Carboxysomes evolved due to rising O2, not falling CO2, enabling planktic cyanobacteria to flourish during earth’s middle age.
Abstract
Ancestral cyanobacteria are assumed to be prominent primary producers after the Great Oxidation Event [≈2.4 to 2.0 billion years (Ga) ago], but carbon isotope fractionation by extant marine cyanobacteria (α-cyanobacteria) is inconsistent with isotopic records of carbon fixation by primary producers in the mid-Proterozoic eon (1.8 to 1.0 Ga ago). To resolve this disagreement, we quantified carbon isotope fractionation by a wild-type planktic β-cyanobacterium (Synechococcus sp. PCC 7002), an engineered Proterozoic analog lacking a CO2-concentrating mechanism, and cyanobacterial mats. At mid-Proterozoic pH and pCO2 values, carbon isotope fractionation by the wild-type β-cyanobacterium is fully consistent with the Proterozoic carbon isotope record, suggesting that cyanobacteria with CO2-concentrating mechanisms were apparently the major primary producers in the pelagic Proterozoic ocean, despite atmospheric CO2 levels up to 100 times modern. The selectively permeable microcompartments central to cyanobacterial CO2-concentrating mechanisms (“carboxysomes”) likely emerged to shield rubisco from O2 during the Great Oxidation Event.
INTRODUCTION
Members of the phylum Cyanobacteria are the only extant bacteria capable of oxygenic photosynthesis, leading to the inference that ancestral cyanobacteria were responsible for the Paleoproterozoic accumulation of atmospheric O2 known as the Great Oxidation Event [GOE; 2.4 to 2.0 billion years (Ga) ago] (1). Although estimates of when oxygenic photosynthesis originated span a billion years—from sometime in the Paleoarchean eon (3.6 to 3.2 Ga ago) to immediately preceding the GOE [Fig. 1 and the Supplementary Materials (SM)] [e.g., (2, 3)]—the oxidative impact of this metabolism across the GOE was profound. Atmospheric O2 concentrations increased by up to 100 million–fold (1, 4) relative to CO2 concentrations (Fig. 1), while primary productivity rose to potentially modern levels (5). Following the GOE, the trajectories of both atmospheric O2 concentrations and primary productivity appear to have stalled, with atmospheric oxygen falling to somewhere between 0.1 and 10% of present atmospheric levels [1 PAL = 210,000 parts per million (ppm) O2; Fig. 1] (1, 6) and oxygenic primary production decreasing to less than 10% of modern values (5). Stabilization of the Earth system at this intermediate state of oxygenic primary production characterized much of the Proterozoic eon (7, 8). There are a variety of hypotheses for why this stasis defined the Proterozoic Earth system [e.g., (9–12)] and the physiology of ancestral cyanobacteria features prominently in all of them.
Fig. 1. Isotopic, atmospheric, and biologic context for the Proterozoic “Age of Cyanobacteria” (25).
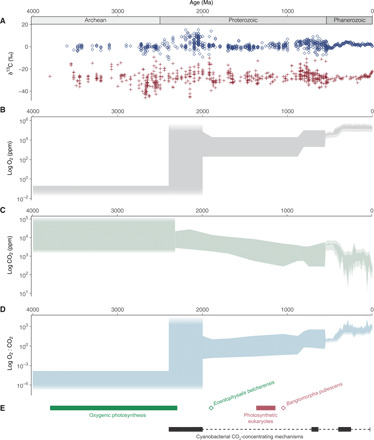
(A) Carbonate δ13C values shown in blue diamonds and total organic carbon δ13C values shown in red crosses (24). Ma, million years. (B) Mass-independent sulfur isotope fractionation restricts Archean pO2 estimates to <10−6 PAL or 2 ppm (4). Proterozoic and Phanerozoic pO2 estimates come from proxies and modeling (8, 59). (C) Archean, Proterozoic, and Phanerozoic CO2 estimates come from proxies and modeling (8, 39, 59). (D) Estimated range of O2-to-CO2 ratios (each expressed in ppm) from the Archean through the Phanerozoic eons. (E) Range of time estimates for the origin of oxygenic photosynthesis (e.g., 2, 3) shown as a green bar and the earliest unambiguous cyanobacterial microfossils (Eoentophysalis belcherensis) shown as a green diamond (13, 14). Age of earliest unambiguous photosynthetic eukaryote (Bangiomorpha pubescens) shown as red diamond with corresponding molecular clock estimates for the primary plastid endosymbiosis shown as a red bar (21). Proposed dates for the emergence of a cyanobacterial CCM shown as black bars [e.g., (34)].
While ancestral cyanobacteria are assumed to play a central role in Proterozoic biogeochemistry, there is limited direct evidence of the ecological niches that they occupied. The oldest unambiguous cyanobacterial microfossils are found in 2.018- to 2.015-Ga peritidal black cherts of the Orosirian Belcher Group (13, 14). When similarly preserved fossil cyanobacteria are found in younger Proterozoic rocks, they are also interpreted as ancient analogs of benthic cyanobacteria in littoral environments (15). If the paleontological record is expanded to include all possible microfossils with cyanobacterial affinities, then benthic forms still dominate, with rare and contentious interpretations of cyanobacteria in planktic habitats (16, 17). The lack of fossil indicators for planktic cyanobacteria may reflect an absence of these cyanobacterial lineages at this time (18) or the improbable preservation of cyanobacterial microfossils in pelagic environments (19). Paired biomarker and nitrogen isotope measurements identify the presence of pelagic cyanobacteria by 1.1 Ga ago (20), but earlier documentation of a pelagic habitat would help evaluate hypotheses for the global influence of cyanobacteria in the Proterozoic Earth system.
If Proterozoic cyanobacteria inhabited a globally important ecological niche, the productivity of the biosphere would be largely dependent on their ability to fix carbon. At the level of the global marine ecosystem, the most continuous evidence of carbon fixation by the dominant primary producers is preserved in sedimentary marine carbon isotope records. The carbon isotopic difference between carbonate minerals and total organic carbon (TOC) (εTOC; eq. S1) in sedimentary rocks has well-resolved coverage between the GOE, the origin of photosynthetic eukaryotes (21), and the ultimate ecological dominance of photosynthetic eukaryotes in the pelagic marine environment (22). Although the isotopic difference summarized by εTOC is imparted initially by the net carbon isotope effect associated with carbon fixation by primary producers (εP; eq. S2), carbon isotope fractionations associated with geologic preservation do not allow for εTOC to be directly substituted for εP (23).
