Abstract
While Parkinson’s disease (PD) and attention-deficit hyperactivity disorder (ADHD) are two distinct conditions, it has been hypothesized that they share several overlapping anatomical and neurochemical changes. In order to investigate that hypothesis, this study used claims data from Taiwan’s Longitudinal Health Insurance Database 2000 to provide the significant nationwide population-based evidence of an increased risk of PD among ADHD patients, and the connection between the two conditions was not the result of other comorbidities. Moreover, this study showed that the patients with PD were 2.8 times more likely to have a prior ADHD diagnosis compared with those without a prior history of ADHD. Furthermore, an animal model of ADHD was generated by neonatally injecting rats with 6-hydroxydopamine (6-OHDA). These rats were subjected to behavior tests and the 99mTc-TRODAT-1 brain imaging at the juvenile stage. Compared to control group rats, the 6-OHDA rats showed a significantly reduced specific uptake ratio in the striatum, indicating an underlying PD-linked pathology in the brains of these ADHD phenotype-expressing rats. Overall, these results support that ADHD shares a number of anatomical and neurochemical changes with PD. As such, improved knowledge of the neurochemical mechanisms underlying ADHD could result in improved treatments for various debilitating neurological disorders, including PD.
Keywords: Parkinson’s disease, attention-deficit hyperactivity disorder, Longitudinal Health Insurance Database 2000, 6-hydroxydopamine
Introduction
Parkinson’s disease (PD), a chronic, progressive neurological disease, is characterized by the drastic reduction of dopamine transporters (DAT) and the dopaminergic neurons upon which they are expressed1–5. Epidemiological data show that the overall age- and gender-adjusted incidence rate of PD is 13.4 per 100,000, with a higher prevalence in men (19.0 per 100,000 men vs. 9.9 per 100,000 women)5. Meanwhile, an age-adjusted incidence rate of PD for all age groups in Taiwan of 10.4 per 100,000 population has also been reported6. In terms of etiology, the progressive loss of 60%–70% of nigral dopaminergic neurons leads to the clinical diagnosis of PD due to the resulting motor symptoms, which include bradykinesia, rigidity, tremor, etc2,3. The dopamine (DA) depletion in PD also causes frontostriatal dysfunction in the striatum and frontal cortex3,7,8. The diagnosis of PD is based on clinical criteria, and a good response to DA agonists is commonly regarded as supporting diagnostic features, while the clinical progression of PD is heterogeneous2.
Attention-deficit hyperactivity disorder (ADHD) is a heterogeneous neurobehavioral disorder of childhood that more frequently affects males than females9,10, affecting approximately 5%–6% of children worldwide, and the disorder is present in 2.5% of the adult population11,12. ADHD is characterized by developmentally inappropriate levels of inattention, hyperactivity, impulsivity, or some combination thereof that impair academic performance, social interactions, emotions, and family functions13–15. It is associated with considerable personal and societal burden. Most neural models for the pathophysiology of ADHD have indicated some dysfunction of the prefrontal cortex, including decreased brain volume, blood flow, and glucose metabolism16–18, while impaired cholinergic system19,20, norepinephrine21,22, serotonin23,24, and, especially, DA25,26 transmission have been reported in ADHD patients. The diagnosis of ADHD is based on the criteria in the Diagnostic and Statistical Manual (DSM-V).
Although the causes of PD and ADHD remain unknown, some genetic susceptibility alleles have been reported in particularly PD2, and there are also established risk factors27. However, it is becoming increasingly clear that beyond its central role in neuronal transmission and cognitive and emotional modulation, DA is a key player in multiple pathophysiological conditions, including PD and ADHD. Aberrant DA receptor signaling and abnormal dopaminergic nerve function is implicated in several neuropsychiatric disorders, including PD and ADHD28. Reuptake of released DA at the synaptic cleft is spatiotemporally regulated by the DAT, which is located in the presynaptic membrane on the terminal of the dopaminergic projection and shuttles DA from neuronal extracellular space into intracellular compartments and, as such, is a marker of DA terminal innervation29,30. The gene encoding the DAT, DAT1 (also known as SLC6A3)31, was reported to be associated with PD and ADHD in several meta-analyses32–34 and genetic linkage studies35,36. Medications, such as methyl-amphetamine, that inhibit the DAT have widely demonstrated efficacy in the treatment of ADHD37, and abusers of amphetamine and methyl-amphetamine are more likely to develop PD38–40. Relatedly, knockout DAT mice have been reported to exhibit motor hyperactivity41. In vivo explorations of DAT density, which can be measured by molecular single photon emission computed tomography (SPECT) and positron emission tomography (PET) with radioligands, have found it to be reduced by 30%–50% in PD, especially early onset PD4,42 while being significantly increased in ADHD43,44. Meanwhile, a meta-analysis of seven studies including a total of 114 patients with ADHD identified a significant relationship between structural brain alterations, especially a significant regional gray matter reduction in the right basal ganglia, and ADHD45. Subjects with ADHD exhibit decreased DA in the striatum, prefrontal cortex, septum, midbrain, and amygdala46–48. Based on these findings, we suspected a potential connection between PD and ADHD.
To test the hypothesis, the present study first utilized claims data from Taiwan’s Longitudinal Health Insurance Database 2000 (LHID2000) to determine whether PD patients exhibited a greater propensity for the prior diagnosis of ADHD than a control group of matched patients without PD. Furthermore, as animal models are an excellent tool to model human neurodevelopmental alterations that occur during disease onset and progression, we adopted a classical ADHD animal model by injecting 6-hydroxydopamine (6-OHDA) into the brains of neonatal rats. When these littermates were in the juvenile period, they clearly demonstrated the hyperlocomotor activity that is a significant behavioral hallmark of the disorder49–51. To clarify the association between the two conditions, these juvenile ADHD model rats were then injected with technetium-99m-[2-[[2-[[[3-(4-chlorophenyl)-8-methyl-8-azabicyclo[3.2.1]oct-2-yl]methyl](2-mercaptoethyl)amino]ethyl]amino]ethanethiolato(3-)-N2, N20, S2, S20] oxo-[1R-(exo-exo)] (99mTc-TRODAT-1) and subjected to brain imaging, with their imaging results compared with those of rats without 6-OHDA injection. Any evidence of PD-like features appearing in the ADHD model rats could then be taken as supporting evidence for any associations between ADHD and PD found among the aforementioned human PD patients, and such associations could, in turn, suggest that it is necessary to closely monitor the onset and progression of ADHD, as some aspects of said onset and progression could potentially yield clues for the diagnosis of PD.
Materials and Methods
Human Study
Data Source
Because it could take several decades, at minimum, to track children with ADHD to see if they subsequently develop PD, this study instead utilized claims data from Taiwan’s LHID2000 to compare patients diagnosed with PD with a control group of matched patients without PD with respect to prior diagnoses of ADHD. The LHID2000 is a sub-dataset of data for one million beneficiaries randomly sampled from Taiwan’s National Health Insurance Research Database (NHIRD), which contains all the claims data, including demographic data, dates of clinical visits, diagnostic codes, prescription details, laboratory and imaging examinations, procedure codes, expenditures, and registration files, for the 23.73 million residents of Taiwan covered by the nation’s National Health Insurance (NHI) program52. Initiated in 1995, a distinctive aspect of the NHI is that it covers roughly 99% of Taiwan’s population of approximately 23 million people, meaning that the NHIRD is a nationally representative dataset. The LHID2000, in turn, has been confirmed by the National Health Research Institutes (NHRI) to be representative of the Taiwanese population53.
Individual and hospital identifiers in the LHID2000 are de-identified and encrypted to protect the privacy of patients before the release of the dataset to the public for research purposes and cannot be used either to trace individual patients or hospitals or linked to other census data, such as the cancer registry or household registry. The encrypting procedure is consistent, so the linkage of claims belonging to the same patient is feasible within the NHIRD datasets. We suggest that readers refer to the NHRI website (http://nhird.nhri.org.tw/en/Data_Subsets.html) for more detailed information on these datasets. This study was approved by the institutional review board at Tungs’ Taichung Metroharbor Hospital, Taiwan, ROC (IRB approval No.: 106053), and given the anonymized nature of data, the need for informed consent was waived. All the protocols used in the human study were performed in accordance with relevant guidelines and regulations.
