Abstract
BACKGROUND:
Regorafenib is an oral multikinase inhibitor targeting angiogenesis, oncogenesis, and cancer proliferation/metastasis. This study evaluated the efficacy of regorafenib in refractory biliary tract cancer (BTC) in a multi-institutional phase 2 study.
METHODS:
Patients with BTC who progressed on at least 1 line of systemic therapy received regorafenib at 160 mg daily for 21 days on and 7 days off. The primary endpoint was 6-month overall survival (OS), and the secondary endpoints were median OS, progression-free survival (PFS), and objective response rates. Pretreatment plasma was collected for cytokine evaluation.
RESULTS:
A total of 39 patients were enrolled, and 33 were evaluable for efficacy. The median PFS and OS were 3.7 and 5.4 months, respectively, with survival rates of 46.2% at 6 months, 35.9% at 12 months, and 25.6% at 18 months for the intention-to-treat population. For the 33 evaluable patients who received regorafenib for at least 3 weeks, the median PFS and OS were 3.9 and 6.7 months, respectively, with survival rates of 51.5% at 6 months, 39.4% at 12 months, and 27.3% at 18 months. The objective response rate was 9.1%, and the disease control rate was 63.6%. Twenty-eight patients (71.8%) experienced grade 3/4 adverse events. Among the 23 cytokines analyzed, elevated baseline vascular endothelial growth factor D (VEGF-D) was associated with shorter PFS, whereas elevated baseline interleukin 6 (IL-6) and glycoprotein 130 (GP130) were associated with shorter OS.
CONCLUSIONS:
Regorafenib demonstrated modest clinical efficacy in heavily pretreated patients with BTC. Further exploration of biomarkers is warranted to identify a group of patients with BTC who may benefit from regorafenib.
Keywords: biliary tract cancer, chemotherapy-refractory, cholangiocarcinoma, gallbladder cancer, regorafenib
INTRODUCTION
Biliary tract cancer (BTC) arises from the epithelial cells of bile ducts and includes intrahepatic cholangiocarcinoma, extrahepatic cholangiocarcinoma, and gallbladder cancer. BTC is a rare disease accounting for approximately 3% of all gastrointestinal cancers.1 However, it is a very aggressive malignancy with a 5-year survival rate of 10%.2 Although local therapy such as surgical resection is effective, most patients have either advanced disease at presentation or relapse disease despite curative-intent surgery.3 Gemcitabine plus cisplatin has demonstrated significant anticancer activity as first-line therapy in patients with advanced BTC,4 and the recent ABC-06 (Advanced Biliary Cancer-06) study fluorouracil, folinic acid and oxaliplatin (FOLFOX) as an option for second-line treatment with the modest improvement of a 1-month survival benefit over active symptom control (6.2 vs 5.3 months).5
Therefore, new effective therapeutic approaches are needed to improve the clinical outcomes of refractory advanced BTC.
Dysregulation of receptor tyrosine kinases (RTKs), including fibroblast growth factor receptor (FGFR), KIT, platelet-derived growth factor receptor (PDGFR), RET, and vascular endothelial growth factor receptor (VEGFR), and their downstream cell signaling pathways, such as the RAS/RAF/MEK/ERK pathway and the PI3K/PTEN/AKT pathway, are implicated in the development, progression, and metastasis of cancer.6 In BTC, aberrant expression of FGFR, vascular endothelial growth factor (VEGF), and platelet-derived growth factor (PDGF) and activation of the RAS/RAF/MEK/ERK and PI3K/PTEN/AKT pathways play essential roles in cancer development, progression, and metastasis.7–9 Furthermore aberrant expressions and activations are associated with a poor prognosis9–12; indicating potential therapeutic targets in BTC of BTC.
Regorafenib is a novel oral multikinase inhibitor that has shown significant antitumor activity in diverse gastrointestinal malignancies such as colorectal cancer, gastrointestinal stromal tumors, and hepatocellular carcinoma13–16 by blocking several angiogenic and stromal RTKs (FGFR1, PDGFR-β, TIE2, VEGFR1, VEGFR2, and VEGFR3), oncogenic RTKs (KIT and RET), and their downstream cell signaling pathways (the RAS/RAF/MEK/ERK pathway and the PI3K/PTEN/AKT pathway).13
Because regorafenib targets multiple RTKs and cell signaling pathways involved in BTC development, progression, and metastasis, we conducted a multicenter phase 2 study to assess the safety and efficacy of regorafenib in patients with advanced and refractory BTC.
