Abstract
Defined as helpful live bacteria that can provide medical advantages to the host when administered in tolerable amounts, oral probiotics might be worth considering as a possible preventive or therapeutic modality to mitigate coronavirus disease 2019 (COVID-19) symptom severity. This hypothesis stems from an emerging understanding of the gut–lung axis wherein probiotic microbial species in the digestive tract can influence systemic immunity, lung immunity, and possibly viral pathogenesis and secondary infection co-morbidities. We review the principles underlying the gut–lung axis, examples of probiotic-associated antiviral activities, and current clinical trials in COVID-19 based on oral probiotics.
Keywords: Coronavirus disease 2019, gut–lung axis, gut microbiome, probiotics, secondary infection, severe acute respiratory syndrome coronavirus 2
Highlights
-
•
Probiotics are known as helpful live bacteria for medical advantages to the host when administered intolerable amounts.
-
•
The study summarizes the embedded cross-road between gut and lung microbiota.
-
•
Probiotics are significant solutions to improve gut health or dysbiosis created by viral infections like SARS-CoV-2.
-
•
Evidence has also been given about extenuating inflammation and antiviral properties of probiotics.
Probiotics can be an adjunctive treatment procedure to battle against COVID-19 and associated secondary infections.
Gut–lung connectivity in infection and immunity
The spike protein of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) uses angiotensin converting enzyme 2 as the receptor for cell entry, which is highly expressed in gut and lung tissues that produce well-described symptoms in coronavirus disease 2019 (COVID-19) [1,2]. In the gut, proximal and distal enterocytes are targets [3], and both the receptor and these cell types are known to be pathologically connected with intestinal inflammation and diarrhoea [4]. Nausea and diarrhoea are reported to be primary symptoms of COVID-19 even before the development of fever and respiratory symptoms, whereas abdominal pain continues to be reported frequently in patients admitted to intensive care [5]. The most severe cases of COVID-19 often involve pneumonia followed by acute respiratory distress syndrome [6], involving hypoxaemic respiratory distress concurrent with lung neutrophilia, mucus and fluid accumulation in bronchi, and bronchiectasis [7].
Besides the shared trait of direct viral targeting in both gut and lung, the two tissues share a relationship influencing inflammatory and immune responses via the gut–lung axis that can be responsive to probiotics through effects on commensal microbial flora. From birth, both gut and lungs share exposure to microbes through the oral route, a process that over time seeds a quasi-stable and complex gut flora, with growing evidence for a much lower level of microbial species in the lung that are different between the upper and lower respiratory tracts [[8], [9], [10]]. Focusing on gut flora, microbe interactions and their products influence innate and adaptive immune signals and cells locally, systemically, and specifically in the lungs, where it has been shown that the gut microbiome affects susceptibility to asthma, lung allergic responses and chronic obstructive pulmonary disease [11,12]. Various insults can cause dysbiosis of the gut microbial flora, including antibiotic treatment or pathogenic infection, which can result in increased gut permeability, in turn leading to microbial translocation and systemic dispersal of toxins and inflammatory products to the circulatory system [13]. Therefore, whether due to pre-existing differences in gut flora, or differences induced upon infection, it is conceivable that the microbiome may influence differences in the inflammatory response between patients that could correspond with COVID-19 severity.
Probiotics improving anti-viral responses
Oral probiotics are live bacteria that can improve gut health in homeostasis, and can exhibit antiviral effects [14,15] via the gut–lung axis [16]. Upon delivery, probiotics are understood to adjust the crosstalk between commensal microbes and the mucosal immune framework, and in this way alter the basal and induced inflammatory balance in response to viral infections [14]. Regarding upper respiratory tract disease, some probiotics have shown anti-viral protective and therapeutic effects, lessening the severity and extent of tissue damage from infection and inflammation [17,18]. Diminished plasma titres of Epstein–Barr virus and antibody titres against cytomegalovirus were observed in upper respiratory tract infections upon treatment with the lactic acid-producing bacterium Lactobacillus casei, by a mechanism dependent on Toll-like receptors [19]. Another probiotic bacterium, Lactococcus lactis JCM 5805, was demonstrated to have antiviral activity against influenza virus infection and to activate plasmacytoid dendritic cells using Toll-like receptor 9 [20]. Treatment with the probiotic Bifidobacterium lactis HN019 was reported to increase mononuclear leucocyte recruitment and elevate phagocytic and lytic activity [21]. The probiotic impact of Lactobacillus gasseri has been shown against respiratory syncytial virus infection in mice by a significant reduction of viral titre in the lungs along with decreases in several pulmonary pro-inflammatory cytokines, but increases in interferon types I and II [22]. Lactic acid-producing bacteria (LAB) have been used in probiotic settings via nasal and oral application, where modulation of cytokine profiles was associated with protection against respiratory syncytial virus [23,24], and wider application for respiratory infections has been suggested [25]. A clinical report in neonates demonstrated that probiotic treatment in early life was associated with decreased rates of subsequent respiratory tract infections [26]. Up to this point, many clinical trials have investigated the potential impacts of probiotics on viral infections (Table 1), with some current trials exploring the effectiveness of probiotics in the context of COVID-19 (Table 2).
