Abstract
Background:
Female runners are at increased risk of stress fractures (SFs) compared with men. Literature is lacking with regard to best practice for preventing and treating SFs in women. The purpose of the study was to compare physiological measures and running-related factors between women of various ages and running abilities with and without a history of running-related SFs.
Hypothesis:
Women with and without SF histories will differ with regard to medical and menstrual history, bone health, body composition, nutrition, and running history.
Study Design:
Prospective cohort study.
Level of Evidence:
Level 2.
Methods:
A total of 20 female runners with SF histories were matched based on age and running distance with 20 women without SF histories. Data included medical, menstrual, running, injury, and nutritional histories; blood histology related to nutritional, hormonal, and bone-related risk factors; and bone density, fat, and lean tissue using dual energy x-ray absorptiometry. Paired t tests were used to examine differences between women with and without SF histories, and Spearmen correlations were conducted to examine relationships between physiological factors.
Results:
Women with SF histories had lower hip bone mineral density compared with women without SF histories (P < 0.05). SF history was moderately correlated with menstrual changes during increased training times (r = 0.580; P < 0.0001) but was not correlated with any other physiological factor. There was a moderate correlation within the SF group (r = 0.65; P = 0.004) for bone markers for resorption and formation both increasing, indicating increased bone turnover.
Conclusion:
Female runners with low hip bone mineral density, menstrual changes during peak training, and elevated bone turnover markers may be at increased risk of SF.
Clinical Relevance:
Female runners need routine screening for risks associated with SF occurrence. As bone mineral density and bone turnover markers are not routinely assessed in this population, important risk factors may be missed.
Keywords: running, female, stress fracture, bone density
Stress fractures (SFs) are nontraumatic incomplete fractures resulting from repetitive loading on normal bone or from normal loading on abnormal bone.11 Running-related SFs account for 69% of all SFs, with 95% occurring in the lower extremities and pelvis.11 Women have at least 2 times greater risk than men,13,16 and more women than men are now running. In the 2018 National Runner Survey, runners were 54% female, 52% of all runners were between the ages of 35 and 54 years, and 60% considered themselves frequent fitness runners.29
The risk factors for SFs in women are multifactorial and include differences in anatomy, body composition, metabolism, the cardiovascular system, hormonal status, and psychological status compared with men.16 Both intrinsic and extrinsic factors contribute to the occurrence of SFs. Intrinsic factors are physiological11 and include bone structure and density, decreased fat in relation to lean tissue, and nutritional, hormonal, and bone-related health status. Menstrual irregularities and energy deficiency due to an imbalance between nutritional intake and activity are often present.22 Women also have greater risks due to the female athlete triad, a negative energy balance between nutritional intake and activity that can lead to menstrual issues and decreased bone mineral density, showing the interrelationships of these factors.20 Both pre- and postmenopausal women are at risk.20,26 Extrinsic factors include training intensity, training surfaces, diet, and footwear.11
The literature is lacking with regard to the best practice for preventing and treating SFs in women. Surprisingly, few studies4,27 directly evaluate women with and without a history of SFs to assist in better assessing risk and developing preventative strategies. There are several articles related to risk factors,11,13,16,20,23 a few case reports with female runners,3,10,12,18 and a few observational15 and experimental studies.4,21,27,30 These studies examine various factors including bone density, nutritional status, biomechanics, and menstrual status. Overall these studies show some relationships between these factors. Some limitations include small sample sizes in most studies, inclusion of only high-level adolescent or young female runners, and mixed populations (male/female or different sports). Because of these limitations and the increased risk for SF for women, there is a significant need to better understand issues related to SFs to prevent and properly treat these injuries to optimize return to running, overall health, and participation. The issue is not limited to women of a specific age as hormonal issues affect all female runners, thus making it important to not limit studies to young, elite runners. Therefore, the objective of this study was to compare important physiological measures between women with and without running-related SF histories of various ages and running abilities. The hypothesis was that there would be differences related to medical and menstrual history, bone health, body composition, nutrition, and running history.
