to metabolic impairment. Several have been proposed (Burke and Small, 2016; Davidson and Swithers, 2004; Pepino, 2015; Sylvetsky and Rother, 2018). The binding of LCS to extra-oral taste receptors in the pancreas and intestine could influence glucose absorption by affecting glucose transporters SGLT-1 and GLUT2 or by altering glucose metabolism by promoting incretin release. Central mechanisms could also play a role. For example, it has been suggested that uncoupling sweet taste from energy receipt leads to a weakening of conditioned responses to sweet taste (Swithers, 2013). In this case, sweetness-elicited conditioned responses, such as release of incretins, which help regulate glucose metabolism, is hypothesized to be reduced, leading to the subsequent development of glucose intolerance (Swithers, 2013). Support for this uncoupling hypothesis comes from a series of studies in rodents reporting weight gain or glucose intolerance in rats consuming yogurts sweetened inconsistently with sucrose and LCS compared to rats consuming yogurts consistently sweetened with only sucrose (Davidson and Swithers, 2004; Davidson et al., 2011; Feijó et al., 2013; Foletto et al., 2016; Swithers et al., 2012, 2013).
In the current study we set out to test the sweet uncoupling hypothesis in humans. Forty-five healthy humans were randomly assigned to consume: (1) beverages sweetened with sucralose (sweet uncoupled from calories - LCS), (2) beverages sweetened with sucrose (sweet coupled with calories - Sugar), or (3) beverages sweetened with sucralose and combined with maltodextrin (Combo). Oral glucose tolerance test (OGTTs) (or single blood draws), sensory tests, and neuroimaging were conducted before and after participants consumed seven of their assigned beverages over 2-weeks in the laboratory. Protocol details and inclusion/exclusion criteria are listed in STAR Methods. We reasoned that if the uncoupling hypothesis is correct, then participants in the LCS, but not the Sugar or Combo groups should have reduced insulin sensitivity coupled with decreased brain and sensory response to sweet, but not sour, salty or savory taste. A parallel study was conducted in adolescents.
Results
Forty-five healthy young adults aged 20-45 who were non-regular consumers of LCS were recruited for the study. A parallel study was conducted with adolescents, aged 13-17, since adolescents go through a period of transient insulin resistance (Moran et al., 1999), a time of increased preference for sweet beverages and of intensive brain development (Casey et al., 2008; Giedd et al., 1999; Mills et al., 2014a, 2014b; Paus et al., 1999), especially for dopaminergic and prefrontal cortical circuits (Reichelt, 2016). In these studies, we assessed glucose tolerance and taste perception before and after participants consumed seven 355ml novel-flavored equi-sweet beverages over two weeks using randomized double-blind designs. These beverages were sweetened with either 0.06g sucralose (0 Kcal, uncoupled stimulus), equi-sweet 30.38g sucrose (120 Kcal, coupled stimulus) or a control beverage containing the same dose of sucralose plus 31.83g of the non-sweet carbohydrate maltodextrin (120 Kcal, coupled stimulus). In addition, we measured brain response to sweet, sour, salty and savory taste using functional magnetic resonance imaging (fMRI). We reasoned that if uncoupling sweet taste from energy affects sweet taste guided feeding and conditioned responses, then uncoupling should result in glucose intolerance and reduced brain and perceptual responses to the sweet taste of sugar relative to other tastes in the LCS group, but not in the other two groups. A study overview is given in Figure 1. Detailed participant demographics are provided in Table S1.
Figure 1: Human Study overview.
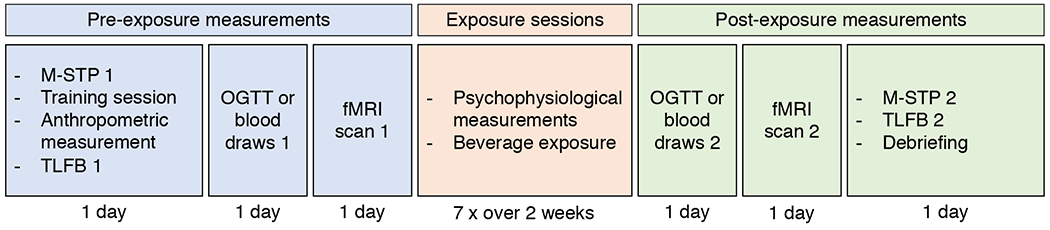
Participants visited the lab 13 times. Measurements were divided into pre-exposure measurements, exposure sessions and post-exposure measurements. NQ: Nutrition Questionnaire; M-STP: Monell forced-choice Sweet Taste Preference test; TLFB: time line follow back; fMRI: functional magnetic resonance imaging; OGTT: Oral Glucose Tolerance Test. See STAR Methods for more details.
Insulin sensitivity is reduced following consumption of sucralose with, but not without, maltodextrin
Glucose tolerance was assessed in young adults using the incremental area under the curve (iAUC) of blood plasma insulin and glucose during an Oral Glucose Tolerance Test (OGTT). An OGTT measures the physiological response to glucose consumption and is used to measure changes in glucose tolerance (see STAR Methods). We found a significant difference between the groups for first phase insulin response (time 0-30 min, F(2,36)=3.88, P=0.03) (Figure 2A) while we found no group differences in the first phase glucose response (F(2,36)=0.43,p=0.65). Contrary to the uncoupling hypothesis, post hoc tests revealed a larger first phase insulin response in the Combo group (i.e., exposed to sucralose plus maltodextrin) compared to the LCS and Sugar groups (exposed to sucrose alone or sucralose alone; false discovery rate corrected t tests; β=37.00%, P=0.03 and β=39.59%, P=0.03, respectively). In addition, when testing for changes across the full 120-minute OGTT period, change in AUC insulin also differed between the LCS and Combo groups (t(1,36)= 3.63, p(fdr)=0.003). Although, we found a baseline difference in sugar sweetened beverage consumption, we found no evidence that beverage consumption prior to the experiment influenced group differences found during the experiment (see Table S1, STAR Methods).
Figure 2. Changes in insulin sensitivity.
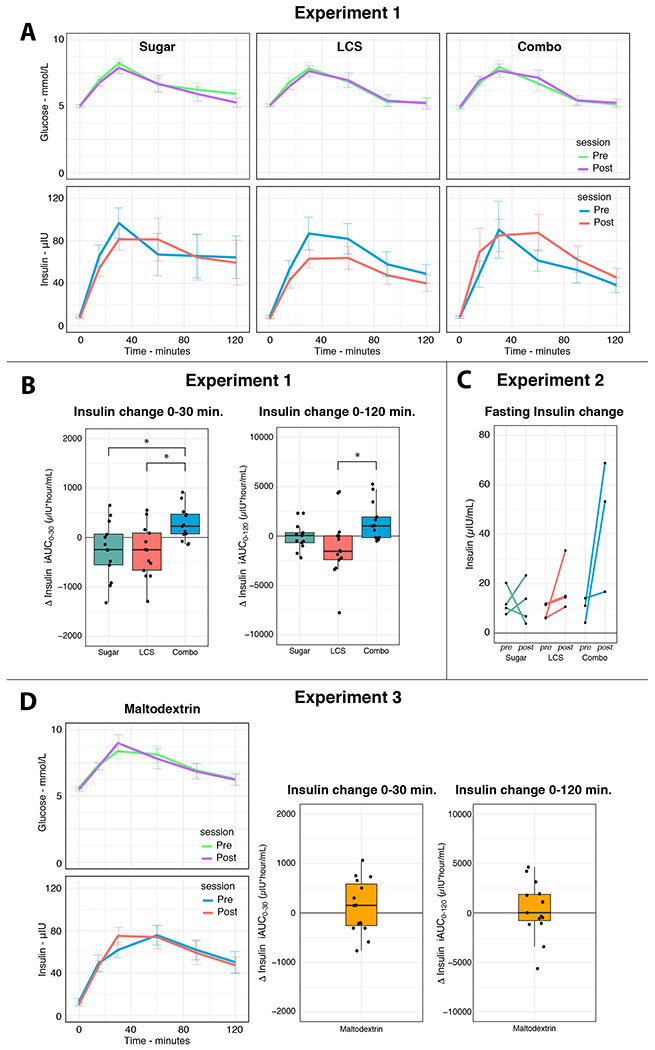
(A) Oral Glucose Tolerance Test (OGTT) blood plasma glucose (top row) and insulin (bottom row) for the pre and post beverage exposure measurements in young adults. (B) Relative change in first phase (i.e., 0-30 min) OGTT plasma insulin incremental area under the curve, iAUC0-30, (left) and OGTT plasma insulin iAUC0-120 (right) from pre to post beverage exposure and in young adults. Post beverage exposure, iAUC0-30 insulin was significantly elevated in the Combo group compared to the sugar and LCS groups (false discovery rate corrected t tests; both P=0.03). For iAUC0-120 insulin, change differed between the LCS and Combo groups (t(1,36)= 3.63, p(fdr)=0.003). (C) Change in plasma insulin plotted per individual in the adolescents study. The adolescent study was terminated because two participants in the Combo group showed highly elevated insulin (and HOMA-IR) levels post beverage exposure. Permutation testing (n=1000) indicated that the difference scores of this group are significantly different from the sucrose and sucralose groups together (p=0.043). Although this result is in line with the results from the adults study, it should be interpreted with care due to the low number of subjects. (D) An extra control group was recruited to rule out that maltodextrin, rather than the combination, leads to changes in insulin sensitivity. Results show no change in first phase OGTT plasma insulin iAUC0-30 nor in plasma insulin iAUC0-120 from pre to post beverage exposure in young adults.
In the adolescent group, we performed single timepoint blood draws to measure fasting blood plasma insulin and glucose. The Yale Human Investigations Committee recommended fasting blood draws for the assessment of glucose metabolism in adolescents rather than OGTTs because it is less invasive. Glucose tolerance in this group was assessed using the Homeostatic Model Assessment of Insulin Resistance (HOMA-IR). HOMA-IR quantifies the dynamic between fasting blood sugar and insulin response and is calculated as fasting insulin (microU/L) x fasting glucose (nmol/L)/22.5. Based on our findings in the adults we contacted the Yale Human Investigations Committee and they recommended halting enrollment in the adolescents, study, to examine the data for adverse effects. We found that HOMA-IR levels elevated from <3.5 to >12.9 in 2 out of 3 participants in the Combo group. This elevation was driven by an increase in fasting blood plasma insulin levels (Figure 2B). We reported this adverse event to the Human Investigations Committee, which recommended trial termination. While the small group numbers currently do not permit us to draw any firm conclusions, permutation testing (n=1000) indicated that the HOMA-IR difference scores of the Combo group are significantly different from the LCS and Sugar groups together (p=0.043).
