Abstract
Background
The proportion of patients infected with SARS-CoV-2 that are prescribed antibiotics is uncertain, and may contribute to patient harm and global antibiotic resistance.
Objective
The aim was to estimate the prevalence and associated factors of antibiotic prescribing in patients with COVID-19.
Data Sources
We searched MEDLINE, OVID Epub and EMBASE for published literature on human subjects in English up to June 9 2020.
Study Eligibility Criteria
We included randomized controlled trials; cohort studies; case series with ≥10 patients; and experimental or observational design that evaluated antibiotic prescribing.
Participants
The study participants were patients with laboratory-confirmed SARS-CoV-2 infection, across all healthcare settings (hospital and community) and age groups (paediatric and adult).
Methods
The main outcome of interest was proportion of COVID-19 patients prescribed an antibiotic, stratified by geographical region, severity of illness and age. We pooled proportion data using random effects meta-analysis.
Results
We screened 7469 studies, from which 154 were included in the final analysis. Antibiotic data were available from 30 623 patients. The prevalence of antibiotic prescribing was 74.6% (95% CI 68.3–80.0%). On univariable meta-regression, antibiotic prescribing was lower in children (prescribing prevalence odds ratio (OR) 0.10, 95% CI 0.03–0.33) compared with adults. Antibiotic prescribing was higher with increasing patient age (OR 1.45 per 10 year increase, 95% CI 1.18–1.77) and higher with increasing proportion of patients requiring mechanical ventilation (OR 1.33 per 10% increase, 95% CI 1.15–1.54). Estimated bacterial co-infection was 8.6% (95% CI 4.7–15.2%) from 31 studies.
Conclusions
Three-quarters of patients with COVID-19 receive antibiotics, prescribing is significantly higher than the estimated prevalence of bacterial co-infection. Unnecessary antibiotic use is likely to be high in patients with COVID-19.
Keywords: Antibiotic Prescribing, Antibiotics, Antimicrobial Stewardship, Antimicrobial therapy, COVID-19, SARS-CoV-2
Introduction
With millions of cases globally, the COVID-19 pandemic has had an immediate and devastating impact on the healthcare system and society as a whole. The long-term repercussions of COVID-19 on antimicrobial resistance have been raised as a grave concern due to elevated antibiotic use in patients infected with SARS-CoV-2 [1,2]. Despite the viral nature of this syndrome, initial studies indicate that antibiotics are prescribed frequently to patients with COVID-19, largely due to suspected bacterial co-infections [[3], [4], [5]].
Despite frequent antibiotic prescribing to patients with COVID-19, the prevalence of bacterial co-infection and secondary infection in patients hospitalized with COVID-19 is relatively low at 3.5% and 14.3%, respectively [5]. The gap between the prevalence of bacterial infection and frequency of antibiotic prescribing highlights the potential for significant antibiotic overuse in these patients. Over-prescribing of antibiotics in patients infected with SARS-CoV-2 can result in increased selective pressure for antimicrobial resistance. Antibiotic misuse coupled with a strained healthcare workforce and a reduced surveillance capacity for antibiotic-resistant organisms may lead to antimicrobial resistance as a lasting consequence of the COVID-19 pandemic [6,7].
Antibiotic stewardship interventions aimed at improving the appropriateness of antibiotic use are associated with reduced antibiotic utilization, and decreased incidence of drug-resistant infections [8]. Understanding patterns and predictors of antibiotic prescribing in COVID-19 can help to identify opportunities for interventions, and target antibiotic stewardship strategies to improve the quality and safety of antibiotic use. Our objective was to determine the prevalence of antibiotic use and identify the predictors of antibiotic use in patients with COVID-19.
Materials and methods
We conducted a rapid review based on modified Cochrane Rapid Reviews Methods Group guidance [9] to determine the proportion of patients with COVID-19 that were prescribed an antibiotic during the course of their illness, herein referred to as prevalence of antibiotic use in patients with confirmed COVID-19 infection. We selected rapid review as the optimal methodology to synthesize knowledge in a timely fashion for this emergent issue because we aimed to help clinicians apply the learnings to COVID-19 management strategies efficiently during the pandemic.
We included studies of humans with laboratory-confirmed SARS-CoV-2 infection, across all healthcare settings (i.e. hospital, community, long-term care) and age groups (paediatric and adult patients) as defined by study authors.
We included cohort studies, case series with ten or more patients and randomized controlled trials (not evaluating antibiotic use as an intervention), but excluded reviews, editorials, letters and case studies. We considered studies to be eligible regardless of experimental or observational design, and irrespective of their primary objective. However, we excluded studies that did not report data on the number and percentage of patients receiving antibiotics. This protocol was registered under PROSPERO, the international registry of systematic reviews (ID CRD42020192286).
Data Sources
We performed systematic searches of MEDLINE, OVID Epub, and EMBASE databases for published literature in the English language from 1 January 2019 to 9 June 2020 with assistance from a medical library information specialist. The limitation to English language articles was based on Cochrane Rapid Review Methods Group guidance [9] aimed at improving the timeliness while maintaining broad representation of the review. The search was structured to include COVID-19 terms and antibiotic, co-infection, bacterial infection, respiratory infection, epidemiology, or descriptive cohort study terms. The complete search strategy is described in the supplementary material. The results of the search were imported into Covidence (Covidence, Melbourne, Australia), an online software tool for systematic reviews. Duplicate records were removed using Covidence.
Study selection
Initial screening of titles and abstracts from the search were shared by two authors (B.L. or V.L.), who independently identified studies that met all inclusion criteria and none of the exclusion criteria. For quality assurance, three other authors (M.S., S.R. or N.D.) then randomly selected 25% of the identified studies for duplicate screening. Disagreements that could not be resolved via consensus were reviewed independently by another author who had not participated in the screening. All full text studies meeting initial criteria were then reviewed by one of the authors (B.L., M.S., S.R. or N.D.) for final inclusion in the rapid review. Studies potentially describing overlapping data were noted (e.g., same hospital and population during an overlapping time period).
Data extraction
One of five authors (B.L., M.S., S.R., V.L. or D.M.) independently extracted data from included studies using a standardized data collection form. For quality assurance, two authors (V.L. or D.W.) then randomly sampled 25% of data extraction forms to confirm accuracy and completeness. We collected data on the following variables for demographics and setting: author; country of study; start and end dates; name(s) of healthcare facility; study design (retrospective vs. prospective); healthcare setting (inpatient ICU vs. non ICU, outpatient); sample size; age group; patient population; mean or median age; and proportion of female patients. Regarding clinical characteristics, we collected information on COVID-19 severity; proportion of patients requiring mechanical ventilation; proportion of patients that were smokers; number of patients with comorbidities (chronic obstructive pulmonary disease, cardiovascular disease or malignancy); and number of patients who were prescribed an antibiotic. We also collected information on the following parameters if reported: antibiotic classes prescribed; duration of therapy; timing of antibiotic initiation (on admission or empiric vs. after admission); antibiotic stewardship interventions; and prevalence of respiratory or bloodstream bacterial co-infections.
Data synthesis
The main outcome of interest was the overall prevalence of antibiotic prescribing among patients with COVID-19. We evaluated the number of patients prescribed an antibiotic at any point during the course of their illness while under study observation as a proportion of all patients with laboratory-proven COVID-19. First, we stratified by region to identify differences in prescribing practices based on geography. Second, we stratified prescribing by severity of COVID-19 illness based on the study's quartile of the proportion of mechanically ventilated patients and by study population: (a) critically ill patients (admitted to intensive care unit); (b) all hospitalized patients; and (c) mixed hospitalized/outpatient population. Third, we stratified by the month in which the study completed follow-up to determine if antibiotic prescribing decreased as the pandemic progressed, as more information became available regarding the low rates of co-infection in patients with COVID-19. Fourth, we stratified by study age group to evaluate differences in antibiotic prescribing between paediatric and adult patients. We pooled proportion data across studies via a random-effects meta-analysis using a generalized linear mixed model (GLMM) with logit link approach [10,11]. Results were illustrated using forest plots. Heterogeneity was assessed by I 2 statistic, with <40% considered low heterogeneity, 30–60% considered moderate heterogeneity, 50–90% considered substantial heterogeneity and 75–100% considered considerable heterogeneity [12]. All analyses were carried out using R version 3.6.0 with the packages metafor and meta. The statistical code for this analysis is made available online [13].
Meta-regression
To predict the effect of specific patient characteristics on antibiotic prescribing, we performed univariable meta-regression evaluating patient demographic characteristics (age, sex, comorbidities), markers of severity (mechanical ventilation, healthcare setting), geography (grouped by China, Middle East, East/Southeast Asia, Europe, North America, multiple countries), Healthcare Access and Quality Index (a novel measure of health system quality for 195 countries based on age-standardized mortality for 32 conditions with largely avoidable cause of death [14]), and end month of study. Prevalence differences in antibiotic prescribing for each variable were described in terms of the prevalence odds ratio (OR).
Assessment of bias
We considered a formal assessment for risk of bias to be of limited utility, given the lack of appropriate assessment tool (e.g. most appraisal questions are not applicable). Although a risk of bias tool has been developed for meta-analyses of disease prevalence [15], there are no tools that are directly relevant to our research question addressing the prevalence of antibiotic prescribing. Therefore, our modified rapid review approach incorporated study quality into our sensitivity analysis by estimating the quality of antibiotic prescribing data based on whether the study reported detail on antibiotic classes. In additional sensitivity analyses, we removed studies focusing exclusively on populations where antibiotic prescribing may differ from the general population (i.e. transplant, malignancy, obstetrics, older age (i.e. 60 years or older), chronic obstructive pulmonary disease (COPD), diabetes, fatal COVID infection, HIV, surgery, dialysis and acute kidney injury), and we removed studies with potentially overlapping patient cohorts (studies occurring in the same patient population in the same hospital during the same time frame).
Role of the funding source
University of Toronto, Department of Medicine, Network Seed Funding Grant supported the role of a research coordinator (D.W.) to provide research project management. The University was not involved in study concept, analysis or synthesis of evidence, nor the decision to publish.
Results
Of 16 378 studies identified, after duplicate removal, we reviewed a total of 7469 studies via title and abstract screening, 523 of which were assessed via full-text screening. We included 154 studies in the final analysis [[16], [17], [18], [19], [20], [21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40], [41], [42], [43], [44], [45], [46], [47], [48], [49], [50], [51], [52], [53], [54], [55], [56], [57], [58], [59], [60], [61], [62], [63], [64], [65], [66], [67], [68], [69], [70], [71], [72], [73], [74], [75], [76], [77], [78], [79], [80], [81], [82], [83], [84], [85], [86], [87], [88], [89], [90], [91], [92], [93], [94], [95], [96], [97], [98], [99], [100], [101], [102], [103], [104], [105], [106], [107], [108], [109], [110], [111], [112], [113], [114], [115], [116], [117], [118], [119], [120], [121], [122], [123], [124], [125], [126], [127], [128], [129], [130], [131], [132], [133], [134], [135], [136], [137], [138], [139], [140], [141], [142], [143], [144], [145], [146], [147], [148], [149], [150], [151], [152], [153], [154], [155], [156], [157], [158], [159], [160], [161], [162], [163], [164], [165], [166], [167], [168], [169]] (Fig. 1 ). Study design was primarily retrospective in nature (n = 135), followed by prospective cohort (n = 11), randomized controlled (n = 6) and mixed prospective and retrospective design (n = 1). Studies were conducted between 8 December 2019 and 21 May 2020.
