Abstract
Characterizing typologies of childhood adversity may inform the development of risk profiles and corresponding interventions aimed at mitigating its lifelong consequences. A neurobiological grounding of these typologies requires systematic comparisons of neural structure and function among individuals with different exposure histories. Using seed-to-whole brain analyses, this study examined associations between childhood adversity and amygdala resting-state functional connectivity (rs-fc) in adolescents aged 11–19 years across three independent studies (N = 223; 127 adversity group) in both general and dimensional models of adversity (comparing abuse and neglect). In a general model, adversity was associated with altered amygdala rs-fc with clusters within the left anterior lateral prefrontal cortex. In a dimensional model, abuse was associated with altered amygdala rs-fc within the orbitofrontal cortex, dorsal precuneus, posterior cingulate cortex, and dorsal anterior cingulate cortex/anterior mid-cingulate cortex, as well as within the dorsal attention, visual, and somatomotor networks. Neglect was associated with altered amygdala rs-fc with the hippocampus, supplementary motor cortex, temporoparietal junction, and regions within the dorsal attention network. Both general and dimensional models revealed unique regions, potentially reflecting pathways by which distinct histories of adversity may influence adolescent behavior, cognition, and psychopathology.
Keywords: Childhood adversity, Childhood maltreatment, Amygdala, Resting-state functional connectivity, Adolescence
1. Introduction
Many psychiatric disorders first emerge during adolescence (Kessler et al., 2005, 2007), and childhood adversity is associated with higher rates of mental health diagnoses in adolescents (McLaughlin et al., 2012). Adolescents who have experienced severe forms of adversity, such as maltreatment, may exhibit clinically and neurobiologically distinct sub-types of psychopathology (Teicher and Samson, 2013) along with varying social, cognitive, and behavioral profiles related to the type(s) of adversity they have experienced (Hildyard and Wolfe, 2002; Kuhlman et al., 2017). Understanding the partly distinct neurobiological mechanisms associated with specific types of adversity may support the identification of unique risk profiles and corresponding intervention targets across development.
Similar to other areas of mental health research (Fair et al., 2012; Feczko and Fair, 2020), investigators began their initial inquiries into understanding the neurobiological consequences of childhood adversity via direct comparisons between those with and without specific exposures (case vs control; e.g., Bremner et al., 1997; De Bellis et al., 1999; Stein et al., 1997). Some analyses further differentiated between subtypes of adversity by examining them in separate models (e.g., Edmiston et al., 2011; Gheorghe et al., 2020; van Rooij et al., 2019), while others turned to cumulative risk models tallying the total number of distinct exposures (Evans et al., 2013). Although historically common, these approaches may fail to account for co-occurrence among exposures and/or assume that diverse types of adverse exposures affect development identically.
This study aims to disentangle mechanistic heterogeneity by systematically comparing the effects of distinct dimensions of adversity on amygdala resting-state functional connectivity (rs-fc), specifically evaluating a general model of adversity (focusing on maltreatment) versus a dimensional one (distinguishing between abuse and neglect). We test predictions that specific neural changes are associated with abuse and neglect using rs-fc magnetic resonance imaging (MRI), which examines low-frequency correlations in blood oxygen level dependent (BOLD) timeseries while subjects are ‘at rest’ (i.e., not performing a task). Rs-fc indirectly relates to spontaneous neuronal activity and organizes into reproducible and stable brain networks (for a review, see Grayson and Fair, 2017). As resting-state MRI scans are noninvasive and fairly stable (Gratton et al., 2018), they are a feasible method for characterizing neurodevelopmental and psychiatric disorders (Di Martino et al., 2014) and individualized neural profiles (Finn et al., 2015; Miranda-Dominguez et al., 2014).
We focus on the rs-fc of the amygdala, a brain region implicated in developmental psychopathology (Pine and Fox, 2015) due to its central role in emotion processing (Phelps and LeDoux, 2005). In addition to supporting the body’s stress response, the amygdala is critical for long-term emotional learning about environmental stimuli (e.g., Hooker et al., 2006) across development (Nelson et al., 2014). It does so via cortical interactions, including connections with medial prefrontal regions (Berretta, 2005). Some studies have found that adversity is associated with reduced amygdala-medial prefrontal cortex rs-fc (Cisler, 2017; Thomason et al., 2015; in females only in Herringa et al., 2013), while others have not (Nooner et al., 2013; Saxbe et al., 2018; van der Werff et al., 2012). The lateral prefrontal and orbitofrontal cortices, insula, cingulate cortex, and other regions have also exhibited altered adversity-related amygdala rs-fc (for a review, see Teicher et al., 2016), although inconsistencies within this literature may be related to small samples sizes and exposure heterogeneity.
When mapping associations between adversity and neural function, changes in neural functioning alone should not be interpreted as deficits (Ellwood-Lowe et al., 2016) and may indeed reflect functionally adaptive changes (Teicher et al., 2016). However, analyses of this type can provide foundational knowledge supporting the integration of past and future investigations linking adversity-related changes, neural functioning, and relevant outcomes, including psychopathology.
1.1. Current study
This study aimed to examine cortico-amygdala rs-fc in general and dimensional models of adversity across three cross-sectional adolescent samples. In the general model, adversity was operationalized as recruitment into foster care or child advocacy center groups and/or exposure to one or more forms of maltreatment (physical, emotional, and sexual abuse as well as physical and emotional neglect) across studies. The dimensional model operationalized threat as exposure to any of these forms of abuse and operationalized deprivation as exposure to any of these forms of neglect. Hypotheses for this study were informed by the Dimensional Model of Adversity and Psychopathology (McLaughlin et al., 2014), a theoretical model that distinguishes between threats to physical integrity and deprivation of complex environmental inputs. We note that abuse and neglect each reflect only single examples of threat and deprivation, respectively, from the constellation of experiences that constitute each of these dimensions. Adversity assessment predominantly relied on retrospective self-report measures; while not uncommon, this approach is limited (see Discussion).
In the general adversity model, we identified cortical regions exhibiting adversity-related changes in amygdala rs-fc using seed to whole-brain analyses. We preregistered hypotheses that adversity would, on average, be related to decreased amygdala rs-fc with clusters within the ventromedial prefrontal cortex (vmPFC), subgenual anterior cingulate cortex (sgACC), and lateral prefrontal cortex (lPFC). We also hypothesized altered amygdala rs-fc with clusters within the orbitofrontal cortex (OFC), posterior cingulate cortex (PCC)/precuneus, and insula (direction unspecified; hypotheses informed by Teicher et al., 2016). In the dimensional model, we identified effects of abuse and neglect on cortico-amygdala rs-fc. Importantly, we identified effects associated with abuse when controlling for neglect (and vice versa). This approach is critical for establishing specificity for widely co-occurring types of adversity (McMahon et al., 2003) and has been used in recent structural neuroimaging work that additionally directly compares such models to cumulative risk models (King et al., 2019; LoPilato et al., 2019). We hypothesized that abuse would be associated with altered amygdala rs-fc with clusters within the vmPFC and sgACC (hypotheses informed by McLaughlin et al., 2014), as well as with regions of the fronto-parietal network (specifically lateral PFC; based on exploratory analyses described in S1 of the Supplementary Materials). We further hypothesized that neglect would be negatively associated with amygdala rs-fc within regions of the dorsal attention network, as well as sensory and somatomotor processing regions (also based on exploratory analyses).
2. Methods
This study examined adolescent amygdala rs-fc changes associated with general and dimensional models of adversity across three cross-sectional adolescent samples. Methods and hypotheses were described in a pre-registration (https://osf.io/u3dey/; see Section 2.6 for a description of deviations from preregistered analyses).
2.1. Participants
Table 1 provides an overview of the three studies used in these analyses (all data were collected in Oregon): University of Oregon’s Teen Decisions Study (TDS; PI Chamberlain and Fisher), Oregon Health & Science University’s Teen Stress Study (TSS; PI Mackiewicz Seghete), and Project MINA (PI Feldstein Ewing). Each study recruited a range of adolescents across different rates of adversity, for a total of 223 adolescents aged 11–19 years across three studies. Recruitment criteria differed by study, most notably in terms of a) age, b) less stringent inclusion criteria for medication and psychiatric disorders in TDS and c) sampling binge drinking youth from the community for Project MINA as compared to recruiting from separate community and adversity groups in TDS and TSS. Neuroimaging studies with adolescents that have experienced significant adversity tend to have a small number of participants, reflecting the resource- and funding-intensive nature of data collection of this type on a special population of minors. Collapsing across heterogeneous samples is a strategy with distinct strengths and limitations (see Discussion) that increases the number of participants in a sample, making it possible to examine the effects of dimensions of adversity. Furthermore, results derived from multiple heterogeneous samples are less likely to be study-specific and could be more generalizable to a larger population.
Table 1.
Overview of three adolescent studies used in this analysis.
| TDS | TSS | Project MINA | |
|---|---|---|---|
| Ages | 11−17 | 13−17 | 14−19 |
| Recruitment | A high adversity group of youth were recruited from the child welfare system in Lane County with caseworker approval. These adolescents were currently in foster or kinship care, or youths with open cases in the child welfare system still living with their biological parents. | A high adversity group of youth with a likely history of or confirmed exposure to abuse and/or neglect were recruited via a child advocacy center in the Portland Metro Area or individuals from the broader community reporting a history of abuse and/or neglect. | Participants reported one or more binge events (girls consuming 3+ drinks, boys consuming 4+ drinks per drinking occasion) in the past two months. |
| A comparison group was recruited from Lane County community with no history of involvement with the child welfare system. | A comparison group was recruited from the Portland Metro Area with no history of trauma. | ||
|
| |||
| Exclusion criteria related to medications and substance exposure | Medications were documented, including their type and dose, as well as frequency and duration of use. | No current use of medications that could affect the central nervous system (e.g., psychotropic medication). No excessive substance use (cutoff scores varied by age and gender, see Appendix A for cutoffs that sought to exclude for moderate to high levels of substance use) or maternal use of alcohol (>2 drinks in a week) and any maternal use of nicotine or other drugs during pregnancy. | No use of recreational drugs more than 3 times total, including prescription medications in the past month (exceptions: cannabis, tobacco, and e-cigarette products). No same day alcohol or cannabis use. |
|
| |||
| Psychiatric or neurological disorders | Participants were excluded for autism spectrum disorder, Asperger syndrome, or pervasive developmental disorder, not otherwise specified; schizophrenia, obsessive compulsive disorder, seizure disorders, central nervous system infection (e.g. meningitis), brain tumors, muscular or myotonic dystrophy, and significant visual impairment. They were not excluded for current or prior mood, behavior, and anxiety disorders, including but not limited to depression, bipolar disorder, ADD/ADHD, and oppositional defiant or conduct disorder. | Participants were excluded if they met criteria for DSM-IV bipolar disorder, history of psychosis, substance use disorder, as assessed by the study team; reported autism spectrum disorder; major neurological or medical illness or significant head trauma (loss of consciousness > 2 min); uncorrectable vision or hearing impairments, or color blindness; intellectual disability. They were also excluded if there was a reported history of psychotic disorders in their biological parents. | Participants were excluded if they had a history of brain injury or neurological diagnoses (including loss of consciousness ≥2 min) or a current psychotic or neurodevelopmental disorder. |
|
| |||
| Misc | Participants must be right-handed. All were fluent in English. | Participants must be right-handed. They were excluded for current trauma, premature birth (<34 weeks gestation), or low birth weight (<5 lbs.). All were fluent in English. | Participants must not be left-handed. All were fluent in English. |
|
| |||
| MRI-related | Participants were excluded for MRI contraindications. Participants were not scanned if they reported that they might be pregnant. | ||
Each sample was either recruited or divided into a high adversity group based on known or reported experiences of childhood maltreatment, and a control group that, to the best of our knowledge from questionnaires and/or corroborating case/caregiver reports, had not had such experiences. Table 2 compares the sample characteristics of the adversity and control groups for each study. Adversity-exposed and non-exposed adolescents were fairly well-matched in age. Table 3 presents the characteristics of individuals that had experienced abuse, neglect, both, or neither across the full sample. Adolescents that had been exposed to adversity had lower parental income, as well as higher rates of mental health diagnoses and/or psychotropic medication use, on average (see S2.1 and S2.2 of the Supplementary Materials for sensitivity analyses examining the impact of these variables). All studies obtained parental and/or caregiver consent as well as minor assent from minors, or participant consent for non-minors.
Table 2.
Number of subjects, age, and IQ by study and adversity group status.
| Control N | Adversity N | Control Age (SD) | Adversity Age (SD) | Control IQ (SD) | Adversity IQ (SD) | |
|---|---|---|---|---|---|---|
| TDS | 39 (19 F) | 58 (30 F) | 14.15 (1.62) | 14.52 (1.53) | 108.33 (12.24) | 101.66 (11.51) |
| TSS | 19 (15 F) | 24 (19 F) | 15.71 (1.15) | 15.21 (1.14) | 114.84 (10.63) | 105.92 (9.94) |
| Project MINA | 38 (25 F) | 45 (28 F) | 18.98 (0.52) | 18.49 (1.27) | NA | NA |
| Total | 96 (59 F) | 127 (77 F) | 16.37 (2.5) | 16.05 (2.28) | NA | NA |
Table 3.
Descriptive statistics for parental income and psychopathology across exposures.
| Age (SD) | Median income in thousands | Mean income in thousands (SD) | N with mental health diagnosis and/or psychotropic medication use (percentage) | |
|---|---|---|---|---|
| General adversity model | ||||
| Adversity (N = 127) | 16.1 (2.3) | 68 | 87 (91) | 56 (44.4 %*) |
| None (N = 96) | 16.4 (2.5) | 90 | 108 (84) | 31 (32.3 %) |
| Dimensional model | ||||
| Abuse only (N = 33) | 16.7 (2.5) | 50 | 89 (95) | 13 (40.6 %*) |
| Neglect only (N = 28) | 15.5 (2.3) | 63 | 70 (60) | 11 (39.3 %) |
| Both (N = 45) | 16.4 (2.3) | 75 | 99 (115) | 21 (46.7 %) |
| Neither (N = 96) | 16.4 (2.5) | 90 | 108 (84) | 31 (32.3 %) |
Note. * Due to a missing datapoint, the denominator for this calculation is N-1 rather than N.