Response to sweet, but not sour, salty, or savory taste in the ventral tegmental area, insula, putamen, and anterior cingulate cortex is inversely associated insulin sensitivity in the Combo group.
The uncoupling hypothesis states that uncoupling sweet taste from energy results in an impaired ability to use sweet taste to guide feeding. If so, we reasoned that brain response to sweet, but not the other tastes should change. To this end, we investigated the effect of beverage exposure on brain response to sweet taste and other basic tastes as control stimuli (sweet, sour, salty and umami – bitter was not used because of its lingering after-taste) by assessing blood-oxygen-level dependent (BOLD) changes in the brain using functional Magnetic Resonance Imaging (fMRI) in the adult study. We calculated fMRI-BOLD difference maps (post minus pre-beverage exposure) per taste on a single-subject level using mass univariate regression. At the group-level, we performed a mass univariate ANCOVA per basic taste to test whether brain response changed as a function of beverage exposure group while assessing the effect of insulin change as a covariate. Contrasting BOLD-difference maps between groups for each basic taste did not show any difference surviving a cluster-wise familywise error (FWE) correction threshold. However, regressing insulin iAUC difference scores on the BOLD-difference maps for sweet taste showed a strong negative relation in several limbic and mesolimbic areas (Figure 3, Table 1) in the Combo group. In this group, the left anterior insula, right middle insula, anterior cingulate, right ventral tegmental area, right putamen, and several cortical areas in the superior temporal gyrus and postcentral gyrus showed a decreased fMRI-BOLD response to sweet taste as a function of iAUC. We found no association between insulin change and central processing of umami, salty, or sour taste nor any associations between insulin change and taste perception in the LCS and Sugar groups.
Figure 3. Changes in brain response to sweet taste in human young adults.
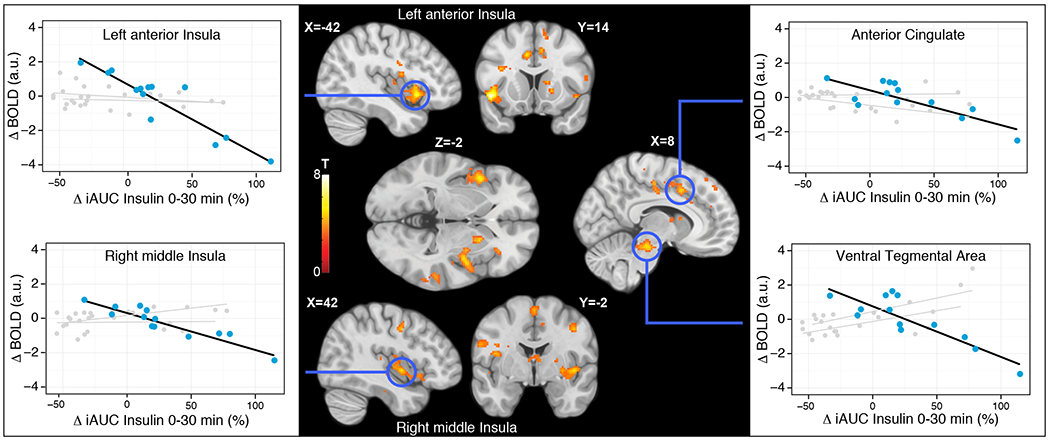
Relative change in plasma insulin iAUC0-30m from pre to post beverage exposure was significantly related to fMRI BOLD change in the young adults Combo group during sucrose ingestion. The relation indicates that percent increase in blood insulin is negatively associated with fMRI BOLD responses to tasting sugar in the anterior cingulate, left anterior insula, right substantia nigra/ventral tegmental area (VTA), and right middle insula. All reported clusters are corrected for a cluster-wise FWE correction threshold of P<0.05. Multiple coronal, sagittal and axial brain slices are shown at highlighted MNI stereotaxic coordinates. Activation color maps are thresholded at p<0.001 (unc.) for visual purposes and based on T-values associated with the negative linear relationship between fMRI BOLD and relative change in plasma insulin iAUC0-30m. Correlational graphs of this relationship are shown for peak voxels in the highlighted areas for the Combo group (blue), and for the sucralose and sucrose groups (grey).
Table 1. Negative relation between Δ insulin iAUC0-30m and brain response to sucrose.
The table shows the cluster-wise FWE corrected peak coordinates that show a decreased response when tasting sucrose as a function of increases in plasma insulin levels during the first 30 minutes of the OGTT in the adult Combo group. The contrast was masked for grey matter only voxels. L: left; R: right; STG: superior temporal gyrus; SN: substantia nigra; VTA: ventral tegmental area; ACC: anterior cingulate cortex; SMA: supplementary motor area; PCG: postcentral gyrus.
| Cluster | MNI {mm} | |||||
|---|---|---|---|---|---|---|
| Region | p(FWE) | size k (2x2x2mm) | T | x | y | z |
| L Insula | < 0.001 | 385 | 8.95 | −46 | 12 | −8 |
| L Insula | 4.73 | −38 | 10 | −14 | ||
| L Rolandic operculum | 4.69 | −58 | 4 | 8 | ||
| R STG | < 0.001 | 901 | 6.1 | 50 | −20 | 6 |
| R STG | 6.02 | 66 | −26 | 6 | ||
| R pInsula | 5.54 | 38 | −4 | −2 | ||
| SN/VTA | < 0.001 | 265 | 5.54 | 8 | −26 | −18 |
| R Cerebellum | 5.49 | 16 | −36 | −20 | ||
| R Cerebellum | 5.29 | 22 | −42 | −26 | ||
| ACC | < 0.001 | 462 | 5.47 | −8 | 14 | 36 |
| ACC | 5.27 | 8 | 12 | 42 | ||
| SMA | 4.79 | 0 | −2 | 62 | ||
| R STG | 0.041 | 103 | 5.44 | 62 | −36 | 20 |
| L PCG | < 0.001 | 255 | 5.25 | −58 | −20 | 20 |
| L PCG | 4.69 | −60 | −20 | 34 | ||
| L PCG | 4.62 | −50 | −22 | 26 | ||
| L PCG | 0.026 | 115 | 4.68 | −30 | −44 | 58 |
| L PCG | 4.25 | −30 | −32 | 62 | ||
| L PCG | 3.67 | −24 | −28 | 72 | ||
Taste intensity perception and preference is unaffected.
As sucralose is a high affinity ligand for the G-protein coupled sweet taste receptor, repeated consumption may result in receptor down regulation and, in turn, alter intensity perception affecting brain responses to sweet taste. We therefore also investigated the effects of LCS consumption on taste perception, and measured taste intensity ratings for sucrose, sucralose, citric acid (sour), sodium chloride (salty), monopotassium glutamate (umami), and sucralose+citric acid prior to each beverage exposure (7 times) across the two-week time period (Figure 1, Figure S2, and STAR Methods). We also assessed sweet concentration preference using a sucrose preference test pre and post beverage exposure. We found no differences in intensity perception or sucrose preference across the groups nor did we find an association between plasma insulin change and these measures (Figure S2 and STAR Methods).
No evidence that maltodextrin changes insulin sensitivity
Since the Combo group was included as a control group, we did not consider including a control group exposed to maltodextrin alone in the initial study. However, given that consuming the Combo stimulus unexpectedly produced changes in brain and insulin response to sugar we performed a follow-up experiment to determine if consuming maltodextrin alone caused changes in the insulin response during an OGTT. We found no evidence that consuming maltodextrin-containing beverages alters insulin sensitivity for either the first phase insulin response (time 0-30 min, t(14)=0.86, P=0.41) or the full 120 min. OGTT period (t(14)=0.55, P=0.59) (see Figure 2D). These results rule out the possibility that consuming maltodextrin alone accounts for the changes in insulin sensitivity observed in the first experiment.
Collectively, the findings from the two human studies refute the hypothesis that uncoupling sweet taste from caloric content causes metabolic dysfunction or decreases in the potency of sweet taste as a conditioned stimulus. Rather, the results reveal that metabolic dysfunction, coupled with reduced central sensitivity to sweet taste, occurs when an LCS is repeatedly consumed with, but not without a carbohydrate. Critically, while these findings fail to support the uncoupling hypothesis, they are nevertheless consistent with the results of the studies on which the hypothesis is based. More specifically, in these studies, LCS were added to yogurts that contained a number of nutrients including carbohydrates and thus metabolic dysfunction followed repeated simultaneous consumption of LCS and carbohydrates (Davidson and Swithers, 2004; Davidson et al., 2011; Feijó et al., 2013; Foletto et al., 2016; Swithers et al., 2012).
Discussion
The results of our study demonstrate that consuming sucralose with, but not without a carbohydrate rapidly impairs glucose metabolism. More specifically, in healthy human adults we observed reduced insulin sensitivity and blunted brain response to sucrose following consumption of seven 355ml beverages over two weeks, whereas no changes were observed following equal consumption of beverages with sucralose, sucrose, or maltodextrin alone. These results do not support the sweet uncoupling hypothesis. Rather, they suggest that sucralose consumption alters the metabolism of simultaneously consumed glucose to rapidly produce deleterious effects on metabolic health. Since the extent of this exposure is very likely experienced in a natural setting, our results provide evidence that LCS consumption contributes to the rise in the incidence of impaired glucose tolerance. They also indicate that the mechanism underlying this relationship involves acute LCS-induced alterations in glucose metabolism that are coupled with longer-term reductions in central sensitivity to sweet taste. Since sweet taste perception was unaffected, we suggest that the altered central responses reflect changes in central regulation of glucose metabolism.
