Abstract
Sleep spindles, defining oscillations of stage 2 non-rapid eye movement sleep (N2), mediate memory consolidation. Schizophrenia is characterized by reduced spindle activity that correlates with impaired sleep-dependent memory consolidation. In a small, randomized, placebo-controlled pilot study of schizophrenia, eszopiclone (Lunesta®), a nonbenzodiazepine sedative hypnotic, increased N2 spindle density (number/minute) but did not significantly improve memory. This larger double-blind crossover study that included healthy controls investigated whether eszopiclone could both increase N2 spindle density and improve memory. Twenty-six medicated schizophrenia outpatients and 29 healthy controls were randomly assigned to have a placebo or eszopiclone (3 mg) sleep visit first. Each visit involved two consecutive nights of high density polysomnography with training on the Motor Sequence Task (MST) on the second night and testing the following morning. Patients showed a widespread reduction of spindle density and, in both groups, eszopiclone increased spindle density but failed to enhance sleep-dependent procedural memory consolidation. Follow-up analyses revealed that eszopiclone also affected cortical slow oscillations: it decreased their amplitude, increased their duration, and rendered their phase locking with spindles more variable. Regardless of group or visit, the density of coupled spindle-slow oscillation events predicted memory consolidation significantly better than spindle density alone, suggesting that they are a better biomarker of memory consolidation. In conclusion, sleep oscillations are promising targets for improving memory consolidation in schizophrenia, but enhancing spindles is not enough. Effective therapies also need to preserve or enhance cortical slow oscillations and their coordination with thalamic spindles, an interregional dialog that is necessary for sleep-dependent memory consolidation.
Subject terms: Non-REM sleep, Circadian rhythms and sleep
Introduction
Cognitive deficits are a core feature of schizophrenia that often predate its onset and persist throughout its course, even after psychotic symptoms have been effectively treated. Consequently, cognitive deficits contribute to the chronic disability that renders the vast majority of individuals with schizophrenia unable to work [1]. Although ameliorating cognitive deficits is a priority of the schizophrenia research community, effective treatments are lacking [2]. This reflects a lack of mechanistic understanding. Clearly, new approaches are needed to illuminate the pathophysiology of cognitive deficits and identify therapeutic targets.
It is now well established that sleep plays a critical role in memory, contributing to its stabilization, enhancement, and integration into cortical networks [3]. In schizophrenia, a growing literature suggests that abnormal sleep impairs memory consolidation and cognition more generally. Specifically, schizophrenia is characterized by a deficit in sleep spindles [4], a defining electroencephalography (EEG) oscillation of non-rapid eye movement (NREM) stage 2 sleep (N2). Sleep spindles are brief (~1 s), powerful bursts of 12–15 Hz activity organized in a waxing/waning envelope. Spindles correlate with sleep-dependent memory consolidation, learning efficiency, and IQ [5]. Recent work supports a causal role for spindles in memory consolidation: optogenetic studies of rodents [6], and pharmacological [7–9] and neurostimulation [10, 11] studies of humans show that increasing spindle activity improves memory, while decreasing it impairs memory [12]. These findings provide an impetus to target spindles to ameliorate cognitive deficits in schizophrenia [13]. The goal of this clinical trial was to determine whether enhancement of spindles using eszopiclone, (Lunesta®), a nonbenzodiazepine sedative hypnotic, improves sleep-dependent memory consolidation in schizophrenia.
In schizophrenia, reduced spindle activity [14–16] is associated with impaired sleep-dependent consolidation of both procedural and declarative memory, and predicts lower IQ and worse executive function [17–19]. Except for increased latency to sleep onset [14, 15], in chronic medicated patients, spindle deficits occur in the context of normal sleep quality and architecture, demonstrating that they are not secondary to sleep disruption [14–16]. Spindle deficits are also seen in adolescents with early-onset schizophrenia spectrum disorder [20], early course antipsychotic-naive schizophrenia patients, and young nonpsychotic first-degree relatives of schizophrenia patients [19, 21]. These findings, along with the high heritability of spindles [22–24] and their association with schizophrenia risk genes [25], suggest that the spindle deficit in schizophrenia is not due to antipsychotic drugs, psychosis, or chronicity and instead is an endophenotype that contributes to cognitive dysfunction [26].
The considerable cross-species knowledge of sleep spindle physiology guided our selection of eszopiclone. Spindles are generated in the thalamic reticular nucleus (TRN) [27], a thin net-like structure comprising entirely γ-aminobutyric acid (GABA)-ergic neurons, which is the major inhibitory nucleus of the thalamus [28]. Spindle initiation by the TRN depends on powerful and prolonged inhibition of thalamocortical neurons [29] followed by rebound spike bursts that entrain cortical neurons to oscillate at spindle frequency [30]. In schizophrenia, postmortem studies reveal TRN abnormalities that may disrupt spindle generation [31–33]. Eszopiclone prolongs inhibitory postsynaptic currents in GABAA receptors of TRN neurons, which increases the burst firing that generates spindles [34]. In our previous randomized, placebo-controlled, double-blind pilot study, eszopiclone increased spindles in schizophrenia but did not significantly improve memory, perhaps reflecting the small sample, between-subjects design, and that memory is a less direct assay of drug effects than spindles [35]. The present, larger, double-blind crossover study, which included healthy controls, investigated whether eszopiclone could both increase spindles and improve memory. Despite increasing spindles, eszopiclone again failed to improve memory. To understand this failure, we investigated whether eszopiclone disrupted cortical slow oscillations (SOs) and their coordination with spindles. SOs are large amplitude ~1 Hz oscillations that reflect the rhythmic depolarization (upstates) and hyperpolarization (downstates) of large populations of cortical pyramidal neurons [36]. SOs group spindles in their excitable upstates [37, 38] and compelling recent evidence indicates that memory consolidation relies on this phase locking [6], including in schizophrenia [38].
Methods
Participants
Thirty-nine outpatients with schizophrenia and 48 healthy individuals enrolled, and 26 patients and 29 controls completed the study (Supplementary Fig. S1). Patients were recruited from an urban mental health center. Diagnoses were confirmed with the Structured Clinical Interview for DSM-IV [39]. Symptom severity was rated with the Positive and Negative Syndrome Scale [40] and the Scale for the Assessment of Negative Symptoms [41]. Two patients were unmedicated and the rest were maintained on stable doses of antipsychotic and adjunctive medications for at least 6 weeks prior to enrolling and throughout the study (Table S1).
Healthy controls, screened to exclude a personal history of mental illness [42] and a family history of schizophrenia spectrum disorder or psychosis, were recruited from the community through advertisements. Potential participants were screened to exclude pregnant and breastfeeding women, and individuals with diagnosed sleep disorders, intellectual disability, hepatic impairment, or a history of significant head injury, neurological disorder, or recent substance abuse (within 6 months). Patient and control groups did not differ significantly in age, sex, handedness, mean parental education, or home-based actigraphy measures of sleep quality (Table 1). The study was approved by the Partners Human Research Committee and participants gave written informed consent. In addition to a base rate of pay, participants received $0.05 for each correctly typed sequence on the memory task.
Table 1.
Participant characteristics.
| Control (n = 29) | Schizophrenia (n = 26) | t | p | |
|---|---|---|---|---|
| M ± SD | M ± SD | |||
| Age (years) | 30 ± 6 | 32 ± 8 | 1.16 | 0.25 |
| Sex | 8F/21M | 5F/21M | χ2 = 0.47 | 0.54 |
| Mean parental education (years) | 15 ± 3 | 14 ± 3 | −1.13 | 0.27 |
| Handednessa | 66 ± 53 | 81 ± 23 | 1.25 | 0.22 |
| Total sleep time (min)b | 533 ± 111 | 553 ± 76 | 0.70 | 0.49 |
| Sleep efficiency (%)b | 81 ± 8 | 83 ± 6 | 0.67 | 0.51 |
| WASO (min)b | 57 ± 26 | 56 ± 26 | 0.18 | 0.86 |
| Rating scale scores/level of severity | ||||
| PANSS total | 69 ± 14 | Mild | ||
| PANSS positive | 16 ± 5 | Mild | ||
| PANSS negative | 20 ± 5 | Mild | ||
| PANSS general | 34 ± 7 | Mild | ||
| SANS | 37 ± 12 | Minimal | ||
PANSS Positive and Negative Syndrome Scale, SANS Scale for the Assessment of Negative Symptoms, WASO wake time after sleep onset.
