Abstract
Activation of transcription factors is a key driver event in cancer. We and others have recently reported that the Krüppel-like transcription factor KLF5 is activated in multiple epithelial cancer types including squamous cancer and gastrointestinal adenocarcinoma, yet the functional consequences and the underlying mechanisms of this activation remain largely unknown. Here we demonstrate that activation of KLF5 results in strongly selective KLF5 dependency for these cancer types. KLF5 bound lineage-specific regulatory elements and activated gene expression programs essential to cancer cells. HiChIP analysis revealed that multiple distal KLF5 binding events cluster and synergize to activate individual target genes. Immunoprecipitation-mass spectrometry assays showed that KLF5 interacts with other transcription factors such as TP63 and YAP1, as well as the CBP/EP300 acetyltransferase complex. Furthermore, KLF5 guided the CBP/EP300 complex to increase acetylation of H3K27, which in turn enhanced recruitment of the bromodomain protein BRD4 to chromatin. The 3D chromatin architecture aggregated KLF5-dependent BRD4 binding to activate Polymerase II (POL2) elongation at KLF5-target genes, which conferred a transcriptional vulnerability to proteolysis-targeting chimera (PROTAC)-induced degradation of BRD4. Our study demonstrates that KLF5 plays an essential role in multiple epithelial cancers by activating cancer-related genes through 3D chromatin loops, providing an evidence-based rationale for targeting the KLF5 pathway.
INTRODUCTION
Transcription factors, interacting with diverse co-activators or co-repressors, bind to cell type-specific regulatory elements throughout the genome, most of which are distal enhancers, to regulate transcription of target genes through 3D chromatin loops that connect distal enhancers and gene promoters (1–4). Dysregulation of transcription factors, caused by myriad genomic and epigenomic mechanisms, is frequently observed in cancer cells and is often associated with global transcriptional changes favoring tumorigenesis (5). However, the mechanisms by which these transcription factors function particularly in the 3D genome structure are largely unclear, which hinders developments of therapeutic strategies targeting them.
The Krüppel-like transcription factor (KLF) family consists of 17 members, all of which contain three highly conserved C2H2 Zinc Finger domains that recognize a CG-enriched DNA binding motif. KLF family members are expressed in many cell lineages and play essential roles in development, metabolism and pluripotency, while their dysregulation is highly involved in development of human diseases including cancer (6). The KLF5 gene, highly expressed in dividing cells, is a driver of diverse cancer-related phenotypes including cell proliferation (7). In addition, KLF5 is required for in vivo tumorigenesis of bladder, colon, pancreatic, gastric, and skin squamous cancers (8–14). For instance, deletion of Klf5 in murine intestinal stem cells leads to complete suppression of intestinal tumors induced by an oncogenic mutant of beta-catenin (15).
In addition to functional assays supporting the cancer-related role of KLF5, genomic alterations of KLF5 have been identified in multiple cancer types. Copy number amplifications of the KLF5 gene or its adjacent super-enhancers are associated with KLF5 overexpression in gastric and colorectal adenocarcinomas, bladder carcinomas, and squamous cancers of lung, esophagus, cervix, and head and neck (15–19). Furthermore, recent studies have identified missense mutations of KLF5 in various cancer types (20,21), which may either stabilize the KLF5 protein by interfering with recognition by the E3 ligase FBXW7 or alter the KLF5 transcription factor activity by changing its DNA binding specificity (17). All these data provide genomic evidence that KLF5 is activated in various types of epithelial cancers.
Despite our knowledge of KLF5 activation and its cancer-associated phenotypes, the mechanisms by which KLF5 contributes to these phenotypes particularly in the context of the 3D genome remains elusive. Here we comprehensively evaluate the importance of KLF5 in cell proliferation of various cancer types, identify KLF5-dependent lineage-specific transcriptional programs, and reveal the mechanisms by which KLF5 activates its target genes through the 3D chromatin architecture. We also utilize these findings to identify a bromodomain degrader as a potential therapeutic avenue for targeting KLF5-dependent cancer cells.
MATERIALS AND METHODS
CRISPR screen analysis:
We analyzed the publicly available CRISPR screen results from the Broad Institute Dependency Map (DepMap) project (URL: https://depmap.org/portal/) (22). Data was downloaded in December of 2019. The CERES CRISPR dependency score was calculated as previously described, with computational correction of copy number effects from CRISPR cutting (23). We used RNA-seq results from the Broad Institute Cancer Cell Line Encyclopedia (CCLE) project (24) to measure the KLF5 expression level in cell lines included in the DepMap project. We applied Spearman correlation to quantify the association between expression and CRISPR dependency for each of the screened genes across the screened cell lines. We calculated the enrichment of KLF5 dependency by cancer type versus all others combined with a Fisher’s Exact Test.
The Likelihood Ratio Test (LRT) scores were calculated as described by project Drive for their shRNA screens (25). Briefly, the distribution of the dependency scores for a gene across all cell lines was fitted to a skewed-t distribution and a normal distribution using the fitdist function from the fitdistrplus package in R. The skewed-t distribution was obtained from the doppelgangR R package. The LRT score was calculated for each gene individually and is defined as two times the log of the likelihood of the fit of the skewed t-distribution over the likelihood of the fit of the normal distribution as described by project Drive. If we failed to fit the dependency scores to either distribution the gene was omitted. The inflection point of the ranked LRT score curve was determined using the extremum distance estimator implemented in the inflection package in R. We performed the Kolmogorov–Smirnov test of normality and calculated exact p-values for all genes using R.
Cell lines:
Cancer cell lines were obtained from the Broad Institute CCLE project (24) in 2017 and 2018 and American Type Culture Collection (ATCC) in 2018 and 2019. Cells were tested negative for mycoplasma using the Lonza MycoAlert Detection kit. Cells were grown in RPMI-1640 supplemented with 10% FBS and 1% of penicillin-streptomycin. Cell line identities were verified by either SNP fingerprinting using an Affymetrix SNP assay as previously described in the CCLE project (24) or Short Tandem Repeats (STR) analysis (24 loci tested) by the DNA Sequencing Core at the University of Utah.
CRISPR-mediated gene knockouts:
Cas9-expressing cells were generated by infecting cells with the lentiCas9-Blast (Addgene: 52962) and selected with Blasticidin (10ug/ml) for five days. Cas9-expressing cells were infected with lentiGuide-Puro (Addgene: 52963) carrying either sgRNAs targeting negative control regions or the coding region of KLF5, and then selected by Puromycin for at least two days before any molecular or cellular assays. All sgRNA sequences are listed in Table S1.
Soft agar assay and Clonogenic assay:
For soft agar assay, 30,000 cells were plated in a layer of select agar in RPMI/10% FBS. After 12 or 15 days, wells were photographed and colonies were counted using the ImageJ software. For the anchorage-dependent clonogenic assays, 1000 cells were seeded in 3 ml of RPMI/10% FBS media. After 12 or 15 days, cells were fixed and stained with crystal violet. Wells were destained using 10% acetic acid, and the signal was read at 595 nm on a EnVision plate-reader.
ChIP-seq and ChIP-qPCR:
5–10 million cells were crosslinked with 1% Formaldehyde and lysed with Lysis Buffer I (5mM PIPES pH8.0, 85mM KCl, 0.5%NP40) and then Lysis Buffer II (1xPBS, 1%NP40, 0.5%Sodium Deoxycholate, 0.1%SDS) supplemented with protease inhibitors. The chromatin extract was sonicated by the QSonica Q800R (30mins, 70%amplitude) and immunoprecipitated with antibodies that were mixed with Dynabeads A and G. Antibodies: KLF5 (Abcam, ab137676), CBP (Cell signaling s7389), H3K27ac (Abcam, ab4729), BRD4 (Bethyl A301–985A100), Ser2-Phosphorylated POL2 (Cell signaling s13499) and Ser5-Phoshporylated POL2 (Cell signaling s13523). Libraries were prepared using the NEB DNA Ultra II library prep kit and sequenced by Illumina HiSeq, NextSeq or NovaSeq. For ChIP-qPCR assays, we designed primers targeting KLF5 binding sites identified from ChIP-seq. We also used sonicated genomic DNA to normalize variance in primer efficiency. All the primers are listed in Table S1.
We used Bowtie2 (26) to align sequencing reads to hg19 and MACS2 (27) to call KLF5 binding sites. ChIP-seq Signal Per Million Reads (SPMR) was calculated by MACS2. For cell lines that have biological replicates, we used the binding sites identified from both replicates for subsequent analysis. Distal KLF5 binding sites were identified by removing KLF5 sites that overlap with transcription start sites (TSS) of RefSeq genes. For clustering cancer cell lines by KLF5 binding profiles, we first merged all the identified KLF5 binding sites and then used the bedtools ‘genomecov’ function (28) to count sequencing reads that are mapped to each of the merged binding sites. Lastly, we used the edgeR package (29) to normalize read counts and identify the 1000 most variable KLF5 binding sites across cell lines, which were used for unsupervised clustering. DNA binding motifs enriched in ATAC-seq peaks and KLF5 binding sites were identified by the Homer de novo motif pipeline (30).
RNA-seq and RT-qPCR:
Cas9-expressing HARA and HT55 cells were infected with negative control or KLF5-targeting sgRNAs (three biological replicates for each condition) and selected by Puromycin for five days before RNA extraction. RNA was extracted using the Zymo Quick-RNA miniprep kit, treated with on-column DNase I. For RNA-seq, mRNA was purified by the NEBNext Poly-A mRNA Magnetic Isolation Module. RNA-seq libraries were prepared using the NEBNext Ultra Directional RNA library prep kit and sequenced by Illumina NovaSeq. We used Bowtie2 (26) to align sequencing reads and RSEM (31) to quantify the expression level (read counts) for each gene. We applied the edgeR package (29) to normalize read counts and identify genes that are differentially expressed after KLF5 knockout. Gene Ontology (GO) analysis was performed using the online David tool (URL: https://david.ncifcrf.gov/) (32). The enriched GO pathways were presented using the online REVIGO tool (URL: http://revigo.irb.hr/) (33). For RT-qPCR assays, the extracted RNA was first converted into cDNA and then processed with real-time PCR using the NEB Luna Universal qPCR Master Mix. Primers used for RT-qPCR were listed in Table S1.