Fig. 1.
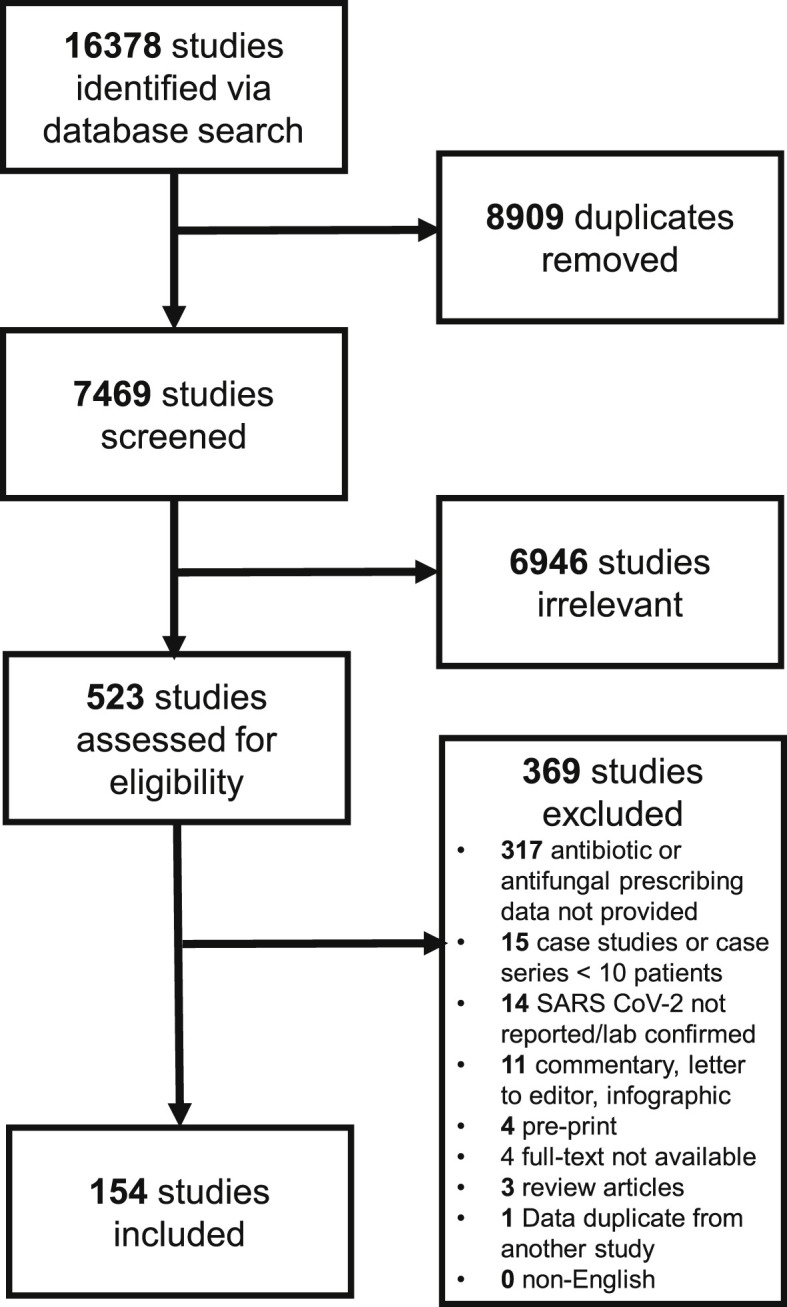
Study flow diagram.
Study geography
Most studies took place in China (n = 115 studies; 21 852 patients), followed by the United States (n = 12 studies, 2302 patients), Italy (n = 11 studies, 3785 patients) and South Korea (n = 4 studies, 5321 patients). A complete list of all study details and locations is available in Table 1 .
Table 1.
Predictors of antibiotic prescribing in patients with COVID-19
| Characteristic | Prevalence odds ratio | 95% confidence interval | Studies included |
|---|---|---|---|
| Region | 154 | ||
| China | reference | 115 | |
| Middle East | 6.05 | 0.29–126.87 | 2 |
| East/Southeast Asia | 1.94 | 0.38–9.88 | 6 |
| Europe | 0.54 | 0.21–1.40 | 18 |
| North America | 0.60 | 0.19–1.85 | 12 |
| Multiple | 0.54 | 0.01–21.63 | 1 |
| Healthcare access/quality score (10-point increase) change) | 0.77 | 0.46–1.28 | 152 |
| End month | 154 | ||
| January | reference | 14 | |
| February | 0.61 | 0.29–1.88 | 72 |
| March | 0.37 | 0.11–1.24 | 33 |
| April | 0.28 | 0.08–0.98 | 29 |
| May | 0.45 | 0.05–3.71 | 4 |
| Not specified | 0.18 | 0.01–2.90 | 2 |
| Setting | 154 | ||
| Hospital | reference | 133 | |
| Hospital ICU | 2.55 | 0.68–9.49 | 9 |
| Hospital/outpatient | 0.51 | 0.16–1.56 | 12 |
| Age group | 154 | ||
| Adults | reference | 91 | |
| Children | 0.10 | 0.03–0.33 | 10 |
| Adults and Children | 0.33 | 0.17–0.65 | 41 |
| Not specified | 0.48 | 0.16–1.39 | 12 |
| Age (10-year change) | 1.45 | 1.18–1.77 | 153 |
| Female (10% change) | 0.97 | 0.78–1.20 | 154 |
| Mechanical Ventilation (10% change) | 1.33 | 1.15–1.54 | 115 |
| Smoker (10% change) | 1.08 | 0.68–1.72 | 51 |
| COPD (10% change) | 1.04 | 0.52–2.05 | 81 |
| CVD (10% change) | 1.01 | 0.83–1.22 | 115 |
| Diabetes (10% change) | 1.15 | 0.90–1.49 | 120 |
| Deaths (10% change) | 1.45 | 1.21–1.74 | 133 |
Patient characteristics
A total of 35 263 patients with laboratory-confirmed COVID-19 were included across the 154 studies. The median patient age was 53 years (IQR 44–61, range 3–72). The majority of studies evaluated exclusively adults (n = 91) whereas a smaller number of studies (n = 10) included only children. Females comprised a median 45% of patients (IQR 39–51%) (Table 1). Commonly reported comorbidities included diabetes (median 12%, IQR 8–21%), cardiovascular disease (median 12%, IQR 6–18%), COPD (median 4%, IQR 2–7%) and malignancy (median 3%, IQR 1–6%). A history of smoking was reported in a median of 10% of patients (IQR 6–19%).
Although most studies evaluated a range of patients with varying demographics and comorbidities, some studies focused on COVID-19 in specific patient subpopulations. They included organ transplant (n = 6 studies, 96 patients); obstetrics (n = 6 studies, 547 patients); healthcare workers (n = 3 studies, 107 patients); older adults (n = 2 studies, 309 patients); malignancies (n = 2 studies, 156 patients); HIV (n = 2 studies, 74 patients); COPD (n = 1 study, 1048 patients); diabetes (n = 1 study, 258 patients); cystic fibrosis (n = 1 study, 40 patients); surgery (n = 1 study, 34 patients); and acute kidney injury (n = 1 study, 30 patients).
Severity of COVID-19 illness
Most studies evaluated hospitalized patients (n = 133 studies, 30 212 patients) including a mix of both general ward and critically ill patients. A smaller group of studies evaluated a mixed inpatient/outpatient population (n = 12 studies, 4084 patients) or patients exclusively in the intensive care unit (n = 9 studies, 967 patients). The median proportion of patients requiring mechanical ventilation support was 16% (IQR 5–27%), based on 114 studies that reported this variable. Among studies that reported mortality (n = 133), 5% (IQR 0–18%) of patients died during follow up.
Antibiotic prescribing in patients with COVID-19
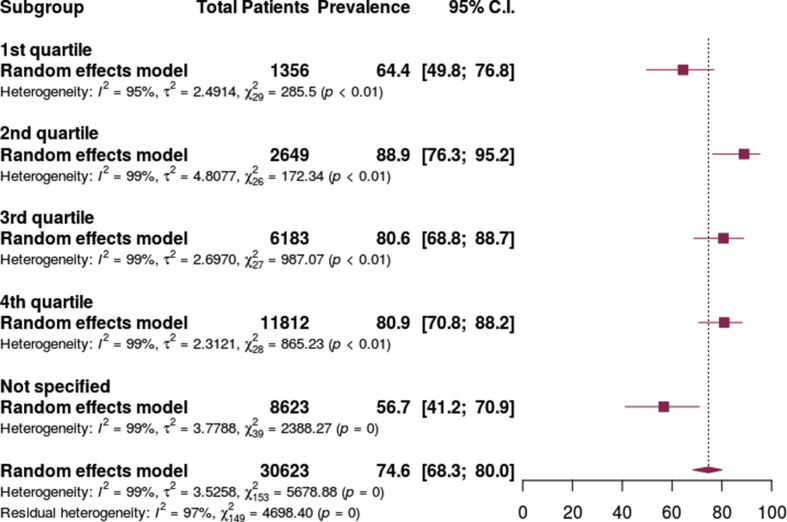
Out of total of 35 263 patients, 30 623 were assessed for antibiotic prescribing, of whom 19 102 (62.4%) received at least one antibiotic agent. The random effects meta-analysis of all combined studies estimated the antibiotic prescribing prevalence 74.6% (95% CI 68.3–80.0%). with considerable heterogeneity (I 2 = 99%) (Fig. S1).