Definition of Research Variables
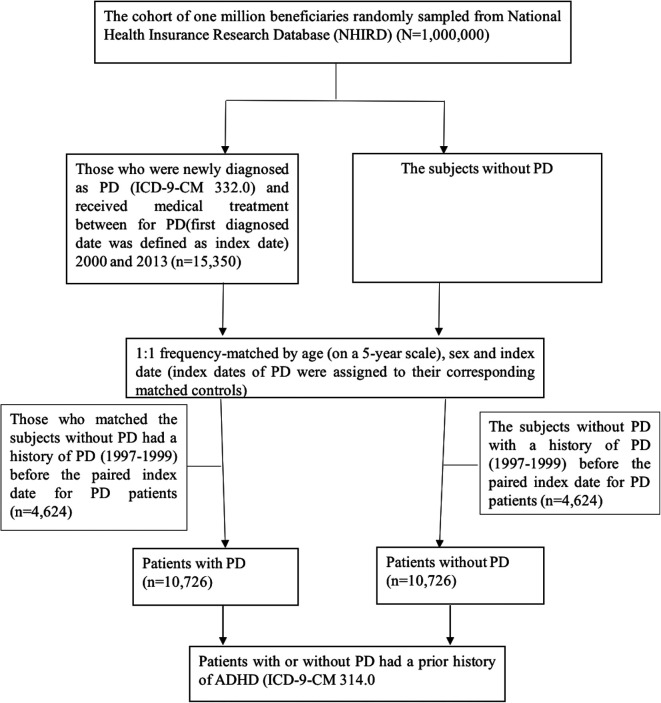
We first identified any hospitalized patients or patients who made outpatient visits who were diagnosed with PD by a qualified physician from January 1, 2000, through December 31, 2013, according to the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) code 332.0 for a given outpatient or inpatient claim. To increase the likelihood that the 332.0 code was a valid indicator of a diagnosis of PD, we only enrolled patients diagnosed with PD if they had at least three or more outpatient visits or hospital admissions and received at least one PD medication (including levodopa, peride, pramipexole, ropinirole, apomorphine, selegiline, entacapone, akineton, trihexyphenidyl, and amantadine). The first date of PD diagnosis after 2000 was defined as the index date for patients with PD. Fifteen thousand three hundred and fifty patients in the LHID2000 dataset met the above inclusion criteria. A control group of patients without PD matched on a 1:1 basis by age (on a 5-year scale), gender, and index date with the PD patients was also randomly selected. Ultimately, final totals of 10,726 PD patients and 10,726 matched control group patients were included in the study (Fig. 1). The main outcome for the human study was the occurrence of ADHD, which was defined as any diagnosis of ADHD (ICD-9-CM code: 314.0) before the index date. Although there is a link between hyperkinetic disorders and PD54, and the ICD-9-CM code 314.x broadly includes hyperkinetic disorders, such as 314.8 and 314.9 for hyperkinetic syndrome and 314.2 for hyperkinetic conduct disorder, however, we specifically used the code 314.0 and excluded other confounding factors in this study. In addition, a Charlson Comorbidity Index (CCI) score was also estimated for each patient according to the comorbid conditions of the patient recorded for at least two ambulatory visits and one hospitalization with the respective diagnosis within 3 years before the index date. The CCI scores were used to exclude the possibility that any association found between PD and ADHD was the result of a comorbidity (or comorbidities), such as diabetes mellitus, hypertension, cancer, etc55.
Fig. 1.
The process used to select and match the patients with and without PD included in the human study. PD: Parkinson’s disease.
Animal Experiment
Research Animals
Sprague-Dawley rats were obtained from BioLASCO Taiwan (Taipei, Taiwan) and kept under consistent temperature conditions (25°C ± 2°C). Since 6-OHDA (Sigma Chemicals, Sigma-Aldrich, St. Louis, MO, USA) cannot cross the blood-brain barrier (BBB), it is necessary that the substance has to be directly injected into the brain by means of stereotaxic surgery to generate effects in the CNS. To produce a group of ADHD model rats, neonatal 6-OHDA lesioning was performed with modifications as in a previous study56. On postnatal day (PND) 1, twenty-one male pups were assigned at random to lactating dams (10 per dam). On PND 3, the 21 male pups were placed on a stereotaxic frame and received desipramine hydrochloride (20 mg/kg body weight; Sigma Chemicals (Sigma-Aldrich, St. Louis, MO, USA), i.p.) 30 min before 6-OHDA injection to protect noradrenergic neurons. After 30 min, the pups were anesthetized by hypothermia (placed on ice for 1 min). Eleven pups received intracisternal injection of 5 µl of 6-OHDA solution [100 µg 6-OHDA, dissolved in 0.9% (w/v) sodium chloride containing 0.1% (w/v) ascorbic acid (Sigma Chemicals, Sigma-Aldrich, St. Louis, MO, USA)] with a glass pipette with air pressure. The injection site of was targeted at 0.6 mm lateral to the medial sagittal suture, 2 mm rostral to the lambda, and 1.3 mm below the skull surface. Ascorbic acid is used as an antioxidant to inhibit the oxidation of 6-OHDA57. The pups were returned to the nursing dams in their home cages after recovery of their consciousness. After weaning, the rats were housed four to five per cage under standard housing conditions with food and water available ad libitum. A control group of 10 rats (i.e., non-ADHD model rats) was subjected to the same conditions as the ADHD model rats, except that they were injected with 5 µl of 0.9% (w/v) sodium chloride containing 0.1% (w/v) ascorbic acid. The rats in both groups were subjected to the testing described below at 4 weeks after the 6-OHDA + ascorbic acid injection or ascorbic acid-only injection, that is, when they were juvenile male rats at PND 20–30. If not stated otherwise, the testing was performed in the light phase of the light/dark cycle (lights on 07:30–19:30). All behavioral and pharmacological studies were performed in a sound-proof room. All experiments were approved by the local institutional animal care and use committee of the National Defense Medical Center, Taipei, Taiwan, ROC (IACUC-12-233).
Animal Behavior Analysis
Testing to determine the effects of the 6-OHDA on locomotor activity was conducted between 10:00 and 16:00 with low illumination to soften stress. Prior to undergoing any behavioral testing, these rats in their home cages were brought to a room next to the testing room and allowed to rest for at least 1 h before the testing commenced. Members in our group strickly obey the rule of careful transportation and handling these animals to reduce their stress. Locomotor, rearing, and tracks of movement were examined in an open field (OF; 48 × 48 × 48 cm) under high illumination (100 lux lamp installed on 45 cm above the floor). The walls and floor of the OF were white to attenuate shadows in the box. The behavior of each animal was videotaped (OmnitechDigiscan Animal Activity Monitor; Model Opto-Varimex, Columbus Co., USA) for 60 min. Frame-by-frame analysis was conducted with a computer-based software (TopScanLite). The floor of the box was divided into 16 squares of equal dimension. The Activity Monitor program automatically recorded and analyzed their locomotion according to previous experimental protocols28,29. The parameters were defined as follows:
Velocity. The mean ambulatory distance (cm) divided by time (seconds).
Distance counts. The number of squares the animal crossed (when the animal passed through a square with its two forepaws) while a subject is engaged in ambulatory (locomotion) movement.
Rearing counts. The number of times that the animal raised on its two hind legs sniffing in the air was defined as rearing whether it leant on the wall or not. Rearing consisted of standing on the hind limbs and stopping ambulation.
Animal Image Data Acquisition and Analysis
99mTc-TRODAT-1/SPECT imaging scans [Triumph™ PET/SPECT/CT System (TriFoil Imaging, Inc., Northridge, CA, USA)] were used to image the brains of ADHD rats and non-ADHD rats. The BBB partially impedes some paracellular diffusion of ions, peptides, immune cells, and chemicals58, and transient BBB disruption with hyperosmolar solutions, such as mannitol, can clinically facilitate target drugs to easily diffuse though the gaps between cells directly into the brain59. Because previous data have demonstrated that 99mTc-TRODAT-1 scarcely penetrates the BBB of rats, resulting in poor striatal uptake, administration of mannitol induced a temporary BBB disruption without brain damage to enhance the 99mTc-TRODAT-1 uptake in the brain of each rat. After fasting overnight, rats were anesthetized with ketamine (10 mg/ kg, intramuscular injection (IM)) followed by passive inhalation of O2 (2 l/min) containing 2% isoflurane. Each SPECT scan was started immediately after a bolus injection of 10 mCi of 99mTc-TRODAT-1. Brain imaging was conducted with a dual-headed SPECT rotating camera equipped with ultra-high resolution fan-beam collimators (Hawkeye, Millennium VG, General Electric Medical Systems, Milwaukee, WI, USA). A dynamic sequence of eight brain images was acquired over 180 min (15 min per image). Imags were reconstructed using back projection with a Metz filter. The data were corrected for photon attenuation using Chang’s first-order method60. Only one person (K.Y., Yeh) analyzed data to avoid bias. For 99mTc-TRODAT-1/SPECT, the intensities on the striatum were measured by regions of interest (ROI)61, drawn on that region which comprised three contiguous slices with the highest activity, based on corresponding computed tomography (CT) images. The CT images were acquired on a 1.5 T instrument (Picker, Cleveland, OH, USA) and resliced, resized, and coregistered to all corresponding SPECT images in planes parallel to the canthomeatal line. The 99mTc-TRODAT-1/SPECT images were processed as described above for the striatum. The specific uptake ratio (SUR) was calculated as follows: SUR = [[(ROI of left CT + ROI of right CT)/2] − ROI of CB]/(ROI of CB)62.
