Abstract
The prevalences of metabolic syndrome (MetS) and vitamin D deficiency are increasing dramatically worldwide. MetS is a major challenge because it can increase the risk of most non-communicable diseases. The beneficial effect of vitamin D on MetS components remains controversial, so the present review focused on the clinical effects of vitamin D supplementation on MetS components. Vitamin D can inhibit the protein expression of nuclear factor beta; improve arterial stiffness; decrease renin-angiotensin-aldosterone system activity, parathyroid hormone levels, inflammatory cytokines, 3-hydroxy-3-methylglutaryl-coenzyme A reductase, and lanosterol 14 α-demethylase enzyme activity; increase the activity of lipoprotein lipase; alter gene expression in C2C12 cells; and improve phospholipid metabolism and mitochondrial oxidation. We tried to elucidate and analyze almost all evidence from randomized controlled trial studies of the efficacy of vitamin D supplementation in patients with MetS. The findings of the present study reported beneficial effects of vitamin D supplementation on mentioned factors. Vitamin D supplementation is recommended in people with vitamin D deficiency even if it has no considerable effect on most MetS factors. However, existing data from interventional studies are insufficient to reach a definitive conclusion about the effect of vitamin D supplementation on MetS components in patients without vitamin D deficiency. Thus, new clinical studies are needed to test the hypothesis that vitamin D supplementation could alleviate MetS components in patients with sufficient intake of vitamin D.
Keywords: Metabolic syndrome, Vitamin D, Obesity, Hypertension
INTRODUCTION
Metabolic syndrome (MetS), also known as syndrome X, visceral adiposity syndrome, or insulin resistance syndrome, includes hyperlipidemia, hypertension, hyperglycemia, insulin resistance, abdominal obesity, and proinflammatory states.1-3 MetS is a major challenge worldwide because it can increase the risk of other diseases, such as a two-fold increase in risk for cardiovascular disease, two- to four-fold for stroke, fivefold or more for type 2 diabetes, and three- to four-fold for myocardial infarction.4,5 According to Table 1, there are several definitions for MetS.3,6,7
Table 1.
| World Health Organization | Insulin resistance or glucose level <6.1 mmol/L, 2-hour OGTT <7.8 mmol/L together with 2 or more of the following factors: HDL-C <35 mg/dL in males, <40 mg/dL in females Triglycerides >150 mg/dL Waist-to-hip ratio >0.9 (males) or >0.85 (females) or BMI >30 kg/m2 Blood pressure >140/90 mmHg |
| National Cholesterol Education Program-Adult Treatment Panel III |
Three or more of the following factors: Blood glucose >100 mg/dL or taking medication for medical care of it HDL-C <40 mg/dL in males, <50 mg/dL in females or taking medication to regulate HDL-C levels Blood triglycerides > 150 mg/dL or taking medication to regulate triglyceride levels Waist circumference >102 cm for males or >88 cm for females Blood pressure >130/85 mmHg or taking medication for hypertension |
| International Diabetes Federation | Waist circumference >94 cm for males or >80 cm for females together with 2 or more of the following factors: Blood glucose >100 mg/dL or diabetes Blood triglycerides >150 mg/dL or taking medication to regulate triglyceride levels SBP >130 mmHg and DBP >85 mmHg or taking medication for hypertension |
MetS, metabolic syndrome; OGTT, oral glucose tolerance test; HDL-C, high-density lipoprotein cholesterol; BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure.
The prevalence of MetS is increasing dramatically across the world.8 According to the International Diabetes Federation (IDF), overall global prevalence of MetS was at about 25% in 2006.9 In 2007, up to 34.7% of the adult population in the Middle East had MetS, based on criteria from the National Cholesterol Education Program-Adult Treatment Panel III.10 In addition, 37.4% of adult population in the Middle East had MetS in 2007 based on the IDF definition.10 The prevalence of MetS increases with age, and the mortality of persons with MetS is higher than that of people without it.4,11
Positive family history, cigarette smoking, higher age, obesity, low socioeconomic status, vitamin D deficiency, consumption of fast food, Mexican-American ethnicity, postmenopausal status, physical inactivity, stress, drinking sweetened beverages, alcohol consumption, Western diet, increasing urbanization, genetic predisposition, low cardiorespiratory fitness, low birth weight, and too much television watching are important risk factors for MetS.12-14
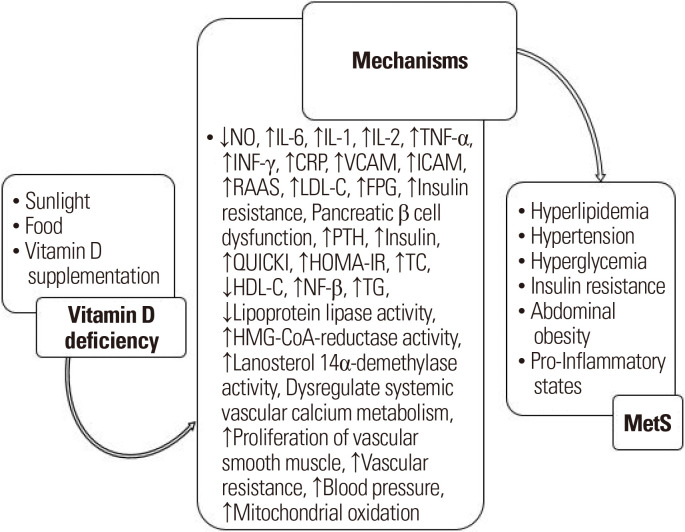
Vitamin D has been called the “sunshine vitamin”.15 The most important constituents of this vitamin in humans, vitamin D3 (cholecalciferol) and vitamin D2 (ergocalciferol), are converted to 25-hydroxyvitamin D in the liver, which is the most important form of vitamin D for determining the level of vitamin D in the bloodstream.16 The prevalence of vitamin D deficiency is also significant in the general population, with reports suggesting that about one billion people worldwide have vitamin D deficiency.17 A low 25-hydroxyvitamin D level has been reported to be associated with obesity, hypertension, diabetes, MetS, and chronic vascular inflammation, even though there has been controversy surrounding these associations.18-21 Several explanations have been suggested for the role of vitamin D in the MetS-related components (Fig. 1).22-30 Vitamin D deficiency affects the components of MetS by affecting various variables such as nitric oxide, interleukin (IL)-6, IL-1 and so on.
Figure. 1.
Overview of the role of vitamin D deficiency in metabolic syndrome (MetS) development. ↓, significant decrease; ↑, significant increase; NO, nitric oxide; IL, interleukin; TNF-α, tumor necrosis factor alpha; INF-γ, interferon gamma; CRP, C-reactive protein; VCAM, vascular cell adhesion molecule; ICAM, intercellular adhesion molecule; RAAS, renin-angiotensin-aldosterone system; LDL-C, lowdensity lipoprotein cholesterol; FPG, fasting plasma glucose; PTH, parathyroid hormone; QUICKI, quantitative insulin sensitivity check index; HOMA-IR, homeostasis model assessment of insulin resistance; TC, total cholesterol; HDL-C, high-density lipoprotein cholesterol; NF-β, nuclear factor beta; TG, triglyceride; HMG-CoA, 3-hydroxy-3-methylglutaryl-coenzyme A.