The sweet uncoupling hypothesis
The current findings are consistent with the results of studies in rodents showing impaired glucose metabolism following repeated consumption of foods with added LCS (e.g., yogurt plus sucralose) (Davidson and Swithers, 2004; Davidson et al., 2011; Feijó et al., 2013; Foletto et al., 2016; Swithers et al., 2012, 2013). However, they refute the hypothesis that the impairment results from a decoupling of sweet taste with energy. First, in healthy adults who are non-regular consumers of LCS, repeated consumption of the sucralose beverage (i.e., group LCS), did not significantly influence glucose metabolism and produced no effects on brain or perceptual responses to sweet taste, despite being clearly rated as sweet-tasting and being decoupled from calories. Rather, in direct contradistinction, consuming a similarly sweet beverage containing the same dose of sucralose appropriately coupled to calories rapidly decreased insulin sensitivity. Second, the magnitude of the reduced insulin sensitivity was closely coupled to decreases in brain response to the sweet taste stimulus, whereas no main effects or correlations were observed with the responses to sweet taste in the LCS, Sugar or Malto groups. Although it is not possible to discern if this association results from altered central responses contributing to reduced insulin sensitivity or vice versa, it does suggest that central circuits, like peripheral glucose tolerance are altered by the exposure to the LCS only when it is coupled, rather than decoupled from calories. However, we can rule out alterations in sweet taste perception as a driver of the brain effects, which include primary gustatory cortex, since neither sweet taste intensity perception nor preference changed following exposure to any of the beverages (see STAR Methods and Figure S2). Third, the data from the adolescent study, though very preliminary, are consistent with the adult findings. While it is of high interest to further study this population, it will be important to establish durability and reversibility of these effects in adults, before further study commences. Collectively, the results from our experiments are consistent and lead to the conclusion that consumption of the LCS sucralose with, but not without a carbohydrate, produces metabolic dysfunction that is coupled with reduced sensitivity to sweet taste in a network of brain regions that includes primary taste and interoceptive regions in the insular cortex (Evrard, 2019).
Possible mechanisms
Our findings argue that uncoupling sweet taste from calories cannot be responsible for associations that are observed between LCS consumption and impaired glucose metabolism. Rather they point towards a mechanism that operates when LCS and carbohydrate are consumed concurrently. LCSs, including sucralose, bind to T1R2/T1R3 sweet taste receptors that are expressed in a variety of tissues including the oral cavity, intestine, liver, pancreas and brain (Laffitte et al., 2014). Activation of sweet taste receptors expressed in the intestine by LCSs produce up-regulation of sodium/glucose co-transporter SGLT-1 (Margolskee et al., 2007), which plays a role in glucose absorption and are implicated in the ability of dietary supplementation of LCS in piglets to increase weight gain (Shirazi-Beechey et al., 2014). The binding of LCS to intestinal taste receptor cells may also influence absorption via the translocation of GLUT2 (Kellett et al., 2008; Mace et al., 2007; Pepino, 2015). Considering the current study, maltodextrin is quickly metabolized into glucose, which would then be available to bind to intestinal taste receptor cells. Simultaneous binding of maltodextrin-derived glucose and sucralose could therefore increase glucose transport (by SLT-1 and/or GLUT2) beyond optimal levels for the amount of glucose present, resulting in acutely perturbed glucose homeostasis. Consistent with this possibility, in obese, but glucose tolerant humans, consuming sucralose compared to water prior to an OGTT, results in higher peak plasma glucose concentrations, increased insulin concentration and AUC, and decreased insulin sensitivity (Pepino et al., 2013). Importantly, this work excluded individuals who self-reported consuming more than the equivalent of 1 diet soda per week. In contrast, studies that have not excluded regular users have failed to find effects of LCS consumption on glucose metabolism (Brown et al., 2011; Ford et al., 2011; Ma et al., 2010; T. et al., 2012). However, as suggested by Pepino and colleagues, negative results would be expected if regular consumption of LCS impaired glucose tolerance. Our findings align with this proposal. Like Pepino and colleagues, we excluded individuals who self-reported consuming LCS more than three times per month. Further, examination of the TLFB questionnaire data indicated that participants consumed an average of 260 mls of diet drinks per week - which is less than three 355ml bottles per month. In this case, our intervention (seven, 355, ml bottles in two weeks) clearly increased consumption above baseline levels and, as would be predicted from the acute effects observed by Pepino and colleagues, resulted in a longer-term decrease in insulin sensitivity. Critically, in our study, sucralose was not consumed prior to the OGTT. Therefore, the observed decrease in insulin sensitivity must be attributed to a chronic effect of consuming the Combo beverage on glucose tolerance.
Another possibility is that change in insulin sensitivity results from alterations in central regulation of glucose metabolism. We observed that changes in brain response to sweet but not sour, salty or savory taste, were proportional to changes in plasma insulin release following the glucose challenge. This suggests that the two effects of consuming the Combo drinks are linked but implies nothing about directionality. The possibilities are that (1) there is a common mechanism affecting peripheral insulin release and brain response to sweet taste; (2) peripheral insulin affects, brain response to sweet taste or; (3) brain response to sweet taste affects insulin secretion. Future studies are needed to test these alternative possibilities, but our findings point to two potential pathways by which consuming the Combo beverages might alter central regulation of insulin secretion.
First, greater reductions in insulin sensitivity were correlated with greater reductions in BOLD response to sweet taste in the midbrain and striatum. The midbrain houses dopamine neurons that project to striatal regions important for encoding oral and post-oral reinforcing signals from food (Tellez et al., 2016). In mice, the sensation of sweet taste increases extracellular dopamine in the striatum (de Araujo et al., 2008) and in humans, changes in dopamine binding potential, indicative of dopamine release, occur in the midbrain and striatum upon consumption of a sweet milkshake and these binding potential effects correlate with BOLD response to milkshake observed in the same subjects. (Thanarajah et al., 2019). It is therefore possible, that the BOLD effects we observed reflect reduced dopaminergic response to sweet taste. This is of interest because manipulating central dopamine circuits can influence peripheral insulin sensitivity (Ter Horst et al., 2018). Accordingly, in humans, peripheral insulin resistance correlates with dopamine type 2 receptor availability (Dunn et al., 2012) and in fruit flies, chronic exposure to sucralose alters the equivalent of insulin and dopamine systems leading to glucose intolerance (Wang et al., 2016). It is therefore possible that repeated consumption of the LCS with the carbohydrate lead to reductions in sweet evoked dopamine responses resulting in reduced insulin sensitivity.
Second, correlations between changes in peripheral insulin sensitivity and BOLD response to sweet taste were observed in the gustatory and interoceptive sensory areas as well as the left anterior agranular output region of the insular cortex (Craig, 2002; Small, 2010). The effect in gustatory cortex does not reflect sweet taste perception, which did not change, and cannot be accounted for by diminished association between sweet taste and nutrients, since similar effects were not observed in LCS group. Interoceptive cortex corresponds to the region where afferents that monitor the ongoing physiological status of the organs and the tissues of the body, including the gut, terminate. These sensory regions form a feed-forward circuit projecting to the anterior agranular insular cortex where Von Economo projection neurons are found (Evrard, 2019). Here the correlation between changes in BOLD response to sweet taste and changes in peripheral insulin response was strongest in this area of left anterior insula. This is of interest because the Von Economo cells target midbrain and brainstem autonomic nuclei and are proposed to convey interoceptive error attenuation commands via top-down sensory gating and subsequent regulation of autonomic control (Critchley and Seth, 2012; Seth and Friston, 2016).
This raises the possibility that repeated consumption of the Combo beverage resulted in reduced sweet taste evoked responses in sensory insula leading to diminished autonomic outflow and, in turn, reduced midbrain response. This proposal is in line with the observation that sweet taste perception regulates dietary induced thermogenesis (DIT) in response to maltodextrin consumption (Veldhuizen et al., 2017). More specifically, DIT, which depends on autonomic outflow (Ahrén, 2000; Rodriguez-Diaz et al., 2011; Taborsky, 2011), is diminished when sweetness is either too sweet or not sweet enough given the caloric load. In this case, the acutely altered DIT may well result from “mismatched” sensory gating of autonomic outflow. Again, future work is needed to test these hypotheses, but the current findings add to accumulating evidence that central responses to sweet taste play a role in glucose metabolism.
Resolving the inconsistencies in the literature.
As mentioned above, although our results fail to support the uncoupling hypothesis, they are nevertheless consistent with the results of the studies on which this hypothesis is based since LCS was added to carbohydrate-containing foods and therefore parallel our Combo groups. In many rodent studies reporting a negative impact of LCS on metabolism, LCS (e.g., saccharin, aspartame, sucralose, AceK) were either added to a carbohydrate or a carbohydrate-containing yogurt stimuli ranging from 0.4 to 0.6 kcal/g (Davidson et al., 2011; Feijó et al., 2013; Foletto et al., 2016; Suez et al., 2014; Swithers et al., 2009). Similarly, in human randomized control trials (RCTs) reporting that LCS consumption impairs metabolism, LCS were consumed concomitant to carbohydrates (Brown et al., 2009; Pepino et al., 2013; Sylvetsky et al., 2016; Temizkan et al., 2015). Critically, when study protocols promote consumption of LCS alone or in capsules at home during meal times, studies fail to find a negative impact on metabolism (Baird et al., 2000; Grotz et al., 2003, 2017b; Steinert et al., 2011). This suggests that LCS may have different effects depending on how they are consumed, with greater likelihood for impairment when LCS are provided in conjunction with carbohydrate.
Another important factor, as proposed by Pepino and colleagues is that results may depend on individual factors like prior experience consuming LCS. More specifically, including individuals who are regular users of LCS may bias towards negative findings because these individuals might already be affected and would therefore be less likely to show a change upon additional limited small exposures. A further important issue is that LCS are biochemically heterogeneous and have diverging bioactive effects. Sucralose is the most commonly used LCS, but there are several other in frequent use and possessing different pharmacokinetics (i.e. absorption, distribution, metabolism and excretion) (Schiffman, 2012). Several studies (reviewed in (Chan et al., 2017)) suggest that the effects of LCS on glucose transporters and subsequent absorption are strongest for Ace-K and weak, or absent for aspartame. For example, sucralose and Ace-K, but not aspartame, increase SGLT-1 mRNA expression, which correlates with absorption rate (Margolskee et al., 2007). In addition, Ace-K and sucralose, but not aspartame, increase insulin secretion (Liang et al., 1987a, 1987b). One reason why aspartame may produce less effects on incretins and glucose absorption is that it is rapidly metabolized in the small intestine and would therefore have less opportunity to bind to taste receptor cells or glucose transporters. Given the potential for insights into mechanisms as well as importance for health, future work should focus on comparing different categories of LCS within the same study.
