Abstract
The British Society of Gastroenterology in collaboration with British Association for the Study of the Liver has prepared this document. The aim of this guideline is to review and summarise the evidence that guides clinical diagnosis and management of ascites in patients with cirrhosis. Substantial advances have been made in this area since the publication of the last guideline in 2007. These guidelines are based on a comprehensive literature search and comprise systematic reviews in the key areas, including the diagnostic tests, diuretic use, therapeutic paracentesis, use of albumin, transjugular intrahepatic portosystemic stent shunt, spontaneous bacterial peritonitis and beta-blockers in patients with ascites. Where recent systematic reviews and meta-analysis are available, these have been updated with additional studies. In addition, the results of prospective and retrospective studies, evidence obtained from expert committee reports and, in some instances, reports from case series have been included. Where possible, judgement has been made on the quality of information used to generate the guidelines and the specific recommendations have been made according to the ‘Grading of Recommendations Assessment, Development and Evaluation (GRADE)’ system. These guidelines are intended to inform practising clinicians, and it is expected that these guidelines will be revised in 3 years’ time.
Keywords: ascites, cirrhosis
Executive summary of recommendations
-
Diagnostic paracentesis in new- onset ascites
1.1. A diagnostic paracentesis is recommended in all patients with new-onset ascites. (Quality of evidence: moderate; Recommendation: strong)
1.2. The initial ascitic fluid analysis should include total protein concentration and calculation of the serum ascites albumin gradient (SAAG). (Quality of evidence: moderate; Recommendation: strong)
1.3. Ascites fluid analysis for cytology, amylase, brain natriuretic peptide (BNP) and adenosine deaminase should be considered based on pretest probability of specific diagnosis (Quality of evidence: moderate; Recommendation: weak)
-
Spontaneous bacterial peritonitis
2.1. Diagnostic paracentesis should be carried out without a delay to rule out spontaneous bacterial peritonitis SBP) in all cirrhotic patients with ascites on hospital admission. (Quality of evidence: moderate; Recommendation: strong)
2.2. A diagnostic paracentesis should be performed in patients with GI bleeding, shock, fever or other signs of systemic inflammation, gastrointestinal symptoms, hepatic encephalopathy, and in patients with worsening liver or renal function. (Quality of evidence: moderate; Recommendation: strong)
2.3. Ascitic neutrophil >250/mm3 count remains the gold standard for the diagnosis of SBP and this can be performed either by manual microscopy or using automated counts, based on flow cytometry for counting and differentiating cells. (Quality of evidence: moderate; Recommendation: strong)
2.4. Ascitic fluid culture with bedside inoculation of blood culture bottles should be performed to guide the choice of antibiotic treatment when SBP is suspected. (Quality of evidence: moderate; Recommendation: strong)
2.5. Immediate empirical antibiotic therapy should be determined with due consideration of context of SBP (community acquired or healthcare associated), severity of infection and local bacterial resistance profile. Cefotaxime has been widely studied, but choice of antibiotic should be guided by local resistance patterns and protocol. (Quality of evidence: moderate; Recommendation: strong)
2.6. A second diagnostic paracentesis at 48 hours from the start of treatment to check the efficacy of antibiotic therapy should be considered in those who have apparently inadequate response or where secondary bacterial peritonitis is suspected. (Quality of evidence: low; Recommendation: weak)
2.7. Patients presenting with gastrointestinal bleeding and underlying ascites due to cirrhosis should receive prophylactic antibiotic treatment (cefotaxime has been widely studied but the antibiotic should be chosen based on local data) to prevent the development of SBP. (Quality of evidence: strong; Recommendation: strong)
2.8. Patients who have recovered from an episode of SBP should be considered for treatment with norfloxacin (400 mg once daily), ciprofloxacin (500 mg once daily, orally) or co-trimoxazole (800 mg sulfamethoxazole and 160 mg trimethoprim daily, orally) to prevent further episode of SBP. (Quality of evidence: low; Recommendation: weak)
2.9. Primary prophylaxis should be offered to patients considered at high risk, as defined by an ascitic protein count <1.5 g/dL. However, it is important that the potential risks and benefits and existing uncertainties are communicated to patients. (Quality of evidence: low; Recommendation: weak)
-
Dietary salt restriction
3.1. Patients with cirrhosis and ascites should have a moderately salt restricted diet with daily salt intake of no more than 5–6.5 g (87–113 mmol sodium). This translates to a no added salt diet with avoidance of precooked meals. (Quality of evidence: moderate; Recommendation: strong)
3.2. Patients with cirrhosis and ascites should receive nutritional counselling on the sodium content in the diet. (Quality of evidence: weak; Recommendation: strong)
-
Diuretics
4.1. In patients with the first presentation of moderate ascites, spironolactone monotherapy (starting dose 100 mg, increased to 400 mg) is reasonable. In those with recurrent severe ascites, and if faster diuresis is needed (for example, if the patient is hospitalised), combination therapy with spironolactone (starting dose 100 mg, increased to 400 mg) and furosemide (starting dose 40 mg, increased to 160 mg) is recommended. (Quality of evidence: moderate; Recommendation: strong)
4.2. All patients initiating diuretics should be monitored for adverse events. Almost half of those with adverse events require diuretic discontinuation or dose reduction. (Quality of evidence: low; Recommendation: weak)
4.3. Hypovolaemic hyponatraemia during diuretic therapy should be managed by discontinuation of diuretics and expansion of plasma volume with normal saline. (Quality of evidence: low; Recommendation: weak)
4.4. Fluid restriction to 1–1.5 L/day should be reserved for those who are clinically hypervolaemic with severe hyponatraemia (serum sodium <125 mmol/L). (Quality of evidence: low; Recommendation: weak)
4.5. Hypertonic sodium chloride (3%) administration should be reserved for those who are severely symptomatic with acute hyponatraemia. Serum sodium should be slowly corrected. (Quality of evidence: low; Recommendation: weak)
4.6. It may be appropriate to consider use of midodrine in refractory ascites on a case by case basis. (Quality of evidence: low; Recommendation: weak)
-
Large volume paracentesis (LVP)
5.1. Patients should give informed consent for a therapeutic or diagnostic paracentesis. (Quality of evidence: low; Recommendation: strong)
5.2. Ultrasound guidance should be considered when available during LVP to reduce the risk of adverse events (Quality of evidence: low; Recommendation: weak)
5.3. Routine measurement of the prothrombin time and platelet count before therapeutic or diagnostic paracentesis and infusion of blood products are not recommended. (Quality of evidence: moderate; Recommendation: strong)
-
Use of human albumin solution (HAS)
6.1. Albumin (as 20% or 25% solution) should be infused after paracentesis of >5 L is completed at a dose of 8 g albumin/L of ascites removed. (Quality of evidence: high; Recommendation: strong)
6.2. Albumin (as 20% or 25% solution) can be considered after paracentesis of <5 L at a dose of 8 g albumin/L of ascites removed in patients with ACLF or high risk of post-paracentesis acute kidney injury. (Quality of evidence: low; Recommendation: weak)
6.3. In patients with SBP and an increased serum creatinine or a rising serum creatinine, infusion of 1.5 g albumin/kg within 6 hours of diagnosis, followed by 1 g/kg on day 3, is recommended. (Quality of evidence: low; Recommendation: weak)
-
Transjugular intrahepatic portosystemic shunt (TIPSS)
7.1. TIPSS should be considered in patients with refractory ascites. (Quality of evidence: high; Recommendation: strong)
7.2. Caution is required if considering TIPSS in patients with age >70 years, serum bilirubin >50 µmol/L, platelet count <75×109/L, model for end-stage liver disease (MELD) score ≥18, current hepatic encephalopathy, active infection or hepatorenal syndrome. (Quality of evidence: moderate; Recommendation: strong)
-
Umbilical hernia
8.1. Suitability and timing of surgical repair of umbilical hernia should be considered in discussion with the patient and multidisciplinary team involving physicians, surgeons and anaesthetists. (Quality of evidence: low; Recommendation: strong)
-
Hepatic hydrothorax (HH)
9.1. TIPSS should be considered in patients with HH after discussion with the multidisciplinary team. (Quality of evidence: low; Recommendation: strong)
9.2. In patients with HH who are not undergoing a TIPSS and/or a liver transplant evaluation, alternative palliative interventions should be considered. (Quality of evidence: low; Recommendation: strong)
-
Non-selective beta-blockers (NSBB) and ascites
10.1. Refractory ascites should not be viewed as a contraindication to NSBB. (Quality of evidence: moderate; Recommendation: strong)
10.2. Patients with refractory ascites who are taking NSBB should be monitored closely, and dose reduction or discontinuation may be appropriate in those who develop hypotension or acute/progressive renal dysfunction. (Quality of evidence: moderate; Recommendation: strong)
-
Automated low-flow ascites pump
11.1. An automated low-flow ascites pump should be considered only in special circumstances with robust arrangements of clinical governance, audit or research. (Quality of evidence: low; Recommendation: weak)
-
Palliative care
12.1. Patients with refractory ascites who are not undergoing evaluation for liver transplant should be offered a palliative care referral. Besides repeated LVP, alternative palliative interventions for refractory ascites should also be considered. (Quality of evidence: weak; Recommendation: strong)
-
Research recommendations
13.1. Randomised controlled trials (RCT) with large sample size should evaluate the role of antibiotics in the secondary prophylaxis for SBP in ascites secondary to cirrhosis.
13.2. Large RCTs should assess the role of midodrine in the management of ascites.
13.3. Cost-effectiveness of long-term administration of albumin to patients with decompensated cirrhosis and ascites should be evaluated.
13.4. Role of nutritional interventions in the management of ascites should be evaluated.
13.5. Large RCT of long-term carvedilol versus no carvedilol in patients with refractory ascites without large oesophageal varices should be carried out.
13.6. Role of TIPSS in the management of hepatic hydrothorax should be compared with other therapeutic interventions.
13.7. The cost-effectiveness and the effect of automated low-flow ascites pumps on the quality of life of patients with refractory ascites should be evaluated.
13.8. Effectiveness and safety of long-term abdominal drains should be assessed in RCTs for the palliative care of patients with cirrhosis and refractory ascites.
Patient summary
These guidelines have been produced on behalf of the British Society of Gastroenterology (BSG) in collaboration with the British Association for the Study of the Liver (BASL). These guidelines are aimed at healthcare professionals who look after patients with cirrhosis and ascites.
Ascites is the build-up of fluid in the belly (abdomen). This occurs when the liver gets irreversibly scarred, a condition known as cirrhosis. Ascites is the most common complication of cirrhosis.
All patients with a new onset of ascites should have the fluid tested. This involves inserting a small needle into the abdomen and removing about two tablespoons of ascitic fluid. The fluid is then analysed for protein and white cell count. Protein count can help differentiate whether the cause of ascites is cirrhosis or whether the ascites is due to other causes like heart disease or cancer. The white cell count indicates whether there is an infection in the ascitic fluid. If infection is present, this is treated with a short course of antibiotics. Infection of ascites should be ruled out at every hospital admission as it carries a high risk of death and should therefore be diagnosed and treated promptly. After this initial treatment, patients are given long-term antibiotics to prevent repeat infections.
No salt should be added at the table to food. The total amount of salt in food per day should not be more than the equivalent of one teaspoon. Patients should read labels on prepared foods to confirm their daily salt intake is within the limit of 5 g of salt. The initial treatment for patients with ascites involves taking medication, commonly known as 'water tablets' (diuretics). These drugs are begun at a small dose, which is gradually increased until the ascites is treated. Diuretics can have side effects such dehydration, confusion, abnormal levels of sodium and potassium and kidney damage. Therefore patients should be monitored while taking these tablets.
As the liver disease progresses the ascites may no longer respond to medication. This is known as untreatable or refractory ascites. This requires the patient to come into hospital every few weeks to have a temporary drain inserted into the abdomen and the ascitic fluid drained. If more than 5 L of fluid is removed, patients are also given a protein solution into the vein to prevent dehydration.
In patients with untreatable ascites, alternatives to repeated hospital drainage include placing a small tube (stent) in the liver. This specialised procedure is known as a transjugular intrahepatic portosystemic shunt (TIPSS). The TIPSS procedure is effective in reducing the need for repeated fluid drainage. Because of potential side effects, patients should be selected carefully for this procedure. This is particularly true for patients with more advanced liver disease, where the insertion of a TIPSS can potentially be harmful.
The only curative option for untreatable ascites is liver transplantation. If the patient is not suitable for liver transplantation, medical care then focuses on controlling the ascites symptoms. This is known as palliative care. The most common palliative treatment for untreatable ascites is repeated hospital drainage. Alternative treatments for untreatable ascites, such as long-term abdominal drains, need further research.
Introduction
Contemporary data from an NHS hospital serving a population of 700 000, found 164 adults with a new diagnosis of ascites over a period of 5 years. Of these, 55% had cirrhosis (alcohol-related liver disease 58, non-alcoholic fatty liver disease 21, chronic viral hepatitis 4, autoimmune liver diseases 3 and cryptogenic cirrhosis 4), 29% had malignancies (gynaecological 12, gastrointestinal 25 and others 11), 6% cardiac failure (CF), 3% end-stage renal disease (ESRD) and 7% other aetiologies.
Development of ascites is an important milestone in the natural history of cirrhosis. About 20% of patients with cirrhosis have ascites at their first presentation, and 20% of those presenting with ascites die in the first year of the diagnosis.1 The aim of this guideline is to review and summarise the evidence that guides clinical diagnosis and management of ascites in patients with cirrhosis.
Pathogenesis
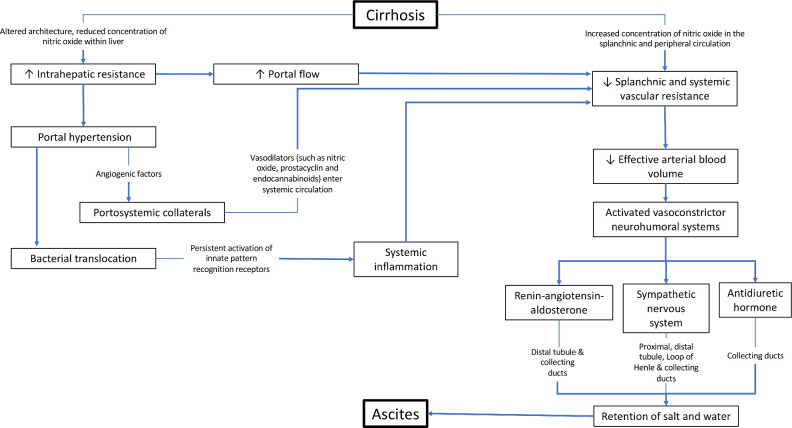
A detailed description of the pathogenesis of ascites formation is beyond the scope of this article, but two key factors involved in the pathogenesis of ascites formation are portal hypertension and retention of sodium and water. This is summarised in figure 1.
Figure 1.
The pathogenesis of ascites in cirrhosis.
An elevated sinusoidal pressure is essential for the development of ascites, as fluid accumulation does not develop at portal pressure gradient below 8 mm Hg, and rising corrected sinusoidal pressure correlates with decreased 24-hour urinary excretion of sodium.2 3 Architectural changes associated with advanced fibrosis are clearly the primary mechanism underlying increased intrahepatic resistance to the portal flow in cirrhosis. In addition, phenotypic changes in hepatic stellate cells and liver sinusoidal endothelial cells contribute to the pathophysiology. Activated stellate cells become contractile, and their recruitment around newly formed sinusoidal vessels increases the vascular resistance. Reduction in the production/bioavailability of nitric oxide (NO) in the cirrhotic liver adds further to the rise in vascular tone. Overall, vasoconstriction has been estimated to account for about 25% of the increased resistance within the liver.4
Increased portal pressure is sensed by intestinal microvasculature that generates angiogenic factors such as vascular endothelial growth factor,5 and these stimulate the development of portosystemic collaterals through the opening of pre-existing vessels or new vessel formation. When the portal pressure rises further, induction of endothelial nitric oxide synthase and over production of NO leads to splanchnic arterial vasodilatation. This, in turn, increases portal blood flow, thus exacerbating portal hypertension. Portosystemic collaterals also permit vasodilators such as NO, prostacyclin and endocannabinoids6 to enter the systemic circulation leading to a state of ‘effective hypovolaemia’.7 This activates sympathetic nervous system stimulating reabsorption of sodium in proximal, distal tubules, loop of Henle and collecting duct as well as the renin–angiotensin–aldosterone system, leading to sodium absorption from distal tubule and collecting duct.8 Renal sodium retention and eventual free water clearance due to non-osmotic release of arginine–vasopressin and its action on V2 receptor in the collecting duct underlie the fluid retention associated with oedema and ascites in cirrhosis.8
More recently, it has been hypothesised that bacterial translocation associated with portal hypertension in cirrhosis and related pathogen-associated, molecular pattern activated innate immune responses lead to systemic inflammation.9 This is associated with vasodilatation as well as release of proinflammatory cytokines, reactive oxygen and nitrogen species, contributing to organ dysfunction.
Definitions
The terms used in this article have been defined by the International Ascites Club10
Uncomplicated ascites
Ascites that is not infected and which is not associated with the development of the hepatorenal syndrome (HRS). Ascites can be graded as mild when ascites is detectable only by ultrasound examination, moderate when it causes moderate symmetrical distension of the abdomen and large when it causes marked abdominal distension.
Refractory ascites
Ascites that cannot be mobilised or the early recurrence of which (ie, after therapeutic paracentesis) cannot be satisfactorily prevented by medical treatment. This includes two different subgroups.
Diuretic-resistant ascites
Ascites that is refractory to dietary sodium restriction and intensive diuretic treatment.
Diuretic-intractable ascites
Ascites that is refractory to treatment owing to the development of diuretic-induced complications that preclude the use of an effective diuretic dosage.
Evaluation of patients with ascites
Clinical evaluation should include history of exposure to risk factors for cirrhosis and physical examination to look for evidence to support chronic liver disease or an alternative diagnosis. Shifting dullness is detectable when about one and a half litres of free fluid accumulate in the abdomen; the physical sign has 83% sensitivity and 56% specificity in detecting ascites.11 12 However, in the presence of obesity or smaller amount of fluid, imaging such as ultrasound or CT is necessary to confirm the presence of ascites.
Diagnostic paracentesis in new-onset ascites
Aspiration of ascitic fluid and its laboratory analysis is an essential step in the management of patients with newly diagnosed ascites. In cirrhosis, hepatic sinusoids are less permeable owing to fibrous tissue deposition, resulting in ascites with low protein content. It is important to estimate total protein level in ascites fluid; a concentration below 1.5 g/dL (or 15 g/L) is a risk factor for the development of spontaneous bacterial peritonitis. In addition, serum ascites albumin gradient (SAAG) should be estimated routinely. A cut-off point of 1.1 g/dL (or 11 g/L) differentiates between causes of ascites with high sensitivity,13–18 although alternative causes should be considered based on the clinical scenario (table 1).
Table 1.
Grouping of aetiology of ascites based on serum albumin ascites gradient (SAAG)
| SAAG ≥11 /L | SAAG <11 /L |
| Portal hypertension | Peritoneal carcinomatosis |
| Cardiac failure | Peritoneal tuberculosis |
| Portal vein thrombosis* | Pancreatitis* |
| Hypothyroidism* | Bowel perforation* |
| Nephrotic syndrome* |
*Limited data27 283 284
Hepatic sinusoids are normally permeable in heart failure, which allows for leakage of protein-rich lymph into the abdominal cavity and therefore, total protein concentration in ascitic fluid is high (>2.5 g/dL) in combination with a high SAAG. In such a situation, measurement of brain natriuretic peptide (BNP) in the serum±ascites is useful. Total protein concentrations >2.5 g/dL within the ascites and serum BNP >364 ng/L are suggestive of underlying or additional cardiac disease, whereas serum protein values <182 ng/L rule out cardiac disease.19
In low SAAG states, clinical context and imaging should guide the investigational approach. The yield for positive cytology in the context of malignancy is variable, ranging from 0% to 96.7%, in part determined by the site of the tumour.20 21 Combining cytology with tumour markers in the ascitic fluid may increase the positive predictive value (PPV), specifically the use of carcinoembryonic antigen (CEA), epithelial cell adhesion molecule (EpCAM), CA 15-3 and CA 19-9.20 However, CA 125 in the serum or ascites has no role as a discriminator and will commonly be elevated by the presence of ascites from any cause.22
Where peritoneal TB is considered plausible, ascites can be sent for acid-fast bacilli smear and culture, although culture positivity occurs in <50% and smear-positive ascites is rare.23 Adenosine deaminase is more useful to distinguish between peritoneal TB and carcinomatosis, with an area under the receiver operating characteristic curve of 0.98; adenosine deaminase levels of <40 IU/mL are used to exclude TB.24 25
Pancreatic ascites is a rare complication of pancreatitis, although more common when a pseudocyst is present. In pancreatic ascites, the amylase level in the ascitic fluid is typically >1000 IU/L or greater than six times the serum amylase, with mean values exceeding 4000 IU/L in a recent cohort of 80 patients.26 Raised polymorphonuclear leucocytes (PMN) count may also be found in pancreatic ascites.27
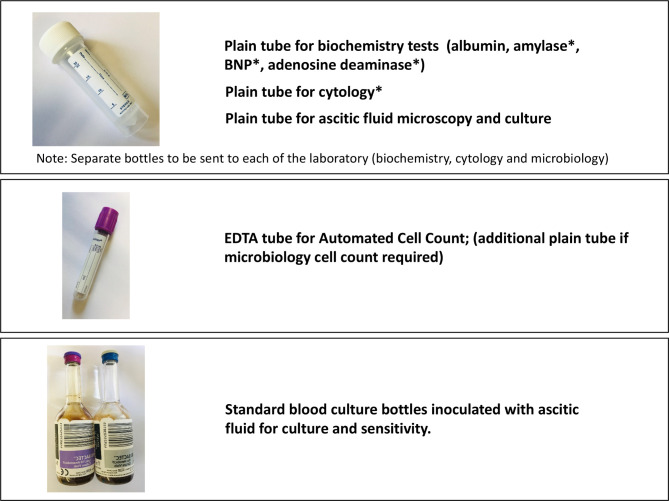
The ascitic fluid samples required from the diagnostic paracentesis is summarised in figure 2.