MATERIALS AND METHODS
This was a multi-institution, open-label, single-arm phase 2 study designed to evaluate the safety, tolerability, and efficacy of regorafenib in patients with advanced, refractory BTC. This study was approved by the local institutional review boards, and all patients provided written informed consent before enrollment.
Patients Selection and Treatment
All eligible patients were required to have histologically or cytologically documented unresectable, locally advanced or metastatic cholangiocarcinoma (intrahepatic and extrahepatic) or gallbladder adenocarcinoma. The ampulla of Vater was excluded. Other inclusion criteria included an age of 18 years or older, an Eastern Cooperative Oncology Group performance status of 0 or 1, and adequate organ function. Patients had to have disease progression on at least 1 line but no more than 2 prior lines of systemic chemotherapy. All patients received 160 mg of regorafenib daily by mouth (21 days on and 7 days off) until disease progression or unacceptable toxicity occurred.
Evaluation
The tumor assessment was performed with computed tomography and/or magnetic resonance imaging at the baseline and every 8 weeks until disease progression or treatment discontinuation. Response and progression were evaluated with the revised Response Evaluation Criteria in Solid Tumors (version 1.1). Toxicities were monitored according to the Common Terminology Criteria for Adverse Events (version 4.0).
Cytokine/Chemokine Analysis
Ethylenediaminetetraacetic acid plasma samples were collected from all patients at the baseline. The plasma levels of 23 biomarkers, including Ang-2, glycoprotein 130 (GP130), HGF, ICAM-1, interleukin 6 (IL-6), IL-6R, OPN, PDGF-AA, PDGF-BB, PlGF, SDF1, TGFβ1, TGFβ2, TIMP-1, TSP-2, VACM-1, VEGF-A, VEGF-D, VEGF-R1, VEGF-R2, and VEGF-R3, were measured with the CiraScan multiplex platform (Quanterix, Billerica, Massachusetts),17 whereas BMP-9 and TGFβ-R3 were tested as described previously.18
Statistical Analysis
The primary endpoint of the study was overall survival (OS) at 6 months. OS was defined as the time from starting on the trial to the date of death due to any cause. This study was designed to test the null hypothesis of ≤30% OS at 6 months against the alternative of ≥50% OS at 6 months (hazard ratio [HR] under exponential model, 0.578). Based on Simon’s 2-stage minimax design, the experimental treatment was deemed to have good activity if ≥16 of 39 evaluable patients survived 6 months or longer. Patients were considered to be evaluable if they had received regorafenib for at least 3 weeks. Among the 39 enrolled patients, 33 were evaluable. With a 10% significance level and 85% power, the sample size was re-evaluated by Simon’s 2-stage minimax design. Twenty-three patients were evaluated in the first stage. If 7 or more of the 23 patients survived 6 months or longer, then the second stage would be opened, and an additional 10 patients would be evaluated in the second stage. The null hypothesis would be rejected if 14 or more of the 33 evaluable patients survived 6 months or longer. The 1-sided P value for the primary endpoint was computed by the conditional distribution,19 and no patient was censored before 6 months. The secondary endpoints in this study included the disease control rate (DCR), which was defined as the percentage of patients who achieved a complete response, a partial response, or stable disease; toxicity; and progression-free survival (PFS). The frequency and severity of adverse events were summarized with descriptive statistics. PFS and OS were estimated with the Kaplan-Meier method. For the correlative biomarker analyses, the prognostic value of each biomarker was tested with the Cox proportional hazards model for both PFS and OS. P values from the prognostic analysis were adjusted for multiple testing by controlling the false discovery rate. All statistical analyses were performed with SPSS Statistics 24 (IBM, Armonk, New York). All statistical tests except the primary endpoint used a 2-sided significance level of 5%.
RESULTS
Baseline Characteristics
Thirty-nine patients were enrolled in this study between June 2014 and October 2017 at 3 institutions. The baseline characteristics are summarized in Table 1. The median age of the patients was 62 years (range, 27–88 years), and the primary tumor sites were intrahepatic (69.2%), extrahepatic (15.4%), and the gallbladder (15.4%). Twenty-five patients (64.1%) received 1 line of systemic therapy, and 14 (35.9%) received 2 lines before regorafenib treatment. A total of 25 patients (64.1%) were treated with first-line gemcitabine/cisplatin, and 5 of the 14 patients who had disease progression on 2 lines of systemic treatment received oxaliplatin-based second-line therapy. Seven patients had received prior local therapy, including radiation (n = 3), yttrium-90 radioembolization (n = 3), and radiofrequency ablation (n = 1).
