Abstract
P. aeruginosa causes mostly both community-acquired and nosocomial infections, which leads to serious therapeutic challenges for treatment and requirement of appropriate therapeutic agent is needed which can combat antibiotic resistance. The research work was performed to investigate the effect of Zinc Oxide nanoparticles (ZnO NPs) in combination with Meropenem, Ciprofloxacin, and Colistin against clinical isolated strains of P. aeruginosa and ATCC 27853 strain.
The minimum inhibitory concentration (MIC) of ZnO NPs and the antibiotics (Meropenem, Ciprofloxacin, and Colistin), was determined by the microdilution method and the results of MIC values were ranging between 1 and 16 µg/mL was found to be shown for antibiotics and ZnO NPs found to showed highest MIC values ranging from 2000 to 4000 µg/mL. The fractional inhibitory concentration index (FICI) was calculated using checkerboard method to test the combinations of ZnO NPs and the antibiotics (Meropenem, Ciprofloxacin, and Colistin), and among all the six P. aeruginosa clinical isolated strains P. aeruginosa (MRO-16-3 and MRO-16-4), showed FICI as 0.24 and 0.39 9, whereas P. aeruginosa ATCC 27853 strain showed FICI as 0.41 which indicates synergistic effect with Colistin.
The time kill growth curve showed synergistic effect for the combination of Colistin and ZnO NPs against P. aeruginosa (MRO-16-3 and MRO-16-) strains. P. aeruginosa (MRO-16-3) was found to be highly sensitive to Colistin with an MIC of 2 µg/mL, which has shown to reduced bacterial growth to zero colonies after 24 h of incubation.
In conclusion, combination of Colistin and ZnO NPs at appropriate dosage intervals might be beneficial as using therapeutic agent in treatment of P. aeruginosa ailments.
Keywords: ZnO NPs, Meropenem, Ciprofloxacin, Colistin, P. aeruginosa
Abbreviations: ZnO NPs, Zinc Oxide nanoparticles; MIC, Minimum inhibitory concentration; FICI, Fractional inhibitory concentration index; ATCC, American Type Culture Collection; CLSI, Clinical Laboratory Standards Institute; DMSO, Dimethyl sulfoxide; MHB, Muller-Hinton Broth; KKUH, King Khalid University hospital
1. Introduction
P. aeruginosa (Pseudomonas aeruginosa) causes most of the hospital and community born infections which causes a serious therapeutic challenges for treatment of both community-acquired and nosocomial infections, it is essential to select and prepare appropriate therapy against resistant strains of P. aeruginosa to optimize the clinical outcome (Bisbe et al., 1988, Micek et al., 2005). The main mechanisms to counter antibiotic attack was mainly classified into intrinsic, acquired and adaptive resistance for P. aeruginosa, whereas intrinsic resistance of P. aeruginosa includes less outer membrane permeability, efflux pumps that expel antibiotics out of the cell and the production of antibiotic deactivating enzymes, acquired resistance of P. aeruginosa could be achieved by either horizontal transfer of resistance genes or may be due to mutational changes and the adaptive resistance of P. aeruginosa involves in formation of biofilm in infected patients lungs where the biofilm act as a diffusion barrier to limit antibiotic contact to the bacterial cells (Drenkard, 2003, Breidenstein et al., 2011).
In the previous study, high level of intrinsic resistance to many antibiotics was found in P. aeruginosa through restricted outer membrane permeability, efflux systems which pumps out antibiotics from the cell and production of antibiotic-inactivating enzymes such as β-lactamases (Breidenstein et al., 2011) whereas, P. aeruginosa possesses an inducible ampC gene that encodes the hydrolytic enzyme β-lactamase which breaks the amide bond in the β-lactam ring, leading to deactivation of β-lactam antibiotics (Wright, 2005). Moreover, the cells which are multidrug-tolerant are able to survive antibiotic attack can form biofilm and these cells are responsible for prolonged and frequent infections in CF patients (Mulcahy et al., 2010).
Nanotechnology represents an innovative approach to develop new formulations based upon the metallic nanoparticles antimicrobial properties, combination of antibiotics and antibiotics. Interactions of antibiotics with nanoparticles is the most common among studies dedicated to the testing of combined action of nanoparticles with antibiotics and moreover some studies have found that the efficacy of antimicrobial agents can be enhanced by combining them with nanoparticles against different pathogens, including Staphylococcus aureus, Pseudomonas aeruginosa, Escherichia coli, etc. As therapeutic roles for zinc in different diseases have been established in recent years and Zinc oxide had been found to be very good potential in the clinic (Shopsin et al., 1999). Previous studies have revealed improved activity of ZnO nanoparticle when used in combination with cephalosporins, beta lactums and amino glycosides against different pathogenic microorganisms (Gaddad et al., 2010, Solomon et al., 2007).
Based on the resistance and recurrent infections of P. aeruginosa, development of alternative therapeutic agent for treatment P. aeruginosa infections is urgently required for the patients whose infections are resistant to many conventional antibiotics. The aim of our present research work is to study the effects of combination of selected antibiotics (Colistin, Ciprofloxacin, Meropenem) and Zinc oxides Nanoparticles (ZnO NPs) against P. aeruginosa clinically isolated bacterial strains and P. aeruginosa ATCC 27853 strain.
2. Materials and methods
2.1. Clinical samples collection and isolation of Pseudomonas aeruginosa strains
All the clinical samples have been collected from department of Microbiology, King Khalid University hospital (KKUH), King Saud University, Riyadh. The different clinical samples as Respiratory/Sputum, blood, urine, abdominal drainage and body fluid have been collected in sterile bottles and shifted to the laboratory for the isolation of P. aeruginosa bacterial strains. Reference bacterial strains, American Type Culture Collection (ATCC) P. aeruginosa ATCC 27853, were used for quality control in all procedures and assays of the study, ensuring that the MIC values of reference strains were in the acceptable range according to Clinical Laboratory Standards Institute (CLSI) guidelines (CLSI, 2018).