Figure 2.
The ascitic fluid samples required from diagnostic paracentesis. *These investigations should be considered based on pretest probability of specific diagnosis. BNP, brain natriuretic peptide.
Recommendations
A diagnostic paracentesis is recommended in all patients with new-onset ascites. (Quality of evidence: moderate; Recommendation: strong)
The initial ascitic fluid analysis should include total protein concentration and calculation of SAAG. (Quality of evidence: moderate; Recommendation: strong)
Ascites fluid analysis for cytology, amylase, BNP and adenosine deaminase should be considered based on pretest probability of specific diagnosis (Quality of evidence: moderate; Recommendation: weak)
Spontaneous bacterial peritonitis (SBP)
Spontaneous bacterial peritonitis is the development of bacterial infection of ascites in the absence of any intra-abdominal surgically treatable source of infection. The prevalence of SBP in outpatients is 1.5–3.5% and approximately 10% in hospitalised patients.28 A recent European study detected a prevalence of 11.3% among inpatients.29 When first described, mortality associated with SBP exceeded 90%, but, in-hospital mortality has been reduced to approximately 20% with early diagnosis and prompt treatment.30 In an observational study, each hour of delay in diagnostic paracentesis after admission was associated with a 3.3% increase of in-hospital mortality after adjusting for model for end-stage liver disease (MELD) score.31 Long-term survival remains poor; 1-year survival after hospitalisation with SBP in a UK study was found to be 34%.32 Patients recovering from an episode of SBP should always be considered as potential candidates for liver transplantation if they have not already been assessed.
Diagnosis of SBP
The diagnosis of SBP is confirmed when ascitic neutrophil count is >250 cells/mm3 in the absence of an intra-abdominal and surgically treatable source of sepsis. A cut-off point of 250 neutrophils/mm3 has the greatest sensitivity, although a cut-off point of 500 neutrophils/mm3 has greater specificity.33 A meta-analysis of primary data from 14 prospective trials has defined the positive and negative likelihood ratios of SBP at different thresholds for total white cell count (WCC) and PMN in ascitic fluid. WCC >1000 cells/µL or PMN ≥500 cells/µL are most accurate and yield positive likelihood ratios of 9.1 (95% CI 5.5 to 15.1) and 10.6 (95% CI 6.1 to 18.3), respectively. Likelihood ratios for WCC >500 cells/µL (5.9; 95% CI 2.3 to 15.5) and PMN >250 cells/µL (6.4; 95% CI 4.6 to 8.8) support routine clinical practice of using lower thresholds, where the greater risk lies with underdiagnosing SBP.18
Historically, ascitic neutrophil counts have been performed by manual microscopy, but, this is time and cost intensive. Automated counts, based on flow cytometry for counting and differentiating cells, are now used in most centres. This technique has been shown to have sensitivity and specificity close to 100%,34 35 allowing a tube containing ethylenediamine tetra-acetate (EDTA; as used for plasma full blood count) to be inoculated with ascitic fluid and processed on a standard blood count analyser. Reagent strips have insufficient sensitivity for reliable use in this context36 and hence cannot be recommended to replace cell count to diagnose SBP.
Ascitic fluid culture
Ascites culture is essential to help guide antibiotic therapy. Patients with ‘culture-negative neutrocytic ascites’ (PMN count >250 cells/mm3) have a similar presentation to those with culture-positive SBP. As both groups of patients have significant morbidity and mortality,37 38 they should be treated in a similar manner. Some patients have ‘bacterascites’ in which cultures are positive, but, ascitic neutrophil count is <250 cells/mm3. In some patients, bacterascites represents a transient and spontaneously reversible colonisation of ascites, in others, particularly those who are symptomatic, it may represent the first step in the development of SBP.33 Discussion with microbiologists about the organism cultured can help differentiate the above two scenarios, and when a positive culture is obtained a repeat tap should be sent to guide management.
Although the identification of pathogen(s) is essential for the management of infectious diseases, ascites fluid cultures often fail to provide positive results, even when using ascites samples from patients who develop clinical manifestations of SBP. Bacterial DNA detection and sequencing have been applied to the diagnosis of several infectious diseases, and molecular techniques can detect small amounts of bacterial DNA within a few hours. These promising techniques have yet to be introduced into routine clinical practice.39
Fungal peritonitis is a rare, less studied complication and observational data suggest a worse prognosis.40 In a large multicentre study of 2743 cirrhotic inpatients, of whom 1052 had infections, 12.7% of infected patients had evidence of fungal infections with a case fatality of 30%. The majority of these were urinary, but the highest mortality was seen with fungaemia and peritonitis (case fatality >50%).41
Secondary bacterial peritonitis
A small proportion of patients with cirrhosis may develop peritonitis secondary to perforation or inflammation of an intra-abdominal organ, known as secondary bacterial peritonitis. In a small retrospective analysis, secondary peritonitis represented 4.5% of all peritonitis in cirrhotic patients.42 This should be suspected in those who have localised abdominal symptoms or signs, very high ascitic neutrophil count, the presence of multiple organisms on ascitic culture or in those with inadequate response to treatment.42 Cross-sectional imaging, such as CT, should be performed with early consideration of surgery in this scenario.
Antibiotic therapy
The most common organisms isolated in patients with SBP include Escherichia coli, Gram-positive cocci (mainly streptococcus species) and enterococci. Empirical antibiotic therapy must be initiated immediately after the diagnosis of SBP.33 In the 1990s, cefotaxime, a third-generation cephalosporin, was extensively investigated in patients with SBP because it was found to cover 95% of organisms and high ascitic fluid concentrations could be achieved.43 44 The take home message from these studies is that matching an effective antibiotic to the cultured organism is key to successful treatment, rather than any apparent superiority of one drug over another. Since these studies, the landscape of bacterial resistance has significantly changed with an increase in antimicrobial resistant organisms,45 and therefore recommending a specific single empirical antibiotic is challenging. Thus, it is crucial to separate community-acquired SBP from healthcare-associated SBP (nosocomial – defined as infection >48 hours after hospital admission)46 and to consider both the severity of infection and the local resistance profile in order to decide the empirical antibiotic treatment of SBP.47 Over recent years there has been a significant increase in the number of infections caused by multidrug-resistant organisms,29 48 defined by an acquired non-susceptibility to at least one agent in three or more antimicrobial categories.49 It is also important to highlight the shift to extensively drug resistant bacteria, defined by non-susceptibility to at least one agent in all but two or fewer antimicrobial categories, or to pan-drug resistance bacteria, defined by non-susceptibility to all agents in all antimicrobial categories.49
A second diagnostic tap should be considered at 48 hours from starting treatment, to check the efficacy of antibiotic therapy in patients who have an apparently inadequate response. If ascitic fluid neutrophil count fails to decrease to less than 25% of the pretreatment value, this should raise suspicion of antibiotic resistance or the presence of ‘secondary peritonitis’.33 50 Specialist microbiology links should be developed within each trust to help guide local policy and patient management and, in addition, de-escalation of anti-microbial agents according to susceptibility of positive cultures is recommended.
The evidence for the use of human albumin solution and recommendations for its use in SBP are discussed in a separate section below.
Prophylactic therapy for SBP
Three groups at high risk of developing SBP have been identified: (i) patients with acute gastrointestinal (GI) haemorrhage; (ii) patients with a low ascitic protein concentration and no prior history of SBP (primary prophylaxis) and (iii) patients with a previous episode of SBP (secondary prophylaxis).51 Although antibiotic prophylaxis to prevent further infection in patients presenting to hospital with upper GI bleeding is established in clinical practice,52–54 there remains uncertainty over prophylaxis in other circumstances. Additional studies related to this area after the Cochrane review53 are summarised in online supplemental table 1.
gutjnl-2020-321790supp001.pdf (462.9KB, pdf)
Primary prophylaxis
Primary prophylaxis is a controversial area and broad recommendations are not straightforward. In 2016 the National Institute for Health and Care Excellence (NICE) recommended offering prophylactic oral ciprofloxacin or norfloxacin for people with cirrhosis and ascites and no history of SBP with an ascitic protein of ≤15 g/L (1.5 g/dL), until the ascites has resolved.55 Six studies were included in their analyses.56–61 The European Association for the Study of Liver (EASL) recommend primary prophylaxis with norfloxacin (400 mg/day) in patients with Child-Pugh score ≥9 and serum bilirubin ≥3 mg/dL, with either impaired renal function or hyponatraemia and ascitic fluid protein lower than 15 g/L.47 The American Association for the Study of Liver Diseases (AASLD) also suggest that antibiotics for primary prophylaxis of SBP should be considered for people at high risk of developing this complication, which was defined as an ascitic fluid protein <1.5 g/dL together with impaired renal function or liver failure.62
In contrast, in a large placebo-controlled randomised clinical trial, the NORFLOCIR trial, norfloxacin did not reduce 6-month mortality in patients with advanced cirrhosis, with >95% of patients included having no history of prior SBP.63 In post-hoc analyses, norfloxacin, appeared to increase survival of patients with low ascites fluid protein concentrations. However, other data have failed to replicate an association of incidence of SBP in patients with pre-existing low total ascitic fluid protein concentration in three large cohorts of hospitalised patients with cirrhosis and ascites.64 65 Furthermore, there are concerns about the potential consequences of long-term oral antibiotic therapy, including resistance, increased risk of Clostridium difficile associated diarrhoea, adverse reactions and drug interactions. In 2019 the Medicines and Healthcare products Regulatory Agency (MHRA) issued updated guidance on new restrictions and precautions for use of fluoroquinolone antibiotics following a detailed EU review of very rare reports of disabling and potentially longlasting or irreversible side effects affecting the musculoskeletal and nervous systems. Although SBP prophylaxis was not specifically considered, renal impairment is considered to increase this risk, and therefore healthcare professionals and patients should be vigilant during treatment with fluoroquinolone antibiotics and discontinue treatment at the first sign of tendon pain or inflammation. Finally, norfloxacin is not widely available in the UK.
In view of the uncertainties outlined above, we advocate primary prophylaxis is offered to patients considered at high risk, as defined by an ascitic protein count <1.5 g/dL. However, it is important that the potential risks and benefits and existing uncertainties are communicated to patients.
It is expected that a large ongoing multicentre UK trial (European Union Drug Regulating Authorities Clinical Trials Database Registration Number: 2019-000581-38) to investigate the efficacy of long-term co-trimoxazole compared with placebo as primary prevention for SBP may deal with these uncertainties.
Secondary prophylaxis
In patients who survive an episode of SBP, the cumulative recurrence rate at 1 year is approximately 70%.33 Probability of survival at 1 year after an episode of SBP is 30–50% and falls to 25–30% at 2 years.66 67 There is only one randomised, double-blind, placebo-controlled trial of norfloxacin (400 mg/day) in patients who had a previous episode of SBP68; treatment reduced the probability of recurrence of SBP from 68% to 20%. A recent systematic review with meta-analysis concluded that rifaximin may be effective for both primary and secondary SBP prophylaxis compared with systemically absorbed antibiotics and compared with no intervention.69 However, additional prospective studies are required before a change in clinical practice can be recommended. It has been suggested that proton pump inhibitor use may increase the risk for the development of SBP and indications for long-term use should be carefully assessed.70 71
We therefore recommend norfloxacin 400 mg once a day as secondary prophylaxis, although in view of limited availability in the UK, many centres use once daily ciprofloxacin 500 mg once a day as an alternative.
Recommendations
Diagnostic paracentesis should be carried out without a delay to rule out SBP in all cirrhotic patients with ascites on hospital admission. (Quality of evidence: moderate; Recommendation: strong)
A diagnostic paracentesis should be performed in patients with GI bleeding, shock, fever or other signs of systemic inflammation, gastrointestinal symptoms, hepatic encephalopathy, and in patients with worsening liver or renal function. (Quality of evidence: moderate; Recommendation: strong)
Ascitic neutrophil >250/mm3 count remains the gold standard for the diagnosis of SBP and this can be performed either by manual microscopy or using automated counts, based on flow cytometry for counting and differentiating cells. (Quality of evidence: moderate; Recommendation: strong)
Ascitic fluid culture with bedside inoculation of blood culture bottles should be performed to guide the choice of antibiotic treatment when SBP is suspected. (Quality of evidence: moderate, Recommendation: strong)
Immediate empirical antibiotic therapy should be determined with due consideration of context of SBP (community acquired or healthcare associated), severity of infection and local bacterial resistance profile. Cefotaxime has been widely studied, but choice of antibiotic should be guided by local resistance patterns and protocol. (Quality of evidence: moderate; Recommendation: strong)
A second diagnostic paracentesis at 48 hours from the start of treatment to check the efficacy of antibiotic therapy should be considered in those who have apparently inadequate response or where secondary bacterial peritonitis is suspected. (Quality of evidence: low; Recommendation: weak)
Patients presenting with gastrointestinal bleeding and underlying ascites due to cirrhosis should receive prophylactic antibiotic treatment (cefotaxime has been widely studied but the antibiotic should be chosen based on local data) to prevent the development of SBP. (Quality of evidence: strong, Recommendation: strong)
Patients who have recovered from an episode of SBP should be considered for treatment with norfloxacin (400 mg once daily), ciprofloxacin (500 mg once daily, orally) or co-trimoxazole (800 mg sulfamethoxazole and 160 mg trimethoprim daily, orally) to prevent further episode of SBP. (Quality of evidence: low; Recommendation: weak)
Primary prophylaxis should be offered to patients considered at high risk, as defined by an ascitic protein count <1.5 g/dL. However, it is important that the potential risks and benefits and existing uncertainties are communicated to patients. (Quality of evidence: low; Recommendation: weak)
Dietary salt restriction
There is little evidence to support salt restriction in patients with cirrhosis in absence of ascites. In patients with cirrhosis and ascites, seven RCTs and one cross-sectional survey have examined the role of salt restriction (online supplemental table 2).72–79 One of the studies has only been published as an abstract.76 Four of the earlier RCTs72–75 found no difference in ascites control in those with and without salt restriction. Two recent RCTs found that a salt unrestricted diet (5–6.5 g/day) in contrast to a salt restricted diet (<5 g/day), resulted in ascites disappearance in a larger proportion (45% vs 16%) over a shorter time period and also significantly reduced the need for large volume paracentesis (LVP).77 78 Additionally, five of the eight above-mentioned studies reported significant adverse events with salt restriction, including hyponatraemia,72 77 reduced caloric intake,76 77 79 higher risk of renal impairment (0% vs 14%),77 hepatic encephalopathy (HE), hepatorenal syndrome (HRS), SBP78 and mortality.77 78 In a study by Sorrentino et al,78 1-year mortality was 45–60% (salt unrestricted diet) versus 82.5% (salt restricted diet).
Thus salt restricted diets (<5 g m of salt, <85 mmol sodium/day) in patients with cirrhosis and ascites do not improve ascites control and, on the contrary, can result in complications. Additionally, such diets are difficult to comply with, especially since the average European ingests about 10 g of salt/day.80–82 A cross-sectional survey79 indicated that only about a third of cirrhotic patients were compliant with salt restriction, with an additional 45% incorrectly stating that they were. Based on these data, patients with cirrhosis and ascites should have a moderately salt restricted diet, with daily salt intake of no more than 5–6.5 g (87 mmol–113 mmol sodium). This translates to a no added salt diet with avoidance of precooked meals. An ongoing systematic review is assessing the role of salt restriction in patients with ascites due to cirrhosis.83
Recommendations
Patients with cirrhosis and ascites should have a moderately salt restricted diet with daily salt intake of no more than 5–6.5 g (87 mmol–113 mmol sodium). This translates to a no added salt diet with avoidance of precooked meals. (Quality of evidence: moderate; Recommendation: strong)
Patients with cirrhosis and ascites should receive nutritional counselling on the sodium content in the diet. (Quality of evidence: weak; Recommendation: strong)
Diuretics
Diuretics remain the main stay in management of ascites, though do not modify its natural history, providing only symptomatic benefit.47 Secondary aldosteronism plays a major role in renal sodium retention in patients with cirrhosis.84 Spironolactone is a specific pharmacological aldosterone antagonist, acting primarily through competitive binding of receptors at the aldosterone-dependent sodium–potassium exchange site in the distal convoluted renal tubule.85 Its hydrophilic derivative is potassium canrenoate. They are usually the first-line diuretics used,84 85 either alone or in combination with a loop diuretic such as furosemide (causing sodium to flood more distal nephron sites).86 Spironolactone appears to be more effective (response rate of 95%) than furosemide (response rate of 52%) in non-azotemic patients with cirrhosis and ascites.87 88 Spironolactone has a long elimination half-life, allowing once a day dosing89–91; dose changes should occur no more frequently than every 3–4 days.86
In those who are intolerant to spironolactone an alternative diuretic is amiloride (acts in the collecting duct). However it is not as effective, an earlier RCT showing response rates of 35% vs 70% in those receiving amiloride versus potassium canrenoate, respectively.92 Other diuretics which have been used in patients with cirrhosis and ascites include bumetanide93 and torasemide.94 95
Sequential versus combined therapy
Three RCTs assessing the role of sequential therapy (spironolactone followed by furosemide) or combination therapy (spironolactone plus furosemide) have given conflicting results (online supplemental table 3). In the first study,88 onset of diuresis was faster in the combination group than in the sequential group. The second RCT mostly included those with first presentation of ascites and found no difference in sequential versus combined therapy for the rapidity of ascites mobilisation and incidence of complications. However, a need for dose reductions was significantly higher in the combination group (68% vs 34%).96 The third RCT included almost two-thirds of patients with prior ascites.97 It reported shorter mean time for ascites resolution, lower risk of adverse events (especially hyperkalaemia), lower treatment failures (24% vs 44%), with ascites resolving in a higher percentage without need for diuretic dose change (76% vs 56%) in the combination versus sequential group, respectively.97
These conflicting results are explained by the heterogeneous patient population as studies by Angeli et al 97 and Fogel et al 88 included those with more advanced disease, explaining the lower response to spironolactone monotherapy. Others have also reported the likelihood of response to spironolactone monotherapy (vs no response) if a first occurrence (56% vs 37%) rather than recurrent (44% vs 63%) or large ascites (16% vs 58%).98 Since in non-azotemic cirrhotic patients with ascites, the distal tubule reabsorbs almost all the sodium delivered, it is unsurprising that the administration of spironolactone alone results in a good natriuretic response in most.96 99 Another advantage of spironolactone monotherapy is its modest diuretic effect,86 as patients with cirrhosis are sensitive to compromises in their intravascular volume.91
Therefore, in patients with first presentation of moderate ascites, starting treatment with spironolactone monotherapy (starting dose 100 mg, increased to 400 mg) is reasonable. In those with persistent or severe ascites, and if faster diuresis is needed (for example, if hospitalised), it may be prudent to use combination therapy with spironolactone and furosemide (starting dose 40 mg, increased to 160 mg). Although maximal daily recommended doses of spironolactone and furosemide are 400 mg and 160 mg respectively,92 97 98 100 these are rarely achieved.88 96 In the largest study until now, which recruited about 2000 patients with ascites, at the time of discharge, mean diuretic units (one unit being 40 mg furosemide and 100 mg spironolactone) varied from 2.5+0.2 to 2.7+0.3.101
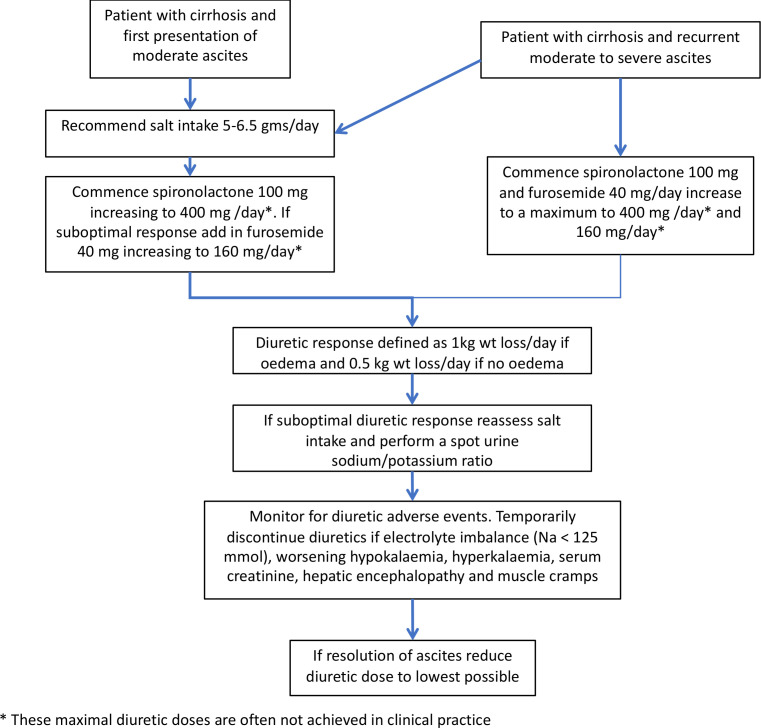
Based on evidence from an earlier RCT, it is recommended that diuretic-induced weight loss should not exceed 0.5 kg/day in patients without peripheral oedema, and 1 kg in the presence of peripheral oedema.47 100 Figure 3 summarises the stepped-up98 approach to diuretic treatment.
