Abstract
Background and purpose
The aim of this study was to look for deviations of cerebral perfusion in patients suffering from pantothenate kinase-associated neurodegeneration, where the globus pallidus is affected by severe accumulation of iron.
Material and methods
Under resting conditions, cerebral blood flow was measured by the magnetic resonance imaging technique of arterial spin labelling in cortical areas and basal ganglia in eight pantothenate kinase-associated neurodegeneration patients and 14 healthy age-matched control subjects and correlated to T2* time of these areas and – in patients – to clinical parameters.
Results
Despite highly significant differences of T2* time of the globus pallidus (20 vs 39 ms, p < 0.001), perfusion values of this nucleus were nearly identical in both groups (32 ± 3.3 vs 31 ± 4.0 ml/min/100 g) as well as in total brain gray matter (both 62 ± 6.7 resp. ±10.3 ml/min/100 g), putamen (41 ± 5.4 vs 40 ± 6.1 ml/min/100 g), in selected cortical regions, and the cerebellum. Correlations between perfusion and T2* time to clinical data did not reach significance (p > 0.05).
Conclusion
The absence of any obvious deviations of perfusion in the group of patients during a resting condition does not support the view that (non-functional) vascular pathology is a major pathogenic factor in pantothenate kinase-associated neurodegeneration in the younger age group. The findings underline the value of the arterial spin technique to measure cerebral blood flow in areas of disturbed susceptibility.
Keywords: Pantothenate kinase-associated neurodegeneration, cerebral blood flow, globus pallidus, tiger’s eye
Introduction
Pantothenate kinase-associated neurodegeneration (PKAN) belongs to the group of orphan diseases with a prevalence of 1:1–3,000,000 cases.1 The primary genetic defect has been localized to the PANK2 gene and induces impairment in the production of acetyl-coenzyme A resulting in formation of deficient mitochondrial acyl carrier protein and finally in dyshomeostasis and accumulation of iron.2,3
In magnetic resonance imaging (MRI), this accumulation of iron together with the primary defect, an area of gliosis in the antero-medial portion of the globus pallidus, is known as the “eye-of-the-tiger” sign. The iron deposits increase with age, but without significant correlation to the severity of dystonia, and might be a secondary phenomenon.4–6 Other findings like the reduction of gray matter (GM) density of the frontal, parietal, and cingular cortex have been speculated to be responsible for progression of symptoms.7
Another factor, cerebral perfusion, might also be involved in the determination of the course of the disease. In the zebrafish, Zizioli et al.8 demonstrated that knock-down of pantothenate kinase 2 severely affects the development of the nervous and also the vascular system. Thus, alteration of cerebral perfusion might be related to progression of PKAN dystonia, similar to Alzheimer’s disease. Because of the histo-pathological findings of iron deposition, loss of myelin, neuro-axonal spheroids, and neuro-fibrillary tangles,9 as well as its progressive course, PKAN has to be regarded as a degenerative condition.
Cerebral blood flow (CBF) has not yet been evaluated systematically in this condition. To the best of our knowledge, there are just two reports in the literature, one single photon emission tomography (SPECT) examination10 and one MRI pilot study, which proved the feasibility of CBF measurement in areas of high content of iron using arterial spin labelling (ASL).11
The present study looked for impairment of CBF in cortical areas, the basal ganglia, and the cerebellum in a group of PKAN patients, who had been characterized previously to suffer from an identical missense mutation of the PANK2 gene (c.680 A>G, p.Y227C) and clinically by a delayed type of onset of symptoms during late infancy or early adolescence.12 T2* time was measured as well to see if a high concentration of extracellular iron as in the “tiger’s eye” does affect CBF results as measured by the ASL technique.
Material and methods
This prospective study was approved by the local Ethics Committee, and informed consent had been received from all participants resp. their parents.
