Graphical abstract
Keywords: Polyethylene glycol, Antidrug antibody, Immunotoxicity, Flow cytometry
Highlights
-
•
A rapid, sensitive, and specific flow cytometry assay was developed to detect anti-PEG IgG and IgM in human blood plasma.
-
•
Using the method, anti-PEG IgG or IgM were detected in 65% of plasma samples from 300 healthy blood donors.
-
•
The presence of anti-PEG IgG and IgM was confirmed using three validation assays.
-
•
The highest prevalence of both anti-IgG and anti-IgM was in individuals 18–24 years of age.
-
•
No correlation was found between anti-PEG IgG and IgM concentrations.
Abstract
Polyethylene glycol (PEG) is a biocompatible polymer used in biotherapeutics to increase bioavailability, reduce the frequency of administration, and optimize pharmacokinetics. Anti-PEG antibodies have been detected in healthy individuals and may decrease efficacy and alter the pharmacokinetics of PEGylated therapeutics; however, the prevalence of anti-PEG antibodies is unclear. In this study, a flow cytometry assay was optimized to detect anti-PEG IgG and IgM in human blood plasma. Three hundred (300) plasma samples from healthy blood donors were screened; anti-PEG IgG or IgM was detected in 65.3% of the total population, with 21.3% having anti-PEG IgG, 19.0% having anti-PEG IgM, and 25.0% having both anti-PEG IgG and IgM. The presence of anti-PEG IgG and IgM was confirmed using a 0.5% Tween-20 interference assay, a 20 kDa PEGylated polystyrene bead binding assay, and Western blotting of purified plasma from human IgG and IgM purification columns. The concentrations of anti-PEG IgG and IgM in positive samples ranged from 39 ng/mL to 18.7 μg/mL and 26 ng/mL to 11.6 μg/mL, respectively. The highest prevalence of both anti-IgG and anti-IgM was in individuals 18–24 years of age. The prevalence of anti-PEG IgG and IgM tended to be higher in women but did not differ among races. Age, sex, and race were not associated with the concentrations of anti-PEG IgG or IgM. No correlation was found between anti-PEG IgG and IgM concentrations. Our study indicates that flow cytometry can be used to detect anti-PEG IgG and IgM antibodies in human plasma.
1. Introduction
Polyethylene glycol (PEG) is a biocompatible synthetic polymer composed of repeating ethylene oxide subunits that is used in PEGylated therapeutics, nonprescription medicines, cosmetics, personal care and household cleaning products, and foods [1,2]. PEGylation is the process of either covalently or non-covalently linking a high-molecular-weight (MW) PEG to an agent (drug or therapeutic peptide/protein), and is commonly used to increase the serum half-life of drugs or proteins/peptides, improve efficacy, and reduce the immunogenicity of the proteins/peptides [3]. To date, at least 20 PEGylated therapeutics have been approved for use in humans [4] and most of them have been used in the treatment of various diseases for over a decade.
Although PEG is generally considered to be non-immunogenic, several reports have demonstrated a potential immunogenicity of PEG [5,6]. An early study observed antibody formation against PEG in rabbits immunized with PEG-linked proteins [7]. A single intravenous administration of PEGylated bovine serum albumin, ovalbumin, or adenovirus produced an anti-PEG IgM response in Wistar rats [8]. Repeat injections of PEGylated solid lipid nanoparticles in mice and beagles induced an unexpected anti-PEG immunogenic phenomenon of accelerated blood clearance [9,10].
Anti-PEG antibodies have been detected in patients after exposure to PEGylated therapeutics [[11], [12], [13], [14], [15]]. In most instances, the anti-PEG antibodies had little to no impact on the safety or efficacy of the therapeutics. However, in some situations, anti-PEG antibodies altered the pharmacokinetics, including half-life, clearance, maximum concentration, and area-under-the-curve, and affected the efficacy of the PEGylated agents [11,[16], [17], [18], [19]]. Moreover, anti-PEG antibodies have been linked to acute severe allergic reactions to the PEGylated RNA aptamer pegnivacogin [20,21]. They have also been associated with adverse effects, such as gout flares and mild-to-moderate pain, cellulitis, and urticaria at the injection site following subcutaneous injection of mammalian PEG-uricase [11]. Thus, anti-PEG antibodies may be a concern for the efficacy and safety of PEGylated therapeutics.
Unlike other antidrug antibodies, anti-PEG antibodies in humans have been found in healthy individuals who have never been treated with PEGylated therapeutics [22]. The existence of anti-PEG antibodies in healthy untreated individuals could also be a significant factor affecting the efficacy and safety of PEGylated therapeutics. Thus, recent FDA guidelines recommend screening of anti-PEG antibodies in patients when evaluating the potential immune response of PEGylated therapeutics [23].
Assays for analyzing and characterizing anti-PEG antibodies are still being developed. The prevalence of anti-PEG antibodies has varied dramatically from < 1% to 72% in healthy untreated individuals [16,20,[24], [25], [26]]. This variation may be due to differences among subjects and to the use of a variety of assays, such as immunodiffusion, passive hemagglutination, bridging assays, and enzyme linked immunosorbent assays (ELISAs), and competitive ELISAs, that can have different sensitivities and specificities [7,8,14,24,[26], [27], [28]]. The lack of positive human anti-PEG antibody standards also makes it difficult to compare results of anti-PEG antibodies from different studies. Recently, a few positive chimeric human anti-PEG antibodies have been generated and characterized [24,26]. In the current study, we optimized a flow cytometry assay [17] to analyze the prevalence and concentrations of anti-PEG antibodies in human plasma and applied the method to 300 plasma samples from healthy blood donors. The detection of anti-PEG antibodies in healthy blood donors was validated using a 0.5% Tween-20 interference assay, a 20 kDa PEGylated polystyrene bead binding assay, and Western blotting of purified plasma from human IgG and IgM purification columns.