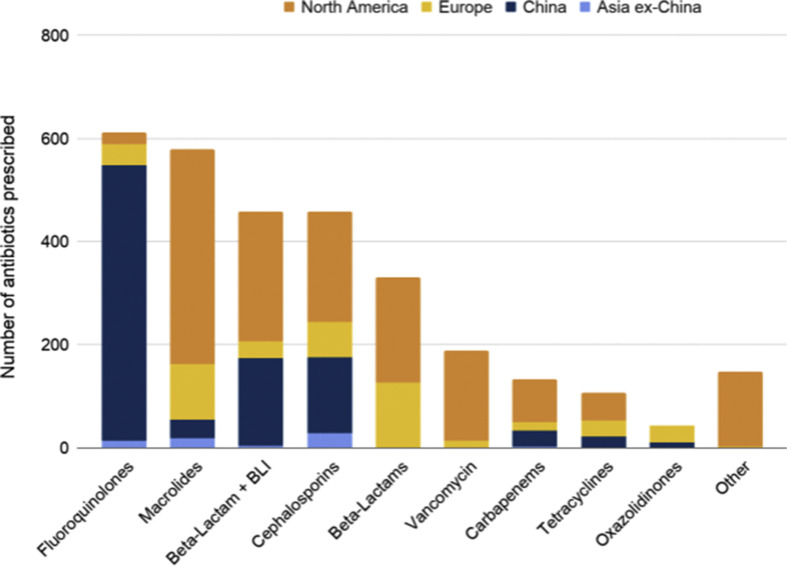
Of all included studies 28 (18.2%) provided data on antibiotic classes prescribed. In these studies, 4721 patients were evaluated and 2482 patients received 3058 antibiotic agents. Of ten classes of antibiotics studied, the most common antibiotic classes prescribed were fluoroquinolones (n = 612, 20.0%), macrolides (n = 579, 18.9%), β-lactam/β-lactamase inhibitors (n = 459, 15.0%) and cephalosporins (n = 459, 15.0%) (Fig. 2 ). All studies provided data on the number of patients receiving antibiotics (n = 154); however, only five studies (3.2%) [29,33,47,51,142] provided additional metrics including duration (n = 5), single vs. combination therapy (n = 1), time to empiric antibiotic therapy (n = 1) and antibiotics started within 48 hr vs. antibiotics continued beyond 48 hr (n = 1). Antibiotic use was classified by authors as empiric or started on admission in 49 studies (31.8%), post-admission in 13 studies (8.4%) and not specified in 92 studies (59.7%). Antibiotic stewardship strategies were reported in three studies (1.9%) [23,24,40], indicating that there were recommendations to avoid antibiotics in patients without suspected co-infection (n = 2) or to de-escalate antibiotics when additional data became available (n = 1).
Fig. 2.
Classes of antibiotic prescribing in patients with COVID-19 by region. BLI, β-lactamase inhibitor. One course of polymyxins was prescribed in China.
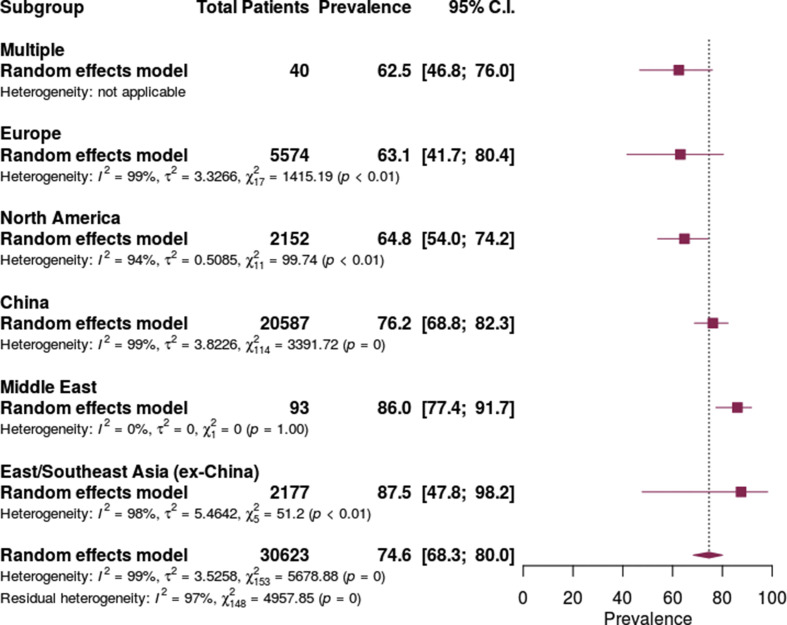
Antibiotic prescribing by region
The prevalence of antibiotic use across regions had considerable heterogeneity with I 2 = 99%. In order of increasing prevalence of use, antibiotic prescribing in Europe was 63.1% (95% CI 41.7–80.4%), in North America (USA) 64.8% (95% CI 54.0–74.2%), in China 76.2% (66.8–82.3%), Middle East 86.0% (95% CI 77.4–91.7%) and in East/Southeast Asia (excluding China) 87.5% (47.8–98.2%) (Fig. 3 ).
Fig. 3.
Antibiotic prescribing in patients with COVID-19 by region.
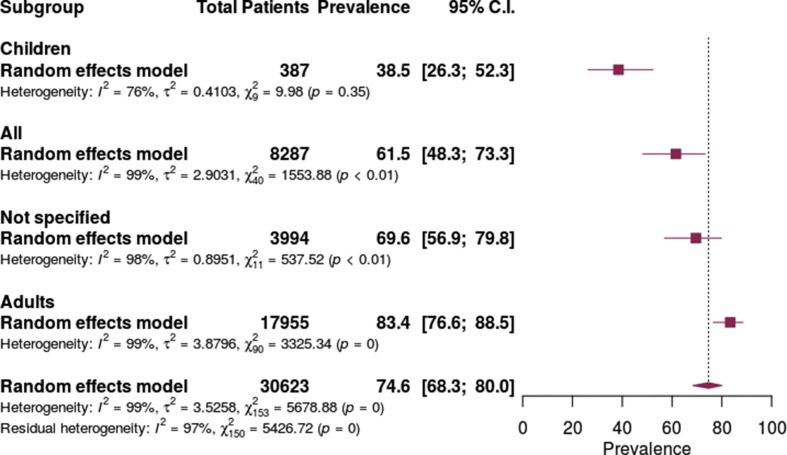
Antibiotic prescribing by age
Antibiotic prescribing prevalence increased along with increasing age. The prevalence of antibiotic prescribing was lowest in children (38.5%, 95% CI 26.3–52.3%), moderate in all age groups combined (61.5%, 95% CI 48.3–73.3%) and highest in adults (83.4, 95% CI 76.6–88.5%). (Fig. 4 ).
Fig. 4.
Antibiotic prescribing in patients with COVID-19 by age group.
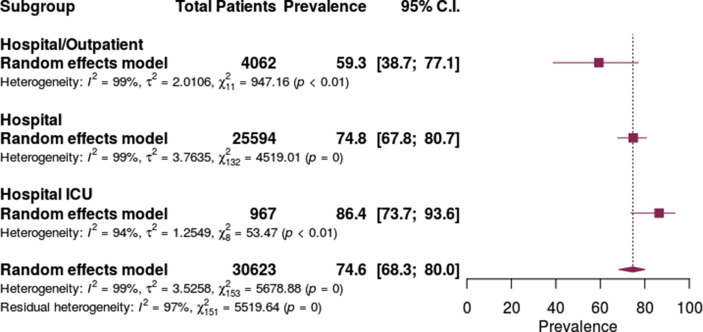
Antibiotic prescribing by setting/severity
Antibiotic prescribing was lowest in the mixed inpatient/outpatient setting at 59.3% (95% CI 38.7% to 77.1%), followed by the inpatient hospital setting at 74.8% (95% CI 67.8–80.7%) and highest in the ICU setting at 86.4% (95% CI 73.7–93.6%) (Fig. 5 ). The prevalence of antibiotic prescribing varied based on the proportion of patients requiring mechanical ventilation. Prescribing was lowest in studies not reporting ventilation status at 56.7% (95% CI 41.2–70.9) and the lowest quartile of patients requiring mechanical ventilation at 64.4 (95% CI 49.8–76.8) but higher in patients in the highest three quartiles of patients requiring mechanical ventilation. (Fig. 6 ).
Fig. 5.
Antibiotic prescribing in patients with COVID-19 by healthcare setting.
Fig. 6.
Antibiotic prescribing in patients with COVID-19 by study quartile of proportion of patients requiring mechanical ventilation.
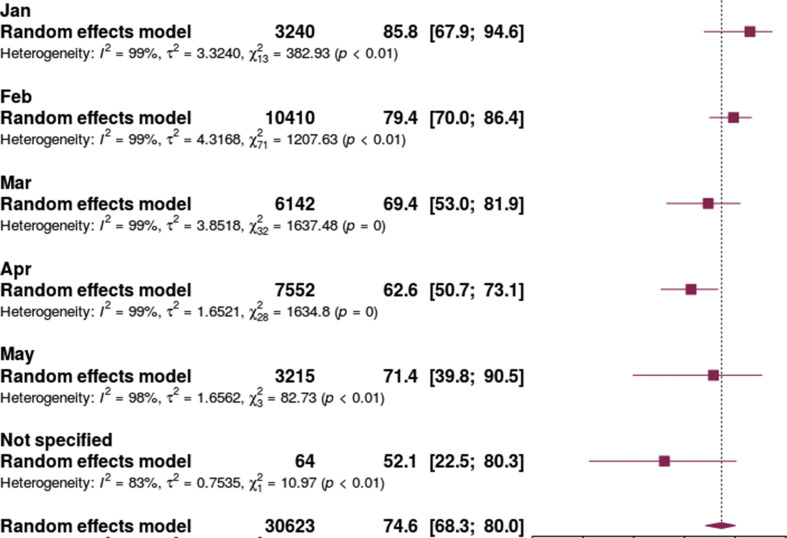
Antibiotic prescribing by date
There was a trend towards reduced antibiotic prescribing as the pandemic progressed. Studies ending enrolment and/or follow-up in January showed the highest prescribing prevalence (85.8%, 95% CI 67.9–94.6%) and studies ending in April showed the lowest (62.6%, 95% CI 50.7–73.1%) (Fig. 7 ).
Fig. 7.
Antibiotic prescribing in patients with COVID-19 by study end date.
Sensitivity analyses
As an estimate of antibiotic prescribing data quality, studies reporting information on antibiotic classes prescribed showed numerically higher prevalence of prescribing (82.1%, 95% CI 67.3–91.1%) compared with the studies that did not report this additional data (72.8%, 95% CI 65.8–78.9%). When removing studies focusing exclusively on patient populations at higher risk of antibiotic use (i.e. transplant, malignancy, obstetrics, older age, COPD, diabetes, fatal infection, HIV, acute kidney injury, surgery, dialysis and acute kidney injury, n = 29 studies) overall antibiotic prescribing did not differ significantly (71.8%, 95% CI 64.4–78.2%) from the overall estimate. Similarly, when removing studies with potentially overlapping patient populations (n = 16 studies) the estimate of antibiotic prescribing (72.5%, 95% CI 65.6–78.5%) was similar to the overall estimate (Figs S2–4).
Meta-regression antibiotic prescribing prevalence in COVID-19
In the meta-regression, geographic region was not identified as a predictor of antibiotic prescribing prevalence differences. In terms of study date, prescribing was noted to be lower in April 2020 (OR 0.28, 95% CI 0.08–0.98) than in January 2020.Antibiotic prescribing was also lower in studies evaluating children (prescribing prevalence OR 0.10, 95% CI 0.03–0.33) and combined children and adults (OR 0.33, 95% CI 0.17–0.65) compared to studies examining only adults. Antibiotic prescribing was higher with increasing median or mean patient age (OR 1.45 per 10 year increase, 95% CI 1.18–1.77), higher with increasing proportion of patients requiring mechanical ventilation (OR 1.33 per 10% increase, 95% CI 1.15–1.54) and higher with increasing proportion of patients that died (OR 1.45 per 10% increase, 95% CI 1.21–1.74) (Table 1). Quantile–quantile plots of univariable model residuals are available in Fig. S5.