Figure 3.
Approach to the use of diuretics in the management of ascites in patients with cirrhosis.
Adverse reactions to diuretics
All patients initiating diuretics should be monitored for adverse events, the prevalence of which ranges from 19%96 to 33%.88 97 Almost half with adverse events require diuretic discontinuation or dose reduction.88 In hospitalised patients treated with diuretics, hepatic encephalopathy is seen in up to 25%102 and renal impairment in 14–20%,97 102 especially in the absence of peripheral oedema.100 Renal impairment is usually of moderate severity and is reversible on discontinuing diuretics.10 Hyponatraemia occurs in 8–30% and is related to impaired ability of the kidneys to excrete free water.10 97 Hypokalaemia is also a frequent side effect of loop diuretics.10 Similarly hyperkalaemia can occur in up to 11%.97
Gynaecomastia is commonly seen with spironolactone, especially with higher doses.86 It occurs less frequently with potassium canrenoate (53% vs 100%).103 Eplerenone can also relieve the gynaecomastia.104 105
A causal relation is found between cirrhosis and muscle cramps, especially in advanced cirrhosis, with prevalence varying between 26% and 72%,.106–108 The cirrhosis-induced arterial underfilling probably plays a role in the pathogenesis of cramps.107 Diuretics accentuate this reduction in effective plasma volume, thereby increasing the prevalence of cramps.107 An earlier systematic review (including only three RCTs) assessed various interventions for muscle cramps, including zinc, 1-α-hydroxyvitamin D, vitamin E, branched chain amino acids, taurine, intravenous albumin and quinidine. Improvements occurred with most interventions with the exception of vitamin E.109 Recent RCTs have reported beneficial effects with methocarbamol,110 taurine111 and baclofen.112
Monitoring of diuretics
The aim of diuretic therapy is to ensure that urinary sodium excretion exceeds 78 mmol/day (88 mmol intake per day – 10 mmol non-urinary excretion per day).62 113 A random spot urine sodium:potassium ratio between 1.8 and 2.5 has a sensitivity of 87.5%, specificity of 56–87.5% and accuracy of 70–85% in predicting a 24-hour urinary sodium excretion of 78 mmol/day.114 115
Hyponatraemia
Recent guidelines define hyponatraemia as a serum sodium <135 mmol/L, with 130–135 mmol/L, 125–129 mmol/L and <125 mmol/L, constituting mild, moderate and severe hyponatraemia, respectively.47 116 A prospective population survey among patients with cirrhosis found serum sodium <130 mmol/L in 21.6%.117 Hyponatraemia has been associated with higher prevalence of refractory ascites, hepatic encephalopathy, SBP, HRS and mortality.117–119 Acknowledging this, the Model for End Stage Liver Disease (MELD) score now incorporates serum sodium (MELD-Na).120 Those with cirrhosis and chronic hyponatraemia are often asymptomatic and seldom need treatment.62
Both hypovolaemic and hypervolaemic hyponatraemia is observed in cirrhosis.47 Hypovolaemic hyponatraemia results from overzealous diuretic therapy, being characterised by a prolonged negative sodium balance with marked loss of extracellular fluid. Its management requires expansion of plasma volume with normal saline and cessation of diuretics.47 Most hepatologists would discontinue diuretics if serum sodium is <125 mmol/L.
A number of studies (which include four RCTs and a retrospective cohort study) have assessed role of intravenous (IV) human albumin solution (HAS) in patients with hyponatraemia (online supplemental table 4).121–126 These studies did not strictly stratify patients as having hypovolaemic hyponatraemia and mostly included those with Child C cirrhosis and refractory ascites undergoing LVP. There were differences in baseline serum sodium levels, use of diuretics, and degree of salt and fluid restriction. Therefore not unsurprisingly results have been conflicting. An earlier meta-analysis that included three of the above RCTs123–125 reported that use of IV HAS versus no IV HAS reduced occurrence of hyponatraemia (3.9% vs 16.5%) but did not affect mortality.127 A later meta-analysis (IV HAS vs no IV HAS) which included only two RCTs123 124 found no beneficial effects on hyponatraemia or mortality.128 In the retrospective cohort study among hospitalised patients with hyponatraemia (only 42% requiring inpatient LVP), those receiving IV HAS were more likely to have resolution of hyponatraemia than those who did not (85.41% vs 44.78%).126 Hyponatraemia resolution was an independent predictor of 30-day survival, even after adjustment for admission sodium and glomerular filtration rate. At present, however, there is insufficient evidence to routinely recommend IV HAS outside of a LVP setting in patients with cirrhosis and ascites and hypovolaemic hyponatraemia.
Hypervolaemic hyponatraemia is more common in cirrhosis, occurring owing to non-osmotic hypersecretion of vasopressin and enhanced proximal nephron sodium reabsorption with impaired free water clearance, both being caused by effective hypovolaemia.47 129 Impaired free water clearance is observed in about 60% of patients with cirrhosis.130
Hypervolaemic hyponatraemia requires a negative water balance.47 Many hepatologists do recommend fluid restriction of between 1 and 1.5 L/day in presence of severe hyponatraemia (serum sodium <125 mmol/L). However, there are few data to support the level of serum sodium at which to initiate fluid restriction and how much fluid to restrict. It is sodium restriction and not fluid restriction that results in weight loss as fluid passively follows the sodium.62 113 131 Although fluid restriction may be helpful in preventing further decrease in serum sodium, it only rarely improves it. This is because on a practical level, fluid restriction to <1 L /day is not tolerated.129 Water restriction should be reserved for those who are clinically hypervolaemic with severe hyponatraemia (serum sodium <125 mmol/L) with normal serum creatinine and not currently receiving diuretics. These recommendations are based on our consensus and consistent with our earlier guidelines.132 It is also our consensus that fluid restriction is unnecessary in absence of hyponatraemia.
Hypertonic sodium chloride (3%) administration may improve hyponatraemia at the cost of worsening fluid overload. It is best reserved for those with severely symptomatic acute hyponatraemia, especially if a transplant is imminent.47 To prevent rapid increase in serum sodium and the risk of developing central pontine myelinolysis,133 guidelines recommend a serum sodium increase of up to 5 mmol/L in the first hour with a limit of 8–10 mmol/L every 24 hours thereafter until the serum sodium concentration reaches 130 mmol/L.47 116 134 135
Vaptans
Vaptans are vasopressin antagonists that competitively bind and block the V2-receptors of arginine vasopressin in the renal collecting ducts and induce a highly hypotonic diuresis without affecting the excretion of electrolytes.136 In three RCTs involving 1200 patients, satavaptan was no more effective than placebo in controlling ascites and need for LVPs, though it improved serum sodium concentration in those with hyponatraemia.137 Two of the studies were terminated owing to an increase in serum bilirubin, higher mortality (31% vs 22%), mostly due to increased cirrhosis complications and other adverse events.137 Two meta-analyses138 139 on vaptans in cirrhosis reported improved serum sodium levels and ascites mobilisation, but without a beneficial effect on cirrhosis-related complications or mortality (RR=1.06, 95% CI 0.90 to 1.26). Current evidence does not support routine use of vaptans in cirrhosis.
Midodrine
Portal hypertension and splanchnic vasodilatation are major contributors to the development of ascites.7 In fact, mean arterial pressure and plasma norepinephrine are two of the best predictors of prognosis in ascites.140 Therefore, vasopressors such as midodrine, an α-adrenergic agonist, have been used in non-azotemic patients with ascites, resulting in significant increase in mean arterial pressure and urine sodium excretion and significant decreases in plasma renin and aldosterone.141
A small RCT in patients with refractory ascites (midodrine 7.5 mg three times a day vs standard medical therapy) showed that at 3 months 94% versus 50% had a complete/partial ascites control, with a trend for a survival benefit in the midodrine group.142 In another small RCT (midodrine vs placebo), significant reduction in body weight and abdominal girth was observed after 2 weeks of midodrine therapy.143 Though larger RCTs are needed to confirm these findings, it may be appropriate to consider use of midodrine in refractory ascites on a case-by-case basis.
Recommendations
In patients with the first presentation of moderate ascites spironolactone monotherapy (starting dose 100 mg, increased to 400 mg) is reasonable. In those with recurrent severe ascites, and if faster diuresis is needed (for example, if the patient is hospitalised), combination therapy with spironolactone (starting dose 100 mg, increased to 400 mg) and furosemide (starting dose 40 mg, increased to 160 mg) is recommended. (Quality of evidence: moderate; Recommendation: strong)
All patients initiating diuretics should be monitored for adverse events. Almost half of those with adverse events require diuretic discontinuation or dose reduction. (Quality of evidence: low; Recommendation: weak)
Hypovolaemic hyponatraemia during diuretic therapy should be managed by discontinuation of diuretics and expansion of plasma volume with normal saline. (Quality of evidence: low; Recommendation: weak)
Fluid restriction to 1–1.5 L/day should be reserved for those who are clinically hypervolaemic with severe hyponatraemia (serum sodium <125 mmol/day). (Quality of evidence: low; Recommendation: weak)
Hypertonic sodium chloride (3%) administration should be reserved for those who are severely symptomatic with acute hyponatraemia. Serum sodium should be slowly corrected. (Quality of evidence: low; Recommendation: weak)
It may be appropriate to consider use of midodrine in refractory ascites on a case-by-case basis. (Quality of evidence: low; Recommendation: weak)
Large volume (therapeutic) paracentesis
Large volume paracentesis (LVP) is the standard of care for managing large volume ascites both in conjunction with diuresis to relieve symptoms of a tense abdomen, as well as in the management of refractory ascites, when diuretics become ineffective or the side effects preclude their continued use. Development of refractory ascites is of prognostic significance,144 therefore, at its onset, suitability of liver transplantation should be considered and assessed as a priority.
Performance standards
An efficient LVP service can be provided safely in a day case or outpatient setting and non-physician healthcare providers such as GI endoscopy assistants145 and specialist nurses146 can be trained to perform therapeutic paracentesis and lead the service effectively. Exemplar training programmes indicate that 10 supervised procedures would be optimal for training to achieve competence in performing therapeutic paracentesis; mean duration of LVP was 97±24 min, and the mean volume of ascitic fluid removed was 8.7±2.8 L.145
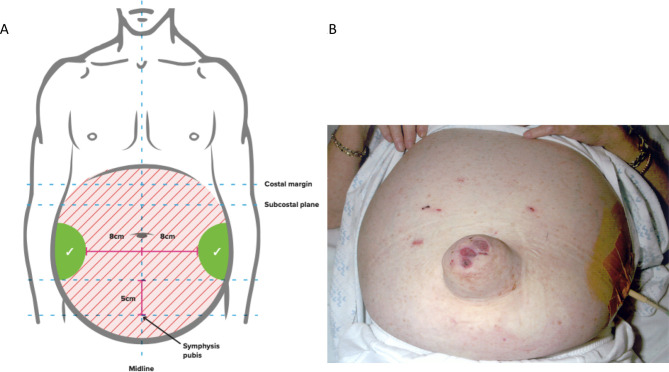
Patients should provide informed consent before the procedure. In 52 patients (15% obese) with ascites due to cirrhosis, ultrasound demonstrated that the left lower quadrant abdominal wall was thinner and depth of ascites greater, therefore, a suitable site for drain insertion.147 To minimise the risk of injury to the inferior epigastric artery (and avoid the liver and spleen) during paracentesis, point of puncture should be at least 8 cm from the midline and 5 cm above the symphysis148–150 (figure 4). All ascitic fluid should be drained to dryness in a single session as rapidly as possible over 1–4 hours assisted by gentle mobilisation of the cannula or turning the patient onto their side, if necessary. After the paracentesis, the patient should lie on the opposite side for 2 hours if there is leakage of any remaining ascitic fluid, and/or a suture (ideally purse-string) inserted around the site of drainage. These steps help to minimise the risk of ascitic fluid leakage.
Figure 4.
Anatomical landmarks for the safe performance of paracentesis [A] and performance of ascites drainage in patients with large ascites [B].
Adverse events
A systematic review of adverse events that can result from a paracentesis reported an overall rate of significant bleeding ranging from 0% to 2.7%, ascitic fluid leak in 0% to 2.35%, perforation in 0.83%, residual catheter tip fragment in 0.41% and death in 0% to 17% among studies that were heterogeneous.18 In a retrospective study published in abstract form, of consecutive 3116 ultrasound guided LVPs, the mean international normalised ratio (INR) was 2.1 (range 1.0–7.0) and MELD score 24 (range 6–40) for inpatients, and 1.5 (range 1‐5–5) and 16 (range 5–40) for outpatients, respectively. With no patients receiving fresh frozen plasma (FFP) before the procedure, a total of six patients (0.19%) had post‐LVP bleeding requiring blood transfusion (one inpatient, five outpatients) and one required angiography with embolisation of a bleeding abdominal wall vessel. No patient died.151 In another study where GI endoscopy assistants performed 1100 large volume paracenteses, with a preprocedure mean INR of 1.7 (range 0.9–8.7) and the mean platelet count was 50.4×109/L (range, 19–341×109/L), there were no significant procedure-related complications.145
Risk factors for haemorrhagic complications after paracentesis in three studies (which included patients with acute on chronic liver failure) were high MELD and Child-Pugh scores and renal impairment.152–154 In a study by Hung et al, acute kidney injury at he time of paracentesis was the only independent predictor of post-paracentesis haemoperitoneum, independent of MELD score, large volume paracentesis, sepsis, platelets, INR and haemoglobin levels.153 While some patients with bleeding complications after paracentesis have low platelet counts, elevated INR and low fibrinogen levels, this is invariably accompanied with high MELD scores (>25) and/or renal impairment.152 154
Ultrasound guidance
Use of ultrasound guidance may reduce the adverse events related to LVP.155 In a study involving 1297 procedures, 723 (56%) with ultrasound guidance and 574 (44%) without where the indications for paracentesis were similar between the two groups, the incidence of adverse events was lower in the ultrasound-guided procedures.156 In another retrospective cohort study, 0.8% of 565 patients undergoing paracentesis experienced bleeding complications. After adjustment, ultrasound guidance was associated with lower risk of bleeding complications by 68%.157
Recommendations
Patients should give informed consent for a therapeutic or diagnostic paracentesis. (Quality of evidence: low; Recommendation: strong)
Ultrasound guidance should be considered when available during LVP to reduce the risk of adverse events. (Quality of evidence: low; Recommendation: weak)
Routine measurement of the prothrombin time and platelet count before therapeutic or diagnostic paracentesis and infusion of blood products are not recommended. (Quality of evidence: moderate and Recommendation: strong)
Use of human albumin solution (HAS)
Plasma expansion after paracentesis
One study evaluating haemodynamic and neurohumoral responses in 12 patients after a single, 5 L total paracentesis concluded that it was safe to omit albumin in these patients.158 However, a subsequent study including 80 patients with acute on chronic liver failure (ACLF) found that albumin significantly reduced complications (renal impairment, hyponatraemia and death) following <5 L paracentesis compared with no administration of fluid.159 Thus in <5 L paracentesis we recommend that plasma expansion is not necessary, unless there is evidence of ACLF. This recommendation is based on consensus rather than evidence and is consistent with other international guidance.47
Plasma volume expansion should always be used for LVP with >5 L of ascites removed. Serial paracenteses with and without albumin replacement have been evaluated in patients with tense ascites.123 124 There was a higher rate of renal impairment, fall in serum sodium levels, and a marked activation of the renin–angiotensin–aldosterone system in those not treated with albumin. However, the pooled risk ratio from these studies, which are more than 25 years old, showed only a tendency toward benefit of albumin (pooled RR=0.23, 95% CI 0.03 to 1.64) (online supplemental table 5). The consensus is that volume expansion should be used with LVP. We recommend large volume paracentesis in one session and discourage repeated low volume paracentesis, which offers no additional benefits and carries a higher risk of procedure-related complications.
Some debate remains over the use of albumin or artificial plasma expanders for volume expansion. Pooled analysis of 10 studies160–169 found that cirrhotic patients undergoing paracentesis who received albumin were no less likely to develop renal dysfunction than patients undergoing paracentesis that received an alternative plasma expander (pooled RR=1.11, 95% CI 0.58 to 2.14) (online supplemental table 5). Analysis from two other independently conducted systematic reviews is consistent with these findings.127 128 Pooled analysis from eight studies160 162–164 166 167 169 170 found that cirrhotic patients undergoing paracentesis who received albumin were no less likely to die than those who received an alternate plasma expander (pooled RR=0.83, 95% CI 0.61 to 1.12) (online supplemental table 6), which is supported by two systematic reviews.127 128 However, when all comparators to albumin (including control and vasoconstrictor alone) are pooled (16 RCTs) the RR is 0.77 (95% CI 0.57 to 1.00). This translates to 57 to 100 fewer patients per 1000 dying after LVP when HAS is used (online supplemental table 6).
Less clinically important outcomes have been shown to improve in patients treated with HAS versus other plasma expanders. There is a decreased incidence of post-paracentesis-induced circulatory dysfunction (defined as a decrease in plasma renin) in patients undergoing LVP treated with albumin compared with an alternative plasma expander in a meta-analysis containing eight RCTs127 (OR=0.34, 95% CI 0.23 to 0.51), and a pooled decrease in hyponatraemia in nine RCTs (OR=0.61, 95% CI 0.40 to 0.93).127 Both are supported in a second independently conducted systematic review.128
Most of the plasma expanders used in the described studies are no longer in use and have been restricted by the European Medicines Agency (eg, polygeline carries risk of prion transmission, dextran the risk of allergic reaction and hydroxyethyl starch association with renal impairment and deranged coagulation). Therefore, consensus is that volume expansion should be with HAS due to availability, familiarity of use and suggested benefits in the available studies.
Two small prospective RCTs compared standard dose (6–8 g/L of ascites drained) albumin after LVP with low-dose albumin (2–4 g/L).171 172 Pooled results from 70 patients suggested no difference in post-paracentesis-induced circulatory dysfunction (RR=2.97, 95% CI 0.89, 9.91) and no development of renal dysfunction (no events in either group). A larger retrospective review of 935 patients found no increase in renal dysfunction when adherence to guidance (8 g/L after 5 L drained) was implemented,173 but significant cost savings were made because less HAS was used.
Potential cost savings have been proposed in relation to length of hospital stay in patients with ascites undergoing LVP who are treated with HAS as compared with an alternative plasma expander.166 However, HAS is more expensive than alternatives and is in worldwide shortage, therefore it should be prescribed according to recommended guidance based on the available evidence.174 There have been no cost-effectiveness analyses in the UK.
Until further studies are undertaken to compare efficacy of albumin against clinically available artificial plasma expanders, we would recommend that albumin remains the preferred plasma expander when paracentesis is undertaken. Albumin (as 20% or 25% solution) should be infused after paracentesis of >5 L is completed at a dose of 8 g albumin/L of ascites removed.
Albumin infusion in SBP
Renal impairment develops in up to 30% of patients with SBP and is one of the strongest predictors of mortality,175 176 alongside progressive liver dysfunction. Three studies176–178 have compared albumin with no intervention, and one RCT179 compared albumin with a plasma expander in order to prevent the development of renal impairment in patients with SBP. Cirrhotic patients with SBP treated with albumin were 72% less likely to develop renal dysfunction than patients with SBP who did not receive albumin (288 patients, pooled RR=0.28, 95% CI 0.16 to 0.50) (online supplemental table 7). There was also a decrease in mortality in patients with SBP treated with albumin, with patients 47% less likely to die than those not receiving albumin (334 patients, pooled RR=0.53, 95% CI 0.36 to 0.79) (online supplemental table 8). Therefore, we recommend the use of albumin in patients with SBP to prevent the development of renal dysfunction and decrease mortality.