As a result, vitamin D deficiencies and MetS often appear to be intertwined. Therefore, it is recommended that vitamin D intake be used in the treatment of MetS, but the effect of vitamin D supplementation on MetS in patients with or without vitamin D deficiency is not completely clear. Furthermore, all the benefits of vitamin D supplementation in improving MetS factors are not an acceptable justification for supplementation among patients with sufficient vitamin D levels. Therefore, there is a need for studies with appropriate design to find differences between the effects of vitamin D on people with different levels of vitamin D. To address this need, we sought to determine the effect of vitamin D deficiency on each of the MetS components and its related mechanisms, based on randomized clinical trial studies in people with MetS with or without vitamin D deficiency (Table 2).
Table 2.
All randomized controlled trial studies about the effect of different doses of vitamin D supplementation in patients with MetS
| Author (year) | Study population | Duration (mo) | Outcome measurement | Study design | Vitamin D level at baseline | Vitamin D level at the end of study | Result |
|---|---|---|---|---|---|---|---|
| Salekzamani et al. (2016)27 | 80 | 4 | FPG, HOMA-IR, QUICKI, LDL-C, HDL-C, TG, TC, dietary intake, sun exposure, weight, BMI, FP, WC, HC, SBP, DBP, WHtR | Intervention group: 50,000 IU/wk vitamin D (n=40) | 16.45±15.50 nmol/L | 78.38±21.71 nmol/L | Intervention group: ↓TG |
| Control group: placebo (n=40) | 23.47±21.34 nmol/L | 21.46±17.74 nmol/L | |||||
| Mahmood et al. (2016)24 | Not mentioned | 6 | SBP, DBP, vitamin D, FPG, insulin, HOMA, QUICKI, BMI, WC | Intervention group: oral 25(OH) D3 supplement 60,000 (IU) per week for 8 weeks followed by 60,000 IU monthly for 4 months | 15.4±9.03 ng/mL | 26.1±11.8 ng/mL | Intervention group: ↓WC, ↓BMI Control group: ↑insulin, ↓QUICKI |
| Control group: placebo | - | - | |||||
| Wongwiwatthananukit et al. (2013)29 | 90 | 2 | WC, SBP, DBP, FPI, 25(OH)D, calcium, HDL-C, HOMA-IR, LDL-C, TC, TG, FPG | (1) 40,000 IU/wk vitamin D2 (n=30) | 14.29±3.35 ng/mL | 30.03±6.97 ng/mL | No positive effect of vitamin D2 on metabolic risk factors |
| (2) 20,000 IU/wk vitamin D2 (n=30) | 15.08±3.16 ng/mL | 26.80±6.37 ng/mL | |||||
| (3) Control group: placebo (n=30) | 16.20±2.99 ng/mL | 18.99±6.71 ng/mL | |||||
| Salekzamani et al. (2017)26 | 80 | 4 | IL-6, E-selectin, BMI, FPG, SBP, DBP, weight, FM, FFM, TBW, TG, HsCRP, sun exposure, PA, VCAM-1, cCIMT, calcium | Intervention group: 50,000 IU/week vitamin D (n=40) | 13.70 nmol/L (0.00–25.50) | 80 nmol/L (67.25–90.50) | Intervention group: ↓IL-6, ↑calcium, (↓E-selectin and ↓VCAM-1 but no significant differences between two groups) |
| Control group: placebo (n=40) | 19.97 nmol/L (11.78–31.25) | 17.82 nmol/L (13.07–28.50) | |||||
| Kelishadi et al. (2014)23 | 50 | 3 | BMI, WC, WHtR, 25(OH)D, insulin, FPG, HOMA-IR, TG, MAP, LDL-C, HDL-C, TC, C-MetS | Intervention group: 300,000 IU/wk vitamin D (n=25) | 18.27±2.04 ng/mL | 32.01±2.14 ng/mL | Intervention group: ↓Insulin, ↓TG, ↓HOMA-IR and ↓C-MetS |
| Control group: placebo (n=25) | 17.91±2.27 ng/mL | 19.07±2.01 ng/mL | |||||
| Farag et al. (2018)22 | 180 | 3 | Weight, BMI, WC, FPG, SBP, DBP, TC, TG, LDL-C, HDL-C, vitamin D, PA, vitamin C | (1) Vitamin C group: 500 mg/day vitamin C (n=30) | Group 1: - | - | Group 1 and 2 (more influence compared to vitamin D): ↓TG, and ↑HDL-C Group 3 and 4 (more influence compared to vitamin C): ↓FPG, ↓TC, ↓LDL-C and ↓blood pressure |
| (2) Vitamin C plus PA group (n=30) | Group 2: - | - | |||||
| (3) Vitamin D group: 2,000 IU/day vitamin D (n=30) | Group 3: 10.8± 2.8 ng/mL | 23.2± 4.9 ng/mL | |||||
| (4) Vitamin D plus PA group (n=30) | Group 4 : 10.4± 3.2 ng/mL | 29±5.5 ng/mL | |||||
| (5) Placebo plus PA group (n=30) | Group 5:12.2±4 ng/mL | 12.6±4 ng/mL | |||||
| (6) Control group: placebo (n=30) | Group 6:11±4 ng/mL | 18.9±4.5 ng/mL | |||||
| Sansanayudh et al. (2014)28 | 90 | 2 | 25(OH)D, calcium, QTc | (1) Control group: 2 capsules of placebo/wk (n=30) | 16.2±3.0 ng/mL | 19.0 ng/mL | Group 2 and 3: ↑25(OH)D |
| (2) Ergocalciferol 20,000 IU/wk (n=30) | 15.1±3.2 ng/mL | 26.8 ng/mL | |||||
| (3) Ergocalciferol 40,000 IU/wk (n=30) | 14.3±3.4 ng/mL | 30.0 ng/mL | |||||
| Yin et al. (2016)30 | 126 | 12 | BMI, WC, FPG, SBP, DBP, LDL-C, HDL-C, PTH, 25(OH)D, HOMA-IR, QUICKI, FPI | Intervention group: 700 IU/day vitamin D (n=63) | 14.6±2.18 ng/mL | 33.1±4.37 ng/mL | Intervention group: ↓PTH |
| Control group: placebo (n=63) | 14.2±2.55 ng/mL | 14.6±2.80 ng/mL | |||||
| Makariou et al. (2017)25 | 50 | 3 | Weight, BMI, SBP, DBP, WC, TC, TG, LDL-C, HDL-C, Apo A1, Apo B, FPG, FPI, HOMA index*, HbA1c, PTH, 25(OH)D | Intervention group: dietary instructions (–500 kcal/day) plus 2,000 IU/day vitamin D (n=25) | 16.0 ng/mL (3.0–35.0) | 30.6 ng/mL (8.4–67.0) | Intervention group: ↓SBP |
| Control group: dietary instructions (–500 kcal/day) (n=25) | 10.0 ng/mL (4.0–39.6) | 13.0 ng/mL (3.5–37.0) |
*HOMA index= fasting insulin× fasting glucose/405.