HiChIP and loop calling:
HiChIP assays were performed based on the previously published protocol (34) with several modifications. ~10 million cells were crosslinked with 1% of Formaldehyde, quenched with 0.125M of Glycine, and lysed with Hi-C lysis buffer (10mM Tris-HCl pH 7.5, 10mM NaCl, 0.2% NP40) supplemented with protease inhibitors. DNA was digested by MboI. The digested overhangs were then filled with dCTP, dGTP, dTTP and biotin-labeled dATP and then ligated with T4 DNA ligase. After ligation, the nuclei were resuspended in Nuclear Lysis Buffer (50mM Tris-HCl pH7.5, 10mM EDTA, 1% SDS) supplemented with protease inhibitors and sonicated by Covaris E220. The sonicated chromatin was then processed with H3K27ac ChIP. The enriched DNA fragments were then captured by the Streptavidin C1 magnetic beads, processed with Nextera DNA library preparation, and sequenced by Illumina NextSeq.
The HiChIP sequencing reads were aligned to the hg19 reference genome by the HiC-Pro pipeline (35). Chromatin loops were identified using the Hichipper pipeline (36). The loops supported by at least three pairs of valid reads (PETs) and associated with an FDR value < 0.05 were considered as high-confidence loops for subsequent analyses. For connecting distal KLF5 binding sites to gene promoters, we first identified loop anchors that overlap with transcription start sites (TSS) of each gene in the genome. Then we use the bedtools ‘pairToBed’ function (28) to link each of the distal KLF5 binding sites to the promoter anchors.
CRISPR-mediated enhancer repression and motif cutting:
The dCas9-KRAB-MeCP2 fusion, including its CMV promoter, was PCR amplified from pcDNA3.3-dCas9-KRAB-MeCP2 (Addgene, 110821) and cloned into the BamHI-NheI sites of lentiCas9-Blast (Addgene, 52962) to generate lenti-dCas9-KRAB-MeCP2-Blast. sgRNAs were designed close to the summits of KLF5 binding peaks and were cloned into lentiGuide-Puro (Addgene, 52963). For repression of KLF5-bound enhancer elements, we first infected HARA cells with lenti-dCas9-KRAB-MeCP2-Blast and then infected the cells with lentiGuide-Puro carrying either negative control sgRNAs or enhancer-targeting sgRNAs. The infected cells were selected with Puromycin for six days before RNA extraction and RT-qPCR assays. Motif cutting assays were performed in Cas9-expressing HARA cells. sgRNAs were designed to cut KLF5 DNA binding motifs identified in each of the tested enhancers. All sgRNA sequences are listed in Table S1.
Immunoprecipitation (IP) and Mass Spectrometry assays:
10 million cells were lysed with NP40 lysis buffer (1% NP40, 150 mM NaCl and 50 mM Tris-HCl pH 8.0) supplemented with protease inhibitors and sonicated by QSonica Q800R (2 mins, 50% amplitude). Sonicated cell lysate was first incubated with 4 ug of the KLF5 antibody (Abcam, ab137676) or rabbit IgG (Cell signaling s2729) overnight at 4°C and then incubated with mixed Dynabeads A and G for 2 hours at 4°C. Next, the beads were washed five times with NP40 lysis buffer. Enriched protein complexes were eluted with the SDS sample buffer (2% SDS, 100mM Tris-HCl pH7.5, 10% glycerol, 0.5mM EDTA) for 20 mins at 65°C. For immunoblotting, the eluted protein samples were mixed with LDS sample buffer (Thermo Fisher, NP007) and DTT (Final: 20mM). For Mass Spectrometry assays, proteins were purified and precipitated with ProteoExtract kit (Millipore Sigma: 539180) and submitted to Harvard Medical School Taplin Biological Mass Spectrometry Facility. For the analysis, the number of peptides uniquely mapped to each of the detected proteins were used to compare the protein complexes pulled down by KLF5 and IgG (Table S2).
Accession codes:
ChIP-seq, HiChIP, and RNA-seq data were deposited to Gene Expression Omnibus (GEO) under the series GSE147853, GSE147854 and GSE147855, respectively.
RESULTS
KLF5 is selectively essential to multiple epithelial cancer types.
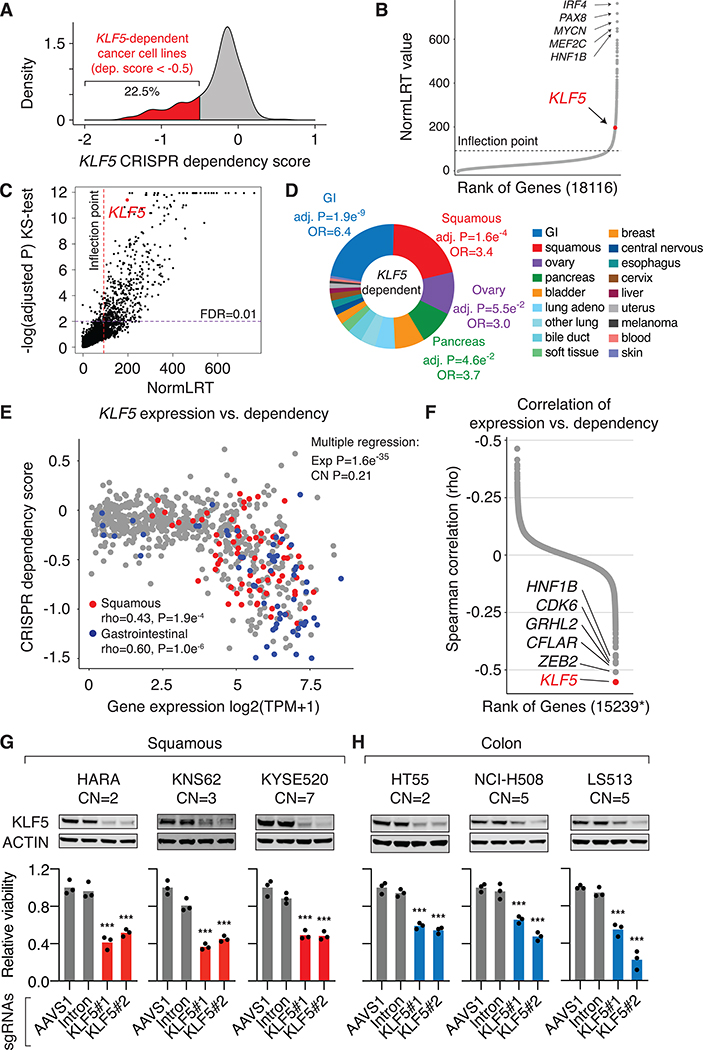
To define the functional significance of KLF5 activation across cancer types, we examined publicly available genome-wide CRISPR-mediated gene knockout screen data from the Broad Institute DepMap project (URL: https://depmap.org). The screens were designed to evaluate the effect of gene knockouts on proliferation of 684 cell lines representing dozens of cancer types over a time period of three weeks (22). Based on the cellular effects, a CRISPR dependency score is assigned to each gene for each cell line, by which a lower score indicates higher dependency (23). The potential copy-number effects on cell proliferation have been computationally corrected in the publicly available dataset (23). The KLF5 dependency level identified from the DepMap project strongly correlates with the Sanger Institute CRISPR screen results (Spearman’s rho=0.66) (37), and also correlates with shRNA screen results from the Broad Institute and Novartis (Spearman’s rho=0.37) (25,38) (Figure S1A). The CRISPR dependency score was normalized such that for each cell line the median effect of a set of known non-essential genes (“negative controls”) is 0, and the median effect of known essential genes (“positive controls”) is −1 (23). Therefore, a cutoff of −0.5 represents half the effect size expected from knocking out genes essential to each cell line. We found that the KLF5 gene is selectively essential to proliferation of a subset of cancer cell lines – 22.5% of cancer cell lines exhibit CRISPR dependency scores < −0.5 (Figure 1A).
Figure 1: KLF5 is selectively essential to multiple epithelial cancer types.
A. Distribution of KLF5 CRISPR dependency score across cancer cell lines from the DepMap project.
B. The screened genes are ranked by the Likelihood Ratio Test (LRT) score that measures their selective dependencies.
C. The relationship between the LRT scores and the Kolmogorov–Smirnov test adjusted P values. The LRT outlier (red) and Kolmogorov–Smirnov significance (purple) (FDR = 0.01) thresholds are shown.
D. The cancer-type distribution of KLF5-dependent cell lines. The pie-chart represents the percentage of KLF5-dependent cell lines in each cancer type. Bonferroni-adjusted P values are derived from Fisher exact tests (enrichment of KLF5 dependency by cancer type versus all others combined).
E. The gene expression value (log2 transformed TPM value) and CRISPR dependency score of KLF5 across cancer cell lines from the DepMap project. Squamous and gastrointestinal cancer cells are highlighted by red and blue colors, respectively. Upper right: P-values derived from multiple regression measuring the effects of KLF5 expression and copy number status on KLF5 dependency.
F. Spearman correlation between gene expression level and CRISPR dependency score for all protein-coding genes included in the DepMap project. *Presented are genes that have available copy number data from the DepMap project and whose CRISPR-dependencies are not affected by their copy number status (FDR>0.05).
G. Cell proliferation assay in squamous cancer cell lines one week post CRISPR knockout of the KLF5 gene. Western blots show the KLF5 protein level changes after KLF5 knockout. Cell number was normalized to cells infected with the negative control sgRNA targeting the AAVS1 locus. The copy number of KLF5 for each cell line is indicated. Three biological replicates were included for each condition. P values are derived from t-tests:*** P value < 0.001.