Patients and controls
Apart from genetic confirmation of PKAN, a main criterion for inclusion of patients was the absence of movement artifacts in the highly susceptible T2*-weighted and other images. Thus, eight patients, five females and three males, with a mean age of 19.0 (13.1–27.4) years and 14 healthy age-matched controls, nine females and five males not related to hospital staff, with a mean age of 18.7 (11.4–38.1) years were included. Mean age of onset of symptoms was 9.0 ± 2.9 years and mean duration of symptoms was 15.9 ± 8.0 years. On neurological examination, patients presented a mean score on the Burke-Fahn-Marsden (BFM) dystonia scale of 53.4 ± 19.2 points and of 16.8 ± 7.7 points on the disability scale (Table 1).
Table 1.
Patients and clinical data.
| Gender | Age of onseta | Durationa | Main affection | Dystonia scale | DisabIity scale | Medication | |
|---|---|---|---|---|---|---|---|
| Patient 1 | F | 8 | 19 | Extremities >trunk | 60.5 | 23 | None |
| Patient 2 | F | 7 | 12 | Oro-pharyngeal >extremities | 48 | 12 | None |
| Patient 3 | M | 5 | 10 | Extremities | 17 | 5 | None |
| Patient 4 | F | 8 | 6 | Extremities >trunk | 75 | 7 | None |
| Patient 5 | F | 11 | 27 | Extremities | 76.5 | 25 | Occasionally paracetamol |
| Patient 6 | F | 14 | 26 | Extremities >oro-pharyngeal | 56 | 22 | Occasionally paracetamol |
| Patient 7 | M | 8 | 8 | Extremities >trunk | 40.5 | 18 | Occasionally coenzyme q10, 50 mg daily |
| Patient 8 | M | 11 | 19 | Extremities >oro-pharyngeal | 54 | 22 | Occasionally paracetamol |
aIn years.
MRI examination
Imaging was carried out on a 3 Tesla Philips scanner with the exception of the ASL data, which for logistical reasons had to be acquired on a 1.5 Tesla General Electric (GE) scanner.
Philips Achieva 3T
Apart from routine T1- and T2-weighted sequences, we measured T2* time using a 3D Fast Field Echo sequence covering the basal ganglia: Time of Repetition (TR) 329 ms, Time of Echo (TE) 2.1 ms, flip angle 12°, 10 echoes with 3.2 ms spacing, slice thickness 4 mm, pixel size 1.3 × 1.3 mm.
GE Signa Artist 1.5T
Pseudo-continuous ASL (pCASL) 3D Fast Spin Echo sequence with 38 slices of a thickness of 4 mm covering whole head; using a stack of spiral readout with 512 readout points and eight arms; acquisition matrix 128 × 128, pixel size 1.9 × 1.9 mm. TR 4877 ms, TE 10.7 ms, label duration 1500 ms, post-label delay 2025 ms.
Postprocessing and further evaluation of data
CBF maps were calculated by vender-provided software as offered on the GE workstation using the following parameters: labeling efficiency 0.6, blood/brain partition coefficient 0.9, T1 of cerebral GM 1.2 s and of arterial blood 1.4 s (both at 1.5T).13
T2* maps were calculated with in-house software developed in Python (www.python.org). Data were fitted to a mono-exponential decay model using the Levenberg-Marquardt algorithm from the Scipy scientific libraries (www.scipy.org).
