INTRODUCTION
A fundamental principle of surgery is performing the optimal operation on the correct patient at the indicated location. This relies on patient selection, a judgement of treatment options, and the technical skill of the surgeon performing the operation. Operating at the correct level in the spine involves more than just familiarity with spinal anatomy and the ability to localize the proper level intraoperatively; it relies on making the proper diagnosis prior to proceeding to the operating room.
The physical exam is traditionally the cornerstone of medical evaluation and diagnosis. However, as more patients arrive at the office with advanced imaging, it is tempting to rely more heavily on imaging findings than clinical examination. This can be particularly misleading in spine surgery, as findings on advanced imaging do not always correlate with symptomatology. This may lead to operations on levels that do not necessarily require surgery, or operating on the spine altogether when the main pathology may be elsewhere.
The purpose of this article is to outline a comprehensive evaluation of the cervical spine and to discuss key principles of applying the history and physical exam to diagnosis and treatment. This will help to ensure that the optimal surgery is done for the correct patient at the correct level(s), as even technically-perfect surgery done at the wrong site will be of no use to the patient.
HISTORY
The beginning of any patient evaluation is the patient history, which often guides proper treatment in spine surgery. Severity and duration of symptoms, as well as prior treatments, can influence the decision to pursue operative versus nonoperative treatment. Several features in the history are particularly important in evaluating for suspected cervical spinal pathology.
1. Importance of the Chief Complaint
Patients often present with multiple complaints, including complaints of radiculopathy, myelopathy, or deformity. Understanding these complaints and the order in which they are bothersome can guide a discussion of possible treatments. For example, a 76-year-old lady presented to the office with multiple complaints including neck and trapezial pain, forward-pitched posture, difficulty with hand coordination, and mild gait disturbance. Given these multiple complaints, after physical exam and review of imaging, the patient was pushed toward various surgical treatment options ranging from laminoplasty, to 2-level anterior cervical discectomy and fusion (ACDF), to circumferential long cervical fusion. Correcting of her posture was a specifically discussed aspect of her prior office visits, with discussion held regarding the necessity of extensive surgery to change her cervical alignment. However, upon further discussion with the patient in our office, her posture was revealed to be a fairly a relatively complaint of hers, and she was not interested in correction of her alignment and did not wish to undergo surgery for her mild myelopathy. Instead, after a thorough discussion of her treatment goals, she elected for nonoperative interventions only. The case illustrates the importance of taking into consideration the right of the patient to make an informed decision based on discussion with the surgeon and the importance of properly elucidating the priorities of the patient, rather than pushing the patient towards an operation that the surgeon might expect the patient to want based on their list of presenting complaints. Indeed, regardless of the surgeon’s bias in evaluating the physical exam and imaging, certain patients, as in this scenario, want more information without necessarily wanting to pursue additional surgery. Having such discussions allows for patient outcomes and expectations to be aligned.
Another case exemplifying the importance of the chief complaint is a 46-year-old surgeon who presented with chronic right upper extremity radiculopathy. He complained of years of pain, but his main concern was numbness in his right index finger that had started making surgery difficult for him to perform. On imaging, the patient had severe right-sided foraminal stenosis from C4-6, and mild to moderate stenosis at the C6–7 level. The imaging led 2 surgeons to offer the patient an ACDF from C4–6.
However, imaging does not always correlate with symptoms. The C7 nerve root typically innervates the second digit, although there can occasionally be anomalous distributions. To operate without including the C6–7 level, however, would be to gamble upon the existence of an anatomically-anomalous innervation, with the chance that the patient could awaken from surgery with his chief complaint unresolved. As such, on presentation to our institution, he was offered an ACDF from C5–7, rather than C4–6, as this would likely result in a higher probability that surgery would resolve his symptoms. Importantly, this patient should be warned that the C4–5 level may need to be addressed in the future, given his already severe foraminal stenosis. Presently, however, particularly in a young patient, it would be preferable to leave C4–5 alone to preserve cervical motion. By contrast, if this were an older patient (over 60 years), it would be a reasonable to consider adding the C4–5 level prophylactically.
2. Multilevel Pathology: Acute on Chronic Complaints
Although patients occasionally present seeking treatment for longstanding symptoms, they more commonly present due to acute complaints. It is vital to elicit whether chronic symptoms have worsened or had instead been a new onset of different symptoms. It is important for the surgeon to precisely elicit the symptoms in detail to differentiate the acute symptoms from the chronic.
Consider the case of a 44-year-old man who presented with chronic neck pain with acute onset of worsening neck pain as well as right upper extremity pain and weakness.