2.1.1. TDS (P50 DA035763, PIs Chamberlain and Fisher)
For the TDS study, 89 subjects were recruited into a community sample and participated in the session protocol. Of these, scans were unusable if adolescents completed a behavioral-only version of the protocol due to participant preference (N = 8), did not complete a resting state scan (N = 8), did not complete a field map (N = 1), or exhibited severe dropout across functional scans (N = 1), leaving a total of 71 potentially usable scans in the community sample. A total of 75 subjects were recruited into a foster-care sample and participated in the session protocol. Of these, scans were unusable if adolescents completed a behavioral-only protocol (N = 3), did not complete a resting state scan (N = 12), or did not complete a field map (N = 2), leaving 58 potentially usable scans. To make the TDS sample more comparable to the other samples, participants who reported that they were currently taking psychotropic medications were further excluded from analyses (community group N = 2, foster care group N = 3). Participants were also excluded if they did not meet an a priori criteria for sufficient resting-state data following motion scrubbing (at least five minutes of data with a framewise displacement of less than 0.2 mm; resulted in excluding 11 from the community group and 16 in the foster care group; more participants were excluded for this reason in TDS as compared to other studies due to a shorter resting-state scan time). Therefore, a total of 97 adolescents were included in the general adversity model, with 58 from the community sample and 39 from the foster care sample. In general adversity models, participants from the foster care sample were included in the adversity group, along with an additional 19 participants from the community sample who reported experiencing adversity in self-report measures (adversity group N = 58, control group N = 39). Despite being a part of the foster care sample, 13 participants did not endorse having experienced abuse or neglect and were excluded from dimensional analyses. (See Section 2.2.3 for more details on defining adversity, and Op de Macks et al., 2018 for additional information on the community sample and overall study).
2.1.2. TSS (K23MH105678, PI Mackiewicz Seghete)
For the TSS study, participants were recruited from a child advocacy center (N = 8) and from the community (N = 44). Of those recruited from the community, 2 participants were randomly selected for removal amongst groups of siblings, such that there were no siblings in the final sample. Participants were also excluded if they did not meet the criteria for a sufficient quantity of data (five minutes; N = 7). This left a total of 43 participants with usable data. A total of 24 adolescents were sorted into the adversity group on the basis of recruitment via a child advocacy center, interview measures, and/or self-reported or parent-reported exposures, leaving 19 adolescents in the control group. Participants from the adversity group (child advocacy center sample N = 1, community sample N = 8) that did not endorse having specifically experienced abuse or neglect were excluded from dimensional analyses.
2.1.3. Project MINA (1R01AA023658-01, PI Feldstein Ewing)
Data collection is currently complete for Project MINA; we preregistered our intent to use data from the first 112 participants that were available at the time image processing began (Feb 2018). Of these, participants were excluded if they did not complete a resting state scan (N = 6) or did not meet the original study exclusion criteria (N = 6; including being over the age of 20 (N = 1), being left-handed (N = 1), taking excluded medications (N = 2), and same-day cannabis use (N = 2)). This study was funded to examine questions around adolescent alcohol use; however, for the purposes of this data analysis we excluded adolescents for consumption of a greater number of drinks in one sitting than the reported within-sample mean plus one standard deviation for their gender (14 drinks for females, 20 for males; N = 10). Participants were also excluded due to excessive dropout (N = 1), poor quality segmentation (N = 1), insufficient resting state data following motion scrubbing (N = 3), poor outlier detection leading to large (>0.95) correlations across the brain (N = 1), and missing data on the entire adversity questionnaire (N = 1). This left a total of 83 subjects with usable data, including 38 adolescents in the control group and 45 adolescents who we categorized as being in the adversity group (see Section 2.2.3 for how this determination was made).
2.2. Measuring adversity and adverse exposure type
2.2.1. Measures
Participants in the TDS study completed a version of the Adverse Childhood Experiences (ACE) questionnaire (Felitti et al., 1998) adapted for use with adolescents. Items on this questionnaire prompted participants to indicate (yes/no) as to whether they had experienced 10 adverse childhood events, including emotional abuse, physical abuse, sexual abuse, emotional neglect, and/or physical neglect. Participants in the TSS and Project MINA studies completed the Childhood Trauma Questionnaire (CTQ; Bernstein et al., 2003), which contained questions assessing exposure to the same five types of adversities. The CTQ included five questions per exposure type that prompted participants to evaluate the frequency with which specific occurrences had happened on a five-point scale ranging from “Never True” to “Very Often True.”
2.2.2. Thresholding CTQ values
We ultimately sought to include participants that had completed both adversity measures in the same models. Therefore, we transformed CTQ responses for each exposure type to a single binary value (exposed or unexposed) for comparability with binary responses on the ACE questionnaire. Although this did not make use of the richer, continuous responses from the CTQ, “downsampling” to the binary ACE questionnaire facilitated comparisons across samples. The ACE questionnaire and CTQ use overlapping language, and degree of overlap varies by exposure type. One example of this imperfect overlap can be seen in their assessments of physical abuse: The single ACE item states, “Did a parent or other adult in the household often…Push, grab, slap, or throw something at you? -or- Ever hit you so hard that you had marks or were injured?" On the CTQ, five items assess the frequency of being physically hit or punished to a noticeable degree with certain objects and/or requiring medical attention, as well as the extent to which participants identified as being a victim of physical abuse (exact wording is copyrighted; additional comparisons are described in Cheng, Teicher, & Mackiewicz Seghete, in prep).
There are several possible ways to transform CTQ responses to binary exposure values. The authors of the CTQ applied cutoff scores to summed responses to characterize exposure severity using four categories (Bernstein and Fink, 1997). Our cutoff of interest (unexposed/exposed) is arguably analogous to the CTQ authors’ cutoff between no exposure (“none to minimal”) and low-severity exposure (“slight to moderate”). Initial published thresholds were developed by comparing CTQ responses to therapist reports in a randomly-selected non-clinical sample of adult women recruited from a health maintenance organization in the 1990s (Bernstein and Fink, 1997), and is not representative of the broader population or of comparability to the ACE questionnaire. To inform cutoff score selection, we examined correspondence between the CTQ and ACE questionnaire in independent samples of young adults that completed both scales (initial/training sample N = 462 (273 female, aged 20–27 20–27 years); validation/hold-out sample N = 64 (43 female, aged 18–19 years; data provided by Dr. Martin Teicher). We evaluated the sensitivity, specificity, and generalizability of several thresholding approaches (described in Cheng et al., in prep; results can be explored within an interactive web application at https://theresacheng.shinyapps.io/ctq_to_aces_shiny/).
Based on these analyses, we selected cutoff scores that were algorithmically determined by a cost-benefit criterion (costs ratio = 0.5; McNeil et al., 1975) for each exposure type (emotional abuse, physical abuse, sexual abuse, emotional neglect, and physical neglect). At this value, false positives and false negatives are considered equally costly, such that the number of misclassified individuals is reduced overall (this is a particularly useful procedure for low-prevalence outcomes; Smits, 2010). This procedure misclassified fewer individuals compared to applying cutoffs (a) between no exposure and low-severity exposure and (b) that maximize sensitivity and specificity. For all exposure types except physical neglect, cost-benefit cutoff scores fell in the middle-to-upper range of the low-severity category scores and exhibited fairly good sensitivity and specificity. This was likely due to lower rates of physical neglect endorsement on the ACE questionnaire and weaker psychometric validity of that CTQ subscale in general (Gerdner and Allgulander, 2009; Klinitzke et al., 2012). We therefore replaced the atypically high physical neglect cutoff score (14) with a score in the upper range of the low severity category (range was 8–9, we selected 9). Our final cutoff scores were 11 for emotional abuse, 9 for physical abuse, 7 for sexual abuse, 14 for emotional neglect, and 9 for physical neglect; when applying these to the young adult sample, sensitivities ranged from 64 to 86 % and specificities ranged from 80 to 97 %. (See histograms of the distribution of CTQ scores relative to this cutoff for each type of adversity in S3 of the Supplementary Materials).
Cost-benefit criterion cutoffs were used to obtain our best approximation of binary adversity status across samples (see next section for more details). We additionally conducted sensitivity analyses to mitigate uncertainty around the appropriate exposure status of participants whose CTQ scores were just below threshold. The no exposure/low-severity thresholds established by the CTQ authors were 0–4 points lower than the cutoff scores used in our analyses. Sensitivity analyses were more conservative in that they only kept participants in no exposure group if they reported CTQ values below these lower thresholds, and excluded participants entirely if their scores fell between these lower thresholds and our higher, cost-benefit criterion- defined thresholds. These analyses removed 20 participants across Project MINA and TSS samples from the general adversity model and 30 participants from the dimensional model. See S2.4 of the Supplementary Materials for reporting of these findings.
2.2.3. Defining adversity status and exposure in each sample
For the TDS sample, adolescents were included in the adversity group if they were (a) recruited through the foster care system or (b) recruited through the community, but endorsed any type(s) of maltreatment (abuse or neglect) on the ACE questionnaire. For the TSS sample, adolescents were included in the adversity group if they (a) were recruited through the child advocacy center for suspected and/or confirmed maltreatment, (b) endorsed any type(s) of maltreatment based on converted CTQ responses (as described in the previous section), or if (c) exposure was corroborated through interview, medical documentation, or parent report. For Project MINA, adolescents were included in the adversity group if they endorsed any type(s) of maltreatment based on converted CTQ responses. Across studies, adolescents not meeting these criteria were part of the control group.
For dimensional models, exposure to abuse and/or neglect was defined in terms of ACE questionnaire or converted CTQ responses. Two adolescents from the TSS sample were missing data (one CTQ item each) pertaining to their physical neglect status. We imputed this score with the average of their other responses pertaining to physical neglect. For both subjects, the other responses on the subscale were quite low (ratings of “Never True” and “Rarely True” only), so they were classified as not having been exposed to physical neglect. Participants in the adversity group that did not endorse exposure to any type(s) of adversity on self-report measures were not included in dimensional analyses due to an inability to determine their exposure type (TDS N = 13; TSS N = 8). However, to maximize power, these participants were included in the adversity group in the general adversity model. Sensitivity analyses suggest that the impact of restricting participants to the subset that endorsed maltreatment on the questionnaires has only a modest impact on adversity effects (within the general model only; see S2.5 of the Supplementary Materials).
2.3. Neuroimaging: processing
2.3.1. Acquisition
The TDS dataset was acquired on a Siemens Skyra 3 T scanner at the Lewis Center for Neuroimaging at the University of Oregon. The data included a T1-weighted MPRAGE structural scan (TE = 3.41 ms, TR = 2500 ms, flip angle = 7°, voxel size = 1.0 × 1.0 × 1.0 mm, slices = 176), a T2*-weighted BOLD-EPI resting-state scan (TE = 30.80 ms, TR = 780 ms, flip angle = 55°, voxel size = 2.5 × 2.5 × 2.5 mm, slices = 60, interleaved, multiband factor = 6), and a double-echo gradient field map (TE=4.37 ms, TR = 639 ms, flip angle = 60°, voxel size = 2.0 mm3, slices = 72). The single resting state scan was 6 min, 50 s long, and took place at the end of the Teen Decisions Study protocol (described in Op de Macks et al., 2018). Participants were instructed to close their eyes, relax, and try not to fall asleep as a white fixation cross was displayed on their viewing screen.
The TSS dataset was acquired across a Siemens Prisma 3 T (N = 17 of final sample) and on a Siemens Trio 3 T scanner (N = 28 of final sample). The data included a T1-weighted MP-RAGE structural scan (TE = 3.61 ms, TR = 2300.0 ms, flip angle = 10°, voxel size = 1.0 × 1.0 × 1.1 mm, slices = 160), two T2*-weighted BOLD-EPI resting-state scans (TE = 30.0 ms, TR = 2500 ms, flip angle = 90°, voxel size = 3.8 × 3.8 × 3.8 mm, slices = 36, interleaved), and no field maps. These scan parameters were consistent between scanners. Participants were told that they could think about whatever they wanted, but to focus on the white fixation cross displayed on their viewing screen, not to fall asleep, and to stay very still. Data were concatenated from two scans each lasting 5 min, 17 s.
The Project MINA dataset was acquired on a Siemens Prisma 3 T. The data included a T1-weighted MP-RAGE structural scan (TE = 2.88 ms, TR = 2500.0 ms, flip angle = 8.0°, voxel size = 1.0 × 1.0 × 1.0 mm, slices = 176), a T2 structural scan (TE =565 ms, TR = 3200 ms, voxel size = 1 mm3, slices = 176), two T2*-weighted BOLD-EPI resting-state scans (TE = 30.0 ms, TR = 800 ms, flip angle = 52°, voxel size = 2.4 mm3, slices = 60, multiband factor = 6), and spin-echo field maps (TE = 80.0 ms, TR = 7030 ms, voxel size = 2.4 × 2.4 × 2.4 mm, slices = 60, interleaved). Participant instructions for the resting state scan were identical to those administered in the TSS study, but consisted of two scans each lasting 5 min, 11 s.
2.3.2. General pre-processing
Pre-processing procedures were adapted from the Human Connectome Project’s minimal pre-processing pipeline (Glasser et al., 2013) with improvements to generalizability and usage (DCAN-Labs/abcd-hcp-pipeline: release v0.0.0; doi:10.5281/zenodo.2587210). Analyses were conducted within the cifti format, which contains approximately ∼92 K grayordinates (a combination of cortical vertices and subcortical gray matter voxels).
2.3.3. Resting-state pre-processing
Magnetization steady state was assumed after the first seven seconds and these initial frames were discarded. To avoid calculating parameters that could be dominated by head movement, we estimated beta weights for nuisance regression and performed detrending based on frames with <0.3 mm framewise displacement (FD) only. This threshold is independent of the FD threshold later used for motion censoring. Regressors included 24 Friston motion regressors (6 motion estimates, estimates from the previous time point, and square terms of each of these values; Parkes et al., 2018), global signal, average white-matter signal, and average signal from the ventricles. Prior to temporal bandpass filtering between 0.009 and 0.080 Hz, discarded frames were interpolated based on low motion (<0.3 mm FD) data only. This interpolation prevents the blurring of spurious signals when filtering in the time domain while maintaining the temporal sequence of the frames.
We applied a notch frequency filter to remove a respiration artifact that is enhanced in acquisition sequences with faster TRs (Fair et al., 2020). This was applied in the TDS and Project MINA samples, which have fast TRs. To define the notch filter, we identified the upper and lower quartiles of the distribution of peak frequencies (within a plausible range of frequencies based on human adolescent respiration rates) in the power spectra of motion parameters for each of the two samples. The quartiles in the TDS study encompassed a wider range of values than those of the Project MINA study. To make the notch more inclusive of potential respiration artifact, we used the TDS study values (rounded) to obtain a notch filter of 0.24 Hz (14.4 bpm) to 0.35 Hz (21.0 bpm).
2.3.4. Motion censoring to create correlation matrices
When creating correlation matrices, we included frames with a more stringent threshold (FD < 0.2 mm) than previously employed for nuisance regression (FD < 0.3 mm). When there were fewer than five contiguous frames between high motion (FD > 0.2 mm) frames, these “in-between”’ frames were removed (Power et al., 2014). We also removed frames with highly variable signal suggestive of motion artifacts, defined as frames with signal standard deviation greater than three scaled median absolute deviations from the median standard deviation of contiguous low-motion frames. Five minutes of subject data were randomly selected from the remaining frames, such that group differences could not be attributed to the influence of data quality on scan length. Subjects with less than five minutes of acceptable data were excluded from analyses (as detailed in Section 2.1). The thresholds for motion censoring did not differ across samples.