MetS, metabolic syndrome; FPG, fasting plasma glucose; HOMA-IR, homeostasis model assessment of insulin resistance; QUICKI, quantitative insulin sensitivity check index; LDL-C, low-density lipoprotein cholesterol; HDL-C, high-density lipoprotein cholesterol; TG, triglyceride; TC, total cholesterol; BMI, body mass index; FP, body fat percent; WC, waist circumference; HC, hip circumference; SBP, systolic blood pressure; DBP, diastolic blood pressure; WHtR, waist-to-height ratio; ↓, significant decrease; ↑, significant increase; HOMA, homeostasis model assessment; 25(OH)D, 25-hydroxyvitamin D; FPI, fasting plasma insulin; IL, interleukin; FM, fat mass; FFM, fat-free mass; TBW, total body water; HsCRP, high-sensitivity C-reactive protein; PA, physical activity; VCAM-1, vascular cell adhesion molecule-1; cCIMT, common carotid intima-media thickness; MAP, mean arterial blood pressure; CMetS, continuous metabolic syndrome; QTc, corrected QT interval; PTH, parathyroid hormone; Apo, apolipoprotein; HbA1c, glycosylated hemoglobin.
VITAMIN D AND ABDOMINAL OBESITY
Vitamin D might affect obesity-associated inflammation and adiposity. Obesity has recently emerged as a public health issue of pandemic proportions.31,32 Abdominal obesity is the most frequently observed component of MetS.33,34 Based on several studies, vitamin D levels are lower in most obese and overweight people, compared to people of normal weight who have less body fat, and obesity can increase the risk of vitamin D deficiency.35 Fat accumulation via increasing oxidative stress36 can increase the expression of nicotinamide adenine dinucleotide phosphate oxidase, decrease the expression of anti-oxidative enzymes, impair glucose uptake in muscle and adipose tissue, decrease insulin secretion from pancreatic β cells, and affect oxidative phosphorylation, glyceraldehyde auto-oxidation, and protein kinase C activation.37-40
Macrophages within the adipose tissue secrete adipocytokines, such as plasminogen activator inhibitor-1 (PAI-1), tumor necrosis factor alpha (TNF-α), resistin, leptin, and adiponectin. PAI-1 leads to thrombosis and insulin resistance.37,41,42 TNF-α can phosphorylate and inactivate insulin receptors in the adipose tissue and smooth muscle cells, induce lipolysis, and inhibit adiponectin release,43 leading to insulin resistance and atherosclerosis. Several studies have demonstrated that levels of IL-6 and C-reactive protein (CRP) are high with MetS, and increased production of IL-6 and CRP results in increases in obesity and insulin resistance.44 Vitamin D through binding to its receptors in monocytes can reduce inflammatory cytokines such as CRP and lead to a reduction in systemic inflammation.45 In addition, receptors of vitamin D are located in the nucleus of macrophages, which produce cytokines such as TNF-α. Vitamin D inhibits the protein expression of nuclear factor beta (NF-β), which has an important effect on the expression of TNF-α.35
In several studies, the effect of different doses of vitamin D on body composition has been investigated in patients with MetS.22-25,27,29,30 In only one of these studies, conducted by Mahmood et al.,24 vitamin D supplementation of 50,000 IU/wk decreased waist circumference (WC) and body mass index (BMI) in adults with MetS after 6 months. In this study, the group that received vitamin D supplementation experienced a significant decrease in WC and BMI (P=0.001) at the end of the study.
In another study, 63 overweight or obese women were assigned to two groups, where intervention group received 600 mg calcium and 200 IU/day vitamin D and the placebo group received a placebo along with a 700 kcal/day energy-deficit diet. This study by Major et al.46 showed that supplementation with calcium and vitamin D for 15 weeks affected WC.
In the study by Makariou et al.,25 weight was reduced by about 1–2 kg after 3 months in both the intervention and control groups, which received dietary instructions (–500 kcal/day from usual intake) with or without 2,000 IU/day vitamin D, respectively. It seems this weight loss is due to the reduction in energy intake in both groups and not the vitamin D supplementation. In another study comparing groups with or without vitamin D deficiency (<20 ng/mL),vitamin D deficiency was associated with higher systolic 24-hour ambulatory blood pressure monitoring (ABPM) (P=0.01), daytime ABPM (P=0.02), and body fat mass.47 In order to reach a definite conclusion, studies with stronger methods are needed to evaluate the effects of vitamin D supplementation on body composition in patients with MetS.
VITAMIN D AND HYPERTENSION
Studies have shown that MetS is more frequently observed in hypertensive populations.48 Increases in plasma volume and cardiac output can lead to peripheral vascular resistance as an important factor in the pathogenesis of hypertension. In turn, increased vascular resistance can result in severe insulin resistance and hyperinsulinemia, leading to a defective cycle. This condition can occur in obesity-related hypertension.49 Several studies have demonstrated that insufficient levels of vitamin D can increase the risk of hypertension and obesity.35
Obesity-related MetS is an inflammatory state.50 Several inflammatory factors have been associated with obesity and other complications such as hypertension in obesity-associated MetS.51 It should be noted that various epidemiological studies have shown a positive association between IL-6 and blood pressure, atherosclerosis, and cardiovascular disease.12
Oxidative stress caused by adiposity can affect vascular wall cells directly and cause hypertension and atherosclerosis as MetS outcomes.37 The relationship between insulin resistance and hypertension is clear.52 Insulin has two roles in hypertension, as a vasodilator and as a factor in sodium reabsorption in the kidney. The setting of insulin resistance destroys the vasodilator role but cannot affect the sodium reabsorption.53
In some studies, the effect of vitamin D on the improvement of endothelial function has been shown.54,55 Vitamin D can participate in the pathogenesis of hypertension through several mechanisms, such as reducing the production of proinflammatory cytokines, decreasing renin-angiotensin-aldosterone system activity, and lowering parathyroid hormone levels.56
In a meta-analysis by Wu et al.,57 which included four intervention studies, vitamin D significantly reduced systolic blood pressure (SBP) by about 2.44 mmHg, but it had no effect on diastolic blood pressure (DBP). However, other meta-analyses indicated that vitamin D supplementation does not affect either SBP or DBP.57 In another meta-analysis in 2018 by Shu and Huang58 on subjects with vitamin D deficiency, vitamin D supplementation resulted in a significant decrease in SBP but not in DBP.
In one randomized controlled trial by Makariou et al.25 in 25 patients with MetS, a hypocaloric diet (–500 kcal/day) plus 2,000 IU/day vitamin D supplements significantly changed SBP. In this study, SBP decreased about 3.7% in the intervention group (P=0.05), whereas it was reduced about 1.5% in the placebo group. In this study, baseline vitamin D levels were 16.0 ng/mL (3.0–35.0 ng/mL) and 10.0 ng/mL(4.0–39.6 ng/mL) in the intervention and placebo groups, respectively.