Although patients with SBP have a higher risk of post-drain renal dysfunction, LVP is not contraindicated. Therefore, if LVP is indicated in a patient with SBP then this should proceed with HAS support. The dose of albumin in original studies was 1.5 g albumin per kg body weight within 6 hours of diagnosis and 1.0 g/kg on day 3, using estimated dry weight, which is often difficult in cirrhotic patients. Some small studies have suggested that lower doses of albumin are as effective in preventing renal dysfunction and mortality in SBP,180 181 and one retrospective review including 88 patients with SBP suggested that doses of HAS in excess of 87.5 g (>4×100 mL 20% HAS) are associated with a worse outcome, possibly secondary to fluid overload.182 Fluid overload has been reported in prospective studies of albumin in patients with cirrhosis and non-SBP infection.183 184 Therefore, if patients have an increased serum creatinine or a rising serum creatinine, we recommend 1.5 g albumin/kg within 6 hours of diagnosis, followed by 1 g/kg on day 3.
Long term regular outpatient HAS therapy
Improving morbidity and mortality by long-term administration of albumin to patients with decompensated cirrhosis and ascites has been explored in six studies with three recent RCTs, in contrasting patient groups, with contradictory findings (online supplemental table 9).185–190
In the ANSWER185 study, 431 patients with uncomplicated ascites receiving diuretics were randomised to weekly outpatient HAS infusions or no additional intervention (standard medical therapy). The study had a pragmatic approach and was unblinded. Overall 18-month survival was significantly higher in the standard therapy plus HAS than in the standard medical therapy group (Kaplan-Meier estimates 77% vs 66%; p=0.028), resulting in a 38% reduction in the mortality hazard ratio (0.62, 95% CI 0.40 to 0.95). There were additional benefits with lower incidence rate ratio (IRR) for infection (SBP and non-SBP) and renal dysfunction. However, unlike the standard therapy group, the HAS group had weekly medical professional contact when IV albumin was administered which could possibly have caused a confounding effect by improving standard of care in this group.
In the MACHT186 study, a double-blind, placebo-controlled trial, patients with advanced cirrhosis (MELD score 17–18) awaiting liver transplantation received outpatient fortnightly treatment with midodrine and albumin. This slightly suppressed vasoconstrictor activity but did not prevent complications of cirrhosis or improve survival. However, only nine patients were treated for the entire year, the median length of treatment was only 80 days and the mortality rate in both arms was very low due to patients undergoing timely liver transplantation. Perhaps, therefore, a greater dose of albumin or longer duration of treatment is required to benefit patients and should be targeted at those who are not close to receiving a liver transplant.
Di Pascoli et al 190 most recently published outcomes of a study of 45 patients with refractory ascites undergoing regular LVP who accepted 20 g twice weekly albumin plus diuretics and sodium restriction versus 25 patients who did not (non-randomised, single centre, not blinded). Cumulative incidence of mortality was 41.6% in the albumin group versus 65.5% in the standard of care group. Albumin-treated patients had a lower probability of hospitalisation. There were no differences in the number of LVPs performed. Follow-up was 400 days in the albumin group and 318 in the standard of care group. Although the study was non-randomised (patient choice to treatment arm) it does provide some additional evidence that using albumin in a longer-term outpatient setting may be beneficial, as in the ANSWER study, even in patients with very advanced disease. Two older studies support the use of outpatient albumin therapy in decreasing hospital admissions and LVP requirement with conflicting results on mortality.187 189
We expect these studies to stimulate further investigation to determine whether long-term albumin administration is feasible, efficacious and cost-effective in patients with cirrhosis and ascites within the NHS. Further research is required to determine which patients could benefit most from treatment, which seems to be those with less advanced disease who could receive treatment for at least 12 months. At present it is not possible to recommend the use of outpatient albumin administration in patients with ascites due to cirrhosis.
Recommendations
Albumin (as 20% or 25% solution) should be infused after paracentesis of >5 L is completed at a dose of 8 g albumin/L of ascites removed. (Quality of evidence: high; Recommendation: strong)
Albumin (as 20% or 25% solution) can be considered after paracentesis of <5 L at a dose of 8 g albumin/L of ascites removed in patients with ACLF or high risk of post-paracentesis acute kidney injury. (Quality of evidence: low; Recommendation: weak)
In patients with SBP and an increased serum creatinine or a rising serum creatinine, infusion of 1.5 g albumin/kg within 6 hours of diagnosis, followed by 1 g/kg on day 3, is recommended. (Quality of evidence: low; Recommendation: weak)
Transjugular intrahepatic portosystemic stent shunt (TIPSS)
TIPSS decompresses the portal system by creating an artificial communication between the portal and the hepatic vein. TIPSS results in an increase in cardiac output and decrease in systemic vascular resistance in the short term.191–193 Consequently, TIPSS leads to improvement in effective hypovolaemia and renal function, resulting in increased urinary sodium excretion.191–195 The increase in urinary sodium correlates with the reduction in plasma renin activity.195 196
The efficacy of TIPSS in the management of ascites has been compared with LVP in seven RCTs (online supplemental table 10).194 197–202 The first six trials used bare metal stents. Shunt dysfunction due to stent stenosis or thrombosis is a common complication of bare stents and develops in up to 80% of patients.199 201 The use of polytetrafluoroethylene (PTFE)-covered stents has significantly increased the long-term patency of the stent to 92% at 1 year and 89% at 2 years.202–204
TIPSS controls ascites better than LVP. Patients treated with TIPSS are more likely to be free of recurrent ascites than those treated with LVP at 12 months.205 However, the impact of TIPSS on survival is less consistent. The initial trial by Lebrec et al 194 showed a better survival with LVP mainly due to the detrimental effect of TIPSS in Child-Pugh C patients. The subsequent three studies did not show any difference in survival between TIPSS and LVP,197–199 whereas the most recent three studies have shown improved survival with TIPSS.200–202 In the two meta-analyses of the six trials using bare stent grafts, Bai et al 205 reported an improved transplant-free survival with TIPSS but this was limited to patients with recurrent ascites and not refractory ascites in the other study.206 All the six trials with bare metal stents consistently showed that TIPSS resulted in higher incidence of hepatic encephalopathy than with LVP.
PTFE-covered stents are now standard of care, and hence the results of the historical RCTs using bare stents are less relevant. Only the most recent RCTs compared PTFE-covered stent with LVP in patients with recurrent ascites. It demonstrated improved 1-year survival with TIPSS without any increased incidence of hepatic encephalopathy.202
TIPS has also been shown to improve the quality of life and nutritional status in patients with refractory ascites,207–209 but the improvement was dependent on the resolution of ascites.
TIPSS technique
A controlled study comparing TIPSS with 8 mm and 10 mm covered stent was stopped early as the 8 mm stent was not effective in controlling portal hypertensive complications (variceal bleeding and ascites).210 The rate of hepatic encephalopathy was equal between the two groups. However, in another study of variceal bleeding, 8 mm stents were as effective as 10 mm stents in preventing rebleeding, with a 50% reduction in the incidence of encephalopathy with 8 mm stents.211 Nonetheless, the applicability of these data in patients with refractory ascites is unclear, and evidence suggests that stent diameter increases over time.212 In a retrospective study of patients with refractory ascites, 10 mm covered stent led to better control of ascites than an 8 mm stent, without any increase in encephalopathy.213 Data from recent TIPSS registry in Germany reported an improved survival with 8 mm than with 10 mm covered stent.214 The optimal diameter of covered TIPS stent in refractory ascites remains unclear.
The volume of TIPSS being performed in an individual hospital influences outcomes. Inpatient mortality was significantly lower in centres performing more than 20 TIPS procedures per year.215
Patient selection
Careful selection of patients with refractory ascites to be treated with TIPSS is vital to maximise the benefits and reduce the harmful effects of the treatment. The exclusion criteria for the insertion of TIPSS in the seven RCTs are reported in online supplemental table 10.
Patients with advanced stages of cirrhosis have been excluded from the trials as indicated by serum bilirubin,197–202 prolonged prothrombin time,198 199 renal dysfunction194 197 198 200–202 and Child-Pugh score.200–202 Presence of chronic hepatic encephalopathy was an exclusion criterion in all the RCTs; the presence of pre-TIPS encephalopathy has been shown to be a predictor of poor outcome following TIPSS.216–218
In retrospective studies, patients with advanced disease had a worse outcome following TIPS insertion. The MELD score was originally developed to predict survival following TIPSS and included serum creatinine, bilirubin, INR and aetiology of cirrhosis.219 The MELD score has now evolved into a prognostic score in patients with cirrhosis.220 Among patients with refractory ascites treated with TIPSS with covered stent, 1-year survival is 84% in those with MELD score <15 compared with 54% for MELD score >18.221 In refractory ascites, a simple model of serum bilirubin and platelet count has been shown to predict 1-year survival.222 The survival rate of patients with serum bilirubin >50 µmol/L or platelet count of <75×109/L was only 31.2% compared with 73.1% in patients with serum bilirubin <50 µmol/L and platelet count of >75×109/L. The exact survival rate quoted here has to be interpreted cautiously as earlier patients in the cohort were treated with a bare metal stent before the introduction of a PTFE-covered stent. Hyponatraemia has also been shown to be related poor survival following TIPSS,223 but subsequent studies have not reproduced this finding.221 224 Up to 50% of patients with decompensated cirrhosis have sarcopenia, which in turn negatively affects clinical outcome in these patients. However, the direct impact of sarcopenia on outcomes following TIPSS is unclear. Sarcopenia has been shown to be independently associated with development of post-TIPSS encephalopathy, but this study included patients with refractory ascites and uncontrolled variceal bleeding.225 A recent retrospective study published in abstract form on patients undergoing TIPSS for refractory ascites reported no impact of sarcopenia on outcomes after TIPSS.226 Furthermore, they reported an improvement in muscle mass following TIPSS insertion.
Although increasing age has been associated with poorer survival following TIPSS,215 227 228 the mean age in these studies was between 54 and 57 years. Patients above the age of 70 were generally excluded from the previously mentioned RCTs. There is a lack of data in the literature for clinical outcome following TIPS in older patients (>70 years). Interestingly, functional disability as measured by patient-reported activities of daily living predicts post-TIPSS mortality adjusted for MELD score.229
The management of hepatic encephalopathy after TIPSS is beyond the scope of this document and is discussed in the recent BSG/BASL TIPSS guidelines.
Recommendations
TIPSS should be considered in patients with refractory ascites. (Quality of evidence: high; Recommendation: strong)
Caution is required if considering TIPSS in patients with age >70 years, serum bilirubin >50 µmol/L, platelet count <75×109/L, MELD core ≥18, current hepatic encephalopathy, active infection or hepatorenal syndrome. (Quality of evidence: moderate; Recommendation: strong)
Management of umbilical hernia in patients with ascites
In the cirrhotic patient, the incidence of abdominal wall hernia is 16% and reaches 24% in the presence of ascites; more than half of these are umbilical hernias.230 These progressively enlarge and are prone to complications, including ulceration of the overlying skin, incarceration, strangulation, and rupture. Non-operative management of complicated hernias with antibiotics and dressing changes might result in mortality rates in the range of 60–88%.231 On the other hand, hernia repair in cirrhosis has often been avoided due to high postoperative morbidity and mortality. Emergency surgery (OR=10.32, 95% CI 3.66 to 47.82), Child-Pugh-Turcotte class C (OR=5.52, 1.67 to 32.45), American Society of Anaesthesiologists (ASA) score ≥3 (OR=8.65, 3.65 to 87.23) and MELD score ≥20 (OR=2.15, 2.71 to 32.68) are associated with mortality.232
A recent retrospective series of 102 patients, who underwent surgical repair of the umbilical hernia in the presence of ascites, included 45 patients having emergency surgery (24 with incarceration, 12 with rupture of the hernia sac, and nine with skin ulceration or necrosis). Morbidity and mortality rates of 37.2% and 3.9%, respectively, were observed.233 Optimising management of ascites, including LVP and TIPSS perioperatively, could reduce the risk of wound dehiscence and recurrence of hernia.234
Recommendations
Suitability and timing of surgical repair of umbilical hernia should be considered in discussion with the patient and multidisciplinary team involving physicians, surgeons and anaesthetists. (Quality of evidence: low; Recommendation: strong)
Hepatic hydrothorax
Hepatic hydrothorax (HH) is accumulation of transudative fluid in the pleural space in the absence of cardiac, pulmonary or pleural disease, affecting approximately 5–12% of patients with advanced liver disease.235 The first-line management of hydrothorax is based on controlling ascites with diuretics and/or LVP as discussed previously. However, pleural effusion can persist despite successful treatment of ascites, defined as refractory hydrothorax.47
Therapeutic thoracentesis is required to provide symptomatic relief from dyspnoea but the effect is transient. Repeated procedures increase the risks of complications, including pneumothorax, bleeding and pleural infection.236 TIPSS has been suggested either as a definitive treatment or as a bridge to transplantation in patients with refractory hydrothorax. Meta-analysis of six retrospective studies with a total of 198 patients reported a response rate of 56%.237 Mortality within 45 days of TIPSS placement was 18%, mainly related to older age and the severity of liver disease. However, without any data on comparison with standard treatment, it is difficult to interpret the impact of TIPSS on survival. It is also worth noting that only one of these studies reported on patients treated with PTFE-covered stents.238 In patients with contraindications to TIPSS or liver transplant, a permanent indwelling pleural catheter can be considered in conjunction with the patient and the multidisciplinary team taking into account the infection risk.
Recommendation
TIPSS should be considered in patients with HH after discussion with the multidisciplinary team. (Quality of evidence: low; Recommendation: strong)
In patients with HH who are not undergoing a TIPSS and/or a liver transplant evaluation, alternative palliative interventions should be considered. (Quality of evidence: low; Recommendation: strong)
Non-selective beta-blockers and ascites
The portal pressure-lowering effects of non-selective beta-blockers (NSBB) have been known to be beneficial in patients with ascites for three decades. Lebrec’s group demonstrated back in 1991 in a meta-analysis of trial data that NSBB reduce the likelihood of first variceal haemorrhage in the ascites subgroup, while in Child’s Pugh B and C cirrhosis the addition of NSBB to band ligation results in less variceal rebleeding and superior survival.239 240 Proven haemodynamic response to NSBB (drop in hepatic venous pressure gradient (HVPG) of ≥10–20% from baseline, or to <12 mm Hg) has been linked with a lower probability of the development of ascites; and in patients already with ascites, a lower probability of refractory ascites and hepatorenal syndrome.241 242 However, it remains possible that non-response in this context is simply a surrogate marker for disease severity.
In recent years, there has been increasing recognition that the benefits of NSBB in patients with cirrhosis may not be exclusively explained by the reduction in portal pressure. NSBB reduce markers of intestinal permeability, bacterial translocation, systemic inflammation and the incidence of spontaneous bacterial peritonitis independently of haemodynamic response, suggesting a direct effect, potentially via intestinal transit time or on the bowel mucosal integrity.243–245 Given the mounting evidence that bacterial translocation and the systemic inflammatory response contribute to the downward spiral of circulatory dysfunction in cirrhosis, it follows that NSBB may also reduce non-bleeding related mortality.61 246
Despite all this, Lebrec et al 194 introduced controversy to the beta-blocker story in 2010 when his team reported that patients with refractory ascites receiving NSBB had greater all-cause mortality during long-term follow-up.247 The window hypothesis has since been proposed, which postulates that refractory ascites represents the tipping point in portal hypertension when the cardio-inhibitory effects of NSBB compromise organ perfusion.246 Krag and coauthors suggest that the ‘window’ of benefit from NSBB opens when grade 2 oesophageal varices develop and closes with the development of refractory ascites. Yet, concerns have been raised about the methodology of the original observational study; and many subsequent studies, including five meta-analyses and three with propensity risk score matching, have shown either similar or superior survival in patients with refractory ascites receiving NSBB.175 240 247–253 Of relevance is that the Lebrec data observed patients receiving a relatively high dose of NSBB compared with many of the other cohorts studied, and the NSBB type (carvedilol vs propranolol) may be important.247 254
We conclude that, until randomised high-quality data are available, the current evidence supports the use of NSBB when indicated in patients with refractory ascites (online supplemental table 11), unless alternative markers of circulatory failure, such as hypotension or reduced glomerular filtration rate, are present.175
Recommendation
Refractory ascites should not be viewed as a contraindication to NSBB. (Quality of evidence: moderate; Recommendation: strong)
Patients with refractory ascites who are taking NSBB should be monitored closely, and dose reduction or discontinuation may be appropriate in those who develop hypotension or acute/progressive renal dysfunction. (Quality of evidence: moderate; Recommendation: strong)
Automated low-flow ascites pump (ALFApump)
The ALFApump system consists of a subcutaneously implanted battery-powered programmable pump. It is connected to catheters that transfer ascites from the peritoneal cavity to the bladder, from which it is eliminated with urine. Initially, two multicentre safety and efficacy studies255 256 reported substantial reduction of the number and volume of paracenteses in patients with advanced cirrhosis and refractory ascites. However, adverse effects directly related to the device occurred in up to 39% of cases. In a multicentre RCT in patients with refractory ascites, the ALFApump significantly reduced the need for paracentesis and was associated with significantly improved chronic liver disease questionnaire, nutritional parameters such as hand-grip strength and body mass index.257 Meta-analysis of the data from the RCT and several case series showed that 62% of the patients did not require LVP following pump insertion.258 Pooled estimate rates were 30% for acute kidney injury, 27% for bacterial peritonitis and 20% for urinary tract infection. The device had no effect on survival. Currently, NICE recommends that the ALFApump should be used only with special arrangements for clinical governance, consent and audit or research.259
Recommendation
Automated low-flow ascites pump should be considered only in special circumstances with robust arrangements of clinical governance, audit or research. (Quality of evidence: low; Recommendation: weak)
Hepatorenal syndrome
Patients with cirrhosis and ascites can develop a specific form of renal dysfunction, which is termed hepatorenal syndrome (HRS). Traditionally HRS was thought to be due to the altered haemodynamic alterations with hyperdynamic circulation as well as overactive endogenous vasoactive system which results in renal hypoperfusion.260 It is now recognised that systemic inflammation also plays an important role in the pathophysiology of HRS.261 262
The traditional classification of HRS-1 and HRS-2 has recently been revised by the International Club of Ascites.263 HRS-1, which reflects a rapid reduction in renal function, has been proposed to be changed to HRS-acute kidney injury (HRS-AKI). The new definition of HRS-AKI includes an increase in serum creatinine of ≥0.3 mg/dL (27 µmol/L) within 48 hours or ≥50% from baseline, without necessitating a final cut-off value of 1.5 mg/dL (133 µmol/L). This allows treatment to be started earlier. HRS-2, which represents renal dysfunction that does not progress rapidly, has been proposed to be changed to HRS-NAKI (non-AKI).263
The mainstay of treatment of HRS-AKI involves HAS and vasoconstrictors, particularly terlipressin. Combination of terlipressin and HAS has been shown in RCTs to significantly improve renal function in HRS-AKI and improve short-term mortality (online supplemental table 12).264–266 Higher baseline serum creatinine is an independent predictor of failure to respond to vasoconstrictor treatment.262 267 A full review of HRS is beyond the scope of this guideline.
Palliative care
Palliative care is integral to the management of advanced non-cancer conditions, such as cardiac, respiratory and renal disease, often supported by practice guidelines.268–270 Patients with cirrhosis and ascites often report a poor quality of life,271 this being an independent predictor of 12-month mortality.272 However, only a minority of patients with advanced cirrhosis receive timely palliative care and this has been discussed in recent reviews.273 274 A British study indicated that from 2013 to 2015, of the 45 000 cirrhosis-related deaths, about a third required LVP in the last year of their life, overall healthcare costs being over £21 000 per person.275
Substantial literature supports the use of palliative tunnelled long-term abdominal drains (LTAD) in individuals with refractory ascites due to malignancy, including NICE medical technology guidance.276–279 These drains are inserted in hospital and allow drainage of small amounts of ascites 2–3 times a week at home. Potential advantages over LVP include symptom-guided drainage and avoidance of repeated hospitalisations. A recent systematic review on LTAD in cirrhosis showed that the majority of patients could be managed in the community.280
Results from a recent feasibility RCT comparing palliative LTAD with LVP in refractory ascites due to cirrhosis have just been published (REDUCe study).281 In this 3-month study, 36 patients were randomised, 19 to LVP and 17 to LTAD. All patients received prophylactic antibiotics for the study duration. Following randomisation, the median number (IQR) of hospital ascitic drains for LTAD versus LVP groups were 0 (0, 1) versus 4 (3, 7), respectively. Only two patients allocated to LTAD required hospital admissions specifically for ascites drainage. Self-limiting cellulitis/leakage occurred in 41% (7/17) in the LTAD vs 11% (2/19) in the LVP group; peritonitis incidence being 6% (1/17) vs 11% (2/19), respectively. Median (IQR) fortnightly community/hospital/social care ascites-related costs were lower in the LTAD group than in the LVP group, £329 (253, 580) versus £843 (603, 1060), respectively. Qualitative data (currently only published as a summary) indicate that LTAD could transform the care pathway.281
The REDUCe study demonstrates feasibility, with preliminary evidence of LTAD acceptability, effectiveness and safety and reduction in health resource use. Future trials should assess LTAD as a palliative intervention for refractory ascites in cirrhosis.