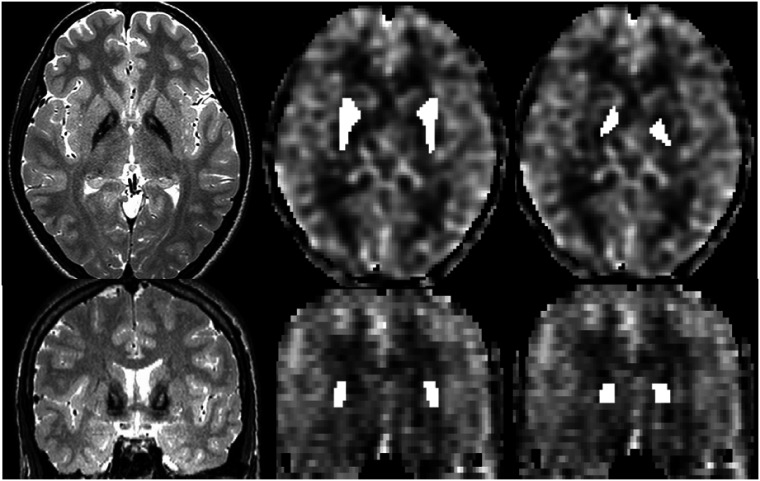
To measure CBF and T2* time, individual regions of interest (ROIs) were drawn on T2-weighted images, co-registered to the CBF maps (Statistical Parametric Mapping (SPM 12), www.fil.ion.ucl.ac.uk/spm) to cover the globus pallidus resp. the putamen, and were transferred to the CBF maps (Figure 1). GM ROIs were taken from co-registered and segmented T2-weighted images. To measure regional cortical CBF, subvolumes of the ROI-MNI-V4-atlas included in the aal-toolbox of SPM were co-registered to normalized and segmented individual CBF maps of the frontal, precentral, parietal, and cingular cortex of both hemispheres, the supplementary motor area (SMA), and the cerebellum. To look for an influence of T2* time on CBF measured within the iron-containing globus pallidus, we correlated CBF values of all participants to T2* time of this nucleus.
Figure 1.
Original (not co-registered) T2W images showing the “tiger’s eye” (left) and cerebral blood flow (CBF) maps with regions of interest (ROIs) covering putamen (middle) and globus pallidus (right) in axial (upper row) and coronal projection (lower row).
Results were compared between patients and controls by using the two-tailed t-test, and CBF was correlated to age and to T2* time measured in corresponding areas. In patients, the CBF of total brain GM and of cortical segmented subvolumes was correlated to clinical data, after correction for age at MRI examination using the SPSS statistical package (www.ibm.com/de-de/analytics/spss-statistics-software). Signif-cance was accepted at a 95% level, corrected for false detection rate (FDR; www.sdmproject.com/utilities/?show=FDR).
Results
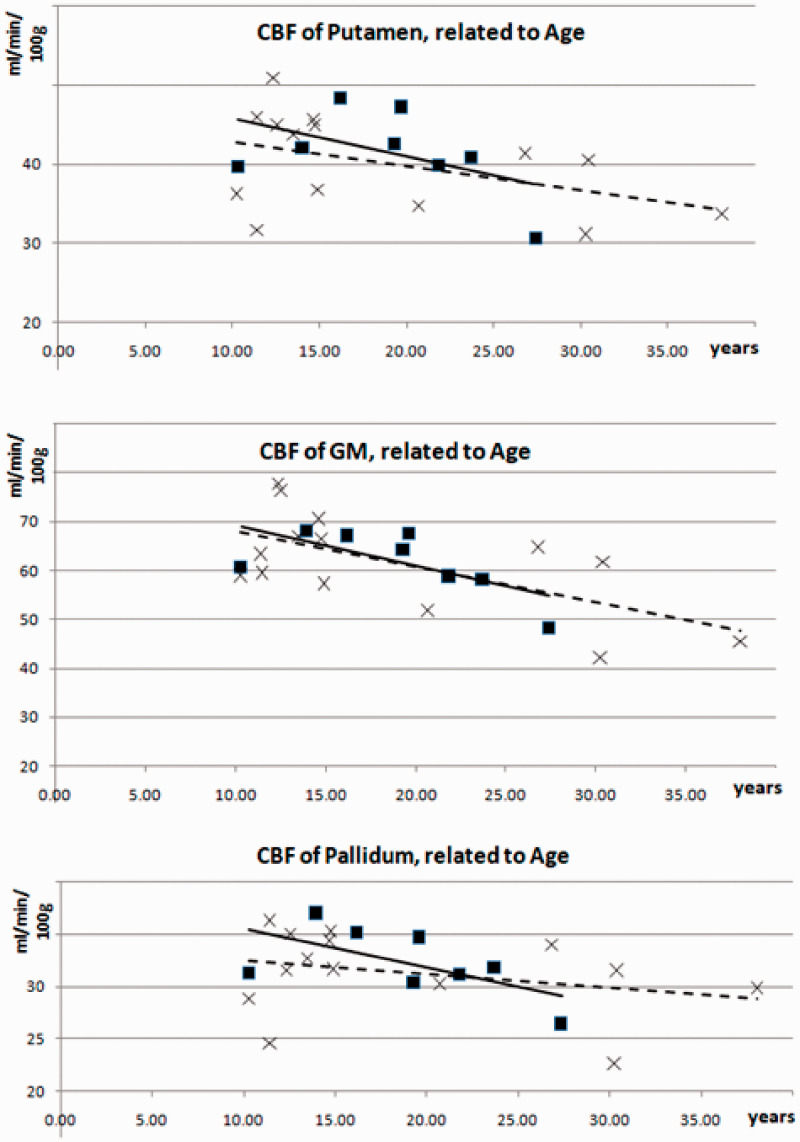