In 2018, Farag et al.22 studied 180 patients with MetS who were assigned to six groups. The first group received a 500 mg/day supplement of vitamin C. The second group received vitamin C (500 mg/day) plus 30 min/day physical activity. The third group received 2,000 IU/day supplements of vitamin D. The fourth group received a 2,000 IU/day supplement of vitamin D along with 30 min/day physical activity. The placebo group received a placebo with 30 min/day physical activity. The control group received a placebo without any physical activity. After 3 months intervention, results showed that vitamin D supplementation has more influence on SBP and DBP compared to vitamin C. SBP decreased from 127.1±11.5 mmHg to 121.3±9.2 mmHg (mean change −5.8±7.1 mmHg), and DBP increased from 79.8±9.5 mmHg to 80.1±5.8 mmHg (mean change 1±6.9 mmHg) in the vitamin D group. Meanwhile, in the vitamin D plus physical activity group, SBP decreased from 129±12.7 mmHg to 126.2±8.9 mmHg, and DBP changed from 83.6±9.4 mmHg at baseline to 81.7±5.3 mmHg after 3 months of intervention. The vitamin D levels in the vitamin D and vitamin D plus physical activity groups were 10.8±2.8 ng/mL and 10.4±3.2 ng/mL, respectively, at the beginning of study. By comparing the significant decrease in blood pressure in the mentioned study with other studies that showed no effect of vitamin D supplementation on blood pressure, we noted that vitamin D supplementation may have a more positive effect in people with vitamin D deficiency than in people with insufficient vitamin D level.24,26,27,29,30
VITAMIN D, HYPERGLYCEMIA, AND INSULIN RESISTANCE
Hyperglycemia and insulin resistance are components of MetS.1,2 Patients with MetS are at increased risk for type 2 diabetes.59 Noting a low level of vitamin D in patients with MetS,60 studies have demonstrated that restoration of serum 25-hydroxyvitamin D levels can improve insulin resistance.61 Diabetes is a type of inflammatory disease, and vitamin D has anti-inflammatory effects, so it follows that vitamin D could be useful in improving islet cell function, insulin release, and insulin resistance.62
In 2016, Mahmood et al.24 designed a study to assess the effects of vitamin D supplements on insulin resistance in patients with MetS who have low vitamin D intake. The intervention group consumed 60,000 IU/wk vitamin D during the first 8 weeks, followed by 60,000 IU/mo over the next 4 months, and the control group consumed a placebo for 6 months. At the end of 6 months, mean insulin levels increased significantly from 10.7±4.81 IU/L to 15.4± 14.0 IU/L (P=0.03), but the mean quantitative insulin sensitivity check index (QUICKI) decreased significantly from 0.34±0.03 to 0.32±0.03 in the placebo group (P=0.02). After 6 months, there were no significant changes in the intervention group, except in serum vitamin D, WC, and BMI. Serum vitamin D increased significantly from 15.4±9.03 ng/mL to 26.1±11.8 ng/mL (P<0.0001), WC changed from 95.9±6.66 cm to 94.6±7.47 cm (P=0.001), and BMI decreased from 29.1±4.06 kg/m2 to 28.5±4.16 kg/m2 (P=0.001). In another randomized controlled trial in 2014, Kelishadi et al.23 demonstrated that 12 weeks supplementation with 300,000 IU/wk vitamin D in 25 children with MetS resulted in decreased insulin and homeostasis model assessment of insulin resistance (HOMA-IR) compared with the baseline (P=0.04) and with the placebo group (P=0.02). Insulin changed from 14.27±1.32 μIU/L to 13.71±1.58 μIU/L, and HOMA-IR decreased from 3.21±0.11 to 2.81±0.25 compared with the baseline. In both Mahmood et al.24 and Kelishadi et al.23 studies, patients in the intervention group had insufficient levels of vitamin D (5.4±9.03 and 18.27±2.04 ng/mL, respectively). The controversy in results is probably due to the use of a high dose of vitamin D in the Kelishadi et al.23 study, compared to the other study.
Farag et al.22 aimed to determine the effect of supplementation of vitamin D and vitamin C on MetS patients. In this study, the results showed that 3 months intervention could decrease fasting plasma glucose (FPG) in the vitamin D (n=30) and vitamin D plus physical activity (n=30) groups, and vitamin D had more of an effect on FPG than did vitamin C. FPG decreased from 108±17.1 mg/dL at baseline to 97.8±7.7 mg/dL in the vitamin D group and changed from 106±11.8 mg/dL to 97.7±8.7 mg/dL in the vitamin D plus 30 min/day physical activity group.
The HOMA, QUICKI, HOMA-IR, FPG, and fasting plasma insulin are the parameters that were measured in recent studies, which assessed the effect of vitamin D supplements in patients with MetS.22-25,27,29,30 Most of these studies showed no significant effect of vitamin D on the mentioned parameters; therefore, further clinical trials in patients with prediabetes and diabetes are needed to test the hypothesis that vitamin D deficiency exacerbates these diseases, and vitamin D supplementation would improve it.
VITAMIN D AND HYPERLIPIDEMIA
Patients with MetS have postprandial lipemia (PPL).63 PPL is characterized by the secretion of triglyceride (TG)-rich lipoproteins. When these lipoproteins accumulate, they can affect activation of systemic leukocytes, impair endothelial cell function, promote the development of atherogenic low-density lipoprotein cholesterol (LDL-C) particles, reduce the concentration of high-density lipoprotein cholesterol (HDL-C), and increase the accumulation of atherogenic lipoproteins, which leads to oxidative stress and inflammation.64,65 Several studies have indicated an inverse association between vitamin D and serum lipid profile.66 In a meta-analysis conducted by Wang et al.67 of 10 randomized clinical trials regarding the influence of vitamin D supplementation on plasma lipid profile, vitamin D supplementation increased LDL-C concentration by about 3.23 mg/dL, but did not significantly affect total cholesterol (TC) (increased by 1.52 mg/dL), HDL-C (decreased by 0.14 mg/dL), or TG (decreased by 1.92 mg/dL). In a study by Jorde et al.68 that included 8,018 nonsmoking and 2,087 smoking subjects, direct associations were observed between serum vitamin D and TC, HDL-C, and LDL-C, but the associations between vitamin D and the LDL-C to HDL-C ratio and between vitamin D and TG were negative. The purpose of this study was to determine the association between serum 25-hydroxyvitamin D and serum lipid profile.
Although the effect of vitamin D on lipid metabolism is known, its mechanisms are still unknown.69 Several studies have shown that vitamin D plays direct and indirect roles in altering lipid profile by affecting lipid metabolism.70 Vitamin D can (1) increase the activity of lipoprotein lipase in adipose tissue;71 (2) alter gene expression in C2C12 cells through effects on proliferation, differentiation, and myotube size;72 (3) improve cytokines,73 the immune system,74 arterial stiffness,75 inflammation,76 phospholipid metabolism, and mitochondrial oxidation;77,78 (4) reduce3-hydroxy-3-methylglutaryl-coenzyme A reductase and lanosterol 14α-demethylase enzyme activity;79 and (5) have anti-atherogenic and anti-inflammatory potency.80,81
In the study conducted by Hirschler et al.,82 supplementation of 5,000 IU/wk of vitamin D for 8 weeks in children with vitamin D deficiency decreased the prevalence of low HDL-C in the intervention group from 35.7% to 5.7% after 1-year follow-up (P<0.01). In this study, HDL-C increased from 38±7 mg/dL at baseline to 46± 7 mg/dL after 1year.In another study on children 6–10 years of age, the relationship between vitamin D and lipid profile was shown.83 Sixty children (29 males) used 100,000 IU/mo of vitamin D, and 36 children used 50,000 IU/mo for 2 months. The mean HDL-C increased significantly in the group that received 100,000 IU of vitamin D, from 39.8 mg/dL to 43.9 mg/dL, while HDL-C increased non-significantly from 44.4 mg/dL to 45.1 mg/dL in the group that received 50,000 IU of vitamin D. No significant changes were found in the median values of TG (117 vs. 111 mg/dL) and TG/HDL-C (3.0 vs. 2.7 mg/dL) in the group that received 100,000 IU of vitamin D, but TG (95–111 mg/dL) and TG/HDL-C (2.2–2.4 mg/dL) increased significantly in the group that received 50,000 IU of vitamin D.81 Islam et al.84 showed that in patients who underwent one year of vitamin D supplementation, there were no significant changes in the TC, LDL-C, HDL-C, or in the LDL-C to HDL-C ratio. It should be noted that in this study, 200 healthy subjects aged 16–36 years with hypovitaminosis D were assigned into four groups. The first group received vitamin D supplements (400 IU/day), the second group received vitamin D plus calcium lactate (600 mg), the third group received multiple micronutrients, vitamin D, and calcium lactate, and the last group received a placebo.