Recommendations
Patients with refractory ascites who are not undergoing evaluation for liver transplant should be offered a palliative care referral. Besides repeated LVP, alternative palliative interventions for refractory ascites should also be considered. (Quality of evidence: weak; Recommendation: strong)
Conclusions
The development of ascites is a landmark in the natural history of cirrhosis. Therefore, it should be considered an important time point at which an individual patient’s suitability for liver transplantation which is a definitive treatment of ascites and its complications, should be determined. Over the years, there has been a substantial improvement in care of patients with cirrhosis, including those with ascites. A study involving over 780 000 hospitalisations of patients with cirrhosis demonstrated an improvement in inpatient survival over a decade despite higher age and more medically complex disease.282 This was remarkably consistent across several cirrhosis complications, suggesting improved cirrhosis care beyond general improvements in inpatient care. Future research should focus on areas of need and questions where there is no high-quality evidence to guide the management of ascites.
Research recommendations
Randomised controlled trials (RCTs) with large sample size should evaluate the role of antibiotics in the secondary prophylaxis for SBP in ascites secondary to cirrhosis.
Large RCTs should assess the role of midodrine in the management of ascites.
Cost-effectiveness of long-term administration of HAS to patients with decompensated cirrhosis and ascites should be evaluated.
Role of nutritional interventions in the management of ascites should be evaluated.
Large RCT of long-term carvedilol versus no carvedilol in patients with refractory ascites without large oesophageal varices should be carried out.
Role of TIPSS in the management of hepatic hydrothorax should be compared with other therapeutic interventions.
The cost-effectiveness and the effect of automated low-flow ascites pumps on the quality of life of patients with refractory ascites should be evaluated.
Effectiveness and safety of long-term abdominal drains should be assessed in RCTs for the palliative care of patients with cirrhosis and refractory ascites.
Acknowledgments
We are grateful to Shani Steer, voluntary peer mentor, Brighton for her involvement and review of these guidelines. We wish to thank the BSG Liver Section for support and internal review of the guideline. We are grateful to BASL for review and endorsement of this guideline. We also thank Peuish Sugathan and Nottingham University Hospitals NHS Trust for providing data on the underlying aetiology in patients with new onset ascites.
Footnotes
Contributors: GA: senior author. NP: co-author (TIPSS, hepatic hydrothorax). LC: co-author (albumin in ascites). SH: co-author (albumin in ascites). LM: co-author (paracentesis). JMR: co-author (spontaneous bacterial peritonitis). EAW: co-author (diagnostic paracentesis). KM: senior author. JAL: co-author (beta-blockers in ascites). PCH: senior author (beta-blockers in ascites). AJO: senior author (albumin in ascites). SV: senior author (diuretics, palliative care). All authors reviewed and approved the final document.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: SV and LM: Received support from Rocket Medical for the NIHR funded REDUce Study.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Patient consent for publication: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: All data relevant to the study are included in the article or uploaded as supplementary information.
References
- 1. Fleming KM, Aithal GP, Card TR, et al. The rate of decompensation and clinical progression of disease in people with cirrhosis: a cohort study. Aliment Pharmacol Ther 2010;32:1343–50. 10.1111/j.1365-2036.2010.04473.x [DOI] [PubMed] [Google Scholar]
- 2. Morali GA, Sniderman KW, Deitel KM, et al. Is sinusoidal portal hypertension a necessary factor for the development of hepatic ascites? J Hepatol 1992;16:249–50. 10.1016/S0168-8278(05)80128-X [DOI] [PubMed] [Google Scholar]
- 3. Casado M, Bosch J, García-Pagán JC, et al. Clinical events after transjugular intrahepatic portosystemic shunt: correlation with hemodynamic findings. Gastroenterology 1998;114:1296–303. 10.1016/S0016-5085(98)70436-6 [DOI] [PubMed] [Google Scholar]
- 4. Iwakiri Y. Pathophysiology of portal hypertension. Clin Liver Dis 2014;18:281–91. 10.1016/j.cld.2013.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Abraldes JG, Iwakiri Y, Loureiro-Silva M, et al. Mild increases in portal pressure upregulate vascular endothelial growth factor and endothelial nitric oxide synthase in the intestinal microcirculatory bed, leading to a hyperdynamic state. Am J Physiol Gastrointest Liver Physiol 2006;290:G980–7. 10.1152/ajpgi.00336.2005 [DOI] [PubMed] [Google Scholar]
- 6. Iwakiri Y, Groszmann RJ. The hyperdynamic circulation of chronic liver diseases: from the patient to the molecule. Hepatology 2006;43:S121–31. 10.1002/hep.20993 [DOI] [PubMed] [Google Scholar]
- 7. Schrier RW, Arroyo V, Bernardi M, et al. Peripheral arterial vasodilation hypothesis: a proposal for the initiation of renal sodium and water retention in cirrhosis. Hepatology 1988;8:1151–7. 10.1002/hep.1840080532 [DOI] [PubMed] [Google Scholar]
- 8. Cárdenas A, Arroyo V. Mechanisms of water and sodium retention in cirrhosis and the pathogenesis of ascites. Best Pract Res Clin Endocrinol Metab 2003;17:607–22. 10.1016/S1521-690X(03)00052-6 [DOI] [PubMed] [Google Scholar]
- 9. Bernardi M, Moreau R, Angeli P, et al. Mechanisms of decompensation and organ failure in cirrhosis: from peripheral arterial vasodilation to systemic inflammation hypothesis. J Hepatol 2015;63:1272–84. 10.1016/j.jhep.2015.07.004 [DOI] [PubMed] [Google Scholar]
- 10. Arroyo V, Ginès P, Gerbes AL, et al. Definition and diagnostic criteria of refractory ascites and hepatorenal syndrome in cirrhosis. International Ascites Club. Hepatology 1996;23:164–76. 10.1002/hep.510230122 [DOI] [PubMed] [Google Scholar]
- 11. Subramanian V, Aithal G. The gastrointestinal system In: In Chamberlain’s symptoms and signs in clinical medicine. 13th edn, 2010. [Google Scholar]
- 12. Cattau EL, et al. The accuracy of the physical examination in the diagnosis of suspected ascites. JAMA 1982;247:1164–6. 10.1001/jama.1982.03320330060027 [DOI] [PubMed] [Google Scholar]
- 13. Runyon BA, Montano AA, Akriviadis EA, et al. The serum-ascites albumin gradient is superior to the exudate-transudate concept in the differential diagnosis of ascites. Ann Intern Med 1992;117:215–20. 10.7326/0003-4819-117-3-215 [DOI] [PubMed] [Google Scholar]
- 14. Rector WG, Reynolds TB. Superiority of the serum-ascites albumin difference over the ascites total protein concentration in separation of "transudative" and "exudative" ascites. Am J Med 1984;77:83–5. 10.1016/0002-9343(84)90440-6 [DOI] [PubMed] [Google Scholar]
- 15. Gupta R, Misra SP, Dwivedi M, et al. Diagnosing ascites: value of ascitic fluid total protein, albumin, cholesterol, their ratios, serum-ascites albumin and cholesterol gradient. J Gastroenterol Hepatol 1995;10:295–9. 10.1111/j.1440-1746.1995.tb01096.x [DOI] [PubMed] [Google Scholar]
- 16. Paré P, Talbot J, Hoefs JC. Serum-ascites albumin concentration gradient: a physiologic approach to the differential diagnosis of ascites. Gastroenterology 1983;85:240–4. 10.1016/0016-5085(83)90306-2 [DOI] [PubMed] [Google Scholar]
- 17. Mauer K, Manzione NC. Usefulness of serum-ascites albumin difference in separating transudative from exudative ascites. another look. Dig Dis Sci 1988;33:1208–12. 10.1007/BF01536667 [DOI] [PubMed] [Google Scholar]
- 18. Wong CL, et al. Does this patient have bacterial peritonitis or portal hypertension? How do I perform a paracentesis and analyze the results? JAMA 2008;299:1166–78. 10.1001/jama.299.10.1166 [DOI] [PubMed] [Google Scholar]
- 19. Farias AQ, Silvestre OM, Garcia-Tsao G, et al. Serum B-type natriuretic peptide in the initial workup of patients with new onset ascites: a diagnostic accuracy study. Hepatology 2014;59:1043–51. 10.1002/hep.26643 [DOI] [PubMed] [Google Scholar]
- 20. Liu F, Kong X, Dou Q, et al. Evaluation of tumor markers for the differential diagnosis of benign and malignant ascites. Ann Hepatol 2014;13:357–63. 10.1016/S1665-2681(19)30865-8 [DOI] [PubMed] [Google Scholar]
- 21. Cascinu S, Del Ferro E, Barbanti I, et al. Tumor markers in the diagnosis of malignant serous effusions. Am J Clin Oncol 1997;20:247–50. 10.1097/00000421-199706000-00007 [DOI] [PubMed] [Google Scholar]
- 22. Collazos J, Genolla J, Ruibal A. CA 125 serum levels in patients with non-neoplastic liver diseases. A clinical and laboratory study. Scand J Clin Lab Invest 1992;52:201–6. 10.3109/00365519209088786 [DOI] [PubMed] [Google Scholar]
- 23. Shakil AO, Korula J, Kanel GC, et al. Diagnostic features of tuberculous peritonitis in the absence and presence of chronic liver disease: a case control study. Am J Med 1996;100:179–85. 10.1016/S0002-9343(97)89456-9 [DOI] [PubMed] [Google Scholar]
- 24. Shen Y-C, Wang T, Chen L, et al. Diagnostic accuracy of adenosine deaminase for tuberculous peritonitis: a meta-analysis. Arch Med Sci 2013;9:601–7. 10.5114/aoms.2013.36904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kang SJ, Kim JW, Baek JH, et al. Role of ascites adenosine deaminase in differentiating between tuberculous peritonitis and peritoneal carcinomatosis. World J Gastroenterol 2012;18:2837–43. 10.3748/wjg.v18.i22.2837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. He W-H, Xion Z-J, Zhu Y, et al. Percutaneous drainage versus peritoneal lavage for pancreatic ascites in severe acute pancreatitis: a prospective randomized trial. Pancreas 2019;48:343–9. 10.1097/MPA.0000000000001251 [DOI] [PubMed] [Google Scholar]
- 27. Hernaez R, Hamilton JP. Unexplained ascites. Clin Liver Dis 2016;7:53–6. 10.1002/cld.537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Evans LT, Kim WR, Poterucha JJ, et al. Spontaneous bacterial peritonitis in asymptomatic outpatients with cirrhotic ascites. Hepatology 2003;37:897–901. 10.1053/jhep.2003.50119 [DOI] [PubMed] [Google Scholar]
- 29. Fernández J, Prado V, Trebicka J, et al. Multidrug-resistant bacterial infections in patients with decompensated cirrhosis and with acute-on-chronic liver failure in Europe. J Hepatol 2019;70:398–411. 10.1016/j.jhep.2018.10.027 [DOI] [PubMed] [Google Scholar]
- 30. Piano S, Fasolato S, Salinas F, et al. The empirical antibiotic treatment of nosocomial spontaneous bacterial peritonitis: results of a randomized, controlled clinical trial. Hepatology 2016;63:1299–309. 10.1002/hep.27941 [DOI] [PubMed] [Google Scholar]
- 31. Kim JJ, Tsukamoto MM, Mathur AK, et al. Delayed paracentesis is associated with increased in-hospital mortality in patients with spontaneous bacterial peritonitis. Am J Gastroenterol 2014;109:1436–42. 10.1038/ajg.2014.212 [DOI] [PubMed] [Google Scholar]
- 32. Lim KHJ, Potts JR, Chetwood J, et al. Long-term outcomes after hospitalization with spontaneous bacterial peritonitis. J Dig Dis 2015;16:228–40. 10.1111/1751-2980.12228 [DOI] [PubMed] [Google Scholar]
- 33. Rimola A, García-Tsao G, Navasa M, et al. Diagnosis, treatment and prophylaxis of spontaneous bacterial peritonitis: a consensus document. International Ascites Club. J Hepatol 2000;32:142–53. 10.1016/S0168-8278(00)80201-9 [DOI] [PubMed] [Google Scholar]
- 34. van de Geijn G-JM, van Gent M, van Pul-Bom N, et al. A new flow cytometric method for differential cell counting in ascitic fluid. Cytometry B Clin Cytom 2016;90:506–11. 10.1002/cyto.b.21171 [DOI] [PubMed] [Google Scholar]
- 35. Fleming C, Brouwer R, van Alphen A, et al. UF-1000I: validation of the body fluid mode for counting cells in body fluids. Clin Chem Lab Med 2014;52:1781–90. 10.1515/cclm-2014-0512 [DOI] [PubMed] [Google Scholar]
- 36. Nousbaum J-B, Cadranel J-F, Nahon P, et al. Diagnostic accuracy of the Multistix 8 SG reagent strip in diagnosis of spontaneous bacterial peritonitis. Hepatology 2007;45:1275–81. 10.1002/hep.21588 [DOI] [PubMed] [Google Scholar]
- 37. Terg R, Levi D, Lopez P, et al. Analysis of clinical course and prognosis of culture-positive spontaneous bacterial peritonitis and neutrocytic ascites. Evidence of the same disease. Dig Dis Sci 1992;37:1499–504. 10.1007/BF01296493 [DOI] [PubMed] [Google Scholar]
- 38. Pelletier G, Salmon D, Ink O, et al. Culture-negative neutrocytic ascites: a less severe variant of spontaneous bacterial peritonitis. J Hepatol 1990;10:327–31. 10.1016/0168-8278(90)90140-M [DOI] [PubMed] [Google Scholar]
- 39. Enomoto H, Inoue S-I, Matsuhisa A, et al. Amplification of bacterial genomic DNA from all ascitic fluids with a highly sensitive polymerase chain reaction. Mol Med Rep 2018;18:2117–23. 10.3892/mmr.2018.9159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Gravito-Soares M, Gravito-Soares E, Lopes S, et al. Spontaneous fungal peritonitis: a rare but severe complication of liver cirrhosis. Eur J Gastroenterol Hepatol 2017;29:1010–6. 10.1097/MEG.0000000000000927 [DOI] [PubMed] [Google Scholar]
- 41. Bajaj JS, Reddy RK, Tandon P, et al. Prediction of fungal infection development and their impact on survival using the NACSELD cohort. Am J Gastroenterol 2018;113:556–63. 10.1038/ajg.2017.471 [DOI] [PubMed] [Google Scholar]
- 42. Soriano G, Castellote J, Alvarez C, et al. Secondary bacterial peritonitis in cirrhosis: a retrospective study of clinical and analytical characteristics, diagnosis and management. J Hepatol 2010;52:39–44. 10.1016/j.jhep.2009.10.012 [DOI] [PubMed] [Google Scholar]
- 43. Runyon BA. Management of adult patients with ascites due to cirrhosis. Hepatology 2004;39:841–56. 10.1002/hep.20066 [DOI] [PubMed] [Google Scholar]
- 44. Runyon BA, Akriviadis EA, Sattler FR, et al. Ascitic fluid and serum cefotaxime and desacetyl cefotaxime levels in patients treated for bacterial peritonitis. Dig Dis Sci 1991;36:1782–6. 10.1007/BF01296625 [DOI] [PubMed] [Google Scholar]
- 45. Piano S, Singh V, Caraceni P, et al. Epidemiology and effects of bacterial infections in patients with cirrhosis worldwide. Gastroenterology 2019;156:1368–80. 10.1053/j.gastro.2018.12.005 [DOI] [PubMed] [Google Scholar]
- 46. Horan TC, Andrus M, Dudeck MA. CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control 2008;36:309–32. 10.1016/j.ajic.2008.03.002 [DOI] [PubMed] [Google Scholar]
- 47. European Association for the Study of the Liver. Electronic address: easloffice@easloffice.eu, European Association for the Study of the Liver . EASL clinical practice guidelines for the management of patients with decompensated cirrhosis. J Hepatol 2018;69:406–60. 10.1016/j.jhep.2018.03.024 [DOI] [PubMed] [Google Scholar]
- 48. Fernández J, Bert F, Nicolas-Chanoine M-H. The challenges of multi-drug-resistance in hepatology. J Hepatol 2016;65:1043–54. 10.1016/j.jhep.2016.08.006 [DOI] [PubMed] [Google Scholar]
- 49. Magiorakos A-P, Srinivasan A, Carey RB, et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect 2012;18:268–81. 10.1111/j.1469-0691.2011.03570.x [DOI] [PubMed] [Google Scholar]
- 50. Fong TL, Akriviadis EA, Runyon BA, et al. Polymorphonuclear cell count response and duration of antibiotic therapy in spontaneous bacterial peritonitis. Hepatology 1989;9:423–6. 10.1002/hep.1840090313 [DOI] [PubMed] [Google Scholar]
- 51. Fernández J, Tandon P, Mensa J, et al. Antibiotic prophylaxis in cirrhosis: good and bad. Hepatology 2016;63:2019–31. 10.1002/hep.28330 [DOI] [PubMed] [Google Scholar]
- 52. Chavez-Tapia NC, Barrientos-Gutierrez T, Tellez-Avila F, et al. Meta-analysis: antibiotic prophylaxis for cirrhotic patients with upper gastrointestinal bleeding - an updated Cochrane review. Aliment Pharmacol Ther 2011;34:509–18. 10.1111/j.1365-2036.2011.04746.x [DOI] [PubMed] [Google Scholar]
- 53. Cohen MJ, Sahar T, Benenson S, et al. Antibiotic prophylaxis for spontaneous bacterial peritonitis in cirrhotic patients with ascites, without gastro-intestinal bleeding. Cochrane Database Syst Rev 2009:CD004791. 10.1002/14651858.CD004791.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Tripathi D, Stanley AJ, Hayes PC, et al. U.K. guidelines on the management of variceal haemorrhage in cirrhotic patients. Gut 2015;64:1680–704. 10.1136/gutjnl-2015-309262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Harrison P, Hogan BJ, Floros L, et al. Assessment and management of cirrhosis in people older than 16 years: summary of NICE guidance. BMJ 2016;354:i2850. 10.1136/bmj.i2850 [DOI] [PubMed] [Google Scholar]
- 56. Grangé JD, Roulot D, Pelletier G, et al. Norfloxacin primary prophylaxis of bacterial infections in cirrhotic patients with ascites: a double-blind randomized trial. J Hepatol 1998;29:430–6. 10.1016/S0168-8278(98)80061-5 [DOI] [PubMed] [Google Scholar]
- 57. Rolachon A, Cordier L, Bacq Y, et al. Ciprofloxacin and long-term prevention of spontaneous bacterial peritonitis: results of a prospective controlled trial. Hepatology 1995;22:1171–4. 10.1016/0270-9139(95)90626-6 [DOI] [PubMed] [Google Scholar]
- 58. Soriano G, Guarner C, Teixidó M, et al. Selective intestinal decontamination prevents spontaneous bacterial peritonitis. Gastroenterology 1991;100:477–81. 10.1016/0016-5085(91)90219-B [DOI] [PubMed] [Google Scholar]
- 59. Terg R, Fassio E, Guevara M, et al. Ciprofloxacin in primary prophylaxis of spontaneous bacterial peritonitis: a randomized, placebo-controlled study. J Hepatol 2008;48:774–9. 10.1016/j.jhep.2008.01.024 [DOI] [PubMed] [Google Scholar]
- 60. Téllez-Ávila F, Sifuentes-Osornio J, Barbero-Becerra V, et al. Primary prophylaxis with ciprofloxacin in cirrhotic patients with ascites: a randomized, double blind study. Ann Hepatol 2013;13:65–74. 10.1016/S1665-2681(19)30906-8 [DOI] [PubMed] [Google Scholar]
- 61. Fernández J, Navasa M, Planas R, et al. Primary prophylaxis of spontaneous bacterial peritonitis delays hepatorenal syndrome and improves survival in cirrhosis. Gastroenterology 2007;133:818–24. 10.1053/j.gastro.2007.06.065 [DOI] [PubMed] [Google Scholar]
- 62. Runyon BA, AASLD . Introduction to the revised American Association for the Study of Liver Diseases Practice Guideline management of adult patients with ascites due to cirrhosis 2012. Hepatology 2013;57:1651–3. 10.1002/hep.26359 [DOI] [PubMed] [Google Scholar]
- 63. Moreau R, Elkrief L, Bureau C, et al. Effects of long-term norfloxacin therapy in patients with advanced cirrhosis. Gastroenterology 2018;155:1816–27. 10.1053/j.gastro.2018.08.026 [DOI] [PubMed] [Google Scholar]