Means, SDs, and group comparisons of demographic data and home-based actigraphy measures of sleep quality in the weeks prior to and between experimental visits. Symptom rating scale scores for patients.
aBased on the modified Edinburgh Handedness Inventory [82]. Laterality scores of −100 and +100 denote exclusive use of the left or right hand, respectively.
bBased on averaged actigraphy data in the week prior to and between experimental visits.
Procedures
Overview
This was a double-blind, placebo-controlled, crossover study (Fig. 1a). One week prior to their first sleep visit, participants had an introductory/screening visit for informed consent, clinical assessments, liver function tests, urine toxicology, and pregnancy screening. They toured the Clinical Research Center and were given sleep diaries to indicate bedtimes and wake-up times, and an actigraphy wrist band to wear until study completion (Supplementary Methods). The MGH Clinical Trials Pharmacy randomly assigned included participants in each group to have an eszopiclone (3 mg) or placebo visit first using www.randomization.com (block size: 4).
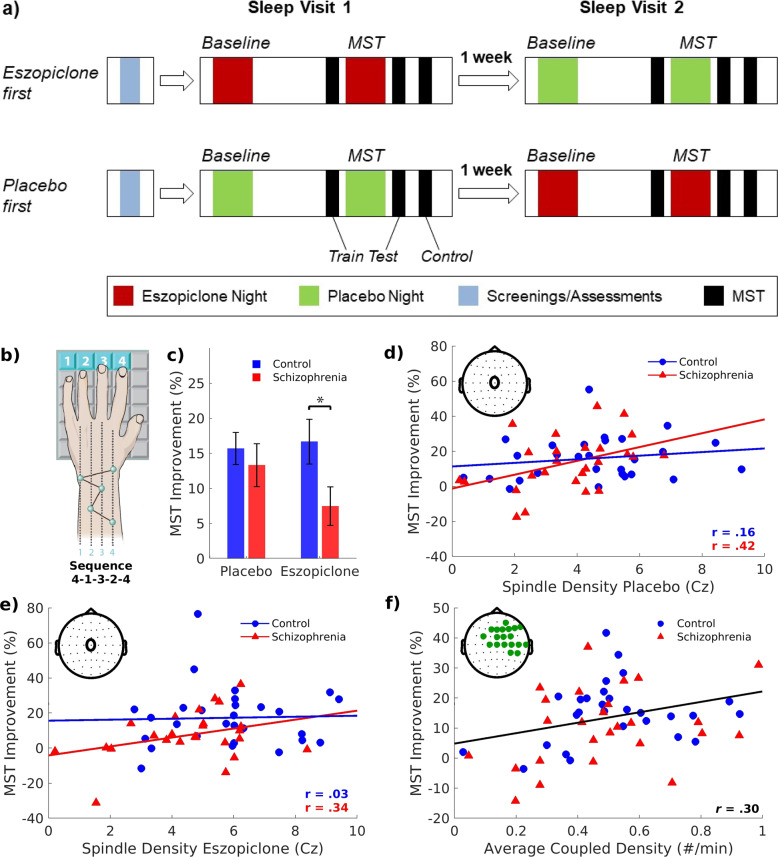
Fig. 1. Finger tapping Motor Sequence Task (MST) administration and results.
a Participants, randomized to the placebo or eszopiclone visit first, had an introductory/screening visit and two sleep visits, a week apart. Sleep visits involved polysomnography on two consecutive nights (Baseline, MST). Different MST sequences were employed for the two sleep visits and their order was counterbalanced within groups. There is no transfer of learning between MST sequences [83]. Participants trained on the MST before bedtime of the second night and were tested in the morning. Ten minutes after testing, participants trained on a new “control” sequence to evaluate the possibility of eszopiclone “hangover” effects on MST performance. Prior to each MST administration, participants completed the Stanford Sleepiness Scale (SSS) to measure subjective alertness [84]. b The MST requires participants to repeatedly type a five-digit sequence (e.g., 4–1–3–2–4) on a numerically labeled keyboard with the left hand, “as quickly and accurately as possible” for twelve 30 s trials separated by 30 s rest periods. The sequence is displayed at the top of the screen and dots appear beneath it with each keystroke. Participants train before sleep and test on an additional 12 trials after sleep. c Bar graphs of group differences in overnight MST improvement on placebo and eszopiclone with SE bars. Asterisk denotes p < 0.05. d Spindle density at electrode location Cz (inset) on the MST night plotted against overnight MST improvement by group for the placebo and e eszopiclone visits. f Density of spindle–SO events on the MST nights plotted against overnight MST improvement, averaged over the placebo and eszopiclone visits, in the cluster showing a significant correlation (inset). The regression line for the combined groups and visits is shown.
Sleep visits (eszopiclone, placebo) were separated by 1 week and each involved two consecutive nights of polysomnography (PSG). Eszopiclone or placebo administration was at 10:00 p.m., 30 m before bedtime. Participants were allowed to sleep up to 10 h. They engaged in their usual daytime activities, but were asked not to nap, as confirmed by actigraphy. On the second night of each visit, participants trained on the Motor Sequence Task (MST), a procedural memory test, at 8:30 p.m. Testing occurred at 9:30 a.m., followed by a control sequence 10 m later. The primary outcome measure was N2 spindle density (number/minute) and the secondary measure was overnight MST improvement.
Motor Sequence Task
The MST is the most extensively validated probe of sleep-dependent memory consolidation. We have reported on its sleep dependence in health [43] and on the failure of sleep-dependent improvement in schizophrenia, despite normal learning during training [16, 18, 44]. Performance is measured as the number of correctly typed sequences per trial (Fig. 1b). Learning during training is calculated as the percent change in correct sequences from the first trial to the average of the last three training trials. The main outcome measure, overnight improvement, is the percent increase in correctly typed sequences from the average of the last three training trials at night to the first three test trials the following morning [43].
Actigraphy
Standard scoring algorithms quantified time in bed (TIB), total sleep time (TST), sleep efficiency (TST/TIB) and wake time after sleep onset (WASO).
Polysomnography
Data were acquired at 400 Hz with an Aura LTM64 system (Grass Technologies, Astro-Med, Inc., RI) and EEG caps (Easycap GmbH, Herrsching, Germany) with 58 EEG, 2 submental electromyography, and 2 electrooculography electrodes. Each 30 s epoch was visually scored according to standard criteria [36] as WAKE, REM, N1, N2, or N3 by raters blind to visit, group, and night. EEG data were pre-processed and analyzed using BrainVision Analyzer 2.0 (BrainProducts, Germany) and Matlab (MathWorks, Natick MA). Data were band-pass filtered at 0.3–35 Hz and notch filtered at 60 Hz. Independent Component Analysis removed cardiac artifacts and remaining artifacts were visually identified and removed. Electrodes displaying significant artifacts for more than 30 m were spatially interpolated.
Spindle measures
After referencing to the common average [45], spindles were automatically detected in the 12–15 Hz band-pass-filtered data at each electrode using a wavelet-based algorithm [18, 46]. The threshold for spindle detection, nine times the median signal amplitude of artifact-free epochs, maximized between class (“spindle” vs. “non-spindle”) variance [47] in schizophrenia and control samples from a previous study [18].
In addition to N2 spindle density, we measured the amplitude, duration, frequency, and sigma power (12–15 Hz) of individual spindles based on 4 s epochs centered on the point of spindle detection. Amplitude was the maximal voltage; frequency was the spectral peak following fast Fourier transform (FFT) decomposition, sigma power was the mean FFT-derived power spectral density, and duration was the half-height width of the wavelet energy. N2 sigma power was calculated as power spectral density (μV2/Hz) by FFT using 8 s Hanning windows with 50% overlap.