H. Cell proliferation assay in colorectal cancer cell lines one week post CRISPR knockout of the KLF5 gene.
McDonald, et al. (25) introduced a likelihood ratio test (LRT) score to identify selective cancer cell dependencies. Briefly, LRT evaluating the fit of the gene dependency scores, across cell lines, to a skew T or normal distribution was applied to identify and nominate strong selective cell dependencies (25). We calculated the LRT score for all the screened genes in the Broad DepMap project. We used the inflection point of the curve of ranked LRT scores to identify an outlier threshold (Figure 1B). The calculated cut-off (LRT=92) corresponds well with Tukey’s method to detect outliers (Figure S1B). The dependency score outliers represent the top 5.6% of genes. We also performed a Kolmogorov–Smirnov test of normality for all genes. The Kolmogorov–Smirnov D statistic is highly correlated with the LRT scores (Spearman rho = 0.79, p < 2.2×10−16) (Figure S1C). A quantile-quantile plot of the Kolmogorov-Smirnov test p-values reveals a significant tail (Figure S1D) in the p-value distribution (FDR < 0.01), which corresponds well with high LRT scores (Figure 1C). We found that the distribution of KLF5 dependency scores is highly skewed with an LRT value of 197 and is ranked 1.3 percentile among all the screened genes and this deviation from normality is confirmed with the Kolmogorov-Smirnov test (FDR= 3.90×10−12) (Figure 1C).
We found the KLF5 gene to be selectively essential to several epithelial cancer types, including gastrointestinal (GI) cancers such as colorectal and gastric adenocarcinomas, squamous cancers of diverse tissue origins such as lung, esophagus and head and neck, and pancreatic adenocarcinomas (Figure 1D and Table S3). In contrast, cancer cells of hematopoietic lineages and the central nervous system tend to be not dependent on the KLF5 gene (Figure 1D and Table S3). Although some cancer types are more likely to be dependent on KLF5, we observed a range in the level of KLF5 dependency across their individual cell lines (Figure 1E). We examined KLF5 expression levels in the CRISPR-screened cell lines using RNA-seq data from Cancer Cell Line Encyclopedia (CCLE) (24). Cancer cell lines with higher KLF5 expression are more likely to be dependent on the KLF5 gene (lower CRISPR dependency score) (Figure 1E), suggesting that KLF5 dependency is attributed to KLF5 overexpression. We also performed a multiple regression analysis to determine the effect of KLF5 RNA expression and KLF5 copy number status on the observed KLF5 dependency. Using quantile normalized dependency scores, we show that KLF5 expression (P value=1.6e−35), but not KLF5 copy number (P value=0.21), is significantly associated with KLF5 dependency. Furthermore, the correlation between KLF5 expression and KLF5 dependency remains strong for cancer cell lines that are diploid for the KLF5 gene (Spearman rho = −0.53, Figure S2A). These results show that KLF5 dependency is not contingent on KLF5 copy number. We also observed strong correlation between the protein level of KLF5 (39) and the KLF5 dependency (Spearman rho = −0.54, Figure S2B), suggesting that the abundance of the KLF5 transcription factor may contribute to KLF5 dependency.
We then applied the analysis to all the protein-coding genes with available RNA expression, copy number, and CRISPR dependency data from the DepMap project and removed the ones whose gene dependencies are affected by their copy number status (Table S4). Among the remaining genes, KLF5 exhibits the strongest correlation between the RNA expression and gene dependency level (Figure 1F). The correlation between KLF5 expression and dependency slightly varies across cancer types, with gastrointestinal cancer cells showing the strongest correlation (Spearman’s rho=−0.6) (Figure S2C), suggesting that additional unknown cancer-type specific factors may contribute to the observed KLF5 dependency. We previously showed that super-enhancer activation drives KLF5 overexpression in cancer cells (17,19). Using published H3K27ac ChIP-seq data from squamous cancer cell lines (17,40,41), we identified super-enhancer elements in KLF5-high and -dependent (CRISPR dependency score < −0.5) HARA and KNS62 cell lines, as well as in KLF5-high but -independent KYSE510 and CAL33, but not in KLF5-low and -independent CALU1 and HCC2450 (Figure S3A–B). Taken together, these results suggest that transcriptional activation of KLF5 can partially predict KLF5 dependency in cancer cells.
We then performed individual CRISPR-mediated knockout of KLF5 in the lung squamous cancer cell lines HARA and KNS62, esophageal squamous cancer cell line KYSE520, and the colorectal adenocarcinoma cell lines HT55, NCI-H508 and LS513. The cell lines are associated with either diploid or copy number amplified KLF5 (Figure 1G–H). All of the selected cell lines are associated with high expression levels of KLF5 based on RNA-seq data (Table S5). We used two separate guide RNAs (sgRNAs) that target distinct positions of the KLF5 gene. As a negative control, we used an sgRNA that recognizes an intronic region within AAVS1 that is not essential to cell proliferation. We also included a sgRNA that targets the first intron of the KLF5 gene to normalize the potential copy number-specific effect from CRISPR knockout. KLF5 knockout results in a significant reduction in proliferation rate of these cancer cell lines (Figure 1G–H), validating the role of KLF5 in cell proliferation of the respective cancer types. To rule out off-target effects from the CRISPR knockout, we ectopically expressed the KLF5 cDNA that harbors a synonymous mutation at the PAM sequence and is resistant to sgKLF5#2-mediated CRISPR targeting (Figure S4A). This sgRNA was included in the DepMap CRISPR screen and also used in our individual validations. Overexpression of the mutant KLF5 rescues the growth-inhibitory phenotypes caused by KLF5 knockout in HARA cells, demonstrating the on-target effect of the CRISPR experiment (Figure S4A).
In contrast, knockout of KLF5 in four KLF5-independent squamous cancer cell lines show little effect on their cell proliferation rate (Figure S4B). Furthermore, we show that KLF5 knockout has no detectable effects on proliferation of the immortalized normal lung epithelial cell line AALE (42), suggesting that KLF5 may represent a cancer-related dependency (Figure S4C). We also assessed the effects of overexpressing KLF5 in KLF5-low and -independent squamous cancer cell lines. While KLF5 overexpression increases the proliferation of SKMES1 cells, it has no detectable effects on RERFLCAI cells (Figure S4D). Taken together, these results suggest that KLF5 is necessary, but may not be sufficient, for promoting cancer cell proliferation.
In addition to cell proliferation, we also tested the effect of KLF5 knockout on other cancer-related phenotypes. We performed soft agar colony formation and clonogenic assays in the KLF5-dependent squamous cancer cell lines HARA, KNS62 and KYSE520 to assess KLF5’s role in cellular transformation and clonogenic expansion. The results show that KLF5 knockout results in reductions in both anchorage-independent (Figure S5A) and -dependent colony formation efficiency (Figure S5B). In contrast, KLF5 knockout has little effects on these phenotypes in KLF5-independent squamous cancer cell lines (Figure S5A–B). These data, together with the cell proliferation results and previous animal studies (8–14), support the critical role of KLF5 in diverse cancer-related phenotypes.
KLF5 drives lineage-specific cancer-related transcriptional programs.
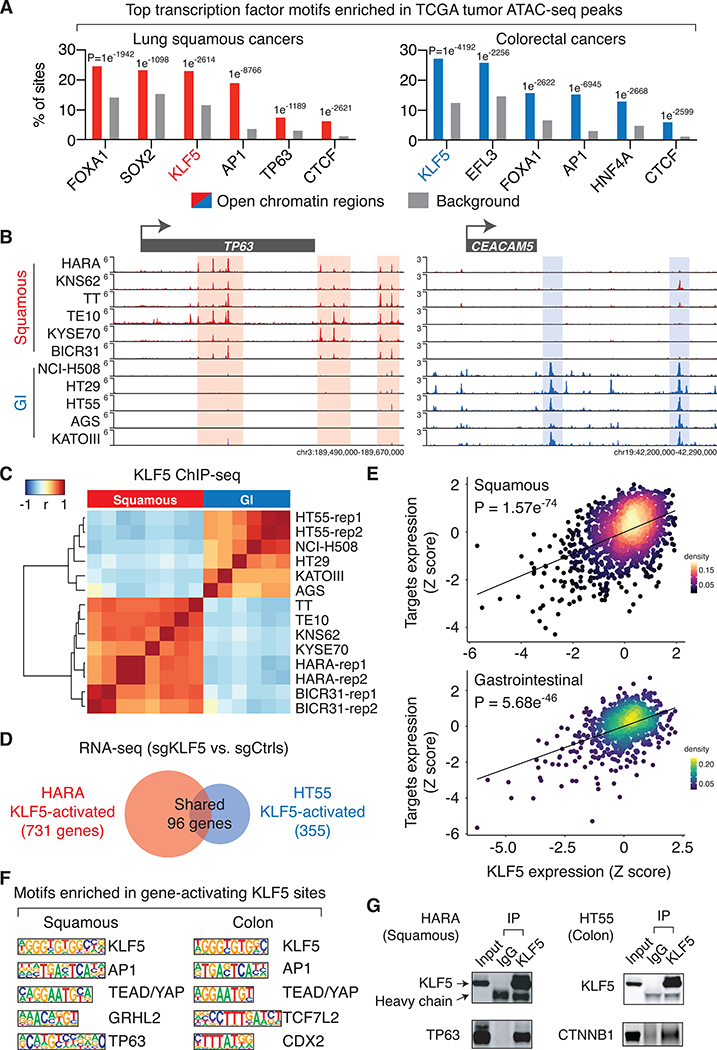
Transcription factors bind to chromatin accessible regions and drive transcriptional programs essential to cancer cells. Motif analyses of chromatin accessible regions in cancer cells have been utilized to predict the activity of these candidate transcription factors (43). We performed HOMER de novo DNA motif analyses (30) in chromatin accessible regions that were identified from the Cancer Genome Atlas (TCGA) ATAC-seq results (43). The analysis reveals that the KLF5 DNA recognition sequence is one of the top three motifs enriched in chromatin accessible regions found in squamous, colorectal and bladder cancers (Figure 2A and S6A), suggesting KLF5 is an active transcription factor in these cancer types.
Figure 2: KLF5 drives lineage-specific transcription programs in cancer cells.
A. Top transcription factor binding motifs that are significantly enriched in chromatin accessible regions identified in the TCGA ATAC-seq datasets. The listed motifs are ranked based on their presence in the percentage of chromatin accessible regions.