Imaging had been notable for spondylosis at C5–6 leading to adjacent-level stress, causing a disc herniation at C6–7. Given his relatively young age, initial surgical considerations would have included 2-level artificial disc replacement at C5–6 and C6–7 to preserve cervical mobility, or potentially a 2-level ACDF at C5–6 and C6–7. However, he instead underwent a single-level C6–7 ACDF, which addressed only the disc herniation but not the spondylosis at C5–6, which left him unsatisfied postoperatively as his chronic neck pain was not relieved. He thus presented to our clinic for a second opinion.
A more thorough history, as well as a comprehensive discussion with the patient, might have helped the patient better understand the goals of the operation with regards to treating his acute versus his chronic pain generators, and allow for the opportunity for a discussion in the clinic to determine whether he would thus want to pursue an operation on one or both levels. It was revealed that addressing his chronic pain resulting from his C5–6 level was a preoperative priority of his, as was addressing his acutely worsening neck pain. Unfortunately, though he was preoperatively a potential candidate for a 2-level artificial disc replacement, he was no longer a candidate for cervical disc arthroplasty at the adjacent C5–6 level after his ACDF, leaving only the option of adjacent level fusion. Therefore, it is important to elicit the timing of the complaints as well as patient goals of care and to understand if spondylosis at one level could be increasing stress and contributing to an adjacent disc herniation to address patients complaints appropriately.
3. Distribution of Pain
It is not enough to diagnose a patient as having neck pain; understanding the exact distribution of pain can lead to more effective treatment of the offending pathology, especially in patients with a multilevel disease on imaging. The following summarizes classic cervical dermatomal distributions; though these are subject to variability (especially C7), they are often reliable in the majority of the patient population [1-6]:
O–C1: Base of a skull and worsens with flexion/extension (rare)
C1–2: Posterior occiput behind mastoid, pain with rotating neck to left or right
C2–3: Posterior head to the back of the eyes
C3–4: Upper neck to behind the ear, or paraspinal neck pain. This is incompletely described in most textbooks. The pain can indeed travel caudally to give classic paraspinal neck pain, but the pain can often be referred cephalad instead, or in addition.
C4–5: Mid-neck to behind the shoulders, sometimes into upper arms. It can be paraspinal and up to the bottom of ears since that’s where the trapezius inserts.
C5–6: Interscapular, lower neck to the radial forearm, thumb, and index finger
C6–7: Most variable dermatome affecting any single or combination of digits, often including the index finger, and causing symptoms in the anterior lateral chest and axillary regions. Typically the most frequently involved nerve root [3-6]
C7–T1: Medial upper extremity; C8 symptoms only comprise about 4%–12% of all cervical cases [5,6]. Also can be axillary or across the chest
T1–2: Also can be axillary or across the chest
Some of these, especially the dermatomes of C3–4 and C6–7, have not been fully described or debated in the literature [7]. However, after examining thousands of patients with cervical radiculopathy, we have found these distributions of pain to be quite reliable.
4. Brachial Plexus Variants
It is also important to note the possibility that patients may have either prefixed (C4 contribution) or postfixed (T2 contribution) brachial plexus variants. Prior cadaveric studies have reported the presence of a prefixed brachial plexus in 18%–25% of cadaveric specimens and a postfixed brachial plexus in 5%–7.5% of specimens, necessitating a careful review of imaging to assess for pathology affecting the C4 and T2 roots as well for patients with exams localizing to the deltoid or the intrinsic hand musculature, respectively [8,9].
PHYSICAL EXAM
1. Inspection
Begin by noting prior cervical scars as well as neck position; if the neck is protruding forward and the patient denies trauma or past surgery, inquire about a history of radiation treatment in the past. Note unilateral atrophy in the upper arm or forearm, as well as the presence of thenar, hypothenar, or intrinsic atrophy. While this varies greatly, we find that the dominant arm should typically be 1 centimeter larger in circumference than the non-dominant arm, both in the upper arm as well as the forearm.
2. Sensation
Light touch and pinwheel testing of the various dermatomes is used for testing of sensation. This should be taken in the context of whether the patient describes any potential dysesthesias as intermittent or constant.
3. Strength
Begin strength testing with a dynamometer assessment of hand grip strength. Patients can alternate hands and check their strength a few times to familiarize themselves with their own strength and fatigue rate. Since dynamometers may be calibrated differently, readings from one device cannot be compared to readings done on another device. If only one device is used consistently, then readings can be tracked over time. If multiple devices are used in the office setting, the main goal is to compare side-to-side differences in grip strength. We find grip strength decreases not only with C8 and T1 radiculopathies, but any cervical radiculopathy from C5–T1. In a typical patient, the grip strength of the dominant arm is somewhere between 10%, or in our experience, approximately 5 kg, higher than the non-dominant arm. So, in a strongly right-arm dominant individual, similar right and left grip strengths would represent a deficit of the right side [10]. This relationship can vary, but we find this is an additional objective measure to assist in the diagnosis [11,12].