Bacterial Co-Infection or secondary infection
The number of patients with COVID-19 and concomitant bacterial infection was reported in 31 studies. Pooled data from all studies reporting bacterial infection indicated that the prevalence was 8.6% (95% CI 4.7–15.2%). There was a considerable degree of heterogeneity between studies I 2 = 96%.
Discussion
In this large rapid review and meta-analysis evaluating patients with COVID-19 in the first 6 months of the global pandemic, we found that nearly three-quarters of patients received antibiotic therapy. Prescribing was very heterogeneous but overall consistently high across healthcare setting and geography. Prescribing was elevated in older age groups and those with higher severity of illness, as marked by proportion of patients requiring mechanical ventilation and fatal infections. Consistent with our data, bacterial co-infection rates for SARS-CoV-2 have been estimated between 6.1% and 8.0% [4,5,170,171]. As such, antibiotic prescribing is significantly higher than the prevalence of bacterial co-infection suggesting a large number of antibiotic prescriptions are unnecessary, increasing the risk of preventable harm including adverse events, Clostridium difficile infection, and antimicrobial resistance.
Although the risk of co-infection appears to be lower in patients with SARS-CoV-2 than in those with influenza, our findings raise similar, if not greater, concerns for antibiotic overuse than in patients with influenza. On average, an estimated 23% of hospitalized patients with influenza experience bacterial co-infection [172], while antibiotic use far exceeds this estimate. In a cohort study of 322 hospitalized patients with influenza, 65.5% of patients received antibiotics on admission and 34.5% of those were continued without evidence of bacterial infection [173].
The impact of COVID-19 on global antimicrobial resistance (AMR) is uncertain, and likely to be unevenly distributed across disease epicentres and populations. Pandemic-associated changes in human behaviour and healthcare practices, including decreased travel, physical distancing, hospital and clinic avoidance, improved infection prevention and control, hand hygiene and environmental cleaning may alleviate some of the impact of COVID-19-mediated antibiotic use on AMR [174]. However, disproportionately high use of antibiotics in patients with COVID-19 has the potential to exacerbate this public health threat [175], particularly in areas where AMR is already a significant problem, such as China [176], Italy [177] and the United States [178].
Regardless of the net impact of COVID-19 on AMR, antimicrobial stewardship principles should guide the antibiotic management of patients with COVID-19 [7]. As there is significant diagnostic uncertainty in identifying bacterial infection in patients with COVID-19, guidelines can support appropriate empiric prescribing decisions. Several guidelines advocate for the use of empiric antibiotics for patients with severe COVID-19 [179,180], whereas others have more tailored approach to antibiotic use based on patient presentation [181]. Our findings support the need for clear and consistent guidance on which patients with COVID-19 would derive greatest benefit from empiric antibiotics, and in which patients the risks of antibacterial therapy exceed the benefits. Prospective studies evaluating the role of initiating antibiotics in patients with severe COVID-19, and to identify appropriate parameters for safe antibiotic discontinuation (e.g. based on imaging, clinical criteria and/or biomarkers) are needed.
Key strengths of our review include the large number of studies spanning several months of the COVID-19 pandemic. The meta-regression identifies key factors associated with antibiotic use and highlights opportunities for improvement. Limitations include the disproportionate representation from Asia, potentially limiting the generalizability of the results to other countries affected by COVID-19. However, representation of over ten studies each from Europe and North America (USA) provides adequate data and it appears antibiotic use is consistently high across regions. Additionally, the antibiotic prescribing data are of uncertain quality and we found limited data on antibiotic prescribing details other than number of patients receiving antibiotics (e.g. antibiotic classes, duration). Further information on indication, selection, timing and duration could be helpful to better estimate the appropriateness of antibiotic therapy and the quality of the data. For example, empiric antibiotic therapy may be appropriate in severely ill patients with COVID-19 but upon follow-up with laboratory, imaging and microbiological data, antibiotic therapy can be re-assessed and discontinued promptly to prevent unnecessary exposure. Finally, we were unable to identify an appropriate risk of bias tool for this research question; however, our sensitivity analyses evaluating the quality and quantity of antibiotic data, different patient populations, and potential for study overlap were robust to our initial estimates.
Opportunities for future work include improved co-infection diagnostics, evaluation of bacterial co-infection, providing additional detail on antibiotic prescribing including timing, duration and indication, estimating antibiotic appropriateness, and identifying successful antimicrobial stewardship initiatives in patients with COVID-19.
Conclusion
Antibiotics are prescribed in three-quarters of patients with COVID-19. Given the low rate of co-infection in these patients, there is a high risk for unnecessary antibiotics. Predictors of increased antibiotic use in COVID-19 include advanced age, use of mechanical ventilation and fatal COVID-19 infections. Antimicrobial stewardship efforts are urgently needed to help mitigate the impact of COVID-19 on antimicrobial resistance.
Transparency declaration
The authors have no conflicts of interest to declare. Funding: University of Toronto, Department of Medicine, Network Seed Funding Grant. Registration: PROSPERO (ID CRD42020192286).
Author contributions
Concept and design: All authors. Acquisition, analysis, or interpretation of data: All authors.Drafting of the manuscript: Langford, So. Critical revision of the manuscript for important intellectual content: All authors. Statistical analysis: Soucy, Langford. Administrative, technical, or material support: All authors.
Acknowledgements
We thank Ashley Farrell, BA, MLIS, AHIP, Information Specialist for her support with formulating and executing the search strategy for this systematic review. We thank the Toronto Antimicrobial Resistance Research Network (TARRN), an interprofessional forum of clinicians and researchers that acted as a venue for bringing together this research team, and a repository for our rapid review.
Editor: Mariska Leeflang
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cmi.2020.12.018.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Nieuwlaat R., Mbuagbaw L., Mertz D., Burrows L., Bowdish D.M.E., Moja L. COVID-19 and antimicrobial resistance: parallel and interacting health emergencies. Clin Infect Dis. 2020:ciaa773. doi: 10.1093/cid/ciaa773. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.van Duin D., Barlow G., Nathwani D. The impact of the COVID-19 pandemic on antimicrobial resistance: a debate. JAC-Antimicrob Resist. 2020;2 doi: 10.1093/jacamr/dlaa053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rawson T.M., Moore L.S.P., Zhu N., Ranganathan N., Skolimowska K., Gilchrist M. Bacterial and fungal co-infection in individuals with coronavirus: a rapid review to support COVID-19 antimicrobial prescribing. Clin Infect Dis. 2020;71:2459–2468. doi: 10.1093/cid/ciaa530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lansbury L., Lim B., Baskaran V., Lim W.S. Co-infections in people with COVID-19: a systematic review and meta-analysis. J Infect. 2020;81:266–275. doi: 10.1016/j.jinf.2020.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Langford B.J., So M., Raybardhan S., Leung V., Westwood D., MacFadden D.R. Bacterial co-infection and secondary infection in patients with COVID-19: a living rapid review and meta-analysis. Clin Microbiol Infect. 2020;26:1622–1629. doi: 10.1016/j.cmi.2020.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rawson T.M., Moore L.S.P., Castro-Sanchez E., Charani E., Davies F., Satta G. COVID-19 and the potential long-term impact on antimicrobial resistance. J Antimicrob Chemother. 2020;75:1681–1684. doi: 10.1093/jac/dkaa194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huttner B., Catho G., Pano-Pardo J.R., Pulcini C., Schouten J. COVID-19: don’t neglect antimicrobial stewardship principles! Clin Microbiol Infect. 2020;26:808–810. doi: 10.1016/j.cmi.2020.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baur D., Gladstone B.P., Burkert F., Carrara E., Foschi F., Döbele S. Effect of antibiotic stewardship on the incidence of infection and colonisation with antibiotic-resistant bacteria and Clostridium difficile infection: a systematic review and meta-analysis. Lancet Infect Dis. 2017;17:990–1001. doi: 10.1016/S1473-3099(17)30325-0. [DOI] [PubMed] [Google Scholar]
- 9.Cochrane Rapid Reviews . 2020. Interim guidance from the Cochrane rapid reviews methods group. [Google Scholar]
- 10.Lin L., Xu C. Arcsine-based transformations for meta-analysis of proportions: pros, cons, and alternatives. Health Sci Rep. 2020;3:e178. doi: 10.1002/hsr2.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lin L., Chu H. Meta-analysis of proportions using generalized linear mixed models. Epidemiology. 2020;31:713–717. doi: 10.1097/EDE.0000000000001232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Deeks J., Higgins J., Altman D. 2019. Cochrane handbook for systematic reviews of interventions.https://training.cochrane.org/handbook Available from: [Google Scholar]
- 13.Soucy J. 2020. Antibiotic prescribing in COVID-19 statistical code.https://github.com/jeanpaulrsoucy/covid-19-antibiotic Available from: [Google Scholar]
- 14.Fullman N., Yearwood J., Abay S.M., Abbafati C., Abd-Allah F., Abdela J. Measuring performance on the healthcare access and quality Index for 195 countries and territories and selected subnational locations: a systematic analysis from the global burden of disease study 2016. Lancet. 2018;391:2236–2271. doi: 10.1016/S0140-6736(18)30994-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hoy D., Brooks P., Woolf A., Blyth F., March L., Bain C. Assessing risk of bias in prevalence studies: modification of an existing tool and evidence of interrater agreement. J Clin Epidemiol. 2012;65:934–939. doi: 10.1016/j.jclinepi.2011.11.014. [DOI] [PubMed] [Google Scholar]