SOs and their coordination with spindles
After referencing to the linked mastoids (6/220 records were excluded due to noise in the mastoids and/or sweat artifacts), SOs were automatically detected using a published method [38, 48] and spindles were detected using the algorithm described above. To identify SOs, the raw EEG at each electrode was filtered at 0.5–4 Hz using a two-way least-squares finite impulse response filter [49]. Consecutive positive-to-negative zero crossings within 0.8–2 s (corresponding to 0.5–1.25 Hz) were marked as candidate SOs (Fig. S2a). After excluding waveforms with peak-to-peak amplitudes >300 μV as artifacts, waveforms with amplitudes in the upper 25% were defined as SOs (Supplementary Fig. S2b) [50, 51]. We measured SO amplitude, duration, and density.
We identified spindle–SO events when a spindle peaked during the SO and measured their density, the SO phase at spindle peak, and the consistency of this phase locking (Supplementary Fig. S2c). We derived the SO phase from the Hilbert transform of the filtered data. The mean SO phase was calculated using circular statistics [52]. The length of the mean SO phase vector indexes the consistency of the spindle–SO phase locking. To ensure reliable measures, SO phase and phase-locking consistency were only calculated at electrodes with ≥20 coupled events.
Statistical analyses
Eszopiclone effects
Linear mixed-effects models investigated eszopiclone effects on sleep parameters with Group, Drug (Placebo, Eszopiclone), and Night (Baseline, MST), and their interactions as fixed effects and Subject as a random effect. As there were no significant effects or interactions with Night and omitting Night from the models did not substantially change the results, analyses are based on the averaged two nights of each sleep visit. Paired and two-sample circular tests (Watson–William F-test) evaluated the effects of Group, Drug, and their interaction on SO phase [52, 53]. Eszopiclone effects on overnight MST improvement were tested with a linear mixed-effects model that included Group, Drug, and their interaction as fixed effects and Subject as a random effect. One patient’s MST data from the eszopiclone visit was excluded from analyses as a statistical outlier (>3 SD).
Relations of sleep parameters with memory consolidation
We used data from the MST nights in Pearson’s correlations of overnight MST improvement with spindle density at electrode Cz, to compare with previous studies [18, 35, 38]. We then regressed overnight MST improvement against spindle density and spindle–SO parameters at each electrode using Group, Drug, and their interactions as fixed effects and Subject as a random effect. To examine MST relations with SO phase, we performed circular-linear correlations for each group and visit.
Multiple comparisons correction
We corrected for multiple comparisons across electrodes using a nonparametric cluster-based method [54] implemented in MATLAB. For each term in the model, clusters were formed from adjacent electrodes that met an uncorrected threshold of p < 0.05. The cluster statistic (tsum) was the sum of the test statistics of the electrodes within the cluster. Permutation distributions were created by randomly shuffling the labels (Group, Drug) 10,000 times at each electrode and retaining the cluster with the maximum statistic for each permutation. The cluster-level corrected p-value is the probability that the observed cluster would be found by chance under the permutation distribution.
Results
As N2 spindle activity differentiates schizophrenia patients from controls and correlates with sleep-dependent MST improvement [18, 38], and spindle and SO parameters differ in N2 and N3 (Stage 3 NREM sleep) [37, 38], we focus on N2. N3 findings are in Supplementary Results and Supplementary Figs. S4–7.
Measurement reliability
As in prior work [37], spindle, SO, and spindle–SO coordination parameters were highly reliable across nights for both groups at both visits (Supplementary Methods and Supplementary Fig. S3).
Eszopiclone effects
Sleep quality and architecture
PSG measures of sleep quality and architecture did not differ by Group (Supplementary Table S2). For both groups, eszopiclone improved sleep quality, increasing TST (p = 0.002) and sleep efficiency (p = 0.003), and decreasing WASO (p = 0.04). Eszopiclone also affected sleep architecture, increasing N2 (percent: p < 0.001; duration: p < 0.001) and decreasing REM (percent: p = 0.001; duration: p = 0.04) for both groups. In patients (t(25) = 2.73, p = 0.01), but not controls (t(28) = −0.13, p = 0.90), eszopiclone decreased N3% (GroupXDrug: p = 0.02). Eszopiclone also decreased N1 duration for controls (t(28) = 2.25, p = 0.03) but increased it for patients (t(25) = −2.15, p = 0.04; GroupXDrug: p = 0.003).
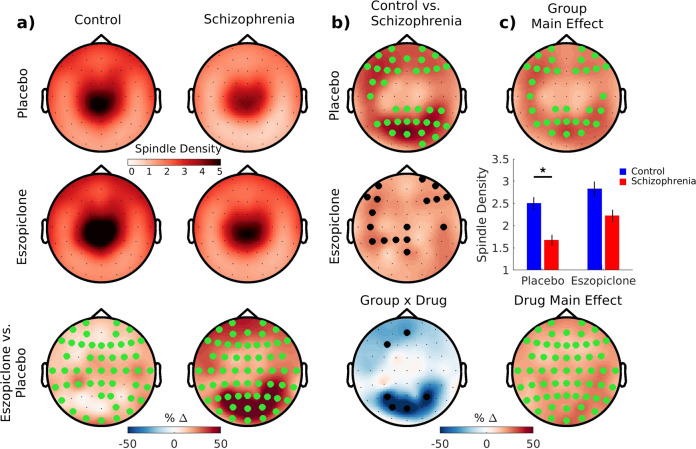
Spindle density
Compared with controls, patients showed widespread reductions in N2 spindle density (Table 2 and Fig. 2). Eszopiclone increased spindle density similarly in both groups at all electrodes (partial-η2 = 0.30; 95% confidence interval (95% CI): 0.15, 0.49; controls: partial-η2 = 0.27; 95% CI: 0.09, 0.53; schizophrenia: partial-η2 = 0.34; 95% CI: 0.15, 0.59).
Table 2.
Group and Drug main effects on spindles, slow oscillations, and their coordination.
| Group main effect | Drug main effect | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control (M ± SD) | Schiz. (M ± SD) | Control vs. Schiz. (partial-η2) | Cluster statistics | Placebo (M ± SD) | Eszopicl. (M ± SD) | Eszopicl. vs. Placebo (partial-η2) | Cluster statistics | |||||
| No. of electrodes | tsum | p | No. of electrodes | tsum | p | |||||||
| Spindles | ||||||||||||
| Density (#/min) | 2.6 ± 1.3 | 1.9 ± 1.0 | 0.10 | 32 | 76 | 0.03* | 2.2 ± 1.3 | 2.6 ± 1.3 | 0.30 | 58 | 277 | <0.001* |
| Amplitude (μV) | 12.4 ± 2.8 | 11.8 ± 3.5 | 0.01 | - | - | - | 12.0 ± 3.2 | 12.3 ± 3.1 | 0.04 | - | - | - |
| Frequency (Hz) | 13.07 ± 0.33 | 13.02 ± 0.38 | 0.01 | - | - | - | 13.14 ± 0.37 | 13.07 ± 0.36 | 0.17 | 33 | −106 | <0.001* |
| Duration (s) | 0.84 ± 0.07 | 0.83 ± 0.09 | 0.01 | - | - | - | 0.83 ± 0.08 | 0.85 ± 0.08 | 0.30 | 49 | 197 | <0.001* |
| Spindle sigma (μV2/Hz) | 8.7 ± 4.2 | 8.0 ± 4.7 | 0.01 | - | - | - | 8.8 ± 4.6 | 9.8 ± 5.3 | 0.13 | 27 | 76 | 0.006* |
| N2 sigma (μV2/Hz) | 0.63 ± 0.34 | 0.54 ± 0.32 | 0.02 | - | - | - | 0.61 ± 0.35 | 0.70 ± 0.40 | 0.16 | 33 | 106 | 0.002* |
| Slow oscillations (SO) | ||||||||||||
| Density (#/min) | 4.5 ± 0.6 | 4.9 ± 1.0 | 0.06 | - | - | - | 4.7 ± 0.8 | 4.8 ± 0.8 | 0.04 | - | - | - |
| Amplitude (μV) | 66 ± 15 | 65 ± 17 | 0.002 | - | - | - | 69 ± 16 | 62 ± 15 | 0.37 | 58 | −329 | <0.001* |
| Duration (s) | 1.06 ± 0.04 | 1.06 ± 0.05 | 0.02 | - | - | - | 1.05 ± 0.04 | 1.07 ± 0.04 | 0.22 | 37 | 144 | 0.002* |
| Spindle–SO coordination | ||||||||||||
| Coupled density (#/min) | 0.48 ± 0.21 | 0.47 ± 0.23 | 0.01 | - | - | - | 0.44 ± 0.21 | 0.51 ± 0.22 | 0.26 | 55 | 238 | <0.001* |
| Consistency | 0.41 ± 0.13 | 0.53 ± 0.15 | 0.18 | 37 | −123 | 0.01* | 0.49 ± 0.15 | 0.44.15 | 0.20 | 54 | −190 | <0.001* |
| Phase (°) | 8 ± 25° | 2 ± 22° | 0.02 | - | - | - | 6 ± 24° | 4 ± 24° | 0.04 | - | - | - |
Means, SDs, and effect sizes are based on data averaged across nights. Descriptive statistics are averaged across electrodes within significant clusters (otherwise across all electrodes).