Table 1.
Probiotics, targeted viral infections, immunostimulatory mode of action, reported medicinal effects and supporting references
| Probiotic bacteria (strain) | Viral infection | Immunostimulatory mode of action | Reported medicinal effects | Ref. |
|---|---|---|---|---|
| Lactobacillus brevis (KB290) | Influenza virus | Increased IFN-α production and augmentation of influenza-virus-specific immunoglobulin A production | Reduced risk of infection | [61] |
| Lactobacillus rhamnosus (GG) | Influenza virus | Increased IFN-γ production in serum | Reduced risk of infection | [62] |
| Lactobacillus bulgaricus (OLL1073R-1) and Streptococcus thermophiles | Rhinovirus | Increased IFN-γ production in serum | No significant difference | [63] |
| Lactobacillus rhamnosus (GG) | Rhinovirus | Not determined | Reduced incidence of respiratory tract infections (RTIs) | [64] |
| Lactobacillus rhamnosus (GG) | Rhinovirus | Not determined | Reduced incidence of RTIs | [65] |
| Lactobacillus rhamnosus (GG) | Rhinovirus, respiratory syncytial virus, parainfluenza virus 1 | Not determined | Reduced number of days with symptoms | [66] |
| Lactobacillus casei (DN-114001) | Rhinopharyngitis, influenza virus | Increased expression of defensins | Decreased duration of common infectious diseases | [67] |
| Lactobacillus rhamnosus (M21) | Influenza virus | Increased IFN-γ and interleukin-2 | Increased host resistance against influenza virus infection | [68] |
| Bacillus subtilis (OKB105) | Transmissible gastroenteritis virus | Inhibition of virus entry by competing with viral entry receptors | Reduced viral entry in vitro | [69] |
| Bifidobacterium animalis | Rhinovirus | Inhibition of CXCL8 response upon viral infection | Decreased viral titres in nasal lavage and viral shedding in the nasal secretions | [70] |
Abbreviations: COVID-19, coronavirus disease 2019; IFN, interferon; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Table 2.
Current clinical trials of probiotics in coronavirus disease 2019 registered at ClinicalTrials.gov
| ClinicalTrials.gov identifier | Study title | Probiotic bacteria (strain) | Procedure synopsis |
|---|---|---|---|
| NCT04458519 | Efficacy of intranasal probiotic treatment to reduce severity of symptoms in COVID-19 infection | Lactococcus lactis (W136) | Nasal irrigation with probiorinse |
| NCT04390477 | Study to evaluate the effect of a probiotic in COVID-19 | Not revealed | Dietary supplementation |
| NCT04366180 | Evaluation of probiotic Lactobacillus coryniformis K8 on COVID-19 prevention in health-care workers | Lactobacillus coryniformis (K8) | Dietary supplementation |
| NCT04517422 | Efficacy of Lactobacillus plantarum and Pediococcus acidilactici in adults with SARS-CoV-2 and COVID-19 | Lactobacillus plantarum (CECT7481) Lactobacillus plantarum (CECT7484) Lactobacillus plantarum (CECT7485) Pediococcus acidilactici (CECT7483) | Not revealed |
Abbreviations: COVID-19, coronavirus disease 2019; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Anti-inflammatory probiotics and COVID-19
Some probiotics enhance regulatory T-cell activity and reduce pro-inflammatory cytokine production [[27], [28], [29], [30]]. For example, the anti-inflammatory activity of Weissella cibaaria (JW15) was assessed upon lipopolysaccharide challenge in mouse macrophages, where the probiotic was associated with reduced induction of interleukin-1β (IL-1β), IL-6 and tumour necrosis factor-α (TNF-α). When the stimulus was changed to heat-killed JW15, the same study observed diminished production of nitric oxide and prostaglandin E2 using down-regulation of inducible nitric oxide synthase and cyclooxygenase 2 [31]. Human commensal strains Lactobacillus rhamnosus GG and GR-1 appear capable of anti-inflammatory effects using down-regulation of TNF-α production in human monocytes and mouse macrophages [32].