2. Methods and materials
2.1. Reagents and antibodies
Human chimeric anti-PEG antibodies cHu6.3-IgG and cAGP4-IgM were obtained as described in previous studies [24,26]. TentaGel™ M OH beads (particle size 10 μm), horseradish peroxidase (HRP)-conjugated goat anti-human IgM (μ-chain specific) secondary antibody, and HRP-conjugated goat anti-human IgG (γ-chain specific) secondary antibody were purchased from Sigma-Aldrich (St. Louis, MO). Fluorescein isothiocyanate (FITC)-conjugated goat anti-human IgM (μ-chain specific) secondary antibody was purchased from Novus Biologicals (Centennial, CO). FITC-conjugated goat anti-human IgG (γ-chain specific) secondary antibody was purchased from Thermo Fisher Scientific (Waltham, MA). Linear methoxy PEG with molecular weights (MWs) of 40 and 60 kDa were purchased from JenKem Technology USA, Inc. (Plano, TX).
2.2. Plasma samples
Plasma samples (n = 300) of healthy blood donors were purchased from BioIVT (Hicksville, NY). The characteristics of the donors are listed in Table 1. The ages of these healthy blood donors ranged from 18 to 77 years (median age of 38 years). Plasma samples were stored at −80 °C until analysis.
Table 1.
Characteristics (sex, age, and race) of healthy blood donors.
| Sex | Number |
|---|---|
| Female | 116 |
| Male | 184 |
| Age* (years) | Number |
| 18 - 24 | 41 |
| 25 - 64 | 253 |
| ≥ 65 | 5 |
| Race | Number |
| Caucasian | 18 |
| Hispanic | 134 |
| Black | 142 |
| Asian | 1 |
| Unknown | 5 |
The age of one male was not known.
2.3. Flow cytometry assay
To analyze anti-PEG antibodies in plasma samples, a previously described flow cytometry assay [17] was slightly modified. Specifically, TentaGel™ M OH beads were used without sonication, the reaction was conducted by shaking at 800 rpm with an Eppendorf Thermomixer R (Eppendorf North America, Hauppauge, NY), and the TentaGel™ M OH beads were incubated with diluted human plasma (10 μL plasma and 40 μL 5% BSA) for 1 h. All other experimental conditions were the same as described by Armstrong et al. [17]. After the beads were stained for bound IgG or IgM with a specific FITC-conjugated anti-human IgG or IgM secondary antibody, the beads were washed three times with PBS and analyzed on a Beckman Coulter CytoFlex flow cytometry (Indianapolis, IN). Data were acquired and analyzed using CytExpert 1.2 Software (Beckman Coulter). The forward-scatter (FSC) signal and the side-scatter (SSC) signal were measured in the linear mode, with a gain of 20 for FSC and 30 for SSC. FITC fluorescence was detected on a logarithmic scale. A total of 10,000 beads was analyzed for each sample. The mean fluorescence intensity was used to analyze the presence or absence of anti-PEG antibodies in plasma. cHu6.3-IgG (1:500) and cAGP4-IgM (1:500) were the positive anti-PEG antibody standards and 5% BSA was used as a negative control. The FITC-conjugated anti-human IgG and IgM secondary antibodies were affinity-purified and further tested by flow cytometry to ensure no cross-reaction with other human immunoglobulins. All wash and incubation steps were performed using PBS without any surfactant.
The intra-assay variation coefficient was < 5% for both positive anti-PEG antibody standards from more than three analyses within 1 day; the inter-assay variation coefficient was 9.7% and 15.9% for cHu6.3-IgG and cAGP4-IgM, respectively, from more than six independent experiments performed over one month.
Both cHu6.3-IgG and cAGP4-IgM standard curves were constructed by plotting the mean fluorescent intensity versus the antibody concentration. The relative concentration of anti-PEG IgG and IgM was calculated by comparison to the cHu6.3-IgG or cAGP4-IgM standard curves, respectively. The detection limit was 35 and 25 ng/mL for cHu6.3-IgG and cAGP4-IgM, respectively.
2.4. Validation assays
Three assays, i.e., a 0.5% Tween-20 interference assay, a 20 kDa PEGylated polystyrene beads binding assay, and Western blotting of purified plasma from human IgG and IgM purification columns, were used to confirm the detection of anti-PEG antibodies in the healthy blood donors.
In the interference assay, all procedures were performed as in the flow cytometry assay described above, except for using PBS containing 0.5% Tween-20 in the incubation of TentaGel™ M OH beads with plasma samples or positive anti-PEG antibody standards.
In the binding assay, 25 μL of 2% (w/v) 20 kDa PEGylated polystyrene beads (particle size 3 μm) (Creative PEG Works, Research Triangle Park, NC) were incubated with human plasma (10 μL plasma and 40 μL 5% BSA) or positive anti-PEG antibody standards (1:500) and 125 μL PBS at room temperature with shaking at 800 rpm for 1 h. The beads were then centrifuged at 5000 g for 2 min and the supernatant (175 μL) was incubated with the TentaGel™ M OH beads as in the flow cytometry assay described above.