Statistical Analysis
For the human study, the baseline characteristics of the study subjects are described as frequencies with percentages and as means with standard deviation for the categorical variables and continuous variables, respectively. The differences between the PD and non-PD groups were compared using t tests and chi-square tests. The age-specific percentages were calculated for the patient groups by age <60 years, age 60–69 years, age 70–79 years, and age ≧80 years, respectively. The logistic regression model in this study was for estimating the odds ratio (OR) and 95% confidence interval (CI) of ADHD in the PD group compared to the non-PD group. A two-sided P value <0.05 is statistical significance in all analyses. All analyses were conducted with the statistical package SAS version 9.4 (SAS Institute Inc., Cary, NC, USA).
For that animal behavioral data, an analysis of variance was used to compare the distribution of the values between the ADHD model rats and the control rats; post-hoc individual comparisons were made using Turkey’s test. Data are presented as mean ± standard deviation.
Results
Human Study Results
A total of 21,452 subjects (10,726 PD patients and 10,726 matched patients without PD) were enrolled in the analysis. Table 1 shows the baseline characteristics of the patients with PD and without PD groups. The patients with PD group had a mean age of 70.1 years and 50.9% were men, while the patients without PD group had a mean age of 70.0 years and 50.9% were men. The average CCI score for the patients with PD group was significantly greater than the average CCI score for the patients without PD group (4.4 vs. 2.7).
Table 1.
The Baseline Characteristics of the Patients With and Without PD.
| Patients with PD | Patients without PD | P value for chi-square test | |
|---|---|---|---|
| Gender | n (%) | n (%) | 1.000 |
| Female | 5,262 (49.1) | 5,262 (49.1) | |
| Male | 5,464 (50.9) | 5,464 (50.9) | |
| Age | |||
| <60 | 1,997 (18.6) | 2,030 (18.9) | 0.623 |
| 60–70 | 2,082 (19.4) | 2,132 (19.9) | |
| 70–80 | 4,162 (38.8) | 4,076 (38.0) | |
| 80+ | 2,485 (23.2) | 2,488 (23.2) | |
| Mean ± SD | 70.1 ± 14.0 | 70.0 ± 14.1 | 0.600 |
| CCI score | 4.4 ± 2.9 | 2.7 ± 3.0 | <0.001 |
PD: Parkinson’s disease; SD: standard deviation.
Table 2 shows that 14 of the patients with PD group were found to have had a prior diagnosis of ADHD, whereas only five of the patients without PD group had a prior ADHD diagnosis. In other words, the patients with PD group had a significantly higher rate of prior ADHD diagnoses than the patients without PD group (PD with prior ADHD vs. PD without prior ADHD: 14/10,726 vs. 5/10,726; P = 0.039). In a further analysis adjusting for age, gender, and CCI score, it was also found that the patients with PD group had a significant 3.65-fold higher OR (95% CI: 2.26–10.50) for ADHD than the patients without PD group (Table 3). This indicated that the association found between PD and ADHD in the PD patients was not the result of a comorbidity (or comorbidities), such as diabetes mellitus, hypertension, cancer, etc.
Table 2.
Comparison of Prior Diagnoses of ADHD for the Patients With and Without PD.
| Patients with PD | Patients without PD | P value | |
|---|---|---|---|
| n (%) | n (%) | 0.039 | |
| Patients with a prior diagnosis of ADHD | 14 (0.1) | 5 (0.1) | |
| Patients without a prior diagnosis of ADHD | 10,712 (99.9) | 10,721 (99.9) | |
| Total | 10,726 (100) | 10,726 (100) |
ADHD: attention-deficit hyperactivity disorder; PD: Parkinson’s disease; SD: standard deviation.
Table 3.
The ORs of ADHD for the Patients With and Without PD.
| Crude OR (95% CI) | P value | Adjusted ORa (95% CI) | P value for logistic regression | |
|---|---|---|---|---|
| PD | ||||
| With vs. without | 2.80 (1.01–7.78) | 0.048 | 3.65 (2.26–10.50) | 0.016 |
| Gender | ||||
| Male vs. female | 1.32 (0.53–3.29) | 0.546 | 1.37 (0.55–3.40) | 0.504 |
| Age | 0.95 (0.93–1.04) | 0.075 | 0.96 (0.93–1.05) | 0.077 |
| CCI score | 0.86 (0.81–1.02) | 0.079 | 0.89 (0.74–1.08) | 0.236 |
ADHD: attention-deficit hyperactivity disorder; CI: confidence interval; OR: odds ratio; PD: Parkinson’s disease; SD: standard deviation.
a A OR was adjusted for gender, age, and CCI score.
With respect to the onset ages, the average age for the PD patients with a prior diagnosis of ADHD was much lower than that for the PD patients without a prior diagnosis of ADHD (ADHD + PD vs. PD: 56.9 ± 21.3 vs. 72.5 ± 11.0; P = 0.016) (Table 4). In a further analysis of the onset ages, it was found that 6 of 14 (42.86%) of the PD patients with a prior diagnosis of ADHD had PD onset ages of less than 50 years old. This indicates that the prior ADHD might cause the onset ages for PD earlier.
Table 4.
The Comparison of the Average Onset Ages Between Patients With and Without PD With and Without a Prior Diagnosis of ADHD.
| Patients with a prior diagnosis of ADHD | Patients without a prior diagnosis of ADHD | P value for t-test | |
|---|---|---|---|
| Patients with PD | 56.9 ± 21.3 | 72.5 ± 11.0 | <0.001 |
| Patients without PD | 69.5 ± 9.8 | 70.3 ± 14.2 | 0.403 |
ADHD: attention-deficit hyperactivity disorder; PD: Parkinson’s disease.
Animal Experiment Results
Animal Behavior Analysis Results
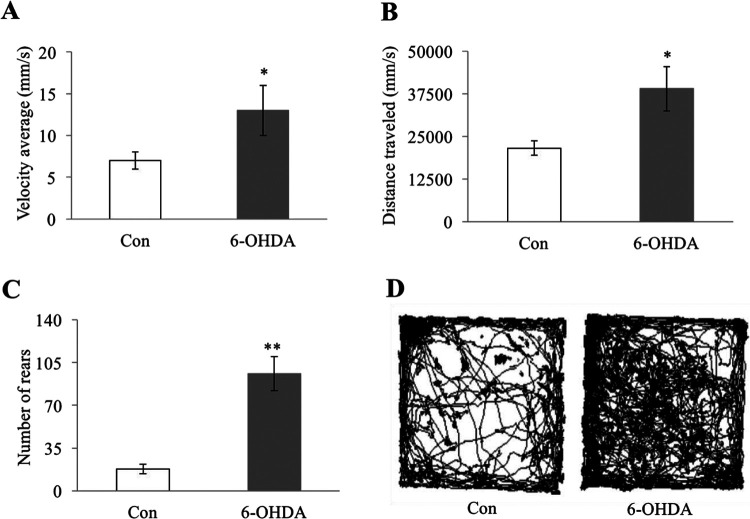
ADHD model rats and control group rats were subjected to behavioral testing at 4 weeks after receiving an injection of 6-OHDA and ascorbic acid (for the ADHD group rats) or ascorbic acid only (for the control group rats). Specifically, the rats were placed into an OF testing box, and their locomotor and rearing activities were measured for 60 min. Because the ADHD group rats and the control group rats displayed similar locomotor activity during the first 10 min of testing (the initial exploratory phase), the data for those first 10 min were omitted. During the remaining 50 min, the ADHD group exhibited, on average, a significantly higher movement velocity (Fig. 2A), greater average distance travelled (Fig. 2B), higher number of rears (Fig. 2C), and farther tracks of movement pattern (P < 0.001) than the non-ADHD rats without injection (Fig. 2D). Taken together, these results indicated that the ADHD group rats did, in fact, constitute an effective ADHD model.