The clinical samples were collected between 2016 and 2018 from different body sites of patients. The clinical isolates that we were able to identify their sites are shown in (Table 1). They were routinely cultured and identified by conventional laboratory techniques based on colony morphology and lactose fermentation on MacConkey agar plates, and by the Micro Scan Walk-Away® system (Beckman Coulter Inc.) by KKUH. Clinical isolates of P. aeruginosa strains which was found to be resistance and showed elevated MIC were kept for further investigation by stored at −80 °C in skim milk media (Oxoid Ltd, Hampshire, UK), with glycerol.
Table 1.
The isolates codes and their isolation sites.
| Site of isolates | Number of isolates | Isolate serial number |
|---|---|---|
| Respiratory/sputum | 1 | P. aeruginosa (MRO-16-3) |
| Blood | 1 | P. aeruginosa (MRO-16-7) |
| Urine | 1 | P. aeruginosa (MRO-17-3) |
| Abdominal drainage | 1 | P. aeruginosa (MRO-17-29) |
| Body fluid | 1 | P. aeruginosa (MRO-16-1) |
| N/A | 1 | P. aeruginosa (MRO-16-4) |
(N/A) not been identified.
2.2. Bacterial suspension preparation
All the work was carried out at the KKUH in Riyadh. Reference bacterial clinical isolated strains and P. aeruginosa ATCC 27853 strain, were used for quality control in all procedures and assays of the study, ensuring that the MIC values of reference strains were in the acceptable range according to Clinical Laboratory Standards Institute (CLSI) guidelines (CLSI, 2018). Positive and negative controls were also used for each experiment. The positive control was used to ensure good growth conditions for the bacteria. And the negative control to ensure the sterility of the media (Microbiology and Diseases, 2003).
2.3. Antibacterial agent and ZnO NPs preparation
Pure powdered antibiotics, namely Meropenem trihydrate ≥ 98.0% (HPLC) (Sigma– Aldrich, St. Louis, USA), Ciprofloxacin ≥ 98.0% (HPLC) (Sigma–Aldrich, St. Louis, USA), Colistin sulphate salt ≥ 15000 U/mg (Sigma–Aldrich, St. Louis, USA), and ZnO NPs (50 nm diameter) (Sigma–Aldrich, St. Louis, USA), were all used as antibacterial agents. To prepare antibiotic stock solutions, these powders were accurately weighed and dissolved in the appropriate diluents to yield the required concentrations.
Antibiotic stock solution was prepared for Meropenem trihydrate by dissolving 1.6 mg of the powdered antibiotic in 1 mL Dimethyl sulfoxide (DMSO) as a solvent, to get a concentration of 1.6 mg/mL, Ciprofloxacin stock solution was prepared by dissolving 1.6 mg of the powdered antibiotic in 1 mL 0.1 N HCL as an aqueous acidic solvent and aqueous solution of Colistin was prepared by dissolving 3.2 mg of the powdered antibiotic in 1 mL sterile distilled water, to get a concentration of 3.2 mg/mL. Aliquots of 100 μL were saved at −20 °C and thawed when needed.
The ZnO/water suspension was prepared immediately before each experiment. After weighing the powdered nanoparticles, 16 mg of the powder was added to a tube containing 1 mL of sterile distilled water the resulting suspension was subjected to vigorous vortex before each use (Ghasemi and Jalal, 2016, Xie et al., 2011).
2.4. Minimum inhibitory concentration (MIC)
For each bacterial sample, the broth microdilution method was used to determine the MIC. We used Costar 96-well Polystyrene Cell Culture Cluster with flat bottom plate (Corning Inc., Corning, N.Y.). Then, antibiotic susceptibility test were determined by MIC value breakpoints (CLSI, 2018).
To prepare the broth microdilution method, 100 μL MHB was added to the microtiter plate, from column two to column twelve; the first column was left empty. Then, the antibiotic stock solution, previously prepared was taken out of the –20 °C storage, and thawed. After thawing, this stock solution was diluted 1/100 in a separate tube containing MHB, with 20 μL of the stock solution added to 1980 μL MHB to get a concentration of 16 μg/mL. Then, 200 μL of this dilution was added to all the wells of the first column in the microtiter plate, and double diluted by taking 100 μL from the first column to the second one using the multichannel pipette, using new tips for each dilution step until reaching the tenth column. The mixing for each step was done separately using the multichannel pipette. Finally, 10 μL of the bacterial suspension, was prepared and added to the whole plate except column number twelve. Column twelve was the negative control, and contained only 100 μL MHB. Column eleven was used as a positive control (CLSI, 2012). MIC was determined by comparing the growth of test bacteria in the wells with the positive and negative controls.
2.5. Checkerboard method
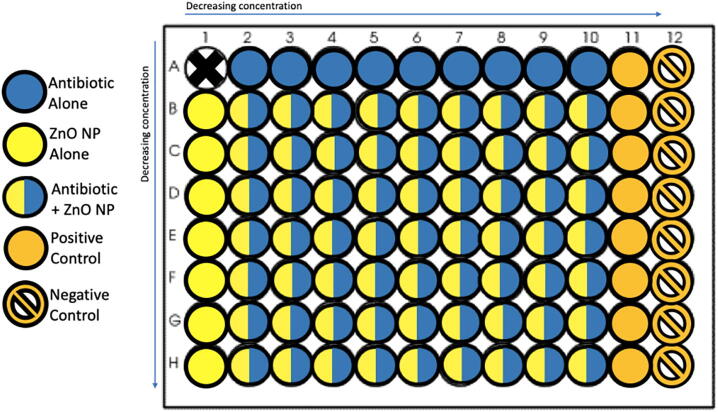
This method is used to assess the interaction between the antibiotic of choice, Meropenem, Ciprofloxacin, or Colistin, and the ZnO NPs. Two-dimensional checkerboard titrations were performed using the broth microdilution method, with the antibiotic having decreasing concentration in the horizontal direction, and the ZnO NPs having decreasing concentration in the vertical direction (Fig. 1).