We used bootstrap resampling and Monte Carlo simulations to produce a new record of εP in the middle of the Proterozoic eon (1.8 to 1.0 Ga ago), taking into account isotopic fractionations that occur as the primary substrates and products of carbon fixation (e.g., dissolved CO2 and photoautotrophic biomass) are transformed into their final geological states (e.g., carbonate rocks and TOC). This new εP record was derived from a curated dataset of carbon isotope measurements from sedimentary rocks from a variety of depositional settings, including open and shallow marine environments (24). The middle Proterozoic shows limited variation in the sedimentary carbon isotope record [e.g., (8, 24)] spanning the proposed “Age of Cyanobacteria” (25). As a result, it represents a favorable target for isolation of any cyanobacterial component of the Proterozoic εP record. Our statistical simulation of middle Proterozoic εP values yielded a distribution in which 95% of the values fall between 8 and 24 per mil (‰) (95th percentile) with a median value of 16‰ (Fig. 2A and the SM). This εP distribution provides a benchmark to compare different autotrophic contributions to global Proterozoic primary production.
Fig. 2. Middle Proterozoic εP estimates as compared to empirical cyanobacterial εP values.
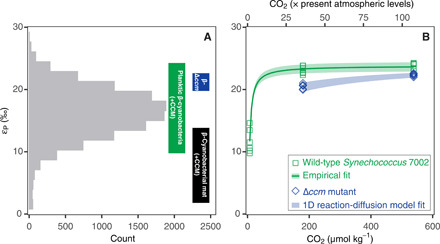
(A) Histogram of estimated εP values between 1.0 and 1.8 Ga. Boxed vertical ranges represents εP values from a cyanobacterial mat system [black (26)] and εP values reported here in cultures of WT (green) and Δccm mutant (blue) Synechococcus sp. PCC 7002 strains. (B) Measured values of εP increase at higher dissolved CO2 levels in cultures of Synechococcus sp. PCC 7002. In the WT strain (green squares), εP values covary with [CO2(aq)]−1 (green line, fig. S5; R2, 0.96). Blue diamonds are experimental results for the Δccm mutant, which requires ≥36 × PAL CO2 to grow under our experimental conditions. The shaded blue band represents calculations from a 1D reaction-diffusion model trained on physiological observations of the Δccm mutant. Horizontal axes refer to CO2 in the culture headspace relative to PAL (1 PAL = 280 ppm CO2; upper axis) and the corresponding dissolved CO2 in the culture medium (micromole per kilogram; lower axis). Data points represent biological replicates (n = 6 for each condition).
Benthic cyanobacteria have, for example, been proposed as ecologically important contributors to Proterozoic primary production (18). In modern cyanobacterial mats, benthic photoautotrophic biomass is commonly enriched in 13C relative to biomass from planktic environments [e.g., (26, 27)]. We used our statistical simulation to quantify the distribution of εP values in a well-characterized modern mat system on the basis of previously published values of δ13Ccarb and δ13Corg (26). In this system, the predicted distribution of εP values has a median value of 8.5‰ and a range of 4 to 13‰ (95th percentile; fig. S3). This exercise suggests that the dynamics of carbon supply in cyanobacterial mats appears to limit the overall εP range that they can preserve, especially in hypersaline environments (26). The εP distribution for this system covers less than 25% of the middle Proterozoic εP record, with the overlap restricted to a small tail in the Proterozoic distribution that extends to εP values less than 10‰ (Fig. 2A). Detailed datasets do not exist that can similarly constrain how εP distributions for cyanobacterial mats might change if CO2 levels approached those proposed for middle Proterozoic (8). Proof-of-concept experiments, however, indicate that mat εP values average ≈11‰ when overlying CO2 levels are <36 × PAL (1 PAL = 280 ppm CO2) and approach ≈25‰ only at CO2 levels of ≈320 to 420 × PAL [table 2 in (27)]. Although benthic cyanobacterial microfossils are common in the Proterozoic eon, εP values associated with cyanobacterial mats appear to be much less than those seen in the middle Proterozoic εP distribution unless CO2 levels were much greater than proposed for the middle Proterozoic (Fig. 2A).
The middle Proterozoic εP distribution also differs from εP values characteristic of planktic cyanobacteria dominant in open ocean ecosystems today (28). Values of εP cluster from ≈15 to 19‰ in physiologically controlled experiments with a planktic member of the monophyletic marine Synechococcus/Prochlorococcus (Syn/Pro) group (28), Synechococcus sp. CCMP838. This tight range spans less than 33% of the middle Proterozoic εP distribution. Experimental Syn/Pro εP values lack sensitivity to CO2 levels [between 6 and 18 μmol kg−1 (28)] or specific growth rate (28), which suggests that variations in these factors cannot be called on to explain the full middle Proterozoic εP distribution. A complete interpretation of the Proterozoic carbon isotope record thus seems to require major contributions by noncyanobacterial primary producers or a shift in our understanding of carbon fixation by Proterozoic cyanobacteria.
It is possible that extant marine cyanobacteria from the Syn/Pro clade may not represent apt physiological analogs for Proterozoic cyanobacteria. All extant cyanobacteria use at least one CO2-concentrating mechanism (CCM) (29) to increase the supply of CO2 to rubisco (ribulose 1,5-bisphosphate carboxylase/oxygenase), the key CO2-fixing enzyme in the Calvin-Benson cycle (30). Cyanobacterial rubisco is partitioned into a selectively permeable protein microcompartment known as a carboxysome along with carbonic anhydrase. Inside the carboxysome, actively accumulated intracellular HCO3− is rapidly interconverted into CO2(aq) through the activity of carbonic anhydrase (29, 31, 32). Examination of cyanobacterial CCMs reveals a clear division within the phylum (29). The marine Syn/Pro clade (α-cyanobacteria) contain α-carboxysomes and Form 1A rubisco that are evolutionarily (29) and structurally distinct (29, 33) from the β-carboxysomes and form 1B rubisco shared by the freshwater, estuarine, and marine species (the β-cyanobacteria) in the remainder of the phylum.