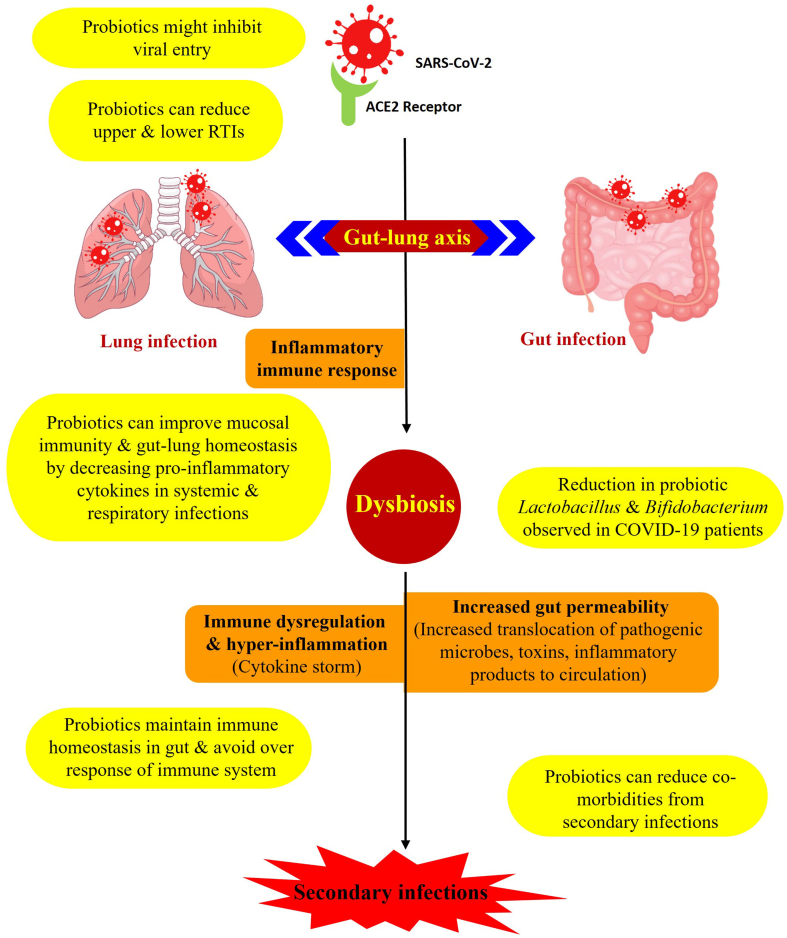
Evidence to date suggests that probiotics with anti-inflammatory or immunomodulatory properties might be predicted to have the most beneficial potential to prevent or alleviate COVID-19 symptoms (Fig. 1). Clinical investigations along with increasing data worldwide suggest that cytokine storm causing hyper-inflammation in the respiratory tract has an apparent causal, positive correlation with COVID-19 disease severity [33]. Blood plasma analysis of 41 individuals with confirmed COVID-19 in Wuhan, China revealed increased levels of various cytokines including IL-1β, IL-7, IL-8, IL-9, IL-10, fibroblast growth factor, granulocyte colony-stimulating factor, granulocyte–macrophage colony-stimulating factor, interferon-γ, IFN-γ-inducible protein-10, monocyte chemoattractant protein-1, macrophage inflammatory protein-1A and -1B, platelet-derived growth factor, TNF-α, and vascular endothelial growth factor in people with COVID-19 compared with healthy individuals [34]. Given these observations, inhibiting or down-regulating this cytokine response may create a healthier immune activation balance to reduce inflammatory symptoms while maintaining adaptive immune engagement against SARS-COV-2 (Fig. 1).
Fig. 1.
Step-by-step progressive schematic illustration of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection and involvement of the gut–lung axis. Potential targets or steps at which the action of probiotics might mitigate coronavirus disease 2019 (COVID-19) are represented, with specific mechanisms of action possible for probiotics highlighted in yellow.
Because probiotics have been studied and recommended in the context of respiratory tract infections, the hypothesis emerges that probiotics might play a positive role against COVID-19. For example, gut dysbiosis during influenza virus infection has been shown to worsen lung pathology and aggravate secondary pneumococcal lung infections [35,36], and gut microbiota dysbiosis has been reported in some COVID-19 patients concurrent with decreases in natural probiotic bacterial species including Lactobacillus and Bifidobacterium [37]. Clinical transcriptome analyses from COVID-19 patients have also indicated a gastrointestinal disease course and potential systemic crosstalk between gut and lungs during SARS-CoV-2 infection [38]. Sufficient rationale has accumulated such that clinical trials of probiotics against COVID-19 are already underway, so far emphasizing probiotics with expected anti-inflammatory effects in the gut–lung axis (Table 2).
