Abstract
Loneliness affects group-living mammals triggering a cascade of stress-dependent physiological disorders. Indeed, social isolation stress is a major risk factor for several neuropsychiatric disorders including anxiety and depression. Furthermore, social isolation has a negative impact on health and fitness. However, the neurobiological consequences of long-term chronic social isolation stress (LTCSIS) manifested during the adulthood of affected individuals are not fully understood. Our study assessed the impact of LTCSIS and social buffering (re-socialization) on the behavioural performance and social-affective brain-related proteins in diurnal, social, and long-lived Octodon degus (degus). Thereby, anxiety-like and social behaviour, and social recognition memory were assessed in male and female animals subjected to a variety of stress-inducing treatments applied from post-natal and post-weaning until their adulthood. Additionally, we evaluated the relationship among LTCSIS, Oxytocin levels (OXT), and OXT-Ca2+-signalling proteins in the hypothalamus, the hippocampus, and the prefrontal cortex. Our findings suggest that LTCSIS induces anxiety like-behaviour and impairs social novelty preference whereas sociability is unaffected. On the other hand, re-socialization can revert both isolation-induced anxiety and social memory impairment. However, OXT and its signalling remained reduced in the abovementioned brain areas, suggesting that the observed changes in OXT-Ca2+ pathway proteins were permanent in male and female degus. Based on these findings, we conclude degus experience social stress differently, suggesting the existence of sex-related mechanisms to cope with specific adaptive challenges.
Keywords: Octodon degus, Chronic stress, Anxiety-like behaviour, Social memory-Oxytocin-Ca2+ signalling, Re-socialization
1. Introduction
Gregarious species form complex social structures, where stable bonds play an important role of their social organization to maintain their health, fitness, and survival (Neumann, 2009; Buwalda et al., 2013). While social interactions are essential for many life processes such as communal rearing of young and cooperation within a group (Neumann, 2009; Blumstein et al., 2010; Ebensperger et al., 2012) they also have a protective role against stress-related events as depredation, resource acquisition or defense and social thermoregulation (Ebensperger, 2001; Gilbert et al., 2009). Social buffering and supportive social contact among highly social mammals (such as humans) lead to substantial mental and physical health benefits in stress situations, reducing the risk of mortality (Taylor, 2006; Taylor et al., 2007; Beery and Kaufer, 2015). Therefore, disruptions of the social environment (i.e., social isolation or social instability) are associated with a series of physiological, neuroendocrine, and behavioural dysfunctions, both in humans and non-human species (Beery and Kaufer, 2015; Mumtaz et al., 2018).
Several factors can influence the response to social isolation, this also varies greatly among individuals. These include the life-span phase of the individual (i.e., early life, adolescence, adulthood, or aging), both intensity and duration of the social isolation event (i.e., acute vs. chronic), past experiences (i.e., maternal separation), sex/gender and/or genetic background (Beery and Kaufer, 2015; Mumtaz et al., 2018). Disruption of the mother-newborn relationship is a well-established model of psychosocial stress (Gilles and Polston, 2017) that causes permanent changes in brain development and plasticity. These include physical- and emotional-behavioural disorders that can modulate stress-response mechanisms in the adulthood, decreasing social communication and increasing aggressiveness (Babygirija et al., 2012; Nishi et al., 2014; Ieraci et al., 2016; Gilles and Polston, 2017). Moreover, social isolation during the post-weaning and peripubertal periods (i.e., mild-to late-adolescence) leads to marked behavioural, emotional, and neuronal consequences that increase anxiety, aggressiveness, and cognitive deficits in the adulthood (Veenema et al., 2008; Buwalda et al., 2013; Mumtaz et al., 2018). Similarly, these effects can be seen in individuals subjected to social isolation in their adulthood, despite having a normal social state in the early stages of their life (Arranz et al., 2009; Gilles and Polston, 2017).
The adaptive response to stress also depends on the type, intensity, and duration of the stressor stimulus, as well as on the physiological state of the individual (Pitman et al., 1990; Rostamkhani et al., 2012). In particular, time-limited stressful events (i.e., acute stress) generally result in adaptive responses to homeostatic changes. However, this response can become harmful if persists chronically, resulting in maladaptive and irreversible changes in neuroendocrine responses, affecting the entire organism (Pitman et al., 1990; Lagraauw et al., 2015; Eisenmann et al., 2016). Consequences range from neuro-molecular impairment in OXT signalling, a social behaviour/memory related hormone, impaired neuroplasticity and memory loss to emotional and affective disorders such as anxiety, depression, schizophrenia, and epilepsy (Anacker and Beery, 2013; Maroun and Wagner, 2016).
The effects of social isolation stress can also differ according to sex. This is a significant variable when assessing the impact of social rearing conditions on later behaviour. Sex differences include innate neurochemical features and response to environmental stressors (Palanza et al., 2001; Martin and Brown, 2010; Brent et al., 2017). In animal models (and humans), females appear to be at a greater risk for early life and peripubertal changes (i.e., physiological and social), leading to affective disorders throughout their life (Andersen and Teicher, 2008; Bale and Epperson, 2015).
In this context, social buffering plays a key role in psychological and physiological wellbeing (Hennessy et al., 2000; Yee et al., 2008; Silk et al., 2010; Stanton and Mann, 2012). The strategies to ameliorate or reverse social disturbances induced by social isolation are known as “re-socialization” and constitute an experimental analogy of behavioural therapy (Tulogdi et al., 2014; An et al., 2017). Re-socialization in humans and murine models can be evidenced by recovery of myelination, normalized behaviour and improvements in cognitive abilities (Liu et al., 2012; An et al., 2017). Previous studies have investigated the effects of early-life social isolation on behavioural parameters in rodents; however only a few studies have focused on long-term persistent effects of stress exposure.
We selected Octodon degus (hereafter simply called degus) as a model for the study of long-term social-affective biological behaviour. Humans and degus share many physiological and behavioural characteristics, including the ability to form social bonds and extended social group families (Silk et al., 2007; Colonnello et al., 2011; Rivera et al., 2016a). On average, degus live 7–8 years in captivity, making them ideal for longitudinal studies (Lee, 2004). Furthermore, following maternal separation and isolation from peers degus display a robust distress response that includes decreased social motivation and impaired emotional behaviour. On the other hand, just an hour a day of re-socialization triggers a positive social-buffer effect that mitigates the effects of social isolation (Braun et al., 2003; Braun, 2011; Colonnello et al., 2011). Recently, we observed that long-term re-socialization of animals after chronic isolation modified physiological, behavioural, functional, and molecular aspects of spatial memory-related tasks (Rivera et al., 2020). We also observed impaired spatial learning and memory processes linked to physiological stress response, and effects on synaptic transmission related to pre-and postsynaptic proteins (Rivera et al., 2020). Herein, we hypothesized that long-term chronic social isolation stress (LTCSIS) would impact social behavioural outcomes (i.e., sociability and social memory) in juvenile, adolescent, and adult degus (female and male). Then, we evaluated the effect of LTCSIS on the OXT signalling pathway in social-related brain regions, and whether the effect of long-term re-socialization in adult animals would mitigate its effects.
2. Material and methods
2.1. Animals and social isolation protocol
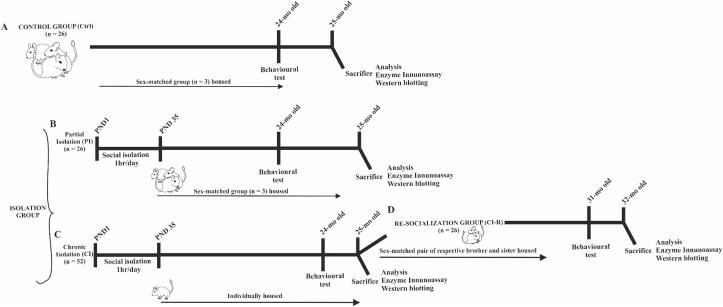
Pregnant female degus obtained from our colony at the Faculty of Biological Sciences, Pontificia Universidad Catόlica de Chile were kept in pairs and housed in clear acrylic aquaria (length x height x depth: 50 × 35 × 23 cm) with bedding of hardwood chips. Each cage contained one nest box made of clear acrylic (22 × 12 × 15 cm). We checked for litters daily, and the day of birth was defined as PND 0. To avoid litter differential parental effect, the whole litter was randomly assigned to one of the following rearing conditions: (i) unstressed controls: the litters were left undisturbed with their family. The siblings remained together until PND 90, and thereafter they were raised as sex-matched groups of three siblings from PND 91 until the end of the experiment (Control group, CTL, Fig. 1A). (ii) Separation stress: From PND 1 to PND 35 (day of weaning), the pups were removed from their mothers and home cage. In the same room, the pups were kept individually in small opaque cages for 1 h daily (between 09:00 a.m. and 12:00 p.m. noon). Thus, during separation, the pups had acoustic and olfactory but no visual and social contact with their siblings. After 1 h, separated pups were returned to their family and home cage and left undisturbed until the next day. After PND 35 the whole litter was randomly assigned to one of the following rearing conditions: (i-a) The litters were left undisturbed with their family. The siblings remained together until PND 90, and thereafter they were raised as sex-matched groups of three siblings (one focal degus and two respective brothers/sisters that were not included in our experimental design) from PND 91 until the end of the experiment when degus reached 25-months old (Partial isolation group, PI, Fig. 1B). (ii-b) From PND 36 until the end of the experiment, female and male degus were individually housed in standard rodent cages, where they had olfactory, acoustic, partial visual, but no physical contact to conspecifics (Chronic isolation group, CI, Fig. 1C).
Fig. 1.
Scheme of experimental design of the stress treatments: (a) unstressed control animals (CTL), where litters were left undisturbed with their family. The siblings remained together until PND 90, and thereafter they were raised in sex-matched groups of three siblings from PND 91 until the end of the experiment (n = 13♀ and 13♂). (b) Partial isolation group (PI), from PND 1 to PND 35 (day of weaning), the degus pups were removed from their mothers and home cage and were kept individually for 1 h daily. After 1-h of separation pups were returned to their family and home cage and left undisturbed until the next day. After PND 35 the whole litter was left undisturbed with their family. The siblings remained together until PND 90, and thereafter they were raised as sex-matched groups of three siblings (one focal degus and two respective brothers/sisters that were not included in our experimental design) from PND 91 until the end of the experiment (n = 13♀ and 13♂). (c) Chronic isolation group (CI), from PND 1 to PND 35 (day of weaning), the degus pups were removed from their mothers and home cage and were kept individually for 1 h daily. After 1-h of separation pups were returned to their family and home cage and left undisturbed until the next day. From PND 36 until the end of the experiment, female and male degus were individually housed (n = 26♀ and 26♂). (d) Re-socialization group (CI-R), after a period of 24-months, half of CI-reared degus were randomly reassigned and housed in sex-matched pairs with CI-reared brothers or sisters (one focal degus and one sibling not included in our experimental design) during a period of 6-month (n = 13♀ and 13♂).
A total of 52 animals (26 female and 26 male degus, n = 13 per group) were analysed per behavioural test (see above) for CTL and PI condition. For the CI condition a total of 52 animals (26 females and 26 males were analysed). To study the protective role of social buffering after LTCSIS (25-months), half of the CI-reared degus (13 females and 13 males) were housed in sex-matched pairs with their respective CI-reared brothers or sisters (one focal degus and one sibling not included in our experimental design) during a re-socialization period of 6-month, when degus had 31-months old (Re-socialization group, CI-R, Fig. 1D).
Degus are long-lived rodents that under captivity conditions reach the age of ~7–8 years. We considered that all our treatments are comparable in time, despite that the CI-R group stayed additional 6-months for the re-socialization purpose. According to our previous studies and over several years of experience working with this animal model, we know that degus older than 3-years old can experiment biochemical changes and impairment in cognitive performance associated with normal ageing (Inestrosa et al., 2015; Rivera et al., 2016b, 2018). Our treatments are performed during the first 2-years of life, the time when maturity is reached and all animals are considered biologically similar.
In our design, we avoided the effect of hormonal fluctuation in 17–21-day regular cycling females, by performing behavioural tests in the diestrous phase of the oestrous cycle. Animals were kept in a ventilated room exposed to a 12:12 h light-dark cycle with temperatures controlled (yearly minimum = 13.4 ± 0.2 °C; yearly maximum = 24.9 ± 0.2 °C). Degus were fed a standard rabbit commercial pellet diet (Champion, Santiago, Chile) and ad libitum water. All animal protocols followed the guidelines of the National Institutes of Health (NIH, Baltimore, MD, USA) and the National Institutes of Health guide for the care and use of laboratory animals (NIH Publications No. 8023, revised 1978). All procedures were approved by the Bioethical and Biosafety Committee of the Faculty of Biological Sciences of the Pontificia Universidad Católica de Chile (CBB-121-2013). The efforts were made to minimize animal suffering and to reduce the number of animals used.
2.2. Behavioural observations
Degus 25-months old from CTL, PI, and CI-reared conditions were subjected to four behavioural tasks, as detailed below. Whereas, for the CI-R group, the behavioural tests were performed at the end of the 6-months re-socialization period (i.e., 31-months). To minimize the effects of behavioural experiences on the results, the experiments were conducted in the order from the less to the more intrusive. The order of experiments was as follows: i) open field; ii) “novel object” open field test; iii) light-dark box test, and iv) three-chambered social interaction test. The animals were subjected to one test per day. All behavioural tests were performed during the active phase of the animals (between 09:00 to 16:00 h). At the end of each session, the animals were returned to their home cages, and the area was wiped clean with a 70% ethanol solution.