TABLE 1.
Patients Characteristics (n = 39)
| Variable | Value |
|---|---|
| Age, median (range), y | 62 (27–88) |
| Sex, No. (%) | |
| Male | 15 (38.5) |
| Female | 24 (61.5) |
| Race, No. (%) | |
| White | 35 (89.7) |
| African American | 1 (2.6) |
| Asian | 1 (2.6) |
| Unknown | 2 (5.1) |
| Location, No. (%) | |
| Intrahepatic | 27 (69.2) |
| Extrahepatic | 6 (15.4) |
| Gallbladder | 6 (15.4) |
| Distant metastasis, No. (%) | |
| No | 7 (17.9) |
| Yes | 32 (82.1) |
| Previous chemotherapy, No. (%) | |
| 1 line | 25 (64.1) |
| 2 lines | 14 (35.9) |
| Previous local therapy, No. (%) | |
| None | 32 (82.1) |
| Radiation | 3 (7.7) |
| Radioembolization | 3 (7.7) |
| Radiofrequency ablation | 1 (2.6) |
| ECOG PS, No. (%) | |
| 0 | 19 (48.7) |
| 1 | 20 (51.3) |
Abbreviations: ECOG, Eastern Cooperative Oncology Group; PS, performance status.
Treatment
Thirty-nine patients received at least 1 dose of regorafenib. Thirty patients discontinued the treatment because of radiological or clinical disease progression, 8 patients discontinued because of an adverse event, and 1 patient discontinued because of consent withdrawal. The median duration of treatment was 2.1 months (range, 0.5–20.6 months). Dose modifications were required in 19 of the 39 patients (48.7%) because of adverse events. Thirteen patients required 1 dose reduction, and 6 required 2 dose reductions. Six patients did not have their tumor response evaluation because of clinical progression (5 patients) or consent withdrawal (1 patient).
Efficacy
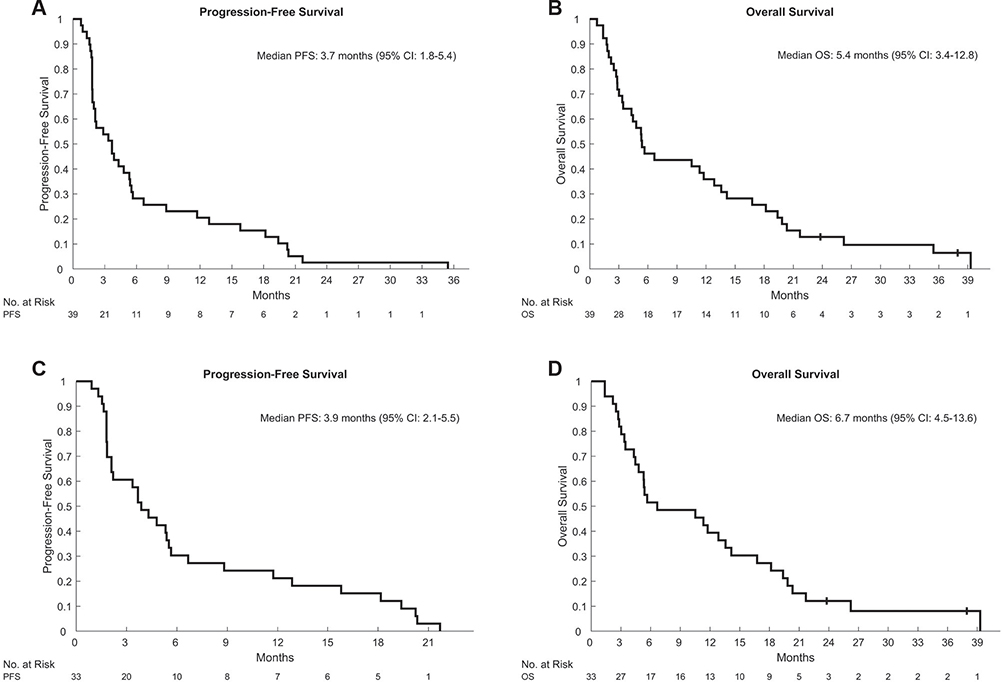
Response analyses were performed on evaluable patients who received regorafenib for at least 3 weeks. Six patients were not evaluable: 1 patient withdrew consent, and 5 had rapid clinical progression within 3 weeks of starting regorafenib. Among the 5 patients with clinical progression, 1 died within 1 week, and 4 died within 8 weeks after discontinuation of regorafenib. Among the 33 evaluable patients, 3 patients (9.1%) achieved a partial response, including 1 unconfirmed partial response, and 18 patients achieved stable disease with a DCR of 63.6%. All 3 responders had intrahepatic cholangiocarcinoma, and 2 responders had distant metastatic disease. All of the responders received 1 line of systemic therapy, and 1 received local radiation to liver lesions before regorafenib. Disease control for ≥12 weeks was achieved in 48% of the patients. Among the 3 responders, 2 required 1 dose reduction, and the other responder required 2 dose reductions. The median PFS and OS were 3.7 months (95% confidence interval [CI], 1.8–5.4 months) and 5.4 months (95% CI, 3.4–12.8 months), respectively, with survival rates of 46.2% at 6 months, 35.9% at 12 months, and 25.6% at 18 months for the intention-to-treat population (Fig. 1). For the evaluable patients, the median PFS was 3.9 months (95% CI, 