Summary and Implications
The results from our studies demonstrate that LCS consumption produces metabolic dysfunction when it is consumed with, rather than uncoupled from, a carbohydrate. This implies that (a) carbohydrate metabolism is altered in the presence of the LCS sucralose and (b) that this alteration leads to decreases in peripheral and central sensitivity to sugar and sweet taste. Of particular relevance to the potential significance of this work, the metabolic changes observed following a very limited exposure that almost certainly occurs in freely living humans - especially if one considers the consumption of a diet drink along with a meal. This raises the possibility that the combination effect may be a major contributor to the rise in the incidence of type two diabetes and obesity. If so, the addition of LCS to increase the sweetness of carbohydrate containing food and beverages should be discouraged and consumption of diet drinks with meals should be counseled against.
Limitations of Study
There are a number of limitations in the current work that should be considered as caveats. Since the adolescent study was terminated by the Yale HIC, the sample size is very small, and may therefore have an artificially inflated effect size that would increase the type 2 error rate. In addition, the duration of exposure was short and did not allow us to determine if the observed changes are transient. Relatedly, we cannot know if these effects are reversible as our design did not include a “wash-out” period. Finally, we only assessed the effects of one LCS. It is possible that similar effects would not be obtained with other LCSs.
STAR METHODS
LEAD CONTACT AND MATERIALS AVAILABILITY
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Dana Small (dana.small@yale.edu). This study did not generate new unique reagents.
EXPERIMENTAL MODEL AND SUBJECT DETAILS
Participants were recruited through advertisements around Yale University and the greater New Haven area. Participants were screened either over the phone or through an online screening form. Participants aged 23-45 years were assigned to groups matched for sex, age, and BMI. Exclusion criteria were obesity (BMI>30), frequent NNS-user (self-report of > 3 times a month), history of psychiatric disorders, eating disorders or head injury with loss of consciousness, being on a diet, alcoholism, tobacco or drug use, use of daily medication other than monophasic birth control, chemosensory impairments, lactose intolerance, food allergies, and ineligibility for an fMRI scan. The study was approved by the Yale Human Investigations Committee and all participants provided written informed consent at the start of their first lab visit. Subjects were assigned to groups by a lab member not involved in data collection. Assignment was semi-random while ensuring there were no significant differences in age, gender and BMI across groups. Exposure beverages were presented double-blind.
This study was funded under an NIH grant with the overarching aim of studying central taste processing in humans. Therefore the sample size was based on the neuroimaging outcome. More specifically, the study was powered to detect between-group differences in amygdala and insula response to sweet taste vs. tasteless using data from six subjects that participated in a pilot study looking at brain response to sweet, sour, salty and savory tastes using the “Fmripower” tool (Mumford and Nichols, 2008), which showed that 27 subjects provided 73-91% power over insula and amygdala.. The primary planned analysis was to be conducted with the SPM software (as described in the Quantification and Statistical Analyses section) using second-level group analyses to enable within-group repeated measures comparisons using flexible factorial random effects models to test the prediction that response would decrease to sweet, but not sour, salty and savory taste. However, data collection was cut short when the fMRI scanner was serviced and upgraded, rendering our acquisition protocol incompatible.
The adolescent study (experiment 2), approved in an addendum under the parent R01, was projected to be 15 subjects per group, as this was a pilot study. Experiment 3 was a control experiment designed to test if consuming maltodextrin alone (rather than in combination with sucralose) alters insulin response. Sample size was projected to be 15 to match the sample sizes in experiment 1.
Experiment 1:
55 adult participants were recruited, 6 dropped out during the experiment, blood sampling during at least one full OGTT failed for 4 participants, and 6 participants later revealed in a timeline follow back measurement that they were regular users of NNS. Perceptual, blood, and brain data of these subjects were discarded before data analysis. Data analysis was performed on data from 39 adult participants (13 per group; 21 women; mean age 27.79 ± 3.96; mean BMI 23.72 ± 3.13). Experiment 1 was registered on 10-24-2014 as Clinical Trial Registration Number NCT02335021 associated with the first submission of R01DC006706 (06-21-2013). The outcome variables included sweet taste perception, brain response to sweet taste and food intake. This experiment was revised on the funded resubmission (10/22/2014), which included sweet taste perception, food intake, neural response to sweet taste as well as GLP-1, insulin and glucose response to glucose ingestion (OGTT) as the main outcome variables. The GLP-1 data was analyzed but results suggested that the samples were contaminated because values were not physiologically possible. The clinical trial registry was not updated to reflect these changes, but the study design is available upon request as the submitted R01. Data were collected from 1-9-2015 to 4-24-2019.
Experiment 2:
17 adolescents were recruited, 2 refused blood sampling, 3 dropped out, and 1 group assignment was lost in a software crash. Participants, aged 13-17 years were recruited similarly to the adult study, with the same inclusion and exclusion criteria, apart from the age range. The outcome variables were sweet taste perception, brain response to sweet taste and fasting insulin and glucose. The study was approved by the Yale Human Investigations Committee and all participants provided written informed assent at the start of their first lab visit together with their parents who provided parental consent. Before the Yale Human Investigations Committee advised study termination, Data analysis was performed on data from 11 adolescents (8 women; mean age 15.95 ± 1.37; mean BMI 22.13 ± 3.63). Data collection occurred from 2-20-2017 to 7-6-2017.
Experiment 3:
16 young adult participants were recruited between 3-5-2018 and 1-3-2019 for a control maltodextrin experiment, 1 participant dropped out. Experiment 3 included all procedures of Experiment 1 with exception of the fMRI measurements/training. The outcome variables were sweet taste perception and glucose and insulin response to glucose ingestion (OGTT). Data analysis was performed on data from 15 participants (8 women, mean age: 29.40±4.81; mean BMI: 24.88±3.54). The additional experiment was approved by the Yale Human Investigations Committee and all participants provided written informed consent at the start of their first lab visit.
METHOD DETAILS
General Procedure
After screening and acquiring informed consent, participants were assigned to either the Sugar, LCS, or Combo group for experiment 1 and 2. Participants completed a nutrition questionnaire (NQ) to screen for inclusion and exclusion criteria, two sucrose preference tests (pre and post), a training session, anthropometric measurement, two Monell forced-choice sweet taste preference tests (M-STP; pre and post), two timeline follow back sessions (TLFB; pre and post), two blood sampling sessions (adults completed oral glucose tolerance tests, OGTTs) while adolescents completed fasting blood draws), two fMRI scanning sessions (pre and post), seven psychophysiological measurements, seven beverage exposures, and a debriefing (Figure 1). Exposure sessions were conducted on separate days within 2 weeks.
On the first pre-exposure session, the exposure sessions, and last post-exposure session, participants arrived at the lab after a 1h fast. For blood sampling, participants arrived after a 10-12 hour overnight fast. For fMRI scans, participants were instructed to arrive neither hungry nor full on each scan day.
Participants in experiment 3 underwent the same protocol with the exception of fMRI scans and training.
Training session & anthropometric measurement
A pregnancy/toxicology screening was performed, and height was measured using a stadiometer. Body weight and body fat percentage were measured using the BodPod body composition tracking system (Dempster and Aitkens, 1995) in minimal attire (spandex shorts and sports bra for women). Following anthropometric measures, participants were trained to make computerized ratings of their internal state as well as the perceptual qualities of various stimuli on computerized scales. Internal state ratings were made up of a series of adapted cross-modal gLMS consisting of a 100mm vertical line scale with the labels “barely detectable” at the lower endpoint and “strongest imaginable sensation” at the upper endpoint (Bartoshuk et al., 2004; Green et al., 1996). Participants were instructed to rate the intensity of their feelings of hunger, fullness, thirst, anxiety, and need to urinate. The perceptual qualities of real and imagined stimuli consisted of ratings of their overall intensity, liking, and wanting to eat. Liking was measured using a labeled hedonic scale consisting of a 100 mm vertical line scale with the labels “most disliked sensation imaginable” at the lower anchor point, “most liked sensation imaginable” at the upper anchor point, and “neutral” in the middle. Wanting to eat was rated on 200 mm visual analog scales labeled on the left with “I would never want to consume this” and “I would want to consume this more than anything” on the right. Participants also rated the perceptual qualities of basic tastes (sucrose, 0.56M; citric acid, 18mM; NaCl, 0.32M; quinine, 0.18mM, and MPG (100mM) alone and when combined as binary taste mixtures (sucrose-citric acid, sucrose-quinine, sucrose-MPG, citric acid-NaCl and NaCl-quinine). Participants rated the sweetness, sourness, saltiness, bitterness, and umami intensity of each taste using the gLMS. In addition, the experimenter assisted the participant in completing a TLFB questionnaire in which all beverages (besides water) consumed over the previous 14 days were written down, including brands and amounts. This questionnaire ensured that participants were not regular users of NNS.
Lastly, participants underwent an fMRI training simulation to familiarize themselves with the paradigm, learn to remain still in the scanner, and reduce anxiety on the day of the scan (see fMRI sessions for more details).
Beverage exposure sessions
Stimuli
For experiment 1 and 2, exposure beverages contained 355ml of a novel-flavored equi-sweet solution. Beverages contained either 0.06g sucralose (0 Kcal, Sigma-Aldrich Inc. MO, USA), 30.38g sucrose (120 Kcal), or 0.06g sucralose and 31.83g of the non-sweet carbohydrate maltodextrin (120Kcal) (Maltodextrin, FCC, M1083, Spectrum Chemical Mfg. Corp.). Beverages were colored and flavored according to the preference of each participant. Participants could choose any color (1-3 drops; McCormick & Co, Inc. MD, USA, Assorted food color & egg dye: Red, Yellow, Green, Blue; McCormick & Co, Inc. MD, USA, NEON! Food color & egg dye: Purple, Green, Pink, Blue) and between an Aloe Vera or Papaya flavor (0,355ml; Aloe Vera, Bell Labs, ID#:141.31480; Papaya, Bell Labs. ID#102.82506). We used novel flavors and colors to reduce the possibility that prior associations with the flavors would influence the predicted outcomes.