B. KLF5 ChIP-seq intensity, Signal Per Million Reads (SPMR)/bp, in the TP63 and CEACAM5 loci.
C. Unsupervised clustering of cancer cell lines based on the correlation of KLF5 binding intensity across the most variable 1000 KLF5 binding sites. ChIP-seq biological replicates were performed for HARA, HT55 and BICR31 cells (rep1 and 2).
D. Comparison of genes that are activated by KLF5 (based on RNA-seq results with and without KLF5 knockout) in the lung squamous cancer cell line HARA and the colorectal cancer cell line HT55.
E. Gene expression correlation between the KLF5 gene and KLF5-target genes. For each cell line, Z-scores are assigned based on the expression level of the KLF5 gene (x-axis) or the average expression level of KLF5-target genes (y-axis).
F. Top transcription factor binding motifs enriched in KLF5 binding sites that are within −/+ 50kb of transcription start sites of KLF5-activated genes.
G. Immunoprecipitation using antibodies recognizing the endogenous KLF5 or IgG, followed by immunoblotting of TP63 in HARA cells, and CTNNB1 in HT55 cells.
We next sought to demonstrate the transcription factor activity of KLF5 in these cancer types. We first performed KLF5 ChIP-seq in multiple cancer cell lines – HARA and KNS62 for lung squamous cancers, TT, TE10 and KYSE70 for esophageal squamous cancers, BICR31 for head and neck squamous cancers, AGS and KATOIII for gastric adenocarcinomas, and HT29, HT55 and NCI-H508 for colorectal adenocarcinomas. The results reveal that squamous cancer cell lines, regardless of their different tissue of origins, exhibit similar KLF5 binding profiles as compared to each other (Figure 2B–C), which aligns with previous findings that squamous cancers are associated with similar transcriptional programs (18,44). Similarly, the GI cancer cells including gastric and colorectal adenocarcinoma cells are associated with similar KLF5 binding patterns (Figure 2B–C). In contrast, the KLF5 binding profiles are distinct between squamous cancers and GI adenocarcinomas (Figure 2B–C), suggesting that KLF5 may regulate different transcriptional programs in these cancer types.
We then performed RNA-seq assays in the KLF5-dependent lung squamous cancer cell line HARA with and without KLF5 knockout (three biological replicates for each condition), which identified 731 KLF5-activated genes. We validated the results by performing RT-qPCR for selected KLF5-activated genes in HARA and KYSE520 cell lines (Figure S6B). In contrast, KLF5 knockout has little effects on expression of these genes in KLF5-independent cell lines or the immortalized lung epithelial cell line AALE (Figure S6B). We also performed the RNA-seq analysis in the colorectal adenocarcinoma cell line HT55 that is dependent on KLF5. In agreement with the distinct KLF5 binding profiles between these two cancer types, the lists of KLF5-activated genes (FDR<0.05, Fold Change > 1.5) are highly cancer type-specific – less than 20% of KLF5-activated genes in HARA lung squamous cells are shared with those found in HT55 colorectal cells (Figure 2D). We then analyzed the TCGA RNA-seq results from squamous cancers (including lung, esophageal, head and neck, and cervical squamous cancer samples) and GI adenocarcinomas and found that KLF5-target genes are significantly and positively correlated with KLF5 on the expression level in the respective cancer types (Figure 2E and S7A), suggesting that they are likely to be KLF5 target genes in human primary tumors. We then performed Gene Ontology pathway analysis (32) using the identified gene lists. Interestingly, although the lists of KLF5-activated genes are largely distinct between the two cancer types, many of their enriched cancer-related pathways are shared, which include cell proliferation, cell adhesion, cell migration, wound-healing and activation of receptor protein tyrosine kinase (RTK) signaling pathways (Figure S7B).
Focusing on the KLF5 binding sites that are adjacent to KLF5-activated genes (+/− 50kb of TSS), we performed HOMER de novo DNA motif analysis, which revealed the enrichment of DNA sequences that are recognized by other transcription factors such as TP63 in squamous cancers and the TCF7L2/CTNNB1 complex in colorectal adenocarcinomas (Figure 2F). We performed immunoprecipitation of KLF5 and detected the physical interaction between KLF5 and TP63 in HARA squamous cells and between KLF5 and CTNNB1 in HT55 colorectal cells (Figure 2G). These results suggest that KLF5 may partner with lineage-specific transcription factors in cancer cells.
Chromatin loops aggregate distal KLF5 binding events to activate their target genes.
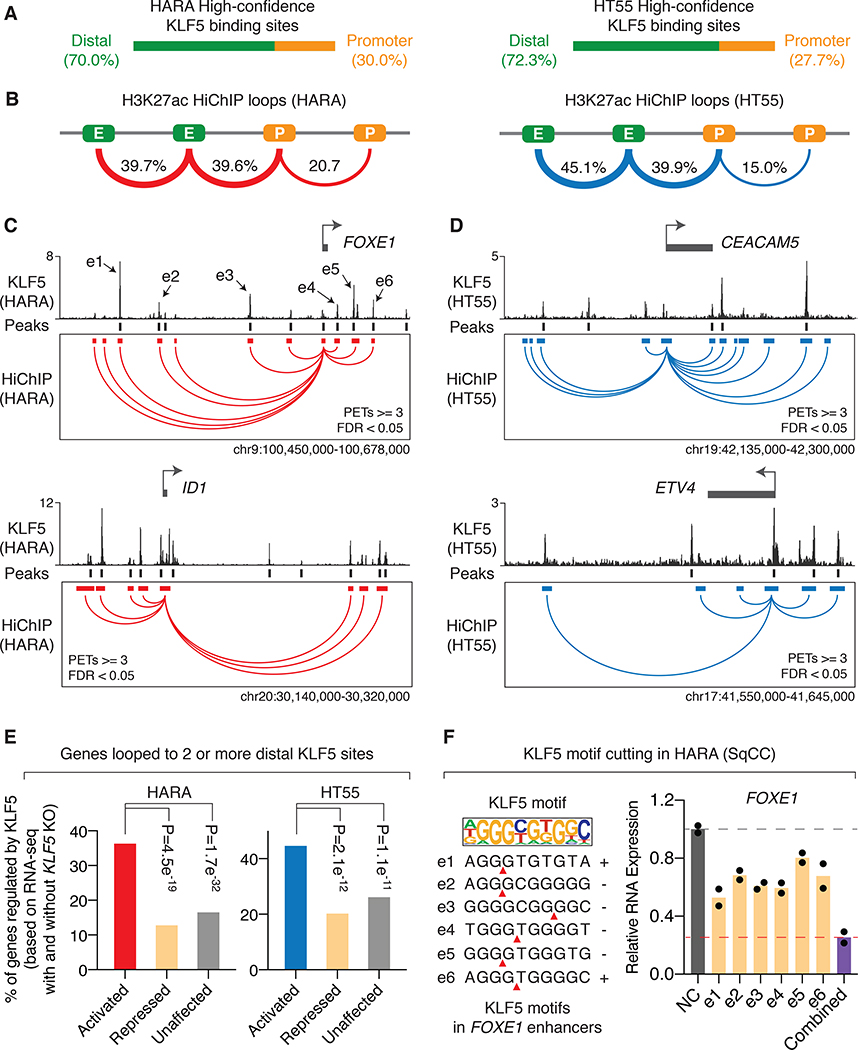
We next sought to understand how KLF5 binding events contribute to regulation of KLF5 target genes using the squamous cancer cell line HARA and colorectal cancer cell line HT55. We selected KLF5 binding events harboring KLF5 DNA recognition motifs as high-confidence sites for the subsequent analysis. The majority of the KLF5 binding sites overlap with ATAC-seq open chromatin regions identified in the corresponding cancer types (Figure S8A). In addition, KLF5 binding sites also tend to overlap with ATAC-seq open chromatin regions in a cancer type-specific manner (Figure S8B). As the majority of KLF5 binding sites are distributed in intergenic or intronic regions instead of gene promoters (Figure 3A), we reasoned that they may regulate gene expression through chromatin loops that connect them to promoter regions of their target genes. Several newly developed 3D genome structure assays such as HiChIP (34) and PLAC-seq (45) combine HiC and ChIP assays to identify high resolution chromatin loops connecting ChIP-captured regions. We performed H3K27ac HiChIP to identify chromatin loops connecting active regulatory elements such as enhancers and promoter in HARA and HT55 cells (Figure 3B and S9A). Among the identified chromatin loops (valid paired-end tags ≥ 3, FDR < 0.05), we found that around 39.6% and 39.9% of them link distal enhancers to gene promoters in HARA and HT55 cells, respectively (Figure 3B).
Figure 3: Chromatin loops aggregate distal KLF5 binding events to activate their target genes.
A. Distribution of high-confidence KLF5 binding sites (KLF5 binding sites that were called in biological replicates of ChIP-seq and harboring KLF5 DNA binding motifs) in HARA and HT55 cells.
B. Distribution of significant chromatin loops called from H3K27ac HiChIP assays (PETs≥3, FDR<0.05) in HARA and HT55 cells. E: enhancers; P: promoters.
C. KLF5-activated genes FOXE1 and ID1 (based on RNA-seq results in HARA cells with and without KLF5 knockout, FDR<0.05) are connected to multiple high-confidence distal KLF5 binding sites through chromatin loops identified from H3K27ac HiChIP assays in HARA squamous cancer cells. HiChIP anchors that are looped to promoters of the target genes are presented.
D. Same as C, but with regards to KLF5-activated genes CEACAM5 and ETV4 in HT55 colorectal cancer cells.
E. Percentage of KLF5-activated, -repressed, and -unaffected genes (based on RNA-seq results with and without KLF5 knockout, FDR<0.05) that are connected to two or more high-confidence distal KLF5 binding sites in HARA and HT55 cells. P values are derived from Fisher Exact Tests.
F. Expression level of the KLF5-activated gene FOXE1 after Cas9-mediated individual disruption of KLF5 motifs within each of FOXE1-connected distal KLF5 binding sites, or simultaneous disruption of the motifs. Gene expression level is normalized to the negative control.