Traditional manual motor testing can be performed on various muscle groups to assess potentially affected nerve roots. Table 1 lists muscle groups that can be tested and their corresponding nerve roots.
Table 1.
Muscle groups with their corresponding innervating nerve roots
| Muscle group | Nerve root |
|---|---|
| Deltoid | C5 |
| Biceps | C5/6 |
| Infraspinatus | C5/6 |
| Subscapularis | C5/C6/C7/C8/T1 |
| Supinator | C5/6 |
| Pronator | C6 |
| Wrist extensor | C6 |
| Triceps | C7 |
| Wrist flexor | C7 |
| Extensor digitorum muscle | C7,8 |
| Interosseous | C8/T1 |
| Opponens | C8/T1 |
| Adductor pollicis brevis | C8/T1 |
| Flexor pollicis brevis | C8/T1 |
4. Reflexes
A standard reflex examination should include testing of the biceps, brachioradialis, triceps, and patellar tendon reflexes, as well as checking for positive Hoffman’s and inverted radial reflexes [13]. Even if initially unremarkable, these may become positive when the neck is flexed or extended [14]. Examination with the neck in flexion/extension may elicit signs of dynamic myelopathy. Additionally, a jaw jerk reflex should be checked on all patients evaluated for cervical myelopathy; if positive, the pathology may be above the foramen magnum [15,16].
5. Other Tests
Several other tests are important in the cervical spine exam. Signs of myelopathy should routinely be assessed, including checking peripheral reflexes, Romberg’s test, single-leg stance, and tandem gait testing. Spurling’s maneuver can also help confirm the existence of radiculopathy originating from the neck. A simple generalization is that a pain that can be replicated by movement of the neck typically is originates from the neck, while pain brought on by the movement of a different part of the body, such as the shoulder, typically originates from that same part of the body.
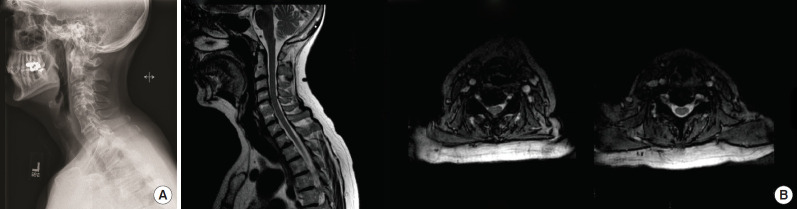
Consider the example of the 62-year-old patient with C5–6 ACDF done 28 years prior to presentation. She complained of several months of pain in her entire left upper extremity, numbness and tingling in her fingertips in the C6 distribution, and excruciating pain involving the neck and left shoulder. On examination, there was a positive Tinel’s sign at the left wrist suggesting peripheral nerve pathology; she was also unable to actively range her left shoulder. Her exam was positive for shoulder impingement, while the neck range of motion, by contrast, was pain-free. Prior to her presentation to our institution, she was recommended for injection to the cervical spine at C4-5, which provided no relief; this was in the setting of imaging demonstrating fusion at the C6–7 level and adjacent level C4–5 degeneration with a right-sided disc-osteophyte complex causing foraminal stenosis at this level (Fig. 1).
Fig. 1.
(A) Sagittal radiograph of the cervical spine demonstrating a fusion of C5–6 with adjacent segment degenerative disease at C4–5. (B) Left image: sagittal T2-weighted image of the cervical spine. Center image: axial T2-weighted imaging of the C4–5 level demonstrating right-sided foraminal disc herniation at the C4–5 level. Right image: axial T2-weighted imaging of the fused C5–6 level does not demonstrate any significant disc pathology to explain the patient’s left-sided symptoms.
Given her cervical spine magnetic resonance imaging (MRI) findings, she was referred by an outside orthopaedist to our institution. On her exam and re-review of imaging, though she did have significant right foraminal stenosis at C4–5, her imaging findings did not correlate with her left-sided complaints. Examination of her shoulder, however, demonstrated a limited active range of motion and positive impingement signs on an exam. As such, she underwent a subsequent MRI imaging of the left shoulder, which demonstrated tenosynovitis and longitudinal split tearing of the long head of the biceps tendon and chronic partial tears of the distal supraspinatus and subscapularis tendons with associated tendinosis, supporting the findings of her physical exam.