The CBF of the GM, putamen, and globus pallidus was similar in patients and controls (slightly above 60 resp. 40 resp. 30 ml/min/100 g) as well as the relationship of pallidal to putaminal CBF (78% resp. 79%, see Table 2). In patients and controls, the CBF of these areas related negatively to age (Figure 2), but only in the control group, the correlation coefficient (CC) between GM CBF and age reached significance (CC = −0.634, p = 0.045 corrected for FDR). All other clinical data such as age of onset, duration, and severity of symptoms as measured by the BFM dystonia and disability scales did not show significant correlations to the CBF of GM.
Table 2.
Cerebral blood flow (CBF) and T2* time of patients and controls of gray matter (GM) and basal ganglia.
| CBF (ml/min/100 g) |
T2* time (ms) |
|||
|---|---|---|---|---|
| Segmented area | Patients | Controls | Patients | Controls |
| Gray matter | 61.66 ± 6.70 | 61.67 ± 10.31a | 61.35 ± 2.68 | 62.97 ± 4.82 |
| Putamen | 41.43 ± 5.39 | 40.16 ± 6.14 | 54.15 ± 3.63 | 51.80 ± 5.75 |
| Pallidum | 32.22 ± 3.30 | 31.35 ± 3.95 | 20.43 ± 3.40b | 39.25 ± 5.96b |
| Pallidum/putamen | 0.78 ± 0.06 | 0.79 ± 0.07 | 0.38 ± 0.05b | 0.76 ± 1.04b |
FDR: false detection rate.
aSignificant correlation to age at examination (Pearson’s correlation, p < 0.05, corrected for FDR).
bSignificant difference between patients and controls (t-test, p < 0.001, corrected for FDR).
Figure 2.
Correlation of cerebral blood flow (CBF) of gray matter (GM) (upper image), putamen (middle image), and globus pallidus (lower image) to age in patients (squares, solid lines) and controls (crosses, broken lines).
The same applied for all cortical and basal ganglia subvolumes, where CBF was nearly the same as in the control group (see Table 3). In the parietal and cingular subvolumes, partial correlation analysis showed a negative relationship of CBF to age at the onset of disease (CC = −0.863 and −0.854, respectively) after correction for age at MRI examination. However, these CCs stayed below the level of significance (p < 0.14) after FDR correction, as did all other CCs of cortical and basal ganglia subvolumes to movement scores.
Table 3.