As α-cyanobacteria diverged from cyanobacterial lineages of β-cyanobacteria at the end of the Proterozoic eon, between 1.0 and 0.5 Ga ago (18), β-carboxysomes appear to be the more ancient basis for a cyanobacterial CCM. Estimates for the initial emergence of CCMs in β-cyanobacteria span over 2 Ga of earth history (34) and are often associated with drops in global CO2 associated with glacial episodes at ca. 2.4 to 2.0 Ga, ca. 0.7 to 0.6 Ga, and, potentially, 0.4 to 0.3 Ga ago (Fig. 1). It is possible that either biochemical differences between α- and β-cyanobacteria or the absence of β-carboxysomes in Proterozoic cyanobacteria could account for the mismatch between εP values from α-cyanobacteria and the middle Proterozoic εP distribution. Potential biochemical differences between α- and β-cyanobacteria include the influx and efflux of rubisco substrates and products from the carboxysome (33) as well the kinetics of rubisco and carbonic anhydrase within the carboxysome (35). These differences would likely alter how whole-cell carbon fixation rates respond to changing environmental conditions (e.g., CO2 concentrations), potentially expanding or contracting the accessible range of cyanobacterial εP values. The possible absence of a β-carboxysome in Proterozoic cyanobacteria would allow freer access of substrates to and from rubisco and carbonic anhydrase, potentially affecting cyanobacterial εP values over a wide range of CO2 concentrations as well.
We propose that primary production by cyanobacteria in the middle Proterozoic might resemble either carbon fixation by extant cyanobacteria with β-carboxysome–based CCMs or a physiologically distinct mode of carbon fixation by ancestral β-cyanobacteria lacking a CCM. To evaluate these possibilities, we determined εP values for a model cyanobacterium containing β-carboxysomes, wild-type (WT) Synechococcus sp. PCC 7002 (Synechococcus 7002), and an engineered mutant of this strain lacking carboxysomes (Δccm) (31, 36, 37) across a range of CO2 concentrations. Net carbon isotope fractionation by WT Synechococcus 7002 allows us to compare εP relationships in β-cyanobacteria to previously published εP values from α-cyanobacteria (fig. S6) (28). The Δccm mutant, which is high CO2 requiring, represents a potential physiological analog for pre-CCM–bearing Proterozoic cyanobacteria.
RESULTS
WT Synechococcus 7002 grew at dissolved CO2 concentrations of 7 to 538 μmol l−1, corresponding to headspace CO2 of 1 to 107 × PAL at pH 6.7 to 8.1. The Δccm mutant failed to grow at CO2 levels of 1, 18, and 30 × PAL but was able to grow at 36 and 107 × PAL at pH 7.3 to 8.1 (fig. S4). These experimental conditions are consistent with both pCO2 [1 to 100 PAL (8)] and pH [6.8 to 8.2 (38, 39)] estimates relevant to the middle Proterozoic marine biosphere (fig. S10). The εP values from acclimated WT batch cultures range from 11.7 ± 2.0‰ to 23.8 ± 0.5‰ over 1 to 107 × PAL, while for Δccm batch cultures, εP values range from 20.5 ± 0.4 to 22.3 ± 0.2 over 36 to 107 × PAL (Fig. 2B). In both the WT and Δccm experiments, values of εP increase with higher concentrations of CO2(aq), in contrast to the insensitivity of εP to CO2(aq) in cyanobacteria with α-carboxysomes (fig. S6A) (28). The positive response of εP to increasing CO2 concentrations indicates that transport limitation is a controlling factor in β-cyanobacterial carbon isotope fractionation, as has been well established for photosynthetic eukaryotes (fig. S6B) (28, 40).
In WT Synechococcus 7002, εP values show a negative covariation with the inverse of dissolved CO2 concentrations (R2 = 0.96; figs. S5 and S6), further confirming similarities between cyanobacterial and algal net carbon isotope fractionation. Although the Δccm mutant did not grow over the full range of experimental CO2 concentrations, it exhibits a 2.5-fold larger decrease in εP values over the same drop in CO2 concentrations when compared to WT (≈1.8‰ versus ≈0.7‰ from 107 to 36 × PAL; Fig. 2B and fig. S5). These different CO2 responses suggest that different mechanisms control CO2 transport to rubisco in the Δccm mutant and WT strains.
To explore the isotopic response of the Δccm mutant to varying CO2 concentrations, we used a one-dimensional (1D) reaction-diffusion model, in which rubisco is uniformly distributed throughout the cytosol (31). This model quantifies the isotopic consequences of the competition between a purely diffusional supply of CO2(aq) to the site of carbon fixation and CO2 fixation into biomass, using three interdependent parameters: (i) the proportion of cellular surface area available for diffusion, (ii) the diffusion coefficient for CO2(aq) into the cell, and (iii) the distance over which CO2(aq) diffuses into the cell until it meets a free rubisco and is fixed (see the SM for detailed model description).
Modeled εP values for the Δccm mutant increase with respect to CO2 concentrations (from ~20‰ at 36 × PAL to ~22‰ at 107 × PAL) with a slightly nonlinear functional dependence (Fig. 2B). Training the model on measured physiological parameters for the Δccm mutant illustrates the inefficiency of carbon fixation by rubisco relative to a purely diffusional supply of CO2(aq). To reproduce our εP-CO2 observations, ≈70 to 90% of the carbon brought into a cyanobacterium without a carboxysome must be lost through back diffusion. This “leakiness” is calculated as the difference between the gross diffusive flux of CO2 into the cell and the net rate of CO2 fixation into biomass. The inability of the Δccm mutant to grow at CO2 levels below 36 PAL during our experiments (Fig. 2B) was likely due to a combination of factors limiting the intracellular accumulation of CO2, including the leakiness of the cell and the lack of an encapsulated carbonic anhydrase to convert accumulated HCO3− into CO2 at the site of carbon fixation.
DISCUSSION
The distribution of εP values extracted from the middle Proterozoic sedimentary record span a range of 8 to 24‰ (95th percentile; Fig. 2A). If cyanobacteria accounted for the majority of primary production at this time, as is commonly asserted, then they should be able to produce a similar range of εP values. Our simulations of a previously characterized mat system (26) suggest that net carbon isotope fractionations by cyanobacteria in benthic settings may only account for the lower 25% of the middle Proterozoic εP distribution (Fig. 2A). Here, we show that net carbon isotope fractionation by β-cyanobacteria without carboxysomes only covers 13% of the middle Proterozoic εP distribution (Fig. 2A). In contrast, the εP range that we determined for planktic cyanobacteria with β-carboxysomes covers >90% of the middle Proterozoic distribution, suggesting that this physiology, in the appropriate ecological niches, could be responsible for a large proportion of Proterozoic primary production (Fig. 2A).