2.2.1. The open field test
To evaluate differences in the locomotor activity and the willingness of the exploratory behaviour between groups, animals were observed for 5 min in the open field test. This test consists in animal observation within a white Plexiglas box (length x height x depth: 100 × 100 × 100 cm). The percentage of time in the middle arena, the speed, and total distance were assessed (Rivera et al., 2016b). At the end of each session, the animals were returned to their home cages, and the area was wiped clean with a 70% ethanol solution. We use this test assessed by quantification of movement frequency and distance.
2.2.2. The “novel object” open field test
This test measures the animal's response towards a novel object placed in the centre of the open field. Specifically, an emotionally relaxed degus would approach the novel object often to investigate, whereas an anxious, hyperreactivity animal would be much less keen to explore the unfamiliar object. Subjects underwent the “novel object” open field test the day following the conventional open field. In the same arena as the open field test, animals were observed for 5 min. The total time spent by the animal exploring this distinct novel stimulus was recorded. In addition, the percentage of time spent in corners and the middle arena, as well as the speed and total distance travelled, was assessed. At the end of each session, the animals were returned to their home cages, and the area was wiped clean with a 70% ethanol solution.
2.2.3. Light-dark box test
The light-dark box test is a frequently used test for anxiety-like behaviour (Crawley, 2005). The Light-dark box consisted of a cage made of Plexiglas box, divided into two equalized brightly illuminated and fully black sections (length x height x depth: 21 × 21 × 21 cm). Both sections were connected by a 7 × 7 cm opening at the floor level. The white (light section) remained uncovered during the test and was illuminated by a 24 V–10W bulb. Whereas, the black (dark section) was covered by a light-proof lid without appreciable illumination. For this test, we followed the light-dark box protocol used for degus by Popović et al. (Popovic et al., 2009). Briefly, the animal was placed in the light section, facing away from the entrance to the dark section, and allowed to explore the apparatus freely for 10 min. The behaviour recorded during the test included: latency to the dark box (i.e. the time it took the animals to enter the dark box (Costall et al., 1989);), number of transitions (i.e. number of times the animals crossed from the light to the dark box, and vice versa) and total duration in the light box (i.e. the amount of time spent by the animal in the light box). At the end of each session, the animals were returned to their home cages, and the apparatus was wiped clean with a 70% ethanol solution.
2.2.4. Three-chambered social interaction test
We used the three-chamber test to assess (i) social affiliation/motivation by comparing the time degus spent interacting with an empty wire cage versus one containing a novel degu and (ii) social memory and preference for social novelty by measuring the time degus spent interacting with an
Unfamiliar (novel) versus a familiar degus. The open field arena was subdivided into three equal compartments using transparent Plexiglas walls, each with a small opening (diameter 2.8 cm), allowing access into each compartment. The degus to be used as social partners were sex-matched, unfamiliar, and unrelated. The test comprised three 20-min sessions. The social test was performed following the protocol previously described by Rivera et al. (2018). Briefly, three phases were performed in the following order: Phase 1 or Habituation: the test animal was placed in the middle compartment and allowed to explore the apparatus. Phase 2 or Session 1 (“Partner I″): the test animal was returned to the middle compartment. Simultaneously, the first social partner (a sex-matched, unfamiliar, and unrelated degus) was placed inside a wire containment cup located in one of the side chambers. The test degus was then free to interact with the social partner or with the empty cup. At the end of Session 1, the test animal was returned to his home cage for 1 h, and the area was wiped clean with a 70% ethanol solution to remove odours. Phase 3 or Session 2 (“Partner II”): The test animal was again returned to the middle compartment, and a second unfamiliar, unrelated degus was placed inside an identical wire containment cup in the opposite side chamber, which had been empty during Session 1. In this part, the test animal is free to choose between the first, already-investigated, unfamiliar degus (Partner I), and a novel unfamiliar animal (Partner II). As measurements of social interaction, we recorded the time spent exploring the partner (i.e., the time where the test animal spent touching the containment cup housing or not housing the new partner for each chamber individually, with the forepaw or nose). To evaluate differences in social affiliation/motivation and social memory, we calculated the recognition index (RI). The RI for Session 1 was defined as the quotient of the time the degus spent with Partner I divided by the sum of the time spent with Partner I and the empty cup. For Session 2, the RI was calculated as the time spent with Partner II divided by the sum of the time spent with Partners I and II. A RI ≤ 0.50 indicates that degus have an absence of social affiliation/motivation during Session 1 and an absence of social memory during Session 2.
In all cases, a digital video camera (LifeCam Studio Full HD, Microsoft Corp, Redmond, WA, USA) was mounted above the test arena, and the performance of each animal was monitored with image tracking software (HVS Image, Hampton, UK).
2.3. Immuno-analysis
2.3.1. Western blot analysis
At the end of the experiment, degus were euthanized by decapitation at the end of the behavioural tests (described previously) after isoflurane deep anaesthesia. Trunk blood was collected immediately and centrifuged at 3500×g for 10 min to collect plasma, which was stored at −150 °C until the determination of Oxytocin enzyme immunoassay (see below). The hypothalamus, hippocampus, and prefrontal cortex (PFC) of CTL, PI, CI, and CI-R degus (n = 3 respectively) were dissected on ice and immediately frozen at −150 °C and processed. Briefly, the brain regions were dissected and removed completely by freehand with curved forceps and razorblade (no punch was used) on a petri dish on ice. First, we dissected the hypothalamus from the middle ventral side of the brain (between both hemispheres). Second, we separated the hemispheres and the hippocampus was visually identified and removed completely. Finally, a coronal section was made, the olfactory bulb was removed, and the PFC was obtained (Wright and Kern, 1992; Spijker et al., 2019). Every tissue was homogenized in RIPA buffer (50 mM Tris-Cl, pH 7.5, 150 mM NaCl, 1% NP-40, 0.5% sodium deoxycholate, and 1% sodium dodecyl sulphate supplemented with a protease inhibitor cocktail (P8340, Sigma-Aldrich, Germany) and phosphatase inhibitors (50 mM NaF, 1 mM Na3VO4, and 30 μM Na4P2O7) using a Potter homogenizer; then, the samples were passed sequentially through different caliber syringes. Protein samples were centrifuged twice at 20.817×g (14000 rpm) at 4 °C for 10 min. The protein concentration was determined using a Bicinchoninic Acid Protein (BCA) Assay Kit (Thermo Scientific). Thirty micrograms of the protein samples were separated by 10% SDS-PAGE and transferred to a polyvinylidene difluoride membrane. The membranes were incubated with primary antibodies: cyclic ADP-ribose cyclase (CD38, Cell Signaling Technology Antibody #14637, MA, USA), and protein kinase C phosphorylated at S660 (p-PKC, Cell Signaling Technology Antibody #9371, MA, USA); total calcium Calmodulin-dependent kinase II (CaMKII, Santa Cruz Biotechnology sc-13141, CA, USA) and total PKC (PKC, Santa Cruz Biotechnology sc-13149, CA, USA); CaMKII phosphorylated at T286 (p-CaMKII, Abcam ab32678, Cambridge, UK). Then washed with phosphate-buffered saline (PBS)-Tween 0.1% or 0.05% and incubated with secondary antibodies conjugated with HRP: anti-mouse, anti-rabbit, and anti-goat IgG peroxidase-conjugated antibodies (Abcam). Later, the membranes were developed using an ECL kit (Biological Industries). To analyse the results, all target protein signals were normalized against the loading control (α-tubulin, β-actin from Sigma-Aldrich, St. Louis, USA, or GAPDH from Santa Cruz Biotechnology). The signal of phosphorylated proteins was normalized against the total protein levels, being referred to as p-CaMKII/total CaMKII levels as “active CaMKII” (or p-CaMKII ratio) while to the ratio p-PKC/total PKC levels as “active PKC” (or p-PKC ratio).
2.3.2. Oxytocin enzyme immunoassay
The determination of OXT was made using 96 plates of commercial OXT ELISA assay kit (cat #ADI-900-153A, Enzo Life Sciences, Farmingdale, NY). The amino acid sequence detected by the assay (UNIPROT ID: P01178 (human); http://www.enzolifesciences.com/fileadmin/reports/Datasheet-ADI-901-153A.pdf, matches a section of the OXT protein in degus (NCBI Reference Sequence: XP_004634307.1; https://www.ncbi.nlm.nih.gov/protein/XP_004634307.1). This OXT ELISA kit is useful for the quantitative determination of OXT in culture supernatants, milk, plasma, and serum from any species. Validated sample types include cerebral spinal fluid, saliva, tissue, and urine. All the precautions, sample handling, and extraction protocols mentioned in the kit manual were taken into consideration (see Manual: https://www.enzolifesciences.com/fileadmin/files/manual/ADI-901-153A_insert.pdf). The protein samples were obtained from tissues of the hypothalamus, the hippocampus, and the prefrontal cortex. Because higher OXT was expected, samples were diluted to 1 μg/μL in homogenization/sample buffer containing protease and phosphatase inhibitors. We used these diluted protein and plasma samples (90 μL) directly to measure OXT by ELISA kit; we did not use a column for the previous extraction of OXT. The optical density (OD) of the plate was read on a microplate reader (Model 680XR, Bio-Rad, Hercules, CA) at 405 nm. The intensity of the colour is inversely proportional to the concentration of OXT in the sample. Standards and samples were run in duplicate. The content (in pg/mL) was determined by plotting the OD of each sample against a standard curve. OXT was determined using a four parameters logistic curve fitting. According to the manufacturer, the lower limit's kit sensitivity is 15.0 pg/mL. The observed intra-assay and inter-assay coefficients are < 12.6% and 20.9%, respectively.
2.4. Statistical analysis
All data are presented as the mean ± standard error of the mean (SEM). In social interaction, the RI was analysed. We used two-way ANOVAs to determine the effects of stress treatments, sex, and a stress treatment by sex interactive effect on behavioural tasks. For the western blot analysis, we used one-way ANOVA to analyse the effect of stress treatment groups in both female and male degus. Where appropriate, Fisher's LSD post-hoc comparisons were performed to examine the individual main effect of stress treatments and sex. The assumptions of normally distributed data and homogeneous variances were confirmed using Shapiro-Wilk and Levene's tests, respectively.
Statistical analyses were performed using the Statistica (StatSoft, Tulsa, OK) software package. Differences were considered statistically significant at p < 0.05.
3. Results
3.1. LTCSIS increases anxiety-like behaviour and is recovered by re-socialization
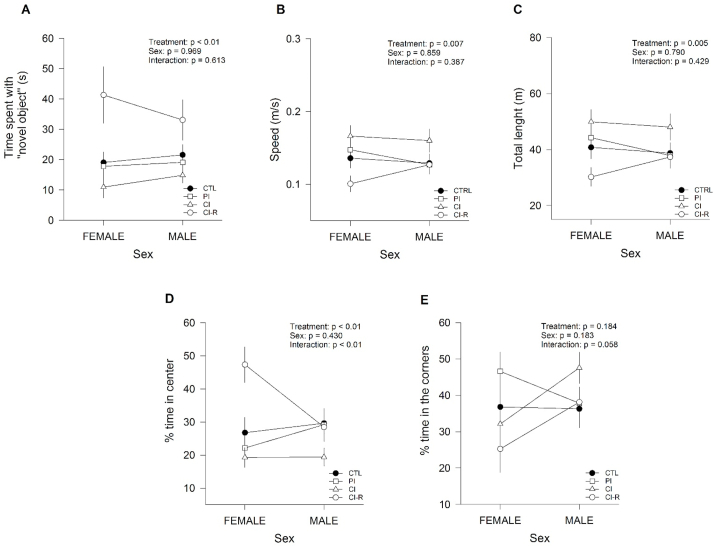
To evaluate the general state of animals, we performed an open field test. In this context, the time spent in the central zone of the arena, speed, and the total distance travelled was not affected by stress treatment, sex, nor interaction was recorded between the two factors, suggesting that general behaviour is not affected by LTCSIS (See Fig. S1 in Supplementary Information (SI)). Next, we evaluated the motivation of female and male degus to interact with a novel object in the “novel object” open field test. Previous evidence indicated that an emotionally relaxed animal approach the novel object more often (Smith et al., 2012). Regarding the time spent with the novel object, our results showed a significant effect of stress treatment [F(3,96) = 8.76, p < 0.01], but was not altered by the sex (p = 0.97), nor was by the interaction between the two factors (p = 0.61; Fig. 2A). The comparisons among groups indicated that CI-R degus spent significantly more time exploring the object than CTL, PI, and CI groups. We also measured the speed and total distance travelled, and we found a significant effect of stress treatment [F(3,96) = 4.29, p < 0.01 and F(3,96) = 4.85, p < 0.01, respectively]. However, these results were not dependent on sex (p = 0.86 and p = 0.79, respectively), and remained no significantly different across the interaction between both factors (average speed, p = 0.39; total distance travelled, p = 0.43; Fig. 2B and C, respectively). Further analysis indicated that CI degus moved faster and travelled more distance than CTL and CI-R groups, strongly suggesting an anxiety-like behaviour. Additionally, we measured the time spent in the central zone of arena, and the two-way ANOVA resulted in a significant effect of stress treatment [F(3,96) = 7.59, p < 0.01], a non-significant effect of sex (p = 0.43), and there was significant interaction between both factors [F(3,96) = 4.19, p < 0.01, Fig. 2D]. The followed analysis showed that CI-R degus spent more time in the central zone than the other groups, whereas CI degus recorded the shortest times in the central zones compared to CTL and CI-R animals. These results suggest that resocialization increases the motivation to interact with a novel object than other groups, and more confidence of the animal to stay in the centre of the arena. Additionally, we measured the percentage of time in the corners, and we found that was not affected by stress treatment (p = 0.18), or sex (p = 0.18), and the interaction between stress treatment x sex just missed significance [F(3,96) = 2.58; p = 0.058; Fig. 2E].