2.3.5. Quality assessment
A total of seven raters visually evaluated the scans, and a second rater assessed any scans identified as potentially unusable. All raters were trained on quality assessment in accordance with the protocol used in the Adolescent Brain Cognitive Development BIDS project (ABCD-BIDS; NDA Collection 3165) and passed training sets with greater than 75 % accuracy. As a part of this protocol, independent raters assessed the quality of each of the following on a 1–3 scale: (1) the T1 to MNI atlas registration; (2) the structural scan and its processing, with attention to the presence of motion artifact as well as segmentation quality; and (3) the resting-state scan, with attention to field of view errors, signal dropout, motion artifact, and alignment with the T1 scan. From this quality control process, two subjects’ data (from the MINA study) were identified as unusable (rating of 3); one due to extreme dropout, and the other due to severe problems with segmentation.
2.3.6. Participants excluded due to data quality and/or head motion
Across studies, a total of 38 participants were excluded due to head motion and/or data quality issues. Due to scan length, more participants were excluded from TDS than the other studies. After mean-centering variables by study to account for differences in rates of exclusion across samples, we found that excluded participants were somewhat younger (by approximately 0.9 years) and had higher mean annual parent income (by approximately $2900) than participants who were included in the study. However, they did not differ in rates of mental health diagnoses/use of psychotropic medications. Please see S4 of the Supplementary Materials for a more detailed comparison.
2.4. Neuroimaging: analyses
2.4.1. Identifying regions associated with adversity and its dimensions
We chose to pursue seed-to-whole brain analyses, as they better reflect the more exploratory nature of dimensional investigations into adversity than region-of-interest approaches. We defined left and right amygdala seeds in each individual participant using the Freesurfer segmentation procedure (Dale et al., 1999). We averaged the pre-processed and motion-corrected BOLD signal of all grayordinates within the boundaries of the seed at each timepoint to obtain a single timecourse per seed. We correlated each seed’s timecourse with every other grayordinate to obtain a vector describing each seed’s connectivity with the whole brain. The resulting Pearson correlation values were then Fisher z transformed.
Models were constructed and analyzed with in-house scripts that use functions within the MPlus software (Developmental Cognition and Neuroimaging Lab, 2020; Muthén and Muthén, 1998). Each model regressed seed-to-grayordinate values on adversity status. In unidimensional adversity analyses, the regressor-of-interest was a binary regressor indicating whether participants belonged to the adversity or control groups. In dimensional analyses, separate binary regressors indicated whether participants had experienced abuse, neglect, or both (interaction term). Dummy coded regressors of no interest accounted for the effects of four separate study protocols across three scanners (1. TDS Siemens Skyra, 2. TSS Siemens Trio, 3. TSS Siemens Prisma, and 4. Project MINA Siemens Prisma; the Siemens Prismas were the same machine). (For visualizations comparing left and right amygdala rs-fc across study, see S5 of the Supplementary Materials.) Although age and sex were similar across comparison groups, exploratory analyses found that sex was associated with larger differences in magnitude and spatial extent than age. Thus, subsequent models included a regressor for sex only. Follow-up sensitivity analyses suggest a minimal impact of additionally controlling for age on adversity-related findings (see S2.3 of the Supplementary Materials). Cluster-based thresholding followed recommendations by (Eklund et al., 2016) to obtain FWER = 0.05 with a primary threshold of p < 0.001. However, these methods have been more rigorously validated in volume-based than in surface-based analyses. Cluster-based thresholding techniques have long held that clusters comprised of a larger number of contiguous voxels are less likely to be due to chance. To be more conservative, we additionally focused on interpreting clusters with >25 mm2 surface area, corresponding to roughly the top ⅓ of clusters by surface area. Tables of smaller clusters are reported in the Supplementary Materials (see S6).
2.4.2. Comparing model results to hypotheses
Our neuroimaging analyses identified clusters associated with adversity in a general adversity model, as well as clusters associated with abuse, neglect, and their interaction in a dimensional model. We report the MNI coordinates and AAL atlas label associated with each cluster’s center-of-gravity. We also report one or more network assignments based on whether any part of the cluster falls within the borders of a parcel of that network, as defined by a parcellation scheme developed by Gordon et al. (2016).
Table 4 provides a summary of our original hypotheses and corresponding region/network definitions. In the results, we state when clusters identified from whole-brain analyses fell within hypothesized regions or networks. The Desikan-Killiany atlas (Desikan et al., 2006) was used when hypothesized regions fell within identifiable labels from that atlas. A number of hypothesized regions within the prefrontal cortex do not have precise anatomical boundaries. Therefore, we defined the ventromedial prefrontal cortex as the combination of three contiguous areas (s32, p32, and 10 r) and the subgenual anterior cingulate cortex as one area (25) from the Human Connectome Project parcellation scheme (Glasser et al., 2016; see Fig. 22A of the Neuroanatomical Supplementary Results: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4990127/bin/NIHMS68870-supplement-Neuroanatomical_Supplementary_Results.pdf). To identify lateral prefrontal cortical regions, we used a manually-defined region-of-interests (used in Vijayakumar et al., 2014). Briefly, this region-of-interest was created by combining atlas regions of the frontal cortex and applying coronal and sagittal cuts bounding the caudal and medial extent (see their manuscript for more details). This is a fairly inclusive definition of lateral PFC that spans its anterior/rostral, dorsolateral, and ventrolateral aspects.
Table 4.
Summary of hypothesized region definitions.
| Hypothesized region(s) | Region definition | Direction hypothesized | Findings |
|---|---|---|---|
| General: Adversity | |||
| Ventromedial prefrontal cortex (vmPFC) | Areas s32, p32, 10 r from the HCP parcellation | Negative | Null |
| Subgenual anterior cingulate cortex (sgACC) | Area 25 from the HCP parcellation | Negative | Null |
| Lateral prefrontal cortex (lPFC) | Manually defined region-of-interest from Vijayakumar et al., 2014 | Negative | General: Clusters within left anterior lPFC were negatively associated with adversity |
| Dimensional: Left dorsal lPFC was negatively associated with neglect | |||
| Orbitofrontal cortex (OFC) | Lateral orbitofrontal or medial orbitofrontal labels within the Desikan-Killiany atlas | Unspecified | General: Null |
| Dimensional: A cluster within right OFC was positively associated with abuse | |||
| Posterior cingulate cortex (PCC)/precuneus | Posterior cingulate, isthmmus cingulate, or precuneus labels within the Desikan-Killiany atlas | Unspecified | General: Null |
| Dimensional: Clusters within the PCC and left precuneus were negatively associated with abuse; a more posterior cluster within the left precuneus was negatively associated with neglect | |||
| Insula | Insula label within the Deskian-Killiany atlas | Unspecified | General: Null |
| Dimensional: A cluster in left anterior insula was negatively associated with neglect | |||
| Dimensional: Abuse | |||
| Ventromedial prefrontal cortex (vmPFC) | Areas s32, p32, 10 r in the HCP parcellation | Unspecified | Null |
| Subgenual anterior cingulate cortex (sgACC) | Area 25 in the HCP parcellation | Unspecified | Null |
| Lateral prefrontal cortex (lPFC) within the frontoparietal network | Manually defined region-of-interest from Vijayakumar et al., 2014 AND within a parcel that is part of the frontoparietal community in the Gordon parcellation scheme | Unspecified | General: Left anterior lPFC clusters within the frontoparietal network were negatively associated with adversity |
| Dimensional: Null | |||
| Dimensional: Neglect | |||
| Dorsal attention network | Within a parcel that is part of the dorsal attention (DA) community in the Gordon parcellation scheme | Negative | General: Left precentral gyrus was negatively associated with adversity; left inferior temporal gyrus was positively associated with adversity |
| Dimensional: Clusters within the dorsal lPFC and intraparietal sulcus were negatively associated with neglect; clusters within the left precentral gyrus were negatively associated with abuse | |||
| Sensory and somatomotor networks | Within a parcel that is part of the visual (Vis), auditory (Aud), or somatomotor (SMh or SMm) communities in the Gordon parcellation scheme | Negative | General: Clusters within the visual network (right parahippocampal gyrus) were positively associated with adversity |
| Dimensional: Clusters within the visual network were positively associated with neglect; more inferior regions within the visual network were positively associated with adversity and/or abuse. No clusters were identified within the auditory network. Clusters within somatomotor networks were negatively associated with abuse only. See section 3.1.5 for more on interactions. | |||
2.5. Sensitivity analyses
We conducted five sensitivity analyses to estimate the impacts of the following on our adversity-related findings: the inclusion of (1) parental income, (2) psychopathology (mental health diagnoses and/or psychotropic medication use), and (3) age as covariates, as well as (4) CTQ thresholding procedures, and (5) excluding participants who had likely experienced adversity but who did not endorse specific maltreatment types from dimensional analyses. Methodological details and results pertaining to sensitivity analyses can be found in S2 of the Supplementary Materials. These analyses found that most of the effects described in this manuscript are generally robust to these model adjustments. However, the inclusion of parental income as a covariate had a relatively larger impact. In addition to a full reporting of results from that model in the Supplementary Materials, the effects of including this variable are mentioned throughout Section 3 (Results & Discussion) when appropriate.
2.6. Deviations from pre-registration
Unplanned deviations from the pre-registration as well as minor clarifications are reported in publicly available addendums (see https://osf.io/u3dey/files/). Notable deviations from the pre-registration are the exclusion of three additional subjects (and corresponding corrections to participant tables). The decisions to focus on clusters with >25 mm2 surface area and the region-of-interest definitions described in 2.4.2 were not pre-registered. The analyses presented here reflect a subset of Aim 1 and 2a from preregistered analyses focusing on cortico-amygdala rs-fc only. Other aspects of the preregistered analyses have yet to be run. Sensitivity analyses were conducted post-hoc and were not pre-registered.
3. Results & discussion
These analyses aimed to examine adolescent amygdala rs-fc with the cortex in a general model of adversity focused on childhood maltreatment, right alongside a dimensional model comparing abuse and neglect. In the general adversity model, we used seed to whole-brain analyses to identify cortical regions exhibiting adversity-related connectivity differences with the left and right amygdala. In the dimensional model, we took the same approach to identify associations with abuse, neglect, and their interaction. While rarely undertaken, accounting for different types of adversity simultaneously in the same model is crucial to understanding the specificity of effects regarding commonly co-occurring types of adversity.
3.1. Hypothesized regions
Table 4 summarizes findings pertaining to our hypotheses. The sections that follow provide a more detailed reporting and discussion of clusters within hypothesized regions and networks, organized by region. Fig. 1 visualizes findings associated with adversity in the general model, while Fig. 2, Fig. 3 visualize findings associated with abuse and neglect. For results associated with the interaction term, see S7 of the Supplementary Materials.
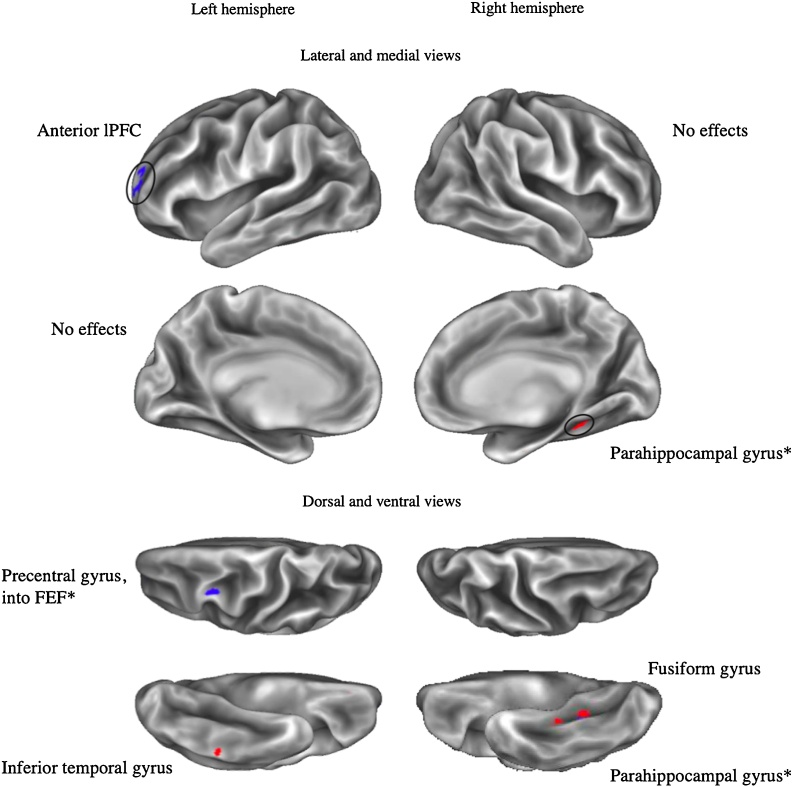
Fig. 1.
General model: Labeled clusters with adversity-related amygdala rs-fc.
Note. *: Cluster overlaps with another cluster from the same seed for a different effect of interest. Clusters displayed exhibit positive (in red) or negative (in blue) adversity-related effects on connectivity with left or right amygdala seeds (vertex wise threshold Z > 3.1, cluster-corrected p < .05, with an additional surface area > 25mm2 threshold). Circles draw attention to clusters that are smaller and/or more difficult to see.
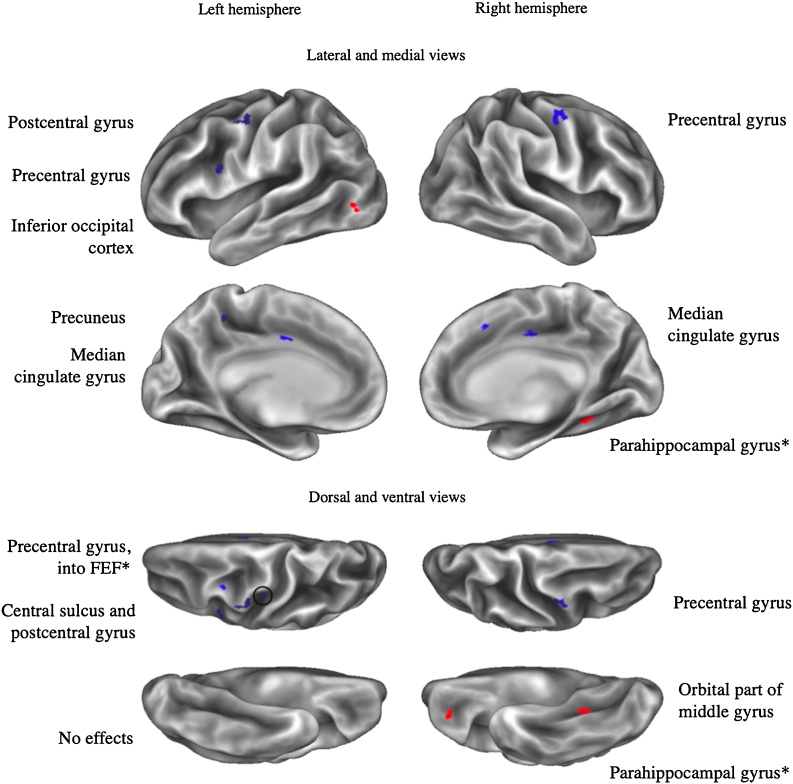
Fig. 2.
Dimensional model: Labeled clusters with abuse-related amygdala rs-fc.