To understand whether evolutionary differences between extant and ancestral rubiscos might allow for β-cyanobacteria without carboxysomes to produce the full middle Proterozoic εP range, we used the Δccm model to calculate the εP relationships that might characterize β-cyanobacteria lacking carboxysomes with ancestral rubisco under middle Proterozoic CO2 levels. We incorporated middle Proterozoic estimates of O2 concentrations [0.1 to 10% PAL, compiled in (8)] in these model simulations as well. Although the timing of evolutionary changes within the rubisco phylogeny remains unconstrained (41), maximum carboxylation rates for ancestral variants of form 1B rubisco are ~50 to 70% of their modern equivalents, while the corresponding Menten constants for CO2(aq) are ~40 to 80% of their modern equivalents (42). Over a wide range of dissolved CO2 and O2 concentrations relevant to the Proterozoic ocean, our calculations suggest that a cyanobacterium without carboxysomes will exhibit a limited range of whole-cell εP values (<~10‰; Fig. 3 and the SM). While lower O2 concentrations slightly contract the range of εP values (by ~3%) relative to those accessible at higher O2 concentrations, the primary control seems to be the mismatch between a fast rate of CO2 supply by diffusion and a slower rate of CO2 fixation, which restricts the accessible range of net carbon isotope fractionation across all CO2 levels in the modeled environment (Fig. 3). In this model, the absolute value of each εP range is set by the intrinsic carbon isotope fractionation factor assumed for rubisco (εfix; Fig. 3 and the SM). We note that resurrected forms of ancient rubisco have not yet been isotopically characterized. However, it appears that the lack of carboxysomes, rather than how reconstructed rubiscos ultimately fractionate carbon isotopes, restricts any one example of this physiological state from producing the full middle Proterozoic εP distribution.
Fig. 3. Modeled relationships between εP and CO2 concentration for β-cyanobacteria without a CCM incorporating estimated middle Proterozoic O2 levels [0.1 to 10% PAL (8)].
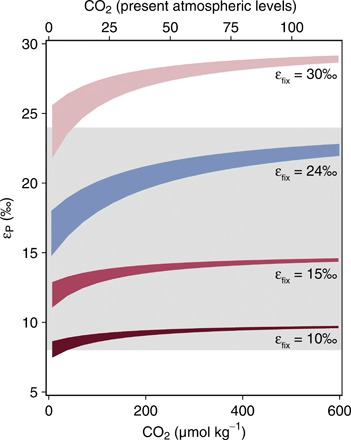
The gray band represents the estimated middle Proterozoic distribution of εP values (95th percentile; 8 to 24‰). The blue field represents calculations extending the observed fractionation by the Δccm mutant across possible Proterozoic CO2 and O2 levels. The red fields represent calculations incorporating the measured kinetics of ancestral form 1B rubisco (table S3) (42) and the full range of known intrinsic isotope effects for rubisco (εfix = 10, 15, and 30‰; the SM).
The middle Proterozoic εP distribution ultimately reflects the interaction between the mode of carbon fixation and CO2 supply for middle Proterozoic autotrophs. Estimates of middle Proterozoic atmospheric pCO2 values range from 1 to 100 PAL (8), but the temporal and spatial resolution of these estimates is extremely coarse. The Our middle Proterozoic εP distribution encompasses a variety of marine environments and atmospheric conditions over the course of 800 million years, and therefore, pCO2 and dissolved CO2 could have exhibited wide variation in time and space over this interval. Estimates of pCO2 over the past ≈70 million years, for example, span a relative range of ≈150-fold (60 to 8900 ppm by volume; https://www.paleo-co2.org), while dissolved CO2 in the modern ocean varies over a relative range of ≈370-fold [8 to 2900 μmol kg−1 (43)]. If atmospheric or marine CO2 in the middle Proterozoic varied similarly then planktic cyanobacteria with β-carboxysomes could produce the full range of middle Proterozoic εP values because of the strong dependence of their net carbon isotope fractionation on CO2 concentrations (Fig. 2).
Although this inference does not rule out alternate forms of carbon fixation, the ranges of εP values produced by other plausible middle Proterozoic primary producers appear to be more restricted even when large variations in middle Proterozoic CO2 concentrations are considered. In the case of β-cyanobacteria lacking CCMs, this is due to the slow rate of CO2 fixation relative to the fast supply of CO2 by diffusion, which restricts the εP response across different CO2 concentrations (Fig. 3). Anoxygenic phototrophs lack carboxysomes (44), suggesting that their isotopic fractionation may show a similar lack of sensitivity to CO2 concentrations as the Δccm mutant investigated here. In cyanobacterial mats, limited CO2 supply appears to restrict εP to low values except, perhaps, when CO2 levels are >300 PAL (27). Hypotheses that call on different carbon fixation modes to explain the middle Proterozoic εP distribution would therefore require the fortuitous preservation of the products of carbon fixation by a diversity of different primary producers.
We recognize that we cannot exclusively rule out these diversity hypotheses, but the genetic, biochemical, environmental, and physiological evidence discussed here points toward a prominent role for ancestral cyanobacteria with β-carboxysome–based CCMs in the middle Proterozoic biosphere. A Paleoproterozoic (or earlier) origin for the CCM in cyanobacteria is consistent with taphonomic inferences of late Mesoproterozoic biomineralization by CCM-bearing cyanobacteria (45). Cyanobacterial CCMs increase the access of rubisco to CO2 to mitigate the enzyme’s dual-substrate specificity for both CO2 and O2 [e.g., (46)]. Under the O2-to-CO2 ratios found in modern environments, competition between carboxylation and oxygenation reactions is metabolically expensive and imposes a wasteful loss of fixed carbon (47). Although Proterozoic pCO2 estimates are higher than modern, spanning ~1 to 100 PAL [compiled in (8)], the jump in atmospheric O2 across the GOE (1, 48) increased the ratio of O2 to CO2 up to 100 million–fold (Fig. 1). These enhanced ratios were sustained throughout the Proterozoic at values at least four orders of magnitude greater than at the end of the Archean.
The transition to higher O2-to-CO2 ratios in the Proterozoic marine environment would have increased O2-to-CO2 ratios within Proterozoic cyanobacteria (49). The carboxysome may therefore have been an evolutionary innovation in response to extreme environmental oxygenation across the GOE. Despite being the principal component of the CCM in all cyanobacteria today, the carboxysome’s original function may have been to shield rubisco from O2 (50), after which it was repurposed as a CCM. This proposed function is consistent with predictions of limited CO2 and O2 permeation through the central pores of carboxysomal shell proteins (33). Early encapsulation inside of a dysoxic carboxysome could further explain why the specificity for CO2 versus O2 is lower in cyanobacterial form 1B rubisco than in form 1B rubisco from Archaeplastida (51), despite a common lineage [e.g., (19)] and over a billion years of shared environmental history (21).