In the third validation assay, human plasma samples (400 μL or various amount of plasma diluted in 5% BSA to 400 μL) or 400 μL of positive anti-PEG antibody standards (1:500) were added to equilibrated microspin columns prefilled with 100 μL LigaTrap® human IgG or IgM purification resin (LigaTrap Technologies, Durham, NC) and mixed overnight at 4 °C. Human IgG or IgM was then purified following the manufacturer’s instructions. The eluent from the microspin columns was diluted in 5% BSA (1:50 – 1:1,000) and subjected to Western blotting.
2.5. Western blotting
PEG with MWs of 40 kDa (125 ng per well) and 60 kDa (62.5 ng per well) was loaded into 6% Bis-Tris gels, separated by gel electrophoresis, and transferred onto a polyvinylidene difluoride membrane. The membranes were blocked with 5% BSA and incubated with human positive anti-PEG antibody standards (cHu6.3-IgG, 1:25,000; cAGP4-IgM, 1:10,000) or the eluent from human IgG or IgM purification microspin columns, followed by a specific HRP-conjugated anti-human IgG (1:30,000) or IgM (1:15,000) secondary antibody. The washing solution did not contain any polyoxyethylene-type nonionic detergents, such as Tween-20. The blots were detected by chemiluminescence using Immobilon Western HRP Substrate (Millipore, Billerica, MA), a FluorChem R System (ProteinSimple, San Jose, CA), and Digital Darkroom software for acquisition & analysis (ProteinSimple).
2.6. Statistical analyses
Statistical analyses were performed using SigmaPlot 13.0. Variations in the prevalence of anti-PEG IgG or IgM antibodies with respect to sex, race, or age were assessed using Chi-square analysis. For the positive plasma samples, a one-way ANOVA was used to analyze the differences in the concentrations of anti-PEG IgG or IgM antibodies with respect to sex, race, or age. If the normality or equal variance tests failed, the analysis was conducted using a Kruskal-Wallis one-way ANOVA on ranks test. A Pearson Product Moment Correlation analysis was conducted to determine the correlation between the levels of anti-PEG IgG and IgM antibodies. The results were considered significant at p < 0.05.
3. Results
3.1. Anti-PEG antibodies in healthy blood donors
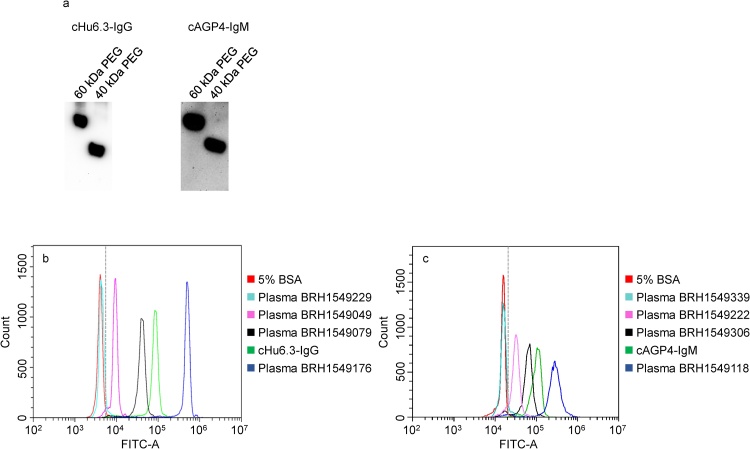
A flow cytometry assay was optimized to measure the prevalence and levels of anti-PEG IgG and IgM in human plasma. As shown in Fig. 1a, positive anti-PEG antibody standards, i.e., cHu6.3-IgG (1:25,000) and cAGP4-IgM (1:10,000), strongly bound to 40 and 60 kDa PEGs. The representative histograms shown in Figs. 1b and 1c demonstrate that cHu6.3-IgG (1:500) and cAGP4-IgM (1:500) had much higher fluorescent intensity than the negative control (5% BSA).
Fig. 1.
(a) Western blotting of positive anti-PEG antibody standards (cHu6.3-IgG, 1:25,000; cAGP4-IgM, 1:10,000). Lane 60 kDa PEG: 62.5 ng; lane 40 kDa PEG: 125 ng. Representative flow cytometry histogram overlay plot showing the detection of anti-PEG IgG (b) and IgM (c). TentaGel™ M OH beads were incubated with negative control (5% BSA, red), cHu6.3-IgG or cAGP4-IgM positive standard (green), and human plasma samples (negative, teal; pink, low-level anti-PEG IgG or IgM; black, medium-level anti-PEG IgG or IgM; blue, high-level anti-PEG IgG or IgM) as described in the Materials and Methods. The dashed line in b and c indicates the cutoff-point.
After the complete analysis of the 300 plasma samples, a cutoff-point was established. The cutoff-point was equal to the mean fluorescent intensity of the negative control of each experiment plus 3 standard deviations, which were calculated from the fluorescent intensity of the negative controls for all the experiments. The plasma was considered positive for anti-PEG IgG or IgM if the mean fluorescent intensity in plasma from a healthy blood donor was higher than the established cutoff-point (Figs. 1b and 1c). Anti-PEG IgG or IgM was detected in 65.3% of the 300 healthy blood donors, with 21.3% having anti-PEG IgG, 19.0% having anti-PEG IgM, and 25.0% having both anti-PEG IgG and IgM.