Salekzamani et al.27 showed that TG levels in patients with MetS were significantly reduced after consumption of 50,000 IU/wk of vitamin D for 12 weeks. No other changes were observed between the two groups in this study. Before the intervention, TG levels were 269±97 mg/dL and significantly decreased to 242±82 mg/dL at the end of study, but it increased from 185±61 mg/dL to 196±72 mg/dL in the placebo group. Farag et al.22 in 2018 designed a randomized controlled study on 180 patients with MetS. In this study, TC and LDL-C levels decreased significantly in groups that received 2,000 IU/day vitamin D (n=30) or 2,000 IU/day vitamin D plus physical activity (n=30). TC levels decreased from 173.5±60.8 mg/dL to 160.5±33.4 mg/dL and from 194.7±32.2 mg/dL to 81.7± 31.3 mg/dL in the vitamin D and vitamin D plus physical activity groups, respectively. LDL-C levels decreased from 120.7±64.4 mg/dL to 107±36.6 mg/dL and from 149.6±35.8 mg/dL to 138.3± 31.4 mg/dL in the vitamin D and vitamin D plus physical activity groups, respectively. Since vitamin D can affect the lipid profile, it follows that vitamin D could probably improve MetS factors. The patients in this study had vitamin D deficiency, and vitamin D supplements could affect their lipid profiles. However, in most studies which recruited patients with insufficient baseline levels of vitamin D, vitamin D supplements did not show any effect,24-26,29,30 although in only a few studies was a significant effect on the lipid profile seen.23,27
CONCLUSION
Across multiple studies, no consensus has been reached regarding the effect of vitamin D supplementation on the components of MetS. Although these studies did not yield similar results, the findings of the present study reported an inverse relationship between vitamin D supplementation and mentioned factors. MetS and vitamin D deficiency are prevalent today, and given the relatively modest price of vitamin D supplementation and the possible mechanisms by which it is suggested, vitamin D supplementation is recommended in people with vitamin D deficiency, even if it has no considerable effect on most MetS factors. However, existing data from interventional studies are insufficient to reach a definitive conclusion about the effect of vitamin D supplementation on MetS components in patients without vitamin D deficiency. Thus, new clinical studies are needed to test the hypothesis that vitamin D supplementation could alleviate MetS components in patients with MetS who consume sufficient amount of vitamin D.
Footnotes
CONFLICTS OF INTEREST
The authors declare no conflict of interest.
AUTHOR CONTRIBUTIONS
Study concept and design: all authors; acquisition of data: SF; drafting of the manuscript: all authors; critical revision of the manuscript: all authors; and study supervision: MA.
REFERENCES
- 1.Lopes HF, Corrêa-Giannella ML, Consolim-Colombo FM, Egan BM. Visceraladiposity syndrome. Diabetol Metab Syndr. 2016;8:40. doi: 10.1186/s13098-016-0156-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Prasad P, Kochhar A. Interplay of vitamin D and metabolic syndrome: a review. Diabetes Metab Syndr. 2016;10:105–12. doi: 10.1016/j.dsx.2015.02.014. [DOI] [PubMed] [Google Scholar]
- 3.Moazzen H, Alizadeh M. Effects of pomegranate juice on cardiovascular risk factors in patients with metabolic syndrome: a double-blinded, randomized crossover controlled trial. Plant Foods Hum Nutr. 2017;72:126–33. doi: 10.1007/s11130-017-0605-6. [DOI] [PubMed] [Google Scholar]
- 4.Samson SL, Garber AJ. Metabolic syndrome. Endocrinol Metab Clin North Am. 2014;43:1–23. doi: 10.1016/j.ecl.2013.09.009. [DOI] [PubMed] [Google Scholar]
- 5.Kaur J. A comprehensive review on metabolic syndrome. Cardiol Res Pract. 2014;2014:943162. doi: 10.1155/2014/943162. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 6.Amirkalali B, Fakhrzadeh H, Sharifi F, Kelishadi R, Zamani F, Asayesh H, et al. Prevalence of metabolic syndrome and its components in the Iranian adult population: a systematic review and meta-analysis. Iran Red Crescent Med J. 2015;17:e24723. doi: 10.5812/ircmj.24723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Saklayen MG. The global epidemic of the metabolic syndrome. Curr Hypertens Rep. 2018;20:12. doi: 10.1007/s11906-018-0812-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Khayyatzadeh SS, Moohebati M, Mazidi M, Avan A, Tayefi M, Parizadeh SM, et al. Nutrient patterns and their relationship to metabolic syndrome in Iranian adults. Eur J Clin Invest. 2016;46:840–52. doi: 10.1111/eci.12666. [DOI] [PubMed] [Google Scholar]
- 9.Nolan PB, Carrick-Ranson G, Stinear JW, Reading SA, Dalleck LC. Prevalence of metabolic syndrome and metabolic syndrome components in young adults: a pooled analysis. Prev Med Rep. 2017;7:211–5. doi: 10.1016/j.pmedr.2017.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Delavari A, Forouzanfar MH, Alikhani S, Sharifian A, Kelishadi R. First nationwide study of the prevalence of the metabolic syndrome and optimal cutoff points of waist circumference in the Middle East: the national survey of risk factors for noncommunicable diseases of Iran. Diabetes Care. 2009;32:1092–7. doi: 10.2337/dc08-1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gluvic Z, Zaric B, Resanovic I, Obradovic M, Mitrovic A, Radak D, et al. Link between metabolic syndrome and insulin resistance. Curr Vasc Pharmacol. 2017;15:30–9. doi: 10.2174/1570161114666161007164510. [DOI] [PubMed] [Google Scholar]
- 12.McCracken E, Monaghan M, Sreenivasan S. Pathophysiology of the metabolic syndrome. Clin Dermatol. 2018;36:14–20. doi: 10.1016/j.clindermatol.2017.09.004. [DOI] [PubMed] [Google Scholar]
- 13.Fiszman M, Rosemblat G, Ahlers CB, Rindflesch TC. Identifying risk factors for metabolic syndrome in biomedical text. AMIA Annu Symp Proc. 2007;2007:249–53. [PMC free article] [PubMed] [Google Scholar]