Note. *: Cluster overlaps with another cluster from the same seed for a different effect of interest. Clusters displayed exhibit positive (in red) or negative (in blue) abuse-related effects on connectivity with left or right amygdala seeds (vertex wise threshold Z > 3.1, cluster-corrected p < .05, plus an additional surface area > 25mm2 threshold). Circles draw attention to clusters that are smaller and/or more difficult to see.
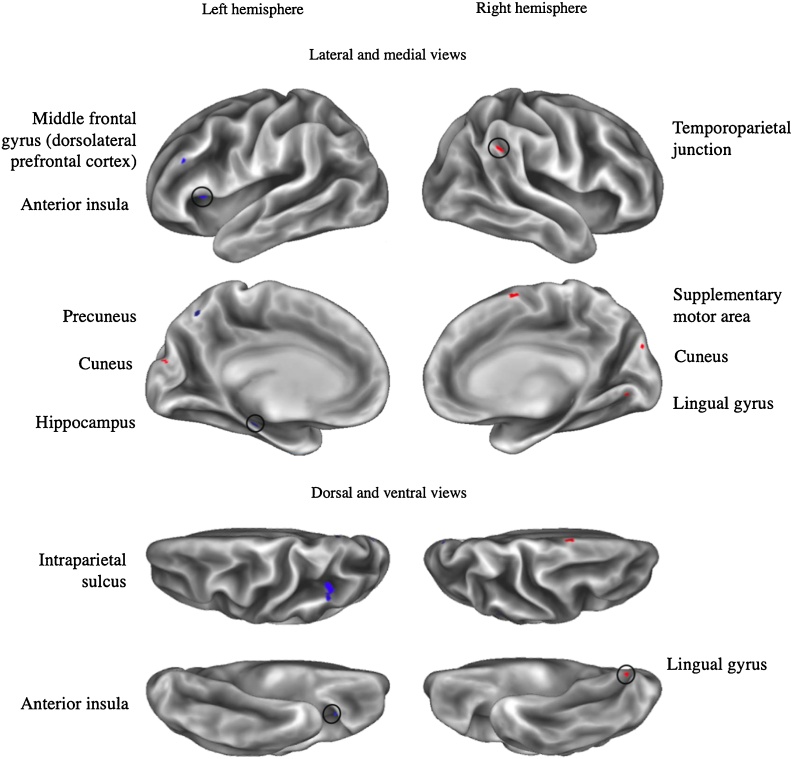
Fig. 3.
Dimensional model: Labeled clusters with neglect-related amygdala rs-fc.
Note. *: Cluster overlaps with another cluster from the same seed for a different effect of interest. Clusters displayed exhibit positive (in red) or negative (in blue) neglect-related effects on connectivity with left or right amygdala seeds (vertex wise threshold Z > 3.1, cluster-corrected p < .05, plus an additional surface area > 25mm2 threshold). Circles draw attention to clusters that are smaller and/or more difficult to see.
To understand how our findings were impacted by covariates, we conducted sensitivity analyses. These analyses suggest modest impacts of including additional linear covariates for psychopathology and age (over and above participant sex and scanner protocol, which were included as covariates in all models; see S2.2 and S2.3 of the Supplementary Materials). Including parental income had relatively greater impact on our findings. As such, we comment on whether effects are robust to the inclusion of this additional covariate throughout (see S2.1 of the Supplementary Materials for full reporting on this model). We consider a cluster to be present in models with and without parental income if there are any overlapping vertices, regardless of whether clusters met an additional 25 mm2 surface area threshold.
To better understand null effects and to provide additional information that might motivate future studies, we also compared effect sizes in all hypothesized regions (see S8 of the Supplementary Materials). We ran additional analyses to qualitatively investigate the specificity of effects found in association with adversity in the general model or with abuse/neglect in the dimensional model. However, we do not statistically test comparisons between the two models, as they were neither independent nor nested. Generally, these analyses suggested that effects identified in association with either abuse or neglect were not associated with adversity in a general model (see S9 of the Supplementary Materials).
3.1.1. Prefrontal cortex
Table 5 provides details pertaining to clusters within hypothesized regions of the prefrontal cortex.
Table 5.
Altered amygdala rs-fc within hypothesized regions of the prefrontal cortex.
| Model and regressor | Seed | Center of gravity AAL label | BA | Comm | Surface area (mm2) | Mean Z | x | y | z |
|---|---|---|---|---|---|---|---|---|---|
| Lateral prefrontal cortex | |||||||||
| General: Adversity | L | L superior frontal gyrus (anterior lPFC)* | FP | 29.3 | −3.39 | −24 | 54 | 9 | |
| L middle frontal gyrus (anterior lPFC) | Def | 48.9 | −3.40 | −23 | 54 | 14 | |||
| R | L superior frontal gyrus (anterior lPFC)* | FP | 83.4 | −3.49 | −24 | 60 | 8 | ||
| Dimensional: Neglect | L | L middle frontal gyrus (dorsolateral PFC) | DA | 28 | −3.73 | −43 | 35 | 21 | |
| Orbitofrontal cortex | |||||||||
| Dimensional: Abuse | L | R orbital part of middle gyrus | 11 | None | 31.4 | 3.73 | 29 | 41 | −10 |
Note. *: Cluster is not present when controlling for income as a covariate. L: left, R: right; x, y, and z coordinates correspond to the cluster’s center of gravity; BA: Brodmann area, if any, associated with the displayed coordinates; Comm: Gordon communities associated with the cluster, if any; Mean Z: average Z-score associated with the regressor across the spatial extent of the cluster. Key for Gordon communities: DA = Dorsal attention, Def = Default mode, FP = Frontoparietal. Cluster reporting is hierarchically organized by hypothesized region, model and regressor, and seed. Reporting is comprehensive for each hypothesized region; i.e., no effects of abuse are reported within the lateral prefrontal cortex because there were no clusters within that region associated with that regressor.
3.1.1.1. Medial PFC
Amygdala-vmPFC connectivity has been a subject of focus in prior adversity investigations, largely due to the vmPFC’s role in regulating amygdala activity (Motzkin et al., 2015) to promote emotion regulation (Blair, 2008) and the extinction of fear processing (Phelps et al., 2004). We hypothesized that adversity and abuse would be associated with amygdala rs-fc with the vmPFC and sgACC. Whole-brain analyses did not identify suprathreshold clusters within these regions. This was true even among clusters smaller than the additional 25 mm2 surface area threshold, as well as in most sensitivity analyses. Controlling for parental income, signal within a vmPFC cluster exhibited a main effect of neglect that was further qualified by an interaction. Closer inspection suggested that experiencing neglect alone was associated with relatively greater connectively between the left amygdala and left vmPFC in models that controlled for parental income (see Fig. S3 of the Supplementary Materials). As rs-fc between these regions typically increases with age (Gabard-Durnam et al., 2014), this may reflect early functional maturation of this circuit, which has previously been associated with institutionalization (Gee et al., 2013; this experience is theorized to be a severe form of neglect). Among younger children, a previous study identified weaker amygdala rs-fc with a similar mPFC region in association with early life stress (Park et al., 2018). Similarly, weaker amygdala connectivity with subregions of the anterior cingulate cortex (particularly pgACC and sgACC) have been associated with adversity (Fan et al., 2014; Herringa et al., 2013; Thomason et al., 2015), but clusters within these regions were not observed in this study. Adversity-related amygdala-mPFC connectivity and associated changes in affective processing have been investigated as both a risk and protective factor for externalizing and internalizing psychopathology (Gee et al., 2013; McLaughlin and Lambert, 2017; Peverill et al., 2019). However, a recent systematic review suggests that studies have been mixed as to whether they find increases, decreases, or no differences in amygdala connectivity with the vmPFC and/or sgACC in association with adversity (McLaughlin et al., 2019). Our findings suggest that amgydala-vmPFC rs-fc may be sensitive to operationalizations of adversity.
3.1.1.2. Lateral PFC
We hypothesized that adversity would be associated with more negative rs-fc between the amygdala and the lPFC in the general adversity model. This hypothesis was supported, as adversity was associated with negative rs-fc between clusters within the left anterior lPFC and both the left and right amygdala. We further hypothesized that abuse would be associated with rs-fc between the amygdala and regions of lPFC within the frontoparietal network. Two of the left anterior lPFC clusters fell within the frontoparietal network, but were associated with adversity in the general model only and also were not present after controlling for parental income. The anterior lPFC is thought to play an integrative role of information processing (Christoff and Gabrieli, 2000) and meta-cognition (Baird et al., 2013; Fleming et al., 2014). We additionally found that neglect was associated with more negative left amygdala rs-fc with a cluster within the left dorsolateral PFC (dlPFC) that is a part of the dorsal attention network. This area of left dlPFC is also implicated in phonological processing and reading ability (Kovelman et al., 2012), and childhood neglect, rather than abuse, is notably associated with language problems and reading performance (see Hildyard and Wolfe, 2002 for a review). The dlPFC is more broadly known as a key cognitive control region that exhibits consistent decreases in volume in association with deprivation, but not with other forms of maltreatment (McLaughlin et al., 2019).
3.1.1.3. Orbitofrontal cortex
Adversity was not associated with amygdala-OFC rs-fc in a general model (although two adversity-related OFC clusters emerged when controlling for parental income). The dimensional model revealed that abuse, rather than neglect, was positively associated with amygdala rs-fc within the medial OFC. The structure of the OFC is altered in individuals that have experienced early life stress (Hanson et al., 2010) or maltreatment (Brito et al., 2013; Dannlowski et al., 2012). This region is broadly implicated in affective decision-making (Krain et al., 2006) and modulates information flow along a widely studied amygdala-medial prefrontal circuit (Chang and Grace, 2018; Kim et al., 2011). These findings are consistent with conceptualizations of abuse on a threat-related dimension of adversity that specifically impacts socioaffective processing. Other studies have found that childhood stress and maltreatment are associated with altered reward and loss processing, potentially underlying differing risk assessment and decision-making patterns that are at times not optimal (Birn et al., 2017; Guyer et al., 2016; Weller and Fisher, 2013).
3.1.1.4. Summary of prefrontal cortex findings
No clusters within the vmPFC or sgACC were found to exhibit amygdala rs-fc in association with adversity or its dimensions in our main models. When additionally controlling for parental income, we identified more positive connectivity between the amygdala and a cluster within the vmPFC in association with neglect only. Generally, accounting for threat and deprivation as putative dimensions of adversity in our analyses did not clarify inconsistencies in the prior literature regarding amygdala connectivity with these regions. Instead, we found that (a) adversity was associated with more negative amygdala rs-fc with a region of the left anterior lPFC, (b) neglect was associated with more negative left amygdala rs-fc with a region of the left dlPFC, and (c) abuse was associated with more positive left amygdala rs-fc with a region of the OFC.
3.1.2. Posterior cingulate cortex/precuneus
Whole brain analyses also identified numerous clusters within hypothesized regions beyond the prefrontal cortex (see Table 6). Contrary to hypotheses, adversity was not associated with amygdala rs-fc with the PCC and precuneus. Instead, abuse was associated with more negative amygdala rs-fc with clusters within the PCC as identified via the Desikan-Killiany atlas, which primarily distinguishes between anterior and posterior cingulate cortex. A more fine-grained lens identifies this cluster as a part of posterior mid-cingulate cortex (pMCC), a region proposed to play a central role in reflexive body orientation to stimuli, and particularly motor withdrawal from painful or noxious stimuli (Vogt, 2016). Because experiences of abuse may be related to a need to withdraw from painful stimuli, it is of interest that this cluster was identified in association with abuse specifically. The impact of childhood maltreatment on nociceptive processes may be particularly important considering the higher incidence of somatic and visceral pain syndromes in adults that have experienced childhood maltreatment (Chandan et al., 2020).
Table 6.
Altered amygdala rs-fc with hypothesized regions beyond the prefrontal cortex.
| Model and regressor | Seed | Center of gravity AAL label (notes on location) | BA | Comm | Surface area (mm2) | Mean Z | x | y | z |
|---|---|---|---|---|---|---|---|---|---|
| Posterior cingulate cortex/precuneus | |||||||||
| Dimensional: Abuse | R | L precuneus | CO | 32.7 | −3.59 | −16 | −45 | 45 | |
| R | R median cingulate and paracingulate gyri (PCC) | CO | 58.9 | −3.67 | 7 | −5 | 41 | ||
| L | L median cingulate and paracingulate gyri (PCC)* | 24 | CO | 33.2 | −3.46 | −2 | −1 | 37 | |
| Dimensional: Neglect | R | L precuneus | 7 | DA | 34.1 | −3.33 | −7 | −63 | 46 |
| Insula | |||||||||
| Dimensional: Neglect | L | L insula (anterior insula)* | CO | 32.5 | −3.39 | −29 | 20 | 10 | |
| Dorsal attention network | |||||||||
| General: Adversity | L | L precentral gyrus**: abuse | DA | 95.5 | −3.54 | −32 | 0 | 57 | |
| R | L inferior temporal gyrus* | DA | 25.6 | 3.56 | −46 | −40 | −17 | ||
| Dimensional: Abuse | L | L precentral gyrus**: adv | 6 | DA | 42.4 | −3.38 | −31 | 0 | 53 |
| R | L precentral gyrus | CO, DA | 71.8 | −3.50 | −50 | 5 | 15 | ||
| Dimensional: Neglect | L | L frontal middle gyrus (dlPFC) | DA | 28 | −3.73 | −43 | 35 | 21 | |
| L | L inferior parietal gyrus (intraparietal sulcus) | DA | 216.4 | −3.47 | −36 | −48 | 37 | ||
| Sensory and somatomotor networks | |||||||||
| General: Adversity | L | R parahippocampal gyrus*; *** | Vis, RT | 39.2 | 3.68 | 33 | −39 | −9 | |
| R | R parahippocampal gyrus*; **: abuse | Vis, RT | 58.9 | 3.65 | 35 | −38 | −11 | ||
| Dimensional: Abuse | R | L inferior occipital cortex | 19 | Vis | 59.3 | 3.33 | −42 | −80 | −7 |
| R | R parahippocampal gyrus*; **: adv | 37 | Vis, RT | 76.9 | 3.61 | 34 | −39 | −11 | |
| R | L frontal lobe, sub-gyral (Central sulcus)* | SMm | 34.7 | −3.52 | −33 | −19 | 40 | ||
| R | L postcentral gyrus | 4 | SMm | 68.3 | −3.51 | −47 | −9 | 49 | |
| R | R precentral gyrus | SMm, CO | 128.4 | −3.62 | 49 | −6 | 48 | ||
| Dimensional: Neglect | R | R lingual gyrus* | Vis | 29.1 | 3.49 | 11 | −70 | −7 | |
| R | R cuneus* | 19 | Vis | 31.4 | 3.32 | 7 | −87 | 32 | |
| R | L cuneus*; **:int | 19 | Vis | 39.3 | 3.29 | −6 | −95 | 20 | |
| Dimensional: Interaction | R | R superior occipital gyrus* | Vis | 30.7 | −3.24 | 11 | −96 | 18 | |
| R | L superior occipital gyrus* | 18 | Vis | 38.7 | −3.29 | −10 | −101 | 12 | |
| R | L cuneus*; **: neglect | 19 | Vis | 41.1 | −3.27 | −5 | −96 | 19 | |
| R | R superior occipital gyrus* | Vis | 89.3 | −3.4168 | 18 | −88 | 21 | ||
| R | R postcentral gyrus* | SMh | 27.2 | −3.25 | 51 | −26 | 54 | ||
| R | R postcentral gyrus* | 40 | SMh | 70.3 | −3.39 | 42 | −32 | 49 | |
Note. *: Cluster is not present when controlling for income as a covariate. **Cluster overlaps with another cluster from the same seed but a different regressor (specified as a superscript). ***Cluster overlaps with a cluster associated with neglect that is just below the 25 mm2 threshold, this finding is therefore not discussed as adversity specific. L: left, R: right; x, y, and z coordinates correspond to the cluster’s center of gravity; BA: Brodmann area, if any, associated with the displayed coordinates; Comm: Gordon communities associated with the cluster, if any; Mean Z: average Z-score associated with the regressor across the spatial extent of the cluster. Key for Gordon communities: CO = Cingulo-opercular, DA = Dorsal attention, RT = Retrosplenial-temporal system, SMh = Somatomotor - hand, SMm = Somatomotor - mouth, Vis = Visual. Cluster reporting is hierarchically organized by hypothesized region, model and regressor, and seed. Reporting is comprehensive for each hypothesized region.