Methods
Female runners (age range, 18-65 years) with and without running-related SF histories were recruited for this study held within an urban university hospital system over a 5-month period via posted flyers and social media. A variety of social media sites were identified to decrease possible selection bias. Women self-identified as runners, with no upper or lower limit set for running intensity, duration, or distance. To control for differences in age and running ability, after each woman with a SF history was enrolled in the study, a woman without SF history was recruited, who was age-matched within 5 years and distance-matched within 10 miles per week.5,31 All enrolled women signed a written informed consent form approved by the governing institutional review board. Women with SF were included if they had an SF at any time as runners. Women with and without SF histories were excluded if they had a neurologic diagnosis or any systemic medical condition that would affect bone health, were pregnant, or were breastfeeding.
Data collection included background information and physiological measures. Participants completed an online questionnaire (Qualtrics) to collect demographics as well as medical, menstrual, running, injury, and nutritional histories. To examine physiological data on nutritional, hormonal, and bone-related risk factors,8 the following nonfasting serum histological measures were collected and processed using standard medical laboratory procedures: complete blood count, vitamin D (25-(OH)D), calcium, albumin, parathyroid hormone, estradiol, testosterone, bone-specific alkaline phosphatase (BALP; measure of bone formation),6 and N-telopeptide (N-Tx; measure of bone resorption).6 To examine bone, fat, and lean tissue, dual energy x-ray absorptiometry (DXA)9 was used to measure areal bone mineral density (aBMD) of the left hip and the lumbar spine, and full body composition was obtained using a Hologic Horizon A scanner (Hologic). The DXA machine was calibrated prior to each testing session to decrease measurement error. A negative pregnancy test was required prior to conducting the DXA for all participants.
To examine differences between women with and without SF histories, paired t tests were conducted using SPSS Statistics (Version 25; IBM Corp). Cohen d was calculated to determine effect size. Spearman correlations were performed to examine possible relationships between group and physiological factors and among different physiological factors. Because of the lack of data available on medical and menstrual history, bone health, body composition, nutrition, and running history that span the age ranges included, a sample of 20 per group was chosen based on differences in bone turnover, body mass, and estradiol levels seen in a study with 37 adolescent runners.2 Effect sizes were thus calculated for measures in this study.
Results
A total of 49 women were screened for inlcusion in this study. Two women with SF histories were excluded due to thyroid disease, and 5 eligible women without SF histories were excluded as they did not match with a woman with an SF. Therefore, 42 women (mean age, 35.0 ± 7.4 years; range, 22-50 years) were enrolled in the study. Two participants withdrew after signing the consent form due to time constraints, and data are therefore complete for 40 participants, or 20 matched pairs. Data were complete for all participants expect for 1 in the SF group who was missing the albumin value and 2 in the non-SF group who were missing N-Tx values. These data and the matched-pair values were thus excluded from data analysis.
The oldest enrolled woman was 50 years old, and she was the only participant who was postmenopausal. Her match with SF history was perimenopausal. Women were highly educated and predominately white (Table 1). Women with SF histories were 2.2 ± 2.6 years after their most recent fracture (range, 0.8-10 years), with 10 having suffered a fracture within the past year, 5 in the past 1 to 3 years, and 5 in more than 5 years prior. Fracture sites included the tibia (n = 15), metatarsal (n = 8), femur (n = 5), cuneiform (n = 1), and sesamoid (n = 1), with 6 participants reporting having had 2 SFs and 2 participants reporting 3 SFs.
Table 1.
Participant demographics
| Variable | Choice | Stress Fracture Group, n | Nonfracture Group, n |
|---|---|---|---|
| Age | Years, mean ± SD | 35.1 ± 7.2 | 34.4 ± 7.7 |
| Highest educational degree | Bachelor’s | 7 | 7 |
| Master’s | 6 | 9 | |
| Doctoral | 7 | 4 | |
| Race | Asian | 0 | 3 |
| Hispanic | 1 | 1 | |
| White | 19 | 16 |
Tables 2 and 3 show self-reported information for running and menstrual status, respectively, and there were no differences (P = 0.57 to >0.999) between groups for these data. Groups were also evenly distributed with regard to birth control use and type, and for the number of participants who had ever gone >3 months without a period other than during pregnancy (6 per group). However, 12 women who had an SF reported that their menstrual periods changed during increased training times, while only 1 reported this occurring in the non-SF group. Age when started running did not differ between groups, yet 9 women with SF histories started running at 18 years or younger, while only 4 without SF histories started this young.