2.1–5.5 months), and the median OS was 6.7 months (95% CI, 4.5–13.6 months) with survival rates of 51.5% at 6 months, 39.4% at 12 months, and 27.3% at 18 months (Fig. 1). The median PFS and OS of the patients who received 1 line of therapy before regorafenib were 5.3 months (95% CI, 2.2–8.8 months) and 10.5 months (95% CI, 4.8–19.4 months), respectively (Supporting Fig. 1). The median PFS and OS of the patients who received 2 lines were 2.0 months (95% CI, 1.6–5.4 months) and 4.9 months (95% CI, 2.7–14.2 months), respectively (Supporting Fig. 1). We have not observed any difference in PFS (HR, 0.51; 95% CI, 0.24–1.07) or OS (HR, 0.53; 95% CI, 0.25–1.15) between 1 and 2 lines before regorafenib, although the comparison should be interpreted cautiously because of the limited sample size (Supporting Fig. 1). The median PFS and OS of the patients with no dose reductions were 4.2 months (95% CI, 1.8–5.5 months) and 6.2 months (95% CI, 4.8–13.6 months), respectively, and the median PFS and OS of the patients with dose reductions were 3.9 months (95% CI, 1.8–12.8 months) and 10.5 months (95% CI, 2.7–19.4 months), respectively (Supporting Fig. 2). Dose modifications were not associated with clinical outcomes (HR for PFS, 0.65; 95% CI, 0.31–1.38; HR for OS, 1.1; 95% CI, 0.54–2.28; Supporting Fig. 2). However, the study was underpowered to detect significant differences.
FIGURE 1.
Kaplan-Meier estimates of (A,C) PFS and (B,D) OS in (A,B) the ITT population (n = 39) and (C,D) the evaluable patients (n = 33). CI, confidence interval; ITT, intention to treat; OS, overall survival; PFS, progression-free survival.
Toxicity
The most common adverse event was fatigue (59%), which was followed by hypertension (53.8%), aspartate aminotransferase (51.3%)/alanine aminotransferase (43.6%) increases, thrombocytopenia (38.5%), hand-foot skin reactions (35.9%), anemia (30.8%), and nausea (30.8%; Table 2). Twenty-eight of the 39 patients (71.8%) experienced grade 3/4 adverse events. The most common grade 3/4 adverse events were hypertension (30.8%), fatigue (10.3%), aspartate aminotransferase (10.3%)/alanine aminotransferase (10.3%) elevations, and hand-foot skin reactions (10.3%; Table 2). There was no treatment-related death.
TABLE 2.
Treatment-Related Adverse Events (n = 39)
| Toxicity | All Grades, No. (%) | Grade ≥ 3, No. (%) |
|---|---|---|
| Fatigue | 23 (59) | 4 (10.3) |
| Hypertension | 21 (53.8) | 12 (30.8) |
| AST increased | 20 (51.3) | 4 (10.3) |
| ALT increased | 17 (43.6) | 4 (10.3) |
| Thrombocytopenia | 15 (38.5) | 2 (5.1) |
| Hand foot skin reaction | 14 (35.9) | 4 (10.3) |
| Anemia | 12 (30.8) | 2 (5.1) |
| Nausea | 12 (30.8) | 1 (2.6) |
| Vomiting | 9 (23.1) | 0 |
| Diarrhea | 6 (15.4) | 0 |
| Rash, acneiform | 3 (7.7) | 0 |
| Thromboembolic event | 2 (5.1) | 2 (5.1) |
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase.