- 64. Terg R, Casciato P, Garbe C, et al. Proton pump inhibitor therapy does not increase the incidence of spontaneous bacterial peritonitis in cirrhosis: a multicenter prospective study. J Hepatol 2015;62:1056–60. 10.1016/j.jhep.2014.11.036 [DOI] [PubMed] [Google Scholar]
- 65. Bruns T, Lutz P, Stallmach A, et al. Low ascitic fluid protein does not indicate an increased risk for spontaneous bacterial peritonitis in current cohorts. J Hepatol 2015;63:527–8. 10.1016/j.jhep.2015.03.040 [DOI] [PubMed] [Google Scholar]
- 66. Titó L, Rimola A, Ginès P, et al. Recurrence of spontaneous bacterial peritonitis in cirrhosis: frequency and predictive factors. Hepatology 1988;8:27–31. 10.1002/hep.1840080107 [DOI] [PubMed] [Google Scholar]
- 67. Altman C, Grangé JD, Amiot X, et al. Survival after a first episode of spontaneous bacterial peritonitis. prognosis of potential candidates for orthotopic liver transplantation. J Gastroenterol Hepatol 1995;10:47–50. 10.1111/j.1440-1746.1995.tb01046.x [DOI] [PubMed] [Google Scholar]
- 68. Ginés P, Rimola A, Planas R, et al. Norfloxacin prevents spontaneous bacterial peritonitis recurrence in cirrhosis: results of a double-blind, placebo-controlled trial. Hepatology 1990;12:716–24. 10.1002/hep.1840120416 [DOI] [PubMed] [Google Scholar]
- 69. Goel A, Rahim U, Nguyen LH, et al. Systematic review with meta-analysis: rifaximin for the prophylaxis of spontaneous bacterial peritonitis. Aliment Pharmacol Ther 2017;46:1029–36. 10.1111/apt.14361 [DOI] [PubMed] [Google Scholar]
- 70. Min YW, Lim KS, Min B-H, et al. Proton pump inhibitor use significantly increases the risk of spontaneous bacterial peritonitis in 1965 patients with cirrhosis and ascites: a propensity score matched cohort study. Aliment Pharmacol Ther 2014;40:695–704. 10.1111/apt.12875 [DOI] [PubMed] [Google Scholar]
- 71. Dam G, Vilstrup H, Watson H, et al. Proton pump inhibitors as a risk factor for hepatic encephalopathy and spontaneous bacterial peritonitis in patients with cirrhosis with ascites. Hepatology 2016;64:1265–72. 10.1002/hep.28737 [DOI] [PubMed] [Google Scholar]
- 72. Reynolds TB, Lieberman FL, Goodman AR. Advantages of treatment of ascites without sodium restriction and without complete removal of excess fluid. Gut 1978;19:549–53. 10.1136/gut.19.6.549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Descos L, Gauthier A, Levy VG, et al. Comparison of six treatments of ascites in patients with liver cirrhosis. A clinical trial. Hepatogastroenterology 1983;30:15–20. [PubMed] [Google Scholar]
- 74. Gauthier A, Levy VG, Quinton A, et al. Salt or no salt in the treatment of cirrhotic ascites: a randomised study. Gut 1986;27:705–9. 10.1136/gut.27.6.705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Bernardi M, Laffi G, Salvagnini M, et al. Efficacy and safety of the stepped care medical treatment of ascites in liver cirrhosis: a randomized controlled clinical trial comparing two diets with different sodium content. Liver 1993;13:156–62. 10.1111/j.1600-0676.1993.tb00624.x [DOI] [PubMed] [Google Scholar]
- 76. Soulsby CT, Madden AM, Morgan MY. The effect of dietary sodium restriction on energy and protein intake in patients with cirrhosis. Hepatology 1997;26:1013. [Google Scholar]
- 77. Gu X-B, Yang X-J, Zhu H-Y, et al. Effect of a diet with unrestricted sodium on ascites in patients with hepatic cirrhosis. Gut Liver 2012;6:355–61. 10.5009/gnl.2012.6.3.355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Sorrentino P, Castaldo G, Tarantino L, et al. Preservation of nutritional-status in patients with refractory ascites due to hepatic cirrhosis who are undergoing repeated paracentesis. J Gastroenterol Hepatol 2012;27:813–22. 10.1111/j.1440-1746.2011.07043.x [DOI] [PubMed] [Google Scholar]
- 79. Morando F, Rosi S, Gola E, et al. Adherence to a moderate sodium restriction diet in outpatients with cirrhosis and ascites: a real-life cross-sectional study. Liver Int 2015;35:1508–15. 10.1111/liv.12583 [DOI] [PubMed] [Google Scholar]
- 80. Powles J, Fahimi S, Micha R, et al. Global, regional and national sodium intakes in 1990 and 2010: a systematic analysis of 24 H urinary sodium excretion and dietary surveys worldwide. BMJ Open 2013;3:e003733. 10.1136/bmjopen-2013-003733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Gonçalves C, Abreu S, Padrão P, et al. Sodium and potassium urinary excretion and dietary intake: a cross-sectional analysis in adolescents. Food Nutr Res 2016;60:29442. 10.3402/fnr.v60.29442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Haberl J, Zollner G, Fickert P, et al. To salt or not to salt?-That is the question in cirrhosis. Liver Int 2018;38:1148–59. 10.1111/liv.13750 [DOI] [PubMed] [Google Scholar]
- 83. Walbaum B, Valda ML, Rada G. Sodium restriction in patients with cirrhotic ascites: a protocol for a systematic review. Syst Rev 2016;5:78. 10.1186/s13643-016-0250-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Bernardi M, Trevisani F, Gasbarrini A, et al. Hepatorenal disorders: role of the renin-angiotensin-aldosterone system. Semin Liver Dis 1994;14:23–34. 10.1055/s-2007-1007295 [DOI] [PubMed] [Google Scholar]
- 85. Bernardi M, Servadei D, Trevisani F, et al. Importance of plasma aldosterone concentration on the natriuretic effect of spironolactone in patients with liver cirrhosis and ascites. Digestion 1985;31:189–93. 10.1159/000199198 [DOI] [PubMed] [Google Scholar]
- 86. Brater DC. Update in diuretic therapy: clinical pharmacology. Semin Nephrol 2011;31:483–94. 10.1016/j.semnephrol.2011.09.003 [DOI] [PubMed] [Google Scholar]
- 87. Pérez-Ayuso RM, Arroyo V, Planas R, et al. Randomized comparative study of efficacy of furosemide versus spironolactone in nonazotemic cirrhosis with ascites. Relationship between the diuretic response and the activity of the renin-aldosterone system. Gastroenterology 1983;84:961–8. [PubMed] [Google Scholar]
- 88. Fogel MR, Sawhney VK, Neal EA, et al. Diuresis in the ascitic patient: a randomized controlled trial of three regimens. J Clin Gastroenterol 1981;3(Suppl 1):73–80. 10.1097/00004836-198100031-00016 [DOI] [PubMed] [Google Scholar]
- 89. Ochs HR, Greenblatt DJ, Bodem G, et al. Spironolactone. Am Heart J 1978;96:389–400. 10.1016/0002-8703(78)90052-2 [DOI] [PubMed] [Google Scholar]
- 90. Overdiek HW, Hermens WA, Merkus FW. New insights into the pharmacokinetics of spironolactone. Clin Pharmacol Ther 1985;38:469–74. 10.1038/clpt.1985.206 [DOI] [PubMed] [Google Scholar]
- 91. Shear L, Ching S, Gabuzda GJ. Compartmentalization of ascites and edema in patients with hepatic cirrhosis. N Engl J Med 1970;282:1391–6. 10.1056/NEJM197006182822502 [DOI] [PubMed] [Google Scholar]
- 92. Angeli P, Dalla Pria M, De Bei E, et al. Randomized clinical study of the efficacy of amiloride and potassium canrenoate in nonazotemic cirrhotic patients with ascites. Hepatology 1994;19:72–9. 10.1002/hep.1840190113 [DOI] [PubMed] [Google Scholar]
- 93. Sarin SK, Sachdev G, Mishra SP, et al. Bumetanide, spironolactone and a combination of the two, in the treatment of ascites due to liver disease. A prospective, controlled, randomized trial. Digestion 1988;41:101–7. 10.1159/000199738 [DOI] [PubMed] [Google Scholar]
- 94. Laffi G, Marra F, Buzzelli G, et al. Comparison of the effects of torasemide and furosemide in nonazotemic cirrhotic patients with ascites: a randomized, double-blind study. Hepatology 1991;13:1101–5. 10.1002/hep.1840130616 [DOI] [PubMed] [Google Scholar]
- 95. Gerbes AL, Bertheau-Reitha U, Falkner C, et al. Advantages of the new loop diuretic torasemide over furosemide in patients with cirrhosis and ascites. A randomized, double blind cross-over trial. J Hepatol 1993;17:353–8. 10.1016/s0168-8278(05)80217-x [DOI] [PubMed] [Google Scholar]
- 96. Santos J, Planas R, Pardo A, et al. Spironolactone alone or in combination with furosemide in the treatment of moderate ascites in nonazotemic cirrhosis. A randomized comparative study of efficacy and safety. J Hepatol 2003;39:187–92. 10.1016/S0168-8278(03)00188-0 [DOI] [PubMed] [Google Scholar]
- 97. Angeli P, Fasolato S, Mazza E, et al. Combined versus sequential diuretic treatment of ascites in non-azotaemic patients with cirrhosis: results of an open randomised clinical trial. Gut 2010;59:98–104. 10.1136/gut.2008.176495 [DOI] [PubMed] [Google Scholar]
- 98. Gatta A, Angeli P, Caregaro L, et al. A pathophysiological interpretation of unresponsiveness to spironolactone in a stepped-care approach to the diuretic treatment of ascites in nonazotemic cirrhotic patients. Hepatology 1991;14:231–6. 10.1002/hep.1840140205 [DOI] [PubMed] [Google Scholar]
- 99. Angeli P, Gatta A, Caregaro L, et al. Tubular site of renal sodium retention in ascitic liver cirrhosis evaluated by lithium clearance. Eur J Clin Invest 1990;20:111–7. 10.1111/j.1365-2362.1990.tb01800.x [DOI] [PubMed] [Google Scholar]
- 100. Pockros PJ, Reynolds TB. Rapid diuresis in patients with ascites from chronic liver disease: the importance of peripheral edema. Gastroenterology 1986;90:1827–33. 10.1016/0016-5085(86)90249-0 [DOI] [PubMed] [Google Scholar]
- 101. Stanley MM, Ochi S, Lee KK, et al. Peritoneovenous shunting as compared with medical treatment in patients with alcoholic cirrhosis and massive ascites. N Engl J Med Overseas Ed 1989;321:1632–8. 10.1056/NEJM198912143212403 [DOI] [PubMed] [Google Scholar]
- 102. Sherlock S, Senewiratne B, Scott A, et al. Complications of diuretic therapy in hepatic cirrhosis. Lancet 1966;1:1049–53. 10.1016/S0140-6736(66)91005-1 [DOI] [PubMed] [Google Scholar]
- 103. Bellati G, Idéo G. Gynaecomastia after spironolactone and potassium canrenoate. Lancet 1986;1:626. 10.1016/S0140-6736(86)92856-4 [DOI] [PubMed] [Google Scholar]
- 104. Mimidis K, Papadopoulos V, Kartalis G. Eplerenone relieves spironolactone-induced painful gynaecomastia in patients with decompensated hepatitis B-related cirrhosis. Scand J Gastroenterol 2007;42:1516–7. 10.1080/00365520701676070 [DOI] [PubMed] [Google Scholar]
- 105. Dimitriadis G, Papadopoulos V, Mimidis K. Eplerenone reverses spironolactone-induced painful gynaecomastia in cirrhotics. Hepatol Int 2011;5:738–9. 10.1007/s12072-010-9235-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Murata A, Hyogo H, Nonaka M, et al. Overlooked muscle cramps in patients with chronic liver disease: in relation to the prevalence of muscle cramps. Eur J Gastroenterol Hepatol 2019;31:375–81. 10.1097/MEG.0000000000001294 [DOI] [PubMed] [Google Scholar]
- 107. Angeli P, Albino G, Carraro P, et al. Cirrhosis and muscle cramps: evidence of a causal relationship. Hepatology 1996;23:264–73. 10.1002/hep.510230211 [DOI] [PubMed] [Google Scholar]
- 108. Peng J-K, Hepgul N, Higginson IJ, et al. Symptom prevalence and quality of life of patients with end-stage liver disease: a systematic review and meta-analysis. Palliat Med 2019;33:24–36. 10.1177/0269216318807051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Vidot H, Carey S, Allman-Farinelli M, et al. Systematic review: the treatment of muscle cramps in patients with cirrhosis. Aliment Pharmacol Ther 2014;40:221–32. 10.1111/apt.12827 [DOI] [PubMed] [Google Scholar]
- 110. Abd-Elsalam S, Arafa M, Elkadeem M, et al. Randomized-controlled trial of methocarbamol as a novel treatment for muscle cramps in cirrhotic patients. Eur J Gastroenterol Hepatol 2019;31:499–502. 10.1097/MEG.0000000000001310 [DOI] [PubMed] [Google Scholar]
- 111. Vidot H, Cvejic E, Carey S, et al. Randomised clinical trial: oral taurine supplementation versus placebo reduces muscle cramps in patients with chronic liver disease. Aliment Pharmacol Ther 2018;48:704–12. 10.1111/apt.14950 [DOI] [PubMed] [Google Scholar]
- 112. Elfert AA, Abo Ali L, Soliman S, et al. Randomized placebo-controlled study of baclofen in the treatment of muscle cramps in patients with liver cirrhosis. Eur J Gastroenterol Hepatol 2016;28:1280–4. 10.1097/MEG.0000000000000714 [DOI] [PubMed] [Google Scholar]
- 113. Eisenmenger WJ, Blondheim SH, Bongiovanni AM, et al. Electrolyte studies on patients with cirrhosis of the liver. J Clin Invest 1950;29:1491–9. 10.1172/JCI102390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. El-Bokl MA, Senousy BE, El-Karmouty KZ, et al. Spot urinary sodium for assessing dietary sodium restriction in cirrhotic ascites. World J Gastroenterol 2009;15:3631–5. 10.3748/wjg.15.3631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Mohii ESM, El Mansy IM, Salah M, et al. Diagnostic usefulness of the random urine Na/K ratio in predicting therapeutic response for diuretics in cirrhotic patients with ascites. J Egypt Soc Parasitol 2013;43:767–76. 10.12816/0006433 [DOI] [PubMed] [Google Scholar]
- 116. Spasovski G, Vanholder R, Allolio B, et al. Clinical practice guideline on diagnosis and treatment of hyponatraemia. Intensive Care Med 2014;40:320–31. 10.1007/s00134-014-3210-2 [DOI] [PubMed] [Google Scholar]
- 117. Angeli P, Wong F, Watson H, et al. Hyponatremia in cirrhosis: results of a patient population survey. Hepatology 2006;44:1535–42. 10.1002/hep.21412 [DOI] [PubMed] [Google Scholar]
- 118. Sersté T, Gustot T, Rautou P-E, et al. Severe hyponatremia is a better predictor of mortality than MELDNa in patients with cirrhosis and refractory ascites. J Hepatol 2012;57:274–80. 10.1016/j.jhep.2012.03.018 [DOI] [PubMed] [Google Scholar]
- 119. Kim WR, Biggins SW, Kremers WK, et al. Hyponatremia and mortality among patients on the liver-transplant waiting list. N Engl J Med 2008;359:1018–26. 10.1056/NEJMoa0801209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Biggins SW, Kim WR, Terrault NA, et al. Evidence-based incorporation of serum sodium concentration into MELD. Gastroenterology 2006;130:1652–60. 10.1053/j.gastro.2006.02.010 [DOI] [PubMed] [Google Scholar]
- 121. McCormick PA, Mistry P, Kaye G, et al. Intravenous albumin infusion is an effective therapy for hyponatraemia in cirrhotic patients with ascites. Gut 1990;31:204–7. 10.1136/gut.31.2.204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Jalan R, Mookerjee R, Cheshire L, et al. Albumin [232] infusion for severe hyponatraemia in patients with refractory ascites: a randomized clinical trial. J Hepatol 2007;46:S95 10.1016/S0168-8278(07)61830-3 [DOI] [Google Scholar]
- 123. Ginès P, Titó L, Arroyo V, et al. Randomized comparative study of therapeutic paracentesis with and without intravenous albumin in cirrhosis. Gastroenterology 1988;94:1493–502. 10.1016/0016-5085(88)90691-9 [DOI] [PubMed] [Google Scholar]
- 124. García-Compeán D, Zacarías Villarreal J, Bahena Cuevas H, et al. Total therapeutic paracentesis (TTP) with and without intravenous albumin in the treatment of cirrhotic tense ascites: a randomized controlled trial. Liver 1993;13:233–8. 10.1111/j.1600-0676.1993.tb00637.x [DOI] [PubMed] [Google Scholar]
- 125. Luca A, García-Pagán JC, Bosch J, et al. Beneficial effects of intravenous albumin infusion on the hemodynamic and humoral changes after total paracentesis. Hepatology 1995;22:753–8. [PubMed] [Google Scholar]
- 126. Bajaj JS, Tandon P, OʼLeary JG, et al. The impact of albumin use on resolution of hyponatremia in hospitalized patients with cirrhosis. Am J Gastroenterol 2018;113:1339–44. 10.1038/s41395-018-0119-3 [DOI] [PubMed] [Google Scholar]
- 127. Bernardi M, Caraceni P, Navickis RJ, et al. Albumin infusion in patients undergoing large-volume paracentesis: a meta-analysis of randomized trials. Hepatology 2012;55:1172–81. 10.1002/hep.24786 [DOI] [PubMed] [Google Scholar]
- 128. Kütting F, Schubert J, Franklin J, et al. Insufficient evidence of benefit regarding mortality due to albumin substitution in HCC-free cirrhotic patients undergoing large volume paracentesis. J Gastroenterol Hepatol 2017;32:327–38. 10.1111/jgh.13421 [DOI] [PubMed] [Google Scholar]
- 129. Bernardi M, Ricci C, Santi L. Hyponatremia in patients with cirrhosis of the liver. J Clin Med 2015;4:85–101. 10.3390/jcm4010085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Bichet D, Szatalowicz V, Chaimovitz C, et al. Role of vasopressin in abnormal water excretion in cirrhotic patients. Ann Intern Med 1982;96:413–7. 10.7326/0003-4819-96-4-413 [DOI] [PubMed] [Google Scholar]
- 131. Eisenmenger WJ, Ahrens EH, Blondheim SH. The effect of rigid sodium restriction in patients with cirrhosis of the liver and ascites. J Lab Clin Med 1949;34:1029–38. [PubMed] [Google Scholar]
- 132. Moore KP, Aithal GP. Guidelines on the management of ascites in cirrhosis. Gut 2006;55:vi1–12. 10.1136/gut.2006.099580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Vizzini G, Asaro M, Miraglia R, et al. Changing picture of central nervous system complications in liver transplant recipients. Liver Transpl 2011;17:1279–85. 10.1002/lt.22383 [DOI] [PubMed] [Google Scholar]
- 134. Verbalis JG, Goldsmith SR, Greenberg A, et al. Diagnosis, evaluation, and treatment of hyponatremia: expert panel recommendations. Am J Med 2013;126:S1–42. 10.1016/j.amjmed.2013.07.006 [DOI] [PubMed] [Google Scholar]
- 135. Hoorn EJ, Zietse R. Diagnosis and treatment of hyponatremia: compilation of the guidelines. J Am Soc Nephrol 2017;28:1340–9. 10.1681/ASN.2016101139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Decaux G, Soupart A, Vassart G. Non-peptide arginine-vasopressin antagonists: the vaptans. Lancet 2008;371:1624–32. 10.1016/S0140-6736(08)60695-9 [DOI] [PubMed] [Google Scholar]
- 137. Wong F, Watson H, Gerbes A, et al. Satavaptan for the management of ascites in cirrhosis: efficacy and safety across the spectrum of ascites severity. Gut 2012;61:108–16. 10.1136/gutjnl-2011-300157 [DOI] [PubMed] [Google Scholar]
- 138. Dahl E, Gluud LL, Kimer N, et al. Meta-analysis: the safety and efficacy of vaptans (tolvaptan, satavaptan and lixivaptan) in cirrhosis with ascites or hyponatraemia. Aliment Pharmacol Ther 2012;36:619–26. 10.1111/apt.12025 [DOI] [PubMed] [Google Scholar]
- 139. Yan L, Xie F, Lu J, et al. The treatment of vasopressin V2-receptor antagonists in cirrhosis patients with ascites: a meta-analysis of randomized controlled trials. BMC Gastroenterol 2015;15:65. 10.1186/s12876-015-0297-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Llach J, Ginès P, Arroyo V, et al. Prognostic value of arterial pressure, endogenous vasoactive systems, and renal function in cirrhotic patients admitted to the hospital for the treatment of ascites. Gastroenterology 1988;94:482–7. 10.1016/0016-5085(88)90441-6 [DOI] [PubMed] [Google Scholar]