Table 2.
Running status a
| Variable | Choice | Stress Fracture Group, n | Nonfracture Group, n | P |
|---|---|---|---|---|
| Days per week | 2 | 0 | 1 | 0.96 |
| 3 | 11 | 7 | ||
| 4 | 4 | 4 | ||
| 5 | 2 | 5 | ||
| 6 | 2 | 1 | ||
| 7 | 1 | 2 | ||
| Miles per week | 0-10 | 1 | 1 | 0.88 |
| 11-20 | 6 | 9 | ||
| 21-30 | 6 | 6 | ||
| 31-40 | 4 | 2 | ||
| 41-50 | 1 | 1 | ||
| >50 | 2 | 1 | ||
| Average running pace, min/mile | <6 | 1 | 0 | 0.98 |
| 6-7 | 0 | 1 | ||
| 7-8 | 6 | 2 | ||
| 8-9 | 2 | 6 | ||
| 9-10 | 7 | 4 | ||
| 10-11 | 4 | 5 | ||
| >11 | 0 | 2 | ||
| Age when started running, y | <10 | 3 | 1 | 0.96 |
| 11-18 | 6 | 3 | ||
| 19-25 | 2 | 9 | ||
| 26-33 | 5 | 7 | ||
| 34-40 | 3 | 0 | ||
| >40 | 1 | 0 |
No differences were found between groups (P > 0.05) for any variable (chi-square analysis).
Table 3.
Menstrual status a
| Variable | Choice | Stress Fracture Group, n | Nonfracture Group, n | P |
|---|---|---|---|---|
| Age at first menstrual cycle, y | 9-10 | 1 | 2 | >0.999 |
| 11-12 | 9 | 8 | ||
| 13-14 | 6 | 8 | ||
| 15-16 | 4 | 2 | ||
| Menstrual cycle length, days | ≤29 | 11 | 13 | >0.999 |
| 30-35 | 2 | 1 | ||
| ≥36 | 1 | 1 | ||
| Irregular | 6 | 4 | ||
| Absent | 0 | 1 | ||
| Menstrual cycle length, days | N/A | 0 | 1 | 0.57 |
| 1-2 | 1 | 2 | ||
| 3-4 | 9 | 9 | ||
| 5-6 | 8 | 4 | ||
| 7-8 | 0 | 3 | ||
| ≥8 | 0 | 0 | ||
| No answer | 2 | 1 |
No differences were found between groups (P > 0.05) for any variable (chi-square analysis). N/A, not applicable.
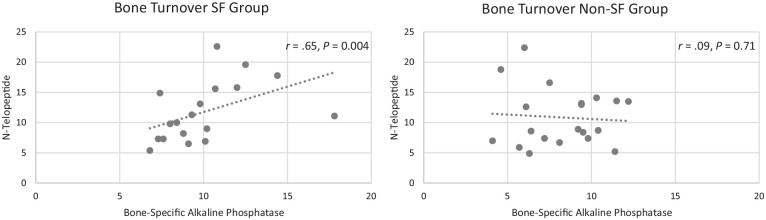
In comparing physiological measures between women with and without SF histories (Table 4), the only statistical difference was for hip aBMD, with lower aBMD in women with an SF history, but the effect size for this difference was low (0.19). The measure with the largest effect size (0.61) was BALP, but the difference between groups was not statistically significant. Correlational analysis showed that time after fracture was unrelated to bone markers (BALP, N-Tx) and that hip aBMD was unrelated to any other physiological factor. SF history was moderately correlated with menstrual changes during increased training times (r = 0.580; P < 0.0001) but was not correlated with any other physiological factor. While there was a low correlation between BALP and N-Tx when looking at all participants together (r = 0.34; P = 0.03), there was a moderate correlation within the SF group with BALP and N-Tx increasing together (r = 0.65; P = 0.004) (Figure 1), indicating increased bone turnover.
Table 4.