Fig. 1.
The microtiter plate of the checkerboard method.
To prepare the checkerboard plate, first, the antibiotic stock solution, previously prepared was taken out of the –20 °C storage, and thawed. After thawing, this stock solution was diluted 1/100 in a separate tube containing MHB, with 40 μL of the stock solution added to 3960 μL MHB to get a concentration of 16 μg/mL. Then, eight tubes were arranged in a rack and each was filled with 2 mL MHB. The first prepared antibiotic concentration (16 μg/mL) was double diluted by placing 2 mL of it in one of the tubes containing 2 mL MHB, making a concentration of 8 μg/mL, and this process was repeated for each further tube until reaching a final concentration of 0.06 μg/mL. Next, 100 μL of the first concentration (16 μg/mL) was added to all the wells in column two of a microtiter plate, except the well in the top row (row A). This process was repeated for the rest of the dilutions, moving across the columns from the highest to the lowest concentration, until column number ten. Then, the wells in row A, which had been left empty, were filled with 200 μL of the antibiotic dilutions, starting from the 2A well containing the first concentration (16 μg/mL), until column number ten, with well 1A remaining empty. Columns eleven and twelve were used as positive and negative controls respectively. The positive control contained 200 μL MHB and the bacterial suspension, while the negative control contained only 200 μL liquid media (MHB).
For the ZnO NP suspension, the following steps were carried out: First, seven tubes were arranged in a rack and filled with 1 mL sterile water. Second, 1 mL of the stock ZnO/water suspension with a concentration depending on the MIC of the organism being tested, was added to the first tube and double diluted in water reaching the seventh tube. Third, 1 mL MHB was added to each tube to support the growth of the bacteria. This gave us seven tubes containing 2 mL of decreasing concentrations of ZnO NP suspension in MHB. The resulting concentration was 4 mg/mL for the first tube, or lower, depending on the MIC of the organism being tested. Next, using the same microtiter plate that already contained the antibiotic solutions, the wells in columns two to ten of the second row (row B) were filled with 100 μL from the concentration of the first tube prepared; the well in column one of that row was filled with 200 μL of the ZnO NP suspension. The same process was followed for the second concentration, filling the next row, and so on until the seventh and final concentration. The final microtiter plate contained a decreasing concentration of the antibiotic in the horizontal direction against a decreasing concentration of the ZnO NPs in the vertical direction, with well 1A remaining empty. Finally, 20 μL of the bacterial suspension prepared was added to the whole plate except column number twelve, which was the negative control. Mixing was done separately for each step, using the multichannel pipette with new tips for each step.
The plate was incubated overnight at 37 °C in a 5% CO2 incubator. Then, MIC for the antibacterial agents were determined by comparing the growth of test bacteria in the wells with the positive and negative controls. The optical density (OD) of the microplate was read before and after incubation using a microplate spectrophotometer (SpectraMax Plus 348) from Molecular Devices at a wavelength equal to 600 nm, and was used for recording. This method was performed for each bacterial sample as three independent experiments on three separate days.
To determine the correlation between the two drugs, FICI was calculated using the equation:
where, FICAb = (MIC of Ab in the presence of NP)/ (MIC of Ab alone)
and FICNP = (MIC of NP in the presence of Ab)/ (MIC of NP alone).
Then, the FICI value was interpreted following the interpretation ranges.
If the result of the FICI is less than or equal to 0.5, it is considered as synergy; if it is >4.0 it is considered as antagonism; if it is between > 0.5 and ≤ 1.0 it is an additive result, and if it is between > 1 and ≤ 4.0, it is considered as indifference. In addition, the microtiter plate was incubated for 16 h in the SpectraMax machine, which automatically read the OD every five minutes, and a growth curve was plotted with the SoftMax Pro Software to show the microbial growth pattern (moleculardevices, 2016).
2.6. Time-kill method
The time-kill method was used to evaluate the bactericidal activity of selected antibiotics Meropenem, Ciprofloxacin, Colistin, and ZnO NPFirst, the antibiotics MIC for the tested strain was determined as described in Section 2.4. Second, the stock solution of the antibiotics of choice was prepared as described in Section 2.3, was diluted to a concentration equal to four times the MIC (4 × MIC) for the tested bacterial strain, as determined earlier in 1 mL MHB. Third, serial double dilutions were done in separate tubes containing 0.5 mL MHB, by adding 0.5 mL of the previous concentration to the next tube, to get concentrations equal to double the MIC (2 × MIC) and the MIC concentration of the antibiotic for the tested strain. Next, The microtiter plate for the time-kill method was prepared, where three wells from the first row (row A) of the 96- well microtiter plate were each filled with 200 μL of the decreasing concentrations of the antibiotic, starting with (4 × MIC), then (2 × MIC), and finally (MIC) concentration. In the same way, three wells from the second row (row B) were also filled with 100 μL of antibiotic concentration (4 × MIC, 2 × MIC, and MIC). In some cases, half the MIC and quarter the MIC (0.5 × MIC, 0.25 × MIC) were also added. Like in the case of highly resistant isolates that had a very high MIC value, using 4 × MIC, and 2 × MIC was not possible because of the possibility of a toxic effect to occur. Also, in the case where the MIC value was equal to the MBC value, half the MIC and quarter the MIC (0.5 × MIC, 0.25 × MIC) were also added.