Thorough upper extremity examination can also rule out other diagnoses such as impingement, bicipital tendonitis, epicondylitis, and carpal or cubital tunnel syndrome. Peripheral nerves must be evaluated, and the presence of decreased sensation and hyporeflexia should also be assessed. Additionally, peripheral nerve entrapments should be ruled out by compressive and stretch testing of those nerves using maneuvers such as the Tinel’s, Durkan’s, or Phalen’s tests.
As a clinical pearl, it is particularly important to understand ulnar nerve innervation to differentiate ulnar nerve pathologies from C8–T1 radiculopathy properly. The ulnar nerve innervates intrinsic hand muscles except for those that are innervated by C8 and T1 through the median nerve. If these 5 muscles are affected (abductor pollicis brevis, flexor pollicis brevis, opponens pollicis, and the first 2 [radial] lumbricals), then the pathology does not arise from the ulnar nerve. Additionally, if sensation at the medial forearm is affected, the medial antebrachial cutaneous nerve is affected, which arises from C8–T1 and not from the ulnar nerve.
IMAGING
The importance of carefully scrutinizing spine imaging first-hand as a spine surgeon cannot be understated. Even experienced surgeons may misinterpret or altogether miss certain radiographic findings. Careful correlation of imaging findings with history and physical exam must be done especially considering the high rate of positive findings shown on advanced imaging of asymptomatic individuals [17]. We have seen patients offered and subsequently undergo surgery for levels appearing the most abnormal on imaging despite a lack of clinical findings localizing to that level.
1. Diagnosing Fusion
Once a level is fused, it is exceedingly rare that this level bothers a patient again; this is generally true regardless of whether the patient is surgically fused or undergoes auto-fusion. However, surgeons often overlook auto-fusions and perform multilevel surgery that includes a fusion of already fused levels. Though it is easiest to determine fusion status on a computed tomography (CT) scan, one can often diagnose a fusion on plain radiographs or MRI. In the noninstrumented spine, an auto-fused facet joint or disc space is easily identified on sagittal MRI images.
Surgeons must be vigilant in diagnosing a failure of fusion or pseudoarthrosis. Patients who have had an anterior cervical arthrodesis at outside institutions often present for the second opinions due to persistent symptoms after being told that they were healed and there was nothing further that could be done for them. The history is often classic: patients note an improvement in symptoms shortly after surgery but then have a recurrence of their symptoms or severe neck pain. Sometimes, these patients have already had radiographs, CT, and/or MRI scans without a diagnosis of pseudoarthrosis by both the spine surgeon and the reading radiologist. A careful review of the imaging will often reveal pseudoarthrosis on any of these 3 imaging modalities. In particular, a cleft can often be seen on CT or MRI, with a lack of extra-graft bridging bone on reconstructed sagittal and coronal CT images previously shown to be highly suggestive of failure of fusion [18]. Importantly, bridging bone that is seen only within the graft or cage is not accurate for determining fusion status [18]. Additionally, flexion/extension x-rays are essential as a dynamic assessment to determine whether motion exists between these extremes of position. The 3 criteria for diagnosing a solid fusion following an anterior arthrodesis procedure are: (1) at least 4 mm of interspinous process motion at a nonarthrodesed level to determine adequate effort, (2) images magnified at least 150% to identify the exact same points on the spinous process on both flexion and extension views, and (3) less than 1 mm of interspinous process motion at the arthrodesis level [18-21].
2. Disc Herniations
Specific types of disc herniations are often missed by spine surgeons and radiologists alike. If these are not specifically sought out on imaging, they can be easily overlooked. One of these is the fresh, well-hydrated disc herniation in the neuroforamen. These often appear almost iso-intense to the remainder of the cerebrospinal fluid in the spinal canal and can be nearly imperceptible on review of axial imaging on MRI. We find these easier to diagnose on parasagittal reconstructions. Herniations at the T1–2 level are also frequently missed. Axial cuts often do not include this level when an MRI of the cervical spine is ordered, but again, they will be evident on sagittal images. Importantly, a patient with weak hand grip can have a herniation at C7–T1 or T1–2. As the C8 and T1 nerve roots are intimately intertwined, it is often difficult to determine the symptomatic level by physical exam or electromyography (EMG); imaging can lend great clarity to the diagnosis. It is worth noting that myelopathy as well as more proximal-level radiculopathies can also lead to decreased grip strength.