Blood histological, bone density, and body composition results
| Measure | Normal Range | Stress Fracture Group, Mean ± SD | Nonfracture Group, Mean ± SD | P | Effect Size |
|---|---|---|---|---|---|
| Albumin, g/dL | 3.2-4.9 g/dL | 4.3 ± 0.3 | 4.4 ± 0.2 | 0.21 | 0.40 |
| Vitamin D, pg/mL | 18-72 pg/mL | 51.0 ± 10.0 | 51.8 ± 21.6 | 0.88 | 0.04 |
| Calcium, mg/dL | 8.5-10.3 | 9.3 ± 0.3 | 9.3 ± 0.3 | 0.73 | 0.11 |
| Estradiol, pg/mL | 12.5-498 a | 76.1 ± 105 | 50.6 ± 67.0 | 0.35 | 0.29 |
| Testosterone, ng/dL | 2-45 | 18.8 ± 8.2 | 19.1 ± 7.8 | 0.90 | 0.03 |
| Parathyroid hormone, pg/mL | 11-67 | 36.7 ± 14.2 | 34.8 ± 9.2 | 0.64 | 0.16 |
| Bone-specific alkaline phosphatase, µg/L | 5.0-18.8 | 9.9 ± 2.7 | 8.3 ± 2.4 | 0.09 | 0.61 |
| N-telopeptide, mg/dL | 6.2-19.0 | 11.8 ± 5.0 | 11.1 ± 4.9 | 0.67 | 0.15 |
| Spine bone mineral density, g/cm2 | N/A b | 1.0 ± 0.09 | 1.0 ± 0.11 | 0.15 | 0.44 |
| Hip bone mineral density, g/cm2 | N/A b | 0.9 ± 0.1 | 1.0 ± 0.1 | 0.03 c | 0.19 |
| Fat percentage | N/A b | 31.2 ± 6.1 | 31.0 ± 5.0 | 0.94 | 0.02 |
| Body mass index, kg/m2 | 18.5-24.9 | 22.4 ± 2.8 | 23.2 ± 2.9 | 0.36 | 0.28 |
N/A, not applicable.
Premenopausal, influenced by menstrual cycle phase.
N/A as normal is based on age and percentiles.
Statistically significant difference.
Figure 1.
Bone turnover for each group. There was a moderate correlation within the stress fracture group between bone resorption (N-telopeptide) and bone formation (bone-specific alkaline phosphatase) but not within the non–stress fracture group. This finding indicates increased bone turnover in the stress fracture group.
Discussion
The main results from this study were that women with an SF history had lower hip aBMD than their matched counterparts without an SF history and that women with an SF history had alterations in their typical menstrual cycles during more intense training times even though current estradiol levels did not differ between groups. The study was conducted during the months of March to June, which represented mainly off- to early-season training for the included women. Within the SF group, there was a correlation between bone formation and resorption that was not seen within the non-SF group, indicating increased bone turnover.17 Of note, DXA for bone density and blood histology for examining bone resorption and formation markers are not routinely performed in this population, thus important information may be missed clinically in these women. As DXA is relatively inexpensive with low radiation exposure, performing DXA in this population may be cost-effective. The more expensive tests for bone resorption and formation markers may then be performed based on concerning findings via DXA. Asking female runners about any menstrual cycle changes during heavier training times may be an important addition to a patient interview. Women who had these changes reported lighter flow, shorter duration, increased spotting, irregularity, and missed cycles.
Several studies have examined menstrual dysfunction in relation to bone but primarily in a younger population. Ackerman et al1 reported decreased spine and whole body aBMD and altered bone structure in 14- to 25-year-old female athletes with oligoamenorrhea (6 cycles or less in prior year), with greater changes seen in participants with more than 1 SF. In a study that included collegiate cross-country runners, Tenforde et al30 reported that oligoamenorrhea or amenorrhea and a prior SF were predictors of subsequent bone stress injuries. A small percentage of participants had low aBMD, with more than half of them being runners. Nose-Ogura et al24 found a relationship between amenorrhea in the teenage years and aBMD in the 20s for female athletes, which included distance runners, suggesting the need for intervention at a younger age. While these studies provide important information for female runners in these younger age groups, women older than 25 years represent a large number of runners. As bone mass starts to decline between 20 and 30 years of age for women,7 issues specific to these women must also be addressed. Micklesfield et al22 studied 613 long-distance (half-marathon and ultramarathon) female runners aged 16 to 62 years, of whom 17.3% had sustained a bone stress injury, but found no differences between these women and women without these injuries with regard to age, weight, body mass index, or menstrual function. They also found that over half of all the 613 women reported menstrual dysfunction. Thus, further study is needed to better understand the risks. These studies that relate menstrual status and aBMD as well as the results of this current study indicate the need to evaluate and treat female runners for these issues early and to continue to evaluate changes over time.