Fig. 2.
ADHD model rats exhibited increased locomotor and rearing activity compared to healthy control rats. ADHD rats subjected to 6-OHDA lesioning and control rats without such lesioning were exposed to an OF box, and their spontaneous motor activity was recorded for 1 h: (A) average movement velocity (millimeters/second) of ADHD rats and control rats, (B) average distance travelled of ADHD rats and control rats, (C) average number of rears of ADHD rats and control rats, and (D) representative tracks of movement patterns of ADHD rats and control rats. All numeric data (A, B, and C) are represented as mean ± SD. * indicates P < 0.001 and ** indicates P < 0.0001 when compared to the control group. (N in the control group = 11 animals per group; N in the 6-OHDA-lesioned group = 10 animals per group). ADHD: attention-deficit hyperactivity disorder; OF: open field; SD: standard deviation.
Animal Image Data Acquisition and Analysis
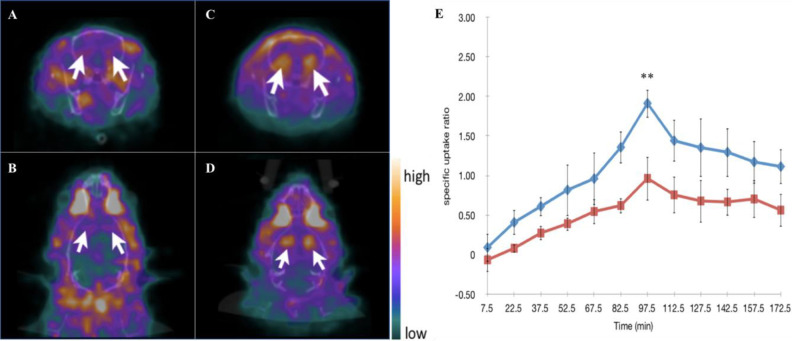
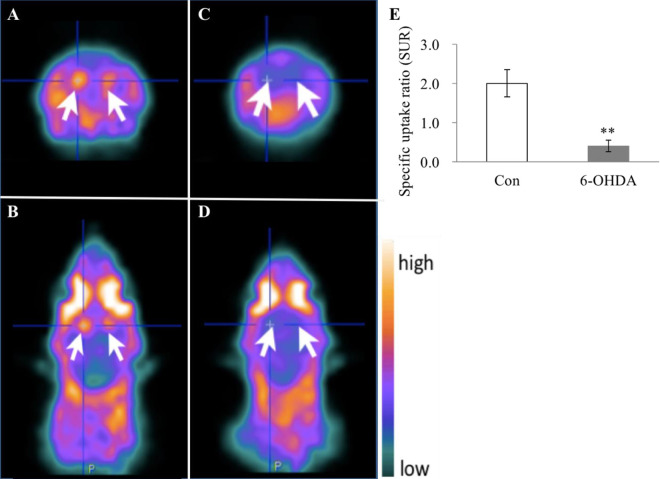
The ADHD model rats and control group rats were subjected to 99mTc-TRODAT-1/SPECT imaging scans at 4 weeks after receiving an injection of 6-OHDA and ascorbic acid (for the ADHD group rats) or ascorbic acid only (for the control group rats). In addition, because previous data have demonstrated that 99mTc-TRODAT-1 scarcely penetrates the BBB of rats, leading to poor striatal uptake5, mannitol was administered to temporarily disrupt BBB without brain damage to facilitate the 99mTc-TRODAT-1 uptake in the brain of each rat. Fig. 3 clearly shows that the 99mTc-TRODAT-1 more effectively penetrated the brains of the rats after pretreatment with mannitol. 97.5 min after the mannitol pretreatment was the optimal time point for the detection of the 99mTc-TRODAT-1. As detailed in Fig. 4, the ADHD model rats showed that the average SUR of the 6-OHDA group was significantly lower than that of the control group (6-OHDA vs. Con: 0.34 ± 0.09 and 1.86 ± 0.28; P < 0.0001), clearly indicating an underlying PD-associated pathology in the brains of the ADHD model rats.
Fig. 3.
Mannitol enhanced the 99mTc-TRODAT-1 signals at the striatum regions. Rats were subjected to 99mTc-TRODAT-1/SPECT imaging scan sat 4 weeks with and without prior mannitol injection. Representative SPECT scans for the rats without mannitol injection at 97.5 min, including transaxial imaging (A) and transverse imaging (B), as well representative transaxial imaging (C) and transverse imaging (D) SPECT scans for the rats with prior mannitol injection. (E) Dynamic analysis of dopamine transporter levels via SPECT imaging. The uptake of 99mTc-TRODAT-1 expressed by SURs was used to compare DAT levels in the rats with and without prior mannitol injection at 4 weeks after their respective injections. The optimal time for pretreatment with mannitol injection to achieve the highest SUR is 99.5 ± 1.5 min. Blue line: rats with prior mannitol injection. Red line: rats without prior mannitol injection. All numeric data are represented as mean ± SD. ** indicates P < 0.0001 when compared to the group without prior mannitol injection. (N in the group without prior mannitol injection = 4 animals per group; N in the group with prior mannitol injection = 5 animals per group.). DAT: dopamine transporter; SD: standard deviation; SPECT: single photon emission computed tomography; SUR: specific uptake ratio.
Fig. 4.
ADHD model rats at juvenile stage exhibited significantly lower DAT levels than healthy control rats. ADHD rats subjected to 6-OHDA lesioning and control rats without such lesioning were subjected to the 99mTc-TRODAT-1/SPECT imaging scans at 4 weeks after receiving their respective injections. Representative SPECT scans for the control rats without 6-OHDA lesioning, including transaxial imaging (A) and transverse imaging (B). Representative SPECT scans for the 6-OHDA-lesioned rats, including transaxial imaging (C) and transverse imaging (D). The uptake of 99mTc-TRODAT-1 expressed by SURs was used to compare DAT levels in rats with and without the 6-OHDA-lesioned at 4 weeks. All numeric data are represented as mean ± SD (E). SUR of the 6-OHDA versus Con: 0.34 ± 0.09 versus1.86 ± 0.28. ** indicates P < 0.0001 when compared to the control group (Con). (N in the control group = 5 animals per group; N in the 6-OHDA-lesioned group = 9 animals per group). ADHD: attention-deficit hyperactivity disorder; DAT: dopamine transporter; SD: standard deviation; SPECT: single photon emission computed tomography; SUR: specific uptake ratio.
Discussion
In this study, we utilized a two-track design to look for an association between PD and ADHD in both a retrospective epidemiological study of human PD patients and a behavioral and a brain imaging study of ADHD model rats. Regarding the human study, despite the fact that several studies have pointed out the apparent connection between PD and ADHD, the results of the present study provide the first nationwide population-based evidence of an increased risk of PD among ADHD patients, revealing that the patients with PD had a significantly higher rate of prior ADHD diagnoses than the patients without PD (P = 0.039), while also demonstrating that the connection between the two conditions was not the result of other comorbidities. Moreover, our study showed that the patients with PD were 2.8 times more likely to have a prior ADHD diagnosis, compared those without a prior history of ADHD. These results were similar to those of a retrospective cohort study in which individuals with ADHD were identified and then followed forward in the historic record for more than two decades showing that ADHD patients had a 2.4 times higher risk of developing PD-like disorders than those without a history of ADHD39. As such, these results provide epidemiological evidence of a link between PD and ADHD. In addition, the average onset age at which the PD patients with a history of ADHD in the present study developed PD was significantly lower than that for the PD patients without a history of ADHD. Relatedly, 42.86% of the PD patients with a prior diagnosis of ADHD had PD onset ages of less than 50 years old. This indicates that the prior ADHD may cause the onset ages for PD earlier.