Fig. 2.
Effect of LTCSIS in anxiety-like behaviour measured in the Novel object open field test. (A) time spent investigating the novel object (B) average speed (C) total distance travelled (D) percentage of time remained in the central zone (E) percentage of time remained in the corners. The data were analysed statistically using two-way ANOVA followed by Fisher's LSD post-hoc test. The statistical effect of stress treatment, sex and the interaction between the two factors are indicated in the top of the figure. Each symbol corresponds to data from a single-sex stress treatment group, represented as the mean ± SEM (n = 13). As is showed in Figures for two-way ANOVA crossing lines indicate the interaction between both factors: stress treatment and sex.
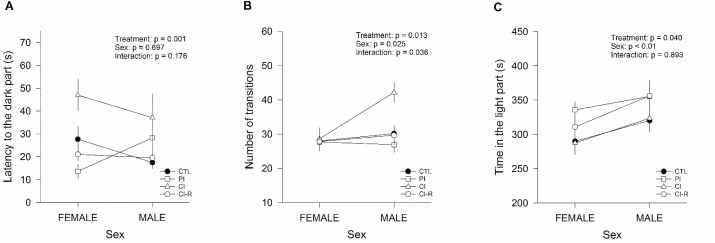
To directly evaluate anxiety-like behaviour, the animals performed the light-dark box test. The latency to enter the dark part of the box showed an effect of stress treatment [two-way ANOVA; F(3,96) = 5.41, p < 0.01], but was not altered by the sex (p = 0.69), nor was an interaction found between the two factors (p = 0.17; Fig. 3A). The followed analysis showed that CI degus had higher latency to enter the dark box than CTL and CI-R animals, whereas CI-R degus behave similarly to CTL. Regarding the total number of transitions between compartments (Fig. 3B), we found an effect of stress treatment [F(3,96) = 3.76, p = 0.01], sex [F(1,96) = 5.15, p = 0.02] and interaction between the two factors [F(3,96) = 2.97, p = 0.03]. The followed analysis demonstrates that all the female groups behave in the same way, whereas CI males presented a higher number of transitions compared to the other groups. More importantly, both CI-R female and male degus behaved similarly to CTL or PI groups. We also measured the total duration in light box, which was different across stress treatment [two-way ANOVA; F(3,96) = 2.88, p = 0.04], sex [F(1,96) = 7.53, p < 0.01], and no interaction was detected between the two factors (p = 0.89; Fig. 3C). In this case, both female and male degus presented a similar time in the light part across the four treatments.
Fig. 3.
Effect of LTCSIS in anxiety-like behaviour measured in the Light-dark box test: (A) latency to the dark box measured as the time it took the animals to enter the dark part of the box. (B) the number of transitions measured as number of times the animals crossed from light to the dark box and vice versa. (C) total duration in light box measured as the amount of time spent by animals in the light compartment. The data were analysed statistically using two-way ANOVA followed by Fisher's LSD post-hoc test. The statistical effect of stress treatment, sex and the interaction between the two factors are indicated in the top of the figure. Each symbol corresponds to data from a single-sex stress treatment group, represented as the mean ± SEM (n = 13). As is showed in Figures for two-way ANOVA crossing lines indicate the interaction between both factors: stress treatment and sex.
Altogether these results suggest that degus under CI displayed more anxiety-like behaviour than the other groups, and more importantly, 6-months of re-socialization was able to normalize social behaviour.
3.2. Effects of LTCSIS on sociability and social recognition memory
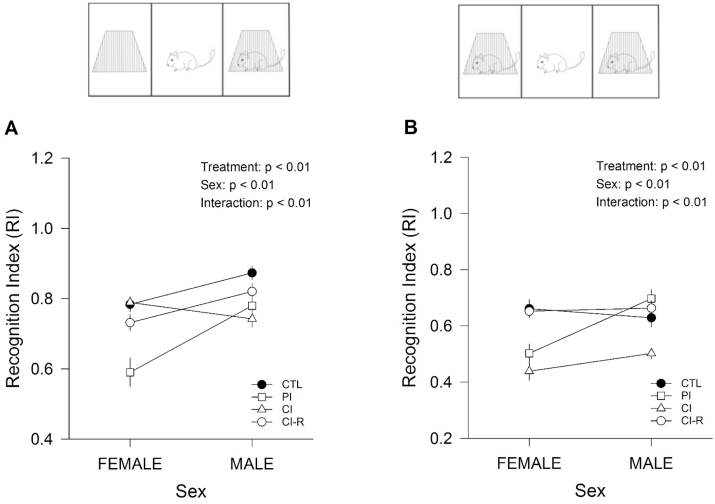
The animals performed a social interaction test, which is a two-session test that evaluates sociability and social recognition memory respectively. The recognition index (RI) showed an effect of stress treatment [RI for session 1: F(3,96) = 11.00, p < 0.01; RI for session 2: F(3,96) = 18.21, p < 0.01]. These differences were dependent on sex [RI for session 1: F(1,96) = 20.17, p < 0.01; RI for session 2: F(1,96) = 8.85, p < 0.01] and are significant across interaction between the two factors [RI for session 1: F(3,96) = 7.38, p < 0.01; RI for session 2: F(3,96) = 6.03, p < 0.01; Fig. 4A and B respectively]. Followed analysis for session 1, showed that PI females were significantly lower than the other female groups, suggesting that earlier separation had a strong effect on socialization in females than males. Whereas in males, CI males had lower values if compared to CTL and CI-R groups, showing that only chronic isolation in males affects socialization (Fig. 4A). During session 2, was evidence that both PI and CI treatments in females affected its social recognition memory, but for males, only CI showed lower RI values (Fig. 4B). These data showed that females are more susceptible to stress than males since early in life (PI group), and in both groups, CI treatment affected socialization and recognition memory. Strikingly, re-socialization in both groups recovers these effects similar to control. The data related to time interaction during session 1 and session 2 is shown in SI.
Fig. 4.
Effect of LTCSIS in social behaviour and social novelty preference measured in the three-chamber social interaction test: (A) RI for session 1 (social affiliation and sociability, expressed as the quotient of the time the subject degus spent with Partner, I divided by the sum of the time spent with Partner I and the empty cup). (B) RI for Session 2 (social memory and novelty, expressed as the quotient of the time the subject degus spent with Partner II divided by the sum of the time spent with Partners I and II). The data were analysed statistically using two-way ANOVA followed by Fisher's LSD post-hoc test. The statistical effect of stress treatment, sex and the interaction between the two factors are indicated in the top of the figure. Each symbol corresponds to data from a single-sex stress treatment group, represented as the mean ± SEM (n = 13). As is showed in Figures for two-way ANOVA crossing lines indicate the interaction between both factors: stress treatment and sex.
3.3. Effects of LTCSIS on social-related signalling of the hypothalamus, hippocampus, and prefrontal cortex
OXT molecular cascade is highly related to social behaviour (Jin et al., 2007; Lopatina et al., 2013). Impairment of this pathway is implicated in the etiology of developmental psychiatric disorders, characterized by deficits in social memory and higher anxiety-related behaviours. OXT, PKC, and CD38 proteins are the main component of the OXT signalling pathway (Lopatina et al., 2013). Additionally, as calcium influx have a key role in this pathway, other Ca2+-related proteins as CaMKII has been related to neuroplasticity processes (Wayman et al., 2008). To determine whether the LTCSIS affects the OXT pathway, we measured their levels in different brain regions.
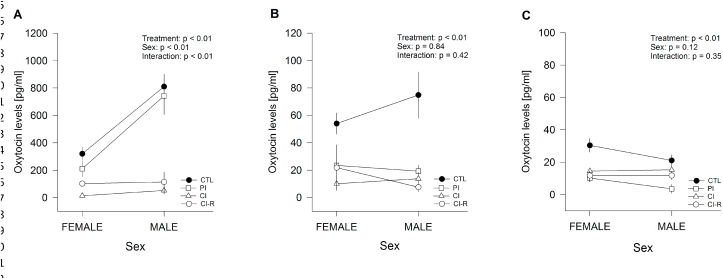
First, we examined OXT across three brain regions, the hypothalamus, hippocampus, and PFC, and the blood plasma concentration of OXT across the four stress treatments. In the hypothalamus of degus, the results of the two-way ANOVA in OXT revealed a significant stress treatment effect [F(3,16) = 29.23, p < 0.01], sex effect [F(1,16) = 36.44, p < 0.01], and treatment × sex interaction effect [F(3,16) = 6.78, p < 0.01; Fig. 5A]. Further analysis indicated that male degus showed higher levels of OXT than females in CTL and PI treatments. Strikingly, the CI treatment reduces the OXT in both females and males, and CI-R treatment was unable to normalize it. Meanwhile, the OXT in the hippocampus and prefrontal cortex of degus, revealed a significant effect of stress treatment [F(3,16) = 10.93, p < 0.01 and F(3,16) = 12.22, p < 0.01, respectively], but no significant sex effect (p = 0.84 and p = 0.12, respectively), nor were an interaction found between the two factors (p = 0.42 and p = 0.35, respectively; Fig. 5B and C). The comparisons among groups indicated that in the hippocampus, the social-stress treatments significantly reduced OXT compared to CTL, and CI-R treatment was unable to normalize it. Similar results were observed in PFC, with lower OXT in all groups under social-stress compared to CTL, with the lowest amount in the PI, and no changes at the CI-R group. These results suggest that different degrees of stress caused permanent dysfunction in the neuroendocrine system of OXT. We also measured OXT in plasma and we did not find significant effects in treatment (p = 0.09), sex (p = 0.59) or interaction effect (p = 0.64; data not shown).
Fig. 5.
Effect of LTCSIS in OXT in different regions of brain degus across the treatments. Absolute values from ELISAs of (A) hypothalamus lysates (B) hippocampal lysates (C) prefrontal cortex lysates. ELISA assay used the same protein extract that for western blot analyses: 90 μL/well (1 μg/μL). Standards and samples were run in duplicate. The data were analysed statistically using two-way ANOVA followed by Fisher's LSD post-hoc test. The statistical effect of stress treatment, sex and the interaction between the two factors are indicated in the top of the figure. Each symbol corresponds to data from a single-sex stress treatment group, represented as the mean ± SEM (n = 3). As is showed in Figures for two-way ANOVA crossing lines indicate the interaction between both factors: stress treatment and sex.
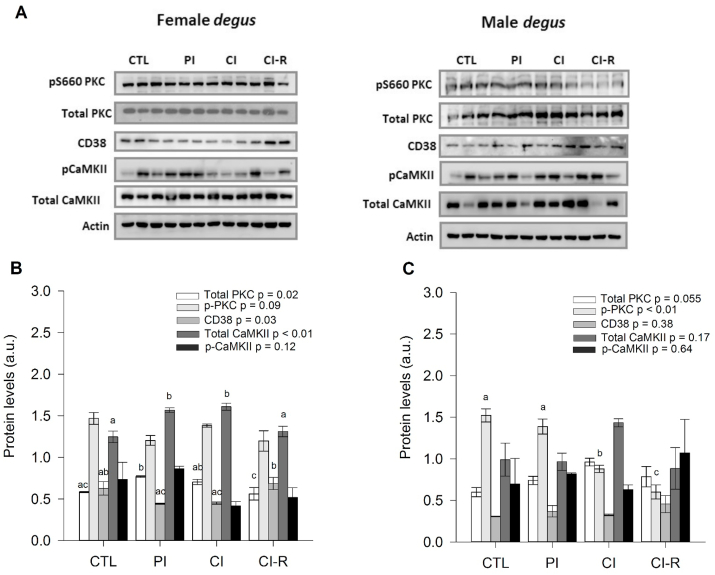
Second, we analysed the downstream signalling of OXT. We measured total and active PKC (total-PKC or p-PKC ratio), CD38, total, and active CaMKII (total-CaMKII or p-CaMKII ratio) in each brain region. In the hypothalamus of female degus, we found a significant effect in total PKC across stress treatments [F(3,8) = 5.89, p = 0.02]. Higher total PKC was found in PI compared to CTL, while lower quantity was found in CI-R compared to PI and CI groups (Fig. 6B). Whereas in males, non-significant differences were found with stress treatment (p = 0.055; Fig. 6C). Meanwhile, in females, no changes were found in the expression of the p-PKC ratio (p = 0.09; Fig. 6B); whereas in males, a significant effect of the stress treatment [F(3,8) = 31.51, p < 0.01], with CI and CI-R degus showing a dramatic decrease compared to CTL and PI groups (Fig. 6C). In terms of CD38, in female degus we found a statistically significant effect of stress treatment [F(3,8) = 4.83, p = 0.03], where CI-R females had significantly more CD38 protein than PI and CI female groups (Fig. 6B); while no significant differences were found in males (p = 0.38; Fig. 6C). We also explored changes in total CaMKII in females was significantly different across stress treatment [F(3,8) = 11.39, p < 0.01], where both CI and PI and PI degus showed higher values of total CaMKII than CTL. Interestingly, these levels were lower in the CI-R group, and comparable to CTL (Fig. 6B). Whereas in male degus we found no changes in total CaMKII across tress treatment (p = 0.17; Fig. 6C). Instead, for the p-CaMKII ratio, no changes were observed in female and male groups (p = 0.11 and p = 0.70, respectively; Fig. 6B and C). These results indicate that both total Ca+2-related proteins in the hypothalamus of females, but not of males, were affected by both LTCSIS and social buffering treatments, suggesting different sex-dependent mechanisms against LTCSIS in the hypothalamus region. See Fig. 9 for a schematic summary of the dynamic of these proteins.