While there were no differences in estrogen levels between women with and without SF histories, some women in the study had very low estrogen levels. The low end of the normal range for estrogen levels is 24 pg/mL. Four women with SF histories and 8 without had very low values (<5 pg/mL), and 2 in each group had low values (8-23 pg/mL). The significance of these low values is difficult to determine in this small sample as the women with and without SF histories were equally affected. Estrogen levels fluctuate during the menstrual cycle,28 and data were not collected regarding menstrual phase in this study. To gather cyclical data on female runners would require measures of estrogen levels to be collected throughout the menstrual cycle to identify patterns.28 Assessing estrogen levels across the menstrual cycle is thus recommended for future studies.
The bone turnover markers of N-Tx and BALP, as measured in this study, are not routinely assessed in female runners but may play a role in assessing risk. While these measures were not statistically significantly different between groups in this study, there was a correlation between increased bone formation and resorption in the SF group, indicating increased bone turnover.17 In a literature review of studies on postmenopausal women by Vasikaran et al,32 several studies reported that an increase in bone turnover markers led to an additive effect on the risk for fractures and that increased bone turnover markers may predict fracture risk independently of aBMD. While the population in that study32 differs from the female runners in this study, the use of these markers may be beneficial and more research is warranted. In a sample of adolescent female cross-country runners, elevated bone markers were associated with a lower body mass index, menstrual irregularities, and lower estradiol and vitamin D levels.2 In contrast, Fujita et al14 measured bone resorption (urine N-Tx) twice per year in a small sample of female runners aged 19 to 34 years and found that while N-Tx values were normal during training, they increased when an SF occurred. These findings suggest that N-Tx may be a noninvasive way to identify SFs and monitor healing. A review article by Papageorgiou et al25 reported that short-term low energy availability can also elevate bone markers, thus several factors need to be considered when using bone markers to guide diagnosis and return to running after an SF. Finally, there is mixed opinion as to the effect of increased turnover. While increased formation temporarily increases bone porosity and decreases stiffness, it may also induce microdamage repair following bone stress.19 Thus, more research is needed on the interpretation of these bone markers clinically.
Clinical Significance
For female runners aged 20 to 50 years of age with varying running abilities, it is recommended that screening of intrinsic and extrinsic risk factors be performed to determine potential risks for SF. Based on the research of others, these factors include nutritional, hormonal,11 and menstrual irregularities; energy deficiency22; training intensity; training surfaces; diet; and footwear.11 Testing of aBMD is also recommended based on this study and others,11 especially for those women who report menstrual changes as intensity, frequency, and/or duration of running increase. While women with these changes may be at increased risk, DXA is encouraged for all female runners to better inform them about potential increased risks and educate them on prevention. Histological measures of bone turnover should also be considered for those with increased risk.
Limitations
In this study, a physical examination was not performed, as the goal was to gather physiological factors rather than specific musculoskeletal impairments. Korpelainen et al21 reported that the risks of recurrent SFs across multiple sites may include a high weekly training mileage, a leg length difference, a high longitudinal arch of the foot, and forefoot varus in addition to menstrual dysfunction. Thus, these factors may be important to consider in the examination of runners clinically along with the measures collected in this study. As the current study controlled for running distance through matching of participants, the impact of mileage cannot be determined.
Other study limitations include the small sample size, which could potentially affect the ability to obtain statistical significance. Matching women based on age and running distance likely reduced some of the impact of small sample size. The sample was also one of convenience and thus may not represent the population of female runners as a whole. The women in this study also spanned a wide age range. However, despite this heterogeneity of age, differences were found between groups.