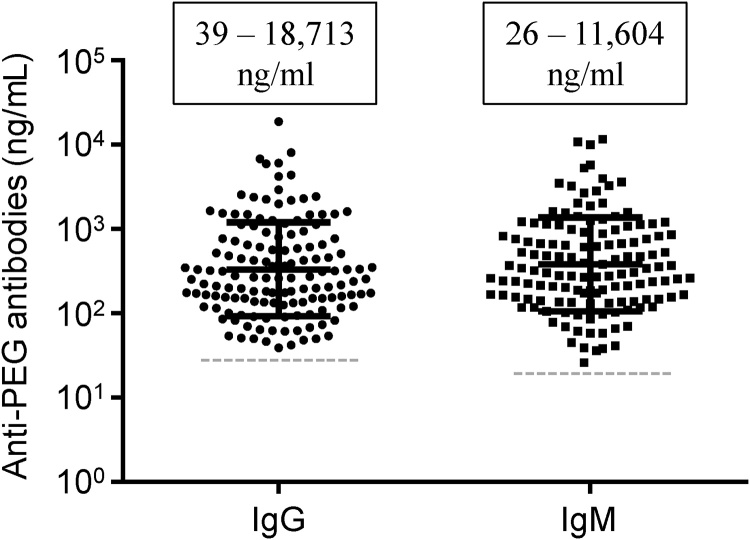
The concentrations of anti-PEG IgG and IgM in human plasma samples were calculated by comparison to the cHu6.3-IgG and cAGP4-IgM standard curves. The standard curves of cHu6.3-IgG and cAGP4-IgM were determined in at least three independent experiments and had a linear correlation coefficient ≥ 0.991 (Supplemental Fig. 1). The concentrations of anti-PEG IgG and IgM in positive human plasma samples ranged widely, with a mean of 0.88 and 0.93 μg/mL (median of 0.26 and 0.31 μg/mL) for anti-PEG IgG and IgM, respectively (Fig. 2). There was no correlation between the concentrations of anti-PEG IgG and IgM.
Fig. 2.
The concentrations of anti-PEG IgG and IgM antibodies in healthy blood donors. Line and bar represent the mean and standard deviation. Boxes indicate the range of antibody concentrations. The dashed line indicates the cutoff-point.
The concentrations of antidrug antibodies have been associated with altered pharmacokinetic parameters in clinical trials of monoclonal antibodies [29]. According to the antibody concentration, positive plasma samples were categorized as low (< 100 ng/mL), medium (100–500 ng/ml), and high (> 500 ng/mL) [29]. The prevalence of blood donors with a low level of anti-PEG IgG and IgM was 8.3% and 5.3%, respectively. The prevalence of blood donors with medium and high levels of anti-PEG IgG and IgM was comparable (medium-level: 22.7% vs 21.7%; high-level: 15.3% vs 17.0%).
3.2. Validation of anti-PEG antibodies in healthy blood donors
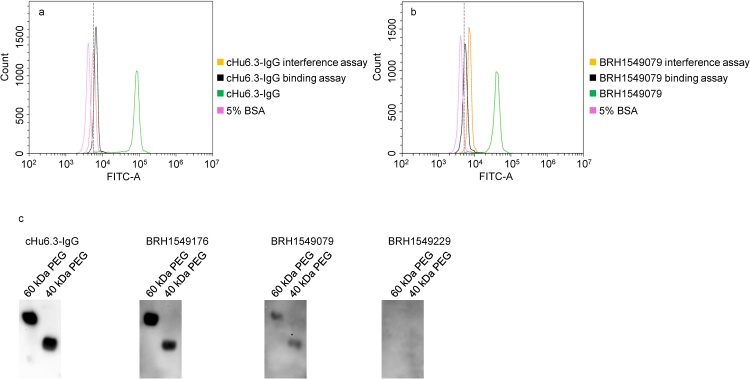
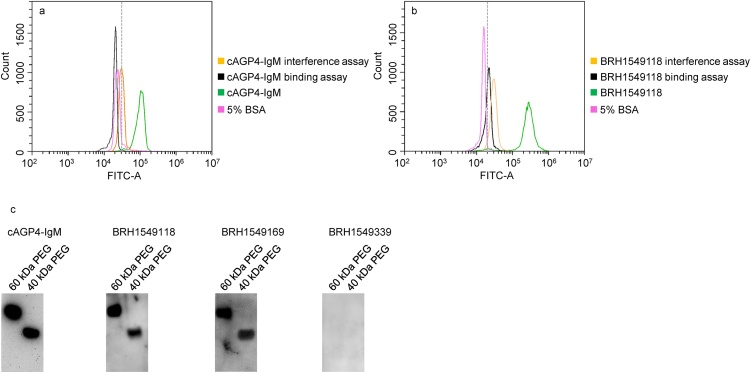
The detection of anti-PEG IgG or IgM in human plasma was validated using three different assays. Due to the structural similarity of PEG and polyoxyethylene type nonionic detergents, 0.5% Tween-20 was used in an interference assay. Tween-20 completely inhibited the binding activity of cHu6.3-IgG to TentaGel™ M OH beads (Fig. 3a) and caused an approximately 70% decrease in the fluorescent intensity of cAGP4-IgM (Fig. 4a). Due to the interference of Tween-20, the fluorescent intensity of anti-PEG IgG or IgM positive plasma samples returned to or near to the background level of the negative control (Figs. 3b and 4b). An interference effect of 0.5% Tween-20 on anti-PEG IgG or IgM negative plasma samples was not observed.
Fig. 3.
Representative flow cytometry histogram overlay plot showing decreased fluorescent intensity of cHu6.3-IgG positive standard (a) and an anti-PEG IgG positive human plasma (b) resulting from the 0.5% Tween-20 interference assay (orange) and 20 kDa PEGylated polystyrene beads binding assay (black). The dashed line in a and b indicates the cutoff-point. (c) Representative Western blotting of elution from human IgG purification column with the application of cHu6.3-IgG positive standard or positive (BRH1549176 and BRH1549079) and negative (BRH1549229) anti-PEG IgG plasma samples. Lane 60 kDa PEG: 62.5 ng; lane 40 kDa PEG: 125 ng.