Possible roles of probiotic antimicrobial peptides
Probiotics can produce direct antimicrobial effects via metabolites and antimicrobial peptides, including bacteriocins, which could potentially contribute beneficial effects against SARS-CoV-2 as a membranous envelope virus. A species of genus Lactococcus is in a current clinical trial for probiotic activity against COVID-19 (Table 2), and this genus includes LAB whose anti-viral effects may be due in part to secreted metabolites and an enormous number of bacteriocins [39], one class of antimicrobial peptides considered to be guard peptides [40]. Nisin is one of the most widely studied bacteriocins and has been approved for many years as an FDA endorsed food additive. Antimicrobial peptides that can be expressed by LAB appear to contribute to probiotic antiviral effects against influenza A virus and other respiratory viruses [41,42]. From a different genus, the probiotic Bacillus subtilis strain was shown to produce an antiviral peptide, P18, which inhibited influenza infection both in vitro and in vivo [43]. Examples of probiotic lipopeptides include lipopeptide detergent-12, subtilisin, curvacin A, sakacin P and lactococcin Gb, which are well described extracellularly expressed products that may block the virus–cell fusion process or other steps of viral entry by mechanisms involving their amphiphilic nature [44,45]. If such probiotic products were to have direct access to SARS-CoV-2 virions in the gut, or perhaps in the lungs through disease-induced dysbiosis, gut permeability, and dissemination, it can be hypothesized that direct antiviral effects may be possible.
Possible probiotic protection against infections secondary to SARS-CoV-2
Because probiotics can mitigate problems of dysbiosis, inflammation and immune function, and can include direct antimicrobial activities, there may be the potential for a positive contribution against secondary infection co-morbidities in COVID-19. New evidence is emerging that infections secondary to SARS-CoV-2 might contribute to COVID-19 pathology or severity [[46], [47], [48], [49]]. Although an early study suggested relatively little concern across the COVID-19 patient population [50], recent reports are finding increased secondary infections in hospitalized individuals with severe disease, observations that may have some association with immunosuppressive drugs in current treatment regimens [[51], [52], [53]]. Outside the COVID-19 context, application of the probiotic strains Lactobacillus rhamnosus GG, Bacillus subtilis and Enterococcus faecalis during clinical trials, showed a significant improvement in patients with ventilator-associated pneumonia, including pathogens of various types, compared with placebo treatment [54,55]. In general, probiotic strains themselves, including LAB strains, are well known to be non-pathogenic and non-immunogenic, and therefore are considered safe and not a source of potential secondary infections, themselves [56]. Along with protective effects reported against influenza A virus, LAB have been reported to promote heterotypic immunity to secondary infections [57,58]. Furthermore, probiotics have been reported to provide some protection against biofilm-forming pathogens in the respiratory tract [59,60]. As secondary infections may rise to more prominence in COVID-19, perhaps commensurate with increasing anti-inflammatory regimens, treatment options that include probiotics may present an even more attractive modality that merits further investigation and attention in clinical trials.
Conflict of interest
There is no conflict of interest.
Funding
PB and AGS were supported by University of Missouri Biomedical Innovation recruitment funds, and AGS was supported by National Institutes of Health grant R01GM103841.
Contributor Information
P. Baindara, Email: pbaindara@health.missouri.edu.
S.M. Mandal, Email: mandalsm@gmail.com.
A.G. Schrum, Email: schruma@health.missouri.edu.
References
- 1.Bourgonje A.R., Abdulle A.E., Timens W., Hillebrands J.L., Navis G.J., Gordijn S.J. Angiotensin-converting enzyme 2 (ACE2), SARS-CoV-2 and the pathophysiology of coronavirus disease 2019 (COVID-19) J Pathol. 2020;251:228–248. doi: 10.1002/path.5471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Letko M., Marzi A., Munster V. Functional assessment of cell entry and receptor usage for SARS-CoV-2 and other lineage B betacoronaviruses. Nat Microbiol. 2020;5:562–569. doi: 10.1038/s41564-020-0688-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liang W., Feng Z., Rao S., Xiao C., Xue X., Lin Z. Diarrhoea may be underestimated: a missing link in 2019 novel coronavirus. Gut. 2020;69:1141–1143. doi: 10.1136/gutjnl-2020-320832. [DOI] [PubMed] [Google Scholar]