* denotes that the cluster meets a corrected threshold of p<.05.
Fig. 2. Eszopiclone effects on N2 sleep spindle density.
a Topographical maps of spindle density averaged across nights of the placebo and eszopiclone visits for each group and the comparison of the two visits. Electrodes with a cluster-corrected p < 0.05 are highlighted in green. b Group difference maps (top and middle rows) show significantly greater spindle density in controls than patients on placebo (two significant clusters), but not eszopiclone. Electrodes highlighted in black correspond to puncorrected < 0.05. Bottom row: Group by Drug interaction showing electrodes where the eszopiclone effect of increasing spindle density was nonsignificantly greater in patients (puncorrected < 0.05). c Top: main effect of Group on spindle density showing a significant cluster. Middle: bar graph of spindle density averaged across the electrodes in the significant cluster with SE bars. Bottom: Drug main effect—eszopiclone increased spindle density at all electrodes.
Spindle characteristics and N2 sigma power
The groups did not differ in individual spindle characteristics or N2 sigma power (Table 2 and Supplementary Fig. S4). Eszopiclone increased the sigma power and duration of individual spindles, decreased their frequency, but did not change their amplitude. N2 sigma power increased on eszopiclone. Eszopiclone effects did not differ by Group.
SOs and their coordination with spindles
Patients did not differ from controls in SO parameters (density, amplitude, duration), the density of spindle–SO events, or SO phase, but showed increased phase consistency (Table 2 and Supplementary Fig. S4). Spindles preferentially peaked during SO upstates at all electrodes regardless of group or visit (Fig. 3a).
Fig. 3. Eszopiclone effects on N2 SOs and their coordination with spindles.
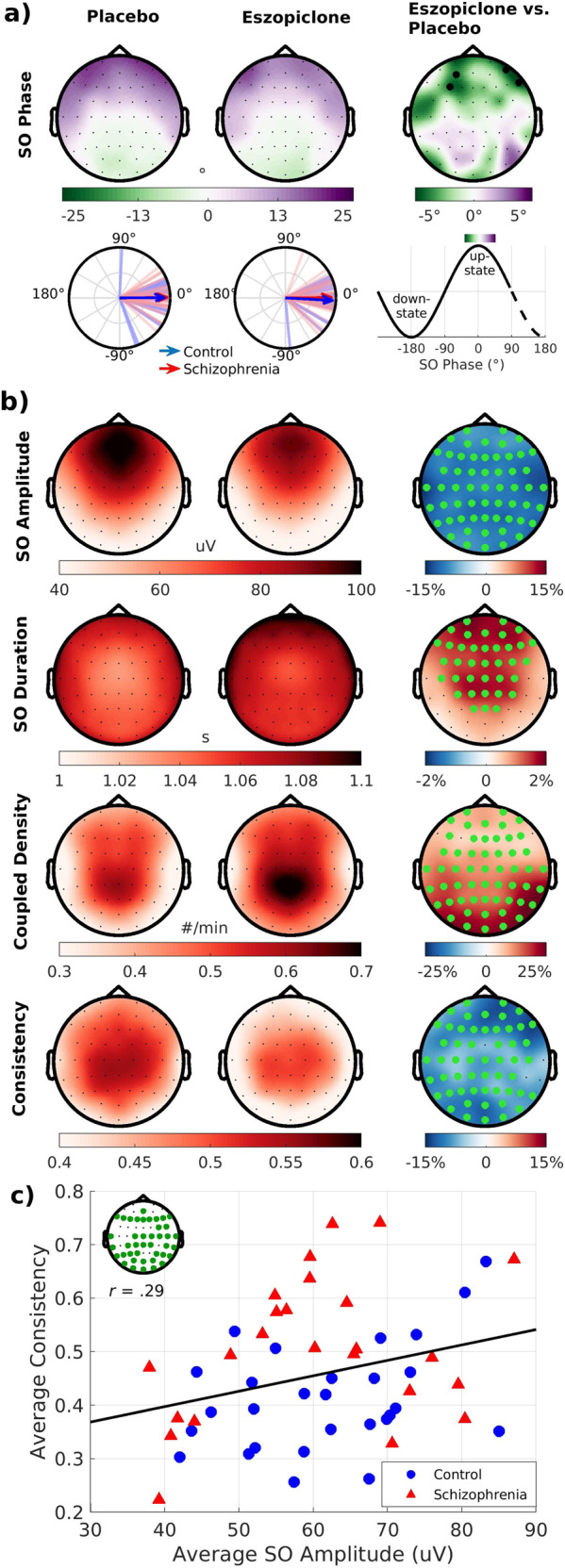
a Topographical maps of mean SO phase at spindle peak during the placebo and eszopiclone visits (averaged across groups and nights) and a statistical map of their difference. Electrodes highlighted in black meet a threshold of puncorrected < 0.05. Circular plots show SO phase at Cz. Lines represent the mean SO phase for each participant. Arrows represent the group means and their lengths represent the consistency of the spindle-SO phase-locking across participants. Right column: mapping of SO phase to topographical maps and circular plots. b Topographical maps of SO and spindle–SO coordination parameters during the placebo and eszopiclone visits and statistical maps of their difference. Electrodes with a cluster-corrected p < 0.05 are highlighted in green. c Plot of the relation of SO amplitude with spindle–SO phase consistency, averaged over the placebo and eszopiclone visits, in the cluster showing a significant correlation (inset shows significant cluster). Regression line is for the combined groups and drug visits.
Eszopiclone did not affect SO density but reduced SO amplitude and increased SO duration (Fig. 3b). Eszopiclone increased the density of spindle–SO events. Although eszopiclone did not change the mean SO phase, the timing of this coupling became less consistent—spindles peaked at more variable phases of the SO. As SOs group spindles in their upstates, weaker (i.e., lower amplitude) SOs might have contributed to less consistent phase locking. Supporting this hypothesis, lower SO amplitude predicted less consistent phase locking (Fig. 3c and tsum(41 electrodes) = 113, p = 0.002), an effect that did not differ by Group or Drug.
Overnight MST improvement
Although they did not differ in learning during training (F(1,50) = 1.37, p = 0.25), patients showed a trend to less overnight improvement than controls (Fig. 1c; F(1,52) = 3.17, p = 0.08; partial-η2 = 0.06; 95% CI: 0, 0.23). Eszopiclone did not significantly affect overnight improvement (F(1,52) = 1.00, p = 0.32; partial-η2 = 0.02; 95% CI: 0, 0.18) and there was no Group by Drug interaction (F(1,52) = 1.97, p = 0.17, partial-η2 = 0.04; 95% CI: 0, 0.17).
Post hoc analyses revealed that patients, but not controls, showed a trend to worse overnight improvement on eszopiclone compared with placebo (44% reduction; t(24) = 1.89, p = 0.07). This is unlikely to reflect an eszopiclone “hangover” effect in the morning, as learning of the control sequences did not differ by drug (F(1,47) = 1.30, p = 0.26; Group × Drug: F(1,47) = 0.14, p = 0.71), nor did subjective level of alertness (Stanford Sleepiness Scale: F(1,45) = 0.03, p = 0.86; Group × Drug: F(1,47) < 0.001, p = 0.98).