Whether or not the carboxysome originated as an O2-exclusion mechanism, its carbon isotope consequences appear to reach back at least 1.8 Ga (Fig. 2). Paleontological interpretations of ancestral cyanobacteria have long been rationalized in terms of morphological and local ecological stasis on geological time scales [e.g., (52)]. The observations reported here extend this working hypothesis of stasis to levels of biological organization—from the global marine ecosystem down to the organellar and, perhaps, biochemical realms—that have not been previously accessible to paleontological insight (17, 19). When viewed in terms of the comprehensive nature of the Proterozoic carbon isotope record, this suggests that, like in the modern ocean, pelagic cyanobacteria were an important component of Proterozoic marine primary productivity. If Proterozoic cyanobacteria were not strictly benthic forms restricted to littoral environments, then a range of hypotheses for limited primary productivity can be ruled out, from environmental hypotheses that rely on an inaccessible pelagic photic zone (53, 54) to evolutionary hypotheses that posit a planktic lifestyle as a derived trait (18, 55). The possibility that Proterozoic cyanobacteria so closely resembled an extant model cyanobacterium opens the door to direct testing of other hypotheses for limiting primary productivity [e.g., (9–12)] through new experiments in comparative physiology and competition under proposed Proterozoic environmental regimes. Cyanobacterial stasis in terms of ecology, morphology, cytology, and biochemistry may have been the foundation behind low Proterozoic productivity (7). The progressive increase of productivity through time could represent a stepwise scaling (56) away from this continuously maintained cyanobacterial state through the introduction of new avenues of primary production in the oceans (19) and, eventually, on land.
MATERIALS AND METHODS
Middle Proterozoic εP values
Our statistical simulations were based on bootstrap resampling of a curated dataset of δ13C values of carbonate minerals and TOC in 1.0- to 1.8-Ga-old sedimentary rocks (24). We sampled uniform distributions representing possible C isotope fractionation during the conversion and preservation of dissolved CO2 as carbonate minerals and primary biomass as TOC. The distribution of equilibrium isotope effects between CO2 (aq) and ranged from 8.9 to 11.7‰ (57) assuming photic zone temperatures of 3° to 30°C [e.g., (38)]. Experimentally determined kinetic isotope effects associated with the precipitation of calcite and aragonite relative to ranged from 0.8 to 3.3‰ (58). Carbon isotope fractionations associated with secondary biological processes such as heterotrophic consumption of primary organic matter (εreworking) ranged from 0 to 1.5‰ (23). Full simulations are detailed in the SM.
Culturing and isotope assays
Synechococcus sp. strain PCC 7002 (Synechococcus 7002) and a previously engineered ∆ccm mutant strain lacking a carboxysome were grown in A+ media, at 37°C under saturating light levels of ~227 ± 5 μmol photons m−2 s−1 provided by cool-white fluorescence lamps. Cultures were grown in 125-ml conical flasks with foam stoppers (Jaece Industries Identi-plug), continuously shaking, in an incubator that kept headspace CO2 constant by continuous replacement with a mixture of CO2 and air during each experiment. Headspace CO2 varied across three experimental conditions: 0.04% (v/v) CO2 (air), 1% (v/v) CO2, and 3% (v/v) CO2, corresponding to CO2(aq) concentrations of 7, 180, and 538 μmol kg−1, respectively. At each CO2 condition, strains were acclimated through the serial inoculation of four consecutive cultures. Each culture grew to an optical density at 730 nm of ~0.2 before inoculating the next culture with 1 to 3% of the final cell density and harvesting biomass. Harvested biomass was kept at −70°C, then centrifuged, and washed twice with ultrapurified water before isotopic analysis. Carbon isotope compositions of biomass were determined by first combusting samples in a Thermo Fisher Scientific FlashEA under a flow of He gas. The resultant CO2 was analyzed with a Thermo Fisher Scientific Delta V Isotope Ratio Mass Spectrometer in continuous-flow mode. Carbon isotope compositions are expressed as the relative per mil difference between the ratio of 13C-12C in the sample (13C/12Csample) and a standard of Vienna Pee Dee Belemnite (13C/12CVPBD). Headspace CO2 gas was purified and analyzed with a Thermo Fisher Scientific 253+ Isotope Ratio Mass Spectrometer in dual-inlet mode.
One-dimensional reaction-diffusion model
A full model description is in the SM. We used a 1D model of steady-state diffusion of CO2 between an infinite extracellular source and an intracellular sink to represent rubisco-catalyzed entry of CO2 into the Calvin-Benson cycle. A fixed distance separates the CO2 source and enzymatic sink. Both the diffusive transport and the sink reaction are isotopically selective. Independent model inputs include the carbon fixation rates observed for the Δccm mutant grown under 1 and 3% CO2 headspace, the calculated concentration of dissolved CO2, and fractionation factors for form 1B rubisco (εfix) and diffusion of CO2 in solution (εdiff). The model has three free parameters: (i) the intracellular distance over which CO2(aq) diffuses, (ii) the intracellular diffusion coefficient for CO2(aq), and (iii) the proportion of cellular surface area available for diffusion. We “trained” the model by selecting interdependent sets of these three parameters that could reproduce experimental εP values at the observed carbon fixation rates in the Δccm mutant. In the trained model, we additionally used previously characterized kinetics of extant rubiscos and reconstructed ancestral rubiscos to determine possible εP values in β-cyanobacteria without a CCM over a range of environmental conditions.
Supplementary Material
Acknowledgments
We thank E. Ellison, P. Crockford, and E. Johnson for valuable discussions, J. Jackson and R. Dunbar for editorial handling, and five anonymous reviewers for thoughtful comments. We acknowledge B. Davidheiser-Kroll of the CUBES-SIL for assistance with stable isotope analyses and data reduction. Funding: This work was supported by an Agouron Institute postdoctoral fellowship to S.J.H., start-up funding from CU Boulder to B.A.W. and J.C.C., NSF-EF1724393 to B.A.W., NIH T32-GM008759 to N.C.H., and by the U.S. Department of Energy (DOE) DE-SC0019306 to J.C.C. Author contributions: S.J.H., B.A.W., and J.C.C. designed the study. S.J.H., C.E.J., and N.C.H. performed the research. S.J.H. and B.A.W. wrote the manuscript with contributions from all authors. Competing interests: The authors declare that they have no competing interests. Data and materials availability: All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials.