- 4.Hashimoto T., Perlot T., Rehman A., Trichereau J., Ishiguro H., Paolino M. ACE2 links amino acid malnutrition to microbial ecology and intestinal inflammation. Nature. 2012;487:477–481. doi: 10.1038/nature11228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yeo C., Kaushal S., Yeo D. Enteric involvement of coronaviruses: is faecal–oral transmission of SARS-CoV-2 possible? Lancet Gastroenterol Hepatol. 2020;5:335–337. doi: 10.1016/S2468-1253(20)30048-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fan E., Beitler J.R., Brochard L., Calfee C.S., Ferguson N.D., Slutsky A.S. COVID-19-associated acute respiratory distress syndrome: is a different approach to management warranted? Lancet Respir Med. 2020;8:816–821. doi: 10.1016/S2213-2600(20)30304-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang J jin, Dong X., Cao Y yuan, Yuan Y dong, Yang Y bin, Yan Y qin. Clinical characteristics of 140 patients infected with SARS-CoV-2 in Wuhan, China. Allergy Eur J Allergy Clin Immunol. 2020;75:1730–1741. doi: 10.1111/all.14238. [DOI] [PubMed] [Google Scholar]
- 8.Grier A., McDavid A., Wang B., Qiu X., Java J., Bandyopadhyay S. Neonatal gut and respiratory microbiota: coordinated development through time and space. Microbiome. 2018;6:193. doi: 10.1186/s40168-018-0566-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Madan J.C., Koestle D.C., Stanton B.A., Davidson L., Moulton L.A., Housman M.L. Serial analysis of the gut and respiratory microbiome in cystic fibrosis in infancy: interaction between intestinal and respiratory tracts and impact of nutritional exposures. MBio. 2012;3 doi: 10.1128/mBio.00251-12. e00251-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu T., Yang Z., Zhang X., Han N., Yuan J., Cheng Y. 16S rDNA analysis of the effect of fecal microbiota transplantation on pulmonary and intestinal flora. 3 Biotech. 2017;7:370. doi: 10.1007/s13205-017-0997-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Enaud R., Prevel R., Ciarlo E., Beaufils F., Wieërs G., Guery B. The gut-lung axis in health and respiratory diseases: a place for inter-organ and inter-kingdom crosstalks. Front Cell Infect Microbiol. 2020;10 doi: 10.3389/fcimb.2020.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dang A.T., Marsland B.J. Microbes, metabolites, and the gut–lung axis. Mucosal Immunol. 2019;12:843–850. doi: 10.1038/s41385-019-0160-6. [DOI] [PubMed] [Google Scholar]
- 13.Openshaw P.J. Crossing barriers: infections of the lung and the gut. Mucosal Immunol. 2009;2:100–102. doi: 10.1038/mi.2008.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kanauchi O., Andoh A., AbuBakar S., Yamamoto N. Probiotics and paraprobiotics in viral infection: clinical application and effects on the innate and acquired immune systems. Curr Pharm Des. 2018;24:710–717. doi: 10.2174/1381612824666180116163411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tiwari S.K., Dicks L.M.T., Popov I.V., Karaseva A., Ermakov A.M., Suvorov A. Probiotics at war against viruses: what is missing from the picture? Front Microbiol. 2020;11 doi: 10.3389/fmicb.2020.01877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Anand S., Mande S.S. Diet, microbiota and gut–lung connection. Front Microbiol. 2018;9 doi: 10.3389/fmicb.2018.02147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Long J.D., Morris A. Probiotics in preventing acute upper respiratory tract infections. Am J Nurs. 2017;117:69. doi: 10.1097/01.NAJ.0000527494.16987.1e. [DOI] [PubMed] [Google Scholar]
- 18.Tapiovaara L., Lehtoranta L., Poussa T., Mäkivuokko H., Korpela R., Pitkäranta A. Absence of adverse events in healthy individuals using probiotics – analysis of six randomised studies by one study group. Benef Microbe. 2016;7:161–169. doi: 10.3920/BM2015.0096. [DOI] [PubMed] [Google Scholar]
- 19.Pimentel-Nunes P., Soares J.B., Roncon-Albuquerque R., Dinis-Ribeiro M., Leite-Moreira A.F. Toll-like receptors as therapeutic targets in gastrointestinal diseases. Expert Opin Ther Targets. 2010;14:347–368. doi: 10.1517/14728221003642027. [DOI] [PubMed] [Google Scholar]
- 20.Shibata T., Kanayama M., Haida M., Fujimoto S., Oroguchi T., Sata K. Lactococcus lactis JCM5805 activates anti-viral immunity and reduces symptoms of common cold and influenza in healthy adults in a randomized controlled trial. J Funct Foods. 2016;24:492–500. doi: 10.1016/j.jff.2016.03.035. [DOI] [Google Scholar]