Relations of overnight MST improvement with spindle density
Replicating prior studies [18, 35, 38], overnight MST improvement correlated with spindle density at Cz in schizophrenia (placebo: r = 0.42, p = 0.03; trend-level for eszopiclone: r = 0.34, p = 0.10; Fig. 1d, e), but not in controls (placebo: r = 0.16, p = 0.41; eszopiclone: r = 0.03, p = 0.87). Although there were no clusters in which spindle density predicted overnight MST improvement, the density of spindle–SO events predicted improvement in a cluster overlying the motor cortex contralateral to the hand that performed the task (tsum(19 electrodes) = 43, p = 0.04; Fig. 1f). This relationship did not differ by Group or Drug. Moreover, spindle–SO density was a significantly better predictor of memory consolidation than spindle density alone (t(57) = 112, p < 0.001) based on comparing the goodness of fit of the two models across all electrodes using the Bayesian Information Criterion [55].
Control and descriptive analyses
In patients, antipsychotic drug dosage (chlorpromazine equivalents) [56] did not correlate with spindle density or overnight MST improvement at either visit, but higher doses predicted a greater increase in spindle density on eszopiclone (tsum(8 electrodes) = 22, p = 0.03). Spindle density did not correlate with any measure of symptom severity. Overnight MST improvement did not correlate with other neuropsychological measures, suggesting that sleep-dependent memory consolidation is a separate domain of cognitive function (Supplementary Results).
Discussion
In this placebo-controlled, randomized, double-blind crossover study, eszopiclone robustly increased N2 sleep spindle density but did not enhance sleep-dependent memory consolidation. This study confirms pilot findings in schizophrenia [35], extends them to healthy people, and raises an important question: if spindles are a mechanism of memory consolidation and patients have reduced spindle density that correlates with worse memory, why does increasing spindles not improve memory? Recent complementary rodent and human studies, along with the present findings, converge on an explanation. Sleep-dependent memory consolidation relies not only on spindles, but on their temporal coordination with cortical SOs. Here we report that spindles coupled to SOs predicted memory consolidation significantly better than spindles alone. Eszopiclone altered SO morphology and reduced the consistency of SO phase locking with spindles. This suggests that eszopiclone disrupted the thalamocortical dialog necessary for the consistent, precisely timed spindle–SO coupling that mediates memory. We conclude that interventions to improve sleep-dependent memory consolidation need to not only increase spindles, but also preserve SOs and the fidelity of their coupling with spindles.
During NREM sleep, cortical SOs coordinate both spindle generation in the TRN and the reactivation of memory representations during hippocampal sharp-wave ripples (100–200 Hz) [57, 58]. Ripples nest in the troughs of spindles, which preferentially peak during SO upstates [37, 38, 48, 51, 59–61]. The orchestration of these three cardinal NREM oscillations mediates the transformation of new memories from temporary indexing in the hippocampus to longer-term representation in the cortex [61, 62]. The importance of this triple phase locking for memory is supported by recent studies. In mice, optogenetic induction of spindles on the rising phase of SOs leads to coupling with ripples and enhanced memory, whereas suppression of spindles at this phase impairs memory [6]. Similarly, ripple-triggered electrical stimulation of the cortex induces triple-coupled spindles and enhances memory [63]. In older people, the degradation of spindle–SO coupling is associated with increased forgetting [50]. In schizophrenia, spindle density, and spindle–SO timing together predict overnight MST improvement significantly better than either alone, suggesting that both are essential [38].
In the present study, spindle density predicted overnight MST improvement at Cz in schizophrenia, replicating previous findings [18, 35, 38], but the density of spindle–SO events correlated with improvement significantly better than spindle density alone in both groups. The correlated cluster overlay motor cortex contralateral to the hand that performed the MST, consistent with studies showing increased spindle activity in brain regions involved in learning that correlates with improved memory consolidation [64–66], including on the MST [67]. Rodent studies provide a higher resolution view of possible mechanisms. Spindle–SO events enhance calcium activity in pyramidal neurons more than isolated spindles and SOs [68] and motor cortex neurons activated during motor learning are reactivated during NREM sleep, promoting dendritic spine formation [69]. Calcium activity and spine formation underlie the synaptic plasticity necessary for memory. Collectively, this work suggests that regionally specific spindle–SO events mediate memory, and that spindle–SO events are a better biomarker of memory than spindles alone.
Eszopiclone reduced spindle–SO phase consistency, suggesting that it disrupted thalamocortical communication. Although the thalamus can generate sleep spindles in isolation, their propagation and synchronization across the cortex requires reciprocal interactions [70]. The synchronous spiking of cortical neurons groups spindles in SO upstates [71]. Less synchronized SO expression, reflected in lower amplitude SOs, may lead to more variable coupling with spindles. Supporting this hypothesis, lower SO amplitude predicted less consistent coupling. This suggests that by altering SOs, eszopiclone disrupted the thalamocortical dialog necessary for consistent, precisely timed spindle–SO coupling.
The present study was limited to studying the role of spindle–SO synchrony in memory, as it is difficult to measure hippocampal ripples without invasive recordings. Our recent study of amnestic patients, however, demonstrates that although an intact hippocampus is not necessary to learn the MST, it is necessary for its sleep-dependent consolidation [72]. In schizophrenia, postmortem studies show a selective loss of hippocampal interneurons [73]. These interneurons contribute to ripple generation [74] and ripple-spindle coordination [75] raising the intriguing possibility that their loss may contribute to impaired sleep-dependent memory in schizophrenia [17, 44, 76]. Although it is not possible to test this hypothesis directly, rodent models of schizophrenia show reduced coordination of ripples with spindles and SOs [77], and impairments in ripple-associated memory replay [78]. We recently found that eszopiclone suppresses hippocampal ripples in rats [79]. Zolpidem, in contrast, increases ripples in rats [80], increases spindles and spindle–SO coupling in humans [9], and improves sleep-dependent memory [7–9]. This illustrates that spindle increases alone do not predict memory improvement. Collectively, these findings reinforce the importance of complementary animal and human studies for measuring all three NREM oscillations and their coordination when evaluating promising new pharmacological and noninvasive brain stimulation approaches to enhancing memory [13].
Several limitations of the present study warrant consideration. Almost all patients took antipsychotic and adjunctive medications. Antipsychotic dosage was not related to spindle density at either sleep visit, but correlated with increased spindle density on eszopiclone. As patients were maintained on stable clinically indicated medication regimens during participation, controls were not medicated, and eszopiclone effects did not differ by group, our findings of increased spindle density but no memory improvement on eszopiclone are unlikely to be confounded by pharmacotherapy.
Another concern is that the present schizophrenia sample differed from previous ones [16, 18, 44, 81] by not showing a significant deficit in overnight MST improvement, despite being similar both demographically and in symptom severity. Previous samples have shown a wide range of improvement and the lack of a significant deficit here may reflect that behavioral measures of memory consolidation are more distal and therefore less sensitive, and more variable indices of thalamocortical integrity than spindles, requiring larger sample sizes for reliable estimates. Sample size, however, does not limit the primary conclusion that increasing spindles with eszopiclone does not improve memory, as both groups showed robust eszopiclone effects on spindle density and either no effect (controls, p = 0.79) or a nonsignificant worsening (patients, p = 0.07) of sleep-dependent memory consolidation. It also replicates our pilot study in which eszopiclone failed to improve memory in a schizophrenia sample that did show a significant memory deficit [35].
In summary, although eszopiclone increased spindles, it disrupted both SO expression and spindle–SO synchrony, and failed to improve memory. We conclude that coordinated NREM sleep oscillations are promising targets for improving cognition in schizophrenia but that enhancing spindles is not enough. Effective therapies will also need to preserve or enhance cortical SOs, hippocampal ripples, and their precise temporal coupling with spindles, an interregional dialog necessary for sleep-dependent memory consolidation.