- 16.Abrishami A., Samavat S., Behnam B., Arab-Ahmadi M., Nafar M., Sanei Taheri M. Clinical course, imaging features, and outcomes of COVID-19 in kidney transplant recipients. Eur Urol. 2020;78:281–286. doi: 10.1016/j.eururo.2020.04.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alberici F., Delbarba E., Manenti C., Econimo L., Valerio F., Pola A. A single center observational study of the clinical characteristics and short-term outcome of 20 kidney transplant patients admitted for SARS-CoV2 pneumonia. Kidney Int. 2020;97:1083–1088. doi: 10.1016/j.kint.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alberici F., Delbarba E., Manenti C., Econimo L., Valerio F., Pola A. A report from the Brescia Renal COVID Task Force on the clinical characteristics and short-term outcome of hemodialysis patients with SARS-CoV-2 infection. Kidney Int. 2020;98:20–26. doi: 10.1016/j.kint.2020.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Argenziano M.G., Bruce S.L., Slater C.L., Tiao J.R., Baldwin M.R., Barr R.G. Characterization and clinical course of 1000 patients with coronavirus disease 2019 in New York: retrospective case series. BMJ. 2020;369:m1996. doi: 10.1136/bmj.m1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barrasa H., Rello J., Tejada S., Martin A., Balziskueta G., Vinuesa C. SARS-Cov-2 in Spanish intensive care: early experience with 15-day survival in Vitoria. Anaesth Crit Care Pain Med. 2020;39:553–561. doi: 10.1016/j.accpm.2020.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Buckner F.S., McCulloch D.J., Atluri V., Blain M., McGuffin S.A., Nalla A.K. Clinical features and outcomes of 105 hospitalized patients with COVID-19 in Seattle, Washington. Clin Infect Dis. 2020;71:2167–2173. doi: 10.1093/cid/ciaa632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Buetti N., Mazzuchelli T., Lo Priore E., Balmelli C., Llamas M., Pallanza M. Early administered antibiotics do not impact mortality in critically ill patients with COVID-19. J Infect. 2020;81:e148–e149. doi: 10.1016/j.jinf.2020.06.004. S0163445320303819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cai J., Xu J., Lin D., Yang Z., Xu L., Qu Z. A Case Series of children with 2019 novel coronavirus infection: clinical and epidemiological features. Clin Infect Dis. 2020;71:1547–1551. doi: 10.1093/cid/ciaa198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cai Q., Huang D., Ou P., Yu H., Zhu Z., Xia Z. COVID-19 in a designated infectious diseases hospital outside Hubei Province, China. Allergy. 2020;75:1742–1752. doi: 10.1111/all.14309. [DOI] [PubMed] [Google Scholar]
- 25.Cao B., Wang Y., Wen D., Liu W., Fan G., Ruan L. A trial of lopinavir-ritonavir in adults hospitalized with severe Covid-19. N Engl J Med. 2020;382:1787–1799. doi: 10.1056/nejmoa2001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cao J., Tu W.-J., Cheng W., Yu L., Liu Y.-K., Hu X. Clinical features and short-term outcomes of 102 patients with corona virus disease 2019 in Wuhan, China. Clin Infect Dis. 2020;71:748–755. doi: 10.1093/cid/ciaa243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cao Y., Wei J., Zou L., Jiang T., Wang G., Chen L. Ruxolitinib in treatment of severe coronavirus disease 2019 (COVID-19): a multicenter, single-blind, randomized controlled trial. J Allergy Clin Immunol. 2020;146:137–146. doi: 10.1016/j.jaci.2020.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cavalli G., De Luca G., Campochiaro C., Della-Torre E., Ripa M., Canetti D. Interleukin-1 blockade with high-dose anakinra in patients with COVID-19, acute respiratory distress syndrome, and hyperinflammation: a retrospective cohort study. Lancet Rheumatol. 2020;2:e325–e331. doi: 10.1016/S2665-9913(20)30127-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chao J.Y., Derespina K.R., Herold B.C., Goldman D.L., Aldrich M., Weingarten J. Clinical Characteristics and outcomes of hospitalized and critically ill children and adolescents with coronavirus disease 2019 (COVID-19) at a tertiary care medical center in New York City. J Pediatr. 2020;223:14–19. doi: 10.1016/j.jpeds.2020.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen T., Wu D., Chen H., Yan W., Yang D., Chen G. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ. 2020;368:m1091. doi: 10.1136/bmj.m1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen G., Wu D., Guo W., Cao Y., Huang D., Wang H. Clinical and immunological features of severe and moderate coronavirus disease 2019. J Clin Invest. 2020;130:2620–2629. doi: 10.1172/JCI137244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen J., Zhang Z.-Z., Chen Y.-K., Long Q.-X., Tian W.-G., Deng H.-J. The clinical and immunological features of pediatric COVID-19 patients in China. Genes Dis. 2020;7:535–541. doi: 10.1016/j.gendis.2020.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen Q., Zheng Z., Zhang C., Zhang X., Wu H., Wang J. Clinical characteristics of 145 patients with corona virus disease 2019 (COVID-19) in Taizhou, Zhejiang, China. Infection. 2020;48:543–551. doi: 10.1007/s15010-020-01432-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen R., Liang W., Jiang M., Guan W., Zhan C., Wang T. Risk factors of fatal outcome in hospitalized subjects with coronavirus disease 2019 from a nationwide analysis in China. Chest. 2020;158:97–105. doi: 10.1016/j.chest.2020.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen M., An W., Xia F., Yang P., Li K., Zhou Q. Clinical characteristics of Re-hospitalized patients with COVID-19 in China. J Med Virol. 2020;92:2146–2151. doi: 10.1002/jmv.26002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chu J., Yang N., Wei Y., Yue H., Zhang F., Zhao J. Clinical characteristics of 54 medical staff with COVID-19: a retrospective study in a single center in Wuhan, China. J Med Virol. 2020;92:807–813. doi: 10.1002/jmv.25793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Colaneri M., Sacchi P., Zuccaro V., Biscarini S., Sachs M., Roda S. Clinical characteristics of coronavirus disease (COVID-19) early findings from a teaching hospital in Pavia, North Italy, 21 to 28 February 2020. Eurosurveillance. 2020;25:2000460. doi: 10.2807/1560-7917.ES.2020.25.16.2000460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cosgriff R., Ahern S., Bell S.C., Brownlee K., Burgel P.-R., Byrnes C. A multinational report to characterise SARS-CoV-2 infection in people with cystic fibrosis. J Cyst Fibros. 2020;19:355–358. doi: 10.1016/j.jcf.2020.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cummings M.J., Baldwin M.R., Abrams D., Jacobson S.D., Meyer B.J., Balough E.M. Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: a prospective cohort study. Lancet. 2020;395:1763–1770. doi: 10.1016/S0140-6736(20)31189-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dai H., Zhang X., Xia J., Zhang T., Shang Y., Huang R. High-resolution chest CT features and clinical characteristics of patients infected with COVID-19 in Jiangsu, China. Int J Infect Dis. 2020;95:106–112. doi: 10.1016/j.ijid.2020.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Deng L., Li C., Zeng Q., Liu X., Li X., Zhang H. Arbidol combined with LPV/r versus LPV/r alone against Corona Virus Disease 2019: a retrospective cohort study. J Infect. 2020;81:e1–e5. doi: 10.1016/j.jinf.2020.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Deng Y., Liu W., Liu K., Fang Y.-Y., Shang J., Zhou L. Clinical characteristics of fatal and recovered cases of coronavirus disease 2019 (COVID-19) in Wuhan, China: a retrospective study. Chin Med J (Engl) 2020;133:1261–1267. doi: 10.1097/CM9.0000000000000824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dong X., Cao Y.-Y., Lu X.-X., Zhang J.-J., Du H., Yan Y.-Q. Eleven faces of coronavirus disease 2019. Allergy. 2020;75:1699–1709. doi: 10.1111/all.14289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Du R.-H., Liu L.-M., Yin W., Wang W., Guan L.-L., Yuan M.-L. Hospitalization and critical care of 109 decedents with COVID-19 pneumonia in Wuhan, China. Ann Am Thorac Soc. 2020;17:839–846. doi: 10.1513/AnnalsATS.202003-225OC. 2020101600811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Duan K., Liu B., Li C., Zhang H., Yu T., Qu J. Effectiveness of convalescent plasma therapy in severe COVID-19 patients. Proc Natl Acad Sci U S A. 2020;117:9490–9496. doi: 10.1073/pnas.2004168117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fadel R., Morrison A.R., Vahia A., Smith Z.R., Chaudhry Z., Bhargava P. Early short course corticosteroids in hospitalized patients with COVID-19. Clin Infect Dis. 2020;71:2114–2120. doi: 10.1093/cid/ciaa601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fan Z., Chen L., Li J., Cheng X., Yang J., Tian C. Clinical features of COVID-19-related liver functional abnormality. Clin Gastroenterol Hepatol. 2020;18:1561–1566. doi: 10.1016/j.cgh.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Feng Y., Ling Y., Bai T., Xie Y., Huang J., Li J. COVID-19 with different severities: a multicenter study of clinical features. Am J Respir Crit Care Med. 2020;201:1380–1388. doi: 10.1164/rccm.202002-0445OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ferguson J., Rosser J.I., Quintero O., Scott J., Subramanian A., Gumma M. Characteristics and outcomes of coronavirus disease patients under nonsurge conditions, Northern California, USA, March-April 2020. Emerg Infect Dis. 2020;26:1679–1685. doi: 10.3201/eid2608.201776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fung M., Chiu C.Y., DeVoe C., Doernberg S.B., Schwartz B.S., Langelier C. Clinical outcomes and serologic response in solid organ transplant recipients with COVID-19: a case series from the United States. Am J Transplant. 2020;20:3225–3233. doi: 10.1111/ajt.16079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gervasoni C., Meraviglia P., Riva A., Giacomelli A., Oreni L., Minisci D. Clinical features and outcomes of HIV patients with coronavirus disease 2019. Clin Infect Dis. 2020;71:2276–2278. doi: 10.1093/cid/ciaa579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Giacomelli A., Ridolfo A.L., Milazzo L., Oreni L., Bernacchia D., Siano M. 30-day mortality in patients hospitalized with COVID-19 during the first wave of the Italian epidemic: a prospective cohort study. Pharmacol Res. 2020;158:104931. doi: 10.1016/j.phrs.2020.104931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gu X., Li X., An X., Yang S., Wu S., Yang X. Elevated serum aspartate aminotransferase level identifies patients with coronavirus disease 2019 and predicts the length of hospital stay. J Clin Lab Anal. 2020;34 doi: 10.1002/jcla.23391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Guan W.-J., Ni Z.-Y., Hu Y., Liang W.-H., Ou C.-Q., He J.-X. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Guo T., Shen Q., Guo W., He W., Li J., Zhang Y. Clinical characteristics of elderly patients with COVID-19 in Hunan Province, China: a multicenter, retrospective study. Gerontology. 2020;7601655:1–9. doi: 10.1159/000508734. [DOI] [PubMed] [Google Scholar]