Fig. 6.
Biochemical analysis of hypothalamus OXT-Ca2+ related proteins. (A) Western blot analysis of OXT-Ca2+ related protein for female and male degus. Densitometric analysis of hypothalamic total PKC, p-PKC, CD38, total CaMKII, and p-CaMKII in: (B) female degus (C) male degus. The data were analysed statistically using one-way ANOVA, with the p-value indicated at the top of the figure. Letters in the top of bars show statistical differences between the same protein across the stress treatment (Fisher's LSD post-hoc test). Results are expressed as mean ± SEM (n = 3). a.u: arbitrary units. Control (CTL), Partial Isolation (PI), Chronic Isolation (CI), and Re-socialization (CI-R) treatment groups.
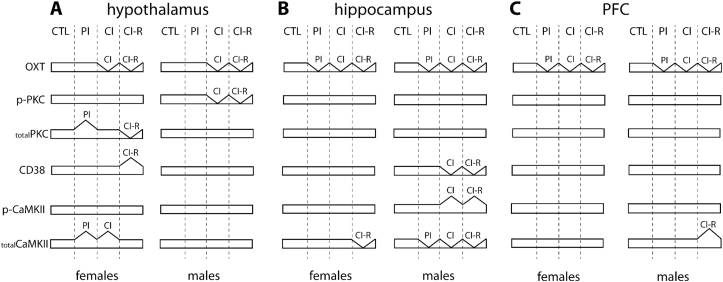
Fig. 9.
Schematic summary of OXT-PKC-CD38 signalling and Ca2+ related proteins dynamic in (a) hypothalamus of female and male (b) hippocampus of female and male (c) prefrontal cortex of female and male degus. Control (CTL), Partial Isolation (PI), Chronic Isolation (CI), and Re-socialization (CI-R) treatment groups.
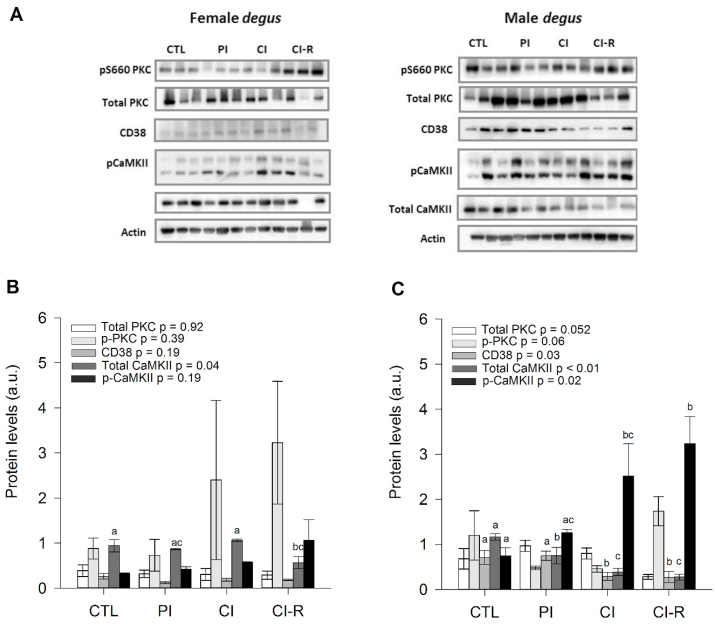
In the hippocampus of female degus, total PKC exhibited a non-significant effect of stress treatment for female degus (p = 0.92; Fig. 7B); additionally, in male degus, almost significant differences with stress treatment (p = 0.052; Fig. 7C). Also, no changes in the p-PKC ratio were found within female or male groups (p = 0.39 and p = 0.06, respectively; Fig. 7B and C). CD38 in female degus did not change across stress treatment (p = 0.18; Fig. 7B); however, in male degus we found a significant stress treatment effect [F(3,8) = 4.61, p = 0.03], with lower levels in CI compared to CTL, while re-socialization process was unable to increase CD38 (Fig. 7C). Moreover, total CaMKII in females revealed a significant effect of stress treatment [F(3,8) = 4.67, p = 0.04], with a decrease in this protein only in the CI-R group compared to CTL and CI (Fig. 7B). Instead in males, we found a statistically significant effect of the stress treatment [F(3,8) = 13.03, p < 0.01], where, lower levels were observed in PI, CI, and CI-R groups compared to CTL (Fig. 7C). Instead in females, p-CaMKII did not show changes within treatments (p = 0.19; Fig. 7B), but a significant effect of the stress treatment was found in males [F(3,8) = 5.68, p = 0.02], where CI and CI-R males showed higher values compared to CTL males (Fig. 7C). These results indicate that despite similar effects that were observed between sexes for total PKC, different outcomes took place in female and male degus for total CaMKII in both isolation and re-socialization treatments, suggesting that both LTCSIS and social buffering, exerts sex-dependent changes in some Ca+2-related proteins in degus. See Fig. 9 for a schematic summary of the dynamic of these proteins.
Fig. 7.
Biochemical analysis of hippocampal OXT-Ca2+ related proteins. (A) Western blot analysis of OXT-Ca2+ related proteins for female and male degus. Densitometric analysis of hippocampal total PKC, p-PKC, CD38, total CaMKII, and p-CaMKII in: (B) female degus (C) male degus. The data were analysed statistically using one-way ANOVA, with the p-value indicated at the top of the figure. Letters in the top of the bars show statistical differences between the same protein across the stress treatment (Fisher's LSD post-hoc test). Results are expressed as mean ± SEM (n = 3). a.u: arbitrary units. Control (CTL), Partial Isolation (PI), Chronic Isolation (CI), and Re-socialization (CI-R) treatment groups.
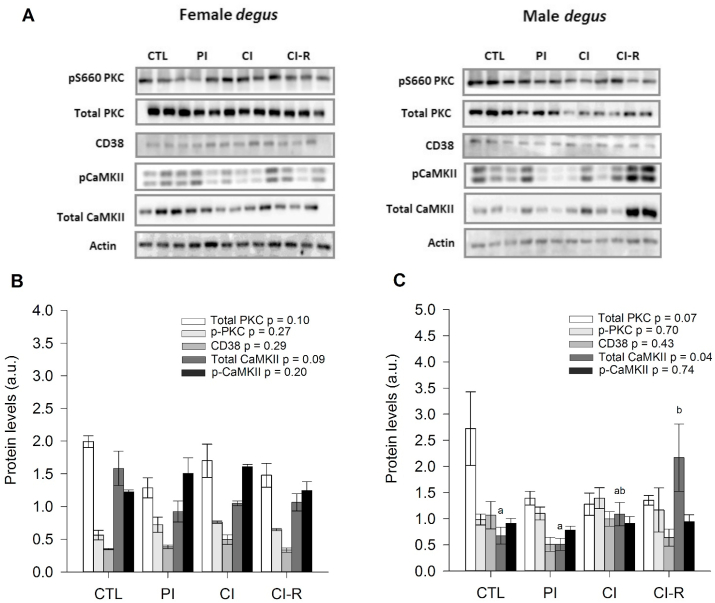
In the PFC, we found no changes in total PKC or p-PKC ratio across stress treatments, neither in female nor male degus (total PKC: p = 0.10 and p = 0.07, p-PKC ratio: p = 0.27 and p = 0.70, respectively; Fig. 8B and C). Similarly, both female and male degus revealed no effect of stress treatment in CD38 protein (p = 0.29 and p = 0.43, respectively; Fig. 8B and C). Furthermore, female degus revealed no significant differences in total CaMKII with stress treatment (p = 0.09; Fig. 8B); however, male degus showed a significant effect of stress treatment [F(3,8) = 4.40, p = 0.04] with CI-R degus showing higher values than CTL and PI groups (Fig. 8C). On the other hand, in the case of p-CaMKII ratio, both female and male degus did not significantly vary with stress treatment (p = 0.20 and p = 0.74, respectively; Fig. 8B and C). These results indicated that only total CaMKII protein of males, but not of females, were affected by social treatments, suggesting a specific sex-dependent effect as a result of LTCSIS in the PFC region. Additionally, this effect was opposite to the observed in the hypothalamus, which shows a clear brain region-dependent effect of LTCSIS. See Fig. 9 for a schematic summary of the dynamic of these proteins.
Fig. 8.
Biochemical analysis of prefrontal cortex (PFC) OXT-Ca2+ related proteins. (A) Western blot analysis of OXT-Ca2+ related proteins for female and male degus. Densitometric analysis of total PKC, p-PKC, CD38, total CaMKII, and p-CaMKII in: (B) female degus PFCs (C) male degus PFCs. The data were analysed statistically using one-way ANOVA, with the p-value indicated at the top of the figure. Letters in the top of the bars show statistical differences between the same protein across the stress treatment (Fisher's LSD post-hoc test). Results are expressed as mean ± SEM (n = 3). a.u: arbitrary units. Control (CTL), Partial Isolation (PI), Chronic Isolation (CI), and Re-socialization (CI-R) treatment groups.
4. Discussion
Our study sought to determine the consequences of LTCSIS in O. degus. For the first time, we evaluated behavioural responses to anxiety and social-related tests, and the profile of some brain-related proteins in adult, female and male individuals previously subjected to LTCSIS (from post-natal and post-weaning until adulthood). We also evaluated the effect of long-term re-socialization on these parameters. The prolonged isolation period used in this study might represent a chronic stress stimulus that mimics some physical or emotional stressful social situations in humans.
As a social species, O. degus is known to establish social bonds, living in a tightly social structure (Colonnello et al., 2011). Female degus under natural conditions, nest and nurse their pups communally (Fulk, 1976; Ebensperger et al., 2002). Moreover, allonursing of young has been observed among captive individuals (Ebensperger et al., 2002, 2007); hence, it seems very unlikely that degus pups under natural conditions stayed alone in the nest. The idea behind our experimental procedure was to leave the pups socially isolated for 1 h away from their mothers, but also the interaction with their siblings. This also included separation from all social groups during adolescence/adulthood.
Previously, we demonstrated that LTCSIS can impact the physiological, behavioural, functional, and molecular aspects of cognitive performance (Rivera et al., 2020). Using the same social stress treatments (i.e., PI, CI, and CI-R) we found impairment of the HPA axis negative feedback loop, in addition to cognitive deficits observed in chronically stressed animals. Remarkably, re-socialization normalizes most of these changes. Chronically stressed males, but not females, showed more efficient synaptic transmission but more deficient plasticity; while for synaptic proteins, we observed sex- and brain structure-dependent modulation (Rivera et al., 2020). Given that chronic stress affected several aspects of the cognitive processes, we wanted to evaluate its impact on affiliative, affective, and emotional parameters implicated in the social interaction affected during isolation stress.
Here, we first evaluated the effect of LTCSIS on anxiety behaviour in female and male degus, using two different tasks: novel object open field and the light-dark box tests, that assess explorative intention or “novelty” (Popovic et al., 2009). Under CI-R treatment, degus spent most of the time exploring the novel object (related to the natural exploratory behaviour). This is usually indicative of a relaxed emotional state after the traumatic LTCSIS experience. Instead, CI degus were less keen to explore the novelty, moved faster and travelled more distance around the arena, suggesting hyperactive behaviour. These results are in agreement with previous reports in rats, where an anxious, hyperreactivity or hyperaroused rats were less keen to explore the novelty, suggesting a deficit in motivation (Rygula et al., 2005; Thorsell et al., 2006; Smith et al., 2012). Similar anxiety-like behaviour and locomotor hyperactivity were observed in socially isolated mice and rats (Levine et al., 2007; Castillo-Gomez et al., 2017; Medendorp et al., 2018). On the other hand, “freezing” or immobility can also be considered an anxiety-like behaviour (Díaz-Morán et al., 2014). In the context of social isolation stress, the evidence is inconsistent, with studies showing increased (Berry et al., 2012; Liu et al., 2019), decreased immobility (Kulesskaya et al., 2011) or no differences (Hilakivi et al., 1989; Sullens et al., 2020). In our experiments, we did not observe freezing or immobility. Instead, our animals were actively exploring novel objects (CTL, PI, and CI-R groups), or moving around the arena displaying a clear anxiety-like hyperactive behaviour (CI group).
The classical open field test has been used to provide an initial screening for anxiety-related behaviour in rodents, measuring novel environment exploration and general locomotor activity. Indeed, the most common measure used to assess exploration/locomotor activity is the total distance travelled (Christmas and Maxwell, 1970). In our study, we used this test and we did not observe differences in the total distance travelled among the stress treatments; however, we did find it in the CI group at the novel object open field test. In another study, Choleris and colleagues (Choleris et al., 2001) found that benzodiazepines consistently reduced the typical anxiety-like behaviour; however, no overall effect was observed on total locomotion in the open field. Instead, these animals shift the locomotor pattern from “high exploration” to “high walking”, suggesting that under the effect of anxiolytics the animals still actively move. In our study, we observed that the speed and the total distance travelled was not affected by stress treatment. The work by Choleris also measured the time spent by the animals in the outer and in the central zone of the arena, and they observed the most behavioural variations in the centre of the open field (Choleris et al., 2001); like in our study, where this is the parameter that changes the most among treatments, despite not reaching significance (Fig. S1A).
On the other hand, a factor influencing anxiety-like behaviour in the open field is the “social isolation” caused by the physical separation from cage mates when performing the test (Christmas and Maxwell, 1970; File, 1980). In our study, all the animals are performing tests (controls and isolated), and the only variable is the time they are in isolated groups (PI, CI, and CI-R). Finally, it has been always suggested to use more than one test to measure anxiety, in order to accurately characterize anxiety-related behaviours and to strengthen the interpretations (Bailey and Crawley, 2009). These are the main reasons why we also used the “novel object” open field and the light-dark box tests in our study.