Cerebral blood flow (CBF) of patients and controls in segmented cortical areas and cerebellum.
| CBF (ml/min/100 g) | ||
|---|---|---|
| Segmented area | Patients | Controls |
| Frontal cortex | 59.49 ± 7.98 | 62.74 ± 11.76 |
| Precentral cortex | 58.37 ± 5.85 | 56.93 ± 10.67 |
| SMA | 57.06 ± 4.78 | 59.68 ± 13.49 |
| Parietal cortex | 60.59 ± 6.60 | 60.67 ± 11.90 |
| Cingular cortex | 64.25 ± 8.92 | 65.47 ± 11.48 |
| Cerebellum | 52.74 ± 5.80 | 50.46 ± 8.33 |
SMA: supplementary motor area.
T2* time of the globus pallidus was reduced significantly in patients (20 vs 39 ms) as well as the pallidal-putaminal quotient (0.38 vs 0.76, p < 0.001 corrected for FDR, Table 2). After correction for age, there was a negative relationship of T2* time of globus pallidus to CBF in this area (CC = –0.571), which did not reach significance (p = 0.181, uncorrected).
CBF values of globus pallidus taken from all participants, correlated negatively to T2* time of this nucleus (CC = −0.080), however, this was far below any level of significance.
Discussion
In contrast to the previously mentioned SPECT perfusion study of one PKAN patient,10 we did not see any significant deviations of global or regional CBF from normal controls. There were no convincing trends towards decreased CBF, either in the cortex or in the basal ganglia. The CBF values of the control group and their correlation to age are well in accordance with results from the literature measured by the pCASL method.14–16 This applies as well to the CBF quotient between globus pallidus and putamen, which was 78% and 79% in our patients and controls and has been reported elsewhere to be 73% in healthy male subjects.17
A second result of this study is that severe deviations of susceptibility, such as those caused by accumulation of iron within the globus pallidus in PKAN, do not have an important impact on the CBF maps. Although a correction of susceptibility-induced alterations as recommended by Madai et al.18 was not applied, the finding of a nearly identical mean CBF coefficient of putamen vs globus pallidus in patients and controls and the lack of correlation between CBF and T2* time supports this conclusion. It might be explained by the fact that the label and control images used in ASL are affected equally by susceptibility-induced effects, and possible artifacts are cancelled by subtraction of both images.19 In this sense, ASL is clearly superior to the dynamic susceptibility contrast method to monitor CBF, if the “tiger’s eye” is included as a ROI in the examination.
We also did not correct for differences between cortical areas and basal ganglia as the slightly lower T1 time and shorter arterial transit times of the latter, where the labeled spins arrive earlier and spend more time relaxing with a shorter T1 time.20,21 However, a possible resulting underestimation of basal ganglia CBF does not affect the comparison of parameters between patients and controls, which is the main aspect of the present study.
Among movement disorders, Parkinson’s disease and related conditions are those most thoroughly examined by imaging techniques.22 Perfusion was reported to be decreased in the cortex and either preserved or decreased in the basal ganglia.23,24 Because of its correlation with patterns of metabolic activity,25 measurement of CBF and might be useful in early diagnosis of individual patients, tracking progression of cognitive and motor status and supporting therapeutic decisions as in levodopa-induced dyskinesia.26–28
Reports about CBF in dystonia caused by PKAN or in other forms of dystonia are not as frequent. As mentioned above, Doi et al.10 (p. 172) found “decreased regional cerebral blood flow in the bilateral fronto-parietal lobes, the globus pallidus, the striatum, and around the ventriculus quartus” in two siblings suffering from PKAN. In transient idiopathic dystonia, basal ganglia and temporo-mesial cortical perfusion was reported to be reduced.29 In two patients with hemidystonia, increased thalamic perfusion normalized or decreased below normal after treatment.30,31 In focal hand dystonia, perfusion was found to be increased in areas related to the motor system32 with corresponding changes of connectivity in resting state networks.33,34