- 21.Gill H.S., Rutherfurd K.J., Cross M.L., Gopal P.K. Enhancement of immunity in the elderly by dietary supplementation with the probiotic Bifidobactedum lactis HN019. Am J Clin Nutr. 2001;74:833–839. doi: 10.1093/ajcn/74.6.833. [DOI] [PubMed] [Google Scholar]
- 22.Eguchi K., Fujitani N., Nakagawa H., Miyazaki T. Prevention of respiratory syncytial virus infection with probiotic lactic acid bacterium Lactobacillus gasseri SBT2055. Sci Rep. 2019;9 doi: 10.1038/s41598-019-39602-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tomosada Y., Chiba E., Zelaya H., Takahashi T., Tsukida K., Kitazawa H. Nasally administered Lactobacillus rhamnosus strains differentially modulate respiratory antiviral immune responses and induce protection against respiratory syncytial virus infection. BMC Immunol. 2013;14:40. doi: 10.1186/1471-2172-14-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Villena J., Chiba E., Tomosada Y., Salva S., Marranzino G., Kitazawa H. Orally administered Lactobacillus rhamnosus modulates the respiratory immune response triggered by the viral pathogen-associated molecular pattern poly(I:C) BMC Immunol. 2012;13:53. doi: 10.1186/1471-2172-13-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Villena J., Oliveira M.L.S., Ferreira P.C.D., Salva S., Alvarez S. Lactic acid bacteria in the prevention of pneumococcal respiratory infection: future opportunities and challenges. Int Immunopharmacol. 2011;11:1633–1645. doi: 10.1016/j.intimp.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 26.Luoto R., Ruuskanen O., Waris M., Kalliomäki M., Salminen S., Isolauri E. Prebiotic and probiotic supplementation prevents rhinovirus infections in preterm infants: a randomized, placebo-controlled trial. J Allergy Clin Immunol. 2014;133:405–413. doi: 10.1016/j.jaci.2013.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yan F., Polk D.B. Probiotics and immune health. Curr Opin Gastroenterol. 2011;27:496–501. doi: 10.1097/MOG.0b013e32834baa4d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Martin Manuel P., Elena B., Carolina M.G., Gabriela P. Oral probiotics supplementation can stimulate the immune system in a stress process. J Nutr Intermed Metab. 2017;8:29–40. doi: 10.1016/j.jnim.2017.06.001. [DOI] [Google Scholar]
- 29.Plaza-Díaz J., Ruiz-Ojeda F.J., Vilchez-Padial L.M., Gil A. Evidence of the anti-inflammatory effects of probiotics and synbiotics in intestinal chronic diseases. Nutrients. 2017;9:555. doi: 10.3390/nu9060555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oh N.S., Joung J.Y., Lee J.Y., Kim Y. Probiotic and anti-inflammatory potential of Lactobacillus rhamnosus 4B15 and Lactobacillus gasseri 4M13 isolated from infant feces. PLoS One. 2018;13:e0192021. doi: 10.1371/journal.pone.0192021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yu H.S., Lee N.K., Choi A.J., Choe J.S., Bae C.H., Paik H.D. Anti-inflammatory potential of probiotic strain Weissella cibaria JW15 isolated from kimchi through regulation of NF-κB and MAPKs pathways in LPS-induced RAW 264.7 cells. J Microbiol Biotechnol. 2019;19:1022–1032. doi: 10.4014/jmb.1903.03014. [DOI] [PubMed] [Google Scholar]
- 32.Kim S.O., Sheikh H.I., Ha S.D., Martins A., Reid G. G-CSF-mediated inhibition of JNK is a key mechanism for Lactobacillus rhamnosus-induced suppression of TNF production in macrophages. Cell Microbiol. 2006;8:1958–1971. doi: 10.1111/j.1462-5822.2006.00763.x. [DOI] [PubMed] [Google Scholar]
- 33.Ragab D., Salah Eldin H., Taeimah M., Khattab R., Salem R. The COVID-19 cytokine storm; what we know so far. Front Immunol. 2020;11:1446. doi: 10.3389/fimmu.2020.01446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sencio V., Barthelemy A., Tavares L.P., Machado M.G., Soulard D., Cuinat C. Gut dysbiosis during influenza contributes to pulmonary pneumococcal superinfection through altered short-chain fatty acid production. Cell Rep. 2020;30:2934–2947. doi: 10.1016/j.celrep.2020.02.013. [DOI] [PubMed] [Google Scholar]
- 36.Budden K.F., Gellatly S.L., Wood D.L.A., Cooper M.A., Morrison M., Hugenholtz P. Emerging pathogenic links between microbiota and the gut-lung axis. Nat Rev Microbiol. 2017;15:55–63. doi: 10.1038/nrmicro.2016.142. [DOI] [PubMed] [Google Scholar]
- 37.Xu K., Cai H., Shen Y., Ni Q., Chen Y., Hu S. Zhejiang Da Xue Xue Bao Yi Xue Ban; 2020. Management of corona virus disease-19 (COVID-19): the Zhejiang experience. 49:147–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ong J., Young B.E., Ong S. COVID-19 in gastroenterology: a clinical perspective. Gut. 2020;69:1144–1145. doi: 10.1136/gutjnl-2020-321051. [DOI] [PubMed] [Google Scholar]