For experiment 3, exposure beverages contained 355ml of a novel-flavored non-sweet solution. The beverage contained 31.83g of maltodextrin (Maltodextrin, FCC, M1083, Spectrum Chemical Mfg. Corp.). Beverages were colored and flavored as described above.
Procedure
Subjects were invited seven times to the lab across a time span of two weeks. Subjects were first asked to perform a psychophysiological measurements measuring perceptual taste thresholds. Subsequently, subjects received their respective exposure beverage and were asked to finish the drink within five minutes.
Blood sampling sessions.
In the young adults (i.e., experiment 1 and 3), we performed OGTTs. Upon arrival, an indwelling intravenous line was placed by an experienced nurse or phlebotomist, followed by a 20 min rest period in order to limit any stress of the catheter placement on the blood measures. Participants were asked to fully consume (within ~2min) an orange-flavored drink containing 75 g of dextrose (10 oz, Trutol, VWR, Radnor, PA). Blood was drawn at 0, and then 15, 30, 60, 90 and 120 min post-drink and immediately placed into tubes. For adolescents, only one fasting blood sample for measurement of HOMA-IR was taken pre and post exposure.
fMRI scans
Stimuli and Delivery
Taste stimuli for fMRI scans included a sweet sucrose solution (0.32 M), a sour citric acid solution (0.0056 M), a salty sodium chloride solution (0.14 M), an umami monopotassium glutamate solution (68 mM), and a tasteless and odorless solution.
A custom-designed gustometer was used to deliver liquid stimuli. This system has been successfully used in past fMRI studies (de Araujo et al., 2013; Bender et al., 2009; Veldhuizen et al., 2007). This gustometer system is a fully portable device that consists of a laptop computer that controls (via a 9-pin serial adaptor and telephone wiring) up to 11 independently programmable BS-8000 syringe pumps (Braintree Scientific, Braintree, MA) to deliver precise amounts of liquids to subjects lying in the mock or real scanner at precisely timed intervals and durations. The pumps, which infuse liquids at rates of 6-15 mL/min, are controlled by programs written using Matlab 7.11 (MathWorks Inc., Sherborn, MA) and Cogent2000 v1.25 (Wellcome Department of Cognitive neurology, London, UK). Each pump holds a 60 mL syringe connected to a 25-foot length of Tygon beverage tubing (Saint-Gobain Performance Plastics, Akron, OH) with an inside diameter of 3/32”. All tubing terminates into a specially designed Teflon, fMRI-compatible gustatory manifold (constructed in the Pierce Laboratory Electronics and Machine Shop), which is anchored to the MRI headcoil and interfaces with the subject. This set-up is depicted in a close-up (Figure S1A), with the subject in the mock scanner (Figure S1B).
The gustometer mouthpiece or “manifold” was designed to deliver up to 11 taste solutions and one tasteless rinse. All tastants and rinses pass through 1-mm channels that converge at a central point at the bottom of the manifold for delivery to the tongue tip. To prevent the subject’s tongue from coming in contact with the 1mm holes, and to ensure the liquids flow directly onto the tongue, a short silicone tube is attached to the outflow point under the 1-mm holes. The subject holds the silicone tube between their lips and teeth, and the tip of the tongue rests up against the lowest point of the tube. A large vent hole prevents subjects from drawing or sucking the stimulant through the manifold at uncontrolled times or rates. Tactile stimulation is held constant across all events (i.e. delivery of the different tastants and the tasteless solutions) by the use of converging outflow – so that the liquid arrives at the same location for each stimulus. The gustometer manifold is mounted by rigid tubing onto an anchoring block that clamps onto the front of the head coil. The anchor height and horizontal positions are adjustable via two knobs accessible to the subject and the experimenter to achieve the most comfortable position. The manifold is then locked in place for the duration of the scanning run. This setup has previously been described by Veldhuizen et al (Veldhuizen et al., 2007).
All scans were scheduled between 10am and 3pm. Sweet, sour, salty, umami, and tasteless stimuli were presented in a block design across two functional imaging runs. During each block, 4 to 8 uncued taste stimulus presentations were presented with a volume of 0.75ml delivered over 2s followed by a 7s swallowing period. Each taste block was presented four times and block length varied between 36 to 54 seconds. The order of blocks was counterbalanced across subjects. Each taste block was followed by a rinsing period (0.75 ml deionized water over 2 seconds). Blocks were separated with a 10 second rest-period.
MRI scans were performed using a Siemens 3.0 Tesla TIM Trio scanner at Yale University Magnetic Resonance Research Center equipped with a 32-channel head coil. A T1-weighted 3D MPRAGE whole brain image was acquired for anatomical reference. Acquisition parameters: TR/TE: 1900ms/2.52ms; flip angle: 9°; FOV: 250; matrix: 256 × 256; slice thickness: 1 mm; number of slices: 176, scan duration = 4:18 min. T2*-weighted functional brain images were acquired using a multiband susceptibility-weighted single-shot echo planar imaging sequence. Acquisition parameters: TR/TE: 1000ms/30ms; MB=4; iPAT=2; FA=60°; FOV=220 mm; matrix=110×110; slice thickness=2 mm; bandwidth:1976 Hz/Px. Each functional taste run lasted for 12:02 minutes. The first 2 volumes of each run allowed the MR signal to equilibrate (“dummy images”).
Following scans, participants were offered a bowl of Annie’s macaroni and cheese to test if treatment influenced food intake, defined as differences in the weight of the bowl before versus after the meal (converted to Kcal). If all the food was consumed, additional portions were served until participants decided they did not want to eat any more.
TLFB Questionnaire
Procedure
The TLFB (Sobell et al., 1996) was used to estimate LCS beverage intake as a proxy for LCS intake. TLFB is procedure that is most commonly used to assess alcohol intake. The TLFB method is superior to straight recall because the time points improve recall. The TLFB was administered by the experimenter who asked participants to retrospectively report their daily beverage intake for the 14 days prior to the interview. The TLFB interviewer used an annotated calendar, started with the day of the interview and asked the participant to report all consumed beverages of that day and estimate the consumed volume by using plastic replicas of different types of drinking glasses as examples. This procedure was repeated 14 times in reverse chronological order. The TLFB was performed pre and post beverage exposure.
Sweet taste preference
Stimuli
We used the sucrose concentrations 3% - 0.09M, 6% - 0.18M, 12% - 0.35M, 24% - 0.70M, and 36% - 1.05M.
Procedure
The M-STP (Mennella et al., 2011) task was performed pre and post beverage exposure to investigate psychophysiological changes in sweet taste preference. Subjects sampled (sip-and-spit) two cups containing 10 ml sucrose dilutions and chose the one they preferred in a forced choice setting. If the subject selected the higher concentration, the next two cups presented was the selected beverage plus the next highest concentration. If the subject selected the weaker concentration, the next two presented cups were the weak solution plus the next lowest concentration. Forced choices were presented until the subject selected the same concentration two times in a row. The procedure was completed twice; starting at 3% and 36% sucrose, respectively.
Psychophysiological measurements
Stimuli
Taste stimuli included a sweet sucrose solution (0.32 M), a sour citric acid solution (0.0056 M), a salty sodium chloride solution (0.14 M), an umami monopotassium glutamate solution (68 mM), a sweet sucralose solution (0.588 mM) and a sweet and sour sucralose + citric acid solution (0.588 mM + 0.009 M).
Procedure
Prior to each beverage exposure, we measured taste intensity perception to test for possible changes as a function of group during each exposure session. Participants were presented with a tray of 18 medicine cups, containing 10 ml of 3x the six taste and one mixture solution (Sucrose, Sucralose, Citric Acid, NaCl, MPG, Sucralose+Citric Acid, see section 1.3). All tastes were presented three times in a randomized order. Participants were asked to sip the solution, swirl it around in their mouth and spit it in the sink, after which they made ratings of the sweetness, saltiness, sourness, umami and general intensity of the solution. A 30 second wait-period between trials was used to rinse at least three times with deionized water. After completing the ratings for all 18 samples, participants were provided with their respective exposure beverage and were asked to finish the drink within five minutes.
Post-test sessions.
At the end of the experiment, participants were invited once more to perform the second M-STP and to fill out another TLFB questionnaire to measure whether participants changed their NNS consumption. Subsequently, participants were debriefed about the goal of the study.
QUANTIFICATION AND STATISTICAL ANALYSIS
Blood sampling sessions.
For experiment 1 and 2, blood samples were centrifuged, frozen immediately and stored at −80°C until analysis. Plasma glucose was analyzed using the YSI Life Sciences 2300 STAT PLUS Glucose and L-Lactate Analyzer. Plasma insulin (sensitivity: 0.1817 mU/L (1.09 pmol/L)) was measured using insulin ELISA Jumbo kits (ALPCO, Salem, NH). All samples were analyzed in duplicate. The sample average was used for statistical analysis.
Statistical analysis was performed in R. For the young adults in experiment 1, 8 out of 480 blood samples were missing at random. Missing values were imputed separately for plasma glucose and insulin using a Principal Components Analysis model (Josse and Husson, 2013) available in the package missMDA (version 1.13). We used this form of imputation as it uses the interrelation across measurement time points for insulin and glucose curves, respectively. Subsequently, incremental area under the curve (iAUC) values for plasma insulin and glucose were calculated for the first 30 minutes of the OGTT using the auc-function in the MESS-package (version 0.5.2). We then calculated absolute and relative iAUC difference scores (%). To test for group differences in insulin levels, ΔInsulin iAUC0-30m was entered in a linear model as dependent variable while group ID was entered as independent variable to test for group differences. In a similar model, we tested for differences in glucose levels by entering ΔGlucose iAUC0-30m as a dependent variable while group ID constituted the independent variable. For completeness, we also investigated the results for the complete OGTT AUC using an identical statistical analysis procedure. Additionally, we calculated the Matsuda index, a measure of whole-body insulin sensitivity (Matsuda and DeFronzo, 1999) and Hepatic insulin resistance index (Abdul-Ghani et al., 2007).