3. Atlantoaxial Arthritis
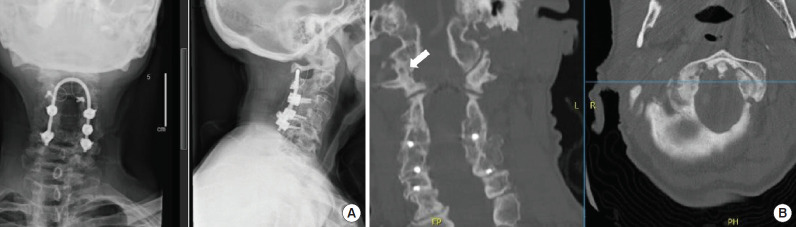
Atlantoaxial arthritis is another frequently missed diagnosis [22,23]. Patients often have a diffusely degenerated cervical spine and present after multilevel cervical fusion that failed to improve their pain. The typical complaint is pain behind the mastoid process exacerbated by rotation of the neck, with rotation to the ipsilateral side usually limited and much more painful than rotation to the contralateral side. In this scenario, careful attention should be paid to imaging of the C1–2 joint. In patients with findings of C1–2 osteoarthritis, a fusion of this level alone, despite other degenerative levels, often provides complete symptomatic relief (Fig. 2). Similar pathology may be seen in patients at the occipitocervical junction, with patients reporting pain at the base of the occiput with flexion/extension of the neck; these also have the potential for improvement with injections and can resolve with auto-fusion of the joint. In one example below, a 73-year-old female presented with excruciating high cervical pain after multiple surgeries on her neck. Although missed on initial radiologic review of the CT scan, we diagnosed her with right atlanto-occipital arthritis (Fig. 3). Her pain improved after the first steroid injection at the right occipitocervical joint and then resolved completely after the second.
Fig. 2.
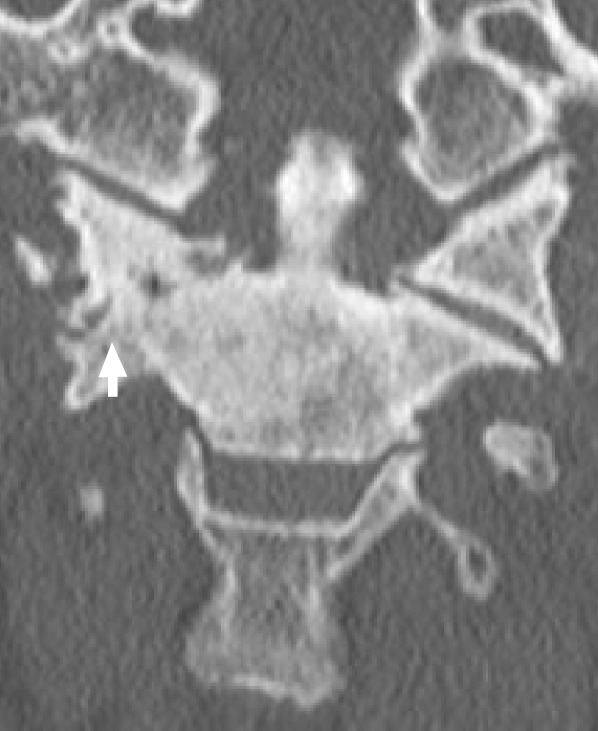
Coronal computed tomography imaging demonstrating C1–2 arthritis on patient’s right side (withe arrow).
Fig. 3.
Imaging from a patient presented after multiple cervical surgeries with persistent high cervical pain at the base of the occiput on the right side. (A) X-rays show prior instrumentation in place (anteroposterior view on left, lateral view on right). (B) Computer tomography images (coronal reformat on left, axial reformat on right) show severe right atlanto-occipital arthritis (white arrow). Pain resolved after right atlanto-occipital steroid injections.
4. Diagnosing Ossification Conditions
Surgeons must rule out ossification of the posterior longitudinal ligament when pathology on MRI does not appear to originate from disc herniation. An x-ray or MRI can be suggestive, but a CT scan is often diagnostic. Though the presence of disc-osteophyte complexes, rather than simple disc herniations alone, is often visualized from careful analysis of MRI, a CT may aid in assessing facet and uncovertebral osteophytes that help with surgical planning. Additionally, hydrated discs and/or squaring of the vertebral bodies in patients should be noted. This finding, along with auto-fusions interpreted from advanced imaging, flexion-extension radiographs, or plain anteroposterior (AP) and lateral films with advanced pathology should raise concerns for ankylosing spondylitis. Finally, single or multiple levels fused with a “wasp-waist” sign may indicate congenital Klippel-Feil auto-fusion or an old prior noninstrumented surgical fusion [24].