Angiogenic and Inflammatory Biomarkers
Baseline levels of 23 angiogenic and inflammatory biomarkers were measured in the plasma of 29 patients (Supporting Table 1). We investigated the association between baseline levels of all biomarkers and PFS and OS to identify potential prognostic markers. A total of 3 markers were observed to be prognostic for either PFS or OS after correction for multiple testing. Elevated VEGF-D levels were associated with worse PFS and exhibited an HR of 7.89 (95% CI, 1.99–31.34). For OS, elevated IL-6 (HR, 1.64; 95% CI, 1.18–2.27) and GP130 levels (HR, 10.49; 95% CI, 2.16–50.87) were associated with shorter OS times (Table 3). In all cases, each biomarker was a negative prognostic marker, which indicated that higher levels of each marker led to an increase in the hazard for either outcome. A complete listing of all biomarkers and prognostic associations is shown in Supporting Tables 2 (PFS) and 3 (OS).
TABLE 3.
Prognostic Markers for PFS and OS
| Marker | Outcome | HR | 95% CI | P | Q |
|---|---|---|---|---|---|
| VEGF-D | PFS | 7.89 | 1.99–31.34 | .0015 | 0.0344 |
| IL-6 | OS | 1.64 | 1.18–2.27 | .0019 | 0.0287 |
| GP130 | OS | 10.49 | 2.16–50.87 | .0025 | 0.0287 |
Abbreviations: CI, confidence interval; GP130, glycoprotein 130; HR, hazard ratio; IL-6, interleukin 6; OS, overall survival; PFS, progression-free survival; VEGF-D, vascular endothelial growth factor D.
DISCUSSION
Currently, there is no standard treatment available for advanced BTC after progression on standard first-line gemcitabine plus cisplatin, even though FOLFOX may be an alternative option on the basis of a recent phase 3 study (ABC-06).5
In our study, we explored the efficacy of regorafenib in refractory BTC on the basis of the hypothesis that regorafenib would inhibit multiple pathways, including angiogenic receptors (VEGFR1, VEGFR2, VEGFR3, and TIE2) and stromal factors receptors (PDGFR-β and FGFR1), which play significant roles in the tumorigenesis, progression, and metastasis of BTC.7,9,11,12
In this phase 2 study, regorafenib demonstrated promising anticancer activity in patients with advanced BTC after progression on first- or second-line systemic therapy.
The study met its primary endpoint with a 6-month survival rate of 51.5% among evaluable patients, and it showed durable disease control and encouraging long-term survival. Disease control for 12 weeks or longer occurred in 48% of the patients with a median OS of 6.7 months. The 6- and 12-month OS rates of 46.2% and 35.9% in the intention-to-treat population of our study are comparable to the findings of the ABC-06 study, in which the 6- and 12-month OS rates were 50.6% and 25.9%, respectively.5 However, a direct comparison of these studies should be interpreted cautiously because of the different sample sizes, different primary objectives, and different patient populations.
Furthermore, the results of our study are comparable to those of 2 other studies of regorafenib in advanced, refractory BTC. The first trial with regorafenib included 43 patients, 34 of whom were evaluable for a response.20 The overall response rate was 11% with a DCR of 56%, and the median OS was 31.8 weeks with a median PFS of 15.6 weeks.20 In the other phase 2 study, 66 patients with refractory BTC were randomized to receive regorafenib or a placebo.21 The study met its endpoint of improving PFS. The median PFS doubled from 1.5 to 3 months (HR, 0.49; 95% CI, 0.29–0.81; P = .005), with the regorafenib arm favored.21 The DCR was 70%.
These phase 2 studies of regorafenib, including our study, have demonstrated encouraging clinical outcomes for advanced, refractory BTC, particularly when effective treatment is lacking in the refractory setting.
Another multi–tyrosine kinase inhibitor (cediranib) that blocks VEGFR1, VEGFR2, VEGFR3, PDGF, and c-KIT was evaluated for patients with advanced, treatment-naive BTC in a randomized phase 2 study.22 In that study, patients were treated with cediranib or a placebo in combination with cisplatin plus gemcitabine, and cediranib did not improve PFS in combination with cisplatin plus gemcitabine in comparison with the placebo (median PFS, 8 vs 7.4 months; HR, 0.93; 95% CI, 0.65–1.35; P = .72).22 However, cediranib in combination with chemotherapy demonstrated significantly higher toxicities, which led to discontinuation of the regimen, and this provided an explanation for the disappointing outcomes in the studies.