For the ZnO NP suspension, the following steps were carried out: First, 1 mL of the stock ZnO/water suspension was prepared as described in Section 2.3., with a concentration equal to (8 × MIC) for the tested organism. Then, serial double dilution was done in separate tubes. To do this, four tubes were arranged in a rack and each filled with 0.5 mL sterile water; the first concentration (8 × MIC) was double diluted by taking 0.5 mL from it and adding this to the next tube, with this process being repeated until reaching a concentration of (MIC). Then, 0.5 mL MHB was added to each of these tubes to support the bacterial growth,which gave four tubes containing 1 mL of decreasing concentrations of ZnO NP suspension in MHB, starting with a concentration equal to four times the MIC (4 × MIC), then double the MIC (2 × MIC), MIC concentration (MIC), and half the MIC (0.5 × MIC) for each tested strain. Next, using the same microtiter plate as for the antibiotic solution, three wells of the second row (row B), which already contained 100 μL antibiotic solution, were filled with 100 μL of (0.5 × MIC) ZnO NP suspension prepared. Then three wells of the third row (row C) were each filled with 200 μL of decreasing concentrations of the ZnO NPs suspension prepared. This resulted in the second row (row B) having different combinations of antibiotic and (0.5 × MIC) of nanoparticle suspension, with a final volume of 200 μL. Additionally, one well was used as a positive control, with 200 μL of the MHB and the bacterial suspension, while another well was used as a negative control, with 200 μL of the MHB only. All the wells except the negative control were inoculated with 20 μL of the prepared bacterial suspension as described in Section 2.2. Finally, the 96-well plate was incubated at 37 °C in a 5% CO2 incubator.
To count the colonies, 10 μL was aspirated from each well at different time intervals, namely after 0, 2, 4, 8, and 24 h of incubation, and diluted 1:10 to 1:10−4 in PBS buffer as required. Then, the diluted samples were streaked in four directions on separate blood agar plates. The streaked plates were incubated for 24 h in a CO2 incubator at 37 °C. After incubation, colonies were counted and recorded in an Excel sheet to plot the time-kill curve as a semi-log plot using Microsoft Excel. To plot the graph, the colony counts as a Log10 of the CFU/mL were plotted on the Y axis in the logarithmic scale, while the time was plotted on the X axis in arithmetic scale.
3. Results and discussion
3.1. Minimum inhibitory concentration of selected antibiotics and ZnO NPs against P. aeruginosa clinical isolates and ATCC 2785 strains
In our present study, the minimum inhibitory concentration of three selected antibiotics Meropenem, Ciprofloxacin, Colistin and ZnO NPs were tested against six clinical isolated P. aeruginosa strains and P. aeruginosa ATCC 2785 strain.
The MIC for Colistin was found to be very effective on four clinical isolated P. aeruginosa strains, whereas P. aeruginosa (MRO-16-1) isolated from body fluids (Table 1) and P. aeruginosa ATCC 2785 showed high sensitivity with MIC value 1 μg/mL with SD ± 1.4 and ± 0. Moreover, P. aeruginosa (MRO-16-3) isolated from respiratory/ Sputum samples and P. aeruginosa (MRO-16-l7) isolated from blood samples also showed good sensitivity with MIC value 2 μg/mL with SD ± 1.1 (Table 2). In correlation with the other research work, our sensitivity MIC values were found to be closely related to their sensitive range MIC values (Malekzadegan et al., 2019). One strain P. aeruginosa (MRO-16-4) found to be resistant with MIC value 4 μg/mL. As reported in earlier study, Colistin is a multicomponent polypeptide antibiotic and relatively old polymyxin antibiotic [Li et al.,2002], it was found to be mainly directed against the bacterial cell membrane results in an increase in the permeability of the cell membrane, leakage of cell contents, and ultimately cell death [Gupta et al., 2009].
Table 2.
Minimum Inhibitory Concentration (MIC) of antibiotics and ZnO NPs.
| Organism | Antibiotic μg/mL |
|||
|---|---|---|---|---|
| Meropenem | Ciprofloxacin | Colistin | ZnO NPs | |
| P. aeruginosa ATCC2785 | 0.6 ± 0.2 | N/T | 1 ± 0 | 2000 ± 0 |
| P. aeruginosa MRO-17-29 | 16 ± 0 | N/T | N/T | 4000 ± 0 |
| P. aeruginosa MRO-17-3 | N/T | 3.33 ± 1.15 | N/T | 4000 ± 0 |
| P. aeruginosa MRO-16-3 | N/T | N/T | 2 (S) ± 1.1 | 2660 ± 1 |
| P. aeruginosa MRO-16-4 | N/T | N/T | 4 (R) ± 0 | 2000 ± 0 |
| P. aeruginosa MRO-16-1 | N/T | N/T | 1 (S) ± 1.4 | 2660 ± 0.86 |
| P. aeruginosa MRO-16-7 | N/T | N/T | 2 (S) ± 1.1 | 2000 ± 1 |
(R) Resistant, (S) Sensitive, (I) Intermediate, (N/T) not been tested.
*MIC value breakpoints for Pseudomonas aeruginosa : Meropenem (S ≤ 2, I = 4, R ≥ 8), Ciprofloxacin (S ≤ 1, I = 2, R ≥ 4), and Colistin (S ≤ 2, R ≥ 4) (CLSI, 2018).
The antibiotics Meropenem was found to be very effective on P. aeruginosa ATCC 2785 with MIC value 0.6 μg/mL with SD ± 0.2 and P. aeruginosa (MRO-17-29) the clinical isolated strain was found to be resistant with MIC value 16 μg/ml with SD ± 0. Whereas, the tested P. aeruginosa (MRO-17-3) strain isolated from urine sample was found to be intermediate susceptible to ciprofloxacin antibiotic with MIC value 3.33 μg/mL with SD ± 1.15.