Furthermore, both abuse and neglect were negatively associated with amygdala rs-fc with clusters within the left dorsal precuneus. More anterior regions of the dorsal precuneus (like the abuse-related cluster) exhibit connectivity with the primary motor cortex and are implicated in spatially guided behaviors; meanwhile, more posterior aspects (like the neglect-related cluster) are implicated in visual imagery (Zhang and Li, 2012). This finding suggests that dimensions of adversity may differentially impact connectivity with anatomical subregions, highlighting the value of whole-brain analyses to detect smaller clusters that might be averaged over in ROI analyses.
3.1.3. Insula
We hypothesized that adversity would be associated with amygdala-insula rs-fc. Instead, we found that a cluster within the left anterior insula exhibited more negative rs-fc with the left amygdala in association with neglect only. This cluster was not present when additionally controlling for parental income; instead, these sensitivity analyses identified a different cluster within the left insula that was associated with abuse. The anterior insula is proposed to anchor a salience (also called cingulo-opercular) network as an integrative hub facilitating higher level task-control (Dosenbach et al., 2007, 2008; Seeley et al., 2007). Core functions ascribed to this region include salience detection, switching between externally- and internally-oriented tasks, and integration of visceral and sensory information sources (Craig, 2003; Menon and Uddin, 2010), with implications for learning and decision-making in affective contexts (Singer et al., 2009). Prior studies examining amygdala-insula rs-fc in adolescents have produced mixed findings (e.g., Thomason et al., 2015 versus Herringa et al., 2013). Our current findings are also mixed, but suggest that heterogeneity both in brain regions and adversity operationalizations may be relevant to explaining inconsistencies in the literature.
3.1.4. Dorsal attention network
In general, this network is thought to support top-down attentional processes, include goal-direction selection and responses (Corbetta and Shulman, 2002; Fox et al., 2006), and can modulate the functioning of visual regions (Vossel et al., 2014). As hypothesized, neglect was associated with more negative amygdala rs-fc with key nodes of the dorsal attention network, including the left dlPFC and left intraparietal sulcus (IPS). The IPS is topographically organized and implicated in higher-order integration of sensory information to inform top-down attentional control (Anderson et al., 2010; Corbetta and Shulman, 2002). Childhood maltreatment is related to the development of attention disorders (Stern et al., 2018), and children exposed to psychosocial neglect via previous institutionalization may be particularly vulnerable to developing attention problems (Stevens et al., 2008; Zeanah et al., 2009). More broadly, changes to IPS connectivity may be related to maltreatment-related impacts on executive functions relevant to academic success, including response inhibition (Osada et al., 2019) and numerical magnitude processing (Bugden et al., 2012).
Adversity and abuse were also associated with more negative amygdala rs-fc with a cluster within the left precentral gyrus (in a region sometimes referred to as the frontal eye fields) that serves as a key node of the dorsal attention network. This region and the IPS are frequently co-activated in studies of visual attention (Corbetta and Shulman, 2002). However, this region is additionally thought to play a critical role in saccadic eye movements (Schall, 2004), which are an important part of orienting to threatening stimuli (Bannerman et al., 2009), and altered amygdala-left precentral gyrus connectivity may be related to attentional biases resulting from abuse that are implicated in the development of anxiety disorders (Pollak et al., 2000; Pollak and Tolley-Schell, 2003; Shackman et al., 2007).
3.1.5. Sensory and somatomotor networks
We hypothesized that neglect would be associated with more negative amygdala rs-fc with regions devoted to sensory and somatomotor networks. Instead, we found effects of adversity, abuse, and neglect on amygdala rs-fc with clusters within these networks. Both adversity and abuse were associated with more positive right amygdala rs-fc with the parahippocampal place area, a region specialized for processing scenes and locations (Weiner et al., 2018); however, these clusters were not present after controlling for parental income. Abuse was associated with more positive right amygdala rs-fc with a cluster within the left lateral occipital cortex associated with shape perception (Larsson and Heeger, 2006), as well as more negative right amygdala connectivity with three clusters within somatosensory and somatomotor cortex (within the Somatomotor - mouth Gordon community; one of these clusters did not persist when controlling for parental income). Neglect was associated with more positive right amygdala rs-fc within upstream visual processing areas in the bilateral cuneus, as well as with the lingual gyrus. The latter cluster is in a right-hemispheric region analogous to the left visual word form area, and is also implicated in both word reading and prosaccades (Zhou and Shu, 2017). These neglect-related clusters were no longer present when controlling for income, suggesting that these effects may be partly explained by socioeconomic status.
3.2. Findings within non-hypothesized regions
We identified a number of findings outside of hypothesized regions; these are reported in Table 7 and displayed in Fig. 1, Fig. 2, Fig. 3. In the general model, adversity was associated with more positive left amygdala rs-fc with a cluster within the right inferior medial temporal cortex, encompassing parahippocampal areas and extending into the entorhinal cortex. As part of the hippocampal memory system, these regions contextually organize and extend representations for learning and memory (Aminoff et al., 2013; Eichenbaum et al., 1996) in coordination with the hippocampus, a region that is sensitive to stress and widely studied in association with childhood adversity (e.g., Dahmen et al., 2018; Pagliaccio et al., 2015), but is not a focus of this investigation of cortico-amygdala connectivity. This cluster was no longer present after controlling for parental income, suggesting that amygdala rs-fc with this region might be partly explained by socioeconomic status.
Table 7.
Altered amygdala resting-state functional connectivity in non-hypothesized regions.
| Model and regressor | Seed | Center of gravity AAL label (notes on location) | BA | Comm | Surface area (mm2) | Mean Z | x | y | z |
|---|---|---|---|---|---|---|---|---|---|
| General: Adversity | L | R fusiform gyrus* | RT | 29.2 | 3.43 | 35 | −24 | −22 | |
| Dimensional: Abuse | L | R median cingulate and paracingulate gyri (dACC/MCC) | 32 | FP | 37.9 | −3.23 | 5 | 21 | 39 |
| Dimensional: Neglect | R | L temporal lobe, sub-gyral (hippocampus)** | NA | 31.1 | −3.75 | −32 | −30 | −13 | |
| R | R supplementary motor area | 6 | CO | 36.5 | 3.63 | 6 | 6 | 65 | |
| R | R superior temporal gyrus (temporoparietal junction) | N/A | 51.1 | 3.33 | 59 | −51 | 19 | ||
| Dimensional: Interaction | R | R rolandic operculum | CO | 62 | −3.43 | 52 | −22 | 17 |
Note. *: Cluster is not present when controlling for income as a covariate. **We choose not to interpret this cluster because the hippocampus is largely represented in volume space along with subcortical structures and bleeds into surface only due to a noisy boundary in FreeSurfer. L: left, R: right; x, y, and z coordinates correspond to the cluster’s center of gravity; BA: Brodmann area, if any, associated with the displayed coordinates; Comm: Gordon communities associated with the cluster, if any; Mean Z: average Z-score associated with the regressor across the spatial extent of the cluster. Key for Gordon communities: CO = Cingulo-opercular, FP = Frontoparietal, RT = Retrosplenial-temporal. Cluster reporting is hierarchically organized by hypothesized region, model and regressor, and seed. Reporting is comprehensive for all suprathreshold clusters outside of hypothesized regions.
In the dimensional model, abuse was associated with more negative left amygdala rs-fc with a region within the dorsal ACC/anterior MCC. Researchers have posed central roles for this region in conflict detection (Bush et al., 2000), motor control (Paus, 2001) and affective distress (Eisenberger and Lieberman, 2004), and amygdala connectivity with this region has been implicated in fear learning (Feng et al., 2014). A recent systematic review identified mixed associations between adversity and functional responses in the ACC (McLaughlin et al., 2019). Neglect was associated with more positive right amygdala connectivity with the supplementary motor area (SMA), a more dorsal region involved in coordinating intentional and complex movement (Nachev et al., 2008) that may play a coordinated role with the amygdala in motor inhibition to emotional cues (Sagaspe et al., 2011). Together, the ACC, insula, and SMA are often considered to be key nodes in the salience/cingulo-opercular network; like the dorsal attention network, we find that nodes of this network are split between regions exhibiting effects associated with abuse versus neglect.
Neglect was additionally associated with more positive connectivity between the right amygdala and right temporoparietal junction (TPJ), a region with roles in flexible attentional control (Vossel et al., 2014) and higher order cognitive processing in the social domain (Saxe and Kanwisher, 2003). Childhood neglect has been associated with lower scores on social cognitive tasks (Kilian et al., 2018). As a part of association cortex supporting higher-order cognition, identifying altered connectivity of the TPJ with neglect rather than abuse is consistent with dimensional conceptualizations of adversity that emphasize neglect as a form of cognitive deprivation (McLaughlin et al., 2014). However, we note that altered connectivity with this region is not widely or consistently identified in association with childhood adversity.
3.3. Strengths and limitations
3.3.1. Strengths
This study is notably among the first resting-state neuroimaging studies to examine general and dimensional models of adversity, with the dimensional model distinguishing between abuse and neglect. Childhood abuse (putatively reflecting one type of threatening experience) and neglect (reflecting one type of deprivation) are associated with distinct and profound social, cognitive, and psychopathological challenges (Hildyard and Wolfe, 2002). Our modeling approach identifies the unique contributions of distinct yet commonly co-occurring dimensions of adversity by examining exposure-specific effects of abuse when controlling for neglect, and vice versa. In contrast, the neuroimaging literature to date has typically examined adversity in cumulative risk models, or in the context of a particular type of trauma history. Preregistered analyses included a fairly large adolescent sample relative to other studies of childhood adversity to date. Studying the impacts of childhood adversity in adolescence is important because this developmental period may offer opportunities for intervention in ameliorating some of its effects (e.g., via pubertal stress recalibration; Gunnar et al., 2019).
3.3.2. Limitations
Our use of a cross-sectional adolescent sample limits opportunities for understanding developmental trajectories. As many studies of adversity on amygdala rs-fc are based on child or adult samples, it is difficult to know if discrepancies between our findings and other studies are age-related. Globally, rs-fc is known to change during adolescence, reflecting numerous underlying neurodevelopmental changes during this period (e.g., Chai et al., 2017; Váša et al., 2020). Cross-sectional analyses have identified age-related changes in amygdala rs-fc with the mPFC, insula, superior temporal sulcus, parahippocampal gyrus, and PCC from childhood to adulthood (Gabard-Durnam et al., 2014).
We further note several limitations pertaining to defining adversity. A number of adolescents recruited based on adversity status (e.g., participation in the foster care system) did not endorse abuse or neglect exposure on questionnaires and were excluded from dimensional analyses. Such minimization or denial is known to occur in widely used self-report questionnaires (MacDonald et al., 2016). Denial may have occurred to a different degree when participants reported experiences of abuse versus neglect, potentially resulting in misclassification. Thresholding CTQ scores into binary adverse exposure outcomes may reflect another source of misclassification, even though our procedures exhibited moderately high sensitivity and specificity values for most types of abuse and neglect (Cheng et al., in prep). This thresholding was undertaken because we were limited to modeling adversity at a low binary resolution due to use of the ACE questionnaire in one of the three samples. Both the severity of adverse experiences (Tozzi et al., 2020) and the developmental timepoints at which they occur are associated with unique changes in amygdala functioning (Luby et al., 2019; Tottenham and Sheridan, 2010); however, we did not have information about frequency and severity for all participants and were unable to incorporate these in our models. Additionally, a number of participants were in foster care, but we were unable to examine effects of foster care due to high collinearity with study (i.e., the majority of these participants came from the TDS sample). While we interpret some of our findings with reference to a dimensional model that distinguishes between threat and deprivation, it is important to recognize that these reflect imperfect mappings to abuse and neglect, respectively. For example, caregiver neglect can be experienced as traumatic (De Bellis, 2005) and may evoke threat-related pathways due to lack of protection from external threats or the absence of species-typical emotional co-regulation (Fareri and Tottenham, 2016). Future dimensional adversity studies would be strengthened by adopting multiple detailed adversity measures, including clinical interviews, case reports, and questionnaires with timing information (e.g., the Maltreatment and Abuse Chronology of Exposure scale; Teicher and Parigger, 2015). More detailed approaches are needed to move the field toward greater understanding of adversity-outcome associations (McMahon et al., 2003) for an array of adversity-related dimensions and health outcomes (Clark et al., 2010; Felitti et al., 1998; Shonkoff and Garner, 2012).
It was critical to pool together samples to obtain a sufficient number of participants exposed to each adversity type. Although we controlled for study protocol, a linear covariate may not have fully accounted for scanner and population differences. Along with adversity measurement, this may have introduced noise, obscuring weaker but still meaningful sub-threshold clusters and/or breaking up regions with similar effects into multiple clusters.
Finally, we employed a single analysis pipeline that was pre-registered to protect against pipeline exploration that would bias us toward positive results. However, variation in analytic processing streams impacts findings and/or data quality in task-based (Botvinik-Nezer et al., 2020) and resting-state (Ciric et al., 2016) functional neuroimaging, and individual studies of between-group rs-fc differences using single analytic approaches may be prone to error (Jia et al., 2018). These same meta-science studies find that meta-analyses examining unthresholded statistical maps across processing streams and replication samples reveal patterns that are robust. For these reasons, we publicly uploaded unthresholded group statistical maps to facilitate further examination of our findings (link: https://github.com/theresacheng/dim_of_adversity_amyg_rsfc) to build toward cumulative knowledge regarding childhood adversity and neurodevelopment.