5. Confirming Accuracy of Reconstruction Imaging
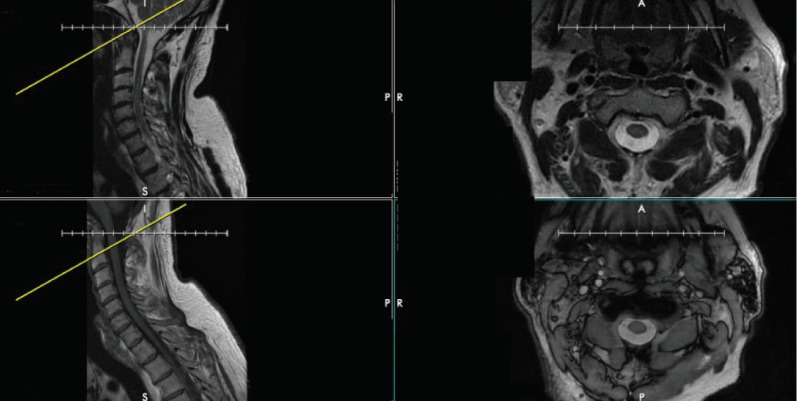
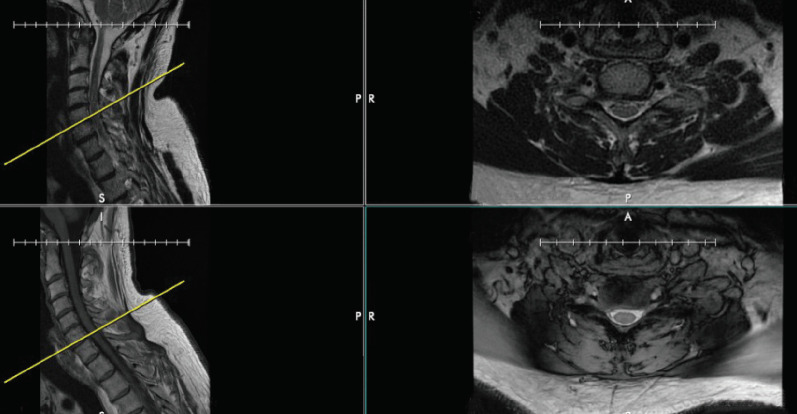
Before analyzing advanced imaging, confirm that the images on axials are correctly referenced by corresponding sagittal cuts. Every several months, we notice that the cut lines on the sagittal plane referencing an axial image have been mislabeled. As an example, the axial images of different series can show the same level – in this example, the base of C2 (Fig. 4). However, the cutline on the T2-weighted sagittal image incorrectly identifies this axial cut as being at the tip of the odontoid process. It is correctly identified on the T1-weighted sagittal image. Though this error is easy to identify at C2 due to the presence of the dens, it can easily be missed in the subaxial cervical spine. The surgeon who does not confirm levels may be misled into operating at the incorrect level. Fig. 5 again demonstrates the importance of verifying correct labeling. Although the axial T1- and T2-weighted images show the same cervical level, the reference line on the sagittal T1- and T2-weighted images differ by one level entirely, with the T2-weighted image referencing the C5–6 level but the T1-weighted image referencing the C6–7 level (Fig. 5).
Fig. 4.
The discrepancy between axial and sagittal cuts on magnetic resonance imaging of the cervical spine. Top left and bottom left show a T2- and T1-weighted sagittal cuts, respectively, demonstrating 2 different cervical spine levels. However, the top right and bottom right images show T2- and T1-weighted axial cuts, respectively, that both are at the same level (at the base of C2).
Fig. 5.
The discrepancy between reference cuts on different MRI sequences of the cervical spine. The top and bottom right images show T2- and T1-weighted axial cuts at the same level in the cervical spine, but the reference lines on the sagittal T2- and T1-weighted images seen on the top left and bottom left images differ by one level entirely, with the T2-weighted image showing the C5-6 level and the T1-weighted image showing the C6-7 level.
DIAGNOSIS AND TREATMENT
Even after a thorough cervical spine and upper extremity examination, the diagnosis may still be nebulous. Parsonage-Turner Syndrome (especially in patients reporting a viral or painful prodrome), multiple sclerosis, amyotrophic lateral sclerosis, and other neurologic conditions must be in the differential to ensure appropriate diagnosis [25-27].