A recent study of focal hand dystonia did not report perfusion deviations of the basal nuclei, but increased CBF in the inferior bifrontal cortex and the anterior part of the putamen, which correlated negatively with the duration of disease. Together with increased gamma aminobutyric acid (GABA)-ergic neurotransmission, this finding was regarded as a compensatory mechanism to control dystonic movements.35 A similar increase of CBF in predominantly motor-related areas was observed during active movements in idiopathic torsion dystonia36 and in dystonia due to PKAN during a motor-activation condition.37
Our patients, however, were lying quietly in the scanner without obvious spells of dystonic movements, and this absence of activity might explain the absence of perfusion deviations in the present study and also the lack of correlation of perfusion to clinical data. Obviously, the defect of pantothenate kinase 2 did not severely affect the the cerebral vascular systems in humans in this rather young group of patients, and pathological reports in PKAN do not mention vascular anomalies.9,38,39
Although we were not able to demonstrate a correlation of CBF changes to progression of age in patients, we cannot rule out that arterial wall degeneration plays a role in later stages of the disease, because PKAN patients suffer from a pyruvate dehydrogenase deficiency,40 and this deficiency is related to increased vascular calcification.41 One reason for the lack of correlation between age progression and decrease of CBF might be the main limitation of this study, that is the rather small number of patients. But considering the rarity of the condition and the necessity to exclude scans with moving artifacts, a larger group size could not be achieved. Further studies about CBF in PKAN are needed to clarify this point.
Conclusion
Our study of cerebral perfusion in dystonia due to PKAN did not demonstrate any obvious deviations between the group of patients and controls during a resting state condition. In accordance with previous histological findings, the results of our study support the hypothesis that vascular pathology probably is not a major pathogenic factor in young PKAN patients. But with progressing age, vascular degeneration cannot be ruled out and should be looked into further with a follow-up study which includes volumetric measurements of the basal ganglia.
Our findings also underline the value of the arterial spin technique to measure CBF in areas of disturbed susceptibility.
Footnotes
Conflict of interest: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Peter Stoeter https://orcid.org/0000-0003-4045-4990
References
- 1.Gregory A, Hayflick SJ. Neurodegeneration with brain iron accumulation. Folia Neuropathol 2005; 43: 286–296. [PMC free article] [PubMed] [Google Scholar]
- 2.Hayflick SJ, Westaway SK, Levinson B, et al. Genetic, clinical, and radiographic delineation of Hallervorden-Spatz syndrome. N Engl J Med 2003; 348: 33–40. [DOI] [PubMed] [Google Scholar]
- 3.Jeong SY, Hogarth P, Placzek A, et al. 4'-Phosphopantetheine corrects CoA, iron, and dopamine metabolic defects in mammalian models of PKAN. EMBO Mol Med 2019; 11: e10489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Savoiardo M, Halliday WC, Nardocci N. Hallervorden-Spatz disease: MR and pathologic findings. AJNR Am J Neuroradiol 1993; 14: 155–162. [PMC free article] [PubMed] [Google Scholar]
- 5.Vilchez-Abreu C, Roa-Sanchez P, Fermin-Delgado R, et al. El signo del “Ojo del Tigre” en resonancia magnética: Cambios relacionados con la edad. Anal Radiol Mexico 2013; 3: 189–196. [Google Scholar]