3.4. Conclusions
This study examined associations between childhood adversity and adolescent cortico-amygdala rs-fc across three samples. In seed-to-whole-brain analyses, we identified regions exhibiting amygdala rs-fc in association with adversity in a general model, as well as with abuse and neglect in a dimensional model. A number of findings related to adversity in the general model, such as left amygdala rs-fc with the left precentral gyrus, were found to be associated with either abuse or neglect when investigated in dimensional models. Adversity-related clusters along the inferior temporal gyrus, including those of the right hippocampal memory system, were no longer significantly associated with adversity in models that controlled for parental income. Amygdala rs-fc with the left anterior lPFC was identified exclusively in the general model only and may be associated with more general psychosocial and/or cumulative risk.
In the dimensional model, abuse was uniquely associated with amygdala rs-fc with clusters within the OFC, dorsal precuneus, PCC/pMCC, and dACC/aMCC, as well as within the dorsal attention (e.g., left precentral gyrus), visual (e.g., parahippocampal place area) and somatomotor networks. Amygdala to dACC/aMCC and OFC rs-fc may be related to different histories of fear learning and conditioning (Greco and Liberzon, 2016). Several abuse-related clusters are within regions that play roles in visual attention (left precentral gyrus; Corbetta and Shulman, 2002; Schall, 2004) and reflexive (PCC/pMCC; Vogt, 2016) as well as visually-guided motor responses (anterior dorsal precuneus; Zhang and Li, 2012). Some changes in adolescent cortico-amygdala rs-fc may be related to altered threat perception and monitoring in children with a history of abuse (e.g., Pollak et al., 2000; Shackman et al., 2007). Additionally, differences in rs-fc with the sensory, somatosensory, and somatomotor cortices may reflect traumatic experiences in a modality-specific manner (e.g., changes in visual processing regions associated with witnessing violence; Tomoda et al., 2012).
In the dimensional model, neglect was associated with amygdala rs-fc with the anterior insula, SMA, temporoparietal junction, and with regions of the dorsal attention (including dlPFC and IPS) and visual networks. Among other functions, these regions are implicated in higher-order cognitive processes critical for academic and social functioning, such as reading (dlPFC and lingual gyrus; Kovelman et al., 2012; Zhou and Shu, 2017), mathematics (IPS, this region is also implicated in visual attention; Bugden et al., 2012; Corbetta and Shulman, 2002), and theory of mind (TPJ; Saxe and Kanwisher, 2003). Neglect-related clusters are also implicated in intentional motor control (SMA; Nachev et al., 2008), as well as salience detection and task maintenance (anterior insula, although this cluster was no longer present when parental income was added as a covariate; Dosenbach et al., 2008; Menon and Uddin, 2010). These amygdala rs-fc findings suggest different pathways for altered integration of information across a wide range of neural systems in adolescents that have experienced neglect. However, the amygdala rs-fc changes identified here may also reflect broader, network-level changes (Cisler, 2017). Changes to the pathways and networks implicated here may help to explain some of the severe social, cognitive, and academic deficits associated with childhood neglect (Hildyard and Wolfe, 2002). Neglect-related clusters within the visual network in the dimensional model were notably absent from sensitivity analyses additionally incorporating income as a covariate, suggesting that poverty and socioeconomic status might account for such effects.
Overall, we found that general and dimensional models each identified unique regions with altered cortico-amygdala rs-fc. This suggests that employing general models only may obscure dimensional effects, and that there may be utility to employing both approaches, (and possibly other dimensional models beyond the one explored here). Neural findings intriguingly parallel some behavioral findings in children that have experienced abuse and/or neglect, suggesting pathways for future inquiry by which specific histories of adversity might relate to functional outcomes across neurodevelopment.
Funding sources
Author TWC was supported by the National Center for Advancing Translational Sciences of the National Institutes of Health under award number TL1TR002371. Author KLM was supported by the Research Council of Norway, grant number 288083. This work was also supported by grants P50 DA035763 (PIs Chamberlain and Fisher), R01 MH107418 (PI Pfeifer), R01 MH115357 (PI Fair), U01 DA041148 (PI Fair), R01 MH096773 (PI Fair), K23MH105678 (PI Mackiewicz Seghete), R01 AA023658 (PI Feldstein Ewing), and K24AA026876-01 (PI Feldstein Ewing). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Declaration of Competing Interest
Damien A. Fair is a patent holder on the Framewise Integrated Real-Time Motion Monitoring (FIRMM) software. He is also a co-founder of Nous Imaging Inc. The nature of this financial interest and the design of the study have been reviewed by two committees at Oregon Health and Science University. They have put in place a plan to help ensure that this research study is not affected by the financial interest.
Acknowledgements
The authors wish to express gratitude to Dr. Eric Feczko and Dr. Robert Hermosillo for providing guidance on analysis strategies and scripts for neuroimaging analysis. The authors additionally wish to thank Moosa Ahmed, Eric Earl, and Emma Schifsky for providing scripts and computing support for neuroimaging analyses. We thank Garrett Ross, Jessi Clare, Leticia Hayes, Ben Hyun, Evelyn Jackson, and Bryan Mantell for assistance with data quality assurance, and thank Evelyn Jackson and Karen Hudson for support with data management.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.dcn.2020.100894.
Appendix A. Substance use cutoffs employed in TSS study
Participants who reported values exceeding these cutoffs were excluded from the TSS study.
| “How many days in your lifetime have you drank something with alcohol?" | “What’s the most drinks you’ve had at any one time?” |
"How many days have you smoked a cigarette?" | "How many days have you used marijuana?" | "How many days have you used another drug or pills to get high?" | ||
|---|---|---|---|---|---|---|
| Age: | Boys | Girls | ||||
| 13 | ≥5 | ≥3 | ≥2 | ≥5 | ≥5 | ≥3 |
| 14 | ≥7 | ≥4 | ≥3 | ≥5 | ≥7 | ≥3 |
| 15 | ≥16 | ≥4 | ≥3 | ≥5 | ≥19 | ≥7 |
| 16 | ≥31 | ≥5 | ≥3 | ≥5 | ≥39 | ≥7 |
| 17 | ≥53 | ≥5 | ≥3 | ≥6 | ≥50 | ≥7 |
Appendix B. Supplementary data
The following is Supplementary data to this article:
References
- Aminoff E.M., Kveraga K., Bar M. The role of the parahippocampal cortex in cognition. Trends Cogn. Sci. 2013;17(8):379–390. doi: 10.1016/j.tics.2013.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson J.S., Ferguson M.A., Lopez-Larson M., Yurgelun-Todd D. Topographic maps of multisensory attention. Proc. Natl. Acad. Sci. 2010;107(46):20110–20114. doi: 10.1073/pnas.1011616107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baird B., Smallwood J., Gorgolewski K.J., Margulies D.S. Medial and lateral networks in anterior prefrontal cortex support metacognitive ability for memory and perception. J. Neurosci. 2013;33(42):16657–16665. doi: 10.1523/JNEUROSCI.0786-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannerman R.L., Milders M., de Gelder B., Sahraie A. Orienting to threat: faster localization of fearful facial expressions and body postures revealed by saccadic eye movements. Proc. R. Soc. B: Biol. Sci. 2009;276(1662):1635–1641. doi: 10.1098/rspb.2008.1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein D.P., Fink L. Psychological Corporation; 1997. Childhood Trauma Questionnaire: a Retrospective Self-Report : Manual. [Google Scholar]
- Bernstein D.P., Stein J.A., Newcomb M.D., Walker E., Pogge D., Ahluvalia T., Stokes J., Handelsman L., Medrano M., Desmond D., Zule W. Development and validation of a brief screening version of the Childhood Trauma Questionnaire. Child Abuse Negl. 2003;27(2):169–190. doi: 10.1016/S0145-2134(02)00541-0. [DOI] [PubMed] [Google Scholar]
- Berretta S. Cortico-amygdala circuits: role in the conditioned stress response. Stress. 2005;8(4):221–232. doi: 10.1080/10253890500489395. [DOI] [PubMed] [Google Scholar]
- Birn R.M., Roeber B.J., Pollak S.D. Early childhood stress exposure, reward pathways, and adult decision making. Proc. Natl. Acad. Sci. 2017;114(51):13549–13554. doi: 10.1073/pnas.1708791114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair R.J.R. The amygdala and ventromedial prefrontal cortex: functional contributions and dysfunction in psychopathy. Philos. Trans. R. Soc. B: Biol. Sci. 2008;363(1503):2557–2565. doi: 10.1098/rstb.2008.0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botvinik-Nezer R., Holzmeister F., Camerer C.F., Dreber A., Huber J., Johannesson M., Kirchler M., Iwanir R., Mumford J.A., Adcock R.A., Avesani P., Baczkowski B.M., Bajracharya A., Bakst L., Ball S., Barilari M., Bault N., Beaton D., Beitner J. Variability in the analysis of a single neuroimaging dataset by many teams. Nature. 2020;582(7810):84–88. doi: 10.1038/s41586-020-2314-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner J.D., Randall P., Vermetten E., Staib L., Bronen R.A., Mazure C., Capelli S., McCarthy G., Innis R.B., Charney D.S. Magnetic resonance imaging-based measurement of hippocampal volume in posttraumatic stress disorder related to childhood physical and sexual abuse—a preliminary report. Biol. Psychiatry. 1997;41(1):23–32. doi: 10.1016/S0006-3223(96)00162-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brito S.A.D., Viding E., Sebastian C.L., Kelly P.A., Mechelli A., Maris H., McCrory E.J. Reduced orbitofrontal and temporal grey matter in a community sample of maltreated children. J. Child Psychol. Psychiatry. 2013;54(1):105–112. doi: 10.1111/j.1469-7610.2012.02597.x. [DOI] [PubMed] [Google Scholar]
- Bugden S., Price G.R., McLean D.A., Ansari D. The role of the left intraparietal sulcus in the relationship between symbolic number processing and children’s arithmetic competence. Dev. Cogn. Neurosci. 2012;2(4):448–457. doi: 10.1016/j.dcn.2012.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush G., Luu P., Posner M. Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn. Sci. 2000;4(6):215–222. doi: 10.1016/S1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- Chai L.R., Khambhati A.N., Ciric R., Moore T., Gur R.C., Gur R.E., Satterthwaite T.D., Bassett D.S. Evolution of brain network dynamics in neurodevelopment. Netw. Neurosci. 2017:1–17. doi: 10.1162/NETN_a_00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandan J.S., Keerthy D., Zemedikun D.T., Okoth K., Gokhale K.M., Raza K., Bandyopadhyay S., Taylor J., Nirantharakumar K. The association between exposure to childhood maltreatment and the subsequent development of functional somatic and visceral pain syndromes. EClinicalMedicine. 2020;23 doi: 10.1016/j.eclinm.2020.100392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C., Grace A.A. Inhibitory modulation of orbitofrontal cortex on medial prefrontal cortex–amygdala information flow. Cereb. Cortex. 2018;28(1):1–8. doi: 10.1093/cercor/bhw342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christoff K., Gabrieli J.D.E. The frontopolar cortex and human cognition: evidence for a rostrocaudal hierarchical organization within the human prefrontal cortex. Psychobiology. 2000;28(2):168–186. doi: 10.3758/BF03331976. [DOI] [Google Scholar]
- Ciric R., Wolf D.H., Power J.D., Roalf D.R., Baum G., Ruparel K., Shinohara R.T., Elliott M.A., Eickhoff S.B., Davatzikos C., Gur R.C., Gur R.E., Bassett D.S., Satterthwaite T.D. 2016. Benchmarking Confound Regression Strategies for the Control of Motion Artifact in Studies of Functional Connectivity.http://arxiv.org/abs/1608.03616 ArXiv:1608.03616 [q-Bio] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cisler J.M. Childhood trauma and functional connectivity between Amygdala and medial prefrontal cortex: a dynamic functional connectivity and large-scale network perspective. Front. Syst. Neurosci. 2017;11:29. doi: 10.3389/fnsys.2017.00029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark C., Caldwell T., Power C., Stansfeld S.A. Does the influence of childhood adversity on psychopathology persist across the lifecourse? A 45-year prospective epidemiologic study. Ann. Epidemiol. 2010;20(5):385–394. doi: 10.1016/j.annepidem.2010.02.008. [DOI] [PubMed] [Google Scholar]
- Corbetta M., Shulman G.L. Control of goal-directed and stimulus-driven attention in the brain. Nat. Rev. Neurosci. 2002;3(3):201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- Craig A. Interoception: the sense of the physiological condition of the body. Curr. Opin. Neurobiol. 2003;13(4):500–505. doi: 10.1016/S0959-4388(03)00090-4. [DOI] [PubMed] [Google Scholar]
- Dahmen B., Puetz V.B., Scharke W., Polier G.Gvon, Herpertz-Dahlmann B., Konrad K. Effects of early-life adversity on hippocampal structures and associated HPA Axis functions. Dev. Neurosci. 2018;40(1):13–22. doi: 10.1159/000484238. [DOI] [PubMed] [Google Scholar]
- Dale A.M., Fischl B., Sereno M.I. Cortical surface-based analysis. I. Segmentation and surface reconstruction. NeuroImage. 1999;9(2):179–194. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- Dannlowski U., Stuhrmann A., Beutelmann V., Zwanzger P., Lenzen T., Grotegerd D., Domschke K., Hohoff C., Ohrmann P., Bauer J., Lindner C., Postert C., Konrad C., Arolt V., Heindel W., Suslow T., Kugel H. Limbic scars: long-term consequences of childhood maltreatment revealed by functional and structural magnetic resonance imaging. Biol. Psychiatry. 2012;71(4):286–293. doi: 10.1016/j.biopsych.2011.10.021. [DOI] [PubMed] [Google Scholar]
- De Bellis M.D. The psychobiology of neglect. Child Maltreat. 2005;10(2):150–172. doi: 10.1177/1077559505275116. [DOI] [PubMed] [Google Scholar]
- De Bellis M.D., Keshavan M.S., Clark D.B., Casey B.J., Giedd J.N., Boring A.M., Frustaci K., Ryan N.D. Developmental traumatology part II: brain development∗∗see accompanying Editorial, in this issue. Biol. Psychiatry. 1999;45(10):1271–1284. doi: 10.1016/S0006-3223(99)00045-1. [DOI] [PubMed] [Google Scholar]
- Desikan R.S., Ségonne F., Fischl B., Quinn B.T., Dickerson B.C., Blacker D., Buckner R.L., Dale A.M., Maguire R.P., Hyman B.T., Albert M.S., Killiany R.J. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. NeuroImage. 2006;31(3):968–980. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- Developmental Cognition and Neuroimaging Lab . 2020. MPlus Master GUI.https://github.com/DCAN-Labs/mastergui [Google Scholar]