We then overlaid the high-confidence KLF5 binding sites on the promoter-enhancer loops (Figure S9B), which connects each of the sites to their potential target genes. We examined genomic loci surrounding KLF5-activated genes from our RNA-Seq data with and without KLF5 knockout. We focused on FOXE1, ID1 and TP63 in HARA squamous cancer cells (Figure 3C and S10A) and CEACAM5 and ETV4 in HT55 colorectal adenocarcinoma cells (Figure 3D). We showed that for each of these genes, multiple KLF5 binding sites (≥2) are connected to their promoter regions. These results suggest potential combinatorial effects of the KLF5 binding events in activating their target genes. Indeed, in both HARA and HT55 cells, genes that are connected to multiple distal KLF5 binding sites (two or more) through chromatin loops (identified by HiChIP data) are more likely to be activated, instead of to be repressed or unaffected, by KLF5 (based on RNA-seq with and without KLF5 knockout) (Figure 3E), while genes that are associated with zero or one distal KLF5 binding site are not likely to be activated (Figure S10B).
To validate the combinatorial effect of KLF5 binding events, we tested two KLF5-target genes FOXE1 and TP63 in HARA squamous cancer cells. We repressed individual KLF5 binding sites that are looped to FOXE1 and TP63 using an improved CRISPR-interference (CRISPRi) system in which an inactivated Cas9 (dCas9) is fused to the KRAB and MeCP2 transcriptional repressors (46). We showed that individually repressing the KLF5 binding sites only results in a modest decrease in expression of FOXE1 or TP63 in HARA cells (Figure S10C). However, simultaneous repression of the KLF5 binding sites leads to a more remarkable reduction in expression (Figure S10C), validating the joint effects of these binding sites. In addition, we applied CRISPR/Cas9 to directly cut KLF5 DNA motifs in each of the enhancers that are looped to the FOXE1 gene (Figure 3F). Similarly, while individually cutting the KLF5 binding motifs yields modest effects in downregulating FOXE1 expression, simultaneous disruption of the motifs gives rise to a more remarkable reduction (Figure 3F, right). Taken together, we showed that chromatin loops connect multiple distal KLF5 binding events to jointly activate their target genes.
KLF5 recruits BRD4 to chromatin through the CBP/EP300 complex.
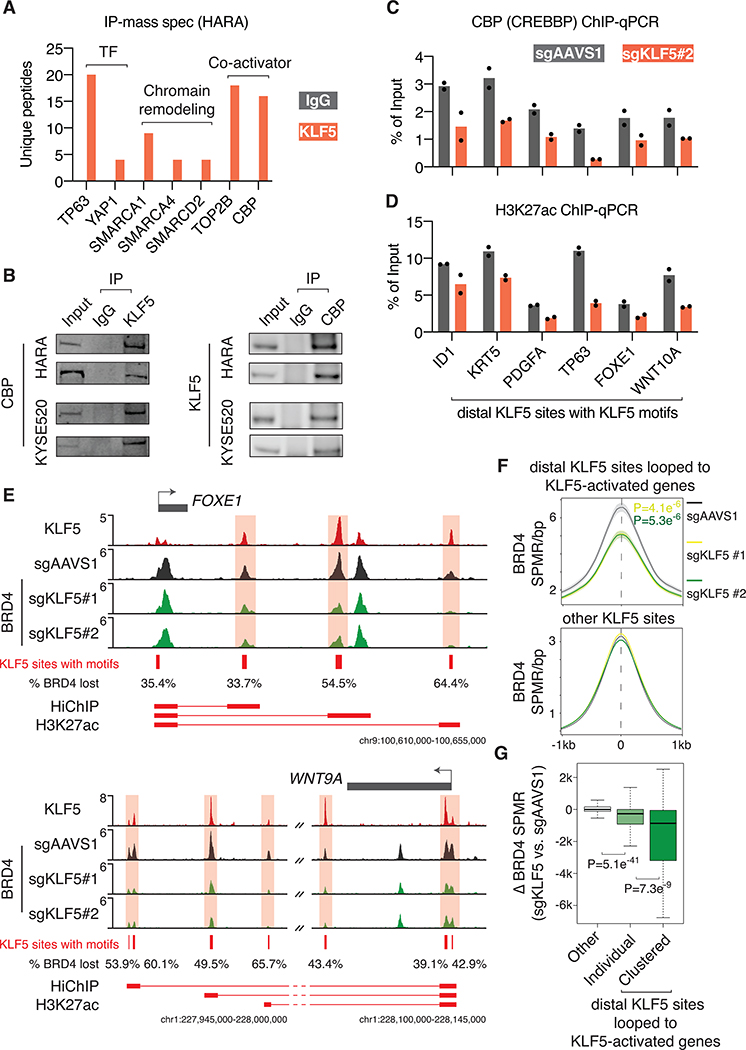
To understand how KLF5 recruitment to distal elements contributes to transcriptional activation, we performed immunoprecipitation of KLF5 followed by mass spectrometry (IP-mass spec) to identify KLF5-interacting coactivators in the lung squamous cancer cell line HARA. Analysis of the IP-mass spec results showed physical interaction of KLF5 with other transcription factors such as TP63 and YAP1 (Figure 4A), which are consistent with our findings that TP63 and YAP1 binding motifs are enriched in KLF5 binding sites in HARA cells (Figure 2F). In addition, we found that KLF5 interacts with multiple members of the SWI/SNF chromatin remodeling complex including SMARCA1, SMARCA4, and SMARCD2 (Figure 4A). Finally, we observed strong interaction between KLF5 and transcriptional coactivators including the topoisomerase II-beta TOP2B and the CREB Binding protein CREBBP, also known as CBP (Figure 4A). CBP forms a histone acetyltransferase complex with EP300, which adds acetylation to histone lysine residues as well as transcription factors. As the CBP/EP300 complex has been associated with activation of regulatory elements (47) and has been previously reported to interact with KLF5 through in vitro GST pull-down assays (48), we focused on this complex for our subsequent analysis. We validated the interaction between KLF5 and CBP by immunoprecipitating KLF5 and CBP in HARA and KYSE520 cells and blotting CBP and KLF5, respectively (two biological replicates for each experiment) (Figure 4B). The other protein interactions such as the association with the SWI/SNF complex and TOP2B need further validation.
Figure 4: KLF5 recruits BRD4 to chromatin through the CBP/EP300 complex.
A. KLF5 IP-mass spec results showing protein complexes interacting with the endogenous KLF5 in HARA squamous cancer cells. Note that the number of IgG-enriched unique peptides that are mapped to the presented proteins are zero.
B. Validation of the interaction between KLF5 and CBP (CREBBP) through immunoprecipitation of KLF5 and CBP followed by immunoblotting CBP and KLF5, respectively, in HARA and KYSE520 cells. Two biological replicates are presented for each immunoprecipitation assay.
C. ChIP-qPCR of CBP in HARA cells with and without KLF5 knockout. The signal was first normalized to DNA concentration of each sample (measured by Qubit) and then to sonicated genomic input. Two biological replicates are presented.
D. ChIP-qPCR of H3K27ac, a substrate of CBP, in HARA cells with and without KLF5 knockout. Two biological replicates are presented.
E. In HARA cells after KLF5 knockout, partial loss of BRD4 binding was observed at high-confidence distal KLF5 binding sites that are looped to KLF5-activated genes FOXE1 and WNT9A. The percentage of BRD4 binding that is lost after KLF5 knockout is indicated for each KLF5 site. HiChIP (H3K27ac) anchors and their connections are presented underneath.
F. The average ChIP-seq SPMR/bp value of BRD4 in HARA cells, with and without KLF5 knockout, at high-confidence distal KLF5 binding sites that are looped to KLF5-activated genes and other KLF5 sites. P values (t-tests) are presented for the comparisons between sgAAVS1 and sgKLF5#1 (yellow), or between sgAAVS1 and sgKLF5#2 (green).
G. The BRD4 ChIP-seq SPMR value that is lost at KLF5 sites looped to KLF5-activated genes and at other KLF5 sites. The lost value is measured for individual KLF5 sites as well as clusters of KLF5 sites that are looped to each of KLF5-activated genes.
We selected several high-confidence distal KLF5 binding sites that are looped to KLF5-activated genes, including squamous cancer-related genes ID1, TP63, FOXE1, PDGFA, WNT10A, and KRT5, for subsequent analysis. We performed ChIP-qPCR of CBP in HARA cells with and without CRISPR knockout of KLF5. We showed that KLF5 knockout decreased the binding of CBP to these regions (Figure 4C), suggesting the role of KLF5 in recruiting CBP to chromatin. H3K27 is known to be a functional substrate of the CBP/EP300 acetyltransferase complex (49). We performed ChIP-qPCR of H3K27ac and showed that KLF5 knockout reduced the acetylation level of this histone lysine residue at the KLF5 binding sites (Figure 4D).
Previous studies have reported that H3K27ac at regulatory elements facilitates recruitment of the bromodomain protein BRD4 to chromatin, which is necessary for activation of downstream target genes (50). In order to globally assess the role of KLF5 in the chromatin recruitment of BRD4, we performed ChIP-seq of BRD4 in HARA cells with and without KLF5 knockout. As exemplified by genomic loci surrounding the KLF5-activated genes FOXE1, ID1, WNT9A and WNT10A, we observed modest yet reproducible decreased BRD4 recruitment at multiple KLF5 binding sites (Figure 4E and S11A). On the genome scale, we show that distal KLF5 binding sites that are looped to KLF5-activated genes lose ~25% of BRD4 binding on average after KLF5 knockout, while other KLF5 sites have barely detectable difference of BRD4 binding (Figure 4F). Interestingly, although the majority of KLF5 binding sites overlap with ATAC-seq open chromatin regions identified in lung squamous cancers (Figure S11B), the ones that are looped to KLF5-activated genes exhibit stronger BRD4 binding as compared to the other KLF5 sites, suggesting these binding events are associated with higher transcriptional regulatory activity (Figure S11B). As multiple KLF5 binding events connect to their target genes through chromatin loops (Figure 4E), they together may recruit stronger level of BRD4 to the chromatin as compared to individual KLF5 binding event. Indeed, we observed that clusters of the looped KLF5 binding events lose significantly more BRD4 binding after KLF5 knockout as compared to individual KLF5 binding events (Figure 4G).