- 6.Lee J-H, Gregory A, Hogarth P, et al. Looking deep into the eye-of-the-tiger in pantothenate kinase-associated neurodegeneration. AJNR Am J Neuroradiol 2018; 39: 583–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rodriguez-Raecke R, Roa-Sanchez P, Speckter H, et al. Grey matter alterations in patients with pantothenate kinase-associated neurodegeneration (PKAN). Parkinsonism Relat Disord 2014; 20: 975–979. [DOI] [PubMed] [Google Scholar]
- 8.Zizioli D, Tiso N, Guglielmi A, Saraceno C, et al. Knock-down of pantothenate kinase 2 severely affects the development of the nervous and vascular system in zebrafish, providing new insights into PKAN disease. Neurobiol Dis 2016; 85: 35–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kruer MC. The neuropathology of neurodegeneration with brain iron accumulation. Int Rev Neurobiol 2013; 110: 165–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Doi H, Koyano S, Miyatake S, et al. Siblings with the adult-onset slowly progressive type of pantothenate kinase-associated neurodegeneration and a novel mutation, Ile346Ser, in PANK2: Clinical features and (99m)Tc-ECD brain perfusion SPECT findings. J Neurol Sci 2010; 290: 172–176. [DOI] [PubMed] [Google Scholar]
- 11.Stoeter P, Roa Sanchez P, Speckter H, et al. Cerebral blood flow in dystonia due to pantothenate kinase-associated neurodegeneration (PKAN) as measured by arterial spin labelling (ASL): A pilot study. ACR J Radiol Med Imaging 2019; 4: 1–5. [Google Scholar]
- 12.Schiessl-Weyer J, Roa P, Laccone F, et al. Acanthocytosis and the c.680 A>G mutation in the PANK2 gene: A study enrolling a cohort of PKAN patients from the Dominican Republic. PLoS One 2015; 10: e0125861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alsop DC, Detre JA, Golay X, et al. Recommended implementation of arterial spin-labeled perfusion MRI for clinical applications: A consensus of the ISMRM perfusion study group and the European consortium for ASL in dementia. Magn Reson Med 2015; 73: 102–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Parkes LM, Rashid W, Chard DT, et al. Normal cerebral perfusion measurements using arterial spin labeling: Reproducibility, stability, and age and gender effects. Magn Reson Med 2004; 51: 736–743. [DOI] [PubMed] [Google Scholar]
- 15.Carsin-Vu A, Corouge I, Commowick O, et al. Measurement of pediatric regional cerebral blood flow from 6 months to 15 years of age in a clinical population. Eur J Radiol 2018; 101: 38–44. [DOI] [PubMed] [Google Scholar]
- 16.Zhang N, Gordon ML, Ma Y, et al. The age-related perfusion pattern measured with arterial spin labeling MRI in healthy subjects. Front Aging Neurosci 2018; 10: 214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hetzer S, Birr P, Fehlner A, et al. Perfusion alters stiffness of deep gray matter. J Cereb Blood Flow Metab 2018; 38: 116–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Madai VI, Martin SZ, von Samson-Himmelstjerna FC, et al. Correction for susceptibility distortions increases the performance of arterial spin labeling in patients with cerebrovascular disease. J Neuroimaging 2016; 26: 436–444. [DOI] [PubMed] [Google Scholar]
- 19.Detre JA, Alsop DC. Perfusion magnetic resonance imaging with continuous arterial spin labeling: Methods and clinical applications in the central nervous system. Eur J Radiol 1999; 30: 115–124. [DOI] [PubMed] [Google Scholar]
- 20.Steen RG, Reddick WE, Kingsley PB. Age-related changes in the pediatric brain: Quantitative MR evidence of maturational changes during adolescence. AJNR Am J Neuroradiol 1997; 18: 819–812. [PMC free article] [PubMed] [Google Scholar]
- 21.Bladt P, den Dekker AJ, Clement P, et al . The costs and benefits of estimating T 1 of tissue alongside cerebral blood flow and arterial transit time in pseudo-continuous arterial spin labeling. NMR Biomed 2019; (online ahead of print): e4182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stoessl AJ, Lehericy S, Strafella AP. Imaging insights into basal ganglia function, Parkinson's disease, and dystonia. Lancet 2014; 384: 532–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fernández-Seara MAVidorreta M, et al. Cortical hypoperfusion in Parkinson's disease assessed using arterial spin labeled perfusion MRI. Neuroimage 2012; 59: 2743–2750. [DOI] [PubMed] [Google Scholar]