Funding and disclosure
This work was supported by National Institutes of Health R01-MH 092638 to DSM and RS, R01-MH 48832 to RS, T32-HL07901 and K01MH114012 to BB, NARSAD Young Investigator Award from the Brain and Behavior Research Foundation to CD, and utilized resources provided by the National Center for Research Resources UL1TR001102-01 to Harvard Clinical and Translational Science Center, and Shared Instrumentation Grants 1S10RR023401, 1S10RR019307, and 1S10RR023043. RS has received compensation for consultation from Eight Sleep, Inc., and Remrise, Inc., DH has research grants from Boehringer Ingelheim, Roche, and Otsuka, and has received honoraria from Alkermes (consultant-Advisory Board), Sunovian-DSMB, NIMH Global DSMB, Council, and Talem Health- CME consultant. The other authors declare no competing financial interests or potential conflicts of interest.
Supplementary information
Acknowledgements
We are grateful to the staff of the Massachusetts General Hospital Translational and Clinical Research Center for their support, to our participants, to Carmen Varela and Matthew Wilson for consultation, and to the anonymous reviewers for constructive critiques.
Author contributions
DSM and RS conceived and designed the trial, and contributed to all aspects of its completion. DM, BB, CD, and RC contributed to conceptualization, methods development, data analysis, and writing. BB, TV, BS, RAF, DC, EP, and CEC contributed to data acquisition, sleep scoring, artifact rejection, and data analysis. AM contributed to task design and programming. DH oversaw all medical aspects of the clinical trial. MV consulted to statistical analysis and study design. All authors reviewed and commented on the finished product.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Dimitrios Mylonas, Bengi Baran
Supplementary information
Supplementary Information accompanies this paper at (10.1038/s41386-020-00833-2).
References
- 1.Insel TR. Translating scientific opportunity into public health impact: a strategic plan for research on mental illness. Arch Gen Psychiatry. 2009;66:128–33. doi: 10.1001/archgenpsychiatry.2008.540. [DOI] [PubMed] [Google Scholar]
- 2.Sinkeviciute I, Begemann M, Prikken M, Oranje B, Johnsen E, Lei WU, et al. Efficacy of different types of cognitive enhancers for patients with schizophrenia: a meta-analysis. NPJ Schizophr. 2018;4:22. doi: 10.1038/s41537-018-0064-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stickgold R. Sleep-dependent memory consolidation. Nature. 2005;437:1272–8. doi: 10.1038/nature04286. [DOI] [PubMed] [Google Scholar]
- 4.Manoach DS, Stickgold R. Abnormal sleep spindles, memory consolidation, and schizophrenia. Annu Rev Clin Psychol. 2019;15:451–79. doi: 10.1146/annurev-clinpsy-050718-095754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fogel SM, Smith CT. The function of the sleep spindle: a physiological index of intelligence and a mechanism for sleep-dependent memory consolidation. Neurosci Biobehav Rev. 2011;35:1154–65. doi: 10.1016/j.neubiorev.2010.12.003. [DOI] [PubMed] [Google Scholar]
- 6.Latchoumane CV, Ngo HV, Born J, Shin HS. Thalamic spindles promote memory formation during sleep through triple phase-locking of cortical, thalamic, and hippocampal rhythms. Neuron. 2017;95:424–35 e6. doi: 10.1016/j.neuron.2017.06.025. [DOI] [PubMed] [Google Scholar]
- 7.Mednick SC, McDevitt EA, Walsh JK, Wamsley E, Paulus M, Kanady JC, et al. The critical role of sleep spindles in hippocampal-dependent memory: a pharmacology study. J Neurosci. 2013;33:4494–504. doi: 10.1523/JNEUROSCI.3127-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kaestner EJ, Wixted JT, Mednick SC. Pharmacologically increasing sleep spindles enhances recognition for negative and high-arousal memories. J Cogn Neurosci. 2013;25:1597–610. doi: 10.1162/jocn_a_00433. [DOI] [PubMed] [Google Scholar]
- 9.Niknazar M, Krishnan GP, Bazhenov M, Mednick SC. Coupling of thalamocortical sleep oscillations are important for memory consolidation in humans. PLoS ONE. 2015;10:e0144720. doi: 10.1371/journal.pone.0144720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marshall L, Helgadottir H, Molle M, Born J. Boosting slow oscillations during sleep potentiates memory. Nature. 2006;444:610–3. doi: 10.1038/nature05278. [DOI] [PubMed] [Google Scholar]
- 11.Lustenberger C, Boyle MR, Alagapan S, Mellin JM, Vaughn BV, Frohlich F. Feedback-controlled transcranial alternating current stimulation reveals a functional role of sleep spindles in motor memory consolidation. Curr Biol. 2016;26:2127–36. doi: 10.1016/j.cub.2016.06.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marshall L, Kirov R, Brade J, Molle M, Born J. Transcranial electrical currents to probe EEG brain rhythms and memory consolidation during sleep in humans. PLoS ONE. 2011;6:e16905. doi: 10.1371/journal.pone.0016905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Manoach DS, Mylonas D, Baxter B. Targeting sleep oscillations to improve memory in schizophrenia. Schizophr Res. 2020;221:63–70. doi: 10.1016/j.schres.2020.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ferrarelli F, Huber R, Peterson MJ, Massimini M, Murphy M, Riedner BA, et al. Reduced sleep spindle activity in schizophrenia patients. Am J Psychiatry. 2007;164:483–92. doi: 10.1176/ajp.2007.164.3.483. [DOI] [PubMed] [Google Scholar]
- 15.Ferrarelli F, Peterson MJ, Sarasso S, Riedner BA, Murphy MJ, Benca RM, et al. Thalamic dysfunction in schizophrenia suggested by whole-night deficits in slow and fast spindles. Am J Psychiatry. 2010;167:1339–48. doi: 10.1176/appi.ajp.2010.09121731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Manoach DS, Thakkar KN, Stroynowski E, Ely A, McKinley SK, Wamsley E, et al. Reduced overnight consolidation of procedural learning in chronic medicated schizophrenia is related to specific sleep stages. J Psychiatr Res. 2010;44:112–20. doi: 10.1016/j.jpsychires.2009.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goder R, Graf A, Ballhausen F, Weinhold S, Baier PC, Junghanns K, et al. Impairment of sleep-related memory consolidation in schizophrenia: relevance of sleep spindles? Sleep Med. 2015;16:564–9. doi: 10.1016/j.sleep.2014.12.022. [DOI] [PubMed] [Google Scholar]
- 18.Wamsley E, Tucker MA, Shinn AK, Ono KE, McKinley S, Ely AV, et al. Reduced sleep spindles and spindle coherence in schizophrenia: mechanisms of impaired memory consolidation? Biol Psychiatry. 2012;71:154–61. doi: 10.1016/j.biopsych.2011.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Manoach DS, Demanuele C, Wamsley EJ, Vangel M, Montrose DM, Miewald J, et al. Sleep spindle deficits in antipsychotic-naïve early course schizophrenia and in non-psychotic first-degree relatives. Front Hum Neurosci. 2014;8:762. doi: 10.3389/fnhum.2014.00762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tesler N, Gerstenberg M, Franscini M, Jenni OG, Walitza S, Huber R. Reduced sleep spindle density in early onset schizophrenia: a preliminary finding. Schizophr Res. 2015;166:355–7. doi: 10.1016/j.schres.2015.04.042. [DOI] [PubMed] [Google Scholar]
- 21.Schilling C, Schlipf M, Spietzack S, Rausch F, Eisenacher S, Englisch S, et al. Fast sleep spindle reduction in schizophrenia and healthy first-degree relatives: association with impaired cognitive function and potential intermediate phenotype. Eur Arch Psychiatry Clin Neurosci. 2016;267:213–24. doi: 10.1007/s00406-016-0725-2. [DOI] [PubMed] [Google Scholar]