Dysregulated dopaminergic pathways may interfere with normal development in children and deteriorate the functions of neurons in adults. Given that the dysregulation of DA is a shared feature of PD and ADHD, in order to further clarify the relationship between these two conditions, we employed a widely used laboratory model of ADHD based on the maximal motor hyperactivity at PNDs 20–30 of juvenile male rats following neonatal lesioning of the forebrain with 6-OHDA. Consistent with previous studies49,63,64, our results showed that 6-OHDA lesioning in rat pups resulted in robust hyperlocomotion as determined by the OF test when the subjects were tested at the juvenile stage (Fig. 2). In addition to hyperactivity, 6-OHDA mice have been reported to exhibit attention deficits, impulsive behavior, and cognitive impairments49,50 that are compatible with human ADHD phenotypes. Next, while routine brain imaging including CT and magnetic resonance imaging cannot detect any specific lesions in ADHD, nuclear medicine molecular imaging scans can demonstrate an increase of DAT signaling in the striatum of humans with ADHD43,44,65. However, when we were using 99mTc-TRODAT-1 SPECT imaging on the 6-OHDA-lesioned ADHD rats, the first challenge we needed to overcome was that the BBB prevented the 99mTc-TRODAT-1 from penetrating into the brain of the rats66. This problem was solved by using mannitol injection to reversibly disrupt the tight junctions between the adjacent endothelial cells in the cerebral microvasculature67, and we are the first group to show that the optimal time point for enhancing 99mTc-TRODAT-1 SPECT imaging in the striatum of the rat through pretreatment with mannitol injection is 99.5 ± 1.5 min (Fig. 3E).
Although we successfully demonstrated that the neonatal 6-OHDA-lesioned rat model could produce ADHD-like behaviors, the results of 99mTc-TRODAT-1 SPECT scanning in the brains of the juvenile rats with neonatal 6-OHDA lesioning exhibited significantly lower DAT levels than the control group rats (P < 0.0001). These findings were not consistent with ADHD but were compatible with PD, in which diminished TRODAT uptake is exhibited in the striatum68–70. Basically, 6-OHDA, a neurotoxin, accumulates in the neuronal cells through DATs and norepinephrine transporters due to its structural similarity with endogenous catecholamines. We thus used the desipramine for blocking the reuptake of norepinephrine and to selectively target dopaminergic neurons71. When infused intracisternally in neonate rats, 6-OHDA evokes cellular oxidative stress; generates excessive reactive oxygen species, hydrogen peroxide, superoxide, and hydroxyl radical (HO·); alters the levels of free radical scavengers; and affects mitochondrial function, causing abnormalities in cell structure and metabolism and, eventually, the overt destruction of dopaminergic nerves72, leading to a significant decrease of the number of DATs (Fig. 4C, D). DAT is a critical component for maintaining sufficient DA levels in the striatum29,30, and DAT, along with dopaminergic neurons, is reduced in PD as well as in other presynaptic parkinsonism disorders29,30,73,74. That being the case, how did the rats in this study with neonatal 6-OHDA lesioning display hyperlocomotion in adulthood? Considering the role of tyrosine hydroxylase (TH) helps to clarify this point. The enzyme is the first rate limiting step in the biosynthesis of the catecholamines, DA, norepinephrine, and epinephrine. A loss of TH-containing nerve endings in the striatum may therefore decrease striatal DA production, causing PD75. However, increased TH activity may be able to sustain sufficient DA for a period of time while in TH loss to sustain cytosolic DA in response to DAT loss.76,77 Therefore, we speculate that DAT activity, like TH activity, may increase as a compensatory mechanism, causing rats with neonatal 6-OHDA lesioning to show hyperlocomotor activity at the juvenile period78.Thus, following the desipramine infusion and neonatal 6-OHDA lesioning of the rats in this study, DA depletion may have activated a cascade of molecules and proteins, increasing corticostriatal glutamatergic transmission, while in the postsynaptic element, aberrant DA–glutamate interaction produced by kinase upregulation may have induced several morphological and physiological changes, especially in striatal neurons, which may have led in turn to the hyperactivity and PD brain changes observed. In any case, it is because of its overall effects that 6-OHDA commonly has been one of the most used neurotoxins in the modeling of PD in animals since 196879.
Neonatal 6-OHDA-lesioned rats have been extensively investigated both neurochemically and behaviorally. Apart from DA, serotonin may also be involved in the hyperactivity seen in neonatal 6-OHDA-lesioned rats. More specifically, the reactive sprouting of serotoninergic innervation of the striatum after neonatal 6-OHDA lesioning has been proposed to be the cause for the hyperactivity of such rats, because several reports have shown that serotoninergic hyperinnervation is prominent in the rostral striatum, caudal striatum, and dorsal and ventral raphe nuclei of adult rats subjected to neonatal 6-OHDA lesioning80–82. DA- and 5-HT-depleted rats exhibited attenuated hyperlocomotor activity after treatment with 5-HT2 agonists80. However, our previous data showed that serotonin levels are decreased after 6-OHDA treatment83,84. In line with our previous findings, Zhang et al. showed that the use of a broad-spectrum 5-HT receptor antagonist did not affect the hyperactivity of rats subjected to neonatal 6-OHDA lesioning64, suggesting that striatal serotoninergic fibers are not the key player in influencing locomotor activity in rats. Currently, no serotonergic medications have been formally recommended as a starndard treatment of ADHD. Taken together, these findings provide two forms of evidence to bolster the case for a link between ADHD and PD. The imaging findings in our animal model showing that a compensatory increase in DAT activity after the number of DATs is decreased following 6-OHDA lesioning in the rat neonatal period may be associated with PD with a previous history of ADHD.
The present study has some potential limitations. First, it would not be ethically acceptable and logistically feasible in terms of the time span to intentionally give humans ADHD to see whether they later develop PD, so a retrospective study of the current type may be an ideal method to investigate a connection between ADHD and PD in humans. However, because the human study was retrospective in nature, it may have been affected due to various biases relating to the required adjustments for confounding factors, such that its data and any conclusions drawn from them are of lower methodological quality and certainty than those of a well-designed randomized trial. Relatedly, our data showed an association between ADHD and PD, that association did not indicate any causality, and it is possible that it is the treatment given to ADHD patients, rather than the ADHD itself, that make ADHD patients more likely to develop PD in later years. Also, many individuals may have met a clinical definition of ADHD before the year 2000 in which electronic data were available and that there may be some classification bias of true ADHD as non-ADHD. Meanwhile, animal models also carry limitations, as few animal models can perfectly manifest typical symptoms and behaviors or execute specific function assays of a given disease (such as ADHD or PD) in humans. For instance, DAT knockout mice may present inherent hyperlocomotion in novel environments and elevated striatal DA levels. However, they showed extraordinarily elevated DA concentrations in the striatum and nucleus accumbens, unlike ADHD patients85,86. Additionally, spontaneously hypertensive rats not only show many major ADHD-like symptoms, such as poor attention, hyperactivity, and impulsivity, but also exhibit hypertension, which is not related to patients with ADHD87. Moreover, rats with hypoxic insult at birth88, such as those exposed to ethanol89, lead90, or cadmium91, also exhibit hyperactivity. Compared with the use of such models alone, therefore, the use of both the LHID and animal models in this study provides a clearer understanding of disease processes without crossing the aforementioned ethical line of causing harm to human subjects.
In conclusion, the human study performed in this overall study analyzed LHID2000 claims data to determine whether people diagnosed with PD were significantly more likely to have had a prior diagnosis of ADHD than a matched control group, while the animal study compared brain images of ADHD model rats and healthy control rats to ascertain whether the former exhibited any underlying PD-associated pathology via significantly reduced levels of DAT. The results suggest that targeting the pathophysiology underlying the loss of DAT may hold therapeutic promise for the treatment of both PD and ADHD.
Footnotes
Author Contributions: Conceptualization: Hueng-Chuen Fan, Kuo-Hsing Ma, and I-Hsun Li; data curation, formal analysis, and methodology: Yu-Kang Chang, Jeng-Dau Tsai, and Kuo-Liang Chiang; investigation, methodology, and software: Jui-Hu Shih and Kuan-Yi Yeh; writing the original draft: Hueng-Chuen Fan; and reviewing the manuscript: Kuo-Hsing Ma and I-Hsun Li.
Ethical Approval: This study was approved by the institutional review board at Tungs’ Taichung MetroHarbor Hospital, Taiwan (IRB approval No.: 106053).
Statement of Human and Animal Rights: All the experimental procedures involving animals were conducted in accordance with the Institutional Animal Care Guidelines of National Defense Medical Center, Taipei, Taiwan, ROC and approved by the local institutional animal care and use committee of the National Defense Medical Center, Taipei, Taiwan, ROC (IACUC-12-233).