- 57.He R., Lu Z., Zhang L., Fan T., Xiong R., Shen X. The clinical course and its correlated immune status in COVID-19 pneumonia. J Clin Virol. 2020;127:104361. doi: 10.1016/j.jcv.2020.104361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.He W., Chen L., Yuan G., Fang Y., Chen W., Wu D. COVID-19 in persons with haematological cancers. Leukemia. 2020;34:1637–1645. doi: 10.1038/s41375-020-0836-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hoek R.A.S., Manintveld O.C., Betjes M.G.H., Hellemons M.E., Seghers L., van Kampen J.A.A. Covid-19 in solid organ transplant recipients: a single center experience. Transpl Int. 2020;33:1099–1105. doi: 10.1111/tri.13662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hong K.S., Lee K.H., Chung J.H., Shin K.C., Choi E.Y., Jin H.J. Clinical features and outcomes of 98 patients hospitalized with SARS-CoV-2 infection in Daegu, South Korea: a brief descriptive study. Yonsei Med J. 2020;61:431–437. doi: 10.3349/ymj.2020.61.5.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hong Y., Wu X., Qu J., Gao Y., Chen H., Zhang Z. Clinical characteristics of Coronavirus Disease 2019 and development of a prediction model for prolonged hospital length of stay. Ann Transl Med. 2020;8:443. doi: 10.21037/atm.2020.03.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hu K., Guan W.-J., Bi Y., Zhang W., Li L., Zhang B. Efficacy and safety of lianhuaqingwen capsules, a repurposed Chinese herb, in patients with coronavirus disease 2019: a multicenter, prospective, randomized controlled trial. Phytomed Int J Phytother Phytopharm. 2020:153242. doi: 10.1016/j.phymed.2020.153242. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hu W., Chen X., He B., Yuan S., Zhang X., Wu G. Clinical characteristics of 16 patients with COVID-19 infection outside of Wuhan, China: a retrospective, single-center study. Ann Transl Med. 2020;8:642. doi: 10.21037/atm-20-3422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Huang Y., Tu M., Wang S., Chen S., Zhou W., Chen D. Clinical characteristics of laboratory confirmed positive cases of SARS-CoV-2 infection in Wuhan, China: a retrospective single center analysis. Travel Med Infect Dis. 2020;36:101606. doi: 10.1016/j.tmaid.2020.101606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Huang Q., Deng X., Li Y., Sun X., Chen Q., Xie M. Clinical characteristics and drug therapies in patients with the common-type coronavirus disease 2019 in Hunan, China. Int J Clin Pharm. 2020;42:837–845. doi: 10.1007/s11096-020-01031-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Huang R., Zhu L., Xue L., Liu L., Wang J., Zhang B. Clinical findings of patients with coronavirus disease 2019 in Jiangsu province, China: a retrospective, multi-center study. PLoS Negl Trop Dis. 2020;14 doi: 10.1371/journal.pntd.0008280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hung I.F.-N., Lung K.-C., Tso E.Y.-K., Liu R., Chung T.W.-H., Chu M.-Y. Triple combination of interferon beta-1b, lopinavir-ritonavir, and ribavirin in the treatment of patients admitted to hospital with COVID-19: an open-label, randomised, phase 2 trial. Lancet. 2020;395:1695–1704. doi: 10.1016/S0140-6736(20)31042-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hur K., Price C.P.E., Gray E.L., Gulati R.K., Maksimoski M., Racette S.D. Factors associated with intubation and prolonged intubation in hospitalized patients with COVID-19. Otolaryngol Head Neck Surg. 2020;163:170–178. doi: 10.1177/0194599820929640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ikitimur H., Borku Uysal B., Cengiz M., Ikitimur B., Uysal H., Ozcan E. Determining host factors contributing to disease severity in a family cluster of 29 hospitalized SARS-CoV-2 patients: could genetic factors be relevant in the clinical course of COVID-19? J Med Virol. 2020 doi: 10.1002/jmv.26106. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ji M., Yuan L., Shen W., Lv J., Li Y., Li M. Characteristics of disease progress in patients with coronavirus disease 2019 in Wuhan, China. Epidemiol Infect. 2020;148:e94. doi: 10.1017/S0950268820000977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jiang Y., He S., Zhang C., Wang X., Chen X., Jin Y. Clinical characteristics of 60 discharged cases of 2019 novel coronavirus-infected pneumonia in Taizhou, China. Ann Transl Med. 2020;8:547. doi: 10.21037/atm.2020.04.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jin X., Lian J.-S., Hu J.-H., Gao J., Zheng L., Zhang Y.-M. Epidemiological, clinical and virological characteristics of 74 cases of coronavirus-infected disease 2019 (COVID-19) with gastrointestinal symptoms. Gut. 2020:320926. doi: 10.1136/gutjnl-2020-320926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jung S.-Y., Choi J.C., You S.-H., Kim W.-Y. Association of renin-angiotensin-aldosterone system inhibitors with COVID-19-related outcomes in Korea: a nationwide population-based cohort study. Clin Infect Dis. 2020;71:2121–2128. doi: 10.1093/cid/ciaa624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Jung H.-Y., Lim J.-H., Kang S.H., Kim S.G., Lee Y.-H., Lee J. Outcomes of COVID-19 among patients on in-center hemodialysis: an experience from the epicenter in South Korea. J Clin Med. 2020;9:1688. doi: 10.3390/jcm9061688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kato H., Shimizu H., Shibue Y., Hosoda T., Iwabuchi K., Nagamine K. Clinical course of 2019 novel coronavirus disease (COVID-19) in individuals present during the outbreak on the Diamond Princess cruise ship. J Infect Chemother. 2020;26:865–869. doi: 10.1016/j.jiac.2020.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kujawski S.A., Wong K.K., Collins J.P., Epstein L., Killerby M.E., Midgley C.M. Clinical and virologic characteristics of the first 12 patients with coronavirus disease 2019 (COVID-19) in the United States. Nat Med. 2020;26:861–868. doi: 10.1038/s41591-020-0877-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lagi F., Piccica M., Graziani L., Vellere I., Botta A., Tilli M. Early experience of an infectious and tropical diseases unit during the coronavirus disease (COVID-19) pandemic, Florence, Italy, February to March 2020. Eurosurveillance. 2020;25:2000556. doi: 10.2807/1560-7917.ES.2020.25.17.2000556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lechien J.R., Chiesa-Estomba C.M., Place S., Van Laethem Y., Cabaraux P., Mat Q. Clinical and epidemiological characteristics of 1,420 European patients with mild-to-moderate coronavirus disease 2019. J Intern Med. 2020;288:335–344. doi: 10.1111/joim.13089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lei S., Jiang F., Su W., Chen C., Chen J., Mei W. Clinical characteristics and outcomes of patients undergoing surgeries during the incubation period of COVID-19 infection. EClinicalMedicine. 2020:100331. doi: 10.1016/j.eclinm.2020.100331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lei Z., Cao H., Jie Y., Huang Z., Guo X., Chen J. A cross-sectional comparison of epidemiological and clinical features of patients with coronavirus disease (COVID-19) in Wuhan and outside Wuhan, China. Travel Med Infect Dis. 2020;35:101664. doi: 10.1016/j.tmaid.2020.101664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Li L., Zhang W., Hu Y., Tong X., Zheng S., Yang J. Effect of convalescent plasma therapy on time to clinical improvement in patients with severe and life-threatening COVID-19: a randomized clinical trial. JAMA. 2020;324:460–470. doi: 10.1001/jama.2020.10044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Li J., Wang X., Chen J., Zuo X., Zhang H., Deng A. COVID-19 infection may cause ketosis and ketoacidosis. Diabetes Obes Metab. 2020;22:1935–1941. doi: 10.1111/dom.14057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Li J., Li M., Zheng S., Li M., Zhang M., Sun M. Plasma albumin levels predict risk for nonsurvivors in critically ill patients with COVID-19. Biomark Med. 2020;14:827–837. doi: 10.2217/bmm-2020-0254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Li H., Tian S., Chen T., Cui Z., Shi N., Zhong X. Newly diagnosed diabetes is associated with a higher risk of mortality than known diabetes in hospitalized patients with COVID-19. Diabetes Obes Metab. 2020;22:1897–1906. doi: 10.1111/dom.14099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Li L., Yang L., Gui S., Pan F., Ye T., Liang B. Association of clinical and radiographic findings with the outcomes of 93 patients with COVID-19 in Wuhan, China. Theranostics. 2020;10:6113–6121. doi: 10.7150/thno.46569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Li N., Han L., Peng M., Lv Y., Ouyang Y., Liu K. Maternal and neonatal outcomes of pregnant women with COVID-19 pneumonia: a case-control study. Clin Infect Dis. 2020;71:2035–2041. doi: 10.1093/cid/ciaa352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Li Y., Wang J., Wang C., Yang Q., Xu Y., Xu J. Characteristics of respiratory virus infection during the outbreak of 2019 novel coronavirus in Beijing. Int J Infect Dis. 2020;96:266–269. doi: 10.1016/j.ijid.2020.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Li S., Jiang L., Li X., Lin F., Wang Y., Li B. Clinical and pathological investigation of severe COVID-19 patients. JCI Insight. 2020;5 doi: 10.1172/jci.insight.138070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Li X., Xu S., Yu M., Wang K., Tao Y., Zhou Y. Risk factors for severity and mortality in adult COVID-19 inpatients in Wuhan. J Allergy Clin Immunol. 2020;146:110–118. doi: 10.1016/j.jaci.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lian J., Jin X., Hao S., Jia H., Cai H., Zhang X. Epidemiological, clinical, and virological characteristics of 465 hospitalized cases of coronavirus disease 2019 (COVID-19) from Zhejiang province in China. Influenza Other Respir Virus. 2020;14:564–574. doi: 10.1111/irv.12758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lian N., Xie H., Lin S., Huang J., Zhao J., Lin Q. Umifenovir treatment is not associated with improved outcomes in patients with coronavirus disease 2019: a retrospective study. Clin Microbiol Infect. 2020;26:917–921. doi: 10.1016/j.cmi.2020.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lim J.-H., Park S.-H., Jeon Y., Cho J.-H., Jung H.-Y., Choi J.-Y. Fatal outcomes of COVID-19 in patients with severe acute kidney injury. J Clin Med. 2020;9:1718. doi: 10.3390/jcm9061718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Liu D., Li L., Wu X., Zheng D., Wang J., Yang L. Pregnancy and perinatal outcomes of women with coronavirus disease (COVID-19) pneumonia: a preliminary analysis. AJR Am J Roentgenol. 2020;215:127–132. doi: 10.2214/AJR.20.23072. [DOI] [PubMed] [Google Scholar]