Although the safety and toxicity profile of regorafenib in our study was similar to that reported in previous phase 3 studies,16,23 19 patients (48.7%) required a dose modification; this rate was higher than that (28%) in Sun et al’s phase 2 study of regorafenib.20 However, in Sun et al’s study, the starting dose was 120 mg, whereas it was 160 mg in our study; this explains more dose modifications in our study. Interestingly, we did not observe any difference in PFS (HR, 0.65; 95% CI, 0.31–1.38) or OS (HR, 1.11; 95% CI, 0.54–2.28; Supporting Fig. 2) between patients with dose modifications and those with no modifications in our study, and the clinical outcomes of our study were similar to those of Sun et al’s phase 2 study of regorafenib. These findings were supported by a retrospective study demonstrating that the initial dose of regorafenib had no significant impact on clinical outcomes in colorectal cancer.24 Recently, the Regorafenib Dose Optimisation Study (ReDOS) has demonstrated that a weekly dose-escalation strategy from 80 to 160 mg is as effective as the standard dose of 160 mg with a lower incidence of adverse events in refractory, metastatic colorectal cancer,25 and this suggests that the dose-escalation strategy be considered to decrease significant adverse events, improve drug compliance, and minimize dose interruptions while maintaining efficacy when clinical studies of regorafenib are designed in the future.
In our study, baseline plasma levels of VEGF-D, IL-6, and GP130 were all identified as negative prognostic biomarkers in patients with BTC treated with regorafenib. High baseline expression of all 3 markers was associated with a worse outcome for either PFS or OS. The negative prognostic value of VEGF-D observed in this study is consistent with other findings in gastrointestinal cancers. VEGF-D has previously been show to act as a negative prognostic marker in both metastatic colorectal cancer and pancreatic cancer.26,27 Interestingly, high baseline expression of VEGF-D has been shown to predict a lack of benefit from bevacizumab in both pancreatic and metastatic colorectal cancers.27,28
IL-6 is another key cytokine that has been shown to have strong negative prognostic value in gallbladder cancer,29 metastatic colorectal cancer,26 and pancreatic cancer.27 IL-6 exerts its function via IL-6R and the binding partner GP130.30 Although we observed that GP130 was associated with OS in this study, GP130 is not as well recognized as IL-6 as a negative prognostic marker in this population. The unusually high HR of GP130 is intriguing and raises speculation that GP130 may have alternative functions in addition to its role as an IL-6 signal transducer. However, because of the limited number of patients and heterogenous groups of BTC in this study, these data should be considered exploratory and hypothesis-generating.
There are several limitations to our study. First, this is a single-arm, nonrandomized study. According to the protocol, we defined evaluable patients as patients who received the study treatment for at least 3 weeks in order to include patients with reasonable exposure to the study treatment to determine the efficacy. We fully acknowledge that this can lead to bias because the clinical outcome of the intention-to-treat population tends to be worse than evaluable patients as defined by the protocol. Second, because of our small sample size, we cannot reach definitive conclusions based on subgroup analysis. Third, BTC represents a heterogeneous group of tumors with variable frequency of alterations in specific molecular pathways based on the site of origin (intrahepatic vs extrahepatic vs gallbladder cancer). In our study, the population included in our study was not selected or enriched on the basis of specific molecular alterations because there are no established predictive biomarkers of regorafenib. Fourth, there is a potential selection bias, and this may not reflect the general population with BTC because this study accrued patients with an Eastern Cooperative Oncology Group performance status of 0 or 1.
Because regorafenib is a pan-FGFR inhibitor and 10% to 16% of patients with intrahepatic cholangiocarcinoma have FGFR2 fusion,31,32 FGFR genetic alteration was evaluated with next-generation sequencing in the patients whose tumor samples were available as a standard of care. Among the 3 patients who achieved a partial response, none had an FGFR genetic aberration according to the next-generation sequencing.
In conclusion, our study suggests that regorafenib can provide modest clinical efficacy and a manageable safety profile in heavily pretreated patients with advanced BTC. Further exploration of biomarkers is warranted to identify a group of patients with BTC who may benefit from regorafenib and to develop potential rational combinations.