- 22.De Gennaro L, Marzano C, Fratello F, Moroni F, Pellicciari MC, Ferlazzo F, et al. The electroencephalographic fingerprint of sleep is genetically determined: a twin study. Ann Neurol. 2008;64:455–60. doi: 10.1002/ana.21434. [DOI] [PubMed] [Google Scholar]
- 23.Ambrosius U, Lietzenmaier S, Wehrle R, Wichniak A, Kalus S, Winkelmann J, et al. Heritability of sleep electroencephalogram. Biol Psychiatry. 2008;64:344–8. doi: 10.1016/j.biopsych.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 24.Purcell SM, Manoach DS, Demanuele C, Cade BE, Mariani S, Cox R, et al. Characterizing sleep spindles in 11,630 individuals from the National Sleep Research Resource. Nat Commun. 2017;8:15930. doi: 10.1038/ncomms15930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Astori S, Wimmer RD, Prosser HM, Corti C, Corsi M, Liaudet N, et al. The Ca(V)3.3 calcium channel is the major sleep spindle pacemaker in thalamus. Proc Natl Acad Sci USA. 2011;108:13823–8. doi: 10.1073/pnas.1105115108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Manoach DS, Pan JQ, Purcell SM, Stickgold R. Reduced sleep spindles in schizophrenia: a treatable endophenotype that links risk genes to impaired cognition? Biol Psychiatry. 2016;80:599–608. doi: 10.1016/j.biopsych.2015.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guillery RW, Harting JK. Structure and connections of the thalamic reticular nucleus: advancing views over half a century. J Comp Neurol. 2003;463:360–71. doi: 10.1002/cne.10738. [DOI] [PubMed] [Google Scholar]
- 28.Houser CR, Vaughn JE, Barber RP, Roberts E. GABA neurons are the major cell type of the nucleus reticularis thalami. Brain Res. 1980;200:341–54. doi: 10.1016/0006-8993(80)90925-7. [DOI] [PubMed] [Google Scholar]
- 29.Steriade M. The corticothalamic system in sleep. Front Biosci. 2003;8:d878–99. doi: 10.2741/1043. [DOI] [PubMed] [Google Scholar]
- 30.Contreras D, Steriade M. Spindle oscillation in cats: the role of corticothalamic feedback in a thalamically generated rhythm. J Physiol. 1996;490:159–79. doi: 10.1113/jphysiol.1996.sp021133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Court J, Spurden D, Lloyd S, McKeith I, Ballard C, Cairns N, et al. Neuronal nicotinic receptors in dementia with Lewy bodies and schizophrenia: alpha-bungarotoxin and nicotine binding in the thalamus. J Neurochem. 1999;73:1590–7. doi: 10.1046/j.1471-4159.1999.0731590.x. [DOI] [PubMed] [Google Scholar]
- 32.Smith RE, Haroutunian V, Davis KL, Meador-Woodruff JH. Expression of excitatory amino acid transporter transcripts in the thalamus of subjects with schizophrenia. Am J Psychiatry. 2001;158:1393–9. doi: 10.1176/appi.ajp.158.9.1393. [DOI] [PubMed] [Google Scholar]
- 33.Steullet P, Cabungcal JH, Bukhari SA, Ardelt MI, Pantazopoulos H, Hamati F, et al. The thalamic reticular nucleus in schizophrenia and bipolar disorder: role of parvalbumin-expressing neuron networks and oxidative stress. Mol Psychiatry. 2018;23:2057–65. doi: 10.1038/mp.2017.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jia F, Goldstein PA, Harrison NL. The modulation of synaptic GABA(A) receptors in the thalamus by eszopiclone and zolpidem. J Pharm Exp Ther. 2009;328:1000–6. doi: 10.1124/jpet.108.146084. [DOI] [PubMed] [Google Scholar]
- 35.Wamsley EJ, Shinn AK, Tucker MA, Ono KE, McKinley SK, Ely AV, et al. The effects of eszopiclone on sleep spindles and memory consolidation in schizophrenia: a randomized placebo-controlled trial. Sleep. 2013;36:1369–76. doi: 10.5665/sleep.2968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Steriade M, Nunez A, Amzica F. Intracellular analysis of relations between the slow (<1 Hz) neocortical oscillation and other sleep rhythms of the electroencephalogram. J Neurosci. 1993;13:3266–83. doi: 10.1523/JNEUROSCI.13-08-03266.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cox R, Mylonas DS, Manoach DS, Stickgold R. Large-scale structure and individual fingerprints of locally coupled sleep oscillations. Sleep. 2018;41:zsy175. doi: 10.1093/sleep/zsy175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Demanuele C, Bartsch U, Baran B, Khan S, Vangel MG, Cox R, et al. Coordination of slow waves with sleep spindles predicts sleep-dependent memory consolidation in schizophrenia. Sleep. 2017;40:zsw013. doi: 10.1093/sleep/zsw013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured clinical interview for DSM-IV axis I disorders, research version, patient edition with psychotic screen (SCID-I/P W/PSY SCREEN) New York: Biometrics Research, New York State Psychiatric Institute; 1997. [Google Scholar]
- 40.Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261–76. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- 41.Andreasen NC, Olsen S. Negative v positive schizophrenia. Definition and validation. Arch Gen Psychiatry. 1982;39:789–94. doi: 10.1001/archpsyc.1982.04290070025006. [DOI] [PubMed] [Google Scholar]
- 42.Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59:22–33. [PubMed] [Google Scholar]
- 43.Walker MP, Brakefield T, Morgan A, Hobson JA, Stickgold R. Practice with sleep makes perfect: sleep-dependent motor skill learning. Neuron. 2002;35:205–11. doi: 10.1016/s0896-6273(02)00746-8. [DOI] [PubMed] [Google Scholar]
- 44.Manoach DS, Cain MS, Vangel MG, Khurana A, Goff DC, Stickgold R. A failure of sleep-dependent procedural learning in chronic, medicated schizophrenia. Biol Psychiatry. 2004;56:951–6. doi: 10.1016/j.biopsych.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 45.Baran B, Karahanoglu FI, Mylonas D, Demanuele C, Vangel M, Stickgold R, et al. Increased thalamocortical connectivity in schizophrenia correlates with sleep spindle deficits: evidence for a common pathophysiology. Biol Psychiatry Cogn Neurosci Neuroimaging. 2019;4:706–14. doi: 10.1016/j.bpsc.2019.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Warby SC, Wendt SL, Welinder P, Munk EG, Carrillo O, Sorensen HB, et al. Sleep-spindle detection: crowdsourcing and evaluating performance of experts, non-experts and automated methods. Nat Methods. 2014;11:385–92. doi: 10.1038/nmeth.2855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Otsu N. Time frequency and wavelets in biomedical signal processing. Piscataway, NJ: Wiley-IEEE; 1979. [Google Scholar]
- 48.Molle M, Born J. Slow oscillations orchestrating fast oscillations and memory consolidation. Prog Brain Res. 2011;193:93–110. doi: 10.1016/B978-0-444-53839-0.00007-7. [DOI] [PubMed] [Google Scholar]
- 49.Delorme A, Makeig S. EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J Neurosci Methods. 2004;134:9–21. doi: 10.1016/j.jneumeth.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 50.Helfrich RF, Mander BA, Jagust WJ, Knight RT, Walker MP. Old brains come uncoupled in sleep: slow wave-spindle synchrony, brain atrophy, and forgetting. Neuron. 2018;97:221–30 e4. doi: 10.1016/j.neuron.2017.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Staresina BP, Bergmann TO, Bonnefond M, van der Meij R, Jensen O, Deuker L, et al. Hierarchical nesting of slow oscillations, spindles and ripples in the human hippocampus during sleep. Nat Neurosci. 2015;18:1679–86. doi: 10.1038/nn.4119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Berens, P. CircStat: A MATLAB Toolbox for Circular Statistics. Journal of Statistical Software. 2009;31:1–21.
- 53.Zar JH. Biostatistical analysis. 4th ed. Prentice Hall; 1999.