- 14.Srimani S, Saha I, Chaudhuri D. Prevalence and association of metabolic syndrome and vitamin D deficiency among postmenopausal women in a rural block of West Bengal, India. PLoS One. 2017;12:e0188331. doi: 10.1371/journal.pone.0188331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nair R, Maseeh A. Vitamin D: the "sunshine" vitamin. J Pharmacol Pharmacother. 2012;3:118–26. doi: 10.4103/0976-500X.95506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang R, Naughton DP. Vitamin D in health and disease: current perspectives. Nutr J. 2010;9:65. doi: 10.1186/1475-2891-9-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yu JR, Lee SA, Lee JG, Seong GM, Ko SJ, Koh G, et al. Serum vitamin d status and its relationship to metabolic parameters in patients with type 2 diabetes mellitus. Chonnam Med J. 2012;48:108–15. doi: 10.4068/cmj.2012.48.2.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vitezova A, Zillikens MC, van Herpt TT, Sijbrands EJ, Hofman A, Uitterlinden AG, et al. Vitamin D status and metabolic syndrome in the elderly: the Rotterdam Study. Eur J Endocrinol. 2015;172:327–35. doi: 10.1530/EJE-14-0580. [DOI] [PubMed] [Google Scholar]
- 19.Amirbaigloo A, Hosseinpanah F, Sarvghadi F, Tohidi M, Eskandary PS, Azizi F. Absence of association between vitamin D deficiency and incident metabolic syndrome: Tehran Lipid and Glucose Study. Metab Syndr Relat Disord. 2013;11:236–42. doi: 10.1089/met.2012.0121. [DOI] [PubMed] [Google Scholar]
- 20.Fung GJ, Steffen LM, Zhou X, Harnack L, Tang W, Lutsey PL, et al. Vitamin D intake is inversely related to risk of developing metabolic syndrome in African American and white men and women over 20 y: the Coronary Artery Risk Development in Young Adults study. Am J Clin Nutr. 2012;96:24–9. doi: 10.3945/ajcn.112.036863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Khan H, Kunutsor S, Franco OH, Chowdhury R. Vitamin D, type 2 diabetes and other metabolic outcomes: a systematic review and meta-analysis of prospective studies. Proc Nutr Soc. 2013;72:89–97. doi: 10.1017/S0029665112002765. [DOI] [PubMed] [Google Scholar]
- 22.Farag HA, Hosseinzadeh-Attar MJ, Muhammad BA, Esmaillzadeh A, Bilbeisi AH. Comparative effects of vitamin D and vitamin C supplementations with and without endurance physical activity on metabolic syndrome patients: a randomized controlled trial. Diabetol Metab Syndr. 2018;10:80. doi: 10.1186/s13098-018-0384-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kelishadi R, Salek S, Salek M, Hashemipour M, Movahedian M. Effects of vitamin D supplementation on insulin resistance and cardiometabolic risk factors in children with metabolic syndrome: a triple-masked controlled trial. J Pediatr (Rio J) 2014;90:28–34. doi: 10.1016/j.jped.2013.06.006. [DOI] [PubMed] [Google Scholar]
- 24.Mahmood SF, Idiculla J, Joshi R, Joshi S, Kulkarni S. Vitamin D supplementation in adults with vitamin D deficiency and its effect on metabolic syndrome: a randomized controlled study. Int J Vitam Nutr Res. 2016;86:121–6. doi: 10.1024/0300-9831/a000426. [DOI] [PubMed] [Google Scholar]
- 25.Makariou SE, Elisaf M, Challa A, Tentolouris N, Liberopoulos EN. No effect of vitamin D supplementation on cardiovascular risk factors in subjects with metabolic syndrome: a pilot randomised study. Arch Med Sci Atheroscler Dis. 2017;2:e52–60. doi: 10.5114/amsad.2017.70504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Salekzamani S, Bavil AS, Mehralizadeh H, Jafarabadi MA, Ghezel A, Gargari BP. The effects of vitamin D supplementation on proatherogenic inflammatory markers and carotid intima media thickness in subjects with metabolic syndrome: a randomized double-blind placebo-controlled clinical trial. Endocrine. 2017;57:51–9. doi: 10.1007/s12020-017-1317-2. [DOI] [PubMed] [Google Scholar]
- 27.Salekzamani S, Mehralizadeh H, Ghezel A, Salekzamani Y, Jafarabadi MA, Bavil AS, et al. Effect of high-dose vitamin D supplementation on cardiometabolic risk factors in subjects with metabolic syndrome: a randomized controlled double-blind clinical trial. J Endocrinol Invest. 2016;39:1303–13. doi: 10.1007/s40618-016-0507-8. [DOI] [PubMed] [Google Scholar]
- 28.Sansanayudh N, Wongwiwatthananukit S, Phetkrajaysang N, Krittiyanunt S. Comparative efficacy and safety of different doses of ergocalciferol supplementation in patients with metabolic syndrome. Int J Clin Pharm. 2014;36:771–8. doi: 10.1007/s11096-014-9958-1. [DOI] [PubMed] [Google Scholar]
- 29.Wongwiwatthananukit S, Sansanayudh N, Phetkrajaysang N, Krittiyanunt S. Effects of vitamin D(2) supplementation on insulin sensitivity and metabolic parameters in metabolic syndrome patients. J Endocrinol Invest. 2013;36:558–63. doi: 10.3275/8817. [DOI] [PubMed] [Google Scholar]
- 30.Yin X, Yan L, Lu Y, Jiang Q, Pu Y, Sun Q. Correction of hypovitaminosis D does not improve the metabolic syndrome risk profile in a Chinese population: a randomized controlled trial for 1 year. Asia Pac J Clin Nutr. 2016;25:71–7. doi: 10.6133/apjcn.2016.25.1.06. [DOI] [PubMed] [Google Scholar]
- 31.Monsey MS, Gerhard DM. Obesity: introduction. Yale J Biol Med. 2014;87:97–8. [PMC free article] [PubMed] [Google Scholar]
- 32.Alizadeh M, Daneghian S, Ghaffari A, Ostadrahimi A, Safaeiyan A, Estakhri R, et al. The effect of hypocaloric diet enriched in legumes with or without L-arginine and selenium on anthropometric measures in central obese women. J Res Med Sci. 2010;15:331–43. [PMC free article] [PubMed] [Google Scholar]
- 33.Alizadeh M, Gharaaghaji R, Gargari BP. The effects of legumes on metabolic features, insulin resistance and hepatic function tests in women with central obesity: a randomized controlled trial. Int J Prev Med. 2014;5:710–20. [PMC free article] [PubMed] [Google Scholar]
- 34.Mozaffarian D. Dietary and policy priorities for cardiovascular disease, diabetes, and obesity: a comprehensive review. Circulation. 2016;133:187–225. doi: 10.1161/CIRCULATIONAHA.115.018585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Khosravi ZS, Kafeshani M, Tavasoli P, Zadeh AH, Entezari MH. Effect of vitamin D supplementation on weight loss, glycemic indices, and lipid profile in obese and overweight women: a clinical trial study. Int J Prev Med. 2018;9:63. doi: 10.4103/ijpvm.IJPVM_329_15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Marseglia L, Manti S, D'Angelo G, Nicotera A, Parisi E, Di Rosa G, et al. Oxidative stress in obesity: a critical component in human diseases. Int J Mol Sci. 2014;16:378–400. doi: 10.3390/ijms16010378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Furukawa S, Fujita T, Shimabukuro M, Iwaki M, Yamada Y, Nakajima Y, et al. Increased oxidative stress in obesity and its impact on metabolic syndrome. J Clin Invest. 2004;114:1752–61. doi: 10.1172/JCI21625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Matsuoka T, Kajimoto Y, Watada H, Kaneto H, Kishimoto M, Umayahara Y, et al. Glycation-dependent, reactive oxygen species-mediated suppression of the insulin gene promoter activity in HIT cells. J Clin Invest. 1997;99:144–50. doi: 10.1172/JCI119126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rudich A, Tirosh A, Potashnik R, Hemi R, Kanety H, Bashan N. Prolonged oxidative stress impairs insulin-induced GLUT4 translocation in 3T3-L1adipocytes. Diabetes. 1998;47:1562–9. doi: 10.2337/diabetes.47.10.1562. [DOI] [PubMed] [Google Scholar]