- 141. Kalambokis G, Fotopoulos A, Economou M, et al. Effects of a 7-day treatment with midodrine in non-azotemic cirrhotic patients with and without ascites. J Hepatol 2007;46:213–21. 10.1016/j.jhep.2006.09.012 [DOI] [PubMed] [Google Scholar]
- 142. Singh V, Dhungana SP, Singh B, et al. Midodrine in patients with cirrhosis and refractory or recurrent ascites: a randomized pilot study. J Hepatol 2012;56:348–54. 10.1016/j.jhep.2011.04.027 [DOI] [PubMed] [Google Scholar]
- 143. Ali A, Farid S, Amin M, et al. Clinical study on the therapeutic role of midodrine in non azotemic cirrhotic patients with tense ascites: a double-blind, placebo-controlled, randomized trial. Hepatogastroenterology 2014;61:1915–24. [PubMed] [Google Scholar]
- 144. Salerno F, Borroni G, Moser P, et al. Survival and prognostic factors of cirrhotic patients with ascites: a study of 134 outpatients. Am J Gastroenterol 1993;88:514–9. [PubMed] [Google Scholar]
- 145. Grabau CM, Crago SF, Hoff LK, et al. Performance standards for therapeutic abdominal paracentesis. Hepatology 2004;40:484–8. 10.1002/hep.20317 [DOI] [PubMed] [Google Scholar]
- 146. Chivinge A, Wilkes E, James M, et al. Implementing a nurse-led paracentesis service to improve patient care and experience in a day case unit. Gastrointestinal Nursing 2015;13:S11–15. 10.12968/gasn.2015.13.Sup10.S11 [DOI] [Google Scholar]
- 147. Sakai H, Sheer TA, Mendler MH, et al. Choosing the location for non-image guided abdominal paracentesis. Liver Int 2005;25:984–6. 10.1111/j.1478-3231.2005.01149.x [DOI] [PubMed] [Google Scholar]
- 148. Hurd WW, Bude RO, DeLancey JO, et al. The location of abdominal wall blood vessels in relationship to abdominal landmarks apparent at laparoscopy. Am J Obstet Gynecol 1994;171:642–6. 10.1016/0002-9378(94)90076-0 [DOI] [PubMed] [Google Scholar]
- 149. Saber AA, Meslemani AM, Davis R, et al. Safety zones for anterior abdominal wall entry during laparoscopy: a CT scan mapping of epigastric vessels. Ann Surg 2004;239:182–5. 10.1097/01.sla.0000109151.53296.07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Joy P, Prithishkumar IJ, Isaac B. Clinical anatomy of the inferior epigastric artery with special relevance to invasive procedures of the anterior abdominal wall. J Minim Access Surg 2017;13:18–21. 10.4103/0972-9941.181331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Rowley MW, Cole L, Aguon PM, et al. Ultrasound imaging guidance and large volume paracentesis: an analysis of safety, cost-effectiveness, and utility in restricting unnecessary transfusion. Hepatology 2018;68:568a. [Google Scholar]
- 152. Pache I, Bilodeau M. Severe haemorrhage following abdominal paracentesis for ascites in patients with liver disease. Aliment Pharmacol Ther 2005;21:525–9. 10.1111/j.1365-2036.2005.02387.x [DOI] [PubMed] [Google Scholar]
- 153. Hung A, Garcia-Tsao G. Acute kidney injury, but not sepsis, is associated with higher procedure-related bleeding in patients with decompensated cirrhosis. Liver Int 2018;38:1437–41. 10.1111/liv.13712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Lin S, Wang M, Zhu Y, et al. Hemorrhagic complications following abdominal paracentesis in acute on chronic liver failure: a propensity score analysis. Medicine 2015;94:e2225. 10.1097/MD.0000000000002225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Cho J, Jensen TP, Reierson K, et al. Recommendations on the use of ultrasound guidance for adult abdominal paracentesis: a position statement of the Society of Hospital Medicine. J Hosp Med 2019;14:E7–15. 10.12788/jhm.3095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Patel PA, Ernst FR, Gunnarsson CL. Evaluation of hospital complications and costs associated with using ultrasound guidance during abdominal paracentesis procedures. J Med Econ 2012;15:1–7. 10.3111/13696998.2011.628723 [DOI] [PubMed] [Google Scholar]
- 157. Mercaldi CJ, Lanes SF. Ultrasound guidance decreases complications and improves the cost of care among patients undergoing thoracentesis and paracentesis. Chest 2013;143:532–8. 10.1378/chest.12-0447 [DOI] [PubMed] [Google Scholar]
- 158. Peltekian KM, Wong F, Liu PP, et al. Cardiovascular, renal, and neurohumoral responses to single large-volume paracentesis in patients with cirrhosis and diuretic-resistant ascites. Am J Gastroenterol 1997;92:394–9. [PubMed] [Google Scholar]
- 159. Arora V, Vijayaraghavan R, Maiwall R, et al. Paracentesis-Induced circulatory dysfunction with modest-volume paracentesis is partly ameliorated by albumin infusion in acute-on-chronic liver failure. Hepatology 2019. 10.1002/hep.31071. [Epub ahead of print: 17 Dec 2019]. [DOI] [PubMed] [Google Scholar]
- 160. Abdel-Khalek EE, Arif SE. Randomized trial comparing human albumin and hydroxyethyl starch 6% as plasma expanders for treatment of patients with liver cirrhosis and tense ascites following large volume paracentesis. Arab Journal of Gastroenterology 2010;11:24–9. 10.1016/j.ajg.2010.01.006 [DOI] [Google Scholar]
- 161. Altman C, Bernard B, Roulot D, et al. Randomized comparative multicenter study of hydroxyethyl starch versus albumin as a plasma expander in cirrhotic patients with tense ascites treated with paracentesis. Eur J Gastroenterol Hepatol 1998;10:5–10. 10.1097/00042737-199801000-00002 [DOI] [PubMed] [Google Scholar]
- 162. Bertrán X, Fernández-Bañares F, Planas R, et al. [Impact on the energetic-proteic nutritional status of total paracentesis combined with infusion of albumin or dextran-70 in the therapy of tension ascites in liver cirrhosis]. Rev Esp Enferm Dig 1991;79:320–3. [PubMed] [Google Scholar]
- 163. Fassio E, Terg R, Landeira G, et al. Paracentesis with dextran 70 vs. paracentesis with albumin in cirrhosis with tense ascites. Results of a randomized study. J Hepatol 1992;14:310–6. 10.1016/0168-8278(92)90176-p [DOI] [PubMed] [Google Scholar]
- 164. García-Compean D, Blanc P, Larrey D, et al. Treatment of cirrhotic tense ascites with dextran-40 versus albumin associated with large volume paracentesis: a randomized controlled trial. Ann Hepatol 2002;1:29–35. 10.1016/S1665-2681(19)32189-1 [DOI] [PubMed] [Google Scholar]
- 165. Pérez REH, Ramírez JRA, López JMH, et al. Paracentesis Masiva Con Reposician de dextran 70 vs Albumina en Pacientes cirrhotics Con ascites a tension. Rev Gastro Max 1995;60:22–6. [PubMed] [Google Scholar]
- 166. Moreau R, Valla D-C, Durand-Zaleski I, et al. Comparison of outcome in patients with cirrhosis and ascites following treatment with albumin or a synthetic colloid: a randomised controlled pilot trial. Liver Int 2006;26:46–54. 10.1111/j.1478-3231.2005.01188.x [DOI] [PubMed] [Google Scholar]
- 167. Planas R, Ginès P, Arroyo V, et al. Dextran-70 versus albumin as plasma expanders in cirrhotic patients with tense ascites treated with total paracentesis. Results of a randomized study. Gastroenterology 1990;99:1736–44. 10.1016/0016-5085(90)90481-F [DOI] [PubMed] [Google Scholar]
- 168. Salerno F, Badalamenti S, Lorenzano E, et al. Randomized comparative study of hemaccel vs. albumin infusion after total paracentesis in cirrhotic patients with refractory ascites. Hepatology 1991;13:707–13. 10.1002/hep.1840130416 [DOI] [PubMed] [Google Scholar]
- 169. Sola-Vera J, Miñana J, Ricart E, et al. Randomized trial comparing albumin and saline in the prevention of paracentesis-induced circulatory dysfunction in cirrhotic patients with ascites. Hepatology 2003;37:1147–53. 10.1053/jhep.2003.50169 [DOI] [PubMed] [Google Scholar]
- 170. Zhao J, Yuan C, Wang D, et al. Mannitolum infusion on cirrhotic patients with tense ascites treated by paracentesis. Chin Med J 2000;113:27–30. [PubMed] [Google Scholar]
- 171. Alessandria C, Elia C, Mezzabotta L, et al. Prevention of paracentesis-induced circulatory dysfunction in cirrhosis: standard vs half albumin doses. A prospective, randomized, unblinded pilot study. Dig Liver Dis 2011;43:881–6. 10.1016/j.dld.2011.06.001 [DOI] [PubMed] [Google Scholar]
- 172. Alsebaey A, Bassuni A, Khalil M, et al. Absno: 2296. Low-dose albumin is equally effective as standard dose albumin in preventing PICD following large volume paracentesis (LVP). Hepatol Int 2013:2296. [Google Scholar]
- 173. Johnson KB, Mueller JL, Simon TG, et al. Reduced albumin dosing during large-volume paracentesis is not associated with adverse clinical outcomes. Dig Dis Sci 2015;60:2190–5. 10.1007/s10620-015-3578-z [DOI] [PubMed] [Google Scholar]
- 174. Mirici-Cappa F, Caraceni P, Domenicali M, et al. How albumin administration for cirrhosis impacts on hospital albumin consumption and expenditure. World J Gastroenterol 2011;17:3479–86. 10.3748/wjg.v17.i30.3479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Mandorfer M, Bota S, Schwabl P, et al. Nonselective β blockers increase risk for hepatorenal syndrome and death in patients with cirrhosis and spontaneous bacterial peritonitis. Gastroenterology 2014;146:1680–90. 10.1053/j.gastro.2014.03.005 [DOI] [PubMed] [Google Scholar]
- 176. Sort P, Navasa M, Arroyo V, et al. Effect of intravenous albumin on renal impairment and mortality in patients with cirrhosis and spontaneous bacterial peritonitis. N Engl J Med 1999;341:403–9. 10.1056/NEJM199908053410603 [DOI] [PubMed] [Google Scholar]
- 177. XUE HP, LIN B, MO JZ, et al. Effect of albumin infusion on preventing the deterioration of renal function in patients with spontaneous bacterial peritonitis. Chin J Dig Dis 2002;3:32–4. 10.1046/j.1443-9573.2002.00062.x [DOI] [Google Scholar]
- 178. Chen T-A, Tsao Y-C, Chen A, et al. Effect of intravenous albumin on endotoxin removal, cytokines, and nitric oxide production in patients with cirrhosis and spontaneous bacterial peritonitis. Scand J Gastroenterol 2009;44:619–25. 10.1080/00365520902719273 [DOI] [PubMed] [Google Scholar]
- 179. Fernández J, Monteagudo J, Bargallo X, et al. A randomized unblinded pilot study comparing albumin versus hydroxyethyl starch in spontaneous bacterial peritonitis. Hepatology 2005;42:627–34. 10.1002/hep.20829 [DOI] [PubMed] [Google Scholar]
- 180. de Araujo A, de Barros Lopes A, Rossi G, et al. Low-dose albumin in the treatment of spontaneous bacterial peritonitis: should we change the standard treatment? Gut 2012;61:1371–2. 10.1136/gutjnl-2011-301739 [DOI] [PubMed] [Google Scholar]
- 181. Weisberg IS, Sharaiha RZ, Goldberg DS, et al. S1848 a kidney function determined treatment algorithm for administration of albumin in the management of spontaneous bacterial peritonitis (SBP). Gastroenterology 2009;136:A-829 10.1016/S0016-5085(09)63821-X [DOI] [Google Scholar]
- 182. Afinogenova Y, Tapper EB. The efficacy and safety profile of albumin administration for patients with cirrhosis at high risk of hepatorenal syndrome is dose dependent. Gastroenterol Rep 2015;3:216–21. 10.1093/gastro/gov032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Thévenot T, Bureau C, Oberti F, et al. Effect of albumin in cirrhotic patients with infection other than spontaneous bacterial peritonitis. A randomized trial. J Hepatol 2015;62:822–30. 10.1016/j.jhep.2014.11.017 [DOI] [PubMed] [Google Scholar]
- 184. Fernandez J, Angeli P, Trebicka J, et al. Albumin administration in the prevention of hepatorenal syndrome (HRS) and death in patients with advanced cirrhosis and non-SBP infections. J Hepatol 2018;68:S253–4. 10.1016/S0168-8278(18)30721-9 [DOI] [Google Scholar]
- 185. Caraceni P, Riggio O, Angeli P, et al. Long-term albumin administration in decompensated cirrhosis (ANSWER): an open-label randomised trial. Lancet 2018;391:2417–29. 10.1016/S0140-6736(18)30840-7 [DOI] [PubMed] [Google Scholar]
- 186. Solà E, Solé C, Simón-Talero M, et al. Midodrine and albumin for prevention of complications in patients with cirrhosis awaiting liver transplantation. A randomized placebo-controlled trial. J Hepatol 2018;69:1250–9. 10.1016/j.jhep.2018.08.006 [DOI] [PubMed] [Google Scholar]
- 187. Gentilini P, Casini-Raggi V, Di Fiore G, et al. Albumin improves the response to diuretics in patients with cirrhosis and ascites: results of a randomized, controlled trial. J Hepatol 1999;30:639–45. 10.1016/S0168-8278(99)80194-9 [DOI] [PubMed] [Google Scholar]
- 188. Vizzutti F, et al. Diuretic and natriuretic effects of long-term albumin infusion in patients with cirrhosis and ascites: a randomised controlled study. J Hepatol 2001;34:17 10.1016/S0168-8278(01)80051-9 [DOI] [Google Scholar]
- 189. Romanelli RG, et al. Long-term albumin infusion improves survival in patients with cirrhosis and ascites: an unblinded randomized trial. World J Gastroenterol 2006;12:1403–7. 10.3748/wjg.v12.i9.1403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Di Pascoli M, Fasolato S, Piano S, et al. Long-term administration of human albumin improves survival in patients with cirrhosis and refractory ascites. Liver Int 2019;39:98–105. 10.1111/liv.13968 [DOI] [PubMed] [Google Scholar]
- 191. Wong F, Sniderman K, Liu P, et al. Transjugular intrahepatic portosystemic stent shunt: effects on hemodynamics and sodium homeostasis in cirrhosis and refractory ascites. Ann Intern Med 1995;122:816–22. 10.7326/0003-4819-122-11-199506010-00002 [DOI] [PubMed] [Google Scholar]
- 192. Wong F, Sniderman K, Liu P, et al. The mechanism of the initial natriuresis after transjugular intrahepatic portosystemic shunt. Gastroenterology 1997;112:899–907. 10.1053/gast.1997.v112.pm9041252 [DOI] [PubMed] [Google Scholar]
- 193. Quiroga J, Sangro B, Núñez M, et al. Transjugular intrahepatic portal-systemic shunt in the treatment of refractory ascites: effect on clinical, renal, humoral, and hemodynamic parameters. Hepatology 1995;21:986–94. [PubMed] [Google Scholar]
- 194. Lebrec D, Giuily N, Hadengue A, et al. Transjugular intrahepatic portosystemic shunts: comparison with paracentesis in patients with cirrhosis and refractory ascites: a randomized trial. French group of clinicians and a group of biologists. J Hepatol 1996;25:135–44. 10.1016/s0168-8278(96)80065-1 [DOI] [PubMed] [Google Scholar]
- 195. Wong W, Liu P, Blendis L, et al. Long-term renal sodium handling in patients with cirrhosis treated with transjugular intrahepatic portosystemic shunts for refractory ascites. Am J Med 1999;106:315–22. [PubMed] [Google Scholar]
- 196. Gerbes AL, Gülberg V, Waggershauser T, et al. Renal effects of transjugular intrahepatic portosystemic shunt in cirrhosis: comparison of patients with ascites, with refractory ascites, or without ascites. Hepatology 1998;28:683–8. 10.1002/hep.510280313 [DOI] [PubMed] [Google Scholar]
- 197. Rössle M, Ochs A, Gülberg V, et al. A comparison of paracentesis and transjugular intrahepatic portosystemic shunting in patients with ascites. N Engl J Med 2000;342:1701–7. 10.1056/NEJM200006083422303 [DOI] [PubMed] [Google Scholar]
- 198. Ginès P, Uriz J, Calahorra B, et al. Transjugular intrahepatic portosystemic shunting versus paracentesis plus albumin for refractory ascites in cirrhosis. Gastroenterology 2002;123:1839–47. 10.1053/gast.2002.37073 [DOI] [PubMed] [Google Scholar]
- 199. Sanyal AJ, Genning C, Reddy KR, et al. The North American study for the treatment of refractory ascites. Gastroenterology 2003;124:634–41. 10.1053/gast.2003.50088 [DOI] [PubMed] [Google Scholar]
- 200. Salerno F, Merli M, Riggio O, et al. Randomized controlled study of tips versus paracentesis plus albumin in cirrhosis with severe ascites. Hepatology 2004;40:629–35. 10.1002/hep.20364 [DOI] [PubMed] [Google Scholar]
- 201. Narahara Y, Kanazawa H, Fukuda T, et al. Transjugular intrahepatic portosystemic shunt versus paracentesis plus albumin in patients with refractory ascites who have good hepatic and renal function: a prospective randomized trial. J Gastroenterol 2011;46:78–85. 10.1007/s00535-010-0282-9 [DOI] [PubMed] [Google Scholar]
- 202. Bureau C, Thabut D, Oberti F, et al. Transjugular intrahepatic portosystemic shunts with covered stents increase transplant-free survival of patients with cirrhosis and recurrent ascites. Gastroenterology 2017;152:157–63. 10.1053/j.gastro.2016.09.016 [DOI] [PubMed] [Google Scholar]
- 203. Geeroms B, Laleman W, Laenen A, et al. Expanded polytetrafluoroethylene-covered stent-grafts for transjugular intrahepatic portosystemic shunts in cirrhotic patients: long-term patency and clinical outcome results. Eur Radiol 2017;27:1795–803. 10.1007/s00330-016-4570-5 [DOI] [PubMed] [Google Scholar]
- 204. Perarnau JM, Le Gouge A, Nicolas C, et al. Covered vs. uncovered stents for transjugular intrahepatic portosystemic shunt: a randomized controlled trial. J Hepatol 2014;60:962–8. 10.1016/j.jhep.2014.01.015 [DOI] [PubMed] [Google Scholar]
- 205. Bai M, Qi X-S, Yang Z-P, et al. Tips improves liver transplantation-free survival in cirrhotic patients with refractory ascites: an updated meta-analysis. World J Gastroenterol 2014;20:2704–14. 10.3748/wjg.v20.i10.2704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206. Chen RP, Zhu Ge XJ, Huang ZM, et al. Prophylactic use of transjugular intrahepatic portosystemic shunt AIDS in the treatment of refractory ascites: metaregression and trial sequential meta-analysis. J Clin Gastroenterol 2014;48:290–9. 10.1097/MCG.0b013e3182a115e9 [DOI] [PubMed] [Google Scholar]
- 207. Campbell MS, Brensinger CM, Sanyal AJ, et al. Quality of life in refractory ascites: transjugular intrahepatic portal-systemic shunting versus medical therapy. Hepatology 2005;42:635–40. 10.1002/hep.20840 [DOI] [PubMed] [Google Scholar]
- 208. Gülberg V, Liss I, Bilzer M, et al. Improved quality of life in patients with refractory or recidivant ascites after insertion of transjugular intrahepatic portosystemic shunts. Digestion 2002;66:127–30. 10.1159/000065593 [DOI] [PubMed] [Google Scholar]
- 209. Allard JP, Chau J, Sandokji K, et al. Effects of ascites resolution after successful tips on nutrition in cirrhotic patients with refractory ascites. Am J Gastroenterol 2001;96:2442–7. 10.1111/j.1572-0241.2001.04051.x [DOI] [PubMed] [Google Scholar]
- 210. Riggio O, Ridola L, Angeloni S, et al. Clinical efficacy of transjugular intrahepatic portosystemic shunt created with covered stents with different diameters: results of a randomized controlled trial. J Hepatol 2010;52:S82–3. 10.1016/S0168-8278(10)60193-6 [DOI] [PubMed] [Google Scholar]
- 211. Wang Q, Lv Y, Bai M, et al. Eight millimetre covered tips does not compromise shunt function but reduces hepatic encephalopathy in preventing variceal rebleeding. J Hepatol 2017;67:508–16. 10.1016/j.jhep.2017.05.006 [DOI] [PubMed] [Google Scholar]
- 212. Mollaiyan A, Bettinger D, Rössle M. The underdilation of nitinol stents at tips implantation: solution or illusion? Eur J Radiol 2017;89:123–8. 10.1016/j.ejrad.2017.01.032 [DOI] [PubMed] [Google Scholar]