The MIC of ZnO NPs against P. aeruginosa ATCC2785, P. aeruginosa (MRO-16-4) and P. aeruginosa (MRO-16-7) strains was found to be 2000 μg/mL with SD ± 0 and ± 1 and in contrast other two clinical isolated strains P. aeruginosa (MRO-17-29 and MRO-17-3) showed highest MIC value 4000 μg/mL with SD ± 0 (Table 2).
As it is generally known, zinc oxide nanoparticles are antibacterial and could inhibit the growth of microorganisms by permeating into the cell membrane and may cause oxidative stress, damages lipids, carbohydrates, proteins, and DNA [Kelly et al., 1998], lipid peroxidation causes modification in cell membrane which finally disrupt vital cellular functions [Rikans and Hornbrook, 1997] as it has been reported in previous research study that gram negative bacteria supports oxidative stress mechanism involves in zinc oxide nanoparticle antibacterial activity [Zhang et al., 2007].
3.2. Fractional inhibitory concentration index (FICI) of ZnO NPs in combination with meropenem, ciprofloxacin, and colistin
In our present study, the effect of ZnO NPs suspension and selected antibiotics (Meropenem, Ciprofloxacin, and Colistin) were assessed using the checkerboard assay and FIC index was calculated by evaluating the degree of interaction between ZnO NPs and the selected antibiotics against clinical isolated P. aeruginosa strains and P. aeruginosa ATCC 27853 strain (Table 3).
Table 3.
Fractional Inhibitory Concentration Index (FICI) for the combination of ZnO NPs with Meropenem, Ciprofloxacin, and Colistin.
| Organism | FICI values |
||
|---|---|---|---|
| Meropenem | Ciprofloxacin | Colistin | |
| P. aeruginosa ATCC 27853 | 1.1 (I) ± 0.2 | N/T | 0.41 (S) ± 0.09 |
| P. aeruginosa MRO-17-29 | 1.8 (I) ± 0.2 | N/T | N/T |
| P. aeruginosa MRO-17-3 | N/T | 0.76 (A) ± 0.2 | N/T |
| P. aeruginosa MRO-16-3 | N/T | N/T | 0.24 (S) ± 0.04 |
| P. aeruginosa MRO-16-4 | N/T | N/T | 0.39 (S) ± 0.1 |
| P. aeruginosa MRO-16-1 | N/T | N/T | 0.64 (A) ± 0.4 |
| P. aeruginosa MRO-16-7 | N/T | N/T | 0.55 (A) ± 0.01 |
(S) Synergy, (A) Additive, (I) Indifference, (N/T) not been tested.
*FICI value interpretation: Synergy ≤ 0.5, additive > 0.5 and ≤ 1.0, Indifference > 1 and ≤ 4.0 and Antagonism > 4.0.
Among the three selected antibiotics used in the study, Colistin found to showed synergistic interaction with ZnO NPs against P. aeruginosa ATCC 27853 and clinical isolates P. aeruginosa (MRO-16-3) and P. aeruginosa (MRO-16-4) with FICI values 0.41 with SD ± 0.09, 0.24 with SD ± 0.04 and 0.39 with SD ± 0.1 which indicates synergistic effect, as reported in the previous study that, Colistin has excellent bactericidal activity against most gram-negative bacilli (Balaji et al.,2011), whereas P. aeruginosa (MRO-16-1 and MRO-16-7) strains showed additive effect with FICI values 0.64 with SD ± 0.4 and 0.55 with SD ± 0.01 (Table 3). As reported in previous research work, Colistin is a cationic polypeptide antibiotic that directly affect the bacterial outer membrane lipid bilayer (Dupuy et al., 2018), and in another latter study have suggested a synergetic effect of Colistin together with conventional and unconventional drugs against MDR bacteria (Vidaillac et al., 2012), where the ability of Colistin to disturb outer membrane permeability facilitates the accumulation of the other drug inside the bacterial cell (Gordon et al., 2010).
The combination of Ciprofloxacin and ZnO NPs showed Additive interaction with FICI value 0.76 with SD ± 0.2 against P. aeruginosa (MRO-17-3) strain (Table 3). Our study results in correlation with the previous study, where they showed combination of ZnO nanoparticles with ciprofloxacin against the ATCC and clinical isolated strains of P. aeruginosa was found to showed partial synergism and they accomplish that it could be possible that Zn2+ ions released from the surface of ZnO NPs may form complexes with ciprofloxacin that may increases its antibacterial activity more over ZnO NPs could also inhibit the efflux transporters which leads to rise in the efficacy of antibiotics against P. aeruginosa (Bayroodi and Jalal, 2016).
Meropenem and ZnO NPs showed indifference effect on P. aeruginosa (MRO-17-29) and P. aeruginosa ATCC 27853. As meropenem found to work binding to Penicillin-binding proteins that are involved in the cell wall synthesis of Gram-negative and Gram-positive bacteria as reported in the previous studies resistance to this antibiotic may result from altered penicillin-binding proteins, enzyme production, membrane impermeability or missing outer membrane protein, as in the case of some P. aeruginosa isolates that lack some outer membrane proteins (Wiseman et al., 1995).