- Di Martino A., Fair D.A., Kelly C., Satterthwaite T.D., Castellanos F.X., Thomason M.E., Craddock R.C., Luna B., Leventhal B.L., Zuo X.-N., Milham M.P. Unraveling the miswired connectome: a developmental perspective. Neuron. 2014;83(6):1335–1353. doi: 10.1016/j.neuron.2014.08.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosenbach N.U., Fair D.A., Miezin F.M., Cohen A.L., Wenger K.K., Dosenbach R.A., Fox M.D., Snyder A.Z., Vincent J.L., Raichle M.E. Distinct brain networks for adaptive and stable task control in humans. Proc. Natl. Acad. Sci. 2007;104(26):11073–11078. doi: 10.1073/pnas.0704320104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosenbach N.U.F., Fair D.A., Cohen A.L., Schlaggar B.L., Petersen S.E. A dual-networks architecture of top-down control. Trends Cogn. Sci. 2008;12(3):99–105. doi: 10.1016/j.tics.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edmiston E.E., Wang F., Mazure C.M., Guiney J., Sinha R., Mayes L.C., Blumberg H.P. Corticostriatal-limbic gray matter morphology in adolescents with self-reported exposure to childhood maltreatment. Arch. Pediatr. Adolesc. Med. 2011;165(12):1069–1077. doi: 10.1001/archpediatrics.2011.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichenbaum H., Schoenbaum G., Young B., Bunsey M. Functional organization of the hippocampal memory system. Proc. Natl. Acad. Sci. 1996;93(24):13500–13507. doi: 10.1073/pnas.93.24.13500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberger N.I., Lieberman M.D. Why rejection hurts: a common neural alarm system for physical and social pain. Trends Cogn. Sci. 2004;8(7):294–300. doi: 10.1016/j.tics.2004.05.010. [DOI] [PubMed] [Google Scholar]
- Eklund A., Nichols T.E., Knutsson H. Cluster failure: why fMRI inferences for spatial extent have inflated false-positive rates. Proc. Natl. Acad. Sci. 2016;113(28):7900–7905. doi: 10.1073/pnas.1602413113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellwood-Lowe M.E., Sacchet M.D., Gotlib I.H. The application of neuroimaging to social inequity and language disparity: a cautionary examination. Dev. Cogn. Neurosci. 2016 doi: 10.1016/j.dcn.2016.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans G.W., Li D., Whipple S.S. Cumulative risk and child development. Psychol. Bull. 2013;139(6):1342–1396. doi: 10.1037/a0031808. [DOI] [PubMed] [Google Scholar]
- Fair D.A., Bathula D., Nikolas M.A., Nigg J.T. Distinct neuropsychological subgroups in typically developing youth inform heterogeneity in children with ADHD. Proc. Natl. Acad. Sci. U.S.A. 2012;109(17):6769–6774. doi: 10.1073/pnas.1115365109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fair D.A., Miranda-Dominguez O., Snyder A.Z., Perrone A., Earl E.A., Van A.N., Koller J.M., Feczko E., Tisdall M.D., van der Kouwe A., Klein R.L., Mirro A.E., Hampton J.M., Adeyemo B., Laumann T.O., Gratton C., Greene D.J., Schlaggar B.L., Hagler D.J. Correction of respiratory artifacts in MRI head motion estimates. NeuroImage. 2020;208 doi: 10.1016/j.neuroimage.2019.116400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan Y., Herrera-Melendez A.L., Pestke K., Feeser M., Aust S., Otte C., Pruessner J.C., Böker H., Bajbouj M., Grimm S. Early life stress modulates amygdala-prefrontal functional connectivity: implications for oxytocin effects. Hum. Brain Mapp. 2014;35(10):5328–5339. doi: 10.1002/hbm.22553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fareri D.S., Tottenham N. Effects of early life stress on amygdala and striatal development. Dev. Cogn. Neurosci. 2016;19:233–247. doi: 10.1016/j.dcn.2016.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feczko E., Fair D.A. Methods and challenges for assessing heterogeneity. Biol. Psychiatry. 2020 doi: 10.1016/j.biopsych.2020.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felitti V.J., Anda R.F., Nordenberg D., Williamson D.F., Spitz A.M., Edwards V., Koss M.P., Marks J.S. Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults: the Adverse Childhood Experiences (ACE) Study. Am. J. Prev. Med. 1998;14(4):245–258. doi: 10.1016/s0749-3797(98)00017-8. [DOI] [PubMed] [Google Scholar]
- Feng P., Feng T., Chen Z., Lei X. Memory consolidation of fear conditioning: Bi-stable amygdala connectivity with dorsal anterior cingulate and medial prefrontal cortex. Soc. Cogn. Affect. Neurosci. 2014;9(11):1730–1737. doi: 10.1093/scan/nst170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn E.S., Shen X., Scheinost D., Rosenberg M.D., Huang J., Chun M.M., Papademetris X., Constable R.T. Functional connectome fingerprinting: identifying individuals using patterns of brain connectivity. Nat. Neurosci. 2015;18(11):1664–1671. doi: 10.1038/nn.4135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming S.M., Ryu J., Golfinos J.G., Blackmon K.E. Domain-specific impairment in metacognitive accuracy following anterior prefrontal lesions. Brain. 2014 doi: 10.1093/brain/awu221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox Michael D., Corbetta Maurizio, Snyder Abraham Z., Vincent Justin L., Raichle Marcus E. Spontaneous neuronalctivity distinguishes human dorsal and ventral attention systems. Proc. Natl. Acad. Sci. 2006;103(26) doi: 10.1073/pnas.0604187103. http://www.pnas.org/content/103/26/10046.full.pdf [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabard-Durnam L.J., Flannery J., Goff B., Gee D.G., Humphreys K.L., Telzer E., Hare T., Tottenham N. The development of human amygdala functional connectivity at rest from 4 to 23 years: a cross-sectional study. NeuroImage. 2014;95:193–207. doi: 10.1016/j.neuroimage.2014.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee D.G., Gabard-Durnam L.J., Flannery J., Goff B., Humphreys K.L., Telzer E.H., Hare T.A., Bookheimer S.Y., Tottenham N. Early developmental emergence of human amygdala–prefrontal connectivity after maternal deprivation. Proc. Natl. Acad. Sci. 2013;110(39):15638–15643. doi: 10.1073/pnas.1307893110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerdner A., Allgulander C. Psychometric properties of the swedish version of the childhood trauma questionnaire-short form (CTQ-SF) Nord. J. Psychiatry. 2009;63(2):160–170. doi: 10.1080/08039480802514366. [DOI] [PubMed] [Google Scholar]
- Gheorghe D.A., Li C., Gallacher J., Bauermeister S. Associations of perceived adverse lifetime experiences with brain structure in UK Biobank participants. J. Child Psychol. Psychiatry. 2020 doi: 10.1111/jcpp.13298. [DOI] [PubMed] [Google Scholar]
- Glasser M.F., Sotiropoulos S.N., Wilson J.A., Coalson T.S., Fischl B., Andersson J.L., Xu J., Jbabdi S., Webster M., Polimeni J.R., Van Essen D.C., Jenkinson M. The minimal preprocessing pipelines for the human connectome project. NeuroImage. 2013;80:105–124. doi: 10.1016/j.neuroimage.2013.04.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasser M.F., Coalson T.S., Robinson E.C., Hacker C.D., Harwell J., Yacoub E., Ugurbil K., Andersson J., Beckmann C.F., Jenkinson M., Smith S.M., Van Essen D.C. A multi-modal parcellation of human cerebral cortex. Nature. 2016;536(7615):171–178. doi: 10.1038/nature18933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon E.M., Laumann T.O., Adeyemo B., Huckins J.F., Kelley W.M., Petersen S.E. Generation and evaluation of a cortical area parcellation from resting-state correlations. Cereb. Cortex (New York, NY) 2016;26(1):288–303. doi: 10.1093/cercor/bhu239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gratton C., Laumann T.O., Nielsen A.N., Greene D.J., Gordon E.M., Gilmore A.W., Nelson S.M., Coalson R.S., Snyder A.Z., Schlaggar B.L., Dosenbach N.U.F., Petersen S.E. Functional brain networks are dominated by stable group and individual factors, not cognitive or daily variation. Neuron. 2018;98(2):439–452. doi: 10.1016/j.neuron.2018.03.035. e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grayson D.S., Fair D.A. Development of large-scale functional networks from birth to adulthood: a guide to the neuroimaging literature. NeuroImage. 2017;160:15–31. doi: 10.1016/j.neuroimage.2017.01.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greco J.A., Liberzon I. Neuroimaging of fear-associated learning. Neuropsychopharmacology. 2016;41(1):320–334. doi: 10.1038/npp.2015.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunnar M.R., DePasquale C.E., Reid B.M., Donzella B., Miller B.S. Pubertal stress recalibration reverses the effects of early life stress in postinstitutionalized children. Proc. Natl. Acad. Sci. 2019;116(48):23984–23988. doi: 10.1073/pnas.1909699116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyer A.E., Silk J.S., Nelson E.E. The neurobiology of the emotional adolescent: from the inside out. Neurosci. Biobehav. Rev. 2016;70:74–85. doi: 10.1016/j.neubiorev.2016.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson J.L., Chung M.K., Avants B.B., Shirtcliff E.A., Gee J.C., Davidson R.J., Pollak S.D. Early stress is associated with alterations in the orbitofrontal cortex: a tensor-based morphometry investigation of brain structure and behavioral risk. J. Neurosci. 2010;30(22):7466–7472. doi: 10.1523/JNEUROSCI.0859-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herringa R.J., Birn R.M., Ruttle P.L., Burghy C.A., Stodola D.E., Davidson R.J., Essex M.J. Childhood maltreatment is associated with altered fear circuitry and increased internalizing symptoms by late adolescence. Proc. Natl. Acad. Sci. U.S.A. 2013;110(47):19119–19124. doi: 10.1073/pnas.1310766110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildyard K.L., Wolfe D.A. Child neglect: developmental issues and outcomes. Child Abuse Negl. 2002;26(6):679–695. doi: 10.1016/s0145-2134(02)00341-1. [DOI] [PubMed] [Google Scholar]
- Hooker C.I., Germine L.T., Knight R.T., D’Esposito M. Amygdala response to facial expressions reflects emotional learning. J. Neurosci. 2006;26(35):8915–8922. doi: 10.1523/JNEUROSCI.3048-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia X.-Z., Zhao N., Barton B., Burciu R., Carrière N., Cerasa A., Chen B.-Y., Chen J., Coombes S., Defebvre L., Delmaire C., Dujardin K., Esposito F., Fan G.-G., Federica D.N., Feng Y.-X., Fling B.W., Garg S., Gilat M. Small effect size leads to reproducibility failure in resting-state fMRI studies [Preprint] Neuroscience. 2018 doi: 10.1101/285171. [DOI] [Google Scholar]
- Kessler R.C., Berglund P., Demler O., Jin R., Merikangas K.R., Walters E.E. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch. Gen. Psychiatry. 2005;62(6):593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- Kessler R.C., Amminger G.P., Aguilar-Gaxiola S., Alonso J., Lee S., Ustün T.B. Age of onset of mental disorders: a review of recent literature. Curr. Opin. Psychiatry. 2007;20(4):359–364. doi: 10.1097/YCO.0b013e32816ebc8c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilian S., Asmal L., Chiliza B., Olivier M.R., Phahladira L., Scheffler F., Seedat S., Marder S.R., Green M.F., Emsley R. Childhood adversity and cognitive function in schizophrenia spectrum disorders and healthy controls: evidence for an association between neglect and social cognition. Psychol. Med. 2018;48(13):2186–2193. doi: 10.1017/S0033291717003671. [DOI] [PubMed] [Google Scholar]
- Kim M.J., Loucks R.A., Palmer A.L., Brown A.C., Solomon K.M., Marchante A.N., Whalen P.J. The structural and functional connectivity of the amygdala: from normal emotion to pathological anxiety. Behav. Brain Res. 2011;223(2):403–410. doi: 10.1016/j.bbr.2011.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King L.S., Humphreys K.L., Camacho M.C., Gotlib I.H. A person-centered approach to the assessment of early life stress: associations with the volume of stress-sensitive brain regions in early adolescence. Dev. Psychopathol. 2019;31(2):643–655. doi: 10.1017/S0954579418000184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klinitzke G., Romppel M., Häuser W., Brähler E., Glaesmer H. [The German Version of the Childhood Trauma Questionnaire (CTQ): psychometric characteristics in a representative sample of the general population] Psychother. Psychosom. Med. Psychol. 2012;62(2):47–51. doi: 10.1055/s-0031-1295495. [DOI] [PubMed] [Google Scholar]
- Kovelman I., Norton E.S., Christodoulou Ja., Gaab N., Lieberman Da., Triantafyllou C., Wolf M., Whitfield-Gabrieli S., Gabrieli J.D.E. Brain basis of phonological awareness for spoken language in children and its disruption in dyslexia. Cereb. Cortex. 2012;22(4):754–764. doi: 10.1093/cercor/bhr094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krain A.L., Wilson A.M., Arbuckle R., Castellanos F.X., Milham M.P. Distinct neural mechanisms of risk and ambiguity: a meta-analysis of decision-making. NeuroImage. 2006;32(1):477–484. doi: 10.1016/j.neuroimage.2006.02.047. [DOI] [PubMed] [Google Scholar]
- Kuhlman K.R., Chiang J.J., Horn S., Bower J.E. Developmental psychoneuroendocrine and psychoneuroimmune pathways from childhood adversity to disease. Neurosci. Biobehav. Rev. 2017;80:166–184. doi: 10.1016/j.neubiorev.2017.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson J., Heeger D.J. Two retinotopic visual areas in human lateral occipital cortex. J. Neurosci. 2006;26(51):13128–13142. doi: 10.1523/JNEUROSCI.1657-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LoPilato A.M., Goines K., Addington J., Bearden C.E., Cadenhead K.S., Cannon T.D., Cornblatt B.A., Mathalon D.H., McGlashan T.H., Seidman L., Perkins D.O., Tsuang M.T., Woods S.W., Walker E.F. Impact of childhood adversity on corticolimbic volumes in youth at clinical high-risk for psychosis. Schizophr. Res. 2019;213:48–55. doi: 10.1016/j.schres.2019.01.048. [DOI] [PubMed] [Google Scholar]