Statement of Informed Consent: Informed consent for patient information to be published in this article was not obtained because of the anonymized nature of data. Therefore, the need for informed consent was waived. All the protocols used in the human study were performed in accordance with relevant guidelines and regulations.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by grants from the Tungs’ MetroHarbor Hospital (TTMHH-106R0022, TTMHH-109R0002 and TTMHH-108R0011), the Ministry of Science and Technology Taiwan (MOST 108-2314-B-016-010), and Tri-Service General Hospital, Taipei, Taiwan (TSGH-D-109145). The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript. The authors declare no conflicts of interest.
ORCID iD: Hueng-Chuen Fan  https://orcid.org/0000-0003-3485-7558
https://orcid.org/0000-0003-3485-7558
References
- 1. Chou CH, Fan HC, Hueng DY. Potential of neural stem cell-based therapy for Parkinson’s disease. Parkinsons Dis. 2015;2015:571475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Fan HC, Chen SJ, Harn HJ, Lin SZ. Parkinson’s disease: from genetics to treatments. Cell Transplant. 2013;22(4):639–652. [DOI] [PubMed] [Google Scholar]
- 3. Mhyre TR, Boyd JT, Hamill RW, Maguire-Zeiss KA. Parkinson’s disease. Subcell Biochem. 2012;65:389–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rizek P, Kumar N, Jog MS. An update on the diagnosis and treatment of Parkinson disease. CMAJ. 2016;188(16):1157–1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Van Den Eeden SK, Tanner CM, Bernstein AL, Fross RD, Leimpeter A, Bloch DA, Nelson LM. Incidence of Parkinson’s disease: variation by age, gender, and race/ethnicity. Am J Epidemiol. 2003;157(11):1015–1022. [DOI] [PubMed] [Google Scholar]
- 6. Chen RC, Chang SF, Su CL, Chen TH, Yen MF, Wu HM, Chen ZY, Liou HH. Prevalence, incidence, and mortality of PD: a door-to-door survey in Ilan County, Taiwan. Neurology. 2001;57(9):1679–1686. [DOI] [PubMed] [Google Scholar]
- 7. Cools R. Dopaminergic modulation of cognitive function-implications for L-DOPA treatment in Parkinson’s disease. Neurosci Biobehav Rev. 2006;30(1):1–23. [DOI] [PubMed] [Google Scholar]
- 8. Tekin S, Cummings JL. Frontal-subcortical neuronal circuits and clinical neuropsychiatry: an update. J Psychosom Res. 2002;53(2):647–654. [DOI] [PubMed] [Google Scholar]
- 9. Polanczyk G, de Lima MS, Horta BL, Biederman J, Rohde LA. The worldwide prevalence of ADHD: a systematic review and metaregression analysis. Am J Psychiatry. 2007;164(6):942–948. [DOI] [PubMed] [Google Scholar]
- 10. Subcommittee on Attention-Deficit/Hyperactivity D, Steering Committee on Quality Improvement and Management; Wolraich M, Brown L, Brown RT, DuPaul G, Earls M, Feldman HM, Ganiats TG, Kaplanek B, Meyer B, Perrin J, Pierce K, et al. ADHD: clinical practice guideline for the diagnosis, evaluation, and treatment of attention-deficit/hyperactivity disorder in children and adolescents. Pediatrics. 2011;128(5):1007–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Franke B, Faraone SV, Asherson P, Buitelaar J, Bau CH, Ramos-Quiroga JA, Mick E, Grevet EH, Johansson S, Haavik J, Lesch K-P, et al. The genetics of attention deficit/hyperactivity disorder in adults, a review. Mol Psychiatry. 2012;17(10):960–987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Simon V, Czobor P, Balint S, Meszaros A, Bitter I. Prevalence and correlates of adult attention-deficit hyperactivity disorder: meta-analysis. Br J Psychiatry. 2009;194(3):204–211. [DOI] [PubMed] [Google Scholar]
- 13. DSM-5 American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. American Psychiatric Press; 2013. [Google Scholar]
- 14. Barkley RA. Major life activity and health outcomes associated with attention-deficit/hyperactivity disorder. J Clin Psychiatry. 2002;63(Suppl 12):10–15. [PubMed] [Google Scholar]
- 15. Fried R, Petty C, Faraone SV, Hyder LL, Day H, Biederman J. Is ADHD a risk factor for high school dropout? A controlled study. J Atten Disord. 2016;20(5):383–389. [DOI] [PubMed] [Google Scholar]
- 16. Curatolo P, D’Agati E, Moavero R. The neurobiological basis of ADHD. Ital J Pediatr. 2010;36(1):79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Halperin JM, Schulz KP. Revisiting the role of the prefrontal cortex in the pathophysiology of attention-deficit/hyperactivity disorder. Psychol Bull. 2006;132(4):560–581. [DOI] [PubMed] [Google Scholar]
- 18. Rubia K, Halari R, Smith AB, Mohammad M, Scott S, Brammer MJ. Shared and disorder-specific prefrontal abnormalities in boys with pure attention-deficit/hyperactivity disorder compared to boys with pure CD during interference inhibition and attention allocation. J Child Psychol Psychiatry. 2009;50(6):669–678. [DOI] [PubMed] [Google Scholar]
- 19. Lee CT, Fuemmeler BF, McClernon FJ, Ashley-Koch A, Kollins SH. Nicotinic receptor gene variants interact with attention deficient hyperactive disorder symptoms to predict smoking trajectories from early adolescence to adulthood. Addict Behav. 2013;38(11):2683–2689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Potter AS, Schaubhut G, Shipman M. Targeting the nicotinic cholinergic system to treat attention-deficit/hyperactivity disorder: rationale and progress to date. CNS Drugs. 2014;28(12):1103–1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Russell V, Allie S, Wiggins T. Increased noradrenergic activity in prefrontal cortex slices of an animal model for attention-deficit hyperactivity disorder--the spontaneously hypertensive rat. Behav Brain Res. 2000;117(1–2):69–74. [DOI] [PubMed] [Google Scholar]
- 22. Viggiano D, Ruocco LA, Arcieri S, Sadile AG. Involvement of norepinephrine in the control of activity and attentive processes in animal models of attention deficit hyperactivity disorder. Neural Plast. 2004;11(1–2):133–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Banerjee E, Nandagopal K. Does serotonin deficit mediate susceptibility to ADHD? Neurochem Int. 2015;82:52–68. [DOI] [PubMed] [Google Scholar]
- 24. Gainetdinov RR, Wetsel WC, Jones SR, Levin ED, Jaber M, Caron MG. Role of serotonin in the paradoxical calming effect of psychostimulants on hyperactivity. Science. 1999;283(5400):397–401. [DOI] [PubMed] [Google Scholar]
- 25. Mehler-Wex C, Riederer P, Gerlach M. Dopaminergic dysbalance in distinct basal ganglia neurocircuits: implications for the pathophysiology of Parkinson’s disease, schizophrenia and attention deficit hyperactivity disorder. Neurotox Res. 2006;10(3–4):167–179. [DOI] [PubMed] [Google Scholar]
- 26. Wu J, Xiao H, Sun H, Zou L, Zhu LQ. Role of dopamine receptors in ADHD: a systematic meta-analysis. Mol Neurobiol. 2012;45(3):605–620. [DOI] [PubMed] [Google Scholar]
- 27. Hansen FH, Skjorringe T, Yasmeen S, Arends NV, Sahai MA, Erreger K, Andreassen TF, Holy M, Hamilton PJ, Neergheen V, Karlsborg M, et al. Missense dopamine transporter mutations associate with adult parkinsonism and ADHD. J Clin Invest. 2014;124(7):3107–3120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Diaz Heijtz R, Wang S, Anuar F, Qian Y, Björkholm B, Samuelsson A, Hibberd ML, Forssberg H, Pettersson S. Normal gut microbiota modulates brain development and behavior. Proc Natl Acad Sci U S A. 2011;108(7):3047–3052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Carli M, Robbins TW, Evendenn JL, Everitt BJ. Effects of lesions to ascending noradrenergic neurons on performance of a 5-choice serial reaction task in rats; implications for theories of dorsal noradrenergic bundle function based on selective attention and arousal. Behav. Brain Res. 1983;9(3):361–380. [DOI] [PubMed] [Google Scholar]