3.3. Effects of Colistin, ZnO NPs alone and in combination of both against P. aeruginosa clinical isolates and P. aeruginosa ATCC 27853
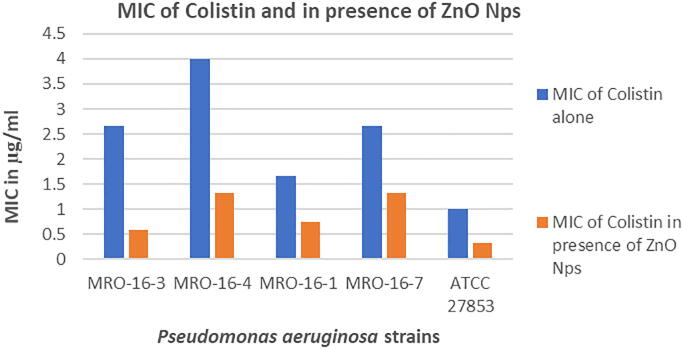
In our present research study, the Colistin and ZnO NPs combination was found to be very effective against the four clinical isolated strains and P. aeruginosa ATCC27853 strains. The MIC of colistin in combination with ZnO NPs was found to be very effective against P. aeruginosa ATCC 27853, P. aeruginosa (MRO-16-3) and P. aeruginosa (MRO-16-1) strains with MIC values 0.33, 0.58 and 0.75 μg/mL.
Whereas, P. aeruginosa (MRO-16-4) and P. aeruginosa (MRO-16-7) strains also found to be sensitive with similar MIC value 1.33 μg/mL. The MIC of colistin alone was not found to be much effective as in comparison with the MIC of colistin in combination with ZnO NPs, but only two strains P. aeruginosa (MRO-16-1) and P. aeruginosa ATCC 27853 was found to be sensitive (Fig. 2).
Fig. 2.
Column chart showing the effect of colistin alone and in the presence of ZnO NPs against P. aeruginosa isolates.
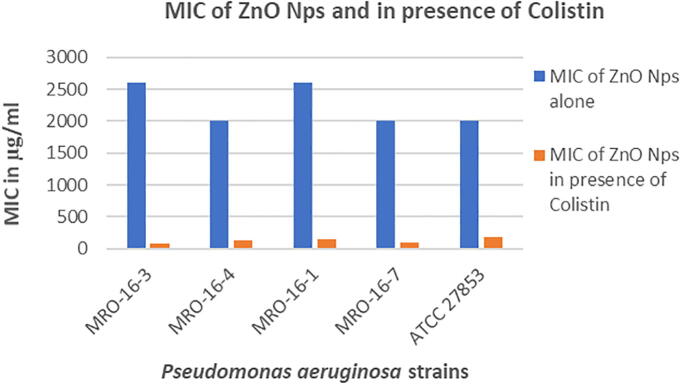
The Fig. 3, represents the results of MIC of ZnO NPs alone and in the presence of Colistin antibiotic, the combination ZnO NPs in presence of Colistin was found to be more effective in comparison with the results of ZnO NPs alone. The combination ZnO NPs in presence of Colistin found to be effective against clinical isolated strain P. aeruginosa (MRO-16-3) with MIC value 80 μg/mL which is found to be least in contrast to the other P. aeruginosa strains. Whereas, ZnO NPs showed high MIC values ranges from 2000 to 2600 μg/mL against all the clinical isolated P. aeruginosa strains and P. aeruginosa ATCC 27853 strain, the effect of colistin on ZnO NPs was found to be more which showed lower MIC values as in contrast to ZnO NPs alone which is found to be less effective as it showed high MIC values.
Fig. 3.
Column chart showing the effect of ZnO NPs alone and in the presence of Colistin against the P. aeruginosa isolates.
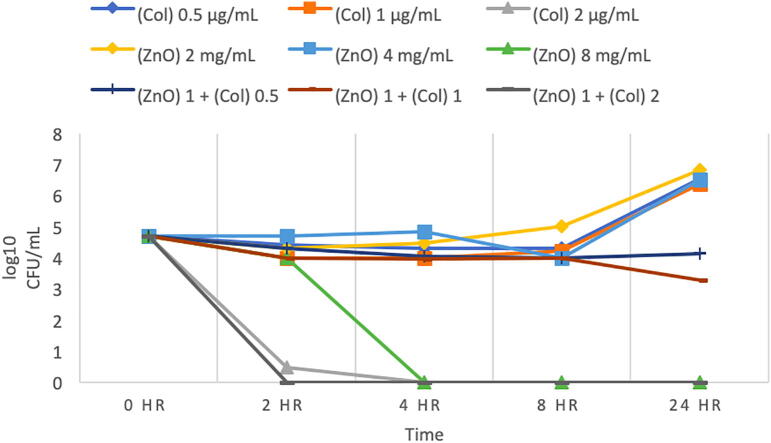
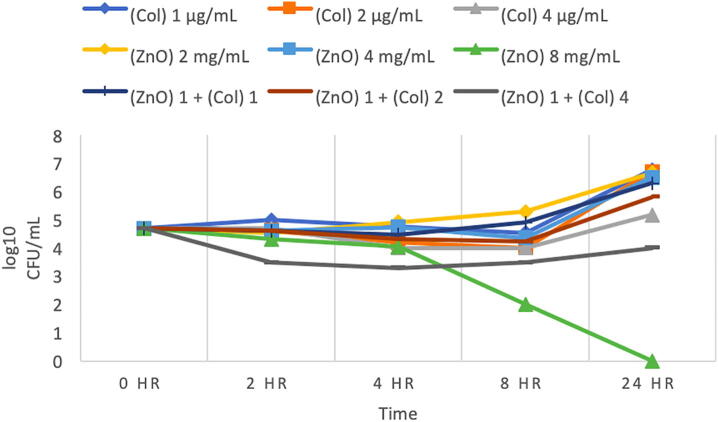
3.4. Time-kill growth curve of P. aeruginosa (MRO-16-3)
P. aeruginosa (MRO-16-3) was found to be highly sensitive to Colistin with an MIC of 2 µg/mL, which has shown to reduced bacterial growth to zero colonies (Fig. 4). And the combination of the same concentration of Colistin 2 µg/mL with 1 mg/mL ZnO NPs also showed no growth. Moreover, other concentrations of Colistin was used (0.5 and 1 µg/mL) there was found to increase in growth relative to the starting inoculum, ZnO NP 8 mg/mL concentration was found to be bactericidal, as shown in (Fig. 4), however the combinations of ZnO NPs 1 mg/ml with 2 µg/mL Colistin indicates synergy.