Conclusion
Based on the results of this study, measurement of aBMD, bone turnover markers, and menstrual change data during training may be important additions to the clinical examination of female runners. More research is needed on the role of bone turnover markers in assessing risk of SFs and return to running after SF.
Supplemental Material
Supplemental material, SF_in_female_runners for Physiological Factors of Female Runners With and Without Stress Fracture Histories: A Pilot Study by Therese E. Johnston, Colleen Dempsey, Frances Gilman, Ryan Tomlinson, Ann-Katrin Jacketti and Jeremy Close in Sports Health: A Multidisciplinary Approach
Acknowledgments
The authors thank the women who participated in the study and Dr Johnston’s graduate research assistants: Mitchell Anhorn, DPT; Kristen N. Frank, DPT; and Ashley Lukacsko, DPT.
Footnotes
The authors report no potential conflicts of interest in the development and publication of this article.
References
- 1. Ackerman KE, Cano SN, DE Nardo MG, et al. Fractures in relation to menstrual status and bone parameters in young athletes. Med Sci Sports Exerc. 2015;47:1577-1586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Barrack MT, Van Loan MD, Rauh MJ, Nichols JF. Physiologic and behavioral indicators of energy deficiency in female adolescent runners with elevated bone turnover. Am J Clin Nutr. 2010;92:652-659. [DOI] [PubMed] [Google Scholar]
- 3. Battaglia M, Guaraldi F, Vannini F, et al. Unusual supero-medial iliac fatigue stress fracture. Skeletal Radiol. 2012;41:103-106. [DOI] [PubMed] [Google Scholar]
- 4. Becker J, James S, Osternig L, Chou LS. Foot kinematics differ between runners with and without a history of navicular stress fractures. Orthop J Sports Med. 2018;6:2325967118767363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bennell KL, Malcolm SA, Brukner PD, et al. A 12-month prospective study of the relationship between stress fractures and bone turnover in athletes. Calcif Tissue Int. 1998;63:80-85. [DOI] [PubMed] [Google Scholar]
- 6. Bergmann P, Body JJ, Boonen S, et al. Evidence-based guidelines for the use of biochemical markers of bone turnover in the selection and monitoring of bisphosphonate treatment in osteoporosis: a consensus document of the Belgian Bone Club. Int J Clin Pract. 2009;63:19-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Borer KT. Physical activity in the prevention and amelioration of osteoporosis in women: interaction of mechanical, hormonal and dietary factors. Sports Med. 2005;35:779-830. [DOI] [PubMed] [Google Scholar]
- 8. Chen YT, Tenforde AS, Fredericson M. Update on stress fractures in female athletes: epidemiology, treatment, and prevention. Curr Rev Musculoskelet Med. 2013;6:173-181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chun KJ. Bone densitometry. Semin Nucl Med. 2011;41:220-228. [DOI] [PubMed] [Google Scholar]
- 10. Cooper LA, Joy EA. Osteoporosis in a female cross-country runner with femoral neck stress fracture. Curr Sports Med Rep. 2005;4:321-322. [DOI] [PubMed] [Google Scholar]
- 11. Denay KL. Stress fractures. Curr Sports Med Rep. 2017;16:7-8. [DOI] [PubMed] [Google Scholar]
- 12. Dugowson CE, Drinkwater BL, Clark JM. Nontraumatic femur fracture in an oligomenorrheic athlete. Med Sci Sports Exerc. 1991;23:1323-1325. [PubMed] [Google Scholar]
- 13. Fields KB. Running injuries—changing trends and demographics. Curr Sports Med Rep. 2011;10:299-303. [DOI] [PubMed] [Google Scholar]
- 14. Fujita S, Sakuraba K, Kubota A, et al. Stress fracture influences bone resorption marker (u-NTX) in female long distance runners. Int J Sports Med. 2017;38:1070-1075. [DOI] [PubMed] [Google Scholar]
- 15. Giffin KL, Knight KB, Bass MA, Valliant MW. Predisposing risk factors and stress fractures in Division I cross country runners [published online November 11, 2017]. J Strength Cond Res. doi: 10.1519/JSC.0000000000002408 [DOI] [PubMed] [Google Scholar]