SUPPLEMENTARY MATERIALS
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/7/2/eabc8998/DC1
REFERENCES AND NOTES
- 1.Canfield D. E., The early history of atmospheric oxygen: Homage to Robert M. Garrels. Annu. Rev. Earth Planet. Sci. 33, 1–36 (2005). [Google Scholar]
- 2.Fischer W. W., Hemp J., Johnson J. E., Evolution of oxygenic photosynthesis. Annu. Rev. Earth Planet. Sci. 44, 647–683 (2016). [Google Scholar]
- 3.Sánchez-Baracaldo P., Cardona T., On the origin of oxygenic photosynthesis and Cyanobacteria. New Phytol. 225, 1440–1446 (2020). [DOI] [PubMed] [Google Scholar]
- 4.Catling D. C., Zahnle K. J., The Archean atmosphere. Sci. Adv. 6, eaax1420 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hodgskiss M. S. W., Crockford P. W., Peng Y., Wing B. A., Horner T. J., A productivity collapse to end Earth’s Great Oxidation. Proc. Natl. Acad. Sci. U.S.A. 116, 17207–17212 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Planavsky N. J., Reinhard C. T., Wang X., Thomson D., McGoldrick P., Rainbird R. H., Johnson T., Fischer W. W., Lyons T. W., Low mid-Proterozoic atmospheric oxygen levels and the delayed rise of animals. Science 346, 635–638 (2014). [DOI] [PubMed] [Google Scholar]
- 7.Crockford P. W., Hayles J. A., Bao H., Planavsky N. J., Bekker A., Fralick P. W., Halverson G. P., Bui T. H., Peng Y., Wing B. A., Triple oxygen isotope evidence for limited mid-Proterozoic primary productivity. Nature 559, 613–616 (2018). [DOI] [PubMed] [Google Scholar]
- 8.Crockford P. W., Kunzmann M., Bekker A., Hayles J., Bao H., Halverson G. P., Peng Y., Bui T. H., Cox G. M., Gibson T. M., Wörndle S., Rainbird R., Lepland A., Swanson-Hysell N. L., Master S., Sreenivas B., Kuznetsov A., Krupenik V., Wing B. A., Claypool continued: Extending the isotopic record of sedimentary sulfate. Chem. Geol. 513, 200–225 (2019). [Google Scholar]
- 9.Kipp M. A., Stüeken E. E., Biomass recycling and Earth’s early phosphorus cycle. Sci. Adv. 3, eaao4795 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Olson S. L., Reinhard C. T., Lyons T. W., Cyanobacterial diazotrophy and Earth’s delayed oxygenation. Front. Microbiol. 7, 1526 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Scott C., Lyons T. W., Bekker A., Shen Y., Poulton S. W., Chu X., Anbar A. D., Tracing the stepwise oxygenation of the Proterozoic ocean. Nature 452, 456–459 (2008). [DOI] [PubMed] [Google Scholar]
- 12.Ozaki K., Thompson K. J., Simister R. L., Crowe S. A., Reinhard C. T., Anoxygenic photosynthesis and the delayed oxygenation of Earth’s atmosphere. Nat. Commun. 10, 3026 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hofmann H. J., Precambrian Microflora, Belcher Islands, Canada: Significance and systematics. J. Paleo. 50, 1040–1073 (1976). [Google Scholar]
- 14.Hodgskiss M. S. W., Dagnaud O. M. J., Frost J. L., Halverson G. P., Schmitz M. D., Swanson-Hysell N. L., Sperling E. A., New insights on the Orosirian carbon cycle, early Cyanobacteria, and the assembly of Laurentia from the Paleoproterozoic Belcher Group. Earth Planet. Sci. Lett. 520, 141–152 (2019). [Google Scholar]
- 15.Kah L. C., Knoll A. H., Microbenthic distribution of Proterozoic tidal flats: Environmental and taphonomic considerations. Geology 24, 79–82 (1996). [DOI] [PubMed] [Google Scholar]
- 16.Sergeev V. N., Sharma M., Shukla Y., Proterozoic fossil cyanobacteria. Palaeobotanist 61, 189–358 (2012). [Google Scholar]
- 17.Demoulin C. F., Lara Y. J., Cornet L., François C., Baurain D., Wilmotte A., Javaux E. J., Cyanobacteria evolution: Insight from the fossil record. Free Radic. Biol. Med. 140, 206–223 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sánchez-Baracaldo P., Origin of marine planktonic cyanobacteria. Sci. Rep. 5, 17418 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.A. H. Knoll, R. E. Summons, J. R. Waldbauer, J. E. Zumberge, The geological succession of primary producers in the oceans, in Evolution of Primary Producers in the Sea (Elsevier, 2007), pp. 133–163. [Google Scholar]
- 20.Gueneli N., McKenna A. M., Ohkouchi N., Boreham C. J., Beghin J., Javaux E. J., Brocks J. J., 1.1-billion-year-old porphyrins establish a marine ecosystem dominated by bacterial primary producers. Proc. Natl. Acad. Sci. U.S.A. 115, E6978–E6986 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gibson T. M., Shih P. M., Cumming V. M., Fischer W. W., Crockford P. W., Hodgskiss M. S. W., Wörndle S., Creaser R. A., Rainbird R. H., Skulski T. M., Halverson G. P., Precise age of Bangiomorpha pubescens dates the origin of eukaryotic photosynthesis. Geology 46, 135–138 (2017). [Google Scholar]
- 22.Brocks J. J., Jarrett A. J. M., Sirantoine E., Hallmann C., Hoshino Y., Liyanage T., The rise of algae in Cryogenian oceans and the emergence of animals. Nature 548, 578–581 (2017). [DOI] [PubMed] [Google Scholar]
- 23.Hayes J. M., Strauss H., Kaufman A. J., The abundance of 13C in marine organic matter and isotopic fractionation in the global biogeochemical cycle of carbon during the past 800 Ma. Chem. Geol. 161, 103–125 (1999). [Google Scholar]
- 24.Krissansen-Totton J., Buick R., Catling D. C., A statistical analysis of the carbon isotope record from the Archean to Phanerozoic and implications for the rise of oxygen. Am. J. Sci. 315, 275–316 (2015). [Google Scholar]
- 25.J. Schopf, M. Walter, in The Biology of Cyanobacteria, N. G. Carr, B. A. Whitton, Eds. (Blackwell, 1982), pp. 543–564. [Google Scholar]
- 26.Schidlowski M., Gorzawski H., Dor I., Carbon isotope variations in a solar pond microbial mat: Role of environmental gradients as steering variables. Geochim. Cosmochim. Acta 58, 2289–2298 (1994). [Google Scholar]
- 27.D. J. Des Marais, D. E. Canfield, The carbon isotope biogeochemistry of microbial mats, in Microbial Mats: Structure, Development and Environmental Significance, L. J. Stal, P. Caumette, Eds. (Springer Berlin Heidelberg, 1994), pp. 289–298. [Google Scholar]