The tendency to avoid stressful situations (i.e., brightly lit places) was measured by the light-dark box test for anxiety (Crawley and Goodwin, 1980). The number of light/dark transitions is used as an index of activity-exploration in mice (Bourin and Hascoet, 2003). We found that CI-R degus were similar to CTL or PI group, confirming that 6-months of re-socialization could normalize behaviour. Interestingly, CI male degus transitioned more times between light and dark chambers versus CI female degus, suggesting that males are more insecure. Moreover, CI female degus had higher latency to enter into the dark chamber for the first time, suggesting more unwilling behaviour to novelty. However, regarding the time spent in the light part females and males behave similarly. This observation reflects the diurnal life of these animals and reinforces the measurement of light/dark transitions as indicative of anxiety-like behaviour. Despite this, some studies have reported that the diurnal version of the dark-light box test would produce less anxiety than other tests for diurnal animals (Popovic et al., 2009; Ashkenazy-Frolinger et al., 2015). Interestingly, our data reported differences between treatments and sex, similarly to previous results in the “novel” open field test. This confirms that classical behavioural tests used for nocturnal animals, can be adapted for diurnal animals. Using both tests we demonstrated high anxiety-like behaviours in socially isolated animals and a reduction in anxiety by re-socialization. More importantly, female and male degus experience social stress behaviour differently.
Social recognition memory reflects the ability to identify and remember conspecifics (Gheusi et al., 1994; Kogan et al., 2000), this is also necessary for the stability of social groups. To evaluate how LTCSIS affects sociability and social novelty preference, we used the three-chamber paradigm. Using the Recognition Index (RI), we found for session 1 that CI females behave similarly to the CTL and CI-R group, whereas CI males had lower values of RI compared to CTL and CI-R. These data suggest, that males’ sociability was negatively affected by stress treatment in contrast females were unaffected. All groups showed a clear preference for the compartment containing the unfamiliar conspecific, in fact they spent about 30% of time interacting with the unfamiliar degus and 8% of the time with the inanimate object (Fig. S2A). Compared to females, males stayed significantly more time interacting with unfamiliar degus (Fig. S2A). Previous evidence demonstrated that chronic stress reduces social motivation and social interaction, particularly in highly anxious animals (Lukkes et al., 2009; Van Der Kooij et al., 2014; Sandi and Haller, 2015). However, our results indicated that despite the LTCSIS, degus maintained social abilities (Van Den Berg et al., 1999; Sandi and Haller, 2015). Studies have suggested that stressed degus are willing to encounter conspecific animals to potentially ameliorate negative emotions to obtain positive neurochemical rewards (Kikusui et al., 2006; Gilles and Polston, 2017). As occurs in humans, stressful situations can promote either affiliative behaviour or group cohesion (Taylor, 2006). Interestingly, only PI females spent equally frequent visits to the compartment with the unfamiliar degu or the empty cage (i.e., lower RI), suggesting that early partial stress in females can affect adult behaviour, as they do not value physical/emotional contact with conspecifics in the same way as other groups.
On the other hand, during session II, social recognition memory assessed the spontaneous approaching behaviour observed in an individual re-exposed to a familiar conspecific. Both female and male CI degus had lower RI values compared to CTL, whereas re-socialization recovered the social memory to CTL. In general, male degus spent significantly more time interacting with Partner II (novel) than females (Fig. S2B). Moreover, we found that both CI female and males spent significantly more time interacting with Partner I but failed to distinguish the novel unfamiliar degus (Partner II), showing that CI treatment affects social memory independently of sex (Fig. S2B). A similar result was reported in PI females, resulting in lower RI compared with CTL degus. This result reinforces the previous idea that early life social isolation causes long-term behavioural alterations in adult female individuals, reaching levels comparable to chronic isolation. Our results are in line with the reported long-lasting effects of early life stress in memory performance in other animal models (Ibi et al., 2008; Mumtaz et al., 2018). Similarly, our study confirms that re-socialized degus were able to recover the impairment in social recognition memory in the LTCSIS animals (Shahar-Gold et al., 2013; Chen et al., 2016; An et al., 2017; Liu et al., 2018). Social interaction can moderate stressful experiences by buffering the adverse impact of isolation stress on social behaviour. Likewise, in humans social support and affiliative behaviours (“befriending”) provide a buffer against stress and improve health and well-being (Oppenheimer et al., 2016; Hsiao et al., 2018).
The OXT peptide has been associated with a variety of behavioural reactions, such as formation, maintenance, and modulation of social relationships (Anacker and Beery, 2013; Lopatina et al., 2013; Beery and Kaufer, 2015; Maroun and Wagner, 2016). In contrast, oxytocin receptors (OXT-Rs) blockade increases anxiety-related behaviours (Bosch and Neumann, 2008). OXT is released from neurosecretory cells at the hypothalamus (Higashida, 2016). Locally released OXT can activate OXT-Rs, triggering the release of intracellular Ca2+ and -PKC-CD38-OXT secretion, resulting in a positive autocrine/paracrine feedback. The release of Ca2+ can also activate CaMKII and its downstream effectors related to neural plasticity, among others (Jin et al., 2007; Lopatina et al., 2013; Higashida, 2016). Therefore, the amount of OXT and its Ca2+-related proteins can be affected in LTCSIS degus brains. Consequently, we analysed several social behaviours and correlated them with OXT signalling status across brain areas. Social behaviours mediated by OXT include social memory, attachment, maternal behaviour, and aggression. In the hypothalamus, OXT was comparable in CTL and PI groups in both female and males, however in males was significantly higher. Instead, re-socialization was unable to bring OXT back to control, showing that LTCSIS treatments cause a permanent reduction in OXT, below the CTL, being unable to overcome this effect. Previous studies have demonstrated that social enrichment/buffering (i.e., group housing) reduces the negative impact of maternal separation for affiliative behaviours. However, these effects were not associated with changes in the number of hypothalamic oxytocin neurons in female or male rats, suggesting changes at the level of OXT-Rs (Gilles and Polston, 2017). Moreover, non-social behaviours mediated by OXT that include learning and memory, and other physiological responses were not assessed in this study and could be indirectly implicated in the differences we observed along with areas (Yang et al., 2013; Rivera et al., 2020). Future studies should assess OXT-Rs by histochemical analyses in order to elucidate the mechanisms involved.
Active PKC and active CaMKII were unaltered in the hypothalamus of female degus subjected to different treatments. However, higher levels of the total PKC and CaMKII were observed in female and male PI groups compared to CTL, suggesting that protein expression is affected by early isolation. In males, hypothalamic CaMKII also remained unchanged, however PKC decreased in CI and CI-R males compared to CTL; this can be explained/or be the cause of the permanent reduction of OXT observed in males. We speculate that LTCSIS induces a sex-determined differential effect on OXT-Ca2+ mechanisms.
The multifunctional protein CD38 plays an important role in the regulation of OXT release Ca+2 influx and downstream pathways, modulating social memory (Jin et al., 2007; Lopatina et al., 2013). Indeed, social behaviour is disrupted when the hypothalamic elevation of intracellular Ca2+ concentration and OXT secretion is reduced in both female and male CD38 knockout mice (Lopatina et al., 2009). Our results showed a significant effect of LTCSIS treatments in hypothalamic CD38, showing an increase at the CI-R female group compared to PI and CI, showing that in females, the signalling pathway can be partially normalized with re-socialization. This result suggested that in females, CD38 can be upregulated to compensate for the reduction in OXT by social stress. Oppositely, no changes were observed in CD38 of male degus.
The OXT is released from the hypothalamus to different brain areas. OXT synthesis in the hypothalamus is followed by its transportation down the axons to accumulate in the terminal buttons until their release. These hypothalamic neurons exhibit a unique sex-specific morphology and functional plasticity; in adult rats, these neurons undergo morphological transformations in response to hormonal and social stimulation (Veening et al., 2010; Carter et al., 2020). OXT injections increase social exploration and the number of OXT neurons in the hypothalamus of males but not female rats (Duarte-Guterman et al., 2019), which can explain the sex differences observed in our study.
Reactions to social stressors include the increased synthesis of OXT, reflected in a high quantity of released OXT. However, OXT can also be accumulated in the hypothalamic neurons due to a reduced release rate. As we did not measure the OXT released in our study, higher levels of hypothalamic OXT can reflect either increased release or increased OXT accumulation. In particular, evidence on alteration of trafficking and/or release of OXT from axonal terminals in social disorders as autism has been observed (Grinevich et al., 2015). Additionally, some mutations lead to prohormones that are not secreted and aggregate to fibrillar proteins leading to cell death. Increased ratios of OXT prohormones have been shown in autistic children (Stoop, 2012). Therefore, the alteration of the axonal release of hypothalamic OXT should be assessed in future studies as a mechanism underlying social alterations.
The exposure to defined stressors activates the classical endocrine stress response (Smith and Wang, 2014). This response involves both intra-hypothalamic and peripheral release of oxytocin, therefore we measured OXT expression on the hippocampus and PFC, two brain structures implicated in social memory and performance. Both hypothalamic OXT-containing axons and OXT receptors are highly present in the hippocampus. Indeed, OXT may affect network oscillations or synchronization or affect synaptic plasticity in this brain area (Stoop, 2012). In our study, hippocampal OXT was significantly decreased in PI, CI, and CI-R groups compared to CTL. Previous studies have established that CD38 is critically involved in social memory (Kim et al., 2016). Indeed, CD38-KO mice showed deficits in several learning-memory and social tasks such as the Morris water maze, contextual fear conditioning, object recognition, and social recognition tests (Kim et al., 2016). In the hippocampus of females, we did not observe changes in CD38, p-PKC, or p-CaMKII across treatments, but lower total CaMKII was observed in the CI-R group compared to CTL and CI, suggesting that re-socialization can to modulate protein expression. Whereas in males, there is a lower expression of CD38 and a higher expression of p-CaMKII in CI and CI-R groups compared to CTL and PI groups. These data suggest that OXT-CD38 signalling can be permanently affected by chronic stress in males and showed that the re-socialization experiment cannot improve the isolation-induced impairments. Moreover, it suggests that the upregulation of p-CaMKII can be an independent effect. Other compensatory mechanisms may replace CD38 functions in the hippocampus of males to recover social memory performance as observed in previous studies (Palop et al., 2007; Murinova et al., 2017).
In PFC, OXT was significantly reduced in all social stress treatments compared to CTL. Due to the importance of OXT in social memory processes and recognition of conspecifics (Uekita and Okanoya, 2011), these results are in agreement with the social memory deficit across CI treatment measured by the three-chamber test. However, the OXT measurements in CI-R groups were not different than CI, showing that, re-socialization was unable to re-establish the normal OXT amounts in this brain region. We cannot rule out the possibility that the re-socialization period is insufficient to re-establish the physiological system in this long-lived animal, or by the opposite, that the isolation period is strong enough to change the system irreversibly. Regarding the other proteins, neither CD38, active PKC, or active CaMKII both in female and male groups were affected in the stress treatments. However, higher total CaMKII was observed only in CI-R males, suggesting that protein expression changes induced by re-socialization in a sex-dependent manner. This protein can be upregulated to compensate for the lack of OXT due to LTCSIS. More qualitatively, we could say that PFC is more resilient to LTCSIS. Recent studies in mice showed different effects of re-socialization in medial PFC (Makinodan et al., 2017). Specifically, early isolated rats exhibited decreased levels of calcium-binding proteins in association with reduced exploratory behaviour (Pascual et al., 2007). Our experiments in the PFC of degus showed that, regardless that OXT decreases under LTCSIS, Ca2+-related proteins remain unchanged, suggesting that their regulation could be compensated to keep the system working.
A previous report demonstrated that emotional stress triggers the release of OXT into the hypothalamic extracellular fluid. In contrast, plasma OXT remained unaltered by the same treatments (Engelmann et al., 1999). Accordingly, we did not observe changes in plasma OXT in animals under stress-inducing treatments. Moreover, these findings are in line with previous studies in animal models, where plasma OXT did not correlate with brain (Landgraf and Neumann, 2004), and in humans where OXT has not been detected in plasma (Taylor et al., 2006; Ditzen et al., 2007; Mcquaid et al., 2016). However, a recent review in this topic suggests that the use of unextracted plasma samples on ELISA tests might overestimate OXT changes in plasma, leading inaccurate OXT measurements due to the interference of high molecular weight factors contained in whole plasma (Leng and Sabatier, 2016). Consequently, since our study used unextracted plasma for OXT measurements we cannot rule out this possibility that the ELISA kit could have failed to detect fluctuations appropriately in response to physiological manipulation among stress treatments.
We agreed that the release of OXT within the hypothalamus is modulated by social (i.e., emotional) stress. Newly, our data suggest that under physiological conditions, the OXT from the hypothalamus, hippocampus, and PFC is differentially regulated by integrative networks at different molecular stages, probably to ensure the correct involvement of this peptide in the stress response of the animals.
Across our study, we observed the dynamic of several proteins by measuring their phosphorylated states in whole brain regions. Studies in CaMKII mutant mice suggested a relevant function of this protein in spatial learning and memory, but also in regulating anxiety-like and aggressive behaviours (Shobe, 2002; Wayman et al., 2008; Matsuo et al., 2009). In the hippocampus, previous studies observed that chronic psychosocial stress modulate both CaMKII and PKC (Gerges et al., 2004); whereas in the PFC, decreased activity of these proteins has been associated with impaired cognition and working memory (Runyan et al., 2005; Yabuki et al., 2013; Ghosh et al., 2015). Additionally, there could be changes at the scale of kinase activity or subcellular localization that were not measured in this study and should be assessed in future studies of LTCSIS (Gardoni et al., 2001; Li et al., 2017; Murinova et al., 2017).