- 30. Huang WS, Lee MS, Lin JC, Chen CY, Yang YW, Lin SZ, Wey SP. Usefulness of brain 99mTc-TRODAT-1 SPET for the evaluation of Parkinson’s disease. Eur J Nucl Med Mol Imaging. 2004;31(2):155–161. [DOI] [PubMed] [Google Scholar]
- 31. Miller GM, Madras BK. Polymorphisms in the 3’-untranslated region of human and monkey dopamine transporter genes affect reporter gene expression. Mol Psychiatry. 2002;7(1):44–55. [DOI] [PubMed] [Google Scholar]
- 32. Gizer IR, Ficks C, Waldman ID. Candidate gene studies of ADHD: a meta-analytic review. Hum Genet. 2009;126(1):51–90. [DOI] [PubMed] [Google Scholar]
- 33. Li D, Sham PC, Owen MJ, He L. Meta-analysis shows significant association between dopamine system genes and attention deficit hyperactivity disorder (ADHD). Hum Mol Genet. 2006;15(14):2276–2284. [DOI] [PubMed] [Google Scholar]
- 34. Zhai D, Li S, Zhao Y, Lin Z. SLC6A3 is a risk factor for Parkinson’s disease: a meta-analysis of sixteen years’ studies. Neurosci Lett. 2014;564:99–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kaiser R, Hofer A, Grapengiesser A, Gasser T, Kupsch A, Roots I, Brockmoller J. L -dopa-induced adverse effects in PD and dopamine transporter gene polymorphism. Neurology. 2003;60(11):1750–1755. [DOI] [PubMed] [Google Scholar]
- 36. Cook EH,, Jr, Stein MA, Krasowski MD, Cox NJ, Olkon DM, Kieffer JE, Leventhal BL. Association of attention-deficit disorder and the dopamine transporter gene. Am J Hum Genet. 1995;56(4):993–998. [PMC free article] [PubMed] [Google Scholar]
- 37. Iacobucci G. ADHD: methylphenidate should be first line drug treatment in children, review confirms. BMJ. 2018;362:k3430. [Google Scholar]
- 38. Moratalla R, Khairnar A, Simola N, Granado N, Garcia-Montes JR, Porceddu PF, Tizabi Y, Costa G, Morelli M. Amphetamine-related drugs neurotoxicity in humans and in experimental animals: main mechanisms. Prog Neurobiol. 2017;155:149–170. [DOI] [PubMed] [Google Scholar]
- 39. Curtin K, Fleckenstein AE, Keeshin BR, Yurgelun-Todd DA, Renshaw PF, Smith KR, Hanson GR. Increased risk of diseases of the basal ganglia and cerebellum in patients with a history of attention-deficit/hyperactivity disorder. Neuropsychopharmacology. 2018;43(13):2548–2555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Christine CW, Garwood ER, Schrock LE, Austin DE, McCulloch CE. Parkinsonism in patients with a history of amphetamine exposure. Mov Disord. 2010;25(2):228–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Giros B, Jaber M, Jones SR, Wightman RM, Caron MG. Hyperlocomotion and indifference to cocaine and amphetamine in mice lacking the dopamine transporter. Nature. 1996;379(6566):606–612. [DOI] [PubMed] [Google Scholar]
- 42. Marek KL, Seibyl JP, Zoghbi SS, Zea-Ponce Y, Baldwin RM, Fussell B, Charney DS, van Dyck C, Hoffer PB, Innis RP. [123I] beta-CIT/SPECT imaging demonstrates bilateral loss of dopamine transporters in hemi-Parkinson’s disease. Neurology. 1996;46(1):231–237. [DOI] [PubMed] [Google Scholar]
- 43. Dresel S, Krause J, Krause KH, LaFougere C, Brinkbaumer K, Kung HF, Hahn K, Tatsch K. Attention deficit hyperactivity disorder: binding of [99mTc]TRODAT-1 to the dopamine transporter before and after methylphenidate treatment. Eur J Nucl Med. 2000;27(10):1518–1524. [DOI] [PubMed] [Google Scholar]
- 44. Dougherty DD, Bonab AA, Spencer TJ, Rauch SL, Madras BK, Fischman AJ. Dopamine transporter density in patients with attention deficit hyperactivity disorder. Lancet. 1999;354(9196):2132–2133. [DOI] [PubMed] [Google Scholar]
- 45. Ellison-Wright I, Ellison-Wright Z, Bullmore E. Structural brain change in Attention Deficit Hyperactivity Disorder identified by meta-analysis. BMC Psychiatry. 2008;8:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Duzel E, Bunzeck N, Guitart-Masip M, Wittmann B, Schott BH, Tobler PN. Functional imaging of the human dopaminergic midbrain. Trends Neurosci. 2009;32(6):321–328. [DOI] [PubMed] [Google Scholar]
- 47. Jucaite A, Fernell E, Halldin C, Forssberg H, Farde L. Reduced midbrain dopamine transporter binding in male adolescents with attention-deficit/hyperactivity disorder: association between striatal dopamine markers and motor hyperactivity. Biol Psychiatry. 2005;57(3):229–238. [DOI] [PubMed] [Google Scholar]
- 48. Volkow ND, Wang GJ, Newcorn J, Fowler JS, Telang F, Solanto MV, Logan J, Wong C, Ma Y, Swanson JM, Schulz K, et al. Brain dopamine transporter levels in treatment and drug naive adults with ADHD. Neuroimage. 2007;34(3):1182–1190. [DOI] [PubMed] [Google Scholar]
- 49. Bouchatta O, Manouze H, Bouali-Benazzouz R, Kerekes N, Ba-M’hamed S, Fossat P, Landry M, Bennis M. Neonatal 6-OHDA lesion model in mouse induces Attention-Deficit/ Hyperactivity Disorder (ADHD)-like behaviour. Sci Rep. 2018;8(1):15349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Davids E, Zhang K, Tarazi FI, Baldessarini RJ. Animal models of attention-deficit hyperactivity disorder. Brain Res Brain Res Rev. 2003;42(1):1–21. [DOI] [PubMed] [Google Scholar]
- 51. Zhang K, Tarazi FI, Davids E, Baldessarini RJ. Plasticity of dopamine D4 receptors in rat forebrain: temporal association with motor hyperactivity following neonatal 6-hydroxydopamine lesioning. Neuropsychopharmacology. 2002;26(5):625–633. [DOI] [PubMed] [Google Scholar]
- 52. Wen YH, Wu SS, Lin CH, Tsai JH, Yang P, Chang YP, Tseng KH. A Bayesian approach to identifying new risk factors for dementia: a nationwide population-based study. Medicine (Baltimore). 2016;95(21):e3658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Cheng TM. Taiwan’s new national health insurance program: genesis and experience so far. Health Aff (Millwood). 2003;22(3):61–76. [DOI] [PubMed] [Google Scholar]
- 54. Jankovic J. Treatment of hyperkinetic movement disorders. Lancet Neurol. 2009;8(9):844–856. [DOI] [PubMed] [Google Scholar]
- 55. Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45(6):613–619. [DOI] [PubMed] [Google Scholar]
- 56. Davids E, Zhang K, Tarazi FI, Baldessarini RJ. Stereoselective effects of methylphenidate on motor hyperactivity in juvenile rats induced by neonatal 6-hydroxydopamine lesioning. Psychopharmacology (Berl). 2002;160(1):92–98. [DOI] [PubMed] [Google Scholar]
- 57. Ben-Shachar D, Eshel G, Finberg JP, Youdim MB. The iron chelator desferrioxamine (Desferal) retards 6-hydroxydopamine-induced degeneration of nigrostriatal dopamine neurons. J Neurochem. 1991;56(4):1441–1444. [DOI] [PubMed] [Google Scholar]
- 58. Huber JD, Egleton RD, Davis TP. Molecular physiology and pathophysiology of tight junctions in the blood-brain barrier. Trends Neurosci. 2001;24(12):719–725. [DOI] [PubMed] [Google Scholar]
- 59. Brown RC, Egleton RD, Davis TP. Mannitol opening of the blood-brain barrier: regional variation in the permeability of sucrose, but not 86Rb+ or albumin. Brain Res. 2004;1014(1–2):221–227. [DOI] [PubMed] [Google Scholar]
- 60. Chang LT. A method for attenuation correction in radionuclide computed tomography. IEEE Trans Nucl Sci. 1978;25(1):6. [Google Scholar]