Fig. 4.
Representative flow cytometry histogram overlay plot showing decreased fluorescent intensity of cAGP4-IgM positive standard (a) and an anti-PEG IgM positive human plasma (b) resulting from the 0.5% Tween-20 interference assay (orange) and 20 kDa PEGylated polystyrene beads binding assay (black). The dashed line in a and b indicates the cutoff-point. (c) Representative Western blotting of elution from human IgM purification column with the application of cAGP4-IgM positive standard or positive (BRH1549118 and BRH1549169) and negative (BRH1549339) anti-PEG IgM plasma samples. Lane 60 kDa PEG: 62.5 ng; lane 40 kDa PEG: 125 ng.
Preincubation of 20 kDa PEGylated polystyrene beads with cHu6.3-IgG or cAGP4-IgM led to a loss > 95% their fluorescent intensity, indicating a strong reactivity of anti-PEG IgG or IgM with 20 kDa PEGylated polystyrene beads (Figs. 3a and 4a). Following the preincubation, most (88%) anti-PEG IgG positive plasma samples exhibited ≥ 70% decrease in fluorescent intensity and other positive plasma samples lost 40–70% in fluorescent intensity (Fig. 3b). With anti-PEG IgM-positive plasma samples, the preincubation decreased their fluorescent intensity > 90% (Fig. 4b). There was no decrease in fluorescent intensity upon preincubation of anti-PEG IgG or IgM negative plasma samples.
To confirm further the detection of anti-PEG IgG or IgM in healthy blood donors, Western blotting assay of purified plasma from human IgG and IgM purification columns was performed. Five plasma samples from the negative and low-level groups and 10 plasma samples from the medium- and high-level groups were purified. As shown in Figs. 3c and 4c, eluent of cHu6.3-IgG, cAGP4-IgM, or medium- and high-level plasma samples from the purification column gave distinct bands of 40 and 60 kDa PEGs. On the other hand, no band was observed from the negative plasma samples eluent (BRH1549229 and BRH1549339) (Figs. 3c and 4c). Of the five low-level plasma samples purified, only one plasma produced faint bands of 40 and 60 kDa PEGs when assessed with anti-PEG IgG and none had detectable bands of 40 and 60 kDa PEGs with anti-PEG IgM (data not shown), indicating that the validation assay might not be sufficiently sensitive to detect low levels of anti-PEG antibodies. These data verified the detection of anti-PEG IgG or IgM human plasma samples as analyzed by the optimized flow cytometry assay.
3.3. The correlation of sex, race, or age with anti-PEG antibodies in healthy blood donors
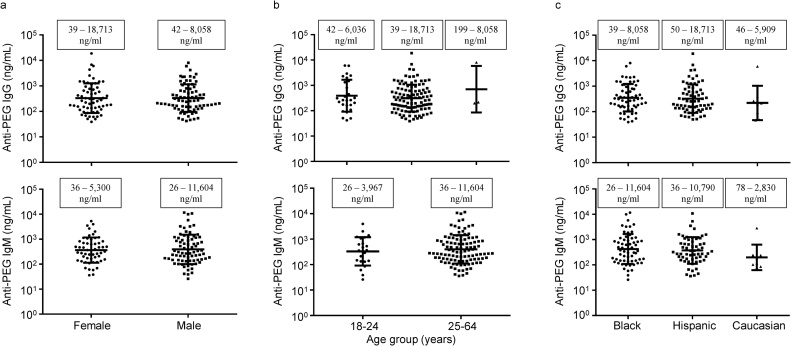
The relationship between the prevalence or concentrations of anti-PEG IgG or IgM and donor characteristics, including sex, age, or race, was analyzed. Of the 300 healthy blood donors, 139 donors (46.3%) and 132 donors (44.0%) had detectable anti-PEG IgG and IgM, respectively. Female donors had a higher prevalence of anti-PEG IgG (53.4%) and IgM (47.4%) antibodies as compared to male donors (41.8% for both IgG and IgM), but the difference did not reach statistical significance. In the positive plasma samples, the concentrations of anti-PEG IgG ranged widely, with a mean of 1.02 μg/mL in females and 0.77 μg/mL in males (Fig. 5a). Similarly, a great variation in the concentrations of anti-PEG IgM also occurred in women and men (Fig. 5a). No statistically significant difference in the concentrations of anti-PEG IgG or IgM was observed between women and men.
Fig. 5.
(a) The concentrations of anti-PEG IgG and IgM in (a) females and males, (b) age groups, and (c) races among healthy blood donors who are positive for anti-PEG IgG and IgM. The concentration of anti-PEG IgM for the ≥ 65 age group is not presented because there was only one individual, who had an anti-PEG IgM level of 146 ng/mL. Line and bar represent the mean and standard deviation. Boxes indicate the range of antibody concentrations.
Based on the classification of age groups by Statistics Canada (https://www.statcan.gc.ca/eng/concepts/definitions/age2), the samples were separated into three age groups (18–24, 25–64, and ≥ 65 years). One male with an unknown age was not included in the analysis. The prevalence of anti-PEG IgG was 65.9%, 43.1%, and 60.0% in age groups 18–24, 25–64, and ≥ 65 years, respectively, and a variation that was significant (p = 0.021, Chi-square analysis). The prevalence of anti-PEG IgM did not vary significantly among age groups and there was not a significant relationship of age with the prevalence of either anti-PEG IgG or IgM when age was considered as a continuous variable. The concentrations of anti-PEG IgG and IgM ranged widely across all age groups (Fig. 5b). There was no difference in the concentrations of anti-PEG IgG or IgM among the age groups.