Overall, our current data evaluated the chronic stress exposure throughout the lifespan, thereby resembling the behavioural and physiological changes of human beings that experience impairments in social behaviours. Our results confirm that LTCSIS across life induces alterations in the social domain, including impaired social memory and increased sociability. We showed evidence that female and male degus experience LTCSIS stress differently, having diverse molecular mechanisms to deal with sex-specific adaptive challenges. Furthermore, consistent with our behavioural findings, LTCSIS also disrupts the OXT pathway in the hypothalamus and at other target regions. This study revealed that re-socialization reverts behavioural alterations; however, it was unable to regulate the OXT in the three regions analysed, inducing long-lasting changes in the OXT-Ca2+ pathway proteins in both female and male degus. Remarkably, our paradigms of LTCSIS caused permanent molecular changes at the OXT signalling in different brain regions, with female and male degus showing homeostatic mechanisms that allow them to overcome their behavioural performance.
Altogether, our data evaluated the exposure to chronic stress throughout the lifespan, thereby resembling the behavioural and physiological changes that suffer human beings when experience disrupted social contact associated stress. In fact, many human conditions can resemble LTCSIS. Several studies have shown that about 75% of adult mental disorders originate during childhood. Later in life, these stressful situations translate into violence, drug abuse, or criminal behaviours (Cacioppo et al., 2006; Mfoafo-M’carthy and Huls, 2014; Wissow et al., 2016; Domènech-Abella et al., 2019; Mullins et al., 2020). Abandonment, poverty, social isolation during critical episodes such as natural disasters are frequently seen in underdeveloped countries. High rates of neglected-parenthood, mainly derived from poverty along with episodes of children separated from their parents during migratory processes, are likely to cause post-traumatic effects/disorders. More recently, the worldwide COVID19 pandemic has imposed long-term quarantines for a large proportion of the population, maintaining individuals socially isolated for several months, increasing anxiety and depression rates. Only time will tell the consequences of isolation-derived stress and anxiety-related effects on the mental health and behaviour of quarantined populations. In summary, our study provides a comprehensive view of the effects of stressful conditions and its consequences on highly social animals. More importantly, we demonstrate that re-socialization plays a significant role as a buffer for stressful situations, normalizing behaviour. However, this is not reflected at the molecular level, at least during the period assessed in our study. The impact of re-socialization in humans is yet to be determined.
CRediT authorship contribution statement
Daniela S. Rivera: Conceptualization, Methodology, Formal analysis, Investigation, Data curation, Writing - original draft, Writing - review & editing, Visualization, Funding acquisition. Carolina B. Lindsay: Methodology, Conceptualization, Writing - original draft, Writing - review & editing. Carolina A. Oliva: Conceptualization, Writing - review & editing, Visualization. Francisco Bozinovic: Writing - review & editing, Supervision, Funding acquisition. Nibaldo C. Inestrosa: Writing - review & editing, Supervision, Funding acquisition.
Declaration of competing interest
The authors declare no competing interests.
Acknowledgments
This work was supported by a postdoctoral grant from Fondo Nacional de Desarrollo Científico y Tecnológico (FONDECYT) N° 11190603 to DSR. NCI was supported by grants from the Basal Centre of Excellence in Science and Technology (CONICYT-PFB12/2007) and AFB 170005. In addition, a grant from CAPES-CONICYT FB 0002–2014 (Line 3) was awarded to FB.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ynstr.2020.100289.
Contributor Information
Daniela S. Rivera, Email: daniela.rivera@umayor.cl.
Nibaldo C. Inestrosa, Email: ninestrosa@bio.puc.cl.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- An D., Chen W., Yu D.Q., Wang S.W., Yu W.Z., Xu H., Wang D.M., Zhao D., Sun Y.P., Wu J.C., Tang Y.Y., Yin S.M. Effects of social isolation, re-socialization and age on cognitive and aggressive behaviors of Kunming mice and BALB/c mice. Anim. Sci. J. 2017;88:798–806. doi: 10.1111/asj.12688. [DOI] [PubMed] [Google Scholar]
- Anacker A.M., Beery A.K. Life in groups: the roles of oxytocin in mammalian sociality. Front. Behav. Neurosci. 2013;7:185. doi: 10.3389/fnbeh.2013.00185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen S.L., Teicher M.H. Stress, sensitive periods and maturational events in adolescent depression. Trends Neurosci. 2008;31:183–191. doi: 10.1016/j.tins.2008.01.004. [DOI] [PubMed] [Google Scholar]
- Arranz L., Gimenez-Llort L., De Castro N.M., Baeza I., De La Fuente M. [Social isolation during old age worsens cognitive, behavioral and immune impairment] Rev. Esp. Geriatr. Gerontol. 2009;44:137–142. doi: 10.1016/j.regg.2008.12.001. [DOI] [PubMed] [Google Scholar]
- Ashkenazy-Frolinger T., Einat H., Kronfeld-Schor N. Diurnal rodents as an advantageous model for affective disorders: novel data from diurnal degu (Octodon degus) J. Neural. Transm. 2015;122(Suppl. 1):S35–S45. doi: 10.1007/s00702-013-1137-3. [DOI] [PubMed] [Google Scholar]
- Babygirija R., Yoshimoto S., Gribovskaja-Rupp I., Bulbul M., Ludwig K., Takahashi T. Social interaction attenuates stress responses following chronic stress in maternally separated rats. Brain Res. 2012;1469:54–62. doi: 10.1016/j.brainres.2012.06.007. [DOI] [PubMed] [Google Scholar]
- Bailey K.R., Crawley J.N. Chapter 5: anxiety-related behaviors in mice. In: Buccafusco J.J., editor. Methods of Behavior Analysis in Neuroscience. 2009. (Frontiers in Neuroscience)). [Google Scholar]
- Bale T.L., Epperson C.N. Sex differences and stress across the lifespan. Nat. Neurosci. 2015;18:1413–1420. doi: 10.1038/nn.4112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beery A.K., Kaufer D. Stress, social behavior, and resilience: insights from rodents. Neurobiol Stress. 2015;1:116–127. doi: 10.1016/j.ynstr.2014.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry A., Bellisario V., Capoccia S., Tirassa P., Calza A., Alleva E., Cirulli F. Social deprivation stress is a triggering factor for the emergence of anxiety- and depression-like behaviours and leads to reduced brain BDNF levels in C57BL/6J mice. Psychoneuroendocrinology. 2012;37:762–772. doi: 10.1016/j.psyneuen.2011.09.007. [DOI] [PubMed] [Google Scholar]
- Blumstein D.T., Ebensperger L.A., Hayes L.D., Vasquez R.A., Ahern T.H., Burger J.R., Dolezal A.G., Dosmann A., Gonzalez-Mariscal G., Harris B.N., Herrera E.A., Lacey E.A., Mateo J., Mcgraw L.A., Olazabal D., Ramenofsky M., Rubenstein D.R., Sakhai S.A., Saltzman W., Sainz-Borgo C., Soto-Gamboa M., Stewart M.L., Wey T.W., Wingfield J.C., Young L.J. Toward an integrative understanding of social behavior: new models and new opportunities. Front. Behav. Neurosci. 2010;4:34. doi: 10.3389/fnbeh.2010.00034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch O.J., Neumann I.D. Brain vasopressin is an important regulator of maternal behavior independent of dams' trait anxiety. Proc. Natl. Acad. Sci. U. S. A. 2008;105:17139–17144. doi: 10.1073/pnas.0807412105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourin M., Hascoet M. The mouse light/dark box test. Eur. J. Pharmacol. 2003;463:55–65. doi: 10.1016/s0014-2999(03)01274-3. [DOI] [PubMed] [Google Scholar]
- Braun K. The prefrontal-limbic system: development, neuroanatomy, function, and implications for socioemotional development. Clin. Perinatol. 2011;38:685–702. doi: 10.1016/j.clp.2011.08.013. [DOI] [PubMed] [Google Scholar]
- Braun K., Kremz P., Wetzel W., Wagner T., Poeggel G. Influence of parental deprivation on the behavioral development in Octodon degus: modulation by maternal vocalizations. Dev. Psychobiol. 2003;42:237–245. doi: 10.1002/dev.10096. [DOI] [PubMed] [Google Scholar]
- Brent L.J.N., Ruiz-Lambides A., Platt M.L. Persistent social isolation reflects identity and social context but not maternal effects or early environment. Sci. Rep. 2017;7:17791. doi: 10.1038/s41598-017-18104-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buwalda B., Stubbendorff C., Zickert N., Koolhaas J.M. Adolescent social stress does not necessarily lead to a compromised adaptive capacity during adulthood: a study on the consequences of social stress in rats. Neuroscience. 2013;249:258–270. doi: 10.1016/j.neuroscience.2012.12.050. [DOI] [PubMed] [Google Scholar]
- Cacioppo J., Hughes M., Waite L., Hawkley L., Thisted R. Loneliness as a specific risk factor for depressive symptoms: cross-sectional and longitudinal analyses. Psychol. Aging. 2006;21(1):140–151. doi: 10.1037/0882-7974.21.1.140. Psychology and Aging 21, 140-151. [DOI] [PubMed] [Google Scholar]
- Carter C.S., Kenkel W.M., Maclean E.L., Wilson S.R., Perkeybile A.M., Yee J.R., Ferris C.F., Nazarloo H.P., Porges S.W., Davis J.M., Connelly J.J., Kingsbury M.A. Is oxytocin "nature's medicine"? Pharmacol. Rev. 2020;72:829–861. doi: 10.1124/pr.120.019398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castillo-Gomez E., Perez-Rando M., Belles M., Gilabert-Juan J., Llorens J.V., Carceller H., Bueno-Fernandez C., Garcia-Mompo C., Ripoll-Martinez B., Curto Y., Sebastia-Ortega N., Molto M.D., Sanjuan J., Nacher J. Early social isolation stress and perinatal NMDA receptor antagonist treatment induce changes in the structure and neurochemistry of inhibitory neurons of the adult amygdala and prefrontal cortex. eNeuro. 2017;4 doi: 10.1523/ENEURO.0034-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colonnello V., Iacobucci P., Fuchs T., Newberry R.C., Panksepp J. Octodon degus. A useful animal model for social-affective neuroscience research: basic description of separation distress, social attachments and play. Neurosci. Biobehav. Rev. 2011;35:1854–1863. doi: 10.1016/j.neubiorev.2011.03.014. [DOI] [PubMed] [Google Scholar]
- Costall B., Jones B.J., Kelly M.E., Naylor R.J., Tomkins D.M. Exploration of mice in a black and white test box: validation as a model of anxiety. Pharmacol. Biochem. Behav. 1989;32:777–785. doi: 10.1016/0091-3057(89)90033-6. [DOI] [PubMed] [Google Scholar]
- Crawley J., Goodwin F.K. Preliminary report of a simple animal behavior model for the anxiolytic effects of benzodiazepines. Pharmacol. Biochem. Behav. 1980;13:167–170. doi: 10.1016/0091-3057(80)90067-2. [DOI] [PubMed] [Google Scholar]
- Crawley J.N. John Wiley & Sons; New Jersey: 2005. Behavioral Neuroscience. Current Protocols in Neuroscience. [Google Scholar]
- Chen W., An D., Xu H., Cheng X., Wang S., Yu W., Yu D., Zhao D., Sun Y., Deng W., Tang Y., Yin S. Effects of social isolation and re-socialization on cognition and ADAR1 (p110) expression in mice. PeerJ. 2016;4 doi: 10.7717/peerj.2306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choleris E., Thomas A.W., Kavaliers M., Prato F.S. A detailed ethological analysis of the mouse open field test: effects of diazepam, chlordiazepoxide and an extremely low frequency pulsed magnetic field. Neurosci. Biobehav. Rev. 2001;25:235–260. doi: 10.1016/s0149-7634(01)00011-2. [DOI] [PubMed] [Google Scholar]
- Christmas A., Maxwell D. A comparison of the effects of some benzodiazepines and other drugs on aggressive and exploratory behaviour in mice and rats. Neuropharmacology. 1970;9:17–29. doi: 10.1016/0028-3908(70)90044-4. [DOI] [PubMed] [Google Scholar]
- Díaz-Morán S., Estanislau C., Ca T., Blázquez G., Ráez A., Tobe A., Fernández-Teruel A. Relationships of open-field behaviour with anxiety in the elevated zero-maze test: focus on freezing and grooming. World J. Neurosci. 2014;4:1–11. [Google Scholar]
- Ditzen B., Neumann I.D., Bodenmann G., Von Dawans B., Turner R.A., Ehlert U., Heinrichs M. Effects of different kinds of couple interaction on cortisol and heart rate responses to stress in women. Psychoneuroendocrinology. 2007;32:565–574. doi: 10.1016/j.psyneuen.2007.03.011. [DOI] [PubMed] [Google Scholar]