- 61. Yin TK, Lee BF, Yang YK, Chiu NT. Differences of various region-of-interest methods for measuring dopamine transporter availability using 99mTc-TRODAT-1 SPECT. Sci World J. 2014;2014:837439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Li IH, Huang WS, Shiue CY, Huang YY, Liu RS, Chyueh SC, Hu SH, Liao MH, Shen LH, Liu JC, Ma K-H. Study on the neuroprotective effect of fluoxetine against MDMA-induced neurotoxicity on the serotonin transporter in rat brain using micro-PET. Neuroimage. 2010;49(2):1259–1270. [DOI] [PubMed] [Google Scholar]
- 63. Shaywitz BA, Yager RD, Klopper JH. Selective brain dopamine depletion in developing rats: an experimental model of minimal brain dysfunction. Science. 1976;191(4224):305–308. [DOI] [PubMed] [Google Scholar]
- 64. Zhang K, Tarazi FI, Baldessarini RJ. Role of dopamine D(4) receptors in motor hyperactivity induced by neonatal 6-hydroxydopamine lesions in rats. Neuropsychopharmacology. 2001;25(5):624–632. [DOI] [PubMed] [Google Scholar]
- 65. Cheon KA, Ryu YH, Kim YK, Namkoong K, Kim CH, Lee JD. Dopamine transporter density in the basal ganglia assessed with [123I]IPT SPET in children with attention deficit hyperactivity disorder. Eur J Nucl Med Mol Imaging. 2003;30(2):306–311. [DOI] [PubMed] [Google Scholar]
- 66. Vanbilloen HP, Kieffer D, Cleynhens BJ, Bormans G, Mortelmans L, Verbruggen AM. Development and biological evaluation of 99mTc-BAT-tropane esters. Nucl Med Biol. 2005;32(6):607–612. [DOI] [PubMed] [Google Scholar]
- 67. Kroll RA, Neuwelt EA. Outwitting the blood-brain barrier for therapeutic purposes: osmotic opening and other means. Neurosurgery. 1998;42(5):1083–1099; discussion 1099–100. [DOI] [PubMed] [Google Scholar]
- 68. Mozley PD, Schneider JS, Acton PD, Plossl K, Stern MB, Siderowf A, Leopold NA, Li PY, Alavi A, Kung HF. Binding of [99mTc]TRODAT-1 to dopamine transporters in patients with Parkinson’s disease and in healthy volunteers. J Nucl Med. 2000;41(4):584–589. [PubMed] [Google Scholar]
- 69. Sasannezhad P, Juibary AG, Sadri K, Sadeghi R, Sabour M, Kakhki VRD, Alizadeh H. (99 m)Tc-TRODAT-1 SPECT imaging in early and late onset Parkinson’s disease. Asia Ocean J Nucl Med Biol. 2017;5(2):114–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Weng YH, Yen TC, Chen MC, Kao PF, Tzen KY, Chen RS, Wey SP, Ting G, Lu CS. Sensitivity and specificity of 99mTc-TRODAT-1 SPECT imaging in differentiating patients with idiopathic Parkinson’s disease from healthy subjects. J Nucl Med. 2004;45(3):393–401. [PubMed] [Google Scholar]
- 71. Smith RD, Cooper BR, Breese GR. Growth and behavioral changes in developing rats treated intracisternally with 6-hydroxydopamine: evidence for involvement of brain dopamine. J Pharmacol Exp Ther. 1973;185(3):609–619. [PubMed] [Google Scholar]
- 72. Segura-Aguilar J, Paris I, Munoz P, Ferrari E, Zecca L, Zucca FA. Protective and toxic roles of dopamine in Parkinson’s disease. J Neurochem. 2014;129(6):898–915. [DOI] [PubMed] [Google Scholar]
- 73. Shih MC, Hoexter MQ, Andrade LA, Bressan RA. Parkinson’s disease and dopamine transporter neuroimaging: a critical review. Sao Paulo Med J. 2006;124(3):168–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Hwang WJ, Yao WJ, Wey SP, Ting G. Reproducibility of 99mTc-TRODAT-1 SPECT measurement of dopamine transporters in Parkinson’s disease. J Nucl Med. 2004;45(2):207–213. [PubMed] [Google Scholar]
- 75. Khan W, Priyadarshini M, Zakai HA, Kamal MA, Alam Q. A brief overview of tyrosine hydroxylase and alpha-synuclein in the Parkinsonian brain. CNS Neurol Disord Drug Targets. 2012;11(4):456–462. [DOI] [PubMed] [Google Scholar]
- 76. Leng A, Mura A, Hengerer B, Feldon J, Ferger B. Effects of blocking the dopamine biosynthesis and of neurotoxic dopamine depletion with 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) on voluntary wheel running in mice. Behav Brain Res. 2004;154(2):375–383. [DOI] [PubMed] [Google Scholar]
- 77. Snyder GL, Keller RW, Jr, Zigmond MJ. Dopamine efflux from striatal slices after intracerebral 6-hydroxydopamine: evidence for compensatory hyperactivity of residual terminals. J Pharmacol Exp Ther. 1990;253(2):867–876. [PubMed] [Google Scholar]
- 78. Chotibut T, Apple DM, Jefferis R, Salvatore MF. Dopamine transporter loss in 6-OHDA Parkinson’s model is unmet by parallel reduction in dopamine uptake. PLoS One. 2012;7(12):e52322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Ungerstedt U, Arbuthnott GW. Quantitative recording of rotational behavior in rats after 6-hydroxy-dopamine lesions of the nigrostriatal dopamine system. Brain Res. 1970;24(3):485–493. [DOI] [PubMed] [Google Scholar]
- 80. Brus R, Nowak P, Szkilnik R, Mikolajun U, Kostrzewa RM. Serotoninergics attenuate hyperlocomotor activity in rats. Potential new therapeutic strategy for hyperactivity. Neurotox Res. 2004;6(4):317–325. [DOI] [PubMed] [Google Scholar]
- 81. Descarries L, Soghomonian JJ, Garcia S, Doucet G, Bruno JP. Ultrastructural analysis of the serotonin hyperinnervation in adult rat neostriatum following neonatal dopamine denervation with 6-hydroxydopamine. Brain Res. 1992;569(1):1–13. [DOI] [PubMed] [Google Scholar]
- 82. Snyder AM, Zigmond MJ, Lund RD. Sprouting of serotoninergic afferents into striatum after dopamine-depleting lesions in infant rats: a retrograde transport and immunocytochemical study. J Comp Neurol. 1986;245(2):274–281. [DOI] [PubMed] [Google Scholar]
- 83. Chiu CH, Li IH, Weng SJ, Huang YS, Wu SC, Chou TK, Huang WS, Liao MH, Shiue CY, Cheng CY, Ma K-H. PET imaging of serotonin transporters with 4-[(18)F]-ADAM in a Parkinsonian Rat Model with porcine neural xenografts. Cell Transplant. 2016;25(2):301–311. [DOI] [PubMed] [Google Scholar]
- 84. Weng SJ, Shiue CY, Huang WS, Cheng CY, Huang SY, Li IH, Tao CC, Chou TK, Liao MH, Chang YP, Ma K-H. PET imaging of serotonin transporters with 4-[18F]-ADAM in a Parkinsonian rat model. Cell Transplant. 2013;22(7):1295–1305. [DOI] [PubMed] [Google Scholar]
- 85. Gainetdinov RR, Jones SR, Fumagalli F, Wightman RM, Caron MG. Re-evaluation of the role of the dopamine transporter in dopamine system homeostasis. Brain Res Brain Res Rev. 1998;26(2–3):148–153. [DOI] [PubMed] [Google Scholar]
- 86. Jones SR, Gainetdinov RR, Wightman RM, Caron MG. Mechanisms of amphetamine action revealed in mice lacking the dopamine transporter. J Neurosci. 1998;18(6):1979–1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Alsop B. Problems with spontaneously hypertensive rats (SHR) as a model of attention-deficit/hyperactivity disorder (AD/HD). J Neurosci Methods. 2007;162(1–2):42–48. [DOI] [PubMed] [Google Scholar]
- 88. Gramatte T, Schmidt J. The effect of early postnatal hypoxia on the effectiveness of drugs influencing motor behaviour in adult rats. Biomed Biochim Acta. 1986;45(8):1069–1074. [PubMed] [Google Scholar]
- 89. Fahlke C, Hansen S. Alcohol responsiveness, hyperreactivity, and motor restlessness in an animal model for attention-deficit hyperactivity disorder. Psychopharmacology (Berl). 1999;146(1):1–9. [DOI] [PubMed] [Google Scholar]
- 90. Silbergeld EK, Goldberg AM. Pharmacological and neurochemical investigations of lead-induced hyperactivity. Neuropharmacology. 1975;14(5–6):431–444. [DOI] [PubMed] [Google Scholar]
- 91. Ruppert PH, Dean KF, Reiter LW. Development of locomotor activity of rat pups exposed to heavy metals. Toxicol Appl Pharmacol. 1985;78(1):69–77. [DOI] [PubMed] [Google Scholar]