Injections can aid with both diagnosis and treatment. We ask patients to take notice of whether their pain improves upon anesthetic injection. If they immediately achieve sufficient pain relief, then the level of interest has typically been found. However, one should be cautious as an epidural steroid injection may give a false positive if the anesthetic is injected at one level and subsequently flows with gravity to lower adjacent levels. In this case, the patient may report that the injected level is the correct level as it provided relief, when the level below was symptomatic. For this reason, a transforaminal injection is optimal for precisely diagnosing the level of the pathology. These should be undertaken and even requested with caution, however, particularly in patients who do not have a normal contralateral vertebral artery given the potential for arterial injury during the injection [28,29].
Once the diagnosis is made based on history, physical exam, and imaging, a treatment plan can be made. The first line in nonurgent cases includes traditional forms of nonoperative management, including medications (such as anti-inflammatories, gabapentin, and oral steroids), physical therapy, and injections. For those who fail nonoperative treatment or are otherwise indicated for surgery (e.g., those who have moderate or severe myelopathy or recalcitrant radiculopathy), surgical intervention can be discussed if the patient is medically fit for an operation.
SUMMARY
The cervical spine can be a particularly difficult region for surgeons to evaluate and treat accurately and effectively. The complexity of this region predisposes to an equally complex and often confounding set of presenting complaints, physical examination results, and imaging findings. Familiarizing one-self with the pearls and pitfalls in the workup and diagnosis of cervical spine pathology is of paramount importance. It is our hope that our prior experience in treating patients with cervical spinal conditions can be of use as a guide for other surgeons in their treatment of the cervical spine.
Footnotes
The authors have nothing to disclose.
REFERENCES
- 1.Schwartz HG. Anastomoses between cervical nerve roots. J Neurosurg. 1956;13:190–4. doi: 10.3171/jns.1956.13.2.0190. [DOI] [PubMed] [Google Scholar]
- 2.Nittby HR, Bendix T. On the variations of cervical dermatomes. Int J Anat Res. 2014:462–9. [Google Scholar]
- 3.Kimura J. Electrodiagnosis in diseases of nerve and muscle: principles and practice. 3rd ed. New York: Oxford University Press; 2001. [Google Scholar]
- 4.Dumitru D, Amato A, Zwarts M. Electrodiagnostic medicine. 2nd ed. Philadelphia (PA): Hanley and Belfus; 2002. [Google Scholar]
- 5.Greathouse DG, Joshi A. Radiculopathy of the eighth cervical nerve. J Orthop Sports Phys Ther. 2010;40:811–7. doi: 10.2519/jospt.2010.3187. [DOI] [PubMed] [Google Scholar]
- 6.Levin KH, Maggiano HJ, Wilbourn AJ. Cervical radiculopathies: comparison of surgical and EMG localization of single-root lesions. Neurology. 1996;46:1022–5. doi: 10.1212/wnl.46.4.1022. [DOI] [PubMed] [Google Scholar]
- 7.Downs MB, Laporte C. Conflicting dermatome maps: educational and clinical implications. J Orthop Sports Phys Ther. 2011;41:427–34. doi: 10.2519/jospt.2011.3506. [DOI] [PubMed] [Google Scholar]
- 8.Tubbs RS, El-Zammar D, Loukas M, et al. Intradural cervical root adjacent interconnections in the normal, prefixed, and postfixed brachial plexus. J Neurosurg Spine. 2009;11:413–6. doi: 10.3171/2009.4.SPINE09104. [DOI] [PubMed] [Google Scholar]
- 9.Guday E, Bekele A, Muche A. Anatomical study of prefixed versus postfixed brachial plexuses in adult human cadaver. ANZ J Surg. 2017;87:399–403. doi: 10.1111/ans.13534. [DOI] [PubMed] [Google Scholar]
- 10.Bechtol CO. Grip test; the use of a dynamometer with adjustable handle spacings. J Bone Joint Surg Am. 1954;36-A:820–4. [PubMed] [Google Scholar]
- 11.Joghataei MT, Arab AM, Khaksar H. The effect of cervical traction combined with conventional therapy on grip strength on patients with cervical radiculopathy. Clin Rehabil. 2004;18:879–87. doi: 10.1191/0269215504cr828oa. [DOI] [PubMed] [Google Scholar]