Supplementary Material
Acknowledgments
FUNDING SUPPORT
This work was supported by Bayer, which did not have any role in the study design; the collection, analysis, or interpretation of data; the writing of the report; or the decision to submit the report for publication. This work was also supported in part by the Biostatistics Core Facility at the H. Lee Moffitt Cancer Center and Research Institute, a National Cancer Institute–designated comprehensive cancer center (P30-CA076292).
CONFLICT OF INTEREST DISCLOSURES
Richard D. Kim has received honoraria from Lilly, Bristol-Myers Squibb, and Bayer outside the submitted work. Hanna K. Sanoff has received research funding from Bayer and Merck. Andrew B. Nixon reports personal fees from Eli Lilly, Kanghong Pharma, Pfizer, and GlaxoSmithKline and grants from Acceleron Pharma, Amgen, AstraZeneca/MedImmune, Eureka Therapeutics, Genentech, Leadiant Biosciences, MedPacto, Novartis, Seattle Genetics, and Tracon Pharma outside the submitted work; in addition, Nixon has a patent issued for methods of developing a prognosis for pancreatic cancer and predicting responsiveness to cancer therapeutics and a patent pending for methods of predicting the responsiveness of a cancer to a VEGF targeting agent and methods of prognosing and treating cancer. The other authors made no disclosures.
Footnotes
Additional supporting information may be found in the online version of this article.
REFERENCES
- 1.Rizvi S, Gores GJ. Pathogenesis, diagnosis, and management of cholangiocarcinoma. Gastroenterology. 2013;145:1215–1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Everhart JE, Ruhl CE. Burden of digestive diseases in the United States part III: liver, biliary tract, and pancreas. Gastroenterology. 2009; 136:1134–1144. [DOI] [PubMed] [Google Scholar]
- 3.Jarnagin WR, Fong Y, DeMatteo RP, et al. Staging, resectability, and outcome in 225 patients with hilar cholangiocarcinoma. Ann Surg. 2001;234:507–517; discussion 517–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Valle J, Wasan H, Palmer DH, et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med. 2010;362:1273–1281. [DOI] [PubMed] [Google Scholar]
- 5.Lamarca A, Palmer DH, Wasan HS, et al. ABC-06 | A randomised phase III, multi-centre, open-label study of active symptom control (ASC) alone or ASC with oxaliplatin/5-FU chemotherapy (ASC+mFOLFOX) for patients (pts) with locally advanced/metastatic biliary tract cancers (ABC) previously-treated with cisplatin/gemcitabine (CisGem) chemotherapy [abstract 4003]. J Clin Oncol. 2019;37(15 suppl):4003. [Google Scholar]
- 6.Sever R, Brugge JS. Signal transduction in cancer. Cold Spring Harb Perspect Med. 2015;5:a006098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pellino A, Loupakis F, Cadamuro M, et al. Precision medicine in cholangiocarcinoma. Transl Gastroenterol Hepatol. 2018;3:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goel HL, Mercurio AM. VEGF targets the tumour cell. Nat Rev Cancer. 2013;13:871–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yothaisong S, Dokduang H, Techasen A, et al. Increased activation of PI3K/AKT signaling pathway is associated with cholangiocarcinoma metastasis and PI3K/mTOR inhibition presents a possible therapeutic strategy. Tumour Biol. 2013;34:3637–3648. [DOI] [PubMed] [Google Scholar]
- 10.Javle M, Bekaii-Saab T, Jain A, et al. Biliary cancer: utility of next-generation sequencing for clinical management. Cancer. 2016; 122:3838–3847. [DOI] [PubMed] [Google Scholar]
- 11.Simone V, Brunetti O, Lupo L, et al. Targeting angiogenesis in biliary tract cancers: an open option. Int J Mol Sci. 2017;18:E418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yoshikawa D, Ojima H, Iwasaki M, et al. Clinicopathological and prognostic significance of EGFR, VEGF, and HER2 expression in cholangiocarcinoma. Br J Cancer. 2008;98:418–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wilhelm SM, Dumas J, Adnane L, et al. Regorafenib (BAY 73–4506): a new oral multikinase inhibitor of angiogenic, stromal and oncogenic receptor tyrosine kinases with potent preclinical antitumor activity. Int J Cancer. 2011;129:245–255. [DOI] [PubMed] [Google Scholar]
- 14.Li J, Qin S, Xu R, et al. Regorafenib plus best supportive care versus placebo plus best supportive care in Asian patients with previously treated metastatic colorectal cancer (CONCUR): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2015;16:619–629. [DOI] [PubMed] [Google Scholar]