Fig. 4.
Time-kill curve for Pseudomonas aeruginosa (MRO-16-3). (This figure shows growth curves against Colistin alone, ZnO alone, and the combination of different concentrations of Colistin (0.5, 1, 2) µg/mL with 1 mg/mL ZnO NPs at different time intervals. (Col) Colistin, (ZnO) ZnO NPs suspension. Bacteriostatic and bactericidal activities are defined as < 3 log10 and ≥ 3 log10 reductions in CFU/mL at 24-hour (CLSI, 1999). Synergy is defined as a ≥ 2 log10 CFU/mL for the combination in comparison to its most active agent after 24 h. Indifference is defined as ≤ 1 log10 CFU/mL compared to the most active agent alone at 24 h. Additive is < 2 log10 CFU/mL after 24- hours.)
3.5. Time-kill growth curve of P. aeruginosa (MRO-16-1)
P. aeruginosa (MRO-16-1) was found to be Colistin sensitive, but regrowth of bacteria was observed after 24 h when treated with all concentrations of Colistin (Fig. 5). Moreover, MIC of ZnO NPs was 8 mg/ml which was found to be bactericidal (Fig. 5). After 24 h of incubation, the combination of 1 mg/mL ZnO with 4 µg/mL Colistin indicates an additive interaction, and the combinations of 1 mg/mL ZnO with 2 and 1 µg/mL of Colistin showed indifference results.
Fig. 5.
Time-kill curve for Pseudomonas aeruginosa (MRO-16-1). (This figure shows growth curves against Colistin alone, ZnO alone, and the combination of different concentrations of Colistin (1, 2, 4) µg/mL with 1 mg/mL ZnO NPs at different time intervals. (Col) Colistin, (ZnO) ZnO NPs suspension. Bacteriostatic and bactericidal activities are defined as < 3 log10 and ≥ 3 log10 reductions in CFU/mL at 24- hour (CLSI, 1999). Synergy is defined as a ≥ 2 log10 CFU/mL for the combination in comparison to its most active agent after 24 h. Indifference is defined as ≤ 1 log10 CFU/mL compared to the most active agent alone at 24 h. Additive is < 2 log10 CFU/mL after 24- hours.)
4. Conclusion
To conclude, recent innovations in the nanotechnology has provided significant changes in the field of medicine using nanoparticles for the treatment of diseases such as in cancer therapy, and in combination therapy for resistant bacterial infections. In this study, the effect of ZnO NPs, was tested alone and in combination with other antibiotics against Pseudomonas aeruginosa standard and isolated strains. Our results showed synergetic and additive interaction for the combination of ZnO NPs and Colistin, this combination at appropriate dosage intervals might be beneficial in clinical practice, by lowering the concentration needed of both agents (ZnO NPs and Colistin), to reduce the possibility of toxic effect on humans to occur. Moreover, given the current-status of Colistin as a last resort in antimicrobial therapy, not only is synergy beneficial for patient care, but an additive result is also desirable, as even a relatively small increase in the activity of both agents may be beneficial with clinically acceptable concentrations. Finally, further studies are needed to explore and fully understand the combination of ZnO NPs and antibiotics, such as using electron microscopy and a living model such as Wax Mouth Larvae, which is going to be investigated in further research.
Authors contribution
AOF: Supervising the research work, designing methodology, analyzing the data and writing the results, manuscript revising and editing. DKA: performed experimental work and compiled the data generated. AM: manuscript writing, compiling and analyzing the data, manuscript revising and editing. AMA: conceived the idea of work, Supervising the bench work and planning and designing the complete research project work.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
The authors acknowledge the support of grant from the “Research Center of the Female Scientific and Medical Colleges”, Deanship of Scientific Research, King Saud University for supporting this research project, and also special thanks to all the authors for their constant support and sincere efforts to carry out the present research work.
Footnotes
Peer review under responsibility of King Saud University.
References:
- Breidenstein E.B., De La Fuente-Nunez C., Hancock R.E. Pseudomonas aeruginosa: all roads lead to resistance. Trends. Microbiol. 2011;19:419–426. doi: 10.1016/j.tim.2011.04.005. [DOI] [PubMed] [Google Scholar]
- Balaji V., Jeremiah S.S., Baliga P.R. Polymyxins: antimicrobial susceptibility concerns and therapeutic options. Indian. J. Med. Microbiol. 2011;29:230–242. doi: 10.4103/0255-0857.83905. [DOI] [PubMed] [Google Scholar]
- Bayroodi E., Jalal R. Modulation of antibiotic resistance in Pseudomonas aeruginosa by ZnO nanoparticles. Iran. J. Microbiol. 2016;8(2):85–92. [PMC free article] [PubMed] [Google Scholar]
- Bisbe J., Gatell J.M., Puig J. Pseudomonas aeruginosa bacteremia: univariate and multivariate analyses of factors influencing the prognosis in 133 episodes. Rev. Infect. Dis. 1988;10:629–635. doi: 10.1093/clinids/10.3.629. [DOI] [PubMed] [Google Scholar]
- CLSI 2012. M07-A9: Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically; Approved Standard—Ninth Edition. Wayne, PA, USA. Clinical and Laboratory Standards Institute.