The KLF5 protein complex activates its target genes by promoting POL2 elongation.
Transcription factors cooperate with their co-activators or co-repressors to modulate the activity of RNA polymerase II (POL2) to control gene expression (1). The process of POL2 activation consists of multiple steps including POL2 recruitment, initiation and elongation, which requires the involvement of distinct transcription factors and chromatin complexes (51). Previous studies have shown that BRD4 is required for assembly of the POL2 elongation complex, which promotes transcription by releasing POL2 from a paused state at gene promoters to an elongating state at gene bodies (52,53). Given the functional link between KLF5 and BRD4, we hypothesized that KLF5 activates expression of its target genes by activating POL2 elongation.
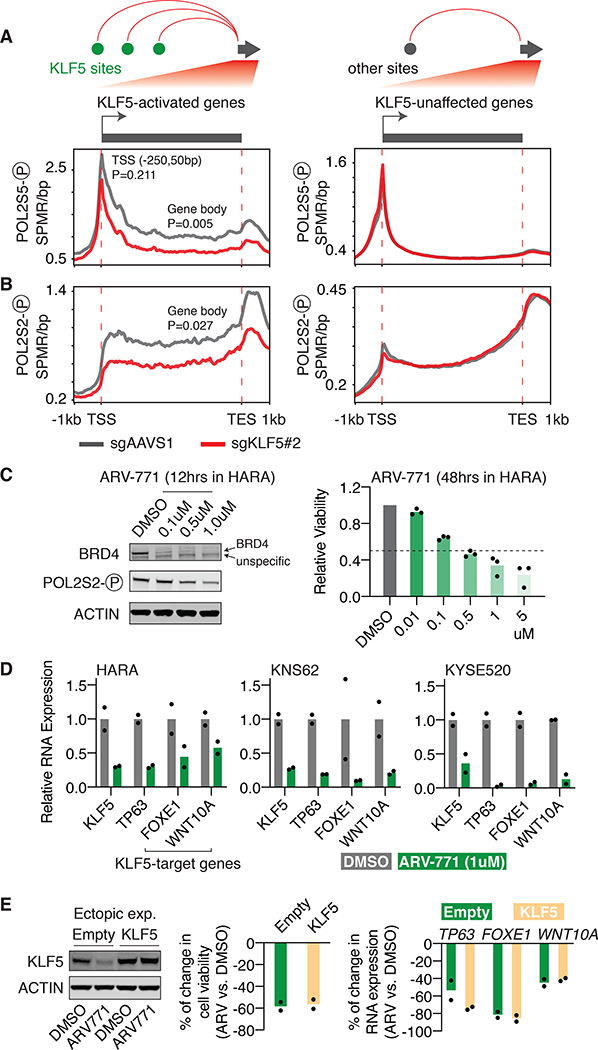
Different phosphorylated forms of POL2 indicate separate steps of POL2 activation. Phosphorylation of Serine 5 (Ser5) at POL2 C-terminal domain (CTD) is essential for POL2 initiation at gene promoters and stays with POL2 during early transcription elongation at gene bodies (51). We performed ChIP-seq using an antibody against Ser5-phosphorylated POL2 in HARA cells with and without KLF5 knockout. Focusing on KLF5-activated genes that are connected to multiple (two or more) distal KLF5 binding sites through chromatin loops, we showed that KLF5 knockout significantly reduces the enrichment of Ser5-phosphorylated POL2 at gene body regions (P=0.005), while having modest and insignificant effect at gene promoter regions (P=0.211), suggesting the role of KLF5 in activating POL2 elongation instead of initiation. No obvious change is observed for Ser5-phosphorylated POL2 level at promoters or gene bodies of genes that are unaffected by KLF5 (Figure 5A). To validate our findings, we then performed ChIP-seq of Ser2-phosphorylated POL2 in HARA cells with and without KLF5 knockout. Different from Ser5-phosphorylation, POL2 CTD Ser2-phosphorylation is solely associated with elongating POL2 at gene bodies (51). Accordingly, we observed a significant reduction in the enrichment of Ser2-phosphorylated POL2 at gene bodies of KLF5-activated genes (P=0.027), but not for genes that are unaffected by KLF5 (Figure 5B). Taken together, our data demonstrates that KLF5 stimulates POL2 elongation at its target genes.
Figure 5: KLF5 activates its target genes by promoting POL2 elongation.
A. Distribution of Ser5-phosphorylated POL2 ChIP-seq signal (SPMR/bp) in HARA cells, with and without KLF5 knockout, throughout gene bodies of KLF5-activated genes that are looped to two or more high-confidence distal KLF5 binding sites, or KLF5-unaffected genes. P values (t-tests) are presented for the difference at gene promoter regions (−250bp to 50bp of TSS) and gene body regions.
B. Same as A, but with regards to distribution of Ser2-phosphorylated POL2 ChIP-seq signal (SPMR/bp).
C. Treatments of the BRD4 PROTAC molecule ARV-771 degrades BRD4 and decreases cell proliferation of HARA cells (48 hours post treatments). Cell number was normalized to cells treated with DMSO. Three biological replicates were included for the assay.
D. The BRD4 degrader ARV-771 reduces RNA expression of the KLF5 gene itself and KLF5-target genes in HARA, KNS62 and KYSE520 cells (12 hours post treatments). Gene expression was first normalized to the internal control gene ACTB and then normalized to cells treated with the control vehicle DMSO.
E. KLF5 protein level (Left), cell proliferation (middle), and KLF5-target gene expression (right, RT-qPCR results) of the wild-type and KLF5-overexpressing HARA cells with DMSO or BRD4 degrader treatments. Cell proliferation and RNA expression reduction is relative to the DMSO control (two biological replicates).
Therapeutic opportunities to target the KLF5 pathway in cancer cells.
Our results show that KLF5 activates its target genes through the recruitment of BRD4 at KLF5 binding sites, which promotes the release of POL2 from promoter regions of KLF5 target genes. Pharmacological inhibitors have been developed to target BRD4 (as summarized in (54)). In addition, our previous work has shown that the KLF5 gene itself is driven by adjacent super-enhancers (17,19), and previous studies have reported that super-enhancer-linked genes tend to be sensitive to BRD4 inhibitors (55). Thus, we reasoned that inhibition of BRD4 may affect both activity of the KLF5 protein and transcription of the KLF5 gene, which may serve as a strategy to inhibit KLF5-dependent cancer cells. We chose the ARV-771 small molecule that was designed based on the proteolysis-targeting chimera technology (PROTAC) to recruit the E3 ligase cereblon to degrade bromodomain proteins including BRD4 (56). Treatment of ARV-771 resulted in efficient degradation of BRD4 as well as a reduction of POL2 Ser2 phosphorylation (Figure 5C), consistent with previous findings that inhibition of BRD4 impairs POL2 activity (52). ARV-771 caused remarkable decreases in proliferation of KLF5-dependent squamous cancer cell lines HARA, KNS62 and KYSE520 within 48 hours of treatments (Figure 5C and S11C).
Furthermore, 12 hours treatment of the BRD4 degrader reduced expression of both the KLF5 gene itself and KLF5-target genes, including FOXE1, TP63, and WNT10A that have been previously associated with tumorigenesis of squamous cancer (57–59), in all the three tested cell lines (Figure 5D), suggesting its inhibitory effects on the KLF5 pathway. We also treated cells with the BRD4 degrader for a shorter period of time (2 hours) and performed ChIP-qPCR of Ser5- and Ser2-phosphorylated POL2 at the FOXE1 and TP63 loci. Two hours of treatment is sufficient to degrade the BRD4 protein but has little effect on the KLF5 protein levels (Figure S12), providing a time point to specifically measure the effect of BRD4. The results show that BRD4 degradation causes a reduction in POL2 elongation activity at the KLF5-target genes (Figure S12), supporting the critical role of BRD4 in the KLF5 pathway. Finally, we reasoned that if the gene regulation function of KLF5 relies on the activity of BRD4, then KLF5 overexpression will fail to rescue the cellular and molecular phenotypes caused by BRD4 degradation (as the overexpressed KLF5 still lacks the co-activator BRD4). To test this hypothesis, we applied the BRD4 degrader ARV-771 to wild-type and KLF5-overexpressing HARA cells. As expected, BRD4 degradation leads to reductions of cell proliferation and expression of KLF5-target genes in KLF5-reconstituted cells to an extent comparable to wild-type cells, demonstrating that BRD4 is indispensable for the function of KLF5 (Figure 5E).
DISCUSSION
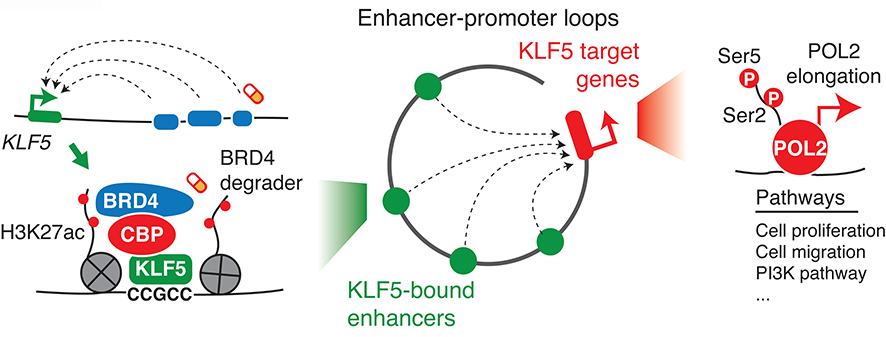
Activation of KLF5 has been associated with cancer-related phenotypes in multiple cancer types, yet the underlying mechanisms remain unclear. We applied integrative omics methodologies to study the role of KLF5 in squamous and gastrointestinal cancers that are associated with KLF5 overexpression and are dependent on the KLF5 gene. We show that KLF5 binds to cancer type-specific regulatory elements and activates gene expression programs essential to these cancer types. KLF5 recruits the CBP acetyltransferase to chromatin to increase levels of the histone modification H3K27ac, which facilitates the recruitment of the bromodomain protein BRD4. Chromatin loops connect these KLF5 binding events to promoters of their target genes, which contributes to activation of POL2 elongation (Figure 6).