- 40.Manna P, Jain SK. Obesity, oxidative stress, adipose tissue dysfunction, and the associated health risks: causes and therapeutic strategies. Metab Syndr Relat Disord. 2015;13:423–44. doi: 10.1089/met.2015.0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yamauchi T, Kamon J, Waki H, Terauchi Y, Kubota N, Hara K, et al. The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nat Med. 2001;7:941–6. doi: 10.1038/90984. [DOI] [PubMed] [Google Scholar]
- 42.Okamoto Y, Kihara S, Ouchi N, Nishida M, Arita Y, Kumada M, et al. Adiponectin reduces atherosclerosis in apolipoprotein E-deficient mice. Circulation. 2002;106:2767–70. doi: 10.1161/01.CIR.0000042707.50032.19. [DOI] [PubMed] [Google Scholar]
- 43.Rochlani Y, Pothineni NV, Kovelamudi S, Mehta JL. Metabolic syndrome: pathophysiology, management, and modulation by natural compounds. Ther Adv Cardiovasc Dis. 2017;11:215–25. doi: 10.1177/1753944717711379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bastard JP, Jardel C, Bruckert E, Blondy P, Capeau J, Laville M, et al. Elevated levels of interleukin 6 are reduced in serum and subcutaneous adipose tissue of obese women after weight loss. J Clin Endocrinol Metab. 2000;85:3338–42. doi: 10.1210/jcem.85.9.6839. [DOI] [PubMed] [Google Scholar]
- 45.Foroughi M, Maghsoudi Z, Ghiasvand R, Iraj B, Askari G. Effect of vitamin D supplementation on C-reactive protein in patients with nonalcoholic fatty liver. Int J Prev Med. 2014;5:969–75. [PMC free article] [PubMed] [Google Scholar]
- 46.Major GC, Alarie F, Doré J, Phouttama S, Tremblay A. Supplementation with calcium + vitamin D enhances the beneficial effect of weight loss on plasma lipid and lipoprotein concentrations. Am J Clin Nutr. 2007;85:54–9. doi: 10.1093/ajcn/85.1.54. [DOI] [PubMed] [Google Scholar]
- 47.Moreira JS, de Paula TP, Sperb LF, Miller ME, Azevedo MJ, Viana LV. Association of plasma vitamin D status with lifestyle patterns and ambulatory blood pressure monitoring parameters in patients with type 2 diabetes and hypertension. Diabetes Res Clin Pract. 2018;139:139–46. doi: 10.1016/j.diabres.2018.02.038. [DOI] [PubMed] [Google Scholar]
- 48.Pierdomenico SD, Cuccurullo F. Ambulatory blood pressure monitoring in type 2 diabetes and metabolic syndrome: a review. Blood Press Monit. 2010;15:1–7. doi: 10.1097/MBP.0b013e3283360ed1. [DOI] [PubMed] [Google Scholar]
- 49.Cabandugama PK, Gardner MJ, Sowers JR. The renin angiotensin aldosterone system in obesity and hypertension: roles in the cardiorenal metabolic syndrome. Med Clin North Am. 2017;101:129–37. doi: 10.1016/j.mcna.2016.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.de Heredia FP, Gómez-Martínez S, Marcos A. Obesity, inflammation and the immune system. Proc Nutr Soc. 2012;71:332–8. doi: 10.1017/S0029665112000092. [DOI] [PubMed] [Google Scholar]
- 51.Cox AJ, West NP, Cripps AW. Obesity, inflammation, and the gut microbiota. Lancet Diabetes Endocrinol. 2015;3:207–15. doi: 10.1016/S2213-8587(14)70134-2. [DOI] [PubMed] [Google Scholar]
- 52.Soleimani M. Insulin resistance and hypertension: new insights. Kidney Int. 2015;87:497–9. doi: 10.1038/ki.2014.392. [DOI] [PubMed] [Google Scholar]
- 53.Horita S, Seki G, Yamada H, Suzuki M, Koike K, Fujita T. Insulin resistance, obesity, hypertension, and renal sodium transport. Int J Hypertens. 2011;2011:391762. doi: 10.4061/2011/391762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sugden JA, Davies JI, Witham MD, Morris AD, Struthers AD. Vitamin D improves endothelial function in patients with type 2 diabetes mellitus and low vitamin D levels. Diabet Med. 2008;25:320–5. doi: 10.1111/j.1464-5491.2007.02360.x. [DOI] [PubMed] [Google Scholar]
- 55.Tarcin O, Yavuz DG, Ozben B, Telli A, Ogunc AV, Yuksel M, et al. Effect of vitamin D deficiency and replacement on endothelial function in asymptomatic subjects. J Clin Endocrinol Metab. 2009;94:4023–30. doi: 10.1210/jc.2008-1212. [DOI] [PubMed] [Google Scholar]
- 56.Beveridge LA, Struthers AD, Khan F, Jorde R, Scragg R, Macdonald HM, et al. Effect of vitamin D supplementation on blood pressure: a systematic review and meta-analysis incorporating individual patient data. JAMA Intern Med. 2015;175:745–54. doi: 10.1001/jamainternmed.2015.0237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wu SH, Ho SC, Zhong L. Effects of vitamin D supplementation on blood pressure. South Med J. 2010;103:729–37. doi: 10.1097/SMJ.0b013e3181e6d389. [DOI] [PubMed] [Google Scholar]
- 58.Shu L, Huang K. Effect of vitamin D supplementation on blood pressure parameters in patients with vitamin D deficiency: a systematic review and meta-analysis. J Am Soc Hypertens. 2018;12:488–96. doi: 10.1016/j.jash.2018.04.009. [DOI] [PubMed] [Google Scholar]
- 59.Nsiah K, Shang VO, Boateng KA, Mensah FO. Prevalence of metabolic syndrome in type 2 diabetes mellitus patients. Int J Appl Basic Med Res. 2015;5:133–8. doi: 10.4103/2229-516X.157170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.von Hurst PR, Stonehouse W, Matthys C, Conlon C, Kruger MC, Coad J. Study protocol: metabolic syndrome, vitamin D and bone status in South Asian women living in Auckland, New Zealand: a randomised, placebo-controlled, double-blind vitamin D intervention. BMC Public Health. 2008;8:267. doi: 10.1186/1471-2458-8-267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Takiishi T, Gysemans C, Bouillon R, Mathieu C. Vitamin D and diabetes. Endocrinol Metab Clin North Am. 2010;39:419–46. doi: 10.1016/j.ecl.2010.02.013. [DOI] [PubMed] [Google Scholar]