- CLSI 2018. M100: Performance Standards for Antimicrobial Susceptibility Testing. Twenty-eighth informational supplement Wayne. P.A. Clinical and Laboratory Standards Institute
- Drenkard E. Antimicrobial resistance of Pseudomonas aeruginosa biofilms. Microbes. Infect. 2003;5:1213–1219. doi: 10.1016/j.micinf.2003.08.009. [DOI] [PubMed] [Google Scholar]
- Dupuy F.G., Pagano I., Andenoro K., Peralta M.F., Elhady Y., Heinrich F., Tristram-Nagle S. Selective interaction of colistin with lipid model membranes. Biophys. J. 2018;114:919–928. doi: 10.1016/j.bpj.2017.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaddad S.M., Thati V., Roy A.S., Ambika Prasad M.V.N., Shivannavar C.T. Nanostructured zinc oxide enhances the activity of antibiotics against Staphylococcus aureus. J. Biosci. Technol. 2010;1:64–69. [Google Scholar]
- Ghasemi F., Jalal R. Antimicrobial action of zinc oxide nanoparticles in combination with ciprofloxacin and ceftazidime against multidrug-resistant Acinetobacter baumannii. J. Glob. Antimicrob. Resist. 2016;6:118–122. doi: 10.1016/j.jgar.2016.04.007. [DOI] [PubMed] [Google Scholar]
- Gordon N.C., Png K., Wareham D.W. Potent synergy and sustained bactericidal activity of a vancomycin-colistin combination versus multidrug-resistant strains of Acinetobacter baumannii. Antimicrob Agents Chemother. 2010;54:5316–5322. doi: 10.1128/AAC.00922-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta S., Govil D., Kakar P.N., Prakash O., Arora D., Das S. Colistin and polymyxin B: a re-emergence. Indian J. Crit. Care Med. 2009;13(2):49–53. doi: 10.4103/0972-5229.56048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly S.A., Havrilla C.M., Brady T.C., Abramo K.H., Levin E.D. Oxidative stress in toxicology: established mammalian and emerging piscine model systems. Environ. Health. Perspect. 1998;106:375–384. doi: 10.1289/ehp.98106375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., Milne R.W., Nation R.L., Turnidge J.D., Coulthard K., Valentine J. Simple method for assaying colistin methanesulfonate in plasma and urine using high-performance liquid chromatography. Antimicrob Agents Chemother. 2002;46(10):3304–3307. doi: 10.1128/AAC.46.10.3304-3307.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malekzadegan Y., Abdi A., Heidari H. In vitro activities of colistin, imipenem and ceftazidime against drug-resistant Pseudomonas aeruginosa and Acinetobacter baumannii isolates in the south of Iran. BMC Res. Notes. 2019;12:301. doi: 10.1186/s13104-019-4344-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micek, S.T., Lloyd, A.E., Ritchie, D.J., Reichley, R.M., Fraser, V.J., Kollef, M.H., 2005. Pseudomonas aeruginosa bloodstream infection: importance of appropriate initial antimicrobial treatment. Antimicrob. Agents. Chemother. 49, 1306–1311. [DOI] [PMC free article] [PubMed]
- Mulcahy L.R., Burns J.L., Lory S., Lewis K. Emergence of Pseudomonas aeruginosa strains producing high levels of persister cells in patients with cystic fibrosis. J. Bacteriol. 2010;192:6191–6199. doi: 10.1128/JB.01651-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- European Committee for Antimicrobial Susceptibility Testing (EUCAST) of the European Society of Clinical Microbiology and Infectious Diseases Determination of minimum inhibitory concentrations (MICs) of antibacterial agents by broth dilution. Clin. Microbiol. Infect. 2003;9:ix–xv. [Google Scholar]
- Molecular Devices, 2016. SpectraMax Plus 384 Microplate Reader A microplate spectrophotometer with a built-in cuvette port [Online]. Molecular Devices. [Accessed June 22 2019].
- Rikans L.E., Hornbrook K.R. Lipid peroxidation, antioxidant protection and aging. Biochim. Biophys. Acta. 1997;1362:116–127. doi: 10.1016/s0925-4439(97)00067-7. [DOI] [PubMed] [Google Scholar]
- Shopsin B., Gomez M., Montgomery S.O., Smith D.H., Waddington M., Dodge D.E., Bost D.A., Riehman M., Naidich S., Krieswirth B.N. Evaluation of protein a gene polymorphic region DNA sequencing for typing of Staphylococcus aureus strains. J. Clin. Microbio. 1999;37:3556–3563. doi: 10.1128/jcm.37.11.3556-3563.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon S.D., Bahadory M., Jeyarajasingam A.V., Rutkowsky S.A., Boritz C. Synthesis and study of silver nanoparticles. J. Chem. Educ. 2007;84(2):322–325. [Google Scholar]
- Vidaillac C., Benichou L., DuvaL R.E. In vitro synergy of colistin combinations against colistin-resistant Acinetobacter baumannii, Pseudomonas aeruginosa, and Klebsiella pneumoniae isolates. Antimicrob. Agents Chemother. 2012;56:4856–4861. doi: 10.1128/AAC.05996-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiseman L.R., Wagstaff A.J., Brogden R.N., Bryson H.M. Meropenem. Drugs. 1995;50:73–101. doi: 10.2165/00003495-199550010-00007. [DOI] [PubMed] [Google Scholar]
- Wright G.D. Bacterial resistance to antibiotics: enzymatic degradation and modification. Adv. Drug. Deliv. Rev. 2005;57:1451–1470. doi: 10.1016/j.addr.2005.04.002. [DOI] [PubMed] [Google Scholar]
- Xie Y., He Y., Irwin P.L., Jin T., Shi X. Antibacterial activity and mechanism of action of zinc oxide nanoparticles against Campylobacter jejuni. Appl. Environ. Microbiol. 2011;77:2325–2331. doi: 10.1128/AEM.02149-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L., Jiang Y., Ding Y., Povey M., York D. Investigation into the antibacterial behaviour of suspensions of ZnO nanoparticles (ZnO nanofluids) J. Nanopart. Res. 2007;9:479–489. [Google Scholar]