Matsuda index:
Hepatic insulin resistance index:
We found no group differences for the Matsuda index. However, there were differences among the groups for the Hepatic insulin resistance index (F(2,36)=3.79, p= 0.03). The effects are similar to the iAUC0-30 results. Furthermore, we found a difference between the LCS and Combo group for the OGTT AUC0-120 and incremental AUC0-120. The results are given in Figure 2 and Table S2.
To explore the influence of sex on the results, we have performed additional model comparisons for the insulin sensitivity analyses on the OGTT data. We found no evidence that adding the interaction group x gender improved the model fits (Δinsulin iAUC0-30m: F(3,33)=0.31, P=0.82; Δinsulin iAUC0-120m: F(3,30)=0.31, P=0.83)
For Experiment 3, glucose samples were analyzed by Yale Center for Clinical Investigation Core Laboratory Services (Glucose, plasma(ace)). Plasma insulin (sensitivity: 0.1817 mU/L (1.09 pmol/L)) was measured using insulin ELISA Jumbo kits (ALPCO, Salem, NH). One glucose sample was missing and imputed using the procedure reported for Experiment 1.
fMRI scans
fMRI data were analysed using SPM12 (v6906, Wellcome Trust Centre for Neuroimaging, http://www.fil.ion.ucl.ac.uk/spm) running in Matlab 2016b (The MathWorks Inc., Natick, MA). The first 2 dummy images from each functional run were removed. Subsequently, functional images from both visits were realigned, co-registered to the T1-weighted anatomical image acquired during the first visit, normalized to MNI space, and smoothed with a 6 mm FWHM kernel. For first-level statistical analysis, we constructed mass-univariate general linear regression models for each participant. The regressors included: 1) conditions ‘Sweet’, ‘Sour’, ‘Salty’, ‘Umami’, and ‘Tasteless’, and 2) the realignment parameters and their first derivatives as covariates (Friston et al., 1996). Task-related regressors were convolved with the canonical hemodynamic response function (HRF) and a high-pass filter of 128 seconds was applied. Prior to group analyses, we calculated difference maps [post scan - pre scan] per taste condition. On group-level we performed a mass univariate ANCOVA on these difference maps per basic taste to investigate whether brain responses changed as a function of beverage exposure group. To test whether changes were attributed to dysregulation in insulin signaling, we entered ΔInsulin iAUC0-30m as covariate. FWE correction for mass univariate analyses was performed on cluster level.
TLFB Questionnaire
In addition to measuring LCS consumption using the TLFB as control exclusion criterion, we tested whether our experimental manipulation affected sugar sweetened beverage (SSB) consumption outside of the experiment. To test for (a) any group differences in SSB consumption, (b) group x time (pre vs post exposure) differences in SSB consumption, and (c) any associations between ΔInsulin iAUC0-30m and SSB consumption, we performed two linear mixed models (LMMs) using package LME4 (version 1.1-7) (Pinheiro and Bates, 2000). Subsequent statistical tests on the LMMs were performed using the Satterthwaite’s approximation for the degrees of freedom, provided in the package ImerTest (version 2.0-11, http://cran.r-project.org/package=ImerTest) (Kuznetsova et al., 2014). As dependent variables, we entered drinking days and amount consumed for the first and second model, respectively. As independent variables, we entered a group x time interaction and a group x ΔInsulin iAUC0-30m interaction. Reported model results are based on the type III Analysis of Variance Table of the LMMs.
As drinking days and consumed volume (in ml) are count variables, we took extra care in optimally fitting the LMMs to the data. As LMMs assume that the residual error distribution of the model is normally distributed, we excluded outliers with a residual error greater than 2.5 standard deviations from 0 using the ‘romr.fnc’-function, available in LMERConvenienceFunctions (version 2.5) (Baayen, 2008; Newman et al., 2012). Subsequently, we refitted the models to the trimmed data if necessary. Residual errors in the LMM fitted on drinking days contained no outliers, whereas 4 outliers (5.1%) were removed based on the LMM fitted on consumed volume. The resulting residual error distributions of both models did not deviate from normality (Shapiro-Wilk W=0.982, p=0.34 and W = 0.9924, p-value = 0.94, for the drinking days and consumed volume model, respectively).
Although the mixed-effects repeated-measures ANOVA table indicated a difference in the total number of SSB drinking days (F(2,32)=4.74, p<0.05), the total amount consumed did not differ across groups (F(2,31)=2.17, p=0.13), nor did we find any group x time interactions (F(1,36)=0.11, p=0.74; F(1,34.64)=0.30, p=0.58 for drinking days and total amount, respectively). Furthermore, we found a significant group x ΔInsulin iAUC0-30m interaction for SSB drinking days (F(2,32)=3.94, p<0.05). Post hoc contrasts indicated a significant negative association between SSB drinking days and ΔInsulin iAUC0-30m in the Combo group (β= −0.0095, t(32)=−2.45, p<0.05). This association was significantly different from the LCS group (β= 0.012, t(32)=−2.78, p<0.01).
Together these results indicate that we found no evidence that our experimental manipulation affected SSB consumption outside the experiment. In contrast, we did find that participants who drink SSBs on a more regular basis had a lower change in plasma insulin between pre and post OGTT measurement in the Combo group.
As there was a difference between groups in SSB consumption prior to the experiment we tested whether the measures [Pre TLFB SSB consumption in ml] and [Pre TLFB SSB consumption in drinking days over 2 weeks] might have influenced Insulin iAUC0-30, Insulin iAUC pretest, Glucose iAUC0-30m, and Glucose iAUC pretest using likelihood ratio tests in R. For each dependent variable we evaluated if the model fit improved by adding either [Pre TLFB SSB consumption in ml] as covariate. All likelihood ratio tests indicated that this was not the case (X2(1), P > 0.37 for all eight ratio tests) suggesting that group differences before the experiment had little influence on the presented results.
Sweet taste preference
Statistical analysis was performed in R (version 3.1.3, 2015-03-09). First, we made the step size in the concentration range equal by converting the preferred concentrations to a log scale (correlation between log concentrations and linear scale, r = 0.996). The preferred level of sucrose was estimated by averaging the two chosen concentrations (on log scale) for the pre and post measurement, separately. Subsequently, difference scores were calculated (post minus pre beverage exposure) and submitted to a type III Analysis of Variance (ANOVA) model to investigate changes in sweet taste preferences across groups and to investigate whether changes in sweet taste preference were associated with changes in insulin sensitivity. ΔLog sucrose preference was entered as dependent variable whereas group * ΔInsulin iAUC0-30mwere entered as independent variables. Results are reported in the Supporting information.
We found no differences across groups (F(2,32)=0.16, p=0.85). We found no association between changes in sucrose preference and changes in insulin sensitivity (F(1,32)=0.05, p=0.82), nor did we find a group * ΔInsulin iAUC0-30m interaction (F(2,32)=2.08, p=0.14). These results indicate that we found no evidence that our experimental manipulation affected sucrose preference. Though, we are unable to rule out that the M-STP was not sensitive enough.
Psychophysiological measurements
To investigate whether beverage exposure affected taste intensity ratings, we performed LMMs in R using packages LME4 and ImerTest. Models were performed for each taste solution separately. Within each model, log transformed intensity ratings were entered as dependent variable, whereas time in days, experimental group, and their interaction were entered as independent variables. Finally, participant ID was entered as random variable. To test for any interaction with ΔInsulin iAUC0-30m we also performed similar models that included an interaction between change in insulin and time in days. We found no time x group interactions nor a time x group x ΔInsulin iAUC0-30m interaction that survived a multiple comparison correction. The results of the intensity ratings together with the group x time interaction F-statistic derived from a mixed-effects repeated-measures ANOVA (type III) are shown in Figure S2.
Food Intake Measure
The difference in the weight of the bowl before versus after the meal at pre-test versus post-test for each of the three groups in experiment 1 was recalculated to Kcal and analyzed using an ANOVA table for linear mixed models. We found no interaction between group and time indicating no group differences (F(35.52,2)=0.23, p=0.79, using Satterthwaite’s degrees of freedom).
DATA AND CODE AVAILABILITY
Raw MRI data, Time line follow back, sucrose preference test, and oral glucose tolerance test: https://openneuro.org/datasets/ds002419
Statistical maps of the human brain: https://neurovault.org/collections/6375/
Psychophysiological measurement: doi:10.17632/3wbc7nc3vv.1
Supplementary Material
Figure S1. Gustatory Manifold & System
Figure S2. Intensity perception changes across taste solutions and groups.
Table S1: Detailed Participant demographics and beverage consumption
Table S2. Group comparisons for the OGTT indices.
Acknowledgements
We would like to acknowledge those volunteers who participated in our study, the MR technicians, as well as several colleagues, including Barry Green, Ivan de Araujo and Wolfgang Meyerhof for insightful discussions and comments on design, interpretation or prior drafts of this manuscript. Funding: NIH NIDCDR01 DC006706-06A1.
Footnotes
Declaration of Interests
Authors declare no competing interests.
ADDITIONAL RESOURCES
Clinical Trial Registration Number NCT02335021 (https://clinicaltrials.gov/ct2/show/NCT02335021).