- 213. Miraglia R, Maruzzelli L, Tuzzolino F, et al. Transjugular intrahepatic portosystemic shunts in patients with cirrhosis with refractory ascites: comparison of clinical outcomes by using 8- and 10-mm PTFE-covered stents. Radiology 2017;284:281–8. 10.1148/radiol.2017161644 [DOI] [PubMed] [Google Scholar]
- 214. Trebicka J, Bastgen D, Byrtus J, et al. Smaller-diameter covered transjugular intrahepatic portosystemic shunt stents are associated with increased survival. Clin Gastroenterol Hepatol 2019;17:2793–9. 10.1016/j.cgh.2019.03.042 [DOI] [PubMed] [Google Scholar]
- 215. Sarwar A, Zhou L, Novack V, et al. Hospital volume and mortality after transjugular intrahepatic portosystemic shunt creation in the United States. Hepatology 2018;67:690–9. 10.1002/hep.29354 [DOI] [PubMed] [Google Scholar]
- 216. Jalan R, Elton RA, Redhead DN, et al. Analysis of prognostic variables in the prediction of mortality, shunt failure, variceal rebleeding and encephalopathy following the transjugular intrahepatic portosystemic stent-shunt for variceal haemorrhage. J Hepatol 1995;23:123–8. 10.1016/0168-8278(95)80325-4 [DOI] [PubMed] [Google Scholar]
- 217. Berlioux P, Robic MA, Poirson H, et al. Pre-transjugular intrahepatic portosystemic shunts (tips) prediction of post-TIPS overt hepatic encephalopathy: the critical flicker frequency is more accurate than psychometric tests. Hepatology 2014;59:622–9. 10.1002/hep.26684 [DOI] [PubMed] [Google Scholar]
- 218. Masson S, Mardini HA, Rose JD, et al. Hepatic encephalopathy after transjugular intrahepatic portosystemic shunt insertion: a decade of experience. QJM 2008;101:493–501. 10.1093/qjmed/hcn037 [DOI] [PubMed] [Google Scholar]
- 219. Malinchoc M, Kamath PS, Gordon FD, et al. A model to predict poor survival in patients undergoing transjugular intrahepatic portosystemic shunts. Hepatology 2000;31:864–71. 10.1053/he.2000.5852 [DOI] [PubMed] [Google Scholar]
- 220. Al Sibae MR, Cappell MS. Accuracy of MELD scores in predicting mortality in decompensated cirrhosis from variceal bleeding, hepatorenal syndrome, alcoholic hepatitis, or acute liver failure as well as mortality after non-transplant surgery or tips. Dig Dis Sci 2011;56:977–87. 10.1007/s10620-010-1390-3 [DOI] [PubMed] [Google Scholar]
- 221. Bercu ZL, Fischman AM, Kim E, et al. Tips for refractory ascites: a 6-year single-center experience with expanded polytetrafluoroethylene-covered stent-grafts. AJR Am J Roentgenol 2015;204:654–61. 10.2214/AJR.14.12885 [DOI] [PubMed] [Google Scholar]
- 222. Bureau C, Métivier S, D'Amico M, et al. Serum bilirubin and platelet count: a simple predictive model for survival in patients with refractory ascites treated by tips. J Hepatol 2011;54:901–7. 10.1016/j.jhep.2010.08.025 [DOI] [PubMed] [Google Scholar]
- 223. Salerno F, Cammà C, Enea M, et al. Transjugular intrahepatic portosystemic shunt for refractory ascites: a meta-analysis of individual patient data. Gastroenterology 2007;133:825–34. 10.1053/j.gastro.2007.06.020 [DOI] [PubMed] [Google Scholar]
- 224. Hamel B, Guillaud O, Roman S, et al. Prognostic factors in patients with refractory ascites treated by transjugular intrahepatic porto-systemic shunt: from the liver to the kidney. Dig Liver Dis 2014;46:1001–7. 10.1016/j.dld.2014.06.013 [DOI] [PubMed] [Google Scholar]
- 225. Nardelli S, Lattanzi B, Torrisi S, et al. Sarcopenia is risk factor for development of hepatic encephalopathy after transjugular intrahepatic portosystemic shunt placement. Clin Gastroenterol Hepatol 2017;15:934–6. 10.1016/j.cgh.2016.10.028 [DOI] [PubMed] [Google Scholar]
- 226. Benmassaoud A, Roccarina D, Yu D, et al. SAT-016-Impact of sarcopenia in patients undergoing transjugular intrahepatic portosystemic shunt insertion for refractory ascites. J Hepatol 2019;70:e632 10.1016/S0618-8278(19)31259-9 [DOI] [Google Scholar]
- 227. Tan HK, James PD, Sniderman KW, et al. Long-term clinical outcome of patients with cirrhosis and refractory ascites treated with transjugular intrahepatic portosystemic shunt insertion. J Gastroenterol Hepatol 2015;30:389–95. 10.1111/jgh.12725 [DOI] [PubMed] [Google Scholar]
- 228. Parvinian A, Bui JT, Knuttinen MG, et al. Transjugular intrahepatic portosystemic shunt for the treatment of medically refractory ascites. Diagn Interv Radiol 2014;20:58–64. 10.5152/dir.2013.13131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229. Grunwald D, Tapper EB, Jiang ZG, et al. A standardized assessment of functional disability predicts 1-year mortality in patients undergoing transjugular intrahepatic portosystemic shunt for refractory ascites. J Clin Gastroenterol 2016;50:75–9. 10.1097/MCG.0000000000000339 [DOI] [PubMed] [Google Scholar]
- 230. Bhangui P, Laurent A, Amathieu R, et al. Assessment of risk for non-hepatic surgery in cirrhotic patients. J Hepatol 2012;57:874–84. 10.1016/j.jhep.2012.03.037 [DOI] [PubMed] [Google Scholar]
- 231. Lemmer JH, Strodel WE, Knol JA, et al. Management of spontaneous umbilical hernia disruption in the cirrhotic patient. Ann Surg 1983;198:30–4. 10.1097/00000658-198307000-00006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232. Salamone G, Licari L, Guercio G, et al. The abdominal wall hernia in cirrhotic patients: a historical challenge. World J Emerg Surg 2018;13:35. 10.1186/s13017-018-0196-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233. Elshoura AF, Elbedewy T. Surgical repair of umbilical hernia in cirrhotic patients with ascites: is it safe? Egypt J Surg 2019;38:52–7. 10.4103/ejs.ejs_110_18 [DOI] [Google Scholar]
- 234. Coelho JCU, Claus CMP, Campos ACL, et al. Umbilical hernia in patients with liver cirrhosis: a surgical challenge. World J Gastrointest Surg 2016;8:476–82. 10.4240/wjgs.v8.i7.476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235. Krowka MJ, Wiesner RH, Heimbach JK. Pulmonary contraindications, indications and MELD exceptions for liver transplantation: a contemporary view and look forward. J Hepatol 2013;59:367–74. 10.1016/j.jhep.2013.03.026 [DOI] [PubMed] [Google Scholar]
- 236. Orman ES, Lok ASF. Outcomes of patients with chest tube insertion for hepatic hydrothorax. Hepatol Int 2009;3:582–6. 10.1007/s12072-009-9136-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237. Ditah IC, Al Bawardy BF, Saberi B, et al. Transjugular intrahepatic portosystemic stent shunt for medically refractory hepatic hydrothorax: a systematic review and cumulative meta-analysis. World J Hepatol 2015;7:1797–806. 10.4254/wjh.v7.i13.1797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238. Dhanasekaran R, West JK, Gonzales PC, et al. Transjugular intrahepatic portosystemic shunt for symptomatic refractory hepatic hydrothorax in patients with cirrhosis. Am J Gastroenterol 2010;105:635–41. 10.1038/ajg.2009.634 [DOI] [PubMed] [Google Scholar]
- 239. Poynard T, Calès P, Pasta L, et al. Beta-adrenergic-antagonist drugs in the prevention of gastrointestinal bleeding in patients with cirrhosis and esophageal varices. An analysis of data and prognostic factors in 589 patients from four randomized clinical trials. Franco-Italian multicenter study group. N Engl J Med 1991;324:1532–8. 10.1056/NEJM199105303242202 [DOI] [PubMed] [Google Scholar]
- 240. Albillos A, Zamora J, Martínez J, et al. Stratifying risk in the prevention of recurrent variceal hemorrhage: results of an individual patient meta-analysis. Hepatology 2017;66:1219–31. 10.1002/hep.29267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241. Villanueva C, Albillos A, Genescà J, et al. β blockers to prevent decompensation of cirrhosis in patients with clinically significant portal hypertension (PREDESCI): a randomised, double-blind, placebo-controlled, multicentre trial. Lancet 2019;393:1597–608. 10.1016/S0140-6736(18)31875-0 [DOI] [PubMed] [Google Scholar]
- 242. Turco L, Villanueva C, La Mura V, et al. Lowering portal pressure improves outcomes of patients with cirrhosis, with or without ascites: a meta-analysis. Clin Gastroenterol Hepatol 2020;18:313–27. 10.1016/j.cgh.2019.05.050 [DOI] [PubMed] [Google Scholar]
- 243. Pérez-Paramo M, Muñoz J, Albillos A, et al. Effect of propranolol on the factors promoting bacterial translocation in cirrhotic rats with ascites. Hepatology 2000;31:43–8. 10.1002/hep.510310109 [DOI] [PubMed] [Google Scholar]
- 244. Senzolo M, Cholongitas E, Burra P, et al. β-Blockers protect against spontaneous bacterial peritonitis in cirrhotic patients: a meta-analysis. Liver Int 2009;29:1189–93. 10.1111/j.1478-3231.2009.02038.x [DOI] [PubMed] [Google Scholar]
- 245. Reiberger T, Ferlitsch A, Payer BA, et al. Non-selective betablocker therapy decreases intestinal permeability and serum levels of LBP and IL-6 in patients with cirrhosis. J Hepatol 2013;58:911–21. 10.1016/j.jhep.2012.12.011 [DOI] [PubMed] [Google Scholar]
- 246. Krag A, Wiest R, Albillos A, et al. The window hypothesis: haemodynamic and non-haemodynamic effects of β-blockers improve survival of patients with cirrhosis during a window in the disease. Gut 2012;61:967–9. 10.1136/gutjnl-2011-301348 [DOI] [PubMed] [Google Scholar]
- 247. Sersté T, Melot C, Francoz C, et al. Deleterious effects of beta-blockers on survival in patients with cirrhosis and refractory ascites. Hepatology 2010;52:1017–22. 10.1002/hep.23775 [DOI] [PubMed] [Google Scholar]
- 248. Leithead JA, Rajoriya N, Tehami N, et al. Non-selective β-blockers are associated with improved survival in patients with ascites listed for liver transplantation. Gut 2015;64:1111–9. 10.1136/gutjnl-2013-306502 [DOI] [PubMed] [Google Scholar]
- 249. Bossen L, Krag A, Vilstrup H, et al. Nonselective β-blockers do not affect mortality in cirrhosis patients with ascites: post hoc analysis of three randomized controlled trials with 1198 patients. Hepatology 2016;63:1968–76. 10.1002/hep.28352 [DOI] [PubMed] [Google Scholar]
- 250. Chirapongsathorn S, Valentin N, Alahdab F, et al. Nonselective β-blockers and survival in patients with cirrhosis and ascites: a systematic review and meta-analysis. Clin Gastroenterol Hepatol 2016;14:1096–104. 10.1016/j.cgh.2016.01.012 [DOI] [PubMed] [Google Scholar]
- 251. Sinha R, Lockman KA, Mallawaarachchi N, et al. Carvedilol use is associated with improved survival in patients with liver cirrhosis and ascites. J Hepatol 2017;67:40–6. 10.1016/j.jhep.2017.02.005 [DOI] [PubMed] [Google Scholar]
- 252. Facciorusso A, Roy S, Livadas S, et al. Nonselective beta-blockers do not affect survival in cirrhotic patients with ascites. Dig Dis Sci 2018;63:1737–46. 10.1007/s10620-018-5092-6 [DOI] [PubMed] [Google Scholar]
- 253. Wong RJ, Robinson A, Ginzberg D, et al. Assessing the safety of beta-blocker therapy in cirrhosis patients with ascites: a meta-analysis. Liver Int 2019;39:1080–8. 10.1111/liv.14040 [DOI] [PubMed] [Google Scholar]
- 254. Njei B, McCarty TR, Garcia-Tsao G. Beta-blockers in patients with cirrhosis and ascites: type of beta-blocker matters. Gut 2016;65:1393–4. 10.1136/gutjnl-2016-312129 [DOI] [PubMed] [Google Scholar]
- 255. Bellot P, Welker M-W, Soriano G, et al. Automated low flow pump system for the treatment of refractory ascites: a multi-center safety and efficacy study. J Hepatol 2013;58:922–7. 10.1016/j.jhep.2012.12.020 [DOI] [PubMed] [Google Scholar]
- 256. Stirnimann G, Berg T, Spahr L, et al. Treatment of refractory ascites with an automated low-flow ascites pump in patients with cirrhosis. Aliment Pharmacol Ther 2017;46:981–91. 10.1111/apt.14331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257. Bureau C, Adebayo D, Chalret de Rieu M, et al. Alfapump® system vs. large volume paracentesis for refractory ascites: a multicenter randomized controlled study. J Hepatol 2017;67:940–9. 10.1016/j.jhep.2017.06.010 [DOI] [PubMed] [Google Scholar]
- 258. Lepida A, Marot A, Trépo E, et al. Systematic review with meta-analysis: automated low-flow ascites pump therapy for refractory ascites. Aliment Pharmacol Ther 2019;50:978–87. 10.1111/apt.15502 [DOI] [PubMed] [Google Scholar]
- 259. NICE Subcutaneous automated low-flow pump implantation for refractory ascites caused by cirrhosis, 2018. https://www.nice.org.uk/guidance/IPG631/chapter/1-Recommendations [Google Scholar]
- 260. Salerno F, Gerbes A, Ginès P, et al. Diagnosis, prevention and treatment of hepatorenal syndrome in cirrhosis. Gut 2007;56:1310–8. 10.1136/gut.2006.107789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261. Trebicka J, Amoros A, Pitarch C, et al. Addressing profiles of systemic inflammation across the different clinical phenotypes of acutely decompensated cirrhosis. Front Immunol 2019;10:476. 10.3389/fimmu.2019.00476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262. Piano S, Schmidt HH, Ariza X, et al. Association between grade of acute on chronic liver failure and response to terlipressin and albumin in patientswith hepatorenal syndrome. Clin Gastroenterol Hepatol 2018;16:1792–800. 10.1016/j.cgh.2018.01.035 [DOI] [PubMed] [Google Scholar]
- 263. Angeli P, Garcia-Tsao G, Nadim MK, et al. News in pathophysiology, definition and classification of hepatorenal syndrome: a step beyond the International Club of Ascites (ICA) consensus document. J Hepatol 2019;71:811–22. 10.1016/j.jhep.2019.07.002 [DOI] [PubMed] [Google Scholar]
- 264. Israelsen M, Krag A, Allegretti AS, et al. Terlipressin versus other vasoactive drugs for hepatorenal syndrome. Cochrane Database Syst Rev 2017;44:CD011532 10.1002/14651858.CD011532.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265. Allegretti AS, Israelsen M, Krag A, et al. Terlipressin versus placebo or no intervention for people with cirrhosis and hepatorenal syndrome. Cochrane Database Syst Rev 2017;4:CD011532 10.1002/14651858.CD005162.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266. Palaniyappan N, Aithal GP. Editorial: treating hepatorenal syndrome-a window and the views. Aliment Pharmacol Ther 2020;52:895–6. 10.1111/apt.15943 [DOI] [PubMed] [Google Scholar]
- 267. Boyer TD, Sanyal AJ, Garcia-Tsao G, et al. Predictors of response to terlipressin plus albumin in hepatorenal syndrome (HRS) type 1: relationship of serum creatinine to hemodynamics. J Hepatol 2011;55:315–21. 10.1016/j.jhep.2010.11.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268. Allen LA, Stevenson LW, Grady KL, et al. Decision making in advanced heart failure: a scientific statement from the American Heart Association. Circulation 2012;125:E587. 10.1161/CIR.0b013e31824f2173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269. Lanken PN, Terry PB, Delisser HM, et al. An official American Thoracic Society clinical policy statement: palliative care for patients with respiratory diseases and critical illnesses. Am J Respir Crit Care Med 2008;177:912–27. 10.1164/rccm.200605-587ST [DOI] [PubMed] [Google Scholar]
- 270. Galla JH. Clinical practice guideline on shared decision-making in the appropriate initiation of and withdrawal from dialysis. The Renal Physicians Association and the American Society of Nephrology. J Am Soc Nephrol 2000;11:1340–2. [DOI] [PubMed] [Google Scholar]
- 271. Day R, Hollywood C, Durrant D, et al. Patient experience of non-malignant ascites and its treatment: a qualitative study. Int J Palliat Nurs 2015;21:372–9. 10.12968/ijpn.2015.21.8.372 [DOI] [PubMed] [Google Scholar]
- 272. Macdonald S, Jepsen P, Alrubaiy L, et al. Quality of life measures predict mortality in patients with cirrhosis and severe ascites. Aliment Pharmacol Ther 2019;49:321–30. 10.1111/apt.15084 [DOI] [PubMed] [Google Scholar]
- 273. Mazzarelli C, Prentice WM, Heneghan MA, et al. Palliative care in end-stage liver disease: time to do better? Liver Transpl 2018;24:961–8. 10.1002/lt.25193 [DOI] [PubMed] [Google Scholar]
- 274. Rakoski MO, Volk ML. Palliative care and end-stage liver disease: a critical review of current knowledge. Curr Opin Gastroenterol 2019;35:155–60. 10.1097/MOG.0000000000000530 [DOI] [PubMed] [Google Scholar]
- 275. Hudson B, Round J, Georgeson B, et al. Cirrhosis with ascites in the last year of life: a nationwide analysis of factors shaping costs, health-care use, and place of death in England. Lancet Gastroenterol Hepatol 2018;3:95–103. 10.1016/S2468-1253(17)30362-X [DOI] [PubMed] [Google Scholar]
- 276. Fleming ND, Alvarez-Secord A, Von Gruenigen V, et al. Indwelling catheters for the management of refractory malignant ascites: a systematic literature overview and retrospective chart review. J Pain Symptom Manage 2009;38:341–9. 10.1016/j.jpainsymman.2008.09.008 [DOI] [PubMed] [Google Scholar]
- 277. White J, Carolan-Rees G. PleurX peritoneal catheter drainage system for vacuum-assisted drainage of treatment-resistant, recurrent malignant ascites: a NICE medical technology guidance. Appl Health Econ Health Policy 2012;10:299–308. 10.1007/BF03261864 [DOI] [PubMed] [Google Scholar]
- 278. Stukan M. Drainage of malignant ascites: patient selection and perspectives. Cancer Manag Res 2017;9:115–30. 10.2147/CMAR.S100210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279. Caldwell J, Edriss H, Nugent K. Chronic peritoneal indwelling catheters for the management of malignant and nonmalignant ascites. Proc (Bayl Univ Med Cent) 2018;31:297–302. 10.1080/08998280.2018.1461525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280. Macken L, Hashim A, Mason L, et al. Permanent indwelling peritoneal catheters for palliation of refractory ascites in end-stage liver disease: a systematic review. Liver Int 2019;39:1594–607. 10.1111/liv.14162 [DOI] [PubMed] [Google Scholar]
- 281. Macken L, Bremner S, Gage H, et al. Randomised clinical trial: palliative long-term abdominal drains vs large-volume paracentesis in refractory ascites due to cirrhosis. Aliment Pharmacol Ther 2020;52:107–22. 10.1111/apt.15802 [DOI] [PubMed] [Google Scholar]
- 282. Schmidt ML, Barritt AS, Orman ES, et al. Decreasing mortality among patients hospitalized with cirrhosis in the United States from 2002 through 2010. Gastroenterology 2015;148:967–77. 10.1053/j.gastro.2015.01.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283. de Castro F, Bonacini M, Walden JM, et al. Myxedema ascites. Report of two cases and review of the literature. J Clin Gastroenterol 1991;13:411–4. 10.1097/00004836-199108000-00010 [DOI] [PubMed] [Google Scholar]
- 284. Seth AK, Rangarao R, Pakhetra R, et al. Accuracy of serum-ascites albumin gradient in the aetiological diagnosis of ascites. Med J Armed Forces India 2002;58:124–6. 10.1016/S0377-1237(02)80044-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
gutjnl-2020-321790supp001.pdf (462.9KB, pdf)