- 15.Bruix J, Qin S, Merle P, et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;389:56–66. [DOI] [PubMed] [Google Scholar]
- 16.Demetri GD, Reichardt P, Kang YK, et al. Efficacy and safety of regorafenib for advanced gastrointestinal stromal tumours after failure of imatinib and sunitinib (GRID): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet. 2013;381:295–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu Y, Starr MD, Bulusu A, et al. Correlation of angiogenic biomarker signatures with clinical outcomes in metastatic colorectal cancer patients receiving capecitabine, oxaliplatin, and bevacizumab. Cancer Med. 2013;2:234–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu Y, Starr MD, Brady JC, et al. Modulation of circulating protein biomarkers in cancer patients receiving bevacizumab and the antiendoglin antibody, TRC105. Mol Cancer Ther. 2018;17:2248–2256. [DOI] [PubMed] [Google Scholar]
- 19.Atkinson EN, Brown BW. Confidence limits for probability of response in multistage phase II clinical trials. Biometrics. 1985;41:741–744. [PubMed] [Google Scholar]
- 20.Sun W, Patel A, Normolle D, et al. A phase 2 trial of regorafenib as a single agent in patients with chemotherapy-refractory, advanced, and metastatic biliary tract adenocarcinoma. Cancer. 2019;125:902–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Demols A, Borbath I, Van Den Eynde M, et al. Regorafenib after failure of gemcitabine and platinum-based chemotherapy for locally advanced (nonresectable) and metastatic biliary tumors: a randomized double-blinded placebo-controlled phase II trial [abstract 345]. J Clin Oncol. 2019;37(4 suppl):345. [Google Scholar]
- 22.Valle JW, Wasan H, Lopes A, et al. Cediranib or placebo in combination with cisplatin and gemcitabine chemotherapy for patients with advanced biliary tract cancer (ABC-03): a randomised phase 2 trial. Lancet Oncol. 2015;16:967–978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grothey A, Van Cutsem E, Sobrero A, et al. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet. 2013;381:303–312. [DOI] [PubMed] [Google Scholar]
- 24.Yoon SE, Lee SJ, Lee J, et al. The use of regorafenib for patients with refractory metastatic colorectal cancer in clinical practice. Onco Targets Ther. 2019;12:225–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bekaii-Saab TS, Ou FS, Ahn DH, et al. Regorafenib dose-optimisation in patients with refractory metastatic colorectal cancer (ReDOS): a randomised, multicentre, open-label, phase 2 study. Lancet Oncol. 2019;20:1070–1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu Y, Starr MD, Brady JC, et al. Biomarker signatures correlate with clinical outcome in refractory metastatic colorectal cancer patients receiving bevacizumab and everolimus. Mol Cancer Ther. 2015;14:1048–1056. [DOI] [PubMed] [Google Scholar]
- 27.Nixon AB, Pang H, Starr MD, et al. Prognostic and predictive blood-based biomarkers in patients with advanced pancreatic cancer: results from CALGB80303 (Alliance). Clin Cancer Res. 2013;19:6957–6966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Weickhardt AJ, Williams D, Lee C, et al. Vascular endothelial growth factor (VEGF) and VEGF receptor expression as predictive biomarkers for benefit with bevacizumab in metastatic colorectal cancer (mCRC): analysis of the phase III MAX study [abstract 3531]. J Clin Oncol. 2011;29(15 suppl):3531. [Google Scholar]
- 29.Wang J, Liu J, Chang Q, Yang B, Li S, Gu C. The association between preoperative serum interleukin-6 levels and postoperative prognosis in patients with T2 gallbladder cancer. J Surg Oncol. 2018;117:1672–1678. [DOI] [PubMed] [Google Scholar]
- 30.Taga T. The interleukin-6 signal transducer, gp130, functioning in immune, hematopoietic, and neural systems [in Japanese]. Nihon Rinsho. 1992;50:1802–1810. [PubMed] [Google Scholar]
- 31.Arai Y, Totoki Y, Hosoda F, et al. Fibroblast growth factor receptor 2 tyrosine kinase fusions define a unique molecular subtype of cholangiocarcinoma. Hepatology. 2014;59:1427–1434. [DOI] [PubMed] [Google Scholar]
- 32.Borad MJ, Champion MD, Egan JB, et al. Integrated genomic characterization reveals novel, therapeutically relevant drug targets in FGFR and EGFR pathways in sporadic intrahepatic cholangiocarcinoma. PLoS Genet. 2014;10:e1004135. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.