The correlation of race with the prevalence or levels of anti-PEG IgG or IgM in healthy blood donors was also analyzed. One Asian male and five “unknown” individuals were not included in the analysis. Differences among races in the prevalence or levels of anti-PEG IgG or IgM (Fig. 5c) were not significant.
4. Discussion
In this study, the presence of anti-PEG IgG and IgM in 300 plasma samples from heathy blood donors was assessed using an optimized flow cytometry assay. The detection of anti-PEG antibodies was verified using three validation assays. Anti-PEG antibodies were detected in healthy blood donors with a prevalence of 21.3%, 19.0%, and 25.0% for anti-PEG IgG only, anti-PEG IgM only, and both anti-PEG IgG and IgM, respectively. The prevalence of anti-PEG IgG was associated with age. The concentrations of anti-PEG IgG and IgM ranged from 39 ng/mL to 18.7 μg/mL and 26 ng/mL to 11.6 μg/mL, respectively. No statistically significant correlation was found for the concentrations of anti-PEG IgG or IgM based up sex, age, or race. Thus, the flow cytometry assay provides an approach to investigate the impact of anti-PEG antibodies on the therapeutic efficacy and safety of PEGylated therapeutics and to screen the concentrations of anti-PEG antibodies in patients before the administration of PEGylated therapeutics.
The analysis of anti-PEG antibodies requires methods with high specificity, sensitivity, rapidity, and reproducibility. The most recent studies have used ELISAs in their analyses [8,24,26,28]. However, competitive ELISAs using PEG as the inhibitor may not detect anti-PEG antibodies accurately because anti-PEG antibodies have been shown to bind less efficiently to unconjugated PEG in solution compared to PEGylated agents [30]. Most animal studies have used only direct ELISAs using microplates coated with the same PEGylated agents that were administered; thus, the specificity of antibodies to the PEG moiety was doubtful. In this study, our optimized flow cytometry assay provided a reliable, accurate, and robust analysis of anti-PEG antibodies. The inter-assay variation coefficient was < 5.0% and the intra-assay variation coefficient was < 16.0%. All secondary antibodies used in this study were tested for specificity and did not show any cross-reactivity with other immunoglobulins. More importantly, the detection of anti-PEG antibodies was validated using three different approaches, which thoroughly confirmed the specificity of anti-PEG antibodies. Since Western blotting generally requires a high quality of antibody with the appropriate concentration [31], the low concentration of anti-PEG antibodies may be a factor leading to the discrepancy in the detection of the low-level anti-PEG antibodies using a flow cytometry assay and Western blotting of purified plasma from human IgG and IgM purification columns.
Our study demonstrated that 0.1% Tween-20 completely inhibited the binding of anti-PEG-positive standards to PEG in the Western blotting (Supplemental Fig. 2) due to the structural homology between PEG and polyoxyethylene type nonionic detergents. The interference assay further showed that 0.5% Tween-20 significantly decreased the binding of both anti-PEG-positive standards and positive plasma samples. These findings agree with other reports [22,26,32] and clearly demonstrate that polyoxyethylene type nonionic detergents should not be used in any immunological methods, including immunohistochemistry assays, to examine anti-PEG antibodies or PEG.
This study focused on a flow cytometry assay to measure the prevalence and concentrations of anti-PEG antibodies in human plasma. Since the current study used the same anti-PEG-positive standards as Yang and colleagues [26], the prevalence of anti-PEG antibodies in the general populations was expected to be similar. The high prevalence of anti-PEG antibodies in our study may partly be due to the high sensitivity of the flow cytometry assay. In our study, 15.3% and 17.0% of blood donors had a high level of anti-PEG IgG and IgM, which differed from the report of Yang and colleagues [26]. Individuals with high levels of anti-PEG antibodies following the treatment of PEGylated agents have accelerated drug clearance and a great risk of adverse reactions [20,33]. Thus, sensitive measurement of anti-PEG antibodies in clinical settings may be of great importance to ensure the efficacy and safe use of PEGylated agents.
We assessed the potential effects of sex, race, and age on the prevalence and concentrations of anti-PEG antibodies in healthy blood donors. Our findings indicated that race did not impact the prevalence and concentrations of anti-PEG antibodies, but that their prevalence was greatest in the 18–24 years age group. These results agree with recent studies [[24], [25], [26]]. The higher prevalence of anti-PEG antibodies in younger heathy individuals may result from age-specific changes in the immune system [34] or other unknown factors.
Our study provides an analytical approach to examine anti-PEG antibodies and demonstrates their presence in most healthy individuals. Considering the impact of anti-PEG antibodies on the therapeutic efficacy and safety of PEGylated agents, it is important to measure regularly anti-PEG antibodies in patients after exposure to PEGylated agents.
Funding
This work was supported by an Interagency Agreement (FDA IAG No. 224-19-0500R) between the National Toxicology Program, National Institute of Environmental Health Sciences, and the U.S. Food and Drug Administration, National Center for Toxicological Research. Yangshun Tang was supported by an appointment to the Postgraduate Research Program in the Division of Biochemical Toxicology at the National Center for Toxicological Research administered by Oak Ridge Institute for Science Education through an interagency agreement between the U.S. Department of Energy and the U.S. Food and Drug Administration.