Figure 6: A proposed model summarizing the function of KLF5 in cancer cells.
The KLF5 gene itself is activated by super-enhancers. The activated KLF5 binds to enhancers and recruits the CBP/EP300 complex to enhance the binding of BRD4. Chromatin loops aggregate the KLF5 binding events to act on promoters of their target genes, which activates POL2 elongation and transcription. The transcription of the KLF5 gene and the activity of the KLF5 protein are sensitive to BRD4 degraders.
Although KLF5 recognizes the same CG-enriched DNA motif in different cancer types, its binding sites vary between squamous cancers and gastrointestinal cancers. This may be due to the differential distribution of chromatin accessible regions, which are bookmarked by the binding of pioneer factors that are associated with chromatin remodeling capacity. Lineage-specific master regulators such as TP63 and CDX2 have been associated with pioneer factor features in the squamous and gastrointestinal lineages, respectively (60,61). Accordingly, we found that KLF5 binding sites are enriched with DNA motifs recognized by these candidate pioneer factors. In addition, we showed that KLF5 physically interacts with TP63 in squamous cancer cells, suggesting that the candidate pioneer factor may also directly recruit KLF5 to chromatin, a mechanism that has been described for the pioneer factor FOXA1 and the androgen receptor AR in prostate cancer (62). Interestingly, our IP-mass spec results showed that KLF5 itself interacts with several members of the SWI/SNF chromatin remodeling complex, suggesting its own role in regulating chromatin accessibility. Future studies will provide more insights into these possibilities.
Activated transcription factors often bind to a large number of DNA regions across the genome, many of which are distal enhancers. However, it is unclear how such processes impact gene regulation, as it is difficult to link binding sites to their target genes on a genome-wide scale. We applied H3K27ac HiChIP to globally map enhancer-promoter loops, which enabled us to study KLF5 binding events in the 3D genome structure. We showed that chromatin loops cluster multiple KLF5 binding events to act on individual genes. Although each KLF5 binding event is only responsible for part of BRD4’s recruitment to chromatin, multiple such events may act in combination to aggregate higher levels of BRD4 to activate their target genes. We expect clustering of binding events by chromatin loops to be a common mechanism underlying cancer-related functions of overexpressed transcription factors. Indeed, a recent study applying HiChIP in breast cancer cells has found that the binding events of another transcription factor NOTCH1 also act together as clusters to regulate their target genes (63).
Our study justifies KLF5 as a potential therapeutic target in cancer cells. Given their unorganized protein structures, transcription factors are difficult drug targets. We show that PROTAC-mediated BRD4 degradation leads to reductions in expression of the KLF5 gene itself as well as KLF5-target genes, providing an alternative strategy to target KLF5-dependent cancer cells. Targeting BRD4 has been applied in other cancer types with activation of oncogenic transcription factors such as MYCN in neuroblastoma (64) and AR in prostate cancer (56), suggesting a common transcriptional vulnerability. Although the bromodomain protein BRD4 is universally important for diverse cell types, cancer cells often exhibit hypersensitivity to BRD4 inhibition or degradation, which is likely due to aberrant activation of diverse transcription factors in cancer cells. This may provide a unique therapeutic window that can be tolerated by normal cells, which is supported by previous animal studies as well as phase I clinical trials (as summarized in (54)). Our study, along with others, reveal the mechanisms underlying the hypersensitivity to BRD4 inhibition in cancer cells, which may provide more cancer-specific targets for future drug development. The PROTAC system (56,65) has shown a stronger efficiency in inhibiting target proteins such as BRD4, as compared to traditional small molecules. In addition, as opposed to small molecules that need to recognize protein enzymatic domains to fulfill their inhibitory function, ligands that bind to any sites on a target protein can be adapted for the PROTAC system, holding great promise for directly targeting transcription factors like KLF5 in the future.
Supplementary Material
SIGNIFICANCE.
An integrative 3D genomics methodology delineates mechanisms underlying the function of KLF5 in multiple epithelial cancers and suggests potential strategies to target cancers with aberrantly activated KLF5.
ACKNOWLEDGEMENT
We thank Aviad Tsherniak from the Broad Institute and members of the Zhang laboratory for discussions, and the Harvard Medical School Taplin Biological Mass Spectrometry Facility for their mass spectrometry service. X. Zhang is supported by the National Cancer Institute (R00CA215244). S.D. Bailey is supported by a chercheur-boursier junior 1 award from the Fonds de Recherche du Québec – Santé (FRQS) and a Thomlinson award from McGill University. S.D. Bailey received the Dr. Ray Chiu distinguish scientist in surgical research award from the Montreal General Hospital Foundation and a Dr. Henry R. Shibata fellowship from the Cedars Cancer Foundation.
Footnotes
Conflict of interest statement: B. Guo reports being a paid consultant of WuXi AppTec. The work presented here has no relevance to the consulting work the author performs atWuXi AppTec and has not been influenced in any manner in regard to contributions to this manuscript. J.M. Francis reports being a paid employee of Repertoire Immune Medicines (formerly known as Cogen Immune Medicine) and holds stock in that company. The work presented here has no relevance to work the author performs at Repertoire and has not been influenced in any manner in regard to contributions to this manuscript. No disclosures were reported by the other authors.
REFERENCES
- 1.Spitz F, Furlong EEM. Transcription factors: from enhancer binding to developmental control. Nat Rev Genet. 2012;13:613–26. [DOI] [PubMed] [Google Scholar]
- 2.Gorkin DU, Leung D, Ren B. The 3D Genome in Transcriptional Regulation and Pluripotency. Cell Stem Cell. 2014;14:762–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee TI, Young RA. Transcriptional regulation and its misregulation in disease. Cell. 2013;152:1237–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dixon JR, Gorkin DU, Ren B. Chromatin Domains: the Unit of Chromosome Organization. Mol Cell. 2016;62:668–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bhagwat AS, Vakoc CR. Targeting Transcription Factors in Cancer. Trends Cancer. 2015;1:53–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McConnell BB, Yang VW. Mammalian Krüppel-like factors in health and diseases. Physiol Rev. 2010;90:1337–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sun R, Chen X, Yang VW. Intestinal-enriched Krüppel-like factor (Krüppel-like factor 5) is a positive regulator of cellular proliferation. J Biol Chem. 2001;276:6897–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen C, Benjamin MS, Sun X, Otto KB, Guo P, Dong X-Y, et al. KLF5 promotes cell proliferation and tumorigenesis through gene regulation and the TSU-Pr1 human bladder cancer cell line. Int J Cancer. 2006;118:1346–55. [DOI] [PubMed] [Google Scholar]
- 9.Chia N-Y, Deng N, Das K, Huang D, Hu L, Zhu Y, et al. Regulatory crosstalk between lineage-survival oncogenes KLF5, GATA4 and GATA6 cooperatively promotes gastric cancer development. Gut. 2015;64:707–19. [DOI] [PubMed] [Google Scholar]
- 10.Ge Y, Gomez NC, Adam RC, Nikolova M, Yang H, Verma A, et al. Stem Cell Lineage Infidelity Drives Wound-Repair and Cancer. Cell. 2017;169:636–650.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.He P, Yang JW, Yang VW, Bialkowska AB. Krüppel-like Factor 5, Increased in Pancreatic Ductal Adenocarcinoma, Promotes Proliferation, Acinar-to-Ductal Metaplasia, Pancreatic Intraepithelial Neoplasia, and Tumor Growth in Mice. Gastroenterology. 2018;154:1494–1508.e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jia L, Zhou Z, Liang H, Wu J, Shi P, Li F, et al. KLF5 promotes breast cancer proliferation, migration and invasion in part by upregulating the transcription of TNFAIP2. Oncogene. 2016;35:2040–51. [DOI] [PubMed] [Google Scholar]
- 13.Nandan MO, McConnell BB, Ghaleb AM, Bialkowska AB, Sheng H, Shao J, et al. Krüppel-like factor 5 mediates cellular transformation during oncogenic KRAS-induced intestinal tumorigenesis. Gastroenterology. 2008;134:120–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Qin J, Zhou Z, Chen W, Wang C, Zhang H, Ge G, et al. BAP1 promotes breast cancer cell proliferation and metastasis by deubiquitinating KLF5. Nat Commun. 2015;6:8471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nakaya T, Ogawa S, Manabe I, Tanaka M, Sanada M, Sato T, et al. KLF5 regulates the integrity and oncogenicity of intestinal stem cells. Cancer Res. 2014;74:2882–91. [DOI] [PubMed] [Google Scholar]