- 95.Liu F., Xu A., Zhang Y., Xuan W., Yan T., Pan K. Patients of COVID-19 may benefit from sustained Lopinavir-combined regimen and the increase of Eosinophil may predict the outcome of COVID-19 progression. Int J Infect Dis. 2020;95:183–191. doi: 10.1016/j.ijid.2020.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Liu J., Chen T., Yang H., Cai Y., Yu Q., Chen J. Clinical and radiological changes of hospitalised patients with COVID-19 pneumonia from disease onset to acute exacerbation: a multicentre paired cohort study. Eur Radiol. 2020;30:5702–5708. doi: 10.1007/s00330-020-06916-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Liu W., Tao Z.-W., Lei W., Ming-Li Y., Kui L., Ling Z. Analysis of factors associated with disease outcomes in hospitalized patients with 2019 novel coronavirus disease. Chin Med J (Engl) 2020;133:1032–1038. doi: 10.1097/CM9.0000000000000775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Liu K., Chen Y., Lin R., Han K. Clinical features of COVID-19 in elderly patients: a comparison with young and middle-aged patients. J Infect. 2020;80:e14–e18. doi: 10.1016/j.jinf.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Liu L., Lei X., Xiao X., Yang J., Li J., Ji M. Epidemiological and clinical characteristics of patients with coronavirus disease-2019 in Shiyan City, China. Front Cell Infect Microbiol. 2020;10:284. doi: 10.3389/fcimb.2020.00284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lo I.L., Lio C.F., Cheong H.H., Lei C.I., Cheong T.H., Zhong X. Evaluation of SARS-CoV-2 RNA shedding in clinical specimens and clinical characteristics of 10 patients with COVID-19 in Macau. Int J Biol Sci. 2020;16:1698–1707. doi: 10.7150/ijbs.45357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Morena V., Milazzo L., Oreni L., Bestetti G., Fossali T., Bassoli C. Off-label use of tocilizumab for the treatment of SARS-CoV-2 pneumonia in Milan, Italy. Eur J Intern Med. 2020;76:36–42. doi: 10.1016/j.ejim.2020.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Nair V., Jandovitz N., Hirsch J.S., Nair G., Abate M., Bhaskaran M. COVID-19 in kidney transplant recipients. Am J Transplant. 2020;20:1819–1825. doi: 10.1111/ajt.15967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Nowak B., Szymanski P., Pankowski I., Szarowska A., Zycinska K., Rogowski W. Clinical characteristics and short-term outcomes of coronavirus disease 2019: retrospective, single-center experience of designated hospital in Poland. Pol Arch Intern Med. 2020;130:407–411. doi: 10.20452/pamw.15361. [DOI] [PubMed] [Google Scholar]
- 104.Okoh A.K., Bishburg E., Grinberg S., Nagarakanti S. COVID-19 pneumonia in patients with HIV – a case series. J Acquir Immune Defic Syndr. 2020;85:e4–e5. doi: 10.1097/QAI.0000000000002411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Palmieri L., Vanacore N., Donfrancesco C., Lo Noce C., Canevelli M., Punzo O. Clinical characteristics of hospitalized individuals dying with covid-19 by age group in Italy. J Gerontol A Biol Sci Med Sci. 2020;75:1796–1800. doi: 10.1093/gerona/glaa146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Pan L., Mu M., Yang P., Sun Y., Wang R., Yan J. Clinical characteristics of COVID-19 patients with digestive symptoms in Hubei, China: a descriptive, cross-sectional, multicenter study. Am J Gastroenterol. 2020;115:766–773. doi: 10.14309/ajg.0000000000000620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Peng H., Gao P., Xu Q., Liu M., Peng J., Wang Y. Coronavirus disease 2019 in children: characteristics, antimicrobial treatment, and outcomes. J Clin Virol. 2020;128:104425. doi: 10.1016/j.jcv.2020.104425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Pereira A., Cruz-Melguizo S., Adrien M., Fuentes L., Marin E., Perez-Medina T. Clinical course of coronavirus disease-2019 (COVID-19) in pregnancy. Acta Obstet Gynecol Scand. 2020;99:839–847. doi: 10.1111/aogs.13921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Pierce-Williams R.A.M., Burd J., Felder L., Khoury R., Bernstein P.S., Avila K. Clinical course of severe and critical COVID-19 in hospitalized pregnancies: a US cohort study. Am J Obstet Gynecol MFM. 2020;2:100134. doi: 10.1016/j.ajogmf.2020.100134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Piva S., Filippini M., Turla F., Cattaneo S., Margola A., De Fulviis S. Clinical presentation and initial management critically ill patients with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection in Brescia. Italy J Crit Care. 2020;58:29–33. doi: 10.1016/j.jcrc.2020.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Pongpirul W.A., Mott J.A., Woodring J.V., Uyeki T.M., MacArthur J.R., Vachiraphan A. Clinical characteristics of patients hospitalized with coronavirus disease, Thailand. Emerg Infect Dis. 2020;26:1580–1585. doi: 10.3201/eid2607.200598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Quartuccio L., Sonaglia A., Pecori D., Peghin M., Fabris M., Tascini C. Higher levels of IL-6 early after tocilizumab distinguish survivors from non-survivors in COVID-19 pneumonia: a possible indication for deeper targeting IL-6. J Med Virol. 2020;92:2852–2856. doi: 10.1002/jmv.26149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Shi M., Chen L., Yang Y., Zhang J., Xu J., Xu G. Analysis of clinical features and outcomes of 161 patients with severe and critical COVID-19: a multicenter descriptive study. J Clin Lab Anal. 2020;34 doi: 10.1002/jcla.23415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Shi Q., Zhang X., Jiang F., Zhang X., Hu N., Bimu C. Clinical characteristics and risk factors for mortality of COVID-19 patients with diabetes in Wuhan, China: a two-center, retrospective study. Diabetes Care. 2020;43:1382–1391. doi: 10.2337/dc20-0598. [DOI] [PubMed] [Google Scholar]
- 115.Shi S., Qin M., Shen B., Cai Y., Liu T., Yang F. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol. 2020;5:802–810. doi: 10.1001/jamacardio.2020.0950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Song C., Xu C., Jin G., Chen Y., Xu X., Ma H. Clinical characteristics of 24 asymptomatic infections with COVID-19 screened among close contacts in Nanjing, China. Sci China Life Sci. 2020;63:706–711. doi: 10.1007/s11427-020-1661-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Song W., Li J., Zou N., Guan W., Pan J., Xu W. Clinical features of pediatric patients with coronavirus disease (COVID-19) J Clin Virol Off Publ Pan Am Soc Clin Virol. 2020;127:104377. doi: 10.1016/j.jcv.2020.104377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Sun D., Chen X., Li H., Lu X.-X., Xiao H., Zhang F.-R. SARS-CoV-2 infection in infants under 1 year of age in Wuhan City, China. World J Pediatr WJP. 2020;16:260–266. doi: 10.1007/s12519-020-00368-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Sun J., Deng X., Chen X., Huang J., Huang S., Li Y. Incidence of adverse drug reactions in COVID-19 patients in China: an active monitoring study by hospital pharmacovigilance System. Clin Pharmacol Ther. 2020;108:791–797. doi: 10.1002/cpt.1866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Sun L., Shen L., Fan J., Gu F., Hu M., An y. Clinical features of patients with coronavirus disease 2019 (COVID-19) from a designated hospital in Beijing, China. J Med Virol. 2020;92:2055–2066. doi: 10.1002/jmv.25966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Tan N.-D., Qiu Y., Xing X.-B., Ghosh S., Chen M.-H., Mao R. Associations between angiotensin converting enzyme inhibitors and angiotensin ii receptor blocker use, gastrointestinal symptoms, and mortality among patients with COVID-19. Gastroenterology. 2020;159:1170–1172. doi: 10.1053/j.gastro.2020.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Tan Y.-P., Tan B.-Y., Pan J., Wu J., Zeng S.-Z., Wei H.-Y. Epidemiologic and clinical characteristics of 10 children with coronavirus disease 2019 in Changsha, China. J Clin Virol. 2020;127:104353. doi: 10.1016/j.jcv.2020.104353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Tian J., Yuan X., Xiao J., Zhong Q., Yang C., Liu B. Clinical characteristics and risk factors associated with COVID-19 disease severity in patients with cancer in Wuhan, China: a multicentre, retrospective, cohort study. Lancet Oncol. 2020;21:893–903. doi: 10.1016/S1470-2045(20)30309-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Tian S., Chang Z., Wang Y., Wu M., Zhang W., Zhou G. Clinical characteristics and reasons for differences in duration from symptom onset to release from quarantine among patients with COVID-19 in Liaocheng, China. Front Med. 2020;7:210. doi: 10.3389/fmed.2020.00210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Tschopp J., L’Huillier A.G., Mombelli M., Mueller N.J., Khanna N., Garzoni C. First experience of SARS-CoV-2 infections in solid organ transplant recipients in the Swiss Transplant Cohort Study. Am J Transplant. 2020;20:2876–2882. doi: 10.1111/ajt.16062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Wan Y., Li J., Shen L., Zou Y., Hou L., Zhu L. Enteric involvement in hospitalised patients with COVID-19 outside Wuhan. Lancet Gastroenterol Hepatol. 2020;5:534–535. doi: 10.1016/S2468-1253(20)30118-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Wang F., Hou H., Wang T., Luo Y., Tang G., Wu S. Establishing a model for predicting the outcome of COVID-19 based on combination of laboratory tests. Travel Med Infect Dis. 2020;36:101782. doi: 10.1016/j.tmaid.2020.101782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Wang X., Liu W., Zhao J., Lu Y., Wang X., Yu C. Clinical characteristics of 80 hospitalized frontline medical workers infected with COVID-19 in Wuhan, China. J Hosp Infect. 2020;105:399–403. doi: 10.1016/j.jhin.2020.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Wang D., Yin Y., Hu C., Liu X., Zhang X., Zhou S. Clinical course and outcome of 107 patients infected with the novel coronavirus, SARS-CoV-2, discharged from two hospitals in Wuhan, China. Crit Care. 2020;24:188. doi: 10.1186/s13054-020-02895-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Wang J., Yang Q., Zhang P., Sheng J., Zhou J., Qu T. Clinical characteristics of invasive pulmonary aspergillosis in patients with COVID-19 in Zhejiang, China: a retrospective case series. Crit Care. 2020;24:299. doi: 10.1186/s13054-020-03046-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Wang L., Duan Y., Zhang W., Liang J., Xu J., Zhang Y. Epidemiologic and clinical characteristics of 26 cases of COVID-19 arising from patient-to-patient transmission in Liaocheng, China. Clin Epidemiol. 2020;12:387–391. doi: 10.2147/CLEP.S249903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Wang R., Pan M., Zhang X., Han M., Fan X., Zhao F. Epidemiological and clinical features of 125 hospitalized patients with COVID-19 in Fuyang, Anhui, China. Int J Infect Dis. 2020;95:421–428. doi: 10.1016/j.ijid.2020.03.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Wang Y., Lu X., Chen H., Chen T., Su N., Huang F. Clinical course and outcomes of 344 intensive care patients with COVID-19. Am J Respir Crit Care Med. 2020;201:1430–1434. doi: 10.1164/rccm.202003-0736LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Wang Z., Yang B., Li Q., Wen L., Zhang R. Clinical features of 69 cases with coronavirus disease 2019 in Wuhan, China. Clin Infect Dis. 2020;71:769–777. doi: 10.1093/cid/ciaa272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Wei X.-S., Wang X.-R., Zhang J.-C., Yang W.-B., Ma W.-L., Yang B.-H. A cluster of health care workers with COVID-19 pneumonia caused by SARS-CoV-2. J Microbiol Immunol Infect. 2020 doi: 10.1016/j.jmii.2020.04.013. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Wu J., Li J., Zhu G., Zhang Y., Bi Z., Yu Y. Clinical features of maintenance hemodialysis patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. Clin J Am Soc Nephrol. 2020;15:1139–1145. doi: 10.2215/CJN.04160320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Wu C., Chen X., Cai Y., Xia J., Xu S., Huang H. Risk Factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020;180:934–943. doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Wu F., Zhou Y., Wang Z., Xie M., Shi Z., Tang Z. Clinical characteristics of COVID-19 infection in chronic obstructive pulmonary disease: a multicenter, retrospective, observational study. J Thorac Dis. 2020;12:1811–1823. doi: 10.21037/jtd-20-1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Wu H., Zhu H., Yuan C., Yao C., Luo W., Shen X. Clinical and immune features of hospitalized pediatric patients with coronavirus disease 2019 (COVID-19) in Wuhan, China. JAMA Netw Open. 2020;3 doi: 10.1001/jamanetworkopen.2020.10895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Wu J., Li W., Shi X., Chen Z., Jiang B., Liu J. Early antiviral treatment contributes to alleviate the severity and improve the prognosis of patients with novel coronavirus disease (COVID-19) J Intern Med. 2020;288:128–138. doi: 10.1111/joim.13063. [DOI] [PubMed] [Google Scholar]