- 39.Al Kassaa I., Hober D., Hamze M., Chihib N.E., Drider D. Antiviral potential of lactic acid bacteria and their bacteriocins. Probiotics Antimicrob Proteins. 2014;6:177–185. doi: 10.1007/s12602-014-9162-6. [DOI] [PubMed] [Google Scholar]
- 40.Cotter P.D., Ross R.P., Hill C. Bacteriocins – a viable alternative to antibiotics? Nat Rev Microbiol. 2013;11:95–105. doi: 10.1038/nrmicro2937. [DOI] [PubMed] [Google Scholar]
- 41.Lange-Starke A., Petereit A., Truyen U., Braun P.G., Fehlhaber K., Albert T. Antiviral potential of selected starter cultures, bacteriocins and d,l-lactic acid. Food Environ Virol. 2014;6:42–47. doi: 10.1007/s12560-013-9135-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Małaczewska J., Kaczorek-ŁUkowska E., Wójcik R., Krzysztof Siwicki A. Antiviral effects of nisin, lysozyme, lactoferrin and their mixtures against bovine viral diarrhoea virus. BMC Vet Res. 2019;15:318. doi: 10.1186/s12917-019-2067-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Starosila D., Rybalko S., Varbanetz L., Ivanskaya N., Sorokulova I. Anti-influenza activity of a Bacillus subtilis probiotic strain. Antimicrob Agents Chemother. 2017;61 doi: 10.1128/AAC.00539-17. e00539-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chowdhury T., Baindara P., Mandal S.M. LPD-12, a promising lipopeptide to control COVID-19. Int J Antimicrob Agents. 2020;57:106218. doi: 10.1016/j.ijantimicag.2020.106218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Manna S., Baindara P., Mandal S.M. Fusion protein targeted antiviral peptides: fragment based drug design (FBDD) guided rational design of dipeptides against SARS-CoV-2. Curr Protein Pept Sci. 2020;21:938–947. doi: 10.2174/1389203721666200908164641. [DOI] [PubMed] [Google Scholar]
- 46.Thaden J.T., Maskarinec S.A. When two for the price of one isn’t a bargain: estimating prevalence and microbiology of bacterial co-infections in patients with COVID-19. Clin Microbiol Infect. 2020;26:1602–1603. doi: 10.1016/j.cmi.2020.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Parrill A., Tsao T., Dong V., Huy N.T. SARS-CoV-2-induced immunodysregulation and the need for higher clinical suspicion for co-infection and secondary infection in COVID-19 patients. J Microbiol Immunol Infect. 2020 doi: 10.1016/j.jmii.2020.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sohal S., Rodriguez-Nava G., Khabbaz R., Chaudry S., Musurakis C., Chitrakar S. SARS-CoV2 and co-infections: a review of two cases. Case Rep Infect Dis. 2020;2020:1–4. doi: 10.1155/2020/8882348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Manna S., Baindara P., Mandal S.M. Molecular pathogenesis of secondary bacterial infection associated to viral infections including SARS-CoV-2. J Infect Public Health. 2020;13:1397–1404. doi: 10.1016/j.jiph.2020.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Langford B.J., So M., Raybardhan S., Leung V., Westwood D., MacFadden D.R. Bacterial co-infection and secondary infection in patients with COVID-19: a living rapid review and meta-analysis. Clin Microbiol Infect. 2020;26:1622–1629. doi: 10.1016/j.cmi.2020.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ripa M., Galli L., Poli A., Oltolini C., Spagnuolo V., Mastrangelo A. Secondary infections in patients hospitalized with COVID-19: incidence and predictive factors. Clin Microbiol Infect. 2020 doi: 10.1016/j.cmi.2020.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kimmig L.M., Wu D., Gold M., Pettit N.N., Pitrak D., Mueller J. IL-6 inhibition in critically ill COVID-19 patients is associated with increased secondary infections. Front Med. 2020;7 doi: 10.3389/fmed.2020.583897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang H., Zhang Y., Wu J., Li Y., Zhou X., Li X. Risks and features of secondary infections in severe and critical ill COVID-19 patients. Emerg Microbe. Infect. 2020;9:1958–1964. doi: 10.1080/22221751.2020.1812437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zeng J., Wang C.T., Zhang F.S., Qi F., Wang S.F., Ma S. Effect of probiotics on the incidence of ventilator-associated pneumonia in critically ill patients: a randomized controlled multicenter trial. Intensive Care Med. 2016;42:1018–1028. doi: 10.1007/s00134-016-4303-x. [DOI] [PubMed] [Google Scholar]