- 16.Deng N, Goh LK, Wang H, Das K, Tao J, Tan IB, et al. A comprehensive survey of genomic alterations in gastric cancer reveals systematic patterns of molecular exclusivity and co-occurrence among distinct therapeutic targets. Gut. 2012;61:673–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang X, Choi PS, Francis JM, Gao GF, Campbell JD, Ramachandran A, et al. Somatic Superenhancer Duplications and Hotspot Mutations Lead to Oncogenic Activation of the KLF5 Transcription Factor. Cancer Discov. 2018;8:108–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Campbell JD, Yau C, Bowlby R, Liu Y, Brennan K, Fan H, et al. Genomic, Pathway Network, and Immunologic Features Distinguishing Squamous Carcinomas. Cell Rep. 2018;23:194–212.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang X, Choi PS, Francis JM, Imielinski M, Watanabe H, Cherniack AD, et al. Identification of focally amplified lineage-specific super-enhancers in human epithelial cancers. Nat Genet. 2016;48:176–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Campbell JD, Alexandrov A, Kim J, Wala J, Berger AH, Pedamallu CS, et al. Distinct patterns of somatic genome alterations in lung adenocarcinomas and squamous cell carcinomas. Nat Genet. 2016;48:607–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Giannakis M, Mu XJ, Shukla SA, Qian ZR, Cohen O, Nishihara R, et al. Genomic Correlates of Immune-Cell Infiltrates in Colorectal Carcinoma. Cell Rep. 2016;15:857–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dempster JM, Pacini C, Pantel S, Behan FM, Green T, Krill-Burger J, et al. Agreement between two large pan-cancer CRISPR-Cas9 gene dependency data sets. Nat Commun. 2019;10:5817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Meyers RM, Bryan JG, McFarland JM, Weir BA, Sizemore AE, Xu H, et al. Computational correction of copy-number effect improves specificity of CRISPR-Cas9 essentiality screens in cancer cells. Nat Genet. 2017;49:1779–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barretina J, Caponigro G, Stransky N, Venkatesan K, Margolin AA, Kim S, et al. The Cancer Cell Line Encyclopedia enables predictive modelling of anticancer drug sensitivity. Nature. 2012;483:603–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McDonald ER, de Weck A, Schlabach MR, Billy E, Mavrakis KJ, Hoffman GR, et al. Project DRIVE: A Compendium of Cancer Dependencies and Synthetic Lethal Relationships Uncovered by Large-Scale, Deep RNAi Screening. Cell. 2017;170:577–592.e10. [DOI] [PubMed] [Google Scholar]
- 26.Langmead B, Salzberg SL. Fast gapped-read alignment with Bowtie 2. Nat Methods. 2012;9:357–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang Y, Liu T, Meyer CA, Eeckhoute J, Johnson DS, Bernstein BE, et al. Model-based Analysis of ChIP-Seq (MACS). Genome Biol. 2008;9:R137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Quinlan AR, Hall IM. BEDTools: a flexible suite of utilities for comparing genomic features. Bioinforma Oxf Engl. 2010;26:841–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Robinson MD, McCarthy DJ, Smyth GK. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinforma Oxf Engl. 2010;26:139–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Heinz S, Benner C, Spann N, Bertolino E, Lin YC, Laslo P, et al. Simple combinations of lineage-determining transcription factors prime cis-regulatory elements required for macrophage and B cell identities. Mol Cell. 2010;38:576–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li B, Dewey CN. RSEM: accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinformatics. 2011;12:323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dennis G, Sherman BT, Hosack DA, Yang J, Gao W, Lane HC, et al. DAVID: Database for Annotation, Visualization, and Integrated Discovery. Genome Biol. 2003;4:P3. [PubMed] [Google Scholar]
- 33.Supek F, Bošnjak M, Škunca N, Šmuc T. REVIGO Summarizes and Visualizes Long Lists of Gene Ontology Terms. PLoS ONE. 2011;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mumbach MR, Rubin AJ, Flynn RA, Dai C, Khavari PA, Greenleaf WJ, et al. HiChIP: Efficient and sensitive analysis of protein-directed genome architecture. Nat Methods. 2016;13:919–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Servant N, Varoquaux N, Lajoie BR, Viara E, Chen C-J, Vert J-P, et al. HiC-Pro: an optimized and flexible pipeline for Hi-C data processing. Genome Biol. 2015;16:259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lareau CA, Aryee MJ. hichipper: a preprocessing pipeline for calling DNA loops from HiChIP data. Nat Methods. 2018;15:155–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Behan FM, Iorio F, Picco G, Gonçalves E, Beaver CM, Migliardi G, et al. Prioritization of cancer therapeutic targets using CRISPR-Cas9 screens. Nature. 2019;568:511–6. [DOI] [PubMed] [Google Scholar]
- 38.Tsherniak A, Vazquez F, Montgomery PG, Weir BA, Kryukov G, Cowley GS, et al. Defining a Cancer Dependency Map. Cell. 2017;170:564–576.e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nusinow DP, Szpyt J, Ghandi M, Rose CM, McDonald ER, Kalocsay M, et al. Quantitative Proteomics of the Cancer Cell Line Encyclopedia. Cell. 2020;180:387–402.e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sato T, Yoo S, Kong R, Sinha A, Chandramani-Shivalingappa P, Patel A, et al. Epigenomic Profiling Discovers Trans-lineage SOX2 Partnerships Driving Tumor Heterogeneity in Lung Squamous Cell Carcinoma. Cancer Res. 2019;79:6084–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jiang Y-Y, Lin D-C, Mayakonda A, Hazawa M, Ding L-W, Chien W-W, et al. Targeting super-enhancer-associated oncogenes in oesophageal squamous cell carcinoma. Gut. 2017;66:1358–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lundberg AS, Randell SH, Stewart SA, Elenbaas B, Hartwell KA, Brooks MW, et al. Immortalization and transformation of primary human airway epithelial cells by gene transfer. Oncogene. 2002;21:4577–86. [DOI] [PubMed] [Google Scholar]
- 43.Corces MR, Granja JM, Shams S, Louie BH, Seoane JA, Zhou W, et al. The chromatin accessibility landscape of primary human cancers. Science. 2018;362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Guan Y, Wang G, Fails D, Nagarajan P, Ge Y. Unraveling cancer lineage drivers in squamous cell carcinomas. Pharmacol Ther. 2020;206:107448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fang R, Yu M, Li G, Chee S, Liu T, Schmitt AD, et al. Mapping of long-range chromatin interactions by proximity ligation-assisted ChIP-seq. Cell Res. 2016;26:1345–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yeo NC, Chavez A, Lance-Byrne A, Chan Y, Menn D, Milanova D, et al. An enhanced CRISPR repressor for targeted mammalian gene regulation. Nat Methods. 2018;15:611–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Visel A, Blow MJ, Li Z, Zhang T, Akiyama JA, Holt A, et al. ChIP-seq accurately predicts tissue-specific activity of enhancers. Nature. 2009;457:854–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang Z, Teng CT. Phosphorylation of Kruppel-like factor 5 (KLF5/IKLF) at the CBP interaction region enhances its transactivation function. Nucleic Acids Res. 2003;31:2196–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jin Q, Yu L-R, Wang L, Zhang Z, Kasper LH, Lee J-E, et al. Distinct roles of GCN5/PCAF-mediated H3K9ac and CBP/p300-mediated H3K18/27ac in nuclear receptor transactivation. EMBO J. 2011;30:249–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Roe J-S, Mercan F, Rivera K, Pappin DJ, Vakoc CR. BET Bromodomain Inhibition Suppresses the Function of Hematopoietic Transcription Factors in Acute Myeloid Leukemia. Mol Cell. 2015;58:1028–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Phatnani HP, Greenleaf AL. Phosphorylation and functions of the RNA polymerase II CTD. Genes Dev. 2006;20:2922–36. [DOI] [PubMed] [Google Scholar]
- 52.Winter GE, Mayer A, Buckley DL, Erb MA, Roderick JE, Vittori S, et al. BET Bromodomain Proteins Function as Master Transcription Elongation Factors Independent of CDK9 Recruitment. Mol Cell. 2017;67:5–18.e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yang Z, Yik JHN, Chen R, He N, Jang MK, Ozato K, et al. Recruitment of P-TEFb for stimulation of transcriptional elongation by the bromodomain protein Brd4. Mol Cell. 2005;19:535–45. [DOI] [PubMed] [Google Scholar]
- 54.Xu Y, Vakoc CR. Targeting Cancer Cells with BET Bromodomain Inhibitors. Cold Spring Harb Perspect Med. 2017;7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lovén J, Hoke HA, Lin CY, Lau A, Orlando DA, Vakoc CR, et al. Selective inhibition of tumor oncogenes by disruption of super-enhancers. Cell. 2013;153:320–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Raina K, Lu J, Qian Y, Altieri M, Gordon D, Rossi AMK, et al. PROTAC-induced BET protein degradation as a therapy for castration-resistant prostate cancer. Proc Natl Acad Sci U S A. 2016;113:7124–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bass AJ, Watanabe H, Mermel CH, Yu S, Perner S, Verhaak RG, et al. SOX2 is an amplified lineage-survival oncogene in lung and esophageal squamous cell carcinomas. Nat Genet. 2009;41:1238–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Long A, Giroux V, Whelan KA, Hamilton KE, Tétreault M-P, Tanaka K, et al. WNT10A promotes an invasive and self-renewing phenotype in esophageal squamous cell carcinoma. Carcinogenesis. 2015;36:598–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rocco JW, Leong C-O, Kuperwasser N, DeYoung MP, Ellisen LW. p63 mediates survival in squamous cell carcinoma by suppression of p73-dependent apoptosis. Cancer Cell. 2006;9:45–56. [DOI] [PubMed] [Google Scholar]
- 60.Santos-Pereira JM, Gallardo-Fuentes L, Neto A, Acemel RD, Tena JJ. Pioneer and repressive functions of p63 during zebrafish embryonic ectoderm specification. Nat Commun. 2019;10:3049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Verzi MP, Shin H, San Roman AK, Liu XS, Shivdasani RA. Intestinal master transcription factor CDX2 controls chromatin access for partner transcription factor binding. Mol Cell Biol. 2013;33:281–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gao N, Zhang J, Rao MA, Case TC, Mirosevich J, Wang Y, et al. The role of hepatocyte nuclear factor-3 alpha (Forkhead Box A1) and androgen receptor in transcriptional regulation of prostatic genes. Mol Endocrinol Baltim Md. 2003;17:1484–507. [DOI] [PubMed] [Google Scholar]
- 63.Petrovic J, Zhou Y, Fasolino M, Goldman N, Schwartz GW, Mumbach MR, et al. Oncogenic Notch Promotes Long-Range Regulatory Interactions within Hyperconnected 3D Cliques. Mol Cell. 2019;73:1174–1190.e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Puissant A, Frumm SM, Alexe G, Bassil CF, Qi J, Chanthery YH, et al. Targeting MYCN in Neuroblastoma by BET Bromodomain Inhibition. Cancer Discov. 2013;3:308–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Winter GE, Buckley DL, Paulk J, Roberts JM, Souza A, Dhe-Paganon S, et al. DRUG DEVELOPMENT. Phthalimide conjugation as a strategy for in vivo target protein degradation. Science. 2015;348:1376–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.