- 142.Wu J., Liu J., Zhao X., Liu C., Wang W., Wang D. Clinical characteristics of imported cases of COVID-19 in Jiangsu Province: a multicenter descriptive study. Clin Infect Dis. 2020;71:706–712. doi: 10.1093/cid/ciaa199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Xi A., Zhuo M., Dai J., Ding Y., Ma X., Wang X. Epidemiological and clinical characteristics of discharged patients infected with SARS-CoV-2 on the Qinghai plateau. J Med Virol. 2020;92:2528–2535. doi: 10.1002/jmv.26032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Xiong F., Tang H., Liu L., Tu C., Tian J.-B., Lei C.-T. Clinical characteristics of and medical interventions for COVID-19 in hemodialysis patients in Wuhan, China. J Am Soc Nephrol. 2020;31:1387–1397. doi: 10.1681/ASN.2020030354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Xiong Z., Xin C., Yan X., Cai Y., Zhou K., Xie C. Clinical characteristics and outcomes of 421 patients with COVID-19 treated in a mobile cabin hospital. Chest. 2020;158:939–946. doi: 10.1016/j.chest.2020.05.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Xu X.-W., Wu X.-X., Jiang X.-G., Xu K.-J., Ying L.-J., Ma C.-L. Clinical findings in a group of patients infected with the 2019 novel coronavirus (SARS-Cov-2) outside of Wuhan, China: retrospective case series. BMJ. 2020;368:m606. doi: 10.1136/bmj.m606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Yan J., Guo J., Fan C., Juan J., Yu X., Li J. Coronavirus disease 2019 in pregnant women: a report based on 116 cases. Am J Obstet Gynecol. 2020;223:111.e1–111.e14. doi: 10.1016/j.ajog.2020.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Yang X., Yu Y., Xu J., Shu H., Xia J., Liu H. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8:475–481. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Yang L., Liu J., Zhang R., Li M., Li Z., Zhou X. Epidemiological and clinical features of 200 hospitalized patients with corona virus disease 2019 outside Wuhan, China: a descriptive study. J Clin Virol. 2020;129:104475. doi: 10.1016/j.jcv.2020.104475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Yang Q., Xie L., Zhang W., Zhao L., Wu H., Jiang J. Analysis of the clinical characteristics, drug treatments and prognoses of 136 patients with coronavirus disease 2019. J Clin Pharm Ther. 2020;45:609–616. doi: 10.1111/jcpt.13170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Yang H., Sun G., Tang F., Peng M., Gao Y., Peng J. Clinical features and outcomes of pregnant women suspected of coronavirus disease 2019. J Infect. 2020;81:e40–e44. doi: 10.1016/j.jinf.2020.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Yao Q., Wang P., Wang X., Qie G., Meng M., Tong X. A retrospective study of risk factors for severe acute respiratory syndrome coronavirus 2 infections in hospitalized adult patients. Pol Arch Intern Med. 2020;130:390–399. doi: 10.20452/pamw.15312. [DOI] [PubMed] [Google Scholar]
- 153.Yu Y., Xu D., Fu S., Zhang J., Yang X., Xu L. Patients with COVID-19 in 19 ICUs in Wuhan, China: a cross-sectional study. Crit Care. 2020;24:219. doi: 10.1186/s13054-020-02939-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Zeng Y., Lin L., Yan Q., Wei W., Yang B.X., Huang R. Update on clinical outcomes of women with COVID-19 during pregnancy. Int J Gynaecol Obstet. 2020;150:264–266. doi: 10.1002/ijgo.13236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Zha L., Li S., Pan L., Tefsen B., Li Y., French N. Corticosteroid treatment of patients with coronavirus disease 2019 (COVID-19) Med J Aust. 2020;212:416–420. doi: 10.5694/mja2.50577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Zhang L., Zhu F., Xie L., Wang C., Wang J., Chen R. Clinical characteristics of COVID-19-infected cancer patients: a retrospective case study in three hospitals within Wuhan, China. Ann Oncol Off J Eur Soc Med Oncol. 2020;31:894–901. doi: 10.1016/j.annonc.2020.03.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Zhang D., Du G., Du R., Zhao J., Jin Y., Fu S. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2020;395:1569–1578. doi: 10.1016/S0140-6736(20)31022-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Zhang L., Feng X., Zhang D., Jiang C., Mei H., Wang J. Deep vein thrombosis in hospitalized patients with COVID-19 in Wuhan, China: prevalence, risk factors, and outcome. Circulation. 2020;142:114–128. doi: 10.1161/CIRCULATIONAHA.120.046702. [DOI] [PubMed] [Google Scholar]
- 159.Zhang H., Shang W., Liu Q., Zhang X., Zheng M., Yue M. Clinical characteristics of 194 cases of COVID-19 in Huanggang and Taian, China. Infection. 2020;48:687–694. doi: 10.1007/s15010-020-01440-5. 0365307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Zhang Y., Cui Y., Shen M., Zhang J., Liu B., Dai M. Association of diabetes mellitus with disease severity and prognosis in COVID-19: a retrospective cohort study. Diabetes Res Clin Pract. 2020;165:108227. doi: 10.1016/j.diabres.2020.108227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Zhao M., Wang M., Zhang J., Gu J., Zhang P., Xu Y. Comparison of clinical characteristics and outcomes of patients with coronavirus disease 2019 at different ages. Aging. 2020;12:10070–10086. doi: 10.18632/aging.103298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Zhao X.-Y., Xu X.-X., Yin H.-S., Hu Q.-M., Xiong T., Tang Y.-Y. Clinical characteristics of patients with 2019 coronavirus disease in a non-Wuhan area of Hubei Province, China: a retrospective study. BMC Infect Dis. 2020;20:311. doi: 10.1186/s12879-020-05010-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Zheng T., Yang C., Wang H.-Y., Chen X., Yu L., Wu Z.-L. Clinical characteristics and outcomes of COVID-19 patients with gastrointestinal symptoms admitted to Jianghan Fangcang Shelter Hospital in Wuhan, China. J Med Virol. 2020;92:2735–2741. doi: 10.1002/jmv.26146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Zheng F., Liao C., Fan Q.-H., Chen H.-B., Zhao X.-G., Xie Z.-G. Clinical characteristics of children with coronavirus disease 2019 in Hubei, China. Curr Med Sci. 2020;40:275–280. doi: 10.1007/s11596-020-2172-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Zheng Y., Sun L., Xu M., Pan J., Zhang Y., Fang X. Clinical characteristics of 34 COVID-19 patients admitted to intensive care unit in Hangzhou, China. J Zhejiang Univ-Sci B. 2020;21:378–387. doi: 10.1631/jzus.B2000174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Zheng Y., Xiong C., Liu Y., Qian X., Tang Y., Liu L. Epidemiological and clinical characteristics analysis of COVID-19 in the surrounding areas of Wuhan, Hubei Province in 2020. Pharmacol Res. 2020;157:104821. doi: 10.1016/j.phrs.2020.104821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Zhou Y., Han T., Chen J., Hou C., Hua L., He S. Clinical and autoimmune characteristics of severe and critical cases of COVID-19. Clin Transl Sci. 2020;13:1077–1086. doi: 10.1111/cts.12805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Zhu L., Wang J., Huang R., Liu L., Zhao H., Wu C. Clinical characteristics of a case series of children with coronavirus disease 2019. Pediatr Pulmonol. 2020;55:1430–1432. doi: 10.1002/ppul.24767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Hughes S., Troise O., Donaldson H., Mughal N., Moore L.S.P. Bacterial and fungal coinfection among hospitalized patients with COVID-19: a retrospective cohort study in a UK secondary-care setting. Clin Microbiol Infect. 2020;26:1395–1399. doi: 10.1016/j.cmi.2020.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Toronto Antimicrobial Resistance Research Network Bacterial co-infection and secondary infection in patients with COVID-19: a rapid systematic review and meta-analysis. Toronto, ON. 2020. https://www.tarrn.org/covid [Google Scholar]
- 172.Klein E.Y., Monteforte B., Gupta A., Jiang W., May L., Hsieh Y.-H. The frequency of influenza and bacterial coinfection: a systematic review and meta-analysis. Influenza Other Respir Virus. 2016;10:394–403. doi: 10.1111/irv.12398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Ghazi I.M., Nicolau D.P., Nailor M.D., Aslanzadeh J., Ross J.W., Kuti J.L. Antibiotic utilization and opportunities for stewardship among hospitalized patients with influenza respiratory tract infection. Infect Control Hosp Epidemiol. 2016;37:583–589. doi: 10.1017/ice.2016.17. [DOI] [PubMed] [Google Scholar]
- 174.Collignon P., Beggs J. CON: COVID-19 will not result in increased antimicrobial resistance prevalence. JAC Antimicrob Resist. 2020;2 doi: 10.1093/jacamr/dlaa051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Clancy C.J., Buehrle D.J., Nguyen M.H. PRO: the COVID-19 pandemic will result in increased antimicrobial resistance rates. JAC Antimicrob Resist. 2020;2 doi: 10.1093/jacamr/dlaa049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Hu F., Guo Y., Yang Y., Zheng Y., Wu S., Jiang X., on behalf of the China Antimicrobial Surveillance Network (CHINET) Study Group Resistance reported from China antimicrobial surveillance network (CHINET) in 2018. Eur J Clin Microbiol Infect Dis. 2019;38:2275–2281. doi: 10.1007/s10096-019-03673-1. [DOI] [PubMed] [Google Scholar]
- 177.Surveillance of antimicrobial resistance in Europe 2018. European Centre for Disease Prevention and Control; Stockholm: 2019. https://www.ecdc.europa.eu/sites/default/files/documents/surveillance-antimicrobial-resistance-Europe-2018.pdf Available from: [Google Scholar]
- 178.Jernigan J.A., Hatfield K.M., Wolford H., Nelson R.E., Olubajo B., Reddy S.C. Multidrug-resistant bacterial infections in U.S. Hospitalized patients, 2012–2017. N Engl J Med. 2020;382:1309–1319. doi: 10.1056/NEJMoa1914433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.World Health Organization . World Health Organization; Geneva, Switzerland: 2020. Clinical management of COVID-19 interim guidance.https://www.who.int/publications-detail/clinical-management-of-severe-acute-respiratory-infection-when-novel-coronavirus-(ncov)-infection-is-suspected Available from: [Google Scholar]
- 180.Alhazzani W., Møller M.H., Arabi Y.M., Loeb M., Gong M.N., Fan E. Surviving sepsis campaign: guidelines on the management of critically ill adults with coronavirus disease 2019 (COVID-19) Crit Care Med. 2020;48:e440–e469. doi: 10.1097/CCM.0000000000004363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.National Institute for Health and Care Excellence (NICE). COVID-19 rapid guideline: antibiotics for pneumonia in adults in hospital. National Institute for Health and Care Excellence (NICE); 2020. https://www.nice.org.uk/guidance/ng173/chapter/4-Assessing-the-ongoing-need-for-antibiotics [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.