- 12.Petersen P, Petrick M, Connor H, et al. Grip strength and hand dominance: challenging the 10% rule. Am J Occup Ther. 1989;43:444–7. doi: 10.5014/ajot.43.7.444. [DOI] [PubMed] [Google Scholar]
- 13.Estanol BV, Marin OS. Mechanism of the inverted supinator reflex. A clinical and neurophysiological study. J Neurol Neurosurg Psychiatry. 1976;39:905–8. doi: 10.1136/jnnp.39.9.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tuchman A, Tan LA, Shillingford JN, et al. Dynamic changes in the reflex exam of patients with sub-axial cervical stenosis. J Clin Neurosci. 2019;60:84–7. doi: 10.1016/j.jocn.2018.09.032. [DOI] [PubMed] [Google Scholar]
- 15.Walker HK. Cranial nerve V: the trigeminal nerve. In: Walker HK, Hall WD, Hurst JW, editors. Clinical methods: the history, physical, and laboratory examinations. 3rd ed. Boston: Butterworths; 1990. Chapter 61. [PubMed] [Google Scholar]
- 16.Bishop B, Hickenbottom RS, Moriarty TM. Identification and assessment of factors contributing to variability of the jaw jerk. Exp Neurol. 1984;84:549–64. doi: 10.1016/0014-4886(84)90203-6. [DOI] [PubMed] [Google Scholar]
- 17.Kato F, Yukawa Y, Suda K, et al. Normal morphology, age-related changes and abnormal findings of the cervical spine. Part II: magnetic resonance imaging of over 1,200 asymptomatic subjects. Eur Spine J. 2012;21:1499–507. doi: 10.1007/s00586-012-2176-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Riew KD, Yang JJ, Chang DG, et al. What is the most accurate radiographic criterion to determine anterior cervical fusion? Spine J. 2019;19:469–75. doi: 10.1016/j.spinee.2018.07.003. [DOI] [PubMed] [Google Scholar]
- 19.Cannada LK, Scherping SC, Yoo JU, et al. Pseudoarthrosis of the cervical spine: a comparison of radiographic diagnostic measures. Spine (Phila Pa 1976) 2003;28:46–51. doi: 10.1097/00007632-200301010-00012. [DOI] [PubMed] [Google Scholar]
- 20.Song KS, Piyaskulkaew C, Chuntarapas T, et al. Dynamic radiographic criteria for detecting pseudarthrosis following anterior cervical arthrodesis. J Bone Joint Surg Am. 2014;96:557–63. doi: 10.2106/JBJS.M.00167. [DOI] [PubMed] [Google Scholar]
- 21.Rhee JM, Chapman JR, Norvell DC, et al. Radiological determination of postoperative cervical fusion: a systematic review. Spine (Phila Pa 1976) 2015;40:974–91. doi: 10.1097/BRS.0000000000000940. [DOI] [PubMed] [Google Scholar]
- 22.Schaeren S, Jeanneret B. Atlantoaxial osteoarthritis: case series and review of the literature. Eur Spine J. 2005;14:501–6. doi: 10.1007/s00586-004-0856-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Buraimoh MA, Massie LW, Montgomery DM. Lateral atlantoaxial osteoarthritis: a narrative literature review. Clin Spine Surg. 2017;30:433–8. doi: 10.1097/BSD.0000000000000592. [DOI] [PubMed] [Google Scholar]
- 24.Nguyen VD, Tyrrel R. Klippel-Feil syndrome: patterns of bony fusion and wasp-waist sign. Skeletal Radiol. 1993;22:519–23. doi: 10.1007/BF00209100. [DOI] [PubMed] [Google Scholar]
- 25.Young WF. Cervical spondylotic myelopathy: a common cause of spinal cord dysfunction in older persons. Am Fam Physician. 2000;62:1064-70, 1073. [PubMed] [Google Scholar]
- 26.Pellerin M, Kimball Z, Tubbs RS, et al. The prefixed and postfixed brachial plexus: a review with surgical implications. Surg Radiol Anat. 2010;32:251–60. doi: 10.1007/s00276-009-0619-3. [DOI] [PubMed] [Google Scholar]
- 27.Mamula CJ, Erhard RE, Piva SR. Cervical radiculopathy or Parsonage-Turner syndrome: differential diagnosis of a patient with neck and upper extremity symptoms. J Orthop Sports Phys Ther. 2005;35:659–64. doi: 10.2519/jospt.2005.35.10.659. [DOI] [PubMed] [Google Scholar]
- 28.Gitkind AI, Olson TR, Downie SA. Vertebral artery anatomical variations as they relate to cervical transforaminal epidural steroid injections. Pain Med. 2014;15:1109–14. doi: 10.1111/pme.12266. [DOI] [PubMed] [Google Scholar]
- 29.Beckworth WJ, Sood R, Katzer AF, et al. Anomalous location of the vertebral artery in relation to the neural foramen. Implications for cervical transforaminal epidural steroid injections. Pain Med. 2013;14:1119–25. doi: 10.1111/pme.12121. [DOI] [PubMed] [Google Scholar]