- 16. Goldring AE, Ashok AP, Casey EK, Mulcahey MK. Key components and potential benefits of a comprehensive approach to women’s musculoskeletal health. Phys Sportsmed. 2016;44:417-424. [DOI] [PubMed] [Google Scholar]
- 17. Hamwi A, Ganem AH, Grebe C, et al. Markers of bone turnover in postmenopausal women receiving hormone replacement therapy. Clin Chem Lab Med. 2001;39:414-417. [DOI] [PubMed] [Google Scholar]
- 18. Hoglund LT, Silbernagel KG, Taweel NR. Distal fibular stress fracture in a female recreational runner: a case report with musculoskeletal ultrasound imaging findings. Int J Sports Phys Ther. [PMC free article] [PubMed] [Google Scholar]
- 19. Hughes JM, Popp KL, Yanovich R, et al. The role of adaptive bone formation in the etiology of stress fracture. Exp Biol Med (Maywood). 2016;242:897-906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Joy EA, Campbell D. Stress fractures in the female athlete. Curr Sports Med Rep. 2005;4:323-328. [DOI] [PubMed] [Google Scholar]
- 21. Korpelainen R, Orava S, Karpakka J, et al. Risk factors for recurrent stress fractures in athletes. Am J Sports Med. 2001;29:304-310. [DOI] [PubMed] [Google Scholar]
- 22. Micklesfield LK, Hugo J, Johnson C, et al. Factors associated with menstrual dysfunction and self-reported bone stress injuries in female runners in the ultra- and half-marathons of the Two Oceans. Br J Sports Med. 2007;41:679-683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Moreira CA, Bilezikian JP. Stress fractures: concepts and therapeutics. J Clin Endocrinol Metab. 2017;102:525-534. [DOI] [PubMed] [Google Scholar]
- 24. Nose-Ogura S, Yoshino O, Dohi M, et al. Low bone mineral density in elite female athletes with a history of secondary amenorrhea in their teens. Clin J Sport Med. 2020;30(3):245-250. [DOI] [PubMed] [Google Scholar]
- 25. Papageorgiou M, Dolan E, Elliott-Sale KJ, Sale C. Reduced energy availability: implications for bone health in physically active populations. Eur J Nutr. 2018;57:847-859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Pegrum J, Crisp T, Padhiar N, Flynn J. The pathophysiology, diagnosis, and management of stress fractures in postmenopausal women. Phys Sportsmed. 2012;40:32-42. [DOI] [PubMed] [Google Scholar]
- 27. Popp KL, McDermott W, Hughes JM, et al. Bone strength estimates relative to vertical ground reaction force discriminates women runners with stress fracture history. Bone. 2017;94:22-28. [DOI] [PubMed] [Google Scholar]
- 28. Redman LM, Loucks AB. Menstrual disorders in athletes. Sports Med. 2005;35:747-755. [DOI] [PubMed] [Google Scholar]
- 29. Running USA. 2018. National Runner Survey. https://www.runningusa.org/RUSA/News/2018/2018_National_Runner_Survey_Results_Released_by_Running_USA. Accessed August 23, 2019.
- 30. Tenforde AS, Carlson JL, Chang A, et al. Association of the female athlete triad risk assessment stratification to the development of bone stress injuries in collegiate athletes. Am J Sports Med. 2017;45:302-310. [DOI] [PubMed] [Google Scholar]
- 31. Tenforde AS, Sayres LC, McCurdy ML, et al. Identifying sex-specific risk factors for stress fractures in adolescent runners. Med Sci Sports Exerc. 2013;45:1843-1851. [DOI] [PubMed] [Google Scholar]
- 32. Vasikaran S, Eastell R, Bruyere O, et al. Markers of bone turnover for the prediction of fracture risk and monitoring of osteoporosis treatment: a need for international reference standards. Osteoporos Int. 2011;22:391-420. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, SF_in_female_runners for Physiological Factors of Female Runners With and Without Stress Fracture Histories: A Pilot Study by Therese E. Johnston, Colleen Dempsey, Frances Gilman, Ryan Tomlinson, Ann-Katrin Jacketti and Jeremy Close in Sports Health: A Multidisciplinary Approach