- 62.Wimalawansa SJ. Associations of vitamin D with insulin resistance, obesity, type 2 diabetes, and metabolic syndrome. J Steroid Biochem Mol Biol. 2018;175:177–89. doi: 10.1016/j.jsbmb.2016.09.017. [DOI] [PubMed] [Google Scholar]
- 63.Freese EC, Gist NH, Acitelli RM, McConnell WJ, Beck CD, Hausman DB, et al. Acute and chronic effects of sprint interval exercise on postprandial lipemia in women at-risk for the metabolic syndrome. J Appl Physiol (1985) 2015;118:872–9. doi: 10.1152/japplphysiol.00380.2014. [DOI] [PubMed] [Google Scholar]
- 64.Kolovou GD, Anagnostopoulou KK, Pavlidis AN, Salpea KD, Iraklianou SA, Tsarpalis K, et al. Postprandial lipemia in men with metabolic syndrome, hypertensives and healthy subjects. Lipids Health Dis. 2005;4:21. doi: 10.1186/1476-511X-4-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Couillard C, Bergeron N, Bergeron J, Pascot A, Mauriège P, Tremblay A, et al. Metabolic heterogeneity underlying postprandial lipemia among men with low fasting high density lipoprotein cholesterol concentrations. J Clin Endocrinol Metab. 2000;85:4575–82. doi: 10.1210/jc.85.12.4575. [DOI] [PubMed] [Google Scholar]
- 66.Jorde R, Grimnes G. Vitamin D and metabolic health with special reference to the effect of vitamin D on serum lipids. Prog Lipid Res. 2011;50:303–12. doi: 10.1016/j.plipres.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 67.Wang H, Xia N, Yang Y, Peng DQ. Influence of vitamin D supplementation on plasma lipid profiles: a meta-analysis of randomized controlled trials. Lipids Health Dis. 2012;11:42. doi: 10.1186/1476-511X-11-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jorde R, Figenschau Y, Hutchinson M, Emaus N, Grimnes G. High serum25-hydroxyvitamin D concentrations are associated with a favorable serum lipid profile. Eur J Clin Nutr. 2010;64:1457–64. doi: 10.1038/ejcn.2010.176. [DOI] [PubMed] [Google Scholar]
- 69.Fernández-Arroyo S, Hernández-Aguilera A, de Vries MA, Burggraaf B, van der Zwan E, Pouw N, et al. Effect of vitamin D3 on the postprandial lipid profile in obese patients: a non-targeted lipidomics study. Nutrients. 2019;11:1194. doi: 10.3390/nu11051194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hafez M, Musa N, Abdel Atty S, Ibrahem M, Abdel Wahab N. Effect of vitamin D supplementation on lipid profile in vitamin D-deficient children with type 1 diabetes and dyslipidemia. Horm Res Paediatr. 2019;91:311–8. doi: 10.1159/000500829. [DOI] [PubMed] [Google Scholar]
- 71.Silvagno F, Pescarmona G. Spotlight on vitamin D receptor, lipid metabolism and mitochondria: some preliminary emerging issues. Mol Cell Endocrinol. 2017;450:24–31. doi: 10.1016/j.mce.2017.04.013. [DOI] [PubMed] [Google Scholar]
- 72.Girgis CM, Clifton-Bligh RJ, Mokbel N, Cheng K, Gunton JE. Vitamin D signaling regulates proliferation, differentiation, and myotube size in C2C12 skeletal muscle cells. Endocrinology. 2014;155:347–57. doi: 10.1210/en.2013-1205. [DOI] [PubMed] [Google Scholar]
- 73.Zhang Y, Leung DY, Richers BN, Liu Y, Remigio LK, Riches DW, et al. Vitamin D inhibits monocyte/macrophage proinflammatory cytokine production by targeting MAPK phosphatase-1. J Immunol. 2012;188:2127–35. doi: 10.4049/jimmunol.1102412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.El-Fakhri N, McDevitt H, Shaikh MG, Halsey C, Ahmed SF. Vitamin D and its effects on glucose homeostasis, cardiovascular function and immune function. Horm Res Paediatr. 2014;81:363–78. doi: 10.1159/000357731. [DOI] [PubMed] [Google Scholar]
- 75.Klop B, van de Geijn GJ, Birnie E, Njo TL, Janssen HW, Jansen HG, et al. Vitamin D3 mediated effects on postprandial leukocyte activation and arterial stiffness in men and women. Eur J Clin Nutr. 2014;68:635–7. doi: 10.1038/ejcn.2014.29. [DOI] [PubMed] [Google Scholar]
- 76.Querfeld U. Vitamin D and inflammation. Pediatr Nephrol. 2013;28:605–10. doi: 10.1007/s00467-012-2377-4. [DOI] [PubMed] [Google Scholar]
- 77.Boyan BD, Sylvia VL, Dean DD, Pedrozo H, Del Toro F, Nemere I, et al. 1,25-(OH)2D3 modulates growth plate chondrocytes via membrane receptor-mediated protein kinase C by a mechanism that involves changes in phospholipid metabolism and the action of arachidonic acid and PGE2. Steroids. 1999;64:129–36. doi: 10.1016/S0039-128X(98)00099-3. [DOI] [PubMed] [Google Scholar]
- 78.Sinha A, Hollingsworth KG, Ball S, Cheetham T. Improving the vitamin D status of vitamin D deficient adults is associated with improved mitochondrial oxidative function in skeletal muscle. J Clin Endocrinol Metab. 2013;98:E509–13. doi: 10.1210/jc.2012-3592. [DOI] [PubMed] [Google Scholar]
- 79.Gupta AK, Sexton RC, Rudney H. Effect of vitamin D3 derivatives on cholesterol synthesis and HMG-CoA reductase activity in cultured cells. J Lipid Res. 1989;30:379–86. [PubMed] [Google Scholar]
- 80.Riek AE, Oh J, Sprague JE, Timpson A, de las Fuentes L, Bernal-Mizrachi L, et al. Vitamin D suppression of endoplasmic reticulum stress promotes an antiatherogenic monocyte/macrophage phenotype in type 2 diabetic patients. J Biol Chem. 2012;287:38482–94. doi: 10.1074/jbc.M112.386912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Cannell JJ, Grant WB, Holick MF. Vitamin D and inflammation. Dermatoendocrinol. 2015;6:e983401. doi: 10.4161/19381980.2014.983401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hirschler V, Maccallini G, Sanchez MS, Castaño L, Molinari C. Improvement in high-density lipoprotein cholesterol levels in argentine Indian school children after vitamin D supplementation. Horm Res Paediatr. 2013;80:335–42. doi: 10.1159/000355511. [DOI] [PubMed] [Google Scholar]
- 83.Hirschler V, Maccallini G, Tamborenea MI, Gonzalez C, Sanchez M, Molinari C, et al. Improvement in lipid profile after vitamin D supplementation in indigenous argentine school children. Cardiovasc Hematol Agents Med Chem. 2014;12:42–9. [PubMed] [Google Scholar]
- 84.Islam MZ, Shamim AA, Akhtaruzzaman M, Kärkkäinen M, Lamberg-Allardt C. Effect of vitamin D, calcium and multiple micronutrients supplementation on lipid profile in pre-menopausal Bangladeshi garment factory workers with hypovitaminosis D. J Health Popul Nutr. 2014;32:687–95. [PMC free article] [PubMed] [Google Scholar]