- 28.Popp B. N., Laws E. A., Bidigare R. R., Dore J. E., Hanson K. L., Wakeham S. G., Effect of phytoplankton cell geometry on carbon isotopic fractionation. Geochim. Cosmochim. Acta 62, 69–77 (1998). [Google Scholar]
- 29.Rae B. D., Long B. M., Badger M. R., Price G. D., Functions, compositions, and evolution of the two types of carboxysomes: Polyhedral microcompartments that facilitate CO2 fixation in cyanobacteria and some proteobacteria. Microbiol. Mol. Biol. Rev. 77, 357–379 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sharkey T. D., Discovery of the canonical Calvin–Benson cycle. Photosynth. Res. 140, 235–252 (2019). [DOI] [PubMed] [Google Scholar]
- 31.Cameron J. C., Wilson S. C., Bernstein S. L., Kerfeld C. A., Biogenesis of a bacterial organelle: The carboxysome assembly pathway. Cell 155, 1131–1140 (2013). [DOI] [PubMed] [Google Scholar]
- 32.Hill N. C., Tay J. W., Altus S., Bortz D. M., Cameron J. C., Life cycle of a cyanobacterial carboxysome. Sci. Adv. 6, eaba1269 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mahinthichaichan P., Morris D. M., Wang Y., Jensen G. J., Tajkhorshid E., Selective permeability of carboxysome shell pores to anionic molecules. J. Phys. Chem. B 122, 9110–9118 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Giordano M., Beardall J., Raven J. A., CO2 concentrating mechanisms in algae: Mechanisms, environmental modulation, and evolution. Annu. Rev. Plant Biol. 56, 99–131 (2005). [DOI] [PubMed] [Google Scholar]
- 35.Whitehead L., Long B. M., Price G. D., Badger M. R., Comparing the in vivo function of α-carboxysomes and β-carboxysomes in two model cyanobacteria. Plant Physiol. 165, 398–411 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gordon G. C., Korosh T. C., Cameron J. C., Markley A. L., Begemann M. B., Pfleger B. F., CRISPR interference as a titratable, trans-acting regulatory tool for metabolic engineering in the cyanobacterium Synechococcus sp. strain PCC 7002. Metab. Eng. 38, 170–179 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Clark R. L., Gordon G. C., Bennett N. R., Lyu H., Root T. W., Pfleger B. F., High-CO2 requirement as a mechanism for the containment of genetically modified cyanobacteria. ACS Synth. Biol. 7, 384–391 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Krissansen-Totton J., Arney G. N., Catling D. C., Constraining the climate and ocean pH of the early Earth with a geological carbon cycle model. Proc. Natl. Acad. Sci. 115, 4105–4110 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Halevy I., Bachan A., The geologic history of seawater pH. Science 355, 1069–1071 (2017). [DOI] [PubMed] [Google Scholar]
- 40.Laws E. A., Popp B. N., Bidigare R. R., Kennicutt M. C., Macko S. A., Dependence of phytoplankton carbon isotopic composition on growth rate and [CO2]aq: Theoretical considerations and experimental results. Geochim. Cosmochim. Acta 59, 1131–1138 (1995). [Google Scholar]
- 41.Kacar B., Hanson-Smith V., Adam Z. R., Boekelheide N., Constraining the timing of the Great Oxidation Event within the Rubisco phylogenetic tree. Geobiology 15, 628–640 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shih P. M., Occhialini A., Cameron J. C., Andralojc P. J., Parry M. A. J., Kerfeld C. A., Biochemical characterization of predicted Precambrian RuBisCO. Nat. Commun. 7, 10382 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lebrato M., Garbe-Schönberg D., Müller M. N., Blanco-Ameijeiras S., Feely R. A., Lorenzoni L., Molinero J.-C., Bremer K., Jones D. O. B., Iglesias-Rodriguez D., Greeley D., Lamare M. D., Paulmier A., Graco M., Cartes J., Barcelos e Ramos J., de Lara A., Sanchez-Leal R., Jimenez P., Paparazzo F. E., Hartman S. E., Westernströer U., Küter M., Benavides R., da Silva A. F., Bell S., Payne C., Olafsdottir S., Robinson K., Jantunen L. M., Korablev A., Webster R. J., Jones E. M., Gilg O., du Bois P. B., Beldowski J., Ashjian C., Yahia N. D., Twining B., Chen X.-G., Tseng L.-C., Hwang J.-S., Dahms H.-U., Oschlies A., Global variability in seawater Mg:Ca and Sr:Ca ratios in the modern ocean. Proc. Natl. Acad. Sci. U.S.A. 117, 22281–22292 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tang K.-H., Tang Y., Blankenship R. E., Carbon metabolic pathways in phototrophic bacteria and their broader evolutionary implications. Front. Microbiol. 2, 165 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kah L. C., Riding R., Mesoproterozoic carbon dioxide levels inferred from calcified cyanobacteria. Geology 35, 799–802 (2007). [Google Scholar]
- 46.Price G. D., Badger M. R., Woodger F. J., Long B. M., Advances in understanding the cyanobacterial CO2-concentrating-mechanism (CCM): Functional components, Ci transporters, diversity, genetic regulation and prospects for engineering into plants. J. Exp. Bot. 59, 1441–1461 (2008). [DOI] [PubMed] [Google Scholar]
- 47.Bauwe H., Hagemann M., Fernie A. R., Photorespiration: Players, partners and origin. Trends Plant Sci. 15, 330–336 (2010). [DOI] [PubMed] [Google Scholar]
- 48.Bachan A., Kump L. R., The rise of oxygen and siderite oxidation during the Lomagundi Event. Proc. Natl. Acad. Sci. U.S.A. 112, 6562–6567 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kihara S., Hartzler D. A., Savikhin S., Oxygen concentration inside a functioning photosynthetic cell. Biophys. J. 106, 1882–1889 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cannon G. C., Bradburne C. E., Aldrich H. C., Baker S. H., Heinhorst S., Shively J. M., Microcompartments in prokaryotes: Carboxysomes and related polyhedra. Appl. Environ. Microbiol. 67, 5351–5361 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Flamholz A. I., Prywes N., Moran U., Davidi D., Bar-On Y. M., Oltrogge L. M., Alves R., Savage D., Milo R., Revisiting trade-offs between Rubisco kinetic parameters. Biochemistry 58, 3365–3376 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schopf J. W., Disparate rates, differing fates: Tempo and mode of evolution changed from the Precambrian to the Phanerozoic. Proc. Natl. Acad. Sci. U.S.A. 91, 6735–6742 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Swanner E. D., Mloszewska A. M., Cirpka O. A., Schoenberg R., Konhauser K. O., Kappler A., Modulation of oxygen production in Archaean oceans by episodes of Fe(II) toxicity. Nat. Geosci. 8, 126–130 (2015). [Google Scholar]