- 54.Maris E, Oostenveld R. Nonparametric statistical testing of EEG- and MEG-data. J Neurosci Methods. 2007;164:177–90. doi: 10.1016/j.jneumeth.2007.03.024. [DOI] [PubMed] [Google Scholar]
- 55.Schwarz GE. Estimating the dimension of a model. Ann Stat. 1978;6:461–64. [Google Scholar]
- 56.Woods SW. Chlorpromazine equivalent doses for the newer atypical antipsychotics. J Clin Psychiatry. 2003;64:663–7. doi: 10.4088/jcp.v64n0607. [DOI] [PubMed] [Google Scholar]
- 57.Wilson MA, McNaughton BL. Reactivation of hippocampal ensemble memories during sleep. Science. 1994;265:676–9. doi: 10.1126/science.8036517. [DOI] [PubMed] [Google Scholar]
- 58.Ji D, Wilson MA. Coordinated memory replay in the visual cortex and hippocampus during sleep. Nat Neurosci. 2007;10:100–7. doi: 10.1038/nn1825. [DOI] [PubMed] [Google Scholar]
- 59.Clemens Z, Molle M, Eross L, Barsi P, Halasz P, Born J. Temporal coupling of parahippocampal ripples, sleep spindles and slow oscillations in humans. Brain. 2007;130:2868–78. doi: 10.1093/brain/awm146. [DOI] [PubMed] [Google Scholar]
- 60.Clemens Z, Molle M, Eross L, Jakus R, Rasonyi G, Halasz P, et al. Fine-tuned coupling between human parahippocampal ripples and sleep spindles. Eur J Neurosci. 2011;33:511–20. doi: 10.1111/j.1460-9568.2010.07505.x. [DOI] [PubMed] [Google Scholar]
- 61.Siapas AG, Wilson MA. Coordinated interactions between hippocampal ripples and cortical spindles during slow-wave sleep. Neuron. 1998;21:1123–8. doi: 10.1016/s0896-6273(00)80629-7. [DOI] [PubMed] [Google Scholar]
- 62.Born J, Wilhelm I. System consolidation of memory during sleep. Psychological Res. 2012;76:192–203. doi: 10.1007/s00426-011-0335-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Maingret N, Girardeau G, Todorova R, Goutierre M, Zugaro M. Hippocampo-cortical coupling mediates memory consolidation during sleep. Nat Neurosci. 2016;19:959–64. doi: 10.1038/nn.4304. [DOI] [PubMed] [Google Scholar]
- 64.Clemens Z, Fabo D, Halasz P. Twenty-four hours retention of visuospatial memory correlates with the number of parietal sleep spindles. Neurosci Lett. 2006;403:52–6. doi: 10.1016/j.neulet.2006.04.035. [DOI] [PubMed] [Google Scholar]
- 65.Johnson LA, Blakely T, Hermes D, Hakimian S, Ramsey NF, Ojemann JG. Sleep spindles are locally modulated by training on a brain-computer interface. Proc Natl Acad Sci USA. 2012;109:18583–8. doi: 10.1073/pnas.1207532109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bang JW, Khalilzadeh O, Hamalainen M, Watanabe T, Sasaki Y. Location specific sleep spindle activity in the early visual areas and perceptual learning. Vis Res. 2014;99:162–71. doi: 10.1016/j.visres.2013.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tamaki M, Huang TR, Yotsumoto Y, Hamalainen M, Lin FH, Nanez JE, Sr, et al. Enhanced spontaneous oscillations in the supplementary motor area are associated with sleep-dependent offline learning of finger-tapping motor-sequence task. J Neurosci. 2013;33:13894–902. doi: 10.1523/JNEUROSCI.1198-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Niethard N, Ngo HV, Ehrlich I, Born J. Cortical circuit activity underlying sleep slow oscillations and spindles. Proc Natl Acad Sci USA. 2018;115:E9220–E29. doi: 10.1073/pnas.1805517115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yang G, Lai CS, Cichon J, Ma L, Li W, Gan WB. Sleep promotes branch-specific formation of dendritic spines after learning. Science. 2014;344:1173–8. doi: 10.1126/science.1249098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Contreras D, Destexhe A, Sejnowski TJ, Steriade M. Control of spatiotemporal coherence of a thalamic oscillation by corticothalamic feedback. Science. 1996;274:771–4. doi: 10.1126/science.274.5288.771. [DOI] [PubMed] [Google Scholar]
- 71.Steriade M, Contreras D, Curro Dossi R, Nunez A. The slow (<1 Hz) oscillation in reticular thalamic and thalamocortical neurons: scenario of sleep rhythm generation in interacting thalamic and neocortical networks. J Neurosci. 1993;13:3284–99. doi: 10.1523/JNEUROSCI.13-08-03284.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Schapiro AC, Reid AG, Morgan A, Manoach DS, Verfaellie M, Stickgold R. The hippocampus is necessary for the consolidation of a task that does not require the hippocampus for initial learning. Hippocampus. 2019;29:1091–100. doi: 10.1002/hipo.23101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Konradi C, Yang CK, Zimmerman EI, Lohmann KM, Gresch P, Pantazopoulos H, et al. Hippocampal interneurons are abnormal in schizophrenia. Schizophr Res. 2011;131:165–73. doi: 10.1016/j.schres.2011.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Donoso JR, Schmitz D, Maier N, Kempter R. Hippocampal ripple oscillations and inhibition-first network models: frequency dynamics and response to GABA modulators. J Neurosci. 2018;38:3124–46. doi: 10.1523/JNEUROSCI.0188-17.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Xia F, Richards BA, Tran MM, Josselyn SA, Takehara-Nishiuchi K, Frankland PW. Parvalbumin-positive interneurons mediate neocortical-hippocampal interactions that are necessary for memory consolidation. eLife. 2017;6:e27868. doi: 10.7554/eLife.27868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Baran B, Correll D, Vuper TC, Morgan A, Durrant SJ, Manoach DS, et al. Spared and impaired sleep-dependent memory consolidation in schizophrenia. Schizophr Res. 2018;199:83–89. doi: 10.1016/j.schres.2018.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Phillips KG, Bartsch U, McCarthy AP, Edgar DM, Tricklebank MD, Wafford KA, et al. Decoupling of sleep-dependent cortical and hippocampal interactions in a neurodevelopmental model of schizophrenia. Neuron. 2012;76:526–33. doi: 10.1016/j.neuron.2012.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Suh J, Foster DJ, Davoudi H, Wilson MA, Tonegawa S. Impaired hippocampal ripple-associated replay in a mouse model of schizophrenia. Neuron. 2013;80:484–93. doi: 10.1016/j.neuron.2013.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Becker L, Penagos H, Manoach DS, Wilson MA, Varela C. Disruption of CA1 Sharp-Wave Ripples by the nonbenzodiazepine hypnotic eszopiclone. Poster No. 610.13. Neuroscience Meeting Planner. Chicago, IL: Society for Neuroscience, 2019. Online. https://www.abstractsonline.com/pp8/#!/7883/presentation/45684.
- 80.Koniaris E, Drimala P, Sotiriou E, Papatheodoropoulos C. Different effects of zolpidem and diazepam on hippocampal sharp wave-ripple activity in vitro. Neuroscience. 2011;175:224–34. doi: 10.1016/j.neuroscience.2010.11.027. [DOI] [PubMed] [Google Scholar]
- 81.Genzel L, Dresler M, Cornu M, Jager E, Konrad B, Adamczyk M, et al. Medial prefrontal-hippocampal connectivity and motor memory consolidation in depression and schizophrenia. Biol Psychiatry. 2015;77:177–86. doi: 10.1016/j.biopsych.2014.06.004. [DOI] [PubMed] [Google Scholar]
- 82.White K, Ashton R. Handedness assessment inventory. Neuropsychologia. 1976;14:261–64. doi: 10.1016/0028-3932(76)90058-0. [DOI] [PubMed] [Google Scholar]
- 83.Walker MP, Brakefield T, Seidman J, Morgan A, Hobson JA, Stickgold R. Sleep and the time course of motor skill learning. Learn Mem. 2003;10:275–84. doi: 10.1101/lm.58503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hoddes E, Zarcone V, Smythe H, Philips R, Dement WC. Quantification of sleepiness: a new approach. Psychophysiology. 1973;10:431–6. doi: 10.1111/j.1469-8986.1973.tb00801.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.