CRediT authorship contribution statement
Jia-Long Fang: Conceptualization, Methodology, Investigation, Validation, Formal analysis, Writing - original draft, Writing - review & editing. Frederick A. Beland: Formal analysis, Writing - review & editing, Supervision. Yangshun Tang: Investigation, Writing - original draft. Steve R. Roffler: Validation, Writing - review & editing.
Declaration of Competing Interest
The authors declare that there are no conflicts of interest.
Edited by Dr. A.M Tsatsaka
Footnotes
The views presented in this article do not necessarily reflect those of the U.S. Food and Drug Administration
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.toxrep.2020.12.022.
Appendix A. Supplementary data
The following is supplementary data to this article:
References
- 1.Fruijtier-Pölloth C. Safety assessment on polyethylene glycols (PEGs) and their derivatives as used in cosmetic products. Toxicology. 2005;214(1–2):1–38. doi: 10.1016/j.tox.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 2.Turecek P.L., Bossard M.J., Schoetens F., Ivens I.A. PEGylation of biopharmaceuticals: a review of chemistry and nonclinical safety information of approved drugs. J. Pharm. Sci. 2016;105(2):460–475. doi: 10.1016/j.xphs.2015.11.015. [DOI] [PubMed] [Google Scholar]
- 3.Morpurgo M., Veronese F.M. Conjugates of peptides and proteins to polyethylene glycols. Methods Mol. Biol. 2004;283:45–70. doi: 10.1385/1-59259-813-7:045. [DOI] [PubMed] [Google Scholar]
- 4.Chang T.-C., Chen B.-M., Lin W.-W. Both IgM and IgG antibodies against polyethylene glycol can alter the biological activity of methoxy polyethylene glycol-epoetin beta in mice. Pharmaceutics. 2019;12(1):15. doi: 10.3390/pharmaceutics12010015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Garay R.P., El-Gewely R., Armstrong J.K., Garratty G., Richette P. Antibodies against polyethylene glycol in healthy subjects and in patients treated with PEG-conjugated agents. Expert Opin. Drug Deliv. 2012;9(11):1319–1323. doi: 10.1517/17425247.2012.720969. [DOI] [PubMed] [Google Scholar]
- 6.Schellekens H., Hennink W.E., Brinks V. The immunogenicity of polyethylene glycol: facts and fiction. Pharm. Res. 2013;30(7):1729–1734. doi: 10.1007/s11095-013-1067-7. [DOI] [PubMed] [Google Scholar]
- 7.Richter A.W., Åkerblom E. Antibodies against polyethylene glycol produced in animals by immunization with monomethoxy polyethylene glycol modified proteins. Int. Arch. Allergy Appl. Immunol. 1983;70(2):124–131. doi: 10.1159/000233309. [DOI] [PubMed] [Google Scholar]
- 8.Shimizu T., Ichihara M., Yoshioka Y., Ishida T., Nakagawa S., Kiwada H. Intravenous administration of polyethylene glycol-coated (PEGylated) proteins and PEGylated adenovirus elicits an anti-PEG immunoglobulin M response. Biol. Pharm. Bull. 2012;35(8):1336–1342. doi: 10.1248/bpb.b12-00276. [DOI] [PubMed] [Google Scholar]
- 9.Abu Lila A.S., Kiwada H., Ishida T. The accelerated blood clearance (ABC) phenomenon: clinical challenge and approaches to manage. J. Control Release. 2013;172(1):38–47. doi: 10.1016/j.jconrel.2013.07.026. [DOI] [PubMed] [Google Scholar]
- 10.Zhao Y., Wang L., Yan M. Repeated injection of PEGylated solid lipid nanoparticles induces accelerated blood clearance in mice and beagles. Int. J. Nanomed. 2012;7:2891–2900. doi: 10.2147/IJN.S30943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ganson N.J., Kelly S.J., Scarlett E., Sundy J.S., Hershfield M.S. Control of hyperuricemia in subjects with refractory gout, and induction of antibody against poly(ethylene glycol) (PEG), in a phase I trial of subcutaneous PEGylated urate oxidase. Arthritis Res. Ther. 2006;8(1):R12. doi: 10.1186/ar1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hershfield M.S., Ganson N.J., Kelly S.J., Scarlett E.L., Jaggers D.A., Sundy J.S. Induced and pre-existing anti-polyethylene glycol antibody in a trial of every 3-week dosing of pegloticase for refractory gout, including in organ transplant recipients. Arthritis Res. Ther. 2014;16(2):R63. doi: 10.1186/ar4500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Myler H., Hruska M.W., Srinivasan S. Anti-PEG antibody bioanalysis: a clinical case study with PEG-IFN-λ-1a and PEG-IFN-α2a in naive patients. Bioanalysis. 2015;7(9):1093–1106. doi: 10.4155/bio.15.36. [DOI] [PubMed] [Google Scholar]
- 14.Richter A.W., Åkerblom E. Polyethylene glycol reactive antibodies in man: titer distribution in allergic patients treated with monomethoxy polyethylene glycol modified allergens or placebo, and in healthy blood donors. Int. Arch. Allergy Appl. Immunol. 1984;74(1):36–39. doi: 10.1159/000233512. [DOI] [PubMed] [Google Scholar]
- 15.Sundy J.S., Ganson N.J., Kelly S.J. Pharmacokinetics and pharmacodynamics of intravenous PEGylated recombinant mammalian urate oxidase in patients with refractory gout. Arthritis Rheum. 2007;56(3):1021–1028. doi: 10.1002/art.22403. [DOI] [PubMed] [Google Scholar]