- Domènech-Abella J., Mundó J., Haro J., Rubio-Valera M. Anxiety, depression, loneliness and social network in the elderly: longitudinal associations from the Irish Longitudinal Study on Ageing (TILDA) J. Affect. Disord. 2019;246:82–88. doi: 10.1016/j.jad.2018.12.043. [DOI] [PubMed] [Google Scholar]
- Duarte-Guterman P., Lieblich S.E., Qiu W., Splinter J.E.J., Go K.A., Casanueva-Reimon L., Galea L.A.M. Oxytocin has sex-specific effects on social behaviour and hypothalamic oxytocin immunoreactive cells but not hippocampal neurogenesis in adult rats. Horm. Behav. 2019;122:104734. doi: 10.1016/j.yhbeh.2020.104734. [DOI] [PubMed] [Google Scholar]
- Ebensperger L.A. A review of the evolutionary causes of rodent group-living. Acta Theriol. 2001;46:115–144. [Google Scholar]
- Ebensperger L.A., Hurtado M.J., León C. An experimental examination of the consequences of communal versus solitary breeding on maternal condition and the early post-natal growth and survival of degu (Octodon degus) pups. Anim. Behav. 2007;73:185–194. [Google Scholar]
- Ebensperger L.A., Rivera D.S., Hayes L.D. Direct fitness of group living mammals varies with breeding strategy, climate and fitness estimates. J. Anim. Ecol. 2012;81:1013–1023. doi: 10.1111/j.1365-2656.2012.01973.x. [DOI] [PubMed] [Google Scholar]
- Ebensperger L.A., Veloso C., Wallem P.K. Do female degus communally nest and nurse their pups? J. Ethol. 2002;20:143–146. [Google Scholar]
- Eisenmann E.D., Rorabaugh B.R., Zoladz P.R. Acute stress decreases but chronic stress increases myocardial sensitivity to ischemic injury in rodents. Front. Psychiatr. 2016;7:71. doi: 10.3389/fpsyt.2016.00071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelmann M., Ebner K., Landgraf R., Holsboer F., Wotjak C.T. Emotional stress triggers intrahypothalamic but not peripheral release of oxytocin in male rats. J. Neuroendocrinol. 1999;11:867–872. doi: 10.1046/j.1365-2826.1999.00403.x. [DOI] [PubMed] [Google Scholar]
- File S. The use of social interaction as a method for detecting anxiolytic activity of chlordiazepoxide-like drugs. J. Neurosci. Methods. 1980;2:219–238. doi: 10.1016/0165-0270(80)90012-6. [DOI] [PubMed] [Google Scholar]
- Fulk G.W. Notes on the activity, reproduction, and social behavior of Octodon degus. J. Mammal. 1976;57:495–505. [Google Scholar]
- Gardoni F., Bellone C., Cattabeni F., Di Luca M. Protein kinase C activation modulates alpha-calmodulin kinase II binding to NR2A subunit of N-methyl-D-aspartate receptor complex. J. Biol. Chem. 2001;276:7609–7613. doi: 10.1074/jbc.M009922200. [DOI] [PubMed] [Google Scholar]
- Gerges N.Z., Aleisa A.M., Schwarz L.A., Alkadhi K.A. Reduced basal CaMKII levels in hippocampal CA1 region: possible cause of stress-induced impairment of LTP in chronically stressed rats. Hippocampus. 2004;14:402–410. doi: 10.1002/hipo.10193. [DOI] [PubMed] [Google Scholar]
- Gheusi G., Bluthe R.M., Goodall G., Dantzer R. Social and individual recognition in rodents: methodological aspects and neurobiological bases. Behav. Process. 1994;33:59–87. doi: 10.1016/0376-6357(94)90060-4. [DOI] [PubMed] [Google Scholar]
- Ghosh S., Reuveni I., Lamprecht R., Barkai E. Persistent CaMKII activation mediates learning-induced long-lasting enhancement of synaptic inhibition. J. Neurosci. 2015;35:128–139. doi: 10.1523/JNEUROSCI.2123-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert C., Mccafferty D., Le Maho Y., Martrette J.M., Giroud S., Blanc S., Ancel A. One for all and all for one: the energetic benefits of huddling in endotherms. Biol. Rev. Camb. Phil. Soc. 2009;85:545–569. doi: 10.1111/j.1469-185X.2009.00115.x. [DOI] [PubMed] [Google Scholar]
- Gilles Y.D., Polston E.K. Effects of social deprivation on social and depressive-like behaviors and the numbers of oxytocin expressing neurons in rats. Behav. Brain Res. 2017;328:28–38. doi: 10.1016/j.bbr.2017.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinevich V., Knobloch-Bollmann H.S., Eliava M., Busnelli M., Chini B. Assembling the puzzle: pathways of oxytocin signaling in the brain. Biol. Psychiatr. 2015;79:155–164. doi: 10.1016/j.biopsych.2015.04.013. [DOI] [PubMed] [Google Scholar]
- Hennessy M.B., Maken D.S., Graves F.C. Consequences of the presence of the mother or unfamiliar adult female on cortisol, ACTH, testosterone and behavioral responses of periadolescent Guinea pigs during exposure to novelty. Psychoneuroendocrinology. 2000;25:619–632. doi: 10.1016/s0306-4530(00)00014-7. [DOI] [PubMed] [Google Scholar]
- Higashida H. Somato-axodendritic release of oxytocin into the brain due to calcium amplification is essential for social memory. J. Physiol. Sci. 2016;66:275–282. doi: 10.1007/s12576-015-0425-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilakivi L.A., Ota M., Lister R.G. Effect of isolation on brain monoamines and the behavior of mice in tests of exploration, locomotion, anxiety and behavioral 'despair'. Pharmacol. Biochem. Behav. 1989;33:371–374. doi: 10.1016/0091-3057(89)90516-9. [DOI] [PubMed] [Google Scholar]
- Hsiao Y.H., Chang C.H., Gean P.W. Impact of social relationships on Alzheimer's memory impairment: mechanistic studies. J. Biomed. Sci. 2018;25:3. doi: 10.1186/s12929-018-0404-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibi D., Takuma K., Koike H., Mizoguchi H., Tsuritani K., Kuwahara Y., Kamei H., Nagai T., Yoneda Y., Nabeshima T., Yamada K. Social isolation rearing-induced impairment of the hippocampal neurogenesis is associated with deficits in spatial memory and emotion-related behaviors in juvenile mice. J. Neurochem. 2008;105:921–932. doi: 10.1111/j.1471-4159.2007.05207.x. [DOI] [PubMed] [Google Scholar]
- Ieraci A., Mallei A., Popoli M. Social isolation stress induces anxious-depressive-like behavior and alterations of neuroplasticity-related genes in adult male mice. Neural Plast. 2016:6212983. doi: 10.1155/2016/6212983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inestrosa N.C., Rios J.A., Cisternas P., Tapia-Rojas C., Rivera D.S., Braidy N., Zolezzi J.M., Godoy J.A., Carvajal F.J., Ardiles A.O., Bozinovic F., Palacios A.G., Sachdev P.S. Age progression of neuropathological markers in the brain of the Chilean rodent Octodon degus, a natural model of alzheimer's disease. Brain Pathol. 2015;25:679–691. doi: 10.1111/bpa.12226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin D., Liu H.X., Hirai H., Torashima T., Nagai T., Lopatina O., Shnayder N.A., Yamada K., Noda M., Seike T., Fujita K., Takasawa S., Yokoyama S., Koizumi K., Shiraishi Y., Tanaka S., Hashii M., Yoshihara T., Higashida K., Islam M.S., Yamada N., Hayashi K., Noguchi N., Kato I., Okamoto H., Matsushima A., Salmina A., Munesue T., Shimizu N., Mochida S., Asano M., Higashida H. CD38 is critical for social behaviour by regulating oxytocin secretion. Nature. 2007;446:41–45. doi: 10.1038/nature05526. [DOI] [PubMed] [Google Scholar]
- Kikusui T., Winslow J.T., Mori Y. Social buffering: relief from stress and anxiety. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2006;361:2215–2228. doi: 10.1098/rstb.2006.1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S., Kim T., Lee H.R., Jang E.H., Ryu H.H., Kang M., Rah S.Y., Yoo J., Lee B., Kim J.I., Lim C.S., Kim S.J., Kim U.H., Lee Y.S., Kaang B.K. Impaired learning and memory in CD38 null mutant mice. Mol. Brain. 2016;9:16. doi: 10.1186/s13041-016-0195-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kogan J.H., Frankland P.W., Silva A.J. Long-term memory underlying hippocampus-dependent social recognition in mice. Hippocampus. 2000;10:47–56. doi: 10.1002/(SICI)1098-1063(2000)10:1<47::AID-HIPO5>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Kulesskaya N., Rauvala H., Voikar V. Evaluation of social and physical enrichment in modulation of behavioural phenotype in C57BL/6J female mice. PloS One. 2011;6 doi: 10.1371/journal.pone.0024755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagraauw H.M., Kuiper J., Bot I. Acute and chronic psychological stress as risk factors for cardiovascular disease: insights gained from epidemiological, clinical and experimental studies. Brain Behav. Immun. 2015;50:18–30. doi: 10.1016/j.bbi.2015.08.007. [DOI] [PubMed] [Google Scholar]
- Landgraf R., Neumann I.D. Vasopressin and oxytocin release within the brain: a dynamic concept of multiple and variable modes of neuropeptide communication. Front. Neuroendocrinol. 2004;25:150–176. doi: 10.1016/j.yfrne.2004.05.001. [DOI] [PubMed] [Google Scholar]
- Lee T.M. Octodon degus: a diurnal, social, and long-lived rodent. ILAR J. 2004;45:14–24. doi: 10.1093/ilar.45.1.14. [DOI] [PubMed] [Google Scholar]
- Leng G., Sabatier N. Measuring oxytocin and vasopressin: bioassays, immunoassays and random numbers. J. Neuroendocrinol. 2016;28 doi: 10.1111/jne.12413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine J.B., Youngs R.M., Macdonald M.L., Chu M., Leeder A.D., Berthiaume F., Konradi C. Isolation rearing and hyperlocomotion are associated with reduced immediate early gene expression levels in the medial prefrontal cortex. Neuroscience. 2007;145:42–55. doi: 10.1016/j.neuroscience.2006.11.063. [DOI] [PubMed] [Google Scholar]
- Li D.P., Zhou J.J., Zhang J., Pan H.L. CaMKII regulates synaptic NMDA receptor activity of hypothalamic presympathetic neurons and sympathetic outflow in hypertension. J. Neurosci. 2017;37:10690–10699. doi: 10.1523/JNEUROSCI.2141-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J., Dietz K., Deloyht J.M., Pedre X., Kelkar D., Kaur J., Vialou V., Lobo M.K., Dietz D.M., Nestler E.J., Dupree J., Casaccia P. Impaired adult myelination in the prefrontal cortex of socially isolated mice. Nat. Neurosci. 2012;15:1621–1623. doi: 10.1038/nn.3263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu N., Wang Y., An A.Y., Banker C., Qian Y.H., O'donnell J.M. Single housing-induced effects on cognitive impairment and depression-like behavior in male and female mice involve neuroplasticity-related signaling. Eur. J. Neurosci. 2019;52:2694–2704. doi: 10.1111/ejn.14565. [DOI] [PubMed] [Google Scholar]
- Liu Y., Lv L., Wang L., Zhong Y. Social isolation induces rac1-dependent forgetting of social memory. Cell Rep. 2018;25:288–295. doi: 10.1016/j.celrep.2018.09.033. [DOI] [PubMed] [Google Scholar]
- Lopatina O., Inzhutova A., Salmina A.B., Higashida H. The roles of oxytocin and CD38 in social or parental behaviors. Front. Neurosci. 2013;6:182. doi: 10.3389/fnins.2012.00182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopatina O., Liu H.X., Amina S., Hashii M., Higashida H. Oxytocin-induced elevation of ADP-ribosyl cyclase activity, cyclic ADP-ribose or Ca(2+) concentrations is involved in autoregulation of oxytocin secretion in the hypothalamus and posterior pituitary in male mice. Neuropharmacology. 2009;58:50–55. doi: 10.1016/j.neuropharm.2009.06.012. [DOI] [PubMed] [Google Scholar]
- Lukkes J.L., Mokin M.V., Scholl J.L., Forster G.L. Adult rats exposed to early-life social isolation exhibit increased anxiety and conditioned fear behavior, and altered hormonal stress responses. Horm. Behav. 2009;55:248–256. doi: 10.1016/j.yhbeh.2008.10.014. [DOI] [PubMed] [Google Scholar]
- Makinodan M., Ikawa D., Yamamuro K., Yamashita Y., Toritsuka M., Kimoto S., Yamauchi T., Okumura K., Komori T., Fukami S.I., Yoshino H., Kanba S., Wanaka A., Kishimoto T. Effects of the mode of re-socialization after juvenile social isolation on medial prefrontal cortex myelination and function. Sci. Rep. 2017;7:5481. doi: 10.1038/s41598-017-05632-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maroun M., Wagner S. Oxytocin and memory of emotional stimuli: some dance to remember, some dance to forget. Biol. Psychiatr. 2016;79:203–212. doi: 10.1016/j.biopsych.2015.07.016. [DOI] [PubMed] [Google Scholar]
- Martin A.L., Brown R.E. The lonely mouse: verification of a separation-induced model of depression in female mice. Behav. Brain Res. 2010;207:196–207. doi: 10.1016/j.bbr.2009.10.006. [DOI] [PubMed] [Google Scholar]