- 54.Mloszewska A. M., Cole D. B., Planavsky N. J., Kappler A., Whitford D. S., Owttrim G. W., Konhauser K. O., UV radiation limited the expansion of cyanobacteria in early marine photic environments. Nat. Commun. 9, 3088 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dick G. J., Grim S. L., Klatt J. M., Controls on O2 production in cyanobacterial mats and implications for Earth’s oxygenation. Annu. Rev. Earth Planet. Sci. 46, 123–147 (2018). [Google Scholar]
- 56.Knoll A. H., Bambach R. K., Directionality in the history of life: Diffusion from the left wall or repeated scaling of the right? Paleobiology 26, 1–14 (2000). [Google Scholar]
- 57.Zhang J., Quay P. D., Wilbur D. O., Carbon isotope fractionation during gas-water exchange and dissolution of CO2. Geochim. Cosmochim. Acta 59, 107–114 (1995). [Google Scholar]
- 58.Romanek C. S., Grossman E. L., Morse J. W., Carbon isotopic fractionation in synthetic aragonite and calcite: Effects of temperature and precipitation rate. Geochim. Cosmochim. Acta 56, 419–430 (1992). [Google Scholar]
- 59.Lenton T. M., Daines S. J., Mills B. J. W., COPSE reloaded: An improved model of biogeochemical cycling over Phanerozoic time. Earth Sci. Rev. 178, 1–28 (2018). [Google Scholar]
- 60.Gumsley A. P., Chamberlain K. R., Bleeker W., Söderlund U., de Kock M. O., Larsson E. R., Bekker A., Timing and tempo of the Great Oxidation Event. Proc. Natl. Acad. Sci. 114, 1811–1816 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bjerrum C. J., Canfield D. E., Ocean productivity before about 1.9 Gyr ago limited by phosphorus adsorption onto iron oxides. Nature 417, 159–162 (2002). [DOI] [PubMed] [Google Scholar]
- 62.Fennel K., Follows M., Falkowski P. G., The co-evolution of the nitrogen, carbon and oxygen cycles in the Proterozoic ocean. Am. J. Sci. 305, 526–545 (2005). [Google Scholar]
- 63.Anbar A. D., Knoll A. H., Proterozoic ocean chemistry and evolution: A Bioinorganic bridge? Science 297, 1137–1142 (2002). [DOI] [PubMed] [Google Scholar]
- 64.Johnston D. T., Wolfe-Simon F., Pearson A., Knoll A. H., Anoxygenic photosynthesis modulated Proterozoic oxygen and sustained Earth’s middle age. Proc. Natl. Acad. Sci. 106, 16925–16929 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Butterfield N. J., Oxygen, animals and oceanic ventilation: An alternative view. Geobiology 7, 1–7 (2009). [DOI] [PubMed] [Google Scholar]
- 66.Sánchez-Baracaldo P., Ridgwell A., Raven J. A., A Neoproterozoic transition in the marine nitrogen cycle. Curr. Biol. 24, 652–657 (2014). [DOI] [PubMed] [Google Scholar]
- 67.Hamilton T. L., Bryant D. A., Macalady J. L., The role of biology in planetary evolution: Cyanobacterial primary production in low-oxygen Proterozoic oceans. Environ. Microbiol. 18, 325–340 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Allen J. F., Thake B., Martin W. F., Nitrogenase inhibition limited oxygenation of Earth’s proterozoic atmosphere. Trends Plant Sci. 24, 1022–1031 (2019). [DOI] [PubMed] [Google Scholar]
- 69.R. E. Summons, J. M. Hayes, Principles of molecular and isotopic biogeochemistry, in The Proterozoic Biosphere, a Multidisciplinary Study, J. W. Schopf, C. Klein, Eds. (Cambridge Univ. Press, 1992), pp. 83–94. [Google Scholar]
- 70.Stevens S. E. Jr., Patterson C. O. P., Myers J., The production of hydrogen peroxide by blue-green algae: A survey1. J. Phycol. 9, 427–430 (1973). [Google Scholar]
- 71.R. E. Zeebe, D. Wolf-Gladrow, CO2 in Seawater: Equilibrium, Kinetics, Isotopes (Oceanography Series, Elsevier, ed. 1, 2001), vol. 65. [Google Scholar]
- 72.Trick C. G., Wilhelm S. W., Physiological changes in the coastal marine cyanobacterium Synechococcus sp. PCC 7002 exposed to low ferric ion levels. Mar. Chem. 50, 207–217 (1995). [Google Scholar]
- 73.Guy R. D., Fogel M. L., Berry J. A., Photosynthetic fractionation of the stable isotopes of oxygen and carbon. Plant Physiol. 101, 37–47 (1993). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.McNevin D. B., Badger M. R., Whitney S. M., von Caemmerer S., Tcherkez G. G. B., Farquhar G. D., Differences in carbon isotope discrimination of three variants of D-ribulose-1,5-bisphosphate carboxylase/oxygenase reflect differences in their catalytic mechanisms. J. Biol. Chem. 282, 36068–36076 (2007). [DOI] [PubMed] [Google Scholar]
- 75.Clark R. L., Cameron J. C., Root T. W., Pfleger B. F., Insights into the industrial growth of cyanobacteria from a model of the carbon-concentrating mechanism. AIChE J. 60, 1269–1277 (2014). [Google Scholar]
- 76.Thomas P. J., Boller A. J., Satagopan S., Tabita F. R., Cavanaugh C. M., Scott K. M., Isotope discrimination by form IC RubisCO from Ralstonia eutropha and Rhodobacter sphaeroides, metabolically versatile members of ‘Proteobacteria’ from aquatic and soil habitats. Environ. Microbiol. 21, 72–80 (2019). [DOI] [PubMed] [Google Scholar]
- 77.Wilkes E. B., Pearson A., A general model for carbon isotopes in red-lineage phytoplankton: Interplay between unidirectional processes and fractionation by RubisCO. Geochim. Cosmochim. Acta 265, 163–181 (2019). [Google Scholar]
- 78.Zeebe R. E., On the molecular diffusion coefficients of dissolved , and and their dependence on isotopic mass. Geochim. Cosmochim. Acta 75, 2483–2498 (2011). [Google Scholar]
- 79.O’Leary M. H., Measurement of the isotope fractionation associated with diffusion of carbon dioxide in aqueous solution. J. Phys. Chem. 88, 823–825 (1984). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/7/2/eabc8998/DC1