References
- Abdul-Ghani MA, Matsuda M, Balas B, and DeFronzo RA (2007). Muscle and liver insulin resistance indexes derived from the oral glucose tolerance test. Diabetes Care 30, 89–94. [DOI] [PubMed] [Google Scholar]
- Ahrén B (2000). Autonomic regulation of islet hormone secretion - Implications for health and disease. Diabetologia 43, 393–410. [DOI] [PubMed] [Google Scholar]
- de Araujo IE, Oliveira-Maia AJ, Sotnikova TD, Gainetdinov RR, Caron MG, Nicolelis MAL, and Simon SA (2008). Food Reward in the Absence of Taste Receptor Signaling. Neuron 57, 930–941. [DOI] [PubMed] [Google Scholar]
- de Araujo IE, Lin T, Veldhuizen MG, and Small DM (2013). Metabolic regulation of brain response to food cues. Curr. Biol 23, 878–883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baayen R (2008). Analyzing linguistic data: A practical introduction to statistics using R (Cambridge University Press; ). [Google Scholar]
- Baird IM, Shephard NW, Merritt RJ, and Hildick-Smith G (2000). Repeated dose study of sucralose tolerance in human subjects. Food Chem. Toxicol 38, 123–129. [DOI] [PubMed] [Google Scholar]
- Bartoshuk LM, Duffy VB, Green BG, Hoffman HJ, Ko CW, Lucchina LA, Marks LE, Snyder DJ, and Weiffenbach JM (2004). Valid across-group comparisons with labeled scales: The gLMS versus magnitude matching. Physiol. Behav 82, 109–114. [DOI] [PubMed] [Google Scholar]
- Bender G, Veldhuizen MG, Meltzer JA, Gitelman DR, and Small DM (2009). Neural correlates of evaluative compared with passive tasting. Eur. J. Neurosci 30, 327–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown AW, Bohan Brown MM, Onken KL, and Beitz DC (2011). Short-term consumption of sucralose, a nonnutritive sweetener, is similar to water with regard to select markers of hunger signaling and short-term glucose homeostasis in women. Nutr. Res 31, 882–888. [DOI] [PubMed] [Google Scholar]
- Brown RJ, Walter M, and Rother KI (2009). Ingestion of diet soda before a glucose load augments glucagon-like peptide-1 secretion. Diabetes Care 32, 2184–2186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke MV, and Small DM (2016). Effects of the modern food environment on striatal function, cognition and regulation of ingestive behavior. Curr. Opin. Behav. Sci 9, 97–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey BJ, Jones RM, and Hare TA (2008). The adolescent brain. Ann. N. Y. Acad. Sci 1124, 111–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan C, Hashemi Z, and Subhan F (2017). The impact of low and no-caloric sweeteners on glucose absorption, incretin secretion and glucose tolerance Journal: Appl. Physiol. Nutr. Metab 42, 793–801. [DOI] [PubMed] [Google Scholar]
- Craig AD (2002). How do you feel? Interoception: the sense ofthe physiological condition ofthe body. Nat. Rev. Neurosci 3, 655–666. [DOI] [PubMed] [Google Scholar]
- Critchley H, and Seth A (2012). Will Studies of Macaque Insula Reveal the Neural Mechanisms of Self-Awareness? Neuron 74, 423–426. [DOI] [PubMed] [Google Scholar]
- Davidson TL, and Swithers SE (2004). A Pavlovian approach to the problem of obesity. Int. J. Obes. Relat. Metab. Disord 28, 933–935. [DOI] [PubMed] [Google Scholar]
- Davidson TL, Martin A. a, Clark K, and Swithers SE (2011). Intake of high-intensity sweeteners alters the ability of sweet taste to signal caloric consequences: implications for the learned control of energy and body weight regulation. Q. J. Exp. Psychol. (Hove). 64, 1430–1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dempster P, and Aitkens S (1995). A new air displacement method for the determination of human body composition. Med. Sci. Sports Exerc 27, 1692–1691. [PubMed] [Google Scholar]
- Dunn JP, Kessler RM, Feuer ID, Volkow ND, Patterson BW, Ansari MS, Marks-Shulman P, and Abumrad NN (2012). Relationship of Dopamine Type 2 Receptor Binding Potential With Fasting Neuroendocrine Hormones and Insulin Sensitivity in Human Obesity. Diabetes Care 35, 1105–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evrard HC (2019). The organization of the primate insular cortex. Front. Neuroanat 13, 1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feijó F. de M., Ballard CR, Foletto KC, Batista BAM, Neves AM, Ribeiro MFM, and Bertoluci MC (2013). Saccharin and aspartame, compared with sucrose, induce greater weight gain in adult Wistar rats, at similar total caloric intake levels. Appetite 60, 203–207. [DOI] [PubMed] [Google Scholar]
- Foletto KC, Melo Batista BA, Neves AM, de Matos Feijó F, Ballard CR, Marques Ribeiro MF, and Bertoluci MC (2016). Sweet taste of saccharin induces weight gain without increasing caloric intake, not related to insulin-resistance in Wistar rats. Appetite 96, 604–610. [DOI] [PubMed] [Google Scholar]
- Ford HE, Peters V, Martin NM, Sleeth ML, Ghatei MA, Frost GS, and Bloom SR (2011). Effects of oral ingestion of sucralose on gut hormone response and appetite in healthy normal-weight subjects. Eur. J. Clin. Nutr 65, 508–513. [DOI] [PubMed] [Google Scholar]
- Fowler SPG (2016). Low-calorie sweetener use and energy balance: Results from experimental studies in animals, and large-scale prospective studies in humans. Physiol. Behav 164, 517–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler SP, Williams K, Resendez RG, Hunt KJ, Hazuda HP, and Stern MP (2008). Fueling the obesity epidemic? Artificially sweetened beverage use and long-term weight gain. Obesity 16, 1894–1900. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Williams S, Howard R, Frackowiak RS, and Turner R (1996). Movement-related effects in fMRI time-series. Magn. Reson. Med 35, 346–355. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenbos A, Paus T, Evans AC, and Rapoport JL (1999). Brain development during childhood and adolescence: a longitudinal MRI study. Nat. Neurosci 2, 861–863. [DOI] [PubMed] [Google Scholar]
- Glendinning JI (2016). Do low-calorie sweeteners promote weight gain in rodents? Physiol. Behav 164, 509–513. [DOI] [PubMed] [Google Scholar]
- Green BG, Dalton P, Cowart B, Shaffer G, Rankin K, and Higgins J (1996). Evaluating the “Labeled Magnitude Scale” for measuring sensations of taste and smell. Chem. Senses 21, 323–334. [DOI] [PubMed] [Google Scholar]
- Greenwood DC, Threapleton DE, Evans CEL, Cleghorn CL, Nykjaer C, Woodhead C, and Burley VJ (2014). Association between sugar-sweetened and artificially sweetened soft drinks and type 2 diabetes: systematic review and dose–response meta-analysis of prospective studies. Br. J. Nutr (2014), 112, 725–734. [DOI] [PubMed] [Google Scholar]
- Grotz VL, Henry RR, Mcgill JB, Prince MJ, Shamoon H, Trout JR, and Pi-Sunyer FX (2003). Lack of effect of sucralose on glucose homeostasis in subjects with type 2 diabetes. J. Am. Diet. Assoc 103, 1607–1612+ 1711. [DOI] [PubMed] [Google Scholar]
- Grotz VL, Pi-Sunyer X, Porte D, Roberts A, and Trout JR (2017a). A 12-week randomized clinical trial investigating the potential for sucralose to affect glucose homeostasis. Regul. Toxicol. Pharmacol 88, 30126–5. [DOI] [PubMed] [Google Scholar]
- Grotz VL, Pi-Sunyer X, Porte D, Roberts A, and Richard Trout J (2017b). A 12-week randomized clinical trial investigating the potential for sucralose to affect glucose homeostasis. Regul. Toxicol. Pharmacol 88, 22–33. [DOI] [PubMed] [Google Scholar]
- Ter Horst KW, Lammers NM, Trinko R, Opland DM, Figee M, Ackermans MT, Booij J, Munckhof P. van den, Schuurman PR, Fliers E, et al. (2018). Striatal dopamine regulates systemic glucose metabolism in humans and mice. Sci. Transl. Med 10, 1–11. [DOI] [PubMed] [Google Scholar]
- Imamura F, O’Connor L, Ye Z, Mursu J, Hayashino Y, Bhupathiraju SN, and Forouhi NG (2015). Consumption of sugar sweetened beverages, artificially sweetened beverages, and fruit juice and incidence of type 2 diabetes: systematic review, meta-analysis, and estimation of population attributable fraction. Bmj 351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josse J, and Husson F (2013). Handling missing values in exploratory multivariate data analysis methods. J. La SFdS 153, 79–99. [Google Scholar]
- Kellett GL, Brot-Laroche E, Mace OJ, and Leturque A (2008). Sugar Absorption in the Intestine: The Role of GLUT2. Annu. Rev. Nutr 28, 35–54. [DOI] [PubMed] [Google Scholar]
- Kuznetsova A, Brockhoff PB, and Christensen RHB (2014). ImerTest: Tests for random and fixed effects for linear mixed effect models (Imer objects of Ime4 package). [Google Scholar]
- Laffitte A, Neiers F, and Briand L (2014). Functional roles of the sweet taste receptor in oral and extraoral tissues. Curr. Opin. Clin. Nutr. Metab. Care 17, 379–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang Y, Steinbach G, Maier V, and Pfeiffer EF (1987a). The effect of artificial sweetener on insulin secretion 1. The Effect of Acesulfame K on Insulin Secretion in the Rat (Studies In Vivo). Horm. Metab. Res 19, 233–238. [DOI] [PubMed] [Google Scholar]
- Liang Y, Maier V, Steinbach G, Lalić L, and Pfeiffer EF (1987b). The Effect of Artificial Sweetener on Insulin Secretion II. Stimulation of Insulin Release from Isolated Rat Islets by Acesulfame K (In Vitro Experiments) Acesulfame K is an artificial sweetener which has been used in the food industry for some years (.Horm. Metab. Res 19, 285–289. [DOI] [PubMed] [Google Scholar]
- Ma J, Chang J, Checklin HL, Young RL, Jones KL, Horowitz M, and Rayner CK (2010). Effect of the artificial sweetener, sucralose, on small intestinal glucose absorption in healthy human subjects. Br. J. Nutr 104, 803–806. [DOI] [PubMed] [Google Scholar]
- Mace OJ, Affleck J, Patel N, and Kellett GL (2007). Sweet taste receptors in rat small intestine stimulate glucose absorption through apical GLUT2. J. Physiol 582, 379–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolskee RF, Dyer J, Kokrashvili Z, Salmon KSH, Ilegems E, Daly K, Maillet EL, Ninomiya Y, Mosinger B, and Shirazi-Beechey SP (2007). T1R3 and gustducin in gut sense sugars to regulate expression of Na+-glucose cotransporter 1. Proc. Natl. Acad. Sci 104, 15075–15080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda M, and DeFronzo RA (1999). Insulin sensitivity indices obtained from oral glucose tolerance testing: Comparison with the euglycemic insulin clamp. Diabetes Care 22, 1462–1470. [DOI] [PubMed] [Google Scholar]