- 16.Armstrong J.K. The occurrence, induction, specificity and potential effect of antibodies against poly(ethylene glycol) In: Veronese F.M., editor. PEGylated Protein Drugs: Basic Science and Clinical Applications. Milestones in Drug Therapy; Birkhäuser Basel: 2009. pp. 147–168. [Google Scholar]
- 17.Armstrong J.K., Hempel G., Koling S. Antibody against poly(ethylene glycol) adversely affects PEG-asparaginase therapy in acute lymphoblastic leukemia patients. Cancer. 2007;110(1):103–111. doi: 10.1002/cncr.22739. [DOI] [PubMed] [Google Scholar]
- 18.Hsieh Y.-C., Wang H.-E., Lin W.-W. Pre-existing anti-polyethylene glycol antibody reduces the therapeutic efficacy and pharmacokinetics of PEGylated liposomes. Theranostics. 2018;8(11):3164–3175. doi: 10.7150/thno.22164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sundy J.S., Baraf H.S.B., Yood R.A. Efficacy and tolerability of pegloticase for the treatment of chronic gout in patients refractory to conventional treatment: two randomized controlled trials. JAMA. 2011;306(7):711–720. doi: 10.1001/jama.2011.1169. [DOI] [PubMed] [Google Scholar]
- 20.Ganson N.J., Povsic T.J., Sullenger B.A. Pre-existing anti-polyethylene glycol antibody linked to first-exposure allergic reactions to pegnivacogin, a PEGylated RNA aptamer. J. Allergy Clin. Immunol. 2016;137(5):1610–1613. doi: 10.1016/j.jaci.2015.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Povsic T.J., Lawrence M.G., Lincoff A.M. Pre-existing anti-PEG antibodies are associated with severe immediate allergic reactions to pegnivacogin, a PEGylated aptamer. J. Allergy Clin. Immunol. 2016;138(6):1712–1715. doi: 10.1016/j.jaci.2016.04.058. [DOI] [PubMed] [Google Scholar]
- 22.FDA . 2014. Guidance for Industry: Immunogenicity Assessment for Therapeutic Protein Products.https://www.fda.gov/media/85017/download [Google Scholar]
- 23.Chen B.-M., Su Y.-C., Chang C.-J. Measurement of pre-existing IgG and IgM antibodies against polyethylene glycol in healthy individuals. Anal. Chem. 2016;88(21):10661–10666. doi: 10.1021/acs.analchem.6b03109. [DOI] [PubMed] [Google Scholar]
- 24.Lubich C., Allacher P., de la Rosa M. The mystery of antibodies against polyethylene glycol (PEG) - what do we know? Pharm. Res. 2016;33(9):2239–2249. doi: 10.1007/s11095-016-1961-x. [DOI] [PubMed] [Google Scholar]
- 25.Yang Q., Jacobs T.M., McCallen J.D. Analysis of pre-existing IgG and IgM antibodies against polyethylene glycol (PEG) in the general population. Anal. Chem. 2016;88(23):11804–11812. doi: 10.1021/acs.analchem.6b03437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Garratty G. Progress in modulating the RBC membrane to produce transfusable universal/stealth donor RBCs. Transfus. Med. Rev. 2004;18(4):245–256. doi: 10.1016/j.tmrv.2004.06.005. [DOI] [PubMed] [Google Scholar]
- 27.Liu Y., Reidler H., Pan J. A double antigen bridging immunogenicity ELISA for the detection of antibodies to polyethylene glycol polymers. J. Pharmacol. Toxicol. Methods. 2011;64(3):238–245. doi: 10.1016/j.vascn.2011.07.003. [DOI] [PubMed] [Google Scholar]
- 28.Zhou L., Hoofring S.A., Wu Y. Stratification of antibody-positive subjects by antibody level reveals an impact of immunogenicity on pharmacokinetics. AAPS J. 2013;15(1):30–40. doi: 10.1208/s12248-012-9408-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Su Y.-C., Chen B.-M., Chuang K.-H., Cheng T.-L., Roffler S.R. Sensitive quantification of PEGylated compounds by second-generation anti-poly(ethylene glycol) monoclonal antibodies. Bioconjug. Chem. 2010;21(7):1264–1270. doi: 10.1021/bc100067t. [DOI] [PubMed] [Google Scholar]
- 30.Gilda J.E., Ghosh R., Cheah J.X., West T.M., Bodine S.C., Gomes A.V. Western blotting inaccuracies with unverified antibodies: need for a Western blotting minimal reporting standard (WBMRS) PLoS One. 2015;10(8) doi: 10.1371/journal.pone.0135392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sherman M.R., Williams L.D., Sobczyk M.A., Michaels S.J., Saifer M.G.P. Role of the methoxy group in immune responses to mPEG-protein conjugates. Bioconjug. Chem. 2012;23(3):485–499. doi: 10.1021/bc200551b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang Q., Lai S.K. Anti-PEG immunity: emergence, characteristics, and unaddressed questions. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2015;7(5):655–677. doi: 10.1002/wnan.1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Longo N., Harding C.O., Burton B.K. Single-dose, subcutaneous recombinant phenylalanine ammonia lyase conjugated with polyethylene glycol in adult patients with phenylketonuria: an open-label, multicentre, phase 1 dose-escalation trial. Lancet. 2014;384(9937):37–44. doi: 10.1016/S0140-6736(13)61841-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Linton P.J., Dorshkind K. Age-related changes in lymphocyte development and function. Nat. Immunol. 2004;5(2):133–139. doi: 10.1038/ni1033. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.