- 24.Pyatigorskaya N, Gallea C, Garcia-Lorenzo D, et al. A review of the use of magnetic resonance imaging in Parkinson's disease. Ther Adv Neurol Disord 2014; 7: 206–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Colloby SJ, McKeith IG, Burn DJ, et al. Cholinergic and perfusion brain networks in Parkinson disease dementia. Neurology 2016; 87: 178–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Teune LK, Renken RJ, de Jong BM, et al. Parkinson's disease-related perfusion and glucose metabolic brain patterns identified with PCASL-MRI and FDG-PET imaging. Neuroimage Clin 2014; 5: 240–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Melzer TR, Watts R, MacAskill MR, et al. Arterial spin labelling reveals an abnormal cerebral perfusion pattern in Parkinson's disease. Brain 2011; 134: 845–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lerner RP, Bimpisidis Z, Agorastos S, et al. Dissociation of metabolic and hemodynamic levodopa responses in the 6-hydroxydopamine rat model. Neurobiol Dis 2016; 96: 31–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.John B, Klemm E, Haverkamp F. Evidence for altered basal ganglia and cortical functions in transient idiopathic dystonia. J Child Neurol 2000; 15: 820–822. [DOI] [PubMed] [Google Scholar]
- 30.Fiedler A, Marienhagen J, Aderbauer J, et al. Follow-up findings in regional cerebral blood flow (r-CBF)-SPECT in a case of idiopathic childhood hemidystonia. Functional neuroimaging and pathophysiological implications. Nuklearmedizin 1999; 38: 72–74. [PubMed] [Google Scholar]
- 31.Hiraga A, Fukutake T, Arai K, et al. SPECT abnormalities with unilateral arm dystonia in a young mentally retarded apprentice cook: Contralateral thalamo-cortical dysfunction. Parkinsonism Relat Disord 2003; 9: 253–256. [DOI] [PubMed] [Google Scholar]
- 32.Odergren T, Stone-Elander S, Ingvar M. Cerebral and cerebellar activation in correlation to the action-induced dystonia in writer's cramp. Mov Disord 1998; 13: 497–508. [DOI] [PubMed] [Google Scholar]
- 33.Mohammadi B, Kollewe K, Samii A, et al. Changes in resting-state brain networks in writer's cramp. Hum Brain Mapp 2012; 33: 840–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mantel T, Meindl T, Li Y, Jochim A, et al. Network-specific resting-state connectivity changes in the premotor-parietal axis in writer's cramp. Neuroimage Clin 2017; 17: 137–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gallea C, Herath P, Voon V, et al. Loss of inhibition in sensorimotor networks in focal hand dystonia. Neuroimage Clin 2017; 17: 90–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Playford ED, Passingham RE, Marsden CD, et al. Increased activation of frontal areas during arm movement in idiopathic torsion dystonia. Mov Disord 1998; 13: 309–318. [DOI] [PubMed] [Google Scholar]
- 37.Stoeter P, Rodriguez-Raecke R, Vilchez C, et al. Motor activation in patients with pantothenate-kinase associated neurodegeneration: A functional magnetic resonance imaging study. Parkinsonism Relat Disord 2012; 18: 1007–1010. [DOI] [PubMed] [Google Scholar]
- 38.Hallervorden J, Spatz H. Eigenartige Erkrankung im extrapyramidalen System mit besonderer Beteiligung des Globus pallidus und der Substantia nigra. Z Gesamte Neurol Psychiatr 1922; 79: 254–302. [Google Scholar]
- 39.Gupta R, Kumar A, Sharma MC, et al. Autopsy always teach and tell: Neurodegeneration with brain iron accumulation: A case report. Indian J Pathol Microbiol 2007; 50: 792–794. [PubMed] [Google Scholar]
- 40.Lambrechts RA, Schepers H, Yu Y, et al. CoA-dependent activation of mitochondrial acyl carrier protein links four neurodegenerative diseases. EMBO Mol Med 2019; 11: e10488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Leem J, Lee IK. Mechanisms of vascular calcification: The pivotal role of pyruvate dehydrogenase kinase 4. Endocrinol Metab (Seoul ) 2016; 31: 52–61. [DOI] [PMC free article] [PubMed] [Google Scholar]