- Luby J.L., Tillman R., Barch D.M. Association of timing of adverse childhood experiences and caregiver support with regionally specific brain development in adolescents. JAMA Network Open. 2019;2(9):e1911426. doi: 10.1001/jamanetworkopen.2019.11426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald K., Thomas M.L., Sciolla A.F., Schneider B., Pappas K., Bleijenberg G., Bohus M., Bekh B., Carpenter L., Carr A., Dannlowski U., Dorahy M., Fahlke C., Finzi-Dottan R., Karu T., Gerdner A., Glaesmer H., Grabe H.J., Heins M. Minimization of childhood maltreatment is common and consequential: results from a large, multinational sample using the childhood trauma questionnaire. PLoS One. 2016;11(1) doi: 10.1371/journal.pone.0146058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin K.A., Lambert H.K. Child trauma exposure and psychopathology: mechanisms of risk and resilience. Curr. Opin. Psychol. 2017;14:29–34. doi: 10.1016/j.copsyc.2016.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin K.A., Greif Green J., Gruber M.J., Sampson N.A., Zaslavsky A.M., Kessler R.C. Childhood adversities and first onset of psychiatric disorders in a national sample of US adolescents. Arch. Gen. Psychiatry. 2012;69(11):1151. doi: 10.1001/archgenpsychiatry.2011.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin K.A., Sheridan M.A., Lambert H.K. Childhood adversity and neural development: deprivation and threat as distinct dimensions of early experience ଝ. Neurosci. Biobehav. Rev. 2014;47:578–591. doi: 10.1016/j.neubiorev.2014.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin K.A., Weissman D., Bitrán D. Childhood adversity and neural development: a systematic review. Annu. Rev. Dev. Psychol. 2019;1(1):277–312. doi: 10.1146/annurev-devpsych-121318-084950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon S.D., Grant K.E., Compas B.E., Thurm A.E., Ey S. Stress and psychopathology in children and adolescents: is there evidence of specificity? J. Child Psychol. Psychiatry. 2003;44(1):107–133. doi: 10.1111/1469-7610.00105. [DOI] [PubMed] [Google Scholar]
- McNeil B.J., Keller E., Adelstein S.J. Primer on certain elements of medical decision making. N. Engl. J. Med. 1975;293(5):211–215. doi: 10.1056/NEJM197507312930501. [DOI] [PubMed] [Google Scholar]
- Menon V., Uddin L.Q. Saliency, switching, attention and control: a network model of insula function. Brain Struct. Funct. 2010;214(5–6):655–667. doi: 10.1007/s00429-010-0262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda-Dominguez O., Mills B.D., Carpenter S.D., Grant K.A., Kroenke C.D., Nigg J.T., Fair D.A. Connectotyping: model based fingerprinting of the functional connectome. PLoS One. 2014;9(11):e111048. doi: 10.1371/journal.pone.0111048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motzkin J.C., Philippi C.L., Wolf R.C., Baskaya M.K., Koenigs M. Ventromedial prefrontal cortex is critical for the regulation of amygdala activity in humans. Biol. Psychiatry. 2015;77(3):276–284. doi: 10.1016/j.biopsych.2014.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthén L., Muthén B. Muthén & Muthén; 1998. Mplus Users Guide (Sixth) [Computer software] [Google Scholar]
- Nachev P., Kennard C., Husain M. Functional role of the supplementary and pre-supplementary motor areas. Nat. Rev. Neurosci. 2008;9(11):856–869. doi: 10.1038/nrn2478. [DOI] [PubMed] [Google Scholar]
- Nelson E.E., Lau J.Y.F., Jarcho J.M. Growing pains and pleasures: how emotional learning guides development. Trends Cogn. Sci. 2014;18(2):99–108. doi: 10.1016/j.tics.2013.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nooner K.B., Mennes M., Brown S., Castellanos F.X., Leventhal B., Milham M.P., Colcombe S.J. Relationship of trauma symptoms to amygdala-based functional brain changes in adolescents. J. Trauma. Stress. 2013;26(6):784–787. doi: 10.1002/jts.21873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Op de Macks Z.A., Flannery J.E., Peake S.J., Flournoy J.C., Mobasser A., Alberti S.L., Fisher P.A., Pfeifer J.H. Novel insights from the Yellow Light Game: safe and risky decisions differentially impact adolescent outcome-related brain function. NeuroImage. 2018;181:568–581. doi: 10.1016/j.neuroimage.2018.06.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osada T., Ohta S., Ogawa A., Tanaka M., Suda A., Kamagata K., Hori M., Aoki S., Shimo Y., Hattori N., Shimizu T., Enomoto H., Hanajima R., Ugawa Y., Konishi S. An essential role of the intraparietal sulcus in response inhibition predicted by parcellation-based network. J. Neurosci. 2019;39(13):2509–2521. doi: 10.1523/JNEUROSCI.2244-18.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagliaccio D., Luby J.L., Bogdan R., Agrawal A., Gaffrey M.S., Belden A.C., Botteron K.N., Harms M.P., Barch D.M. Amygdala functional connectivity, HPA axis genetic variation, and life stress in children and relations to anxiety and emotion regulation. J. Abnorm. Psychol. 2015;124(4):817–833. doi: 10.1037/abn0000094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park A.T., Leonard J.A., Saxler P.K., Cyr A.B., Gabrieli J.D.E., Mackey A.P. Amygdala–medial prefrontal cortex connectivity relates to stress and mental health in early childhood. Soc. Cogn. Affect. Neurosci. 2018;13(4):430–439. doi: 10.1093/scan/nsy017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkes L., Fulcher B., Yücel M., Fornito A. An evaluation of the efficacy, reliability, and sensitivity of motion correction strategies for resting-state functional MRI. NeuroImage. 2018;171:415–436. doi: 10.1016/j.neuroimage.2017.12.073. [DOI] [PubMed] [Google Scholar]
- Paus T. Primate anterior cingulate cortex: where motor control, drive and cognition interface. Nat. Rev. Neurosci. 2001;2(6):417–424. doi: 10.1038/35077500. [DOI] [PubMed] [Google Scholar]
- Peverill M., Sheridan M.A., Busso D.S., McLaughlin K.A. Atypical prefrontal-amygdala circuitry following childhood exposure to abuse: links with adolescent psychopathology. Child Maltreat. 2019;24(4):411–423. doi: 10.1177/1077559519852676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelps E.A., LeDoux J.E. Contributions of the amygdala to emotion processing: from animal models to human behavior. Neuron. 2005;48(2):175–187. doi: 10.1016/j.neuron.2005.09.025. [DOI] [PubMed] [Google Scholar]
- Phelps E.A., Delgado M.R., Nearing K.I., LeDoux J.E. Extinction learning in humans: role of the amygdala and vmPFC. Neuron. 2004;43(6):897–905. doi: 10.1016/j.neuron.2004.08.042. [DOI] [PubMed] [Google Scholar]
- Pine D.S., Fox N.A. Childhood antecedents and risk for adult mental disorders. Annu. Rev. Psychol. 2015;66(1):459–485. doi: 10.1146/annurev-psych-010814-015038. [DOI] [PubMed] [Google Scholar]
- Pollak S.D., Tolley-Schell S.A. Selective attention to facial emotion in physically abused children. J. Abnorm. Psychol. 2003;112(3):323–338. doi: 10.1037/0021-843X.112.3.323. [DOI] [PubMed] [Google Scholar]
- Pollak S.D., Cicchetti D., Hornung K., Reed A. Recognizing emotion in faces: developmental effects of child abuse and neglect. Dev. Psychol. 2000;36(5):679–688. doi: 10.1037/0012-1649.36.5.679. [DOI] [PubMed] [Google Scholar]
- Power J.D., Mitra A., Laumann T.O., Snyder A.Z., Schlaggar B.L., Petersen S.E. Methods to detect, characterize, and remove motion artifact in resting state fMRI. NeuroImage. 2014;84:320–341. doi: 10.1016/j.neuroimage.2013.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagaspe P., Schwartz S., Vuilleumier P. Fear and stop: a role for the amygdala in motor inhibition by emotional signals. NeuroImage. 2011;55(4):1825–1835. doi: 10.1016/j.neuroimage.2011.01.027. [DOI] [PubMed] [Google Scholar]
- Saxbe D., Khoddam H., Stoycos S.A., Margolin G., Kaplan J.T., Piero L.D., Gimbel S.I. Community violence exposure in early adolescence: longitudinal associations with hippocampal and amygdala volume and resting state connectivity. Dev. Sci. 2018;21(6) doi: 10.1111/desc.12686. N.PAG-N.PAG. [DOI] [PubMed] [Google Scholar]
- Saxe R., Kanwisher N. People thinking about thinking people the role of the temporo-parietal junction in “theory of mind”. NeuroImage. 2003;19:1835–1842. doi: 10.1016/S1053-8119(03)00230-1. [DOI] [PubMed] [Google Scholar]
- Schall J.D. On the role of frontal eye field in guiding attention and saccades. Vision Res. 2004;44(12):1453–1467. doi: 10.1016/j.visres.2003.10.025. [DOI] [PubMed] [Google Scholar]
- Seeley W.W., Menon V., Schatzberg A.F., Keller J., Glover G.H., Kenna H., Reiss A.L., Greicius M.D. Dissociable intrinsic connectivity networks for salience processing and executive control. J. Neurosci. 2007;27(9):2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shackman J.E., Shackman A.J., Pollak S.D. Physical abuse amplifies attention to threat and increases anxiety in children. Emotion. 2007;7(4):838–852. doi: 10.1037/1528-3542.7.4.838. [DOI] [PubMed] [Google Scholar]
- Shonkoff J.P., Garner A.S. The lifelong effects of early childhood adversity and toxic stress. Pediatrics. 2012;129(1):e232–246. doi: 10.1542/peds.2011-2663. [DOI] [PubMed] [Google Scholar]
- Singer T., Critchley H.D., Preuschoff K. A common role of insula in feelings, empathy and uncertainty. Trends Cogn. Sci. 2009;13(8):334–340. doi: 10.1016/j.tics.2009.05.001. [DOI] [PubMed] [Google Scholar]
- Smits N. A note on Youden’s J and its cost ratio. BMC Med Res Method. 2010;10:89. doi: 10.1186/1471-2288-10-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein M.B., Koverola C., Hanna C., Torchia M.G., McClarty B. Hippocampal volume in women victimized by childhood sexual abuse. Psychol. Med. 1997;27(4):951–959. doi: 10.1017/S0033291797005242. [DOI] [PubMed] [Google Scholar]
- Stern A., Agnew-Blais J., Danese A., Fisher H.L., Jaffee S.R., Matthews T., Polanczyk G.V., Arseneault L. Associations between abuse/neglect and ADHD from childhood to young adulthood: a prospective nationally-representative twin study. Child Abuse Negl. 2018;81:274–285. doi: 10.1016/j.chiabu.2018.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens S.E., Sonuga-Barke E.J.S., Kreppner J.M., Beckett C., Castle J., Colvert E., Groothues C., Hawkins A., Rutter M. Inattention/Overactivity following early severe institutional deprivation: presentation and associations in early adolescence. J. Abnorm. Child Psychol. 2008;36(3):385–398. doi: 10.1007/s10802-007-9185-5. [DOI] [PubMed] [Google Scholar]
- Teicher M.H., Parigger A. The “Maltreatment and Abuse Chronology of Exposure” (MACE) scale for the retrospective assessment of abuse and neglect during development. PLoS One. 2015;10(2):e0117423. doi: 10.1371/journal.pone.0117423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teicher M.H., Samson J.A. Childhood maltreatment and psychopathology: a case for ecophenotypic variants as clinically and neurobiologically distinct subtypes. Am. J. Psychiatry. 2013;170(10):1114–1133. doi: 10.1176/appi.ajp.2013.12070957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teicher M.H., Samson J.A., Anderson C.M., Ohashi K. The effects of childhood maltreatment on brain structure, function and connectivity. Nat. Rev. Neurosci. 2016;17(10):652–666. doi: 10.1038/nrn.2016.111. [DOI] [PubMed] [Google Scholar]
- Thomason M.E., Marusak H.A., Tocco M.A., Vila A.M., McGarragle O., Rosenberg D.R. Altered amygdala connectivity in urban youth exposed to trauma. Soc. Cogn. Affect. Neurosci. 2015;10(11):1460–1468. doi: 10.1093/scan/nsv030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomoda A., Polcari A., Anderson C.M., Teicher M.H. Reduced visual cortex gray matter volume and thickness in young adults who witnessed domestic violence during childhood. PLoS One. 2012;7(12):e52528. doi: 10.1371/journal.pone.0052528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tottenham N., Sheridan M.A. A review of adversity, the amygdala and the hippocampus: a consideration of developmental timing. Front. Hum. Neurosci. 2010;3 doi: 10.3389/neuro.09.068.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tozzi L., Garczarek L., Janowitz D., Stein D.J., Wittfeld K., Dobrowolny H., Lagopoulos J., Hatton S.N., Hickie I.B., Carballedo A., Brooks S.J., Vuletic D., Uhlmann A., Veer I.M., Walter H., Bülow R., Völzke H., Klinger-König J., Schnell K. Interactive impact of childhood maltreatment, depression, and age on cortical brain structure: mega-analytic findings from a large multi-site cohort. Psychol. Med. 2020;50(6):1020–1031. doi: 10.1017/S003329171900093X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Werff S.J.A., Pannekoek J.N., Veer I.M., van Tol M.-J., Aleman A., Veltman D.J., Zitman F.G., Rombouts S.A.R.B., Elzinga B.M., van der Wee N.J.A. Resting-state functional connectivity in adults with childhood emotional maltreatment. Psychol. Med.; Cambridge. 2012;43(9):1825–1836. doi: 10.1017/S0033291712002942. [DOI] [PubMed] [Google Scholar]
- van Rooij S.J.H., Smith R.D., Stenson A.F., Ely T.D., Yang X., Tottenham N., Stevens J.S., Jovanovic T. Increased activation of the fear neurocircuitry in children exposed to violence. Depress. Anxiety. 2019;37(4):303–312. doi: 10.1002/da.22994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Váša F., Romero-Garcia R., Kitzbichler M.G., Seidlitz J., Whitaker K.J., Vaghi M.M., Kundu P., Patel A.X., Fonagy P., Dolan R.J., Jones P.B., Goodyer I.M., Consortium the N., Vértes P.E., Bullmore E.T. Conservative and disruptive modes of adolescent change in human brain functional connectivity. Proc. Natl. Acad. Sci. 2020;117(6):3248–3253. doi: 10.1073/pnas.1906144117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijayakumar N., Whittle S., Yücel M., Dennison M., Simmons J., Allen N.B. Thinning of the lateral prefrontal cortex during adolescence predicts emotion regulation in females. Soc. Cogn. Affect. Neurosci. 2014;9(11):1845–1854. doi: 10.1093/scan/nst183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt B.A. Midcingulate cortex: structure, connections, homologies, functions and diseases. J. Chem. Neuroanat. 2016;74:28–46. doi: 10.1016/j.jchemneu.2016.01.010. [DOI] [PubMed] [Google Scholar]
- Vossel S., Geng J.J., Fink G.R. Dorsal and ventral attention systems. Neuroscientist. 2014;20(2):150–159. doi: 10.1177/1073858413494269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner K.S., Barnett M., Witthoft N., Golarai G., Stigliani A., Kay K.N., Gomez J., Natu V.S., Amunts K., Zilles K., Grill-Spector K. Defining the most probable location of the parahippocampal place area using cortex-based alignment and cross-validation. NeuroImage. 2018;170:373–384. doi: 10.1016/j.neuroimage.2017.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weller J.A., Fisher P.A. Decision-making deficits among maltreated children. Child Maltreat. 2013;18(3):184–194. doi: 10.1177/1077559512467846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeanah C.H., Egger H.L., Smyke A.T., Nelson C.A., Fox N.A., Marshall P.J., Guthrie D. Institutional rearing and psychiatric disorders in Romanian preschool children. Am. J. Psychiatry. 2009;166(7):777–785. doi: 10.1176/appi.ajp.2009.08091438. [DOI] [PubMed] [Google Scholar]
- Zhang S., Li C.R. Functional connectivity mapping of the human precuneus by resting state fMRI. NeuroImage. 2012;59(4):3548–3562. doi: 10.1016/j.neuroimage.2011.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou W., Shu H. A meta-analysis of functional magnetic resonance imaging studies of eye movements and visual word reading. Brain Behav. 2017;7(5):e00683. doi: 10.1002/brb3.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.