- Mennella JA, Lukasewycz LD, Griffith JW, and Beauchamp GK (2011). Evaluation of the monell forced-choice, paired-comparison tracking procedure for determining sweet taste preferences across the lifespan. Chem. Senses 36, 345–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller PE, and Perez V (2014). Low-calorie sweeteners and body weight and composition: a meta-analysis of randomized controlled trials and prospective cohort studies. Am. J. Clin. Nutr 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills KL, Lalonde F, Clasen LS, Giedd JN, and Blakemore SJ (2014a). Developmental changes in the structure of the social brain in late childhood and adolescence. Soc. Cogn. Affect. Neurosci 9, 123–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills KL, Goddings AL, Clasen LS, Giedd JN, and Blakemore SJ (2014b). The developmental mismatch in structural brain maturation during adolescence. Dev. Neurosci 36, 147–160. [DOI] [PubMed] [Google Scholar]
- Moran A, Jacobs DR, Steinberger J, Hong CP, Prineas R, Luepker R, and Sinaiko AR (1999). Insulin resistance during puberty: Results from clamp studies in 357 children. Diabetes 48, 2039–2044. [DOI] [PubMed] [Google Scholar]
- Mumford JA, and Nichols TE (2008). Power calculation for group fMRI studies accounting for arbitrary design and temporal autocorrelation. Neuroimage 39, 261–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nettleton J, Lutsey P, Wang Y, Lima J, Michos E, and Jacobs D (2009). Diet Soda Intake and Risk of Incident Metabolic Syndrome and Type 2 Diabetes in the Multi-Ethnic Study of Atherosclerosis. Diabetes Care 32, 688–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman AJ, Tremblay A, Nichols ES, Neville HJ, and Ullman MT (2012). The Influence of Language Proficiency on Lexical Semantic Processing in Native and Late Learners of English. J. Cogn. Neurosci 24, 1205–1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paus T, Zijdenbos A, Worsley K, Collins DL, Blumenthal J, Giedd JN, Rapoport JL, and Evans AC (1999). Structural Maturation of Neural Pathways in Children and Adolescents: In Vivo Study. Science (80-. ). 283, 1908–1911. [DOI] [PubMed] [Google Scholar]
- Pepino MY (2015). Metabolic effects of non-nutritive sweeteners. Physiol. Behav 152, 450–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepino M, Tiemann C, Patterson B, Wice B, and Klein S (2013). Sucralose affects glycemic and hormonal responses to an oral glucose load. Diabetes Care 36, 2530–2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinheiro J, and Bates D (2000). Mixed effects models in S and S-PLUS (Springer; ). [Google Scholar]
- Reichelt AC (2016). Adolescent Maturational Transitions in the Prefrontal Cortex and Dopamine Signaling as a Risk Factor for the Development of Obesity and High Fat/High Sugar Diet Induced Cognitive Deficits. Front. Behav. Neurosci 10, 189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Diaz R, Abdulreda MH, Formoso AL, Gans I, Ricordi C, Berggren PO, and Caicedo A (2011). Innervation patterns of autonomic axons in the human endocrine pancreas. Cell Metab. 14, 45–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers PJ, Hogenkamp PS, de Graaf C, Higgs S, Lluch A, Ness AR, Penfold C, Perry R, Putz P, Yeomans MR, et al. (2016). Does low-energy sweetener consumption affect energy intake and body weight? A systematic review, including meta-analyses, of the evidence from human and animal studies. Int. J. Obes 40, 381–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiffman SS (2012). Rationale for further medical and health research on high-potency sweeteners. Chem. Senses 37, 671–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seth AK, and Friston KJ (2016). Active interoceptive inference and the emotional brain. Philos. Trans. R. Soc. B Biol. Sci 371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirazi-Beechey SP, Daly K, Al-Rammahi M, Moran AW, and Bravo D (2014). Role of nutrient-sensing taste 1 receptor (T1R) family members in gastrointestinal chemosensing. Br. J. Nutr 111, S8–S15. [DOI] [PubMed] [Google Scholar]
- Small DM (2010). Taste representation in the human insula. Brain Struct. Funct 214, 551–561. [DOI] [PubMed] [Google Scholar]
- Sobell LC, Brown J, Leo GI, and Sobell MB (1996). The reliability of the Alcohol Timeline Followback when administered by telephone and by computer. Drug Alcohol Depend. 42, 49–54. [DOI] [PubMed] [Google Scholar]
- Steinert RE, Frey F, Tpfer A, Drewe J, and Beglinger C (2011). Effects of carbohydrate sugars and artificial sweeteners on appetite and the secretion of gastrointestinal satiety peptides. Br. J. Nutr 105, 1320–1328. [DOI] [PubMed] [Google Scholar]
- Suez J, Korem T, Zeevi D, Zilberman-Schapira G, Thaiss CA, Maza O, Israeli D, Zmora N, Gilad S, Weinberger A, et al. (2014). Artificial sweeteners induce glucose intolerance by altering the gut microbiota. Nature 514, 181–186. [DOI] [PubMed] [Google Scholar]
- Swithers SE (2013). Artificial sweeteners produce the counterintuitive effect of inducing metabolic derangements. Trends Endocrinol. Metab 24, 431–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swithers SE, Baker CR, and Davidson TL (2009). General and Persistent Effects of High-Intensity Sweeteners on Body Weight Gain and Caloric Compensation in Rats. Behav. Neurosci 123, 772–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swithers SE, Laboy AF, Clark K, Cooper S, and Davidson TL (2012). Experience with the high-intensity sweetener saccharin impairs glucose homeostasis and GLP-1 release in rats. Behav. Brain Res 233, 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swithers SE, Sample CH, and Davidson TL (2013). Adverse effects of high-intensity sweeteners on energy intake and weight control in male and obesity-prone female rats. Behav. Neurosci 127, 262–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sylvetsky AC, and Rother KI (2018). Nonnutritive Sweeteners in Weight Management and Chronic Disease: A Review. Obesity 26, 635–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sylvetsky AC, Welsh JA, Brown RJ, and Vos MB (2012). Low-calorie sweetener consumption is increasing in the United States. Am. J. Clin. Nutr 96, 640–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sylvetsky AC, Brown RJ, Blau JE, Walter M, and Rother KI (2016). Hormonal responses to non-nutritive sweeteners in water and diet soda. Nutr. Metab. (Lond). 13, 71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- W T, Z. BR, B. MJ, C. HL, B. M, L. TJ, Y. RL, J. KL, and H. M (2012). Effects of different sweet preloads on incretin hormone secretion, gastric emptying, and postprandial glycemia in healthy humans. Am. J. Clin. Nutr 95, 78–83. [DOI] [PubMed] [Google Scholar]
- Taborsky GJ (2011). Islets have a lot of nerve! or do they? Cell Metab. 14, 5–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tellez LA, Han W, Zhang X, Ferreira TL, Perez IO, Shammah-lagnado SJ, Pol A.N. Van Den, and Araujo I.E. De (2016). Separate circuitries encode the hedonic and nutritional values of sugar. Nat. Neurosci [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temizkan S, Deyneli O, Yasar M, Arpa M, Gunes M, Yazici D, Sirikci O, Haklar G, Imeryuz N, and Yavuz DG (2015). Sucralose enhances GLP-1 release and lowers blood glucose in the presence of carbohydrate in healthy subjects but not in patients with type 2 diabetes. Eur. J. Clin. Nutr 69, 162–166. [DOI] [PubMed] [Google Scholar]
- Thanarajah SE, Backes H, DiFeliceantonio AG, Albus K, Cremer AL, Hanssen R, Lippert RN, Cornely OA, Small DM, Brüning JC, et al. (2019). Food Intake Recruits Orosensory and Post-ingestive Dopaminergic Circuits to Affect Eating Desire in Humans. Cell Metab. 29, 695–706.e4. [DOI] [PubMed] [Google Scholar]
- Toews I, Lohner S, Küllenberg De Gaudry D, Sommer H, and Meerpohl JJ (2019). Association between intake of non-sugar sweeteners and health outcomes: systematic review and meta-analyses of randomised and non-randomised controlled trials and observational studies. Bmj 364, 4718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veldhuizen MG, Bender G, Constable RT, and Small DM (2007). Trying to detect taste in a tasteless solution: modulation of early gustatory cortex by attention to taste. Chem. Senses 32, 569–581. [DOI] [PubMed] [Google Scholar]
- Veldhuizen MG, Babbs RKRK, Patel BP, Fobbs W, Kroemer NB, Garcia E, Yeomans MR, and Small DM (2017). Integration of Sweet Taste and Metabolism Determines Carbohydrate Reward. Curr. Biol 27, 2476–2485.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang QP, Lin YQ, Zhang L, Wilson YA, Oyston LJ, Cotterell J, Qi Y, Khuong TM, Bakhshi N, Planchenault Y, et al. (2016). Sucralose Promotes Food Intake through NPY and a Neuronal Fasting Response. Cell Metab. 24, 75–90. [DOI] [PubMed] [Google Scholar]
- Wiebe N, Padwal R, Field C, Marks S, Jacobs R, and Tonelli M (2011). A systematic review on the effect of sweeteners on glycemic response and clinically relevant outcomes Natasha. BMC Med. 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Gustatory Manifold & System
Figure S2. Intensity perception changes across taste solutions and groups.
Table S1: Detailed Participant demographics and beverage consumption
Table S2. Group comparisons for the OGTT indices.
Data Availability Statement
Raw MRI data, Time line follow back, sucrose preference test, and oral glucose tolerance test: https://openneuro.org/datasets/ds002419
Statistical maps of the human brain: https://neurovault.org/collections/6375/
Psychophysiological measurement: doi:10.17632/3wbc7nc3vv.1