- Matsuo N., Yamasaki N., Ohira K., Takao K., Toyama K., Eguchi M., Yamaguchi S., Miyakawa T. Neural activity changes underlying the working memory deficit in alpha-CaMKII heterozygous knockout mice. Front. Behav. Neurosci. 2009;3:20. doi: 10.3389/neuro.08.020.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mcquaid R.J., Mcinnis O.A., Paric A., Al-Yawer F., Matheson K., Anisman H. Relations between plasma oxytocin and cortisol: the stress buffering role of social support. Neurobiol Stress. 2016;3:52–60. doi: 10.1016/j.ynstr.2016.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medendorp W.E., Petersen E.D., Pal A., Wagner L.M., Myers A.R., Hochgeschwender U., Jenrow K.A. Altered behavior in mice socially isolated during adolescence corresponds with immature dendritic spine morphology and impaired plasticity in the prefrontal cortex. Front. Behav. Neurosci. 2018;12:87. doi: 10.3389/fnbeh.2018.00087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mfoafo-M’carthy M., Huls S. SAGE Open; 2014. Human Rights Violations and Mental Illness: Implications for Engagement and Adherence. [Google Scholar]
- Mullins T., Campbell E., Hogeveen J. Neighborhood deprivation shapes motivational-neurocircuit recruitment in children. Psychol. Sci. 2020;31:881–889. doi: 10.1177/0956797620929299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mumtaz F., Khan M.I., Zubair M., Dehpour A.R. Neurobiology and consequences of social isolation stress in animal model-A comprehensive review. Biomed. Pharmacother. 2018;105:1205–1222. doi: 10.1016/j.biopha.2018.05.086. [DOI] [PubMed] [Google Scholar]
- Murinova J., Hlavacova N., Chmelova M., Riecansky I. The evidence for altered BDNF expression in the brain of rats reared or housed in social isolation: a systematic review. Front. Behav. Neurosci. 2017;11:101. doi: 10.3389/fnbeh.2017.00101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann I.D. The advantage of social living: brain neuropeptides mediate the beneficial consequences of sex and motherhood. Front. Neuroendocrinol. 2009;30:483–496. doi: 10.1016/j.yfrne.2009.04.012. [DOI] [PubMed] [Google Scholar]
- Nishi M., Horii-Hayashi N., Sasagawa T. Effects of early life adverse experiences on the brain: implications from maternal separation models in rodents. Front. Neurosci. 2014;8:166. doi: 10.3389/fnins.2014.00166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oppenheimer C.W., Ladouceur C.D., Waller J.M., Ryan N.D., Allen K.B., Sheeber L., Forbes E.E., Dahl R.E., Silk J.S. Emotion socialization in anxious youth: parenting buffers emotional reactivity to peer negative events. J. Abnorm. Child Psychol. 2016;44:1267–1278. doi: 10.1007/s10802-015-0125-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palanza P., Gioiosa L., Parmigiani S. Social stress in mice: gender differences and effects of estrous cycle and social dominance. Physiol. Behav. 2001;73:411–420. doi: 10.1016/s0031-9384(01)00494-2. [DOI] [PubMed] [Google Scholar]
- Palop J.J., Chin J., Roberson E.D., Wang J., Thwin M.T., Bien-Ly N., Yoo J., Ho K.O., Yu G.Q., Kreitzer A., Finkbeiner S., Noebels J.L., Mucke L. Aberrant excitatory neuronal activity and compensatory remodeling of inhibitory hippocampal circuits in mouse models of Alzheimer's disease. Neuron. 2007;55:697–711. doi: 10.1016/j.neuron.2007.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual R., Zamora-Leon P., Catalan-Ahumada M., Valero-Cabre A. Early social isolation decreases the expression of calbindin D-28k and dendritic branching in the medial prefrontal cortex of the rat. Int. J. Neurosci. 2007;117:465–476. doi: 10.1080/00207450600773459. [DOI] [PubMed] [Google Scholar]
- Pitman D.L., Ottenweller J.E., Natelson B.H. Effect of stressor intensity on habituation and sensitization of glucocorticoid responses in rats. Behav. Neurosci. 1990;104:28–36. doi: 10.1037//0735-7044.104.1.28. [DOI] [PubMed] [Google Scholar]
- Popovic N., Bano-Otalora B., Rol M.A., Caballero-Bleda M., Madrid J.A., Popovic M. Aging and time-of-day effects on anxiety in female Octodon degus. Behav. Brain Res. 2009;200:117–121. doi: 10.1016/j.bbr.2009.01.001. [DOI] [PubMed] [Google Scholar]
- Rivera D.S., Inestrosa N.C., Bozinovic F. On cognitive ecology and the environmental factors that promote Alzheimer disease: lessons from Octodon degus (Rodentia: octodontidae) Biol. Res. 2016;49:10. doi: 10.1186/s40659-016-0074-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera D.S., Lindsay C.B., Codocedo J.F., Carreno L.E., Cabrera D., Arrese M.A., Vio C.P., Bozinovic F., Inestrosa N.C. Long-term, fructose-induced metabolic syndrome-like condition is associated with higher metabolism, reduced synaptic plasticity and cognitive impairment in Octodon degus. Mol. Neurobiol. 2018;55:9169–9187. doi: 10.1007/s12035-018-0969-0. [DOI] [PubMed] [Google Scholar]
- Rivera D.S., Lindsay C.B., Codocedo J.F., Morel I., Pinto C., Cisternas P., Bozinovic F., Inestrosa N.C. Andrographolide recovers cognitive impairment in a natural model of Alzheimer's disease (Octodon degus) Neurobiol. Aging. 2016;46:204–220. doi: 10.1016/j.neurobiolaging.2016.06.021. [DOI] [PubMed] [Google Scholar]
- Rivera D.S., Lindsay C.B., Oliva C.A., Codocedo J.F., Bozinovic F., Inestrosa N.C. Effects of long-lasting social isolation and re-socialization on cognitive performance and brain activity: a longitudinal study in Octodon degus. Sci. Rep. 2020 doi: 10.1038/s41598-020-75026-4. [Accepted for publication]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rostamkhani F., Zardooz H., Zahediasl S., Farrokhi B. Comparison of the effects of acute and chronic psychological stress on metabolic features in rats. J. Zhejiang Univ. - Sci. B. 2012;13:904–912. doi: 10.1631/jzus.B1100383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Runyan J.D., Moore A.N., Dash P.K. A role for prefrontal calcium-sensitive protein phosphatase and kinase activities in working memory. Learn. Mem. 2005;12:103–110. doi: 10.1101/lm.89405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rygula R., Abumaria N., Flugge G., Fuchs E., Ruther E., Havemann-Reinecke U. Anhedonia and motivational deficits in rats: impact of chronic social stress. Behav. Brain Res. 2005;162:127–134. doi: 10.1016/j.bbr.2005.03.009. [DOI] [PubMed] [Google Scholar]
- Sandi C., Haller J. Stress and the social brain: behavioural effects and neurobiological mechanisms. Nat. Rev. Neurosci. 2015;16:290–304. doi: 10.1038/nrn3918. [DOI] [PubMed] [Google Scholar]
- Shahar-Gold H., Gur R., Wagner S. Rapid and reversible impairments of short- and long-term social recognition memory are caused by acute isolation of adult rats via distinct mechanisms. PloS One. 2013;8 doi: 10.1371/journal.pone.0065085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shobe J. The role of PKA, CaMKII, and PKC in avoidance conditioning: permissive or instructive? Neurobiol. Learn. Mem. 2002;77:291–312. doi: 10.1006/nlme.2001.4022. [DOI] [PubMed] [Google Scholar]
- Silk J.B., Beehner J.C., Bergman T.J., Crockford C., Engh A.L., Moscovice L.R., Wittig R.M., Seyfarth R.M., Cheney D.L. Strong and consistent social bonds enhance the longevity of female baboons. Curr. Biol. 2010;20:1359–1361. doi: 10.1016/j.cub.2010.05.067. [DOI] [PubMed] [Google Scholar]
- Silk J.S., Vanderbilt-Adriance E., Shaw D.S., Forbes E.E., Whalen D.J., Ryan N.D., Dahl R.E. Resilience among children and adolescents at risk for depression: mediation and moderation across social and neurobiological contexts. Dev. Psychopathol. 2007;19:841–865. doi: 10.1017/S0954579407000417. [DOI] [PubMed] [Google Scholar]
- Smith A.S., Wang Z. Hypothalamic oxytocin mediates social buffering of the stress response. Biol. Psychiatr. 2014;76:281–288. doi: 10.1016/j.biopsych.2013.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith C.B., Williams-Jones P., Lewis-Trabeaux S., Mitchell D. Facilitators and barriers to success among ethnic minority students enrolled in a predominately white baccalaureate nursing program. J. Natl. Black Nurses' Assoc. JNBNA. 2012;23:41–51. [PubMed] [Google Scholar]
- Spijker S., Faliagkas L., Rao-Ruiz P. Dissection of Rodent Brain Regions: Guided Free-Hand Slicing and Dissection of Frozen Tissue. second ed. Humana Press Inc); 2019. pp. 7–19. (Neuroproteomics). [Google Scholar]
- Stanton M.A., Mann J. Early social networks predict survival in wild bottlenose dolphins. PloS One. 2012;7 doi: 10.1371/journal.pone.0047508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoop R. Neuromodulation by oxytocin and vasopressin. Neuron. 2012;76:142–159. doi: 10.1016/j.neuron.2012.09.025. [DOI] [PubMed] [Google Scholar]
- Sullens D.G., Gilley K., Jensen K., Sekeres M.J. 2020. Girls Gone Wild: Social Isolation Induces Hyperactivity and Exploration in Aged Female Mice. bioRxiv. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor S.E. Tend and befriend: biobehavioral bases of affiliation under stress. Curr. Dir. Psychol. Sci. 2006;15:273–277. [Google Scholar]
- Taylor S.E., Gonzaga G.C., Klein L.C., Hu P., Greendale G.A., Seeman T.E. Relation of oxytocin to psychological stress responses and hypothalamic-pituitary-adrenocortical axis activity in older women. Psychosom. Med. 2006;68:238–245. doi: 10.1097/01.psy.0000203242.95990.74. [DOI] [PubMed] [Google Scholar]
- Taylor S.E., Welch W.T., Kim H.S., Sherman D.K. Cultural differences in the impact of social support on psychological and biological stress responses. Psychol. Sci. 2007;18:831–837. doi: 10.1111/j.1467-9280.2007.01987.x. [DOI] [PubMed] [Google Scholar]
- Thorsell A., Slawecki C.J., El Khoury A., Mathe A.A., Ehlers C.L. The effects of social isolation on neuropeptide Y levels, exploratory and anxiety-related behaviors in rats. Pharmacol. Biochem. Behav. 2006;83:28–34. doi: 10.1016/j.pbb.2005.12.005. [DOI] [PubMed] [Google Scholar]
- Tulogdi A., Toth M., Barsvari B., Biro L., Mikics E., Haller J. Effects of resocialization on post-weaning social isolation-induced abnormal aggression and social deficits in rats. Dev. Psychobiol. 2014;56:49–57. doi: 10.1002/dev.21090. [DOI] [PubMed] [Google Scholar]
- Uekita T., Okanoya K. Hippocampus lesions induced deficits in social and spatial recognition in Octodon degus. Behav. Brain Res. 2011;219:302–309. doi: 10.1016/j.bbr.2011.01.042. [DOI] [PubMed] [Google Scholar]
- Van Den Berg C.L., Pijlman F.T., Koning H.A., Diergaarde L., Van Ree J.M., Spruijt B.M. Isolation changes the incentive value of sucrose and social behaviour in juvenile and adult rats. Behav. Brain Res. 1999;106:133–142. doi: 10.1016/s0166-4328(99)00099-6. [DOI] [PubMed] [Google Scholar]
- Van Der Kooij M.A., Fantin M., Rejmak E., Grosse J., Zanoletti O., Fournier C., Ganguly K., Kalita K., Kaczmarek L., Sandi C. Role for MMP-9 in stress-induced downregulation of nectin-3 in hippocampal CA1 and associated behavioural alterations. Nat. Commun. 2014;5:4995. doi: 10.1038/ncomms5995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veenema A.H., Reber S.O., Selch S., Obermeier F., Neumann I.D. Early life stress enhances the vulnerability to chronic psychosocial stress and experimental colitis in adult mice. Endocrinology. 2008;149:2727–2736. doi: 10.1210/en.2007-1469. [DOI] [PubMed] [Google Scholar]
- Veening J.G., De Jong T., Barendregt H.P. Oxytocin-messages via the cerebrospinal fluid: behavioral effects; a review. Physiol. Behav. 2010;101:193–210. doi: 10.1016/j.physbeh.2010.05.004. [DOI] [PubMed] [Google Scholar]
- Wayman G.A., Lee Y.S., Tokumitsu H., Silva A.J., Soderling T.R. Calmodulin-kinases: modulators of neuronal development and plasticity. Neuron. 2008;59:914–931. doi: 10.1016/j.neuron.2008.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wissow L., Ginneken N.V., Chandna J., Rahman A. Integrating children's mental health into primary care. Pediatr. Clin. 2016;63:97–113. doi: 10.1016/j.pcl.2015.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright J.W., Kern M.D. Stereotaxic atlas of the brain of Octodon degus. J. Morphol. 1992;214:299–320. doi: 10.1002/jmor.1052140306. [DOI] [PubMed] [Google Scholar]
- Yabuki Y., Nakagawasai O., Moriguchi S., Shioda N., Onogi H., Tan-No K., Tadano T., Fukunaga K. Decreased CaMKII and PKC activities in specific brain regions are associated with cognitive impairment in neonatal ventral hippocampus-lesioned rats. Neuroscience. 2013;234:103–115. doi: 10.1016/j.neuroscience.2012.12.048. [DOI] [PubMed] [Google Scholar]
- Yang H.P., Wang L., Han L., Wang S.C. Nonsocial functions of hypothalamic oxytocin. ISRN Neurosci. 2013;2013:179272. doi: 10.1155/2013/179272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yee J.R., Cavigelli S.A., Delgado B., Mcclintock M.K. Reciprocal affiliation among adolescent rats during a mild group stressor predicts mammary tumors and lifespan. Psychosom. Med. 2008;70:1050–1059. doi: 10.1097/PSY.0b013e31818425fb. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.