- 55.Morrow L.E., Kollef M.H., Casale T.B. Probiotic prophylaxis of ventilator-associated pneumonia: a blinded, randomized, controlled trial. Am J Respir Crit Care Med. 2010;182:1058–1064. doi: 10.1164/rccm.200912-1853OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wells J.M., Mercenier A. Mucosal delivery of therapeutic and prophylactic molecules using lactic acid bacteria. Nat Rev Microbiol. 2008;6:349–362. doi: 10.1038/nrmicro1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yu J.Y., Young T.L., Eun J.K., Ji H.J., Min K.P., Cheol H.K. Novel mechanisms of conferring broad protection against influenza by Lactobacillus casei lactic acid bacteria by mobilizing innate and adaptive immune cells (INC1P.354) J Immunol. 2015;194 [Google Scholar]
- 58.Jung Y.J., Lee Y.T., Ngo V Le, Cho Y.H., Ko E.J., Hong S.M. Heat-killed Lactobacillus casei confers broad protection against influenza A virus primary infection and develops heterosubtypic immunity against future secondary infection. Sci Rep. 2017;7:17360. doi: 10.1038/s41598-017-17487-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bidossi A., De Grandi R., Toscano M., Bottagisio M., De Vecchi E., Gelardi M. Probiotics Streptococcus salivarius 24SMB and Streptococcus oralis 89a interfere with biofilm formation of pathogens of the upper respiratory tract. BMC Infect Dis. 2018;18:653. doi: 10.1186/s12879-018-3576-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Santagati M., Scillato M., Patanè F., Aiello C., Stefani S. Bacteriocin-producing oral streptococci and inhibition of respiratory pathogens. FEMS Immunol Med Microbiol. 2012;65:23–31. doi: 10.1111/j.1574-695X.2012.00928.x. [DOI] [PubMed] [Google Scholar]
- 61.Waki N., Matsumoto M., Fukui Y., Suganuma H. Effects of probiotic Lactobacillus brevis KB290 on incidence of influenza infection among schoolchildren: an open-label pilot study. Lett Appl Microbiol. 2014;59:565–571. doi: 10.1111/lam.12340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kinoshita T., Maruyama K., Suyama K., Nishijima M., Akamatsu K., Jogamoto A. The effects of OLL1073R-1 yogurt intake on influenza incidence and immunological markers among women healthcare workers: a randomized controlled trial. Food Funct. 2019;10:8129–8136. doi: 10.1039/c9fo02128k. [DOI] [PubMed] [Google Scholar]
- 63.Jacobs S.E., Lamson D.M., Kirsten S., Walsh T.J. Human rhinoviruses. Clin Microbiol Rev. 2013;26:135–162. doi: 10.1128/CMR.00077-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tapiovaara L., Kumpu M., Mäkivuokko H., Waris M., Korpela R., Pitkäranta A. Human rhinovirus in experimental infection after peroral Lactobacillus rhamnosus GG consumption, a pilot study. Int Forum Allergy Rhinol. 2016;6:848–853. doi: 10.1002/alr.21748. [DOI] [PubMed] [Google Scholar]
- 65.Kumpu M., Kekkonen R.A., Korpela R., Tynkkynen S., Järvenpää S., Kautiainen H. Effect of live and inactivated Lactobacillus rhamnosus GG on experimentally induced rhinovirus colds: randomised, double blind, placebo-controlled pilot trial. Benef Microbe. 2015;6:631–639. doi: 10.3920/BM2014.0164. [DOI] [PubMed] [Google Scholar]
- 66.Kumpu M., Lehtoranta L., Roivainen M., Rönkkö E., Ziegler T., Söderlund-Venermo M. The use of the probiotic Lactobacillus rhamnosus GG and viral findings in the nasopharynx of children attending day care. J Med Virol. 2013;85:1632–1638. doi: 10.1002/jmv.23623. [DOI] [PubMed] [Google Scholar]
- 67.Guillemard E., Tondu F., Lacoin F., Schrezenmeir J. Consumption of a fermented dairy product containing the probiotic Lactobacillus casei DN-114001 reduces the duration of respiratory infections in the elderly in a randomised controlled trial. Br J Nutr. 2010;103:58–68. doi: 10.1017/S0007114509991395. [DOI] [PubMed] [Google Scholar]
- 68.Song J.A., Kim H.J., Hong S.K., Lee D.H., Lee S.W., Song C.S. Oral intake of Lactobacillus rhamnosus M21 enhances the survival rate of mice lethally infected with influenza virus. J Microbiol Immunol Infect. 2016;49:16–23. doi: 10.1016/j.jmii.2014.07.011. [DOI] [PubMed] [Google Scholar]
- 69.Wang X., Hu W., Zhu L., Yang Q. Bacillus subtilis and surfactin inhibit the transmissible gastroenteritis virus from entering the intestinal epithelial cells. Biosci Rep. 2017;37 doi: 10.1042/BSR20170082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Turner R.B., Woodfolk J.A., Borish L., Steinke J.W., Patrie J.T., Muehling L.M. Effect of probiotic on innate inflammatory response and viral shedding in experimental rhinovirus infection – a randomised controlled trial. Benef Microbe. 2017;8:207–215. doi: 10.3920/BM2016.0160. [DOI] [PMC free article] [PubMed] [Google Scholar]